Abstract
We classified 100 Enterocytozoon bieneusi isolates into five genotypes by a PCR-restriction fragment length polymorphism method. Type I strains were encountered only in human immunodeficiency virus (HIV)-infected patients, whereas type II strains were more frequently found in non-HIV-infected patients (75 versus 10%, respectively; P < 10−4), suggesting differences in the epidemiology of E. bieneusi among these patients.
The microsporidium Enterocytozoon bieneusi causes chronic diarrhea and weight loss in human immunodeficiency virus (HIV)-infected patients with severe immunodeficiency (2). Whereas intestinal microsporidiosis in HIV infection is now less frequent due to the use of antiretroviral therapy, E. bieneusi infections are increasingly observed in organ transplant recipients and other immunocompromised patients as well as in immunocompetent individuals (4, 5). However, the sources of this infection and its mode of transmission to humans are still uncertain (2). In an attempt to better define the genotypic diversity of E. bieneusi, we analyzed the ribosomal DNA (rDNA) internal transcribed spacers (ITSs) of 100 different isolates by a PCR-restriction fragment length polymorphism (PCR-RFLP) method.
Stool specimens were obtained over a 6-year period (1994 to 2000) from 100 patients, among whom 88 were infected with HIV type 1 and 12 were not. Among the 12 non-HIV-infected patients, 8 were organ transplant recipients (6 renal, 1 liver, and 1 heart-lung), 1 had lymphoma, 1 had myeloma, and 2 were immunocompetent hosts with a history of traveler's diarrhea. Some of these patients have been previously reported (7). The diagnosis of microsporidial infection was made by detection of typical spores in stools as previously described (7). Species-level identification of E. bieneusi was made by PCR using a specific primer set (8). For PCR-RFLP analysis, microsporidial DNA was extracted from stored stools in distilled water with a High Pure PCR Template Preparation Kit (Boehringer Mannheim, Meylan, France) according to the manufacturer's instructions. The E. bieneusi rDNA ITS was amplified using Eb.gc and Eb.gt primers, which amplify a 210-bp fragment of the E. bieneusi ITS region (13). All PCR products were then digested with two restriction endonucleases, NlaIII and Fnu4HI (New England Biolabs, Beverly, Mass.), which we selected in our previous study (7). For all samples, enzymatic digestion of amplicons with NlaIII and Fnu4HI produced distinctive bands detectable in ethidium bromide-stained polyacrylamide gels (data not shown). Among the 100 stool specimens we found five genetically unrelated lineages (I to V). Type I strains of E. bieneusi were found in 66 patients, all of whom were HIV infected. Type II strains were encountered in 18 patients, type III strains were seen in 3 patients, type IV strains were observed in 12 patients, and a new strain, designated type V, was seen in 1 patient (Table 1). By this method, the four E. bieneusi genotypes reported by Rinder et al. (10) could be assigned to three of the types described in this study (genotypes A and B belonging to type I, genotype C belongs to type II, and genotype D belongs to type IV). Interestingly, the distribution of genotypes was found to be significantly different among HIV-infected patients compared to non-HIV-infected patients (P < 10−4 [χ2 test]) (Table 1). Type I strains were strongly associated with HIV infection (P < 10−3 [Fischer's exact test]). Type II strains were more frequently seen in non-HIV-infected patients (75 versus 10%; P < 10−4 [Fischer's exact test]). We did not find any relationship between genotype and either age, sex, or year of diagnosis (data not shown).
TABLE 1.
Prevalence of E. bieneusi strain genotypes in stool specimens
| Clinical status of patients (n) | No. (%) of isolates of type:
|
||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| HIV infected (88) | 66 (75) | 9 (10) | 3 (4) | 9 (10) | 1 (1) |
| Not HIV infected (12) | |||||
| Transplant recipients (8) | 0 (0) | 7 (87.5) | 0 (0) | 1 (12.5) | 0 (0) |
| Lymphoma (1) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| Myeloma (1) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
| Immunocompetent (2) | 0 (0) | 1 (50) | 0 (0) | 1 (50) | 0 (0) |
| Total (100) | 66 (66) | 18 (18) | 3 (3) | 12 (12) | 1 (1) |
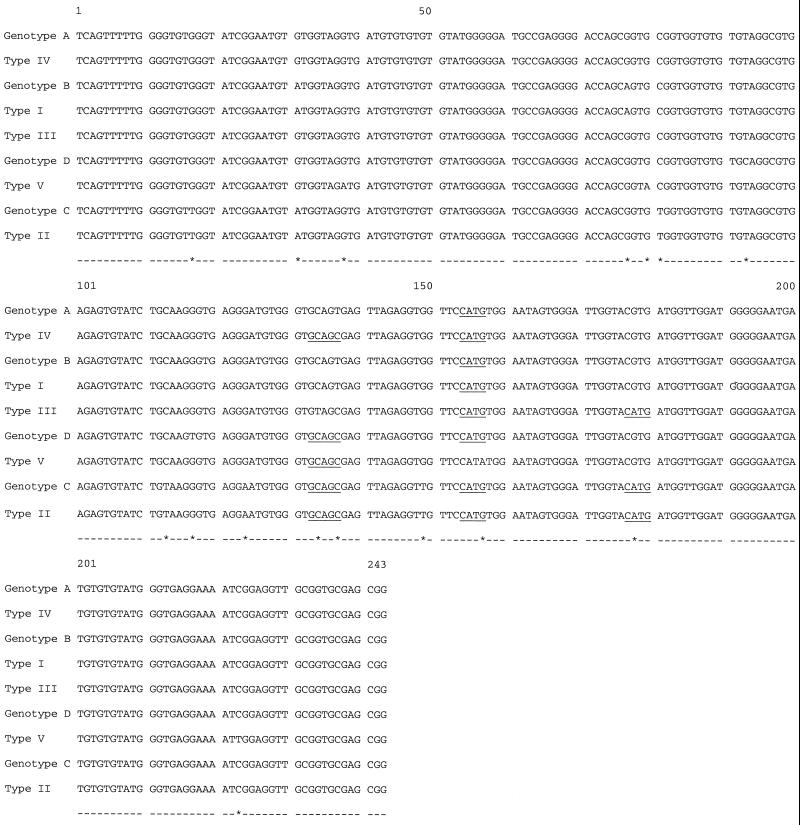
For a more definitive analysis of the genetic diversity of these strains, DNA sequencing of the entire ITS was performed for eight isolates representative of the five PCR-RFLP genotypes. The ITS region of the rRNA gene was amplified by PCR in a Hybaid touchdown thermal cycler (Teddington, Middlesex, United Kingdom) with the MSP3-MSP4 primer set (6). These primers amplify a 508-bp fragment of E. bieneusi containing 122 bp of the 3′ end of the small-subunit rRNA gene, 243 bp of the ITS, and 143 bp of the 5′ region of the large-subunit rRNA gene. Both strands of the PCR products were then sequenced by automated means (ABI PRISM 377 system; Perkin-Elmer, Courtaboeuf, France), and sequences were edited with the Sequence Navigator (Perkin-Elmer) program. All sequence analyses were performed at the website of the Belozersky Institute of Physico-Chemical Biology (http://www.genebee.msu.su/index.html) with phylogenetic tree prediction using the cluster algorithm setting. The nucleotide sequences from the three type II strains analyzed were 100% identical, as were the sequences from the two strains belonging to type IV. Sequences were compared with other available E. bieneusi ITS sequences of human and animal origin (1, 3, 9, 11, 12). Alignment of our sequences with the four human E. bieneusi sequences (GenBank accession numbers AF101197 to AF101200) previously described by Rinder et al. (10) showed a total of 16 polymorphic sites within the 243 bp of the rDNA ITS despite the strong homology (>97%) among isolates, offering the possibility of epidemiologic studies within this particular group of strains (Fig. 1). Sequence analyses of our isolates revealed that only types I and II could be assigned to one of the four genotypes described by Rinder et al. (10). Specifically, isolates of type I belong to genotype B, and isolates of type II belong to genotype C.
FIG. 1.
DNA sequence alignment of the nine human E. bieneusi rDNA ITS sequences available in GenBank. Genotypes A to D have GenBank accession numbers AF101197 to AF1001200, respectively; types I to V have accession numbers of AF242475 to AF242479, respectively. The NlaIII (CATG) and Fnu4HI (GCnGC) digestion sites used in RFLP analysis are underlined. Polymorphic sites are represented by asterisks. Genotype B and type I as well as genotype C and type II shared 100% homology.
We also confirmed that E. bieneusi strains from naturally infected animals differ from those identified so far in humans (ITS homology of >95% for pig, cat, and cattle strains but of only 47% for the dog strain [data not shown]). Strains from pigs, cats, and cattle, however, fell into genotypes I and IV. The dog isolate, which did not carry any restriction site, would belong to a sixth genotype.
Using a PCR-RFLP method with a large number of strains isolated from humans, we found that the distribution of genotypes was different for HIV-infected and non-HIV-infected patients. The possibility that the patients included in this study contracted their infections from a common source seems unlikely, since the material was collected over a 6-year period and since the patients lived in different geographic areas. Thus, despite the small number of isolates obtained from non-HIV-infected patients in this study, this particular distribution of genotypes may suggest differences in the epidemiology of the infection according to HIV infection status or differences in the virulence of the strain. Further studies of a greater number of E. bieneusi strains isolated from non-HIV-infected patients are warranted to confirm our data.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences of types I to V described here are AF242475 to AF242479, respectively.
Acknowledgments
We thank M. Rabodonirina and S. Bretagne for providing us with stool specimens from organ transplant recipients.
Grant support was from Ensemble contre le Sida (SIDACTION), Agence Nationale de Recherches sur le SIDA (ANRS essais 034, 054, 090), and Centre d'Etude et de Recherche en Infectiologie.
REFERENCES
- 1.Breitenmoser A C, Mathis A, Bürgi E, Weber R, Deplazes P. High prevalence of Enterocytozoon bieneusi in swine with four genotypes that differ from those identified in humans. Parasitology. 1999;118:447–453. doi: 10.1017/s0031182099004229. [DOI] [PubMed] [Google Scholar]
- 2.Bryan R T, Schwartz D A. Epidemiology of microsporidiosis. In: Wittner M, editor. The microsporidia and microsporidiosis. Washington, D.C.: ASM Press; 1999. pp. 502–516. [Google Scholar]
- 3.del Aguila C, Izquierdo F, Navajas R, Pieniazek N J, Miro G, Alonso A I, Da Silva A J, Fenoy S. Enterocytozoon bieneusi in animals: rabbits and dogs as new hosts. J Eukaryot Microbiol. 1999;46:8S–9S. [PubMed] [Google Scholar]
- 4.Fournier S, Liguory O, Garrait V, Gangneux J P, Sarfati C, Derouin F, Molina J M. Microsporidiosis due to Enterocytozoon bieneusi as possible cause of traveller's diarrhea. Eur J Clin Microbiol Infect Dis. 1998;10:743–744. doi: 10.1007/s100960050176. [DOI] [PubMed] [Google Scholar]
- 5.Guerard A, Rabodonirina M, Cotte L, Liguory O, Piens M A, Daoud S, Picot S, Touraine J L. Intestinal microsporidiosis occurring in two renal transplant recipients treated with mycophenolate mofetil. Transplantation. 1999;68:699–707. doi: 10.1097/00007890-199909150-00017. [DOI] [PubMed] [Google Scholar]
- 6.Katzwinkel-Wladarsch S, Lieb M, Heise W, Löscher T, Rinder H. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop Med Int Health. 1996;1:373–378. doi: 10.1046/j.1365-3156.1996.d01-51.x. [DOI] [PubMed] [Google Scholar]
- 7.Liguory O, David F, Sarfati C, Derouin F, Molina J M. Determination of types of Enterocytozoon bieneusi strains isolated from patients with intestinal microsporidiosis. J Clin Microbiol. 1998;36:1882–1885. doi: 10.1128/jcm.36.7.1882-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liguory O, David F, Sarfati C, Schuitema A R J, Hartskeerl R A, Derouin F, Modai J, Molina J M. Diagnosis of infections caused by Enterocytozoon bieneusi and Encephalitozoon intestinalis using polymerase chain reaction in stool specimens. AIDS. 1997;11:723–726. doi: 10.1097/00002030-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Mathis A, Breitenmoser A C, Deplazes P. Detection of new Enterocytozoon genotypes in faecal samples of farm dogs and a cat. Parasite. 1999;6:189–193. doi: 10.1051/parasite/1999062189. [DOI] [PubMed] [Google Scholar]
- 10.Rinder H, Katzwinkel-Wladarsch S, Löscher T. Evidence for the existence of genetically distinct strains of Enterocytozoon bieneusi. Parasitol Res. 1997;83:670–676. doi: 10.1007/s004360050317. [DOI] [PubMed] [Google Scholar]
- 11.Rinder H, Thomschke A, Dengjel B, Gothe R, Löscher T, Zahler M. Close genotypic relationship between Enterocytozoon bieneusi from humans and pigs and first detection in cattle. J Parasitol. 2000;86:185–188. doi: 10.1645/0022-3395(2000)086[0185:CGRBEB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Rinder H, Katzwinkel-Wladarsch S, Thomschke A, Löscher T. Strain differentiation in microsporidia. Tokai J Exp Clin Med. 1999;23:433–437. [PubMed] [Google Scholar]
- 13.Velasquez J N S, Carnevale E A, Guarnera E A, Labbé J H, Chertcoff A, Cabrera M G, Rodriguez M I. Detection of the microsporidian parasite Enterocytozoon bieneusi in specimens from patients with AIDS by PCR. J Clin Microbiol. 1996;34:3230–3232. doi: 10.1128/jcm.34.12.3230-3232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]