Abstract
Purpose:
To describe the in vivo confocal microscopic and clinico-pathologic correlations in Lisch corneal dystrophy.
Methods:
This is a retrospective case series of 2 patients with Lisch corneal dystrophy. The diagnosis was made based on clinical findings in both cases and was confirmed histopathologically following epithelial debridement in case 1. In vivo laser scanning confocal microscopy using the Heidelberg Retina Tomograph III with the Rostock Cornea Module was carried out in both cases.
Results:
Clinical examination of the corneas revealed areas of epithelial opacification that were sharply demarcated in juxtaposition with normal corneal epithelium. The gray feathery appearance of the epithelial lesions in both cases was characteristic of Lisch corneal dystrophy. The central visual axis was involved in case 1, and corneal topography showed irregular astigmatism. Histological analysis of the epithelial cells in this case showed intracytoplasmic vacuoles, confirming the diagnosis of Lisch corneal dystrophy. In vivo confocal microscopy in both cases demonstrated highly hyperreflective epithelial cytoplasm with hypo-reflective nuclei. There was involvement of all epithelial layers extending to the limbus and findings on imaging were confined to the clinically observed areas of corneal opacity. The lesion in case 1 recurred after epitheliectomy of the central cornea without removal of affected limbal cells.
Conclusions:
The unique features on in vivo confocal microscopy correlated with the clinical and histopathologic features of Lisch corneal dystrophy may be used to distinguish this disorder from other corneal epithelial conditions. The affected epithelial cells appear to originate from abnormal limbal stem cells.
Keywords: In vivo confocal microscopy, Lisch corneal microcystic dystrophy, limbal stem cells, band-shaped corneal opacity, corneal opacity
Microcystic corneal dystrophy was first described by Lisch et al in 1992, hence the name Lisch corneal dystrophy.1 It is transmitted by an X-linked dominant pattern and is clinically characterized by isolated gray punctate or cystic epithelial opacities presenting in a band-like distribution.1,2 There is no associated pigmentation, deposition of iron, bulbar or palpebral conjunctival abnormalities. The presentation may be unilateral or bilateral, and there is no characteristic location or size. The salient features of this inherited dystrophy include characteristic band-shaped feathery opacities and devoid of recurrent erosions, which is unlike other corneal epithelial abnormalities with a similar appearance.3 However, they are visually significant if present within the visual axis. Histopathologic and electron microscopic features include vacuolated epithelial cytoplasm,1,4,5 though the partially homogeneous and lamellar intracytoplasmic material has not been further characterized.1,6 Electron-dense whorled inclusions and reduced tonofilaments have been noted.5
In vivo confocal microscopic findings for this rare corneal epithelial dystrophy have not been previously reported. This imaging modality offers an alternative non-invasive method of characterizing and diagnosing this condition. In this study, we report the in vivo confocal microscopic findings in 2 cases of Lisch corneal dystrophy.
CASE 1
Patient 1 was a 37-year-old myopic man with a history of visually disabling corneal opacification in the right eye requiring epithelial debridement of the central cornea and repeat scraping with application of mitomycin C several years later. He subsequently presented with recurrence of blurry vision approximately 2 years following the last debridement. Corrected visual acuities were 20/20 in both eyes. Clinical examination of the right eye revealed a fairly well-demarcated paracentral area of gray, feathery corneal opacification with a microcystic pattern that extended to the limbus in a tapering fashion (Figs. 1A–B). The visual axis was partly involved. There was another thin linear opacity inferiorly that had the same appearance as the larger lesion. The left cornea was completely clear. The remainder of the examination in both eyes was unremarkable. Corneal topography showed irregular astigmatism in the right eye (Fig. 2).
FIGURE 1.
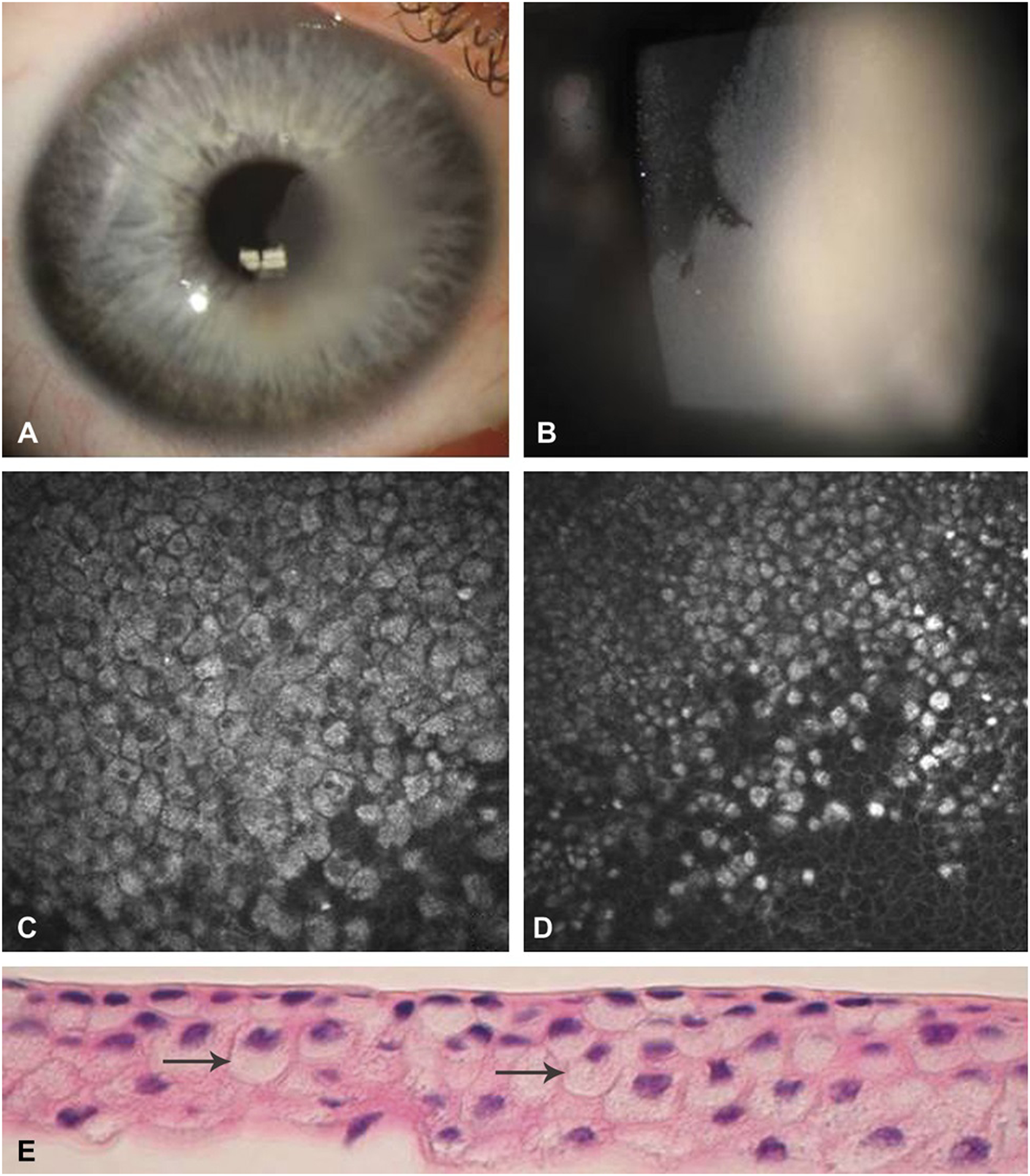
Clinical presentation of Case 1. A, Slit-lamp photograph showing localized corneal epithelial opacification involving the visual axis. B, Higher magnification slit-lamp photograph of the corneal lesion in sharp contrast to adjacent normal epithelium. C-D, In vivo confocal microscopy of epithelial cells with hyperreflective cytoplasm and hyporeflective nuclei. E, Histopathologic demonstration of vacuolization in the cytoplasm of the involved epithelial cells (arrows).
FIGURE 2.
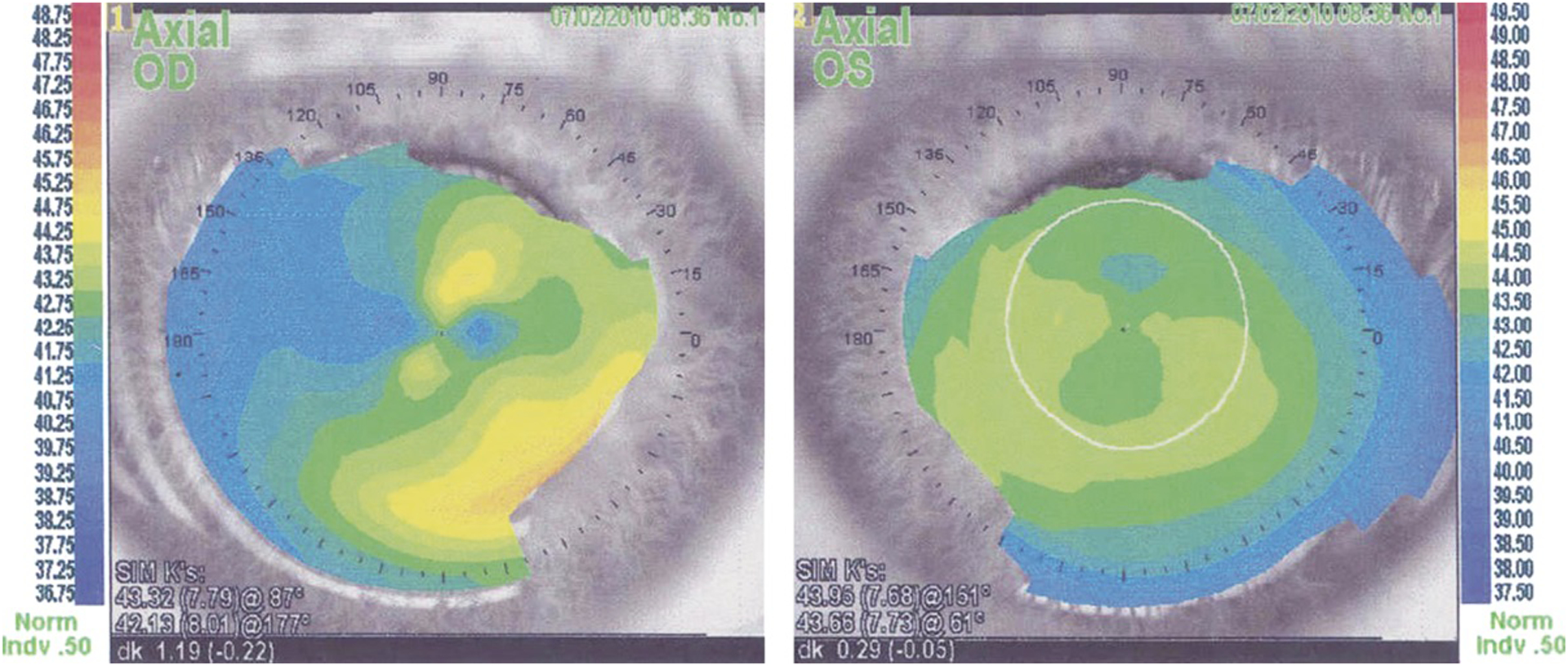
Case 1. Corneal topography of the affected right eye demonstrating irregular astigmatism, in contrast to the unaffected left eye.
In vivo confocal microscopy using the Heidelberg Retina Tomograph III with the Rostock Cornea Module (Heidelberg Engineering GmbH, Dossenheim, Germany) revealed a well-demarcated hyperreflective cytoplasm and hyporeflective nuclei (Figs. 1C–D). The cells were highly compact and no intraepithelial inclusions were present. The cell size and shape of the abnormal epithelium were similar to those of the normal cells. The abnormal cells were noted to be densely packed with sharply demarcated cell borders, with appearance in distinct contrast to the surrounding normal epithelial cells. The sheet of affected epithelium corresponded to the clinically involved area and showed a discrete border with normal epithelium. The hyperreflective cells appeared to originate from the limbus, with the overall lesion increasing in dimension as the central cornea was approached.
The patient underwent an alcohol-facilitated epitheliectomy of the central cornea for diagnostic purposes and to rule out a dysplastic condition. Histopathologic examination revealed cytoplasmic vacuolization confirming the diagnosis of Lisch corneal dystrophy (Fig. 1E). On subsequent examination 2 months following epitheliectomy, the lesion recurred at the same location and was approximately one-third the size of the original lesion. A repeat confocal microscopy showed the characteristic highly hyperreflective cytoplasm and hyporeflective nuclei in the affected epithelium in a well-demarcated pattern, confirming the recurrence of the lesion. Again, all layers of the epithelium were affected within the abnormal area. Since the central visual axis was not involved, further observation for possible visually significant recurrence was resumed.
CASE 2
A 40-year-old man with prior bilateral LASIK surgery and history of retinal tear was referred for a non-visually significant corneal lesion in the right eye. Uncorrected visual acuities were 20/20 in both eyes. Slit-lamp examination revealed well-positioned LASIK flaps and a superficial band-shaped, gray, feathery opacity with well-demarcated distribution in the periphery of the right cornea (Figs. 3A–B). The lesion was within the LASIK hinge region; therefore, the integrity of the lesion had not been affected during the refractive procedure. The lesion had a microcystic appearance and resembled the features from the first case. The opacity extended to the limbus where the width of the lesion was significantly narrower, in a tapered fashion. There was no visual axis involvement. No epithelial defect or staining with fluorescein was noted, and the conjunctiva was not inflamed. The remainder of the examination in both eyes was remarkable only for myopic degeneration and a retinal hole with laser demarcation of the left eye.
FIGURE 3.
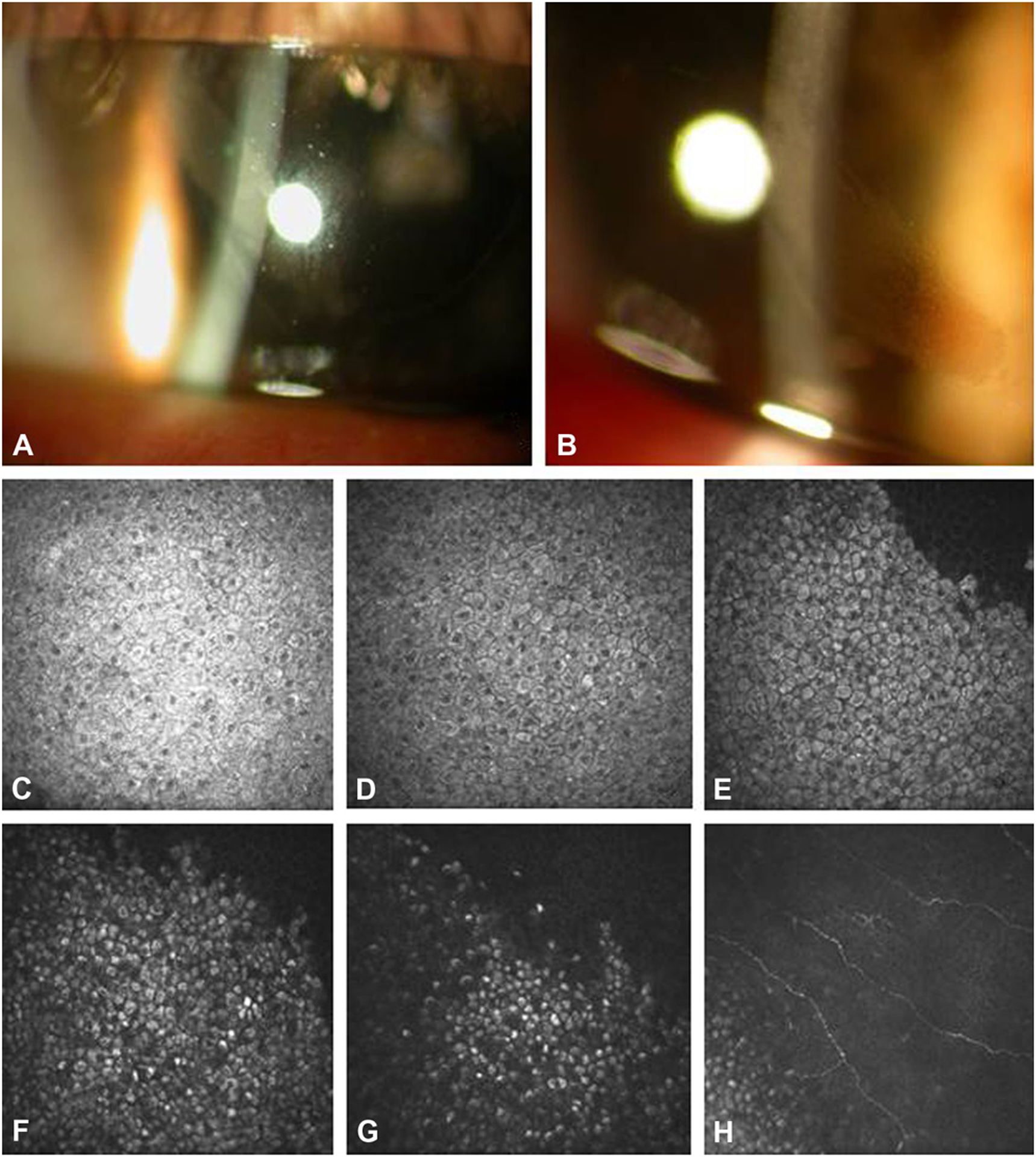
Clinical Presentation of Case 2. A, Slit-lamp photograph showing a band shaped corneal epithelial lesion radiating from the limbus. B, Higher magnification slit-lamp photograph showing microcystic pattern corneal opacification. C-G, In-vivo confocal microscopy from superficial to the wing epithelial layers (C-E) and basal epithelial layers (F-H) demonstrating cells with hyperreflective cytoplasm and hyporeflective nuclei. The nuclei are less prominent in the basal cells (F-G).
In vivo confocal microscopy of the cornea revealed an identical phenotype to case 1. The affected epithelial cells had hyperreflective cytoplasm and hyporeflective nuclei (Figs. 3C–E). The cells in the basal layer were more uniformly hyperreflective with less obvious nuclei (Figs. 3F–3H). A montage of confocal images of the entire lesion demonstrated the tapered configuration, suggesting that the lesion may have originated from the limbus (Fig. 4). Within the affected area, all layers of the epithelial cells were involved in both the limbus and central cornea. Given paucity of visual symptoms and lack of proven treatment, observation was elected.
FIGURE 4.
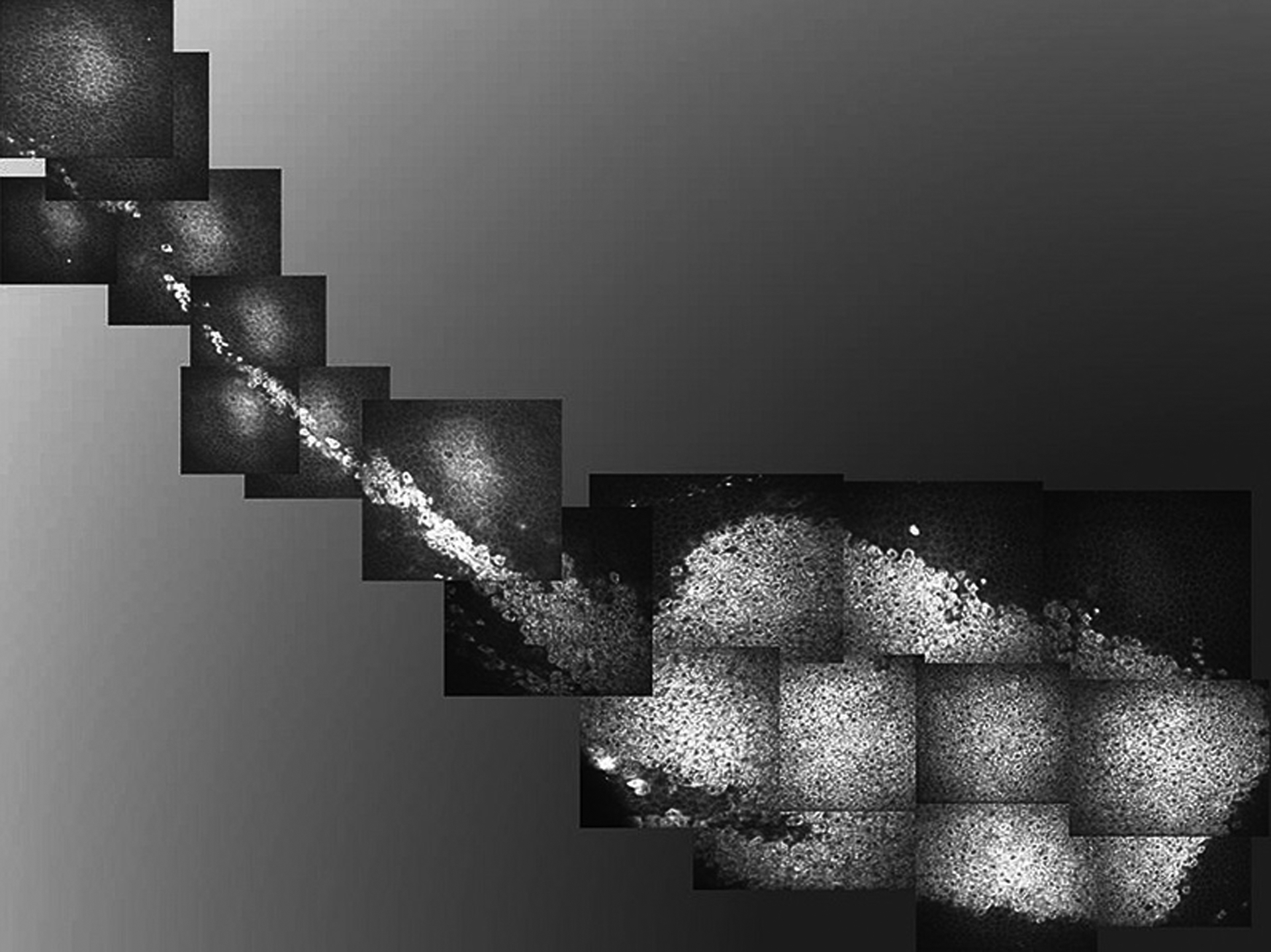
Case 2. In vivo confocal microscopy composite image demonstrating localized, well-demarcated distribution of affected epithelial cells with hyperreflective cytoplasm. The configuration of the confocal image corresponds to the clinical appearance and location of the epithelial opacification. The tapered appearance of the lesion is near the limbus (pictured at the left side of the image). The basal limbal epithelial cells were also involved.
DISCUSSION
In vivo confocal microscopy in the cases described herein showed 4 characteristic features of the abnormal epithelial cells: 1) highly hyperreflective cytoplasm and hyporeflective nuclei; 2) uniform involvement of all layers of the epithelial cells within the affected area; 3) well-demarcated borders with adjacent normal epithelium; and 4) involvement of the limbal area. The lesions imaged with confocal microscopy were present only in the areas directly corresponding to the clinically observed opacities. The shape and size of the affected cells were similar to those of normal epithelial cells. No distinct intracellular deposits were present, though the cytoplasm was hyperreflective in a granular pattern. The non-uniform nature of hyperreflectivity may be due to the partially lamellar intracytoplasmic material1,6 or secondary to whorled inclusions noted on electron microscopy.5 The features observed with in vivo confocal imaging correspond with the pathologic findings: abnormal cells with intracytoplasmic vacuoles and involvement of all epithelial layers on light microscopy,1,4,6 as noted in the sample obtained in case 1 (Fig. 1E). This very distinct pattern of confocal characteristics appears to be unique to this epithelial dystrophy.
The clinical presentation of band-shaped whorled keratopathy in Lisch corneal dystrophy may mimic other lesions, such as epithelial dysplasia, and it is imperative to rule out conjunctival or limbal epithelial dysplasia with corneal involvement. Confocal microscopy findings in corneal epithelial dysplasia show a cluster of cells with highly reflective nuclei and cytoplasm in all layers of the corneal epithelium,7 in contrast to the hyporeflective nuclei in Lisch corneal dystrophy. Meesmann’s corneal dystrophy, which is genetically distinct from Lisch dystrophy,2 presents with intracytoplasmic microcysts. The patients often suffer from recurrent erosions due to rupture of the superficial cysts. Unlike the discrete uniform dense lesions seen in Lisch corneal dystrophy, the clinically affected areas in Meesmann’s are diffuse, bilateral, and found in an interpalpebral distribution. Confocal microscopy shows hyporeflective intraepithelial cysts that are generally larger than 30 μm.8 There is hyperreflective material within the intraepithelial cysts, and other reports have speculated that these hyperreflective intracytoplasmic dots represent cell nuclei,9,10 which is in contrast to the hyporeflective nuclei in Lisch corneal dystrophy.
Epithelial basement membrane dystrophy, which may present with smaller cystic lesions, is also known to cause painful recurrent epithelial erosions. Confocal microscopy studies have revealed subepithelial linear changes,11 including curved ridges and ring-like structures in the basal epithelial cell layers, as well as islands of cells with intracellular hyperreflective deposits.12 Other reports have also commented on the presence of round hyperreflective structures, thought to be the nuclei of multinucleate epithelial giant cells.9 Among other similar corneal epithelial conditions, corneal verticillata in Fabry disease and in drug-related corneal deposits may occasionally present with confounding clinical findings. These bilateral lesions have a characteristic appearance and may often be discerned with the help of historical factors and systemic associations, as well as by laboratory evaluation. Confocal microscopy findings in amiodarone-induced verticillata reveal circular intracellular microdeposits; the presence of inclusions is most prominent at the level of basal epithelium.11,13 These pinpoint scattered hyperreflective deposits are not present in Lisch corneal dystrophy. Taken together, the affected epithelium in Lisch corneal dystrophy has a very distinct phenotype on confocal imaging that seems to be pathognomonic of this condition.
Although observation is often the elected mode of management, it is important to rule out epithelial dysplasia, when suspected. The lesions in Lisch corneal dystrophy may be progressive and recurrence following epitheliectomy often occurs. In case 1, the affected limbal epithelium was not removed during the debridement, and the corneal lesion recurred within 2 months of the procedure. This phenomenon may be attributed to the presence of epithelial limbal stem cells that are affected by this condition, leading to repopulation of the corneal surface with abnormal cells despite debridement of clinically affected areas in the central cornea.
Although Lisch microcystic corneal dystrophy is normally asymptomatic, there is no proven management for visually significant lesions that preclude recurrence. One suggested modality by Lisch et al14 is contact lens wear, which presumably reduces the corneal opacification by inducing thinning of the epithelial layers. Progression of lesions after discontinuation of contact lens wear has been noted.4,6,14 A recent report indicated the possible utility of surface ablation with use of photorefractive keratectomy and mitomycin C.5 If lesions indeed originate from abnormal epithelial stem cells; however, only removal of the affected limbal epithelial stem cells may eradicate the disease.
In conclusion, pathognomonic in vivo confocal microscopic findings in Lisch corneal dystrophy may allow a non-invasive diagnostic modality and may help distinguish this condition from other clinically similar corneal lesions. In addition, the natural history of recurrence and the morphologic features confirmed with confocal microscopy introduce the possibility that abnormal limbal stem cells may be implicated in the pathogenesis of Lisch corneal dystrophy.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Ben Glasgow for the contribution and interpretation of the histopathology slide. This study was supported in part by a Prevent Blindness American Investigator Award (SXD).
Footnotes
Financial disclosures/conflicts of interest: None reported.
REFERENCES
- 1.Lisch W, Steuhl KP, Lisch C, et al. A new, band-shaped and whorled microcystic dystrophy of the corneal epithelium. Am J Ophthalmol. 1992;114:35–44. [DOI] [PubMed] [Google Scholar]
- 2.Lisch W, Büttner A, Oeffner F, et al. Lisch corneal dystrophy is genetically distinct from Meesmann corneal dystrophy and maps to Xp22.3. Am J Ophthalmol. 2000;130:461–468. [DOI] [PubMed] [Google Scholar]
- 3.Butros S, Lang GK, Alvarez de Toledo J, et al. [The different opacity patterns of Lisch corneal dystrophy]. Klin Monbl Augenheilkd. 2006;223: 837–840. [DOI] [PubMed] [Google Scholar]
- 4.Charles NC, Young JA, Kumar A, et al. Band-shaped and whorled microcystic dystrophy of the corneal epithelium. Ophthalmology. 2000; 107:1761–1764. [DOI] [PubMed] [Google Scholar]
- 5.Wessel MM, Sarkar JS, Jakobiec FA, et al. Treatment of Lisch corneal dystrophy with photorefractive keratectomy and mitomycin C. Cornea. 2011;30:481–485. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Fischer M, de Toledo JA, Barraquer RI. Lisch corneal dystrophy. Cornea. 2005;24:494–495. [DOI] [PubMed] [Google Scholar]
- 7.Wakuta M, Chikama T, Takahashi N, et al. A case of bilateral corneal epithelial dysplasia characterized by laser confocal biomicroscopy and cytokeratin immunofluorescence. Cornea. 2008;27:107–110. [DOI] [PubMed] [Google Scholar]
- 8.Szaflik JP, Ołdak M, Maksym RB, et al. Genetics of Meesmann corneal dystrophy: a novel mutation in the keratin 3 gene in an asymptomatic family suggests genotype-phenotype correlation. Mol Vis. 2008;14: 1713–1718. [PMC free article] [PubMed] [Google Scholar]
- 9.Hernández-Quintela E, Mayer F, Dighiero P, et al. Confocal microscopy of cystic disorders of the corneal epithelium. Ophthalmology. 1998;105: 631–636. [DOI] [PubMed] [Google Scholar]
- 10.Javadi MA, Rezaei Kanavi M, Javadi A, et al. Meesmann corneal dystrophy; a clinico-pathologic, ultrastructural and confocal scan report. J Ophthalmic Vis Res. 2010;5:122–126. [PMC free article] [PubMed] [Google Scholar]
- 11.Szaflik JP. Comparison of in vivo confocal microscopy of human cornea by white light scanning slit and laser scanning systems. Cornea. 2007;26: 438–445. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg ME, Tervo TM, Petroll WM, et al. In vivo confocal microscopy of patients with corneal recurrent erosion syndrome or epithelial basement membrane dystrophy. Ophthalmology. 2000;107:565–573. [DOI] [PubMed] [Google Scholar]
- 13.Ciancaglini M, Carpineto P, Zuppardi E, et al. In vivo confocal microscopy of patients with amiodarone-induced keratopathy. Cornea. 2001;20: 368–373. [DOI] [PubMed] [Google Scholar]
- 14.Lisch W, Wasielica-Poslednik J, Lisch C, et al. Contact lens-induced regression of Lisch epithelial corneal dystrophy. Cornea. 2010;29:342–345. [DOI] [PubMed] [Google Scholar]


