Abstract
Wastewater-based epidemiology has been used to measure SARS-CoV-2 prevalence in cities worldwide as an indicator of community health, however, few longitudinal studies have followed SARS-CoV-2 in wastewater in small communities from the start of the pandemic or evaluated the influence of tourism on viral loads. Therefore the objective of this study was to use measurements of SARS-CoV-2 in wastewater to monitor viral trends and variants in a small island community over a twelve-month period beginning May 1, 2020, before the community re-opened to tourists. Wastewater samples were collected weekly and analyzed to detect and quantify SARS-CoV-2 genome copies. Sanger sequencing was used to determine genome sequences from total RNA extracted from wastewater samples positive for SARS-CoV-2. Visitor data was collected from the local Chamber of Commerce. We performed Poisson and linear regression to determine if visitors to the Cedar Key Chamber of Commerce were positively associated with SARS-CoV-2-positive wastewater samples and the concentration of SARS-CoV-2 RNA. Results indicated that weekly wastewater samples were negative for SARS-CoV-2 until mid-July when positive samples were recorded in four of five consecutive weeks. Additional positive results were recorded in November and December 2020, as well as January, March, and April 2021. Tourism data revealed that the SARS-CoV-2 RNA concentration in wastewater increased by 1.06 Log10 genomic copies/L per 100 tourists weekly. Sequencing from six positive wastewater samples yielded two complete sequences of SARS-CoV-2, two overlapping sequences, and two low yield sequences. They show arrival of a new variant SARS-CoV-2 in January 2021. Our results demonstrate the utility of wastewater surveillance for SARS-CoV-2 in a small community. Wastewater surveillance and viral genome sequencing suggest that population mobility likely plays an important role in the introduction and circulation of SARS-CoV-2 variants among communities experiencing high tourism and who have a small population size.
Keywords: Wastewater-based epidemiology, SARS-CoV-2, COVID-19, Whole genome sequence, Variants, Tourism
1. Introduction
Since wastewater contains pathogens excreted from symptomatic as well as asymptomatic individuals, wastewater-based epidemiology (WBE) is a highly innovative approach to measure the prevalence of pathogens in a population served by a given wastewater treatment plant (Sinclair et al., 2008). Early application of WBE showed it to be an effective tool for monitoring community-level virus presence in the global poliovirus eradication campaign (Asghar et al., 2014; Falman et al., 2019; Lodder et al., 2012; Matrajt et al., 2018). Since then, wastewater surveillance has proven successful for monitoring the presence and circulation of enteric viruses such as hepatovirus A, E virus, human adenovirus, norovirus and rotavirus within a community (Hellmér et al., 2014; Iaconelli et al., 2020; Prado et al., 2014; Sidhu et al., 2013). Recently, spurred by the global COVID-19 pandemic, WBE has been applied to detect and quantify SARS-CoV-2 RNA in influent wastewater as a measure of SARS-CoV-2 viral infection trends and prevalence in a community (Daughton, 2020). Reports from North America (Gonzalez et al., 2020; Melvin et al., 2021; Peccia et al., 2020a; Sherchan et al., 2020; Wu et al., 2021), Australia (Ahmed et al., 2020), Europe (La Rosa et al., 2020; Rimoldi et al., 2020; Wurtzer et al., 2020), and India (Kumar et al., 2020) suggest that testing for SARS-CoV-2 in wastewater could serve as an indicator of infection trends within communities.
Most WBE-based studies to date have focused on sampling from wastewater treatment plants (WWTPs) in urban areas. Zhou et al. (2021) reported that 93.9% of environmental surveillance studies focused on sampling from WWTPs that served populations between 50,000 and 500,000. Few studies have examined the effectiveness of this approach in small rural communities with population sizes less than 50,000 (Zhou et al., 2021). Very few studies to date have used detection and sequencing of SARS-CoV-2 from samples collected directly from wastewater to identify introduction of SARS-CoV-2 variants in a small community setting (Fontenele et al., 2021). However, we are not aware of any studies that have utilized this approach in a small community setting that sees significant changes in the local population attributable to tourism. By leveraging WBE and tourism data, such communities present unique opportunities, as a type of natural experiment, to explore the role of tourism on local transmission in the midst of a pandemic (Doorley et al., 2021). Such information can inform whether restrictions on tourism to control local transmission may be effective. The influence of population movement, in the form of tourists, has not been studied during the COVID-19 pandemic. Therefore, the objectives of this study were to examine the use of wastewater-based monitoring of SARS-CoV-2 to track the presence of this virus and introduction of virus variants, and to assess the influence of tourists on SARS-CoV-2 infection trends in a small (<1000 people) coastal community. If WBE can be used to effectively monitor the presence of SARS-CoV-2 in smaller communities, the utility of this method can help officials in rural areas direct public health resources that are often limited in these settings.
2. Materials and methods
2.1. Study site
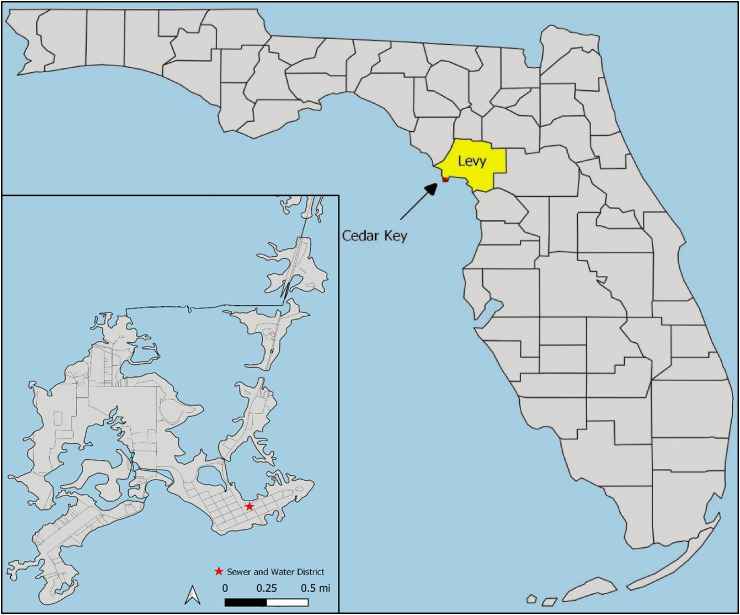
The city of Cedar Key is a collection of small islands in Levy County on the Gulf coast of Florida (Fig. 1 ). The city encompasses approximately two square miles of land and water. According to US Census Bureau estimates, the resident population was 720 in 2019. However, because of its rich artistic community, abundant natural attractions, and productive fishing grounds, Cedar Key also has a large transient tourist population that visits the island year-round. While commercial fishing is the number one economic driver in the city, tourism is a close second. The entire city of Cedar Key is served by a single WWTP that is owned and operated by the Cedar Key Water and Sewer District (Fig. 1). All wastewater from the city flows through this plant and septic systems are not permitted in Cedar Key.
Fig. 1.
Cedar Key wastewater treatment plant service area. Map of Florida showing location of Levy County and Cedar Key. Cedar Key Water and Sewer treatment plant is marked with a star.
2.2. Sample collection
Fifty-two influent wastewater samples were collected between May 1, 2020 and April 30, 2021 from the Cedar Key WWTP. Influent samples were collected as 24-h composites using a flow proportional peristaltic pump. A 1 L subsample of the composite collected by plant personnel was obtained each week. Samples that were collected and delivered to the lab on the same day were maintained at 2-8 °C on wet ice until they arrived at the laboratory for analysis. Samples that required storage for more than 24 h were maintained at −20 °C until transportation to the laboratory. All samples were analyzed within 14 days of collection. Sample volumes that remained after all tests were performed were frozen and stored at −80 °C for future analysis as needed.
2.3. Water quality
Upon receipt of the samples at the laboratory, measurements of water quality were recorded and the mean values for the samples are shown in Supplemental Table 1. Conductivity and salinity were measured using the YSI Professional Plus Multiparameter Instrument (YSI Inc., Yellow Springs, OH, USA), pH was measured using the Fisher brand Accumet AB15 Basic pH meter (Thermo Fisher Scientific, Grand Island, NY, USA), and absorbance was measured using the Hach DR1900 Portable Spectrophotometer (Hach Company, Loveland, CO, USA).
2.4. Virus concentration and extraction of nucleic acids
SARS-CoV-2 was concentrated from the initial influent wastewater sample following a modification of the method described by Haramoto et al. (2020). A 50 mL aliquot of each sample was brought to a final concentration of 25 mM MgCl2 by the addition of 2 M MgCl2 and mixed thoroughly. The sample was then passed through a 0.45 μm pore size mixed cellulose ester electronegative membrane filter disc (Millipore Sigma, Burlington, MA, USA) under vacuum using a 47 mm filter holder with stainless steel support (VWR International, Radnor, PA, USA). The membrane filter was immediately placed into a 5 mL bead beating tube containing 1.5 g of a 1:1 mixture of 1 mm and 0.5 mm garnet shards (BioSpec, Bartlesville, OK, USA) and 1 mL of 1X Zymo DNA/RNA Shield solution (Zymo Research, Irvine, CA, USA). The filter was vortexed in the bead beating tube at maximum speed (3200 RPM) for 5 min using the Qiagen Vortex-Genie 2 Vortex Adapter (Qiagen, Valencia, CA, USA). The bead beating tube was then centrifuged at 4000 g for 3 min at 4 °C. After centrifugation, the sample lysate was transferred from the bead beating tube into a clean 1.5 mL tube and centrifuged at 16,000 g for 1 min to remove additional beads and debris. 600 μL of sample lysate was then added to an Eppendorf Deepwell Plate 96/2000 μL (Eppendorf, Hamburg, Germany) and total nucleic acids were extracted using the Zymo Quick-DNA/RNA Viral MagBead Kit (Zymo Research, Irvine, CA, USA). The only modification of the manufacturer's protocol was that the initial volume of viral DNA/RNA buffer was adjusted to 1200 μL to accommodate 600 μL of sample. Following extraction, the 50 μL extracted and eluted nucleic acid was passed through a Zymo OneStep PCR Inhibitor Removal Kit column (Zymo Research, Irvine, CA, USA) following the manufacturer's protocol. For quality control, a negative control filter blank was processed through the concentration and extraction steps and a positive extraction control of RNA from lysed SARS-CoV-2 (virus isolated from a SARS-CoV-2 positive patient and grown in Vero-E6 cells) was used in the extraction step. Final nucleic acid extracts were analyzed by reverse transcription quantitative polymerase chain reaction (rRT-qPCR) analyses and remaining aliquots stored at −80 °C. Our spike recovery studies indicate this method yields approximately 29.21% recovery of the SARS-CoV-2 surrogate, Human Coronavirus OC-43, RNA from wastewater (manuscript in preparation). Genomic copies reported were not corrected for recovery and are therefore an underestimation of viral genomic copies in the wastewater samples.
2.5. rRT-qPCR
The Centers for Disease Control and Prevention (CDC) N2 genetic target was used for SARS-CoV-2 detection and quantification by rRT-qPCR (CDC, 2020). Primer sequences for this target were: CDC N2 Forward: 5′ TTACAAACATTGGCCGCAAA 3’; CDC N2 Reverse: 5′ GCGCGACATTCCGAAGAA 3’; CDC N2 Probe: 5’ FAM-ACA ATT TGC/ZEN/CCC CAG CGC TTC AG-3IABkFQ. For sample quantification, an 8-point standard curve of 1:5 dilutions ranging from 100,000 to 1.28 CDC N2 genomic copies/μL was generated using the 2019-nCoV_N_positive control DNA plasmid from Integrated DNA Technologies (Integrated DNA Technologies, Coralville, IA). rRT-qPCR assays were performed in 25 μL reaction mixtures using the SuperScript III Platinum One-Step rRT-qPCR Kit (Thermo Fisher Scientific, Grand Island, NY, USA). Each reaction mixture contained 12.5 μL of 2X Reaction Mix, 0.5 μL of the SuperScript III/Platinum Taq Mix, 1.8 μL of 2019-nCoV_N2 Combined Primer/Probe Mix (Integrated DNA Technologies, Coralville, IA) consisting of 6.7 μM CDC N2 forward and reverse primers and 1.7 μM of CDC N2 probe, 5.2 μL of molecular grade water, and 5 μL of sample template. A negative filter blank control and positive extraction control (described above) were used in each rRT-qPCR assay. The rRT-qPCR assay was performed using the Bio-Rad CFX96 Thermal Cycler (Bio-Rad Laboratories, Richmond, CA) using the following cycle conditions: 50 °C for 20 min, 95 °C for 2 min, followed by 45 cycles of 95 °C for 15 s, 57 °C for 30 s, and 68 °C for 10 s. The assay limit of detection was set at a cycle threshold (Cq) cutoff value ≤ 40 cycles, as recommended by the CDC for wastewater surveillance (https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/wastewater-surveillance/data-reporting-analytics.html), to determine a positive sample. The Cq was calculated using a regression threshold determination method. The assay limit of quantification was the lowest detectable dilution concentration of the standard curve. Assays were included for data analysis if the standard curve amplification efficiency was within the accepted range (90–110%), and passed negative filter blank and positive extraction quality control measures. Quantifiable samples were back calculated to Log10 genomic copies/L Positive and quantifiable wastewater sample results were reported as data representing an entire week (Monday-Sunday) for comparisons with weekly reported Cedar Key chamber of commerce visitor totals.
2.6. Weekly tourism frequency data
The City of Cedar Key tracks visitor traffic on the island by keeping a tally of daily visitors to the city's Chamber of Commerce Visitor Center. These daily visitor logs were made available to researchers upon request. Daily visitor totals were summed for each week to serve as our proxy indicator for weekly number of tourists to the City of Cedar Key.
2.7. Study covariates
Since Cedar Key experiences high visitor traffic from Floridians that likely contributes to the wastewater SARS-CoV-2 concentrations, state level vaccination data was used to account for changes in vaccination rates during the study period among Florida resident visitors to Cedar Key. Florida's vaccination rates may serve as a temporal confounder in our study because vaccination rates are plausibly linked with both the exposure of interest (level of tourism) and the outcome of interest (presence of SARS-CoV-2 in wastewater). We used the daily FL DOH COVID-19 vaccination summaries (http://ww11.doh.state.fl.us/comm/_partners/covid19_report_archive/vaccine/) to obtain the weekly total number of individuals in the state of Florida who either were partially or fully vaccinated against COVID-19 from.
Ambient temperature may also serve as a plausible temporal confounder in our study because ambient temperature has previously been found to be associated with COVID-19 cases (Mecenas, Bastos, Vallinoto and Normando, 2020; Xie and Zhu, 2020) and temperature can influence levels of tourism during different seasons (Matzarakis, 2006). Hence, we gathered daily ambient temperature data from the National Oceanic and Atmospheric Administration online climate data search (https://www.ncdc.noaa.gov/cdo-web/search) from Lower Suwannee, FL (Field Station USR0000FLSU) and used the daily average temperatures to generate the weekly average temperature reported in degrees Celsius.
2.8. Statistical analysis
For the key study variables described above, we computed summary statistics such as frequencies, means, and their distributions. We performed unadjusted and adjusted Poisson and linear regression analysis to test our hypothesis that the number of weekly visitors to the Cedar Key Chamber of Commerce is associated with SARS-CoV-2-positive wastewater sample outcomes and also associated with the quantified concentration of SARS-CoV-2 RNA in wastewater. Statewide vaccinations and ambient temperature were included in adjusted models to control for possible confounding (Li et al., 2021). To substantiate the link between SARS-CoV-2 transmission and local tourism in Cedar Key, analyses were conducted using a fully adjusted model containing both statewide vaccination and ambient temperature, as well as models adjusted for only statewide vaccination or ambient temperature. Each model was analyzed to determine if the weekly total number of individuals vaccinated in Florida is associated with wastewater surveillance SARS-CoV-2 outcomes (SARS-CoV-2 positive sample detection and quantified SARS-CoV-2 wastewater concentration) in Cedar Key during the study period. All regression analyses were conducted using the mgcv package in R (Wood, 2006).
2.9. Sanger sequencing of SARS-CoV-2 directly from wastewater samples
In order to detect the presence of SARS-CoV-2 variants, we used Sanger sequencing of virus RNA that had been purified directly from wastewater samples with Cq values of 20–40. RNA was extracted from wastewater samples (as above) and stored at −80 °C. Amplicons for RT-PCR were designed to be ∼1500 bp, based on the efficiency of the high-fidelity reverse transcriptase that was used (AccuScript RT). cDNA synthesis reactions were catalyzed by AccuScript High Fidelity 1st Strand cDNA Synthesis Kit (Agilent, Santa Clara, CA). The completed first-strand cDNA synthesis reaction was placed on ice for use in downstream applications and any remainder stored at −80 °C. PCR reactions were carried out with Q5 Hot Start high fidelity polymerase (New England Biolabs, Ipswich, MA). RACE reactions (FirstChoice™ RLM-RACE Kit, ThermoFisher Scientific) were performed following the manufacturer's instructions, with the following modification: 3′ RACE was performed with 3′ primers 3′RACEF and 3′RACER. The resulting PCR amplicons were cloned using a Zero Blunt PCR cloning kit with TOP10 chemically competent E. coli (ThermoFisher Scientific). Inserts in at least three resulting plasmids were sequenced for each PCR amplicon cloned. Sequencing primers are listed in Supplemental Table 2 and are based on primers described by Cotten et al., (2021).
2.10. Virus phylogeny cladogram
A rectangular cladogram was created using the program Complete Tree available through the NCBI SARS-CoV-2 Data Hub using parameters specified by the program. This program aligns sequences to NCBI Reference sequence NC_045512.2, which is the complete genomic sequence of SARS-CoV-2 Hu/Wuhan-1. As specified by the program, distances are computed between pairs of sequences as the number of different mutations, where non-ambiguous different mutations are counted as 1, and ambiguous vs. non-ambiguous different mutations are counted as 0.1 (one sequence has a non-ambiguous mutation and another sequence has an ambiguous mutation in the same place).
3. Results
3.1. Detection of SARS-CoV-2 in wastewater
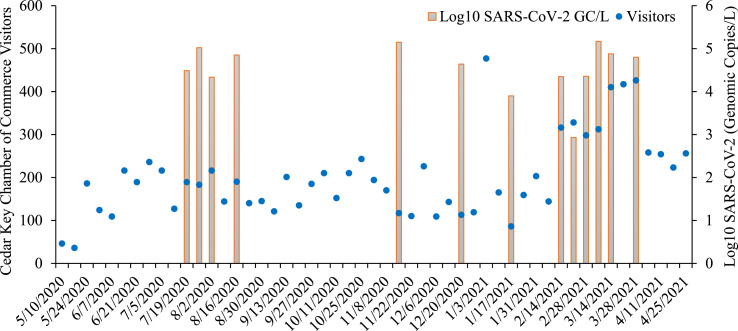
Among the 52 wastewater samples tested, 15/52 (28.8%) were positive for SARS-CoV-2 and 13/15 (86.7%) of the positive samples yielded quantifiable values. Positive, quantifiable sample values are shown in Fig. 2 . The first wastewater sample to test positive for SARS-CoV-2 was July 18, 2020. The mean quantified value of CDC N2 genomic copies (GC) among the positive samples was 4.63 Log10 GC/L. There were two samples that were detectable by rRT-qPCR but were unable to be quantified, as their threshold cycles were higher than the lowest concentration standard on the standard curve. These two samples were taken November 11, 2020 and December 4, 2020.
Fig. 2.
Time series plot of SARS-Co-V-2 positive influent wastewater sample genomic copy concentrations (Log10 GC/L) and the weekly Chamber of Commerce visitors in Cedar Key, FL.
3.2. Analysis of SARS-CoV-2 measurements in wastewater and Cedar Key visitors
Results from the measurements of SARS-CoV-2 in Cedar Key wastewater and the weekly number of visitors to the Cedar Key Chamber of Commerce are presented in Fig. 2. The descriptive statistics of the variables used in the statistical analysis are listed in Table 1 . The unadjusted (Model 1) and adjusted models (Models 3–4) for obtaining a positive wastewater sample from weekly number of visitors to the Cedar Key Chamber of Commerce and study covariates are in Table 2 . Under the unadjusted model (Model 1) for Cedar Key Chamber of Commerce visitors, the odds of obtaining a SARS-CoV-2 positive wastewater sample increased by 96% for every 100 weekly visitors (OR = 1.01; 95% CI: 1.00,1.01) (Table 2). Under the fully adjusted (Model 4), controlling for both ambient temperature and total number of vaccinated individuals, there was an increased odds of obtaining a SARS-CoV-2 positive wastewater sample for each weekly visitor to the Cedar Key Chamber of Commerce (aOR = 1.04; 95%CI:1.00,1.15) (Table 2). The unadjusted (Model 1) and adjusted models (Models 2–4) parameter estimates of the linear association with SARS-CoV-2 Log10 GC/L with weekly number of visitors to the Cedar Key Chamber of Commerce and study covariates are in Table 3 . In the unadjusted model (Model 1), there was a positive linear association between Cedar Key Chamber of Commerce visitors and the SARS-CoV-2 viral wastewater concentration increased by 0.44 (95%CI: 0.01,0.86) for every 100 weekly visitors to the Cedar Key Chamber of Commerce (Table 3). Under the fully adjusted (Model 4), controlling for both ambient temperature and total number of vaccinated individuals, for every 100 weekly visitors to the Cedar Key Chamber of Commerce the SARS-CoV-2 viral concentration increased by 1.06 Log10 GC/L (95% CI: 0.11,2.01) (Table 3).
Table 1.
Descriptive statistics for wastewater association analyses between May 1, 2020 and April 30, 2021 in Cedar Key, Florida.
| Parameter | Mean (±SD) |
|---|---|
| Number of weekly visitors according to Cedar Key Chamber of Commerce (n = 51 weeks) | 196.90 (94) |
| Wastewater SARS-CoV-2 concentration (Log10 Genomic Copies/L) (n = 51 weeks) | 2.05 (1.49) |
| Weekly Average Ambient Temperature (°C) (n = 51 weeks) | 21.23 (5.69) |
| Weekly Total Individuals Vaccinated in Floridaa (n = 14 weeks) | 4,429,324 (2,422,500.8) |
Vaccination data are from January 18 to April 30, 2021.
Table 2.
Unadjusted and adjusted odds ratios of weekly visitors, weekly ambient temperature and weekly vaccination levels for positive SARS-CoV-2 detection in wastewater between May 1, 2020 and April 30, 2021 in Cedar Key, Florida (Ct ≤ 40). OR=Odds Ratio; aOR = adjusted Odds Ratio.
| Parameter | OR (95%CI)a | aOR (95%CI)b | aOR (95%CI)c | aOR (95%CI)d |
|---|---|---|---|---|
| Cedar Key Chamber of Commerce Weekly Visitors | 1.01 (1.00,1.01) | 1.01 (0.99,1.01) | 1.04 (1.01,1.15) | 1.04 (1.00,1.15) |
| Ambient Temperature | 0.96 (0.86,1.08) | 0.97 (0.86,1.10) | – | 2.08 (0.47,29.93 |
| Weekly Total Individuals Vaccinated in Florida | 0.99 (0.99,1.00) | – | 0.99 (0.99,0.99) | 0.99 (0.99,0.99) |
Model 1-Unadjusted models.
Model 2-Cedar Key Chamber of Commerce Weekly Visitors adjusted for Ambient Temperature.
Model 3-Cedar Key Chamber of Commerce Weekly Visitors adjusted for Weekly Total Individuals Vaccinated in Florida.
Model 4-Cedar Key Chamber of Commerce Weekly Visitors adjusted for Ambient Temperature and Weekly Total Individuals Vaccinated in Florida.
Table 3.
Unadjusted and adjusted parameter estimates of weekly visitors, weekly ambient temperature, and weekly vaccination levels for SARS-CoV-2 concentrations in wastewater between May 1, 2020 and April 30, 2021 in Cedar Key, Florida. (Log10Genomic Copes/L of Wastewater).
| Parameter | Parameter Estimate (95%CI)a | Parameter Estimate (95%CI)b | Parameter Estimate (95%CI)c | Parameter Estimate (95%CI)d |
|---|---|---|---|---|
| Cedar Key Chamber of Commerce Weekly Visitors (Per 100 Visitors) | 0.44 (0.01,0.86) | 0.43 (−0.003,0.87) | 1.33 (0.54,2.11) | 1.06 (0.11,2.01) |
| Ambient Temperature (Per 10 °C) | −0.15 (−0.89,0.58) | −0.09 (−0.80,0.63) | – | 2.19 (−2.10,6.47) |
| Weekly Total Individuals Vaccinated in Florida (Per 100 vaccinated individuals) | −7.02e-05 (−5.64e-05, -2.15e-05 | – | −2.62e-05 (−5.55e-05, -3.17e-06) | −4.39e-05 (--8.93e-05, -1.56e-06) |
Model 1-Unadjusted models.
Model 2-Cedar Key Chamber of Commerce Weekly Visitors adjusted for Ambient Temperature.
Model 3-Cedar Key Chamber of Commerce Weekly Visitors adjusted for Weekly Total Individuals Vaccinated in Florida.
Model 4-Cedar Key Chamber of Commerce Weekly Visitors adjusted for Ambient Temperature and Weekly Total Individuals Vaccinated in Florida.
3.3. Sequencing of SARS-CoV-2 isolates from wastewater
Sanger sequencing was used to obtain SARS-CoV-2 genome sequences from wastewater for two reasons: 1) next-generation sequencing (NGS) platforms typically require a SARS-CoV-2 RT-qPCR Cq value ≤ 20 to obtain adequate sequence coverage, which is rare in environmental samples, and 2) when different virus lineages are present, computer-assisted programs can reconstruct a false consensus virus genomic sequence(s). By using Sanger sequencing, we are able to obtain sequences using lower amounts of virus RNA (the procedure has worked for samples up to a Cq of 38), and non-overlapping amplicons can be cloned and authentic sequences assembled with a high degree of confidence. Based on our experience and due to the emergence of SARS-CoV-2 variants, we chose primers described by Cotten et al., (2021) for our sequencing strategy (Supplemental Table 2). Only one primer (primer MR10-39) required modification.
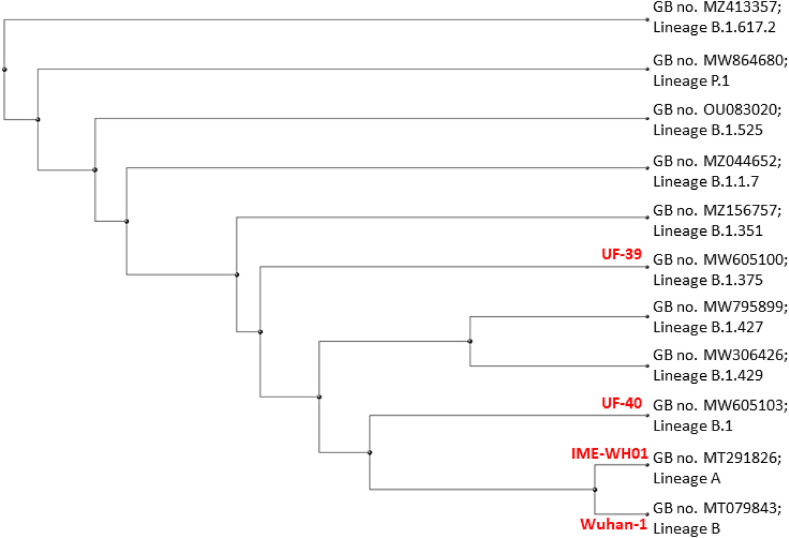
Results from sequencing are presented in Table 4 . Virus sequences were compared to known SARS-CoV-2 sequences from GISAID (Table 5 ) and genetic relatedness is presented on a rectangular cladogram (Fig. 3 ). SARS-CoV-2/environment/USA/UF-40/2020 – Genome sequence alignment of UF-40 with the SARS-CoV-2 reference strain Wuhan-Hu-1 (GenBank no. NC_045512.2) revealed 99.95% identity (29881/29895 nt) and no sequence gaps. The deduced amino acid substitutions in UF-40 are: Spike D614G, Spike T29I, NS3 Q57H, NSP2 T85I, NSP12 A656S, NSP12 P323L, NSP13 I195T, and NSP13 T599I. The D614G mutation in the spike protein is dominant in circulating SARS-CoV-2 strains around the world (Korber et al., 2020; Plante et al., 2021). The virus has specific rnt markers C241T, C3037T, A23403G, G25563T S-D614G + NS3-Q57H, which indicate that the virus belongs in GISAID clade GH, which along with clade G, was one of the two predominant clades in North America in August 2020 [https://gisaid.org/hcov-19-analysis-update]. The genome sequence of UF-40 conforms to Phylogenetic Assignment of Named Global Outbreak Lineages (PANGO) (Rambaut et al., 2020) lineage B.1 (version: 2021-04-21), which is a large European lineage that originated in Northern Italy in 2020 and one that was detected in the United States early in 2020 [https://cov-lineages.org/lineages/lineage_B.1.html]. Not surprisingly, NCBI BLAST analyses indicated UF-40 has high rnt identity with SARS-CoV-2 strains that were in circulation in the United States early in 2020, such as SARS-CoV-2/human/USA/AZ-ASU2936/2020 [GenBank MT339041.1; rnt identity 29887/29895 (99.97%)], from a human nasopharyngeal specimen collected in Arizona on March 17, 2020.
Table 4.
Wastewater samples sequenced.
| Date composite sample collected | Sequencing Result | Virus strain designation [GenBank accession no.] |
|---|---|---|
| 8/11/20 | Successful | SARS-CoV-2/environment/USA/UF-40/2020 [MW605103] |
| 11/13/20 | Overlapping sequences indicate multiple virus strains present | N/A |
| 12/13/20 | Overlapping sequences indicate multiple virus strains present | N/A |
| 1/12/21 | Successful | SARS-CoV-2/environment/USA/UF-39/2021 [MW605100] |
Table 5.
Virus strain and accession numbers used for construction of rectangular cladogram.
| SARS-CoV-2 strain | Notes | GenBank no. | GISAID no. | GISAID clade | PANGO lineage |
|---|---|---|---|---|---|
| Wuhan-Hu-01 | NCBI reference strain; from China. | NC_045512.2 | EPI_ISL_402124 | L | B |
| Wuhan/IME-WH01/2019 | Early strain from China. | MT291826 | EPI_ISL_529213 | S | A |
| Environment/USA/FL-UF-40/2020 | This study. | MW605103 | EPI_ISL_1273075 | GH | B.1 |
| USA/CA-CZB-12872/2020 | Variant first detected in California, USA. | MW306426.1 | EPI_ISL_648527 | GH | B.1.429 |
| hCoV-19/USA/UT-UPHL-2103140561/2021 | Variant first detected in Utah, USA. | MW795899.1 | EPI_ISL_1313734 | GH | B.1.427 |
| Environment/USA/FL-UF-39/2021 | This study. | MW605100 | EPI_ISL_1273074 | GH | B.1.375 |
| SARS-CoV-2/human/USA/VSP 1974/2021 | Variant first detected in S. Africa. | MZ156757.1 | EPI_ISL_2003978 | GH | B.1.351 |
| hCoV-19/England/NORW- 22B902/2021 | Variant first detected in the UK. | MZ044652.1 | EPI_ISL_1178459 | GRY | B.1.1.7 |
| England/CAMC-C769B3/2020 | Variant jointly detected in the UK and Nigeria. | OU083020.1 | EPI_ISL_760883 | G | B.1.525 |
| SARS-CoV-2/human/USA/MA-CDC-STM-000029211/202 | Variant first detected in Brazil. | MW864680.1 | EPI_ISL_2494055 | GR | P.1 |
| India/GJ-GBRC814/2021 | Variant first detected in India. | MZ413357.1 | EPI_ISL_2562091 | G | B.1.617.2 |
Fig. 3.
Rectangular cladogram for genomic sequences of SARS-CoV-2 UF-39 and UF-40 and representative virus genomic sequences of key SARS-CoV-2 clades in circulation in the USA as of June 2021. The dimensions of the horizontal branches indicate changes over time within evolutionary lineages. The vertical dimension has no meaning and is used simply to lay out the tree visually with the labels evenly spaced vertically. SARS-CoV-2 UF-39 and -40 fall within clades B.1.375 and B.1, and their genomes differ from SARS-CoV-2 Wuhan-1 by 27 and 14 rnt, respectively, and from IME-WH01 by 30 and 17 nt.
SARS-CoV-2 environment/USA/UF-39/2021 – Genome sequence alignment of UF-39 with SARS-CoV-2 strain Wuhan-Hu-1 revealed 99.9% identity (29,863/29,890 nt). There is a 6 nt gap in the spike protein gene coding sequence corresponding to Spike H69del, Spike V70del. The deduced amino acid substitutions in UF-39 are Spike D614G, Spike H69del, Spike V70del, M I48V, N L139F, N T205I, NS3 G49V, NS3 Q57H, NS3 T151I, NSP2 T85I, NSP3 K412N, NSP3 T1010A, NSP10 A104V, NSP12 P323L, NSP13 E341D, NSP16 A258V, and NSP16 R216C. The virus has specific rnt markers C241T, C3037T, A23403G, G25563T S-D614G + NS3-Q57H, which indicate that the virus belongs in GISAID clade GH, which was the predominant clade in North America in January 2021 [https://www.gisaid.org/hcov19-variants/], when UF-39 was collected from wastewater. It has D614G, which is dominant in currently circulating global SARS-CoV-2 strains. The genome sequence of UF-39 conforms to PANGO lineage B.1.375 (version: 2021-04-21), whose peak circulation in the United States was in January 2021 [https://cov-lineages.org/lineages/lineage_B.1.375.html]. Noteworthy, Spike H69del and V70del are hypothesized to increase transmissibility (Kemp et al., 2021; McCarthy et al., 2021). Nucleotide BLAST analysis indicated that UF-39 is highly related to SARS-CoV-2 strains such as SARS-CoV-2/human/USA/PA-CDC-Q2EF-8401/2021 (GenBank MW646945.1; nt identity of 29880/29884) and SARS-CoV-2/human/USA/TN-CDC-8630/2020 (GenBank MW490628.1; nt identity of 29880/29884).
4. Discussion
Wastewater surveillance for SARS-CoV-2 has emerged as a significant tool to provide information about presence, prevalence, and emergence of new variants around the world. The goal of this practice is to inform public health responses by gaining more information about transmission in local communities. However, few studies have focused the use of this tool to investigate how these measurements may be influenced by visitors to small communities. Our study conducted weekly wastewater surveillance for SARS-CoV-2 in the small coastal community of Cedar Key, FL. While the earliest detection of the virus in wastewater in the US occurred in April 2020 (Sherchan et al., 2020), Cedar Key did not see its first SARS-CoV-2 positive wastewater sample until July 18th, 2020, indicating that introduction of the virus to this community may have lagged behind the rest of the US. There were only 4 cumulative clinically confirmed COVID-19 cases in the zip code encompassing Cedar Key before our first positive wastewater sample, demonstrating the analytical sensitivity of SARS-CoV-2 detection in wastewater. Additionally, we detected the presence of the virus in a wastewater sample when there were no clinically confirmed COVID-19 cases in the reporting region (FL DOH, 2020). A contributing factor to this delay was likely the city's restriction of access only to Cedar Key residents between March 24th to May 5th, 2020. Because Cedar Key only has a single road for access (Florida State Road 24), they were able to initiate a checkpoint for confirmation of residence before providing access. The first wastewater sample was collected from the Cedar Key Water and Sewer District treatment plant influent on May 1st before the city was re-opened to the general population, and weekly thereafter. The first positive detection in July 2020 followed weeks of increased tourist traffic as measured by Cedar Key Chamber of Commerce visitors (Fig. 2). Following this initial positive sample there were four weeks of SARS-CoV-2 detection in wastewater coinciding with the summer tourist season. From the week of 8/23/2020 through June 2, 2021 there was only intermittent detection of the virus in wastewater samples. However, beginning the week of 2/14/2021 there was a marked increase in visitor traffic to the Cedar Key Chamber of Commerce, which coincided with eight consecutive weeks of SARS-CoV-2 positive wastewater samples. Beginning the week of April 4, 2021, there was a sharp drop in visitor traffic, which also coincided with negative samples until the end of the study on 4/30/2021.
While numerous studies have found positive associations between COVID-19 cases and SARS-CoV-2 detection in wastewater (Gerrity et al., 2021; Graham et al., 2021; Peccia et al., 2020b; Randazzo et al. 2020a, 2020b), no published studies have investigated whether tourists or transient visitors can influence SARS-CoV-2 in municipal wastewater. However, positive associations between tourism and wastewater detection of virus are likely considering the documented associations between COVID-19 cases and tourism. For example, Correa-Martínez et al. (2020) found that visitors to a local ski area likely sparked an outbreak of COVID-19 cases in Ischgl, Austria. A review of tourism and COVID-19 cases in 90 countries estimated that every 1% of inbound and outbound tourists results in a 1.2% and 1.4% increase in confirmed COVID-19 cases, respectively (Farzanegan et al., 2021). We were not able to determine a significant association between measurement of SARS-CoV-2 in a wastewater sample by 96% for every 100 visitors to the Cedar Key Chamber of Commerce. Our unadjusted model (Model 1) results revealed the odds of obtaining a positive measurement of SARS-CoV-2 in a wastewater sample by 96% for every 100 visitors to the Cedar Key Chamber of Commerce, however, this significant association went away after adjusted for all potential confounders (Table 2). While initially, the linear association between Chamber of Commerce visitors and the SARS-CoV-2 wastewater concentration was relatively small (Model 1), after adjustment for all potential confounders (Model 4), the positive linear association between these two variables increased to 1.06 Log10 GC/L for every 100 visitors to Cedar Key (Table 3). We examined statewide vaccination rates as a potential confounder on our wastewater results as vaccinations influence population mobility and overall SARS-CoV-2 infection rates within the community. Adjusting only for statewide vaccination (Model 3), the association between visitors to Cedar Key and the SARS-CoV-2 wastewater concentration was 1.33 Log10 GC/L for every 100 weekly Chamber of Commerce Visitors (Table 3). We also examined ambient temperature as a factor in genomic copies of the virus in wastewater due to the influence of ambient temperature on population mobility and tourism. Results from this analysis indicated that adjusting for ambient temperature (Model 2), there was a 0.43 Log10 GC/L increase in SARS-CoV-2 wastewater concentrations for every 100 weekly Chamber of Commerce visitors (Table 3). These results indicate that both vaccinations and ambient temperature may confound the association between wastewater measurements of SARS-CoV-2 and detection of SARS-CoV-2 in Cedar Key wastewater. Our findings of a non-significant association between ambient temperature and SARS-Cov-2 wastewater outcomes are not consistent with a previous study that described a significant positive association (Li et al., 2021). Ambient temperature as an explanatory variable for SARS-CoV-2 wastewater outcomes should be further investigated to determine its usefulness in future analyses. While no one has examined the relationship between SARS-CoV-2 measurements in wastewater and vaccination rates, multiple studies have found that vaccines are effectively decreasing COVID-19 cases across the world, so we might expect our results to indicate as much. An increasingly vaccinated population may be a contributing factor to a drop in SARS-CoV-2 positive samples starting on April 4, 2021 as the vaccine became available to all persons over 40 on 3/26/2021 and all persons over 18 years of age on May 4, 2021 in Florida.
Given our observation that weekly tourism levels are positively associated with SARS-CoV-2 measurements in Cedar Key weekly wastewater samples, it follows that COVID-19 cases would also be positively associated within the city. Unfortunately, this analysis was not possible due to a lack of resolution in FL DOH COVID-19 case data (discussed below in limitations section). However, our wastewater samples did provide an opportunity to examine the introduction of new viral variants within the City of Cedar Key. Many studies have used wastewater to examine the introduction of SARS-CoV-2 variants (Fontenele et al., 2021; Izquierdo-Lara et al., 2021; La Rosa et al., 2020; Nemudryi et al., 2020; Rios et al., 2021; Rothman et al., 2021). Crits-Christoph et al. (2021) sequenced multiple variants of the virus in wastewater and found that most variants were similar to those found in regional clinical samples. However, they were able to periodically detect variants from outside of the region indicating that wastewater could be used to identify introduction of new variants to the region. As part of this study we successfully sequenced a viral genome (designated as UF-40) and were able to show it is representative of SARS-CoV-2 strains that originated in Italy and spread throughout the United States early during the COVID-19 pandemic, and belongs in PANGO SARS-CoV-2 genetic lineage B.1. This particular SARS-CoV-2 genetic lineage has been supplanted by others and is now infrequently encountered in the United States, but its detection in a sample collected in August 2020 is consistent with its previous detection and circulation patterns. There was no clinically identified COVID-19 outbreak on the island after UF-40 was detected, indicating that infection control and quarantine practices may have stemmed a local outbreak. Wastewater positive samples from 11/14/2020 and 12/14/2020 were successfully sequenced, but were unable to be annotated due to overlapping sequences in areas of nucleotide divergence indicating the presence of multiple strains. However, the sample from December 1, 2021 was successfully sequenced and annotated (GenBank Accession No. MW605100). This virus variant (designated as UF-39) was likely spread to Cedar Key by a visitor from another state, since its PANGO genetic lineage is more closely related to that of the UK variant of concern (VOC) designated B.1.1.7 that was first detected in mid-2020 in other states. However, SARS-CoV-2 UF-39 is not considered a variant of concern (VOC), as it retains 484 E and 501 N in its spike protein. While we cannot be sure this was the first introduction of variants of SARS-CoV-2 to Cedar Key, since the previous two previous samples contained ambiguous sequences indicating multiple variants, it is a good indication that the first of the more virulent variants likely spread quickly to Cedar Key as the first B.1.1.7 variant was first detected in Florida 12/31/2020 and we detected a closely related variant just two weeks later.
While our study has observed a significant relationship between tourism and SARS-CoV-2 in wastewater and the utility of identifying new viral variants with the community, and equally important objective of our study was to inform city leaders so that they could make educated decisions regarding commerce, tourism, and safety precautions throughout the pandemic. From the start, we provided data and interpretations of the data to the City of Cedar key stakeholders on a weekly basis. Weekly data were reviewed at the city commission meetings and posted on the city's social media sites to keep residents informed about the presence of the virus in wastewater, and how these results related to the presence of SARS-CoV-2 in their community. These posts were accompanied by suggested public health precautions including social distancing, masking, and other recommendations. For example, while the city initially closed their roads to visitors (discussed above), monitoring of SARS-CoV-2 increased the confidence of the city council to re-open for commercial and consumer operations in the beginning of May. The highest weekly new case count for the Cedar Key zip code (32625) throughout the study period was 16 and the average weekly new case count was 2.05 ± 2.87 cases/week. However, not all these cases can be attributed to Cedar Key as postal ZIP code 32625 encompasses a larger geographic area than the city (discussed below). While we cannot be sure that SARS-CoV-2 wastewater surveillance data used by the city influenced these low case numbers, we can speculate that the recommendations based on these data was likely a contributing factor.
There were numerous limitations noted during our study that should be considered for future studies. First, we were only able to sample from the wastewater treatment plant once per week throughout the study. More frequent sampling may have enabled more robust analysis and possible significant associations between our wastewater measurements and other exploratory variables that were tested as transient visitors may not stay for a week at a time. Second, the Chamber of Commerce visitor data is used by the City of Cedar Key to track visitors to the city, however, this is not an absolute measurement of the number of visitors to the island. Third, while traffic data were not available for Cedar Key, future studies should consider installation of counters and/or Bluetooth tracking devices for a more accurate count of visitors. Finally, we would have liked to analyze the relationships between wastewater measurements of SARS-CoV-2, Chamber of Commerce visitors, and COVID-19 cases. However, the FL DOH codes the city of Cedar Key with the zip code of 32625, which encompasses a much larger area than the city of Cedar Key and the catchment area of the Cedar Key Water and Sewer District. Approximately 40% of the residents of the 32625 ZIP code live within the city limits of Cedar Key, which means that up to 60% of cases reported by the FL DOH are not represented in our wastewater surveillance catchment. As a result, we decided to exclude these analyses from this study.
5. Conclusions
Results of our study indicate that wastewater surveillance of SARS-CoV-2 is a viable tool for monitoring viral trends among small communities. We detected the virus multiple times throughout the 52-week study period and showed that the presence of virus in wastewater was statistically correlated with visitors to the City of Cedar Key. Results from these studies were used by the city to provide public messaging about public health precautions during times of positive detection in wastewater, potentially staving off outbreaks. Sequencing results indicated that initial samples from 2020 represented variants that were regionally widespread during that time. New variants that were closely related to the more virulent UK variant likely spread to Cedar Key shortly after they arrived in Florida at the end of 2020. While numerous studies have established the relationships between wastewater measurements of SARS-CoV-2 and COVID-19 cases, few have exhibited how these data can be used for decision making in small communities. Our partnership with Cedar Key demonstrates that WBE can be effectively applied in small rural communities.
Author contributions statement
Andrew L. Rainey: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Julia C. Loeb: Investigation. Sarah, E. Robinson: Investigation. John A. Lednicky: Formal analysis, Resources, Visualization, Writing – original draft. John McPherson: Investigation, Resources, Writing – review & editing. Sue Colson: Investigation, Writing – review & editing. Michael Allen: Resources. Eric S. Coker: Formal analysis. Tara Sabo-Attwood: Conceptualization, Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing. Anthony T. Maurelli: Conceptualization, Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing. Joseph H. Bisesi Jr.: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project Administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding source
University of Florida Department of Environmental and Global Health for funding.
Ethics statement
There were no animals or humans subjects used in this research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge the UF Department of Environmental and Global Health for funding. The authors would like to thank Sarah Waldo for her preparation of the map used in Fig. 1.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.112496.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., et al. First confirmed detection of sars-cov-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of covid-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar H., Diop O.M., Weldegebriel G., Malik F., Shetty S., El Bassioni L., et al. Environmental surveillance for polioviruses in the global polio eradication initiative. J. Infect. Dis. 2014;210(Suppl. 1):S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC 2019-novel coronavirus (2019-ncov) real-time rrt-pcr panel primers and probes. 2020. https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf Available:
- Correa-Martínez C.L., Kampmeier S., Kümpers P., Schwierzeck V., Hennies M., Hafezi W., et al. A pandemic in times of global tourism: superspreading and exportation of covid-19 cases from a ski area in Austria. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00588-20. e00588-00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Lule Bugembe D., Kaleebu P., Vtp M. Alternate primers for whole-genome sars-cov-2 sequencing. Virus Evol. 2021;7:veab006. doi: 10.1093/ve/veab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crits-Christoph A., Kantor R.S., Olm M.R., Whitney O.N., Al-Shayeb B., Lou Y.C., et al. Genome sequencing of sewage detects regionally prevalent sars-cov-2 variants. mBio. 2021;12 doi: 10.1128/mBio.02703-20. e02703-02720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide covid-19: the present and future. Sci. Total Environ. 2020;736:139631. doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorley R.M., Berke A., Noyman A., Alonso L.A., Ribo J., Arroyo V., et al. medRxiv; 2021. Mobility and Covid-19 in andorra: Country-Scale Analysis of High-Resolution Mobility Patterns and Infection Spread. [DOI] [PubMed] [Google Scholar]
- Falman J.C., Fagnant-Sperati C.S., Kossik A.L., Boyle D.S., Meschke J.S. Evaluation of secondary concentration methods for poliovirus detection in wastewater. Food Environ Virol. 2019;11:20–31. doi: 10.1007/s12560-018-09364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzanegan M.R., Gholipour H.F., Feizi M., Nunkoo R., Andargoli A.E. International tourism and outbreak of coronavirus (covid-19): a cross-country analysis. J. Trav. Res. 2021;60:687–692. [Google Scholar]
- FL Doh . 2020. Florida Covid-19 Dashboard. [Google Scholar]
- Fontenele R.S., Kraberger S., Hadfield J., Driver E.M., Bowes D., Holland L.A., et al. High-throughput sequencing of sars-cov-2 in wastewater provides insights into circulating variants. Water Res. 2021;205:117710. doi: 10.1016/j.watres.2021.117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity D., Papp K., Stoker M., Sims A., Frehner W. Early-pandemic wastewater surveillance of sars-cov-2 in southern Nevada: Methodology, occurrence, and incidence/prevalence considerations. Water Res. X. 2021;10:100086. doi: 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., et al. Covid-19 surveillance in southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186:116296. doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K.E., Loeb S.K., Wolfe M.K., Catoe D., Sinnott-Armstrong N., Kim S., et al. Sars-cov-2 rna in wastewater settled solids is associated with covid-19 cases in a large urban sewershed. Environ. Sci. Technol. 2021;55:488–498. doi: 10.1021/acs.est.0c06191. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. medRxiv; 2020. First Environmental Surveillance for the Presence of Sars-Cov-2 Rna in Wastewater and River Water in japan. 2020.2006.2004.20122747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., et al. Detection of pathogenic viruses in sewage provided early warnings of hepatitis a virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80:6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaconelli M., Bonanno Ferraro G., Mancini P., Suffredini E., Veneri C., Ciccaglione A.R., et al. Nine-year nationwide environmental surveillance of hepatitis e virus in urban wastewaters in Italy (2011–2019) Int. J. Environ. Res. Publ. Health. 2020;17:2059. doi: 10.3390/ijerph17062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Lara R., Elsinga G., Heijnen L., Munnink B.B.O., Schapendonk C.M., Nieuwenhuijse D., et al. Monitoring sars-cov-2 circulation and diversity through community wastewater sequencing, The Netherlands and Belgium. Emerg. Infect. Dis. 2021;27:1405. doi: 10.3201/eid2705.204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp S., Harvey W., Lytras S., Carabelli A., Robertson D., Gupta R. 2021. Recurrent Emergence and Transmission of a Sars-Cov-2 Spike Deletion H69/v70; p. 422555. bioRxiv:2020.2012.2014. [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking changes in sars-cov-2 spike: evidence that d614g increases infectivity of the covid-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., et al. First proof of the capability of wastewater surveillance for covid-19 in India through detection of genetic material of sars-cov-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., et al. First detection of sars-cov-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kulandaivelu J., Zhang S., Shi J., Sivakumar M., Mueller J., et al. Data-driven estimation of covid-19 community prevalence through wastewater-based epidemiology. Sci. Total Environ. 2021;789:147947. doi: 10.1016/j.scitotenv.2021.147947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W.J., Buisman A.M., Rutjes S.A., Heijne J.C., Teunis P.F., de Roda Husman A.M. Feasibility of quantitative environmental surveillance in poliovirus eradication strategies. Appl. Environ. Microbiol. 2012;78:3800–3805. doi: 10.1128/AEM.07972-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrajt G., Naughton B., Bandyopadhyay A.S., Meschke J.S. A review of the most commonly used methods for sample collection in environmental surveillance of poliovirus. Clin. Infect. Dis. 2018;67:S90–s97. doi: 10.1093/cid/ciy638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzarakis A. Weather-and climate-related information for tourism. Tourism Hospit. Plann. Dev. 2006;3:99–115. [Google Scholar]
- McCarthy K.R., Rennick L.J., Nambulli S., Robinson-McCarthy L.R., Bain W.G., Haidar G., et al. Recurrent deletions in the sars-cov-2 spike glycoprotein drive antibody escape. Science. 2021;371:1139–1142. doi: 10.1126/science.abf6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin R.G., Hendrickson E.N., Chaudhry N., Georgewill O., Freese R., Schacker T.W., et al. A novel wastewater-based epidemiology indexing method predicts sars-cov-2 disease prevalence across treatment facilities in metropolitan and regional populations. Sci. Rep. 2021;11:21368. doi: 10.1038/s41598-021-00853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., et al. Temporal detection and phylogenetic assessment of sars-cov-2 in municipal wastewater. Cell Reports Medicine. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., et al. Measurement of sars-cov-2 rna in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38:1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., et al. medRxiv; 2020. Sars-cov-2 Rna Concentrations in Primary Municipal Sewage Sludge as a Leading Indicator of Covid-19 Outbreak Dynamics. 2020.2005.2019.20105999. [Google Scholar]
- Plante J.A., Liu Y., Liu J., Xia H., Johnson B.A., Lokugamage K.G., et al. Spike mutation d614g alters sars-cov-2 fitness. Nature. 2021;592:116–121. doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Gaspar A.M.C., Miagostovich M.P. Detection of enteric viruses in activated sludge by feasible concentration methods. Braz. J. Microbiol. 2014;45:343–349. doi: 10.1590/s1517-83822014000100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A., Holmes E.C., O'Toole Á., Hill V., McCrone J.T., Ruis C., et al. A dynamic nomenclature proposal for sars-cov-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Cuevas-Ferrando E., Sanjuán R., Domingo-Calap P., Sánchez G. Metropolitan wastewater analysis for covid-19 epidemiological surveillance. Int. J. Hyg Environ. Health. 2020;230:113621. doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. Water Research; 2020. Sars-cov-2 Rna in Wastewater Anticipated Covid-19 Occurrence in a Low Prevalence Area; p. 115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., et al. Presence and infectivity of sars-cov-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios G., Lacoux C., Leclercq V., Diamant A., Lebrigand K., Lazuka A., et al. Monitoring sars-cov-2 variants alterations in nice neighborhoods by wastewater nanopore sequencing. The Lancet Regional Health - Europe. 2021;10:100202. doi: 10.1016/j.lanepe.2021.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.A., Loveless T.B., Kapcia J., Adams E.D., Steele J.A., Zimmer-Faust A.G., et al. Rna viromics of southern California wastewater and detection of sars-cov-2 single-nucleotide variants. Appl. Environ. Microbiol. 2021;87 doi: 10.1128/AEM.01448-21. e01448-01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., et al. First detection of sars-cov-2 rna in wastewater in north America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu J.P.S., Ahmed W., Toze S. Sensitive detection of human adenovirus from small volume of primary wastewater samples by quantitative pcr. J. Virol Methods. 2013;187:395–400. doi: 10.1016/j.jviromet.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Sinclair R.G., Choi C.Y., Riley M.R., Gerba C.P. Pathogen surveillance through monitoring of sewer systems. Adv. Appl. Microbiol. 2008;65:249–269. doi: 10.1016/S0065-2164(08)00609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., et al. Wastewater surveillance of sars-cov-2 across 40 u.S. States from february to june 2020. Water Res. 2021;202:117400. doi: 10.1016/j.watres.2021.117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., et al. Evaluation of lockdown effect on sars-cov-2 dynamics through viral genome quantification in waste water, greater paris, France, 5 march to 23 april 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N.A., Tharpe C., Meschke J.S., Ferguson C.M. Survey of rapid development of environmental surveillance methods for sars-cov-2 detection in wastewater. Sci. Total Environ. 2021;769:144852. doi: 10.1016/j.scitotenv.2020.144852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.