Abstract
Despite multiple prior pharmacological trials in traumatic brain injury (TBI), the search for an effective, safe, and practical treatment of these patients remains ongoing. Given the ease of delivery and rapid absorption into the systemic circulation, inhalational gases that have neuroprotective properties will be an invaluable resource in the clinical management of TBI patients. In this review, we perform a systematic review of both pre-clinical and clinical reports describing inhalational gas therapy in the setting of TBI. Hyperbaric oxygen, which has been investigated for many years, and some of the newest developments are reviewed. Also, promising new therapies such as hydrogen gas, hydrogen sulfide gas, and nitric oxide are discussed. Moreover, novel therapies such as xenon and argon gases and delivery methods using microbubbles are explored.
Keywords: head trauma, oxidative stress, traumatic brain injury
Introduction
Traumatic brain injury (TBI) results in death and disability in millions of individuals throughout the world annually. Although various pharmacological agents have been trialed and many of them are still under development, an exciting field of therapeutic intervention is the use of inhalational gases that have various neuroprotective effects. Given the ease of delivery and lack of discomfort in mild TBI patients, as well as direct control of exact concentration of delivered gas in intubated severe TBI patients, inhalational gases that have neuroprotective effects have advantages as a medical intervention. This review will discuss four major inhalational gases shown to have neuroprotective effect in traumatic brain injury: oxygen (O2), hydrogen (H2), hydrogen sulfide (H2S), and nitric oxide (NO). Additionally, new advances in the field of targeted gas delivery will also be discussed. Thus far, the majority of evidence in support of their neuroprotective role is in the pre-clinical setting. Although clinical trials using hyperbaric oxygen are under way, there is a lack of robust clinical data on majority of other gases. Further validation of their therapeutic efficacy in clinical trials is much needed to advance their utility and impact the current management algorithm for patients with TBI.
To perform a comprehensive review of inhalational gases that have been used in either TBI patients or animal models of TBI, a literature search was conducted in September of 2020 using PubMed database. Search terminology was performed with the phrases (‘nitric oxide’ [MeSH Major Topic] or ‘inhalational gas’ [MeSH Major Topic] or ‘hyperbaric oxygen’ [MeSH Major Topic] or ‘hydrogen sulfide’[MeSH Major Topic] or ‘xenon’[MeSH Major Topic] or ‘hydrogen gas’[MeSH Major Topic]) AND (‘traumatic brain injury’[MeSH Major Topic] or ‘TBI’ [Title/Abstract] or ‘traumatic brain injury’ [Title/Abstract]). Appropriate articles under these search terms were included. Additional searches of articles that were referred by these articles were also included. This resulted in approximately 150 unique articles that reported the use of various inhalational gases or related chemical compounds used for treatment of TBI or other associated conditions that were relevant to the discussion in this review.
Normobaric Hyperoxia
In severe TBI, multiple mechanisms may contribute to decreased oxygen supply to the brain tissue such as increased intracranial pressure, systemic hypotension, and acute respiratory distress syndrome.1-3 As TBI can result in cerebral ischemia and hypoxia,4 optimization of arterial oxygen level has been suggested as a potential strategy to mitigate secondary insult.5
Supplemental oxygen is given readily in the intensive care unit, but the effect of potential hyperoxia on the outcome of TBI patients has always been a question among neurointensivists and neurosurgeons. Prior studies that looked at normobaric hyperoxia showed mixed results but the level of evidence was not very strong, as most were retrospective studies.6–9 Thus far, there has not been any randomized prospective study looking at the effect of normobaric hyperoxia.
In a study of a large cohort of severe TBI patients,6 hyperoxia with arterial partial pressure of oxygen (PaO2) > 300 mm Hg or hypoxia with PaO2 < 60 mm Hg was not associated with any difference in Glasgow Outcome Scale-Extended (GOSE) at 6, 12, and 24 months. However, a more focused approach looking at several PaO2 cutoff values showed that mild levels of hyperoxia were associated with better outcomes.7 In this study, PaO2 value >150 and >200 mm Hg (but not >250, > 300, or >350 mm Hg) within 24 h of hospital admission were associated with higher GOSE at 6 months, as well as improved neuropsychological outcome on a battery of eight tests. However, other retrospective studies of large cohort of patients showed that higher mortality8 and lower discharge GCS9 may result from higher PaO2 levels.
Whether normobaric hyperoxia can result in neuroprotection or not is controversial.7 In a small, focused study that looked at the effects of increasing fraction of inspired oxygen (FiO2) supplied to the severe TBI patients who are on ventilators (from 35% to 60% then 100% over a period of 6 h in 14 patients), microdialysate lactate levels decreased significantly.10 Additionally, 3-month clinical outcome was better among patients with better brain tissue oxygenation. Another prospective study on 52 severe TBI patients treated for 24 h with 100% oxygen resulted in lower intracranial pressure (ICP) and lower lactate/pyruvate ratio.11 Aside from the lack of large-scale, prospective randomized study, the lack of clarity on the effects of normobaric hyperoxia after TBI in these prior randomized studies may also be due to the fact that the optimal level of oxygenation is difficult to determine in severe TBI.5 As cerebral metabolic rate is reduced in severe TBI, oxygen extraction is reduced. Targeting high levels of arterial oxygen than required by cerebral metabolism may theoretically result in oxygen toxicity due to increased risk of oxidative stress.
Hyperbaric Oxygen
Perhaps the oldest and most rigorously tested inhalational gas for various purposes is hyperbaric oxygen therapy (HBOT). It is defined as inhalation of pure oxygen in a pressurized chamber that is greater than 1 atmospheric pressure. During more than 50 years of clinical use, HBOT has been primarily used for treatment of carbon monoxide poisoning and air embolism. In addition, HBOT has also been used empirically in the treatment of cerebral edema, spinal cord injury, stroke, and other ischemia-reperfusion injuries.12-14 The mechanisms of its benefit are thought to be multi-fold: by increasing PaO2, there is a subsequent increase in oxygen supply to the brain tissue. There is also a potential secondary effect of reducing tissue edema, thereby attenuating intracranial pressure increase and reducing secondary ischemia and resulting neuroinflammation.15
The pre-clinical evidence of benefits of HBOT is extensive, as summarized in Table 1. In a weight drop model of TBI,15–18 fluid percussion injury,19,20 blast injury,21 and controlled cortical impact,22 improvements in cell survival and functional outcome were demonstrated. In other forms of trauma, such as stab injury23 and dynamic cortical deformation24-26 improved cell survival, reduced inflammatory, and apoptotic changes were shown. In line with these findings, early clinical trials of HBOT in severe TBI patients also showed various improvements in oxidative metabolism, cerebral blood flow (CBF), reduced ICP, and functional outcomes as reported in Table 2.27-29
Table 1.
Pre-Clinical Data on Hyperbaric Oxygen Therapy in TBI
| Inhaled gas study | Form | Timing after injury (duration) | TBI type (severity) | Subject | Outcomes assessed | Findings |
|---|---|---|---|---|---|---|
| Hyperbaric oxygen15 | Inhaled 100% O2 at 2 ATA | 3 h, 7 days (60 min each session for 4 days) | Weight drop (moderate, 70 g from 80 cm height) | Mice | Cerebral cortex, hippocampus injury | Attenuated myelin basic protein loss, neuronal loss, reactive astrocytosis; improvement in learning |
| Hyperbaric oxygen20 | Inhaled 100% O2 at 1.5 ATA | 6 h, 60 days (90 min each session for 15 days) | Fluid percussion injury (severe, >120-sec time LOC) | Rats | Hippocampus injury | Improved memory, Reduced cell death, HIF-1α expression |
| Hyperbaric oxygen22 | Inhaled 100% O2 at 2 ATA | Immediately post-injury (1h each session for 3d) | Controlled cortical impact (mild, 5 m/sec, 0.8 mm depth) | Mice | Cerebral cortex injury | Reduced astrocyte and microglial activation |
| Hyperbaric oxygen23 | Inhaled 100% O2 at 2.5 ATA | Immediately post-injury (1 h each session for 10 days) | Cortical stab injury (N/A) | Rats | Cerebral cortex injury | Attenuated astrocyte markers, inflammation |
| Hyperbaric oxygen16 | Inhaled 100% O2 at 0.2 MPa | 6 h (1 h each session for 1 week, 2 weeks) | Weight drop (moderate, 25 g from 30 cm height) | Rats | Hippocampus injury | Reduced cell death and reactive astrocytosis; improved cognitive function |
| Hyperbaric oxygen24 | Inhaled 100% O2 at 2.8 ATA | 3 h, 24 h (2 consecutive 45-min sessions separated by 5-min pause) | Dynamic cortical deformation (0.605 ATA vacuum pressure) | Rats | Cerebral cortex injury | Reduced neuronal loss, axonal loss, caspase activity |
| Hyperbaric oxygen26 | Inhaled 100% O2 at 2.8 ATA | 3 h (2 consecutive 45-min sessions separated by 5-min pause each day for 3 days) | Dynamic cortical deformation (0.605 ATA vacuum pressure) | Rats | Cerebral cortex injury | Reduced cell death, neutrophilic infiltration, MMP-9 expression |
| Hyperbaric oxygen25 | Inhaled 100% O2 at 2.8 ATA | 3 h (2 consecutive 45-min sessions separated by 5-min pause each day for 3 days) | Dynamic cortical deformation (0.605 ATA vacuum pressure) | Rats | Cerebral cortex injury | Reduced cell death |
| Hyperbaric oxygen18 | Inhaled 100% O2 at 3 ATA | 3 h, 6 h, 12 h, 24 h, 48 h, 72 h (1 h) 6, 24 h, 48 h (1 h session for 1 day, 3 days, 5 days) |
Weight drop (moderate, 20g from 30-cm height) | Rats | Hippocampus injury | Improved spatial learning and memory, increased neurons, reduced reactive astroglia when treated at 3-12h post-injury |
| Hyperbaric oxygen19 | Inhaled 100% O2 at 1.5 ATA or normobaric 100% O2 or normobaric 30% O2 |
15 min (4 h normobaric 100% O2, or 1 h 100% O2 at 1.5 ATA +3 h normobaric 100% O2, or 4 h normobaric 30% O2) |
Fluid percussion injury (moderate, 2.25 atm) | Rats | Cerebral cortex ATP levels Hippocampus (neuronal loss) |
Hyperbaric O2 group had improved spatial learning, higher ATP levels, attenuated neuronal loss Normobaric 100% O2 group had higher ATP levels. |
| Hyperbaric oxygen21 | Inhaled 100% O2 at 2 ATA | 12 h (1 h session once) | Blast TBI (paper detonator-600 mg TNT equivalent at 6.5 cm distance) | Rabbit | Cerebral cortex (caspase-3, TNF-α, IL-8) | Attenuated metabolic dysfunction by MRS Reduced cerebral edema, contusion volume, apoptosis (caspase-3), inflammation (TNF-α, IL-8), preserved BBB integrity, |
| Hyperbaric oxygen17 | Inhaled 100% O2 at 0.25 MPa | 30 mins (1 h) | Weight drop (moderate, 20 g from 20cm height) | Rats | Unspecified | Attenuated apoptotic factor expression |
TBI, traumatic brain injury; ATA, atmosphere absolute; LOC, loss of consciousness; HIF, hypoxia-inducible factor; N/A, not applicable; ATP, adenosine triphosphate; BBB, blood–brain barrier; MRS, magnetic resonance spectroscopy; TNF, tumor necrosis factor; IL, interleukin.
Table 2.
Clinical Reports on Hyperbaric Oxygen Therapy for TBI
| Inhaled gas study | Study design | Intervention | Time after injury (therapy duration) | Subject number, injury type | Assessment | Findings |
|---|---|---|---|---|---|---|
| Hyperbaric oxygen33 | Single group, pre- and post-testing | Inhaled 100% O2 at 2 ATA | 6 months-27 years (90-min session for 60 days) | 15 subjects with TBI (7 moderate/severe, 8 mild) with PCS | DSC, DTI, multiple neurocognitive tests | Improved white matter integrity, brain perfusion, neurocognitive function |
| Hyperbaric oxygen27 | Single group, pre- and post-testing pilot study | Inhaled 100% O2 at 1.5 ATA | 9-24 h (1-h session for 7 days) | 37 subjects with severe TBI | CMRO2, ICP, microdialysis | Increased CMRO2, Decreased CSF lactate |
| Hyperbaric oxygen28 | Prospective, randomized | Inhaled 100% O2 at 1.5 ATA followed by normobaric 100% O2 | < 24 h (daily session with HBOT for 1 h then normobaric O2 for 3 h for 3 days) | 69 subjects with severe TBI | CMRO2, CBF, microdialysis | Improved CBF, CMRO2, Decreased CSF lactate, lactate/pyruvate ratio |
| Hyperbaric oxygen29 | Prospective, randomized | Inhaled 100% O2 at 1.5 ATA followed by normobaric 100% O2 | < 24 h (daily session with HBOT for 1 h then normobaric O2 for 3 h for 3 days) | 42 subjects with severe TBI | Sliding dichotomized GOS, microdialysis, ICP | Reduced mortality, ICP; Improved microdialysis markers of oxidative metabolism, sliding dichotomized GOS |
| Hyperbaric oxygen35 | Prospective, randomized | Inhaled 100% O2 at 2.8 ATA | 3 month-3 year (1 h sessions for 40 days) | 61 subjects with mild TBI from military combat with PCS | Rivermead questionnaire | No difference in Rivermead questionnaire |
| Hyperbaric oxygen34 | Prospective randomized | Inhaled 100% O2 at 1.5 ATA | 24.9 ± 18.1 month for sham 26.3 ± 16.5 months for HBOT (1-h sessions for 40 days) |
72 subjects with mild TBI from military TBI with PCS | Rivermead questionnaire | No difference in Rivermead questionnaire |
| Hyperbaric oxygen31 | Single group pre- and post- testing pilot study | Inhaled 100% O2 at 1.5 ATA | 1.0 year-4.75 years (1 h sessions, 2 times daily for 40 days) | 16 subjects with mild-moderate TBI from military combat with PCS | QOL questionnaire, multiple psychometric tests, SPECT | Improved QOL, working memory, global intellectual function, executive function, attention, motor speed, fine motor coordination; Improved activity on SPECT |
| Hyperbaric oxygen32 | Prospective, randomized | Inhaled 100% O2 at 1.5 ATA | 1-5 year (1 h sessions for 40 days) | 56 subjects with mild TBI with PCS | QOL questionnaire, multiple psychometric tests, SPECT | Improved information processing speed, attention, memory, executive function, quality; Improved activity on SPECT imaging |
| Hyperbaric oxygen38 | Prospective matched case-controlled | Inhaled 100% O2 at 2 ATA | 27.5 ± 5.8 days (90-min sessions for 20 days) | 44 subjects (45% mild-moderate, 55% severe in control; 55% mild-moderate, 45% severe in HBOT) | GCS, GOS | Larger improvement of GCS, Improvement among GOS 4 patients |
TBI, traumatic brain injury; N/A, not applicable; ATA, atmosphere absolute; PCS, post-concussion syndrome; SPECT, single photon emission computed tomography; GOS, Glasgow Outcome Score; ICP, intracranial pressure; CSF, cerebrospinal fluid; CMRO2, cerebral metabolic rate of O2; DSC, dynamic susceptibility contrast-enhanced magnetic resonance imaging; DTI, diffusion tensor imaging; GCS, Glasgow Coma Score; GOS, Glasgow Outcome Scale; HBOT, hyperbaric oxygen therapy; QOL, quality of life.
The beneficial effect of HBOT may be dependent on the timing of the therapy. As reduced oxygenation in TBI occurs acutely after injury, comparison of initiation times for HBOT demonstrates a therapeutic window of 6 h after TBI, but not at 60 days after TBI in rats.20 Markers of cell death and injury in the hippocampus and performance on Morris water maze improved only when intervention was performed prior to 6 h. Another detailed study looking at the therapeutic window for HBOT showed that if multiple sessions of HBOT are given, initiation of HBOT even at 48 h after TBI can be therapeutic.18 However, HBOT at more delayed initiation times may also improve memory and histological measures of injury.15
In mice subjected to weight drop model of TBI, HBOT administration for 4 days initiated either immediately (3 h following injury) or at delayed times (7 days following injury) resulted in significant improvement in novel object recognition and Y-maze test, as well as attenuated astrogliosis and losses in neurons and myelin.15 Thus, therapeutic window may be longer than the initial few hours, as cerebral oxygenation study in TBI patients also showed that a large proportion of patients have hypoxic episodes later than 24 h.30 In this earlier study by Gopinath and colleagues looking at severe TBI patients who had jugular venous oxygen saturation monitored continuously, 36.8% of desaturation episodes were within 24 h, but the rest of the episodes were in subsequent days with 12-16% of the episodes each day during Days 2-5.
Some clinical studies on patients with post-concussion syndrome show benefit up to several years after injury.31,32 As shown in Table 2, it is possible that enhanced oxygenation even after resolution of acute injury can still improve neurological function. Also, in another study of clinical trial of HBOT assessed by diffusion tensor imaging (DTI) and dynamic susceptibility contrast-enhanced (DSC) MRI, 15 subjects were administered 60 daily sessions.33 The imaging analysis showed increased fractional anisotropy and decreased mean diffusivity, which signify preserved axonal integrity, as well as increased blood flow and volume. Cognitive testing showed that subjects exposed to HBOT had improvement in major cognitive functions including memory, executive function, and information processing.
Despite these findings, the true benefit of HBOT in post-concussion syndrome has been debated, as two prospective randomized studies showed no benefit.34,35 As these studies used rigorous control groups with 1.2-1.3 atmosphere absolute (ATA) of room air, which could theoretically increase plasma oxygen by 30%, the difference between control group and experimental group in these studies may also have been difficult to detect.36 There is no arteriolar oxygen level that has been reported in these studies for confirmation, but future clinical trials could benefit from confirmation of blood oxygen content difference between the groups to ensure an adequate treatment effect.
Also, despite the reports showing therapeutic potential, there are a number of complications of HBOT that should be considered, including middle ear barotrauma, upper respiratory infection, and availability of equipment and specialized staff.31 In terms of side effects of the 16 subjects with concussion who were treated with HBOT,31 five (31%) experienced mild reversible middle ear barotrauma. Additionally, four of the subjects (25%) also experienced upper respiratory infection, possibly due to the mild immunosuppression that has been previously reported.37 Others have reported seizures in TBI patients treated with HBOT at 9%, but this may be due to their severe TBI and higher dose of oxygen at 2.0 ATA for 90 min.38 Among severe TBI patients who have risk of seizure and hemodynamic instability, treatment inside a hyperbaric chamber may pose a greater risk than other patients. Thus, a closer monitoring and access to rapid treatment should be in place before HBOT is initiated.
Neurotoxic effects can result when HBOT is applied at high pressures around 4.9 ATA.39 This is a major concern in HBOT, as high levels of oxygen can generate reactive oxygen species (ROS), as major source of oxidative stress. However, at 2.5 ATA for 75 min, there was no increase in lipid peroxidation in pre-clinical studies of global cerebral ischemia.40 Despite these concerns for generation of ROS, HBOT protocols in TBI studies have durations ranging in hours. During this short period of time, antioxidant chemicals stored within cells (superoxide dismutase, catalase, glutathione dependent peroxidase, vitamin C) may be able to prevent damage from ROS.41 However, there is much evidence that TBI alone reduces antioxidant reserve and potential added effect of oxidative stress from HBOT must be considered. Cerebrospinal fluid of severe pediatric TBI patients showed reduction of ascorbate and glutathione.42 In the post-TBI setting where antioxidant supply is largely depleted, there is a higher risk of oxidative stress from HBOT and a more thorough characterization of oxidative stress at different durations of HBOT exposure is needed in the future.
Additional insights can be gained from some reports that looked at the effects of atmospheric pressure change to neural tissue in TBI. The effect of post-TBI exposure to hypobaric conditions, for example during flight transport following military blast, have been explored previously.43 Although hyperbaric conditions were shown to increase oxygen delivery to brain tissue and reduce intracranial pressure,44 hypobaric exposure for 6 h in rats after TBI showed that there was significantly worse cognitive function, hippocampal neuronal loss, and glial activation at 7 days post-injury.45 The mechanism of deleterious effects of hypobaric condition is decreased CBF and brain tissue oxygenation, as shown by intracranial monitor in Yorkshire swine that underwent hypobaric condition for 4 h.46 However, based on these few studies, it is difficult to conclude whether the deleterious effect of hypobaric condition is due to limited oxygen delivery, or any other unknown effect of atmospheric decompression and compression of nervous tissue. Further mechanistic studies are needed on the effect of high and low atmospheric pressure asides from oxygen delivery in the setting of TBI.
However, much of the literature on HBOT have flawed methodology and do not describe factors that can lead to placebo effect in their study subjects.47 For example, there is paucity of description on social experience, environment that the patient was treated in, and the expectation of the patient and staff in terms of group assignment. Some of the prior studies often did not randomize patients or perform blinded analysis, introducing high risk for bias.48 Additionally, given wide range of protocols throughout these prior studies, an optimal parameter regarding the pressure and frequency of treatment remains unclear.
To address some of the controversies about the efficacies of HBOT, a new multi-center phase II clinical trial supported by Strategies to Innovate Emergency Care Clinical Trials Network (SIREN) across 15 centers in the United States have been recently initiated.49 Multiple arms with various pressure and duration of HBOT will be used to determine if HBOT has efficacy in severe TBI in this phase II clinical trial named Hyperbaric Oxygen Brain Injury Treatment (HOBIT).
Hydrogen
As ROS play an important role in the pathophysiology of TBI, free radical scavengers and antioxidants have been extensively studied as one of the critical strategies in treatment of TBI. One of the major oxidative stress scavengers of recent interest is molecular hydrogen (H2) which is in a gaseous state. Given its small size, it can freely penetrate cellular membranes and reduce damaging effects of ROS.50 It also has limited side effect profile and its safety has been demonstrated even in prolonged exposures of mice for 72 h,51 as well as human subjects in a 14-day treatment.52 Mixture of inhalational gases with H2 content up to 56% has been previously demonstrated as safe among deep sea divers.53 The additional advantage of H2 use is that it can also be administered in a dissolved state in saline or water with potentially similar therapeutic effect.54 This would allow the potential option of delivery via injection or oral intake.
The key factor to consider in reducing oxidative species is that there are also ROS that have important physiological roles at low concentrations such as superoxide anions (O2∙-) or hydrogen peroxide (H2O2). These agents function in signal transduction of major cellular functions such as growth, differentiation, and apoptosis.55,56 In cell free and cell culture experiments, H2 was shown to specifically reduce hydroxyl radicals (OH∙) but did not react with ROS that have major physiological roles such as H2O2, NO∙, O2∙-, and ROS inside mitochondria.57 Moreover, in these experiments it reduced peroxynitrite, another highly damaging agent after hydroxyl radical.57
Hydroxyl radicals (OH∙) may be one of the most potent ROS, reacting with nucleic acids, proteins, and lipids. It directly kills erythrocytes, damages cell membranes as well as nuclear DNA.12 As H2 can specifically reduce OH∙, the therapeutic efficacy for H2 has been investigated in the setting of high oxidative stress conditions such as reperfusion injury.57,58 Other organs with reperfusion injury such as the heart59 and liver60 have also been studied, with a therapeutic benefit of H2 demonstrated. As oxidative stress is a major driver of pathophysiology in neurodegenerative diseases, intracranial hemorrhage, and TBI, H2 has also been investigated in these settings.61–64
In an ischemia-reperfusion injury model of mice, H2 administration improved survival rate of animals and reduced hippocampal neuronal injury by reduction of oxidative stress.58 Similarly in a study of 7-day-old neonatal rats that underwent common carotid artery ligation and hypoxic injury, intraperitoneal injection of H2 in saline resulted in reduced caspase activity, inflammation, and cognitive performance.54 Intracerebral hemorrhage model of rats also showed that H2 administration reduced cerebral edema and functional impairment.62 As expected with the time course of oxidative stress after neural injury, there was a window of therapeutic effect when administered at 24 h but not at 72 h. In addition to free radical scavenging effects, there may be additional effects of H2 which are indirectly related to its function as an antioxidant. Anti-inflammatory effects of H2 also have been demonstrated.65,66
Although there have been many reports on neurodegenerative and cerebrovascular pathologies in which H2 was shown to be protective, TBI studies have not extensively explored H2 as a therapeutic agent shown in Table 3. There are three rodent studies that showed H2 given through hydrogen rich water63 and inhalation at 2%64,67 to be neuroprotective. The H2 administration resulted in reduced cerebral edema and infarct volume, as well as protection of cognitive function in rats.64,67 Histological analysis of hippocampal sections, cytokine levels in brain tissue, and magnetic resonance spectroscopy showed reduction of inflammatory markers.63
Table 3.
Molecular Hydrogen Use in Experimental TBI
| Inhaled gas study | Form | Timing after injury (duration) | TBI type (severity) | Subject | Outcomes assessed | Findings |
|---|---|---|---|---|---|---|
| Hydrogen gas63 | Hydrogen rich water (0.63-0.82 mmol/L) IP | 5 min (IP injection daily for 1 day, 2 days, 3 days, 14 days duration) | Controlled cortical impact (moderate) | Rats | Whole–brain (inflammatory cytokines), hippocampus (Iba-1, synapsin I) | Reduced TNF-α, IL-1β, HGMB1, Iba-1, inflammatory metabolites on MRS; increased IL-10, synapsin 1, improved cognitive function |
| Hydrogen gas67 | Inhaled 2% H2 | 30 min (4.5 h) | Controlled cortical impact (moderate) | Rats | Pericontusional microRNA-21 | Reduced microRNA-21, cerebral edema, contusion volume, blood–brain barrier permeability, oxidative stress; improved cognitive function |
| Hydrogen gas64 | Inhaled 2% H2 | Immediately post-injury (5 h) | Controlled cortical impact (moderate) | Rats | Cerebral cortex Injury | Reduced blood–brain barrier permeability, cerebral edema, lesion volume, improved cognitive function, reduced markers of oxidative stress |
TBI, traumatic brain injury; IP, intraperitoneal; TNF, tumor necrosis factor; HGMBI1, High mobility group box 1; MRS, magnetic resonance spectroscopy.
Although efficacy remains to be determined, the potential use of H2 as a therapy in TBI would be versatile (it can be used in either inhaled form or injected form dissolved in liquid). It functions via oxygen radical scavenging agent, and it has the most therapeutic effect when administered early after injury. It is one of the newly discovered therapies in TBI that can be further explored with much potential for future studies.
Hydrogen Sulfide
Hydrogen sulfide is endogenously expressed in areas such as the ileum, portal vein, and aorta, and have various effects in many systems, such as both pro- and anti-inflammatory effects, vasodilatory effects, anti-apoptotic effects.68,69 Its role in various processes is incompletely understood, but it is an agent that warrants further investigation given its numerous implications for cytoprotection. As it can be delivered by inhalation70 or hydrogen sulfide donor,71,72 it also has many versatile potential applications for clinical use similar to hydrogen gas.
Neuroprotective effect of hydrogen sulfide has recently been explored in several animal studies of TBI, but the body of data is much smaller than that of other gases as shown in Table 4. It has previously been considered a toxic substance but was shown to have protective effects in stroke73,74 and neurodegenerative states.75 Much of the neuroprotective effect is considered to involve anti-apoptotic and anti-inflammatory function.76 Additionally, it is known to attenuate excitotoxicity in models of ischemic brain injury77 and Parkinson's disease78 by functioning as an N-methyl-D-aspartate (NMDA) receptor antagonist.
Table 4.
Hydrogen Sulfide Use in Experimental TBI
| Inhaled Gas Study | Form | Timing after injury (duration) | TBI type (severity) | Subject | Outcomes assessed | Findings |
|---|---|---|---|---|---|---|
| Hydrogen sulfide gas79 | NaHS in saline IP injection | 30 min prior to injury (1-45 μM/kg) | Weight drop (40 g from 20 cm height) | Mice | Cerebral cortex, Hippocampus injury | Reduced cerebral edema, apoptosis and authophagy markers; improved motor function and memory |
| Hydrogen sulfide gas71 | NaHS in saline IP injection | 3 mg/kg, 5 mg/kg 5 min prior to injury |
Controlled cortical impact (moderate, 4 m/sec, 2.5 mm depth) | Rats | N/A | Improved spatial memory |
| Hydrogen sulfide gas72 | NaHS in saline injection | 3 mg/kg 5 min prior to injury | Controlled cortical impact (moderate, 4 m/sec, 2 mm depth) | Rats | Cerebral cortex injury | Improved blood brain barrier, reduced cerebral edema, contusion volume; increased antioxidant activity, reduced oxidative products, improved motor performance |
TBI, traumatic brain injury; NaHS, sodium hydrosulfide (H2S donor); IP, intraperitoneal; N/A, not applicable.
Intraperitoneal injection of H2S by a donor: sodium hydrosulfide (NaHS) has also been demonstrated as a feasible delivery method. Rats subjected to controlled cortical impact had significant attenuation of spatial memory deficits when pretreated with NaHS injection.71 Another report using this model of injury showed reduced lesion volume, cerebral edema, and improved blood–brain barrier function as well as motor behavioral function.72 In a mouse weight drop model of TBI, H2S was also administered via intraperitoneal injection of NaHS.79 This ameliorated the surge in markers of apoptosis and autophagy, as well as edema and cognitive deficits. Although autophagy has been reported to be both harmful,80 as well as protective,81,82 in TBI, the authors of this report interpreted this finding as H2S preventing oxidative stress to the degree that it reduced an autophagic burden of the injured tissue. Similar to H2 use, hydrogen sulfide has the advantage of usage as gas or liquid delivery forms. Its mechanism of action is more complex however, as it does not function as free radical scavenger but affect various pathways as outlined.
One of the proposed mechanisms of H2S having neuroprotective effects is by reducing metabolic rate, as inhaled H2S was shown to induce hypothermia and reduced metabolism in rodents.83 However, when the same effect was explored in a mechanically ventilated piglets, there was a lack of hypometabolic effects.84 Similarly, in a pig model of hemorrhagic shock,85 there was no difference in hemodynamics, temperature, and metabolic rate of pigs exposed to H2S compared with control pigs. As increasing the dose was associated with lung toxicity without further reduction in metabolism in this study, the efficacy of H2S in TBI will need to be thoroughly assessed in clinical trials.
Nitric Oxide
Unlike many of the other inhaled gases in this report, nitric oxide (NO) has a much more complicated effect as it is used in various physiological processes. Whether nitric oxide can be therapeutic or cytotoxic in the setting of injury has been a subject of debate for many years. This is due to the multiple roles it can serve as an oxidant or antioxidant, and its byproducts that can have various different influences on the vascular dynamics and oxidative stress of the cells. Although NO has scavenging function of free radicals, its byproduct can induce stress pathways.
Nitric oxide functions as a neurotransmitter, essential for dilation of blood vessels.86,87 However, in the presence of O2∙- it is converted into a strong oxidant peroxynitrite (ONOO∙-)88 and can induce oxidative damage in various components of intracellular processes.89 The toxicity of ONOO- has been proposed to be one of the major contributors to reperfusion injury in stroke.90 Also, nitrite (NO2-, byproduct of NO) in the presence of H2O2 can cause oxidative burden on cells, having an opposite effect of NOs protection from oxidative stress.91
The effect of nitric oxide is complex, as it also can also have therapeutic effects. Nitric oxide has a direct scavenging function on hydroxyl radicals.89 Additionally, it indirectly reduces oxidative stress by binding to metals such as iron, copper, and nickel. Formation of this metal-nitrosyl complex inhibits the effect of peroxide on the metal and thus prevents Fenton-type reactions. Moreover, the various cytotoxic level of reactive nitrogen species has been summarized by the following: H2O2 > HNO/NO∙ >> ONOO∙-, which shows that the oxidative stress by reactive nitrogen species may be small.92 The damaging effect of ONOO- may also not be significant given the short lifetime of this species.89
In neuronal cultures, membrane damage induced by H2O2 was prevented by administration of NO donor complex.91,93,94 In these in vitro experiments, donor complexes named NONOates (chemical formula R1R2N−(NO−)−N = O) were used to release NO without the need for enzyme activation. There was a significant increase in cell survival when cells were treated with NO. In a setting of high oxidative stress such as reperfusion injury in a rat model of cerebral ischemia, intravenous delivery of NO donor lead to 10-fold decrease in free radical production and dramatic decrease in infarct size.94 Also, another report demonstrated that various different NO donor complexes were all shown to be protective against ROS induced toxicity.93
In prior observations of cat vasculature by both intravital and postmortem microscopy, inhibition of NO production by agents such as NG-monomethyl-L-arginine (L-NMMA) or NG-nitro-L-arginine methyl ester (L-NAME) increased leukocyte adhesion to endothelium95,96 and extravasation.97 These studies also demonstrate that P-selectin and ICAM-1 mediate leukocyte adhesion and extravasation when NO is inhibited. Additionally, it can activate NADPH oxidase to inhibit O2∙- formation in neutrophils.98 Thus, NO serves an important role in preventing pro-inflammatory changes in leukocytes which can potentially lead to cell damage.
At 4 h after moderate fluid percussion injury in rats, pericontusional tissue measurements of NO showed significant NO decrease.99 However, another study using fluid percussion injury showed that there is an upregulation of NO levels in cerebral cortex, hippocampus and cerebellum after severe injury.100 This increase in NO also resulted in pericontusional tyrosine nitration and was associated with decreased mitochondrial respiration, specifically complex I dysfunction. As prior studies showed that high levels of NO or ONOO∙- can directly inactivate complex I by tyrosine nitration, acute NO increase was suggested as the driver of mitochondrial dysfunction.101,102
Studies detailing the activities of NO synthesis enzyme: nitric oxide synthase (NOS) showed that the actual time course of NOS activity may be more complicated.103-105 There are multiple isoforms of NOS: constitutive NOS, neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS), and each of them may have a different role and time course of activity after TBI. Constitutive NOS activity is temporarily increased from 5 min to 30 min after TBI, but there was a subsequent decrease from 1 day up to 7 days.103 Individual isoforms of NOS may also have a different role in TBI pathophysiology. Specific inhibition of nNOS by administration of 3-bromo-7-nitroindazole (7-NI) after TBI decreased contusion volume.103 However, eNOS deficient mice had greater perfusion deficits in pericontusional tissue 104 as well as reduced arteriolar diameter.105 Thus, while eNOS activity may be neuroprotective, nNOS activity may exacerbate injury.
In iNOS deficient mice, TBI led to decreased CBF in multiple regions including the hippocampus, thalamus, and amygdala as detected by arterial spin-labeling MRI.106 This demonstrated the important role that iNOS may play in post-TBI perfusion in brain tissue. Experiments using iNOS deficient mice subjected to TBI also showed increased markers of oxidative stress.107 Similarly, when iNOS inhibitors aminoguanidine and L-N-iminoethyl-lysine were administered immediately following TBI in rats, there was worse functional outcome and neuronal loss.108 However, another study contrasted this finding by showing that administration of iNOS inhibitor 1400 W starting at 18 h or 24 h after TBI resulted in improved histological outcomes.109 The exact role of iNOS in injury may be more complicated, as it seems to show time dependent effect if no major secondary effect by the iNOS inhibitor drugs can be assumed in both studies. Overall, whether NO serves a neuroprotective or damaging role in the setting of TBI depends on the source and thereby location of its effect, as well as the timing of its effect.
Nitric oxide has also been explored as a therapeutic agent focused on improving CBF given its vasodilatory effects.110-112 When tested at a range of 3-300 parts per million (ppm), inhaled NO does not cause major decrease in systemic blood pressure over a wide range until high concentrations such as 300 ppm.113 In a mouse model of stroke, inhaled NO at 50 ppm was carried to the brain in the form of nitrite and S-nitrosohemoglobin and specifically increased the penumbral perfusion without increasing perfusion in other areas of the brain.114 Similarly in animals that had TBI, perfusion in pericontusional site was increased without any significant increase in ICP when L-arginine, NO precursor was administered.115,116 Moreover, it reduced inflammatory markers and lead to functional improvement when administered to mice immediately after TBI.117
Large animal experiments also show improved CBF: pigs that were subjected to contusion TBI and inhaled NO (at 30 ppm for male animals and 20 ppm for female animals) showed increase in CBF measured via radioactive microspheres injected via arterial catheter.118 Using the pig model of TBI, the effect of inhaled NO on cerebrovascular regulation was also studied.119 Potassium channel agonists (cromakalin, NS1619, prostaglandin E2) induce vasodilation of cerebral blood vessels, but following TBI this effect is blunted. Exposure of injured pigs to inhaled NO reversed this loss of vasodilatory effect.119 Moreover, inhaled NO reduced cerebrospinal fluid levels of ET-1, ERK, and IL-6, the major agents in cerebral autoregulation and inflammation.120
In a randomized, blinded study of mice that underwent controlled cortical impact, inhaled NO was administered following injury.110 There was a significant increase in CBF immediately, and ICP increase seen in control group was attenuated in the inhaled NO group over the course of 90 min of monitoring. Additionally, in a detailed study of cerebral perfusion of sheep and mice using 18F-fluorodeoxyglucose micropositron emission tomography and intravital microscopy, a specific vasodilatory effect of inhaled NO to hypoperfused brain tissue was found.114 Inhaled NO selectively increased CBF in areas of low perfusion without affecting normally perfused brain regions. This inverse steal phenomenon has been termed “Robin Hood Effect” by the authors. These reports showing selective targeting of hypoperfused areas by inhaled NO argue against global cerebral vasodilation leading to potential ICP crisis. As several early studies using nitroprusside, a NO donor, in patients with intracranial pathologies raised concerns about the possibility of risk of increased ICP,121-123 this is an important point to be noted in these more recent studies using inhaled NO.
A classic study from 1980s that raised a concern for the use of nitroprusside is a case report from of a 14-year-old girl who suffered a respiratory tract infection, who then became comatose.121 She was found to have elevated transaminases and ammonia level, and ICP was noted to be at 30 mm Hg when intracranial pressure monitor was placed. The combination of pentobarbital, mannitol, and hyperventilation reduced the ICP. However, on Day 5 of hospitalization, arterial blood pressure rose to 200/100 mm Hg, and sodium nitroprusside drip was initiated. Immediate elevation of ICP from 20-22 to 38 mm Hg was reported with subsequent blood pressure falling to 170/90. This case report is concerning for nitroprusside causing ICP elevation, but it is also possible that ICP had been up-trending just prior to this event. If the cerebral edema had begun already and nitroprusside reduced arterial blood pressure, cerebral hypoperfusion would be exacerbated leading to worsening hypoperfusion injury. Unfortunately, the detailed timeline of events is not described in this study.
Other reports from the 1970s also showed significant decrease in blood pressure when nitroprusside was used.122,123 Administration of nitroprusside in ten intracranial tumor patients was associated with ICP increase from 14.5 to 27 mm Hg while mean arterial pressure was reduced by 33%.123 Decreased systemic blood pressure during the ICP crisis may have contributed to reduced cerebral perfusion. In a descriptive study of Mongrel cats that were treated with sodium nitroprusside, ICP had an early fluctuation for one minute after initiation of treatment while mean arterial pressure was reduced from the baseline of 100 mm Hg to 65 mm Hg.122 Soon afterwards ICP was maintained at baseline and was unchanged as nitroprusside administration continued. The lack of ICP elevation during the time nitroprusside was administered argues against the direct cause and effect relationship of nitroprusside to increase ICP. In a more recent study, which explored the use of nitroprusside in ischemic stroke patients, it was applied only at a dose that caused 10 mm Hg fall in mean arterial pressure, and improvement in CBF was seen.124
In the last 3 decades, neurointensivists and neurosurgeons avoided using nitroprusside in patients with risk of increased ICP, but inhaled NO should be approached with a different stance. Inhaled NO, as shown in the studies reviewed in this section induced no significant hypotension. While high NO concentrations (at 300 ppm) decreased mean arterial blood pressure from 62 to 55 mm Hg in rabbits,113 the doses of 10-30 ppm used in the recent studies resulted in no significant effect on blood pressure. Although the dose-dependent relationship of inhaled NO and parameters such as cerebral blood volume, mean arterial pressure, and ICP would need to be clarified in future studies, these prior studies indicate that inhaled NO may be a useful therapy in TBI patients. Additionally, other mechanisms of its beneficial effect should be explored as it is theoretically possible that inhaled NO could improve blood flow without increasing cerebral blood volume by altering blood rheology.
To date, there have not been any large-scale clinical studies completed on use of inhaled NO in human subjects. As approximately 20-30% of patients with severe TBI develop acute lung injury125,126 the use of NO as a selective pulmonary vasodilator has been explored in several case reports. In a 10-year-old child who suffered from a motor vehicle accident found with subarachnoid hemorrhage, diffuse axonal injury, as well as pulmonary hypertension, a trial of 10-35 ppm dose of inhaled NO resulted in decreased pulmonary artery pressure, improved cardiac index without major changes in intracranial pressure.127 Other promising case reports have supported the potential utility of using inhaled NO in TBI patients with acute respiratory distress syndrome as shown in Table 5.128,129
Table 5.
Pre-Clinical and Clinical Data on Inhaled NO in TBI
| Inhaled gas study | Concentration | Time after injury (duration) | Mode of injury | Subject | Outcomes assessed | Findings |
|---|---|---|---|---|---|---|
| Nitric oxide110 | Inhaled 50 ppm in 30% O2, 70% air | 10 min (24 h) | Controlled cortical impact (severe) | Mice | Pericontusional Injury | Improved NSS outcome, reduced contusion volume/ICP, improved rCBF |
| Nitric oxide117 | Inhaled 10 ppm in 30% oxygen 70% nitrogen | Immediately post-TBI (4 h or 8 h) | Weight drop (mild) | Mice | Cerebral cortex, hippocampus Injury | Improved short term memory, reduced microglia/astrocyte activation |
| Nitric oxide118 | Inhaled 30 ppm (males), 20 ppm (females) | 30 min, 2 h (1.5 h) | Fluid percussion injury (2 atm; moderate) | Pigs | Hippocampus injury | Reduced neuronal necrosis, protected cerebral autoregulation |
| Nitric oxide119 | Inhaled 30 ppm (males), 20 ppm (females) | 2 h (1.5 h) | Fluid percussion injury (1.9-2.3 atm; moderate) | Pigs | Pericontusional injury | Preservation of pial artery dilatation to cromakalin, NS1619, PGE2 |
| Nitric oxide130 | Inhaled 20 ppm | 7 days (several days*) | Motor vehicle accident | Human (37 years old) | N/A** | Decreased ICP from 50 mm Hg to 15 mm Hg. Discharged to rehabilitation center on Day 34 with amnesia, right hemiparesis, cognitive deficits |
| Nitric oxide129 | Inhaled 10 ppm | 1 day (22 days—gradual wean) | Fall from 10 meters | Human (37 years old) | N/A** | Stable head CT |
| Nitric oxide127 | Inhaled 10 ppm | 4 days (not reported) | Moto vehicle accident | Human (10 years old) | N/A** | Discharged to rehabilitation facility on Day 14 with Glasgow Outcome Score: 4 |
Exact length of inhaled NO administration was not reported. **These studies were focused on targeting respiratory complications and not brain injury.
TBI, traumatic brain injury; NSS, Neurological Severity Score; ICP, intracranial pressure; rCBF, relative cerebral blood flow as measured by laser doppler fluxmetry; PGE2, prostaglandin E 2; N/A, not applicable; CT, computed tomography.
In a case of a patient who fell 10 m and sustained intracranial bleeding, lumbar spine fracture, as well as development of acute respiratory distress syndrome, inhaled NO was administered at 10 ppm to treat the respiratory impairment.129 There was successful recovery of oxygenation and no adverse effect on intracranial pressure. Thus, NO was considered as a potentially useful agent in a population of patients with TBI who also suffer from acute respiratory distress syndrome. In another case, a patient who suffered a motor vehicle accident with multiple brain parenchymal contusions and developed acute respiratory distress syndrome (ARDS) was treated with 20 ppm inhaled NO.130 There was significant improvement of oxygenation, as well as significant decrease in intracranial pressure. Although there is relative lack of data on human subjects, these case reports give hopeful outlook for larger clinical studies using inhaled NO for TBI patients.
Nitric oxide use has different safety profiles across various ages. It has a favorable safety profiles in newborns without major side effects such as systemic hypotension.131 Among newborns treated with inhaled NO, abrupt cessation of therapy can lead to severe hypoxemia as endogenous NO production is downregulated.132 Thus, a gradual down titration is necessary after a course of inhaled NO therapy. Also, a minor increase in methemoglobin levels (less than 10% increase in 90% of the neonates) has been reported.133 Aside from these, in pediatric patients randomized trials of inhaled NO in ARDS reported no major side effects.134,135 However, more significant side effects have been demonstrated in adults. In Cochrane review of inhaled NO use for ARDS, adult patients had increased risk of renal dysfunctions.136 Thus, in future TBI clinical studies using inhaled NO, limited dosing in patients with renal impairments and close monitoring of renal function would be necessary.
Nitric oxide is a naturally expressed chemical with its role in blood vessel dilatation as well as antioxidant and anti-inflammatory function. The use of NO in TBI has been limited as it was only reported in several case reports, in which patients had both TBI and ARDS. In ARDS, high positive ventilation pressures for alveolar recruitment and permissive hypercapnia are tolerated. However, these strategies may increase intracranial pressure in a TBI patient. In these patient populations with conflicting therapeutic strategies for each disease entity, NO was shown to be able to increase pulmonary ventilation while not significantly affecting intracranial pressures. It may also improve perfusion in cerebral blood vessels without increasing intracranial pressure. Moreover, as mentioned above it may have additional benefits in preventing the most damaging oxidative stress by H2O2. Nitric oxide therapy is an exciting avenue of TBI research that has great future potential for clinical use.
Noble Gases
Noble gases such as helium, neon, argon, krypton, and xenon have very low chemical reactivity given their stability due to full valence electron shells. However, many in vitro and in vivo experiments have shown that they certainly have biological activity, as xenon and argon can be neuroprotective as listed in Table 6. Other noble gases such as helium, neon, and krypton have been trialed in in-vitro hippocampal model of TBI, without notable neuroprotective effect.137
Table 6.
Reports of Noble Gases Used in Experimental TBI
| Inhaled gas study | Concentration | Time after injury (duration) | Mode of injury | Subject | Outcomes assessed | Findings |
|---|---|---|---|---|---|---|
| Xenon gas147 | 50% Xe | 1 h (24 h, 48 h, 72 h) | Compressed air blast (55 kPa) | Mouse hippocampal slice | Hippocampal slice Injury | Reduced membrane permeability by propidium iodide |
| Xenon gas146 | 75% Xe | Immediately post-injury, 2 h, 3 h (24 h) | Focal stylus drop | Mouse hippocampal slice | Hippocampal slice injury | Reduced membrane permeability by propidium iodide |
| Xenon gas148 | 75% Xe | 15 min (3 h) | Controlled cortical impact (moderate-severe) | Mice | Hippocampus, corpus callosum, hypothalamus, amygdala, cerebral cortex Injury | Reduced lesion volume, CC white matter injury, hippocampal neuronal loss, astroglia/microglial stains; improved vestibulomotor function, reduced delayed memory deficits |
| Xenon gas149 | 50% or 30% Xe | 15 min, 1 h, 3 h, 6 h (3 h) | Controlled cortical impact (moderate) | Mice | Pericontusional Injury | Reduced contusion volume, Improved locomotion and neurological outcome score |
| Xenon gas, argon gas137 | 50% Xe or 50% Ar |
Immediately post-injury (24 h, 48 h, 72 h) | Focal stylus drop | Mouse hippocampal spice | Hippocampal slice injury | Reduced membrane permeability by propidium iodide |
| Argon gas167 | 50% Ar gas with 6% desflurane | Immediately post-injury (2 h) | Focal stylus drop | Mouse hippocampal slice | Hippocampal slice Injury | No improvement in injury assessed by propidium iodide |
| Argon gas166 | 25%, 50%, or 74% Ar gas | Immediately post-injury (72 h) | Focal stylus drop | Mouse hippocampal slice | Hippocampal slice Injury | Reduced membrane permeability by propidium iodide |
TBI, traumatic brain injury; Xe, xenon; Ar, argon.
Of particular interest in the recent years has been application of xenon in several neuropathological conditions such as stroke, perinatal asphyxia, and TBI.137-139 In an intrauterine perinatal asphyxia model of rats, 35% xenon provided neuroprotection, as demonstrated by reduced apoptotic cell death in hippocampal neurons and improved cognitive function on Morris water maze at 50 days.140 Application of 50% atmospheric xenon also prevented damage from hypoxic-ischemic injury in hippocampal brain slices.141 This study also demonstrated that NMDA receptor glycine binding site may be the focus of xenon's activity, as application of glycine blocked the neuroprotection by xenon. The amount of xenon that can be given in inhalation is limited by supplemental oxygen that is needed for the patient. Prior trials of xenon as an anesthetic agent utilized 30% oxygen and 70% xenon combination or 20% oxygen and 80% xenon combination.142 However, when acute lung injury such as ARDS is present in addition to TBI, higher oxygen supplementation may limit the amount of xenon that can be administered.
Xenon exists in a monoatomic form with low reactivity, stable at a gaseous state.143 It also exhibits little to no toxicity in hepatic and renal function, platelet function, coagulation activity, and cardiac hemodynamics.144 Additionally, it is eliminated quickly from the body given its low blood-gas partition coefficient that allows it to diffuse through the blood–brain barrier with ease.145 The neuroprotective effect of xenon gas has been demonstrated initially in hippocampal slices experiments. In this in vitro study, hippocampal slices were subjected to focal weight drop of a stylus.146 Xenon gas at 75% applied immediately after injury, or with a 2- to 3-h therapeutic window reduced injury as measured by propidium iodide staining. Also, similar to the previous study on neonatal asphyxia model,141 glycine application blocked the xenon's neuroprotection in hippocampal slice TBI model.137 Neuroprotection by xenon was also confirmed using a blast injury model of in vitro TBI, with reduced injury assessed by propidium iodide staining.147 In a mouse model of controlled cortical impact, xenon administration at 75%148 and 30-50%149 were shown to reduce injury volume and improve neurological outcomes. Histological analysis showed reduced astrogliosis and microglia in hypothalamus and amygdala, respectively. Also, there was an attenuation of hippocampal neuronal loss.147
Xenon has additionally been explored as an anesthetic agent due to its analgesic effect and due to subjects having faster emergence from anesthesia compared with other gaseous anesthetics.150,151 However, its high costs currently at $10 per liter is prohibitive in its widespread use.152 During a 2-h anesthetic procedure, reaching an anesthesia level at 70% using 0.5 L/min would result in an efficiency lower than 20% with 34 L of xenon gas to be wasted into the atmosphere.153 To reach a much more cost-effective solution, closed circuit rebreathing devices are currently explored by various companies to reach 70-90% of the xenon being delivered into the system. However, even with the use of a closed-circuit system, xenon at the current prices would be 2-5 times more costly than isoflurane for a typical anesthetic procedure.154,155 There is also limitation in annual production of xenon153 and each liter of xenon requires 220 watt-hours of energy to produce (million times that of nitrous oxide).155 Thus, an efficient delivery technology that can further increase the efficiency of xenon delivery is much needed.
In addition to closed circuit inhalational system, methods to deliver xenon in various lipid emulsions have been studied, as approximately 20-fold more xenon can deliver in lipid solutions compared with aqueous solutions.156 The xenon gas dissolved in lipid emulsion was also shown to successfully inhibit NMDA receptors.157 Moreover, specific lipid emulsion carriers were shown to enhance NMDA receptor antagonist activity of xenon.156 Liposomes that encapsulate xenon and release them upon ultrasonic stimulation have also been devised as a novel delivery method to the area of injury.158 As specific delivery to an injured tissue will help in limiting the dose needed for the therapeutic benefit, this strategy may enhance potential efficacy in TBI. With additional progress in xenon delivery methods, xenon encapsulated lipid bubbles, which also have clear ultrasound visualization have been developed.159 This is an exciting field as further development may allow theranostic (simultaneously diagnostic and therapeutic) use of xenon encapsulated lipid bubbles in ischemic tissue as well as TBI.160-162
Although less extensively studied than xenon, argon gas also was shown to have narcotic as well as neuroprotective effects.163 It is believed to operate by a different mechanism compared with xenon, likely by stimulation of γ-aminobutyric acid type-A receptors (GABAAR).164 When applied in hippocampal brain slices for oxygen-glucose deprivation, its neuroprotective effect was not blocked by glycine application.165 Similarly in a hippocampal slice model of TBI, application of argon reduced cell damage measured by propidium iodide.166 The duration of argon administration is important for neuroprotection, as administration for 2 h failed to show significant neuroprotection,167 but 72-h exposure was able to attenuate cellular injury.166 Argon exhibits neuroprotective effect via NMDA-receptor independent mechanism in TBI: when hippocampal slice was subjected to trauma, glycine administration blocked the neuroprotective effect of xenon but not argon.166
Use of noble gases as therapeutic intervention in TBI is a recently discovered field of interest. As they are generally inert chemicals with specific mechanisms of neuroprotection, they may be great candidates for future clinical trials in TBI. As the price of these agents are high, efficient delivery mechanisms are necessary before making them widely used in clinical settings. Novel delivery mechanisms of noble gases via microbubbles and localized release at the site of injury may also make them achieve therapeutic effects even at lower doses.
Systemic administration of neuroprotective gas agents can result in systemic side effects, reduce local therapeutic efficacy and may be expensive. To overcome these limitations, studies have examined whether targeted, ultrasound-guided delivery of gas loaded ultrasound contrast agents, shown to be safer than other imaging contrast agents in vivo, can achieve neuroprotection.168 The concept behind therapeutic use of microbubbles is to load neuroprotective gas into ultrasound contrast agents that serve as stable carriers which can undergo acoustically triggered cavitation, a process in which high acoustic power induces oscillation and subsequent collapse of the microbubble for localized gas release. This would achieve high concentration of gas in the brain in a short amount of time, in the range of minutes, without the need for hours of gas inhalation therapy. For therapeutic applications, these microbubbles can be modified such that the inner core can encapsulate any gas agent of choice. In fact, a study recently showed that Xenon encapsulated within the liposome bilayers when released via high acoustic power at the level of the carotid can locally deliver Xenon to the injured site in models of stroke and achieve neuroprotective efficacy.158,169,170 More studies will be needed to validate therapeutic efficacy of these gas loaded microbubbles in large animal models of TBI. The acoustic visibility of these microbubbles also raises the possibility of using these agents as potential theranostic agents.
Conclusions
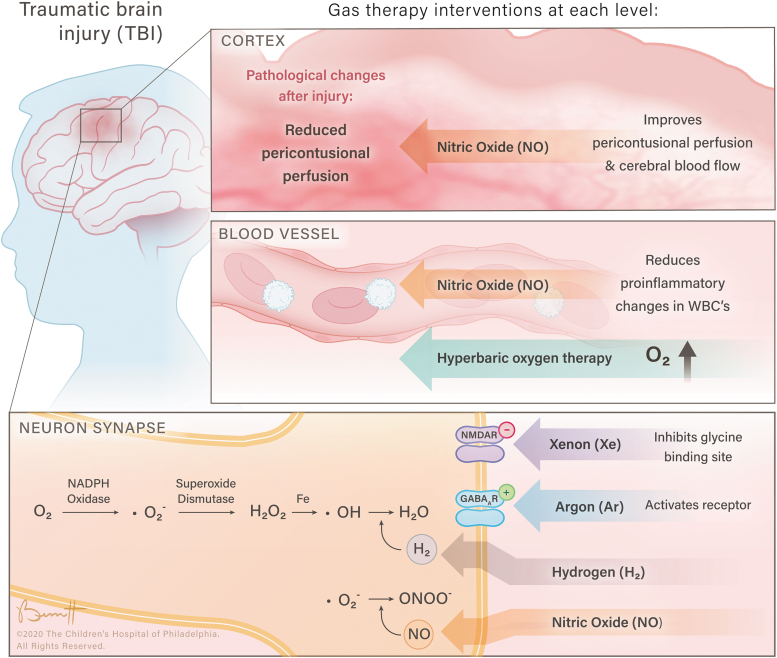
Thus far, much of the pre-clinical and some clinical data on these inhalational gases hold a very promising outlook on their widespread uses as summarized in Fig. 1. Although both normobaric and hyperbaric oxygen treatments have been investigated for many years, a rigorous clinical trial is needed to clarify their efficacy in TBI. Hydrogen gas, hydrogen sulfide, and NO have shown promising effects in some pre-clinical studies and have had limited clinical investigation. They have, however, yet to be able to be translated into clinical research. Additionally, xenon and argon gases also have promising pre-clinical results but are also in need of clinical validation. The specific mechanisms of each of these agents will be further clarified in future research, but given their ease of delivery many of these will likely be seen in clinical trials in the near future. As TBI is a heterogenous injury involving multiple concurrent mechanisms, a potential avenue of future exploration is using each of these agents for different injury types as the TBI community is working on categorizing TBI subtypes via various imaging and blood biomarkers.171 Also with continued advances in the field of targeted gas delivery, these interventions may play an important role in clinical TBI management in the near future.
FIG. 1.
The neuroprotective effects of inhalational gases at various physiological levels.
Funding Information
T.K. is supported by NIH, NHLBI R01HL141386. M.H. is supported by Foerderer Grant from Children's Hospital of Philadelphia and Junior Faculty Pilot Grant.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Abdelmalik, P.A., Draghic, N., and Ling, G.S.F. (2019). Management of moderate and severe traumatic brain injury. Transfusion 59, 1529–1538. [DOI] [PubMed] [Google Scholar]
- 2. Godoy, D.A., Lubillo, S., and Rabinstein, A.A. (2018). Pathophysiology and management of intracranial hypertension and tissular brain hypoxia after severe traumatic brain injury: an integrative approach. Neurosurg. Clin. N. Am. 29, 195–212. [DOI] [PubMed] [Google Scholar]
- 3. Aisiku, I.P., Yamal, J.M., Doshi, P., Rubin, M.L., Benoit, J.S., Hannay, J., Tilley, B.C., Gopinath, S., and Robertson, C.S. (2016). The incidence of ARDS and associated mortality in severe TBI using the Berlin definition. J. Trauma Acute Care Surg. 80, 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouma, G.J., Muizelaar, J.P., Stringer, W.A., Choi, S.C., Fatouros, P., and Young, H.F. (1992). Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J. Neurosurg. 77, 360–368. [DOI] [PubMed] [Google Scholar]
- 5. Diringer, M.N., Zazulia, A.R., and Powers, W.J. (2011). Does ischemia contribute to energy failure in severe TBI? Transl. Stroke Res. 2, 517–523. [DOI] [PubMed] [Google Scholar]
- 6. Weeden, M., Bailey, M., Gabbe, B., Pilcher, D., Bellomo, R., and Udy, A. (2020). Functional outcomes in patients admitted to the intensive care unit with traumatic brain injury and exposed to hyperoxia: a retrospective multicentre cohort study. Neurocrit. Care 34, 441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alali, A.S., Temkin, N., Vavilala, M.S., Lele, A.V., Barber, J., Dikmen, S., and Chesnut, R.M. (2019). Matching early arterial oxygenation to long-term outcome in severe traumatic brain injury: target values. J. Neurosurg. 132, 537–544. [DOI] [PubMed] [Google Scholar]
- 8. Rincon, F., Kang, J., Vibbert, M., Urtecho, J., Athar, M.K., and Jallo, J. (2014). Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J. Neurol. Neurosurg. Psychiatry 85, 799–805. [DOI] [PubMed] [Google Scholar]
- 9. Brenner, M., Stein, D., Hu, P., Kufera, J., Wooford, M., and Scalea, T. (2012). Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch. Surg. 147, 1042–1046. [DOI] [PubMed] [Google Scholar]
- 10. Menzel, M., Doppenberg, E.M., Zauner, A., Soukup, J., Reinert, M.M., Clausen, T., Brockenbrough, P.B., and Bullock, R. (1999). Cerebral oxygenation in patients after severe head injury: monitoring and effects of arterial hyperoxia on cerebral blood flow, metabolism and intracranial pressure. J. Neurosurg. Anesthesiol. 11, 240–251. [DOI] [PubMed] [Google Scholar]
- 11. Tolias, C.M., Reinert, M., Seiler, R., Gilman, C., Scharf, A., and Bullock, M.R. (2004). Normobaric hyperoxia–induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J. Neurosurg. 101, 435–444. [DOI] [PubMed] [Google Scholar]
- 12. Ding, Z., Tong, W.C., Lu, X.X., and Peng, H.P. (2014). Hyperbaric oxygen therapy in acute ischemic stroke: a review. Interv. Neurol. 2, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nighoghossian, N., Trouillas, P., Adeleine, P., and Salord, F. (1995). Hyperbaric oxygen in the treatment of acute ischemic stroke. A double-blind pilot study. Stroke 26, 1369–1372. [DOI] [PubMed] [Google Scholar]
- 14. Gill, A.L. and Bell, C.N. (2004). Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM 97, 385–395. [DOI] [PubMed] [Google Scholar]
- 15. Baratz-Goldstein, R., Toussia-Cohen, S., Elpaz, A., Rubovitch, V., and Pick, C.G. (2017). Immediate and delayed hyperbaric oxygen therapy as a neuroprotective treatment for traumatic brain injury in mice. Mol. Cell. Neurosci. 83, 74–82. [DOI] [PubMed] [Google Scholar]
- 16. Liu, S., Shen, G., Deng, S., Wang, X., Wu, Q., and Guo, A. (2013). Hyperbaric oxygen therapy improves cognitive functioning after brain injury. Neural Regen. Res. 8, 3334–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu, Z., Jiao, Q.F., You, C., Che, Y.J., and Su, F.Z. (2006). Effect of hyperbaric oxygen on cytochrome C, Bcl-2 and Bax expression after experimental traumatic brain injury in rats. Chin. J. Traumatol. 9, 168–174. [PubMed] [Google Scholar]
- 18. Wang, G.H., Zhang, X.G., Jiang, Z.L., Li, X., Peng, L.L., Li, Y.C., and Wang, Y. (2010). Neuroprotective effects of hyperbaric oxygen treatment on traumatic brain injury in the rat. J. Neurotrauma 27, 1733–1743. [DOI] [PubMed] [Google Scholar]
- 19. Zhou, Z., Daugherty, W.P., Sun, D., Levasseur, J.E., Altememi, N., Hamm, R.J., Rockswold, G.L., and Bullock, M.R. (2007). Protection of mitochondrial function and improvement in cognitive recovery in rats treated with hyperbaric oxygen following lateral fluid-percussion injury. J. Neurosurg. 106, 687–694. [DOI] [PubMed] [Google Scholar]
- 20. Yang, Y., Zhang, Y.G., Lin, G.A., Xie, H.Q., Pan, H.T., Huang, B.Q., Liu, J.D., Liu, H., Zhang, N., Li, L., and Chen, J.H. (2014). The effects of different hyperbaric oxygen manipulations in rats after traumatic brain injury. Neurosci. Lett. 563, 38–43. [DOI] [PubMed] [Google Scholar]
- 21. Zhang, Y., Yang, Y., Tang, H., Sun, W., Xiong, X., Smerin, D., and Liu, J. (2014). Hyperbaric oxygen therapy ameliorates local brain metabolism, brain edema and inflammatory response in a blast-induced traumatic brain injury model in rabbits. Neurochem. Res. 39, 950–960. [DOI] [PubMed] [Google Scholar]
- 22. Huang, L., Obenaus, A., Hamer, M., and Zhang, J.H. (2016). Neuroprotective effect of hyperbaric oxygen therapy in a juvenile rat model of repetitive mild traumatic brain injury. Med. Gas Res. 6, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lavrnja, I., Parabucki, A., Brkic, P., Jovanovic, T., Dacic, S., Savic, D., Pantic, I., Stojiljkovic, M., and Pekovic, S. (2015). Repetitive hyperbaric oxygenation attenuates reactive astrogliosis and suppresses expression of inflammatory mediators in the rat model of brain injury. Mediators Inflamm. 2015, 498405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palzur, E., Zaaroor, M., Vlodavsky, E., Milman, F., and Soustiel, J.F. (2008). Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res. 1221, 126–133. [DOI] [PubMed] [Google Scholar]
- 25. Palzur, E., Vlodavsky, E., Mulla, H., Arieli, R., Feinsod, M., and Soustiel, J.F. (2004). Hyperbaric oxygen therapy for reduction of secondary brain damage in head injury: an animal model of brain contusion. J. Neurotrauma 21, 41–48. [DOI] [PubMed] [Google Scholar]
- 26. Vlodavsky, E., Palzur, E., and Soustiel, J.F. (2006). Hyperbaric oxygen therapy reduces neuroinflammation and expression of matrix metalloproteinase-9 in the rat model of traumatic brain injury. Neuropathol. Appl. Neurobiol. 32, 40–50. [DOI] [PubMed] [Google Scholar]
- 27. Rockswold, S.B., Rockswold, G.L., Vargo, J.M., Erickson, C.A., Sutton, R.L., Bergman, T.A., and Biros, M.H. (2001). Effects of hyperbaric oxygenation therapy on cerebral metabolism and intracranial pressure in severely brain injured patients. J. Neurosurg. 94, 403–411. [DOI] [PubMed] [Google Scholar]
- 28. Rockswold, S.B., Rockswold, G.L., Zaun, D.A., Zhang, X., Cerra, C.E., Bergman, T.A., and Liu, J. (2010). A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J. Neurosurg. 112, 1080–1094. [DOI] [PubMed] [Google Scholar]
- 29. Rockswold, S.B., Rockswold, G.L., Zaun, D.A., and Liu, J. (2013). A prospective, randomized Phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. J. Neurosurg. 118, 1317–1328. [DOI] [PubMed] [Google Scholar]
- 30. Gopinath, S.P., Robertson, C.S., Contant, C.F., Hayes, C., Feldman, Z., Narayan, R.K., and Grossman, R.G. (1994). Jugular venous desaturation and outcome after head injury. J. Neurol. Neurosurg. Psychiatry 57, 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harch, P.G., Andrews, S.R., Fogarty, E.F., Amen, D., Pezzullo, J.C., Lucarini, J., Aubrey, C., Taylor, D.V., Staab, P.K., and Van Meter, K.W. (2012). A phase I study of low-pressure hyperbaric oxygen therapy for blast-induced post-concussion syndrome and post-traumatic stress disorder. J. Neurotrauma 29, 168–185. [DOI] [PubMed] [Google Scholar]
- 32. Boussi-Gross, R., Golan, H., Fishlev, G., Bechor, Y., Volkov, O., Bergan, J., Friedman, M., Hoofien, D., Shlamkovitch, N., Ben-Jacob, E., and Efrati, S. (2013). Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury—randomized prospective trial. PLoS One 8, e79995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tal, S., Hadanny, A., Sasson, E., Suzin, G., and Efrati, S. (2017). Hyperbaric oxygen therapy can induce angiogenesis and regeneration of nerve fibers in traumatic brain injury patients. Front. Hum. Neurosci. 11, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller, R.S., Weaver, L.K., Bahraini, N., Churchill, S., Price, R.C., Skiba, V., Caviness, J., Mooney, S., Hetzell, B., Liu, J., Deru, K., Ricciardi, R., Fracisco, S., Close, N.C., Surrett, G.W., Bartos, C., Ryan, M., and Brenner, L.A.; HOPPS Trial Team. (2015). Effects of hyperbaric oxygen on symptoms and quality of life among service members with persistent postconcussion symptoms: a randomized clinical trial. JAMA Intern. Med. 175, 43–52. [DOI] [PubMed] [Google Scholar]
- 35. Cifu, D.X., Walker, W.C., West, S.L., Hart, B.B., Franke, L.M., Sima, A., Graham, C.W., and Carne, W. (2014). Hyperbaric oxygen for blast-related postconcussion syndrome: three-month outcomes. Ann. Neurol. 75, 277–286. [DOI] [PubMed] [Google Scholar]
- 36. Hu, Q., Manaenko, A., Guo, Z., Huang, L., Tang, J., and Zhang, J.H. (2015). Hyperbaric oxygen therapy for post concussion symptoms: issues may affect the results. Med. Gas Res. 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rossignol, D.A. (2007). Hyperbaric oxygen therapy might improve certain pathophysiological findings in autism. Med. Hypotheses 68, 1208–1227. [DOI] [PubMed] [Google Scholar]
- 38. Lin, J.W., Tsai, J.T., Lee, L.M., Lin, C.M., Hung, C.C., Hung, K.S., Chen, W.Y., Wei, L., Ko, C.P., Su, Y.K., and Chiu, W.T. (2008). Effect of hyperbaric oxygen on patients with traumatic brain injury. Acta Neurochir. Suppl 101, 145–149. [DOI] [PubMed] [Google Scholar]
- 39. Blenkarn, G.D., Schanberg, S.M., and Saltzman, H.A. (1969). Cerebral amines and acute hyperbaric oxygen toxicity. J. Pharmacol. Exp. Ther. 166, 346–353. [PubMed] [Google Scholar]
- 40. Mink, R.B. and Dutka, A.J. (1995). Hyperbaric oxygen after global cerebral ischemia in rabbits does not promote brain lipid peroxidation. Crit. Care Med. 23, 1398–1404. [DOI] [PubMed] [Google Scholar]
- 41. Thom, S.R. (2009). Oxidative stress is fundamental to hyperbaric oxygen therapy. J. Appl. Physiol. (1985) 106, 988-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bayir, H., Kagan, V.E., Tyurina, Y.Y., Tyurin, V., Ruppel, R.A., Adelson, P.D., Graham, S.H., Janesko, K., Clark, R.S., and Kochanek, P.M. (2002). Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr. Res. 51, 571–578. [DOI] [PubMed] [Google Scholar]
- 43. Goodman, M.D., Makley, A.T., Lentsch, A.B., Barnes, S.L., Dorlac, G.R., Dorlac, W.C., Johannigman, J.A., and Pritts, T.A. (2010). Traumatic brain injury and aeromedical evacuation: when is the brain fit to fly? J. Surg. Res. 164, 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu, Q., Manaenko, A., Xu, T., Guo, Z., Tang, J., and Zhang, J.H. (2016). Hyperbaric oxygen therapy for traumatic brain injury: bench-to-bedside. Med. Gas Res. 6, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Skovira, J.W., Kabadi, S.V., Wu, J., Zhao, Z., DuBose, J., Rosenthal, R., Fiskum, G., and Faden, A.I. (2016). Simulated aeromedical evacuation exacerbates experimental brain injury. J. Neurotrauma 33, 1292–1302. [DOI] [PubMed] [Google Scholar]
- 46. Scultetus, A.H., Haque, A., Chun, S.J., Hazzard, B., Mahon, R.T., Harssema, M.J., Auker, C.R., Moon-Massat, P., Malone, D.L., and McCarron, R.M. (2016). Brain hypoxia is exacerbated in hypobaria during aeromedical evacuation in swine with traumatic brain injury. J. Trauma Acute Care Surg. 81, 101–107. [DOI] [PubMed] [Google Scholar]
- 47. Crawford, C., Teo, L., Yang, E., Isbister, C., and Berry, K. (2017). Is hyperbaric oxygen therapy effective for traumatic brain injury? A rapid evidence assessment of the literature and recommendations for the field. J. Head Trauma Rehabil. 32, E27–E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Daly, S., Thorpe, M., Rockswold, S., Hubbard, M., Bergman, T., Samadani, U., and Rockswold, G. (2018). Hyperbaric oxygen therapy in the treatment of acute severe traumatic brain injury: a systematic review. J. Neurotrauma 35, 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gajewski, B.J., Berry, S.M., Barsan, W.G., Silbergleit, R., Meurer, W.J., Martin, R., and Rockswold, G.L. (2016). Hyperbaric oxygen brain injury treatment (HOBIT) trial: a multifactor design with response adaptive randomization and longitudinal modeling. Pharm. Stat. 15, 396–404. [DOI] [PubMed] [Google Scholar]
- 50. Liu, C., Cui, J., Sun, Q., and Cai, J. (2010). Hydrogen therapy may be an effective and specific novel treatment for acute radiation syndrome. Med. Hypotheses 74, 145–146. [DOI] [PubMed] [Google Scholar]
- 51. Cole, A.R., Raza, A., Ahmed, H., Polizzotti, B.D., Padera, R.F., Andrews, N., and Kheir, J.N. (2019). Safety of inhaled hydrogen gas in healthy mice. Med. Gas Res. 9, 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ono, H., Nishijima, Y., Ohta, S., Sakamoto, M., Kinone, K., Horikosi, T., Tamaki, M., Takeshita, H., Futatuki, T., Ohishi, W., Ishiguro, T., Okamoto, S., Ishii, S., and Takanami, H. (2017). Hydrogen gas inhalation treatment in acute cerebral infarction: a randomized controlled clinical study on safety and neuroprotection. J. Stroke Cerebrovasc. Dis. 26, 2587–2594. [DOI] [PubMed] [Google Scholar]
- 53. Rostain, J.C., Gardette-Chauffour, M.C., Lemaire, C., and Naquet, R. (1988). Effects of a H2-He-O2 mixture on the HPNS up to 450 msw. Undersea Biomed Res 15, 257–270. [PubMed] [Google Scholar]
- 54. Cai, J., Kang, Z., Liu, K., Liu, W., Li, R., Zhang, J.H., Luo, X., and Sun, X. (2009). Neuroprotective effects of hydrogen saline in neonatal hypoxia-ischemia rat model. Brain Res. 1256, 129–137. [DOI] [PubMed] [Google Scholar]
- 55. Sauer, H., Wartenberg, M., and Hescheler, J. (2001). Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 11, 173–186. [DOI] [PubMed] [Google Scholar]
- 56. Liu, H., Colavitti, R., Rovira, II, and Finkel, T. (2005). Redox-dependent transcriptional regulation. Circ. Res. 97, 967–974. [DOI] [PubMed] [Google Scholar]
- 57. Ohsawa, I., Ishikawa, M., Takahashi, K., Watanabe, M., Nishimaki, K., Yamagata, K., Katsura, K., Katayama, Y., Asoh, S., and Ohta, S. (2007). Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 13, 688–694. [DOI] [PubMed] [Google Scholar]
- 58. Nagatani, K., Wada, K., Takeuchi, S., Kobayashi, H., Uozumi, Y., Otani, N., Fujita, M., Tachibana, S., and Nawashiro, H. (2012). Effect of hydrogen gas on the survival rate of mice following global cerebral ischemia. Shock 37, 645–652. [DOI] [PubMed] [Google Scholar]
- 59. Hayashida, K., Sano, M., Ohsawa, I., Shinmura, K., Tamaki, K., Kimura, K., Endo, J., Katayama, T., Kawamura, A., Kohsaka, S., Makino, S., Ohta, S., Ogawa, S., and Fukuda, K. (2008). Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 373, 30–35. [DOI] [PubMed] [Google Scholar]
- 60. Fukuda, K., Asoh, S., Ishikawa, M., Yamamoto, Y., Ohsawa, I., and Ohta, S. (2007). Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 361, 670–674. [DOI] [PubMed] [Google Scholar]
- 61. Hou, C., Peng, Y., Qin, C., Fan, F., Liu, J., and Long, J. (2018). Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Abeta clearance. Free Radic. Res. 52, 1311–1322. [DOI] [PubMed] [Google Scholar]
- 62. Manaenko, A., Lekic, T., Ma, Q., Ostrowski, R.P., Zhang, J.H., and Tang, J. (2011). Hydrogen inhalation is neuroprotective and improves functional outcomes in mice after intracerebral hemorrhage. Acta Neurochir. Suppl 111, 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tian, R., Hou, Z., Hao, S., Wu, W., Mao, X., Tao, X., Lu, T., and Liu, B. (2016). Hydrogen-rich water attenuates brain damage and inflammation after traumatic brain injury in rats. Brain Res. 1637, 1–13. [DOI] [PubMed] [Google Scholar]
- 64. Ji, X., Liu, W., Xie, K., Liu, W., Qu, Y., Chao, X., Chen, T., Zhou, J., and Fei, Z. (2010). Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res. 1354, 196–205. [DOI] [PubMed] [Google Scholar]
- 65. Kajiya, M., Silva, M.J., Sato, K., Ouhara, K., and Kawai, T. (2009). Hydrogen mediates suppression of colon inflammation induced by dextran sodium sulfate. Biochem. Biophys. Res. Commun. 386, 11–15. [DOI] [PubMed] [Google Scholar]
- 66. Zhang, Y., Sun, Q., He, B., Xiao, J., Wang, Z., and Sun, X. (2011). Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int. J. Cardiol. 148, 91–95. [DOI] [PubMed] [Google Scholar]
- 67. Wang, L., Zhao, C., Wu, S., Xiao, G., Zhuge, X., Lei, P., and Xie, K. (2018). Hydrogen gas treatment improves the neurological outcome after traumatic brain injury via increasing miR-21 expression. Shock 50, 308–315. [DOI] [PubMed] [Google Scholar]
- 68. Yu, Y.P., Chi, X.L., and Liu, L.J. (2014). A hypothesis: hydrogen sulfide might be neuroprotective against subarachnoid hemorrhage induced brain injury. Sci. World J. 2014, 432318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Che, X., Fang, Y., Si, X., Wang, J., Hu, X., Reis, C., and Chen, S. (2018). The role of gaseous molecules in traumatic brain injury: an updated review. Front. Neurosci. 12, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li, H., Zhu, L., Feng, J., Hu, X., Li, C., and Zhang, B. (2018). Hydrogen sulfide decreases blood-brain barrier damage via tegulating protein kinase C and tight junction after cardiac arrest in rats. Cell. Physiol. Biochem. 47, 994–1006. [DOI] [PubMed] [Google Scholar]
- 71. Karimi, S.A., Hosseinmardi, N., Janahmadi, M., Sayyah, M., and Hajisoltani, R. (2017). The protective effect of hydrogen sulfide (H2S) on traumatic brain injury (TBI) induced memory deficits in rats. Brain Res. Bull. 134, 177–182. [DOI] [PubMed] [Google Scholar]
- 72. Jiang, X., Huang, Y., Lin, W., Gao, D., and Fei, Z. (2013). Protective effects of hydrogen sulfide in a rat model of traumatic brain injury via activation of mitochondrial adenosine triphosphate-sensitive potassium channels and reduction of oxidative stress. J. Surg. Res. 184, e27–35. [DOI] [PubMed] [Google Scholar]
- 73. Wei, X., Zhang, B., Cheng, L., Chi, M., Deng, L., Pan, H., Yao, X., and Wang, G. (2015). Hydrogen sulfide induces neuroprotection against experimental stroke in rats by down-regulation of AQP4 via activating PKC. Brain Res. 1622, 292–299. [DOI] [PubMed] [Google Scholar]
- 74. Panthi, S., Chung, H.J., Jung, J., and Jeong, N.Y. (2016). Physiological importance of hydrogen sulfide: emerging potent neuroprotector and neuromodulator. Oxid. Med. Cell Longev. 2016, 9049782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu, Y., Liao, S., Quan, H., Lin, Y., Li, J., and Yang, Q. (2016). Involvement of microRNA-135a-5p in the protective effects of hydrogen sulfide against Parkinson's disease. Cell. Physiol. Biochem. 40, 18–26. [DOI] [PubMed] [Google Scholar]
- 76. Wang, Y.Z., Li, T.T., Cao, H.L., and Yang, W.C. (2019). Recent advances in the neuroprotective effects of medical gases. Med. Gas Res. 9, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Marutani, E., Kosugi, S., Tokuda, K., Khatri, A., Nguyen, R., Atochin, D.N., Kida, K., Van Leyen, K., Arai, K., and Ichinose, F. (2012). A novel hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonist prevents ischemic neuronal death. J. Biol. Chem. 287, 32124–32135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marutani, E., Sakaguchi, M., Chen, W., Sasakura, K., Liu, J., Xian, M., Hanaoka, K., Nagano, T., and Ichinose, F. (2014). Cytoprotective effects of hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonists are mediated by intracellular sulfane sulfur. Medchemcomm 5, 1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang, M., Shan, H., Chang, P., Wang, T., Dong, W., Chen, X., and Tao, L. (2014). Hydrogen sulfide offers neuroprotection on traumatic brain injury in parallel with reduced apoptosis and autophagy in mice. PLoS One 9, e87241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wu, Q., Gao, C., Wang, H., Zhang, X., Li, Q., Gu, Z., Shi, X., Cui, Y., Wang, T., Chen, X., Wang, X., Luo, C., and Tao, L. (2018). Mdivi-1 alleviates blood-brain barrier disruption and cell death in experimental traumatic brain injury by mitigating autophagy dysfunction and mitophagy activation. Int. J. Biochem. Cell. Biol. 94, 44–55. [DOI] [PubMed] [Google Scholar]
- 81. Niu, F., Dong, J., Xu, X., Zhang, B., and Liu, B. (2019). Mitochondrial Division Inhibitor 1 Prevents Early-Stage Induction of Mitophagy and Accelerated Cell Death in a Rat Model of Moderate Controlled Cortical Impact Brain Injury. World Neurosurg. 122, e1090–e1101. [DOI] [PubMed] [Google Scholar]
- 82. Wang, C., Hu, Z., Zou, Y., Xiang, M., Jiang, Y., Botchway, B.O.A., Huo, X., Du, X., and Fang, M. (2017). The post-therapeutic effect of rapamycin in mild traumatic brain-injured rats ensuing in the upregulation of autophagy and mitophagy. Cell Biol. Int. 41, 1039–1047. [DOI] [PubMed] [Google Scholar]
- 83. Blackstone, E., Morrison, M., and Roth, M.B. (2005). H2S induces a suspended animation-like state in mice. Science 308, 518. [DOI] [PubMed] [Google Scholar]
- 84. Li, J., Zhang, G., Cai, S., and Redington, A.N. (2008). Effect of inhaled hydrogen sulfide on metabolic responses in anesthetized, paralyzed, and mechanically ventilated piglets. Pediatr. Crit. Care Med 9, 110–112. [DOI] [PubMed] [Google Scholar]
- 85. Drabek, T., Kochanek, P.M., Stezoski, J., Wu, X., Bayir, H., Morhard, R.C., Stezoski, S.W., and Tisherman, S.A. (2011). Intravenous hydrogen sulfide does not induce hypothermia or improve survival from hemorrhagic shock in pigs. Shock 35, 67–73. [DOI] [PubMed] [Google Scholar]
- 86. Murad, F. (2004). Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci. Rep. 24, 452–474. [DOI] [PubMed] [Google Scholar]
- 87. Terpolilli, N.A., Feiler, S., Dienel, A., Muller, F., Heumos, N., Friedrich, B., Stover, J., Thal, S., Scholler, K., and Plesnila, N. (2016). Nitric oxide inhalation reduces brain damage, prevents mortality, and improves neurological outcome after subarachnoid hemorrhage by resolving early pial microvasospasms. J. Cereb. Blood Flow Metab. 36, 2096–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhu, L., Gunn, C. and Beckman, J.S. (1992). Bactericidal activity of peroxynitrite. Arch. Biochem. Biophys. 298, 452–457. [DOI] [PubMed] [Google Scholar]
- 89. Wink, D.A., Miranda, K.M., Espey, M.G., Pluta, R.M., Hewett, S.J., Colton, C., Vitek, M., Feelisch, M., and Grisham, M.B. (2001). Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox. Signal 3, 203–213. [DOI] [PubMed] [Google Scholar]
- 90. Dawson, D.A. (1994). Nitric oxide and focal cerebral ischemia: multiplicity of actions and diverse outcome. Cerebrovasc. Brain Metab. Rev. 6, 299–324. [PubMed] [Google Scholar]
- 91. Wink, D.A., Hanbauer, I., Krishna, M.C., DeGraff, W., Gamson, J., and Mitchell, J.B. (1993). Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 90, 9813–9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wink, D.A., Miranda, K.M., and Espey, M.G. (2001). Cytotoxicity related to oxidative and nitrosative stress by nitric oxide. Exp. Biol. Med. (Maywood) 226, 621–623. [DOI] [PubMed] [Google Scholar]
- 93. Wink, D.A., Cook, J.A., Pacelli, R., DeGraff, W., Gamson, J., Liebmann, J., Krishna, M.C., and Mitchell, J.B. (1996). The effect of various nitric oxide-donor agents on hydrogen peroxide-mediated toxicity: a direct correlation between nitric oxide formation and protection. Arch. Biochem. Biophys. 331, 241–248. [DOI] [PubMed] [Google Scholar]
- 94. Mason, R.B., Pluta, R.M., Walbridge, S., Wink, D.A., Oldfield, E.H., and Boock, R.J. (2000). Production of reactive oxygen species after reperfusion in vitro and in vivo: protective effect of nitric oxide. J. Neurosurg. 93, 99–107. [DOI] [PubMed] [Google Scholar]
- 95. Kubes, P., Suzuki, M., and Granger, D.N. (1991). Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U. S. A. 88, 4651–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ma, X.L., Weyrich, A.S., Lefer, D.J., and Lefer, A.M. (1993). Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ. Res. 72, 403–412. [DOI] [PubMed] [Google Scholar]
- 97. Kurose, I., Wolf, R., Grisham, M.B., Aw, T.Y., Specian, R.D., and Granger, D.N. (1995). Microvascular responses to inhibition of nitric oxide production. Role of active oxidants. Circ. Res. 76, 30–39. [DOI] [PubMed] [Google Scholar]
- 98. Clancy, R.M., Leszczynska-Piziak, J., and Abramson, S.B. (1992). Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J. Clin. Invest. 90, 1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ahn, M.J., Sherwood, E.R., Prough, D.S., Lin, C.Y., and DeWitt, D.S. (2004). The effects of traumatic brain injury on cerebral blood flow and brain tissue nitric oxide levels and cytokine expression. J. Neurotrauma 21, 1431–1442. [DOI] [PubMed] [Google Scholar]
- 100. Ucal, M., Kraitsy, K., Weidinger, A., Paier-Pourani, J., Patz, S., Fink, B., Molcanyi, M., and Schafer, U. (2017). Comprehensive profiling of modulation of nitric oxide levels and mitochondrial activity in the injured brain: an experimental study based on the fluid percussion injury model in rats. J. Neurotrauma 34, 475–486. [DOI] [PubMed] [Google Scholar]
- 101. Brown, G.C. and Borutaite, V. (2007). Nitric oxide and mitochondrial respiration in the heart. Cardiovasc. Res. 75, 283–290. [DOI] [PubMed] [Google Scholar]
- 102. Singh, I.N., Sullivan, P.G., and Hall, E.D. (2007). Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J. Neurosci. Res. 85, 2216–2223. [DOI] [PubMed] [Google Scholar]
- 103. Wada, K., Chatzipanteli, K., Busto, R., and Dietrich, W.D. (1998). Role of nitric oxide in traumatic brain injury in the rat. J. Neurosurg. 89, 807–818. [DOI] [PubMed] [Google Scholar]
- 104. Hlatky, R., Lui, H., Cherian, L., Goodman, J.C., O'Brien, W.E., Contant, C.F., and Robertson, C.S. (2003). The role of endothelial nitric oxide synthase in the cerebral hemodynamics after controlled cortical impact injury in mice. J. Neurotrauma 20, 995-1006. [DOI] [PubMed] [Google Scholar]
- 105. Schwarzmaier, S.M., Terpolilli, N.A., Dienel, A., Gallozzi, M., Schinzel, R., Tegtmeier, F., and Plesnila, N. (2015). Endothelial nitric oxide synthase mediates arteriolar vasodilatation after traumatic brain injury in mice. J. Neurotrauma 32, 731–738. [DOI] [PubMed] [Google Scholar]
- 106. Foley, L.M., Hitchens, T.K., Melick, J.A., Bayir, H., Ho, C., and Kochanek, P.M. (2008). Effect of inducible nitric oxide synthase on cerebral blood flow after experimental traumatic brain injury in mice. J. Neurotrauma 25, 299–310. [DOI] [PubMed] [Google Scholar]
- 107. Bayir, H., Kagan, V.E., Borisenko, G.G., Tyurina, Y.Y., Janesko, K.L., Vagni, V.A., Billiar, T.R., Williams, D.L., and Kochanek, P.M. (2005). Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J. Cereb. Blood Flow. Metab. 25, 673–684. [DOI] [PubMed] [Google Scholar]
- 108. Sinz, E.H., Kochanek, P.M., Dixon, C.E., Clark, R.S., Carcillo, J.A., Schiding, J.K., Chen, M., Wisniewski, S.R., Carlos, T.M., Williams, D., DeKosky, S.T., Watkins, S.C., Marion, D.W., and Billiar, T.R. (1999). Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J. Clin. Invest. 104, 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jafarian-Tehrani, M., Louin, G., Royo, N.C., Besson, V.C., Bohme, G.A., Plotkine, M., and Marchand-Verrecchia, C. (2005). 1400W, a potent selective inducible NOS inhibitor, improves histopathological outcome following traumatic brain injury in rats. Nitric Oxide 12, 61–69. [DOI] [PubMed] [Google Scholar]
- 110. Terpolilli, N.A., Kim, S.W., Thal, S.C., Kuebler, W.M., and Plesnila, N. (2013). Inhaled nitric oxide reduces secondary brain damage after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 33, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Terpolilli, N.A., Zweckberger, K., Trabold, R., Schilling, L., Schinzel, R., Tegtmeier, F., and Plesnila, N. (2009). The novel nitric oxide synthase inhibitor 4-amino-tetrahydro-L-biopterine prevents brain edema formation and intracranial hypertension following traumatic brain injury in mice. J. Neurotrauma 26, 1963–1975. [DOI] [PubMed] [Google Scholar]
- 112. Hlatky, R., Goodman, J.C., Valadka, A.B., and Robertson, C.S. (2003). Role of nitric oxide in cerebral blood flow abnormalities after traumatic brain injury. J. Cereb. Blood Flow Metab. 23, 582-588. [DOI] [PubMed] [Google Scholar]
- 113. Hogman, M., Frostell, C., Arnberg, H., Sandhagen, B., and Hedenstierna, G. (1994). Prolonged bleeding time during nitric oxide inhalation in the rabbit. Acta Physiol. Scand. 151, 125-129. [DOI] [PubMed] [Google Scholar]
- 114. Terpolilli, N.A., Kim, S.W., Thal, S.C., Kataoka, H., Zeisig, V., Nitzsche, B., Klaesner, B., Zhu, C., Schwarzmaier, S., Meissner, L., Mamrak, U., Engel, D.C., Drzezga, A., Patel, R.P., Blomgren, K., Barthel, H., Boltze, J., Kuebler, W.M., and Plesnila, N. (2012). Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ. Res. 110, 727-738. [DOI] [PubMed] [Google Scholar]
- 115. Cherian, L. and Robertson, C.S. (2003). L-arginine and free radical scavengers increase cerebral blood flow and brain tissue nitric oxide concentrations after controlled cortical impact injury in rats. J. Neurotrauma 20, 77–85. [DOI] [PubMed] [Google Scholar]
- 116. Liu, H., Goodman, J.C., and Robertson, C.S. (2002). The effects of L-arginine on cerebral hemodynamics after controlled cortical impact injury in the mouse. J Neurotrauma 19, 327-334. [DOI] [PubMed] [Google Scholar]
- 117. Liu, P., Li, Y.S., Quartermain, D., Boutajangout, A., and Ji, Y. (2013). Inhaled nitric oxide improves short term memory and reduces the inflammatory reaction in a mouse model of mild traumatic brain injury. Brain Res. 1522, 67–75. [DOI] [PubMed] [Google Scholar]
- 118. Hekierski, H., Pastor, P., Curvello, V., and Armstead, W.M. (2019). Inhaled nitric oxide protects cerebral autoregulation and reduces hippocampal neuronal cell becrosis after traumatic brain injury in newborn and juvenile pigs. J. Neurotrauma 36, 630–638. [DOI] [PubMed] [Google Scholar]
- 119. Pastor, P., Curvello, V., Hekierski, H.. and Armstead, W.M. (2019). Inhaled nitric oxide protects cerebral autoregulation through prevention of impairment of ATP and calcium sensitive K channel mediated cerebrovasodilation after traumatic brain injury. Brain Res. 1711, 1–6. [DOI] [PubMed] [Google Scholar]
- 120. Curvello, V., Pastor, P., Hekierski, H., and Armstead, W.M. (2019). Inhaled nitric oxide protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury through inhibition of ET-1, ERK MAPK and IL-6 upregulation in pigs. Neurocrit. Care 30, 467–477. [DOI] [PubMed] [Google Scholar]
- 121. Griswold, W.R., Reznik, V., and Mendoza, S.A. (1981). Nitroprusside-induced intracranial hypertension. JAMA 246, 2679–2680. [PubMed] [Google Scholar]
- 122. Weiss, M.H., Spence, J., Apuzzo, M.L., Heiden, J.S., McComb, J.G., and Kurze, T. (1979). Influence of nitroprusside on cerebral pressure autoregulation. Neurosurgery 4, 56–59. [DOI] [PubMed] [Google Scholar]
- 123. Cottrell, J.E., Patel, K., Turndorf, H., and Ransohoff, J. (1978). Intracranial pressure changes induced by sodium nitroprusside in patients with intracranial mass lesions. J. Neurosurg. 48, 329–331. [DOI] [PubMed] [Google Scholar]
- 124. Butterworth, R.J., Cluckie, A., Jackson, S.H., Buxton-Thomas, M., and Bath, P.M. (1998). Pathophysiological assessment of nitric oxide (given as sodium nitroprusside) in acute ischaemic stroke. Cerebrovasc. Dis. 8, 158–165. [DOI] [PubMed] [Google Scholar]
- 125. Bratton, S.L. and Davis, R.L. (1997). Acute lung injury in isolated traumatic brain injury. Neurosurgery 40, 707–712. [DOI] [PubMed] [Google Scholar]
- 126. Holland, M.C., Mackersie, R.C., Morabito, D., Campbell, A.R., Kivett, V.A., Patel, R., Erickson, V.R., and Pittet, J.F. (2003). The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J. Trauma 55, 106–111. [DOI] [PubMed] [Google Scholar]
- 127. Vavilala, M.S., Roberts, J.S., Moore, A.E., Newell, D.W., and Lam, A.M. (2001). The influence of inhaled nitric oxide on cerebral blood flow and metabolism in a child with traumatic brain injury. Anesth. Analg. 93, 351–353. [DOI] [PubMed] [Google Scholar]
- 128. Papadimos, T.J. (2008). The beneficial effects of inhaled nitric oxide in patients with severe traumatic brain injury complicated by acute respiratory distress syndrome: a hypothesis. J. Trauma Manag. Outcomes 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gritti, P., Lanterna, L.A., Re, M., Martchenko, S., Olivotto, P., Brembilla, C., Agostinis, C., Paganoni, G., and Lorini, F.L. (2013). The use of inhaled nitric oxide and prone position in an ARDS patient with severe traumatic brain injury during spine stabilization. J. Anesth. 27, 293-297. [DOI] [PubMed] [Google Scholar]
- 130. Papadimos, T.J., Medhkour, A., and Yermal, S. (2009). Successful use of inhaled nitric oxide to decrease intracranial pressure in a patient with severe traumatic brain injury complicated by acute respiratory distress syndrome: a role for an anti-inflammatory mechanism? Scand. J. Trauma Resusc. Emerg. Med. 17, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Peliowski, A., Canadian Paediatric Society, Fetus and Newborn Committee (2012). Inhaled nitric oxide use in newborns. Paediatr. Child Health 17, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Davidson, D., Barefield, E.S., Kattwinkel, J., Dudell, G., Damask, M., Straube, R., Rhines, J., and Chang, C.T. (1999). Safety of withdrawing inhaled nitric oxide therapy in persistent pulmonary hypertension of the newborn. Pediatrics 104, 231–236. [DOI] [PubMed] [Google Scholar]
- 133. Roberts, J.D.Jr., Fineman, J.R., Morin, F.C., 3rd, Shaul, P.W., Rimar, S., Schreiber, M.D., Polin, R.A., Zwass, M.S., Zayek, M.M., Gross, I., Heymann, M.A., and Zapol, W.M. (1997). Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N. Engl. J. Med. 336, 605–610. [DOI] [PubMed] [Google Scholar]
- 134. Dobyns, E.L., Cornfield, D.N., Anas, N.G., Fortenberry, J.D., Tasker, R.C., Lynch, A., Liu, P., Eells, P.L., Griebel, J., Baier, M., Kinsella, J.P., and Abman, S.H. (1999). Multicenter randomized controlled trial of the effects of inhaled nitric oxide therapy on gas exchange in children with acute hypoxemic respiratory failure. J. Pediatr. 134, 406–412. [DOI] [PubMed] [Google Scholar]
- 135. Bronicki, R.A., Fortenberry, J., Schreiber, M., Checchia, P.A., and Anas, N.G. (2015). Multicenter randomized controlled trial of inhaled nitric oxide for pediatric acute respiratory distress syndrome. J. Pediatr. 166, 365–369 e361. [DOI] [PubMed] [Google Scholar]
- 136. Gebistorf, F., Karam, O., Wetterslev, J., and Afshari, A. (2016). Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst. Rev. CD002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Harris, K., Armstrong, S.P., Campos-Pires, R., Kiru, L., Franks, N.P., and Dickinson, R. (2013). Neuroprotection against traumatic brain injury by xenon, but not argon, is mediated by inhibition at the N-methyl-D-aspartate receptor glycine site. Anesthesiology 119, 1137–1148. [DOI] [PubMed] [Google Scholar]
- 138. Luo, Y., Ma, D., Ieong, E., Sanders, R.D., Yu, B., Hossain, M., and Maze, M. (2008). Xenon and sevoflurane protect against brain injury in a neonatal asphyxia model. Anesthesiology 109, 782–789. [DOI] [PubMed] [Google Scholar]
- 139. Zhuang, L., Yang, T., Zhao, H., Fidalgo, A.R., Vizcaychipi, M.P., Sanders, R.D., Yu, B., Takata, M., Johnson, M.R., and Ma, D. (2012). The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Crit. Care Med. 40, 1724–1730. [DOI] [PubMed] [Google Scholar]
- 140. Yang, T., Zhuang, L., Rei Fidalgo, A.M., Petrides, E., Terrando, N., Wu, X., Sanders, R.D., Robertson, N.J., Johnson, M.R., Maze, M., and Ma, D. (2012). Xenon and sevoflurane provide analgesia during labor and fetal brain protection in a perinatal rat model of hypoxia-ischemia. PLoS One 7, e37020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Banks, P., Franks, N.P., and Dickinson, R. (2010). Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor mediates xenon neuroprotection against hypoxia-ischemia. Anesthesiology 112, 614-622. [DOI] [PubMed] [Google Scholar]
- 142. Joyce, J.A. (2000). Xenon: anesthesia for the 21st century. AANA J 68, 259-264. [PubMed] [Google Scholar]
- 143. Lavaur, J., Lemaire, M., Pype, J., Le Nogue, D., Hirsch, E.C., and Michel, P.P. (2016). Xenon-mediated neuroprotection in response to sustained, low-level excitotoxic stress. Cell Death Discov. 2, 16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Esencan, E., Yuksel, S., Tosun, Y.B., Robinot, A., Solaroglu, I., and Zhang, J.H. (2013). XENON in medical area: emphasis on neuroprotection in hypoxia and anesthesia. Med. Gas Res. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Winkler, D.A., Thornton, A., Farjot, G., and Katz, I. (2016). The diverse biological properties of the chemically inert noble gases. Pharmacol. Ther. 160, 44–64. [DOI] [PubMed] [Google Scholar]
- 146. Coburn, M., Maze, M., and Franks, N.P. (2008). The neuroprotective effects of xenon and helium in an in vitro model of traumatic brain injury. Crit. Care Med. 36, 588–595. [DOI] [PubMed] [Google Scholar]
- 147. Campos-Pires, R., Koziakova, M., Yonis, A., Pau, A., Macdonald, W., Harris, K., Edge, C.J., Franks, N.P., Mahoney, P.F., and Dickinson, R. (2018). Xenon protects against blast-induced traumatic brain injury in an in iitro model. J. Neurotrauma 35, 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Campos-Pires, R., Hirnet, T., Valeo, F., Ong, B.E., Radyushkin, K., Aldhoun, J., Saville, J., Edge, C.J., Franks, N.P., Thal, S.C., and Dickinson, R. (2019). Xenon improves long-term cognitive function, reduces neuronal loss and chronic neuroinflammation, and improves survival after traumatic brain injury in mice. Br. J. Anaesth. 123, 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Campos-Pires, R., Armstrong, S.P., Sebastiani, A., Luh, C., Gruss, M., Radyushkin, K., Hirnet, T., Werner, C., Engelhard, K., Franks, N.P., Thal, S.C., and Dickinson, R. (2015). Xenon improves neurologic outcome and reduces secondary injury following trauma in an in vivo model of traumatic brain injury. Crit. Care Med. 43, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Goto, T., Saito, H., Nakata, Y., Uezono, S., Ichinose, F., and Morita, S. (1997). Emergence times from xenon anaesthesia are independent of the duration of anaesthesia. Br. J. Anaesth. 79, 595–599. [DOI] [PubMed] [Google Scholar]
- 151. Goto, T., Saito, H., Shinkai, M., Nakata, Y., Ichinose, F., and Morita, S. (1997). Xenon provides faster emergence from anesthesia than does nitrous oxide-sevoflurane or nitrous oxide-isoflurane. Anesthesiology 86, 1273–1278. [DOI] [PubMed] [Google Scholar]
- 152. Stoppe, C., Rimek, A., Rossaint, R., Rex, S., Stevanovic, A., Schalte, G., Fahlenkamp, A., Czaplik, M., Bruells, C.S., Daviet, C., and Coburn, M. (2013). Xenon consumption during general surgery: a retrospective observational study. Med. Gas Res. 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lynch, C., 3rd, Baum, J., and Tenbrinck, R. (2000). Xenon anesthesia. Anesthesiology 92, 865-868. [DOI] [PubMed] [Google Scholar]
- 154. Neice, A.E. and Zornow, M.H. (2016). Xenon anesthesia for all, or only a select few? Anaesthesia 71, 1267–1272. [DOI] [PubMed] [Google Scholar]
- 155. Goto, T., Nakata, Y., and Morita, S. (2003). Will xenon be a stranger or a friend?: the cost, benefit, and future of xenon anesthesia. Anesthesiology 98, 1–2. [DOI] [PubMed] [Google Scholar]
- 156. Weigt, H.U., Georgieff, M., Fohr, K.J., and Adolph, O. (2009). In vitro-evaluation of lipid emulsions as vehicles for the administration of xenon: interaction with NMDA receptors. Acta Neurobiol. Exp. (Wars) 69, 207–216. [DOI] [PubMed] [Google Scholar]
- 157. Weigt, H.U., Georgieff, M., Beyer, C., Wachter, U., and Fohr, K.J. (2003). Xenon incorporated in a lipid emulsion inhibits NMDA receptor channels. Acta Anaesthesiol. Scand. 47, 1119–1124. [DOI] [PubMed] [Google Scholar]
- 158. Britton, G.L., Kim, H., Kee, P.H., Aronowski, J., Holland, C.K., McPherson, D.D., and Huang, S.L. (2010). In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation 122, 1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chattaraj, R., Hwang, M., Zemerov, S.D., Dmochowski, I.J., Hammer, D.A., Lee, D., and Sehgal, C.M. (2020). Ultrasound responsive noble gas microbubbles for applications in image-guided gas delivery. Adv. Healthc. Mater 9, e1901721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shin, S.S., Huisman, T., and Hwang, M. (2018). Ultrasound imaging for traumatic brain injury. J. Ultrasound Med. 37, 1857–1867. [DOI] [PubMed] [Google Scholar]
- 161. Hwang, M. (2019). Introduction to contrast-enhanced ultrasound of the brain in neonates and infants: current understanding and future potential. Pediatr. Radiol. 49, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hwang, M., Sridharan, A., Darge, K., Riggs, B., Sehgal, C., Flibotte, J., and Huisman, T. (2019). Novel quantitative contrast-enhanced ultrasound detection of hypoxic ischemic injury in neonates and infants: pilot study 1. J. Ultrasound Med. 38, 2025–2038. [DOI] [PubMed] [Google Scholar]
- 163. Nowrangi, D.S., Tang, J., and Zhang, J.H. (2014). Argon gas: a potential neuroprotectant and promising medical therapy. Med. Gas Res. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Abraini, J.H., Kriem, B., Balon, N., Rostain, J.C., and Risso, J.J. (2003). Gamma-aminobutyric acid neuropharmacological investigations on narcosis produced by nitrogen, argon, or nitrous oxide. Anesth. Analg. 96, 746–749. [DOI] [PubMed] [Google Scholar]
- 165. Koziakova, M., Harris, K., Edge, C.J., Franks, N.P., White, I.L., and Dickinson, R. (2019). Noble gas neuroprotection: xenon and argon protect against hypoxic-ischaemic injury in rat hippocampus in vitro via distinct mechanisms. Br. J. Anaesth. 123, 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Loetscher, P.D., Rossaint, J., Rossaint, R., Weis, J., Fries, M., Fahlenkamp, A., Ryang, Y.M., Grottke, O., and Coburn, M. (2009). Argon: neuroprotection in in vitro models of cerebral ischemia and traumatic brain injury. Crit. Care 13, R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Grusser, L., Blaumeiser-Debarry, R., Krings, M., Kremer, B., Hollig, A., Rossaint, R., and Coburn, M. (2017). Argon attenuates the emergence of secondary injury after traumatic brain injury within a 2-h incubation period compared with desflurane: an in vitro study. Med. Gas Res. 7, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kogan, P., Gessner, R.C., and Dayton, P.A. (2010). Microbubbles in Imaging: Applications Beyond Ultrasound. Bubble Sci. Eng. Technol. 2, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Miao, Y.F., Peng, T., Moody, M.R., Klegerman, M.E., Aronowski, J., Grotta, J., McPherson, D.D., Kim, H., and Huang, S.L. (2018). Delivery of xenon-containing echogenic liposomes inhibits early brain injury following subarachnoid hemorrhage. Sci. Rep. 8, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Dandekar, M.P., Peng, T., McPherson, D.D., Quevedo, J., Soares, J.C., and Huang, S.L. (2018). Intravenous infusion of xenon-containing liposomes generates rapid antidepressant-like effects. Prog. Neuropsychopharmacol. Biol. Psychiatry 86, 140–149. [DOI] [PubMed] [Google Scholar]
- 171. Yue, J.K., Vassar, M.J., Lingsma, H.F., Cooper, S.R., Okonkwo, D.O., Valadka, A.B., Gordon, W.A., Maas, A.I., Mukherjee, P., Yuh, E.L., Puccio, A.M., Schnyer, D.M., and Manley, G.T.; TRACK-TBI Investigators. (2013). Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma 30, 1831–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]