Abstract
A recent study suggested that proton pump inhibitor (PPI) use in patients with advanced non-small-cell lung cancer (NSCLC) receiving immune checkpoint inhibitors (ICIs) was associated with poor clinical outcomes. However, the clinical impact of PPI use on the outcome of patients receiving ICIs for postoperative recurrent NSCLC is unknown. The outcomes of 95 patients with postoperative recurrence of NSCLC receiving ICIs at 3 medical centers in Japan were analyzed. We conducted adjusted Kaplan–Meier survival analyses with the log-rank test, a Cox proportional hazards regression analysis, and a logistic regression analysis using inverse probability of treatment weighting (IPTW) to minimize the bias arising from the patients’ backgrounds. The IPTW-adjusted Kaplan–Meier curves revealed that the progression-free survival (PFS), but not the overall survival (OS), was significantly longer in patients who did not receive PPIs than in those who did receive them. The IPTW-adjusted Cox regression analysis revealed that PPI use was an independent poor prognostic factor for the PFS and OS. Furthermore, in the IPTW-adjusted logistic regression analysis, PPI non-use was an independent predictor of disease control. In this multicenter and retrospective study, PPI use was associated with poor clinical outcomes in patients with postoperative recurrence of NSCLC who were receiving ICIs. PPIs should not be prescribed indiscriminately to patients with postoperative recurrence of NSCLC who intend to receive ICIs. These findings should be validated in a future prospective study.
Introduction
Immune checkpoint inhibitors (ICIs) have been approved for use in patients with advanced or recurrent non-small-cell lung cancer (NSCLC), and they are the standard treatment options for these patients globally. Programmed cell death-ligand 1 (PD-L1) tumor expression is a strong predictor for selecting patients who might benefit from ICIs. However, the identification of reliable biomarkers other than PD-L1 tumor expression is considered crucial, and many potential predictive markers for ICI efficacy in NSCLC have been examined.
Recently, several studies have revealed the influence of the gastrointestinal microbiota on the response to cancer immunotherapy [1–3]. Therefore, drugs associated with gastrointestinal dysbiosis and bacterial richness, such as antibiotics, proton pump inhibitors (PPIs), and probiotics, might affect the efficacy of ICIs [1–5]. Antibiotics are representative drugs that affect the gastrointestinal microbiome, which may be an important factor associated with the immune response [1–3]. PPIs also affect the gastrointestinal microbiome, and several studies have shown that the changes induced by PPIs were more prominent than those induced by antibiotics [4–6]. PPI use is associated with gastrointestinal dysbiosis, decreased bacterial richness, and promotion of T cell tolerance [4, 5]. Therefore, PPI use may be associated with the efficacy of ICIs, and these drugs may reduce the efficacy of ICIs. According to previous reports, the use of antibiotics and PPIs were significantly associated with poor clinical outcomes in patients with advanced-stage cancers, including NSCLC, who received ICIs [1–3, 7–9]. However, the clinical impact of these medications on the outcomes of postoperative recurrent NSCLC in patients receiving ICIs is unknown. The survival outcome differs between advanced-stage cancers and postoperative recurrent cancers in NSCLC [10, 11]. Therefore, it is important to independently conduct analyses in patients with advanced-stage cancers and those with postoperative recurrent cancers in NSCLC. The present findings might be informative for clinicians, including thoracic surgeons, involved in the treatment of patients with NSCLC.
We investigated the influence of these medications, especially PPIs, on the clinical outcomes of postoperative recurrent NSCLC in patients receiving ICIs in this study.
Materials and methods
Patients and samples in this study
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The current study was a retrospective cross-sectional one and was approved by our institutional review boards (Kyushu University, IRB No. 2020–76, Kyushu Cancer Center, IRB No. 2019–45, and Kitakyushu Municipal Medical Center, IRB No. 202008008). The requirement for informed consent from the patients enrolled in this study was waived because of the retrospective design of the study, and patient information was protected.
We retrospectively identified and enrolled 95 patients with postoperative recurrent NSCLC receiving ICIs (nivolumab, pembrolizumab, or atezolizumab monotherapy or combination therapy) between January 2016 and December 2019 at 3 medical centers in Japan: Kyushu University Hospital, National Hospital Organization Kyushu Cancer Center, and Kitakyushu Municipal Medical Center. We examined the following patients’ clinicopathological features in this study: age at the time of treatment initiation, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking history, driver oncogene mutation status, histology, PD-L1 expression status, and medications (probiotics and PPIs). We were unable to obtain sufficient information about antibiotic use from patients’ medical records, and we did not include the factor in this study. Probiotics included Bifidobacterium, Clostridium butyricum, and antibiotic-resistant lactic acid bacteria, and PPIs included omeprazole, lansoprazole, rabeprazole, esomeprazole, and vonoprazan fumarate. For both probiotics and PPIs, their use at the time of treatment initiation was examined, and whether or not these medications continued to be prescribed afterward was not investigated in this study. We usually assessed the tumor response by computed tomography every six to eight weeks according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [12], defining “complete response (CR) + partial response (PR) + stable disease (SD)” as "disease control" according to the RECIST criteria. We obtained all clinical information and follow-up data from patients’ medical records, and the end of the follow-up period of this study was April 30, 2020.
PD-L1, epidermal growth factor receptor (EGFR), and anaplastic lymphoma kinase (ALK) analyses
PD-L1 immunohistochemistry was performed using the pharmDx antibody (clone 22C3, Dako North America, Inc., Agilent/Dako, Carpinteria, CA, USA) [13]. PD-L1 data was categorized using the tumor proportion score (TPS). The EGFR status was assessed in tumor samples using the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp method (Mitsubishi Chemical Medience, Tokyo, Japan) [14], and the ALK status was determined in tumor tissue using fluorescence in situ hybridization (FISH) with a Vysis ALK Break Apart FISH Probe Kit (Abbott Molecular, Des Plaines, IL, USA) [15]. We extracted all data on the PD-L1 tumor expression and mutation status of driver oncogenes (EGFR and ALK) from patients’ medical records.
Statistical analyses
All statistical analyses in this study were performed using the JMP® 14.0 or SAS® 9.4 (SAS Institute, Cary, NC, USA) software programs, and P < 0.05 was considered statistically significant.
Patient demographics and baseline characteristics were summarized using descriptive statistics or contingency tables. We conducted adjusted Kaplan–Meier survival analyses with the log-rank test, a Cox proportional hazards regression analysis, and a logistic regression analysis using inverse probability of treatment weighting (IPTW) to minimize the bias arising from the patients’ backgrounds in this study [16, 17]. Associations between PPI use and clinical factors before and after weighting were examined using the χ2 test. We defined the progression-free survival (PFS) and overall survival (OS) as follows: the PFS was defined as the period from the initial treatment to clinical or radiographic progression or death, and the OS was defined as the period from the initial treatment to the date of last follow-up or death. We constructed survival curves using the Kaplan–Meier method with the log-rank test. A Cox proportional hazards regression analysis was used to estimate the hazard ratios for risk factors. Univariate and multivariate analyses of the relationships between disease control and clinical factors were performed via a logistic regression analysis. The backward elimination method was used in the multivariate analyses of the PFS, OS, and relationship between disease control and clinical factors. The model was run with all variables, and the variable with the highest P value was excluded. This process was repeated until all remaining variables yielded P values of < 0.05.
Results
Patient characteristics in this study
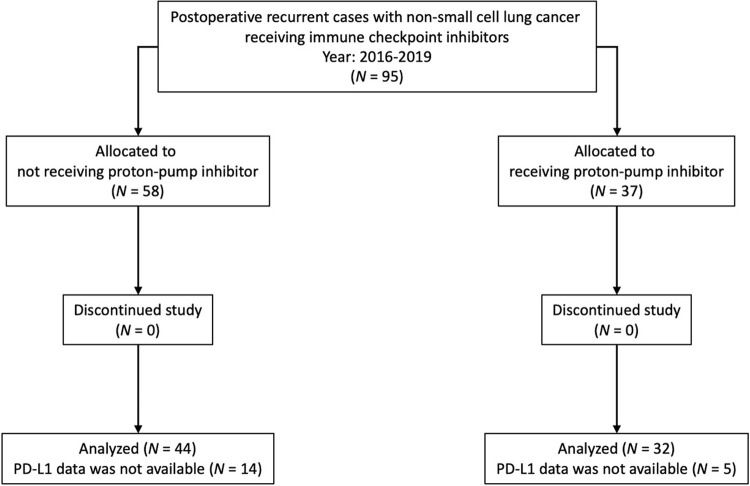
Table 1 shows the clinical characteristics of the 95 patients included in this study. Among the 95 patients, 12 (12.6%) and 37 (38.9%) received probiotics and PPIs, respectively. EGFR or ALK status data were available for 82 patients (86.3%), and PD-L1 tumor expression data were available for 76 patients (80.0%). The CONSORT diagram in this study is presented in Fig 1, and the characteristics of the patients before and after weighting are shown in Table 2. Important factors associated with the efficacy of ICIs, namely the smoking history, driver oncogene mutation status, and PD-L1 tumor expression status, were used in the IPTW treatment-allocation model. The standardized mean differences of the whole model before and after weighting were 0.4257 and −0.0527, respectively.
Table 1. Clinicopathological characteristics of all patients (N = 95).
| Factors | Value or no. of patients | |
|---|---|---|
| Age (years) | Median | 69 |
| Maximum and minimum | 88, 43 | |
| Sex | Female | 17 (17.9%) |
| Male | 78 (82.1%) | |
| ECOG PS | 0 | 53 (55.8%) |
| 1 | 36 (37.9%) | |
| 2 | 4 (4.2%) | |
| 3 | 2 (2.1%) | |
| Smoking history | Never-smoker | 17 (17.9%) |
| Ex-smoker | 61 (64.2%) | |
| Current smoker | 17 (17.9%) | |
| Mutation status (EGFR or ALK) | Wild-type | 72 (75.8%) |
| Mutanta | 10 (10.5%) | |
| Unknown | 13 (13.7%) | |
| Histology | Adenocarcinoma | 66 (69.5%) |
| Squamous cell carcinoma | 20 (21.0%) | |
| Others or unknownb | 9 (9.5%) | |
| Immune checkpoint inhibitor | Nivolumab | 41 (43.2%) |
| Pembrolizumab | 34 (35.8%) | |
| Pembrolizumab + chemotherapy | 10 (10.5%) | |
| Atezolizumab | 9 (9.5%) | |
| Atezolizumab + chemotherapy | 1 (1.0%) | |
| PD-L1 tumor proportion score | <1% | 23 (24.2%) |
| ≥1% and <50% | 24 (25.3%) | |
| ≥50% | 29 (30.5%) | |
| Unknown | 19 (20.0%) | |
| Probiotics | No | 83 (87.4%) |
| Yes | 12 (12.6%) | |
| Proton pump inhibitor | No | 58 (61.1%) |
| Yes | 37 (38.9%) |
aTen patients were EGFR-positive.
bEight patients with sarcomatoid carcinoma and one patient with adenosquamous carcinoma.
ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death-ligand 1; PS, performance status.
Fig 1. CONSORT diagram.
CONSORT diagram in this study.
Table 2. Characteristics of the patients according to the use of PPIs before and after weighting.
| Factors | Unweighted, N (%) | Weighted, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PPI | No | Yes | P value | SMD | No | Yes | P value | SMD | |
| Age (years) | <65 | 15 (34.1%) | 9 (28.1%) | 0.5807 | −0.1274 | 34.1% | 24.9% | 0.3444 | −0.1958 |
| ≥65 | 29 (65.9%) | 23 (71.9%) | 65.9% | 75.1% | |||||
| Sex | Female | 9 (20.5%) | 4 (12.5%) | 0.3632 | 0.2128 | 20.5% | 26.0% | 0.5374 | −0.1482 |
| Male | 35 (79.5%) | 28 (87.5%) | 79.5% | 74.0% | |||||
| ECOG PS | 0 | 26 (59.1%) | 18 (56.3%) | 0.8044 | −0.0567 | 59.1% | 49.6% | 0.3696 | −0.1898 |
| 1–3 | 18 (40.9%) | 14 (43.7%) | 40.9% | 50.4% | |||||
| Smoking history | Never-smoker | 10 (22.7%) | 3 (9.4%) | 0.1270 | 0.3699 | 22.7% | 23.8% | 0.9027 | −0.0305 |
| Smoker | 34 (77.3%) | 29 (90.6%) | 77.3% | 76.2% | |||||
| Mutation status (EGFR or ALK) | Others | 10 (22.7%) | 5 (15.6%) | 0.4425 | −0.1811 | 22.7% | 25.6% | 0.7562 | 0.0721 |
| Wild-type | 34 (77.3%) | 27 (84.4%) | 77.3% | 74.4% | |||||
| Histology | Non-Sq | 36 (81.8%) | 26 (81.3%) | 0.9497 | −0.0144 | 81.8% | 82.6% | 0.9249 | 0.0195 |
| Sq | 8 (18.2%) | 6 (18.7%) | 18.2% | 17.4% | |||||
| PD-L1 tumor proportion score | <50% | 26 (59.1%) | 21 (65.6%) | 0.5626 | −0.1352 | 59.1% | 59.3% | 0.9821 | −0.0049 |
| ≥50% | 18 (40.9%) | 11 (34.4%) | 40.9% | 40.7% | |||||
| Probiotics | No | 41 (93.2%) | 27 (84.4%) | 0.2168 | −0.2777 | 93.2% | 81.0% | 0.0882 | −0.3837 |
| Yes | 3 (6.8%) | 5 (15.6%) | 6.8% | 19.0% | |||||
ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death-ligand 1; PPI, proton pump inhibitor; PS, performance status; SMD, standardized mean difference; Sq, squamous cell carcinoma.
S1 Table shows the breakdown ratios of the PPIs used for patients in this study, and S2 Table summarizes the reasons for PPI use in the 37 patients. Approximately half of these patients (N = 18 [48.7%]) were prescribed PPIs without obvious justification.
Effects of PPI use on the survival
First, we investigated the effects of PPI use on the survival using the original values. The median follow-up time was 331 days (range, 7–1363). The Kaplan–Meier curves revealed that patients who did not receive PPIs had a significantly longer PFS, but not OS, than those treated with PPIs (P = 0.0163 and P = 0.0555, respectively; S1A and S1B Fig). Multivariate analyses revealed that the ECOG PS, mutation status, PPI use, and PD-L1 expression status were independent prognostic factors for the PFS (P = 0.0075, P = 0.0316, P = 0.0002, and P = 0.0047, respectively; S3 Table), whereas the ECOG PS and histology were independent prognostic factors for the OS (P = 0.0018 and P = 0.0042, respectively; S3 Table).
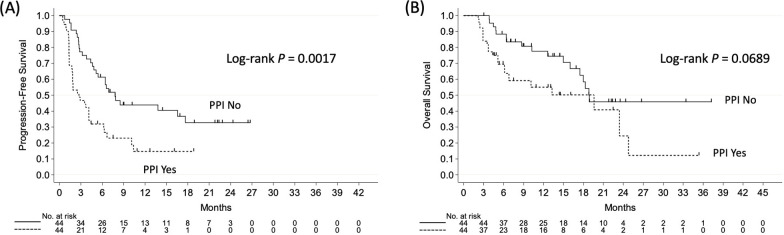
Next, we investigated the effects of PPI use on the survival using IPTW-adjusted values. The IPTW-adjusted Kaplan–Meier curves showed that patients who did not receive PPIs had a significantly longer PFS, but not OS, than those treated with PPIs (P = 0.0017 and P = 0.0689, respectively; Fig 2A and 2B). The IPTW-adjusted Cox analyses showed that the ECOG PS, mutation status, PPI use, and PD-L1 expression status were independent prognostic factors for the PFS (P = 0.0021, P = 0.0006, P < 0.0001, and P = 0.0009, respectively; Table 3), whereas the ECOG PS, mutation status, probiotics use, and PPI use were independent prognostic factors for the OS (P = 0.0499, P = 0.0202, P = 0.0076, and P = 0.0061, respectively; Table 3). As shown in Table 3, PPI use had a stronger impact on the survival than the PD-L1 expression status.
Fig 2. The inverse probability of treatment weighting-adjusted Kaplan–Meier curves according to the use or non-use of proton pump inhibitors.
(A) The progression-free survival and (B) the overall survival.
Table 3. Inverse probability of treatment weighting-adjusted univariate and multivariate analyses of PFS and OS.
| Factors | PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (years) | ≥65/<65 | 1.12 (0.65–1.94) | 0.6820 | 1.04 (0.53–2.04) | 0.9167 | |||||
| Sex | Female/Male | 1.80 (1.04–3.12) | 0.0350 | 0.85 (0.37–1.97) | 0.7079 | |||||
| ECOG PS | 1–3/0 | 2.41 (1.46–4.00) | 0.0006 | 2.25 (1.34–3.78) | 0.0021 | 1.44 (0.77–2.70) | 0.2598 | 1.94 (1.00–3.75) | 0.0499 | |
| Smoking history | Never-smoker/Smoker | 1.68 (0.96–2.92) | 0.0673 | 0.63 (0.26–1.54) | 0.3118 | |||||
| Mutation status (EGFR or ALK) | Others/Wild-type | 2.25 (1.30–3.91) | 0.0039 | 2.96 (1.60–5.47) | 0.0006 | 1.79 (0.88–3.66) | 0.1079 | 2.46 (1.15–5.25) | 0.0202 | |
| Histology | Sq/Non-Sq | 1.55 (0.85–2.83) | 0.1520 | 2.78 (1.43–5.39) | 0.0026 | |||||
| Probiotics | No/Yes | 1.15 (0.52–2.52) | 0.7279 | 2.75 (0.83–9.14) | 0.0984 | 5.93 (1.60–21.92) | 0.0076 | |||
| Proton pump inhibitor | Yes/No | 2.34 (1.41–3.90) | 0.0011 | 4.12 (2.28–7.46) | <0.0001 | 1.94 (1.02–3.67) | 0.0425 | 2.55 (1.31–4.99) | 0.0061 | |
| PD-L1 tumor proportion score | <50%/≥50% | 2.52 (1.47–4.31) | 0.0008 | 2.64 (1.49–4.68) | 0.0009 | 1.50 (0.77–2.95) | 0.2376 | |||
ALK, anaplastic lymphoma kinase; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; HR, hazard ratio; OS, overall survival; PD-L1, programmed cell death-ligand 1; PFS, progression-free survival; PS, performance status; Sq, squamous cell carcinoma.
Effects of PPI use on disease control
Finally, we examined the associations between disease control and clinical factors. The disease control status was CR, PR, SD, and disease progression in 2 (2.1%), 21 (22.1%), 26 (27.4%), and 40 patients (42.1%), respectively, and the status was not evaluable in 6 patients (6.3%). Therefore, the objective response (CR + PR) and disease control (CR + PR + SD) rates were 25.8% (23/89) and 55.1% (49/89), respectively, in this study. Because only 23 patients had objective responses, we only analyzed the association between disease control and clinical factors, and analyses of the association between objective response and clinical factors were not conducted. In the multivariate analyses using the original values, PPI non-use and PD-L1 TPS ≥ 50% were independent predictors of disease control (P = 0.0166 and P = 0.0277, respectively; S4 Table).
The IPTW-adjusted logistic analyses demonstrated that smoking, wild-type mutation status, PPI non-use, and PD-L1 TPS ≥ 50% were independent predictors of disease control (P = 0.0237, P = 0.0110, P = 0.0006, and P = 0.0243, respectively; Table 4). As presented in Table 4, PPI use had a stronger impact on disease control than did the PD-L1 expression status.
Table 4. Inverse probability of treatment weighting-adjusted univariate and multivariate analyses of the relationship between disease control (CR + PR + SD) and clinical factors.
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (years) | ≥65/<65 | 0.62 (0.24–1.62) | 0.3333 | ||
| Sex | Female/Male | 0.40 (0.14–1.14) | 0.0849 | ||
| ECOG PS | 1–3/0 | 0.28 (0.11–0.69) | 0.0057 | ||
| Smoking history | Never-smoker/Smoker | 0.30 (0.10–0.87) | 0.0270 | 0.21 (0.05–0.81) | 0.0237 |
| Mutation status (EGFR or ALK) | Others/Wild-type | 0.24 (0.08–0.71) | 0.0097 | 0.19 (0.05–0.68) | 0.0110 |
| Histology | Sq/Non-Sq | 0.96 (0.32–2.87) | 0.9388 | ||
| Probiotics | No/Yes | 1.39 (0.40–4.87) | 0.6041 | ||
| Proton pump inhibitor | Yes/No | 0.19 (0.08–0.49) | 0.0005 | 0.14 (0.05–0.43) | 0.0006 |
| PD-L1 tumor proportion score | <50%/≥50% | 0.36 (0.14–0.89) | 0.0278 | 0.28 (0.09–0.85) | 0.0243 |
ALK, anaplastic lymphoma kinase; CI, confidence interval; CR, complete response; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; OR, odds ratio; PD-L1, programmed cell death-ligand 1; PR, partial response; PS, performance status; SD, stable disease; Sq, squamous cell carcinoma.
Discussion
In this multicenter, retrospective study, we evaluated the impact of PPI use on clinical outcomes in patients with postoperative recurrent NSCLC receiving ICIs. The results of this study demonstrated that PPI use was significantly associated with poor clinical outcomes and that PPI use might reduce the efficacy of ICIs. These findings were similar to those of a previous report [9], but this is the first report demonstrating the effects of PPI use on the efficacy of ICIs in patients with postoperative recurrent NSCLC.
In the present study, the IPTW-adjusted Cox analyses showed that the ECOG PS, mutation status, PPI use, and PD-L1 expression status were independent prognostic factors for the PFS, whereas the ECOG PS, mutation status, probiotics use, and PPI use were independent prognostic factors for the OS. Furthermore, the IPTW-adjusted logistic analyses demonstrated that smoking, the wild-type mutation status, PPI non-use, and PD-L1 TPS ≥ 50% were independent predictors of disease control. The ECOG PS, smoking, mutation status, and PD-L1 were all previously reported to be significant predictive biomarkers of the efficacy of ICIs in NSCLC patients [18–25], which is similar to our results. PPI use had a stronger impact on the survival and disease control than the PD-L1 expression status, which is a strong predictor for selecting patients who might benefit from ICIs. Given these findings, PPIs should not be prescribed indiscriminately to patients with NSCLC who are expected to receive ICIs. The detailed mechanisms underlying the association between PPI use and ICI efficacy should be investigated in a future study.
Probiotics also influence the gastrointestinal microbiome, but the clinical impact of probiotic use on the outcomes of patients with NSCLC who receive ICIs is poorly understood. Therefore, we assessed the impact of probiotics in this study. Probiotic use was an independent favorable prognostic factor for the OS, but there was no significant association between probiotic use and the PFS or disease control. However, only 12 (12.6%) patients received probiotics in this study, and the results for probiotics only served as a reference in this study. In the future, we plan to analyze the impact of probiotic use on the efficacy of ICIs in a larger study cohort.
According to a previous report, PPI use alters both the intestinal flora and oral microbiome [26]. In the study, Mishiro et al. revealed the alterations of the microbiota in the oral carriage microbiome along with bacterial overgrowth (Streptococcus) along with decreases in the abundance of distinct bacterial species (Neisseria and Veillonella) [26]. For PPI use to be a useful factor in cancer immunotherapy, we should investigate the role of the oral microbiome in predicting the efficacy of ICIs.
There were several limitations associated with the current study. First, this was a retrospective study with a small sample size despite being a multicenter study. However, we conducted adjusted Kaplan–Meier survival analyses with the log-rank test, a Cox proportional hazards regression analysis, and a logistic regression analysis using IPTW to minimize the bias arising from the patients’ backgrounds in this study [16, 17]. The standardized mean differences of the whole model before and after weighting were 0.4257 and −0.0527, respectively, suggesting that the patients’ backgrounds were better balanced after IPTW. This study should be repeated in a larger cohort, and prospective studies may also be warranted. Second, we conducted analyses of the influence of PPIs on the clinical outcomes of postoperative recurrent NSCLC only in patients receiving ICIs in this study. We should conduct the same analyses including a control group comprising patients who were not treated with ICIs, as these results would be extremely informative. We also plan to repeat this analysis in a control group of patients who did not receive immunotherapy. Third, we did not examine how PPIs reduce the efficacy of ICIs in patients with recurrent NSCLC in this study, although we did mention the possible influence of PPIs on the gastrointestinal microbiome based on previous reports. We should clarify the detailed mechanisms underlying the influence of PPIs on the gastrointestinal microbiome in a future study. Fourth, approximately half of the patients who were prescribed PPIs were given the medicine without any apparent justification, a markedly high percentage. Most of them were given PPIs from another hospital, and we might be unable to obtain sufficient information about PPI use from these patients’ medical records. PPIs should not be prescribed indiscriminately to patients, regardless of ICI administration, as indiscriminate prescription of PPIs might cause various adverse events.
Conclusions
In this study, PPI use in patients with postoperative recurrent NSCLC who received ICIs was significantly associated with poor clinical outcomes. PPIs are widely, and often excessively, used drugs. Some patients receiving PPIs had cancer pain, and they also received non-steroidal anti-inflammatory drugs; in addition, other patients had histories of reflux esophagitis or gastroduodenal ulcer. However, approximately half of the patients received PPI without obvious justification in this study. PPIs should not be prescribed indiscriminately to patients with postoperative recurrent NSCLC who are expected to receive ICIs. Furthermore, as indiscriminate prescription of PPIs might cause various adverse events, PPIs should not be prescribed indiscriminately to patients regardless of ICIs administration. The finding should be validated prospectively in a future study.
Supporting information
PPI, proton pump inhibitor.
(DOCX)
PPI, proton pump inhibitor.
(DOCX)
PFS, progression-free survival; OS, overall survival.
(DOCX)
CR, complete response; PR, partial response; SD, stable disease.
(DOCX)
(A) The progression-free survival and (B) the overall survival.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. Epub 2017/11/04. doi: 10.1126/science.aan4236 ; PubMed Central PMCID: PMC5827966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–8. Epub 2018/01/06. doi: 10.1126/science.aao3290 ; PubMed Central PMCID: PMC6707353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7. Epub 2017/11/04. doi: 10.1126/science.aan3706 . [DOI] [PubMed] [Google Scholar]
- 4.Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–8. Epub 2015/12/15. doi: 10.1136/gutjnl-2015-310376 ; PubMed Central PMCID: PMC4853569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–56. Epub 2016/01/01. doi: 10.1136/gutjnl-2015-310861 ; PubMed Central PMCID: PMC4853574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vesper BJ, Jawdi A, Altman KW, Haines GK 3rd, Tao L, Radosevich JA. The effect of proton pump inhibitors on the human microbiota. Curr Drug Metab. 2009;10(1):84–9. Epub 2009/01/20. doi: 10.2174/138920009787048392 . [DOI] [PubMed] [Google Scholar]
- 7.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2018;29(6):1437–44. Epub 2018/04/05. doi: 10.1093/annonc/mdy103 ; PubMed Central PMCID: PMC6354674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen S, Carmagnani Pestana R, Hess K, Viola GM, Subbiah V. Impact of antibiotic use on survival in patients with advanced cancers treated on immune checkpoint inhibitor phase I clinical trials. Annals of oncology: official journal of the European Society for Medical Oncology. 2018;29(12):2396–8. Epub 2018/10/12. doi: 10.1093/annonc/mdy453 ; PubMed Central PMCID: PMC6311953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalabi M, Cardona A, Nagarkar DR, Dhawahir Scala A, Gandara DR, Rittmeyer A, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Annals of oncology: official journal of the European Society for Medical Oncology. 2020;31(4):525–31. Epub 2020/03/03. doi: 10.1016/j.annonc.2020.01.006 . [DOI] [PubMed] [Google Scholar]
- 10.Sekine I, Nokihara H, Yamamoto N, Kunitoh H, Ohe Y, Tamura T. Comparative chemotherapeutic efficacy in non-small cell lung cancer patients with postoperative recurrence and stage IV disease. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2009;4(4):518–21. Epub 2009/04/07. doi: 10.1097/jto.0b013e31819c7bc9 . [DOI] [PubMed] [Google Scholar]
- 11.Moore S, Leung B, Wu J, Ho C. Survival Implications of De Novo Versus Recurrent Metastatic Non-Small Cell Lung Cancer. Am J Clin Oncol. 2019;42(3):292–7. Epub 2019/01/05. doi: 10.1097/COC.0000000000000513 . [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer (Oxford, England: 1990). 2009;45(2):228–47. Epub 2008/12/23. doi: 10.1016/j.ejca.2008.10.026 . [DOI] [PubMed] [Google Scholar]
- 13.Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2017;12(12):1798–805. Epub 2017/09/25. doi: 10.1016/j.jtho.2017.08.022 . [DOI] [PubMed] [Google Scholar]
- 14.Nagai Y, Miyazawa H, Huqun, Tanaka T, Udagawa K, Kato M, et al. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer research. 2005;65(16):7276–82. Epub 2005/08/18. doi: 10.1158/0008-5472.CAN-05-0331 . [DOI] [PubMed] [Google Scholar]
- 15.Marchetti A, Di Lorito A, Pace MV, Iezzi M, Felicioni L, D’Antuono T, et al. ALK Protein Analysis by IHC Staining after Recent Regulatory Changes: A Comparison of Two Widely Used Approaches, Revision of the Literature, and a New Testing Algorithm. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2016;11(4):487–95. Epub 2016/02/27. doi: 10.1016/j.jtho.2015.12.111 . [DOI] [PubMed] [Google Scholar]
- 16.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089–110. Epub 2005/09/29. doi: 10.1002/sim.2174 . [DOI] [PubMed] [Google Scholar]
- 17.Sugihara M. Survival analysis using inverse probability of treatment weighted methods based on the generalized propensity score. Pharm Stat. 2010;9(1):21–34. Epub 2009/02/10. doi: 10.1002/pst.365 . [DOI] [PubMed] [Google Scholar]
- 18.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res. 2016;22(18):4585–93. Epub 2016/05/27. doi: 10.1158/1078-0432.CCR-15-3101 ; PubMed Central PMCID: PMC5026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudnik E, Moskovitz M, Daher S, Shamai S, Hanovich E, Grubstein A, et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: The real-life data. Lung cancer (Amsterdam, Netherlands). 2018;126:217–23. Epub 2017/12/20. doi: 10.1016/j.lungcan.2017.11.015 . [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Kim YH, Tomii K, et al. Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: A multicenter retrospective cohort study. Lung cancer (Amsterdam, Netherlands). 2018;119:14–20. Epub 2018/04/17. doi: 10.1016/j.lungcan.2018.02.017 . [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Nakachi I, Naoki K, Satomi R, Nakamura M, Inoue T, et al. Real-world Efficacy and Safety of Nivolumab for Advanced Non-Small-cell Lung Cancer: A Retrospective Multicenter Analysis. Clinical lung cancer. 2018;19(3):e349–e58. Epub 2018/02/06. doi: 10.1016/j.cllc.2018.01.001 . [DOI] [PubMed] [Google Scholar]
- 22.Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA oncology. 2018;4(2):210–6. Epub 2017/12/23. doi: 10.1001/jamaoncol.2017.4427 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2018;13(8):1138–45. Epub 2018/06/07. doi: 10.1016/j.jtho.2018.03.035 ; PubMed Central PMCID: PMC6063769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim R, Keam B, Hahn S, Ock CY, Kim M, Kim TM, et al. First-line Pembrolizumab Versus Pembrolizumab Plus Chemotherapy Versus Chemotherapy Alone in Non-small-cell Lung Cancer: A Systematic Review and Network Meta-analysis. Clinical lung cancer. 2019;20(5):331–8.e4. Epub 2019/06/06. doi: 10.1016/j.cllc.2019.05.009 . [DOI] [PubMed] [Google Scholar]
- 25.Banna GL, Cortellini A, Cortinovis DL, Tiseo M, Aerts J, Barbieri F, et al. The lung immuno-oncology prognostic score (LIPS-3): a prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small-cell lung cancer. ESMO open. 2021;6(2):100078. Epub 2021/03/19. doi: 10.1016/j.esmoop.2021.100078 ; PubMed Central PMCID: PMC7988288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishiro T, Oka K, Kuroki Y, Takahashi M, Tatsumi K, Saitoh T, et al. Oral microbiome alterations of healthy volunteers with proton pump inhibitor. J Gastroenterol Hepatol. 2018;33(5):1059–66. Epub 2017/11/07. doi: 10.1111/jgh.14040 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PPI, proton pump inhibitor.
(DOCX)
PPI, proton pump inhibitor.
(DOCX)
PFS, progression-free survival; OS, overall survival.
(DOCX)
CR, complete response; PR, partial response; SD, stable disease.
(DOCX)
(A) The progression-free survival and (B) the overall survival.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.