Primary open-angle glaucoma as a causal factor for circadian disruption: Living by the clock, in alignment with external time cues is an important condition for human health and well-being. Periodic changes in the ambient light serve as a key factor to synchronize the endogenously generated circadian rhythms. The retina perceives the photic signals and transmits them to the central body clock, the suprachiasmatic nuclei (SCN), via the retinohypothalamic tract.
Primary open angle glaucoma (POAG) is an optic neuropathy, in which disease progression can be monitored by assessing damage to the retinal ganglion cells (RGCs) (Pérez-Rico et al., 2010; Feigl et al., 2011; Kankipati et al., 2011). Damage of retinal ganglion cells, particularly of intrinsically photosensitive RGCs (ipRGCs), is also one of the causes of circadian disruption.
Pathophysiological mechanisms of POAG are complex, including elevated intraocular pressure (IOP), which adds mechanical stress, causing damage, dysfunction, and death of the RGCs (Figure 1). Glaucoma progression affects both image-forming and non-image-forming visual functions of RGCs. A central role of ipRGCs is to convey non-image-forming photic information to the clock. Their damage reduces light signaling to the SCN. Already in early stages of glaucoma, ipRGCs are dysfunctional (Pérez-Rico et al., 2010; Feigl et al., 2011; Kankipati et al., 2011).
Figure 1.
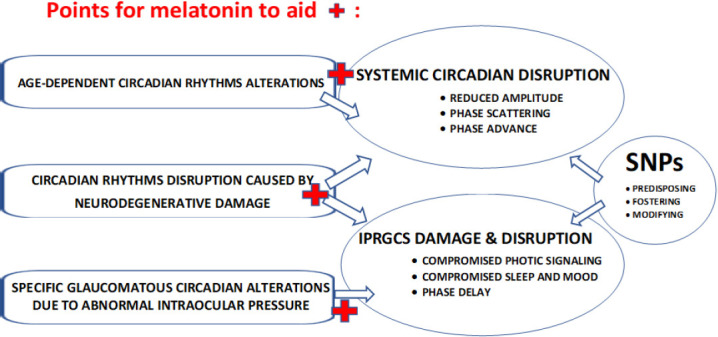
Melatonin potential to counteract complex circadian alterations with aging, neurodegeneration, specifically in glaucoma.
ipRGCs: Intrinsically photosensitive retinal ganglion cells; SNPs: single nucleotide polymorphisms.
As RGCs are progressively altered, and non-image-forming function is affected, circadian rhythms are disrupted, sleep is impaired, and mood is altered (Graticelli et al., 2015; Gubin et al., 2019, 2021). Circadian rhythm alterations are found in POAG as compared to age-matched healthy peers (Gubin et al., 2019). Circadian disruption worsens in advanced POAG (Gubin et al., 2019, Neroev et al., 2020), correlating to the increasing loss of ipRGCs with disease progression (Obara et al., 2016).
Circadian rhythms also change with increasing age. Age-dependent circadian alterations are not necessarily related to retinal damage, as photic transduction to the central clock is not always compromised. When they are related to retinal damage, they can be due to either neurodegenerative ipRGCs damage, or to ipRGC damage caused by increased mean or deregulated circadian IOP. The intriguing principal difference between the presence or absence of retinal damage in aging is that the reduced light transmitted to the SCN by damaged ipRGCs phase-delays circadian rhythms, but ipRGC-uncompromised aging is commonly associated with phase-advanced circadian rhythms (Gubin et al., 2019). Since individual differences in sensitivity to light, and/or in endogenous melatonin production may interfere with this theoretical modeling, the search for specific genetic factors that may determine such individual differences constitutes a promising approach.
In conditions where photic entrainment is compromised, not only is the alignment with external time cues altered, so can be the variability of overt physiologic functions. We showed that large inter-individual variability obscured the circadian IOP rhythm in POAG (Neroev et al., 2020). Circadian IOP rhythms had specific alterations manifested in advanced, but not in mild POAG, which were associated with the progressive damage and dysfunction of RGCs. In patients with RGCs’ global loss volume above 15%, as assessed by high-definition optical coherence tomography, the 24-hour IOP rhythm peaked during the night, whereas in patients with stable POAG and a two-eye mean RGCs’ global loss volume less than 10%, the IOP peaked predominantly during the daytime. Misalignment between circadian rhythms in body temperature and IOP increased as a function of global loss volume loss. Higher nocturnal IOP in POAG may adversely affect the disease state, fostering damage to RGCs (Neroev et al., 2020).
Depending on individual genetic factors, these changes may manifest themselves to a different degree. Individual clock properties depend on numerous genetic factors, comprising clock genes and melatonin receptor genes, melatonin nuclear receptor 1b (MTNR1b) in particular, which may account for large individual differences in light sensitivity.
Our pilot study of gene polymorphisms in POAG showed that the D-allele of the Angiotensin-converting enzyme holding a deletion of the 16th intron Alu repeat was significantly associated with alterations of the circadian IOP rhythm. It may also account for the resistance to IOP-lowering therapy (Neroev et al., 2020).
Endogenous melatonin production in primary open-angle glaucoma: Glaucoma patients experienced reduced post-illumination pupil response (Kankipati et al., 2011) and reduced nocturnal melatonin suppression by light (Pérez-Rico et al., 2010). Clinical evidence for changes in the timing and mean values of endogenous melatonin production in POAG was also evident (reviewed in Gubin et al., 2021): in POAG, salivary melatonin can be lower than in age-matched controls without POAG; even greater alterations were observed In advanced stages of the disease. The main alteration concerned the time of maximal secretion of melatonin. Such altered melatonin production in POAG and other neurodegenerative pathologies can stem from different factors, including diminished light signaling due to a reduced sensitivity to light. The presence of certain gene polymorphisms can increase the susceptibility of carriers to these factors.
We investigated 24-hour profiles of salivary melatonin under controlled lighting conditions and analyzed several clock genes and polymorphisms of the melatonin receptor gene MTNR1b (Gubin et al., 2021). Patients diagnosed with stable POAG had unaltered circadian rhythms of salivary melatonin and body temperature, which peaked at the anticipated time. Circadian rhythms of both variables were delayed, however, in patients diagnosed with advanced POAG (Gubin et al., 2019, 2021). Their 24-hour mean value and circadian amplitude of melatonin were also reduced (Gubin et al., 2021). Analysis of selected polymorphisms in clock and melatonin receptor genes revealed that these changes were observed specifically in carriers of the MTNR1B rs10830963 G-allele with advanced POAG. Overt changes of circadian phenotypes in POAG patients occur when several factors are present in combination: for example, when RGC loss exceeds a certain threshold in carriers of those genotypes, known to be associated with a prolonged duration of melatonin production. The MTNR1B rs10830963 G-allele is mainly known for its association with an elevated fasting glucose and the risk of type 2 diabetes, but it is also listed as a factor predicting POAG independently of diabetes (Shen et al., 2016), a fact supporting the assumption that melatonin may have pleiotropic physiological functions in the development of POAG.
Melatonin to counteract non-image-forming visual function deterioration in primary open-angle glaucoma: To enhance circadian entrainment, morning light therapy and evening melatonin administration can both be effective. While studies aimed at estimating the merit of morning light therapy or outdoor light exposure in POAG are lacking, some studies provide evidence for a beneficial effect of exogenously administered melatonin in glaucoma and neurodegenerative pathologies (González Fleitas et al., 2021; Gubin et al., 2021).
Melatonin transmits environmental light signals, thus facilitating the synchronization of peripheral clocks. It can thus mitigate several conditions such as glaucoma and its progression: disruption of circadian rhythms, compromised sleep, and mood (Tosini et al., 2012; Gubin et al., 2021) (Figure 1). Melatonin improves internal synchronization, ameliorating circadian alignment between local (IOP) and systemic (temperature) circadian rhythms (Gubin et al., 2021), which were progressively desynchronized with greater RGCs loss in POAG (Neroev et al., 2020).
Melatonin is produced endogenously with a pronounced 24-hour rhythm governed by the SCN. Peak production occurs at night. Its specific timing may differ among individuals. Exact endogenous factors that predetermine these differences are not known but may include single nucleotide polymorphisms within candidate genes or melatonin receptors that influence sensitivity to light. Melatonin receptors (MTNR1B) are widespread in numerous brain regions. Their structure may determine the specific response to (both endogenous and exogenous) melatonin. We investigated the effect of oral melatonin administration (daily at 10:30 p.m. for 90 days) on the circadian rhythms of IOP, body temperature, and the pattern electroretinogram in patients diagnosed with stable or advanced POAG, also assessing effects on sleep and mood (Gubin et al., 2021). Melatonin administration increased the stability of the circadian body temperature rhythm, improving its alignment with the circadian IOP rhythm. Melatonin decreased IOP to a different extent at different times of the day and decreased the standard deviation of IOP with statistical significance. Larger changes were found in patients with initially higher 24-hour mean values of IOP. Melatonin improved RGCs function in patients with advanced POAG by increasing the amplitude of pattern electroretinogram that correlated positively with the degree of RGCs loss. Melatonin had more pronounced positive effects on sleep and mood in patients with advanced POAG, who had greater damage of their RGCs. Taken together melatonin has the potential to restore disrupted circadian rhythms in POAG. Its systemic effect is distinct from its local effect on the retinal circadian rhythms. Similar to light exposure, physiological effects of melatonin depend on the time of its administration. Personalizing melatonin administration in terms of its timing and dosing, accounting for the genetic profile, is expected to further refine its multiple benefits.
Melatonin may provide beneficial effects in POAG stemming from both its ability to reduce IOP and its potential to prevent RGC damage derived from mechanisms of neurodegeneration (Hardeland, 2021) (Figure 1). These effects may not only mitigate circadian disruption but also improve other aspects of health and well-being. Circadian alignment may strengthen human physiological functions and help slow neurodegeneration. The choice of an optimal melatonin dosing, however, is not yet clear (Hardeland, 2021). Consideration of the best timing should be based on internal circadian parameters and on genes that may account for personal differences in melatonin efficacy.
Concluding remarks: In assessing disruptions in the non-image-forming visual system in POAG patients, one needs to discriminate between different situations. There may be complex, non-specific changes in circadian rhythms with age. Changes related to neurogenerative disease, including Alzheimer’s disease, Parkinson’s disease, and POAG, might promote alterations in neural structures: SCN, pineal, retina. Changes specific to POAG include additional RGC damage caused by the elevated IOP, together with abnormal circadian patterns of physiological variables such as IOP, body temperature, pattern electroretinogram, and melatonin (Figure 1). The circadian IOP pattern with relatively higher values during the resting span may foster harmful effects of IOP on RGCs, since tissue sensitivity may vary depending on circadian time (Neroev et al., 2020). Numerous candidate gene polymorphisms may play a role, alone or in combination with others, affecting the susceptibility to POAG itself (as a primary pathology of vision), or POAG-associated alterations of circadian rhythms, sleep, and mood, linked to non-visual pathways. To answer this question, clinical data combined with circadian profiles of melatonin and other physiological variables, chronotype questionnaires, sleep and mood information, to be checked against single nucleotide polymorphisms databases, should be collected on large cohorts. Constructive collaboration among ophthalmologists, chronobiologists, and geneticists is therefore advocated.
The present work was supported by the Russian Foundation for Basic Research (grant No. 19-015-00329) (to DG), and by Government of Tyumen District, Decree of 20.11.2020 No. 928-rp (to DG).
The authors have no proprietary or commercial interest in any materials discussed in this article.
Footnotes
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Feigl B, Mattes D, Thomas R, Zele AJ. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:4362–7. doi: 10.1167/iovs.10-7069. [DOI] [PubMed] [Google Scholar]
- 2.González Fleitas MF, Devouassoux J, Aranda ML, Dieguez HH, Calanni JS, Iaquinandi A, Sande PH, Dorfman D, Rosenstein RE. Melatonin prevents non-image-forming visual system alterations induced by experimental glaucoma in rats. Mol Neurobiol. 2021;58:3653–3664. doi: 10.1007/s12035-021-02374-1. [DOI] [PubMed] [Google Scholar]
- 3.Gracitelli CP, Duque-Chica GL, Roizenblatt M, Moura AL, Nagy BV, Ragot de Melo G, Borba PD, Teixeira SH, Tufik S, Ventura DF, Paranhos A., Jr Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology. 2015;122:1139–48. doi: 10.1016/j.ophtha.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Gubin D, Neroev V, Malishevskaya T, Cornelissen G, Astakhov SY, Kolomeichuk S, Yuzhakova N, Kabitskaya Y, Weinert D. Melatonin mitigates disrupted circadian rhythms, lowers intraocular pressure, and improves retinal ganglion cells function in glaucoma. J Pineal Res. 2021;70:e12730. doi: 10.1111/jpi.12730. [DOI] [PubMed] [Google Scholar]
- 5.Gubin DG, Malishevskaya TN, Astakhov YS, Astakhov SY, Cornelissen G, Kuznetsov VA, Weinert D. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiology Int. 2019;36:564–577. doi: 10.1080/07420528.2019.1566741. [DOI] [PubMed] [Google Scholar]
- 6.Hardeland R. Divergent importance of chronobiological considerations in high- and low-dose melatonin therapies. Diseases. 2021;9:18. doi: 10.3390/diseases9010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kankipati L, Girkin CA, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011;52:2287–2292. doi: 10.1167/iovs.10-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neroev V, Malishevskaya T, Weinert D, Astakhov S, Kolomeichuk S, Cornelissen G, Kabitskaya Y, Boiko E, Nemtsova I, Gubin D. Disruption of 24-hour rhythm in intraocular pressure correlates with retinal ganglion cell loss in glaucoma. Int J Mol Sci. 2020;22:359. doi: 10.3390/ijms22010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obara EA, Hannibal J, Heegaard S, Fahrenkrug J. Loss of melanopsin-expressing retinal ganglion cells in severely staged glaucoma patients. Invest Ophthalmol Vis Sci. 2016;57:4661–4667. doi: 10.1167/iovs.16-19997. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Rico C, de la Villa P, Arribas-Gómez I, Blanco R. Evaluation of functional integrity of the retinohypothalamic tract in advanced glaucoma using multifocal electroretinography and light-induced melatonin suppression. Exp Eye Res. 2010;91:578–83. doi: 10.1016/j.exer.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Walter S, Melles RB, Glymour MM, Jorgenson E. Diabetes pathology and risk of primary open-angle glaucoma: evaluating causal mechanisms by using genetic information. Am J Epidemiol. 2016;183:147–155. doi: 10.1093/aje/kwv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res. 2012;103:82–89. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


