Abstract
OBJECTIVES:
Mixed cardiogenic-septic shock is common and associated with high mortality. There are limited contemporary data on concomitant sepsis in acute myocardial infarction complicated by cardiogenic shock (AMI-CS).
DESIGN:
Observational study.
SETTING:
Twenty percent stratified sample of all community hospitals (2000–2014) in the United States.
PARTICIPANTS:
Adults (> 18 yr) with AMI-CS with and without concomitant sepsis.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Outcomes of interest included inhospital mortality, development of noncardiac organ failure, complications, utilization of guideline-directed procedures, length of stay, and hospitalization costs. Over 15 years, 444,253 AMI-CS admissions were identified, of which 27,057 (6%) included sepsis. The sepsis cohort had more comorbidities and had higher rates of noncardiac multiple organ failure (92% vs 69%) (all p < 0.001). In 2014, compared with 2000, the prevalence of sepsis increased from 0.5% versus 11.5% with an adjusted odds ratio (aOR) 11.71 (95% CI, 9.7–14.0) in ST-segment elevation myocardial infarction and 24.6 (CI, 16.4–36.7) (all p < 0.001) in non-ST segment elevation myocardial infarction. The sepsis cohort received fewer cardiac interventions (coronary angiography [65% vs 68%], percutaneous coronary intervention [43% vs 48%]) and had greater use of mechanical circulatory support (48% vs 45%) and noncardiac support (invasive mechanical ventilation [65% vs 41%] and acute hemodialysis [12% vs 3%]) (p < 0.001). The sepsis cohort had higher inhospital mortality (44.3% vs 38.1%; aOR, 1.21; 95% CI, 1.18–1.25; p < 0.001), longer length of stay (14.0 d [7–24 d] vs 7.0 d [3–12 d]), greater hospitalization costs (×1,000 U.S. dollars) ($176.0 [$85–$331] vs $77.0 [$36–$147]), fewer discharges to home (22% vs 44%) and more discharges to skilled nursing facilities (51% vs 28%) (all p < 0.001).
CONCLUSIONS:
In AMI-CS, concomitant sepsis is associated with higher mortality and morbidity highlighting the need for early recognition and integrated management of mixed shock.
Keywords: acute myocardial infarction, cardiogenic shock, circulatory shock, critical care cardiology, sepsis
Cardiogenic shock (CS) is a leading cause of death among patients suffering acute myocardial infarction (AMI) and the prevalence may be increasing (1–3). Patients with AMI complicated by CS (AMI-CS) have very high short-term mortality (up to 35–50%), with no interventions demonstrated to improve survival during the past 2 decades (4). AMI-CS patients are at substantial risk of major nonfatal complications during hospitalization, including infection and organ failure (4). One particularly important subgroup within the broader CS population includes patients with mixed cardiogenic-vasodilatory shock, who have an elevated risk of adverse outcomes (5).
AMI-CS patients are at risk of development of an inflammatory phenotype accompanied by pathological vasodilation (6). Systemic inflammation appears common in patients with AMI-CS, either triggered by AMI itself, as a response to organ hypoperfusion, due to preceding cardiac arrest, or due to infection (7). In a seminal analysis of the SHould we emergently revascularize Occluded Coronaries for Cardiogenic shocK (SHOCK) trial, approximately 20% of patients with AMI-CS developed suspected sepsis (8). CS patients with concomitant sepsis are at elevated risk of adverse outcomes, which is not unexpected considering that sepsis by itself is a lethal condition that can trigger multiple organ failure (9, 10). However, no large studies have examined the epidemiology or outcomes associated with sepsis occurring in patients with AMI-CS.
Given these knowledge gaps, we sought to assess the temporal trends, management, cost, and outcomes of concomitant sepsis and AMI-CS. We hypothesized that an increasing prevalence of sepsis in AMI-CS would be paralleled by higher rates of critical care therapy utilization, temporary mechanical circulatory support (MCS) use and multiple organ failure.
MATERIALS AND METHODS
Study Population, Variables, and Outcomes
The National (Nationwide) Inpatient Sample (NIS) is a database and software created through a Federal-State-industry partnership sponsored by the Agency for Healthcare Research and Quality for the Healthcare Cost and Utilization Project (HCUP). The HCUP-NIS is the largest all-payer database of hospital inpatient stays in the United States and contains discharges approximating 20% of the stratified sample of the U.S. hospitals (11). The database extracts information from all the states participating in HCUP, which covers more than 97% of the U.S. population. De-identified patient information such as demographics, primary payer, comorbidities, principal diagnosis, up to 29 secondary diagnoses, and procedural diagnoses are available for each discharge. Institutional Review Board approval was not required due to the de-identified nature of the publicly available database. We identified observations as “admissions” rather than considering them as individual patients, restricted study details to inpatient variables as HCUP-NIS does not include validated outpatient information, and used administrative codes previously validated and used for similar studies.
We used the HCUP-NIS data from January 1, 2000, to December 31, 2014, to identify adults (> 18 yr) with AMI in the primary diagnosis field (International Classification of Diseases, 9th Edition, Clinical Modification [ICD-9-CM] 410×) (4, 12, 13). The administrative codes for AMI have high sensitivity (98%), specificity (91%), positive predictive value (95%), and negative predictive value (97%). A secondary diagnosis of CS was identified using ICD-9-CM 785.51, and administrative codes for CS have high positive predictive value (> 90%) and specificity (> 95%) but lower sensitivity (> 50%) (14, 15). While there are several systematic methods to identify sepsis in the literature, most of these algorithms were created prior to integration of sepsis and septic shock ICD codes. To add, since CS itself contributes to organ dysfunction, using combination of sepsis with organ dysfunction would reduce the specificity of our cohort. Therefore, we identified the sepsis diagnosis by using the ICD-9 codes for severe sepsis (Supplementary Table 1, http://links.lww.com/CCX/A912) and septic shock (ICD-9-CM 785.52). These codes have been reciprocated in numerous prior studies (16–20) and are reported to have a sensitivity of ~50% and specificity of 99% (21). It is to be noted that identification of sepsis should be extrapolated as a sepsis “diagnoses,” as changes in the identification could be related to increasing recognition of the disease entity. Comorbidities were identified using the Deyo’s modification of the Charlson Comorbidity Index (22). Information such as age, sex, race, hospital details, comorbidities, cardiac procedures, and other noncardiac organ support use was identified using previously used methodologies from our group (Supplementary Table 2, http://links.lww.com/CCX/A912) (4, 12, 13, 23–29). Noncardiac organ failure was defined using our previously used definition, and multiple organ failure was defined as involvement of greater than or equal to one organ system other than cardiovascular failure (4).
The primary outcomes of interest were temporal trends in prevalence, characteristics, and inhospital mortality of AMI-CS with sepsis. The secondary outcomes of interests were temporal trends in single organ failure, multiple organ failures and the utilization of coronary angiography (CA), percutaneous coronary intervention (PCI), pulmonary artery catheter (PAC), MCS, hospitalization costs, hospital length of stay, and discharge disposition in the cohorts with and without sepsis in AMI-CS.
Statistical Analysis
Discharge weights provided by HCUP were used for statistical analysis, which helped in generating the national estimates (11). Using the trend weights issued by HCUP-NIS, we reweighted the samples from 2000 to 2011 to adjust for the 2012 HCUP-NIS redesign (30). One-way analysis of variance and two-sided t tests were used to compare categorical and continuous variables, respectively. Temporal trends in the prevalence of sepsis and use of CA, PCI, MCS, PAC, acute hemodialysis and invasive, and noninvasive mechanical ventilation were plotted after substratifying for the presence of sepsis and type of AMI. We evaluated temporal trends in prevalence of single and multiple organ failure from 2000 to 2014. Univariable analysis for trends and outcomes was performed and represented as odds ratio (OR) with 95% CI. Multivariable logistic regression was performed to analyze trends over time (compared with the year 2000 as the referent), and OR with 95% CI were calculated for each year adjusting for age, sex, race, income status, comorbidities, primary payer, hospital characteristics, acute organ failure, cardiac arrest, CA, PCI, coronary artery bypass grafting, PAC, MCS, invasive and noninvasive mechanical ventilation, and acute hemodialysis. For the multivariable modeling, regression analysis with purposeful selection of statistically (liberal threshold of p < 0.20 in univariate analysis) and clinically relevant variables was conducted. A sensitivity analysis was performed for admissions with sepsis versus septic shock and adjusted for age, gender, race, insurance, hospital location, zip code, region, type of AMI, sepsis, comorbidities, organ failure, acute kidney injury, and use of invasive cardiac procedures. Two-tailed p value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Version 28.0 (IBM Corp, Armonk NY).
RESULTS
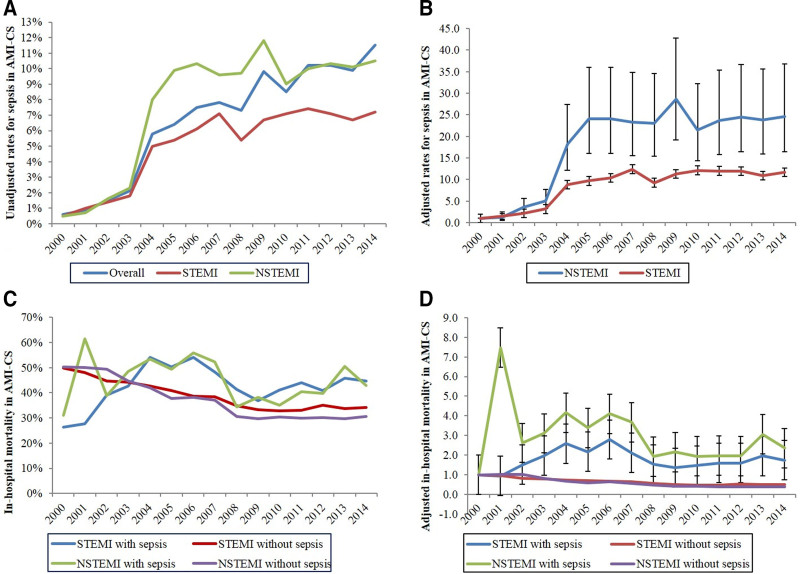
During the study period between January 1, 2000, and December 31, 2014, we identified a total of 444,253 admissions with AMI-CS, of which 27,057 (6.1%) developed concomitant sepsis, including 13,066 (2.9%) labeled as septic shock. Among admissions with sepsis, ST-segment elevation myocardial infarction (STEMI) and non-ST segment elevation myocardial infarction (NSTEMI) accounted for 55.7% and 44.3%, respectively. Temporal trends revealed a steady increase in the prevalence of sepsis, with higher rates in NSTEMI-CS compared with STEMI-CS (Fig. 1, A and B). Admissions with sepsis were younger and more frequently male and White, with greater comorbidities (p < 0.001) (Table 1).
Figure 1.
Trends in the prevalence of sepsis and inhospital mortality of acute myocardial infarction complicated by cardiogenic shock (AMI-CS) admissions. A, Unadjusted temporal trends in the prevalence of AMI-CS admissions with concurrent sepsis stratified by type of AMI (p < 0.001 for trend over time). B, Adjusted odds ratio for prevalence of sepsis in ST-segment elevation myocardial infarction (STEMI)-CS and non-ST segment elevation myocardial infarction (NSTEMI)-CS admissions (with 2000 as the referent)*; p < 0.001 for trend over time. C, Unadjusted inhospital mortality in AMI-CS admissions stratified by the presence of sepsis and type of AMI (p < 0.001 for trend over time). D, Adjusted odds ratio for inhospital mortality by year (2000 as the referent) among AMI-CS with or without presence of sepsis, further stratified based on type of AMI**; p < 0.001 for trend over time. *Adjusted for age, sex, race, comorbidity, primary payer, income status, hospital region, hospital location and teaching status, and hospital bed size. **Adjusted for age, sex, race, income status, comorbidity, primary payer, hospital region, hospital location, teaching status, hospital bed size, acute organ failure, atrial fibrillation, atrial flutter, cardiac arrest, coronary angiography, percutaneous coronary intervention, coronary artery bypass grafting, pulmonary artery catheterization, mechanical circulatory support, invasive and noninvasive mechanical ventilation, and acute hemodialysis (p < 0.001 for trend over time).
TABLE 1.
Baseline Characteristics of Acute Myocardial Infarction Complicated by Cardiogenic Shock Admissions With and Without Sepsis
| Characteristic (n = 444,253) | Sepsis (n = 27,057) | No Sepsis (n = 417,195) | p |
|---|---|---|---|
| Age (yr) | 68.24 ± 12.6 | 69.23 ± 13 | < 0.001 |
| Female | 35.9 | 39.4 | < 0.001 |
| Race | |||
| White | 60.0 | 63.3 | < 0.001 |
| Black | 8.2 | 5.6 | |
| Othersa | 31.8 | 31.1 | |
| Primary payer | |||
| Medicare | 61.0 | 61.5 | < 0.001 |
| Medicaid | 9.1 | 6.1 | |
| Private | 21.9 | 24.1 | |
| Othersb | 8.0 | 8.3 | |
| Quartile of median household income for zip code | |||
| 0–25th | 27.1 | 23.0 | < 0.001 |
| 26–50th | 25.3 | 26.6 | |
| 51st–75th | 23.4 | 25.1 | |
| 75–100th | 24.3 | 25.3 | |
| Charlson Comorbidity Index | |||
| 0–3 | 19.9 | 24.5 | < 0.001 |
| 4–6 | 55.6 | 55.5 | |
| ≥ 7 | 24.5 | 20.0 | |
| Cardiac arrest | 30.0 | 28.3 | < 0.001 |
| Hypertension | 45.1 | 50.6 | < 0.001 |
| Hyperlipidemia | 23.1 | 32.3 | < 0.001 |
| Multiple organ failure | 91.6 | 64.6 | < 0.001 |
| Heart failure | 19.8 | 12.5 | < 0.001 |
| Hospital teaching status and location | |||
| Rural | 4.1 | 7.7 | < 0.001 |
| Urban nonteaching | 33.1 | 41.1 | |
| Urban teaching | 62.8 | 51.2 | |
| Hospital bed size | |||
| Small | 6.6 | 7.9 | < 0.001 |
| Medium | 20.8 | 22.3 | |
| Large | 72.6 | 69.9 | |
| Hospital region | |||
| Northeast | 21.2 | 18.4 | < 0.001 |
| Midwest | 19.9 | 23.1 | |
| South | 36.7 | 38.4 | |
| West | 22.2 | 20.1 | |
aHispanic, Asian or Pacific Islander, Native American, others.
bSelf-pay, no charge, others.
Represented as percentage or mean ± sd.
Compared with admissions without sepsis, the sepsis cohort had a higher prevalence of cardiac arrest, ventricular arrhythmias, atrial flutter, systolic heart failure, stroke (specifically ischemic stroke), and noncardiac single and multiple organ failure (Table 2). The sepsis cohort had higher prevalence of hemorrhage requiring blood transfusions (Table 2). While the sepsis cohort received higher rates of noncardiac interventions and supportive critical care interventions such as PAC, MCS, acute hemodialysis, invasive and noninvasive mechanical ventilation, they also received lower rates of CA and PCI when compared with the cohort without sepsis (p < 0.001), respectively (Table 2). Those with sepsis received more palliative care interventions and had more frequent do-not-resuscitate orders (Table 2).
TABLE 2.
Inhospital Characteristics of Acute Myocardial Infarction Complicated by Cardiogenic Shock Admissions With and Without Sepsis
| Characteristic (n = 444,253) | Sepsis (n = 27,057) | No Sepsis (n = 417,195) | p |
|---|---|---|---|
| Acute myocardial infarction type | |||
| ST-segment elevation myocardial infarction | 55.7 | 68.9 | < 0.001 |
| Non-ST segment elevation myocardial infarction | 44.3 | 31.1 | |
| Acute noncardiac organ failure | |||
| Overall | 91.6 | 64.6 | < 0.001 |
| Respiratory | 65.9 | 41.9 | < 0.001 |
| Hepatic | 20.2 | 7.1 | < 0.001 |
| Renal | 64.9 | 33.2 | < 0.001 |
| Hematologic | 20.4 | 10.4 | < 0.001 |
| Neurologic | 24.3 | 12.6 | < 0.001 |
| Inhospital events | |||
| Cardiac arrest | 30.0 | 28.3 | < 0.001 |
| Ventricular arrhythmias | 27.8 | 26.5 | < 0.001 |
| Atrial fibrillation | 24.8 | 24.3 | 0.048 |
| Atrial flutter | 6.1 | 3.5 | < 0.001 |
| Systolic heart failure | 19.8 | 12.5 | < 0.001 |
| Stroke | 5.8 | 2.8 | < 0.001 |
| Ischemic stroke | 5.1 | 2.5 | < 0.001 |
| Intracranial hemorrhage | 1.0 | 0.4 | < 0.001 |
| Acute pulmonary embolism | 1.7 | 0.7 | < 0.001 |
| Cardiac procedures | |||
| Coronary angiography | 64.7 | 68.0 | < 0.001 |
| Percutaneous coronary intervention | 43.3 | 47.6 | < 0.001 |
| Coronary artery bypass grafting | 19.4 | 17.2 | < 0.001 |
| Mechanical circulatory support | 48.1 | 44.9 | < 0.001 |
| Pulmonary artery catheterization | 12.3 | 7.8 | < 0.001 |
| Noncardiac procedures | |||
| Invasive mechanical ventilation | 65.1 | 40.6 | < 0.001 |
| Noninvasive mechanical ventilation | 5.4 | 2.9 | < 0.001 |
| Acute hemodialysis | 11.6 | 2.9 | < 0.001 |
| Complications | |||
| Ventricular septal defect | 1.3 | 1.0 | 0.001 |
| Papillary muscle rupture | 0.7 | 0.4 | < 0.001 |
| Hemopericardium | 0.2 | 0.3 | 0.172 |
| Cardiac tamponade | 0.9 | 0.5 | < 0.001 |
| Hemorrhage | 5.7 | 3.4 | < 0.001 |
| Vascular injury | 1.8 | 1.7 | 0.531 |
| Blood transfusion | 22.8 | 12.7 | < 0.001 |
| High degree heart block | 5.2 | 6.7 | < 0.001 |
| Palliative care consultation | 7.4 | 4.3 | < 0.001 |
| Do-not-resuscitate status | 6.0 | 4.3 | < 0.001 |
aHispanic, Asian or Pacific Islander, Native American, others.
bSelf-pay, no charge, others.
Represented as percentage or mean ± sd.
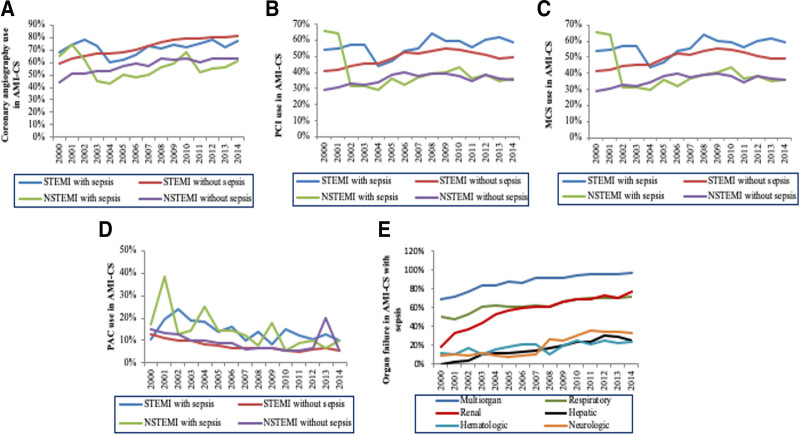
When stratified based on the type of AMI, use of CA, PCI, and MCS increased in STEMI gradually but declined in NSTEMI (Fig. 2A–C). A decline in PAC use was seen across AMI-CS subgroups over the 15 years (Fig. 2D). A higher percentage of sepsis admissions had concomitant multiple organ failure with a steady increase in trend during this 15-year period (Fig. 2E). The baseline and inhospital characteristics of those with sepsis versus septic shock are presented in Supplementary Table 3 (http://links.lww.com/CCX/A912).
Figure 2.
Temporal trends in the use of cardiac procedures, noncardiac organ failure and support device in acute myocardial infarction complicated by cardiogenic shock (AMI-CS) admissions. A, Temporal trends of the proportion of AMI-CS admissions receiving coronary angiography (A), percutaneous coronary intervention (PCI) (B), mechanical circulatory support (MCS) (C) pulmonary artery catheterization (PAC) (D), and single and multiple organ failure (E), stratified by type of AMI (p < 0.001 for trend over time for all). NSTEMI = non-ST segment elevation myocardial infarction, STEMI = ST-segment elevation myocardial infarction.
Inhospital mortality was higher in those with sepsis compared with those without (44.3% vs 38.1%; unadjusted OR, 1.29; 95% CI, 1.26–1.32; adjusted OR, 1.21; 95% CI, 1.18–1.25; p < 0.001). Temporal trends revealed an increase in inhospital mortality among the admissions with sepsis, whereas there was a decline in mortality for those without sepsis (p < 0.001) (Fig. 1, C and D). Admissions with sepsis had a significantly longer length of hospital stay, higher hospitalization costs, less frequent discharges to home, and higher rates of discharge to skilled nursing facilities (p < 0.001) (Table 3). In a sensitivity analysis (Supplementary Table 4, http://links.lww.com/CCX/A912), admissions coded as septic shock had higher inhospital mortality in the subgroups of age, gender, race, organ failure, and cardiac arrest.
TABLE 3.
Clinical Outcomes of Acute Myocardial Infarction Complicated by Cardiogenic Shock Admissions With and Without Sepsis
| Characteristic (n = 444,253) | Sepsis (n = 27,057) | No Sepsis (n = 417,195) | p |
|---|---|---|---|
| Inhospital mortality | 44.3 | 38.1 | < 0.001 |
| Length of stay (d) | 14.0 (7–24) | 7.0 (3–12) | < 0.001 |
| Hospitalization costs (×1,000 U.S. dollars) | 176.0 (85–331) | 77.0 (36–147) | < 0.001 |
| Discharge disposition | |||
| Home | 21.5 | 43.6 | < 0.001 |
| Transfer | 10.8 | 11.5 | |
| Skilled nursing facility | 51.0 | 27.9 | |
| Home with home health care | 16.3 | 16.6 | |
| Against medical advice | 0.4 | 0.4 | |
Represented as percentage or median (interquartile range).
DISCUSSION
In the largest study assessing the epidemiology and outcomes of sepsis in AMI-CS, sepsis was present in 6% of all AMI-CS admissions, with a steadily increasing prevalence over time paralleled by a rising use of invasive therapies. The sepsis cohort was sicker, with higher burden of comorbidities, inhospital complications, higher rates of noncardiac organ failure, cardiac arrest, and higher rates of organ support therapies; while this suggests a real association between sepsis and adverse outcomes in AMI-CS, we could conclusively determine that residual confounding by severity of illness did not mediate this association. Although all patients had AMI-CS, admissions with sepsis consistently received CA and PCI less frequently as compared with those without sepsis. The cohort with sepsis had higher inhospital mortality, hospitalization costs, and longer hospital length of stay than those without sepsis, even after adjusting for their greater illness severity and utilization of critical care therapies. The presence of sepsis was associated with differences in AMI-CS management, as evidenced by lower utilization of guideline-directed cardiac procedures and more palliative interventions, which in turn may have contributed to higher mortality. Inhospital mortality rose during the study period for AMI-CS patients with sepsis despite declining mortality among AMI-CS patients without sepsis, highlighting a critical need to identify improved care strategies for this high-risk subgroup.
Sepsis is among the leading causes of hospitalization, organ failure, and death worldwide (10). Therefore, it is not surprising that patients with concomitant AMI-CS and sepsis were at elevated risk for a broad array of adverse outcomes. This phenomenon was first demonstrated in a secondary analysis of the SHOCK trial, which found that 18% of patients with AMI-CS subsequently developed suspected sepsis (three-quarters had positive blood cultures) and these patients had a two-fold higher adjusted hazard of death (8). A recent single-center study from the Mayo Clinic likewise found that patients with concomitant CS and sepsis (who accounted for 19% of all patients with CS) had higher illness severity and a greater risk of short-term mortality (9). In the multicenter Cardiac Critical Care Trials Network cardiac ICU (CICU) registry, patients with mixed vasodilatory-CS accounted for 20% of shock patients, and they were sicker and at higher risk of dying during hospitalization (5). An analysis of Mayo Clinic CICU patients found that the prevalence of sepsis increased in parallel with the severity of shock, from 6% in patients with mild shock to 37% in patients with severe shock; this implicates concomitant sepsis as a potential driver of more severe CS (31). A subsequent analysis using this same CICU cohort demonstrated that most patients met systemic inflammatory response syndrome (SIRS) criteria on admission, with an increasing prevalence as the severity of shock increased; these patients were more likely to die across the spectrum of shock severity (32). By contrast, only 2.4% of patients with AMI (but without CS) enrolled in the Pexelizumab in Conjunction With Angioplasty trial developed a serious infection during hospitalization; these patients had nearly five-fold higher rates of 90-day adverse events (33). It is notable that the prevalence of sepsis identified in this nationally representative cohort is substantially lower (6% vs 18%) than that observed in more selected CS cohorts; whether this reflects differences in population composition or variability in disease recognition or documentation is uncertain (5, 8, 9). Changes in recognition or documentation of sepsis over time may, in part, explain the multi-fold increase of sepsis prevalence in AMI-CS in addition to the aforementioned reasons.
Critically ill patients with AMI-CS who require invasive cardiopulmonary support will be at elevated risk of developing healthcare-associated infections (HAIs), mandating diligent infection prevention practices (34). Indeed, the longer length of stay and greater use of critical care interventions in the sepsis group could have predisposed this group to development of nosocomial sepsis, as opposed to development of sepsis contributing to greater care needs directly. The prevalence of HAIs in patients hospitalized with acute cardiovascular disease is not insignificant and was highest among patients with CS (35). Patients who developed HAI had higher inhospital mortality, longer length of stay, and higher hospital costs, as we observed among AMI-CS patients with concomitant sepsis.
The role of systemic inflammation in the development of progressive CS and multiple organ failure after AMI has been recognized for many years, and it is likely that the same pathophysiological processes linking inflammation from sepsis to multiple organ and circulatory failure overlap. Myocyte necrosis from AMI triggers a multitude of immune-mediated inflammatory processes, frequently leading to sterile systemic inflammation that may mimic sepsis; indeed, up to one-quarter of patients with STEMI meet SIRS criteria on admission (36). The systemic ischemia-reperfusion injury occurring after resuscitation from cardiac arrest often produces a profound inflammatory response, in addition to the increased risk of infection these patients face (37). Regardless of the cause, systemic inflammation can produce pathologic vasodilation aggravating hemodynamic compromise as well as promoting direct tissue and organ injury. Levels of inflammatory mediators such as C-reactive protein and interleukin-6 have been identified as important prognostic biomarkers in patients with AMI-CS and out-of-hospital cardiac arrest and correlate with the severity of shock in the latter group (38–40).
Patients with sepsis in the SHOCK trial had lower systemic vascular resistance, and patients with mixed shock in the Mayo Clinic and Cardiac Critical Care Trials Network cohorts had greater vasopressor requirements (8). Considering that temporary MCS devices augment arterial pressure by increasing cardiac output, it is expected that the presence of vasoplegia from sepsis and systemic inflammation could decrease the hemodynamic efficacy of these devices (6, 41). This represents a major challenge impeding the stabilization of shock in these patients with high shock severity, who may represent a clinically relevant CS subphenotype in whom a different treatment strategy may be necessary. Although we observed decreasing use of the PAC over time in our cohort, recent studies have highlighted the potential benefit of invasive hemodynamic monitoring with a PAC in patients with CS (42, 43).
Despite the HCUP-NIS database’s efforts to minimize errors in analysis by utilizing internal and external quality control measures, the HCUP-NIS database has fundamental restrictions that may limit research designing, data interpretations, and data analysis that we took into account (30). There are several established approaches to identifying sepsis using administrative codes, each of which has important limitations; we used explicit ICD-CM coding for sepsis rather than implicit sepsis ICD-9-CM coding (i.e., the combination of infection and organ dysfunction diagnosis codes) because CS itself can cause organ dysfunction, which would reduce the specificity of implicit coding for sepsis (10). Because all ICD-9-CM codes used in this analysis are hospitalization discharge diagnoses, we cannot definitively established whether AMI-CS or sepsis occurred first; however, included patients had a primary discharge diagnosis of AMI, making it likely that this was the initial indication for admission. Given the administrative nature of this database, we cannot accurately distinguish type 1 from type 2 AMIs. However, this study included admissions with a primary diagnosis of AMI (i.e., the reason most likely for the admission) and, therefore, is less likely to include type 2 AMIs, which often have an alternate primary diagnosis.
Information about shock severity, the source of sepsis, laboratory results, radiographic evidence, and additional details about the medical management could not be reliably identified from the NIS-HCUP database. This precludes use from definitively concluding that all patients labeled as sepsis had a true infection, as opposed to a sterile systemic inflammatory response. Similarly, septic shock may occur as a part of mixed shock in clinical practice, but the ICD codes for mixed shocks are not validated. Additionally, there is a possibility of residual confounding despite careful attempts to adjust for confounders through multivariable analysis.
Although we studied the data worth 15 years duration, we included admissions until 2014. Since there have been substantial changes in definition and treatment of sepsis by introduction of Third International Consensus Definitions for Sepsis and Septic Shock and Surviving Sepsis Campaign guidelines, we believe future analysis of recent data will be helpful in determining trends (44, 45). Several studies published after 2015 have highlighted the limitations of using discharge ICD-9 codes for case identification. The increase in coding of sepsis diagnoses seems to be independent of the increase in prevalence of sepsis diagnosis recognized by clinical criteria (46–49). Therefore, we cannot determine whether the observed trends toward more frequent sepsis diagnoses over time were due to changes in disease prevalence, recognition or coding. As such, our results regarding temporal trends should be interpreted with caution. Our study period includes some admissions prior to the consensus definition of sepsis and original Surviving Sepsis Campaign guidelines were published, and the publication of these important documents could have increased awareness of sepsis and influenced the observed trends in sepsis diagnoses.
Last, our results are only representative of inhospital outcomes and cannot be correlated with long-term consequences given the nature of the database; this is salient insofar as survivors of sepsis hospitalization are known to be at elevated risk of subsequent death and adverse cardiovascular events (50).
CONCLUSIONS
In conclusion, in this 15-year national study, we found a steady rise in the prevalence and associated consequent inhospital mortality in sepsis complicating AMI-CS. Admissions that developed sepsis in AMI-CS had worse comorbidity, higher rates of multiple organ failure and cardiac arrest and a greater risk of death even when accounting for these factors. The increased utilization of invasive support modalities paralleled the growing prevalence of sepsis, raising important questions regarding whether a rising prevalence of sepsis necessitated greater use of these therapies versus whether the increased use of invasive support devices put patients at a higher risk of sepsis. Further clinical research is warranted to understand the causes and pathophysiology of sepsis in AMI-CS to identify improved prevention and treatment strategies.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Jentzer and Bhat contributed equally to this article as co-first authors.
Drs. Jentzer, Bhat, Patlolla, and Vallabhajosyula involved in study design, literature review, and statistical analysis. Drs. Bhat, Patlolla, and Vallabhajosyula involved in data management, data analysis, and drafting article. Drs. Sinha, Miller, Lawler, van Diepen, Khanna, Zhao, and Vallabhajosyula involved in article revision, intellectual revisions, and mentorship. All authors involved in access to data and final approval.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Chioncel O, Parissis J, Mebazaa A, et al. : Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22:1315–1341 [DOI] [PubMed] [Google Scholar]
- 2.Omer MA, Tyler JM, Henry TD, et al. : Clinical characteristics and outcomes of STEMI patients with cardiogenic shock and cardiac arrest. JACC Cardiovasc Interv 2020; 13:1211–1219 [DOI] [PubMed] [Google Scholar]
- 3.Kolte D, Khera S, Aronow WS, et al. : Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 2014; 3:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallabhajosyula S, Dunlay SM, Prasad A, et al. : Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol 2019; 73:1781–1791 [DOI] [PubMed] [Google Scholar]
- 5.Berg DD, Bohula EA, van Diepen S, et al. : Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes 2019; 12:e005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Diepen S, Katz JN, Albert NM, et al. ; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline: Contemporary management of cardiogenic shock: A scientific statement from the American Heart Association. Circulation 2017; 136:e232–e268 [DOI] [PubMed] [Google Scholar]
- 7.Cuinet J, Garbagnati A, Rusca M, et al. : Cardiogenic shock elicits acute inflammation, delayed eosinophilia, and depletion of immune cells in most severe cases. Sci Rep 2020; 10:7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohsaka S, Menon V, Lowe AM, et al. ; SHOCK Investigators: Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch Intern Med 2005; 165:1643–1650 [DOI] [PubMed] [Google Scholar]
- 9.Jentzer JC, Ahmed AM, Vallabhajosyula S, et al. : Shock in the cardiac intensive care unit: Changes in epidemiology and prognosis over time. Am Heart J 2021; 232:94–104 [DOI] [PubMed] [Google Scholar]
- 10.Rudd KE, Johnson SC, Agesa KM, et al. : Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020; 395:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HCUP National Inpatient Sample (NIS): Healthcare Cost and Utilization Project (HCUP). Rockville, MD, Agency for Healthcare Research and Quality. Available at: https://www.hcup-us.ahrq.gov/. Accessed January 24, 2022. [Google Scholar]
- 12.Vallabhajosyula S, Kumar V, Vallabhajosyula S, et al. : Acute myocardial infarction-cardiogenic shock in patients with prior coronary artery bypass grafting: A 16-year national cohort analysis of temporal trends, management and outcomes. Int J Cardiol 2020; 310:9–15 [DOI] [PubMed] [Google Scholar]
- 13.Vallabhajosyula S, Patlolla SH, Dunlay SM, et al. : Regional variation in the management and outcomes of acute myocardial infarction with cardiogenic shock in the United States. Circ Heart Fail 2020; 13:e006661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert L, Blais C, Hamel D, et al. : Evaluation of care and surveillance of cardiovascular disease: Can we trust medico-administrative hospital data? Can J Cardiol 2012; 28:162–168 [DOI] [PubMed] [Google Scholar]
- 15.Lauridsen MD, Gammelager H, Schmidt M, et al. : Positive predictive value of International Classification of Diseases, 10th revision, diagnosis codes for cardiogenic, hypovolemic, and septic shock in the Danish National Patient Registry. BMC Med Res Methodol 2015; 15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar G, Kumar N, Taneja A, et al. ; Milwaukee Initiative in Critical Care Outcomes Research (MICCOR) Group of Investigators: Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest 2011; 140:1223–1231 [DOI] [PubMed] [Google Scholar]
- 17.Martin GS, Mannino DM, Eaton S, et al. : The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 18.Angus DC, Linde-Zwirble WT, Lidicker J, et al. : Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 19.Bouza C, Lopez-Cuadrado T, Amate-Blanco JM: Use of explicit ICD9-CM codes to identify adult severe sepsis: Impacts on epidemiological estimates. Crit Care 2016; 20:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallabhajosyula S, Deshmukh AJ, Kashani K, et al. : Tako-Tsubo cardiomyopathy in severe sepsis: Nationwide trends, predictors, and outcomes. J Am Heart Assoc 2018; 7:e009160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolley RJ, Sawka KJ, Yergens DW, et al. : Validity of administrative data in recording sepsis: A systematic review. Crit Care 2015; 19:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quan H, Sundararajan V, Halfon P, et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139 [DOI] [PubMed] [Google Scholar]
- 23.Vallabhajosyula S, Dunlay SM, Barsness GW, et al. : Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol 2019; 124:491–498 [DOI] [PubMed] [Google Scholar]
- 24.Vallabhajosyula S, Dunlay SM, Barsness GW, et al. : Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS One 2019; 14:e0222894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallabhajosyula S, Dunlay SM, Kashani K, et al. : Temporal trends and outcomes of prolonged invasive mechanical ventilation and tracheostomy use in acute myocardial infarction with cardiogenic shock in the United States. Int J Cardiol 2019; 285:6–10 [DOI] [PubMed] [Google Scholar]
- 26.Vallabhajosyula S, Dunlay SM, Prasad A, et al. : Cardiogenic shock and cardiac arrest complicating ST-segment elevation myocardial infarction in the United States, 2000-2017. Resuscitation 2020; 155:55–64 [DOI] [PubMed] [Google Scholar]
- 27.Vallabhajosyula S, Kashani K, Dunlay SM, et al. : Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000-2014. Ann Intensive Care 2019; 9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallabhajosyula S, Prasad A, Bell MR, et al. : Extracorporeal membrane oxygenation use in acute myocardial infarction in the United States, 2000 to 2014. Circ Heart Fail 2019; 12:e005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallabhajosyula S, Prasad A, Dunlay SM, et al. : Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: A 15-year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc 2019; 8:e011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khera R, Angraal S, Couch T, et al. : Adherence to methodological standards in research using the national inpatient sample. JAMA 2017; 318:2011–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jentzer JC, van Diepen S, Barsness GW, et al. : Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol 2019; 74:2117–2128 [DOI] [PubMed] [Google Scholar]
- 32.Jentzer JC, Lawler PR, van Diepen S, et al. : Systemic inflammatory response syndrome is associated with increased mortality across the spectrum of shock severity in cardiac intensive care patients. Circ Cardiovasc Qual Outcomes 2020; 13:e006956. [DOI] [PubMed] [Google Scholar]
- 33.Truffa AA, Granger CB, White KR, et al. : Serious infection after acute myocardial infarction: Incidence, clinical features, and outcomes. JACC Cardiovasc Interv 2012; 5:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fordyce CB, Katz JN, Alviar CL, et al. ; American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; and Stroke Council: Prevention of complications in the cardiac intensive care unit: A scientific statement from the American Heart Association. Circulation 2020; 142:e379–e406 [DOI] [PubMed] [Google Scholar]
- 35.Miller PE, Guha A, Khera R, et al. : National trends in healthcare-associated infections for five common cardiovascular conditions. Am J Cardiol 2019; 124:1140–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Diepen S, Vavalle JP, Newby LK, et al. : The systemic inflammatory response syndrome in patients with ST-segment elevation myocardial infarction. Crit Care Med 2013; 41:2080–2087 [DOI] [PubMed] [Google Scholar]
- 37.Jentzer JC, Chonde MD, Dezfulian C: Myocardial dysfunction and shock after cardiac arrest. Biomed Res Int 2015; 2015:314796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceglarek U, Schellong P, Rosolowski M, et al. : The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur Heart J 2021; 42:2344–2352 [DOI] [PubMed] [Google Scholar]
- 39.Bro-Jeppesen J, Johansson PI, Kjaergaard J, et al. : Level of systemic inflammation and endothelial injury is associated with cardiovascular dysfunction and vasopressor support in post-cardiac arrest patients. Resuscitation 2017; 121:179–186 [DOI] [PubMed] [Google Scholar]
- 40.Bro-Jeppesen J, Kjaergaard J, Wanscher M, et al. : Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: A substudy of the target temperature management trial. Crit Care Med 2015; 43:1223–1232 [DOI] [PubMed] [Google Scholar]
- 41.Rihal CS, Naidu SS, Givertz MM, et al. ; Society for Cardiovascular Angiography and Interventions (SCAI), Heart Failure Society of America (HFSA), Society of Thoracic Surgeons (STS), American Heart Association (AHA), and American College of Cardiology (ACC): 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiología Intervencionista; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol 2015; 65:2140–2141 [DOI] [PubMed] [Google Scholar]
- 42.Garan AR, Kanwar M, Thayer KL, et al. : Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in-hospital mortality. JACC Heart Fail 2020; 8:903–913 [DOI] [PubMed] [Google Scholar]
- 43.O’Neill WW, Grines C, Schreiber T, et al. : Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J 2018; 202:33–38 [DOI] [PubMed] [Google Scholar]
- 44.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhodes A, Evans LE, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43:304–377 [DOI] [PubMed] [Google Scholar]
- 46.Rhee C, Dantes R, Epstein L, et al. ; CDC Prevention Epicenter Program: Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA 2017; 318:1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhee C, Kadri S, Huang SS, et al. : Objective sepsis surveillance using electronic clinical data. Infect Control Hosp Epidemiol 2016; 37:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhee C, Murphy MV, Li L, et al. ; Centers for Disease Control and Prevention Epicenters Program: Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin Infect Dis 2015; 60:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhee C, Murphy MV, Li L, et al. ; Centers for Disease Control and Prevention Epicenters Program: Improving documentation and coding for acute organ dysfunction biases estimates of changing sepsis severity and burden: A retrospective study. Crit Care 2015; 19:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mankowski RT, Yende S, Angus DC: Long-term impact of sepsis on cardiovascular health. Intensive Care Med 2019; 45:78–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.