Abstract
The overarching objective is to review how early exposure to adversity interacts with inflammation to alter brain maturation. Both adversity and inflammation are significant risk factors for psychopathology. Literature relevant to the effects of adversity in children and adolescents and brain development is reviewed. These studies are supported by research in animals exposed to species-relevant stressors during development. While it is known that exposure to adversity at any age increases inflammation, the effects of inflammation are exacerbated at developmental stages when the immature brain is uniquely sensitive to experiences. Microglia play a vital role in this process, as they scavenge cellular debris and prune synapses to optimize performance. In essence, microglia modify the synapse to match environmental demands, which is necessary for someone with a history of adversity. To achieve the main objective, clinical and preclinical research areas are pieced together to show how adversity uniquely sculpts the brain. Microglia interactions with the inhibitory neurotransmitter GABA (specifically, the subtype expressing parvalbumin) are discussed within contexts of development and adversity. A review of inflammation markers in individuals with a history of abuse is combined with preclinical studies to describe their effects on maturation. Inconsistencies within the literature are discussed, with a call for standardizing methodologies in the areas of the age of assessment of adversity effects, measures to quantify stress and inflammation, and more brain-based measures of biochemistry. Preclinical studies pave the way to interventions for anti-inflammation-based agents (COX-2 inhibitors, CB2 agonists, meditation/yoga) by identifying where, when, and how the developmental trajectory goes awry.
Keywords: accelerated aging, maltreatment, microglia, prevention, sensitive period
Introduction
Exposure to early life adversity affects brain development differentially, depending on the type of adversity, the sex/gender of the individual, and the timing of exposure. When an individual is exposed to a real or potential threat, the body perceives that event as stressful. Such responses to stressors are found in all mammalian systems that are relevant to each species and activate the hypothalamic-pituitary-adrenal axis (HPA). Sources of stress that occur during childhood and adolescence and associated with psychopathology, including physical and sexual abuse or neglect that occurs before the age of 18 years, are considered for the current review. The studies included here on adversity exposure in humans use standardized measures such as the Adverse Childhood Experiences Scale (ACES:1) or the Childhood Trauma Questionnaire (CTQ).2
For animal models, developmental stressors include separation from parents3 or peers4, or physical stress5, or limited bedding model6; we did not include the resident intruder for late adolescents.7 Comparisons of these various stressors reveal similarities and differences8 that are discussed in more detail.9 Differences are found across the maternal separation paradigm, with paradigms varying in duration of separation, the timing of ages of separation, and the use of males and females within each litter.
No standard scale exists for animal studies to quantify adversity. The activation of the HPA axis is used as evidence of the stress, as measured by corticosterone (the animal analog of cortisol), corticotropin-releasing hormone (CRH), adrenal corticotropin hormone (ACTH). However, like in humans, these measures show tremendous variation and are subject to the time of collection. The nature of the stressors used in animals may or not reflect the same phenomenon in humans. Most paradigms alter maternal behavior (e.g., maternal separation or limited nesting); other stressors, like shock, are more physical than we would expect to model child sexual abuse; the resident intruder model is only effective in adolescents and adult animals.7 Despite these issues, modifying the mother’s behavior has been useful as a model and is used herein, unless described otherwise.
If sufficiently potent, exposure to these stressors increases the risk of psychopathology. Anxiety, depression, and post-traumatic stress disorder (PTSD) are expected outcomes of adversity, as are increased substance use disorders, schizophrenia, obesity, and heart disease.10 Animal studies find parallel behavioral outcomes of adversity exposure, including increased fear and anxiety11, depressive-like behavior4, and increased drug use, especially alcohol.12 Adversity exposure is a risk factor for these disorders whose negative effects can be mitigated with novel treatments to prevent serious outcomes.
The objective of the current review is to integrate knowledge about the effects of neuroinflammation, early life adversity (keywords: maltreatment, stress, abuse, deprivation), and brain development. Additional key terms used for the literature search include microglia, phagocytosis, inflammation, maturation, and brain. An emphasis was placed on how preclinical research can inform the neurophysiological mechanisms and how these mechanisms may be used to develop possible interventions of the human condition. The following two sections focus on brain maturation and explain how exposure to adversity and its effects on development depend on the timing of the stressor and the age of assessment. In the three sections after that, the interconnections between early life stress, microglia, and inflammation are discussed in relation to maturation and the potent effects of stress experienced during sensitive periods of development. Finally, the review surveys what is known about specific inflammation markers and developmental stress.
Sensitive periods are experience-expectant stages in development when events can influence and sculpt brain maturation. They are not experience-dependent, such as the necessity of light for the formation of the visual system during a critical period of development.13 The greatest impact of childhood or adolescent adversity on brain development occurs during sensitive periods by producing regional effects on the brain that are associated with the timing of exposure.14,15 As discussed below, sensitive periods are related to changes in plasticity, including the overproduction and elimination of synapses/gray matter in a process that is highly conserved across species.15–17
Factors important in predicting clinical outcomes include the nature of experience, the brain regions underlying a given function (e.g., fear, emotion, reward), and the timing of exposure and assessment. Attention to these factors is critical if a comprehensive timeline of the course of the sequelae of adversity is to be ascertained. When possible, the age/stage when the adversity occurred is presented for the clinical studies. By raising awareness of the importance of tracking the stages, it is the author’s hope that we can arrive at more consensus findings of the effects of “adversity” on brain maturation.
The effects of early adversity on behavior and brain maturation
Depression occurs in ~ 67% of an early life stress population (reviewed by18,19) with an age of onset that is an average of nine years earlier than the non-early life stress exposed population.20,21 Early life adversity preferentially impacts brain circuits associated with threat detection, emotional regulation, reward anticipation, executive functioning, autonomic functions, and sleep/wake regulation.22,23 Elevated inflammation exacerbates the underlying brain regions associated with these behaviors.24
The age when the abuse occurs is associated with different developmental trajectories; children with abuse occurring before 6 years of age exhibit significant levels of depression and anxiety later in life compared to children with abuse occurring between 6–11 years of age, who were more at risk for externalizing disorders.25 This earlier abuse/depression track is associated with changes in emotional regulation pathways. Exposure to maltreatment during adolescence results in abnormal development of cortical structures that results in immediate manifestation of depression in humans26 and in animals following a significant social stressor.4,27 Additional research on older stressed animals is outside the scope of this review.
Physical, sexual abuse, and neglect have unique effects on brain development, suggesting that different mechanisms underlie the enduring consequences28,29 that are partially due to the brain regions that are activated. An early manifestation of early sexual abuse exposure includes fear processing, which typically appears in infancy. For example, the human amygdala, which is involved in fear processing, is reduced in volume following childhood sexual abuse.30 In rodent models of adversity (e.g., the limited bedding model), offspring show reduced fear expression before adolescence, mediated by changes in the amygdala.11 Behaviorally, both human and rodent species exhibit anxiety following exposure to adversity.9,31,32
The expression of more complex behavioral symptoms is delayed after the initiation of childhood abuse, likely due to the engagement (or lack thereof) of higher cortical areas involved in regulation.4,11,20 Adversity-associated depression involves regions regulating emotional responses33, including the anterior cingulate cortex, dorsolateral cortex, and orbital cortex.26 Increased risk for depression in the early abuse window occurs at the same stage when elevated amygdala activity is observed in children with an early life stress history.34,35
The adolescent emergence of depression reflects a dysfunction in emotional regulatory circuits36 as they mature, with delayed effects as maturity unmasks earlier the consequences of abuse (childhood). Cortical connections to subcortical regions must play a role. For example, the younger vulnerability to depression coincides with the stage when the prefrontal cortex (PFC) innervates the amygdala11 and amygdala innervation into the PFC37, which increases in female rodents exposed to early stress.
Abuse-related changes in connectivity are also found in the hippocampus, where early childhood maltreatment reduces hippocampal volume in adolescence, but not earlier.38,39 Studies in animals exposed to early stress (maternal separation) show reduced overproduction of hippocampal synapses during adolescence.16 The hippocampus informs us about the context which is used by the prefrontal cortex to interpret our emotional responses.40 In children with a maltreatment history, connectivity between the hippocampus and prefrontal cortex and cortical activity to threat (as revealed by the angry faces MRI paradigm) is reduced.41 The sensitive period for environmental manipulations to affect the connectivity between the hippocampus and cortical structures is during early adolescence in animals.42 As a result, early abuse manifests when these hippocampal/cortical connections are becoming functional later in adolescence.
Processes of brain maturation at the synaptic level
The process of how exposure to early adversity alters the course of development at the anatomical/functional level is not well understood. Figure 1 illustrates the fundamental players in brain maturation (left side) and the impact of exposure to early adversity (right side). Typical synaptogenesis results in a balance between excitatory (E) information (mainly glutamate) and inhibitory (I) information (mainly GABA). This balance has been referred to as the E/I balance. During a sensitive period of development, the balance shifts to more excitatory activity (encoding the environment) and less inhibitory activity leading to increased brain responsiveness to the environment.43 Increases in the fast-spiking GABA neuron that expresses parvalbumin ends the sensitive period by increasing inhibition.44,45 The timing of parvalbumin expression is unique for each brain region46 and reports differ across laboratories47–49, complicating the story. Nevertheless, parvalbumin rises in concert with brain-derived growth factor (and its receptor Tropomyosin receptor kinase B; [TrkB]), which facilitates synaptogenesis.44,45 Finally, a perineuronal net wraps around the parvalbumin neuron, limiting its plasticity by acting as a physical barrier.50
Figure 1.
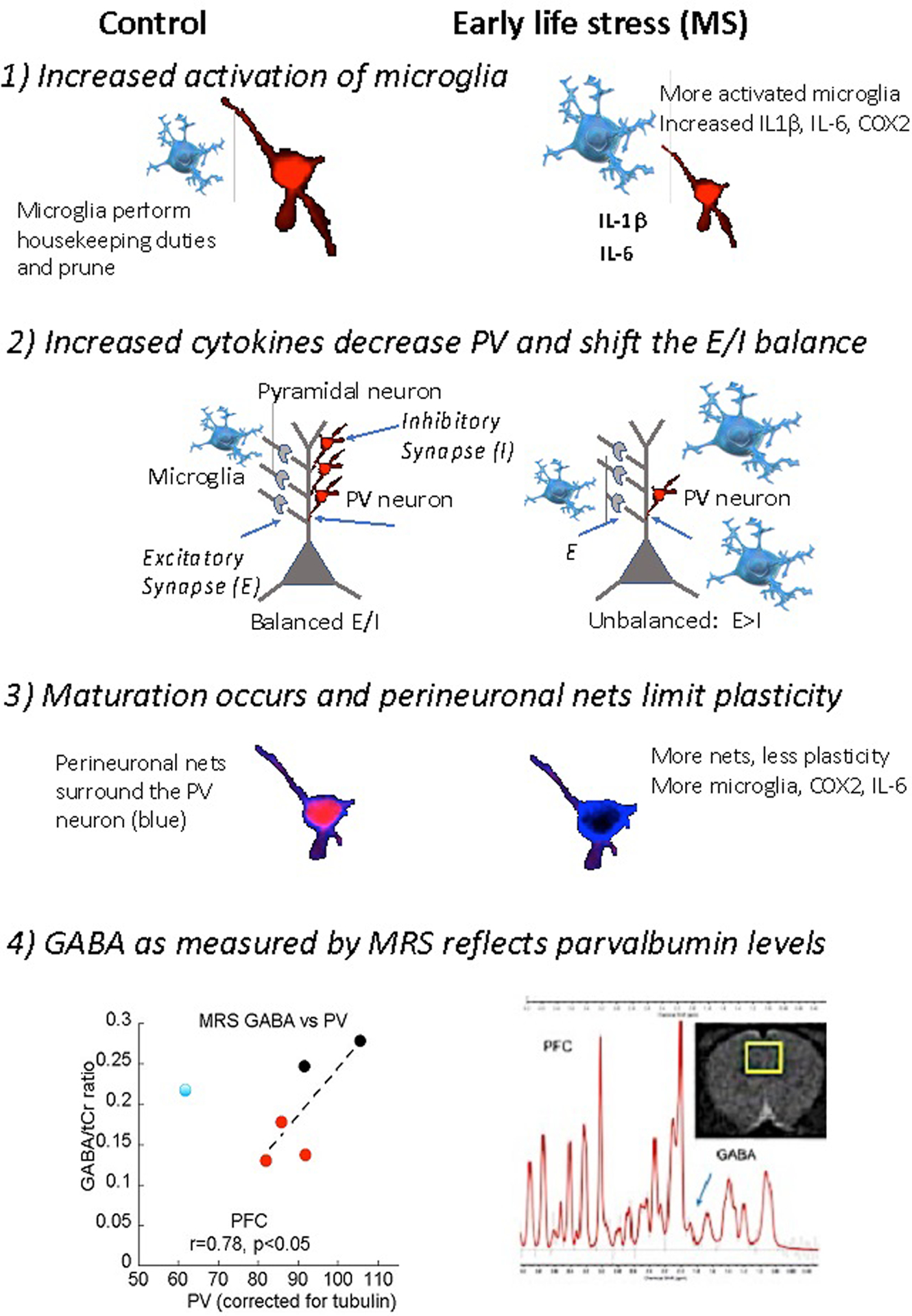
A simplified model of the interactions of microglia (blue), parvalbumin neurons, and pyramidal neurons (gray) across age. Typically, microglia aid in pruning but will switch to an activated form and increase inflammatory factors. Second, cytokines decrease parvalbumin (PV; red) neurons and shift the balance to more excitation. Third, eventually, the neurons are surrounded by perineuronal nets (blue), and plasticity is limited. (Bottom) Voxel placement on the right in adolescent male rats. MRS data of GABA/tCr versus PV levels from the same animals as assayed by Western immunoblot152. We can detect GABA levels with magnetic resonance spectroscopy (MRS) and compare them to PV levels later. Shown are controls animals (black dots), rats with a history of maternal separation (red dots). Levels of PV were quantified with Western Immunoblot, using our standard methods. The spectrograph and the location of the voxel are on the right. Credit to Dr. Dionyssios Mintzopoulos for the MRS data.
In keeping with a sensitive period framework, the overall impact of early adversity depends partly on the timing of exposure and the brain region examined. Differences across the timing of stress exposure and assessment can explain discrepant results across studies. Maternal separation reduces parvalbumin and TrkB levels in the prefrontal cortex in adolescence (35 days of age) in males51 and earlier in females.52 The stress-related reduction in parvalbumin occurs earlier than the control subject, and levels rise to control groups with age. The maturational timeline is also advanced after early stress and closes the amygdala and PFC sensitive periods earlier than controls as indicated by increased parvalbumin expression.11,48 By adulthood, parvalbumin levels appear normal, with no quantitative difference between experimental and control subjects. Thus, if the timing of assessment is too late (adulthood), it would appear as if there was no effect.
Maternal separation in animals also increases the glutamate receptors GluN2A NMDA53, mGluR554, further shifting the E/I balance. Glutamatergic projections from the amygdala to the prefrontal cortex increase after exposure to maternal separation or the limited bedding rodent models.11,37 Finally, the perineuronal nets are more robust in animals exposed to early life stress55, but occur later than in controls.56 Together, low parvalbumin, increased glutamate, and delayed perineuronal net expression suggest that early life stress extends the sensitive period, allowing the neuron to remain vulnerable to “experiences” longer by starting earlier.
Clinically, the underlying mechanism of these changes is difficult to ascertain, but proxies exist. For example, magnetic resonance spectroscopy reveals that adolescents with anhedonia have lower levels of GABA in prefrontal cortical areas.57 Figure 1 shows how GABA, as measured with spectroscopy, reflects changes in parvalbumin in rats. Parvalbumin activity in humans is also reflected in cortical gamma activity, whereby the strength and coherence of the electroencephalogram reflect the E/I balance of the region58, but see.59 Gamma activity is lower in the superior temporal gyrus in a population with elevated Childhood Trauma Questionnaire (CTQ) scores.60 Similarly, exposure to adversity increases connectivity between the amygdala and cortical regions, presumably via glutamatergic projections–although the nature of these projections is not known in humans.61,62 These imaging studies used resting state and functional connectivity measures. Whether these changes are indeed glutamatergic could be ascertained with magnetic resonance spectroscopy.
Data from animals with maternal separation demonstrate either increased63 or decreased glutamate (relative to the stable neuro metabolite creatine) in the hippocampus.64 In a different study, GABA and glutamate + glutamine (Glu+Gln) levels in the prefrontal cortex were reduced (a trend).63 These animals were adults, leaving open the possibility that glutamate measured during adolescence is elevated. In the single study on the subject, hippocampal glutamate levels were inversely correlated with the degree of stress exposure indexed by ACES.65
Neuroinflammation
Inflammation, independent of early life stress, alters developmental processes in several ways. Studies that examine the effects of inflammation produced by an immune challenge, such as lipopolysaccharide (LPS), find reduced cell proliferation and migration; synaptogenesis, and pruning (reviewed in66). Changes in myelination and expression of other support cells are also observed.67,68 It is the tenet of this paper that inflammation resulting from stress during a sensitive period is a trifecta for exerting significant and enduring damage.
As the story builds, the next objective of this review is to discuss the role of inflammation on the effects of early life adversity on synaptic plasticity. In general, stress challenges the body causing it to react by increasing the sympathetic nervous system and the HPA system. As part of the sympathetic nervous system response, norepinephrine phosphorylates mitogen-activated protein kinases (MAPKs) that in turn influence the immune system.69 The HPA system releases glucocorticoids that stimulate both anti- and pro-inflammatory responses. Acute stressors increase the release of glucocorticoids to reduce inflammation.70 Over time, chronic stress shifts the response to pro-inflammatory71, allowing the organism to respond to sustained threat.72 Indeed, glucocorticoid levels or glucocorticoid responses in humans73 and animals74 are suppressed in some, but not all, individuals following chronic stress.75 The inconsistent findings can be due to methodology of sample ascertainment, age of assessment, types or the age of abuse, or other underlying differences in subjects.76,77 With prolonged immune activation, markers including C-reactive protein78 and interleukin-6 can be measured readily (discussed in more detail below).
The interaction of stress that occurs during a sensitive period of development to effect inflammation produces a permanent change in neural circuitry. Inflammation associated with the stress79 affects neurons at all ages by reducing dendritic branching.80 However, these early descriptions show that dendrites resume their exuberance when the stress abates. In contrast, dendritic branching following exposure to early adversity does not return to normal. Loss of gray matter/synapses is exaggerated following stress at a specific stage or may not be detectable until age-appropriate pruning occurs.16 The structural/functional changes in the brain following early life stress exposure are more pronounced, however, if the stressor occurs during a sensitive period.15 Early life stress-induced inflammation interacts with synaptogenesis in two ways. First, inflammation activates microglia, which are one of several cells in the brain involved in inflammatory responses and the pruning of neurons. Microglia express glucocorticoid receptors81, which are highly expressed in the PFC and hippocampus at the ages coinciding with the sensitive periods discussed here.15,82 Second, inflammation directly impacts GABA/parvalbumin neurons to shift the E/I balance (Figure 1), further exacerbating the effects of stress on regional development.
Inflammation is associated with exposure to early life adversity
Physicians have long recognized the link between psychological stress and healing in their patients.83–86 Multiple reviews establish a role for inflammatory processes in adverse outcomes66,87–91, and the relationship between inflammation and depression has been established for some time in adults.92 Inflammation is associated with depression in teens93,94, and mood dysregulation following vaccinations in children95 Individuals with a history of maltreatment or animals exposed to maternal separation have increased inflammation.48,96,97
The objective of the following section is to synthesize what is known about neuroinflammation and integrate this information with developmental processes. Currently, little of this vast knowledge is applied to developmental processes. The proposed framework explains why inflammation during a sensitive period may be vital to producing a permanent effect on circuitry versus the more temporary changes when inflammation occurs at other stages. Stress increases inflammation via microglia (the focus here), astrocytes98, and by causing the blood-brain barrier to leak, allow peripheral cytokines to enter the brain.99 Changes in HPA axis activity, inflammation, and most importantly, circuit changes, will produce sustained vulnerability to depression and other emotion-regulating conditions in individuals with maltreatment. As a result, psychopathology associated with adversity, especially depression, is generally more severe, recurring, and more resistant to treat as reviewed by.100
The role of microglia and inflammation to facilitate pruning
There is still limited understanding of how inflammation permanently alters the immature brain following exposure to adversity. Recent investigations shine a light on microglia as critical cells of interest due to their dual role in inflammation and phagocytotic pruning that occurs during normal development.101–103 Contact between microglia and a synapse activates the cell under normal conditions and synchronizes their activity; such synchrony is absent when the cell is inflamed.104 Microglia have been called the “guards of homeostasis” and are activated when homeostasis is disrupted.105 When an environmental disruption such as adversity occurs, microglia and neurons undergo asynchronous development.
Microglia transition between two fundamental (and oversimplified) states: M1 during inflammation and M2 when the cell is involved in phagocytosis. Microglia produce cytokines such as TNF-α and IL-1β during the pro-inflammatory phase (M1). Microglia in the M2 phase are neuroprotective and produce the anti-inflammatory cytokines IL-10 and TGF-β.106,107 A ramified morphology characterizes the resting (M2) phenotype that constantly scans its environment.106,108 The ramified morphology increases microglia/neuron interactions and is observed following chronic stress or elevated glutamate activity.109–112 However, studies in neurodegenerative models show that ramification is reduced as the microglia cell is no longer able to perform its neuroprotective role.113
During development, microglia in the M2 state aid in the pruning of synapses102,114 and other markers (e.g., dopamine signaling101,115 to optimize neural circuits and signaling pathways to match the demands of the environment.102,116–118 The synchronized activity of the microglia/synapse pair support synaptogenesis. In other words, the microglia/neuron pair that fires together wires together. Exposure to adversity desynchronizes activity, resulting in abnormal development.
A meta-analysis of microglia number in animal models of stress not restricted to development (e.g., including adulthood) and seven different types of social stressors found an increase in microglia number using the marker ionised calcium-binding adaptor molecule 1 (Iba-1).81 In the case of early maternal separation in animals, the cell count of microglia in prefrontal structures did not differ overall from control animals119, but did in cortical subregions.120 This observation, however, is misleading given that the state of the cell (M1 or M2, which is based on morphology) is vital to microglia impact. By adolescence, not at a younger age, the number of processes on each microglial cell (M2) is elevated in females (but not males) following exposure to adversity and an immune challenge. Soma size is increased in both sexes after stress. This study118 highlights several important details that can influence the interpretation of the results: age of assessment, sex, and cell state. It further suggests that additional metrics of microglia activity are needed to study the effects of inflammation on microglia/synapse interactions in development.
The signal for pruning is mediated in part by interactions between the Complement component 1q (C1q), part of the innate immune system, and complement 3 fractalkine (C3) and its receptor (C3R).102,121 C1q and C3 promote microglia engulfment of developing synapses during pruning marked by a “tag” containing the complement protein C3. The tag molecule binds to a C3 receptor, CD11b, that is expressed exclusively on microglia and is directly involved in synaptic elimination (pruning).102 CD11b orchestrates inflammatory and anti-inflammatory activity. Under typical developmental conditions, the innate immune signaling protects synapses from being pruned erroneously.122 A loss of C1q and C3 prevents pruning, resulting in excess synapses in adulthood102,116,121; we would expect the opposite whereby increased C1q or C3R enhances pruning. Evidence is emerging suggesting that early life stress alters C1q and C3R.123
Phagocytic activity by microglia increases earlier in life (e.g., childhood in mice) in animals that underwent early maternal separations compared with controls.124 Given the role that the CD11b receptor plays in pruning125, it is not surprising that animals exposed to early life stress have elevated CD11b receptors (Andersen, unpublished observation). Increased CD11b provides a mechanism for explaining enhanced pruning in animals exposed to early life stress.
Another microglia marker, fractalkine (CX3CL1; CX3 chemokine ligand [L] 1), is associated with pruning during a sensitive period. Changes in CX3CR are necessary for glutamatergic synaptogenesis during development.126–128 CX3CL1 activation of microglia modulates the overproduction of inducible nitric oxide synthase (iNOS), IL-1β, TNFα, and IL-6. Decreased CX3CR1 (CX3 chemokine receptor [R] 1) signaling in young mice (early childhood) enhances long-term depression in the hippocampus, but reductions in CX3CR1 at adolescence have no effect.129
One way chemokines work is to modify glutamate. Reduced expression of the chemokine CXCR1 during a sensitive period is associated with less glutamatergic AMPA activity and delays the developmental switch in GluN2B to GluN2A NMDA receptors.105 Animal studies show that early stress increases GluN2A NMDA receptor expression, but GluN2B was not measured in this study.53 GluN2B was decreased in the adult hippocampus after maternal separation in rats.130 Early stress exposure is expected to reduce plasticity as the synapse matures, as suggested by the GluN2B: Glu N2A ratio.
Manipulating the CX3CR1 changes developmental plasticity and the effects of inflammation, but the result is not universal across all brain regions.131 Since every brain region has a unique period of plasticity, the possibility exists that the timing of assessment prevented the detection of CX3CR1 changes in various brain regions. The single study that examined CX3R after early stress exposure failed to find an effect within a single age group in one brain area.132 However, increased inflammation at four days of age in mice (due to LPS treatment) elevated microglia engulfment of synapses via a CX3CR1-mediated mechanism in the adolescent prefrontal cortex.133 Possible reasons for the lack of CX3CR1 change following early stress include differences in the timing of stress, LPS activates the HPA or immune systems more than maternal separation, or the timing of assessment was not appropriate for the sensitive period of the region. These pervasive issues in the field hamper our progress to detect important factors relevant to psychiatry. By aiming for a more systematic approach across laboratories that includes the use of key ages of a stressor that produces a maximal effect, duration of stress manipulation, and key ages to measure outcome, we will improve our ability to identify important changes. The paper by11 provides an example of such a foundational analysis.
In addition to microglia, astrocytes enable synaptic pruning by directly engulfing synapses and indirectly by increasing the release of different measures of the complement system and IL-33.134–136 For a more expanded review of the role of inflammatory markers involved in synaptogenesis in other conditions, such as Parkinson’s disease, Alzheimer’s disease, and schizophrenia, the reader is referred to a review by.105
Pathways of inflammation
The objective of the following section is to review the limited number of studies that examine the effects of inflammation that affect brain development following early adversity. Several inflammation markers that are associated with adversity, depression, or aging have been identified. Behaviorally, increased inflammation and stress are associated with depression, memory impairment, and cognitive dysfunction in humans66,137,138 and animals.48,51,139 Inflammation is also associated with neurodegeneration, as evidenced by structural brain changes in gray and white matter.
Figure 2 focuses on markers that have been examined clinically or preclinically or are of potential interest. The signal 1 initiating cascade includes pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). DAMPs are responsible for the activation of the TLR pathway.140 The interleukins (IL) IL-6, IL-1b, IL-10, and tumor necrotic factor-alpha (TNFa) are studied frequently. C-reactive protein (CRP) is a systemic marker of inflammation and is considered a downstream product of the IL-6 cascade.141
Figure 2.
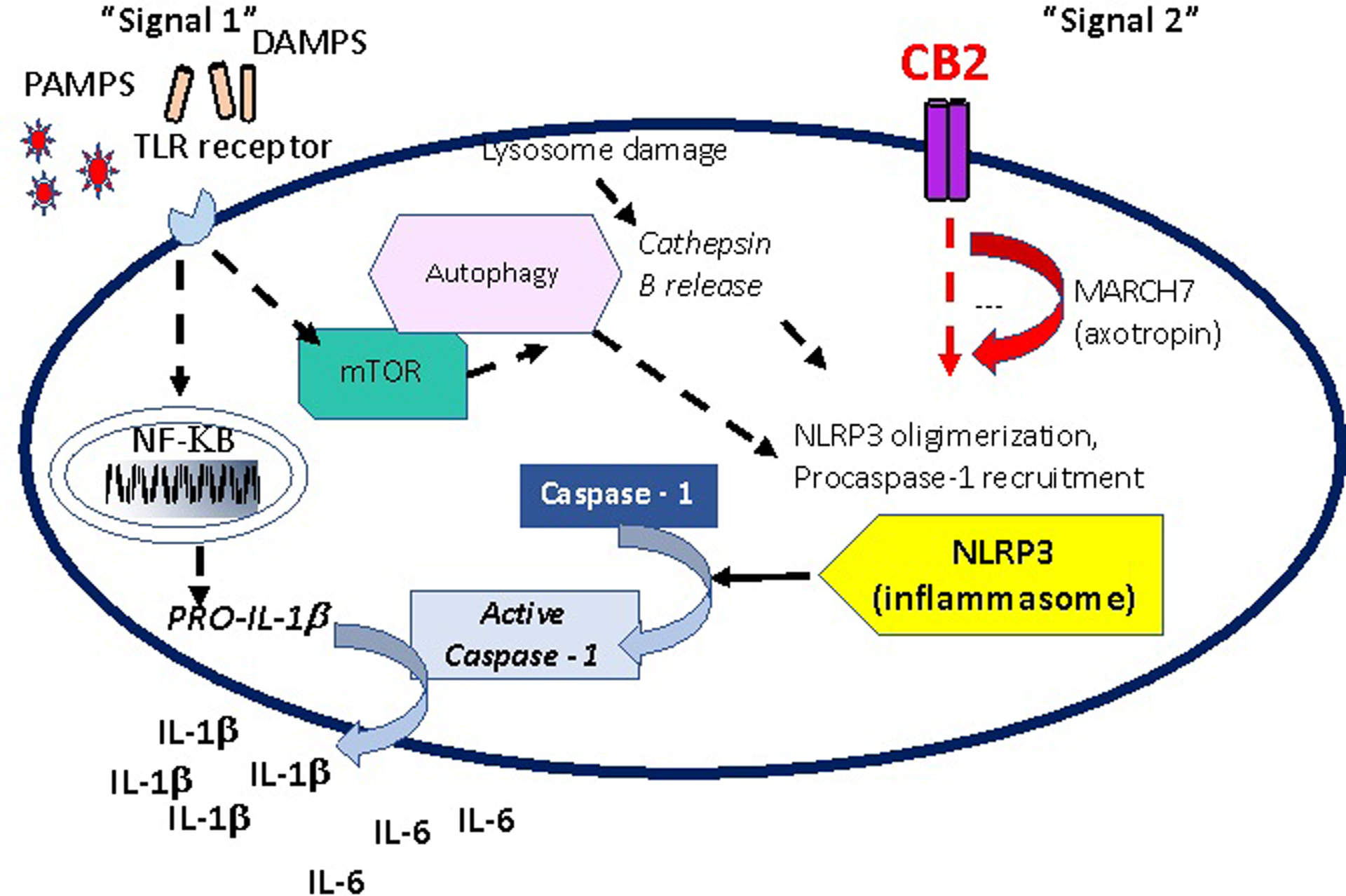
Two different types of signals activate the innate immune system. The first pathway, mediated by the toll receptors (TLRs), recognizes an initiating pro-inflammatory signal. The second is mediated by the inflammasome, which has a well-recognized role in depression. The key protein complex, (Nod)-like receptor family pyrin domain-containing 3 (NLRP3), is a target of novel therapeutics. This complex is modulated by the cannabinoid receptor 2 (CB2), MARCH7 (axotropin). Signal 1 via the TLRs initiates the synthesis of NF-KB, which is involved in the synthesis of a Pro-IL-1b. The NLRP3 activates Caspase-1, which then results in a cleaved and active IL-1b. This simplified diagram suggests that there are two different points of access to regulate inflammation.
Inflammation, adversity, and development
The following discussion focuses on the relationship between early adversity, inflammation, and how they relate to brain and behavior changes when data are available. Changes in systemic inflammatory markers in populations with a history of maltreatment are reviewed elsewhere.66,87–91 Keeping in mind that stress allows peripheral cytokines to enter the brain99, these references are important when considering biomarkers. The review here discusses clinical findings of inflammation on the brain and will be supported further with preclinical studies that are experimental and not correlational.142,143
Inflammation (IL-6)
IL-6 is one of the most well-known interleukins and can be measured readily in blood samples. Elevated levels of IL-6 are reported in children with a maltreatment history in several studies. IL-6 decreases CX3CR1 levels144,145 resulting in reduced plasticity. For example, IL-6 dose-dependently inhibits synaptic plasticity in the hippocampus, whereas blocking IL-6 activity improves long-term memory.146,147
Maltreatment-related depression is often associated with inflammation. The level of childhood trauma as measured by the CTQ correlates positively with serum levels of IL-6 for physical abuse and emotional abuse.148 IL-6 levels are inversely associated with the volume of the hippocampus and prefrontal cortex regions in humans.149
Studies in rats that underwent maternal separation show increased IL-6 levels in the hippocampus, but not the prefrontal cortex.120 Mechanistically, preclinical studies show that IL-6 identifies parvalbumin cells as “sick” and dying150. Animal studies reveal an inverse correlation between IL-6 in the plasma and parvalbumin in the prefrontal cortex.53 IL-6 levels are increased in the prefrontal cortex and hippocampus of males, but not female rats exposed to maternal separation.151 Notably, the time course of parvalbumin loss in an animal model is delayed until adolescence in females and occurs earlier in males.49 These data suggest that increased IL-6 opens the sensitive period early, making the individual more plastic (vulnerable?) to their experiences.152
IL-1β
IL-1β is another cytokine that inhibits cell proliferation153 ,154,155, reduces CX3CR1 levels in vivo144,145 and reduces LTP and calcium currents in culture. In addition, IL-1β induces the expression of cyclo-oxygenase-2 (COX2;156), the target of non-steroidal anti-inflammatories. An interaction between the IL-1β genotype, the NR3C1 haplotype (a variant that encodes the glucocorticoid receptor), and adversity exposure is associated with thinning of the left subgenual anterior cingulate cortex in humans.157
IL-1β is often, but not always, associated with depression158 and impair memory-related behaviors in humans.144,145 Depressive symptoms in males, but not females, with a history of adversity, were higher if they had the single nucleotide polymorphism GG IL-1β polymorphism in the rs16944.159 IL-1β correlates with gray matter volumes in the left occipitotemporal area, left superior occipital gyrus, and left inferior parietal lobule in older adults (non-abused).160
Changes in IL-1β are also associated with increased GABA-A receptor expression in animals.155,161 The GABA-A receptor is the site of action for benzodiazepines. GABA-A receptor activity is very dynamic the first month of life in the rat (corresponding to childhood in humans;162), and thus susceptible to changes in maternal care.163 Reduced GABA activity during peri-adolescence increases amygdala innervation into the prefrontal cortex in animals164–167 This observation is consistent with the lower GABA/glutamate balance found in affective disorders in humans168 and increased glutamate activity in individuals with a history of maltreatment.169
Studies in animals show that this GABA loss can be rescued by increasing GABA during the first two weeks of life.170 In depression-resilient humans, greater cortical activity and less amygdala activity facilitate adapting to stress quickly.62 Thus, intervening early in life (see below) may mitigate the effects of maltreatment. In animal models of early adversity (e.g., the maternal separation model), IL-1β levels inversely correlate with performance on the win-shift maze task (a memory task;49) and are elevated in the hippocampus.171
Pro-inflammatory tumor necrosis factor-alpha (TNFα)
TNFα regulates glutamate and GABA receptor trafficking and neuronal connectivity.172–174 Children with a history of trauma have elevated levels of TNFα.175 Decreases in gray matter in individuals with a history of childhood trauma negatively correlate with a composite measure of IL-6, CRP, and TNFα. Affected regions the bilateral posterior cingulate cortex/precunei, postcentral gyri, inferior/superior parietal lobules.176 Changes in these regions implicate social processing. These results are supported by a second study, finding TNFα significantly correlated with gray matter volumes of the left occipitotemporal area, left superior occipital gyrus, left inferior parietal lobule, and the medial prefrontal cortices160 Finally, the effects of inflammation can be mediated via social means. Increased inflammation in caregivers is associated with elevated children’s inflammation markers (a composite measure of IL-1B, Il-6, IL-8, TNFα, but not CRP) where both had adverse experiences.177 A significant interest in caregiver mediation is building in the field, in general.
While individuals with an abuse history showed deficits in both the inhibitory control when tested with a Go-No Go task and a behavioral rating scale, levels of TNFα were associated with deficits only at a trend level with the Go-No Go performance.158 The effects of treatment with inflixamib, which targets the TNFα receptors, is moderated by a history of physical abuse in bipolar patients.178 These data highlight the vital role that maltreatment and inflammation play in mediated psychopathology later in life.
Toll-like receptors
The TLR pathway is the subject of extensive research in other fields179, but emerging evidence suggests this pathway changes in response to early experiences. TLR activity increases nuclear factor kappa B (NF-κB)-mediated signaling to increase pro- IL-1β formation (Figure 1). Basic research from slice culture shows that the TLR pathway, namely TLR2 and TLR3, is activated by lipopolysaccharide, causing microglia to release IL-6 and TNFa. Maternal separation increases TLR4 activity in the hypothalamus180 and the hippocampus.181 Voluntary, but not mandatory, exercise reduces TLR4 levels in these rats.
NF-kB
Lymphocytes can be collected from blood in humans and used to assay systemic, but not brain, immune function. Studies of NF-kB activity following exposure to early adversity show a pattern of gate keeping, where only high levels of NF-kB are associated with extreme stress in the form of PTSD. Cellular levels of NF-kB are elevated in women with child abuse-related PTSD.182 However, when not stimulated (or the trauma experienced does not lead to PTSD), peripheral levels of NF-kB in a population defined by Adverse Childhood Events (ACEs) were not altered relative to controls.183 NF-kB in lymphocytes need to be stimulated in resilient, healthy adolescents with a history of maltreatment to evoke an increase relative to controls.184 For example, a history of childhood physical neglect, but not CTQ scores, are associated with NF-kB expression in response to the Trier Social Stress Test.185 These studies illustrate the importance of defining the population of interest (discussed below).
Animals separated from their mothers (a model of early life stress) also show elevated levels of NF-kB186 and greater NF-kB responsivity to challenge (i.e., to cocaine) than controls.187 Together, these data suggest that NF-kB requires a minimum threshold of activity for activation following early adversity.
Inflammasome (NLRP3)
The inflammasome is a multi-protein, intracellular complex that detects outside pathogens as part of the innate immune system. While much is known about the inflammasomes and their role in depression, the inflammasome may represent a novel avenue of investigation to treat abuse. The inflammasome can detect danger signals, including stress and metabolic distress188 Microglia contain inflammasomes.189 The nucleotide-binding and oligomerization domain (Nod)-like receptor family pyrin domain-containing 3 (NLRP3) is one of many different proteins that makes up the inflammasome. The inflammasome is integral to the cascade of IL-1b synthesis.190 Maternal separation increases NLRP3 expression in the prefrontal cortex, secondary to a 20% reduction in MARCH7 (an enzyme that regulates caspase activity). Inflammasome expression in the hippocampus is elevated in an animal model of social isolation and reversed by the inhibitor, MCC950.191 MCC950 reduced cognitive impairment also.
Preventing the inflammatory cascade
COX-2 inhibitors
Cyclo-oxygenase-2 (COX-2), an inflammation marker, is elevated in individuals with depression and in early life stress animals with depressive behaviors.48,51,192,193 Treatment with a COX-2 inhibitor reduces depressive behavior, restores working memory impairment, and increases parvalbumin levels in early stress animals.48,51,53 At the same time, COX-2 inhibition demonstrated positive effects as a depression intervention; the drug influences other mechanisms. Some COX-2 inhibitors (e.g., rofecoxib) have been deemed unsafe by Merck but remain supported by the Food and Drug Administration. In any event, new and safer targets are needed to alter depression following exposure to early life stress.
IL-10
Neuronal development and synaptic function are modulated by microglial interleukin-10 (IL-10), which binds to IL-10 receptors on neurons.194 IL-10 downregulates the expression of Th1 cytokines, major histocompatibility class II antigens, and co-stimulatory molecules on macrophages.195 It also enhances B cell survival, proliferation, and antibody production. IL-10 can block NF-κB activity and regulates the JAK-STAT signaling pathway.196 Taken together, the effects of IL-10 are beneficial.
IL-10 abrogates the IL-1β -induced inhibition of glutamate release and LTP.196 In animal models of early adversity (e.g., the maternal separation model), IL-10 levels positively correlate with performance on the win-shift maze task.49 Microinjections of IL-10 into the ventricles also prevented memory impairment in maternally separated animals.53
CB2 Receptors:
Maltreatment during early childhood is a significant predictor of cannabis dependence197–199, even after adjustment for genetic vulnerability.200 Cannabis use by depressed individuals thus can be considered self-medication.201 CB2 receptors play a role in social behavior202 and modulate GABA activity.203 Studies in late adolescent rats show that CB2 agonist treatment reduces anxiety levels. The effect is partly due to a normalization of cortical glucocorticoid receptors in both males and females.204
The CB2 receptor is found on microglia.205–208 CB2 activity reduces inflammation by inhibiting the inflammasome protein NLRP3209 Activation of CB2 receptors increases IL-10210,211, which we have found prevents PV loss in MS animals.53 CB2 agonists activate the MAPK-ERK pathway via a Gβγ subunit and alter cell migration and increases neurogenesis.212–215
While a cannabis intervention has yet to be examined in an animal model of early maltreatment, we know that treatment with a CB1/2 agonist during a sensitive period can impair function. Treatment in early or mid-adolescence, but not in late adolescence or adulthood, alters GABA-A activity; frequency-dependent activity in the prefrontal cortex is disinhibited to juvenile-like levels in adults (arrested development).216 Increased CB2 receptor expression, on the other hand, produces an anti-depressant-like action.217 Consistent with an intervention occurring before pruning begins, the existing literature on adolescent THC/CB1 exposure in animals suggests that a sensitive period occurs before adulthood, with most studies suggesting exposure before puberty onset in males as critical.216,218–220
Non-pharmacological interventions that reduce inflammation
A host of other approaches are under development to reduce inflammation/increase GABA levels as a means of treatment for a maltreated population. Yoga and meditation have been studied for years. Meditation can reduce brain aging221, and reductions of stress-induced changes in emotional regulation dysfunction222, and IL-6 are reported.223 A recent review of the appropriateness of mindfulness for maltreatment patients, including pros and cons, of mindfulness-based interventions, exists.224,225. Researchers find that yoga is helpful.226
Another approach is to manipulate the microbiome to reduce inflammation.227,228 Studies associating food intake with major depression exist, but studies including maltreatment, hopefully, are forthcoming. Finally, there is nothing better than human touch. Adoption studies in humans229 or manipulations in rats230 show that social interaction can have a positive impact.
Discussion
Early adversity mediates part of its effects on brain development by increased inflammation. One objective of the current review was to discuss the differential impact that the occurrence of adversity during a sensitive period has on maturation, and to highlight its importance for predicted effects and maximum vulnerability. The second objective was to introduce the role of microglia as a key mediator for both developmental changes and inflammation. Finally, the third objective presents specific examples of neuroinflammatory agents, adversity, and neuroanatomical and behavioral change.
One important facet to discuss is the role of individual differences. Not all individuals exposed to early adversity show changes in brain development, increased inflammation, or psychopathology. Clinical studies that examine group differences may miss the effect of adversity by assuming otherwise. Data showing individual data points show this plainly. Such differentiation is not always possible, with MRI analyses requiring group analyses as an example. Markers that can help segregate groups to allow for a more refined analysis are key to both predictions of who will be adversely affected and those who are resilient. These markers are also the key to identify the middle group: those whose trajectory of development predicts adverse consequences, but intervention can redirect this course. Possible biomarkers are GABA/parvalbumin and inflammation in combination.
We also need to get the timing right, in a sex-dependent manner. When individual data points are published, it is easier to see why certain group differences are not significant. More importantly, individual data allow us to see those subjects that are more vulnerable231 The animal data, with more experimental control than human studies, also show trends to two groups of outcomes after early exposure to adversity.11,51,119 What additional data might we need? Maybe some of the markers are found here.
Conclusions
Stress is known to increase inflammation. Depending on the state of development, microglia increase or decrease inflammation. If the region of interest is undergoing active synaptogenesis, the microglia are activated and over-prune the synapses. While details of this process are an active area of investigation, we know that elevated inflammation adversely affects the GABAergic parvalbumin cells. As they are lost, the excitatory/inhibitory balance shifts, and the brain over-prunes. The rationale is that the brain matures in a way to match its environment. Here, the brain exposed to maltreatment has fewer synapses, is less plastic, and is less flexible in its behavioral repertoire.
Acknowledgments:
The author would like to acknowledge and thank Dr. Dionyssios Mintzopoulos for the GABA MRS data and figure.
Conflicts of Interest and Source of Funding:
Dr. Andersen has received funding from NIDA (DA-R56 043991). She declares no conflict of interest.
Reference
- 1.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245–58. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry 1997;36:340–8. [DOI] [PubMed] [Google Scholar]
- 3.Levine S Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 1967;156:258–60. [DOI] [PubMed] [Google Scholar]
- 4.Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse 2008;62:22–30. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi LK, Kalin NH. Early developmental and temporal characteristics of stress-induced secretion of pituitary-adrenal hormones in prenatally stressed rat pups. Brain Res 1991;558:75–8. [DOI] [PubMed] [Google Scholar]
- 6.Walker CD, Bath KG, Joels M, et al. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress 2017;20:421–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winslow JT, Miczek KA. Habituation of aggression in mice: pharmacological evidence of catecholaminergic and serotonergic mediation. Psychopharmacology (Berl) 1983;81:286–91. [DOI] [PubMed] [Google Scholar]
- 8.Demaestri C, Pan T, Critz M, Ofray D, Gallo M, Bath KG. Type of early life adversity confers differential, sex-dependent effects on early maturational milestones in mice. Horm Behav 2020;124:104763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen SL. Exposure to early adversity: Points of cross-species translation that can lead to improved understanding of depression. Dev Psychopathol 2015;27:477–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippard ETC, Nemeroff CB. The Devastating Clinical Consequences of Child Abuse and Neglect: Increased Disease Vulnerability and Poor Treatment Response in Mood Disorders. Am J Psychiatry 2020;177:20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzano Nieves G, Bravo M, Baskoylu S, Bath KG. Early life adversity decreases pre-adolescent fear expression by accelerating amygdala PV cell development. Elife 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman E, Nylander I. The impact of emotional stress early in life on adult voluntary ethanol intake-results of maternal separation in rats. Stress 2005;8:157–74. [DOI] [PubMed] [Google Scholar]
- 13.Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev 1987;58:539–59. [PubMed] [Google Scholar]
- 14.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 2008;20:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci 2008;31:183–91. [DOI] [PubMed] [Google Scholar]
- 16.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 2004;29:1988–93. [DOI] [PubMed] [Google Scholar]
- 17.Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci 2006;1071:313–23. [DOI] [PubMed] [Google Scholar]
- 18.Vachon DD, Krueger RF, Rogosch FA, Cicchetti D. Assessment of the Harmful Psychiatric and Behavioral Effects of Different Forms of Child Maltreatment. JAMA Psychiatry 2015;72:1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. J Consult Clin Psychol 2002;70:6–20. [DOI] [PubMed] [Google Scholar]
- 20.Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J Clin Psychiatry 2009;70:684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Harmelen AL, van Tol MJ, Dalgleish T, et al. Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci 2014;9:2026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 2016;17:652–66. [DOI] [PubMed] [Google Scholar]
- 23.Insana SP, Banihashemi L, Herringa RJ, Kolko DJ, Germain A. Childhood maltreatment is associated with altered frontolimbic neurobiological activity during wakefulness in adulthood. Dev Psychopathol 2016;28:551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muscatell KA, Dedovic K, Slavich GM, et al. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun 2015;43:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplow JB, Widom CS. Age of onset of child maltreatment predicts long-term mental health outcomes. J Abnorm Psychol 2007;116:176–87. [DOI] [PubMed] [Google Scholar]
- 26.Teicher MH, Samson JA. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry 2016;57:241–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leussis MP, Lawson K, Stone K, Andersen SL. The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse 2008;62:185–92. [DOI] [PubMed] [Google Scholar]
- 28.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry 2013;170:1114–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teicher MH, Khan A. Childhood Maltreatment, Cortical and Amygdala Morphometry, Functional Connectivity, Laterality, and Psychopathology. Child Maltreat 2019;24:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage 2014;97:236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress 2005;8:209–19. [DOI] [PubMed] [Google Scholar]
- 32.Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–73. [DOI] [PubMed] [Google Scholar]
- 33.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 2011;15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci U S A 2013;110:18274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology 2015;40:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience 2008;154:1178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honeycutt JA, Demaestri C, Peterzell S, et al. Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. Elife 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiatr Clin N Am 2003;12:271–92. [DOI] [PubMed] [Google Scholar]
- 39.De Bellis MD, Keshavan MS, Clark DB, et al. A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol Psychiatry 1999;45:1271–84. [DOI] [PubMed] [Google Scholar]
- 40.Schriber RA, Anbari Z, Robins RW, Conger RD, Hastings PD, Guyer AE. Hippocampal volume as an amplifier of the effect of social context on adolescent depression. Clin Psychol Sci 2017;5:632–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambert HK, Sheridan MA, Sambrook KA, Rosen ML, Askren MK, McLaughlin KA. Hippocampal Contribution to Context Encoding across Development Is Disrupted following Early-Life Adversity. J Neurosci 2017;37:1925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang S, Tseng KY. Maturation of Corticolimbic Functional Connectivity During Sensitive Periods of Brain Development. Curr Top Behav Neurosci 2021. [DOI] [PubMed] [Google Scholar]
- 43.Danese A, S JL. Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology 2017;42:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res 2013;207:3–34. [DOI] [PubMed] [Google Scholar]
- 45.Cabungcal JH, Steullet P, Morishita H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A 2013;110:9130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caballero A, Diah KC, Tseng KY. Region-specific upregulation of parvalbumin-, but not calretinin-positive cells in the ventral hippocampus during adolescence. Hippocampus 2013;23:1331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seidel K, Helmeke C, Poeggel G, Braun K. Repeated neonatal separation stress alters the composition of neurochemically characterized interneuron subpopulations in the rodent dentate gyrus and basolateral amygdala. Dev Neurobiol 2008;68:1137–52. [DOI] [PubMed] [Google Scholar]
- 48.Brenhouse HC, Andersen SL. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry 2011;70:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, Brenhouse HC. Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology 2016;71:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sorg BA, Berretta S, Blacktop JM, et al. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J Neurosci 2016;36:11459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lukkes JL, Meda S, Norman KJ, Andersen SL. Anhedonic behavior and gamma-amino butyric acid during a sensitive period in female rats exposed to early adversity. J Psychiatr Res 2018;100:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holland FH, Ganguly P, Potter DN, Chartoff EH, Brenhouse HC. Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neurosci Lett 2014;566:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wieck A, Andersen SL, Brenhouse HC. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain Behav Immun 2013;28:218–26. [DOI] [PubMed] [Google Scholar]
- 54.Tsotsokou G, Nikolakopoulou M, Kouvelas ED, Mitsacos A. Neonatal maternal separation affects metabotropic glutamate receptor 5 expression and anxiety-related behavior of adult rats. Eur J Neurosci 2021;54:4550–64. [DOI] [PubMed] [Google Scholar]
- 55.Murthy S, Kane GA, Katchur NJ, et al. Perineuronal Nets, Inhibitory Interneurons, and Anxiety-Related Ventral Hippocampal Neuronal Oscillations Are Altered by Early Life Adversity. Biol Psychiatry 2019;85:1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gildawie KR, Honeycutt JA, Brenhouse HC. Region-specific Effects of Maternal Separation on Perineuronal Net and Parvalbumin-expressing Interneuron Formation in Male and Female Rats. Neuroscience 2020;428:23–37. [DOI] [PubMed] [Google Scholar]
- 57.Gabbay V, Mao X, Klein RG, et al. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry 2012;69:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reh RK, Dias BG, Nelson CA 3rd, et al. Critical period regulation across multiple timescales. Proc Natl Acad Sci U S A 2020;117:23242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bader K, Schafer V, Nissen L, Schenkel M. Heightened beta EEG activity during nonrapid eye movement sleep in primary insomnia patients with reports of childhood maltreatment. J Clin Neurophysiol 2013;30:188–98. [DOI] [PubMed] [Google Scholar]
- 60.Kim S, Kim JS, Shim M, Im CH, Lee SH. Altered cortical functional network during behavioral inhibition in individuals with childhood trauma. Sci Rep 2018;8:10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan Y, Herrera-Melendez AL, Pestke K, et al. Early life stress modulates amygdala-prefrontal functional connectivity: implications for oxytocin effects. Hum Brain Mapp 2014;35:5328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gee DG, Gabard-Durnam LJ, Flannery J, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A 2013;110:15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guan J, Ding Y, Rong Y, et al. Early Life Stress Increases Brain Glutamate and Induces Neurobehavioral Manifestations in Rats. ACS Chem Neurosci 2020;11:4169–78. [DOI] [PubMed] [Google Scholar]
- 64.Hui J, Zhang Z, Liu S, et al. Adolescent escitalopram administration modifies neurochemical alterations in the hippocampus of maternally separated rats. Eur Neuropsychopharmacol 2010;20:875–83. [DOI] [PubMed] [Google Scholar]
- 65.Poletti S, Locatelli C, Falini A, Colombo C, Benedetti F. Adverse childhood experiences associate to reduced glutamate levels in the hippocampus of patients affected by mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 2016;71:117–22. [DOI] [PubMed] [Google Scholar]
- 66.Reid B, Danese A. Challenges in researching the immune pathways between early life adversity and psychopathology. Dev Psychopathol 2020;32:1597–624. [DOI] [PubMed] [Google Scholar]
- 67.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry 2004;56:80–5. [DOI] [PubMed] [Google Scholar]
- 68.Lim L, Howells H, Radua J, Rubia K. Aberrant structural connectivity in childhood maltreatment: A meta-analysis. Neurosci Biobehav Rev 2020;116:406–14. [DOI] [PubMed] [Google Scholar]
- 69.Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology 2012;37:1397–416. [DOI] [PubMed] [Google Scholar]
- 70.Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron 2009;64:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int 2008;52:40–51. [DOI] [PubMed] [Google Scholar]
- 72.Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem 2011;286:38703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 2008;33:693–710. [DOI] [PubMed] [Google Scholar]
- 74.Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic- pituitary-adrenal responses to stress. Science 1997;277:1659–62. [DOI] [PubMed] [Google Scholar]
- 75.Struber N, Struber D, Roth G. Impact of early adversity on glucocorticoid regulation and later mental disorders. Neurosci Biobehav Rev 2014;38:17–37. [DOI] [PubMed] [Google Scholar]
- 76.Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Dev 2010;81:252–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doom JR, Cicchetti D, Rogosch FA, Dackis MN. Child maltreatment and gender interactions as predictors of differential neuroendocrine profiles. Psychoneuroendocrinology 2013;38:1442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nusslock R, Miller GE. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol Psychiatry 2016;80:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu YZ, Wang YX, Jiang CL. Inflammation: The Common Pathway of Stress-Related Diseases. Front Hum Neurosci 2017;11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci 1997;821:271–84. [DOI] [PubMed] [Google Scholar]
- 81.Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 2016;233:1637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perlman WR, Webster MJ, Herman MM, Kleinman JE, Weickert CS. Age-related differences in glucocorticoid receptor mRNA levels in the human brain. Neurobiol Aging 2007;28:447–58. [DOI] [PubMed] [Google Scholar]
- 83.Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology 1993;133:2523–30. [DOI] [PubMed] [Google Scholar]
- 84.Sutherland AG, Alexander DA, Hutchison JD. Disturbance of pro-inflammatory cytokines in post-traumatic psychopathology. Cytokine 2003;24:219–25. [DOI] [PubMed] [Google Scholar]
- 85.Saxe G, Geary M, Bedard K, et al. Separation anxiety as a mediator between acute morphine administration and PTSD symptoms in injured children. Ann N Y Acad Sci 2006;1071:41–5. [DOI] [PubMed] [Google Scholar]
- 86.Stoddard FJ Jr., Sorrentino EA, Ceranoglu TA, et al. Preliminary evidence for the effects of morphine on posttraumatic stress disorder symptoms in one- to four-year-olds with burns. J Burn Care Res 2009;30:836–43. [DOI] [PubMed] [Google Scholar]
- 87.Kerr DM, McDonald J, Minnis H. The association of child maltreatment and systemic inflammation in adulthood: A systematic review. PLoS One 2021;16:e0243685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuzminskaite E, Penninx B, van Harmelen AL, Elzinga BM, Hovens J, Vinkers CH. Childhood Trauma in Adult Depressive and Anxiety Disorders: An Integrated Review on Psychological and Biological Mechanisms in the NESDA Cohort. J Affect Disord 2021;283:179–91. [DOI] [PubMed] [Google Scholar]
- 89.Reilly EB, Gunnar MR. Neglect, HPA axis reactivity, and development. Int J Dev Neurosci 2019;78:100–8. [DOI] [PubMed] [Google Scholar]
- 90.Nettis MA, Pariante CM, Mondelli V. Early-Life Adversity, Systemic Inflammation and Comorbid Physical and Psychiatric Illnesses of Adult Life. Curr Top Behav Neurosci 2020;44:207–25. [DOI] [PubMed] [Google Scholar]
- 91.Ports KA, Holman DM, Guinn AS, et al. Adverse Childhood Experiences and the Presence of Cancer Risk Factors in Adulthood: A Scoping Review of the Literature From 2005 to 2015. J Pediatr Nurs 2019;44:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maes M Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 1995;19:11–38. [DOI] [PubMed] [Google Scholar]
- 93.Gabbay V, Klein RG, Alonso CM, et al. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord 2009;115:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindqvist D, Janelidze S, Hagell P, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry 2009;66:287–92. [DOI] [PubMed] [Google Scholar]
- 95.O’Connor TG, Moynihan JA, Wyman PA, et al. Depressive symptoms and immune response to meningococcal conjugate vaccine in early adolescence. Dev Psychopathol 2014;26:1567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perkeybile AM, Schiml-Webb PA, O’Brien E, Deak T, Hennessy MB. Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinology 2009;34:1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ganguly P, Brenhouse HC. Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Dev Cogn Neurosci 2015;11:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Albrecht A, Ivens S, Papageorgiou IE, et al. Shifts in excitatory/inhibitory balance by juvenile stress: A role for neuron-astrocyte interaction in the dentate gyrus. Glia 2016;64:911–22. [DOI] [PubMed] [Google Scholar]
- 99.Menard C, Pfau ML, Hodes GE, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci 2017;20:1752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Bellis MD, Nooner KB, Scheid JM, Cohen JA. Depression in Maltreated Children and Adolescents. Child Adolesc Psychiatr Clin N Am 2019;28:289–302. [DOI] [PubMed] [Google Scholar]
- 101.Kopec AM, Smith CJ, Ayre NR, Sweat SC, Bilbo SD. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun 2018;9:3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012;74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brenhouse HC, Schwarz JM. Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci Biobehav Rev 2016;70:288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akiyoshi R, Wake H, Kato D, et al. Microglia Enhance Synapse Activity to Promote Local Network Synchronization. eNeuro 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front Cell Neurosci 2018;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Olah M, Biber K, Vinet J, Boddeke HW. Microglia phenotype diversity. CNS Neurol Disord Drug Targets 2011;10:108–18. [DOI] [PubMed] [Google Scholar]
- 107.Walker FR, Beynon SB, Jones KA, et al. Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain Behav Immun 2014;37:1–14. [DOI] [PubMed] [Google Scholar]
- 108.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005;308:1314–8. [DOI] [PubMed] [Google Scholar]
- 109.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol 2010;8:e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex 2012;22:1442–54. [DOI] [PubMed] [Google Scholar]
- 111.Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets 2013;14:1262–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 2014;42:50–9. [DOI] [PubMed] [Google Scholar]
- 113.Mecca C, Giambanco I, Donato R, Arcuri C. Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hong S, Stevens B. Microglia: Phagocytosing to Clear, Sculpt, and Eliminate. Dev Cell 2016;38:126–8. [DOI] [PubMed] [Google Scholar]
- 115.Grier BD, Belluscio L, Cheetham CE. Olfactory Sensory Activity Modulates Microglial-Neuronal Interactions during Dopaminergic Cell Loss in the Olfactory Bulb. Front Cell Neurosci 2016;10:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paolicelli RC, Gross CT. Microglia in development: linking brain wiring to brain environment. Neuron Glia Biol 2011;7:77–83. [DOI] [PubMed] [Google Scholar]
- 117.Miyamoto A, Wake H, Moorhouse AJ, Nabekura J. Microglia and synapse interactions: fine tuning neural circuits and candidate molecules. Front Cell Neurosci 2013;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bechade C, Cantaut-Belarif Y, Bessis A. Microglial control of neuronal activity. Front Cell Neurosci 2013;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gildawie KR, Orso R, Peterzell S, Thompson V, Brenhouse HC. Sex differences in prefrontal cortex microglia morphology: Impact of a two-hit model of adversity throughout development. Neurosci Lett 2020;738:135381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Banqueri M, Mendez M, Gomez-Lazaro E, Arias JL. Early life stress by repeated maternal separation induces long-term neuroinflammatory response in glial cells of male rats. Stress 2019;22:563–70. [DOI] [PubMed] [Google Scholar]
- 121.Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007;131:1164–78. [DOI] [PubMed] [Google Scholar]
- 122.Lehrman EK, Wilton DK, Litvina EY, et al. CD47 Protects Synapses from Excess Microglia-Mediated Pruning during Development. Neuron 2018;100:120–34 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith KL, Kassem MS, Clarke DJ, et al. Microglial cell hyper-ramification and neuronal dendritic spine loss in the hippocampus and medial prefrontal cortex in a mouse model of PTSD. Brain Behav Immun 2019;80:889–99. [DOI] [PubMed] [Google Scholar]
- 124.Delpech JC, Wei L, Hao J, et al. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav Immun 2016;57:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci 2008;28:8138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bertollini C, Ragozzino D, Gross C, Limatola C, Eusebi F. Fractalkine/CX3CL1 depresses central synaptic transmission in mouse hippocampal slices. Neuropharmacology 2006;51:816–21. [DOI] [PubMed] [Google Scholar]
- 127.Ragozzino D, Di Angelantonio S, Trettel F, et al. Chemokine fractalkine/CX3CL1 negatively modulates active glutamatergic synapses in rat hippocampal neurons. J Neurosci 2006;26:10488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoshiko M, Arnoux I, Avignone E, Yamamoto N, Audinat E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J Neurosci 2012;32:15106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011;333:1456–8. [DOI] [PubMed] [Google Scholar]
- 130.Lesuis SL, Lucassen PJ, Krugers HJ. Early life stress impairs fear memory and synaptic plasticity; a potential role for GluN2B. Neuropharmacology 2019;149:195–203. [DOI] [PubMed] [Google Scholar]
- 131.Schecter RW, Maher EE, Welsh CA, Stevens B, Erisir A, Bear MF. Experience-Dependent Synaptic Plasticity in V1 Occurs without Microglial CX3CR1. J Neurosci 2017;37:10541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baldy C, Fournier S, Boisjoly-Villeneuve S, Tremblay ME, Kinkead R. The influence of sex and neonatal stress on medullary microglia in rat pups. Exp Physiol 2018;103:1192–9. [DOI] [PubMed] [Google Scholar]
- 133.Cao P, Chen C, Liu A, et al. Early-life inflammation promotes depressive symptoms in adolescence via microglial engulfment of dendritic spines. Neuron 2021. [DOI] [PubMed] [Google Scholar]
- 134.Bosworth AP, Allen NJ. The diverse actions of astrocytes during synaptic development. Curr Opin Neurobiol 2017;47:38–43. [DOI] [PubMed] [Google Scholar]
- 135.Pekny M, Wilhelmsson U, Bogestal YR, Pekna M. The role of astrocytes and complement system in neural plasticity. Int Rev Neurobiol 2007;82:95–111. [DOI] [PubMed] [Google Scholar]
- 136.Vainchtein ID, Chin G, Cho FS, et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 2018;359:1269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carvalho AF, Miskowiak KK, Hyphantis TN, et al. Cognitive dysfunction in depression - pathophysiology and novel targets. CNS Neurol Disord Drug Targets 2014;13:1819–35. [DOI] [PubMed] [Google Scholar]
- 138.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry 2015;172:1075–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hennessy MB, Schiml PA, Berberich K, Beasley NL, Deak T. Early Attachment Disruption, Inflammation, and Vulnerability for Depression in Rodent and Primate Models. Front Behav Neurosci 2018;12:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gadani SP, Walsh JT, Lukens JR, Kipnis J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron 2015;87:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bettcher BM, Wilheim R, Rigby T, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun 2012;26:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brydges NM, Reddaway J. Neuroimmunological effects of early life experiences. Brain Neurosci Adv 2020;4:2398212820953706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dutcher EG, Pama EAC, Lynall ME, et al. Early-life stress and inflammation: A systematic review of a key experimental approach in rodents. Brain Neurosci Adv 2020;4:2398212820978049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goshen I, Kreisel T, Ounallah-Saad H, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 2007;32:1106–15. [DOI] [PubMed] [Google Scholar]
- 145.Rogers JT, Morganti JM, Bachstetter AD, et al. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci 2011;31:16241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Balschun D, Wetzel W, Del Rey A, et al. Interleukin-6: a cytokine to forget. FASEB J 2004;18:1788–90. [DOI] [PubMed] [Google Scholar]
- 147.Tancredi V, D’Antuono M, Cafe C, et al. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem 2000;75:634–43. [DOI] [PubMed] [Google Scholar]
- 148.Pedrotti Moreira F, Wiener CD, Jansen K, et al. Childhood trauma and increased peripheral cytokines in young adults with major depressive: Population-based study. J Neuroimmunol 2018;319:112–6. [DOI] [PubMed] [Google Scholar]
- 149.Kakeda S, Watanabe K, Katsuki A, et al. Relationship between interleukin (IL)-6 and brain morphology in drug-naive, first-episode major depressive disorder using surface-based morphometry. Sci Rep 2018;8:10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dugan LL, Ali SS, Shekhtman G, et al. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One 2009;4:e5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gonzalez-Pardo H, Arias JL, Gomez-Lazaro E, Lopez Taboada I, Conejo NM. Sex-Specific Effects of Early Life Stress on Brain Mitochondrial Function, Monoamine Levels and Neuroinflammation. Brain Sci 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lukkes JL, Meda S, Thompson BS, Freund N, Andersen SL. Early life stress and later peer distress on depressive behavior in adolescent female rats: Effects of a novel intervention on GABA and D2 receptors. Behav Brain Res 2017;330:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baartman TL, Swanepoel T, Barrientos RM, Laburn HP, Mitchell D, Harden LM. Divergent effects of brain interleukin-1ss in mediating fever, lethargy, anorexia and conditioned fear memory. Behav Brain Res 2017;324:155–63. [DOI] [PubMed] [Google Scholar]
- 154.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A 2008;105:751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem 2002;78:379–89. [DOI] [PubMed] [Google Scholar]
- 156.Fang X, Chen P, Moore SA. The oxygen radical scavenger pyrrolidine dithiocarbamate enhances interleukin-1beta-induced cyclooxygenase-2 expression in cerebral microvascular smooth muscle cells. Microvasc Res 2002;64:405–13. [DOI] [PubMed] [Google Scholar]
- 157.Gupta A, Labus J, Kilpatrick LA, et al. Interactions of early adversity with stress-related gene polymorphisms impact regional brain structure in females. Brain Struct Funct 2016;221:1667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Peters AT, Ren X, Bessette KL, et al. Interplay between pro-inflammatory cytokines, childhood trauma, and executive function in depressed adolescents. J Psychiatr Res 2019;114:1–10. [DOI] [PubMed] [Google Scholar]
- 159.McQuaid RJ, Gabrys RL, McInnis OA, Anisman H, Matheson K. Understanding the Relation Between Early-Life Adversity and Depression Symptoms: The Moderating Role of Sex and an Interleukin-1beta Gene Variant. Front Psychiatry 2019;10:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang H, Sachdev PS, Wen W, et al. The relationship between inflammatory markers and voxel-based gray matter volumes in nondemented older adults. Neurobiol Aging 2016;37:138–46. [DOI] [PubMed] [Google Scholar]
- 161.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res 1993;628:227–34. [DOI] [PubMed] [Google Scholar]
- 162.Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol 2013;110:926–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology 2003;28:1950–9. [DOI] [PubMed] [Google Scholar]
- 164.Berretta S, Benes FM. A rat model for neural circuitry abnormalities in schizophrenia. Nat Protoc 2006;1:833–9. [DOI] [PubMed] [Google Scholar]
- 165.Berretta S, Lange N, Bhattacharyya S, Sebro R, Garces J, Benes FM. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus 2004;14:876–94. [DOI] [PubMed] [Google Scholar]
- 166.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 2001;25:1–27. [DOI] [PubMed] [Google Scholar]
- 167.Gisabella B, Cunningham MG, Bolshakov VY, Benes FM. Amygdala-dependent regulation of electrical properties of hippocampal interneurons in a model of schizophrenia. Biol Psychiatry 2009;65:464–72. [DOI] [PubMed] [Google Scholar]
- 168.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci 2006;29:272–9. [DOI] [PubMed] [Google Scholar]
- 169.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res 2006;40:1–21. [DOI] [PubMed] [Google Scholar]
- 170.Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron 2014;83:894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lorigooini Z, Boroujeni SN, Sayyadi-Shahraki M, Rahimi-Madiseh M, Bijad E, Amini-Khoei H. Limonene through Attenuation of Neuroinflammation and Nitrite Level Exerts Antidepressant-Like Effect on Mouse Model of Maternal Separation Stress. Behav Neurol 2021;2021:8817309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Filiano AJ, Xu Y, Tustison NJ, et al. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 2016;535:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem 1998;70:1876–86. [DOI] [PubMed] [Google Scholar]
- 174.Konefal SC, Stellwagen D. Tumour necrosis factor-mediated homeostatic synaptic plasticity in behavioural models: testing a role in maternal immune activation. Philos Trans R Soc Lond B Biol Sci 2017;372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bucker J, Fries GR, Kapczinski F, et al. Brain-derived neurotrophic factor and inflammatory markers in school-aged children with early trauma. Acta Psychiatr Scand 2015;131:360–8. [DOI] [PubMed] [Google Scholar]
- 176.Quide Y, Bortolasci CC, Spolding B, et al. Systemic inflammation and grey matter volume in schizophrenia and bipolar disorder: Moderation by childhood trauma severity. Prog Neuropsychopharmacol Biol Psychiatry 2021;105:110013. [DOI] [PubMed] [Google Scholar]
- 177.Huffhines L, Jackson Y, McGuire A, Schreier HMC. The intergenerational interplay of adversity on salivary inflammation in young children and caregivers. Psychoneuroendocrinology 2021;128:105222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mansur RB, Delgado-Peraza F, Subramaniapillai M, et al. Extracellular Vesicle Biomarkers Reveal Inhibition of Neuroinflammation by Infliximab in Association with Antidepressant Response in Adults with Bipolar Depression. Cells 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Garcia Bueno B, Caso JR, Madrigal JL, Leza JC. Innate immune receptor Toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci Biobehav Rev 2016;64:134–47. [DOI] [PubMed] [Google Scholar]
- 180.Tang HL, Zhang G, Ji NN, et al. Toll-Like Receptor 4 in Paraventricular Nucleus Mediates Visceral Hypersensitivity Induced by Maternal Separation. Front Pharmacol 2017;8:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sadeghi M, Peeri M, Hosseini MJ. Adolescent voluntary exercise attenuated hippocampal innate immunity responses and depressive-like behaviors following maternal separation stress in male rats. Physiol Behav 2016;163:177–83. [DOI] [PubMed] [Google Scholar]
- 182.Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun 2012;26:13–7. [DOI] [PubMed] [Google Scholar]
- 183.Chase KA, Melbourne JK, Rosen C, et al. Traumagenics: At the intersect of childhood trauma, immunity and psychosis. Psychiatry Res 2019;273:369–77. [DOI] [PubMed] [Google Scholar]
- 184.do Prado CH, Grassi-Oliveira R, Daruy-Filho L, Wieck A, Bauer ME. Evidence for Immune Activation and Resistance to Glucocorticoids Following Childhood Maltreatment in Adolescents Without Psychopathology. Neuropsychopharmacology 2017;42:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schreier HMC, Kuras YI, McInnis CM, et al. Childhood Physical Neglect Is Associated With Exaggerated Systemic and Intracellular Inflammatory Responses to Repeated Psychosocial Stress in Adulthood. Front Psychiatry 2020;11:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ye Y, Yao S, Wang R, et al. PI3K/Akt/NF-kappaB signaling pathway regulates behaviors in adolescent female rats following with neonatal maternal deprivation and chronic mild stress. Behav Brain Res 2019;362:199–207. [DOI] [PubMed] [Google Scholar]
- 187.Orso R, Creutzberg KC, Centeno-Silva A, et al. NFkappaB1 and NFkappaB2 gene expression in the prefrontal cortex and hippocampus of early life stressed mice exposed to cocaine-induced conditioned place preference during adolescence. Neurosci Lett 2017;658:27–31. [DOI] [PubMed] [Google Scholar]
- 188.Martinez-Micaelo N, Gonzalez-Abuin N, Pinent M, Ardevol A, Blay M. Dietary fatty acid composition is sensed by the NLRP3 inflammasome: omega-3 fatty acid (DHA) prevents NLRP3 activation in human macrophages. Food Funct 2016;7:3480–7. [DOI] [PubMed] [Google Scholar]
- 189.Gustin A, Kirchmeyer M, Koncina E, et al. NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes. PLoS One 2015;10:e0130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun 2014;41:90–100. [DOI] [PubMed] [Google Scholar]
- 191.Niu L, Luo SS, Xu Y, et al. The critical role of the hippocampal NLRP3 inflammasome in social isolation-induced cognitive impairment in male mice. Neurobiol Learn Mem 2020;175:107301. [DOI] [PubMed] [Google Scholar]
- 192.Sukoff Rizzo SJ, Neal SJ, Hughes ZA, et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry 2012;2:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hennessy MB, Paik KD, Caraway JD, Schiml PA, Deak T. Proinflammatory activity and the sensitization of depressive-like behavior during maternal separation. Behav Neurosci 2011;125:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lim SH, Park E, You B, et al. Neuronal synapse formation induced by microglia and interleukin 10. PLoS One 2013;8:e81218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Uzonna JE, Kaushik RS, Zhang Y, Gordon JR, Tabel H. Experimental murine Trypanosoma congolense infections. II. Role of splenic adherent CD3+Thy1.2+ TCR-alpha beta- gamma delta- CD4+8- and CD3+Thy1.2+ TCR-alpha beta- gamma delta- CD4–8- cells in the production of IL-4, IL-10, and IFN-gamma and in trypanosome-elicited immunosuppression. J Immunol 1998;161:6189–97. [PubMed] [Google Scholar]
- 196.Kelly A, Lynch A, Vereker E, et al. The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. J Biol Chem 2001;276:45564–72. [DOI] [PubMed] [Google Scholar]
- 197.Handley ED, Rogosch FA, Guild DJ, Cicchetti D. Neighborhood Disadvantage and Adolescent Substance Use Disorder: The Moderating Role of Maltreatment. Child Maltreat 2015;20:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Green AJ, De-Vries K. Cannabis use in palliative care - an examination of the evidence and the implications for nurses. J Clin Nurs 2010;19:2454–62. [DOI] [PubMed] [Google Scholar]
- 199.Oshri A, Rogosch FA, Cicchetti D. Child maltreatment and mediating influences of childhood personality types on the development of adolescent psychopathology. J Clin Child Adolesc Psychol 2013;42:287–301. [DOI] [PubMed] [Google Scholar]
- 200.Duncan AE, Sartor CE, Scherrer JF, et al. The association between cannabis abuse and dependence and childhood physical and sexual abuse: evidence from an offspring of twins design. Addiction 2008;103:990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dubowitz H, Thompson R, Arria AM, English D, Metzger R, Kotch JB. Characteristics of Child Maltreatment and Adolescent Marijuana Use: A Prospective Study. Child Maltreat 2016;21:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Argue KJ, VanRyzin JW, Falvo DJ, Whitaker AR, Yu SJ, McCarthy MM. Activation of Both CB1 and CB2 Endocannabinoid Receptors Is Critical for Masculinization of the Developing Medial Amygdala and Juvenile Social Play Behavior. eNeuro 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology 2009;57:356–68. [DOI] [PubMed] [Google Scholar]
- 204.Alteba S, Korem N, Akirav I. Cannabinoids reverse the effects of early stress on neurocognitive performance in adulthood. Learn Mem 2016;23:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) 2006;30 Suppl 1:S13–8. [DOI] [PubMed] [Google Scholar]
- 206.Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. Br J Pharmacol 2008;153:240–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vilalta A, Brown GC. Neurophagy - the phagocytosis of live neurons and synapses by glia - contributes to brain development and disease. FEBS J 2017. [DOI] [PubMed] [Google Scholar]
- 208.Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol 2003;139:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gao F, Xiang HC, Li HP, et al. Electroacupuncture inhibits NLRP3 inflammasome activation through CB2 receptors in inflammatory pain. Brain Behav Immun 2018;67:91–100. [DOI] [PubMed] [Google Scholar]
- 210.Eljaschewitsch E, Witting A, Mawrin C, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 2006;49:67–79. [DOI] [PubMed] [Google Scholar]
- 211.Correa F, Hernangomez M, Mestre L, et al. Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF-kappaB. Glia 2010;58:135–47. [DOI] [PubMed] [Google Scholar]
- 212.Mackie K Cannabinoid receptors: where they are and what they do. J Neuroendocrinol 2008;20 Suppl 1:10–4. [DOI] [PubMed] [Google Scholar]
- 213.Rogers N Cannabinoid receptor with an ‘identity crisis’ gets a second look. Nat Med 2015;21:966–7. [DOI] [PubMed] [Google Scholar]
- 214.Cassano T, Calcagnini S, Pace L, De Marco F, Romano A, Gaetani S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front Neurosci 2017;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Motaghinejad M, Motevalian M, Abdollahi M, Heidari M, Madjd Z. Topiramate Confers Neuroprotection Against Methylphenidate-Induced Neurodegeneration in Dentate Gyrus and CA1 Regions of Hippocampus via CREB/BDNF Pathway in Rats. Neurotox Res 2017;31:373–99. [DOI] [PubMed] [Google Scholar]
- 216.Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry 2014;19:536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ferber SG, Namdar D, Hen-Shoval D, et al. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr Neuropharmacol 2020;18:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Garcia-Cabrerizo R, Garcia-Fuster MJ. Opposite regulation of cannabinoid CB1 and CB2 receptors in the prefrontal cortex of rats treated with cocaine during adolescence. Neurosci Lett 2016;615:60–5. [DOI] [PubMed] [Google Scholar]
- 219.Silva L, Black R, Michaelides M, Hurd YL, Dow-Edwards D. Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: The unique susceptibility of the prepubescent animal. Neurotoxicol Teratol 2016;58:88–100. [DOI] [PubMed] [Google Scholar]
- 220.Higuera-Matas A, Soto-Montenegro ML, del Olmo N, et al. Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology 2008;33:806–13. [DOI] [PubMed] [Google Scholar]
- 221.Kurth F, Cherbuin N, Luders E. Promising Links between Meditation and Reduced (Brain) Aging: An Attempt to Bridge Some Gaps between the Alleged Fountain of Youth and the Youth of the Field. Front Psychol 2017;8:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Robins CJ, Keng SL, Ekblad AG, Brantley JG. Effects of mindfulness-based stress reduction on emotional experience and expression: a randomized controlled trial. J Clin Psychol 2012;68:117–31. [DOI] [PubMed] [Google Scholar]
- 223.Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun 2013;27:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Joss D, Teicher MH. Clinical effects of mindfulness-based interventions for adults with a history of childhood maltreatment: a scoping review. Curr Treat Options Psychiatry 2021;8:31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Benito A, Silva M, Grillot D, Nunez G, Fernandez-Luna JL. Apoptosis induced by erythroid differentiation of human leukemia cell lines is inhibited by Bcl-XL. Blood 1996;87:3837–43. [PubMed] [Google Scholar]
- 226.Clark CJ, Lewis-Dmello A, Anders D, et al. Trauma-sensitive yoga as an adjunct mental health treatment in group therapy for survivors of domestic violence: a feasibility study. Complement Ther Clin Pract 2014;20:152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Slyepchenko A, Maes M, Jacka FN, et al. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother Psychosom 2017;86:31–46. [DOI] [PubMed] [Google Scholar]
- 228.Bremner JD, Moazzami K, Wittbrodt MT, et al. Diet, Stress and Mental Health. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nelson CA 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science 2007;318:1937–40. [DOI] [PubMed] [Google Scholar]
- 230.Hennessy MB, Jacobs S, Schiml PA, Hawk K, Stafford N, Deak T. Maternal inhibition of infant behavioral response following isolation in novel surroundings and inflammatory challenge. Dev Psychobiol 2013;55:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gabbay V, Bradley KA, Mao X, Ostrover R, Kang G, Shungu DC. Anterior cingulate cortex gamma-aminobutyric acid deficits in youth with depression. Transl Psychiatry 2017;7:e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]


