Introduction
Alport syndrome is characterized by hematuria, progressive kidney failure, hearing loss, and ocular abnormalities.1 X-linked inheritance caused by pathogenic COL4A5 variants is much more common than recessive disease with 2 pathogenic variants in COL4A3 or COL4A4. Individuals with a heterozygous pathogenic COL4A3 or COL4A4 variant (sometimes called “autosomal dominant Alport syndrome” or “thin basement membrane nephropathy”) have hematuria but rarely develop kidney failure, hearing loss, or the ocular features.2 The COL4A3-COL4A5 genes correspond to the collagen IV α3 to α5 chains that form the major structural network within the basement membranes of the kidney, cochlea, and eye, which explains the typical clinical manifestations.
Overall, X-linked Alport syndrome affects about 1 in 2000 of the population and heterozygous COL4A3 or COL4A4 variants occur in 1 in 100.3 However, even X-linked Alport syndrome is often unrecognized because affected individuals have milder disease or an atypical phenotype. Milder disease occurs in women, and in men with pathogenic variants located in certain regions of the collagen IV α5 chain, or involving substitutions with certain amino acids.3
Atypical phenotypes with Alport syndrome are recognized increasingly. Thus, many affected individuals present with proteinuria rather than hematuria and are often initially diagnosed with steroid-resistant nephrotic syndrome or focal segmental glomerular sclerosis (FSGS).4 Focal segmental glomerular sclerosis probably occurs with pathogenic COL4A3-COL4A5 variants because the abnormal glomerular basement membrane results in loss of the overlying podocytes5 and the development of secondary glomerular hyperfiltration. Similar changes also occur in the cornea6 and presumably in the retina where the temporal retina is thinned overlying Bruch’s membrane.7 Pathogenic changes in the genes affected in Alport syndrome are the most common variants found in cohorts with focal segmental glomerular sclerosis.4 In addition, pathogenic COL4A3 to COL4A5 variants are common in familial IgA glomerulonephritis where the thinned glomerular basement membrane may enable IgA to pass from the glomerular capillaries into the mesangium.
Kidney cysts have also been reported more often in individuals with pathogenic COL4A3-COL4A5 variants and the diagnosis of Alport syndrome.8,9 We describe here a man with renal impairment and multiple bilateral kidney cysts in whom genetic testing identified a pathogenic COL4A5 variant consistent with the diagnosis of X-linked Alport syndrome.
Case Presentation
A 49-year-old man was referred for management of kidney failure by his family doctor. He had hypertension and gout but no other significant medical history. He had not been aware of his kidney function until this time, but his current serum creatinine level was 297 μmol/l (normal 60–110) corresponding to an estimated glomerular filtration rate of 20 ml/min per 1.73 m2 (normal >90), with a urinary red blood cell count of 109 red blood cells × 106/l (normal <10) and urinary albumin:creatinine of 128.6 (normal <2.5). The patient had a family history of kidney disease, with a maternal grandfather who developed kidney failure and a 69-year-old mother, a brother, and a sister all with mildly impaired kidney function. None of the affected family members knew the cause of their kidney impairment, and in particular, none was known to have kidney cysts.
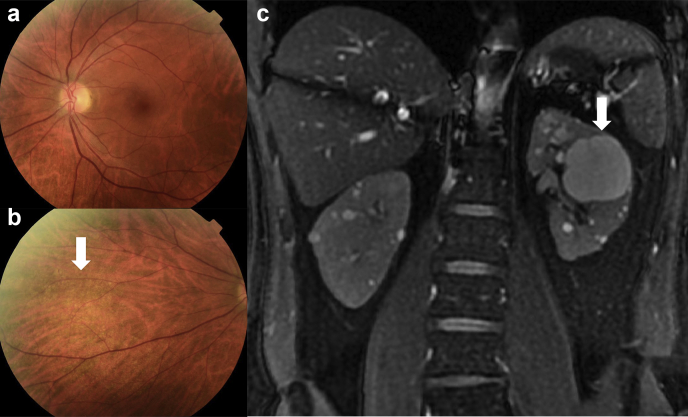
The patient was evaluated for Alport syndrome and stated that his hearing was normal, and he did not undergo formal audiometry. He had no central fleck retinopathy on retinal imaging or temporal retinal thinning on optical coherence tomography (Heidelberg instruments) (Figure 1a). There was a subtle nasal fleck retinopathy but its significance was not clear (Figure 1b).
Figure 1.
(a) Central view of the patient’s left retina that demonstrates no central or peripheral fleck retinopathy. (b) Nasal view revealing a subtle peripheral fleck retinopathy (arrow) that suggests Alport syndrome. (c) Coronal T2 MR image revealing multiple bilateral cortical and medullary cysts of varying sizes, with the largest on the left measuring 40 mm across (arrow). There were no solid renal lesions found and no cysts in the liver, pancreas, or spleen. MR, magnetic resonance.
An abdominal magnetic resonance imaging scan revealed multiple bilateral kidney cysts (Figure 1c). The kidney volume as evaluated by the ellipsoid calculation method (volume = length × width × depth × Pi / 6) was 188 ml and 190 ml for the right and left kidneys, where the normal values were 219 ml and 213 ml, respectively. There were no cysts in the liver, pancreas, or spleen.
The patient then underwent whole genomic screening using a “Cystic and ciliopathy” and a “Glomerulopathy” gene panel that included COL4A3-COL4A5. A COL4A5 variant was identified (c.358G>A, p.Gly120Ser) that was considered Likely Pathogenic based on the criteria of the American College of Medical Genetics and Genomics (PM1, PM2, PP2, PP3), consistent with the diagnosis of X-linked Alport syndrome. No pathogenic variant was found in the PKD1 or PKD2 genes that are affected in autosomal dominant polycystic kidney disease (ADPKD), nor in HNF1b, GANAB, or DNAJB11, nor in any of the other genes in the cyst and ciliopathy panel.
The patient had a fistula constructed and hemodialysis was commenced, as well as a workup for kidney transplantation. His 2 children and other family members were referred for counseling and genetic testing.
Discussion
The management of this individual demonstrates the advantages of genetic testing to identify the cause of inherited kidney disease. The presence of multiple kidney cysts and late-onset kidney failure, with inheritance consistent with AD disease, together with apparently normal hearing, suggested a cystic kidney disease, most likely ADPKD. The cysts were probably too numerous for age-related cystic change.
The patient also had hematuria and a family history of kidney impairment that was consistent with X-linked Alport syndrome. He did not have the typical hearing loss or central fleck retinopathy, but the subtle peripheral flecks were a possible clue to the diagnosis.
Kidney cysts, however, have been reported previously in Alport syndrome. They occur in males and females with a pathogenic COL4A5 variant and X-linked disease, and those with a heterozygous COL4A3 or COL4A4 variant and thin basement membrane nephropathy or AD Alport syndrome.8,9 Cysts in COL4A3-COL4A5-associated disease are distinguished from those in ADPKD by being fewer, smaller, and not substantially distorting the kidney outline or increasing the kidney volume. They affect both the cortex and medulla. They are associated with any pathogenic COL4A3 to COL4A5 variants, first occur before the age of 50 years, and may be associated with normal kidney function but are more common when proteinuria is also present. These features imply that cysts occur with more severe disease, but too few pathogenic variants have been reported for genotype-phenotype correlations. The cysts are not large enough to worsen kidney function themselves, and, at present, no specific treatment is required. Cysts have not been described in the liver or other abdominal organs with COL4A3- to COL4A5-associated disease.
The explanation for the cysts in Alport syndrome is unclear, but the collagen IV α3 to α5 chains are found in the basement membranes in the glomerulus, Bowman’s capsule, and distal tubule. Kidney cysts occur too in a canine model of AD Alport syndrome.10 Pathogenic variants in COL4A1 affecting the collagen IV α1 chain also result in kidney cysts in hereditary angiopathy, with nephropathy, aneurysms and muscle cramps syndrome (“HANAC”).11 This rare condition is more typically associated with cerebral small vessel disease and porencephaly, than kidney cysts. COL4A1 is expressed in the vascular basement membranes in the brain and elsewhere, including membranes in the glomerulus and proximal and distal kidney tubules. Increased fluid pressure or hypertension in addition to thinned and weakened membranes12 may result in cyst formation.
The cysts in Alport syndrome can be distinguished from those in ADPKD which are typically more numerous, larger, and associated with cysts in the liver, and sometimes spleen and pancreas. Inheritance in ADPKD is clearly AD with affected individuals in each generation having multiple cysts. Conversely, in Alport syndrome, there is dysmorphic hematuria, and proteinuria, and, in the case of X-linked or recessive disease, hearing loss, and ocular abnormalities.
The importance of describing this patient is that hematuria and a family history of renal impairment suggest Alport syndrome (Table 1). The demonstration of a hearing loss or ocular abnormalities makes the diagnosis of Alport syndrome even more likely. Finding kidney cysts should not discourage the clinician or the laboratory from considering the diagnosis of Alport syndrome and that the cysts are caused by pathogenic COL4A3-COL4A5 variants (Table 1). This means that cystic and ciliopathy gene panels should also include the COL4A3, COL4A4 and COL4A5 genes. Genetic testing (often whole-genome sequencing) for ADPKD detects COL4A3-COL4A5 pathogenic variants, but whole-exome sequencing performed for a glomerulonephritis or Alport gene panel may not detect a variant in PKD1 or PKD2 when the diagnosis is actually ADPKD.
Table 1.
Teaching points
|
|
|
|
|
It is still unclear how often cysts occur with pathogenic COL4A3-COL4A5 variants and the age when cysts can first be found. Making the distinction between Alport syndrome and ADPKD or age-related kidney cysts is important because their management is different. All males with X-linked Alport sydnrome should be treated with renin-angiotensin-aldosterone blockade from the time of diagnosis, and all women from the onset of proteinuria. Those with ADPKD may require specific treatment to slow down cyst expansion. In addition, the prognosis is different. Individuals with ADPKD develop kidney failure by their late 50s, and 90% of men with X-linked Alport syndrome reach kidney failure by 40 years and 30% of women by 70 years. The prognosis for family members is also different. On average, half of the sons and half of the daughters of an individual with ADPKD inherit the mutation and develop kidney failure. In contrast, a man with X-linked Alport syndrome has none of his sons affected but all his daughters inherit the mutation. Although the risk of kidney failure for these women is small, they pass on the pathogenic variant to half their own sons who will themselves develop kidney failure.
This patient illustrates the extended clinical phenotype associated with pathogenic COL4A3 -COL4A5 variants and indicates that the finding of kidney cysts where Alport syndrome is suspected should not discourage genetic testing for variants in the COL4A3, COL4A4 and COL4A5 genes (Table 1). Indeed, the presence of cysts in a young person with a family history of hematuria may suggest a pathogenic COL4A3-COL4A5 variant. These cysts are not currently considered to contribute significantly to kidney impairment, and no cyst-specific treatment is required.
Disclosure
All the authors declared no competing interests.
Patient Consent
The patient provided written permission for this manuscript, and its submission was approved by the Northern Health Low Risk Ethics Committee.
Acknowledgments
The authors thank our patient whose medical history is described here.
References
- 1.Gubler M., Levy M., Broyer M., et al. Alport’s syndrome. A report of 58 cases and a review of the literature. Am J Med. 1981;70:493–505. doi: 10.1016/0002-9343(81)90571-4. [DOI] [PubMed] [Google Scholar]
- 2.Savige J., Rana K., Tonna S., Buzza M., Dagher H., Wang Y.Y. Thin basement membrane nephropathy. Kidney Int. 2003;64:1169–1178. doi: 10.1046/j.1523-1755.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 3.Gibson J., Fieldhouse R., Chan M.M.Y., et al. Prevalence estimates of predicted pathogenic COL4A3-COL4A5 variants in a population sequencing database and their implications for Alport syndrome. J Am Soc Nephrol. 2021;32:2273–2290. doi: 10.1681/ASN.2020071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gast C., Pengelly R.J., Lyon M., et al. Collagen (COL4A) mutations are the most frequent mutations underlying adult focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2016;31:961–970. doi: 10.1093/ndt/gfv325. [DOI] [PubMed] [Google Scholar]
- 5.Wickman L., Hodgin J.B., Wang S.Q., Afshinnia F., Kershaw D., Wiggins R.C. Podocyte depletion in thin GBM and Alport syndrome. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicklason E., Mack H., Beltz J., et al. Corneal endothelial cell abnormalities in X-linked Alport syndrome. Ophthal Genet. 2020;41:13–19. doi: 10.1080/13816810.2019.1709126. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed F., Kamae K.K., Jones D.J., et al. Temporal macular thinning associated with X-linked Alport syndrome. JAMA Ophthalmol. 2013;131:777–782. doi: 10.1001/jamaophthalmol.2013.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sevillano A.M., Gutierrez E., Morales E., et al. Multiple kidney cysts in thin basement membrane disease with proteinuria and kidney function impairment. Clin Kidney J. 2014;7:251–256. doi: 10.1093/ckj/sfu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulati A., Sevillano A.M., Praga M., et al. Collagen IV gene mutations in adults with bilateral renal cysts and CKD. Kidney Int Rep. 2020;5:103–108. doi: 10.1016/j.ekir.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hood J.C., Dowling J., Bertram J.F., et al. Correlation of histopathological features and renal impairment in autosomal dominant Alport syndrome in bull terriers. Nephrol Dial Transplant. 2002;17:1897–1908. doi: 10.1093/ndt/17.11.1897. [DOI] [PubMed] [Google Scholar]
- 11.Plaisier E., Gribouval O., Alamowitch S., et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 12.Khoshnoodi J., Pedchenko V., Hudson B.G. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]