Abstract
A 67-year-old man with metastatic lung adenocarcinoma was initially treated with whole-brain radiotherapy for intracranial metastases, followed by chemotherapy and pembrolizumab. After completing 2 years of systemic therapy, the primary right lung lesion was biopsy-proven to have residual adenocarcinoma, which was then treated with radiation (6000 cGy in 15 fractions). Follow-up serial FDG PET/CT showed radiation fibrosis. Eighteen months after radiotherapy, the patient received 2 doses of an mRNA COVID-19 vaccine. FDG PET/CT performed 4 days following his second vaccine dose showed FDG-avid multistation lymphadenopathy and radiation recall pneumonitis, likely vaccination-induced and mimicking recurrent disease. This resolved spontaneously without therapy.
Key Words: COVID-19, pneumonitis, radiation, recall, vaccination
FIGURE 1.
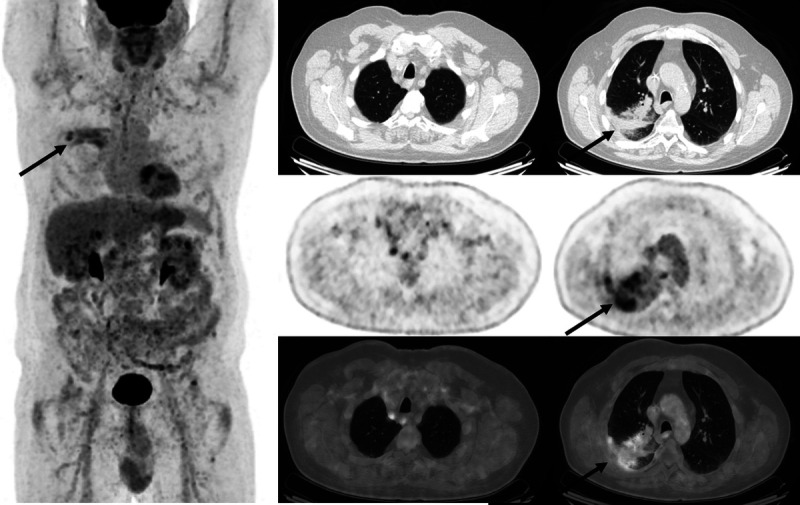
A 67-year-old man was undergoing serial FDG PET/CT scans for restaging evaluations of metastatic lung cancer. Postradiotherapy changes are seen in the right lung on FDG PET/CT performed 15 months following completion of radiotherapy, with somewhat heterogeneous mildly FDG-avid linear consolidative opacification at the treatment site (arrows).
FIGURE 2.
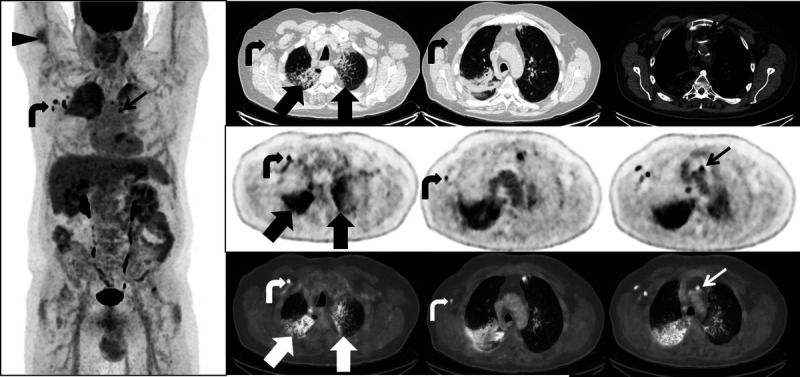
FDG PET/CT performed 3 months later and 4 days after the second dose of mRNA COVID-19 vaccination shows expected postvaccination changes of FDG uptake at the injection site in the right deltoid (arrowhead) and ipsilateral FDG-avid axillary and subpectoral lymph nodes (curved arrows). However, newly FDG-avid mediastinal lymph nodes (thin arrow) were also noted, as well as new FDG-avid ground-glass and interlobular septal thickening in the right upper lobe, superior segment right lower lobe, and left upper lobes (thick arrows). The differential diagnosis for the lung parenchymal findings included recurrent disease manifesting as lymphangitis carcinomatosis; however, given the atypical pattern of spread and the well-demarcated field of involvement, a diagnosis of radiation recall triggered by COVID-19 vaccination was proposed. The patient remained asymptomatic, and further investigation with bronchoscopy was therefore deferred.
FIGURE 3.
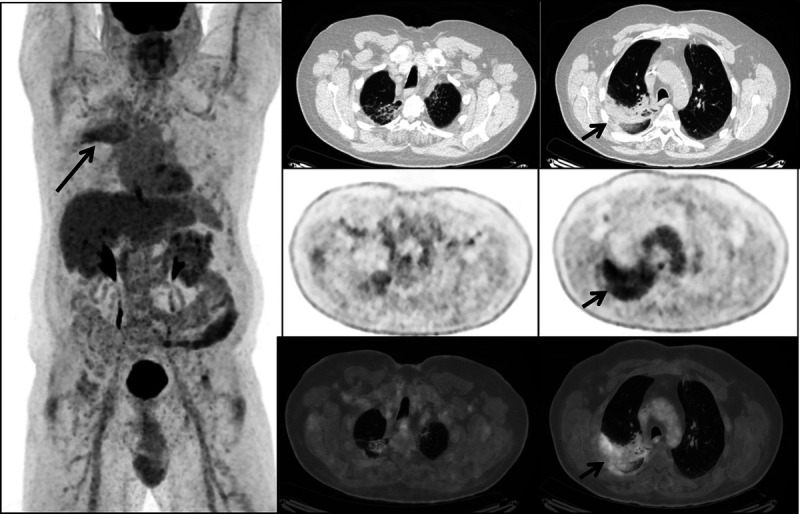
A short-interval follow-up FDG PET/CT was performed 2 months later, while the patient was still off therapy. This showed decreasing intensity of FDG uptake in both upper lobes (arrows), improving lung parenchymal findings on CT, and resolution of FDG uptake seen previously in multistation lymph nodes and at the intramuscular injection site, confirming improving inflammatory changes. While vaccination-induced lymphadenopathy and FDG uptake in ipsilateral regional lymph nodes at the vaccine injection site in the deltoid muscle have been widely reported,1 there are fewer case reports of other inflammatory responses to COVID-19 vaccination, including systemic inflammatory response syndrome2 and cutaneous radiation recall.3 Here, we present an illustrative case of radiation recall pneumonitis induced by COVID-19 vaccination. Radiation recall is an inflammatory response confined to the radiation port site and may be triggered by chemotherapeutic agents, including immunotherapy, but also antibiotics, antitubercular medications, nonsteroidal anti-inflammatory drugs, and statins.4,5 The etiology is poorly understood, and the most commonly affected site is the skin, but the inflammatory response can occur in any previously irradiated tissue including the lung.4 Radiation recall pneumonitis in patients with lung cancer can be mistaken for progressive malignancy on imaging,6 so it is important that the interpreter be aware of this entity. Steber et al7 also reported a case of COVID-19 vaccination–induced radiation recall pneumonitis in a patient with lung cancer who developed rapidly progressive symptoms, and Soyfer et al3 reported 2 cases of COVID-19 vaccination–induced radiation recall involving the skin. To date, the inflammatory nodal responses to COVID-19 vaccinations have been well-documented, and multidisciplinary guidelines have been published to aid imaging interpretation, reporting, and management,8 but the guidance is less clear beyond these manifestations, and it is important that interpreters be aware of and consider the possibility of other inflammatory responses induced by the vaccination.
Footnotes
Author Contribution: N.M.H. interpreted images and drafted, revised, and approved the final manuscript. M.M.H. and H.A.J. interpreted images and revised and approved the final manuscript. M.M.A. contributed data and revised and approved the final manuscript.
Conflicts of interest and sources of funding: M.M.A. reports grants and personal fees from Genentech, grants and personal fees from Bristol-Myers Squibb, personal fees from Merck, grants and personal fees from AstraZeneca, grants from Lilly, personal fees from Maverick, personal fees from Blueprint Medicine, personal fees from Syndax, personal fees from Ariad, personal fees from Nektar, personal fees from Gritstone, personal fees from ArcherDX, personal fees from Mirati, personal fees from NextCure, personal fees from Novartis, personal fees from EMD Serono, personal fees from Panvaxal/NovaRx, outside the submitted work. H.A.J. reports honoraria from Janssen Pharmaceuticals, Bayer Healthcare; research support paid to institution: Siemens Healthcare Inc, GTx Inc, Blue Earth Diagnostics; consulting: Advanced Accelerator Applications; Blue Earth Diagnostics; royalties: Cambridge Publishing; NIH/NCI grant support not related to this work as coinvestigator 1R01CA235589-01A1. N.M.H. and M.M.H. report no disclosures.
Contributor Information
Nicola M. Hughes, Email: nicolahughes@rcsi.ie.
Mark M. Hammer, Email: mmhammer@bwh.harvard.edu.
Mark M. Awad, Email: Mark_Awad@dfci.harvard.edu.
REFERENCES
- 1.Keshavarz P Yazdanpanah F Rafiee F, et al. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021;28:1058–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg J, Thomas A, Iravani A. 18F-fluorodeoxyglucose PET/CT findings in a systemic inflammatory response syndrome after COVID-19 vaccine. Lancet. 2021;397:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soyfer V Gutfeld O Shamai S, et al. COVID-19 vaccine–induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys. 2021;110:957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burris HA, 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGovern K Ghaly M Esposito M, et al. Radiation recall pneumonitis in the setting of immunotherapy and radiation: a focused review. Future Sci OA. 2019;5:FSO378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousin F Desir C Ben Mustapha S, et al. Incidence, risk factors, and CT characteristics of radiation recall pneumonitis induced by immune checkpoint inhibitor in lung cancer. Radiother Oncol. 2021;157:47–55. [DOI] [PubMed] [Google Scholar]
- 7.Steber CR Ponnatapura J Hughes RT, et al. Rapid development of clinically symptomatic radiation recall pneumonitis immediately following COVID-19 vaccination. Cureus. 2021;13:e14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker AS Perez-Johnston R Chikarmane SA, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: Radiology Scientific Expert Panel. Radiology. 2021;300:E323–E327. [DOI] [PMC free article] [PubMed] [Google Scholar]


