Abstract
Subdural hematomas (SDHs) are increasingly common and can cause ischemic brain injury. Previous work has suggested that this is driven largely by vascular compression from herniation, although this work was done before the era of magnetic resonance imaging (MRI). We thus sought to study SDH-related ischemic brain injury by looking at patterns of cytotoxic edema on diffusion-weighted MRI. To do so, we identified all SDH patients at a single institution from 2015 to 2019 who received an MRI within 2 weeks of presentation. We reviewed all MRIs for evidence of restricted diffusion consistent with cytotoxic edema. Cases were excluded if the restricted diffusion could have occurred as a result of alternative etiologies (e.g., cardioembolic stroke or diffuse axonal injury). We identified 450 SDH patients who received an MRI within 2 weeks of presentation. Twenty-nine patients (∼6.5% of all MRIs) had SDH-related cytotoxic edema, which occurred in two distinct patterns. In one pattern (N = 9), patients presented as comatose with severe midline shift and were found to have cytotoxic edema in the vascular territories of the anterior and posterior cerebral artery, consistent with herniation-related vascular compression. In the other pattern (N = 19), patients often presented as awake with less midline shift and developed cytotoxic edema in the cortex adjacent to the SDH outside of typical vascular territories (peri-SDH cytotoxic edema). Both patterns occurred in 1 patient. The peri-SDH cytotoxic edema pattern is a newly described type of secondary injury and may involve direct toxic effects of the SDH, spreading depolarizations, or other mechanisms.
Keywords: ischemia, secondary insult, subdural hematoma
Introduction
Subdural hematomas (SDHs) are a major source of morbidity and mortality in the United States, leaving up to 50% of patients dead or disabled at 3 months.1 Whereas other forms of intracranial hemorrhage are stable in incidence,2,3 SDHs are projected to become the most common neurosurgical condition by 2030.4,5 Much of this rising incidence has been attributed to minor traumas in an aging population with increased antithrombotic use.6 Apart from surgical decompression in appropriate patients, there are few evidence-based interventions to improve outcomes from this increasingly common disease.
Ischemic neuronal injury accounts, in part, for SDH-associated morbidity and mortality,7 although the mechanisms by which this occurs remain poorly understood. Animal models have argued that SDHs directly cause ischemic injury to the underlying cortex through mechanisms like regional hypoperfusion, toxic effects from blood breakdown products, and spreading depolarizations.8–12 A direct, clinically significant effect of SDHs on the underlying cortex would have major therapeutic implications, given that it could provide a target for medical interventions and help further clarify surgical indications.
In contrast to the animal data, work in human subjects has not consistently identified a direct SDH-related effect. The largest study suggested that SDHs caused ischemic injury indirectly through herniation-related compression of the posterior (PCA) and anterior cerebral arteries (ACA).13 This earlier work was limited by its use of radionuclide imaging, a technique with limited sensitivity for smaller regions of ischemic injury.14 Diffusion-weighted magnetic resonance imaging (MRI) is exquisitely sensitive for even small areas of cytotoxic edema, an indicator of neuronal injury most commonly caused by ischemia.15 Cytotoxic edema is known to complicate traumatic brain injury, although the potential mechanisms of this phenomenon have not been fully worked out,16 and there has been no large systematic study of MRI patterns of cytotoxic edema in SDH patients. We thus sought to characterize the radiological and clinical characteristics of SDH patients found to have cytotoxic edema on MRI in the acute period.
Methods
Study design and participants
In this retrospective observational study, all patients presenting to a single level 1 trauma center from October 2015 through September 2020 were screened for incident SDH presentations using discharge diagnosis codes from the International Classification of Diseases, Tenth Revision (S06.5, I62.0). These codes have been previously validated and reliably identify patients with SDHs of all radiological acuities.17 From these incident SDH cases, we identified all cases that met the following inclusion criteria: 1) age >18 and 2) availability of MRI data within 2 weeks of SDH symptom onset. Patients who experienced acute symptomatic expansion of a previously diagnosed SDH were also included. Patients were enrolled based upon clinical time of symptom onset irrespective of radiological SDH characteristics. Patients with potential neoplasms, infections, post-operative subdural collections, or hemorrhages from arteriovenous malformations/aneurysms were excluded. Patients who underwent surgery with any significant manipulation of the cortex (e.g., clot extractions) were also excluded. Surgical decision making and medical management were at the discretion of the treating neurosurgeon.
All MRIs were obtained for clinical purposes at the discretion of the treating physician; at our institution, MRIs are typically obtained for the purpose of prognostication or evaluation of focal neurological deficits. This study was approved by the local institutional review board at the University of Cincinnati (Cincinnati, OH) with a waiver from informed consent.
Data abstraction
For all identified patients, an electronic standardized case report form was used to collect clinical data, including demographics, labs, medical history, initial Glasgow Coma Scale (GCS) scores, neurological examination findings, surgical management/timing, and hospital complications including seizures. Data were abstracted by a single reviewer (D.R.), and 10% of data were double-abstracted by a second reviewer to confirm accuracy of the process. Initial computed tomography (CT) imaging was reviewed for all patients in order to calculate SDH volume18 and assess the radiological characteristics of the SDHs in a standardized fashion as acute (homogeneous and hyperdense on the initial CT) versus all other SDHs (including subacute, mixed density, or chronic radiological characteristics). Basal cisterns were graded as either effaced (including both complete and partial effacement) or not effaced. Midline shift was assessed at the level of the septum pellucidum. Data from clinically obtained CT angiograms within 4 weeks on incident SDH were abstracted where available. For patients without dedicated vascular imaging, MRI T2 flow voids were examined to rule out significant proximal vasospasm. Modified Rankin scales at 3 months were determined by chart review and were available for all but 1 patient.
Magnetic resonance imaging data
All MRIs were performed on 1.5 Tesla systems (GE Optima MR450W, GE Signa Explorer, or GE Signa HDxt; GE Healthcare, Chicago, IL). All images were initially reviewed by a neuroradiologist. Imaging reports were reviewed for all patients and marked for any occurrence of the following phrases: “restricted diffusion”; “acute ischemia”; or “hyperintense signal on diffusion-weighted imaging.” Images for all MRIs that were identified by the above method were then directly reviewed by a board-certified neurologist (D.R.) to further adjudicate and characterize the cytotoxic edema. Equivocal cases were reviewed by a second neurologist (B.F.), and disagreements were resolved by discussion. The following sequences were reviewed for all patients: T2 fluid attenuated inversion recovery (T2 FLAIR); diffusion-weighted imaging (DWI); apparent diffusion coefficient (ADC); and either gradient echo (GRE) or susceptibility-weighted angiography (SWAN). Cytotoxic edema was defined as brain parenchyma that was bright on DWI and dark on ADC.15 FLAIR and GRE/SWAN sequences were used to evaluate for vasogenic edema and hemorrhage, respectively.
To be considered SDH-related cytotoxic edema, patients could not have an alternative explanation for the identified cytotoxic edema (e.g., stroke from cardioembolism, blunt cerebrovascular injury, or large-vessel vasospasm, based on chart review). We also excluded patients with extensive intraparenchymal hemorrhage in the region of the cytotoxic edema that could confound interpretation.19 Finally, we excluded any patients with cytotoxic edema in a pattern consistent with diffuse axonal injury (i.e., punctate lesions at the gray-white border or multi-focal lesions in the deep white matter with associated hemorrhage).20
Statistical analysis
Descriptive statistics are provided using median (interquartile range; IQR) for all continuous variables (and GCS), whereas categorical data are presented as number (percentage). Continuous variables were compared using Wilcoxon's rank-sum test, and Fisher's exact test was used for categorical data considering the small sample size. All statistical analyses were conducted in R (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria). A p value of 0.05 was used as the threshold for significance.
Results
Identification of the cohort
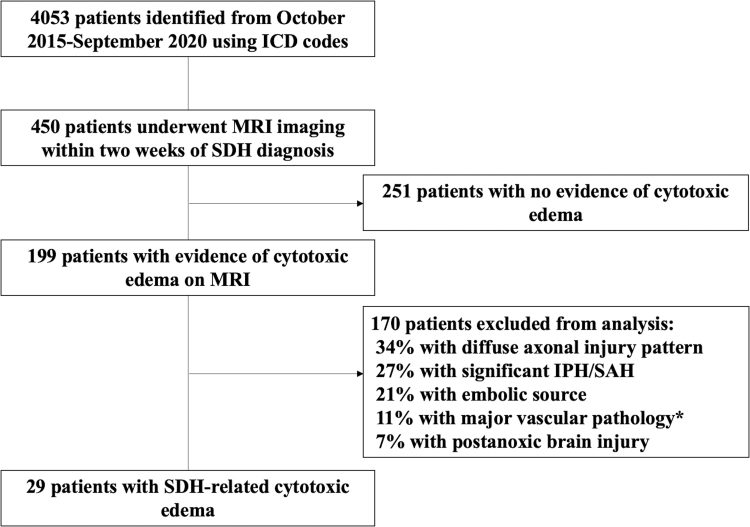
The study flow diagram is shown in Figure 1. Over the 5-year study period, 4053 SDH patients were identified. Four hundred fifty (∼11%) of these patients received an MRI within 2 weeks of diagnosis, with restricted diffusion identified in the reports of 199 cases. Of these 199 cases, 29 patients met all of our criteria for SDH-related cytotoxic edema (∼6.5% of all patients receiving an MRI).
FIG. 1.
Flow diagram of study. *Includes atherosclerosis, moderate-severe vasospasm, and blunt cerebrovascular injury from trauma. ICD, International Classification of Diseases; IPH, intraparenchymal hemorrhage; MRI, magnetic resonance imaging; SAH, subarachnoid hemorrhage SDH, subdural hematoma.
Patterns of subdural hematoma–related cytotoxic edema
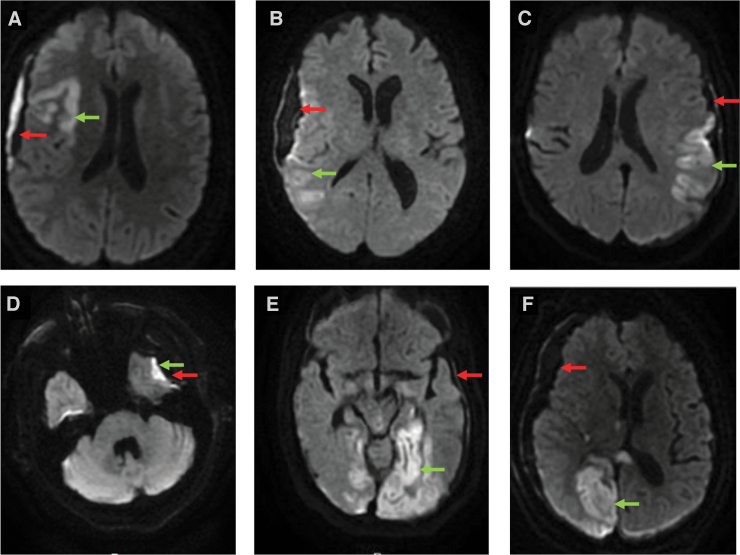
MRIs of patients with SDH-related cytotoxic edema were reviewed in depth and two distinct patterns were identified (Fig. 2). In one pattern (N = 19), cytotoxic edema occurred primarily in the cortex near the SDH and not in traditional vascular distributions (peri-SDH pattern, shown in Fig. 2A–D). In the other pattern (N = 9), cytotoxic edema occurred distant from the SDH, typically in the vascular distribution of the ACA, PCA, or watershed regions (ACA/PCA pattern, Fig. 2E,F). Both patterns were identified in 1 patient.
FIG. 2.
Patterns of cytotoxic edema. Cytotoxic edema is identified with green arrows; SDHs are identified with red arrows. (A-D) Representative patients with peri-SDH cytotoxic edema. (E,F) Representative patients with ACA/PCA pattern of cytotoxic edema. ACA, anterior cerebral artery; PCA, posterior cerebral artery; SDH, subdural hematoma.
Characteristics of patients with subdural hematoma–related cytotoxic edema
Comparison of patients with the peri-SDH pattern of cytotoxic edema and the ACA/PCA pattern of cytotoxic edema are shown in Table 1 (with the patient with both peri-SDH and ACA/PCA patterns excluded from this analysis). MRIs were obtained ∼3.5 days after trauma or symptom onset in both groups. SDHs in both groups most commonly had acute radiological characteristics (58% in the peri-SDH group and 67% in the ACA/PCA group). Some differences were identified between the two groups. Patients with the ACA/PCA pattern were younger (median age of 57 in the ACA/PCA group vs. 71 in the peri-SDH group; p < 0.01), more likely to present with low initial scores on the GCS (median GCS score of 5 vs. 14; p < 0.01), more likely to have effacement of their basal cisterns on the initial imaging (100% vs. 42%; p < 0.01), had more midline shift (14 vs. 6.5 mm; p = 0.02), were more likely to undergo emergent surgery (78% vs. 5%; p < 0.01), and more commonly received osmolar therapy (89% vs. 26%; p < 0.01). Seizures were more common in patients with the peri-SDH pattern (37% vs. 11%), but this was not statistically significant (p = 0.21). There was also a non-significant increase in frequency of subarachnoid hemorrhage in the peri-SDH cohort (58% vs. 33%). Functional outcome at 3 months, as assessed by the modified Rankin Scale, was not significantly different between the two groups (median score of 2 in the peri-SDH pattern vs. 4 in the ACA/PCA pattern; p = 0.47).
Table 1.
Comparison of Cohorts with Peri-SDH and ACA/PCA Patterns of Cytotoxic Edemaa
| Peri-SDH pattern | ACA/PCA pattern | p value | |
|---|---|---|---|
| No. of patients | 19 | 9 | |
| Age, years | 71 (58.0, 74.5) | 57 (49, 60) | <0.01 |
| Male sex | 8 (42) | 7 (78) | 0.11 |
| Days between initial symptom onset/trauma and MRI | 3.8 (3.2, 7.3) | 3.5 (2.5, 6.5) | 0.71 |
| Initial Glasgow Coma Scale | 14 (12.5, 15.0) | 5 (3, 6) | <0.01 |
| Trauma history | 13 (68) | 7 (78) | 1.0 |
| Fall <3 feet | 10 (53) | 3 (33) | 0.43 |
| High-velocity traumab | 3 (16) | 4 (44) | 0.16 |
| Midline shift, mm | 6.5 (3.5, 8.5) | 14 (7, 16) | 0.02 |
| Volume, mL | 54 (40, 112) | 89.5 (65, 131) | 0.14 |
| Effacement of the basilar cisterns | 8 (42) | 9 (100) | <0.01 |
| Acute radiological characteristics | 11 (58) | 6 (67) | 1.0 |
| Subacute, mixed, or chronic radiological characteristics | 8 (42) | 3 (33) | 1.0 |
| Subarachnoid hemorrhage | 11 (58) | 3 (33) | 0.4 |
| Distant intraparenchymal hemorrhage | 7 (37) | 5 (56) | 0.44 |
| Hyperosmolar treatment | 5 (26) | 8 (89) | <0.01 |
| Hypotension (SBP <90 mm Hg) | 5 (26) | 3 (33) | 1.0 |
| Hypoxia (SpO2 <88%) | 3 (15) | 1 (11) | 1.0 |
| Surgery | 7 (37) | 9 (100) | <0.01 |
| Hemicraniectomy | 1 (5) | 6 (67) | <0.01 |
| Surgery within 24 h of presentation | 1 (5) | 7 (78) | <0.01 |
| Seizures | 7 (37) | 1 (11) | 0.21 |
| Modified Rankin Scale at 3 months | 2 (1, 5) | 4 (1, 6) | 0.47 |
Data are median (IQR) or n (%), unless otherwise indicated. Bolded values are statistically significant at p < 0.05.
Patient with both patterns was excluded from this analysis.
Includes motor vehicle collision, pedestrian struck, and falls from >3 feet.
SDH, subdural hematoma; ACA, anterior cerebral artery; PCA, posterior cerebral artery; MRI, magnetic resonance imaging; SBP, systolic blood pressure; SpO2, oxygen saturation; IQR, interquartile range.
Clinical presentation and evaluation of patients with peri–subdural hematoma cytotoxic edema
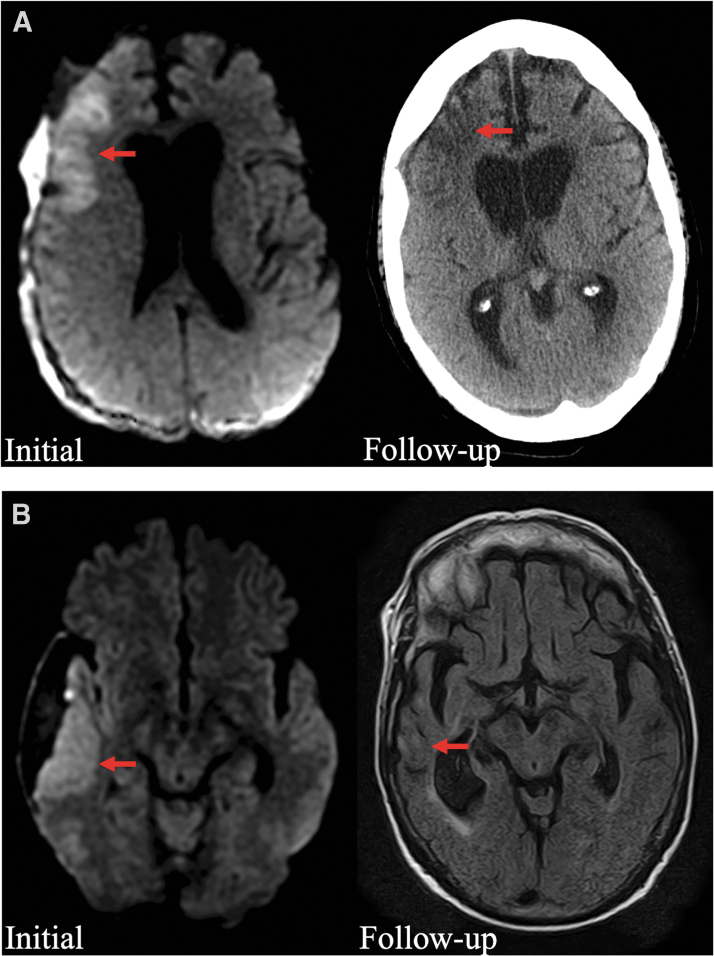
Descriptions of all patients with peri-SDH ischemia are shown in Table 2 (including the patient with both the peri-SDH and ACA/PCA patterns). Most patients (80%) had clear focal neurological deficits during their hospital course. There were waxing and waning symptoms or delayed onset of symptoms after presentation in 10 of 20 patients (50%). Seizures occurred in 8 patients; among the patients without seizures, 6 of 12 (50%) underwent continuous electroencephalographic (EEG) monitoring to confirm the absence of ictal abnormalities. Vessel imaging was obtained in 13 of 20 patients (65%), with a median time between the MRI and vessel imaging of 0 days (IQR, 0, 7). Among these patients, 3 had mild atherosclerosis, 3 had mild vasospasm, 1 had evidence of blunt cerebrovascular injury in a separate territory from the location of the cytotoxic edema, and 1 had evidence of a prominent superficial vein running through the SDH that was indeterminate as to whether or not it had thrombosed. Sixteen patients had long-term follow-up imaging >1 month after presentation; in 10 of these patients, there was evidence of persistent encephalomalacia and/or atrophy at the original site of cytotoxic edema (Fig. 3).
Table 2.
Presentation and Evaluation of Patients with the Peri-SDH Pattern of Cytotoxic Edema
| Patient | Clinical synopsis | Vessel imaging | Seizures | EEG | Follow-up image | Persistent injury |
|---|---|---|---|---|---|---|
| 1 | Presented with contralateral hemiparesis, electrographic seizures developed on day 5 | Normal | + | + | No | |
| 2 | Presented with encephalopathy, then had GTC and new hemiparesis on day 2 | Not performed | + | + | MRI | – |
| 3 | Presented with hemiparesis | Not performed | – | + | CT | – |
| 4 | Waxing and waning encephalopathy, isolated GTC | Mild atherosclerosis | + | + | MRI | – |
| 5 | Presented after a fall, developed new aphasia roughly 24 h into admission | Possible small thrombosed superficial vein in SDH | – | + | MRI | + |
| 6 | Waxing and waning encephalopathy | BCVI in wrong vascular territory | – | + | CT | + |
| 7 | Presented after a fall, developed new hemiparesis on day 3 | Not performed | – | – | MRI | + |
| 8 | Presented after a fall, developed new hemiparesis on day 3; EEG initially negative, but focal motor seizures developed on day 4. | Not performed | + | + | MRI | + |
| 9 | Presented after a fall, developed new hemineglect on day 4 | Normal | – | – | CT | – |
| 10 | Presented with encephalopathy, developed new hemiparesis on day 3; EEG initially negative, but focal motor seizures developed subsequently. | Not performed | + | + | CT | + |
| 11 | Presented with encephalopathy and hemiparesis | Mild atherosclerosis | – | – | No | |
| 12 | Presented after a fall, developed new aphasia on day . | Mild vasospasm | – | + | CT | + |
| 13 | Presented with hemiparesis | Normal | + | + | MRI | + |
| 14 | Presented with hemiparesis | Normal | – | – | CT | – |
| 15 | Presented with encephalopathy | Mild vasospasm | – | – | CT | + |
| 16 | Presented with hemisensory changes | Not performed | – | – | CT | – |
| 17 | Presented with focal motor seizure followed by hemiparesis | Normal | + | + | No | |
| 18 | Presented with encephalopathy | Mild atherosclerosis | – | + | No | |
| 19 | Presented with encephalopathy, developed new aphasia on day 3 | Not performed | – | + | CT | + |
| 20a | Presented as comatose, hemiparesis noted after surgical decompression on day 1; focal motor seizures developed on day 4. | Mild vasospasm | + | + | CT | + |
This patient had both the ACA/PCA pattern and the peri-SDH pattern.
GTC, generalized tonic-clonic seizure; SDH, subdural hematoma; BCVI, blunt cerebrovascular injury; CT, computed tomography; MRI, magnetic resonance image; EEG, electroencephalogram; ACA, anterior cerebral artery; PCA, posterior cerebral artery.
FIG. 3.
Representative long-term follow-up images for 2 patients with peri-SDH cytotoxic edema. (A) Initial diffusion-weighted MRI image shows cytotoxic edema in the right frontal lobe (left panel, red arrow), and follow-up CT at 2 months shows persistent encephalomalacia (right panel, red arrow). (B) Initial diffusion-weighted MRI shows cytotoxic edema in the right lateral temporal lobe (left panel, red arrow), and follow-up MRI at 1 year shows encephalomalacia and focal atrophy (right panel, red arrow). CT, computed tomography; MRI, magnetic resonance imaging, SDH, subdural hematoma.
Discussion
In this large retrospective cohort of SDH patients imaged with MRI within 2 weeks of incident diagnosis, two distinct patterns of SDH-related cytotoxic edema were identified. In one pattern, cytotoxic edema occurred distant from the SDH in the vascular territories of the anterior and posterior cerebral arteries (ACA/PCA pattern). This pattern predominantly occurred in comatose patients with significant midline shift and basal cistern effacement. This is consistent with ischemic brain injury from vascular compression in the setting of cerebral herniation across the falx or tentorium.13,21 In a second and more common pattern, cytotoxic edema was mostly limited to the cortex near the SDH (peri-SDH pattern). This peri-SDH pattern was not within a consistent vascular territory and occurred more frequently in non-comatose patients with lesser degrees of midline shift; it also appeared to be clinically significant, given that it was commonly associated with focal deficits (80%) and permanent brain injury on follow-up imaging (63%). This previously undescribed pattern does not appear to result from cerebral herniation and represents a novel type of secondary brain injury to target in future studies.
Cytotoxic edema develops when perturbed cellular metabolism leads to osmotic expansion of the intracellular space.22 Although our study design cannot determine the exact mechanism by which this occurred in the peri-SDH pattern, spreading depolarizations (SDs) are likely involved. SDs are pathological waves in cerebral gray matter defined by near-complete breakdown of electrochemical membrane gradients and consequent silencing of electrical activity (spreading depression).23 Breakdown of the electrochemical gradient leads to cellular swelling and thus cytotoxic edema.24–26 Although this is initially reversible,27 SDs are metabolically demanding, and repetitive SDs can lead to critical ischemia through vasoconstriction from inverse neurovascular coupling (i.e., spreading ischemia).28 This could lead to the long-term injury we observed in many patients with peri-SDH cytotoxic edema.
Several pieces of evidence argue for the importance of SDs in peri-SDH cytotoxic edema. Rodent SDH models have found that SDs contribute to cortical lesion development.11 In humans, SDs have been observed in ∼60% of patients who undergo emergency craniectomy for acute SDHs29 and contribute to post-operative neurological deterioration in chronic SDH patients.30 Finally, the peri-SDH pattern of cytotoxic edema bears a striking resemblance to the laminar infarcts observed in aneurysmal SAH patients,31–33 which have been attributed to SDs.28 Both SDs34–36 and SDH-induced lesions37,38 are, to a certain degree, treatable with N-methyl-d-aspartate receptor antagonists, and thus the potential clinical relevance of SDs is considerable; however, further studies are needed to determine optimal treatments and their impact on outcomes.
Seizures are a related pathophysiology that could also be involved in the peri-SDH pattern of cytotoxic edema. Seizures were common among patients with the peri-SDH cytotoxic edema, and the pattern resembles that described in some patients with focal seizures.39–41 This observation is consistent with the proposed role of SDs, given that seizures are observed mainly in patients with SDs after brain injury.42,43 There are several reasons why it is unlikely that seizures alone account for the observed cytotoxic edema. First, some of the patients manifested focal weakness days before seizures were apparent clinically or on EEG. Second, 10 of the peri-SDH patients had evidence of long-term injury on the MRI, which is not typical for seizure-related MRI changes.44 Third, more than half of the patients with the peri-SDH pattern did not have seizures (with many of these patients undergoing extensive EEG monitoring), suggesting that other processes are likely necessary to produce this pattern.
Finally, none of the patients in this study had other findings typical of seizure-related MRI changes, like restricted diffusion in the thalamus or hippocampus.44 Instead, the isolated neocortical pattern observed in this study has more commonly been associated with SDs28,45 or with seizures that occur in conjunction with episodes of systemic hypotension or hypoxia.40,46,47 Systemic hypotension and hypoxia were rare in patients with peri-SDH cytotoxic edema, but SDHs are known to significantly impair perfusion of the underlying cortex48; although previous studies have suggested that the resultant hypoperfusion is typically not in the true ischemic range,16,49 borderline hypoperfusion may put these patients at particular risk for seizure-related injury.
Peri-SDH cytotoxic edema was only identified in 4.5% of all MRIs and <1% of the overall SDH cohort. This likely underestimates the true burden of this injury pattern for several reasons. First, relatively few SDH patients received MRIs, and thus cases of cytotoxic edema may have been missed. Second, MRIs may not have been optimally timed to detect cytotoxic edema, given that it could be reversible in some cases27 and in others could develop in a delayed fashion. Third, we applied relatively strict exclusion criteria. Specifically, we excluded patients with evidence of diffusion restriction near a significant intraparenchymal hemorrhage. This was done because restricted diffusion can occur in the setting of traumatic contusions,50 but may have led to the exclusion of some patients who actually had ischemia-reperfusion injury with hemorrhagic conversion.9 Regardless, our data show that peri-SDH cytotoxic edema likely does not occur in the majority of SDH patients. There was not an obvious characteristic present in all patients with the peri-SDH pattern to explain why many SDH patients do not manifest this form of secondary brain injury. That said, this study is not designed to determine risk factors, which would be best examined in a prospective fashion.
There are potential limitations to this study. First, this study was conducted at a single level 1 trauma center and thus may be prone to selection bias. For instance, osmotherapy is frequently used in our institution and was common in both cohorts. Although this could affect the overall incidence of cytotoxic edema and may limit the generalizability of this study, this should not impact the distinct patterns that we observed. Second, the retrospective design of our study could bias our population toward severely injured patients, given that they are more likely to receive MRIs for prognostication. There was little evidence for this in the peri-SDH cohort (median GCS of 14), but nevertheless our findings will need to be confirmed prospectively. Third, the use of diffusion-weighted MRI to identify cytotoxic edema is potentially problematic, given that restricted diffusion can be caused by highly cellular tumors and some infectious processes51; however, this is unlikely to explain our results given that we excluded patients who were suspected of having neoplasms or cerebral infections.
Finally, although patients with evidence of moderate-severe large-vessel vasospasm were excluded from this study, a role for vasospasm cannot be ruled out given that 1) vessel imaging may not have been timed appropriately in all cases, and 2) the main method for vessel imaging (CT angiogram) may be less sensitive for distal spasm.52
Conclusion
SDH-related cytotoxic edema was found in 6.5% of SDH patients who received an MRI and can occur in two distinct patterns: a peri-SDH pattern that does not clearly follow vascular territories and an ACA/PCA pattern that is consistent with vascular compression from herniation. These results suggest that cytotoxic edema can occur in SDH patients by multiple mechanisms. Future research is needed on the mechanism, prevention, and clinical significance of peri-SDH cytotoxic edema.
Acknowledgments
The authors thank Emily Orth for her assistance gathering the initial data.
Authors' Contributions
D.R.: Conception or design of work; data collection; data analysis and interpretation; drafting the article; and final approval of the version to be published. N.K.: Conception or design of work; data analysis and interpretation; critical revision of the article; and final approval of the version to be published. L.B.N.: Conception or design of work; data analysis and interpretation; critical revision of the article; and final approval of the version to be published. O.A.: Conception or design of work; critical revision of the article; and final approval of the version to be published. D.W.: Conception or design of work; data analysis and interpretation; critical revision of the article; and final approval of the version to be published. J.H.: Conception or design of work; data analysis and interpretation; critical revision of the article; and final approval of the version to be published. B.P.F.: Conception or design of work; data collection; data analysis and interpretation; critical revision of the article; and final approval of the version to be published.
Funding Information
David Robinson reports relevant funding for his research time from the National Institute of Neurological Disorders and Stroke, 5T32NS047996-14. The funders had no role in the review or analysis of the data.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Weimer, J.M., Gordon, E., and Frontera, J.A. (2017). Predictors of functional outcome after subdural hematoma: a prospective study. Neurocrit. Care 26, 70–79. [DOI] [PubMed] [Google Scholar]
- 2. de Rooij, N.K., Linn, F.H., van der Plas, J.A., Algra, A., and Rinkel, G.J. (2007). Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 78, 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flaherty, M., Woo, D., and Broderick, J. (2010). The epidemiology of intracerebral hemorrhage, in Intracerebral Hemorrhage. J.R. Carhuapoma, S.A. Mayer, and D.A. Hanley (Eds.), Cambridge University Press: Cambridge, UK, pps. 1–10. [Google Scholar]
- 4. Frontera, J.A., Egorova, N., and Moskowitz, A.J. (2011). National trend in prevalence, cost, and discharge disposition after subdural hematoma from 1998–2007. Crit. Care Med. 39, 1619-1625. [DOI] [PubMed] [Google Scholar]
- 5. Balser, D., Farooq, S., Mehmood, T., Reyes, M., and Samadani, U. (2015). Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J. Neurosurg. 123, 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaist, D., Garcia Rodriguez, L.A., Hellfritzsch, M., Poulsen, F.R., Halle, B., Hallas, J., and Pottegard, A. (2017). Association of antithrombotic drug use with subdural hematoma risk. JAMA, 317, 836–846. [DOI] [PubMed] [Google Scholar]
- 7. Graham, D.I., Adams, J.H., and Doyle, D. (1978). Ischaemic brain damage in fatal non-missile head injuries. J. Neurol. Sci. 39, 213–234. [DOI] [PubMed] [Google Scholar]
- 8. Miller, J.D., Bullock, R., Graham, D.I., Chen, M.H., and Teasdale, G.M. (1990). Ischemic brain damage in a model of acute subdural hematoma. Neurosurgery 27, 433–439. [DOI] [PubMed] [Google Scholar]
- 9. Yokobori, S., Nakae, R., Yokota, H., Spurlock, M.S., Mondello, S., Gajavelli, S., and Bullock, R.M. (2018). Subdural hematoma decompression model: a model of traumatic brain injury with ischemic-reperfusional pathophysiology: a review of the literature. Behav. Brain Res. 340, 23–28. [DOI] [PubMed] [Google Scholar]
- 10. Jussen, D., Krenzlin, H., Papaioannou, C., Ens, S., Kempski, O., and Alessandri, B. (2017). Blood aggravates histological and functional damage after acute subdural hematoma in rats. J. Neurotrauma 34, 906–913. [DOI] [PubMed] [Google Scholar]
- 11. Krenzlin, H., Jussen, D., Plath, M., Tretzel, S. J., Krämer, T., Kempski, O., and Alessandri, B. (2019). Occurrence of spontaneous cortical spreading depression is increased by blood constituents and impairs neurological recovery after subdural hematoma in rats. J. Neurotrauma, 36, 395–402. [DOI] [PubMed] [Google Scholar]
- 12. Baechli, H., Behzad, M., Schreckenberger, M., Buchholz, H.G., Heimann, A., Kempski, O., and Alessandri, B. (2010). Blood constituents trigger brain swelling, tissue death, and reduction of glucose metabolism early after acute subdural hematoma in rats. J. Cereb. Blood Flow Metab. 30, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abe, M., Udono, H., Tabuchi, K., Uchino, A., Yoshikai, T., and Taki, K. (2003). Analysis of ischemic brain damage in cases of acute subdural hematomas. Surg. Neurol. 59, 464–472; discussion, 472. [DOI] [PubMed] [Google Scholar]
- 14. Brass, L.M., Walovitch, R.C., Joseph, J.L., Léveillé, J., Marchand, L., Hellman, R.S., Tikofsky, R.S., Masdeu, J.C., Hall, K.M., and Van Heertum, R.L. (1994). The role of single photon emission computed tomography brain imaging with 99mtc-bicisate in the localization and definition of mechanism of ischemic stroke. J Cereb Blood Flow Metab, 14, Suppl. 1, S91–S98. [PubMed] [Google Scholar]
- 15. Kim, B.J., Kang, H.G., Kim, H.J., Ahn, S.H., Kim, N.Y., Warach, S., and Kang, D.W. (2014). Magnetic resonance imaging in acute ischemic stroke treatment. J. Stroke 16, 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marmarou, A., Signoretti, S., Fatouros, P.P., Portella, G., Aygok, G.A., and Bullock, M.R. (2006). Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J. Neurosurg. 104, 720–730. [DOI] [PubMed] [Google Scholar]
- 17. Poulsen, F.R., Halle, B., Pottegard, A., Garcia Rodriguez, L.A., Hallas, J., and Gaist, D. (2016). Subdural hematoma cases identified through a danish patient register: diagnosis validity, clinical characteristics, and preadmission antithrombotic drug use. Pharmacoepidemiol. Drug Saf. 25, 1253–1262. [DOI] [PubMed] [Google Scholar]
- 18. Gebel, J.M., Sila, C.A., Sloan, M.A., Granger, C.B., Weisenberger, J.P., Green, C.L., Topol, E.J., and Mahaffey, K.W. (1998). Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 Trial. Stroke, 29, 1799–1801. [DOI] [PubMed] [Google Scholar]
- 19. Lin, D.D., Filippi, C.G., Steever, A.B., and Zimmerman, R.D. (2001). Detection of intracranial hemorrhage: comparison between gradient-echo images and b(0) images obtained from diffusion-weighted echo-planar sequences. AJNR Am. J. Neuroradiol. 22, 1275–1281. [PMC free article] [PubMed] [Google Scholar]
- 20. Skandsen, T., Kvistad, K.A., Solheim, O., Strand, I.H., Folvik, M., and Vik, A. (2010). Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J. Neurosurg. 113, 556–563. [DOI] [PubMed] [Google Scholar]
- 21. Mirvis, S.E., Wolf, A.L., Numaguchi, Y., Corradino, G., and Joslyn, J.N. (1990). Posttraumatic cerebral infarction diagnosed by CT: prevalence, origin, and outcome. AJR Am. J. Roentgenol. 154, 1293–1298. [DOI] [PubMed] [Google Scholar]
- 22. Liang, D., Bhatta, S., Gerzanich, V., and Simard, J.M. (2007). Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurg. Focus, 22, E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hartings, J.A., Shuttleworth, C.W., Kirov, S.A., Ayata, C., Hinzman, J.M., Foreman, B., Andrew, R.D., Boutelle, M.G., Brennan, K.C., Carlson, A.P., Dahlem, M.A., Drenckhahn, C., Dohmen, C., Fabricius, M., Farkas, E., Feuerstein, D., Graf, R., Helbok, R., Lauritzen, M., Major, S., Oliveira-Ferreira, A.I., Richter, F., Rosenthal, E.S., Sakowitz, O.W., Sánchez-Porras, R., Santos, E., Schöll, M., Strong, A.J., Urbach, A., Westover, M.B., Winkler, M.K., Witte, O.W., Woitzik, J., and Dreier, J.P. (2017). The continuum of spreading depolarizations in acute cortical lesion development: examining Leão's legacy. J. Cereb. Blood Flow Metab. 37, 1571–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dreier, J.P., Lemale, C.L., Kola, V., Friedman, A., and Schoknecht, K. (2018). Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology 134, 189–207. [DOI] [PubMed] [Google Scholar]
- 25. Van Harreveld, A., and Schade, J.P. (1959). Chloride movements in cerebral cortex after circulatory arrest and during spreading depression. J. Cell. Comp. Physiol. 54, 65–84. [DOI] [PubMed] [Google Scholar]
- 26. de Crespigny, A., Röther, J., van Bruggen, N., Beaulieu, C., and Moseley, M.E. (1998). Magnetic resonance imaging assessment of cerebral hemodynamics during spreading depression in rats. J. Cereb. Blood Flow Metab. 18, 1008–1017. [DOI] [PubMed] [Google Scholar]
- 27. Kirov, S.A., Fomitcheva, I.V., and Sword, J. (2020). Rapid neuronal ultrastructure disruption and recovery during spreading depolarization-induced cytotoxic edema. Cereb. Cortex 30, 5517–5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dreier, J.P., Major, S., Manning, A., Woitzik, J., Drenckhahn, C., Steinbrink, J., Tolias, C., Oliveira-Ferreira, A.I., Fabricius, M., Hartings, J.A., Vajkoczy, P., Lauritzen, M., Dirnagl, U., Bohner, G., and Strong, A. J. (2009). Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 132, 1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hartings, J.A., Andaluz, N., Bullock, M.R., Hinzman, J.M., Mathern, B., Pahl, C., Puccio, A., Shutter, L.A., Strong, A.J., Vagal, A., Wilson, J.A., Dreier, J.P., Ngwenya, L.B., Foreman, B., Pahren, L., Lingsma, H., and Okonkwo, D.O. (2020). Prognostic value of spreading depolarizations in patients with severe traumatic brain injury. JAMA Neurol. 77, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohammad, L.M., Abbas, M., Shuttleworth, C.W., Ahmadian, R., Bhat, A., Hill, D.A., and Carlson, A.P. (2020). Spreading depolarization may represent a novel mechanism for delayed fluctuating neurological deficit after chronic subdural hematoma evacuation. J. Neurosurg. doi: 10.3171/2020.1.JNS192914. [DOI] [PubMed] [Google Scholar]
- 31. Birse, S.H., and Tom, M.I. (1960). Incidence of cerebral infarction associated with ruptured intracranial aneurysms. A study of 8 unoperated cases of anterior cerebral aneurysm. Neurology, 10, 101–106. [DOI] [PubMed] [Google Scholar]
- 32. Dreier, J.P., Sakowitz, O.W., Harder, A., Zimmer, C., Dirnagl, U., Valdueza, J.M., and Unterberg, A.W. (2002). Focal laminar cortical mr signal abnormalities after subarachnoid hemorrhage. Ann. Neurol. 52, 825–829. [DOI] [PubMed] [Google Scholar]
- 33. Weidauer, S., Vatter, H., Beck, J., Raabe, A., Lanfermann, H., Seifert, V., and Zanella, F. (2008). Focal laminar cortical infarcts following aneurysmal subarachnoid haemorrhage. Neuroradiology, 50, 1–8. [DOI] [PubMed] [Google Scholar]
- 34. Carlson, A.P., Abbas, M., Alunday, R.L., Qeadan, F., and Shuttleworth, C.W. (2018). Spreading depolarization in acute brain injury inhibited by ketamine: a prospective, randomized, multiple crossover trial. J. Neurosurg. doi: 10.3171/2017.12.JNS171665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santos, E., Olivares-Rivera, A., Major, S., Sánchez-Porras, R., Uhlmann, L., Kunzmann, K., Zerelles, R., Kentar, M., Kola, V., Aguilera, A.H., Herrera, M.G., Lemale, C.L., Woitzik, J., Hartings, J.A., Sakowitz, O.W., Unterberg, A.W., and Dreier, J.P. (2019). Lasting s-ketamine block of spreading depolarizations in subarachnoid hemorrhage: a retrospective cohort study. Crit. Care, 23, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hartings, J.A., Ngwenya, L.B., Carroll, C.P., and Foreman, B. (2018). Letter to the editor. Ketamine sedation for the suppression of spreading depolarizations. J. Neurosurg. doi: 10.3171/2018.6.JNS18235. [DOI] [PubMed] [Google Scholar]
- 37. Chen, M.H., Bullock, R., Graham, D.I., Miller, J.D., and McCulloch, J. (1991). Ischemic neuronal damage after acute subdural hematoma in the rat: effects of pretreatment with a glutamate antagonist. J. Neurosurg. 74, 944–950. [DOI] [PubMed] [Google Scholar]
- 38. Inglis, F.M., Bullock, R., Chen, M.H., Graham, D.I., Miller, J.D., and McCulloch, J. (1990). Ischaemic brain damage associated with tissue hypermetabolism in acute subdural haematoma: reduction by a glutamate antagonist. Acta Neurochir. Suppl. (Wien), 51, 277–279. [DOI] [PubMed] [Google Scholar]
- 39. Kim, S.E., Lee, B.I., Shin, K.J., Ha, S.Y., Park, J., Park, K.M., Kim, H.C., Lee, J., Bae, S.Y., Lee, D., and Kim, S.E. (2017). Characteristics of seizure-induced signal changes on mri in patients with first seizures. Seizure 48, 62–68. [DOI] [PubMed] [Google Scholar]
- 40. Milligan, T.A., Zamani, A., and Bromfield, E. (2009). Frequency and patterns of MRI abnormalities due to status epilepticus. Seizure 18, 104–108. [DOI] [PubMed] [Google Scholar]
- 41. Briellmann, R.S., Wellard, R.M., and Jackson, G.D. (2005). Seizure-associated abnormalities in epilepsy: evidence from mr imaging. Epilepsia 46, 760–766. [DOI] [PubMed] [Google Scholar]
- 42. Dreier, J.P., Major, S., Pannek, H.W., Woitzik, J., Scheel, M., Wiesenthal, D., Martus, P., Winkler, M.K., Hartings, J.A., Fabricius, M., Speckmann, E.J., and Gorji, A. (2012). Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain 135, 259–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fabricius, M., Fuhr, S., Willumsen, L., Dreier, J.P., Bhatia, R., Boutelle, M.G., Hartings, J.A., Bullock, R., Strong, A.J., and Lauritzen, M. (2008). Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin. Neurophysiol. 119, 1973–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cianfoni, A., Caulo, M., Cerase, A., Della Marca, G., Falcone, C., Di Lella, G.M., Gaudino, S., Edwards, J., and Colosimo, C. (2013). Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur. J. Radiol. 82, 1964–1972. [DOI] [PubMed] [Google Scholar]
- 45. Dreier, J.P., Ebert, N., Priller, J., Megow, D., Lindauer, U., Klee, R., Reuter, U., Imai, Y., Einhäupl, K.M., Victorov, I., and Dirnagl, U. (2000). Products of hemolysis in the subarachnoid space inducing spreading ischemia in the cortex and focal necrosis in rats: a model for delayed ischemic neurological deficits after subarachnoid hemorrhage. J. Neurosurg. 93, 658–666. [DOI] [PubMed] [Google Scholar]
- 46. Vespa, P., Prins, M., Ronne-Engstrom, E., Caron, M., Shalmon, E., Hovda, D.A., Martin, N.A., and Becker, D.P. (1998). Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J. Neurosurg. 89, 971–982. [DOI] [PubMed] [Google Scholar]
- 47. Akgun, B., Cakin, H., Ozturk, S., Yildirim, H., Okcesiz, I., Kazan, S., and Erol, F.S. (2018). Evaluation of cortical brain parenchyma by diffusion and perfusion MRI before and after chronic subdural hematoma surgery. Turk. Neurosurg. 28, 405–409. [DOI] [PubMed] [Google Scholar]
- 48. Verweij, B.H., Muizelaar, J.P., and Vinas, F.C. (2001). Hyperacute measurement of intracranial pressure, cerebral perfusion pressure, jugular venous oxygen saturation, and laser doppler flowmetry, before and during removal of traumatic acute subdural hematoma. J. Neurosurg. 95, 569–572. [DOI] [PubMed] [Google Scholar]
- 49. Slotty, P.J., Kamp, M.A., Steiger, H.J., Cornelius, J.F., Macht, S., Stummer, W., and Turowski, B. (2013). Cerebral perfusion changes in chronic subdural hematoma. J. Neurotrauma 30, 347–351. [DOI] [PubMed] [Google Scholar]
- 50. Newcombe, V.F., Williams, G.B., Outtrim, J.G., Chatfield, D., Gulia Abate, M., Geeraerts, T., Manktelow, A., Room, H., Mariappen, L., Hutchinson, P.J., Coles, J.P., and Menon, D. K. (2013). Microstructural basis of contusion expansion in traumatic brain injury: insights from diffusion tensor imaging. J. Cereb. Blood Flow Metab. 33, 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Förster, A., Griebe, M., Gass, A., Kern, R., Hennerici, M.G., and Szabo, K. (2012). Diffusion-weighted imaging for the differential diagnosis of disorders affecting the hippocampus. Cerebrovasc. Dis. 33, 104–115. [DOI] [PubMed] [Google Scholar]
- 52. Anderson, G.B., Ashforth, R., Steinke, D.E., and Findlay, J.M. (2000). CT angiography for the detection of cerebral vasospasm in patients with acute subarachnoid hemorrhage. AJNR Am. J. Neuroradiol. 21, 1011–1015. [PMC free article] [PubMed] [Google Scholar]