Key Words: activity of daily living, early stage treatment, emotional disorder, limb dysfunction, neural regeneration, randomized controlled clinical trial, rehabilitation, safety of treatment, stroke, transcutaneous auricular-vagus nerve stimulation
Abstract
Transcutaneous auricular vagus nerve stimulation (ta-VNS) is a novel noninvasive treat-ment for stroke that directly stimulates the peripheral auricular branch of the vagus nerve. There have been recent reports that ta-VNS combined with conventional rehabilitation training promotes the recovery of neurological function of patients with acute stroke. However, these were small-sample-sized studies on the recovery of neurological function in patients after percutaneous vagus nerve stimulation in the subacute and chronic phases after stroke. This double-blinded randomized controlled trial involved 60 acute ischemic or hemorrhagic stroke patients aged 18–80 years who received treatment in the Second Affiliated Hospital of Chongqing Medical University. The subjects were randomly assigned to receive ta-VNS or sham ta-VNS combined with conventional rehabilitation training. The follow-up results over 1 year revealed that ta-VNS combined with conventional rehabilitation training greatly improved the recovery of motor and sensory functions and emotional responses compared with sham ta-VNS combined with conventional rehabilitation training. There were no obvious side effects. These findings suggest that ta-VNS combined with conventional rehabilitation training for the treatment of acute ischemic or hemorrhagic stroke patients is safe and effective.
Introduction
The purpose of rehabilitation training is to promote adaptive circuit changes, but often insufficient or inappropriate plasticity blocks neurofunctional recovery post-stroke (Furie, 2020; Le Danseur, 2020). The development of adjunctive treatment strategies broadly supports neuroplasticity to facilitate brain rehabilitation to improve stroke prognosis (Le Danseur, 2020; Stinear et al., 2020). Transcutaneous auricular vagus nerve stimulation (ta-VNS) is a recent non-invasive method of vagus nerve stimulation (VNS) by directly stimulating the peripheral auricular branch of the vagus nerve (Ay et al., 2015; Wu et al., 2020), making it more accessible for treating stroke. However, there is only limited clinical evidence for its effectiveness (Yuan and Silberstein, 2016; Wu et al., 2020). The latter report showed that ta-VNS promoted upper limb motor function recovery (Wu et al., 2020).
Researchers have pointed out that neural plasticity can be enhanced during the early stages post stroke (Coleman et al., 2017; Zeiler, 2019; Yang et al., 2020; Hu et al., 2021; Wang et al., 2021), indicating that the earlier post-stroke rehabilitative strategies commence, the better the outcome. At present, most of the research has centered on subacute and chronic stroke patients (Capone et al., 2017; Wu et al., 2020). Although it was found that simple rehabilitation training can promote the recovery of neurological function after stroke (Khodaparast et al., 2016), the effect of rehabilitation training combined with electrical stimulation on the improvement of neurological function after stroke is still not clear. In addition, there have been very few trials to explore the safety and efficacy of electrical stimulation therapy on the overall motor and sensory functions and the emotional assessments in patients with acute stroke.
We propose that ta-VNS can enhance the benefit to the rehabilitation therapy on the recovery of motor and sensory functions and emotional response following acute ischemic or hemorrhagic stroke. This double-blind randomized placebo-controlled study would test this hypothesis.
Subjects and Methods
Study design
The study was a two-group, pragmatic, double-blinded, randomized controlled trial. Eligible acute stroke patients were enrolled and then randomly assigned (1:1) to one of two groups, ta-VNS group or control group, and were assessed periodically during the first year after intervention. The patients all signed informed consent forms (Additional file 1 (148KB, pdf) ). The partici-pants and researchers were blinded to the therapy process of the entire research. This project was approved by the Ethics Committee of The Second Affiliated Hospital of Chongqing Medical University (approval No. 2018(216)) on May 7, 2018 (Additional file 2 (202.5KB, pdf) ) and retrospective registered in Chinese Clinical Trial Registry (registration No. ChiCTR1800018962) on October 19, 2019. This study followed the CONsolidated Standards Of Reporting Trials (CONSORT) guidance (Schulz et al., 2010; Additional file 3).
CONSORT 2010 checklist of information to include when reporting a randomised trial *
| Section/Topic | It em No | Checklist item | Reported on page No |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | 1 | |
| Background and | 2a | Scientific background and explanation of rationale | 2 |
| objectives | 2b | Specific objectives or hypotheses | 2 |
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 3 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | None | |
| Participants | 4a | Eligibility criteria for participants | 3 |
| 4b | Settings and locations where the data were collected | 4 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 4 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 5 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | None | |
| Sample size | 7a | 5 | |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | None | |
| Randomisation: | |||
| Sequence | 8a | Method used to generate the random allocation sequence | 4 |
| generation | 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 4 |
| Allocation | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), | 4 |
| concealment | describing any steps taken to conceal the sequence until interventions were assigned | ||
| mechanism | |||
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 4 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 4 |
| 11b | If relevant, description of the similarity of interventions | 4 | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 6 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 6 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | 17 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | 6 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 3 |
| 14b | Why the trial ended or was stopped | 6 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 17 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 6 |
| Outcomes estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 6-7 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | 6-7 | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | 6-7 |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | 7 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 11 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 10-11 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 8-11 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | 1 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | None |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 12 |
*We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see .www.consort-statement.org
The individual deidentified participant data will be shared; the additional, related documents will be available (such as study protocol and statistical analysis plan). The data will become available in the future of 5 years. Research colleagues can access the data through the China Clinical Trials Registry.
Subjects
Study participants were recruited from November 2018 to March 2020 in The Second Affiliated Hospital of Chongqing Medical University. All eligible participants (aged 18–80 years) had a history of first-time symptomatic ischemic or hemorrhagic stroke, demonstrated by imaging, that had occurred within the previous month to be included in the trial (Table 1). Participants with any of the following symptoms were excluded from our study: (1) a progressive decline in cardiac, pulmonary, liver, kidney function; (2) apraxia; (3) resting heart rate (< 50 beats/min); (4) a previous operation on the vagus nerve; (5) alcohol or drug abuse; (6) participation in other clinical experiments; and (7) other diagnoses that might interfere with rehabilitation or outcome assessments. A CONSORT diagram is shown in Figure 1. Free treatment was provided for these patients throughout the clinical study.
Table 1.
Baseline demographics for participants in ta-VNS and control groups
| Characteristics | ta-VNS (n = 30) | Control (n = 30) | P-value |
|---|---|---|---|
| Male | 15(50) | 14(47) | 0.79 |
| Age (yr) | 69.2±12.3 | 68.3±12.1 | 0.48 |
| Type of stroke (ischemic/hemorrhagic) | 27/3 | 28/2 | 0.64 |
| Days post-stroke | 10.8±7.7 | 10.4±6.9 | 0.90 |
| Handedness (right/left/ambidextrous) | 27/2/1 | 29/0/1 | 0.35 |
| Stroke hemisphere (right/left) | 12/18 | 10/20 | 0.59 |
| FMA-U | 31.4±5.7 | 30.8±5.3 | 0.74 |
| FMA-L | 17.6±3.0 | 17.2±2.9 | 0.62 |
| FMA-S | 13.2±4.0 | 12.9±3.8 | 0.92 |
| Stroke impact scale | 143.1±57.2 | 129.7±53.2 | 0.06 |
| HADS-A | 10.9±1.9 | 11.3±2.0 | 0.05 |
| HADS-D | 8.3±1.4 | 8.5±1.5 | 0.43 |
Data are expressed as number (percentage) (male), number (type of stroke, handedness, stroke hemisphere) or mean ± SD (other data). Student’s t-test was used for continuous variables, and the Chi-square test was used for categorical variables. FMA-L: Fugl-Meyer assessment–lower extremity; FMA-S: Fugl-Meyer assessment–sensory; FMA-U: Fugl-Meyer assessment–upper extremity; HADS-A: Hospital Anxiety and Depression Scale–Anxiety scale; HADS-D: Hospital Anxiety and Depression Scale–Depression scale.
Figure 1.
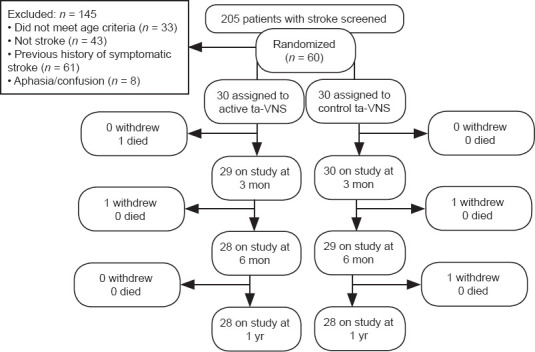
A CONsolidated Standards Of Reporting Trials (CONSORT) diagram.
ta-VNS: Transcutaneous auricular vagus nerve stimulation.
Randomization
Participants were enrolled by a third-party physiotherapist. Participants were randomized to receive active or sham ta-VNS in addition to conventional rehabilitation training by using a system of muddled sealed envelopes including a password for the stimulator. The electrical nerve stimulation instrument (Suzhou Medical supplies Factory Co., Ltd., Suzhou, China) was manipulated by a third party, to ensure that participants, physical therapists, and researchers are all blinded.
Intervention
All participants received a 4-week course of 20-minute therapy sessions five times per week in the hospital. Ta-VNS was delivered by an auricular transcutaneous electrical nerve stimulation apparatus (Suzhou Medical supplies Factory Co., Ltd.). Two carbon-impregnated silicone electrodes of the diameter of 4 mm were placed in the left auricular cavum conchae of the stroke patients and stimulation of the auricular concha innervated by afferent auricular branch of the vagus nerve was delivered via an electric stimulator. For ta-VNS, one electrode was clipped on the concha cavity and the other on the concha cymba. The stimulation parameters were as follows: 0.3-ms square pulses at 20 Hz for 30 seconds and repeated every 5 minutes. The intensity of the current was adjusted according to the tolerance of each patient. The electrical stimulation was given for 20 minutes a day for 20 working days (5 days a week for 4 weeks). The average current intensity was 1.71 ± 0.5 mA to the ta-VNS group. The same procedure was performed on the control group but with no current. Traditional rehabilitation therapy was used on the limbs and torso, depending on the capacity of the patient. Conventional rehabilitation therapy included postural control, neuromuscular facilitation and sensory integration exercises (Wu et al., 2020). Immediately after the active or control ta-VNS session finished, the rehabilitation therapy session began, lasting about 0.5 hours to standardize the intervention and strengthen the effect of the rehabilitation treatment. The apparatus was manipulated by a third party, to ensure that participants, physical therapists, and researchers were all blinded.
Outcome measures
The results evaluated were the Wolf Motor Function Test (WMFT) (Edwards et al., 2012), the upper limb section (FMA-U), the lower limb section (FMA-L), and the somatosensory section (FMA-S) of the Fugl-Meyer Assessment (Bushnell et al., 2015), the Stroke Impact Scale (SIS) (English et al., 2017) and Hospital Anxiety and Depression Scale (HADS) (Mijajlović et al., 2017). These assessments were performed at baseline (on the day of enrollment) and 2 weeks, 1, 3, 6 months, and 1 year from the beginning of ta-VNS treatment. The primary functional outcome was the stroke patients’ motor and sensory functions at a given time after the treatment, as evaluated by WMFT and FMA. WMFT score is ranged from 0 to 75 (Edwards et al., 2012), and FMA score (FMA-U: 0–66; FMA-L: 0–34; FMA-S: 0–24) ranged from 0 to 124 (Bushnell et al., 2015). The higher the WMFT and FMA scores the better the function. The secondary functional outcome was the stroke patients’ activities of daily living and emotional state measured by SIS, and HADS. SIS score is ranged from 64 to 420: the higher the score the better the recovery (English et al., 2017). HADS anxiety (HADS-A) and HADS depression scale (HADS-D) scores ranged from 0 to 21 (Mijajlović et al., 2017). The lower the HADS-A and HADS-D scores the less intense the symptoms.
The safety monitoring assessment was the number of side reactions associated with the equipment or treatment, these included adverse reactions of derma (e.g., ache, red spots on the skin), vocal cord palsy, nausea, and dysphagia. To test the tolerance to ta-VNS treatment, participants were asked whether they experienced any bad feelings or other dis-comforts. Vital signs were carefully measured before and after each treatment session to monitor any cardiovascular risk.
Sample size
The sample size was measured to detect a between-group difference of 5 points on the FMA-U with 80% power at a two-tailed significance level of 0.05. The calculation used by the PASS 15 software (NCSS, LLC, Kaysville, UT, USA) was based on the mean scores and standard deviations of the sample studied in a randomized controlled clinical pilot study (Dawson et al., 2016). We planned to recruit 60 participants, anticipating 20% attrition over the 12 months of the trial.
Statistical analysis
To contrast the baselines of each group, the Student’s t-test was used for continuous varia-bles, and the Chi-square test was used for categorical variables. A per-protocol analysis was conducted. Outcomes (physiological parameter, FMA-U, FMA-L, FMA-S, WMFT score, WMFT time, HADS and SIS) were analyzed using a two-way analysis of variance followed by Šidák’s post hoc multiple comparison test. All data were expressed as mean ± standard deviation (SD). To monitor the security of the electrical stimulations, we measured the BP and HR before and after each stimulation for 20 consecutive days. We performed a repeated-measures analysis of variance followed by Tukey’s post hoc multiple comparison test with pre-post-treatment measurements, days as within-subject factors, and groups as between-subject factors. Statistical significance was accepted at P < 0.05. SPSS 23 software (IBM, Armonk, NY, USA) was used to analyze the data. The statistical methods of this study were reviewed by the epidemiologist of Chongqing Medical University, China.
Results
Study participants
All randomized participants attended the therapeutic process. Four participants left the study before it finished (two withdrew because of improvement in symptoms about 3 months after the stimulus began and no longer wished to carry on). One participant in the control group had another stroke between 6 months and 1 year after the study had begun and was excluded after the 6-month assessment. One participant in the ta-VNS group died of serious lung infection between the 1- and 3-month monitorings and was excluded after the 1-month assessment. In total, 28 participants in each group were included in the per-protocol analysis (Figure 1). Participant characteristics are listed in Table 1. At the start, ta-VNS and control groups were similar in functional assessments.
Physiological parameter
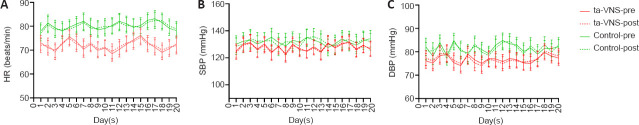
No statistically significant difference was found within each group for heart rate, systolic blood pressure, and diastolic blood pressure before and after treatment (P > 0.05; Figure 2).
Figure 2.
The effects of ta-VNS on cardiovascular measurements in acute stroke patients.
Measurements were taken every day for the 20 days of the experimental therapy. (A) HR, (B) SBP and (C) DBP. Data are expressed as mean ± SD and were analyzed by repeated-measures analysis of variance. DBP: Diastolic blood pressure; HR: heart rate; post: post-treatment; pre: pre-treatment; SBP: systolic blood pressure; ta-VNS: transcutaneous auricular vagus nerve stimulation.
Functional outcomes
There were no significant differences in WMFT (time and score), FMA-U, FMA-L, and FMA-S between the ta-VNS and control groups at the start (P > 0.05). Surprisingly, a significant, continuous improvement trend in neurofunctional recovery was found from the baseline to the 1-year assessment in both groups. The improvements in WMFT (time and score), FMA-U, FMA-L, and FMA-S in the ta-VNS group were significantly higher than those in the control group at each follow-up time point after the 20 sessions of treatment (Figure 3).
Figure 3.
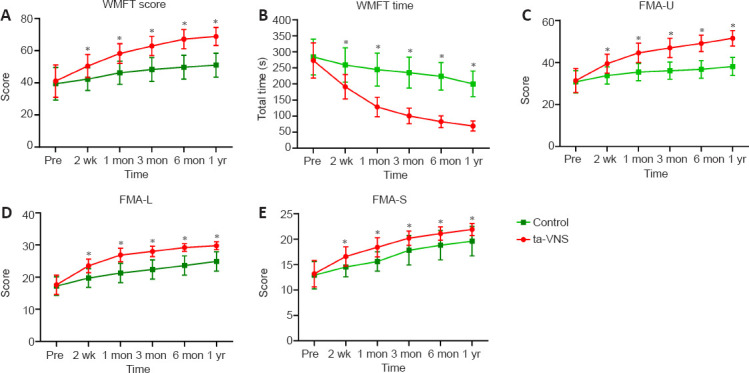
Changes in neurological function scores of acute stroke patients over the year after ta-VNS treatment.
(A–E) WMFT score, WMFT time, FMA-U, FMA-L, and FMA-S. Data are expressed as mean ± SD (n = 28). *P < 0.05, vs. control group (repeated-measures analysis of variance). FMA-L: Fugl-Meyer assessment–lower extremity; FMA-S: Fugl-Meyer assessment–sensory; FMA-U: Fugl-Meyer assessment–upper extremity; WMFT: Wolf Motor Function Test.
Self-assessment outcome
The HADS and SIS revealed remarkable results of “treatment time” and “time-by-treatment”. The HADS-A and HADS-D in the ta-VNS group were lower than those in the control group from the first month onwards. Participants also reported higher SIS in the ta-VNS group from the third month compared with the control group (Figure 4).
Figure 4.
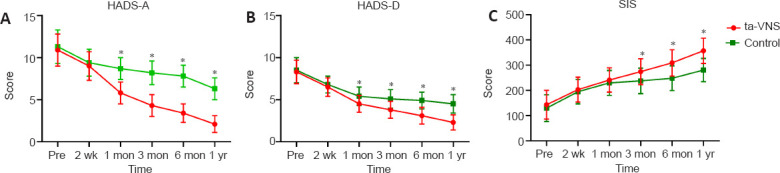
Changes in self-assessment results of acute stroke patients during 1 year after ta-VNS treatment.
(A–C) HADS-A, HADS-D, and SIS. Data are expressed as mean ± SD (n = 28). *P < 0.05, vs. control group (repeated-measures analysis of variance). HADS-A: Hospital Anxiety and Depression Scale—Anxiety scale; HADS-D: Hospital Anxiety and Depression Scale—Depression scale; SIS: stroke impact scale.
Safety
No major side effects or bad experiences were found in the study. Only two participants (both in the ta-VNS group) reported skin redness. But after adjusting the current, the red-ness quickly and completely subsided. These findings are similar to those reported in the previous studies (Zeiler, 2019; Wu et al., 2020).
Discussion
The aim of our study was to research the efficacy and safety of ta-VNS on functional movement, sensory outcomes, and emotional response through the first year of their recov-ery in both acute ischemic and hemorrhagic stroke patients. The primary finding of our re-search is that ta-VNS is safe and, in combination with conventional rehabilitation therapy, significantly improved the neurofunctional outcomes of stroke patients. The measurements of motor function impairment of upper and lower limbs (FMA-U, WMFT, and FMA-L), sensory disturbance rating (FMA-S), emotional state (HADS), and quality of life (SIS) all improved for at least a year after intervention. Our research showed that ta-VNS was well tolerated, none of the participants asked to stop the treatment. Two cases of side effects were found that related to the contact of the ear electrodes. The results are consistent with the recent literature in the field (Wu et al., 2020). Vagal control is crucial for myocardial function (Murray et al., 2016), therefore, we checked heart rate and blood pressure during the therapeutic process to assess cardiovascular risks. Baseline differences in general vital signs existed between the two groups. In theory, this does not affect the comparison of efficacy outcomes. This study revealed that there were no significant clinical changes in physiological parameters during the therapeutic process. In sum, ta-VNS combined with conventional rehabilitation therapy is effective and well-tolerated.
VNS is currently used to treat epilepsy (Wheless et al., 2018), traumatic brain injury (Neren et al., 2016), Parkinson’s disease (Farrand et al., 2017), stroke, and other neurological dis-orders. The potential side effects of VNS therapy, including vocal cord palsy and dysphagia, have been reported after device implantation (Dawson et al., 2016). The damage may be caused by surgical operation or vagus nerve direct stimulation. A second operation had to be performed in approximately 50% of cases. The most common reason is battery replacement, followed by surgical complications. Recently, ta-VNS has been shown to influence afferent vagal networks in animal stroke models (Ay et al., 2015) and the postcentral gyrus, bilateral insula, frontal cortex, and cerebellum in normal adults (Kraus et al., 2007). The data revealed that ta-VNS may activate similar pathways as VNS. ta-VNS therapy that stimulates the external ear is efficient and effective, as confirmed by functional imaging assessments (Yakunina et al., 2017). This new, noninvasive technique of ta-VNS holds promise for treatment after stroke (Redgrave et al., 2018).
Although both the ta-VNS and control groups advanced after the treatment the improve-ment in functional and sensory outcomes was significantly higher for the ta-VNS group. The addition of electrical stimulation therapy provides significantly greater benefits than rehabilitative therapy alone. Our data support the view that the conjunction effect of VNS with rehabilitation is dependent on neural plasticity, a time-dependent phenomenon, and that the effect persisted for a year, even after the stimulus stopped. In this trial, comprehensive rehabilitation therapy was carried out immediately after electrical stimulation therapy. Previously, an animal study demonstrated that the combination of electrical stimulation and rehabilitation together was necessary to obtain the best improvement. VNS alone had no effect on the injury area and had little effect when electrical stimulation therapy was performed after the rehabilitation training had finished (Khodaparast et al., 2016). This research supports the idea confirming that ta-VNS delivered before rehabilitation training can ameliorate neurological function. The cumulative effect of ta-VNS sessions in the stroke patient has previously been described (Capone et al., 2017; Wu et al., 2020), but did not include acute stroke, and the follow-up was never for as long as a year. Other studies have explored the role of ta-VNS in the subacute phase of ischemic stroke (Wu et al., 2020), who discovered a difference of about 46% (at 3 months) in the recovery of FMA-U after 16 consecutive days of ta-VNS. Another study found that after 10 sessions there was a difference of about 28% in the chronic phase of stroke (Capone et al., 2017). In our acute stroke study, after 20 consecutive days of ta-VNS the degree of improvements at 3 months was extremely encouraging, not only in functional (FMA-U: 52%/FMA-L: 62%) but also in sensory outcomes (FMA-S: 72%). The underlining mechanisms of VNS are not necessarily the same as they may depend on the different phases of stroke recovery and these need to be clarified. In the chronic phase of stroke, VNS is thought not to reduce lesion size but rather improve recovery after brain injury by enhancing neural plasticity thereby supporting the benefits of rehabilitative therapy (Khodaparast et al., 2016). In the acute stage of stroke, neural protection, nerve regeneration, angiogenesis, and neural plasticity were seen as the underlying mechanisms of VNS action (Jiang et al., 2015; Zhang et al., 2017; Li et al., 2020a, b). A noticeable improvement of FMA-U, FMA-L, FMA-S, and WMFT scores was significant in the ta-VNS group compared with the control group from the second week onwards. In addition, we found that the cumulative improvement in limb function did not begin to affect the overall quality of life until the third month. These data reveal that early rehabilitative strategies are effective for stroke patients. It is worth noting that the somatosensory results in the ta-VNS group remained stable after 1 month. This may be be-cause of the ceiling effect, or that the somatosensory function was completely restored.
The regions of the brain in connection with the vagus nerve include the locus coeruleus, hippocampus, orbito-frontal cortex, amygdala, and insular cortex that are also responsible for coping with the psycho-somatic components of depression and anxiety (Kar and Sarkar, 2016; Carreno and Frazer, 2017). Post-stroke depression and anxiety have an adverse im-pact on the rehabilitation recovery of motor and cognitive impairments after stroke and sig-nificantly increase the risk of the recurrence of cerebrovascular disease (Das and G, 2018). Not surprisingly, this is supported by the obvious impact of ta-VNS on the HADS scale from the first month until the final evaluation, which is accompanied by the recovery of neurological function. Alternatively, it is possible that the recovery of neurological function also contributes to the improvement of depression and anxiety, and the two are mutually causal.
Not only were the effects of ta-VNS objectively assessed by researchers, but each patient’s self-reported results were also significantly influenced by ta-VNS. The SIS is an appraisal of life quality designed specifically for stroke patients. The results reached statistical significance after 3 months post-onset, and the differences persisted through to the end of the trial. The results indicate that ta-VNS has a promoting effect on the overall neurofunctional recovery in acute stroke patients.
In this study, we recorded quite large standard deviations in most of the tests. In part, this could result from the heterogeneity of the site of brain injury and stroke types, but could also arise from the individual differences in response to ta-VNS. However, the difference diminishes with time, suggesting a ceiling effect.
There are several advantages to this study. The study was randomized, reducing the risk of selection bias. The double-blind experimental design reduces the influence of subjective factors. We had 93% follow-up, and all participants completed the therapy protocol. The control group received intense rehabilitation, the same as that in the ta-VNS group, rather than the typical standard of clinical care. There are also limitations to consider. The potential complexity of self-functional recovery and individual differences were more obvious in acute stroke patients; therefore, a future multicentric study involving more participants may be required for further studies. Furthermore, the lack of an active stimulation control group means any effects of treatment could potentially be attributed to effects of placebo, and no activation of vagal nerve fibers. Finally, the parameters of electrical stimulation could influence the final therapeutic effect. The initiation of electrical stimulation therapy time and many optimal therapeutic parameters, stimulation electrode, and waveform setting have not been identified. It is essential to explore these therapeutic parameters in future research.
We succeeded in our first aim to demonstrate the clinical effect and safety of ta-VNS. Our findings indicate that ta-VNS combined with conventional rehabilitation training can promote better neurofunctional outcomes in acute stroke patients.
Additional files:
Additional file 1 (148KB, pdf) : Informed consent form (Chinese).
Additional file 2 (202.5KB, pdf) : Hospital ethics approval (Chinese).
Additional file 3: CONSORT checklist.
Additional file 4: Open peer review reports 1 (93.3KB, pdf) –3 (96.3KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewers: Fioravante Capone, Campus Bio-Medico University Hospital, Italy; Robert Andrew Morrison, The University of Texas at Dallas, USA; Jonathan M. Borkum, University of Maine, USA.
Funding: This work was supported by the Medical Scientific Research Project of Chongqing Municipal Health Commission of China, Nos. 2018ZDXM022, 2019MSXM017 and 2020MSXM106; and a grant from Chongqing General Hospital of China, No. 2019ZDXM03 (all to LCN and JXM).
P-Reviewers: Capone F, Morrison RA, Borkum JM; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Dawes EA, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Ay I, Napadow V, Ay H. Electrical stimulation of the vagus nerve dermatome in the external ear is protective in rat cerebral ischemia. Brain Stimul. 2015;8:7–12. doi: 10.1016/j.brs.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bushnell C, Bettger JP, Cockroft KM, Cramer SC, Edelen MO, Hanley D, Katzan IL, Mattke S, Nilsen DM, Piquado T, Skidmore ER, Wing K, Yenokyan G. Chronic stroke outcome measures for motor function intervention trials: expert panel recommendations. Circ Cardiovasc Qual Outcomes. 2015;8:S163–169. doi: 10.1161/CIRCOUTCOMES.115.002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capone F, Miccinilli S, Pellegrino G, Zollo L, Simonetti D, Bressi F, Florio L, Ranieri F, Falato E, Di Santo A, Pepe A, Guglielmelli E, Sterzi S, Di Lazzaro V. Transcutaneous vagus nerve stimulation combined with robotic rehabilitation improves upper limb function after stroke. Neural Plast. 2017;2017:7876507. doi: 10.1155/2017/7876507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics. 2017;14:716–727. doi: 10.1007/s13311-017-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman ER, Moudgal R, Lang K, Hyacinth HI, Awosika OO, Kissela BM, Feng W. Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep. 2017;19:59. doi: 10.1007/s11883-017-0686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das J, G KR. Post stroke depression: The sequelae of cerebral stroke. Neurosci Biobehav Rev. 2018;90:104–114. doi: 10.1016/j.neubiorev.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, Hilmi O, McLean J, Forbes K, Kilgard MP, Rennaker RL, Cramer SC, Walters M, Engineer N. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke. 2016;47:143–150. doi: 10.1161/STROKEAHA.115.010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards DF, Lang CE, Wagner JM, Birkenmeier R, Dromerick AW. An evaluation of the Wolf Motor Function Test in motor trials early after stroke. Arch Phys Med Rehabil. 2012;93:660–668. doi: 10.1016/j.apmr.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 9.English C, Hillier SL, Lynch EA. Circuit class therapy for improving mobility after stroke. Cochrane Database Syst Rev. 2017;6 doi: 10.1002/14651858.CD007513.pub3. CD007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrand AQ, Helke KL, Gregory RA, Gooz M, Hinson VK, Boger HA. Vagus nerve stimulation improves locomotion and neuronal populations in a model of Parkinson’s disease. Brain Stimul. 2017;10:1045–1054. doi: 10.1016/j.brs.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furie K. Epidemiology and primary prevention of stroke. Continuum (Minneap Minn) 2020;26:260–267. doi: 10.1212/CON.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 12.Hu J, Liu PL, Hua Y, Gao BY, Wang YY, Bai YL, Chen C. Constraint-induced movement therapy enhances AMPA receptor-dependent synaptic plasticity in the ipsilateral hemisphere following ischemic stroke. Neural Regen Res. 2021;16:319–324. doi: 10.4103/1673-5374.290900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y, Li L, Tan X, Liu B, Zhang Y, Li C. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J Neurochem. 2015;134:173–181. doi: 10.1111/jnc.13097. [DOI] [PubMed] [Google Scholar]
- 14.Kar SK, Sarkar S. Neuro-stimulation techniques for the management of anxiety disorders: an update. Clin Psychopharmacol Neurosci. 2016;14:330–337. doi: 10.9758/cpn.2016.14.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, Rennaker RL, 2nd, Hays SA. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats. Neurorehabil Neural Repair. 2016;30:676–684. doi: 10.1177/1545968315616494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 17.Le Danseur M. Stroke rehabilitation. Crit Care Nurs Clin North Am. 2020;32:97–108. doi: 10.1016/j.cnc.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Zhang K, Zhang Q, Zhou X, Wen L, Ma J, Niu L, Li C. PPAR-γ mediates ta-VNS-induced angiogenesis and subsequent functional recovery after experimental stroke in rats. Biomed Res Int. 2020a;2020:8163789. doi: 10.1155/2020/8163789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Zhang Q, Li S, Niu L, Ma J, Wen L, Zhang L, Li C. α7nAchR mediates transcutaneous auricular vagus nerve stimulation-induced neuroprotection in a rat model of ischemic stroke by enhancing axonal plasticity. Neurosci Lett. 2020b;730:135031. doi: 10.1016/j.neulet.2020.135031. [DOI] [PubMed] [Google Scholar]
- 20.Mijajlović MD, Pavlović A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB, Hermann DM, Assayag EB, Richard E, Thiel A, Kliper E, Shin YI, Kim YH, Choi S, Jung S, Lee YB, Sinanović O, Levine DA, Schlesinger I, Mead G, et al. Post-stroke dementia -a comprehensive review. BMC Med. 2017;15:11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray AR, Atkinson L, Mahadi MK, Deuchars SA, Deuchars J. The strange case of the ear and the heart: The auricular vagus nerve and its influence on cardiac control. Auton Neurosci. 2016;199:48–53. doi: 10.1016/j.autneu.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Neren D, Johnson MD, Legon W, Bachour SP, Ling G, Divani AA. Vagus nerve stimulation and other neuromodulation methods for treatment of traumatic brain injury. Neurocrit Care. 2016;24:308–319. doi: 10.1007/s12028-015-0203-0. [DOI] [PubMed] [Google Scholar]
- 23.Redgrave J, Day D, Leung H, Laud PJ, Ali A, Lindert R, Majid A. Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain Stimul. 2018;11:1225–1238. doi: 10.1016/j.brs.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19:348–360. doi: 10.1016/S1474-4422(19)30415-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang WJ, Zhong YB, Zhao JJ, Ren M, Zhang SC, Xu MS, Xu ST, Zhang YJ, Shan CL. Transcranial pulse current stimulation improves the locomotor function in a rat model of stroke. Neural Regen Res. 2021;16:1229–1234. doi: 10.4103/1673-5374.301018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheless JW, Gienapp AJ, Ryvlin P. Vagus nerve stimulation (VNS) therapy update. Epilepsy Behav. 2018;88s:2–10. doi: 10.1016/j.yebeh.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Wu D, Ma J, Zhang L, Wang S, Tan B, Jia G. Effect and safety of transcutaneous auricular vagus nerve stimulation on recovery of upper limb motor function in subacute ischemic stroke patients: a randomized pilot study. Neural Plast. 2020;2020:8841752. doi: 10.1155/2020/8841752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yakunina N, Kim SS, Nam EC. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation. 2017;20:290–300. doi: 10.1111/ner.12541. [DOI] [PubMed] [Google Scholar]
- 30.Yang YW, Pan WX, Xie Q. Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity. Neural Regen Res. 2020;15:1986–1994. doi: 10.4103/1673-5374.282239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part II. Headache. 2016;56:259–266. doi: 10.1111/head.12650. [DOI] [PubMed] [Google Scholar]
- 32.Zeiler SR. Should we care about early post-stroke rehabilitation. Not yet, but soon. Curr Neurol Neurosci Rep. 2019;19:13. doi: 10.1007/s11910-019-0927-x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Ma J, Jin X, Jia G, Jiang Y, Li C. L-PGDS mediates vagus nerve stimulation-induced neuroprotection in a rat model of ischemic stroke by suppressing the apoptotic response. Neurochem Res. 2017;42:644–655. doi: 10.1007/s11064-016-2121-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.