Abstract
In order to differentiate species within the Borrelia burgdorferi sensu lato complex, LightCyler PCR and melting-curve analysis of the amplicons of two genes with intraspecies variability, the p66 gene and the recA gene, were performed. It was demonstrated that nested LightCycler PCR amplification of p66 is more sensitive in the detection of borrelia DNA than amplification of the recA gene. B. burgdorferi sensu stricto could be differentiated from Borrelia garinii and Borrelia afzelii by melting-curve analysis of the p66 gene amplicon. B. garinii could be differentiated from B. afzelii and B. burgdorferi sensu stricto by melting-curve analysis of the recA gene amplicon. Therefore, the PCRs complement each other in subtyping different Borrelia species, and combined LightCycler PCR and melting-curve analysis of both target genes is a rapid method to distinguish the three species of B. burgdorferi sensu lato.
Lyme disease is the most prevalent tick-borne disease of the Northern Hemisphere (3). Its etiologic agent, Borrelia burgdorferi sensu lato, has been divided into different species. B. burgdorferi sensu stricto, Borrelia afzelii, and Borrelia garinii are the common human pathogenic species (4, 21). The infection leads to a variety of clinical symptoms involving the skin, nervous system, heart, and joints (19).
Erythema migrans (EM) and acrodermatitis chronica atrophicans (ACA) represent common cutaneous manifestations of an infection by B. burgdorferi sensu lato (1, 13, 21), whereas the role of this spirochete in the pathogenesis of morphea and lichen sclerosus et atrophicus is controversial (13, 15, 23, 25).
Since PCR has proved to be a sensitive and fast method for the diagnosis of microrganisms which are difficult to culture, the technique has been applied to the detection of B. burgdorferi sensu lato DNA in infected ticks (6) as well as in human specimens, such as cerebrospinal fluid (4) or synovial fluid (5, 20), urine (2, 16), and skin (9, 24). Established PCR protocols amplify different segments of borrelial chromosomal genes, such as the flagellin gene (10, 15), the one-copy 16S rRNA gene (7), the 23S rRNA gene (17), the p66 gene segment encoding a 66-kDa protein (14), the recA gene (8), and the plasmid-encoded ospA gene (9, 20).
Common PCR with a conventional thermocycler and subsequent separation of the amplicon by agarose gel electrophoresis allows the detection of B. burgdorferi sensu lato DNA. Subtyping of Borrelia species DNA is not possible, since the intraspecies sequence polymorphisms of PCR amplicons are only a few base pairs long.
Several postamplification methods were employed to identify Borrelia species commonly associated with Lyme borreliosis, e.g., oligonucleotide typing with PCR fragments (5), randomly amplified polymorphic DNA fingerprinting analysis (22), pulsed-field gel electrophoresis (4), single-strand conformation polymorphism (18), and subtype-specific PCR targeting the 16S rRNA gene (6). These techniques are usually time-consuming, and some of them require high technical standards and experience.
LightCycler PCR with melting-curve analysis is a new, rapid method to perform PCR and to analyze sequence variations of the amplified fragments without the need of additional techniques by performing a melting-temperature (Tm) analysis immediately after amplification is completed. The specific Tm of a DNA template is defined as the temperature at which 50% of the duplices become single stranded. It is influenced by the GC content, length, and nucleotide sequence of the amplified product (27, 28).
A recent report showed that the amplification of the recA gene with a single primer set leads to the differentiation of B. garinii DNA from B. afzelii and B. burgdorferi sensu stricto DNA by its lower specific Tm. However, the difference in Tm between B. afzelii and B. burgdorferi sensu stricto was too small to distinguish the two species (11). To improve species differentiation, we evaluated a PCR of another target gene with intraspecies variability on the LightCycler system and analyzed the melting curves derived from three species within the B. burgdorferi sensu lato complex, B. burgdorferi sensu stricto, B. afzelii, and B. garinii, and compared these melting profiles with the results obtained by analyzing Borrelia recA gene PCR products of the same samples.
For this purpose, we chose a nested PCR targeting the p66 gene segment, which was originally described by Rosa and Schwan (14), modified by Wienecke et al. (24), and which proved to be highly specific and sensitive (12, 25). The 92-bp amplified target sequence of this gene segment differs at various positions among the three Borrelia species, as indicated in Fig. 1. Sequences were obtained from GenBank; the accession numbers are as follows: B. burgdorferi target sequence, M58431.1; B. garinii, X87727.1; B. burgdorferi sensu stricto, X87725.1; and B. afzelii, X87726.1. Potential Tms for these amplicons were calculated by the oligoapplet program available from TIB MOLBIOL, Berlin, Germany, which revealed Tms of 79.0°C for B. afzelii, 79.0°C for B. garinii, and 81.7°C for B. burgdorferi sensu stricto (Table 1).
FIG. 1.
Sequence comparison of the 92-bp amplicon of the p66 genes of B. garinii, B. afzelii, and B. burgdorferi sensu stricto (as obtained from GenBank) with the results of patients' samples (as obtained by sequencing the amplified PCR products). Variant base positions are indicated by underlining. The primer positions are shown below.
TABLE 1.
Tm of p66 and recA gene fragmentsa
| Species (strain) or patient no. | Diagnosis | Tm (°C) calculated | Mean Tm (± SD) (°C)
|
|
|---|---|---|---|---|
| p66 gene amplicon | recA gene amplicon | |||
| B. burgdorferi sensu stricto (B31) | 81.7 | 78.72 ± 0.28 | 84.67 ± 0.28 | |
| B. afzelii (NE 632) | 79.0 | 77.27 ± 0.33 | 84.20 ± 0.40 | |
| B. garinii | 79.0 | 77.24 ± 0.37 | 82.89 ± 0.49 | |
| 1 | EM | 77.65 | 84.24 | |
| 2 | EM | 77.37 | 84.26 | |
| 3 | EM | 77.57 | 84.62 | |
| 4 | EM | 77.63 | 84.26 | |
| 5 | ACA | 77.95 | NAb | |
| 6 | EM | 76.87 | NA | |
| 7 | EM | 77.15 | NA | |
| 8 | EM | 79.23 | NA | |
Tms of the p66 gene fragment were calculated by the oligoapplet program and obtained by LightCycler PCR analysis of the p66 gene amplicon and the recA gene amplicon of the three reference strains of B. burgdorferi sensu lato and of patients with EM or ACA. For the three reference strains, mean Tms ± SD for 10 independent experiments are given.
NA, no amplification of Borrelia DNA could be detected.
We analyzed DNA of Borrelia control strains and eight patient samples. The DNA of the species B. burgdorferi sensu stricto (strain B31), B. afzelii (strain NE 632) (kindly provided by W. Bautsch, Hannover Medical University, Hannover, Germany), B. garinii, and Borrelia hermsii (purchased from Deutsche Sammlung von Mikroorganismen and Zellkulturen, Braunschweig, Germany) was extracted with a QIA Amp DNA isolation kit (Qiagen, Hilden, Germany).
B. hermsii served as a negative control, since it is not amplified by the primers used in this study. The skin biopsy specimens were obtained from eight patients with clear diagnosis of cutaneous borreliosis. The diagnosis was based on clinical data, histological data, and serological detection of elevated B. burgdorferi immunglobulin M and immunoglobulin G antibodies. Fresh frozen biopsy specimens were cut into small pieces, and genomic DNA extraction was performed with the QIA Amp DNA isolation kit.
PCR was performed on a LightCycler (Roche Molecular Biochemicals, Mannheim, Germany). The primers nTM17F and nTM17R (8) were used to amplify a 222-bp product of the recA gene. The PCR conditions and the LightCycler amplification and melting-curve program were reproduced exactly as described previously (11). For amplification of a 170-bp segment of the p66 gene, the outer primer pair (Bb1 and Bb2) was used. Subsequently, 2 μl of this PCR mixture was used as a template for a second run with the inner primer pair (Bb3 and Bb4) to amplify a 92-bp fragment (25) (Fig. 1).
Master mixes were based on a ready-to-use kit (Roche Diagnostics GmbH) containing Taq DNA polymerase, SYBR-Green I, and deoxynucleoside triphosphate mix (with UTP instead of TTP) and supplemented with 0.5 pmol of each primer and 3 mM MgCl2.
Cycling was performed for both the outer and inner primer pairs for 40 cycles of denaturation (95°C for 1 s), annealing (55°C for 5 s), and extension (72°C for 12 s). After the final PCR cycle, the products were denatured at 95°C, annealed at 68°C, and then slowly heated to 95°C. During the slow heating process, fluorescence was measured continously at every 0.1°C. For analysis of the melting curves, the LightCycler instrument's software automatically converts them into melting peaks. The Tms of the peaks were analyzed using the best-fit analysis software provided by Roche Molecular Biochemicals, and the mean Tms are given for each sample in Table 1.
To assess the correct lengths of the fragments, 10 μl of the LightCycler PCR products was separated by agarose gel electrophoresis. As the DNA quality control, all skin samples were screened for human beta actin amplification with a primer set described by Wienecke et al. (25). To confirm Borrelia species identifications by their sequence-dependent Tms, the p66 gene products obtained by LightCycler PCR were purified using the Qia-quick PCR purification kit (Qiagen) and sequenced by BigDye terminator cycle sequencing (AB Applied Biosystems, Weiterstadt, Germany) on an automated PRISM 3700 capillary sequencer (AB Applied Biosystems).
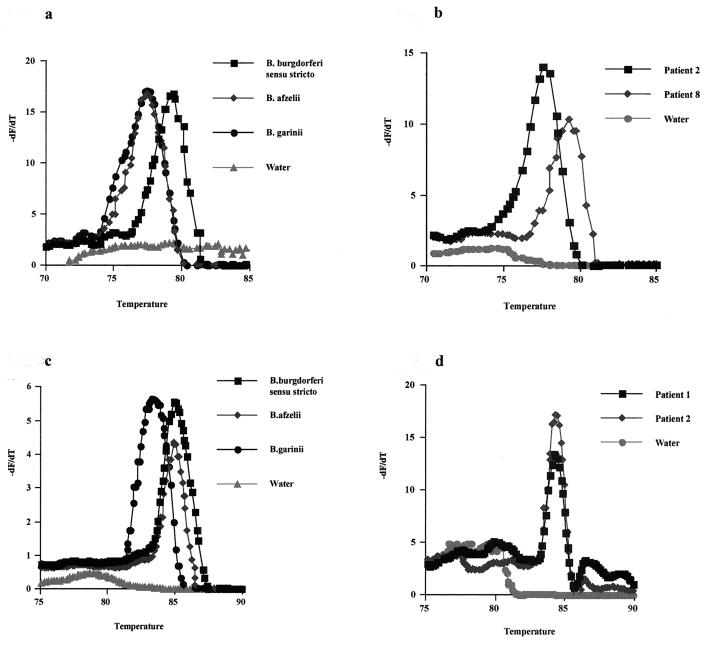
Tms of 77.27°C (standard deviation [SD], ±0.33) for B. afzelii, 77.24°C (SD, ±0.37) for B. garinii, and 78.72°C (SD, ±0.28) for B. burgdorferi sensu stricto were registered by LightCycler PCR and melting-curve analysis of the p66 gene of Borrelia control strain DNA (Table 1 and Fig. 2a). In comparison with those of B. afzelii and B. garinii, the mean Tm of B. burgdorferi sensu stricto was shifted to 1.5°C higher due to the higher GC content of the amplified sequence. This was in accordance with the calculated Tms showing a 2.7°C-higher Tm for B. burgdorferi sensu stricto than for B. afzelii and B. garinii. No amplification was observed with B. hermsii DNA as the target.
FIG. 2.
Melting-curve analyses of amplification products of p66 and recA genes from subspecies of B. burgdorferi sensu lato and two representative DNA samples of patients with skin manifestation of Lyme borreliosis. The Tm of the double-stranded fragment is visualized by plotting the negative derivative of the change of fluorescence (dF) divided by the change of temperature (dT) in relation to the absolute temperature. The turning point of this converted melting curve results in a peak and permits easy identification of the fragment-specific Tm. (a) Separation of B. burgdorferi sensu stricto from B. afzelii and B. garinii by melting-curve analysis of the p66 gene amplicon. (b) Determination of B. burgdorferi sensu stricto in patient 8 and B. afzelii or B. garinii in patient 2 by melting-curve analysis of the p66 gene amplicon. (c) Separation of B. garinii fom B. burgdorferi sensu stricto and B. afzelii by melting-curve analysis of the recA gene amplicon. (d) Determination of B. burgdorferi sensu stricto or B. afzelii in patients 1 and 2 by melting-curve analysis of the recA gene amplicon.
The LightCycler analysis was then applied to eight DNA samples from fresh frozen tissues of patients with serological, clinical, and histological diagnosis of cutaneous borreliosis. For six patients (patients 1, 2, 3, 4, 6, and 7) with a diagnosis of EM and one (patient 5) with a diagnosis of ACA, we found mean Tms in a range of 77.15 to 77.95°C, similar to the values for the controls B. afzelii and B. garinii. One patient (patient 8) with a diagnosis of EM had a mean Tm of 79.23°C, which correlated with the melting profile of the control B. burgdorferi sensu stricto. Tm-defined groups were distinguished by a clear-cut separation of the melting curves (Fig. 2b). Unspecific products or primer dimers could be separated from specific products due to their Tms, which were more than 5°C lower. A Tm around 72°C for the very small peak of the water control, caused by melting of unspecific primer dimers, was registered (Fig. 2b). Subsequent agarose gel electrophoresis of the LightCycler PCR products showed that bands of the appropriate size, 92 bp, were detected for positive results (data not shown). The 92-bp LightCycler PCR products of all patient samples were sequenced. All products which had mean Tms between 77.15 and 77.95°C were identified as B. afzelii. The sample with a mean Tm of 79.23°C was identified as B. burgdorferi sensu stricto (Fig. 1).
Using nTM17F and nTM17R as primers, DNAs from the three Borrelia controls and from the eight patients were subjected to recA gene LightCycler PCR and melting-curve analysis. Mean Tms for the controls B. burgdorferi sensu stricto B32, B. afzelii NE 632, and B. garinii of 84.67, 84.20, and 82.89°C, respectively, were obtained. Therefore, this PCR could differentiate B. garinii from B. afzelii and B. burgdorferi sensu stricto by its 1.3- to 1.8°C-lower Tm (Fig. 2c). These results confirm the data published by Pietila et al. (11).
Out of eight patient samples tested, only four gave positive results for amplification of the recA gene. Therefore, recA gene amplification is less sensitive in detecting borrelial DNA in skin samples than PCR amplification of the p66 gene, which can be explained by the higher sensitivity generally yielded with nested PCR and with different DNA concentrations in the samples.
The Borrelia strains from patients 1, 2, 3, and 4 had mean Tms in a range of 84.24 to 84.62°C. This result, in combination with the results obtained with the p66 gene, would subtype them as B. afzelii. This result was confirmed by nucleotide sequencing of the p66 amplicon (Fig. 1). Melting-curve analysis of the p66 gene shows peaks for B. burgdorferi sensu stricto versus B. garinii and B. afzelii, whereas recA gene analysis could distinguish B. garinii from B. afzelii and B. burgdorferi sensu stricto; thus, the PCRs complement each other in subtyping different Borrelia species.
The choice of target is a crucial parameter for detecting and subtyping species within the B. burgdorferi sensu lato complex. The fragment of the p66 gene (used here for the first time in LightCycler PCR, to our knowledge) has a wide heterogeneity in the three species and can be amplified in two steps with nested primers (14, 24). Molecular subtyping was performed with the same sequence of the p66 gene by analysis of cRNA single-strand conformation polymorphisms (26). Interestingly, a published PCR protocol yielded greater sensitivities for most clinical samples using the p66 nested primer set compared to another nested primer set targeting the plasmid gene ospA (12).
In conclusion, LightCycler nested PCR and melting-curve analysis of the p66 gene enhance the sensitivity of detection of B. burgdorferi sensu lato DNA and are able to differentiate between melting peaks of B. burgdorferi sensu stricto and those of B. afzelii, which could not be separated by the previously reported amplification of the recA gene (11). The amplification of the two target genes, p66 and recA, by LightCycler PCR and subsequent melting-curve analysis is a fast and reliable method to detect borrelial DNA in skin samples and to differentiate the three Borrelia species commonly associated with Lyme disease.
REFERENCES
- 1.Asbrink E, Hovmark A. Early and late cutaneous manifestations in Ixodes-borne borreliosis (erythema migrans borreliosis, Lyme borreliosis) Ann N Y Acad Sci. 1988;539:4–15. doi: 10.1111/j.1749-6632.1988.tb31833.x. [DOI] [PubMed] [Google Scholar]
- 2.Brettschneider S, Bruckbauer H, Klugbauer N, Hofmann H. Diagnostic value of PCR for detection of Borrelia burgdorferi in skin biopsy and urine samples from patients with skin borreliosis. J Clin Microbiol. 1998;36:2658–2665. doi: 10.1128/jcm.36.9.2658-2665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 4.Busch U, Hizo-Teufel C, Boehmer R, Fingerle V, Nitschko H, Wilske B, Preac-Mursic V. Three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. afzelii, and B. garinii) identified from cerebrospinal fluid isolates by pulsed-field gel electrophoresis and PCR. J Clin Microbiol. 1996;34:1072–1078. doi: 10.1128/jcm.34.5.1072-1078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaulhac B, Heller R, Limbach F X, Hansmann Y, Lipsker D, Monteil H, Sibilia J, Piemont Y. Direct molecular typing of Borrelia burgdorferi sensu lato species in synovial samples from patients with Lyme arthritis. J Clin Microbiol. 2000;38:1895–1900. doi: 10.1128/jcm.38.5.1895-1900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liebisch G, Sohns B, Bautsch W. Detection and typing of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks attached to human skin by PCR. J Clin Microbiol. 1998;36:3355–3358. doi: 10.1128/jcm.36.11.3355-3358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marconi R T, Garon C F. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J Clin Microbiol. 1992;30:2830–2834. doi: 10.1128/jcm.30.11.2830-2834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison T B, Ma Y, Weis J H, Weis J J. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J Clin Microbiol. 1999;37:987–992. doi: 10.1128/jcm.37.4.987-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moter S E, Hofmann H, Wallich R, Simon M M, Kramer M D. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospA-specific PCR. J Clin Microbiol. 1994;32:2980–2988. doi: 10.1128/jcm.32.12.2980-2988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pahl A, Kuehlbranst U, Brune K, Roellinghoff M, Gessner A. Quantitative detection of Borrelia burgdorferi by real-time PCR. J Clin Microbiol. 1999;37:1958–1963. doi: 10.1128/jcm.37.6.1958-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pietila J, He Q, Oksi J, Viljanen M K. Rapid differentiation of Borrelia garinii from Borrelia afzelii and Borrelia burgdorferi sensu stricto by LightCycler fluorescence melting curve analysis of a PCR product of the recA gene. J Clin Microbiol. 2000;38:2756–2759. doi: 10.1128/jcm.38.7.2756-2759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priem S, Rittig M R, Kamradt T, Burmester G R, Krause A. An optimized PCR leads to rapid and highly sensitive detection of Borrelia burgdorferi in patients with Lyme borreliosis. J Clin Microbiol. 1997;35:685–690. doi: 10.1128/jcm.35.3.685-690.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranki A, Aavik E, Peterson P, Schauman K, Nurmilaakso P. Successful amplification of DNA specific for Finnish Borrelia burgdorferi isolates in erythema chronicum migrans but not in circumscribed scleroderma lesions. J Investig Dermatol. 1994;102:339–345. doi: 10.1111/1523-1747.ep12371793. [DOI] [PubMed] [Google Scholar]
- 14.Rosa P A, Schwan T G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989;160:1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- 15.Schempp C, Bocklage H, Lange R, Kolmel H W, Orfanos C E, Gollnick H. Further evidence for Borrelia burgdorferi infection in morphea and lichen sclerosus et atrophicus confirmed by DNA amplification. J Investig Dermatol. 1993;100:717–720. doi: 10.1111/1523-1747.ep12472369. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt B, Muellegger R R, Stockenhuber C, Soyer H P, Hoedl S, Luger A, Kerl H. Detection of Borrelia burgdorferi-specific DNA in urine specimens from patients with erythema migrans before and after antibiotic therapy. J Clin Microbiol. 1996;34:1359–1363. doi: 10.1128/jcm.34.6.1359-1363.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz I, Wormser G P, Schwartz J J, Cooper D, Weissensee P, Gazumyan A, Zimmermann E, Goldberg N S, Bittker S, Campbell G L, et al. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J Clin Microbiol. 1992;30:3082–3088. doi: 10.1128/jcm.30.12.3082-3088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Situm M, Grahovac B, Markovic S, Lipozencic J, Poje G, Dobric I, Marinovic B, Bolanca-Bumber S, Misic-Majerus L. Detection and genotyping of Borrelia burgdorferi sensu lato by polymerase chain reaction. Croat Med J. 2000;41:47–53. [PubMed] [Google Scholar]
- 19.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 20.Vasiliu V, Herzer P, Roessler D, Lehnert G, Wilske B. Heterogeneity of Borrelia burgdorferi sensu lato demonstrated by an ospA-type-specific PCR in synovial fluid from patients with Lyme arthritis. Med Microbiol Immunol. 1998;187:97–102. doi: 10.1007/s004300050079. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, van Dam A P, Schwartz I, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin Microbiol Rev. 1999;12:633–653. doi: 10.1128/cmr.12.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G, van Dam A P, Spanjaard L, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato by randomly amplified polymorphic DNA fingerprinting analysis. J Clin Microbiol. 1998;36:768–776. doi: 10.1128/jcm.36.3.768-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weide B, Walz T, Garbe C. Is morphoea caused by Borrelia burgdorferi? A review. Br J Dermatol. 2000;142:636–644. doi: 10.1046/j.1365-2133.2000.03407.x. [DOI] [PubMed] [Google Scholar]
- 24.Wienecke R, Neubert U, Volkenandt M. Molecular detection of Borrelia burgdorferi in formalin-fixed, paraffin-embedded lesions of Lyme disease. J Cutan Pathol. 1993;20:385–388. doi: 10.1111/j.1600-0560.1993.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 25.Wienecke R, Schlupen E M, Zochling N, Neubert U, Meurer M, Volkenandt M. No evidence for Borrelia burgdorferi-specific DNA in lesions of localized scleroderma. J Investig Dermatol. 1995;104:23–26. doi: 10.1111/1523-1747.ep12613456. [DOI] [PubMed] [Google Scholar]
- 26.Wienecke R, Zochling N, Neubert U, Schlupen E M, Meurer M, Volkenandt M. Molecular subtyping of Borrelia burgdorferi in erythema migrans and acrodermatitis chronica atrophicans. J Investig Dermatol. 1994;103:19–22. doi: 10.1111/1523-1747.ep12388947. [DOI] [PubMed] [Google Scholar]
- 27.Wittwer C T, Herrmann M G, Moss A A, Rasmussen R P. Continuous fluorescence monitoring of rapid cycle DNA amplification. BioTechniques. 1997;22:130–131. doi: 10.2144/97221bi01. , 134–138. [DOI] [PubMed] [Google Scholar]
- 28.Wittwer C T, Ririe K M, Andrew R V, David D A, Gundry R A, Balis U J. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. BioTechniques. 1997;22:176–181. doi: 10.2144/97221pf02. [DOI] [PubMed] [Google Scholar]