Summary
The article shows that skeletal muscle plays a dominant role in the catabolism of branched-chain amino acids (BCAAs; valine, leucine, and isoleucine) and the pathogenesis of their decreased concentrations in liver cirrhosis, increased concentrations in diabetes, and nonspecific alterations in disorders with signs of systemic inflammatory response syndrome (SIRS), such as burn injury and sepsis. The main role of skeletal muscle in BCAA catabolism is due to its mass and high activity of BCAA aminotransferase, which is absent in the liver. Decreased BCAA levels in liver cirrhosis are due to increased use of the BCAA as a donor of amino group to α-ketoglutarate for synthesis of glutamate, which in muscles acts as a substrate for ammonia detoxification to glutamine. Increased BCAA levels in diabetes are due to alterations in glycolysis, citric acid cycle, and fatty acid oxidation. Decreased glycolysis and citric cycle activity impair BCAA transamination to branched-chain keto acids (BCKAs) due to decreased supply of amino group acceptors (α-ketoglutarate, pyruvate, and oxaloacetate); increased fatty acid oxidation inhibits flux of BCKA through BCKA dehydrogenase due to increased supply of NADH and acyl-CoAs. Alterations in BCAA levels in disorders with SIRS are inconsistent due to contradictory effects of SIRS on muscles. Specifically, increased proteolysis and insulin resistance tend to increase BCAA levels, whereas activation of BCKA dehydrogenase and glutamine synthesis tend to decrease BCAA levels. The studies are needed to elucidate the role of alterations in BCAA metabolism and the effects of BCAA supplementation on the outcomes of specific diseases.
Keywords: Ammonia, Insulin, Glutamate, Glutamine, Alanine, Pyruvate, Insulin resistance, Systemic inflammatory response syndrome, Cataplerosis, Ketoglutarate
Introduction
Branched-chain amino acids (BCAAs; valine, leucine, and isoleucine) are nutritionally essential amino acids that serve as substrates for protein synthesis and energy production, perform several signaling functions, mainly via the mammalian target of rapamycin (mTOR) pathway, and act as a nitrogen source for the synthesis of dispensable amino acids, particularly glutamate (GLU), glutamine (GLN), alanine (ALA), and aspartate (ASP). The use of BCAAs as a nutritional supplement, especially to promote anabolic pathways, has been investigated in a wide range of diseases, including cancer, liver cirrhosis, trauma, burn injury, sepsis, and renal failure, for several decades (Holeček 2018, De Bandt and Cynober 2006, Mascarenhas and Mobarhan 2004). However, to date, there are uncertainties regarding the metabolism and pathogenesis of alterations in BCAA levels in these circumstances.
BCAA concentrations decrease uniquely in hyperammonaemic states, such as liver cirrhosis and urea cycle disorders (UCD) (Holecek et al. 2000 and Holecek et al. 2011, Rodney and Boneh 2013, Hayashi et al. 1981, Leweling et al. 1996), and increase markedly in diabetes mellitus – in both type 1 (T1DM, insulin-dependent) and type 2 (T2DM, insulin-independent) (Brosnan et al. 1983, Aftring et al. 1988, Wijekoon et al. 2004, Rodriguez et al. 1997, Borghi et al. 1985, She et al. 2013). In liver cirrhosis, BCAAs are recommended as a nutritional supplement mainly due to the adverse role of decreased BCAA levels in the development of fatigue, muscle wasting, and hepatic encephalopathy (Fischer et al. 1975, Marchesini et al. 2003, Nakaya et al. 2007, Urata et al. 2007). On the other hand, increased BCAA concentrations in diabetes are considered to be a factor contributing to the development of insulin resistance and increasing the risk of a number of complications of diabetes (Newgard 2012, Shah et al. 2011).
In addition to hyperammonaemic states and diabetes, significant alterations in BCAA metabolism occur frequently in other diseases, such as burn, trauma, cancer, and sepsis, whose common feature is the presence of signs of systemic inflammatory response syndrome (SIRS) (Beutler and Cerami 1986, Kaukonen et al. 2015, Rangel-Frausto et al. 1995). It is well documented that in these circumstances, BCAAs are used to a greater extent as an energy substrate (Beutler and Cerami 1986, Yang et al. 1997, Tischler and Fagan 1983, Ryan 1976, Holeček 1996, Holeček et al. 1997). However, the alterations in BCAA concentrations are not consistent, and increased, decreased, and unchanged levels have been reported (Askanazi et al. 1980, Vente et al. 1989, Cynober et al. 1982, Hirose et al. 2014, Mierzchala-Pasierb et al. 2020, Druml et al. 2001, Liu et al. 2016, Su et al. 2015).
There is accumulating evidence that skeletal muscle, the main site of BCAA metabolism (Harper et al. 1984, Suryawan et al. 1998), is a crucial player in the pathogenesis of altered BCAA concentrations in liver cirrhosis, diabetes, and disorders associated with SIRS. In the first part of this article, I will give a brief overview of the role of skeletal muscle in the metabolism of BCAAs. In the second part, I will try to explain its role in the pathogenesis of decreased BCAA concentrations in hyperammonaemic states and increased BCAA concentrations in diabetes, and why alterations in BCAA levels are inconsistent in most other disorders, particularly those with marked signs of SIRS.
BCAA catabolism in skeletal muscle
Skeletal muscle plays a dominant role in BCAA metabolism because it is the largest tissue in the body and has the highest activity of BCAA amino-transferase (BCAAT), the first enzyme of BCAA catabolism, which is almost absent in the liver (Harper et al. 1984, Suryawan et al. 1998). Hence, whereas most of the other amino acids provided by food are metabolized in the liver, most BCAAs are extracted by muscles. After amino acid infusion via the peripheral vein, skeletal muscle is responsible for the extraction of up to 30 % of all amino acids and up to 70 % of infused BCAAs (Gelfand et al. 1986).
The pathways of BCAA metabolism have been described in detail in several reviews (Harper et al. 1984, Harris et al. 2004, Harris et al. 2005, Shimomura et al. 2001, Mattick et al. 2013, Holeček 2018). Here, I will provide only information essential for understanding the role of skeletal muscle in the catabolism of BCAAs (Fig. 1).
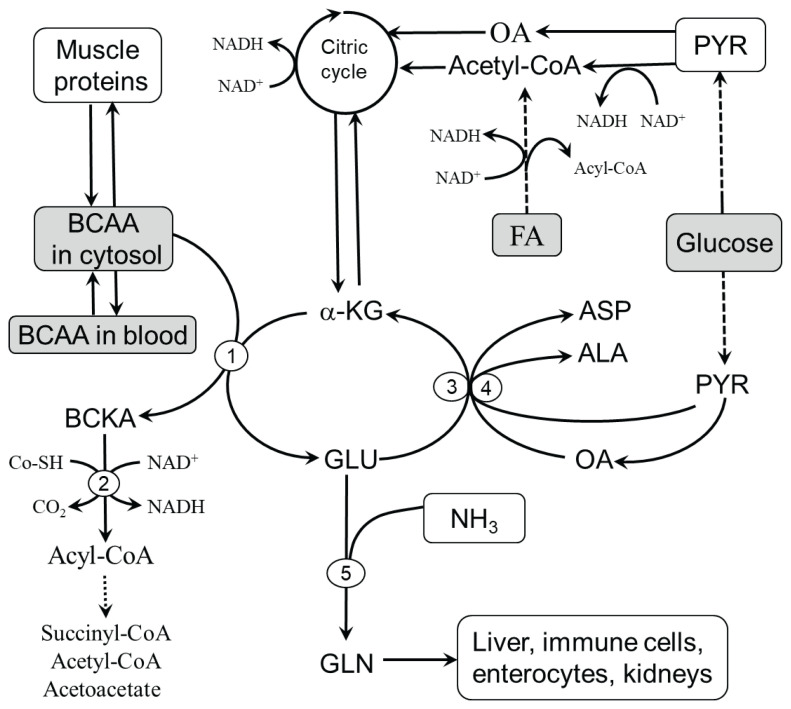
Fig. 1.
Pathways of BCAA catabolism in skeletal muscle. Three aminotransferases, BCAAT, ALT, and AST, catalyze the transport of amino groups between BCAA/BCKA, PYR/ALA, and OA/ASP with the partner pair GLU/α-KG to form BCKA and avoid the drainage of α-KG (cataplerosis) from the citric acid cycle. The supply of amino group acceptors (α-KG, OA, and PYR) is determined by glycolysis, citric acid cycle activity, and GLN synthesis. The BCKAs produced in the BCAAT reaction are released into the circulation or undergo oxidative decarboxylation catalyzed by BCKAD regulated by multiple mechanisms, including NAD+/NADH, CoA-SH/acyl-CoA, and ATP. 1, BCAAT; 2, BCKAD; 3, ALT; 4, AST; 5, GLN synthetase. ALA, alanine; ASP, aspartic acid; FA, fatty acids; GLU, glutamic acid; GLN, glutamine; OA, oxaloacetate; PYR, pyruvate.
BCAA transamination
The first step in BCAA catabolism is reversible transamination by BCAAT in mitochondria to their respective branched-chain keto acids (BCKA), α-ketoisocaproate (ketoleucine), α-keto-β-methylvalerate (ketoisoleucine), and α-ketoisovalerate (ketovaline). The flux of BCAAs through BCAAT is sensitive to the supply of BCAAs and acceptors of amino nitrogen, i.e. α-ketoglutarate (α-KG), pyruvate (PYR), and oxaloacetate (OA) and removal of products of transamination, glutamate (GLU) and BCKA. Hence, BCAA transamination might be influenced by:
BCAA supply – BCAA transamination is activated by food intake and increased breakdown of muscle proteins (Ruderman and Berger 1974, Durschlag and Smith 1985, Holecek et al. 2016).
The activity of the citric acid cycle – determines the supply of α-KG. The citric acid cycle might be activated by increased glycolysis and inhibited by increased levels of NADH (Beatty et al. 1958, Spydevold et al. 1976).
Activity of ALT and AST reactions – ALT and AST enable the regeneration of α-KG from GLU to be available for BCAAT and to attenuate the drain of α-KG (cataplerosis) from the citric acid cycle. Both ALT and AST reactions are reversible and sensitive to the availability of reactants, such as BCAAT reaction.
GLU level – depends on GLU input from the blood, breakdown of muscle proteins, and removal of GLU by GLN synthetase, ALT, and AST.
The disappearance of BCKA – depends on the activity of BCKAD and the release of BCKA into circulation by multiple monocarboxylate transporters, which also control the transport of lactate, pyruvate, and ketone bodies (Bonen et al. 2006).
BCKA decarboxylation
The second step in BCAA catabolism is irreversible decarboxylation of BCKA by the branched-chain keto acid dehydrogenase (BCKAD) complex at the inner mitochondrial membrane to branched-chain acyl-CoA esters. Enzymatic activity levels in humans are high in the liver, kidneys, and brain and low in skeletal muscle, gut, and adipose tissue (Harper et al. 1984, Suryawan et al. 1998).
There are multiple ways to regulate the activity of BCKAD. Long-term regulation occurs through changes in the expression of the enzyme, and short-term regulation by reversible phosphorylation. Phosphory-lation mediated by a specific kinase results in inactivation, and dephosphorylation by a specific phosphatase activates the enzyme. Therefore, ATP depletion activates the enzyme (Harper et al. 1984). Products of the BCKAD reaction, NADH and acyl-CoA derivatives, are competitive inhibitors of the enzyme. The flux of BCKA through BCKAD is activated by exercise, ammonia, glucagon, glucocorticoids, endotoxin, TNF-α, phenylbutyrate, and fibrates (Harper et al. 1984, Holecek 1996, Holeček and Vodeničarovová 2018, Holeček and Vodeničarovová 2020, Pacy et al. 1990, Zimmerman et al. 1989, Shimomura et al. 2006, Keller et al. 2002).
Catabolism of branched-chain acyl-CoA esters
Beyond the BCKAD reaction, BCAA metabo-lism diverges into separate pathways. Catabolism of leucine leads to acetyl-CoA and acetoacetate (leucine is ketogenic), valine is catabolized to succinyl-CoA (valine is glucogenic), and isoleucine is catabolized to acetyl-CoA and succinyl-CoA (isoleucine is both glycogenic and ketogenic). Since leucine degradation gives rise to acetoacetate and acetyl-CoA, unlike valine and isoleucine, this amino acid cannot act as an anaplerotic agent.
Skeletal muscle and decreased BCAA levels in liver cirrhosis and UCD
The decrease in BCAA concentration, as occurs in liver cirrhosis and UCD, is a characteristic feature of hyperammonemia (Holecek et al. 2000, Holecek et al. 2011, Rodney and Boneh 2013, Hayashi et al. 1981, Leweling et al. 1996, Holecek 2020). In liver cirrhosis, hyperammonemia is mainly due to portal systemic shunts, as indicated by increased levels of ammonia following transjugular intrahepatic portal-systemic shunts (He et al. 2020). Although ammonia may increase markedly in acute liver damage, decrease in BCAAs is not observed due to the leakage of amino acids from injured hepatocytes (Rosen et al. 1977, Shi et al. 1984, Holeček et al. 1996 and 1999). In UCD, a marked decrease in BCAAs is observed in acute metabolic decompensation and in subjects treated with phenylbutyrate (Rodney and Boneh 2013, Scaglia et al. 2004, Holecek et al. 2017).
Decreased BCAA levels are believed to play a role in the pathogenesis of hepatic encephalopathy, muscle wasting, and impaired liver regeneration (Fischer et al. 1975, Holecek 1999, Marchesini et al. 2003). Hence, although the pathogenesis of decreased BCAA levels is not entirely clear, BCAAs and BCAA-enriched supplements have been extensively investigated and frequently recommended as therapies for liver disease (Fischer et al. 1975, Marchesini et al. 2003, Nakaya et al. 2007, Urata et al. 2007). In patients with UCD, attempts to use BCAA supplements are rare and have inconclusive results (Adam et al. 2013).
Pathogenesis of decreased BCAA levels
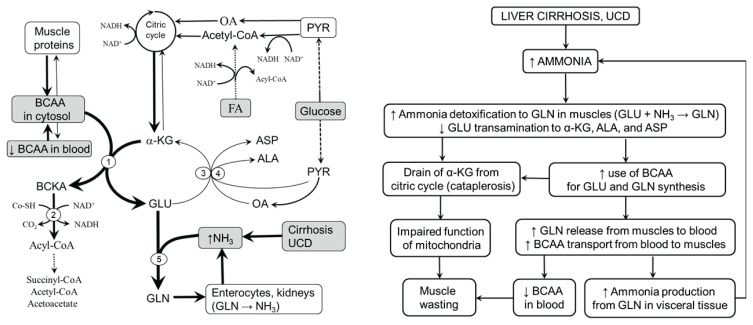
It is supposed that the main role in the pathogenesis of decreased BCAA levels in hyperammonaemic states is increased ammonia detoxification to GLN in muscles (Fig. 2). BCAAs act as the main donor of amino nitrogen to α-KG to form GLU, which is used for ammonia detoxification to GLN in the GLN synthetase reaction (Clemmesen et al. 2000, Girard and Butterworth 1992, Holecek et al. 2011). Hence, in hyperammonemia, increased removal of GLU for ammonia detoxification to GLN enhances the flux of the BCAA through BCAAT and GLN synthetase:
Fig. 2.
Role of skeletal muscle in the pathogenesis of decreased blood plasma BCAA concentrations in hyperammonaemic states. Under hyperammonaemic conditions, such as liver cirrhosis and UCD, ammonia detoxification to GLN in muscles is activated. The needs of GLU for the GLN synthetase reaction are covered by GLU synthesis from BCAA and α-KG. The results are depletion of BCAAs, diversion of GLU from ALT and AST reactions to GLN synthesis, and the drainage of α-KG from the citric acid cycle. Most of GLN produced in muscles is released into the blood via exchange with BCAAs, resulting in a BCAA decrease in blood plasma. Catabolism of GLN in visceral tissues results in increased ammonia production, which, when its detoxification to urea in the liver is insufficient, is detoxified to GLN in reactions consuming BCAA and α-KG in muscles again. 1, BCAAT; 2, BCKAD; 3, ALT; 4, AST; 5, GLN synthetase. ALA, alanine; ASP, aspartic acid; FA, fatty acids; GLU, glutamic acid; GLN, glutamine; OA, oxaloacetate; PYR, pyruvate.
Several studies have shown a stimulatory influence of ammonia on GLN synthesis in muscles (Smith et al. 1984, Clemmesen et al. 2000, Girard and Butterworth 1992, Holeček et al. 2011) and increased GLN concentration in blood plasma in subjects with liver cirrhosis and UCD (Maestri et al. 1992, Holeček and Vodeničarovová 2018). Apparently, a negligible role in ammonia detoxification in muscles is GLU synthesis from α-KG (α-KG + NH3 → GLU) because of extremely low glutamate dehydrogenase expression (Mavrothalassitis et al. 1988).
Enhanced use of GLU for GLN synthesis results in the drain of GLU from ALT and AST reactions and decreased synthesis of ALA and ASP, as demonstrated in hyperammonaemic states by increased GLN and decreased ALA concentrations in blood plasma and muscles (Rodney and Boneh 2013, Holecek et al. 2000, Holeček and Vodeničarovová 2018). A detrimental consequence of the preferential use of GLU for GLN synthesis is the loss of α-KG from the citric acid cycle (cataplerosis), which might impair the activity of the citric acid cycle and subsequently decrease ATP production by mitochondria. Marked decreases in α-KG and ATP were found in the muscles of cirrhotic rats (Holeček and Vodeničarovová 2018). ATP and the total level of adenine nucleotides were markedly reduced in skeletal muscle biopsy specimens of patients with liver cirrhosis (Moller et al. 1984, Davuluri et al. 2016). Hyperammonemia decreased mitochondrial respiration, the NAD+/NADH ratio, the concentrations of citric acid cycle intermediates, and ATP content in C2C12 myotubes (Davuluri et al. 2016). In addition to cataplerosis of α-KG, a role in ATP depletion may be malfunction of mitochondria due to the toxic properties of ammonia and the activated GLN synthetase reaction, which is ATP-consuming (Jayakumar and Norenberg 2016).
Increased formation of BCKA due to increased flux of BCAAs through BCAAT, an increased ratio of NAD+ to NADH, and decreased ATP levels should increase the activity of BCKAD, resulting in irreversible loss of essential BCAAs. Increased oxidation of the BCAA has been demonstrated in rats after ammonia infusion and muscles incubated in medium with high ammonia concentration (Holeček et al. 2000, Holeček et al. 2011). The leucine-oxidized fraction (ratio between leucine oxidation and leucine appearance from protein breakdown) was higher in rats with liver cirrhosis than in controls (Holeček et al. 1996).
The last step towards a decline in BCAAs and a rise in GLN concentration in blood plasma in cirrhosis and UCD is increased influx of BCAAs from extracellular fluid to muscles (attenuates BCAA depletion in the cytosol) by exchange with GLN via the L-transporter system (Meier et al. 2002). Increased expression of the leucine/glutamine exchanger SLC7A5 (LAT1) mRNA has been reported in cirrhotic patients and C2C12 cells during hyperammonemia (Davuluri et al. 2016).
Consequences of increased GLN release from muscles
Ammonia detoxification to GLN in muscles can potentially reduce ammonia concentration in the blood and its detrimental influence on the brain. Unfortunately, this method of ammonia detoxification is only temporary. It has been shown that enhanced GLN concentrations activate glutaminase in visceral tissues, particularly in enterocytes and kidneys, and subsequently increase the release of ammonia into circulation (Souba et al. 1990, Tietze et al. 1992). If ammonia detoxification to urea in the liver is impaired, as occurs in liver injury and UCD, ammonia is returned to muscles for GLN synthesis, resulting in a further decrease in plasma BCAAs. Hence, a vicious cycle, characterized by increased GLN synthesis in muscles and its breakdown in visceral tissues, has been suggested to play a role in the pathogenesis of hyperammonemia and BCAA depletion in liver cirrhosis (Holecek 2014).
Skeletal muscle and increased BCAA levels in diabetes
Skeletal muscle is not only the main site of BCAA catabolism but also the predominant tissue for insulin-mediated glucose disposal (De Fronzo et al. 1985). In muscles, BCAA concentrations are increased markedly in both T1DM and T2DM (Brosnan et al. 1983, Aftring et al. 1988, Wijekoon et al. 2004, Rodriguez et al. 1997, Borghi et al. 1985, She et al. 2013, Holecek et al. 2020). Hence, the role of muscle tissue in the pathogenesis of increased BCAA concentrations in diabetes is obvious.
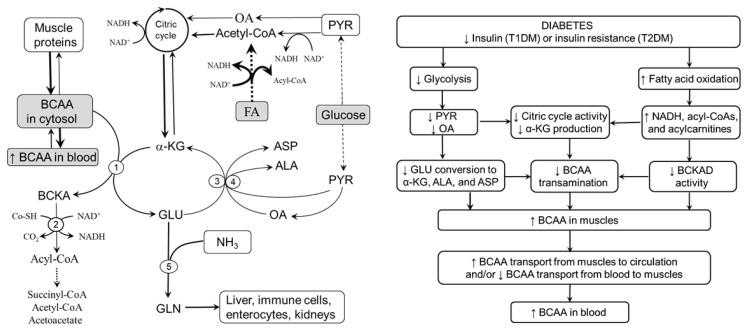
In Figure 3, it is envisaged that metabolic alterations induced by decreased glycolysis and increased fatty acid oxidation, characteristic features of both types of diabetes, might decrease BCAA transamination and BCKA decarboxylation and subsequently increase BCAA levels in muscles. Impaired glycolysis may decrease BCAA transamination by decreasing the supply of PYR and OA, which limits the supply of α-KG for BCAAT via decreased citric acid cycle activity and decreased regeneration of α-KG from GLU in ALT and AST reactions. Increased oxidation of fatty acids may decrease BCAA catabolism in three ways. First, the surplus of NADH produced during fatty acid oxidation inhibits NADH-producing enzymes of the citric acid cycle and subsequently decreases the supply of α-KG for BCAAT. Second, increased NADH/NAD+ inhibits BCKAD. Third, BCKAD should also be inhibited by increased production of acyl-CoA derivatives from increased but, due to impaired entrance of acetyl-CoA into the citric acid cycle, incomplete fatty acid oxidation. Increased BCAA concentrations in muscles can lead to increased BCAA concentrations in blood plasma by their decreased disappearance from the blood after food intake or by increased release from muscles in the postabsorptive state (Felig 1975).
Fig. 3.
Supposed pathogenesis of increased BCAA levels in diabetes. Insulin deficiency and insulin resistance are characterized by impaired glycolysis and increased, but incomplete, fatty acid oxidation. The consequences are decreased BCAA transamination due to decreased supply of all acceptors of amino nitrogen and decreased flux through BCKAD due to the inhibitory influence of enhanced levels of NADH and acyl-CoA. Increased BCAA concentrations in muscles should lead to increased plasma BCAA levels via decreased BCAA disappearance from blood in the postprandial state and/or their increased release from muscles in the fasted state. 1, BCAAT; 2, BCKAD; 3, ALT; 4, AST; 5, GLN synthetase. ALA, alanine; ASP, aspartic acid; FA, fatty acids; GLU, glutamic acid; GLN, glutamine; OA, oxaloacetate; PYR, pyruvate.
In summary, it is hypothesized that decreased flux of BCAAs through BCAAT and BCKAD in muscles due to decreased glycolysis and increased fatty acid oxidation plays a main role in the pathogenesis of increased BCAA levels in diabetes. The role of decreased BCAA transamination is supported by findings of increased BCAA and decreased ALA concentrations in muscles of subjects with diabetes (Brosnan et al. 1983, Rodriguez et al. 1997, Aftring et al. 1988, Jensen-Waern et al. 2009, Borghi et al. 1985). The role of impaired flux through BCKAD indicates increased BCKA concentra-tions in blood plasma and muscles in animals with T1DM induced by alloxan and in obese Zucker rats (Hutson and Harper 1981, She et al. 2013).
A detailed review focused on the differences in the pathogenesis of increased BCAA levels in T1DM and T2DM and early starvation, also characterized by decreased glycolysis, increased fatty acid oxidation and increased plasma BCAA levels (Holeček and Mičuda 2017), has been published recently (Holeček 2020).
Skeletal muscle and BCAA levels in other diseases
Altered, mostly activated, catabolism of BCAA is well documented in serious disorders such as sepsis, burn injury, ischemia, malignancy, and trauma (Beutler and Cerami 1986, Yang et al. 1997, Tischler and Fagan 1983, Ryan 1976). However, unlike hyperammonemia and diabetes, alterations in plasma BCAA concentrations are nonspecific (Vente et al. 1989, Askanazi et al. 1980, Vente et al. 1989, Cynober et al. 1982, Hirose et al. 2014, Mierzchala-Pasierb et al. 2020, Druml et al. 2001, Liu et al. 2016, Su et al. 2015).
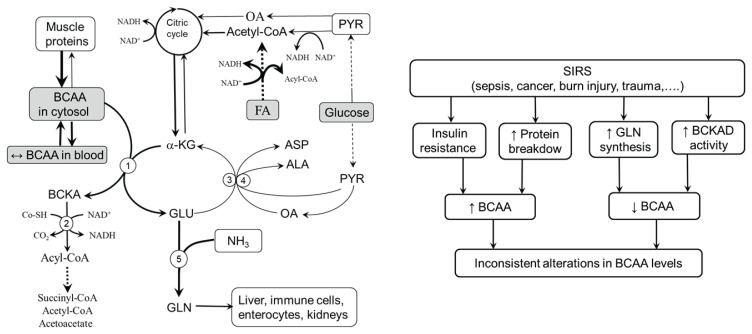
It is the consensus that a wide range of neuro-humoral abnormalities associated with the exaggerated response of the whole body to an infectious or noninfectious insult, referred to as SIRS, have a central role in the pathogenesis of metabolic alterations in many serious diseases. Among the most important are the activation of the sympathetic nervous system and cortisol and cytokine production leading to anorexia, rise in body temperature, insulin resistance, increased use of lipids as an energy source, activated protein breakdown in muscles, and increased protein synthesis in visceral tissues (Beutler and Cerami 1986, Kaukonen et al. 2015, Rangel-Frausto et al. 1995). Several studies have shown that TNF-α, IL-6, glucagon, and cortisol activate BCKAD and that increased BCAA catabolism in muscle tissue is associated with the release of high amounts of GLN to the systemic circulation to be used primarily by enterocytes and lymphatic tissue (Holeček 1996, Nawabi et al. 1990, Pacy et al. 1990, Harper et al. 1984). Enhanced GLN availability can help fight infections and increase the ability to survive in seriously ill patients (Hardy and Hardy 2008).
Figure 4 suggests that alterations in BCAA levels in stress disorders are inconsistent due to the contradictory influence of alterations induced by SIRS in muscles. Specifically, activated proteolysis and insulin resistance tend to increase BCAA concentrations, whereas increased activity of BCKAD and increased synthesis of GLN tend to decrease BCAA concentrations. Roles should also be played by altered food intake, muscle mass, and nutritional status, among other factors.
Fig. 4.
Contradictory alterations in BCAA metabolism in muscles in disorders with pronounced influence of SIRS. Increased proteolysis and insulin resistance tend to increase BCAA levels whereas activation of BCKAD and synthesis of GLN tend to decrease BCAA levels. Increased GLN release might be recognized as beneficial for the patient due to its positive effects on the gut and immune system. 1, BCAAT; 2, BCKAD; 3, ALT; 4, AST; 5, GLN synthetase. ALA, alanine; ASP, aspartic acid; FA, fatty acids; GLU, glutamic acid; GLN, glutamine; OA, oxaloacetate; PYR, pyruvate.
Although increased BCAA oxidation provides a rationale for the use of BCAA-enriched supplements for the therapy of serious stress disorders, such as sepsis, trauma, and cancer, reports on clinical outcomes are controversial (Teasley and Buss 1989, Okada et al. 1988, Vente et al. 1991, Scholten et al. 1990, Sandstedt et al. 1992). A surprising shortcoming of most studies is the lack of information about the influence of illness and therapy on BCAA concentrations. Hence, the differences in study results might be partly due to differences in BCAA levels and their metabolism depending on the type, extent, and stage of illness.
Conclusions
Skeletal muscle plays a central role in BCAA metabolism, and there are several pathways by which muscles can affect BCAA concentrations in blood and tissues. It is suggested that:
Decreased BCAA levels in liver cirrhosis and UCD are due to increased use of the BCAA as a source of amino group for the synthesis of GLU, which is an immediate substrate for ammonia detoxification to GLN by GLN synthetase reaction in muscles. The decrease in BCAA levels is associated with detrimental alterations in mitochondrial function and the catabolism of GLN released from muscles to ammonia in visceral tissues.
Increased BCAA levels in diabetes are due to decreased glycolysis and increased fatty acid oxidation, which decrease BCAA transamination and BCKA decarboxylation via decreased supply of acceptors of amino group (α-KG, PYR, and OA) and inhibitory influence of NADH and acyl-CoA derivatives on BCKAD.
Alterations in BCAA levels in disorders characterized by a significant influence of SIRS, such as burn injury, trauma injury, cancer, and sepsis, are inconsistent due to contradictory effects of SIRS on muscles. Specifically, increased proteolysis and insulin resistance tend to increase BCAA levels, whereas activations of BCKAD and GLN synthesis tend to decrease BCAA levels.
In conclusion, altered profiles of plasma BCAAs reflect alterations in their metabolism, particularly in skeletal muscle, and may be used as diagnostic and prognostic tools. Careful studies are needed to elucidate the role of alterations in BCAA metabolism in the development of specific illnesses and to determine how providing extra amounts of BCAAs affects the outcome of patients in most indications.
Acknowledgements
Supported by the program PROGRES Q40/02 of Charles University, Czech Republic.
Abbreviations
- ALA
alanine
- ALT
alanine aminotransferase
- ASP
aspartic acid
- AST
aspartate aminotransferase
- BCAA
branched-chain amino acids (valine, leucine, and isoleucine)
- BCAAT
branched-chain amino acid aminotransferase
- BCKA
branched-chain keto acids
- BCKAD
branched-chain keto acid dehydrogenase
- GLU
glutamic acid
- GLN
glutamine
- OA
oxaloacetate
- SIRS
systemic inflammatory response syndrome
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- UCD
urea cycle disorders
- α-KG
α-ketoglutarate
Footnotes
This paper is dedicated to the 70th anniversary of the founding of Physiologia Bohemoslovaca (currently Physiological Research)
Conflict of Interest
There is no conflict of interest.
References
- ADAM S, ALMEIDA MF, ASSOUN M, BARUTEAU J, BERNABE SM, BIGOT S, CHAMPION H, DALY A, DASSY M, DAWSON S, ET AL. Dietary management of urea cycle disorders: European practice. Mol Genet Metab. 2013;110:439–445. doi: 10.1016/j.ymgme.2013.09.003. [DOI] [PubMed] [Google Scholar]
- AFTRING RP, MILLER WJ, BUSE MG. Effects of diabetes and starvation on skeletal muscle branched-chain alpha-keto acid dehydrogenase activity. Am J Physiol. 1988;254:E292–E300. doi: 10.1152/ajpendo.1988.254.3.E292. [DOI] [PubMed] [Google Scholar]
- ASKANAZI J, CARPENTIER YA, MICHELSEN CB, ELWYN DH, FURST P, KANTROWITZ LR, GUMP FE, KINNEY JM. Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann Surg. 1980;192:78–85. doi: 10.1097/00000658-198007000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEATTY CH, WEST ES, BOCEK RM. Effect of succinate, fumarate, and oxalacetate on ketone body production by liver slices from non-diabetic and diabetic rats. J Biol Chem. 1958;230:725–733. doi: 10.1016/S0021-9258(18)70495-X. [DOI] [PubMed] [Google Scholar]
- BEUTLER B, CERAMI A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986;320:584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- BONEN A, HEYNEN M, HATTA H. Distribution of monocarboxylate transporters MCT1-MCT8 in rat tissues and human skeletal muscle. Appl Physiol Nutr Metab. 2006;31:31–39. doi: 10.1139/h05-002. [DOI] [PubMed] [Google Scholar]
- BORGHI L, LUGARI R, MONTANARI A, DALL’ARGINE P, ELIA GF, NICOLOTTI V, SIMONI I, PARMEGGIANI A, NOVARINI A, GNUDI A. Plasma and skeletal muscle free amino acids in type I, insulin-treated diabetic subjects. Diabetes. 1985;34:812–815. doi: 10.2337/diabetes.34.8.812. [DOI] [PubMed] [Google Scholar]
- BROSNAN JT, MAN KC, HALL DE, COLBOURNE SA, BROSNAN ME. Interorgan metabolism of amino acids in streptozotocin-diabetic ketoacidotic rat. Am J Physiol. 1983;244:E151–E158. doi: 10.1152/ajpendo.1983.244.2.E151. [DOI] [PubMed] [Google Scholar]
- CLEMMESEN JO, KONDRUP J, OTT P. Splanchnic and leg exchange of amino acids and ammonia in acute liver failure. Gastroenterology. 2000;118:1131–1139. doi: 10.1016/S0016-5085(00)70366-0. [DOI] [PubMed] [Google Scholar]
- CYNOBER L, DINH FN, BLONDE F, SAIZY R, GIBOUDEAU J. Plasma and urinary amino acid pattern in severe burn patients-evolution throughout the healing period. Am J Clin Nutr. 1982;36:416–425. doi: 10.1093/ajcn/36.3.416. [DOI] [PubMed] [Google Scholar]
- DAVULURI G, ALLAWY A, THAPALIYA S, RENNISON JH, SINGH D, KUMAR A, SANDLERS Y, Van WAGONER DR, FLASK CA, HOPPEL C, KASUMOV T, DASARATHY S. Hyperammonaemia-induced skeletal muscle mitochondrial dysfunction results in cataplerosis and oxidative stress. J Physiol. 2016;594:7341–7360. doi: 10.1113/JP272796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVULURI G, KROKOWSKI D, GUAN BJ, KUMAR A, THAPALIYA S, SINGH D, HATZOGLOU M, DASARATHY S. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J Hepatol. 2016;65:929–937. doi: 10.1016/j.jhep.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De BANDT JP, CYNOBER L. Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis. J Nutr. 2006;136:308S–313S. doi: 10.1093/jn/136.1.308S. [DOI] [PubMed] [Google Scholar]
- DEFRONZO RA, GUNNARSSON R, BJÖRKMAN O, OLSSON M, WAHREN J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUML W, HEINZEL G, KLEINBERGER G. Amino acid kinetics in patients with sepsis. Am J Clin Nutr. 2001;73:908–913. doi: 10.1093/ajcn/73.5.908. [DOI] [PubMed] [Google Scholar]
- DURSCHLAG RP, SMITH RJ. Regulation of glutamine production by skeletal muscle cells in culture. Am J Physiol. 1985;248:C442–C448. doi: 10.1152/ajpcell.1985.248.5.C442. [DOI] [PubMed] [Google Scholar]
- FELIG P. Amino acid metabolism in man. Annu Rev Biochem. 1975;44:933–955. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- FISCHER JE, FUNOVICS JM, AGUIRRE A, JAMES JH, KEANE JM, WESDORP RI, YOSHIMURA N, WESTMAN T. The role of plasma amino acids in hepatic encephalopathy. Surgery. 1975;78:276–290. [PubMed] [Google Scholar]
- GELFAND RA, GLICKMAN MG, JACOB R, SHERWIN RS, DEFRONZO RA. Removal of infused amino acids by splanchnic and leg tissues in humans. Am J Physiol. 1986;250:E407–E413. doi: 10.1152/ajpendo.1986.250.4.E407. [DOI] [PubMed] [Google Scholar]
- GIRARD G, BUTTERWORTH RF. Effect of portacaval anastomosis on glutamine synthetase activities in liver, brain, and skeletal muscle. Dig Dis Sci. 1992;37:1121–1126. doi: 10.1007/BF01300297. [DOI] [PubMed] [Google Scholar]
- HARDY G, HARDY IJ. Can glutamine enable the critically ill to cope better with infection? JPEN J Parenter Enteral Nutr. 2008;32:489–491. doi: 10.1177/0148607108319796. [DOI] [PubMed] [Google Scholar]
- HARPER AE, MILLER RH, BLOCK KP. Branched-chain amino acid metabolism. Ann Rev Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- HARRIS RA, JOSHI M, JEOUNG NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun. 2004;313:391–396. doi: 10.1016/j.bbrc.2003.11.007. [DOI] [PubMed] [Google Scholar]
- HARRIS RA, JOSHI M, JEOUNG NH, OBAYASHI M. Overview of the molecular and biochemical basis of branched-chain amino acid catabolism. J Nutr. 2005;135:1527S–1530S. doi: 10.1093/jn/135.6.1527S. [DOI] [PubMed] [Google Scholar]
- HAYASHI M, IKEZAWA K, ONO A, OKABAYASHI S, HAYASHI Y, SHIMIZU S, MIZUNO T, MAEDA K, AKASAKA T, NAITO M, MICHIDA T, UESHIMA D, NADA T, KAWAGUCHI K, NAKAMURA T, KATAYAMA K. Evaluation of the effects of combination therapy with branched-chain amino acid and zinc supplements on nitrogen metabolism in liver cirrhosis. Hepatol Res. 2007;37:615–619. doi: 10.1111/j.1872-034X.2007.00095.x. [DOI] [PubMed] [Google Scholar]
- HE FL, QI RZ, ZHANG YN, ZHANG K, ZHU-GE YZ, WANG M, WANG Y, JIA JD, LIU FQ. Transjugular intrahepatic portosystemic shunt and splenectomy are more effective than endoscopic therapy for recurrent variceal bleeding in patients with idiopathic noncirrhotic portal hypertension. World J Clin Cases. 2020;8:1871–1877. doi: 10.12998/wjcc.v8.i10.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIROSE T, SHIMIZU K, OGURA H, TASAKI O, HAMASAKI T, YAMANO S, OHNISHI M, KUWAGATA Y, SHIMAZU T. Altered balance of the aminogram in patients with sepsis - the relation to mortality. Clin Nutr. 2014;33:179–182. doi: 10.1016/j.clnu.2013.11.017. [DOI] [PubMed] [Google Scholar]
- HOLECEK M. Leucine metabolism in fasted and tumor necrosis factor-treated rats. Clin Nutr. 1996;15:91–93. doi: 10.1016/S0261-5614(96)80028-8. [DOI] [PubMed] [Google Scholar]
- HOLECEK M, TILSER I, SKOPEC F, SPRONGL L. Leucine metabolism in rats with cirrhosis. J Hepatol. 1996;24:209–216. doi: 10.1016/S0168-8278(96)80031-6. [DOI] [PubMed] [Google Scholar]
- HOLEČEK M, MRÁZ J, TILŠER I. Plasma amino acids in four models of experimental liver injury in rats. Amino Acids. 1996;10:229–241. doi: 10.1007/BF00807325. [DOI] [PubMed] [Google Scholar]
- HOLECEK M, SPRONGL L, SKOPEC F, ANDRÝS C, PECKA M. Leucine metabolism in TNF-alpha- and endotoxin-treated rats: contribution of hepatic tissue. Am J Physiol. 1997;273:E1052–E1058. doi: 10.1152/ajpendo.1997.273.6.E1052. [DOI] [PubMed] [Google Scholar]
- HOLECEK M. Nutritional modulation of liver regeneration by carbohydrates, lipids, and amino acids: a review. Nutrition. 1999;15:784–788. doi: 10.1016/S0899-9007(99)00158-6. [DOI] [PubMed] [Google Scholar]
- HOLECEK M, SKALSKÁ H, MRÁZ J. Plasma amino acid levels after carbon tetrachloride induced acute liver damage. A dose-response and time-response study in rats. Amino Acids. 1999;16:1–11. doi: 10.1007/BF01318880. [DOI] [PubMed] [Google Scholar]
- HOLECEK M, SPRONGL L, TICHÝ M. Effect of hyperammonemia on leucine and protein metabolism in rats. Metabolism. 2000;49:1330–1334. doi: 10.1053/meta.2000.9531. [DOI] [PubMed] [Google Scholar]
- HOLECEK M, KANDAR R, SISPERA L, KOVARIK M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids. 2011;40:575–584. doi: 10.1007/s00726-010-0679-z. [DOI] [PubMed] [Google Scholar]
- HOLECEK M. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy-therapeutic perspectives. Metab Brain Dis. 2014;29:9–17. doi: 10.1007/s11011-013-9428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLECEK M, SIMAN P, VODENICAROVOVA M, KANDAR R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr Metab (Lond) 2016;13:12. doi: 10.1186/s12986-016-0072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLEČEK M, MIČUDA S. Amino acid concentrations and protein metabolism of two types of rat skeletal muscle in postprandial state and after brief starvation. Physiol Res. 2017;66:959–967. doi: 10.33549/physiolres.933638. [DOI] [PubMed] [Google Scholar]
- HOLECEK M, VODENICAROVOVA M, SIMAN P. Acute effects of phenylbutyrate on glutamine, branched-chain amino acid and protein metabolism in skeletal muscles of rats. Int J Exp Pathol. 2017;98:127–133. doi: 10.1111/iep.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLEČEK M, VODENIČAROVOVÁ M. Effects of branched-chain amino acids on muscles under hyperammonemic conditions. J Physiol Biochem. 2018;74:523–530. doi: 10.1007/s13105-018-0646-9. [DOI] [PubMed] [Google Scholar]
- HOLEČEK M, VODENIČAROVOVÁ M. Muscle wasting and branched-chain amino acid, alpha-ketoglutarate, and ATP depletion in a rat model of liver cirrhosis. Int J Exp Pathol. 2018;99:274–281. doi: 10.1111/iep.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLEČEK M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond) 2018;15:33. doi: 10.1186/s12986-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLEČEK M. Influence of histidine administration on ammonia and amino acid metabolism: A review. Physiol Res. 2020;69:555–564. doi: 10.33549/physiolres.934449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLEČEK M, VODENIČAROVOVÁ M. Effects of low and high doses of fenofibrate on protein, amino acid, and energy metabolism in rat. Int J Exp Pathol. 2020;101:171–182. doi: 10.1111/iep.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLEČEK M, VODENIČAROVOVÁ M, FINGROVÁ R. Dual effects of beta-hydroxy-beta-methylbutyrate (HMB) on amino acid, energy, and protein metabolism in the liver and muscles of rats with streptozotocin-induced type 1 diabetes. Biomolecules. 2020;10:E1475. doi: 10.3390/biom10111475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLEČEK M. Why are branched-chain amino acids increased in starvation and diabetes? Nutrients. 2020;12:3087. doi: 10.3390/nu12103087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTSON SM, HARPER AE. Blood and tissue branched-chain amino and alpha-keto acid concentrations: effect of diet, starvation, and disease. Am J Clin Nutr. 1981;34:173–183. doi: 10.1093/ajcn/34.2.173. [DOI] [PubMed] [Google Scholar]
- JAYAKUMAR AR, NORENBERG MD. Glutamine synthetase: Role in neurological disorders. Adv Neurobiol. 2016;13:327–350. doi: 10.1007/978-3-319-45096-4_13. [DOI] [PubMed] [Google Scholar]
- JENSEN-WAERN M, ANDERSSON M, KRUSE R, NILSSON B, LARSSON R, KORSGREN O, ESSÉN-GUSTAVSSON B. Effects of streptozotocin-induced diabetes in domestic pigs with focus on the amino acid metabolism. Lab Anim. 2009;43:249–254. doi: 10.1258/la.2008.008069. [DOI] [PubMed] [Google Scholar]
- KAUKONEN KM, BAILEY M, PILCHER D, COOPER DJ, BELLOMO R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- KELLER U, TURKALJ I, LAAGER R, BLOESCH D, BILZ S. Effects of medium- and long-chain fatty acids on whole body leucine and glucose kinetics in man. Metabolism. 2002;51:754–760. doi: 10.1053/meta.2002.32806. [DOI] [PubMed] [Google Scholar]
- LEWELING H, BREITKREUTZ R, BEHNE F, STAEDT U, STRIEBEL JP, HOLM E. Hyperammonemia-induced depletion of glutamate and branched-chain amino acids in muscle and plasma. J Hepatol. 1996;25:756–762. doi: 10.1016/S0168-8278(96)80249-2. [DOI] [PubMed] [Google Scholar]
- LIU Z, YIN P, AMATHIEU R, SAVARIN P, XU G. Application of LC-MS-based metabolomics method in differentiating septic survivors from non-survivors. Anal Bioanal Chem. 2016;408:7641–7649. doi: 10.1007/s00216-016-9845-9. [DOI] [PubMed] [Google Scholar]
- MAESTRI NE, McGOWAN KD, BRUSILOW SW. Plasma glutamine concentration: a guide in the management of urea cycle disorders. J Pediatr. 1992;121:259–261. doi: 10.1016/S0022-3476(05)81200-4. [DOI] [PubMed] [Google Scholar]
- MARCHESINI G, BIANCHI G, MERLI M, AMODIO P, PANELLA C, LOGUERCIO C, ROSSI FANELLI F, ABBIATI R ITALIAN BCAA STUDY GROUP. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/S0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- MASCARENHAS R, MOBARHAN S. New support for branched-chain amino acid supplementation in advanced hepatic failure. Nutr Rev. 2004;62:33–38. doi: 10.1301/nr.2004.jan.33-38. [DOI] [PubMed] [Google Scholar]
- MATTICK JSA, KAMISOGLU K, IERAPETRITOU MG, ANDROULAKIS IP, BERTHIAUME F. Branched-chain amino acid supplementation: impact on signaling and relevance to critical illness. Wiley Interdiscip Rev Syst Biol Med. 2013;5:449–460. doi: 10.1002/wsbm.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAVROTHALASSITIS G, TZIMAGIORGIS G, MITSIALIS A, ZANNIS V, PLAITAKIS A, PAPAMATHEAKIS J, MOSCHONAS N. Isolation and characterization of cDNA clones encoding human liver glutamate dehydrogenase: evidence for a small gene family. Proc Natl Acad Sci U S A. 1988;85:3494–3498. doi: 10.1073/pnas.85.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIER C, RISTIC Z, KLAUSER S, VERREY F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002;21:580–589. doi: 10.1093/emboj/21.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIERZCHALA-PASIERB M, LIPINSKA-GEDIGA M, FLESZAR MG, LESNIK P, PLACZKOWSKA S, SEREK P, WISNIEWSKI J, GAMIAN A, KRZYSTEK-KORPACKA M. Altered profiles of serum amino acids in patients with sepsis and septic shock - Preliminary findings. Arch Biochem Biophys. 2020;691:108508. doi: 10.1016/j.abb.2020.108508. [DOI] [PubMed] [Google Scholar]
- MÖLLER P, BERGSTRÖM J, FÜRST P, HELLSTRÖM K. Muscle biopsy studies in patients with moderate liver cirrhosis with special reference to energy-rich phosphagens and electrolytes. Scand J Gastroenterol. 1984;19:267–272. doi: 10.1080/00365521.1984.12005719. [DOI] [PubMed] [Google Scholar]
- NAKAYA Y, OKITA K, SUZUKI K, MORIWAKI H, KATO A, MIWA Y, SHIRAISHI K, OKUDA H, ONJI M, KANAZAWA H, TSUBOUCHI H, KATO S, KAITO M, WATANABE A, HABU D, ITO S, ISHIKAWA T, KAWAMURA N, ARAKAWA Y HEPATIC NUTRITIONAL THERAPY (HNT) STUDY GROUP. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113–120. doi: 10.1016/j.nut.2006.10.008. [DOI] [PubMed] [Google Scholar]
- NAWABI MD, BLOCK KP, CHAKRABARTI MC, BUSE MG. Administration of endotoxin, tumor necrosis factor, or interleukin 1 to rats activates skeletal muscle branched-chain alpha-keto acid dehydrogenase. J Clin Invest. 1990;85:256–263. doi: 10.1172/JCI114421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWGARD CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA A, MORI S, TOTSUKA M, OKAMOTO K, USUI S, FUJITA H, ITAKURA T, MIZOTE H. Branched-chain amino acids metabolic support in surgical patients: a randomized, controlled trial in patients with subtotal or total gastrectomy in 16 Japanese institutions. JPEN J Parenter Enteral Nutr. 1988;12:332–337. doi: 10.1177/0148607188012004332. [DOI] [PubMed] [Google Scholar]
- PACY PJ, CHENG KN, FORD GC, HALLIDAY D. Influence of glucagon on protein and leucine metabolism: a study in fasting man with induced insulin resistance. Br J Surg. 1990;77:791–794. doi: 10.1002/bjs.1800770723. [DOI] [PubMed] [Google Scholar]
- RANGEL-FRAUSTO MS, PITTET D, COSTIGAN M, HWANG T, DAVIS CS, WENZEL RP. The natural history of the systemic inflammatory response syndrome (SIRS): A prospective study. JAMA. 1995;273:117–123. doi: 10.1001/jama.273.2.117. [DOI] [PubMed] [Google Scholar]
- RODNEY S, BONEH A. Amino acid profiles in patients with urea cycle disorders at admission to hospital due to metabolic decompensation. JIMD Rep. 2013;9:97–104. doi: 10.1007/8904_2012_186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRÍGUEZ T, ALVAREZ B, BUSQUETS S, CARBÓ N, LÓPEZ-SORIANO FJ, ARGILÉS JM. The increased skeletal muscle protein turnover of the streptozotocin diabetic rat is associated with high concentrations of branched-chain amino acids. Biochem Mol Med. 1997;61:87–94. doi: 10.1006/bmme.1997.2585. [DOI] [PubMed] [Google Scholar]
- ROSEN HM, YOSHIMURA N, HODGMAN JM, FISCHER JE. Plasma amino acid patterns in hepatic encephalopathy of differing etiology. Gastroenterology. 1977;72:483–487. doi: 10.1016/S0016-5085(77)80261-8. [DOI] [PubMed] [Google Scholar]
- RUDERMAN NB, BERGER M. The formation of glutamine and alanine in skeletal muscle. J Biol Chem. 1974;249:5500–5506. doi: 10.1016/S0021-9258(20)79756-5. [DOI] [PubMed] [Google Scholar]
- RYAN NT. Metabolic adaptations for energy production during trauma and sepsis. Surg Clin North Am. 1976;56:1073–1090. doi: 10.1016/S0039-6109(16)41032-7. [DOI] [PubMed] [Google Scholar]
- SANDSTEDT S, JORFELDT L, LARSSON J. Randomized, controlled study evaluating effects of branched chain amino acids and alpha-ketoisocaproate on protein metabolism after surgery. Br J Surg. 1992;79:217–220. doi: 10.1002/bjs.1800790308. [DOI] [PubMed] [Google Scholar]
- SCAGLIA F, CARTER S, O’BRIEN WE, LEE B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab. 2004;81:S79–S85. doi: 10.1016/j.ymgme.2003.11.017. [DOI] [PubMed] [Google Scholar]
- SCHOLTEN DJ, MORGAN RE, DAVIS AT, ALBRECHT RM. Failure of BCAA supplementation to promote nitrogen retention in injured patients. J Am Coll Nutr. 1990;9:101–106. doi: 10.1080/07315724.1990.10720357. [DOI] [PubMed] [Google Scholar]
- SHAH SH, CROSSLIN DR, HAYNES CS, NELSON S, TURER CB, STEVENS RD, MUEHLBAUER MJ, WENNER BR, BAIN JR, LAFERRÈRE B, GORROOCHURN P, TEIXEIRA J, BRANTLEY PJ, STEVENS VJ, HOLLIS JF, APPEL LJ, LIEN LF, BATCH B, NEWGARD CB, SVETKEY LP. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55:321–330. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHE P, OLSON KC, KADOTA Y, INUKAI A, SHIMOMURA Y, HOPPEL CL, ADAMS SH, KAWAMATA Y, MATSUMOTO H, SAKAI R, LANG CH, LYNCH CJ. Leucine and protein metabolism in obese Zucker rats. PLoS One. 2013;8:e59443. doi: 10.1371/journal.pone.0059443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHI ZQ, CHANG TM. Amino acid disturbances in experimental hepatic coma rats. Int J Artif Organs. 1984;7:197–202. doi: 10.1177/039139888400700409. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA Y, HONDA T, SHIRAKI M, MURAKAMI T, SATO J, KOBAYASHI H, MAWATARI K, OBAYASHI M, HARRIS RA. Branched-chain amino acid catabolism in exercise and liver disease. J Nutr. 2006;136:250S–253S. doi: 10.1093/jn/136.1.250S. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA Y, OBAYASHI M, MURAKAMI T, HARRIS RA. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care. 2001;4:419–423. doi: 10.1097/00075197-200109000-00013. [DOI] [PubMed] [Google Scholar]
- SMITH RJ, LARSON S, STRED SE, DURSCHLAG RP. Regulation of glutamine synthetase and glutaminase activities in cultured skeletal muscle cells. J Cell Physiol. 1984;120:197–203. doi: 10.1002/jcp.1041200213. [DOI] [PubMed] [Google Scholar]
- SOUBA WW, HERSKOWITZ K, SALLOUM RM, CHEN MK, AUSTGEN TR. Gut glutamine metabolism. JPEN J Parenter Enteral Nutr. 1990;14(Suppl 4):45S–50S. doi: 10.1177/014860719001400403. [DOI] [PubMed] [Google Scholar]
- SPYDEVOLD S, DAVIS EJ, BREMER J. Replenishment and depletion of citric acid cycle intermediates in skeletal muscle. Indication of pyruvate carboxylation. Eur J Biochem. 1976;71:155–165. doi: 10.1111/j.1432-1033.1976.tb11101.x. [DOI] [PubMed] [Google Scholar]
- SU L, LI H, XIE A, LIU D, RAO W, LAN L, LI X, LI F, XIAO K, WANG H, YAN P, LI X, XIE L. Dynamic changes in amino acid concentration profiles in patients with sepsis. PLoS One. 2015;10:e0121933. doi: 10.1371/journal.pone.0121933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SURYAWAN A, HAWES JW, HARRIS RA, SHIMOMURA Y, JENKINS AE, HUTSON SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- TEASLEY KM, BUSS RL. Do parenteral nutrition solutions with high concentrations of branched-chain amino acids offer significant benefits to stressed patients? DICP. 1989;23:411–416. doi: 10.1177/106002808902300510. [DOI] [PubMed] [Google Scholar]
- TIETZE IN, SØRENSEN SS, EISKJAER H, THOMSEN K, PEDERSEN EB. Tubular handling of amino acids after intravenous infusion of amino acids in healthy humans. Nephrol Dial Transplant. 1992;7:493–500. [PubMed] [Google Scholar]
- TISCHLER ME, FAGAN JM. Response to trauma of protein, amino acid, and carbohydrate metabolism in injured and uninjured rat skeletal muscles. Metabolism. 1983;32:853–868. doi: 10.1016/0026-0495(83)90198-1. [DOI] [PubMed] [Google Scholar]
- URATA Y, OKITA K, KORENAGA K, UCHIDA K, YAMASAKI T, SAKAIDA I. The effect of supplementation with branched-chain amino acids in patients with liver cirrhosis. Hepatol Res. 2007;37:510–516. doi: 10.1111/j.1872-034X.2007.00081.x. [DOI] [PubMed] [Google Scholar]
- VENTE JP, Von MEYENFELDT MF, Van EIJK HM, Van BERLO CL, GOUMA DJ, Van der LINDEN CJ, SOETERS PB. Plasma-amino acid profiles in sepsis and stress. Ann Surg. 1989;209:57–62. doi: 10.1097/00000658-198901000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENTE JP, SOETERS PB, Von MEYENFELDT MF, ROUFLART MM, Van der LINDEN CJ, GOUMA DJ. Prospective randomized double-blind trial of branched chain amino acid enriched versus standard parenteral nutrition solutions in traumatized and septic patients. World J Surg. 1991;15:128–133. doi: 10.1007/BF01658984. [DOI] [PubMed] [Google Scholar]
- WIJEKOON EP, SKINNER C, BROSNAN ME, BROSNAN JT. Amino acid metabolism in the Zucker diabetic fatty rat: effects of insulin resistance and of type 2 diabetes. Can J Physiol Pharmacol. 2004;82:506–514. doi: 10.1139/y04-067. [DOI] [PubMed] [Google Scholar]
- YANG Q, BIRKHAHN RH. Branched-chain transaminase and keto acid dehydrogenase activities in burned rats: evidence for a differential adaptation according to sex. Nutrition. 1997;13:640–645. doi: 10.1016/S0899-9007(97)83006-7. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN T, HORBER F, RODRIGUEZ N, SCHWENK WF, HAYMOND MW. Contribution of insulin resistance to catabolic effect of prednisone on leucine metabolism in humans. Diabetes. 1989;38:1238–1244. doi: 10.2337/diabetes.38.10.1238. [DOI] [PubMed] [Google Scholar]