Abstract
Myeloperoxidase is an important inflammatory factor in the myeloid system, primarily expressed in neutrophils and microglia. Myeloperoxidase and its active products participate in the occurrence and development of hemorrhagic and ischemic stroke, including damage to the blood-brain barrier and brain. As a specific inflammatory marker, myeloperoxidase can be used in the evaluation of vascular disease occurrence and development in stroke, and a large amount of experimental and clinical data has indicated that the inhibition or lack of myeloperoxidase has positive impacts on stroke prognosis. Many studies have also shown that there is a correlation between the overexpression of myeloperoxidase and the risk of stroke. The occurrence of stroke not only refers to the first occurrence but also includes recurrence. Therefore, myeloperoxidase is significant for the clinical evaluation and prognosis of stroke. This paper reviews the potential role played by myeloperoxidase in the development of vascular injury and secondary brain injury after stroke and explores the effects of inhibiting myeloperoxidase on stroke prognosis. This paper also analyzes the significance of myeloperoxidase etiology in the occurrence and development of stroke and discusses whether myeloperoxidase can be used as a target for the treatment and prediction of stroke.
Key Words: blood-brain barrier, hemorrhagic stroke, inflammation, ischemic stroke, microglia, myeloperoxidase, neutrophils, secondary brain injury, stroke
Introduction
Stroke refers to a series of cerebrovascular diseases that cause brain damage due to changes in blood flow and oxygen delivery mediated by blood vessels. Depending on whether the cause of stroke is central nervous system hemorrhage or thrombotic ischemia, stroke can be classified as ischemic stroke and hemorrhagic stroke (Bedard and Krause, 2007; Liang et al., 2020), and ischemic stroke is the most common type, accounting for approximately 70% of all strokes. According to the 2017 Global Burden of Disease study (GBD), stroke is responsible for more than 5% of all disability-adjusted life years, and stroke was responsible for 11% of all deaths worldwide, which is equivalent to 6.17 million deaths due to stroke each year, ranking third among all causes of death (GBD 2016 DALYs and HALE Collaborators, 2017; GBD 2017 Causes of Death Collaborators, 2018; Avan et al., 2019; Deuschl et al., 2020). The GBD 2016 Lifetime Risk of Stroke Collaborators (2018), involving GBDs in various regions of the world, was published by the New England Journal of Medicine. The results showed over the past 26 years, the global risk of lifelong stroke among adults increased by 8.9% to 24.9% (95% confidence interval [CI] 23.5–26.2%), with a male risk of 24.7% (95% CI 23.3–26.0%) and a female risk of 25.1% (95% CI 23.7–26.5%), indicating that almost one-quarter of all adults are at risk of experience stroke during their lifetimes. Among all adults included in relevant studies, 18.3% are likely to experience an ischemic stroke, and 8.2% are likely to experience a hemorrhagic stroke. During the period from 1990 to 2016, the stroke incidence in China increased from 204.52 to 403.08 per 100,000 population, and mortality increased from 122.09 to 130.94 per 100,000 population (Wang et al., 2020). High blood pressure, heart disease, diabetes, atherosclerosis, lack of exercise, high blood fat, high-salt diet, smoking, alcoholism, and age have been identified as risk factors for stroke (George, 2020; Mai and Liang, 2020; Zhang et al., 2020). After stroke, the inflammatory system is activated. During the early stages of hemorrhagic stroke, the brain tissue surrounding the hematoma is characterized by the infiltration of inflammatory cells and inflammatory factors, such as free radicals and proteases, produced by neurons. These early inflammatory factors, including myeloperoxidase (MPO), continue to damage the brain during the whole process of the hematoma incident (Wang, 2010).
MPO is an important inflammatory factor in the myeloid system (Klebanoff, 2005). Agner (1941) first isolated and purified the heme peroxidase-containing MPO from the green purulent fluid obtained from tuberculosis patients; due to its green appearance, MPO is also known as verdoperoxidase (Klebanoff, 2005; Ray and Katyal, 2016). MPO is abundantly expressed in neutrophils and other myeloid cells, such as Ly-6Chigh monocytes (Swirski et al., 2009; Grishkovskaya et al., 2017), macrophages, and microglia (Gray et al., 2008; Gellhaar et al., 2017). After acute cerebral ischemia, due to the destruction of the blood–brain barrier (BBB), the infiltration of a large number of neutrophils attacks the central nervous system. Studies have shown that the large growth in the neutrophil population is accompanied by a large increase in MPO production (Gorudko et al., 2017; Reber et al., 2017; Pleskova et al., 2018; Maestrini et al., 2020). Large amounts of inflammation are observed during the early stages of stroke, and the activation of phagosomes represents an important form of inflammation. MPO is an important enzyme in phagocytic vacuoles. Among the antimicrobial systems present in the phagosome, a significant proportion consists of MPO, hydrogen peroxide (H2O2, formed during the respiratory burst), and a halide (X–), particularly chloride (Cl–) (Iana and Sirbu, 2020; Marcinkiewicz and Walczewska, 2020). In the MPO-H2O2-Cl− sterilization system, the oxidant chlorous acid/hypochlorite ion (HOCl/OCl−) plays an important role; under pathological conditions, a sustained inflammatory effect is exerted due to the activation of this system. Here, we outline the etiology of MPO production and its contributions during the occurrence and development of stroke, evaluate its feasibility for use as an indicator in clinical applications, and discuss whether it can serve as a target for stroke treatment and prognostic prediction.
Search Strategy
In the MEDLINE database, we searched for related articles using the English search terms “stroke, myeloperoxidase” in the limited time range from January 1990 to December 2020, and a total of 315 related articles were retrieved. The inclusion criteria were articles directly related to myeloperoxidase-associated stroke research and corresponding previous basic research; and similar research ideas selected from the latest articles published in authoritative journals. Exclusion criteria were repetitive or retrospective studies. Two researchers (YCW and YBL) independently read and screened the articles by reading the titles and abstracts and then combined the screening results. After the readers reported controversial documents, YNZ and YWP discussed whether to include them. Any articles unrelated to stroke and myeloperoxidase and among highly similar studies, only the most recently published article was retained. In the end, 106 articles were included in the reference catalog.
The Etiology of Myeloperoxidase in Stroke
The role of MPO in vascular injury before stroke
Stroke is classified as a vascular disease. When blood vessels are damaged due to malformations in arteriovenous blood vessels, a thrombus can form, leading to ischemic stroke, whereas hemorrhagic stroke results from the rupture of cerebral vessels. As a cornerstone of the pathophysiological mechanisms of these vascular diseases, MPO damages the arterial wall through either direct oxidation reactions with components of the arterial wall or indirect damage exerted on the integrity and function of the blood vessel (Figure 1). Indirect damage includes (i) the promotion of atherosclerotic plaque formation of foamy macrophages, resulting in the formation of a core rich in low-density lipoproteins; (ii) changes in serum cholesterol function, distribution, and flow due to lipid peroxidation; (iii) promoting the rupture and instability of atherosclerotic plaques due to matrix metalloproteinase (MMP) activation; (iv) the stimulation of local occlusive thrombosis through P-selectin interactions; and (v) the impairment of vascular reactivity through the depletion of endothelial-derived nitric oxide (NO), damaging vasodilatation and anti-inflammatory properties (Vita et al., 2004; Lau and Baldus, 2006; Nicholls and Hazen, 2009).
Figure 1.
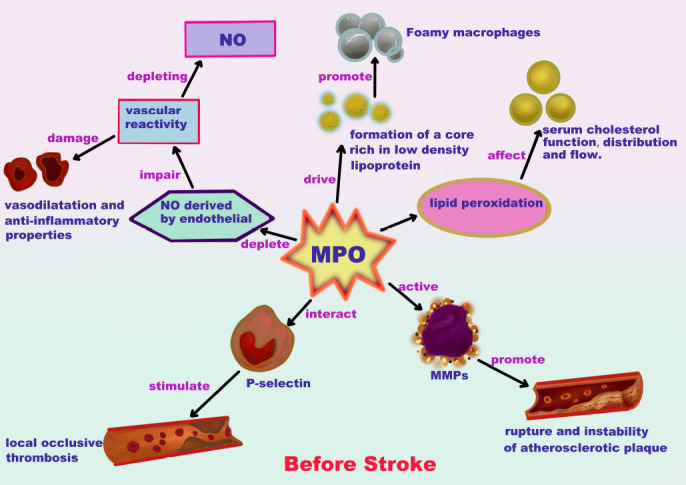
Response caused by myeloperoxidase (MPO) in blood vessels before stroke.
MPO may promote stroke due to damage to the arterial wall through direct oxidation and indirect effects on blood vessel integrity and function, driving a core rich in low-density lipoprotein and promoting the formation of atherosclerotic plaques by foamy macrophages. These plaques affect the function, distribution, and flow of serum cholesterol due to lipid peroxidation. The activation of matrix metalloproteinases leads to atherosclerotic plaque rupture and instability, which can stimulate local occlusive thrombosis through P-selectin interactions. Depleting nitric oxide (NO) can impair vasodilation resistance against inflammation and impair vascular reactivity.
Molecular damage mechanisms mediated by MPO after stroke
The BBB is a highly specialized system for restricting interactions between the brain parenchyma and the bloodstream, promoting the maintenance of brain homeostasis. After a stroke, damage to the BBB is an important contributor to cerebral edema and hemorrhagic transformation (Lin et al., 2018). BBB damage can promote lacunar infarction, white matter lesions, and microhemorrhages in deep brain structures and trigger the production of a large number of neurotoxic substances, which damage synapses and neuronal function (Lin et al., 2018). The BBB becomes compromised after a stroke, allowing many immune cells to enter the central nervous system, where they interact with central immune cells to further aggravate the inflammatory response. Üllen et al. (2013) demonstrated that MPO produced by neutrophils induces the dysfunction of primary brain microvascular endothelial cells (BMVEC) in vitro, exacerbating the damage to the BBB. Klinke et al. (2011) revealed a previously unknown neutrophil recruitment mechanism induced by the electrostatic activity of MPO. These findings indicate that MPO and neutrophils have an interaction relationship in addition to a simple cascade reaction. El Kebir et al. (2008) further revealed that MPO could delay neutrophil apoptosis by signaling the adhesion molecule CD11b/CD18, prolonging the inflammatory response. Therefore, MPO serves to enhance the duration of the inflammatory response, which can cause the brain tissue to undergo continual inflammatory damaged long after the stroke has resolved (Babior, 1984). Kang et al. (2020) showed that neutrophils accumulate in the peri-infarct cortex during all stages of ischemic stroke. Neutrophils produce intravascular and intraparenchymal extracellular neutrophil traps, which peak at 3–5 days. Extracellular neutrophil traps release many cytotoxic proteases, such as histones, elastase, and MPO, which directly induce endothelial cell damage to increase vascular permeability (Villanueva et al., 2011). The infiltration of neutrophils can upregulate peptidyl arginine deiminase 4, stimulator of interferon genes, and interferon regulatory factor 3. Peptidyl arginine deiminase 4 is a key enzyme involved in chromatin decondensation (Wang et al., 2009; Martinod et al., 2013). Stimulator of interferon genes is a DNA sensor, and the upregulation of interferon regulatory factor 3 can induce the production of interferon-β in large quantities, which can disrupt vascular reconstruction and vascular repair after stroke (Kang et al., 2020).
Microglia are another innate immune cell type in the nervous system associated with MPO, which plays a crucial role after stroke occurrence (Qin et al., 2019; Xu et al., 2021). After a stroke occurs, the function of microglia primarily depends on the activation signal received (Ma et al., 2017; Al Mamun et al., 2018). M1 type microglia represent a pro-inflammatory cell type, which primarily contributes to the early stages of stroke and can produce tumor necrosis factor-α (Feng et al., 2017), interleukin (IL)-1β (Facci et al., 2018), interferon-γ (Hwang and Bergmann, 2020), inducible NO synthase (Maksoud et al., 2021), and proteolytic enzymes (MMP9 and MMP3) (Bonetti et al., 2019). During the later stage of stroke, M2 type microglia exert anti-inflammatory effects (Jiang et al., 2018), producing IL-10 (Lobo-Silva et al., 2017), transforming growth factor β (Spittau et al., 2020), insulin-like growth factor (Li et al., 2020), and vascular endothelial growth factor (Ju et al., 2019), which are pro-angiogenic and anti-inflammatory (Ponomarev et al., 2013). Therefore, MPO-related damage is primarily mediated by M1 microglia (Figure 2).
Figure 2.
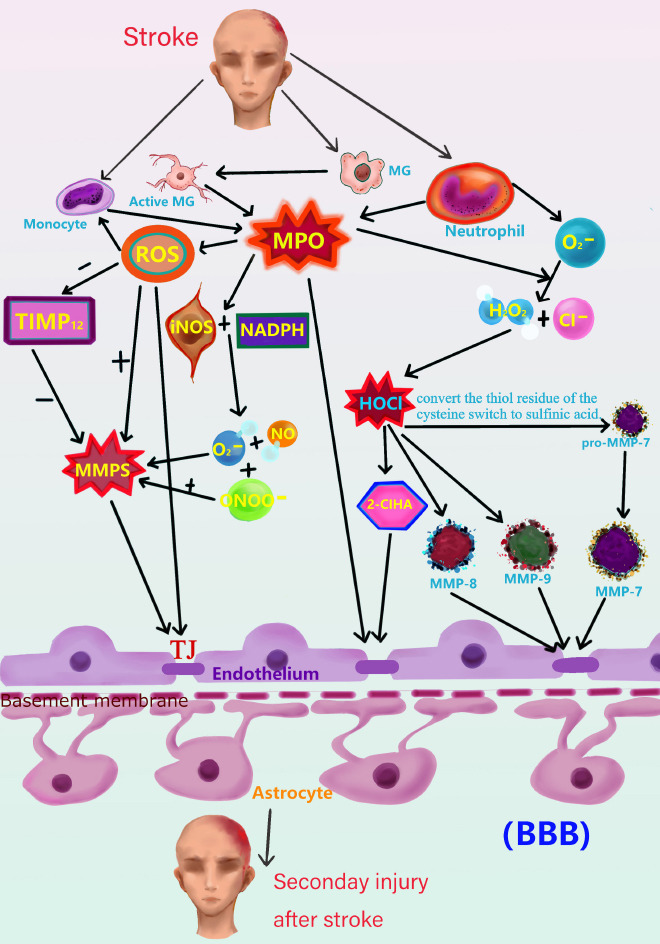
Myeloperoxidase (MPO)-related cascade after stroke.
Active microglia, monocytes, and neutrophils produce MPO when a stroke occurs. In addition to direct damage to the BBB, MPO can cause indirect damage. After a stroke occurs, myeloid immune cells, such as monocytes, neutrophils, and microglia, produce MPO, which participates in the reaction between H2O2 and Cl–, generating HOCl and activating MMP7, MMP8, MMP9, and other MMPs, further damaging the BBB. MPO may also induce the production of 2-ClHA, which further damages the BBB. MPO can increase the activity of ROS, which can directly attack the BBB. ROS can also enhance the activity of MMP and reduce the activity of TIMP12. The simultaneous effects of these positive and negative regulatory actions can activate MMPs to a greater extent. MPO can promote the production of O2– and NO by iNOS and NADPH. In addition to increasing MMP activity, MPO can produce ONOO–, and ONOO– can further increase the activity of MMPs. This series of reactions will cause varying degrees of damage to the BBB, increasing the BBB permeability and aggravating secondary brain damage. 2-ClHA: 2-Chlorohexadecanoic acid; BBB: blood-brain barrier; Cl–: chloride; H2O2: hydrogen peroxide; HOCl: oxidant chlorous acid; iNOS: inducible nitric oxide synthase; MG: microglia; NADPH: nicotinamide adenine dinucleotide phosphate; NO: nitric oxide; O2–: superoxide anion; ONOO–: peroxynitrite ion; ROS: reactive oxygen species; TIMP12: tissue inhibitor of metalloproteinase 12; TJ: tight junction.
MPO can form HOCl/OCl– in the presence of chloride ions and H2O2. These products are important substances that allow the body to resist microbial attacks (Babior, 1984; Nybo et al., 2019). However, excessive HOCl produced by the MPO-H2O2-Cl– system in neutrophils and monocytes can damage various biological tissues, including the BBB (Klebanoff, 2005). Low-dose HOCl can trigger cell apoptosis, whereas high-dose HOCl can induce cell necrosis, including in neuronal cells and astrocytes, which are the main components of the BBB (Pullar et al., 2000; Whiteman et al., 2005). As a weak acid (acid dissociation constant [pKa] of 7.5) (Morris, 1966; Wei et al., 2020), HOCl-induced cellular acidosis is unlikely to be the cause of HOCl neurotoxicity. Recent studies have shown that the production of HOCl can activate an increase in the concentration of calpain. The activation of platelets can induce changes in platelet morphology. Similar to caspase-mediated cell apoptosis (Wolf et al., 1999), the activation of calpain can also rupture cell lysosomes (Yap et al., 2006), resulting in the robust occurrence of secondary injury in the central nervous system after stroke. BMVECs forms the morphological basis of the BBB through the formation of tight junction complexes (Swastika et al., 2019). Bernhart et al. (2018) showed that peripheral blood leukocytes produce HOCl through the MPO-H2O2-Cl– system, which in turn produces chlorinated inflammatory mediators, such as 2-chlorohexadecanoic acid. 2-Chlorohexadecanoic acid can produce a lipid-toxic reaction in BMVECs, destroying the basic BBB structure, further aggravating secondary damage following stroke. Secondary injuries after stroke include hematoma expansion, perihematomal edema, and neurological deterioration (Castellazzi et al., 2010).
In addition to the direct and indirect destruction of the BBB by HOCl, MMPs are crucial for BBB destruction. MMPs are proteolytic, zinc-containing enzymes responsible for the degradation of the extracellular matrices surrounding the blood vessels and neurons in the central nervous system (Zhang and Kim, 2009; Fazal and Al-Ghoul, 2017; Yeo et al., 2020). The activation of MMPs can also induce tight junction degradation, leading to BBB breakdown following cerebral ischemia-reperfusion injury (Anctil et al., 2005; Nalamolu et al., 2020). Fu et al. (2001) showed that HOCl oxidizes the conversion of cysteine into thiol residues, which activates pro-MMP7. Studies showed that HOCl significantly enhanced the proteolytic activity of MMP8 and MMP9 (Weiss et al., 1985; Peppin and Weiss, 1986). Furthermore, after the 4-aminobenzoic acid amide-mediated inhibition of MPO, the expression of MMP9 was reduced (Kim et al., 2016). Therefore, HOCl can trigger molecular cascades that mediate the activation of MMPs, leading to BBB disruption. HOCl itself can also exacerbate oxidative stress, promote the translocation of p67(phox) and p47(phox), activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and mediating the production of superoxide, peroxynitrite, and oxidize endothelial NO synthase dimer in endothelial cells (Xu et al., 2006). Together, these compounds increase the damage to the central nervous system.
H2O2 is the final product of free oxygen radicals. Chloride ions act as a substrate for the catalytic reaction mediated by MPO, resulting in the formation of hypochlorous acid. The toxicity of hypochlorous acid is 50 times that of H2O2 (Graham et al., 2007). Therefore, excessive MPO increases the catalysis of H2O2 into hypochlorous acid, greatly enhancing cellular toxicity, especially in neuronal cells and astrocytes after stroke. In addition to these direct cytotoxic effects of MPO activity, elevated MPO activity increases reactive oxygen species (ROS) formation, MMP activation, and the production of inducible NO synthase, and inflammatory cytokines (i.e., IL-1β and tumor necrosis factor-α) (Ekdahl et al., 2003; Monje et al., 2003; Brovkovych et al., 2008; Cacci et al., 2008). These factors might cause indirect damage to neurons and astrocytes after stroke. ROS and MMPs can destroy tight junctions and disrupt the BBB, further aggravating stroke damage. Furthermore, MPO inhibition can reduce these inflammatory mediators (Monje et al., 2003; Cacci et al., 2008; El Kebir et al., 2008), indicating that MMPs act as downstream molecules during MPO-mediated inflammation (Fu et al., 2004; Cheng et al., 2018).
ROS avidly interact with large numbers of molecules, including other small inorganic molecules, as well as proteins, lipids, carbohydrates, and nucleic acids. Through such interactions, ROS may irreversibly destroy or alter the function of the target molecules. Consequently, ROS have been increasingly identified as major contributors to damage in biological organisms (Bedard and Krause, 2007; Diwanji and Bergmann, 2020). Related research has shown that ROS production increased BBB permeability and monocyte migration, and ROS activated MMP1, MMP2, and MMP9 (Haorah et al., 2007). The protein tyrosine kinase (PTK)-dependent pathway reduces the activity of tissue inhibitor of metalloproteinase 12, and increased MMP and PTK activity is closely related to the degradation of tight junctions in BMVEC proteins (Song et al., 2018). MMPs, PTKs, and antioxidant inhibitors can prevent monocyte migration, suggesting that oxidative stress causes BBB damage through the activation of MMPs and the PTK-mediated degradation of BMVEC proteins (Haorah et al., 2007). NADPH and inducible NO synthase produce superoxide anion (O2–) and NO after stroke, which in turn produce peroxynitrite ion (ONOO–) and further produces factors that increase the activity of MMPs (Chen et al., 2016), which destroy tight junctions and the BBB (Gu et al., 2011). NADPH oxidase is a very important pro-oxidase that induces superoxide anion (O2–) and H2O2 and is a significant source of ROS (Bedard and Krause, 2007). Free radicals play an important role in cerebral ischemia/reperfusion injury. The accumulation of toxic free radicals, such as ROS and reactive nitrogen, increases brain tissue susceptibility to ischemic injury and triggers various molecular cascades, resulting in increased BBB permeability, brain edema, bleeding, inflammation, and neuronal death. Furthermore, free radicals can activate MMPs, which is a critical step in damaging the BBB (Figure 2).
The inflammatory response and oxidative stress both damage the BBB and disrupt neurogenesis. Disorders of learning, language memory, and execution ability are most likely to occur after a stroke, primarily due to damage in corresponding brain areas, such as the cerebral cortex and hippocampus. Functional damage to these brain areas occurs due to repeated ischemia and inflammatory infiltration, which gradually reduces the recruitment of stem cells, affecting neurogenesis (Lin et al., 2018; Deng et al., 2021).
Potential of Myeloperoxidase in Stroke
The genetic risk and predictive value of MPO in stroke
The genetic contributions of MPO levels to ischemic stroke and recurrent stroke risk have been demonstrated in all races (Liu et al., 2012). More specifically, the high expression of MPO-related genes may increase the susceptibility to stroke. Manso et al. (2011) analyzed differences in the expression levels of MPO-related genes between a stroke group and a control group and found a positive correlation between the rs8178406 sequence in the MPO gene and stroke occurrence, providing the additional evidence that MPO is involved in stroke susceptibility and demonstrating a significant correlation between the MPO gene and stroke occurrence. Furthermore, the high expression of MPO protein also increases the risk of stroke, which has been confirmed by other studies (Palm et al., 2018; Pravalika et al., 2019; Ramachandra et al., 2020). Another study from Phuah et al. (2017) examined a large sample cohort that included 1409 cases of primary intracerebral hemorrhage from three studies; a cohort containing 1624 controls and 12,577 ischemic stroke patients from the NINDSSiGN study; an expanded cohort of 25,643 controls; METASTROKE Constatium’s 10,307 ischemic stroke cases; and a validation cohort of 29,326 controls. The results revealed that genetic determinants of elevated MPO levels and the risk of primary intracerebral hemorrhage (odds ratio 1.07, P = 0.04) were associated with the risk of recurrent intracerebral hemorrhage (hazard ratio 1.45, P = 0.006). In the analysis of ischemic stroke subtypes, MPO with increased genetic risk score was only closely related to the cavity subtype (odds ratio 1.05, P = 0.0012). These results suggest that increased genetic variations in MPO levels increased the risk of primary intracerebral hemorrhage and lacunar stroke, proving that MPO is correlated with the risk of small vessel stroke (Phuah et al., 2017).
High expression of the MPO gene can increase the risk of stroke. Simultaneously, MPO plays an important inflammatory role. The effects of a lack or low expression of the MPO gene were examined by Lanza (1998), who indicated that the lack of MPO does not significantly impact human life. Although MPO is an important molecule produced by neutrophils and is involved in the killing of certain microorganisms, no data or research has shown that a lack of MPO results in increased susceptibility to severe or persistent infections. Although serious infections occasionally occur in patients with MPO deficiency, these affect fewer than 5% of patients with MPO deficiency, indicating a low incidence (Kitahara et al., 1981). Visceral candida infections have been reported in patients with MPO deficiency; however, Stendahl et al. (1984) have shown that the microbicidal and fungicidal activities of MPO-deficient neutrophils are only slightly weakened compared with normal neutrophils. Some studies have shown that MPO-deficient neutrophils have prolonged respiratory bursts, resulting in increased H2O2 production in response to stimulation. These factors may compensate for the lack of peroxidase (Cramer et al., 1982). Therefore, on the basis of research performed in MPO knockout models, further relevant research can be performed to observe bodily changes in response to the loss of MPO production, to determine whether the MPO blockade will cause serious damage to the body. Such research should also seek to observe changes in the physiological regulation mechanisms mediated by MPO. If MPO knockout or knockdown shows little effect on the body, MPO inhibition could be applied to animal models of stroke to determine the therapeutic effects of MPO inhibition.
MPO as a therapeutic target in stroke
Malle et al. (2007) found that MPO can be used as a target for future drug development. Related drugs inhibit MPO activity and inhibit substrate production by combining halide binding sites with an aromatic substrate or inhibitor binding sites. They included 4-aminobenzoic acid amide, N-phenylacetamide, and melatonin (Malle et al., 2007).
On the basis of the hypothesis that MPO targets can be used as drugs, many animal experiments have been performed to examine the application of MPO inhibitors to stroke models in recent years. The classic MPO-specific inhibitor, 4-aminobenzoic acid amide, is a common drug used in stroke treatment research, and neurogenesis following ischemic stroke increased after 4-aminobenzoic acid amide treatment. The inhibition of MPO also increased the levels of brain-derived neurotrophic factor, phosphorylated C-reactive protein, acetylated H3 receptor, Cys-X-Cys receptor 4, and neuronal core antigen and reduced inflammatory cell infiltration mediated by MMP9. These results underscore the detrimental role of MPO activity in post-ischemia neurogenesis. A series of experiments demonstrated that MPO activity is inversely proportional to neurogenesis after stroke, and the inhibition of MPO activity increases cell proliferation and improves neurogenesis after ischemic stroke (Drexelius et al., 2019; Kim et al., 2019; Qiu et al., 2021). They further found that the protective environment induced by MPO inhibition or the knockout of MPO genes can reduce inflammatory cell aggregation and increase survival factors, which can improve stroke outcomes. MPO inhibition may represent a promising therapeutic target for stroke therapy, possibly even days after the stroke has occurred (Kim et al., 2016).
New MPO inhibitors are being discovered continuously. For example, N-acetyl lysyl-tyrosyl cysteine amide can inhibit the activity of MPO, which can reduce the numbers of M1 microglia and N1 neutrophils in the brains of stroke mouse models, protecting neuronal function (Yu et al., 2018). Many drugs can also exert antioxidant and anti-inflammatory effects and inhibit MPO. For example, in the study of ischemic stroke, after using rosmarinic acid (Fonteles et al., 2016), melatonin (Pei and Cheung, 2004), tropisetron (Daneshmand et al., 2011), and the traditional Chinese medicine extract Leonurus heterophyllus (Liang et al., 2011), a significant decrease in the amount and activity of MPO was observed. Importantly, cerebral infarction and neuronal damage were improved. Another example is in the study of hemorrhagic stroke. Lee et al. (2006) induced cerebral hemorrhage by injecting collagenase into the rat basal ganglia and administered memantine to inhibit inflammation. They found that the number of MPO-positive cells around the hematoma was significantly reduced in the memantine-treated group, which induced functional recovery after cerebral hemorrhage (Lee et al., 2006).
Although no MPO inhibitors are currently approved for use in clinical stroke patients, many preclinical candidate drugs are under development, and one candidate drug has completed Phase IIa clinical trials (Churg et al., 2012; Forbes et al., 2013; Ward et al., 2013). On the basis of the above review, MPO plays a vital role in stroke occurrence and development. After MPO inhibition, neurogenesis becomes active, and stroke recovery improves; therefore, MPO is expected to become a new target for stroke treatment.
Conclusions
MPO leads to a significant increase in stroke occurrence and development. The overexpression of MPO typically results in impaired BBB permeability. For patients with congenital or acquired loss of MPO expression, the effects on their immunity are not significant. Therefore, MPO can be targeted clinically for stroke treatment and potentially other inflammation-related diseases. Currently, no MPO inhibitors have been approved for clinical use, and the most commonly used specific inhibitor of MPO, ABAH, has a strong toxic effect on the human body. Many other inhibitors, including those mentioned in our article, are not specific inhibitors, and few studies have been performed on these inhibitors, none of which have reached the level of clinical trial. Whether these non-specific inhibitors have side effects on the human body remains unclear. The specific damage mechanism of MPO also remains unclear, and more research is necessary to clarify the underlying mechanisms. The specific etiological mechanism that leads to the activation of MPO during the occurrence and development of stroke also requires further clarification. Neutrophils are a key source of MPO production, and central immune cells can also produce MPO. Additional MPO-targeting drugs that are safe for clinical use must be developed.
Acknowledgments:
We express special thank to Zhao-Xuan Yan, School of Mathematics and Statistics, Lanzhou University, for her guidance on this review; we express special thank to Professor Yu-Hong Jing, School of Basic Medicine, Lanzhou University, for his guidance in setting up the topic of this review; we express special thank to Qin-Qin Ruan, School of Art, Lanzhou University, for her guidance in the figures in this review.
Footnotes
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open peer reviewer: Rayudu Gopalakrishna, University of Southern California, USA.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81771297 (to YNZ); CuiYing Scientific and Technological Innovation Program of Lanzhou University Second Hospital of China, No. CY2017-MS04 (to YNZ); Hui-Chun Chin and Tsung-Dao Lee Chinese Undergraduate Research Endowment of China, No. LZU-JZH2224 (to YCW); National Innovation and Entrepreneurship Training Program for Undergraduate of China, No. 201910730212 (to YCW); and CuiYing Scientific Training Program for Undergraduates of Lanzhou University Second Hospital of China, No. CYXZ2019-06 (to YCW).
P-Reviewer: Gopalakrishna R; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Giles L, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Agner K. Verdoperoxidase: a ferment isolated from leucocytes. Acta Physiol Scand. 1941;2:1–62. [Google Scholar]
- 2.Al Mamun A, Chauhan A, Yu H, Xu Y, Sharmeen R, Liu F. Interferon regulatory factor 4/5 signaling impacts on microglial activation after ischemic stroke in mice. Eur J Neurosci. 2018;47:140–149. doi: 10.1111/ejn.13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anctil M, Poulain I, Pelletier C. Nitric oxide modulates peristaltic muscle activity associated with fluid circulation in the sea pansy Renilla koellikeri. J Exp Biol. 2005;208:2005–2017. doi: 10.1242/jeb.01607. [DOI] [PubMed] [Google Scholar]
- 4.Avan A, Digaleh H, Di Napoli M, Stranges S, Behrouz R, Shojaeianbabaei G, Amiri A, Tabrizi R, Mokhber N, Spence JD, Azarpazhooh MR. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019;17:191. doi: 10.1186/s12916-019-1397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babior BM. The respiratory burst of phagocytes. J Clin Invest. 1984;73:599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Bernhart E, Kogelnik N, Prasch J, Gottschalk B, Goeritzer M, Depaoli MR, Reicher H, Nusshold C, Plastira I, Hammer A, Fauler G, Malli R, Graier WF, Malle E, Sattler W. 2-Chlorohexadecanoic acid induces ER stress and mitochondrial dysfunction in brain microvascular endothelial cells. Redox Biol. 2018;15:441–451. doi: 10.1016/j.redox.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonetti NR, Diaz-Cañestro C, Liberale L, Crucet M, Akhmedov A, Merlini M, Reiner MF, Gobbato S, Stivala S, Kollias G, Ruschitzka F, Lüscher TF, Beer JH, Camici GG. Tumour necrosis factor-α inhibition improves stroke outcome in a mouse model of rheumatoid arthritis. Sci Rep. 2019;9:2173. doi: 10.1038/s41598-019-38670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brovkovych V, Gao XP, Ong E, Brovkovych S, Brennan ML, Su X, Hazen SL, Malik AB, Skidgel RA. Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L96–103. doi: 10.1152/ajplung.00450.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–425. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- 11.Castellazzi M, Tamborino C, De Santis G, Garofano F, Lupato A, Ramponi V, Trentini A, Casetta I, Bellini T, Fainardi E. Timing of serum active MMP-9 and MMP-2 levels in acute and subacute phases after spontaneous intracerebral hemorrhage. Acta Neurochir Suppl. 2010;106:137–140. doi: 10.1007/978-3-211-98811-4_24. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Guan B, Shen J. Targeting ONOO-/HMGB1/MMP-9 signaling cascades: potential for drug development from chinese medicine to attenuate ischemic brain injury and hemorrhagic transformation induced by thrombolytic treatment. Integr Med Int. 2016;3:32–52. [Google Scholar]
- 13.Cheng Z, Wang L, Qu M, Liang H, Li W, Li Y, Deng L, Zhang Z, Yang GY. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J Neuroinflammation. 2018;15:135. doi: 10.1186/s12974-018-1153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Churg A, Marshall CV, Sin DD, Bolton S, Zhou S, Thain K, Cadogan EB, Maltby J, Soars MG, Mallinder PR, Wright JL. Late intervention with a myeloperoxidase inhibitor stops progression of experimental chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:34–43. doi: 10.1164/rccm.201103-0468OC. [DOI] [PubMed] [Google Scholar]
- 15.Cramer R, Soranzo MR, Dri P, Rottini GD, Bramezza M, Cirielli S, Patriarca P. Incidence of myeloperoxidase deficiency in an area of northern Italy: histochemical, biochemical and functional studies. Br J Haematol. 1982;51:81–87. doi: 10.1111/j.1365-2141.1982.tb07292.x. [DOI] [PubMed] [Google Scholar]
- 16.Daneshmand A, Mohammadi H, Rahimian R, Habibollahi P, Fakhfouri G, Talab SS, Mehr SE, Dehpour AR. Chronic lithium administration ameliorates 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis in rats; potential role for adenosine triphosphate sensitive potassium channels. J Gastroenterol Hepatol. 2011;26:1174–1181. doi: 10.1111/j.1440-1746.2011.06719.x. [DOI] [PubMed] [Google Scholar]
- 17.Deng YH, Dong LL, Zhang YJ, Zhao XM, He HY. Enriched environment boosts the post-stroke recovery of neurological function by promoting autophagy. Neural Regen Res. 2021;16:813–819. doi: 10.4103/1673-5374.297084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deuschl G, Beghi E, Fazekas F, Varga T, Christoforidi KA, Sipido E, Bassetti CL, Vos T, Feigin VL. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 2020;5:e551–e567. doi: 10.1016/S2468-2667(20)30190-0. [DOI] [PubMed] [Google Scholar]
- 19.Diwanji N, Bergmann A. Basement membrane damage by ROS- and JNK-mediated Mmp2 activation drives macrophage recruitment to overgrown tissue. Nat Commun. 2020;11:3631. doi: 10.1038/s41467-020-17399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drexelius JA, Kanne CK, Tran HD, Hyacinth HI, Sheehan VA. Plasma BDNF levels are associated with stroke in children with SCD. Blood. 2019;134:3565. [Google Scholar]
- 21.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Kebir D, József L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res. 2008;103:352–359. doi: 10.1161/01.RES.0000326772.76822.7a. [DOI] [PubMed] [Google Scholar]
- 23.Facci L, Barbierato M, Zusso M, Skaper SD, Giusti P. Serum amyloid A primes microglia for ATP-dependent interleukin-1β release. J Neuroinflammation. 2018;15:164. doi: 10.1186/s12974-018-1205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazal N, Al-Ghoul W. Abstract 2662: Statins modulate MMP-9 and beta catenin in epithelial cells. Cancer Res. 2017;77:2662. [Google Scholar]
- 25.Feng W, Wang Y, Liu ZQ, Zhang X, Han R, Miao YZ, Qin ZH. Microglia activation contributes to quinolinic acid-induced neuronal excitotoxicity through TNF-α. Apoptosis. 2017;22:696–709. doi: 10.1007/s10495-017-1363-5. [DOI] [PubMed] [Google Scholar]
- 26.Fonteles AA, de Souza CM, de Sousa Neves JC, Menezes AP, Santos do Carmo MR, Fernandes FD, de Araújo PR, de Andrade GM. Rosmarinic acid prevents against memory deficits in ischemic mice. Behav Brain Res. 2016;297:91–103. doi: 10.1016/j.bbr.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Forbes LV, Sjögren T, Auchère F, Jenkins DW, Thong B, Laughton D, Hemsley P, Pairaudeau G, Turner R, Eriksson H, Unitt JF, Kettle AJ. Potent reversible inhibition of myeloperoxidase by aromatic hydroxamates. J Biol Chem. 2013;288:36636–36647. doi: 10.1074/jbc.M113.507756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 29.Fu X, Kao JL, Bergt C, Kassim SY, Huq NP, d’Avignon A, Parks WC, Mecham RP, Heinecke JW. Oxidative cross-linking of tryptophan to glycine restrains matrix metalloproteinase activity: specific structural motifs control protein oxidation. J Biol Chem. 2004;279:6209–6212. doi: 10.1074/jbc.C300506200. [DOI] [PubMed] [Google Scholar]
- 30.GBD 2016 DALYs and HALE Collaborators (2017) Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study. Lancet. 2016;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GBD 2016 Lifetime Risk of Stroke Collaborators. Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd-Allah F, Abejie AN, Abyu GY, Ademi Z, Agarwal G, Ahmed MB, Akinyemi RO, Al-Raddadi R, Aminde LN, Amlie-Lefond C, Ansari H, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379:2429–2437. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GBD 2017 Causes of Death Collaborators (2018) Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study. Lancet. 2017;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gellhaar S, Sunnemark D, Eriksson H, Olson L, Galter D. Myeloperoxidase-immunoreactive cells are significantly increased in brain areas affected by neurodegeneration in Parkinson’s and Alzheimer’s disease. Cell Tissue Res. 2017;369:445–454. doi: 10.1007/s00441-017-2626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George MG. Risk Factors for ischemic stroke in younger adults: a focused update. Stroke. 2020;51:729–735. doi: 10.1161/STROKEAHA.119.024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorudko IV, Mikhalchik EV, Sokolov AV, Grigorieva DV, Kostevich VA, Vasilyev VB, Cherenkevich SN, Panasenko OM. The production of reactive oxygen and halogen species by neutrophils in response to monomeric forms of myeloperoxidase. Biophysics. 2017;62:919–925. [Google Scholar]
- 36.Graham DB, Robertson CM, Bautista J, Mascarenhas F, Diacovo MJ, Montgrain V, Lam SK, Cremasco V, Dunne WM, Faccio R, Coopersmith CM, Swat W. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCgamma2 signaling axis in mice. J Clin Invest. 2007;117:3445–3452. doi: 10.1172/JCI32729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol. 2008;18:86–95. doi: 10.1111/j.1750-3639.2007.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grishkovskaya I, Paumann-Page M, Tscheliessnig R, Stampler J, Hofbauer S, Soudi M, Sevcnikar B, Oostenbrink C, Furtmüller PG, Djinović-Carugo K, Nauseef WM, Obinger C. Structure of human promyeloperoxidase (proMPO) and the role of the propeptide in processing and maturation. J Biol Chem. 2017;292:8244–8261. doi: 10.1074/jbc.M117.775031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu Y, Dee CM, Shen J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front Biosci (Schol Ed) 2011;3:1216–1231. doi: 10.2741/222. [DOI] [PubMed] [Google Scholar]
- 40.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 41.Hwang M, Bergmann CC. Neuronal ablation of alpha/beta interferon (IFN-α/β) signaling exacerbates central nervous system viral dissemination and impairs IFN-γ responsiveness in microglia/macrophages. J Virol. 2020;94:e00422–20. doi: 10.1128/JVI.00422-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iana A, Sirbu E. Linking myeloperoxidase with subclinical atherosclerosis in adults with metabolic syndrome. Wien Klin Wochenschr. 2020;132:150–154. doi: 10.1007/s00508-019-01602-y. [DOI] [PubMed] [Google Scholar]
- 43.Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47:864–878. doi: 10.1159/000490078. [DOI] [PubMed] [Google Scholar]
- 44.Ju S, Xu C, Wang G, Zhang L. VEGF-C induces alternative activation of microglia to promote recovery from traumatic brain injury. J Alzheimers Dis. 2019;68:1687–1697. doi: 10.3233/JAD-190063. [DOI] [PubMed] [Google Scholar]
- 45.Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, Cao Y, Xu H, Luo H, Lu L, Shi MJ, Tian Y, Fan W, Zhao BQ. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. 2020;11:2488. doi: 10.1038/s41467-020-16191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H, Wei Y, Lee JY, Wu Y, Zheng Y, Moskowitz MA, Chen JW. Myeloperoxidase inhibition increases neurogenesis after ischemic stroke. J Pharmacol Exp Ther. 2016;359:262–272. doi: 10.1124/jpet.116.235127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HJ, Wei Y, Wojtkiewicz GR, Lee JY, Moskowitz MA, Chen JW. Reducing myeloperoxidase activity decreases inflammation and increases cellular protection in ischemic stroke. J Cereb Blood Flow Metab. 2019;39:1864–1877. doi: 10.1177/0271678X18771978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitahara M, Eyre HJ, Simonian Y, Atkin CL, Hasstedt SJ. Hereditary myeloperoxidase deficiency. Blood. 1981;57:888–893. [PubMed] [Google Scholar]
- 49.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 50.Klinke A, Nussbaum C, Kubala L, Friedrichs K, Rudolph TK, Rudolph V, Paust HJ, Schröder C, Benten D, Lau D, Szocs K, Furtmüller PG, Heeringa P, Sydow K, Duchstein HJ, Ehmke H, Schumacher U, Meinertz T, Sperandio M, Baldus S. Myeloperoxidase attracts neutrophils by physical forces. Blood. 2011;117:1350–1358. doi: 10.1182/blood-2010-05-284513. [DOI] [PubMed] [Google Scholar]
- 51.Lanza F. Clinical manifestation of myeloperoxidase deficiency. J Mol Med (Berl) 1998;76:676–681. doi: 10.1007/s001090050267. [DOI] [PubMed] [Google Scholar]
- 52.Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther. 2006;111:16–26. doi: 10.1016/j.pharmthera.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 53.Lee ST, Chu K, Jung KH, Kim J, Kim EH, Kim SJ, Sinn DI, Ko SY, Kim M, Roh JK. Memantine reduces hematoma expansion in experimental intracerebral hemorrhage, resulting in functional improvement. J Cereb Blood Flow Metab. 2006;26:536–544. doi: 10.1038/sj.jcbfm.9600213. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Yu W, Guan Y, Zou H, Liang Z, Huang M, Zhao R, Zhao C, Ren Z, Chen Z. Peripheral circulation and astrocytes contribute to the MSC-mediated increase in IGF-1 levels in the infarct cortex in a dMCAO rat model. Stem Cells Int. 2020;2020:8853444. doi: 10.1155/2020/8853444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang H, Liu P, Wang Y, Song S, Ji A. Protective effects of alkaloid extract from Leonurus heterophyllus on cerebral ischemia reperfusion injury by middle cerebral ischemic injury (MCAO) in rats. Phytomedicine. 2011;18:811–818. doi: 10.1016/j.phymed.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Liang YF, Hao P, Duan HM, Zhao W, Gao YD, Li XG, Yang CY. Pathological and behavioral changes after ischemic stroke in adult mice. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:5625–5631. [Google Scholar]
- 57.Lin R, Lang M, Heinsinger N, Stricsek G, Zhang J, Iozzo R, Rosenwasser R, Iacovitti L. Stepwise impairment of neural stem cell proliferation and neurogenesis concomitant with disruption of blood-brain barrier in recurrent ischemic stroke. Neurobiol Dis. 2018;115:49–58. doi: 10.1016/j.nbd.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Liu C, Xie G, Huang W, Yang Y, Li P, Tu Z. Elevated serum myeloperoxidase activities are significantly associated with the prevalence of ACS and high LDL-C levels in CHD patients. J Atheroscler Thromb. 2012;19:435–443. doi: 10.5551/jat.9704. [DOI] [PubMed] [Google Scholar]
- 59.Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. Interferon-β regulates the production of IL-10 by toll-like receptor-activated microglia. Glia. 2017;65:1439–1451. doi: 10.1002/glia.23172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2017;157:247–272. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Maestrini I, Tagzirt M, Gautier S, Dupont A, Mendyk AM, Susen S, Tailleux A, Vallez E, Staels B, Cordonnier C, Leys D, Bordet R. MPO is partially associated with neutrophil deleterious effect in acute cerebral ischemia. Neurology. 2020 doi: 10.1212/WNL.0000000000009179. doi: 101212/WNL0000000000009179. [DOI] [PubMed] [Google Scholar]
- 62.Mai X, Liang X. Risk factors for stroke based on the national health and nutrition examination survey. J Nutr Health Aging. 2020;24:791–795. doi: 10.1007/s12603-020-1430-4. [DOI] [PubMed] [Google Scholar]
- 63.Maksoud MJE, Tellios V, Xiang YY, Lu WY. Nitric oxide displays a biphasic effect on calcium dynamics in microglia. Nitric Oxide. 2021;108:28–39. doi: 10.1016/j.niox.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Malle E, Furtmüller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development. Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manso H, Krug T, Sobral J, Albergaria I, Gaspar G, Ferro JM, Oliveira SA, Vicente AM. Variants in the inflammatory IL6 and MPO genes modulate stroke susceptibility through main effects and gene-gene interactions. J Cereb Blood Flow Metab. 2011;31:1751–1759. doi: 10.1038/jcbfm.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marcinkiewicz J, Walczewska M. Neutrophils as sentinel cells of the immune system: a role of the MPO-halide-system in innate and adaptive immunity. Curr Med Chem. 2020;27:2840–2851. doi: 10.2174/0929867326666190819123300. [DOI] [PubMed] [Google Scholar]
- 67.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 69.Morris JC. The acid ionization constant of HOCl from 5 to 35°. J Phys Chem. 1966;70:3798–3805. [Google Scholar]
- 70.Nalamolu KR, Challa SR, Mohandass A, Mussman JP, Ilahi SB, Bedadala MR, Klopfenstein JD, Pinson DM, Wang DZ, Kalyanasundaram R, Vemuganti R, Veeravalli KK. ShRNA-mediated gene silencing of t-PA prevents BBB disruption and elevation of MMP-12 after ischemic stroke. FASEB J. 2020;34:1. [Google Scholar]
- 71.Nicholls SJ, Hazen SL. Myeloperoxidase, modified lipoproteins, and atherogenesis. J Lipid Res. 2009;50(Suppl):S346–351. doi: 10.1194/jlr.R800086-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nybo T, Dieterich S, Gamon LF, Chuang CY, Hammer A, Hoefler G, Malle E, Rogowska-Wrzesinska A, Davies MJ. Chlorination and oxidation of the extracellular matrix protein laminin and basement membrane extracts by hypochlorous acid and myeloperoxidase. Redox Biol. 2019;20:496–513. doi: 10.1016/j.redox.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palm F, Pussinen PJ, Safer A, Tervahartiala T, Sorsa T, Urbanek C, Becher H, Grau AJ. Serum matrix metalloproteinase-8, tissue inhibitor of metalloproteinase and myeloperoxidase in ischemic stroke. Atherosclerosis. 2018;271:9–14. doi: 10.1016/j.atherosclerosis.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 74.Pei Z, Cheung RT. Pretreatment with melatonin exerts anti-inflammatory effects against ischemia/reperfusion injury in a rat middle cerebral artery occlusion stroke model. J Pineal Res. 2004;37:85–91. doi: 10.1111/j.1600-079X.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 75.Peppin GJ, Weiss SJ. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc Natl Acad Sci U S A. 1986;83:4322–4326. doi: 10.1073/pnas.83.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phuah CL, Dave T, Malik R, Raffeld MR, Ayres AM, Goldstein JN, Viswanathan A, Greenberg SM, Jagiella JM, Hansen BM, Norrving B, Jimenez-Conde J, Roquer J, Pichler A, Enzinger C, Montaner J, Fernandez-Cadenas I, Lindgren A, Slowik A, Schmidt R, et al. Genetic variants influencing elevated myeloperoxidase levels increase risk of stroke. Brain. 2017;140:2663–2672. doi: 10.1093/brain/awx220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pleskova SN, Mikheeva ER, Razumkova EV, Gornostaeva EE. The effect of magnetite nanoparticles and bacteria on the activity of NADPH-oxidase and myeloperoxidase in neutrophils of human blood. Cell Tiss Biol. 2018;12:120–126. [Google Scholar]
- 78.Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61:91–103. doi: 10.1002/glia.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pravalika K, Sarmah D, Kaur H, Vats K, Saraf J, Wanve M, Kalia K, Borah A, Yavagal DR, Dave KR, Bhattacharya P. Trigonelline therapy confers neuroprotection by reduced glutathione mediated myeloperoxidase expression in animal model of ischemic stroke. Life Sci. 2019;216:49–58. doi: 10.1016/j.lfs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 80.Pullar JM, Vissers MC, Winterbourn CC. Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life. 2000;50:259–266. doi: 10.1080/713803731. [DOI] [PubMed] [Google Scholar]
- 81.Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, Bosco DB, Wu LJ, Tian DS. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35:921–933. doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qiu Z, Yang J, Deng G, Li D, Zhang S. Angiopoietin-like 4 promotes angiogenesis and neurogenesis in a mouse model of acute ischemic stroke. Brain Res Bull. 2021;168:156–164. doi: 10.1016/j.brainresbull.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 83.Ramachandra CJA, Ja K, Chua J, Cong S, Shim W, Hausenloy DJ. Myeloperoxidase as a multifaceted target for cardiovascular protection. Antioxid Redox Signal. 2020;32:1135–1149. doi: 10.1089/ars.2019.7971. [DOI] [PubMed] [Google Scholar]
- 84.Ray RS, Katyal A. Myeloperoxidase: Bridging the gap in neurodegeneration. Neurosci Biobehav Rev. 2016;68:611–620. doi: 10.1016/j.neubiorev.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 85.Reber LL, Gillis CM, Starkl P, Jönsson F, Sibilano R, Marichal T, Gaudenzio N, Bérard M, Rogalla S, Contag CH, Bruhns P, Galli SJ. Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide. J Exp Med. 2017;214:1249–1258. doi: 10.1084/jem.20161238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song J, Tang J, Guo F. Identification of inhibitors of MMPS enzymes via a novel computational approach. Int J Biol Sci. 2018;14:863–871. doi: 10.7150/ijbs.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spittau B, Dokalis N, Prinz M. The role of TGFβ signaling in microglia maturation and activation. Trends Immunol. 2020;41:836–848. doi: 10.1016/j.it.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Stendahl O, Coble BI, Dahlgren C, Hed J, Molin L. Myeloperoxidase modulates the phagocytic activity of polymorphonuclear neutrophil leukocytes. Studies with cells from a myeloperoxidase-deficient patient. J Clin Invest. 1984;73:366–373. doi: 10.1172/JCI111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swastika, Chaturvedi S, Kaul A, Hazari PP, Jha P, Pal S, Lal S, Singh B, Barthélémy P, Mishra AK. Evaluation of BBB permeable nucleolipid (NL(DPU)): A di-C15-ketalised palmitone appended uridine as neuro-tracer for SPECT. Int J Pharm. 2019;565:269–282. doi: 10.1016/j.ijpharm.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 90.Swirski FK, Weissleder R, Pittet MJ. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1424–1432. doi: 10.1161/ATVBAHA.108.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Üllen A, Singewald E, Konya V, Fauler G, Reicher H, Nusshold C, Hammer A, Kratky D, Heinemann A, Holzer P, Malle E, Sattler W. Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction in vitro and in vivo. PLoS One. 2013;8:e64034. doi: 10.1371/journal.pone.0064034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vita JA, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH, Penn MS, Keaney JF, Jr, Hazen SL. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Zhou L, Guo J, Wang Y, Yang Y, Peng Q, Gao Y, Lu W. Secular trends of stroke incidence and mortality in China, 1990 to 2016: The Global Burden of Disease Study 2016. J Stroke Cerebrovasc Dis. 2020;29:104959. doi: 10.1016/j.jstrokecerebrovasdis.2020.104959. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ward J, Spath SN, Pabst B, Carpino PA, Ruggeri RB, Xing G, Speers AE, Cravatt BF, Ahn K. Mechanistic characterization of a 2-thioxanthine myeloperoxidase inhibitor and selectivity assessment utilizing click chemistry--activity-based protein profiling. Biochemistry. 2013;52:9187–9201. doi: 10.1021/bi401354d. [DOI] [PubMed] [Google Scholar]
- 98.Wei P, Liu L, Yuan W, Yang J, Li R, Yi T. A fluorescent probe operating under weak acidic conditions for the visualization of HOCl in solid tumors in vivo. Sci China Chem. 2020;63:1153–1158. [Google Scholar]
- 99.Weiss SJ, Peppin G, Ortiz X, Ragsdale C, Test ST. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227:747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- 100.Whiteman M, Rose P, Siau JL, Cheung NS, Tan GS, Halliwell B, Armstrong JS. Hypochlorous acid-mediated mitochondrial dysfunction and apoptosis in human hepatoma HepG2 and human fetal liver cells: role of mitochondrial permeability transition. Free Radic Biol Med. 2005;38:1571–1584. doi: 10.1016/j.freeradbiomed.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 101.Wolf BB, Goldstein JC, Stennicke HR, Beere H, Amarante-Mendes GP, Salvesen GS, Green DR. Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood. 1999;94:1683–1692. [PubMed] [Google Scholar]
- 102.Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26:2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 103.Xu Y, Jin MZ, Yang ZY, Jin WL. Microglia in neurodegenerative diseases. Neural Regen Res. 2021;16:270–280. doi: 10.4103/1673-5374.290881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yap YW, Whiteman M, Bay BH, Li Y, Sheu FS, Qi RZ, Tan CH, Cheung NS. Hypochlorous acid induces apoptosis of cultured cortical neurons through activation of calpains and rupture of lysosomes. J Neurochem. 2006;98:1597–1609. doi: 10.1111/j.1471-4159.2006.03996.x. [DOI] [PubMed] [Google Scholar]
- 105.Yeo H, Lee JY, Kim J, Ahn SS, Jeong JY, Choi JH, Lee YH, Shin SY. Transcription factor EGR-1 transactivates the MMP1 gene promoter in response to TNFα in HaCaT keratinocytes. BMB Rep. 2020;53:323–328. doi: 10.5483/BMBRep.2020.53.6.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu G, Liang Y, Zheng S, Zhang H. Inhibition of myeloperoxidase by N-acetyl lysyltyrosylcysteine amide reduces oxidative stress-mediated inflammation, neuronal damage, and neural stem cell injury in a murine model of stroke. J Pharmacol Exp Ther. 2018;364:311–322. doi: 10.1124/jpet.117.245688. [DOI] [PubMed] [Google Scholar]
- 107.Zhang C, Kim SK. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: the current situation and future prospects. Mar Drugs. 2009;7:71–84. doi: 10.3390/md7020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Q, Liu Y, Jiang M, Liu Y, Gu S, Tong H, Liu H. Temporal trends in the risk factors and clinical characteristics of ischemic stroke in young adults. J Stroke Cerebrovasc Dis. 2020;29:104914. doi: 10.1016/j.jstrokecerebrovasdis.2020.104914. [DOI] [PubMed] [Google Scholar]


