Abstract
In 2001, the concept of the neurovascular unit was introduced at the Stroke Progress Review Group meeting. The neurovascular unit is an important element of the health and disease status of blood vessels and nerves in the central nervous system. Since then, the neurovascular unit has attracted increasing interest from research teams, who have contributed greatly to the prevention, treatment, and prognosis of stroke and neurodegenerative diseases. However, additional research is needed to establish an efficient, low-cost, and low-energy in vitro model of the neurovascular unit, as well as enable noninvasive observation of neurovascular units in vivo and in vitro. In this review, we first summarize the composition of neurovascular units, then investigate the efficacy of different types of stem cells and cell culture methods in the construction of neurovascular unit models, and finally assess the progress of imaging methods used to observe neurovascular units in recent years and their positive role in the monitoring and investigation of the mechanisms of a variety of central nervous system diseases.
Key Words: 3D printing, blood-brain barrier, computational biology, encephalopathy, imaging techniques, microfluidic on-chip methods, nerve cell co-culture, neurovascular unit, review, stem cells
Introduction
The neurovascular unit (NVU) consists of neurons, nerve glia cells (astrocytes, microglia, oligodendrocytes), vascular cells (brain microvascular endothelial cells (BMECs), pericytes, and smooth muscle cells (SMCs)), and brain-specific extracellular matrix (ECM). Its dynamic cellular composition and structure play an indispensable role in maintaining the stability of the central nervous system (CNS) microenvironment (Heneka et al., 2015; Caffery et al., 2021; Ye et al., 2021). BMECs line the capillaries of the brain, form densely arranged tight junctions (TJs) with pericytes, and form the brain’s unique barrier structure, the blood-brain barrier (BBB). As a core structure of the NVU, the BBB acts as a highly dynamic and functional interface between systemic circulation and the CNS. The BBB strictly and accurately regulates the transport of metabolic molecules and nutrients while maintaining a stable brain environment and protecting the CNS from potentially harmful chemical or systemic fluctuations (Birnbaum and Weinberger, 2017). Injury or destruction of the BBB occurs in a variety of neurological diseases, such as stroke (Zhou et al., 2017; Maoz et al., 2018; Segarra et al., 2018), as well as neurodegenerative diseases such as Alzheimer’s disease (AD) (Giovacchini et al., 2011; Zlokovic, 2011; Urban et al., 2017; Zhou et al., 2017), vascular dementia (Arsava et al., 2018), Parkinson’s disease (PD) (Zhang et al., 2019), Huntington’s disease (Dong et al., 2018), and amyotrophic lateral sclerosis (Zhang et al., 2019). To examine the etiology and pathogenesis of these diseases, many new imaging techniques and in vitro models have been developed.
Noninvasive imaging techniques such as magnetic resonance imaging (MRI) and positron emission tomography (PET) enable researchers and clinicians to assess the structure and function of the NVU, and thus are widely used in animal experiments and clinical diagnostic processes (Islam and Mohamed, 2015). PET can be combined with computed tomography (CT) and MRI for more reliable detection of various diseases in different parts of the nervous system, and can be used to quantitatively analyze certain endogenous substances and metabolic activities in the brain (Keogh et al., 2018). Angiography can be used to measure the degree of arterial stenosis, which can reflect dynamic changes in cerebral microvessels, and can be used to diagnose early changes in arterioles in patients with ischemic stroke and other diseases (Xiao et al., 2018). In addition to these principal imaging techniques used for clinical diagnosis and in vivo research, two-photon imaging, transmission electron microscopy, and other techniques can be used to observe the ultrastructure of lesions (Lo et al., 2003; Tedesco et al., 2018).
In in vitro models of the BBB, primary cells and immortalized BMECs are generally used as the source of cell cultures. However, stem cells (embryonic stem cells, neural stem cells, induced multifunctional stem cells, and bone marrow mesenchymal stem cells (MSCs)) have also been used to model the BBB in simulations of the NVU (Garofalo et al., 2015). Cell culture methods such as the two-dimensional Transwell cell culture are constantly being developed, and these represent a substantial improvement from BMECs cultured to simulate the BBB (Ferrini et al., 2013). Meanwhile, with the continuous innovation of molecular materials, the application of hydrogel and three-dimensional (3D) printing technology has enabled the co-culture of 3D model cells, which may play an important role in simulating the microenvironment and characteristics of the BBB in vitro (Benakis et al., 2014). Another in vitro technology, in silico biology, uses big data and cloud computing to summarize previous results, facilitate drug design, and enable precise medical care for nervous system diseases with reduced costs and improved time efficiency (Allen and Lyons, 2018). In this review, we systematically examined studies of the composition of the NVU, including in vitro and in vivo models, as well as new imaging technologies applied in NVU research.
Retrieval Strategy
For this narrative review, literature searches were performed using PubMed and the China National Knowledge Infrastructure from 2001 to 2021. The following keywords were used: “neurovascular unit” AND “imaging technology” OR “stem cell” OR “cell co-culture” OR “3D printing” OR “microfluidic” OR “computational biology”. The articles included in this review were selected based on their relevance to the topic. The results were further screened according to the title and abstract, and whether they included animal experiments, in vitro studies, clinical trials, and database or software applications.
Basics of the Neurovascular Unit
Neurons are connected to each other via dendrites and axons to form a network that can transmit signals and thus enable communication. This structure plays an important role in maintaining the normal physiological function of the human brain. Before the 21st century, the repair of neuronal damage was considered the main target for the treatment of brain diseases such as neurodegenerative diseases and ischemic stroke (Vasic et al., 2019). In 2001, the concept of the NVU was proposed by American scientists (https://www.ninds.nih.gov/About-NINDS/Strategic-Plans-Evaluations/Strategic-Plans/Stroke-Progress-Review-Group). The NVU includes neurons, astrocytes, vascular cells (BMECs, pericytes, and SMCs), the basal lamina matrix, microglia, and astrocytes (Yang et al., 2005) (Figure 1). Thurgur and Pinteaux (Thurgur and Pinteaux, 2019) proposed that these cells and structures are closely linked via dynamic interactions to form the NVU.
Figure 1.
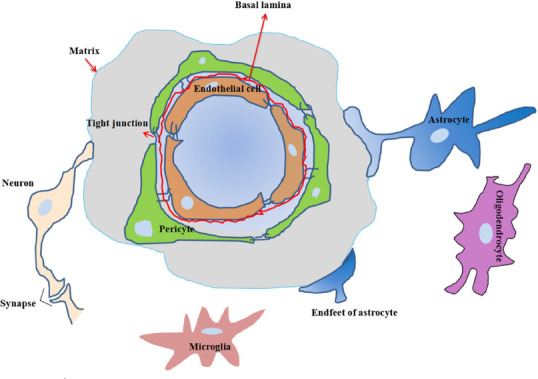
Schematic of the neurovascular unit.
The neurovascular unit is composed of neurons-glial cells-blood vessels interactions, and includes neurons, astrocytes, microglia, vascular endothelial cells, perivascular cells, basement membrane, and extracellular matrix. The model emphasizes the importance of the interconnection and mutual influence between neurons, glial cells, and the cerebrovascular system, and offers a three-dimensional environment suitable for studying neuronal damage and protective mechanisms, as well as searching for new potential targets for clinical treatment.
Glial cells
Microglia
All components of the NVU play an important role in maintaining CNS homeostasis. Neurons are surrounded by glial cells, which provide nutrients and stabilize the microenvironment of neurons by preventing the invasion of vessel cells and blood-derived substances (Dietz et al., 2020). Microglia secrete brain-derived neurotrophic factors, which can promote neuronal nutrition and repair during inflammation caused by brain injury (Prinz et al., 2019). With their ability to dynamically monitor pathogens, microglia serve as “full-time” phagocytes in the CNS and can eliminate apoptotic cells and necrotic debris (Subhramanyam et al., 2019). After 24 hours of cerebral ischemia, amoebic microglia can form a protective barrier at the infarction site, thus preventing further damage (Rodríguez-Gómez et al., 2020). Meanwhile, globular microglia are distributed in the core of the lesion and play a role in reducing injury and inflammation (Voet et al., 2019).
Astrocytes
Astrocytes, the most abundant glial cells in the CNS, play an important role in maintaining the structure and function of the brain. They have various important physiological functions, such as structural support, formation of the BBB, neuronal metabolism, maintenance of extracellular environmental stability, regulation of cerebral blood flow (CBF), stabilization of cell-cell communication, synthesis of neurotransmitters, and protection against oxidative stress (Niu et al., 2013). In particular, astrocytes act as a bridge for glial crosstalk and link glial structures with vascular structures in the CNS (Liu et al., 2020a). Astrocyte proliferation is a common pathological reaction in many neurodegenerative diseases. It is accompanied by cell swelling, neurite lengthening, and the enhanced expression of glial fibrillary acidic protein. In recent years, an increasing number of studies (Al-Qattan et al., 2018; Recasens et al., 2019) have confirmed this point. When the activation of Sonic hedgehog signal is elevated, astrocytes can be activated and transformed into mature neurons (Bardehle et al., 2013). This neuronal transformation ability is affected by the microenvironment and is regulated by related molecular signaling pathways, including the Notch signaling pathway (Saab et al., 2013), the osteopontin signaling pathway (Iijima et al., 2015), and the microvascular contact signaling pathway (Takase et al., 2018). Astrocytes play a crucial role in maintaining the microenvironment homeostasis of the NVU.
Oligodendrocytes
Oligodendrocytes are differentiated from pluripotent neural stem cells on the ventral side of the neural tube. They form the myelin sheaths around axons, speeding up signal transduction. This makes the development of complex and tight neural circuits possible, and also plays an important role in the maintenance and survival of axons and neurons (Takahashi et al., 2011). An increasing number of studies has shown that oligodendrocyte abnormalities play an important role in the pathogenesis of CNS demyelinating diseases (Bennett et al., 2019), neuronal damage (Rafalski et al., 2018), and psychiatric disorders. Oligodendrocytes may be a potential therapeutic target for reversing cognitive impairment in neuropsychiatric disorders (depression, autism, and schizophrenia) (Ma et al., 2018).
Pericytes
Pericytes are an important part of the NVU because they regulate vasomotion and CBF, and also maintain the stability of the BBB (Zeisel et al., 2019). They regulate CBF by contracting and relaxing, but during the acute stage of cerebral ischemia, they may aggravate cerebral ischemia-reperfusion injury via excessive contraction. Pericytes participate in the formation of capillaries and regulate the stability of microvessels. The interaction between pericytes and endothelial cells (ECs) is mainly mediated by paracrine and juxtacrine, and the molecules involved are mainly platelet-derived growth factor and their receptors, transforming growth factor-β, vascular endothelial growth factor, angiopoietin-1, among others. Through these cytokines, pericytes can participate in regulating the initiation, budding, and termination of angiogenesis (Bennett et al., 2019). Pericytes promote the formation of TJs and maintain the permeability of the BBB. Microvessels are surrounded by pericytes, and their aggregation and coverage are crucial for the formation and penetration of the BBB (Bergers and Song, 2005).
Tight junctions
BMECs, pericytes, and astrocytes interconnect around the basal lamina (Zhao et al., 2011). TJs are found between adjacent BMECs, whereas ECs and pericytes are connected by gap junctions at the level of “peg and socket” contacts (Tsukita et al., 2019). The TJs between adjacent BMECs maintain the extremely low permeability and high electrical resistance of the BBB, and they monitor the migration of polar solutes and macromolecules across the BBB. Many of the endothelial TJs in the CNS form the structural basis of the barrier system. The ECM produced by BMECs and pericytes forms the basal lamina of brain microvessels (Caporarello et al., 2019). The BBB, as the core of the NVU, is composed of BMECs, pericytes, astrocytes, and the basal lamina.
TJs have an atresia structure, and they consist of specific transmembrane proteins that are fused with one another, such as membrane-spanning proteins (claudins, occludins, and junctional adhesion molecules), zonula occludens, and F-actin (Dörfel and Huber, 2012). Changes in the structure, distribution, and expression of these proteins can all lead TJs to open to varying degrees, thereby altering the permeability of the BBB. In a pathological state, some other proteins and pathways, such as Rho-associated coiled-coil containing kinases (Huang et al., 2020a), protein kinase C (Geribaldi-Doldán et al., 2019), and mitogen-activated protein kinase (Olateju et al., 2021), regulate TJ function and paracellular permeability. The permeability of the BBB is related to the character of the compounds that encounter it, such as whether they are lipophilic substances, which can easily pass through TJs. BMECs regulate transcytosis between vessels and the brain via endocytic vesicles, and this activity has a strong impact on whether macromolecules (proteins) are able to cross the BBB (Lecrux and Hamel, 2011).
Microvasculature
The CNS requires a large amount of energy to maintain normal life activities. The capillaries are filled with CFS, which ensures neuron activity. Cerebrovascular BMECs regulate the interface of vascular lumen and smooth muscle, and thus actively regulate CBF (Attwell et al., 2010). Neurons regulate the biosynthesis of astrocytes through the release of glutamate from chemical synapses. Astrocytes are widely recognized as agents of coupling between blood vessels and neurons (Zlokovic et al., 1983; Iadecola and Nedergaard, 2007). The end-feet of astrocytes are in close contact with pericytes and vascular SMCs, and astrocytes regulate the expansion of pericytes and vascular SMCs by secreting specific vasodilator factors. In this way, neurons regulate CBF according to their energy and metabolism needs (Venkat et al., 2016; Tarumi and Zhang, 2018).
BMECs, pericytes, and astrocytes work closely together to construct TJs, and the ECM secreted by BMECs and pericytes originates in the basement membranes of capillaries. The cerebral microvascular system, which is anatomically similar to neural networks, is a complex 3D network of microarterioles, venule vessels, and capillaries (Campisi et al., 2018; Park et al., 2019) that is functionally coupled to glial cells and neurons. In-depth assessments of BMECs and pericytes have revealed that their unique structural and functional changes are highly specialized and closely related to pathological processes (Zhang and Fisher, 2012). However, the molecular mechanisms of these changes are not clear. The highly complicated TJs between the adjacent cerebral microvascular BMECs on the BBB have a high resistance, approximately 1800–2000 Ω·cm2, which mainly restricts the pathway of paraventricular diffusion between BMECs (Sifat et al., 2017). In addition to this barrier function, TJs also maintain the polarity of BBB BMECs by restricting the movement of lipids and proteins between the surface of the apex and basolateral cells (Bagchi et al., 2019). The integrity of microvascular ECs in BMECs varies with the structure and function of the disease process, which depends mainly on changes in the local microenvironment (ion concentration disorder, inflammation, oxidation and nitrosative stress, enzyme activity, and angiogenesis) (Uwamori et al., 2019; Liu et al., 2021).
NVU coupling
NVU, the coupling of neural activity, and CBF are the focus of CNS pathology. CBF is important for maintaining the normal physiological function of the brain. The brain lacks energy reserves, and thus must regularly deliver oxygen and glucose to activated areas (Cai et al., 2019). Increased CBF can also contribute to the removal of potential toxic byproducts of brain activity (such as lactic acid, carbon dioxide, and tau), which is also important for brain temperature regulation (Muzik et al., 2018). Currently, there are two main theories regarding neurovascular coupling: those related to “feedback” and to “feedforward” mechanisms. Feedback is regulated by some metabolites, such as potent vasodilators, adenosine, carbon dioxide, hydrogen ions, and lactate, according to their own needs, whereas feedforward often leads to an excessive blood oxygen supply, and the mechanism is not clear (Alarcon-Martinez et al., 2020). Some recent studies have suggested that the feedforward mechanism induces excessive blood flow, while the feedback mechanism regulates CBF based on tissue metabolism demands. Although the mechanisms of neurovascular coupling are very complex, evidence indicates that it serves to maintain brain homeostasis.
Over the last several decades, studies have confirmed that neurons, astrocytes, BMECs, vascular SMCs, and pericytes are involved in neurovascular coupling (Noor et al., 2020). Neurons initiate local vascular responses following the release of neurotransmitters, such as nitric oxide, neuropeptides, prostaglandins, and adenosine, as well as changes in the cellular microenvironment (adenosine triphosphatase, K+, and hypoxia) (Nwokocha et al., 2020). Astrocytes are mainly responsible for the coupling of signal transduction. BMECs regulate blood flow in capillaries and arterioles via retrograde transmission. Finally, the contraction and relaxation of vascular smooth muscle is mediated by Ca2+ concentration, contractile proteins, and hyperpolarization, which further controls CBF (Kisler et al., 2017). Thus, each component in the NVU plays a positive role in the control of CBF.
The NVU mainly contains astrocytes, neurons, ECs, and pericytes. The interactions among these cells are very complex. As shown in Figure 2, it is important to clarify the interactions between cells and matrices when establishing an NVU model.
Figure 2.
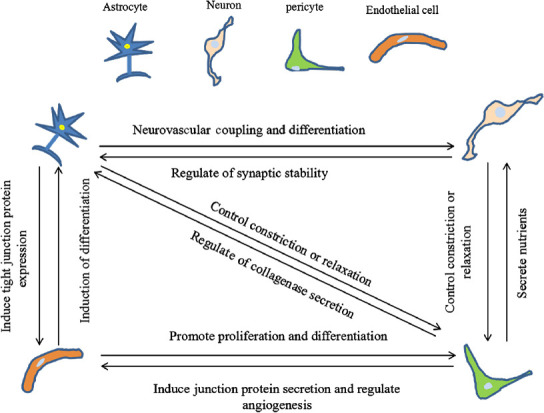
Interaction between cells in the neurovascular unit (NVU).
NVU coupling is important for studying the operation of the nervous system under different physiological and pathological conditions, especially in terms of the relationship between neurons, astrocytes, endothelial cells, pericytes, and blood vessels to reveal the mechanisms underlying central nervous system diseases in the microenvironment.
The Application of Stem Cells in the Neurovascular Unit
Previously, an in vitro BBB model was developed from primary and immortalized BMECs (Stebbins et al., 2016). In recent years, stem cell technology has been applied to the establishment of in vitro BBB models. Because they can differentiate into various cells in the NVU, and are used to model BBB pathology in simulations of the structure and function of the human NVU (Huang et al., 2020b), stem cells provide new opportunities for examining the molecular mechanisms of the NVU, as well as for screening CNS diseases. Therefore, in this review, we summarized the differentiation ability, culture conditions, advantages, and disadvantages of several stem cell approaches in the field of neurology. Examples of the application of stem cells in CNS diseases are shown in Additional Table 1.
Additional Table 1.
Differentiation, culture conditions, and clinical functions of three kinds of stem cells
| Stem cell | Type of differentiated cells | Specific culture condition | Clinical function | References |
|---|---|---|---|---|
| Embryonic stem cells | Motor neuron, dopaminergic neuron, cholinergic neuron, oligodendrocyte, gamma-aminobutyric acid neurons, Schwann cells, glutaminergic neurons | Retinoic acid, Sonic hedgehog, fibroblast growth factor 8, astrocyte, insulin, fibroblast growth factor 2, dibutylcyclic adenylate, neuromodulin 1β, N2 supplement, basic fibrobast growth factor, brain-derived neurotrophic factor | Amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s disease, Huntington’s disease | Dekmak et al., 2018; Glicksman, 2018; Sugaya and Vaidya, 2018 |
| Induced pluripotent stem cells | Astrocyte, neurons, microglia, iPS-ML/NEP2 | mTeSr medium, N2B27, B27 without vitamin A and heparin, β-mercaptoethanol, Glutamax, penicillin, streptomycin | Alzheimer’s disease, Amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease | Vatine et al., 2019; Kolagar et al., 2020; Pong et al., 2020; Tang et al., 2020 |
| Mesenchymal stem cells | Neurons, neuroectodermal like cells, astrocytes dimethyl sulfoxide, basic fibrobast growth factor, brain-derived neurotrophic factor, low lever laser therapy, astragaloside, danshansu, ligustrazine | All-trans retinoic acid, dimethyl sulfoxide, disease, Alzheimer’s disease, cerebral palsy in children, stroke | Spinal cord injury, Parkinson’s | Davidoff, 2019; Ross et al., 2019; Tsai et al., 2021 |
Embryonic stem cells
Embryonic stem cells (ESCs) derived from human or mouse fetal brains have been used to treat traumatic brain injury in various animal models (Dekmak et al., 2018). As ESCs can differentiate into many types of brain cells, they may provide new treatment options for various nervous system diseases (Sugaya and Vaidya, 2018). One recent study showed that vascular endothelial growth factor can induce human ESCs to differentiate into blood vessels with BBB characteristics, and that Wnt7a can further induce the maturation of vascular system components in cultured brain tissue (Liu et al., 2020b). However, in long-term culture, the strong expansion of blood vessels in brain organs is limited because of the low density of vascular-like structures. Moreover, the shape of the open loop in such systems, such as in a 4-month-old organic-like endothelium, may be distorted because of the lack of blood pressure (Omulecki et al., 2011). Besides, in in vitro culture, human ESCs do not self-renew, and they must be harvested from human embryos. Thus, there are some complex ethical questions regarding this type of research (Kolagar et al., 2020).
Gene targeting technology with mouse ESCs has become the “gold standard” for analyzing gene function and establishing disease models (Martin Gonzalez et al., 2018). This technique has been widely used in neurodegenerative disease research (Zhou et al., 2020), leading to the successful development of a new embryonic stem cell line from an AD model mouse containing three mutant genes (APPswe, TauP301L, and PS1M146V) with a complex genetic background. Overexpression of the mutant Presenilin 1 gene in human ESCs leads to synaptic electrophysiological abnormalities, and thus, this model can be used for AD disease research and drug development (Prè et al., 2014). Recently, MSCs-ESCs were successfully applied in an animal model of AD via intra-arterial injection, resulting in higher cell activity and an autophagy induction effect that matched that of bone marrow MSCs. Furthermore, ESCs could significantly inhibit the death of hippocampal cells induced by amyloid β-protein, promote the formation of autophagolysosomes to clear amyloid β-protein, and improve learning and memory in a mouse model of dementia (Wang et al., 2018).
Neural stem cells
Neural stem cells (NSCs) are multifunctional stem cells in the brain, and their potential for self-renewal is relatively low. Normally, NSCs can differentiate into neurons, and so they can be used as candidate cell lines for the production of NVU models in vitro, including microvasculature and brain tissue models (Stebbins et al., 2019). Despite the potential for establishing in vitro models using NSCs, their practical application has been limited by immune incompatibility and ethical problems related to allogeneic transplantation.
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) have potential for use in human cell models as an alternative to ESCs and NSCs. The main advantages of iPSCs are as follows: (1) they are associated with fewer ethical concerns compared to other options, (2) they can independently produce new iPSCs, and so can be transplanted without a risk of immune rejection (Glicksman, 2018), (3) they can be indefinitely differentiated into a large number of cell types in the NVU and are not restricted by mutation and cultivation, and (4) because they are based on a clear genetic background and susceptibility to disease, they can be used to establish an NVU model. Studies of BBB models have made great progress using iPSCs. For example, iPSCs were used to make human BMECs monolayers that were then used to evaluate the role of shear stress in regulating morphology, motility, proliferation, apoptosis, and protein and gene expression (Pong et al., 2020). Furthermore, iPSCs have been used to study many neurodegenerative diseases (AD, PD, and amyotrophic lateral sclerosis), specifically, to deconstruct changes in nerves and blood vessels during the course of the disease (Kolagar et al., 2020). For example, one in vitro evaluation of disease mechanisms used iPSC-derived neurons from sporadic AD patients replicated using a beta-amyloid precursor protein gene (Tang et al., 2020). The process of iPSC differentiation into ECs, neurons, astrocytes, and pericytes has been described in detail in terms of methods for measuring quart endothelial resistance and cell permeability (Vatine et al., 2019). However, iPSC technology has some major limitations. For instance, because of the inefficiency of viral infection and reprogramming in the production of these cells, there is a risk of tumorigenesis. Furthermore, there is a narrow experimental window when using iPSC-derived cells, as they tend to dedifferentiate very rapidly (several days after full differentiation) in vitro.
MSCs
MSCs are not only are easy to isolate, but can also be easily extracted from patient tissue without ethical concerns. As a result, MSCs are now commonly used to treat brain diseases. Growth factors secreted by MSCs are important for the repair of damaged neurons, unlike ESCs, which can induce tumor formation (Tsai et al., 2021). Based on these advantages, MSCs have great potential in stem cell tissue engineering for gene therapy, cancer biology, and other applications (Lah et al., 2020), which is of great significance for the construction of an in vitro NVU model. Pericytes, as one of the key cells in the NVU, surround capillaries and form TJs with ECs, which play an important role in maintaining the balance of material exchange and functional stability between nerves and blood vessels (Huang, 2020). Recent studies (Davidoff, 2019; Ross et al., 2019; Ahmed et al., 2020) have shown that MSCs are similar to pericytes (many cell phenotypic markers are the same), which suggests that MSCs can be used as a multifunctional stem cell type for differentiating pericytes. Uwamori et al. (Uwamori et al., 2019) described an in vitro BBB model of brain capillary ECs co-cultured with MSCs (a potential substitute for pericytes) that was used to study the contribution of MSCs to BBB structure and function. The contribution of this synapse solves the problem of primary cell culturing of pericytes, and MSCs can be used as a substitute for pericytes in various in vitro neurological studies (Huang et al., 2018; An et al., 2020).
The Application of Cell Co-culture in the Neurovascular Unit
A stable model comprising the main structural and functional cells of the NVU in vitro is very important for studying neurovascular diseases and evaluating the effects of therapeutic drugs. In addition to clinical and in vivo experiments, in-depth examinations of CNS diseases must be conducted in accordance with the culturing of nerve cells in vitro. In this review, we considered the experimental conditions, cell lines, and advantages and disadvantages of the prominent technical strategies for establishing NVU models in vitro, as shown in Additional Table 2.
Additional Table 2.
Technical strategies applied to the establishment of in vitro neurovascular unit (NVU) models
| Model | Materials of membrane | Cell types | Advantages | Limited | References |
|---|---|---|---|---|---|
| Transwell static | Poly tetra fluoroethylene, Porous polycarbonate (PC) | Rat brain endothelial cells GP8, RBE4, hCMEC/D3 | Easy to set up, moderately scalable, low-cost | Unable to simulate NVU dynamic model, lack of physiological shear stress to limit endothelial cell blood-brain barrier (BBB) phenotypic differentiation, relatively low endothelial resistance, and high permeability of hydrophilic substances | Bian et al., 2019; |
| Li et al., 2019; | |||||
| Brown et al., 2020; Rumianek and Greaves, 2020 | |||||
| Dynamic microfluidic device | PC | B.end3 endothelial cells or co-culture with astrocytes | Real-time visualization BBB and transmembrane endothelial resistance (TEER) | Weak ability to regulate microenvironment | Booth and Kim, 2014 |
| Dynamic synthetic microvascular device | Polydimethylsiloxane (PDMS) | RBE4 (intra luminal), astrocytes (extra luminal) | Low cost, simulated microcirculation environment, physiological fluid flow, shear stress, allow long-term cell culture, real-time optical monitoring | High throughput drug screening and TEER real-time detection are not available | Prabhakarpandian et al., 2013 |
| Dynamic NVU chip device | PC | RBE4, neurons, astrocytes and microglia | Low cost, realize NVU interaction | The apparatus is complicated and it is difficult to culture cells for a long time | Achyuta et al., 2013 |
| Dynamic New baby BBB chip device | PDMS | Rat brain endothelial cells, astrocytes | Simulation of shear flow in vivo environment, real-time visualization, direct measurement of dynamic process | Technology is not mature enough | Deosarkar et al., 2015 |
| Microfluidic-on-chip | PDMS chip, porous polycarbonate film | Human brain neurons, astrocytes, pericytes, and BMECs | Low reagent consumption, high throughput, easy integration. Controllable physical parameters, accurate simulation of central nervous system environment. Low cost fabrication, flexibility in the design, visualization of cells is possible, consider the effect of sheer stress. Immediate permeability measurements. Improvement in paracellular barrier functions | Highly specialized equipment, limited materials and expensive reagent consumption make it impossible to quantify cavity membrane shear stress and transendothelial resistance, lack of high-throughput, complex process technically, not ideal linear kinetic | Sankar et al., 2017; Mittal et al., 2019; Bhalerao et al., 2020; Guo et al., 2021; Staicu et al., 2021 |
| Three-dimensional printing | Gelatin/alginate hydrogel, swarm hydrogel, collagen, gelatin, fibrin, gellan gum, hyaluronan, self-assembling peptide, elastinlike polypeptide, polyethylene glycol | Neuroblastoma cell line (SH-SY5Y), induced pluripotent stem cells, and neural stem cells, Schwann cells | Quickly, high cost performance, complex geometric features, low cost, multi-material and multi-function, elastic, high resolution | High cell survival rate cannot be guaranteed. Bio-inks have a limited shelf life. A variety of cells in the system may cause an immune response. The mechanical strength of tissue scaffolds is low | Potjewyd et al., 2018; Fantini et al., 2019; Gong et al., 2020 |
| In-Silico | Short time, low cost, low risk. Real-time observation of the interaction between NVU cells. Quantized molecular signal | In vivo or in vitro verify are needed | Katara, 2014; Li et al., 2020a |
Static Transwell culture system
At present, given the associated convenience and maturity of the technology, the Transwell culture system is widely used to build BBB/NVU models (Brown et al., 2020). In this system, one or more types of nerve cells are cultured on either side of a semi-permeable microporous membrane, which enables the exchange of small molecular substances and prevents transmembrane migration (Bian et al., 2019). According to the types of cells cultured in a Transwell system, a BBB model can be divided into single-cell model, or a two-, three-, or four-cell co-culture model. Although the simplest BBB model in vitro is the single-cell model, in which BMECs are cultured on the upper side of the Transwell chamber, it lacks the coupling between ECs and other cells in the BBB (Bagchi et al., 2019). The main co-culture model for two cells is BMECs-astrocyte contact culture (Kulczar et al., 2017), which is more in line with the physiological structure of the BBB, and has improved BBB permeability (Stone et al., 2019). Methods for co-culturing three cells can be divided into BMECs-astrocytes-pericytes co-culture and BMECs-astrocytes-neurons co-culture models. BMECs are cultured on the upper side of the Transwell chamber, and astrocytes and pericytes are mixed and cultured on the underside of the Transwell model microporous membrane. This model more closely reflects the physiological structure of the BBB, and allows for direct contact between cells, as well as the exchange of growth factors required for cell growth and development (Stone et al., 2019). Co-culturing four cell types using a Transwell system, i.e., BMECs-astrocytes-pericytes-neurons, creates a simple NVU. In a recent study, researchers created a static four-dimensional system using the Transwell method by co-culturing four cell types to simulate an NVU in vitro. In their study, each cell type in the NVU was differentiated from the same donor iPSC source, yielding an isogenic model that could enable enhanced personalized modeling of the NVU for human health and disease research (Canfield et al., 2019). In another study, the researchers established an NVU using a four-dimensional model, and verified that hyodeoxycholic acid can protect oxygen glucose privatization and reoxygenation-induced injury in vitro (Li et al., 2019).
Because it is easy to establish, has moderate scalability, and is low cost, the Transwell system is used in a variety of complex research environments, including basic research and high-throughput screening (Rumianek and Greaves, 2020). However, this static in vitro model has many disadvantages, such as the lack of a general 3D model in vivo, low physiological shear stress, which limits the phenotypic differentiation of ECs in the BBB, relatively low endothelial resistance, and high permeability of hydrophilic substances. These limitations are considerable for NVU research (Katt et al., 2016).
Dynamic Transwell culture system
The shear stress generated by blood flow can promote the expression of some tight junction proteins (claudins, occludins, junction adhesion molecules, and zonula occludens) and transporters, enhance the barrier function of the BBB, and more closely mimic conditions in the NVU (Siddharthan et al., 2007). Therefore, the NVU dynamic model employs various materials and devices to simulate an environment for the cultured cells that is similar to blood flow in the body. Through its development, the NVU system has become more integrated and miniaturized.
In the dynamic NVU system, brain ECs are cultured in hollow fiber lumen and exposed to fluid flowing through an artificial capillary stent, while other cells are seeded in an outdoor compartment (Nishibori et al., 2020). Low permeability, high transendothelial resistance, negligible protein extravasation, specific transporters for polar molecules in closed chambers and ion channels, and ejection system expression are several significant advantages of the in vitro NVU (Lauranzano et al., 2019). Researchers using a 96-well plate microfluidic device found that synthetic drugs could penetrate the EC monolayer within 3 hours without causing cytotoxicity (Lee et al., 2018). However, this strategy is not suitable for high-throughput screening research because the experiment has poor operability and it is not possible to visualize the morphological or phenotypic changes of cells in the lumen (Agarwal et al., 2021).
Currently, many dynamic NVU in vitro models, devices, and materials are used in in vitro experiments, and the scope of application of the NVU has expanded. Therefore, it is difficult to systematically classify and describe the dynamic models. Here, we introduce the models that have been reported. First, a BBB system was generated via microfluidic device cultivation, and it could be optically imaged with good results. Booth and Kim (2014) used the C6 glial cell line to co-culture b.end3 cells in two channels, and the measured permeability coefficient was closely related to the brain-plasma ratio in vivo, which demonstrated that the model could predict the BBB drug clearance rate. Second, a synthetic microvascular device was created with a glass channel structure composed of glass and polydimethyl siloxane, which enabled simultaneous imaging inside and outside the chamber. Rat BMECs were cultured in the cavity under fluid shear conditions, and they continuously contacted the conditioned medium of astrocytes in the outer cavity (Prabhakarpandian et al., 2013). Third, an NVU-chip was created from a nerve module and a blood vessel module, which could be cultured separately and then assembled together to study the interaction between nerves and blood vessels (Achyuta et al., 2013). This NVU model could be used to examine brain tissue regeneration and screen for drug interference toxicity. Fourth, Deosarkar et al. (2015) developed an in vitro neonatal BBB model device. It comprised a chip consisting of a tissue chamber and a blood vessel channel placed side by side. Under shear flow conditions, newborn rat BMECs were inoculated in the blood vessel channel, astrocytes were cultured in the tissue chamber, and the two interacted through a porous interface.
Microfluidic-on-chip incubation
Microfluidic-on-chip incubation is an efficient tool that combines the disciplines of chemistry, physics, and bioengineering, and has the characteristics of low reagent consumption, high throughput, and easy integration (Liao et al., 2019). Currently, it can be used to simulate a variety of human organs in vitro, and liver chips (Meng et al., 2021), intestinal chips (Bein et al., 2018), and combination chips (Benam et al., 2020) have been developed. Organ chips enable experimenters to change local cellular, molecular, chemical, biological, and physical parameters in a controlled manner, increasing the accuracy of simulations of the microenvironment of the human body (Mittal et al., 2019). Because microfluidic chips provide precise control of cells and fluids on the microscale and enable multifunctional integration, they have been used to simulate the NVU microenvironment in various experiments (Staicu et al., 2021). After exposing ECs on microfluidic chips to brain tissue in living organisms, as well as improving the physical and chemical parameters of the BBB model, the resulting system is expected to provide an ideal 3D cell co-culture environment for studying the NVU (Bhalerao et al., 2020).
Given that it can be used to precisely control, detect, and manipulate features of the cellular microenvironment, the microfluidic chip has been widely used in biology and clinical medicine. It offers a new powerful strategy for researching the mechanisms of CNS disease (inflammatory response, tumor migration, and intervention) and related drug screening and delivery (Richardson et al., 2020). The other advantages of this model are its simple design, low cost, and BBB permeability. Furthermore, with this method, ECs, neurons, and capillaries do not undergo hemolysis (Bhalerao et al., 2020).
Microfluidic-on-chip incubation requires highly specialized equipment, and materials limit the development of this technology. For example, polyester has poor chemical absorption. Although polydimethyl siloxane is widely used in this field, the low adsorption of this compound makes it unsuitable for drug-related research (Sankar et al., 2017). Furthermore, the basement membrane collagen and matrix gel used to support cell culture and barrier construction are relatively expensive. As a final limitation, it is not possible to quantitatively study some core experimental parameters, such as cavity membrane shear stress and transendothelial resistance (Guo et al., 2021). However, as long as it is applied properly, the microfluidic chip provides great convenience for CNS research.
3D printing
3D printed NVU models produced via tissue engineering can be used to precisely analyze the significance of different cells with respect to functional abnormalities in neurovascular diseases. For instance, the interactions between different cell types and NVU cells, spatially distributed with biodyes based on hydrogel, can be examined in terms of neurovascular miscoupling, abnormal expression patterns and/or secretions of trophic factors from different cell types, and high-throughput drug screening. This method is conducive to examinations of cell migration and adhesion, vascular regeneration and neurogenesis, and the interactions between nerves and blood vessels. This method can incorporate the four main structural characteristics of the NVU: crosslinking, mechanics, porosity, and cell adhesion.
The conversion of matrix solutions to colloids requires physical or chemical interactions between molecules, physical processes through noncovalent bonding, and chemical processes through covalent bonding. When constructing a 3D NVU model, special attention should be paid to the oxidation-reduction reaction caused by chemical interactions, which produces free radicals and exposes cells to free radicals prematurely (Sivandzade and Cucullo, 2018). Another important factor when tissue engineering 3D NVU models is mechanical stress, which is related to the amount of CBF and the shear stress of the ECM that regulates the expression of TJ proteins and promotes the functional phenotype of NVU molecules (Sokolova et al., 2020). Physical and mechanical properties in living organisms can be simulated by choosing bioinks with similar properties. Studies have compared the selection and application of bioinks, with the goal of making the microenvironment in vitro more and more similar to the original ECM (Lougiaki et al., 2019). As discussed, the BBB is the core of the NVU structure, and BBB research is very important for understanding brain diseases and drug screening. The porosities of bioinks in vitro directly determine the permeability of the model, and pore size plays an important role in promoting angiogenesis and neuronal differentiation (Sivandzade and Cucullo, 2018). The characteristics of cell adhesion are very important for maintaining the stability of the microenvironment of the NVU model. Adhesion between specific receptors on the cell membrane and the surrounding environment maintains the interaction between the cell and the environment. This is usually modulated by amino acid sequences Arg-Gly-Asp and Ile-Lys-Val-Ala-Val, the former is the binding site of many different basement membrane proteins, and the latter is found in laminin glycoproteins and promotes axonal growth, synaptic stability, and BBB characteristics (Sokolova et al., 2020).
Because of its complex structure and function, it is difficult to establish a complete in vitro model of the NVU. Most methods have focused on co-culturing some components of BBB cells, namely, BMECs, pericytes, astrocytes, and vascular SMCs, with neurons. These methods cannot directly simulate holistic changes in the NVU, but can be used to effectively study the characteristics of the BBB while avoiding the methodological challenges associated with co-culturing five types of cells. A recent reported indicated that a NVU biological model constructed via 3D printing had good viability (Fantini et al., 2019). The core of 3D printing technology is bioassembly. To establish a complete and systematic NVU model, it is very important that the vascular system of the NVU constitutes an interactive network that is organized based on the characteristics of cell recombination. There have been many reports on tissue engineering bioprinting technology (Abdollahiyan et al., 2020; Matai et al., 2020), and these can generally be divided into two categories: indirect bioprinting technology and direct bioprinting technology. The application of these techniques was made possible by the emergence of multilayered 3D NVU models, with far-reaching implications for the study of cell-to-cell interactions, especially in vitro interactions between cells and vascular systems. A promising area in the development of NVU models is biological processing via 3D biological tissues. A multicomponent NVU can be prepared via 3D printing, and the contribution of different cell types to neurovascular function and dysfunction can be studied at the molecular and cellular levels (Figure 3).
Figure 3.
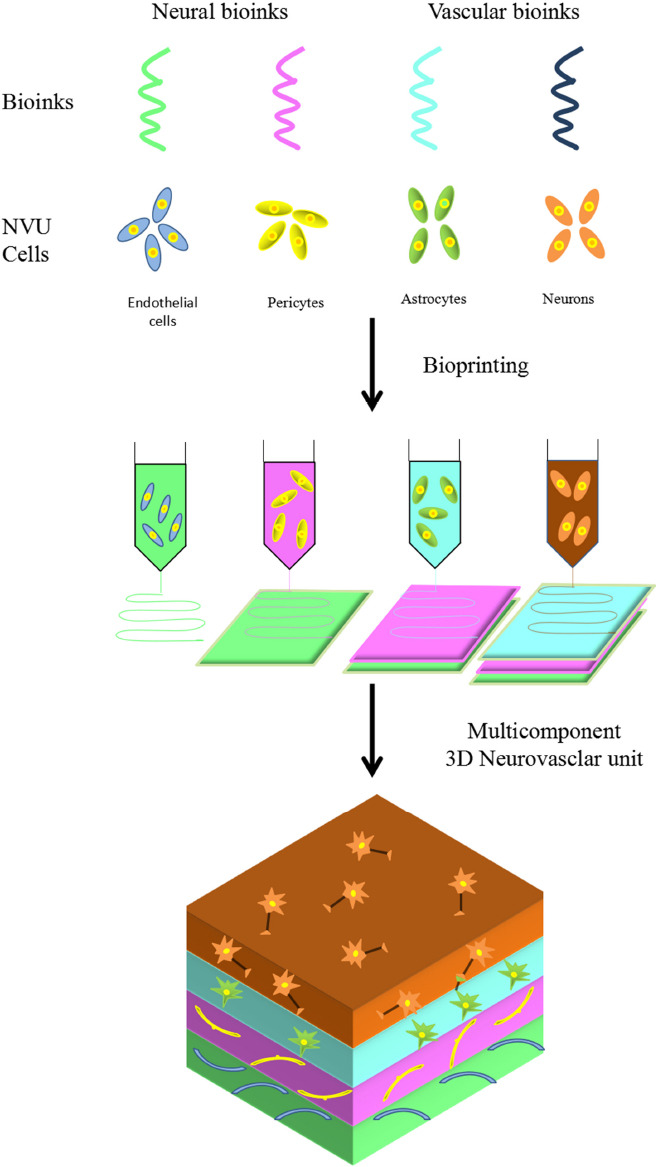
Pattern of three-dimensional (3D) printing for an neurovascular unit (NVU) model.
Different types of NVU cells can be combined with specialized biomaterials called bioink, and then bioprinted to produce multicomponent 3D NVU models. Nerve cells and vascular cells can be encapsulated in the biological inks. Bioimprinting can accurately position nerve cells and vascular cells to form appropriate interfaces for simulating an in vivo model.
3D printing has led to achievements in the prevention, diagnosis, and treatment of neurological diseases. It can reproduce the specific anatomy of complex intracranial tumors for neurosurgery with high accuracy, allowing surgical procedures to be simulated in vitro, and thus improving the effects of intracranial tumor treatment. Furthermore, damaged host axons can regenerate on 3D-printed bionic scaffolds to form synapses on human neural precursor cells implanted in the device, and these axons can extend out of the scaffold and enter the host spinal cord under the injured region to restore synapses (Kosterhon et al., 2020). NVU dysfunction is a central element of the pathogenesis of stroke and AD. 3D printing can be used to screen drugs to improve neurological dysfunction caused by stroke, AD, and other dementias (Potjewyd et al., 2018). Additionally, 3D printing-based approaches offer compatibility with 3D scanning, computer modeling, enhanced selection of input material, and increased control over hierarchical integration, and clinical 3D-printed implants can be used to treat neurological diseases and injuries (Joung et al., 2020). For example, a composite conduit filled with Engelbreth-Holm Swarm hydrogel was found to promote the repair of peripheral nerves, and thus may become a promising way to treat peripheral nerve defects (Gong et al., 2020).
There are many challenges involved in the application of 3D printing technology to NVU research (Potjewyd et al., 2018). First, new types of bio-ink must be developed with mechanical, rheological, chemical, and biological properties that meet the requirements for printing organs and tissues. Because tissues with diverse printing functions and complex structures usually require simultaneous printing of multiple types of cells, the lack of standardized bio-inks means that researchers cannot guarantee the survival rate of cells during the printing process. Second, the viability of bio-inks is limited, and long-term storage is extremely difficult, which greatly limits the application and development of 3D printing. Third, the source of cell donors for 3D printing technology may affect the immune response of the recipient. Therefore, the source of primary cells is another limitation affecting the popularity of this technology.
In-Silico Approach
Computational biology is a new field that has emerged in recent decades. It integrates pharmacology through assisted drug design and delivery. This technology is becoming more and more closely related to CNS disease and drug research. In drug design, the traditional methods are time-consuming, risky, and costly. Katara (Katara, 2014) specifically describes the role of bioinformatics and pharmacogenomics in drug design and delivery. Pharmacogenomics refers to the effects of single nucleotide polymorphisms and copy number variation on drug response. Knowledge regarding single nucleotide polymorphisms and copy number variation can facilitate selection of the best drug, dosage, and course of treatment, and reduce the chance of adverse drug reactions (Qidwai, 2020). Donson et al. (Donson et al., 2018) used in silico models to predict drug sensitivity for ependymoma-related compounds (using dose curves and time-course models). According to drug genome data, P-glycoprotein has been selected as a therapeutic target for optimizing drug delivery in the CNS (Pulido et al., 2020). PharmGKB (https://www.pharmgkb.org/) is a pharmacogenomics resource that provides information about clinical gene-drug associations and gene-phenotype associations (Mlakar et al., 2016; Han et al., 2018). STITCH 5.0 (http://stitch.embl.de/) is a searchable database that uses text mining to summarize information about metabolic pathways, drug-target relationships, and structural similarities (Li et al., 2020a). TTD (http://db.idrblab.net/ttd/) is a therapeutic target database that provides information about known proteins and nucleic acids (Cui et al., 2020). The applications, advantages, and disadvantages of the above-mentioned databases, as well as examples of their application in CNS diseases, are summarized in Additional Table 3. The main advantage of bioinformatics is the use of data mining to classify biological activities and potential candidate drugs and to predict and identify their biological phenomena. The storage and analysis of these massive amounts of data is not simple, and scientists have come up with a solution called cloud computing.
Additional Table 3.
Introduce in detail several databases used in neurovascular unit (NVU) research
| Database | URL | Application category | Advantages and drawbacks | References |
|---|---|---|---|---|
| PharmGKB | https://www.pharmgkb.org/ | Literature data extraction; gene-drug data extraction; | The most complete genotype and phenotype information database related to the drug genome | Mlakar et al., 2016; |
| Clinical annotation and implementation | Han et al., 2018 | |||
| STITCH 5.0 | http://stitch.embl.de/ | Retrieve and predict the interaction between chemicals and proteins; Prediction of 3D molecular models | Structure-guided memetic, cellular, and multiscale evolutionary algorithm for mapping protein conformation spaces | Li et al., 2020a |
| TTD | http://db.idrblab.net/ttd/ | Provide information about known and yet to be explored therapeutic protein and nucleic acid targets, targeted diseases, and pathways | Widely used and updated in a timely manner, but there is less information in the database | Cui et al., 2020 |
| NeuroMorphoVis | https://github.com/BlueBrain/NeuroMorphoVis | Visualization, analysis and automated repair of digitally reconstructed neuronal morphology skeletons from optical microscopy stacks; building highly realistic three-dimensional neuronal somata on a physically plausible basis; creating high fifidelity polygonal mesh models of neurons using the repaired morphology skeletons; Creating high resolution volumetric models of neurons that express their optical and spectroscopic characteristics | Provide an analysis and visualization of the neuron morphology skeleton construction system; subject to the limitation of resolution | Abdellah et al., 2018; Cui et al., 2020; Abdellah et al., 2021 |
In silico models are also valuable for capturing the “virtual tissue model” of embryonic BBB development or in vitro platforms representing all NVU cells. For example, Nyúl-Tóth et al. (2016) described the vascular regeneration process of cerebral vessels using virtual in silico results. This technique enables researchers to predict the possibility of future tissue toxicity using a minimal number of animal experiments. For example, an in silico model was used to predict the binding of tanshinone IIA and cryptotanshinone to nuclear factor-k-gene binding in vitro, which could improve symptoms in patients with AD, and this was validated by nongenetic mouse experiments (Maione et al., 2018).
Establishing computational models will help us to understand how the brain performs computations, although high-fidelity data regarding neural cell morphology will be required. For this purpose, Abdellah et al. (2018) has established NeuroMorphoVis (https://github.com/BlueBrain/NeuroMorphoVis), which consists of five main modules: (I) data processing, (II) simulation of 3D somatic cell contours, (III) restoration and analysis of the morphological skeleton, (IV) creation of polygon surface mesh, and (V) reconstruction of volume models that reflect skeletal geometry. The team promoted the development of morphological research on neurons using in silico models (Abdellah et al., 2021).
Similar to in vitro models, in silico NVU models enable real-time observation of intercellular interactions leading to NVU/BBB development, and they also offer characterization and quantification of molecular signaling events behind cell behavior. There are many differences in the timing of BBB formation between rodents (the main source of BBB development data) and humans. Recognizing this difference should help researchers focus on the advantages of existing animal model data (describing molecular interactions and sequences of events during early embryonic development) and prevent overprediction regarding human outcomes. At this stage, the in silico model cannot be considered a stand-alone tool because further in vivo and in vitro studies are needed to validate the results and/or improve the assumptions underlying the original algorithm.
The Application of Noninvasive Imaging Techniques to the Neurovascular Unit
Noninvasive imaging techniques such as MRI and PET are widely used in animal experiments and clinical diagnosis to assess the structure and function of the NVU. New imaging techniques have also emerged that combine assessments of function, imaging, and anatomy. These include blood oxygenation level dependent MRI, diffusion tensor imaging, and perfusion functional magnetic resonance imaging (fMRI), which can involve diffusion weighted imaging and magnetic resonance spectroscopy (Netto et al., 2018). fMRI has a high temporal resolution and spatial resolution, enabling real-time, repeatable dynamic observation in noninvasive situations. This technology has been useful in clinical applications. fMRI has been use to image various whole-body systems, especially the brain, and can be used for coronal, sagittal, and cross-sectional imaging (Shukla et al., 2017). It is also widely used in animal experiments. fMRI can be used to detect morphological changes in astrocytes and capillary coverage in the hippocampus (Anzabi et al., 2018). Structural changes in the NVU (BBB integrity and the state of microvasculature) can also be diagnosed using fMRI techniques, further guiding clinical treatment (Zhao et al., 2019). MRI usually involves T1-weighted imaging to detect the extravasation of the damaged BBB (Kidwell et al., 2004). As technology continues to evolve, increased imaging resolution and sophistication of software systems enable the use of MRI to monitor NVU components with more sensitivity and accuracy (Hsu et al., 2018).
PET
In addition to measuring CBF and glucose metabolism in the brain, PET can now be used to quantitatively analyze other metabolic parameters, such as receptor occupancy and endogenous release (Judenhofer et al., 2008; Boss et al., 2010). With respect to the NVU, researchers aim to objectively monitor dynamic changes in neurons, glial cells, microvascular systems, and barrier structures. Although fluorodeoxyglucose (FDG) PET can indirectly reflect synaptic function by showing the uptake of 18 F-FDG by neurons and glia (Chen, 2007), PET has certain limitations in accuracy and positioning. However, the multimodal application of PET/CT enables the integration of detailed anatomical information and PET data with CT imaging. The resulting information regarding changes in metabolism and molecular levels in the body is more substantial than the sum of the data obtained using the two separate imaging modalities (Keogh et al., 2018). Combined PET/MRI is used in brain neuroscience research; the good resolution and tissue contrast provided by MRI can be combined with spectroscopy analyses to help detect changes in metabolic function (Herholz, 2003). These techniques can be used for the localization of epileptic foci, early and differential diagnosis of AD, evaluation of PD, and judgments regarding tissue damage and survival after cerebral infarction.
AD, vascular dementia, frontotemporal lobe degeneration, and Lewy body dementia can be differentiated using 18 F-FDG PET according to the uptake of 18 F-FDG in different brain regions. For example, typical AD patients show a symmetrical decrease in 18 F-FDG uptake in the bilateral temporal and parietal lobes, while those with vascular dementia exhibit diffuse decreased uptake of 18 F-FDG in the cerebral cortex and subcortical areas, individuals with frontotemporal lobe degeneration display an asymmetric decrease in 18 F-FDG uptake in the bilateral frontotemporal lobes, and only the occipital lobe is involved in Lewy body dementia (Gallivanone et al., 2016). 18 F-FDG PET is helpful in identifying mild cognitive impairment and predicting the development of AD with higher sensitivity and specificity (Ding et al., 2019; Chung et al., 2020; Li et al., 2020b). Furthermore, FDG-PET has been used to detect inflammation associated with FDG absorption (platelets) in patients with early stroke, and this has facilitated the prevent of recurrent stroke (Jacobs et al., 2005). The combination of PET with other modalities has provided a unique opportunity to link neurochemistry with hemodynamics, and has facilitated research regarding NVU neurovascular coupling in the brain. PET/MRI imaging has been successfully applied to the monitoring of intracranial tumors (Wey et al., 2014), the staging of gliomas, and the early study of dementia, mild cognitive impairment, degenerative changes, brain functional nuclei, and other topics (Almansory and Fraioli, 2019; Zhu and Zhu, 2019; Frantellizzi et al., 2021).
Dynamic contrast-enhanced MRI and dynamic susceptibility contrast MRI
NVU dysfunction, including changes in BBB permeability, is implicated in a variety of conditions including vascular cognitive impairment, dementia, Binswanger’s disease, stroke, type 2 diabetes, and aging (Yu et al., 2020). MRI can clearly show increases in perivascular space, subcortical infarction, and cerebral microbleeds (Debette et al., 2019). As a type of multi-dimensional MRI, dynamic contrast-enhanced MRI (DCE-MRI) has become a preferred imaging technique for evaluating BBB disruption at all levels of BBB leakiness (Brighi et al., 2020; Houston et al., 2020). It has been used for quantitative analysis of cerebrovascular lesions in various animal models, such as focal cerebral ischemia and tumor, and clinically for BBB transfer rate analysis of focal ischemia, multiple sclerosis, and cerebral cavernous malformation (Piotrowski et al., 2020). In recent years, BBB leakage is considered to play an important role in the early stage and progression of AD, although many in vivo studies have failed to detect signs of AD-related BBB decomposition, even with the application of DCE-MRI (Dickie et al., 2019). This suggests that AD-induced damage to the BBB is subtle, and that these negative results are due to the low sensitivity of measurement methods (Nation et al., 2019).
Dynamic susceptibility contrast MRI (DSC-MRI) is a gadolinium-based contrast agent perfusion imaging technology that is commonly used in the clinical detection of brain tumors (Huhndorf et al., 2016). When the contrast agent passes through a blood vessel and enters the extravascular space, it changes the T1, T2, and T2* relaxation rates of the tissue fluid, and thus changes the intensity of the MR signal. Unlike DCE-MRI, in which changes in tissue T1 are evaluated after the contrast agent is injected, DSC-MRI mainly depends on T2 and T2* changes for evaluation (Kang et al., 2021; Sanders et al., 2021). When assessing the growth and treatment of brain tumors, tissue perfusion, tissue permeability (Ktrans), and extravascular extracellular volume fraction (νe) are the main parameters used to analyze DCE-MRI data (Lingala et al., 2020). Blood volume, blood flow, and mean transit time are the main DSC-MRI parameters used to evaluate the hemodynamics of brain, breast, and prostate tumors. Moreover, blood volume and blood flow data obtained by DSC-MR ae correlated with tumor grade and treatment response (Manning et al., 2020). A recent study showed that under the same physiological conditions of Ktrans and νe, the sensitivity of DSC-MRI was the same as that of DCE-MRI (Wu et al., 2009).
Angiography
Angiography is used to measure the degree of arterial stenosis (Dowd, 2021). The way in which the degree of arterial stenosis is defined in patients with acute ischemic stroke is significant for determining the efficacy of thrombolytic therapy (Tomkins et al., 2016). Angiography involves a non-penetrating X-ray that enables intuitive assessment of dynamic changes in brain blood vessels (Frösen et al., 2019). For diagnosing spontaneous intracranial subarachnoid hemorrhage, CT angiography and digital subtraction angiography have a better diagnostic rate than MRI (Ghoneim et al., 2020). The visualization of 3D reconstruction using CT angiography to analyze morphological parameters in 638 patients with MCA aneurysms was important for assessing the condition and risk in patients with brain damage (Lang et al., 2020). This technique has also been used in animal experiments, such as in an experimental carotid arteriovenous fistula (AVF) model in minipigs, and the application of angiography to the monitoring of CBF changes revealed that extracranial arterial production could be induced, thereby promoting the repair process after ischemic brain injury (Ideguchi et al., 2017).
Conclusion and Final Remarks
This paper examined the relationships between NVU components. We comprehensively and systematically introduced current methods of clinical diagnosis, treatment, and drug screening in the study of brain CNS diseases in vivo and in vitro. Noninvasive imaging technology is of great significance for modern encephalopathy research. In addition to the application of MRI, CT, angiography, and others in the diagnosis of clinical patients, these techniques are also important in the monitoring and characterization of many CNS diseases.
Stem cells are increasingly used as cell sources for in vitro experiments. In recent years, human induced pluripotent stem cells have enabled remarkable success in neurodegenerative disease and stroke research. Accordingly, new technologies have been developed, including dynamic in vitro BBB, NVU microfluidic chip technology, and 3D printing technology. These technologies and strategies will likely expand to the mainstream of research on the structure and function of the BBB and NVU, with a focus on drug metabolism, absorption, and transportation, the differentiation and proliferation of stem cells, and the proliferation of cancer cells to other tissues.
This review systematically discussed various technologies used to explore the NVU. However, some technologies are still undergoing rapid development, and there are many drawbacks, such as the restriction of biomimetic materials, technical bottlenecks, and ethical supervision. For emerging technologies in particular, specific patterns can be employed in CNS research, but technical barriers and adaptability will need to be continuously assessed.
Additional files:
Additional Table 1: Differentiation, culture conditions, and clinical functions of three kinds of stem cells.
Additional Table 2: Technical strategies applied to the establishment of in vitro neurovascular unit (NVU) models.
Additional Table 3: Introduce in detail several databases used in neurovascular unit (NVU) research.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewer: Ratnakar TRipathi, University of Missouri, USA.
Funding: This work was financially supported by the National Natural Science Foundation of China, Nos. 82104412 (to TD), 81873023 (to JW); Natural Science Basic Research Program of Shaanxi Province of China, No. 2020JQ-865 (to TD); Education Department of Shaanxi Province of China, No. 20JK0597 (to TD); and the Subject Innovation Team of Shaanxi University of Chinese Medicine of China, No. 2019-QN02 (to PW).
P-Reviewer: TRipathi R; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Abdellah M, Hernando J, Eilemann S, Lapere S, Antille N, Markram H, Schürmann F. NeuroMorphoVis: a collaborative framework for analysis and visualization of neuronal morphology skeletons reconstructed from microscopy stacks. Bioinformatics. 2018;34:i574–i582. doi: 10.1093/bioinformatics/bty231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdellah M, Foni A, Zisis E, Guerrero NR, Lapere S, Coggan JS, Keller D, Markram H, Schürmann F. Metaball skinning of synthetic astroglial morphologies into realistic mesh models for visual analytics and in silico simulations. Bioinformatics. 2021;37:i426–i433. doi: 10.1093/bioinformatics/btab280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdollahiyan P, Oroojalian F, Mokhtarzadeh A, de la Guardia M. Hydrogel-based 3D bioprinting for bone and cartilage tissue engineering. Biotechnol J. 2020;15:e2000095. doi: 10.1002/biot.202000095. [DOI] [PubMed] [Google Scholar]
- 4.Achyuta AK, Conway AJ, Crouse RB, Bannister EC, Lee RN, Katnik CP, Behensky AA, Cuevas J, Sundaram SS. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip. 2013;13:542–553. doi: 10.1039/c2lc41033h. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal S, Sair HI, Pillai JJ. The problem of neurovascular uncoupling. Neuroimaging Clin N Am. 2021;31:53–67. doi: 10.1016/j.nic.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed TA, Shousha WG, Abdo SM, Mohamed IK, El-Badri N. Human adipose-derived pericytes: biological characterization and reprogramming into induced pluripotent stem cells. Cell Physiol Biochem. 2020;54:271–286. doi: 10.33594/000000219. [DOI] [PubMed] [Google Scholar]
- 7.Al-Qattan MM, Abd-Alwahed MM, Shier MK. Effect of TGFβ1 and nAG on astrocyte cultures: a study of astrocyte proliferation and the expression of GFAP, CSPG4, S100B and IL-6. J Coll Physicians Surg Pak. 2018;28:928–933. doi: 10.29271/jcpsp.2018.12.928. [DOI] [PubMed] [Google Scholar]
- 8.Alarcon-Martinez L, Villafranca-Baughman D, Quintero H, Kacerovsky JB, Dotigny F, Murai KK, Prat A, Drapeau P, Di Polo A. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature. 2020;585:91–95. doi: 10.1038/s41586-020-2589-x. [DOI] [PubMed] [Google Scholar]
- 9.Allen NJ, Lyons DA. Glia as architects of central nervous system formation and function. Science. 2018;362:181–185. doi: 10.1126/science.aat0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almansory KO, Fraioli F. Combined PET/MRI in brain glioma imaging. Br J Hosp Med (Lond) 2019;80:380–386. doi: 10.12968/hmed.2019.80.7.380. [DOI] [PubMed] [Google Scholar]
- 11.An H, Li Q, Wen J. Bone marrow mesenchymal stem cells encapsulated thermal-responsive hydrogel network bridges combined photo-plasmonic nanoparticulate system for the treatment of urinary bladder dysfunction after spinal cord injury. J Photochem Photobiol B. 2020;203:111741. doi: 10.1016/j.jphotobiol.2019.111741. [DOI] [PubMed] [Google Scholar]
- 12.Anzabi M, Ardalan M, Iversen NK, Rafati AH, Hansen B, Østergaard L. Hippocampal atrophy following subarachnoid hemorrhage correlates with disruption of astrocyte morphology and capillary coverage by AQP4. Front Cell Neurosci. 2018;12:19. doi: 10.3389/fncel.2018.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arsava EM, Arat A, Topcuoglu MA, Peker A, Yemisci M, Dalkara T. Angiographic microcirculatory obstructions distal to occlusion signify poor outcome after endovascular treatment for acute ischemic stroke. Transl Stroke Res. 2018;9:44–50. doi: 10.1007/s12975-017-0562-2. [DOI] [PubMed] [Google Scholar]
- 14.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagchi S, Chhibber T, Lahooti B, Verma A, Borse V, Jayant RD. In-vitro blood-brain barrier models for drug screening and permeation studies: an overview. Drug Des Devel Ther. 2019;13:3591–3605. doi: 10.2147/DDDT.S218708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 17.Bein A, Shin W, Jalili-Firoozinezhad S, Park MH, Sontheimer-Phelps A, Tovaglieri A, Chalkiadaki A, Kim HJ, Ingber DE. Microfluidic organ-on-a-chip models of human intestine. Cell Mol Gastroenterol Hepatol. 2018;5:659–668. doi: 10.1016/j.jcmgh.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benakis C, Garcia-Bonilla L, Iadecola C, Anrather J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front Cell Neurosci. 2014;8:461. doi: 10.3389/fncel.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benam KH, Novak R, Ferrante TC, Choe Y, Ingber DE. Biomimetic smoking robot for in vitro inhalation exposure compatible with microfluidic organ chips. Nat Protoc. 2020;15:183–206. doi: 10.1038/s41596-019-0230-y. [DOI] [PubMed] [Google Scholar]
- 20.Bennett C, Mohammed F, Álvarez-Ciara A, Nguyen MA, Dietrich WD, Rajguru SM, Streit WJ, Prasad A. Neuroinflammation, oxidative stress, and blood-brain barrier (BBB) disruption in acute Utah electrode array implants and the effect of deferoxamine as an iron chelator on acute foreign body response. Biomaterials. 2019;188:144–159. doi: 10.1016/j.biomaterials.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhalerao A, Sivandzade F, Archie SR, Chowdhury EA, Noorani B, Cucullo L. In vitro modeling of the neurovascular unit: advances in the field. Fluids Barriers CNS. 2020;17:22. doi: 10.1186/s12987-020-00183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bian Y, Du Y, Wang R, Chen N, Du X, Wang Y, Yuan H. A comparative study of HAMSCs/HBMSCs transwell and mixed coculture systems. IUBMB Life. 2019;71:1048–1055. doi: 10.1002/iub.2074. [DOI] [PubMed] [Google Scholar]
- 24.Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18:727–740. doi: 10.1038/nrn.2017.125. [DOI] [PubMed] [Google Scholar]
- 25.Booth R, Kim H. Permeability analysis of neuroactive drugs through a dynamic microfluidic in vitro blood-brain barrier model. Ann Biomed Eng. 2014;42:2379–2391. doi: 10.1007/s10439-014-1086-5. [DOI] [PubMed] [Google Scholar]
- 26.Boss A, Bisdas S, Kolb A, Hofmann M, Ernemann U, Claussen CD, Pfannenberg C, Pichler BJ, Reimold M, Stegger L. Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. J Nucl Med. 2010;51:1198–1205. doi: 10.2967/jnumed.110.074773. [DOI] [PubMed] [Google Scholar]
- 27.Brighi C, Reid L, Genovesi LA, Kojic M, Millar A, Bruce Z, White AL, Day BW, Rose S, Whittaker AK, Puttick S. Comparative study of preclinical mouse models of high-grade glioma for nanomedicine research: the importance of reproducing blood-brain barrier heterogeneity. Theranostics. 2020;10:6361–6371. doi: 10.7150/thno.46468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown JA, Faley SL, Shi Y, Hillgren KM, Sawada GA, Baker TK, Wikswo JP, Lippmann ES. Advances in blood-brain barrier modeling in microphysiological systems highlight critical differences in opioid transport due to cortisol exposure. Fluids Barriers CNS. 2020;17:38. doi: 10.1186/s12987-020-00200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caffrey TM, Button EB, Robert J. Toward three-dimensional in vitro models to study neurovascular unit functions in health and disease. Neural Regen Res. 2021;16:2132–2140. doi: 10.4103/1673-5374.310671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai R, Zhang Y, Simmering JE, Schultz JL, Li Y, Fernandez-Carasa I, Consiglio A, Raya A, Polgreen PM, Narayanan NS, Yuan Y, Chen Z, Su W, Han Y, Zhao C, Gao L, Ji X, Welsh MJ, Liu L. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J Clin Invest. 2019;129:4539–4549. doi: 10.1172/JCI129987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018;180:117–129. doi: 10.1016/j.biomaterials.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canfield SG, Stebbins MJ, Faubion MG, Gastfriend BD, Palecek SP, Shusta EV. An isogenic neurovascular unit model comprised of human induced pluripotent stem cell-derived brain microvascular endothelial cells, pericytes, astrocytes, and neurons. Fluids Barriers CNS. 2019;16:25. doi: 10.1186/s12987-019-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caporarello N, D’Angeli F, Cambria MT, Candido S, Giallongo C, Salmeri M, Lombardo C, Longo A, Giurdanella G, Anfuso CD, Lupo G. Pericytes in microvessels: from “mural” function to brain and retina regeneration. Int J Mol Sci. 2019;20:6351. doi: 10.3390/ijms20246351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W. Clinical applications of PET in brain tumors. J Nucl Med. 2007;48:1468–1481. doi: 10.2967/jnumed.106.037689. [DOI] [PubMed] [Google Scholar]
- 35.Chung JH, Kang SY, Wu HG, Seo YS, Kim DW, Kang KW, Kim HJ, Cheon GJ. Risk stratification of symptomatic brain metastases by clinical and FDG PET parameters for selective use of prophylactic cranial irradiation in patients with extensive disease of small cell lung cancer. Radiother Oncol. 2020;143:81–87. doi: 10.1016/j.radonc.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Cui Q, Zhang YL, Ma YH, Yu HY, Zhao XZ, Zhang LH, Ge SQ, Zhang GW, Qin XD. A network pharmacology approach to investigate the mechanism of Shuxuening injection in the treatment of ischemic stroke. J Ethnopharmacol. 2020;257:112891. doi: 10.1016/j.jep.2020.112891. [DOI] [PubMed] [Google Scholar]
- 37.Davidoff MS. The pluripotent microvascular pericytes are the adult stem cells even in the testis. Adv Exp Med Biol. 2019;1122:235–267. doi: 10.1007/978-3-030-11093-2_13. [DOI] [PubMed] [Google Scholar]
- 38.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. 2019;76:81–94. doi: 10.1001/jamaneurol.2018.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dekmak A, Mantash S, Shaito A, Toutonji A, Ramadan N, Ghazale H, Kassem N, Darwish H, Zibara K. Stem cells and combination therapy for the treatment of traumatic brain injury. Behav Brain Res. 2018;340:49–62. doi: 10.1016/j.bbr.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Deosarkar SP, Prabhakarpandian B, Wang B, Sheffield JB, Krynska B, Kiani MF. A novel dynamic neonatal blood-brain barrier on a chip. PLoS One. 2015;10:e0142725. doi: 10.1371/journal.pone.0142725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickie BR, Vandesquille M, Ulloa J, Boutin H, Parkes LM, Parker GJM. Water-exchange MRI detects subtle blood-brain barrier breakdown in Alzheimer’s disease rats. Neuroimage. 2019;184:349–358. doi: 10.1016/j.neuroimage.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7:272–281. doi: 10.1016/S2215-0366(19)30302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Y, Sohn JH, Kawczynski MG, Trivedi H, Harnish R, Jenkins NW, Lituiev D, Copeland TP, Aboian MS, Mari Aparici C, Behr SC, Flavell RR, Huang SY, Zalocusky KA, Nardo L, Seo Y, Hawkins RA, Hernandez Pampaloni M, Hadley D, Franc BL. A deep learning model to predict a diagnosis of alzheimer disease by using (18)F-FDG PET of the brain. Radiology. 2019;290:456–464. doi: 10.1148/radiol.2018180958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong T, Chen N, Ma X, Wang J, Wen J, Xie Q, Ma R. The protective roles of L-borneolum, D-borneolum and synthetic borneol in cerebral ischaemia via modulation of the neurovascular unit. Biomed Pharmacother. 2018;102:874–883. doi: 10.1016/j.biopha.2018.03.087. [DOI] [PubMed] [Google Scholar]
- 45.Donson AM, Amani V, Warner EA, Griesinger AM, Witt DA, Levy JMM, Hoffman LM, Hankinson TC, Handler MH, Vibhakar R, Dorris K, Foreman NK. Identification of FDA-approved oncology drugs with selective potency in high-risk childhood ependymoma. Mol Cancer Ther. 2018;17:1984–1994. doi: 10.1158/1535-7163.MCT-17-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dörfel MJ, Huber O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J Biomed Biotechnol. 2012;2012:807356. doi: 10.1155/2012/807356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dowd CF. Cerebral angiography: techniques and practice. Handb Clin Neurol. 2021;176:107–119. doi: 10.1016/B978-0-444-64034-5.00006-7. [DOI] [PubMed] [Google Scholar]
- 48.Fantini V, Bordoni M, Scocozza F, Conti M, Scarian E, Carelli S, Di Giulio AM, Marconi S, Pansarasa O, Auricchio F, Cereda C. Bioink composition and printing parameters for 3D modeling neural tissue. Cells. 2019;8:830. doi: 10.3390/cells8080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrini F, Trang T, Mattioli TA, Laffray S, Del’Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl⁻ homeostasis. Nat Neurosci. 2013;16:183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frantellizzi V, Conte M, De Vincentis G. Hybrid imaging of vascular cognitive impairment. Semin Nucl Med. 2021;51:286–295. doi: 10.1053/j.semnuclmed.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Frösen J, Cebral J, Robertson AM, Aoki T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus. 2019;47:E21. doi: 10.3171/2019.5.FOCUS19234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallivanone F, Della Rosa PA, Castiglioni I. Statistical voxel-based methods and [18F]FDG PET brain imaging: frontiers for the diagnosis of AD. Curr Alzheimer Res. 2016;13:682–694. doi: 10.2174/1567205013666151116142039. [DOI] [PubMed] [Google Scholar]
- 53.Garofalo S, D’Alessandro G, Chece G, Brau F, Maggi L, Rosa A, Porzia A, Mainiero F, Esposito V, Lauro C, Benigni G, Bernardini G, Santoni A, Limatola C. Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat Commun. 2015;6:6623. doi: 10.1038/ncomms7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geribaldi-Doldán N, Gómez-Oliva R, Domínguez-García S, Nunez-Abades P, Castro C. Protein kinase C: targets to regenerate brain injuries. Front Cell Dev Biol. 2019;7:39. doi: 10.3389/fcell.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghoneim A, Straiton J, Pollard C, Macdonald K, Jampana R. Imaging of cerebral venous thrombosis. Clin Radiol. 2020;75:254–264. doi: 10.1016/j.crad.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Giovacchini G, Squitieri F, Esmaeilzadeh M, Milano A, Mansi L, Ciarmiello A. PET translates neurophysiology into images: A review to stimulate a network between neuroimaging and basic research. J Cell Physiol. 2011;226:948–961. doi: 10.1002/jcp.22451. [DOI] [PubMed] [Google Scholar]
- 57.Glicksman MA. Induced pluripotent stem cells: the most versatile source for stem cell therapy. Clin Ther. 2018;40:1060–1065. doi: 10.1016/j.clinthera.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Gong H, Fei H, Xu Q, Gou M, Chen HH. 3D-engineered GelMA conduit filled with ECM promotes regeneration of peripheral nerve. J Biomed Mater Res A. 2020;108:805–813. doi: 10.1002/jbm.a.36859. [DOI] [PubMed] [Google Scholar]
- 59.Guo QR, Zhang LL, Liu JF, Li Z, Li JJ, Zhou WM, Wang H, Li JQ, Liu DY, Yu XY, Zhang JY. Multifunctional microfluidic chip for cancer diagnosis and treatment. Nanotheranostics. 2021;5:73–89. doi: 10.7150/ntno.49614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han ZJ, Xue WW, Tao L, Zhu F. Identification of novel immune-relevant drug target genes for Alzheimer’s Disease by combining ontology inference with network analysis. CNS Neurosci Ther. 2018;24:1253–1263. doi: 10.1111/cns.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herholz K. PET studies in dementia. Ann Nucl Med. 2003;17:79–89. doi: 10.1007/BF02988444. [DOI] [PubMed] [Google Scholar]
- 63.Houston ZH, Bunt J, Chen KS, Puttick S, Howard CB, Fletcher NL, Fuchs AV, Cui J, Ju Y, Cowin G, Song X, Boyd AW, Mahler SM, Richards LJ, Caruso F, Thurecht KJ. Understanding the uptake of nanomedicines at different stages of brain cancer using a modular nanocarrier platform and precision bispecific antibodies. ACS Cent Sci. 2020;6:727–738. doi: 10.1021/acscentsci.9b01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu AL, Hou P, Johnson JM, Wu CW, Noll KR, Prabhu SS, Ferguson SD, Kumar VA, Schomer DF, Hazle JD, Chen JH, Liu HL. IClinfMRI software for integrating functional MRI techniques in presurgical mapping and clinical studies. Front Neuroinform. 2018;12:11. doi: 10.3389/fninf.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang H. Pericyte-endothelial interactions in the retinal microvasculature. Int J Mol Sci. 2020;21:7413. doi: 10.3390/ijms21197413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Y, Wang J, Cai J, Qiu Y, Zheng H, Lai X, Sui X, Wang Y, Lu Q, Zhang Y, Yuan M, Gong J, Cai W, Liu X, Shan Y, Deng Z, Shi Y, Shu Y, Zhang L, Qiu W, et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics. 2018;8:5929–5944. doi: 10.7150/thno.28029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang YQ, Peng ZR, Huang FL, Yang AL. Mechanism of delayed encephalopathy after acute carbon monoxide poisoning. Neural Regen Res. 2020a;15:2286–2295. doi: 10.4103/1673-5374.284995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Z, Wong LW, Su Y, Huang X, Wang N, Chen H, Yi C. Blood-brain barrier integrity in the pathogenesis of Alzheimer’s disease. Front Neuroendocrinol. 2020b;59:100857. doi: 10.1016/j.yfrne.2020.100857. [DOI] [PubMed] [Google Scholar]
- 69.Huhndorf M, Moussavi A, Kramann N, Will O, Hattermann K, Stadelmann C, Jansen O, Boretius S. Alterations of the blood-brain barrier and regional perfusion in tumor development: MRI insights from a rat C6 glioma model. PLoS One. 2016;11:e0168174. doi: 10.1371/journal.pone.0168174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 71.Ideguchi M, Kajiwara K, Yoshikawa K, Goto H, Sugimoto K, Inoue T, Nomura S, Suzuki M. Avoidance of ischemic complications after resection of a brain lesion based on intraoperative real-time recognition of the vasculature using laser speckle flow imaging. J Neurosurg. 2017;126:274–280. doi: 10.3171/2016.1.JNS152067. [DOI] [PubMed] [Google Scholar]
- 72.Iijima K, Kurachi M, Shibasaki K, Naruse M, Puentes S, Imai H, Yoshimoto Y, Mikuni M, Ishizaki Y. Transplanted microvascular endothelial cells promote oligodendrocyte precursor cell survival in ischemic demyelinating lesions. J Neurochem. 2015;135:539–550. doi: 10.1111/jnc.13262. [DOI] [PubMed] [Google Scholar]
- 73.Islam MM, Mohamed Z. Computational and pharmacological target of neurovascular unit for drug design and delivery. Biomed Res Int. 2015;2015:731292. doi: 10.1155/2015/731292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobs AH, Kracht LW, Gossmann A, Rüger MA, Thomas AV, Thiel A, Herholz K. Imaging in neurooncology. NeuroRx. 2005;2:333–347. doi: 10.1602/neurorx.2.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joung D, Lavoie NS, Guo SZ, Park SH, Parr AM, McAlpine MC. 3D printed neural regeneration devices. Adv Funct Mater. 2020;30 doi: 10.1002/adfm.201906237. 10.1002/adfm.201906237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M, Klingel K, Reischl G, Widmaier S, Röcken M, Nutt RE, Machulla HJ, Uludag K, Cherry SR, Claussen CD, Pichler BJ. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 77.Kang KM, Choi SH, Chul-Kee P, Kim TM, Park SH, Lee JH, Lee ST, Hwang I, Yoo RE, Yun TJ, Kim JH, Sohn CH. Differentiation between glioblastoma and primary CNS lymphoma: application of DCE-MRI parameters based on arterial input function obtained from DSC-MRI. Eur Radiol. 2021 doi: 10.1007/s00330-021-08044-z. doi: 101007/s00330-021-08044-z. [DOI] [PubMed] [Google Scholar]
- 78.Katara P. Single nucleotide polymorphism and its dynamics for pharmacogenomics. Interdiscip Sci. 2014;6:85–92. doi: 10.1007/s12539-013-0007-x. [DOI] [PubMed] [Google Scholar]
- 79.Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front Bioeng Biotechnol. 2016;4:12. doi: 10.3389/fbioe.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keogh MJ, Wei W, Aryaman J, Walker L, van den Ameele J, Coxhead J, Wilson I, Bashton M, Beck J, West J, Chen R, Haudenschild C, Bartha G, Luo S, Morris CM, Jones NS, Attems J, Chinnery PF. High prevalence of focal and multi-focal somatic genetic variants in the human brain. Nat Commun. 2018;9:4257. doi: 10.1038/s41467-018-06331-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, Butman JA, Patronas N, Alger JR, Latour LL, Luby ML, Baird AE, Leary MC, Tremwel M, Ovbiagele B, Fredieu A, Suzuki S, Villablanca JP, Davis S, Dunn B, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–1830. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 82.Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, Boas DA, Sakadžić S, Zlokovic BV. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017;20:406–416. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kolagar TA, Farzaneh M, Nikkar N, Khoshnam SE. Human pluripotent stem cells in neurodegenerative diseases: potentials, advances and limitations. Curr Stem Cell Res Ther. 2020;15:102–110. doi: 10.2174/1574888X14666190823142911. [DOI] [PubMed] [Google Scholar]
- 84.Kosterhon M, Neufurth M, Neulen A, Schäfer L, Conrad J, Kantelhardt SR, Müller WEG, Ringel F. Multicolor 3D Printing of Complex Intracranial Tumors in Neurosurgery. J Vis Exp. 2020:e60471. doi: 10.3791/60471. [DOI] [PubMed] [Google Scholar]
- 85.Kulczar C, Lubin KE, Lefebvre S, Miller DW, Knipp GT. Development of a direct contact astrocyte-human cerebral microvessel endothelial cells blood-brain barrier coculture model. J Pharm Pharmacol. 2017;69:1684–1696. doi: 10.1111/jphp.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lah TT, Novak M, Breznik B. Brain malignancies: glioblastoma and brain metastases. Semin Cancer Biol. 2020;60:262–273. doi: 10.1016/j.semcancer.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 87.Lang S, Hoelter P, Schmidt M, Eisenhut F, Kaethner C, Kowarschik M, Lücking H, Doerfler A. Evaluation of an artificial intelligence-based 3D-angiography for visualization of cerebral vasculature. Clin Neuroradiol. 2020;30:705–712. doi: 10.1007/s00062-019-00836-7. [DOI] [PubMed] [Google Scholar]
- 88.Lauranzano E, Campo E, Rasile M, Molteni R, Pizzocri M, Passoni L, Bello L, Pozzi D, Pardi R, Matteoli M, Ruiz-Moreno A. A microfluidic human model of blood-brain barrier employing primary human astrocytes. Adv Biosyst. 2019;3:e1800335. doi: 10.1002/adbi.201800335. [DOI] [PubMed] [Google Scholar]
- 89.Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 90.Lee Y, Choi JW, Yu J, Park D, Ha J, Son K, Lee S, Chung M, Kim HY, Jeon NL. Microfluidics within a well: an injection-molded plastic array 3D culture platform. Lab Chip. 2018;18:2433–2440. doi: 10.1039/c8lc00336j. [DOI] [PubMed] [Google Scholar]
- 91.Li CX, Wang XQ, Cheng FF, Yan X, Luo J, Wang QG. Hyodeoxycholic acid protects the neurovascular unit against oxygen-glucose deprivation and reoxygenation-induced injury in vitro. Neural Regen Res. 2019;14:1941–1949. doi: 10.4103/1673-5374.259617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li T, Hu E, Li P, Yang Z, Wu Y, Ding R, Zhu X, Tang T, Wang Y. Metabolomics deciphers potential targets of Xuefu Zhuyu decoction against traumatic brain injury in rat. Front Pharmacol. 2020a;11:559618. doi: 10.3389/fphar.2020.559618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Y, Khamou M, Schaarschmidt BM, Umutlu L, Forsting M, Demircioglu A, Haubold J, Koch AK, Bruckmann NM, Sawicki LM, Herrmann K, Boone JH, Langhorst J. Comparison of (18)F-FDG PET-MR and fecal biomarkers in the assessment of disease activity in patients with ulcerative colitis. Br J Radiol. 2020b;93:20200167. doi: 10.1259/bjr.20200167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liao Z, Li Y, Gu L, Lei R, Miao Y, Lan H, Deng Y, Geng L. Advances in microfluidic chip-based extracellular vesicle separation. Se pu = Chinese journal of chromatography. 2019;37:343–347. doi: 10.3724/SP.J.1123.2018.11045. [DOI] [PubMed] [Google Scholar]
- 95.Lingala SG, Guo Y, Bliesener Y, Zhu Y, Lebel RM, Law M, Nayak KS. Tracer kinetic models as temporal constraints during brain tumor DCE-MRI reconstruction. Med Phys. 2020;47:37–51. doi: 10.1002/mp.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu LR, Liu JC, Bao JS, Bai QQ, Wang GQ. Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol. 2020a;11:1024. doi: 10.3389/fimmu.2020.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y, Li M, Wei C, Tang L, Sheng Y, Liu Y, Li D, Ding D, Qiu J, Zhu X. TSP1-CD47-SIRPα signaling facilitates the development of endometriosis by mediating the survival of ectopic endometrium. Am J Reprod Immunol. 2020b;83:e13236. doi: 10.1111/aji.13236. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y, Zhou C, Su Z, Chang Q, Qiu Y, Bei J, Gaitas A, Xiao J, Drelich A, Khanipov K, Jin Y, Golovko G, Saito TB, Gong B. Endothelial Exosome Plays a Functional Role during Rickettsial Infection. mBio. 2021;12:e00769–00721. doi: 10.1128/mBio.00769-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 100.Lougiaki P, Tsolaki M, Pantazaki A. Bioinks and in vitro neurovascular unit production - new prospects in Alzheimer’s disease research. Hell J Nucl Med. 2019;22(Suppl):209–222. [PubMed] [Google Scholar]
- 101.Ma Q, Zhao Z, Sagare AP, Wu Y, Wang M, Owens NC, Verghese PB, Herz J, Holtzman DM, Zlokovic BV. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol Neurodegener. 2018;13:57. doi: 10.1186/s13024-018-0286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maione F, Piccolo M, De Vita S, Chini MG, Cristiano C, De Caro C, Lippiello P, Miniaci MC, Santamaria R, Irace C, De Feo V, Calignano A, Mascolo N, Bifulco G. Down regulation of pro-inflammatory pathways by tanshinone IIA and cryptotanshinone in a non-genetic mouse model of Alzheimer’s disease. Pharmacol Res. 2018;129:482–490. doi: 10.1016/j.phrs.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 103.Manning P, Daghighi S, Rajaratnam MK, Parthiban S, Bahrami N, Dale AM, Bolar D, Piccioni DE, McDonald CR, Farid N. Differentiation of progressive disease from pseudoprogression using 3D PCASL and DSC perfusion MRI in patients with glioblastoma. J Neurooncol. 2020;147:681–690. doi: 10.1007/s11060-020-03475-y. [DOI] [PubMed] [Google Scholar]
- 104.Maoz BM, Herland A, FitzGerald EA, Grevesse T, Vidoudez C, Pacheco AR, Sheehy SP, Park TE, Dauth S, Mannix R, Budnik N, Shores K, Cho A, Nawroth JC, Segrè D, Budnik B, Ingber DE, Parker KK. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol. 2018;36:865–874. doi: 10.1038/nbt.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin Gonzalez J, Baudet A, Abelechian S, Bonderup K, d’Altri T, Porse B, Brakebusch C, Juliusson G, Cammenga J. A new genetic tool to improve immune-compromised mouse models: Derivation and CRISPR/Cas9-mediated targeting of NRG embryonic stem cell lines. Genesis. 2018;56:e23238. doi: 10.1002/dvg.23238. [DOI] [PubMed] [Google Scholar]
- 106.Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 107.Meng Q, Wang Y, Li Y, Shen C. Hydrogel microfluidic-based liver-on-a-chip: mimicking the mass transfer and structural features of liver. Biotechnol Bioeng. 2021;118:612–621. doi: 10.1002/bit.27589. [DOI] [PubMed] [Google Scholar]
- 108.Mittal R, Woo FW, Castro CS, Cohen MA, Karanxha J, Mittal J, Chhibber T, Jhaveri VM. Organ-on-chip models: implications in drug discovery and clinical applications. J Cell Physiol. 2019;234:8352–8380. doi: 10.1002/jcp.27729. [DOI] [PubMed] [Google Scholar]
- 109.Mlakar V, Huezo-Diaz Curtis P, Satyanarayana Uppugunduri CR, Krajinovic M, Ansari M. Pharmacogenomics in pediatric oncology: review of gene-drug associations for clinical use. Int J Mol Sci. 2016;17:1502. doi: 10.3390/ijms17091502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Muzik O, Reilly KT, Diwadkar VA. “Brain over body”-A study on the willful regulation of autonomic function during cold exposure. Neuroimage. 2018;172:632–641. doi: 10.1016/j.neuroimage.2018.01.067. [DOI] [PubMed] [Google Scholar]
- 111.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Netto JP, Iliff J, Stanimirovic D, Krohn KA, Hamilton B, Varallyay C, Gahramanov S, Daldrup-Link H, d’Esterre C, Zlokovic B, Sair H, Lee Y, Taheri S, Jain R, Panigrahy A, Reich DS, Drewes LR, Castillo M, Neuwelt EA. Neurovascular unit: basic and clinical imaging with emphasis on advantages of ferumoxytol. Neurosurgery. 2018;82:770–780. doi: 10.1093/neuros/nyx357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nishibori M, Wang D, Ousaka D, Wake H. High mobility group box-1 and blood-brain barrier disruption. Cells. 2020;9:2650. doi: 10.3390/cells9122650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Noor MS, Yu L, Murari K, Kiss ZH. Neurovascular coupling during deep brain stimulation. Brain Stimul. 2020;13:916–927. doi: 10.1016/j.brs.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 116.Nwokocha CR, Bafor EE, Ajayi OI, Ebeigbe AB. The malaria-high blood pressure hypothesis: revisited. Am J Hypertens. 2020;33:695–702. doi: 10.1093/ajh/hpaa051. [DOI] [PubMed] [Google Scholar]
- 117.Nyúl-Tóth Á, Suciu M, Molnár J, Fazakas C, Haskó J, Herman H, Farkas AE, Kaszaki J, Hermenean A, Wilhelm I, Krizbai IA. Differences in the molecular structure of the blood-brain barrier in the cerebral cortex and white matter: an in silico, in vitro, and ex vivo study. Am J Physiol Heart Circ Physiol. 2016;310:H1702–1714. doi: 10.1152/ajpheart.00774.2015. [DOI] [PubMed] [Google Scholar]
- 118.Olateju OI, Morè L, Arthur JSC, Frenguelli BG. Mitogen and stress-activated protein kinase 1 negatively regulates hippocampal neurogenesis. Neuroscience. 2021;452:228–234. doi: 10.1016/j.neuroscience.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Omulecki W, Bartela J, Synder A, Pałenga-Pydyn D, Wilczyński M. Results of implantation a new type of foldable anterior chamber intraocular lens. Klin Oczna. 2011;113:216–222. [PubMed] [Google Scholar]
- 120.Park TE, Mustafaoglu N, Herland A, Hasselkus R, Mannix R, FitzGerald EA, Prantil-Baun R, Watters A, Henry O, Benz M, Sanchez H, McCrea HJ, Goumnerova LC, Song HW, Palecek SP, Shusta E, Ingber DE. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat Commun. 2019;10:2621. doi: 10.1038/s41467-019-10588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Piotrowski SL, Wilson L, Maldonado KL, Tailor R, Hill LR, Bankson JA, Lai S, Kasper FK, Young S. Effect of radiation on DCE-MRI pharmacokinetic parameters in a rabbit model of compromised maxillofacial wound healing: a pilot study. J Oral Maxillofac Surg. 2020;78:1034e1–1034e10. doi: 10.1016/j.joms.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 122.Pong S, Lizano P, Karmacharya R. Derivation, expansion, cryopreservation and characterization of brain microvascular endothelial cells from human induced pluripotent stem cells. J Vis Exp. 2020:e61629. doi: 10.3791/61629. [DOI] [PubMed] [Google Scholar]
- 123.Potjewyd G, Moxon S, Wang T, Domingos M, Hooper NM. Tissue engineering 3D neurovascular units: a biomaterials and bioprinting perspective. Trends Biotechnol. 2018;36:457–472. doi: 10.1016/j.tibtech.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 124.Prabhakarpandian B, Shen MC, Nichols JB, Mills IR, Sidoryk-Wegrzynowicz M, Aschner M, Pant K. SyM-BBB: a microfluidic blood brain barrier model. Lab Chip. 2013;13:1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prè D, Nestor MW, Sproul AA, Jacob S, Koppensteiner P, Chinchalongporn V, Zimmer M, Yamamoto A, Noggle SA, Arancio O. A time course analysis of the electrophysiological properties of neurons differentiated from human induced pluripotent stem cells (iPSCs) PLoS One. 2014;9:e103418. doi: 10.1371/journal.pone.0103418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311. doi: 10.1016/j.cell.2019.08.053. [DOI] [PubMed] [Google Scholar]
- 127.Pulido RS, Munji RN, Chan TC, Quirk CR, Weiner GA, Weger BD, Rossi MJ, Elmsaouri S, Malfavon M, Deng A, Profaci CP, Blanchette M, Qian T, Foreman KL, Shusta EV, Gorman MR, Gachon F, Leutgeb S, Daneman R. Neuronal activity regulates blood-brain barrier efflux transport through endothelial circadian genes. Neuron. 2020;108:937–952.e7. doi: 10.1016/j.neuron.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qidwai T. Exploration of copy number variation in genes related to anti-malarial drug resistance in Plasmodium falciparum. Gene. 2020;736:144414. doi: 10.1016/j.gene.2020.144414. [DOI] [PubMed] [Google Scholar]
- 129.Rafalski VA, Merlini M, Akassoglou K. Pericytes: the brain’s very first responders. Neuron. 2018;100:11–13. doi: 10.1016/j.neuron.2018.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Recasens M, Shrivastava K, Almolda B, González B, Castellano B. Astrocyte-targeted IL-10 production decreases proliferation and induces a downregulation of activated microglia/macrophages after PPT. Glia. 2019;67:741–758. doi: 10.1002/glia.23573. [DOI] [PubMed] [Google Scholar]
- 131.Richardson LS, Kim S, Han A, Menon R. Modeling ascending infection with a feto-maternal interface organ-on-chip. Lab Chip. 2020;20:4486–4501. doi: 10.1039/d0lc00875c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rodríguez-Gómez JA, Kavanagh E, Engskog-Vlachos P, Engskog MKR, Herrera AJ, Espinosa-Oliva AM, Joseph B, Hajji N, Venero JL, Burguillos MA. Microglia: agents of the CNS pro-inflammatory response. Cells. 2020;9:1717. doi: 10.3390/cells9071717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ross CL, Ang DC, Almeida-Porada G. Targeting mesenchymal stromal cells/pericytes (MSCs) with pulsed electromagnetic field (PEMF) has the potential to treat rheumatoid arthritis. Front Immunol. 2019;10:266. doi: 10.3389/fimmu.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rumianek AN, Greaves DR. How have leukocyte in vitro chemotaxis assays shaped our ideas about macrophage migration. Biology (Basel) 2020;9:439. doi: 10.3390/biology9120439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saab AS, Tzvetanova ID, Nave KA. The role of myelin and oligodendrocytes in axonal energy metabolism. Curr Opin Neurobiol. 2013;23:1065–1072. doi: 10.1016/j.conb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 136.Sanders JW, Chen HS, Johnson JM, Schomer DF, Jimenez JE, Ma J, Liu HL. Synthetic generation of DSC-MRI-derived relative CBV maps from DCE MRI of brain tumors. Magn Reson Med. 2021;85:469–479. doi: 10.1002/mrm.28432. [DOI] [PubMed] [Google Scholar]
- 137.Sankar GG, Murthy PS, Das A, Sathya S, Nankar R, Venugopalan VP, Doble M. Polydimethyl siloxane based nanocomposites with antibiofilm properties for biomedical applications. J Biomed Mater Res B Appl Biomater. 2017;105:1075–1082. doi: 10.1002/jbm.b.33650. [DOI] [PubMed] [Google Scholar]
- 138.Segarra M, Aburto MR, Cop F, Llaó-Cid C, Härtl R, Damm M, Bethani I, Parrilla M, Husainie D, Schänzer A, Schlierbach H, Acker T, Mohr L, Torres-Masjoan L, Ritter M, Acker-Palmer A. Endothelial Dab1 signaling orchestrates neuro-glia-vessel communication in the central nervous system. Science. 2018;361:eaao2861. doi: 10.1126/science.aao2861. [DOI] [PubMed] [Google Scholar]
- 139.Shukla G, Alexander GS, Bakas S, Nikam R, Talekar K, Palmer JD, Shi W. Advanced magnetic resonance imaging in glioblastoma: a review. Chin Clin Oncol. 2017;6:40. doi: 10.21037/cco.2017.06.28. [DOI] [PubMed] [Google Scholar]
- 140.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sifat AE, Vaidya B, Abbruscato TJ. Blood-brain barrier protection as a therapeutic strategy for acute ischemic stroke. AAPS J. 2017;19:957–972. doi: 10.1208/s12248-017-0091-7. [DOI] [PubMed] [Google Scholar]
- 142.Sivandzade F, Cucullo L. In-vitro blood-brain barrier modeling: a review of modern and fast-advancing technologies. J Cereb Blood Flow Metab. 2018;38:1667–1681. doi: 10.1177/0271678X18788769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sokolova V, Nzou G, van der Meer SB, Ruks T, Heggen M, Loza K, Hagemann N, Murke F, Giebel B, Hermann DM, Atala AJ, Epple M. Ultrasmall gold nanoparticles (2 nm) can penetrate and enter cell nuclei in an in vitro 3D brain spheroid model. Acta Biomater. 2020;111:349–362. doi: 10.1016/j.actbio.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 144.Staicu CE, Jipa F, Axente E, Radu M, Radu BM, Sima F. Lab-on-a-chip platforms as tools for drug screening in neuropathologies associated with blood-brain barrier alterations. Biomolecules. 2021;11:916. doi: 10.3390/biom11060916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stebbins MJ, Wilson HK, Canfield SG, Qian T, Palecek SP, Shusta EV. Differentiation and characterization of human pluripotent stem cell-derived brain microvascular endothelial cells. Methods. 2016;101:93–102. doi: 10.1016/j.ymeth.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stebbins MJ, Gastfriend BD, Canfield SG, Lee MS, Richards D, Faubion MG, Li WJ, Daneman R, Palecek SP, Shusta EV. Human pluripotent stem cell-derived brain pericyte-like cells induce blood-brain barrier properties. Sci Adv. 2019;5:eaau7375. doi: 10.1126/sciadv.aau7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stone NL, England TJ, O’Sullivan SE. A novel transwell blood brain barrier model using primary human cells. Front Cell Neurosci. 2019;13:230. doi: 10.3389/fncel.2019.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Subhramanyam CS, Wang C, Hu Q, Dheen ST. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol. 2019;94:112–120. doi: 10.1016/j.semcdb.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 149.Sugaya K, Vaidya M. Stem cell therapies for neurodegenerative diseases. Adv Exp Med Biol. 2018;1056:61–84. doi: 10.1007/978-3-319-74470-4_5. [DOI] [PubMed] [Google Scholar]
- 150.Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93:13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Takase H, Washida K, Hayakawa K, Arai K, Wang X, Lo EH, Lok J. Oligodendrogenesis after traumatic brain injury. Behav Brain Res. 2018;340:205–211. doi: 10.1016/j.bbr.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 152.Tang Y, Han Y, Yu H, Zhang B, Li G. Increased GABAergic development in iPSC-derived neurons from patients with sporadic Alzheimer’s disease. Neurosci Lett. 2020;735:135208. doi: 10.1016/j.neulet.2020.135208. [DOI] [PubMed] [Google Scholar]
- 153.Tarumi T, Zhang R. Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem. 2018;144:595–608. doi: 10.1111/jnc.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tedesco MT, Di Lisa D, Massobrio P, Colistra N, Pesce M, Catelani T, Dellacasa E, Raiteri R, Martinoia S, Pastorino L. Soft chitosan microbeads scaffold for 3D functional neuronal networks. Biomaterials. 2018;156:159–171. doi: 10.1016/j.biomaterials.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 155.Thurgur H, Pinteaux E. Microglia in the neurovascular unit: blood-brain barrier-microglia interactions after central nervous system disorders. Neuroscience. 2019;405:55–67. doi: 10.1016/j.neuroscience.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 156.Tomkins AJ, Hood RJ, Pepperall D, Null CL, Levi CR, Spratt NJ. Thrombolytic recanalization of carotid arteries is highly dependent on degree of stenosis, despite sonothrombolysis. J Am Heart Assoc. 2016;5:e002716. doi: 10.1161/JAHA.115.002716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsai SC, Lin FC, Chang KH, Li MC, Chou RH, Huang MY, Chen YC, Kao CY, Cheng CC, Lin HC, Hsu YC. The intravenous administration of skin-derived mesenchymal stem cells ameliorates hearing loss and preserves cochlear hair cells in cisplatin-injected mice: SMSCs ameliorate hearing loss and preserve outer hair cells in mice. Hear Res. 2021:108254. doi: 10.1016/j.heares.2021.108254. [DOI] [PubMed] [Google Scholar]
- 158.Tsukita S, Tanaka H, Tamura A. The Claudins: from tight junctions to biological systems. Trends Biochem Sci. 2019;44:141–152. doi: 10.1016/j.tibs.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 159.Urban A, Golgher L, Brunner C, Gdalyahu A, Har-Gil H, Kain D, Montaldo G, Sironi L, Blinder P. Understanding the neurovascular unit at multiple scales: advantages and limitations of multi-photon and functional ultrasound imaging. Adv Drug Deliv Rev. 2017;119:73–100. doi: 10.1016/j.addr.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 160.Uwamori H, Ono Y, Yamashita T, Arai K, Sudo R. Comparison of organ-specific endothelial cells in terms of microvascular formation and endothelial barrier functions. Microvasc Res. 2019;122:60–70. doi: 10.1016/j.mvr.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vasic V, Barth K, Schmidt MHH. Neurodegeneration and neuro-regeneration-Alzheimer’s disease and stem cell therapy. Int J Mol Sci. 2019;20:4272. doi: 10.3390/ijms20174272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, Barthakur S, Kasendra M, Lucchesi C, Kerns J, Wen N, Spivia WR, Chen Z, Van Eyk J, Svendsen CN. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell. 2019;24:995–1005.e6. doi: 10.1016/j.stem.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 163.Venkat P, Chopp M, Chen J. New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat Med J. 2016;57:223–228. doi: 10.3325/cmj.2016.57.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Voet S, Prinz M, van Loo G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med. 2019;25:112–123. doi: 10.1016/j.molmed.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 165.Wang P, Zheng X, Guo Q, Yang P, Pang X, Qian K, Lu W, Zhang Q, Jiang X. Systemic delivery of BACE1 siRNA through neuron-targeted nanocomplexes for treatment of Alzheimer’s disease. J Control Release. 2018;279:220–233. doi: 10.1016/j.jconrel.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 166.Wey HY, Catana C, Hooker JM, Dougherty DD, Knudsen GM, Wang DJ, Chonde DB, Rosen BR, Gollub RL, Kong J. Simultaneous fMRI-PET of the opioidergic pain system in human brain. Neuroimage 102 Pt. 2014;2:275–282. doi: 10.1016/j.neuroimage.2014.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu S, Thornhill RE, Chen S, Rammo W, Mikulis DJ, Kassner A. Relative recirculation: a fast, model-free surrogate for the measurement of blood-brain barrier permeability and the prediction of hemorrhagic transformation in acute ischemic stroke. Invest Radiol. 2009;44:662–668. doi: 10.1097/RLI.0b013e3181ae9c40. [DOI] [PubMed] [Google Scholar]
- 168.Xiao W, Zhang R, Sohrabi A, Ehsanipour A, Sun S, Liang J, Walthers CM, Ta L, Nathanson DA, Seidlits SK. Brain-mimetic 3D culture platforms allow investigation of cooperative effects of extracellular matrix features on therapeutic resistance in glioblastoma. Cancer Res. 2018;78:1358–1370. doi: 10.1158/0008-5472.CAN-17-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang SH, Liu R, Perez EJ, Wang X, Simpkins JW. Estrogens as protectants of the neurovascular unit against ischemic stroke. Curr Drug Targets CNS Neurol Disord. 2005;4:169–177. doi: 10.2174/1568007053544174. [DOI] [PubMed] [Google Scholar]
- 170.Ye LX, An NC, Huang P, Li DH, Zheng ZL, Ji H, Li H, Chen DQ, Wu YQ, Xiao J, Xu K, Li XK, Zhang HY. Exogenous platelet-derived growth factor improves neurovascular unit recovery after spinal cord injury. Neural Regen Res. 2021;16:765–771. doi: 10.4103/1673-5374.295347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yu X, Ji C, Shao A. Neurovascular unit dysfunction and neurodegenerative disorders. Front Neurosci. 2020;14:334. doi: 10.3389/fnins.2020.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastrointestinal and liver disease. Gut. 2019;68:547–561. doi: 10.1136/gutjnl-2018-316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang C, Tabatabaei M, Bélanger S, Girouard H, Moeini M, Lu X, Lesage F. Astrocytic endfoot Ca(2+) correlates with parenchymal vessel responses during 4-AP induced epilepsy: An in vivo two-photon lifetime microscopy study. J Cereb Blood Flow Metab. 2019;39:260–271. doi: 10.1177/0271678X17725417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang Q, Fisher K. Tight junction-related barrier contributes to the electrophysiological asymmetry across vocal fold epithelium. PLoS One. 2012;7:e34017. doi: 10.1371/journal.pone.0034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao LN, Yang ZH, Liu YH, Ying HQ, Zhang H, Xue YX. Vascular endothelial growth factor increases permeability of the blood-tumor barrier via caveolae-mediated transcellular pathway. J Mol Neurosci. 2011;44:122–129. doi: 10.1007/s12031-010-9487-x. [DOI] [PubMed] [Google Scholar]
- 176.Zhao YH, Liu NW, Ke CC, Liu BW, Chen YA, Luo C, Zhang Q, Xia ZY, Liu RS. Combined treatment of sodium ferulate, n-butylidenephthalide ,and ADSCs rehabilitates neurovascular unit in rats after photothrombotic stroke. J Cell Mol Med. 2019;23:126–142. doi: 10.1111/jcmm.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou H, Su J, Hu X, Zhou C, Li H, Chen Z, Xiao Q, Wang B, Wu W, Sun Y, Zhou Y, Tang C, Liu F, Wang L, Feng C, Liu M, Li S, Zhang Y, Xu H, Yao H, et al. Glia-to-neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell. 2020;181:590–603.e16. doi: 10.1016/j.cell.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 178.Zhou T, Zhu H, Fan Z, Wang F, Chen Y, Liang H, Yang Z, Zhang L, Lin L, Zhan Y, Wang Z, Hu H. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science. 2017;357:162–168. doi: 10.1126/science.aak9726. [DOI] [PubMed] [Google Scholar]
- 179.Zhu Y, Zhu X. MRI-driven PET image optimization for neurological applications. Front Neurosci. 2019;13:782. doi: 10.3389/fnins.2019.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zlokovic BV, Begley DJ, Chain DG. Blood-brain barrier permeability to dipeptides and their constituent amino acids. Brain Res. 1983;271:65–71. doi: 10.1016/0006-8993(83)91365-3. [DOI] [PubMed] [Google Scholar]


