Key Words: apoptosis, brain, central nervous system, ischemia/reperfusion, middle cerebral artery occlusion, necroptosis, oxygen and glucose deprivation, PANoptosis, pyroptosis, regulated cell death
Abstract
Some scholars have recently developed the concept of PANoptosis in the study of infectious diseases where pyroptosis, apoptosis and necroptosis act in consort in a multimeric protein complex, PANoptosome. This allows all the components of PANoptosis to be regulated simultaneously. PANoptosis provides a new way to study the regulation of cell death, in that different types of cell death may be regulated at the same time. To test whether PANoptosis exists in diseases other than infectious diseases, we chose cerebral ischemia/reperfusion injury as the research model, collected articles researching cerebral ischemia/reperfusion from three major databases, obtained the original research data from these articles by bibliometrics, data mining and other methods, then integrated and analyzed these data. We selected papers that investigated at least two of the components of PANoptosis to check its occurrence in ischemia/reperfusion. In the cell model simulating ischemic brain injury, pyroptosis, apoptosis and necroptosis occur together and this phenomenon exists widely in different passage cell lines or primary neurons. Pyroptosis, apoptosis and necroptosis also occurred in rat and mouse models of ischemia/reperfusion injury. This confirms that PANoptosis is observed in ischemic brain injury and indicates that PANoptosis can be a target in the regulation of various central nervous system diseases.
Introduction
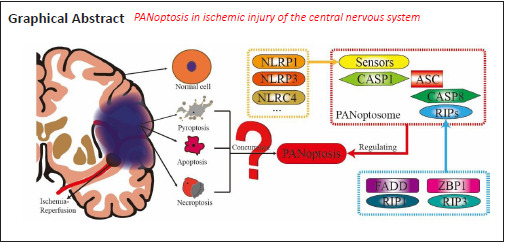
Researchers studying forms of cell death found that the main processes of regulated cell death (RCD) included pyroptosis, apoptosis and regulated necrosis (including necroptosis) (Chen et al., 2021; Hu et al., 2021; Yan et al., 2021). The majority of the research topics on RCD focused on one of these three forms of cell death alone, but a few focused on the simultaneous interaction of these three forms of cell death. Some previous reports into cancer or bacterial/viral infection found that the key regulatory proteins of pyroptosis, apoptosis and necroptosis interacted with each other (Malireddi et al., 2010; Gurung et al., 2014, 2016; Malireddi et al., 2018, 2020b; Jiang et al., 2021; Meng et al., 2021). However, it was not clear how the regulatory mechanisms of pyroptosis, apoptosis and necroptosis intersected. The later research indicated that an innate immune response can simultaneously regulate pyroptosis, apoptosis and necroptosis after the transforming growth factor beta-activated kinase 1 (TAK1) was suppressed or knocked out (Malireddi et al., 2019, 2020b). This view was confirmed in research on coronavirus disease 2019 (COVID-19) (Karki et al., 2021). This suggests that, in the pathophysiological process of some diseases, pyroptosis, apoptosis and necroptosis can occur and be regulated at the same time. In a study by the team of Professor Kanneganti (Malireddi et al., 2019), this phenomenon when pyroptosis (P), apoptosis (A) and necroptosis (N) are regulated at the same time was named PANoptosis, and they showed that there is a multimeric protein complex, named a PANoptosome (Christgen et al., 2020; Samir et al., 2020), that can regulate the occurrence of PANoptosis.
A series of studies on PANoptosis reported by the Kanneganti team (Karki et al., 2020b, 2021; Kesavardhana et al., 2020; Malireddi et al., 2020a; Zheng et al., 2020; Briard et al., 2021) suggest that, in diseases caused by bacterial, fungal or viral infection, pathogens induce the autoimmune response and produce various inflammatory cytokines. These inflammatory cytokines activate the promoter proteins of pyroptosis, apoptosis and necroptosis through specific pathways, and drive them to assemble inflammasomes that are specific to different RCD forms (Cain et al., 2000; Chu et al., 2001; Acehan et al., 2002; Martinon et al., 2002; Agostini et al., 2004; Ogura et al., 2006; Kanneganti et al., 2007; Wallach et al., 2011; Lu et al., 2019b), and further assemble a protein complex, PANoptosome (Samir et al., 2020), that can simultaneously drive pyroptosis, apoptosis and necroptosis to aggravate cell death caused by the pathogens. Apart from diseases caused by pathogens, most other diseases or pathological conditions are more or less related to an immune response, which suggests that PANoptosis associated with an immune response is highly probable. For example, one study found that interferon regulatory factor 1, as the upstream regulator of PANoptosis, can induce cell death in the process of tumorigenesis in colorectal cancer (Karki et al., 2020a). In addition, in the exploration of the treatment of melanoma, a compound of metformin and doxorubicin initiated pyroptosis, apoptosis and necroptosis (PANoptosis) of melanoma cells, reducing the development of the melanoma (Song et al., 2021).
Published studies related to PANoptosis mainly focus on diseases induced by bacterial or viral infections plus a few types of tumors (Karki et al., 2020a, b; Malireddi et al., 2020b; Song et al., 2021). It is unknown whether PANoptosis and PANoptosomes exist in other types of diseases but it is worth further investigation. Many central nervous system (CNS) diseases involve the death of nerve cells, including PANoptosis (Yuan and Yankner, 2000; McKenzie et al., 2020; Yan et al., 2021). All these diseases or pathological conditions are generally associated with inflammatory reactions (Pender and Rist, 2001; Hoffmann et al., 2009; Degterev et al., 2019; Voet et al., 2019; Yuan et al., 2019; Lünemann et al., 2021). The expression of cell death and the pathophysiological mechanism related to inflammation in these CNS diseases are similar to the phenotype and mechanism in the existing studies of PANoptosis, which provides basic evidence for the possible existence of PANoptosis and PANoptosomes in CNS diseases.
In the Web of Science database, we investigated the experimental research articles about pyroptosis, apoptosis and necroptosis in the field of the nervous system and sorted the related articles according to the citation frequency, from high to low. Selecting the top 2% articles (referring to and expanding Essential Science Indicators standards) for keyword extraction and analysis, it was found that ischemia accounted for the highest proportion among the three death forms of PANoptosis in nervous system. Stroke is the second major cause of disability and death in adults, with ischemic stroke accounting for the majority of all stroke cases (Virani et al., 2020), and the main injury of ischemic stroke is caused by ischemia/reperfusion (I/R) (Meschia and Brott, 2018; Campbell et al., 2019; Yan et al., 2020a). The pathophysiological state of I/R can cause serious brain damage, and the pathophysiological process frequently involves an inflammatory reaction and immune system activation (Chamorro et al., 2016; Lambertsen et al., 2019; Shi et al., 2019; Yan et al., 2020b). Following the above argument we chose ischemia injury of the CNS as the analysis object.
We use bibliometrics, knowledge discovery and data-mining methods to capture evidence and analyze bibliometrics on the research of RCD related to ischemic injury of the CNS (Yan et al., 2020b) to assess the experimental research evidence on the involvement of PANoptosis in nervous system diseases. The demonstration of PANoptosis in ischemic injury of the CNS broadens the scope of PANoptosis research. This study takes a new approach to RCD research by exploring multiple RCD synchronously, pluralistically and comprehensively in ischemic injury of the CNS, and explores new ways to improve the intervention efficiency of RCD in nervous system diseases.
Materials and Methods
Data source
We chose PubMed, Scopus and Web of Science as the target databases. The key words were divided into three groups: (1) RCD, including pyroptosis, apoptosis and necroptosis; (2) CNS and their MeSH appositive words, hyponyms or hypernyms; and (3) ischemia. The refining function of the database limited the retrieval field to neuroscience or neurosurgery or neurology. The article type was limited to research articles. The retrieval of literature was completed on June 20, 2021. The end time of the publishing time range of the literature collections retrieved, with three cell death forms as the core theme, was June 20, 2021 but their start times differed as follows: (1) PubMed database: pyroptosis was on November 1, 2018; apoptosis was on May 1, 1995; necroptosis was on January 12, 2007. (2) Scopus database: The starting time of pyroptosis was on July 1, 2008; apoptosis on December 24, 1993. necroptosis started on July 1, 2005. (3) Web of Science database: The starting time of pyroptosis was on April 1, 2014; apoptosis on December 24, 1993; necroptosis on July 1, 2005. The retrieval strategy of each database was customized according to the usage standard of the database and the scale of the retrieved documents. Articles retrieved from each database were merged according to the three forms of cell death, and duplicate documents were screened and removed according to the inclusion criteria. The process of literature screening was shown in Figure 1.
Figure 1.
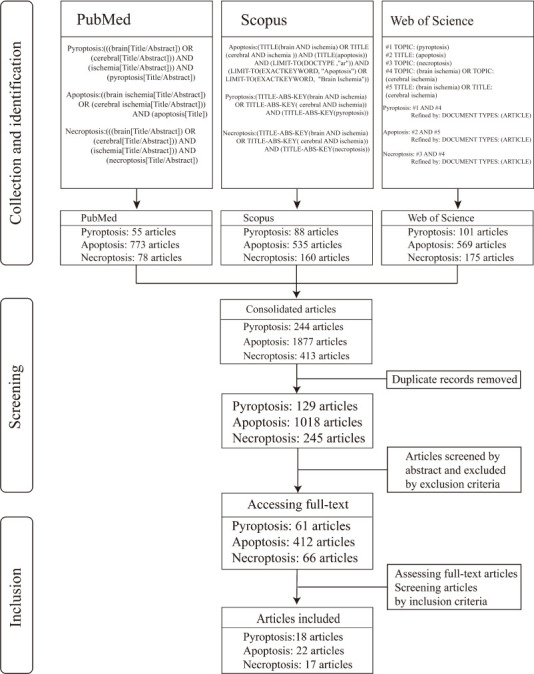
Flow chart of literature screening.
Inclusion/exclusion criteria
Studies were potentially included if they met the following criteria: (1) The core content of the paper was to study ischemia or I/R injury or animal or cell models that can represent ischemia or I/R; (2) Rodents or primary cells or subculture cell lines were used as the experimental materials; (3) The target organ damaged in the experiment was either the brain or primary cells and subculture cells that can represent neurons; (4) The experimental results included two or more corresponding detection results that proved the existence of the three kinds of cell death: pyroptosis, apoptosis and necroptosis, one of which must be the key protein detection results of these three kinds of cell death forms; and (5) Damage treatment group and blank control group were included in the experimental design.
Studies were excluded if they met any of the criteria: (1) Drug-induced animal model or cell model; (2) The target cells of the experimental study were non-neuronal cells (glial cells, endothelial cells, etc.); (3) The process and standard description of establishing the model were not given; and (4) The experimental evidence to prove the existence of any of the three cell death forms was insufficient.
Data mining and sorting analysis
Data such as cell types, animal species, modeling methods, evaluation of cell death and detection results of representative molecules of different cell death types were extracted from the included literature. The literature items exported from the database were imported into the literature management software, and two researchers with medical and biological knowledge independently read the literature one by one, conducted article selection and data mining, and obtained relevant data from the literature. The data obtained by the two researchers were compared, and the consistent results were summarized in a table. When any inconsistent results occurred, the discussion and decision for inclusion involved the participation of the third researcher. The cluster analysis of in vitro experiments was based on the cell type and had to be that used in the study of pyroptosis, apoptosis and necroptosis. Cluster analysis of in vivo experiments of animals was carried out according to the classification of common rodents, ensuring that the I/R operations performed on animals were of the same class. To summarize, the acquired core data was collated and analyzed using EndNote software (version X7.8, Clarivate Analytics, Boston, MA, USA) and Microsoft Excel software (version 2016, Microsoft Corporation, Redmond, WA, USA).
Results
A total of 57 articles were included in this study (18 articles in pyroptosis, 22 articles in apoptosis, and 17 articles in necroptosis; Figure 1), of which 22 were conducted on rodents only (including rats and mice) and 31 were conducted on primary cultured cells or cell lines only and 4 studies included both in vitro cell and in vivo rodent experiments. One of the 31 articles that had experimented on two types of cell. From the included literature, we extracted 62 experiments that assessed pyroptosis or apoptosis or necroptosis. Of these studies, it was necessary to satisfy two conditions that would determine whether I/R injury in the experiment induced the occurrence of pyroptosis or apoptosis or necroptosis. One condition was that commonly used or academically recognized detection methods were used in the experiment, such as propidium iodide staining, terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay, flow cytometry, cell counting kit-8 assay or lactate dehydrogenase assay to evaluate the degree of cell death induced by I/R injury. The other condition was that the key proteins of pyroptosis or apoptosis or necroptosis were detected (Table 1) (Fink and Cookson, 2005; Bergsbaken et al., 2009; Kaczmarek et al., 2013; Nikoletopoulou et al., 2013; Czabotar et al., 2014; Kovacs and Miao, 2017; Hu et al., 2021) and that they should contain at least two or more key proteins. Both the conditions mentioned above had to give results that were statistically significant compared to the control group and be clearly stated in the paper.
Table 1.
The key proteins of three forms of cell death in PANoptosis
| Cell death type | Key proteins |
|---|---|
| Pyroptosis | NLRP1, NLRP3, ASC, CASP-1, 4, 5, 11, C-CASP-1, GSDMD, IL-1β, IL-18 |
| Apoptosis | CASP-3, 7, 8, 9, C-CASP-3, Bcl-2, Bax |
| Necroptosis | RIP1, p-RIP1, RIP3, p-RIP3, MLKL, p-MLKL |
ASC: Apoptosis-associated speck-like protein containing a caspase recruit domain; Bax: B-cell lymphoma 2-associated X; Bcl-2: B-cell lymphoma 2; CASP: cysteinyl aspartate-specific protease; C-CASP: cleaved CASP; GSDMD: gasdermin D; IL: interleukin; MLKL: mixed lineage kinase domain-like pseudokinase; NLRP: nucleotide-binding domain and leucine-rich repeat pyrin-domain containing protein; p-MLKL: phosphorylation of MLKL; p-RIP: phosphorylation of RIP; RIP: receptor-interacting protein kinase.
In the 36 cell-model-based experiments, oxygen and glucose deprivation (OGD) or OGD/recovery was used in most cell experiments to simulate ischemia or I/R injury. The researchers used primary hippocampal cells, primary cortical cells, PC12 cells (rat adrenal pheochromocytoma cells) and SH-SY5Y (human neuroblastoma cells) cells for the experiments. We show the results according to the cell types used in the experiments (Tables 2–5). In the included studies, most were based on rodent models, middle cerebral artery occlusion (MCAO) or modified MCAO to simulate ischemia or I/R injury but some used the method of electric shock cardiac arrest and resuscitation. These modeling methods simulate cerebral I/R injury in experimental animals and are recognized in the research field. The studies used Sprague-Dawley rats or C57 mice, and we tabulated the results according to animal type and modeling method used in the experiment (Tables 6 and 7). In the process of data mining, we found that experimental models, apart from MCAO and OGD models, did not meet the condition that pyroptosis, apoptosis and necroptosis were studied simultaneously. We extracted 62 experiments from the 57 included papers. According to the experimental results included in our analysis it appears that in the same cell model or animal disease model three kinds of RCD, i.e., pyroptosis, apoptosis, necroptosis, were likely to occur simultaneously, which would mean that PANoptosis occurs in these experiments.
Table 2.
Oxygen-glucose deprivation/reperfusion (OGD/R) induces death of primary hippocampal cells
| Sources | Treatments | Injure duration | Reperfusion duration | Death type | Assessment | Critical protein | Reference |
|---|---|---|---|---|---|---|---|
| SD rats | OGD/R | 1.5 h | 20 h | Pyroptosis | Hoechst 33342 | NLRP3, ASC, CASP-1, IL-1β, IL-18 | Zhang et al., 2021 |
| SD rats | H/R | 12 h | 24 h | Pyroptosis | CCK-8 | NLRP3, ASC, CASP-1, C-CASP-1, CASP-11, C-CASP-11, GSDMDp30, IL-1β, IL-18 | Diao et al., 2020 |
| Mongolian gerbils | H/R | 4 h | 24 h | Pyroptosis | Hoechst 33342, MTT | NLRP1, NLRP3, pro-CASP-1, CASP-1, GSDMD, IL-1β, IL-18 | Zhu et al., 2019 |
| C57BL/6 mice | OGD/R | 3 h | 24 h | Apoptosis | TUNEL, LDH | CASP-3, C-CASP-3, Bcl-2, Bax | Yu et al., 2018 |
| C57BL/6 mice | OGD/R | 2 h | 48 h | Necroptosis | PI, LDH | RIPK1, MLKL, p-MLKL | Yang et al., 2017 |
| SD rats | OGD/R | 2 h | 24 h | Necroptosis | PI (Nec-1), LDH | RIP1, RIP3, CASP-8 | Vieira et al., 2014 |
ASC: Apoptosis-associated speck-like protein containing a caspase recruit domain; Bax: B-cell lymphoma 2-associated X; Bcl-2: B-cell lymphoma 2; CASP: cysteinyl aspartate-specific protease; C-CASP: cleaved CASP; CCK-8: cell counting kit-8; GSDMD: gasdermin D; H/R: hypoxia/reoxygenation; IL: interleukin; LDH: lactate dehydrogenase; MLKL: mixed lineage kinase domain-like pseudokinase; MTT: methylthiazolyldiphenyl-tetrazolium bromide; Nec-1: necrostatin-1; NLRP: nucleotide-binding domain and leucine-rich repeat pyrin-domain containing protein; OGD/R: oxygen and glucose deprivation/recovery; PI: propidium iodide staining; PI (Nec-1): Nec-1 inhibited necroptosis, and after the addition of Nec-1, PI staining was performed to verify the occurrence of necroptosis by comparing with the control group; p-MLKL: phosphorylation of MLKL; RIP: receptorinteracting protein kinase; SD rats: Sprague-Dawley rats; TUNEL: TdTmediated dUTP nick-end labeling.
Table 5.
Oxygen-glucose deprivation/reperfusion (OGD/R) induces death of SH-SY5Y cells
| Treatment | Injury duration | Reperfusion duration | Death type | Assessment | Critical protein | Reference |
|---|---|---|---|---|---|---|
| OGD/R | 2, 4, 8, 12 h | 24 h | Pyroptosis | MTT | C-CASP-1, ASC, GSDMD-N, IL-1β, IL-18 | Liang et al., 2020a |
| OGD/R | 6 h | 24 h | Pyroptosis | LDH, Flow cytometry | NLRP3, CASP-1, ASC, GSDMD-N | Wang et al., 2020d |
| OGD | 6 h | 2 h | Apoptosis | Hoechst 33342 | CASP-3, Bcl-2, Bax | Wang et al., 2013 |
| OGD/R | 4 h | 48 h | Apoptosis | MTT | CASP-3, Bcl-2, Bax | Zhang et al., 2016 |
| OGD | 4 h | 0 | Apoptosis | MTT | Act-CASP-3, Bcl-2 | Chang et al., 2017 |
| OGD/R | 8 h | 24 h | Apoptosis | MTT, LDH, Flow cytometry | C-CASP-3, CASP-9, PARP, Bcl-2, Bax | Cai et al., 2020 |
| OGD/R | 4 h | 24 h | Apoptosis | MTT, LDH, Hoechst 33342, TEM | C-CASP-3, Bcl-2, Bax | Yang et al., 2021 |
| OGD/R | 6 h | 2 h | Necroptosis | CCK-8, LDH, Hoechst 33342 | RIP3, MLKL, p-MLKL, CaMKII, p-CaMKII | Li et al., 2021c |
ASC: Apoptosis-associated speck-like protein containing a caspase recruit domain; Bax: B-cell lymphoma 2-associated X; Bcl-2: B cell lymphoma 2; CaMKII: Calcium/calmodulin-dependent kinase II; CASP: cysteinyl aspartate-specific protease; C-CASP: cleaved CASP; CCK-8: cell counting kit-8; C-GSDMD: cleaved GSDMD; GSDMD: gasdermin D; GSDMD-N: N-terminal domain of gasdermin D; IL: interleukin; LDH: Lactate dehydrogenase; MLKL: mixed lineage kinase domain-like pseudokinase; MTT: Methylthiazolyldiphenyl-tetrazolium bromide; Nec-1: Necrostatin-1; NLRP: nucleotide-binding domain and leucine-rich repeat pyrin-domain containing protein; OGD: oxygen and glucose deprivation; OGD/R: OGD/recovery; PARP: poly ADP-ribose polymerase; p-CaMKII: phosphorylation of CaMKII; PI: propidium iodide staining; PI (Nec-1): Nec-1 inhibited necroptosis, and after the addition of Nec-1, PI staining was performed to verify the occurrence of necroptosis by comparing with the control group; p-MLKL: phosphorylation of MLKL; RIP: receptor-interacting protein kinase; SD rats: Sprague- Dawley rats; TEM: transmission electron microscope; TUNEL: TdT-mediated dUTP nick-end labeling.
Table 6.
Brain injury induced by ischemia/reperfusion (I/R) injury in rats
| Treatment | Injury duration | Reperfusion duration | Death type | Assessment | Critical protein | Reference |
|---|---|---|---|---|---|---|
| MCAO | 2 h | 0 | Pyroptosis | Nissl, TUNEL | NLRP3, ASC, C-CASP-1 | An et al., 2019 |
| MCAO | 1.5 h | 0 | Pyroptosis | Nissl, TUNEL | NLRP3, ASC, pro-CASP-1, CASP-1, IL-1β | She et al., 2019 |
| MCAO/R | 2 h | 1, 3, 6, 24 h | Pyroptosis | TUNEL, Flow cytometry | NLRP3, total-CASP-1, IL-1β, IL-18 | Jiang et al., 2020b |
| MCAO/R | 2 h | 24 h | Pyroptosis | HE, TUNEL | ASC, C-CASP-1, GSDMD-N, IL-1β, IL-18 | Liang et al., 2020a |
| MCAO | 6, 12 h, 1, 2, 3 d | 0 | Pyroptosis | IF, TEM | NLRP3, ASC, CASP-1, C-CASP-1, GSDMD, C- GSDMD | Liang et al., 2020b |
| CA/CPR | 7 min | 24 h | Pyroptosis | HE, IHC, TEM | NLRP3, GSDMD, GSDMDp30, IL-1β | Zou et al., 2020 |
| MCAO/R | 2 h | 24 h | Pyroptosis | HE, TUNEL | pro-CASP-1, CASP-1p20, GSDMD, GSDMD-N | Li et al., 2021b |
| CA/CPR | 7 min | 24 h | Pyroptosis | HE, IF | NLRP3, ASC, pro-CASP-1, CASP-1p20, | Zou et al., 2021 |
| IL-1β, GSDMD, GSDMD-N | ||||||
| MCAO/R | 1 h | 2, 12, 24 h | Apoptosis | TUNEL | CASP-3, C-CASP-3, IL-18 | Yuan et al., 2013 |
| MCAO/R | 1 h | 24 h | Apoptosis | TUNEL | CASP-3, C-CASP-3, Bcl-2 | Zhang et al., 2013 |
| MCAO/R | 2 h | 24 h | Apoptosis | TUNEL, Hoechst 33258, TEM | CASP-3, CASP-9, Bcl-2, Bax | Gao et al., 2016 |
| MCAO/R | 2 h | 14 d | Apoptosis | TUNEL, TEM | CASP-3, Cyt-c, Bcl-2, Bax | Chen et al., 2017 |
| MCAO/R | - | 24 h | Apoptosis | Nissl, TUNEL | C-CASP-3, Cyt-c, Bcl-2, Bax | Yang et al., 2021 |
| 4VO/R* | 0.5 h | 1, 3, 6, 12, 24, 48 h | Necroptosis | IF | RIP1, RIP3 | Ryan et al., 2018 |
| MCAO/R | 0.5 h | 12, 24, 72 h | Necroptosis | IHC | RIP1, p-RIP1, RIP3, MLKL, p-MLKL | Deng et al., 2019 |
| 4VO/R | 20 min | 5 d | Necroptosis | HE, IF | RIP3 | Hu et al., 2020 |
| CA/CPR | 7 min | 24 h | Necroptosis | HE, Nissl, TEM | RIP1, RIP3, MLKL, p-MLKL | Wang et al., 2021a |
*Wistar rats were used in this study, and SD rats were used in all other experiments. 4VO/R: 4-Vessel occlusion and reperfusion; ASC: apoptosis-associated speck-like protein containing a caspase recruit domain; Bax: B-cell lymphoma 2-associated X; Bcl-2: B cell lymphoma 2; CA/CPR: cardiac arrest/cardiopulmonary resuscitation; CASP: cysteinyl aspartate-specific protease; C-CASP: cleaved CASP; C-GSDMD: cleaved GSDMD; Cyt-c: cytochrome C; GSDMD: gasdermin D; GSDMD-N: N-terminal domain of gasdermin D; HE: hematoxylin-eosin staining; IF: immunofluorescence; IHC: immunohistochemistry; IL: interleukin; LDH: lactate dehydrogenase; MCAO: middle cerebral artery occlusion; MCAO/R: MCAO/reperfusion; MLKL: mixed lineage kinase domain-like pseudokinase; MTT: methylthiazolyldiphenyl-tetrazolium bromide; Nec-1: Necrostatin-1; Nissl: Nissl staining; NLRP: nucleotide-binding domain and leucine-rich repeat pyrin-domain containing protein; p-MLKL: phosphorylation of MLKL; p-RIP: phosphorylation of RIP; RIP: receptor-interacting protein kinase; SD rats: Sprague-Dawley rats; TEM: transmission electron microscope; TUNEL: TdT-mediated dUTP nick-end labeling.
Table 7.
Brain injury induced by ischemia/reperfusion (I/R) injury in mice
| Species | Treatment | Injury duration | Reperfusion duration | Death type | Assessment | Critical protein | Reference |
|---|---|---|---|---|---|---|---|
| Mongolian gerbils | CAO/R | 5 min | 7 d | Pyroptosis | Nissl, TUNEL | NLRP1, NLRP3, GSDMD, CASP-1, C-CASP-1, IL-1β, IL-18 | Zhu et al., 2019 |
| C57BL/6 mice | PT | 5 min | 1, 3, 7 d | Pyroptosis | IF, TEM | NLRP1, NLRP3, ASC, GSDMD, CASP-1, C-CASP-1, IL-1β, IL-18 | Li et al., 2020a |
| ICR mice | tMCAO | 45 min | 24 h | Pyroptosis | IF | NLRP3, ASC, C-CASP-1, IL-1β, GSDMD, GSDMD-N | Wang et al., 2021c |
| ICR mice | MCAO/R | 2 h | 24 h | Apoptosis | TUNEL | CASP-3, Bcl-2, Bax | Wu et al., 2015 |
| C57BL/6 mice | MCAO/R | 1 h | 2, 6, 12, 24 h | Apoptosis | TUNEL | CASP-3, C-Casp-3 | Ma et al., 2017 |
| Swiss albino mice | MCAO/R | 45 min | 23 h | Apoptosis | TUNEL, PI | CASP-3, Bcl-2, Bax | Asadi et al., 2018 |
| ICR mice | MCAO/R | 1 h | 24 h | Necroptosis | PI (Nec-1) | RIP1, RIP3, MLKL, p-MLKL | Chen et al., 2018 |
| C57BL/6J mice | MCAO | 1 h | 12, 24, 72 h, 5, 7 d | Necroptosis | TUNEL | RIP1, p-RIP1, RIP3, p-RIP3, MLKL, p-MLKL | Li et al., 2020b |
| C57BL/6J mice | MCAO | 1 h | 24 h | Necroptosis | TUNEL | RIP1, RIP3, MLKL | Zhang et al., 2020 |
ASC: Apoptosis-associated speck-like protein containing a caspase recruit domain; Bax: B-cell lymphoma 2-associated X; Bcl-2: B cell lymphoma 2; CAO: carotid arteries occluded; CASP: cysteinyl aspartate-specific protease; C-CASP: cleaved CASP; C-GSDMD: cleaved GSDMD; Cyt-c: cytochrome C; GSDMD: gasdermin D; GSDMD-N: N-terminal domain of gasdermin D; IF: immunofluorescence; IL: interleukin; MCAO: middle cerebral artery occlusion; MLKL: mixed lineage kinase domain-like pseudokinase; Nec-1: Necrostatin-1; Nissl: Nissl staining; NLRP: nucleotide-binding domain and leucine-rich repeat pyrin-domain containing protein; PI: propidium iodide staining; PI (Nec-1): Nec-1 inhibited necroptosis, and after the addition of Nec-1, PI staining was performed to verify the occurrence of necroptosis by comparing with the control group; p-MLKL: phosphorylation of MLKL; p-RIP: phosphorylation of RIP; PT: photothrombotic model; RIP: receptor-interacting protein kinase; SD rats: Sprague-Dawley rats; TEM: transmission electron microscope; tMCAO; transient middle cerebral artery occlusion; TUNEL: TdT-mediated dUTP nick-end labeling.
Table 3.
Oxygen-glucose deprivation/reperfusion (OGD/R) induces death of primary cortical cells
| Sources | Treatments | Injury duration | Reperfusion duration | Death type | Assessment | Critical protein | Reference |
|---|---|---|---|---|---|---|---|
| C57BL/6 mice | OGD/R | 2 h | 24 h | Pyroptosis | Hoechst 33342, PI, CCK-8 | NLRP3, ASC, CASP-1, C-CASP-1, GSDMD-N, IL-1β,IL-18 | Sun et al., 2020 |
| C57BL/6 mice | H/R | 1.5 h | 4 h | Pyroptosis | PI, Flow cytometry | CASP-1, GSDMD, C-GSDMD, IL-1β, IL-18 | Tang et al., 2018 |
| Wistar Rats | OGD/R | 4 h | 24 h | Apoptosis | CCK-8, LDH, TUNEL | C-CASP-3 | Wu et al., 2020a |
| SD Rats | OGD/R | 2 h | 24 h | Apoptosis | CCK-8, TUNEL | C-CASP-3, IL-1β | Mo et al., 2020b |
| SD Rats | OGD/R | 2 h | 48 h | Apoptosis | TUNEL, MTT, Flow cytometry | Bcl-2, Bax | Zhou et al., 2019 |
| SD Rats | OGD/R | 3 h | 24 h | Apoptosis | TUNEL | C-CASP-3, Bcl-2, Bax | He et al., 2019 |
| SD Rats | OGD/R | 3 h | 6, 24, 48, 72 h | Apoptosis | LDH, TUNEL | C-CASP-3, Bcl-2, Bax | He et al., 2016 |
| Balb/C mice | OGD/R | 3 h | 21 h | Apoptosis | Flow cytometry | C-CASP-3, Bcl-2, Bax | Huang et al., 2014 |
| C57BL/6J mice | OGD/R | 1 h | 24 h | Necroptosis | PI | RIP1, RIP3, MLKL | Yuan et al., 2020 |
| C57BL/6J mice | OGD/R | 1 h | 24 h | Necroptosis | PI (Nec-1) | RIP1, RIP3, MLKL | Li et al., 2019 |
| SD Rats | OGD | 2 h | 0 | Necroptosis | PI (Nec-1) | RIP1, RIP3, p-RIP3, MLKL, p-MLKL | Wang et al., 2018 |
| SD Rats | OGD | 6 h | 0 | Necroptosis | LDH | RIP1, RIP3 | Ni et al., 2018 |
| SD Rats | OGD | 6 h | 24 h | Necroptosis | PI | RIP1, RIP3 | Li et al., 2018 |
| SD Rats | OGD/R | 2 h | 48 h | Necroptosis | PI (Nec-1) | RIP1, RIP3 | Kong et al., 2017 |
ASC: Apoptosis-associated speck-like protein containing a caspase recruit domain; Bax: B-cell lymphoma 2-associated X; Bcl-2: B-cell lymphoma 2; CASP: cysteinyl aspartate-specific protease; C-CASP: cleaved CASP; CCK-8: cell counting kit-8; C-GSDMD: cleaved GSDMD; GSDMD: gasdermin D; GSDMD-N: N-terminal domain of gasdermin D; H/R: hypoxia/reoxygenation; IL: interleukin; LDH: lactate dehydrogenase; MLKL: mixed lineage kinase domain-like pseudokinase; MTT: methylthiazolyldiphenyl-tetrazolium bromide; Nec-1: Necrostatin-1; NLRP: nucleotide-binding domain and leucine-rich repeat pyrin-domain containing protein; OGD: oxygen and glucose deprivation; OGD/R: OGD/recovery; PI: propidium iodide staining; PI (Nec-1): Nec-1 inhibited necroptosis, and after the addition of Nec-1, PI staining was performed to verify the occurrence of necroptosis by comparing with the control group; p-MLKL: phosphorylation of MLKL; p-RIP: phosphorylation of RIP; RIP: receptorinteracting protein kinase; SD rats: Sprague-Dawley rats; TUNEL: TdT-mediated dUTP nick-end labeling.
Table 4.
Oxygen-glucose deprivation/reperfusion (OGD/R) induces death of PC12 cells
| Treatments | Injury duration | Reperfusion duration | Death type | Assessment | Critical protein | Reference |
|---|---|---|---|---|---|---|
| OGD/R | 12 h | 1 h | Pyroptosis | Hoechst 33342, PI, CCK-8 | NLRP3, C-CASP-1, GSDMD-N | Zeng et al., 2020 |
| OGD/R | 2 h | 2 h | Pyroptosis | MTT, LDH, TUNEL | CASP-1, CASP-1p20, GSDMD, GSDMD-N | Li et al., 2021b |
| OGD/R | 12 h | 4 h | Pyroptosis | MTT, TEM | NLRP3, ASC, C-CASP-1, GSDMD, GSDMD-N | Liu et al., 2021a |
| OGD | 2, 4, 8, 12, 24 h | 0 | Apoptosis | MTT | C-CASP-3, Bcl-2, Bax | Lin et al., 2015 |
| OGD | 12 h | 0 | Apoptosis | Hoechst 33342, MTT, Flow cytometry | C-CASP-3, CASP-12, Bcl-2 | Cao et al., 2016 |
| OGD/R | 0, 2, 4 h | 24 h | Apoptosis | TUNEL, CCK-8 | Bcl-2, Bax | Ren et al., 2019 |
| OGD | 8 h | 0 | Necroptosis | PI (Nec-1) | RIP1, RIP3, MLKL | Wang et al., 2018 |
| H/R | 8 h | 24 h | Necroptosis | LDH, Flow cytometry | RIP1, RIP3, MLKL/p-MLKL | Zhang et al., 2019b |
ASC: Apoptosis-associated speck-like protein containing a caspase recruit domain; Bax: B-cell lymphoma 2-associated X; Bcl-2: B-cell lymphoma 2; CASP: cysteinyl aspartate-specific protease; C-CASP: cleaved CASP; CCK-8: cell counting kit-8; C-GSDMD: cleaved GSDMD; GSDMD: gasdermin D; GSDMD-N: N-terminal domain of gasdermin D; H/R: Hypoxia/reoxygenation; IL: interleukin; LDH: lactate dehydrogenase; MLKL: mixed lineage kinase domain-like pseudokinase; MTT: methylthiazolyldiphenyl-tetrazolium bromide; Nec-1: Necrostatin-1; NLRP: nucleotide-binding domain and leucine-rich repeat pyrin-domain containing protein; OGD: oxygen and glucose deprivation; OGD/R: OGD/recovery; PI: propidium iodide staining; PI (Nec-1): Nec-1 inhibited necroptosis, and after the addition of Nec-1, PI staining was performed to verify the occurrence of necroptosis by comparing with the control group; p-MLKL: phosphorylation of MLKL; RIP: receptorinteracting protein kinase; SD rats: Sprague-Dawley rats; TEM: transmission electron microscope; TUNEL: TdT-mediated dUTP nick-end labeling.
Discussion
In this study we selected MCAO and OGD as in vivo and in vitro experimental models, respectively, that can simulate I/R injury and its pathophysiology in the CNS. These two methods are the most widely used and generally recognized by researchers (Ryou and Mallet, 2018; Salvador et al., 2018). Many have studied RCD induced by I/R injury of CNS using MCAO and OGD (Yanamoto et al., 2003; Tuttolomondo et al., 2009; McBride and Zhang, 2017; Ryou and Mallet, 2018; Wang et al., 2018; Li et al., 2019; Zhang et al., 2019a), and these two methods have often been used to study the inflammatory reaction related to this kind of injury (Tuttolomondo et al., 2009; Rizzo and Leaver, 2010; Mo et al., 2020a; Stanzione et al., 2020; Huang et al., 2021). Therefore, it is pertinent to discuss PANoptosis in MCAO and OGD models.
Kanneganti’s proposal is that PANoptosis is a newly defined form of cell death in diseases related to the immune response and can be regulated by a multimeric protein complex, named PANoptosome (Malireddi et al., 2019). This new form of cell death includes pyroptosis, apoptosis and necroptosis. He proposes that a PANoptosome can interfere with pyroptosis, apoptosis and necroptosis, each of which have been studied independently by other investigators. The existing research on PANoptosis suggests cysteinyl aspartate-specific protease (CASP) 1 and CASP-11 that drive pyroptosis, CASP-8 that drives apoptosis and RIP3 that drives necroptosis can all be assembled into a PANoptosome, together with other components. The process of PANoptosis can be regulated by Z-DNA-binding protein 1 and TAK1 (Christgen et al., 2020; Samir et al., 2020). To support the theory that PANoptosis is a major factor in the I/R injury of the CNS first it is necessary to confirm that pyroptosis, apoptosis and necroptosis have been shown to occur simultaneously from reports in existing literature on I/R injury. Second, a PANoptosome has to have been identified in I/R injury, and have been confirmed that it can simultaneously initiate the three kinds of RCD. Third, there must be a regulatory system that controls PANoptosome activity.
The data we mined from the literature showed that in the study of cerebral I/R, under the same model condition, the three forms of cell death could occur simultaneously. According to our integrated data, after MCAO induced I/R injury in rat or mouse brain tissue and OGD induced ischemia-hypoxia injury in neurons or cell lines derived from nerve cells, pyroptosis, apoptosis and necroptosis coexisted. This phenomenon accords with the first condition of the PANoptosis definition, and suggests that it is very possible that PANoptosis exists in nervous system diseases from the phenomenon level or the phenotype level of cerebral ischemia injury. We can see from the related studies of the three kinds of RCD—pyroptosis, apoptosis and necroptosis—that the molecular mechanisms of these three kinds of cell death all have inflammation-related parts (Linkermann et al., 2013; Lu et al., 2019a; Guo et al., 2020; Wang et al., 2020c, 2021b; Chen et al., 2021; Liu et al., 2021c). There are also reports that glial cells can interfere with these three forms of cell death after being stimulated by injury (Zhao et al., 2017; Xu et al., 2019; Naito et al., 2020; Wang et al., 2020a; Li et al., 2021a; Liu et al., 2021b; Lu et al., 2021) and these overlap with the inflammation-related and immune-related reports of existing studies of PANoptosis. This suggests the possibility of PANoptosis in CNS diseases at the pathological mechanism level.
The latest research suggests that a PANoptosome includes three kinds of protein: (1) Z-DNA-binding protein 1, a nucleotide-binding domain and a leucine-rich repeat pyrin-domain containing protein 3 that play the role of sensor, (2) an apoptosis-associated speck-like protein, containing a caspase recruit domain, and a Fas-associated protein with death domain that are composite adapters and (3) a receptor-interacting protein kinase (RIP) 1, RIP3, CASP-1 and CASP-8 that have a catalytic effect (Christgen et al., 2020; Samir et al., 2020; Zheng and Kanneganti, 2020a, b). These studies on the PANoptosome are related to infectious diseases and cancer, but there has been no study on PANoptosomes in the study of I/R injury of CNS. It can be seen from the data mined by us that nucleotide-binding domain and leucine-rich repeat pyrin-domain containing protein 3, CASP-1 and apoptosis-associated speck-like protein containing a caspase recruit domain related to pyroptosis, CASP-8 and Fas-associated protein with death domain related to apoptosis, RIP1 and RIP3 related to necroptosis have all been detected as marker proteins in animal models of I/R and/or cell models of OGD/recovery (Tables 2–7). All these proteins are considered to be components of a PANoptosome in infectious diseases. Although there is no study on the assembly of components of a PANoptosome in I/R injury of CNS, the existing data of the “raw materials” that make up a PANoptosome are highly expressed, indicating that there is a molecular basis for finding PANoptosomes in ischemia-induced brain injury.
There are studies that showed there are some molecules that can interfere with two of the components of PANoptosis simultaneously under the condition of I/R injury. For example, nucleotide oligomerization domain-like receptors with caspase activation and recruitment domain 4 inflammasome complex can regulate apoptosis and pyroptosis (Poh et al., 2019). Also blocking thromboxane A synthase/thromboxane A2/thromboxane prostanoid signal can inhibit apoptosis and pyroptosis at the same time (Chueh et al., 2020). RIPK3, as the key molecule of necroptosis (Kikuchi et al., 2012; Sun et al., 2012; Kim and Li, 2013; Thapa et al., 2013; Guo et al., 2020; Wang et al., 2020b; Liao et al., 2021), can interact with the Jun N-terminal kinase-mediated inflammatory signaling pathway (Hu et al., 2020) that is closely related to neuronal apoptosis induced by ischemia (Wang et al., 2011; Liu et al., 2016, 2018) and to cell pyroptosis (Chen et al., 2019; Jiang et al., 2020a). All this information suggests that pyroptosis, apoptosis and necroptosis (PANoptosis) induced by I/R injury could be subject to intervention and regulation simultaneously.
The existing studies on PANoptosis show that TAK1 and Z-DNA-binding protein 1 intervene in PANoptosome activity, and thus participate in the regulation of PANoptosis (Malireddi et al., 2019; Banoth et al., 2020; Kesavardhana et al., 2020; Samir et al., 2020; Zheng and Kanneganti, 2020b). We have not found any internal or external molecules that can interfere with all three of pyroptosis, apoptosis and necroptosis in cerebral ischemia injury, but some studies have shown that inhibiting TAK1 can reduce neuronal death induced by cerebral I/R (Neubert et al., 2011). This indicates that TAK1 can be used as an important target in RCD induced by hypoxia-reperfusion injury (Neubert et al., 2011; Ridder and Schwaninger, 2013; Wang et al., 2019; Wu et al., 2020b). TAK1 can affect the function of microglia and interact with inflammatory pathway, thus affecting neuronal apoptosis and pyroptosis (Gong et al., 2015; Zeyen et al., 2020). It also plays an important role in the interaction between programmed necrosis and apoptosis of neurons mediated by RIP3 during cerebral I/R injury (Naito et al., 2020). All these data suggest that there may be molecules, like TAK1, that can regulate PANoptosomes in a brain subject to I/R injury.
Limitations
Although this paper verifies the possibility of PANoptosis in cerebral ischemia reperfusion injury by collecting data from cell experiments and animal experiments, we admit that this paper has some limitations. First, the limits of paper length and research scale meant we could not conduct data mining for all CNS diseases, therefore we selected only cerebral I/R injury as the research object. This limited the outcome to only showing whether PANoptosis exists in cerebral I/R injury. Whether PANoptosis occurs in other CNS diseases remains to be studied. Second, the data we mined were mainly cell experiments and animal experiments, without clinical samples. Whether PANoptosis occurs in actual clinical stroke needs further verification. Third, the disease models we analyzed were only MCAO and OGD, therefore other ischemia/reperfusion models would need to be studied. Fourth, we only selected three databases for retrieval, whereas there are other databases. Fifth, our retrieval fields are mainly from title, abstract and keywords, so some relevant papers may have been missed. These limitations need to be addressed in future studies.
Summary and future directions
Analysis of existing research highlights how important PANoptosis is and shows how its interaction network of processes is associated with RCD in infectious diseases. The concept of PANoptosis improves our understanding of RCD, suggesting that we should treat and understand RCD systematically, plurally and as a network. Although the current research focuses mainly on infectious diseases, this review proposes expanding investigations of PANoptosis to other diseases. In the pathophysiological mechanism of CNS diseases the inflammatory response and immune response play important roles that are similar to their effects in infectious diseases. Moreover, there are interactions between regulatory proteins that regulate the disease response and immune response of CNS diseases. However, systematic and comprehensive research on these interactions still needs further study. In future, the research on PANoptosis in CNS diseases should examine the interaction network of key regulatory proteins, identify a PANoptosome linked to CNS diseases, find the target of PANoptosis that can intervene in neurons and find new treatment strategies for diseases related to RCD.
Footnotes
Conflicts of interest: The authors declare that there is no potential conflict of interest.
Funding: The study was supported by the National Natural Science Foundation of China, Nos. 81772134 (to KX), 81971891 (to KX), 82172196 (to KX), 81571939 (to KX); the Fundamental Research Funds for the Central Universities of Central South University of China, No. 2020zzts218, (to WTY); Hunan Provincial Innovation Foundation For Postgraduate of China, Nos. CX20200116 (to WTY), CX20190139 (to LSL).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–432. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 2.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 3.An P, Xie J, Qiu S, Liu Y, Wang J, Xiu X, Li L, Tang M. Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sci. 2019;232:116599. doi: 10.1016/j.lfs.2019.116599. [DOI] [PubMed] [Google Scholar]
- 4.Asadi Y, Gorjipour F, Behrouzifar S, Vakili A. Irisin peptide protects brain against ischemic injury through reducing apoptosis and enhancing BDNF in a rodent model of stroke. Neurochem Res. 2018;43:1549–1560. doi: 10.1007/s11064-018-2569-9. [DOI] [PubMed] [Google Scholar]
- 5.Banoth B, Tuladhar S, Karki R, Sharma BR, Briard B, Kesavardhana S, Burton A, Kanneganti TD. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis) J Biol Chem. 2020;295:18276–18283. doi: 10.1074/jbc.RA120.015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briard B, Malireddi RKS, Kanneganti TD. Role of inflammasomes/pyroptosis and PANoptosis during fungal infection. PLoS Pathog. 2021;17:e1009358. doi: 10.1371/journal.ppat.1009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai HA, Tao X, Zheng LJ, Huang L, Peng Y, Liao RY, Zhu YM. Ozone alleviates ischemia/reperfusion injury by inhibiting mitochondrion-mediated apoptosis pathway in SH-SY5Y cells. Cell Biol Int. 2020;44:975–984. doi: 10.1002/cbin.11294. [DOI] [PubMed] [Google Scholar]
- 9.Cain K, Bratton SB, Langlais C, Walker G, Brown DG, Sun XM, Cohen GM. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J Biol Chem. 2000;275:6067–6070. doi: 10.1074/jbc.275.9.6067. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 11.Cao G, Zhou H, Jiang N, Han Y, Hu Y, Zhang Y, Qi J, Kou J, Yu B. YiQiFuMai powder injection ameliorates cerebral ischemia by inhibiting endoplasmic reticulum stress-mediated neuronal apoptosis. Oxid Med Cell Longev. 2016;2016:5493279. doi: 10.1155/2016/5493279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 13.Chang CF, Lai JH, Wu JC, Greig NH, Becker RE, Luo Y, Chen YH, Kang SJ, Chiang YH, Chen KY. (-)-Phenserine inhibits neuronal apoptosis following ischemia/reperfusion injury. Brain Res. 2017;1677:118–128. doi: 10.1016/j.brainres.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Zuo Y, Huang L, Sherchan P, Zhang J, Yu Z, Peng J, Zhang J, Zhao L, Doycheva D, Liu F, Zhang JH, Xia Y, Tang J. The MC(4) receptor agonist RO27-3225 inhibits NLRP1-dependent neuronal pyroptosis via the ASK1/JNK/p38 MAPK pathway in a mouse model of intracerebral haemorrhage. Br J Pharmacol. 2019;176:1341–1356. doi: 10.1111/bph.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Zhang X, Xue L, Hao C, Liao W, Wan Q. Treatment with enriched environment reduces neuronal apoptosis in the periinfarct cortex after cerebral ischemia/reperfusion injury. Cell Physiol Biochem. 2017;41:1445–1456. doi: 10.1159/000468368. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Zhang L, Yu H, Song K, Shi J, Chen L, Cheng J. Necrostatin-1 improves long-term functional recovery through protecting oligodendrocyte precursor cells after transient focal cerebral ischemia in mice. Neuroscience. 2018;371:229–241. doi: 10.1016/j.neuroscience.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Li Y, Guo L, Hong J, Zhao W, Hu X, Chang C, Liu W, Xiong K. Bibliometric analysis of the inflammasome and pyroptosis in brain. Front Pharmacol. 2021;11:626502. doi: 10.3389/fphar.2020.626502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B, Place DE, Briard B, Sharma BR, Tuladhar S, Samir P, Burton A, Kanneganti TD. Identification of the PANoptosome: a molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis) Front Cell Infect Microbiol. 2020;10:237. doi: 10.3389/fcimb.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu ZL, Pio F, Xie Z, Welsh K, Krajewska M, Krajewski S, Godzik A, Reed JC. A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis. J Biol Chem. 2001;276:9239–9245. doi: 10.1074/jbc.M006309200. [DOI] [PubMed] [Google Scholar]
- 20.Chueh TH, Cheng YH, Chen KH, Chien CT. Thromboxane A2 synthase and thromboxane receptor deletion reduces ischaemia/reperfusion-evoked inflammation, apoptosis, autophagy and pyroptosis. Thromb Haemost. 2020;120:329–343. doi: 10.1055/s-0039-3400304. [DOI] [PubMed] [Google Scholar]
- 21.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 22.Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci U S A. 2019;116:9714–9722. doi: 10.1073/pnas.1901179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng XX, Li SS, Sun FY. Necrostatin-1 prevents necroptosis in brains after ischemic stroke via inhibition of RIPK1-mediated RIPK3/MLKL signaling. Aging Dis. 2019;10:807–817. doi: 10.14336/AD.2018.0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diao MY, Zhu Y, Yang J, Xi SS, Wen X, Gu Q, Hu W. Hypothermia protects neurons against ischemia/reperfusion-induced pyroptosis via m6A-mediated activation of PTEN and the PI3K/Akt/GSK-3β signaling pathway. Brain Res Bull. 2020;159:25–31. doi: 10.1016/j.brainresbull.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Chen T, Lei X, Li Y, Dai X, Cao Y, Ding Q, Lei X, Li T, Lin X. Neuroprotective effects of polydatin against mitochondrial-dependent apoptosis in the rat cerebral cortex following ischemia/reperfusion injury. Mol Med Rep. 2016;14:5481–5488. doi: 10.3892/mmr.2016.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong J, Li ZZ, Guo S, Zhang XJ, Zhang P, Zhao GN, Gao L, Zhang Y, Zheng A, Zhang XF, Xiang M, Li H. Neuron-specific tumor necrosis factor receptor-associated factor 3 is a central regulator of neuronal death in acute ischemic stroke. Hypertension. 2015;66:604–616. doi: 10.1161/HYPERTENSIONAHA.115.05430. [DOI] [PubMed] [Google Scholar]
- 28.Guo LM, Wang Z, Li SP, Wang M, Yan WT, Liu FX, Wang CD, Zhang XD, Chen D, Yan J, Xiong K. RIP3/MLKL-mediated neuronal necroptosis induced by methamphetamine at 39°C. Neural Regen Res. 2020;15:865–874. doi: 10.4103/1673-5374.268902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurung P, Burton A, Kanneganti TD. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1β-mediated osteomyelitis. Proc Natl Acad Sci U S A. 2016;113:4452–4457. doi: 10.1073/pnas.1601636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He G, Xu W, Tong L, Li S, Su S, Tan X, Li C. Gadd45b prevents autophagy and apoptosis against rat cerebral neuron oxygen-glucose deprivation/reperfusion injury. Apoptosis. 2016;21:390–403. doi: 10.1007/s10495-016-1213-x. [DOI] [PubMed] [Google Scholar]
- 32.He GQ, Xu WM, Liao HJ, Jiang C, Li CQ, Zhang W. Silencing Huwe1 reduces apoptosis of cortical neurons exposed to oxygen-glucose deprivation and reperfusion. Neural Regen Res. 2019;14:1977–1985. doi: 10.4103/1673-5374.259620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann O, Zipp F, Weber JR. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) in central nervous system inflammation. J Mol Med (Berl) 2009;87:753–763. doi: 10.1007/s00109-009-0484-x. [DOI] [PubMed] [Google Scholar]
- 34.Hu W, Wu X, Yu D, Zhao L, Zhu X, Li X, Huang T, Chu Z, Xu Y. Regulation of JNK signaling pathway and RIPK3/AIF in necroptosis-mediated global cerebral ischemia/reperfusion injury in rats. Exp Neurol. 2020;331:113374. doi: 10.1016/j.expneurol.2020.113374. [DOI] [PubMed] [Google Scholar]
- 35.Hu XM, Li ZX, Lin RH, Shan JQ, Yu QW, Wang RX, Liao LS, Yan WT, Wang Z, Shang L, Huang Y, Zhang Q, Xiong K. Guidelines for regulated cell death assays: a systematic summary, a categorical comparison, a prospective. Front Cell Dev Biol. 2021;9:634690. doi: 10.3389/fcell.2021.634690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang T, Gao D, Jiang X, Hu S, Zhang L, Fei Z. Resveratrol inhibits oxygen-glucose deprivation-induced MMP-3 expression and cell apoptosis in primary cortical cells via the NF-κB pathway. Mol Med Rep. 2014;10:1065–1071. doi: 10.3892/mmr.2014.2239. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Wang S, Huang F, Zhang Q, Qin B, Liao L, Wang M, Wan H, Yan W, Chen D, Liu F, Jiang B, Ji D, Xia X, Huang J, Xiong K. c-FLIP regulates pyroptosis in retinal neurons following oxygen-glucose deprivation/recovery via a GSDMD-mediated pathway. Ann Anat. 2021;235:151672. doi: 10.1016/j.aanat.2020.151672. [DOI] [PubMed] [Google Scholar]
- 38.Jiang C, Shi R, Chen B, Yan X, Tang G. Casticin elicits inflammasome-induced pyroptosis through activating PKR/JNK/NF-κB signal in 5-8F cells. Biomed Pharmacother. 2020a;123:109576. doi: 10.1016/j.biopha.2019.109576. [DOI] [PubMed] [Google Scholar]
- 39.Jiang M, Qi L, Li L, Wu Y, Song D, Li Y. Caspase-8: A key protein of cross-talk signal way in “PANoptosis” in cancer. Int J Cancer. 2021;149:1408–1420. doi: 10.1002/ijc.33698. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Q, Geng X, Warren J, Eugene Paul Cosky E, Kaura S, Stone C, Li F, Ding Y. Hypoxia inducible factor-1α (HIF-1α) mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following ischemic stroke. Neuroscience. 2020b;448:126–139. doi: 10.1016/j.neuroscience.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 41.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Núñez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Karki R, Sharma BR, Lee E, Banoth B, Malireddi RKS, Samir P, Tuladhar S, Mummareddy H, Burton AR, Vogel P, Kanneganti TD. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight. 2020a;5:e136720. doi: 10.1172/jci.insight.136720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby R, Jonsson CB, Kanneganti TD. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. bioRxiv. 2020b doi: 10.1016/j.cell.2020.11.025. doi: 101101/20201029361048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby R, Jonsson CB, Kanneganti TD. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kesavardhana S, Malireddi RKS, Burton AR, Porter SN, Vogel P, Pruett-Miller SM, Kanneganti TD. The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J Biol Chem. 2020;295:8325–8330. doi: 10.1074/jbc.RA120.013752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kikuchi M, Kuroki S, Kayama M, Sakaguchi S, Lee KK, Yonehara S. Protease activity of procaspase-8 is essential for cell survival by inhibiting both apoptotic and nonapoptotic cell death dependent on receptor-interacting protein kinase 1 (RIP1) and RIP3. J Biol Chem. 2012;287:41165–41173. doi: 10.1074/jbc.M112.419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SJ, Li J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 2013;4:e716. doi: 10.1038/cddis.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong D, Zhu J, Liu Q, Jiang Y, Xu L, Luo N, Zhao Z, Zhai Q, Zhang H, Zhu M, Liu X. Mesenchymal stem cells protect neurons against hypoxic-ischemic injury via inhibiting parthanatos, necroptosis, and apoptosis, but not autophagy. Cell Mol Neurobiol. 2017;37:303–313. doi: 10.1007/s10571-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation-target or tool for therapy. Acta Neuropathol. 2019;137:693–714. doi: 10.1007/s00401-018-1930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, Sui C, Wang W, Yan J, Deng N, Du X, Cheng F, Ma X, Wang X, Wang Q. Baicalin attenuates oxygen-glucose deprivation/reoxygenation-induced injury by modulating the BDNF-TrkB/PI3K/Akt and MAPK/Erk1/2 signaling axes in neuron-astrocyte cocultures. Front Pharmacol. 2021a;12:599543. doi: 10.3389/fphar.2021.599543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li F, Xu D, Hou K, Gou X, Lv N, Fang W, Li Y. Pretreatment of indobufen and aspirin and their combinations with clopidogrel or ticagrelor alleviates inflammasome mediated pyroptosis via inhibiting NF-κB/NLRP3 pathway in ischemic stroke. J Neuroimmune Pharmacol. 2021b doi: 10.1007/s11481-020-09978-9. doi: 101007/s11481-020-09978-9. [DOI] [PubMed] [Google Scholar]
- 54.Li HN, Zheng RF, Du YW, Wang W, Sun FL, Liu DW, Xing JG. Effect and mechanism of tilianin against necroptosis on cerebral ischemia-reperfusion. Zhongcaoyao. 2021c;52:1974–1980. [Google Scholar]
- 55.Li J, Zhang J, Zhang Y, Wang Z, Song Y, Wei S, He M, You S, Jia J, Cheng J. TRAF2 protects against cerebral ischemia-induced brain injury by suppressing necroptosis. Cell Death Dis. 2019;10:328. doi: 10.1038/s41419-019-1558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Hao JH, Yao D, Li R, Li XF, Yu ZY, Luo X, Liu XH, Wang MH, Wang W. Caspase-1 inhibition prevents neuronal death by targeting the canonical inflammasome pathway of pyroptosis in a murine model of cerebral ischemia. CNS Neurosci Ther. 2020a;26:925–939. doi: 10.1111/cns.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, Liu J, Chen JR, Zhu YM, Gao X, Ni Y, Lin B, Li H, Qiao SG, Wang C, Zhang HL, Ao GZ. Neuroprotective effects of DTIO, a novel analog of Nec-1, in acute and chronic stages after ischemic stroke. Neuroscience. 2018;390:12–29. doi: 10.1016/j.neuroscience.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 58.Li X, Cheng S, Hu H, Zhang X, Xu J, Wang R, Zhang P. Progranulin protects against cerebral ischemia-reperfusion (I/R) injury by inhibiting necroptosis and oxidative stress. Biochem Biophys Res Commun. 2020b;521:569–576. doi: 10.1016/j.bbrc.2019.09.111. [DOI] [PubMed] [Google Scholar]
- 59.Liang J, Wang Q, Li JQ, Guo T, Yu D. Long non-coding RNA MEG3 promotes cerebral ischemia-reperfusion injury through increasing pyroptosis by targeting miR-485/AIM2 axis. Exp Neurol. 2020a;325:113139. doi: 10.1016/j.expneurol.2019.113139. [DOI] [PubMed] [Google Scholar]
- 60.Liang Y, Song P, Chen W, Xie X, Luo R, Su J, Zhu Y, Xu J, Liu R, Zhu P, Zhang Y, Huang M. Inhibition of caspase-1 ameliorates ischemia-associated blood-brain barrier dysfunction and integrity by suppressing pyroptosis activation. Front Cell Neurosci. 2020b;14:540669. doi: 10.3389/fncel.2020.540669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao LS, Lu S, Yan WT, Wang SC, Guo LM, Yang YD, Huang K, Hu XM, Zhang Q, Yan J, Xiong K. The role of HSP90α in methamphetamine/hyperthermia-induced necroptosis in rat striatal neurons. Front Pharmacol. 2021;12:716394. doi: 10.3389/fphar.2021.716394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin Y, Cai B, Xue XH, Fang L, Wu ZY, Wang N. TAT-mediated delivery of neuroglobin attenuates apoptosis induced by oxygen-glucose deprivation via the Jak2/Stat3 pathway in vitro. Neurol Res. 2015;37:531–538. doi: 10.1179/1743132814Y.0000000420. [DOI] [PubMed] [Google Scholar]
- 63.Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant. 2013;13:2797–2804. doi: 10.1111/ajt.12448. [DOI] [PubMed] [Google Scholar]
- 64.Liu H, Zhao Z, Wu T, Zhang Q, Lu F, Gu J, Jiang T, Xue J. Inhibition of autophagy-dependent pyroptosis attenuates cerebral ischaemia/reperfusion injury. J Cell Mol Med. 2021a;25:5060–5069. doi: 10.1111/jcmm.16483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu J, Wang Q, Yang S, Huang J, Feng X, Peng J, Lin Z, Liu W, Tao J, Chen L. Electroacupuncture inhibits apoptosis of peri-ischemic regions via modulating p38, extracellular signal-regulated kinase (ERK1/2), and c-Jun N terminal kinases (JNK) in cerebral ischemia-reperfusion-injured rats. Med Sci Monit. 2018;24:4395–4404. doi: 10.12659/MSM.908473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X, Lv X, Liu Z, Zhang M, Leng Y. MircoRNA-29a in astrocyte-derived extracellular vesicles suppresses brain ischemia reperfusion injury via TP53INP1 and the NF-κB/NLRP3 axis. Cell Mol Neurobiol. 2021b doi: 10.1007/s10571-021-01040-3. doi: 101007/s10571-021-01040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X, Zhang M, Liu H, Zhu R, He H, Zhou Y, Zhang Y, Li C, Liang D, Zeng Q, Huang G. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol. 2021c;341:113700. doi: 10.1016/j.expneurol.2021.113700. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, Jiang S, Yang PY, Zhang YF, Li TJ, Rui YC. EF1A1/HSC70 cooperatively suppress brain endothelial cell apoptosis via regulating JNK activity. CNS Neurosci Ther. 2016;22:836–844. doi: 10.1111/cns.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu S, Yang Y, Liao L, Yan W, Xiong K, Yan J. iTRAQ-based proteomic analysis of the rat striatum in response to methamphetamine preconditioning. Acta Biochim Biophys Sin (Shanghai) 2021;53:636–639. doi: 10.1093/abbs/gmab024. [DOI] [PubMed] [Google Scholar]
- 70.Lu S, Liao L, Zhang B, Yan W, Chen L, Yan H, Guo L, Lu S, Xiong K, Yan J. Antioxidant cascades confer neuroprotection in ethanol, morphine, and methamphetamine preconditioning. Neurochem Int. 2019a;131:104540. doi: 10.1016/j.neuint.2019.104540. [DOI] [PubMed] [Google Scholar]
- 71.Lu YY, Liu XL, Huang Y, Liao Y, Xi T, Zhang YN, Zhang LL, Shu SN, Fang F. Short-lived AIM2 inflammasome activation relates to chronic MCMV infection in BALB/c mice. Curr Med Sci. 2019b;39:899–905. doi: 10.1007/s11596-019-2121-4. [DOI] [PubMed] [Google Scholar]
- 72.Lünemann JD, Malhotra S, Shinohara ML, Montalban X, Comabella M. Targeting inflammasomes to treat neurological diseases. Ann Neurol. 2021;90:177–188. doi: 10.1002/ana.26158. [DOI] [PubMed] [Google Scholar]
- 73.Ma YL, Zhang LX, Liu GL, Fan Y, Peng Y, Hou WG. N-Myc downstream-regulated gene 2 (Ndrg2) is involved in ischemia-hypoxia-induced astrocyte apoptosis: a novel target for stroke therapy. Mol Neurobiol. 2017;54:3286–3299. doi: 10.1007/s12035-016-9814-5. [DOI] [PubMed] [Google Scholar]
- 74.Malireddi RK, Ippagunta S, Lamkanfi M, Kanneganti TD. Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J Immunol. 2010;185:3127–3130. doi: 10.4049/jimmunol.1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malireddi RKS, Kesavardhana S, Kanneganti TD. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis) Front Cell Infect Microbiol. 2019;9:406. doi: 10.3389/fcimb.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malireddi RKS, Kesavardhana S, Karki R, Kancharana B, Burton AR, Kanneganti TD. RIPK1 distinctly regulates yersinia-induced inflammatory cell death, PANoptosis. ImmunoHorizons. 2020a;4:789–796. doi: 10.4049/immunohorizons.2000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malireddi RKS, Gurung P, Mavuluri J, Dasari TK, Klco JM, Chi H, Kanneganti TD. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med. 2018;215:1023–1034. doi: 10.1084/jem.20171922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malireddi RKS, Gurung P, Kesavardhana S, Samir P, Burton A, Mummareddy H, Vogel P, Pelletier S, Burgula S, Kanneganti TD. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis ,necroptosis, and inflammatory disease. J Exp Med. 2020b;217 doi: 10.1084/jem.20191644. jem20191644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 80.McBride DW, Zhang JH. Precision stroke animal models: the permanent MCAO model should be the primary model, not transient MCAO. Transl Stroke Res. 2017 doi: 10.1007/s12975-017-0554-2. doi: 101007/s12975-017-0554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McKenzie BA, Dixit VM, Power C. Fiery cell death: pyroptosis in the central nervous system. Trends Neurosci. 2020;43:55–73. doi: 10.1016/j.tins.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Meng H, Wu G, Zhao X, Wang A, Li D, Tong Y, Jin T, Cao Y, Shan B, Hu S, Li Y, Pan L, Tian X, Wu P, Peng C, Yuan J, Li G, Tan L, Wang Z, Li Y. Discovery of a cooperative mode of inhibiting RIPK1 kinase. Cell Discov. 2021;7:41. doi: 10.1038/s41421-021-00278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meschia JF, Brott T. Ischaemic stroke. Eur J Neurol. 2018;25:35–40. doi: 10.1111/ene.13409. [DOI] [PubMed] [Google Scholar]
- 84.Mo Y, Sun YY, Liu KY. Autophagy and inflammation in ischemic stroke. Neural Regen Res. 2020a;15:1388–1396. doi: 10.4103/1673-5374.274331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mo ZT, Liao YL, Zheng J, Li WN. Icariin protects neurons from endoplasmic reticulum stress-induced apoptosis after OGD/R injury via suppressing IRE1α-XBP1 signaling pathway. Life Sci. 2020b;255:117847. doi: 10.1016/j.lfs.2020.117847. [DOI] [PubMed] [Google Scholar]
- 86.Naito MG, Xu D, Amin P, Lee J, Wang H, Li W, Kelliher M, Pasparakis M, Yuan J. Sequential activation of necroptosis and apoptosis cooperates to mediate vascular and neural pathology in stroke. Proc Natl Acad Sci U S A. 2020;117:4959–4970. doi: 10.1073/pnas.1916427117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neubert M, Ridder DA, Bargiotas P, Akira S, Schwaninger M. Acute inhibition of TAK1 protects against neuronal death in cerebral ischemia. Cell Death Differ. 2011;18:1521–1530. doi: 10.1038/cdd.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ni Y, Gu WW, Liu ZH, Zhu YM, Rong JG, Kent TA, Li M, Qiao SG, An JZ, Zhang HL. RIP1K contributes to neuronal and astrocytic cell death in ischemic stroke via activating autophagic-lysosomal pathway. Neuroscience. 2018;371:60–74. doi: 10.1016/j.neuroscience.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 89.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 91.Pender MP, Rist MJ. Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia. 2001;36:137–144. doi: 10.1002/glia.1103. [DOI] [PubMed] [Google Scholar]
- 92.Poh L, Kang SW, Baik SH, Ng GYQ, She DT, Balaganapathy P, Dheen ST, Magnus T, Gelderblom M, Sobey CG, Koo EH, Fann DY, Arumugam TV. Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav Immun. 2019;75:34–47. doi: 10.1016/j.bbi.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Ren Q, Hu Z, Jiang Y, Tan X, Botchway BOA, Amin N, Lin G, Geng Y, Fang M. SIRT1 protects against apoptosis by promoting autophagy in the oxygen glucose deprivation/reperfusion-induced injury. Front Neurol. 2019;10:1289. doi: 10.3389/fneur.2019.01289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ridder DA, Schwaninger M. TAK1 inhibition for treatment of cerebral ischemia. Exp Neurol. 2013;239:68–72. doi: 10.1016/j.expneurol.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 95.Rizzo MT, Leaver HA. Brain endothelial cell death: modes, signaling pathways, and relevance to neural development, homeostasis, and disease. Mol Neurobiol. 2010;42:52–63. doi: 10.1007/s12035-010-8132-6. [DOI] [PubMed] [Google Scholar]
- 96.Ryan F, Khodagholi F, Dargahi L, Minai-Tehrani D, Ahmadiani A. Temporal pattern and crosstalk of necroptosis markers with autophagy and apoptosis associated proteins in ischemic hippocampus. Neurotox Res. 2018;34:79–92. doi: 10.1007/s12640-017-9861-3. [DOI] [PubMed] [Google Scholar]
- 97.Ryou MG, Mallet RT. An in vitro oxygen-glucose deprivation model for studying ischemia-reperfusion injury of neuronal cells. Methods Mol Biol. 2018;1717:229–235. doi: 10.1007/978-1-4939-7526-6_18. [DOI] [PubMed] [Google Scholar]
- 98.Salvador E, Burek M, Förster CY. An in vitro model of traumatic brain injury. Methods Mol Biol. 2018;1717:219–227. doi: 10.1007/978-1-4939-7526-6_17. [DOI] [PubMed] [Google Scholar]
- 99.Samir P, Malireddi RKS, Kanneganti TD. The PANoptosome: a deadly protein complex driving pyroptosis, apoptosis, and necroptosis (PANoptosis) Front Cell Infect Microbiol. 2020;10:238. doi: 10.3389/fcimb.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.She Y, Shao L, Zhang Y, Hao Y, Cai Y, Cheng Z, Deng C, Liu X. Neuroprotective effect of glycosides in Buyang Huanwu Decoction on pyroptosis following cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol. 2019;242:112051. doi: 10.1016/j.jep.2019.112051. [DOI] [PubMed] [Google Scholar]
- 101.Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18:1058–1066. doi: 10.1016/S1474-4422(19)30078-X. [DOI] [PubMed] [Google Scholar]
- 102.Song M, Xia W, Tao Z, Zhu B, Zhang W, Liu C, Chen S. Self-assembled polymeric nanocarrier-mediated co-delivery of metformin and doxorubicin for melanoma therapy. Drug Deliv. 2021;28:594–606. doi: 10.1080/10717544.2021.1898703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stanzione R, Forte M, Cotugno M, Bianchi F, Marchitti S, Rubattu S. Role of DAMPs and of leukocytes infiltration in ischemic stroke: insights from animal models and translation to the human disease. Cell Mol Neurobiol. 2020 doi: 10.1007/s10571-020-00966-4. doi: 101007/s10571-020-00966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 105.Sun R, Peng M, Xu P, Huang F, Xie Y, Li J, Hong Y, Guo H, Liu Q, Zhu W. Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. J Neuroinflammation. 2020;17:330. doi: 10.1186/s12974-020-01988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang M, Li X, Liu P, Wang J, He F, Zhu X. Bradykinin B2 receptors play a neuroprotective role in Hypoxia/reoxygenation injury related to pyroptosis pathway. Curr Neurovasc Res. 2018;15:138–144. doi: 10.2174/1567202615666180528073141. [DOI] [PubMed] [Google Scholar]
- 107.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, Licata G. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem. 2009;9:1240–1260. doi: 10.2174/156802609789869619. [DOI] [PubMed] [Google Scholar]
- 109.Vieira M, Fernandes J, Carreto L, Anuncibay-Soto B, Santos M, Han J, Fernández-López A, Duarte CB, Carvalho AL, Santos AE. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol Dis. 2014;68:26–36. doi: 10.1016/j.nbd.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 111.Voet S, Srinivasan S, Lamkanfi M, van Loo G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med. 2019;11:e10248. doi: 10.15252/emmm.201810248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wallach D, Kovalenko A, Kang TB. ‘Necrosome’-induced inflammation: must cells die for it. Trends Immunol. 2011;32:505–509. doi: 10.1016/j.it.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 113.Wang K, Sun Z, Ru J, Wang S, Huang L, Ruan L, Lin X, Jin K, Zhuge Q, Yang S. Ablation of GSDMD improves outcome of ischemic stroke through blocking canonical and non-canonical inflammasomes dependent pyroptosis in microglia. Front Neurol. 2020a;11:577927. doi: 10.3389/fneur.2020.577927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang L, Wu D, Xu Z. USP10 protects against cerebral ischemia injury by suppressing inflammation and apoptosis through the inhibition of TAK1 signaling. Biochem Biophys Res Commun. 2019;516:1272–1278. doi: 10.1016/j.bbrc.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 115.Wang M, Wan H, Wang S, Liao L, Huang Y, Guo L, Liu F, Shang L, Huang J, Ji D, Xia X, Jiang B, Chen D, Xiong K. RSK3 mediates necroptosis by regulating phosphorylation of RIP3 in rat retinal ganglion cells. J Anat. 2020b;237:29–47. doi: 10.1111/joa.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang N, Wu L, Cao Y, Wang Y, Zhang Y. The protective activity of imperatorin in cultured neural cells exposed to hypoxia re-oxygenation injury via anti-apoptosis. Fitoterapia. 2013;90:38–43. doi: 10.1016/j.fitote.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 117.Wang Q, Yin XH, Liu Y, Zhang GY. K252a suppresses neuronal cells apoptosis through inhibiting the translocation of Bax to mitochondria induced by the MLK3/JNK signaling after transient global brain ischemia in rat hippocampal CA1 subregion. J Recept Signal Transduct Res. 2011;31:307–313. doi: 10.3109/10799893.2011.592536. [DOI] [PubMed] [Google Scholar]
- 118.Wang Q, Wu J, Zeng Y, Chen K, Wang C, Yang S, Sun N, Chen H, Duan K, Zeng G. Pyroptosis: a pro-inflammatory type of cell death in cardiovascular disease. Clin Chim Acta. 2020c;510:62–72. doi: 10.1016/j.cca.2020.06.044. [DOI] [PubMed] [Google Scholar]
- 119.Wang QS, Luo XY, Fu H, Luo Q, Wang MQ, Zou DY. MiR-139 protects against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced nerve injury through targeting c-Jun to inhibit NLRP3 inflammasome activation. J Stroke Cerebrovasc Dis. 2020d;29:105037. doi: 10.1016/j.jstrokecerebrovasdis.2020.105037. [DOI] [PubMed] [Google Scholar]
- 120.Wang W, Xie L, Zou X, Hu W, Tian X, Zhao G, Chen M. Pomelo peel oil suppresses TNF-α-induced necroptosis and cerebral ischaemia-reperfusion injury in a rat model of cardiac arrest. Pharm Biol. 2021a;59:401–409. doi: 10.1080/13880209.2021.1903046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang WY, Xie L, Zou XS, Li N, Yang YG, Wu ZJ, Tian XY, Zhao GY, Chen MH. Inhibition of extracellular signal-regulated kinase/calpain-2 pathway reduces neuroinflammation and necroptosis after cerebral ischemia-reperfusion injury in a rat model of cardiac arrest. Int Immunopharmacol. 2021b;93:107377. doi: 10.1016/j.intimp.2021.107377. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y, Guan X, Gao CL, Ruan W, Zhao S, Kai G, Li F, Pang T. Medioresinol as a novel PGC-1α activator prevents pyroptosis of endothelial cells in ischemic stroke through PPARα-GOT1 axis. Pharmacol Res. 2021c;169:105640. doi: 10.1016/j.phrs.2021.105640. [DOI] [PubMed] [Google Scholar]
- 123.Wang Z, Guo LM, Wang Y, Zhou HK, Wang SC, Chen D, Huang JF, Xiong K. Inhibition of HSP90α protects cultured neurons from oxygen-glucose deprivation induced necroptosis by decreasing RIP3 expression. J Cell Physiol. 2018;233:4864–4884. doi: 10.1002/jcp.26294. [DOI] [PubMed] [Google Scholar]
- 124.Wu CX, Wang TF, Yu JQ. Lycium barbarum polysaccharide pretreatment attenuates cerebral ischemic reperfusion injury by inhibiting apoptosis in mice. Zhong Yao Cai. 2015;38:1454–1459. [PubMed] [Google Scholar]
- 125.Wu F, Zhang R, Feng Q, Cheng H, Xue J, Chen J. (-)-Clausenamide alleviated ER stress and apoptosis induced by OGD/R in primary neuron cultures. Neurol Res. 2020a;42:730–738. doi: 10.1080/01616412.2020.1771040. [DOI] [PubMed] [Google Scholar]
- 126.Wu X, Lin L, Qin JJ, Wang L, Wang H, Zou Y, Zhu X, Hong Y, Zhang Y, Liu Y, Xin C, Xu S, Ye S, Zhang J, Xiong Z, Zhu L, Li H, Chen J, She ZG. CARD3 promotes cerebral ischemia-reperfusion injury via activation of TAK1. J Am Heart Assoc. 2020b;9:e014920. doi: 10.1161/JAHA.119.014920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu P, Zhang X, Liu Q, Xie Y, Shi X, Chen J, Li Y, Guo H, Sun R, Hong Y, Liu X, Xu G. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10:555. doi: 10.1038/s41419-019-1777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan PJ, Hou LS, Li ME, Lu ZX, Zhan FY, Ran MD, Li JJ, Zhang L, Yang R, Zhou MK, Zhu CR. Associations between lesion locations and stroke recurrence in survivors of first-ever ischemic stroke: a prospective cohort study. Curr Med Sci. 2020a;40:708–718. doi: 10.1007/s11596-020-2240-y. [DOI] [PubMed] [Google Scholar]
- 129.Yan W, Wang Z, Lu S, Li J, Chen Q, Wang L, Chen S, Wang X, Xiong K, Yan J. Analysis of factors related to prognosis and death of fish bile poisoning in China: a retrospective study. Basic Clin Pharmacol Toxicol. 2020b;127:419–428. doi: 10.1111/bcpt.13447. [DOI] [PubMed] [Google Scholar]
- 130.Yan WT, Lu S, Yang YD, Ning WY, Cai Y, Hu XM, Zhang Q, Xiong K. Research trends, hot spots and prospects for necroptosis in the field of neuroscience. Neural Regen Res. 2021;16:1628–1637. doi: 10.4103/1673-5374.303032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yanamoto H, Nagata I, Niitsu Y, Xue JH, Zhang Z, Kikuchi H. Evaluation of MCAO stroke models in normotensive rats: standardized neocortical infarction by the 3VO technique. Exp Neurol. 2003;182:261–274. doi: 10.1016/s0014-4886(03)00116-x. [DOI] [PubMed] [Google Scholar]
- 132.Yang M, Lv Y, Tian X, Lou J, An R, Zhang Q, Li M, Xu L, Dong Z. Neuroprotective effect of β-caryophyllene on cerebral ischemia-reperfusion injury via regulation of necroptotic neuronal death and inflammation: in vivo and in vitro. Front Neurosci. 2017;11:583. doi: 10.3389/fnins.2017.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang Y, Gao H, Liu W, Liu X, Jiang X, Li X, Wu Q, Xu Z, Zhao Q. Arctium lappa L. roots ameliorates cerebral ischemia through inhibiting neuronal apoptosis and suppressing AMPK/mTOR-mediated autophagy. Phytomedicine. 2021;85:153526. doi: 10.1016/j.phymed.2021.153526. [DOI] [PubMed] [Google Scholar]
- 134.Yu Y, Wu X, Pu J, Luo P, Ma W, Wang J, Wei J, Wang Y, Fei Z. Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation-induced apoptosis and autophagic cell death via the PI3K/Akt/mTOR signaling pathway in primary cultured hippocampal neurons. Biochem Biophys Res Commun. 2018;495:1187–1194. doi: 10.1016/j.bbrc.2017.11.165. [DOI] [PubMed] [Google Scholar]
- 135.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 136.Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20:19–33. doi: 10.1038/s41583-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuan P, Liu Z, Liu M, Huang J, Li X, Zhou X. Up-regulated tumor necrosis factor-associated factor 6 level is correlated with apoptosis in the rat cerebral ischemia and reperfusion. Neurol Sci. 2013;34:1133–1138. doi: 10.1007/s10072-012-1199-2. [DOI] [PubMed] [Google Scholar]
- 138.Yuan Z, Yi-Yun S, Hai-Yan Y. Triad3A displays a critical role in suppression of cerebral ischemic/reperfusion (I/R) injury by regulating necroptosis. Biomed Pharmacother. 2020;128:110045. doi: 10.1016/j.biopha.2020.110045. [DOI] [PubMed] [Google Scholar]
- 139.Zeng Q, Zhou Y, Liang D, He H, Liu X, Zhu R, Zhang M, Luo X, Wang Y, Huang G. Exosomes secreted from bone marrow mesenchymal stem cells attenuate oxygen-glucose deprivation/reoxygenation-induced pyroptosis in PC12 cells by promoting AMPK-dependent autophagic flux. Front Cell Neurosci. 2020;14:182. doi: 10.3389/fncel.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zeyen T, Noristani R, Habib S, Heinisch O, Slowik A, Huber M, Schulz JB, Reich A, Habib P. Microglial-specific depletion of TAK1 is neuroprotective in the acute phase after ischemic stroke. J Mol Med (Berl) 2020;98:833–847. doi: 10.1007/s00109-020-01916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang D, Qian J, Zhang P, Li H, Shen H, Li X, Chen G. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J Neurosci Res. 2019a;97:645–660. doi: 10.1002/jnr.24385. [DOI] [PubMed] [Google Scholar]
- 142.Zhang JF, Shi LL, Zhang L, Zhao ZH, Liang F, Xu X, Zhao LY, Yang PB, Zhang JS, Tian YF. MicroRNA-25 negatively regulates cerebral ischemia/reperfusion injury-induced cell apoptosis through Fas/FasL pathway. J Mol Neurosci. 2016;58:507–516. doi: 10.1007/s12031-016-0712-0. [DOI] [PubMed] [Google Scholar]
- 143.Zhang P, Zhang Y, Zhang J, Wu Y, Jia J, Wu J, Hu Y. Early exercise protects against cerebral ischemic injury through inhibiting neuron apoptosis in cortex in rats. Int J Mol Sci. 2013;14:6074–6089. doi: 10.3390/ijms14036074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Y, Wang H, Li H, Nan L, Xu W, Lin Y, Chu K. Gualou Guizhi granule protects against OGD/R-induced injury by inhibiting cell pyroptosis via the PI3K/Akt signaling pathway. Evid Based Complement Alternat Med. 2021;2021:6613572. doi: 10.1155/2021/6613572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Y, Li M, Li X, Zhang H, Wang L, Wu X, Zhang H, Luo Y. Catalytically inactive RIP1 and RIP3 deficiency protect against acute ischemic stroke by inhibiting necroptosis and neuroinflammation. Cell Death Dis. 2020;11:565. doi: 10.1038/s41419-020-02770-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang YY, Liu WN, Li YQ, Zhang XJ, Yang J, Luo XJ, Peng J. Ligustroflavone reduces necroptosis in rat brain after ischemic stroke through targeting RIPK1/RIPK3/MLKL pathway. Naunyn Schmiedebergs Arch Pharmacol. 2019b;392:1085–1095. doi: 10.1007/s00210-019-01656-9. [DOI] [PubMed] [Google Scholar]
- 147.Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F. Regulation of microglial activation in stroke. Acta Pharmacol Sin. 2017;38:445–458. doi: 10.1038/aps.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zheng M, Kanneganti TD. Newly identified function of caspase-6 in ZBP1-mediated innate immune responses, NLRP3 inflammasome activation, PANoptosis, and host defense. J Cell Immunol. 2020a;2:341–347. doi: 10.33696/immunology.2.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zheng M, Kanneganti TD. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis) Immunol Rev. 2020b;297:26–38. doi: 10.1111/imr.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng M, Williams EP, Malireddi RKS, Karki R, Banoth B, Burton A, Webby R, Channappanavar R, Jonsson CB, Kanneganti TD. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J Biol Chem. 2020;295:14040–14052. doi: 10.1074/jbc.RA120.015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou L, Ao LY, Yan YY, Li WT, Ye AQ, Li CY, Shen WY, Liang BW, Xiong Z, Li YM. JLX001 ameliorates ischemia/reperfusion injury by reducing neuronal apoptosis via down-regulating JNK signaling pathway. Neuroscience. 2019;418:189–204. doi: 10.1016/j.neuroscience.2019.08.053. [DOI] [PubMed] [Google Scholar]
- 152.Zhu S, Zhang Z, Jia LQ, Zhan KX, Wang LJ, Song N, Liu Y, Cheng YY, Yang YJ, Guan L, Min DY, Yang GL. Valproic acid attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-pyroptosis pathways. Neurochem Int. 2019;124:141–151. doi: 10.1016/j.neuint.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 153.Zou X, Xie L, Wang W, Zhao G, Tian X, Chen M. FK866 alleviates cerebral pyroptosis and inflammation mediated by Drp1 in a rat cardiopulmonary resuscitation model. Int Immunopharmacol. 2020;89:107032. doi: 10.1016/j.intimp.2020.107032. [DOI] [PubMed] [Google Scholar]
- 154.Zou XS, Xie L, Wang WY, Zhao GY, Tian XY, Chen MH. Pomelo peel oil alleviates cerebral NLRP3 inflammasome activation in a cardiopulmonary resuscitation rat model. Exp Ther Med. 2021;21:233. doi: 10.3892/etm.2021.9664. [DOI] [PMC free article] [PubMed] [Google Scholar]


