Abstract
Incremental peritoneal dialysis (PD), defined as less than “standard dose” PD prescription, has a number of possible benefits, including better preservation of residual kidney function (RKF), reduced risk of peritonitis, lower peritoneal glucose exposure, lesser environmental impact, and reduced costs. Patients commencing PD are often new to kidney replacement therapy and possess substantial RKF, which may allow safe delivery of an incremental prescription, often in the form of lower frequency or duration of PD. This has the potential to help improve quality of life (QOL) and life participation through reducing time requirements and burden of treatment. Alternatively, incremental PD could potentially contribute to reduced small solute clearance, fluid overload, or patient reluctance to increase dialysis prescription when later needed. This review discusses the definition, rationale, uptake, potential advantages and disadvantages, and clinical trial evidence pertaining to the use of incremental PD.
Keywords: incremental dialysis, patient-centered care, peritoneal dialysis, personalized medicine, quality of life
PD is chosen as the first modality of dialysis by many patients newly diagnosed with having kidney failure and is currently used to treat approximately 11% of patients on dialysis worldwide.1 Frequently, PD is the first form of kidney replacement therapy for patients with kidney failure in whom substantial RKF supports fluid and solute removal. In these circumstances, it is reasonable to contend that these patients may not require “standard dose” PD to maintain health.
There are many purported advantages of incremental PD, including reduced peritonitis risk, glucose exposure, environmental waste and cost, preservation of RKF, and improved QOL. However, there are also possible disadvantages, including reduced small solute clearance, increased risk of fluid overload, increased mortality risk, and reluctance to increase PD prescription over time.
The practice of incremental PD aligns closely to the latest guideline from the International Society for Peritoneal Dialysis on prescribing high-quality, goal-directed PD, which emphasizes that dialysis should be prescribed using shared decision-making, with the aim of minimizing symptoms and treatment burden and maintaining QOL, in light of the lack of evidence that small solute clearance affects patient outcomes.2 Thus, the aim of this review is to provide a comprehensive update on incremental PD, including its definition, uptake, potential advantages and disadvantages, and outcomes.
Definition
Although the concept of incremental PD has been recognized by national bodies since the 1990s3 and has been used by health practitioners ad hoc before this, the definition of incremental PD is not clearly established. In general terms, incremental PD may be considered as any PD prescription that is less than the “standard dose,” achieves a combined peritoneal and kidney clearance target, and is intended to increase as needed if and when RKF declines.4
However, what is considered a standard PD prescription is subject to regional variation, often in the context of differences in the population, particularly body habitus. In China and Hong Kong, a standard prescription may be considered to be 3 exchanges of 2 l/d.5,6 However, in many western countries, a standard PD prescription is typically 4 exchanges of 2 l/d on continuous ambulatory PD (CAPD)7,8 or equivalent on automated PD, incorporating a long dwell.9 As such, incremental PD has been defined using both of these values as a threshold across various studies10, 11, 12, 13, 14 (Tables 1 and 2). Incremental PD can be delivered in numerous ways, including varying fill volume, number of exchanges, or incorporating dry periods or days off PD,10,13 often with a goal to meet patients’ lifestyle and clinical needs (Table 3).
Table 1.
Characteristics of previous studies comparing IncrPD and stPD
| Publication details | N | Study design | Study population details | Incremental PD intervention | Standard PD intervention | Duration on incremental PD |
|---|---|---|---|---|---|---|
| Ankawi et al.10 | N = 106
|
|
Prevalent patients on PD achieving target total weekly Kt/V of ≥1.7 |
|
|
NR (cross-sectional study) |
| Jeloka et al.14 | N = 41
|
|
Adult patients with urinary Kt/V of approximately 1 were offered incremental PD based on local practice. Excluded patients with HIV, hepatitis B, and hepatitis C. IncrPD patients changed to stPD if Kt/V <1.7 or on clinician judgment |
|
|
9.6 mo (IQR NR) 18.8 ± 14.7 mo |
| Lee et al.12 | N = 347
|
|
Incident patients on PD, ≥16 yr old, follow-up ≥ 6 mo, urine volume ≥ 200 ml. Excluded patients with previous HD |
|
|
2.6 yr (IQR 1.6–4.5) |
| Sandrini et al.13 | N = 105
|
|
Incident patients on PD, follow-up ≥6 mo, RKF 3–10 ml/min per 1.73 m2 |
|
|
17 mo (IQR 10–30) |
| Yan et al.11 | N = 139
|
|
Incident patients on PD on CAPD, 18–80 yr old, GFR ≥ 2 ml/min, urine volume ≥ 500 ml/d. Excluded patients with previous HD or kidney transplantation, life expectancy <6 mo, active malignancy, acute infection, significant heart failure, or other severe diseases at enrollment |
|
|
NR (12/70 patients allocated to incrPD changed to stPD during the study) |
| Yu et al.6 | N = 87,183
|
|
Patients commenced on PD between 2005 and 2015, enrolled in Baxter Patient Support Program, established on PD at least 90 d and not received APD previously |
|
|
NR |
APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; HD, hemodialysis; incrPD, incremental PD; IQR, interquartile range; NR, not reported; PD, peritoneal dialysis; RKF, residual kidney function; stPD, standard PD.
Table 2.
Outcomes reported in previous studies comparing IncrPD and stPD
| Publication details | Peritonitis | Glucose exposure | RKF | Cost | QOL | Solute clearance | Technique survival | Mortality |
|---|---|---|---|---|---|---|---|---|
| Ankawi et al.10 | IncrPD peritonitis rate for the 3 yr before the study was 0.34, 0.48, and 0.30 episodes per patient-year. Rate for year of the study was 0.27 episodes per patient-year. Peritonitis for stPD NR. | NR | Among those achieving Kt/V >1.7, residual renal creatinine clearance was significantly greater in incrPD (6.2 ± 3.4 vs. 2.7 ± 2.4 ml/min, P < 0.0001). | NR | NR | Among those achieving Kt/V > 1.7, no significant difference in peritoneal Kt/V (incrPD 1.15 ± 0.3 vs. stPD 1.62 ± 0.4, P < 0.0001). | Duration on PD was significantly less in incremental group (15 ± 14 vs. 27 ± 26 mo, P < 0.001). | NR |
| Jeloka et al.14 | Peritonitis rate incrPD 0.21 vs. stPD 0.47 episodes per patient-year (P value NR) | NR | NR | NR | NR | NR | NR | Patient survival significantly longer in the incrPD group (incrPD 42.84 ± 7 vs. stPD 25.29 ± 9.2 mo, P = 0.01) |
| Lee et al.12 | No difference in incidence rate of first peritonitis (incrPD 0.10 episodes per patient-year, 95% CI 0.08–0.13 vs. stPD 0.10, 95% CI 0.08–0.12). Recurrent events of peritonitis NR. Median time to first episode of peritonitis overall 2.3 yr (NR for incrPD vs. stPD). No difference in probability of remaining peritonitis-free (P = 0.860). |
NR | Reduced risk of anuria in incremental group (HR 0.61, 95% CI 0.43–0.88, P = 0.007) | NR | NR | NR | No difference in time to technique failure (incrPD 2.7 vs. stPD 2.9 yr, P = 0.332) | No difference in death from any cause (10.9 vs. 7.6 events per 1000 person-years, P = 0.449) |
| Sandrini et al.13 | Incidence of peritonitis was 0.09 episodes per patient-year in incrPD vs. 0.23 episodes per patient-year in stPD. No difference in probability of remaining peritonitis-free (P value NR). | NR | Residual renal function was lower in the stPD at 6 mo (incrPD 6.20 ± 2.02 vs. stPD 4.48 ± 2.96 ml/min per 1.73 m2, P < 0.001) and at the end of treatment (incrPD 4.36 ± 2.96 vs. stPD 2.03 ± 2.55 ml/min per 1.73 m2, P < 0.001) | NR | NR | No difference in twKt/V at 6 mo (incrPD 2.13 ± 0.45 vs. stPD 2.20 ± 0.43 ml/min per 1.73 m2, P = 0.527) but stPD significantly greater at end of treatment (incrPD 1.77 ± 0.50 vs. stPD 2.01 ± 0.35 ml/min per 1.73 m2, P = 0.007). | NR | No difference in patient survival by intention-to-treat (P = 0.057) or as-treated (P value NR) |
| Yan et al.11 | Nominally lower proportion of incrPD patients who experienced peritonitis (incrPD 13% vs. stPD 26%, P = 0.06). Nominally longer peritonitis-free survival in incrPD (log-rank = 3.811, P = 0.05). |
Glucose exposure significantly lower in the incrPD (incrPD 100 vs. stPD 127 g/d, P < 0.001) | No difference in GFR at follow-up (incrPD 1.6 ± 2.0 vs. stPD 1.7 ± 1.9 ml/min, P = 0.8) No difference in GFR decline rates (incrPD 0.17 ± 0.13 vs. stPD 0.20 ± 0.11 ml/min per mo, P = 0.2). No difference in urine volume at follow-up (incrPD 505 ± 522 vs. stPD 474 ± 442 ml/d, P = 0.8). No difference in anuria-free survival between groups (log-rank = 0.055, P = 0.8). |
NR | NR | Total Kt/V significantly less in incrPD (incrPD 1.95 ± 0.39 vs. stPD 2.19 ± 0.48 ml/min, P = 0.03). | No difference in technique survival (log-rank = 0.347, P = 0.6) | No difference in patient survival (log-rank = 0.978, P = 0.3) |
| Yu et al.6 | NR | NR | NR | NR | NR | NR | NR | Significantly lower mortality risk in standard group (HR 0.64, 95% CI 0.62–0.66) |
HR, hazard ratio; incrPD, incremental PD; NR, not reported; PD, peritoneal dialysis; QOL, quality of life; RKF, residual kidney function; stPD, standard PD; twKt/V, total weekly Kt/V.
Table 3.
Examples of using incremental PD prescriptions4
| CAPD | APD |
|---|---|
|
|
APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; IPD, intermittent peritoneal dialysis; PD, peritoneal dialysis.
Uptake of Incremental PD
Incremental PD is currently being used in some centers as routine practice10,13,15; however, its use is not standardized and is largely determined by physician practice patterns and patient choice. Incremental PD uptake seems to be increasing over time, with a study of Italian centers finding that the proportion of patients using incremental PD, defined as CAPD 1 to 2 exchanges per day or automated PD 3 to 4 sessions per week, increased from 11.9% of incident patients in 2005 to 27.5% in 2012.16
The current rates of incremental PD use worldwide are not well-known; however, its uptake may be driven by necessity in some countries. The Global Kidney Health Atlas surveyed 313 participants from 121 countries and found that 24% of respondent countries were not able to reliably provide adequate PD, defined as 3 to 4 exchanges per day or equivalent on automated PD.17
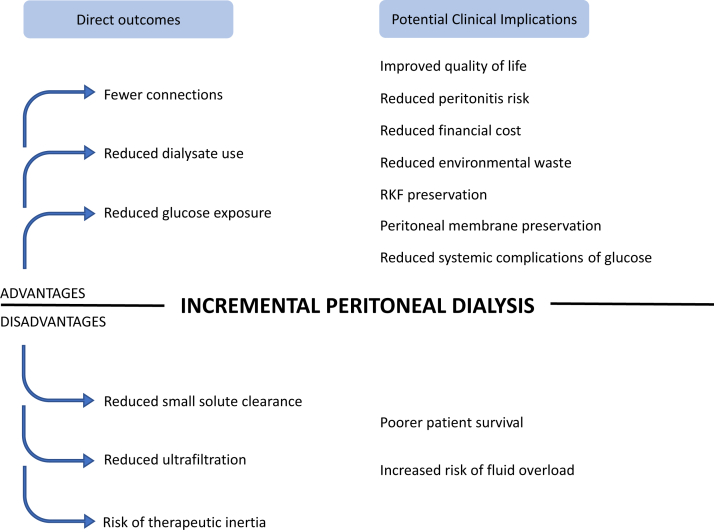
Potential Advantages of Incremental PD (Figure 1)
Figure 1.
Potential advantages and disadvantages of incremental PD. PD, peritoneal dialysis; RKF, residual kidney function.
Peritonitis
Owing to the reduced dose of dialysis which may include fewer connections, it is biologically plausible that incremental PD may decrease the risk of peritonitis in patients performing CAPD. The only prospective randomized controlled trial (RCT) of incremental versus standard PD to date compared 3 versus 4 CAPD exchanges per day in 139 adult incident patients on PD from a single Chinese center.11 In the 24-month study period, 9 patients in the incremental group experienced 15 episodes of peritonitis (0.13 episodes/patient-year) compared with 18 patients in the standard group who experienced 23 episodes of peritonitis (0.20 episodes/patient-year). Although peritonitis-free survival was nominally longer in the incremental group (duration not reported, log-rank 3.81, P = 0.05), the difference was not statistically significant, and the study was underpowered to evaluate peritonitis.
Observational studies have also provided very low certainty evidence pertaining to the effect of incremental PD on peritonitis risk. An Italian single-center, retrospective cohort study compared CAPD 1 to 2 exchanges per day with CAPD 3 to 5 exchanges per day, or daily automated PD, in 105 incident patients on PD.13 On the basis of the initial treatment regimen, the peritonitis incidence was 0.09 episodes/patient-year in those patients treated with incremental PD and 0.23 episodes/patient-year in those with standard PD. Despite the difference in peritonitis rate, peritonitis-free survival was comparable (P value not reported). A retrospective, single-center Korean study of 347 incident patients on PD in an 8-year period also found no significant difference in incidence of first peritonitis or peritonitis-free survival (P = 0.86) in patients who were initiated on 1 to 2 CAPD exchanges daily versus 3 or more CAPD exchanges daily.12 Finally, Jeloka et al.14 compared a once-daily icodextrin exchange with 3 exchanges per day of standard glucose-based solution in 46 adult incident patients on PD. The peritonitis rate in those receiving once-daily icodextrin was 0.21 episodes/patient-year compared with 0.47 episodes/patient-year in those who were initiated on standard PD. Unfortunately, the study did not analyze whether the difference in peritonitis rate between the incremental and standard PD groups was statistically significant, nor report the number of episodes of peritonitis or the total time at risk per group.
Currently, the evidence for incremental PD to reduce risk of peritonitis remains uncertain. The studies to date have been underpowered to detect differences in peritonitis occurrence and largely limited to observational designs. In addition, each of the studies evaluating peritonitis risk defined incremental and standard PD differently, thereby preventing comparison.
Glucose Exposure
Conventional PD solutions contain between 5.5 and 42.5 g of glucose per liter.18 Glucose absorption from PD solutions may be in excess of 60%19,20 and constitute 12% to 34% of daily caloric intake.21 This can lead to adverse systemic effects, including weight and fat gain,22,23 hyperglycemia,24, 25, 26 dyslipidemia,25,27, 28, 29, 30 and metabolic syndrome.27 Moreover, glucose and glucose degradation products can exert harmful effects on the peritoneum through cytotoxicity and formation of advanced glycosylation end products, leading to inflammation, vasculopathy, membrane thickening,31, 32, 33, 34 and accelerated decline in RKF.35,36 Through reduced number or volume of PD exchanges, or use of icodextrin-only regimens, incremental PD can reduce peritoneal glucose exposure, which may be beneficial for membrane preservation37,38 and mitigating the systemic effects of high glucose exposure.
Only one study has evaluated the effects of incremental PD on glucose exposure, a RCT which compared 3 (n = 70) versus 4 CAPD exchanges per day (n = 69) and evaluated glucose exposure as a secondary outcome.11 Baseline glomerular filtration rate (GFR) (5.7 ± 2.9 vs. 6.4 ± 2.8 ml/min, P = 0.1) and urine volume (1465 ± 525 vs. 1602 ± 570 ml/d, P = 0.2) were similar between incremental and standard groups. At 24 months, glucose exposure was significantly lower in the incremental group compared with the standard group (100 [range 82–118] vs. 127 g/d [range 109–145], P < 0.001). However, the study did not find any significant difference between groups in dialysate-to-plasma creatinine ratio (0.57 ± 0.13 vs. 0.55 ± 0.10, P = 0.6), decline in GFR (0.17 ± 0.13 vs. 0.20 ± 0.11 ml/min per month, P = 0.2), or urine volume (40 ± 28 vs. 49 ± 22 ml/mo, P = 0.1) at follow-up. This is in contrast to the findings from the balANZ study which, although not specifically designed to evaluate the effects of incremental PD, found lower glucose exposure to be an independent predictor for higher RKF.35 Multivariable modeling revealed that each 10 g/d increase in dialysate glucose exposure was independently associated with a 4% lower RKF at any study time point. Differing peritoneal glucose exposures may have contributed to these differences in observed outcomes, as daily glucose exposure was much lower in the study by Yan et al.11 (at 24 months, median 100 g/d in incremental, 127 g/d in standard) compared with the balANZ study (at 24 months, mean 160.7 g/d).
To date, no studies have compared the rates of glucose exposure-related effects, such as weight gain, hyperglycemia, or dyslipidemia, between incremental and standard PD regimens. More evidence is required to understand how reduced glucose exposure through use of incremental PD regimens may affect patient-level outcomes.
Residual Kidney Function
Previous studies have revealed the importance of clearance contributed by RKF compared with peritoneal clearance. In the Canada–United States of America study, each 5 l/wk per 1.73 m2 increase in GFR was associated with a 12% decrease in the relative risk of death (relative risk 0.88, 95% CI 0.83–0.94), and each 250 ml increase in urine volume was associated with a 36% decrease in the risk of death (relative risk 0.64, 95% CI 0.51–0.80).39 Preservation of RKF has in turn been associated with improved fluid status, improved blood pressure control,40 reduced left ventricular hypertrophy,41,42 improved biochemical parameters,43, 44, 45 and reduced risk of peritonitis.46,47 Frequency and duration of dialysis and intradialytic hypotension have been associated with loss of RKF in patients on hemodialysis (HD),48 with observational study outcomes revealing better RKF preservation through incremental HD.49, 50, 51, 52, 53 Consequently, it has been hypothesized that incremental PD might be beneficial for the purposes of preserving RKF compared with standard PD.
Sandrini et al.13 compared 1 to 2 exchanges per day with 3 or more exchanges per day in 105 patients with comparable RKF at baseline (6.08 ± 1.74 vs. 5.61 ± 1.49 ml/min per 1.73 m2, P = 0.16). At the end of the treatment (median 17 months), RKF was significantly greater in the incremental group compared with the standard group (4.36 ± 2.96 vs. 2.03 ± 2.55 ml/min per 1.73 m2, P < 0.001). Similarly, in a cohort study of 347 patients in South Korea comparing a CAPD regimen of 1 to 2 versus ≥3 exchanges per day, there was a reduced risk of anuria in the incremental group (hazard ratio 0.61, 95% CI 0.43–0.88, P = 0.007).12 These studies were both limited by being single-center, retrospective, observational studies and were therefore at risk of selection bias and confounding.
A systematic review and meta-analysis of incremental PD and HD by Garofalo et al.54 found a lower mean loss of RKF with incremental PD and HD (−0.13 ml/min per month, 95% CI −0.18 to −0.08) compared with standard dialysis (−0.74 ml/min per month, 95% CI −1.15 to −0.33; mean difference 0.58 ml/min per month, 95% CI 0.16–1.01, P = 0.007). Although the systematic review identified 75,292 participants in 22 nonrandomized studies, only 1573 patients in 5 studies were included in the analysis evaluating RKF. Moreover, 3 of these included studies were in incremental HD, 1 compared incremental PD with HD, and only 1 nonrandomized study of 105 patients compared incremental PD with standard PD. The limitations of this meta-analysis included high heterogeneity (I2 = 97.45%, P < 0.001), pooled analysis of patients on PD and HD, and inclusion of only cohort studies with small sample sizes, short follow-up durations, and high risks of bias.
The only RCT of incremental versus standard PD found that, in a 24-month follow-up period, the primary outcomes of GFR (1.6 ± 2.0 vs. 1.7 ± 1.9 ml/min, P = 0.8), GFR decline rate (0.17 ± 0.13 vs. 0.20 ± 0.11 ml/min per mo, P = 0.2), urine volume (505 ± 522 vs. 474 ± 442 ml/d, P = 0.8), and anuria-free survival (log-rank test statistic 0.055, P = 0.8) were not significantly different between the incremental and standard PD groups.11 Demographic characteristics, including age, sex, body mass index, cause of kidney failure, and comorbidities, were comparable between the incremental and standard PD groups, and there were no significant differences in baseline GFR (5.7 ± 2.9 vs. 6.4 ± 2.8 ml/min, P = 0.1) or urine volume (1465 ± 525 vs. 1602 ± 570 ml/min, P = 0.2). This study was limited in that no formal sample size calculation was performed, and thus, the study may have been underpowered to detect differences in RKF. In addition, this study was performed in a single Chinese centre, with the study population having relatively low body mass index (mean 21.4 ± 3.0 in incremental vs. 21.9 ± 3.2 kg/m2 in standard) and a young age (mean 53.2 years in the incremental group and 53.0 in the standard group), so results may not be generalizable to other populations.
Although observational studies suggest a possible benefit for RKF preservation with incremental PD, this has not been demonstrated in an RCT, and currently, there is very low certainty evidence that incremental PD may lead to little or no difference in RKF.
Cost
Kidney replacement therapy results in a significant direct cost burden to the health system and, despite PD being significantly cheaper than HD, data from the United States Renal Data System approximate the cost of PD to be $76,177 per person, per year.55 Globally, the annual cost of PD seems to increase with increasing country income, but it varies greatly from $5520 in Tunisia to $99,280 in the United Arab Emirates.56 Irrespective of the local costs, reduction in bag use with no impact on patient outcomes (e.g., technique survival, transplant, mortality) would translate into a decrease in economic burden on both health systems and patients, especially in countries where free public health care is unavailable and large disparities in access to health care exist. For example, in Indonesia, the out-of-pocket cost to a patient is US$4.50 per PD bag,57 adding up to $6570 per year on a standard 4 exchanges per day prescription, well in excess of the per-capita national income of US$4135.58 In other words, by performing one less exchange per day, a patient may be able to receive a further 4 months of PD treatment for the same cost. As such, recent guidelines of the International Society for Peritoneal Dialysis recommend that PD prescriptions should take into account local resource availability2 and advocate for the increased uptake of incremental PD in low- and middle-income countries to try to achieve patient well-being at the lowest cost.57
Incremental PD also has the potential to reduce indirect financial costs. Patients who are performing fewer exchanges per day or are incorporating days off PD may experience less loss of productivity and employment hours. Waste disposal costs and accommodating the physical footprint of PD solutions are also indirect costs that patients must bear and could be reduced through use of an incremental prescription.
The cost-effectiveness of incremental PD has not been formally studied. However, a cross-sectional cost analysis from a Canadian center where incremental PD was routinely practiced predicted a 16% increase in treatment cost per patient if the PD regimen was to be modified to meet small solute clearance targets.59 Incremental PD may therefore deliver acceptable treatment while being cost-effective when compared with standard PD, especially with recent changes in practice patterns and decreasing emphasis on small solute clearance target-driven PD.2,57
Environmental Effects
The use of fewer bags through incremental PD would be beneficial from the perspective of environmental cost. Although data on water consumption for PD solution generation are not readily available,60 estimated water use may exceed 720 l/wk for a prescription of 4 exchanges of 2l/day.61 In addition, plastic waste comprising the solution bags, tubing, drainage set, and disinfection cap needed for PD has been estimated to range from 188 to 301 kg per patient per year.62 Finally, reduction in the frequency or load of supplies being delivered may lead to reduced carbon emissions. As a result, incremental PD may reduce carbon footprint and waste generation, although this has not been formally evaluated in any study to date.
Quality of Life
Incremental PD may have important benefits on QOL. Standard PD treatment requires a significant time commitment and care burden. In principle, less time being spent performing PD exchanges and flexibility of timing of treatment may help promote life participation. An incremental approach may also allow patients and their families to gradually adjust to the changes in lifestyle and responsibility that result from dialysis treatment.
Incremental PD, through reduced dwell volumes or use of dry periods, may also provide symptomatic benefit to patients who experience discomfort or complications related to the volume, pressure, and weight of their indwelling PD fluid, such as back pain, decreased mobility, gastroesophageal reflux, early satiety, hernia, and abdominal fluid leaks. On commencement of PD, patients may perceive incremental PD to be less intimidating than standard PD owing to fewer exchanges and fewer supplies required. This may provide benefits from a psychological perspective. In addition, this may encourage more patients to choose PD, such as those with limited living space (e.g., living in apartments or shared housing). In the longer term, it is possible that incremental prescriptions may improve tolerability and help reduce the treatment burden of PD, thereby reducing burnout. Although these putative benefits seem highly plausible, QOL, burden, and mood have not been evaluated in any trial comparing incremental and standard PD to date, nor in any observational studies.
Potential Disadvantages of Incremental PD (Figure 1)
Small Solute Clearance
Incremental PD, by virtue of providing a lesser dose of dialysis compared with standard PD, is associated with reduced small solute clearance, and incremental prescriptions may therefore require increases over time as RKF declines to maintain small solute clearance. However, observational studies have revealed substantial small solute clearance attributable to the presence of RKF at dialysis commencement, contributing a Kt/V of between 0.71 and 0.85.63,64 A standard prescription may therefore achieve a Kt/V of >2 or even >3 in some patients,65 despite 2 RCTs revealing augmenting small solute clearance is not associated with improved patient outcomes.66,67 As such, guidelines have moved away from clearance-based prescription goals to focus on patient priorities and management of symptoms.2
Patients may be maintained on an incremental prescription for some time before requiring an increase to standard PD for clinical or biochemical reasons. Yan et al.15 reported that in their Canadian center, which did not routinely monitor Kt/V, only 32.6% of patients who commenced PD with an incremental prescription (defined as exchange volume of no >6 l/d or PD performed <7 d/wk) subsequently transitioned to a standard prescription, and this was at a median time of 10.3 months (interquartile range 6.2–15.7).
Even in centers where small solute targets are being used, many patients may be able to be maintained on an incremental regimen for a substantial period of time. A recent observational study by Navaratnarajah et al.68 revealed that, in their UK center, 51% of their patients were performing PD <7 days per week at initiation. This increased to 71% at 12 months, yet 98% achieved a creatinine clearance of >50 l/wk per 1.73 m2. The previously described cohort study by Lee et al.12 found that the 176 incident patients treated with CAPD 1 to 2 daily exchanges, targeting a total Kt/V of ≥1.7, were able to be maintained on this incremental prescription for a median duration of 2.6 years (interquartile range 1.6–4.5 years) and as long as 9.2 years. In the observational study by Sandrini et al.,13 median duration on an incremental PD prescription on CAPD 1 to 2 exchanges per day was 17 months (interquartile range 10–30 months). Moreover, some patients may be maintained on even a single daily PD exchange for a considerable period. In the study by Jeloka et al.,14 incident patients were maintained on a once-daily icodextrin-alone regimen for a median period of 9.6 months. Although these studies indicate that many patients can be treated with incremental prescriptions while still attaining small solute clearance targets successfully, these studies were observational and prone to confounding by indication.
In summary, reduced small solute clearance is not an obstacle to performing incremental PD and inability to meet historical Kt/V targets does not necessitate a conversion to standard PD if the patient is otherwise well. This is due to both the lack of convincing evidence for improved patient outcomes through clearance targets and the ability for most incident patients on PD to maintain considerable Kt/V on incremental prescriptions owing to RKF.
Fluid Overload
Compared with standard PD, incremental prescriptions have a reduced margin of peritoneal clearance to compensate as RKF falls. Failure to escalate the prescription to maintain combined peritoneal and kidney ultrafiltration in a timely fashion may therefore result in patients suffering complications, such as fluid overload. Monitoring loss of RKF and making prescription adjustments may be challenging when managing patients who live in rural and remote areas, or who have difficulty attending frequent appointments owing to issues such as poor mobility or lack of transport. In these cases, home visits and telehealth may assist in management, but clinicians may need extra vigilance in continuously monitoring their patients’ volume status, RKF, biochemical parameters, and most importantly, symptoms, so that necessary adjustments to the prescription can be made.
Patient Survival
Incremental PD may potentially lead to reduced patient survival from underdialysis through mechanisms such as electrolyte derangement, fluid overload, and poorer nutritional state. A large registry analysis of 87,183 Chinese patients found that those treated with ≥4 CAPD exchanges per day had a lower risk of death compared with those treated with <4 exchanges per day (hazard ratio 0.64, 95% CI 0.62–0.66).6 However, this analysis was unable to adjust for important factors associated with mortality, which could have influenced PD prescribing, including comorbidities, peritoneal small solute clearance, urine output, and RKF. In contrast, once-daily icodextrin was found to be associated with significantly longer median patient survival compared with thrice-daily glucose-based exchanges in 41 patients on PD at a single Indian center (42.84 vs. 25.29 months, P = 0.01).14 However, this was a small observational study prone to selection bias, and the analysis was limited in that it did not adjust for risk factors, such as RKF or comorbidities, and did not include competing risks analysis. Although the icodextrin-alone group had significantly higher urine output at baseline compared with the standard PD group (1265 ± 316 vs. 551 ± 504 ml/d, P < 0.001), only unadjusted survival analysis was performed, leading to potential for confounding by indication.
No other study comparing incremental and standard PD has revealed a difference in mortality. The single-center Italian cohort study of 105 incident patients on PD comparing patients performing 1 to 2 exchanges per day with those performing 3 to 5 exchanges per day13 found no significant difference in patient survival when analyzed by their regimen at initiation (P = not significant) or their as-treated PD regimen (P = 0.057). A Korean cohort study compared 176 patients on PD performing 1 to 2 CAPD exchanges per day to 171 performing 3 or more exchanges per day and found no difference in mortality (incremental 10.9, 95% CI 5.2–20.0 vs. 7.6, 95% CI 3.3–15.0 events per 1000 person-years; P = 0.449).12 Limitations of this study included its observational design and the presence of significant baseline differences between groups, as the incremental PD group was older with higher urine output, RKF, and Kt/V, although the authors did adjust for these differences in their analyses. The systematic review and meta-analysis by Garofalo et al.54 did not identify any difference in all-cause mortality between incremental and standard dialysis (hazard ratio 1.14, 95% CI 0.85–1.52). However, this meta-analysis included only 1 study that was performed in patients on PD,13 with the other 10 included studies being of patients on HD only, and there was significant heterogeneity (I2 = 86.4%, P < 0.001). Finally, the RCT performed by Yan et al.11 comparing 3 versus 4 PD exchanges per day also failed to demonstrate any difference in patient survival in the 24-month follow-up period (log-rank test statistic 0.978, P = 0.3). Data were censored at death, transfer to HD, and kidney transplantation. Although the study’s strengths included being a prospective randomized trial, it was a single-center study of relatively small sample size with inadequate statistical power, short follow-up duration, and high risk of bias owing to its open-label design, unclear allocation concealment, and dropout rate of 19%.
Currently, there is low certainty evidence that incremental PD may result in no worse patient survival or technique survival compared with standard PD.
Therapeutic Inertia
Therapeutic inertia may be defined as a failure to initiate intensifying therapy when therapeutic goals are not being reached.69 This may be due to clinician factors, for example a prescriber failing to adequately monitor and/or escalate dialysis prescription in response to a patient’s declining RKF. However, this may also occur due to patient factors, whereby a patient may be reluctant to have their PD prescription be increased or changed from that to which they are accustomed.70 Although there is no research specifically addressing therapeutic inertia in PD, it has been associated with poorer long-term outcomes in people with diabetes mellitus, including progression of diabetic retinopathy71 and increased risk of cardiovascular events.72 In fields outside nephrology, failure to follow medication recommendations has been associated with patients feeling disempowered in clinical decision-making73 and feeling that their preferences are not being taken into account,74 and it is possible that these may also be factors contributing to resistance from patients to increase dialysis prescription.
Shared decision-making, with discussion of advantages and disadvantages, may help alleviate patient resistance to necessary treatment changes. Both clinicians and patients must be mindful of the anticipated trajectory of PD treatment. Thus, education and clear communication regarding the need for monitoring and expected changes should be undertaken before, and continue to be reinforced after, commencement on dialysis to achieve optimal patient outcomes.
Summary and Future Directions
Incremental PD has been used in clinical practice for over two decades. However, there is currently no standardized definition and global prescription practices vary. Despite the importance of evaluating the benefits and harms of incremental PD to patients, caregivers, clinicians, and policymakers, especially in resource-limited settings, the evidence for incremental PD remains uncertain because of the limited number, size, duration, and quality of studies performed to date. There are many possible benefits of incremental PD, including preservation of RKF, reduced risk of peritonitis, mitigation of glucose-related membrane and metabolic complications, reduced environmental impact, decreased health care costs, and improved QOL and life participation. These benefits need to be weighed against potential disadvantages, including suboptimal dialysis small solute clearance and fluid removal, effects on patient survival, and a reluctance of patients to increase their PD prescription.
It is clear that a large RCT of incremental versus standard PD is warranted. Although incremental PD should not currently be prescribed solely for economic reasons or for a palliative goal, if incremental PD were found to have comparable safety outcomes to standard PD, it could be used as routine practice, because it would provide equivalent treatment for a lower financial and environmental cost. This would provide benefits to both high-income countries, owing to reduced indirect costs and health care utilization, and low-income countries, by allowing more patients to access dialysis and reducing the out-of-pocket costs to the patients. Importantly, understanding the effect of incremental PD on patient-reported outcomes, including life participation which was identified as a priority outcome in the Standardised Outcomes in Nephrology-PD consensus workshop,75 is essential to inform shared decision-making globally. Until a time when such RCT data are available, incremental PD will still provide high-quality patient care when used in accordance with individual patients’ preferences and priorities and should be considered for use in more patients.
Disclosure
MSC has received travel support from Amgen and is supported by a Queensland Advancing Clinical Research Fellowship. AKJ has received consultancy fees, research grants, speaker’s honoraria, and/or travel sponsorship from Baxter Healthcare and AWAK Technologies. NB has received consultancy fees, research grants, speaker’s honoraria, or travel sponsorship from Baxter Healthcare, Vifor Pharmaceuticals, AstraZeneca, Roche, and Amgen. DWJ has received consultancy fees, research grants, speaker’s honoraria, and travel sponsorships from Baxter Healthcare and Fresenius Medical Care; consultancy fees from AstraZeneca, Bayer, and AWAK; speaker’s honoraria from Ono and BI & Eli Lilly; and travel sponsorships from Ono and Amgen; and is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant. YC has received research grants and speaker’s honoraria from Baxter Healthcare and Fresenius Medical Care. All the other authors declared no competing interests.
References
- 1.Fresenius annual report 2020 Fresenius Medical Care. https://www.fresenius.com/media_library/Fresenius_Annual_Report_2020.pdf Published 2020. Accessed April 15, 2021.
- 2.Brown E.A., Blake P.G., Boudville N., et al. International Society for Peritoneal Dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. 2020;40:244–253. doi: 10.1177/0896860819895364. [DOI] [PubMed] [Google Scholar]
- 3.NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. National Kidney Foundation. Am J Kidney Dis. 1997;30(suppl 2):S67–S136. doi: 10.1016/s0272-6386(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 4.Blake P.G., Dong J., Davies S.J. Incremental peritoneal dialysis. Perit Dial Int. 2020;40:320–326. doi: 10.1177/0896860819895362. [DOI] [PubMed] [Google Scholar]
- 5.Lai K.N., Lo W.K. Optimal peritoneal dialysis for patients from Hong Kong. Perit Dial Int. 1999;19(suppl 3):S26–S34. [PubMed] [Google Scholar]
- 6.Yu X., Chen J., Ni Z., et al. Number of daily peritoneal dialysis exchanges and mortality risk in a Chinese population. Perit Dial Int. 2018;38(suppl 2):S53–S63. doi: 10.3747/pdi.2017.00283. [DOI] [PubMed] [Google Scholar]
- 7.Gillis L., Wilkie M. Peritoneal dialysis. Medicine. 2019;47:603–608. doi: 10.1016/j.mpmed.2019.06.003. [DOI] [Google Scholar]
- 8.Kidney Health Australia . Kidney Health Australia; 2016. An Introduction to Peritoneal Dialysis. [Google Scholar]
- 9.Dombros N., Dratwa M., Feriani M., et al. European best practice guidelines for peritoneal dialysis. 6 Automated peritoneal dialysis. Nephrol Dial Transplant. 2005;20(suppl 9):ix21–ix23. doi: 10.1093/ndt/gfi1120. [DOI] [PubMed] [Google Scholar]
- 10.Ankawi G.A., Woodcock N.I., Jain A.K., Garg A.X., Blake P.G. The use of incremental peritoneal dialysis in a large contemporary peritoneal dialysis program. Can J Kidney Health Dis. 2016;3 doi: 10.1177/2054358116679131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan H., Fang W., Lin A., Cao L., Ni Z., Qian J. Three versus 4 daily exchanges and residual kidney function decline in incident CAPD patients: a randomized controlled trial. Am J Kidney Dis. 2017;69:506–513. doi: 10.1053/j.ajkd.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y., Chung S.W., Park S., et al. Incremental peritoneal dialysis may be beneficial for preserving residual renal function compared to full-dose peritoneal dialysis. Sci Rep. 2019;9:10105. doi: 10.1038/s41598-019-46654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandrini M., Vizzardi V., Valerio F., et al. Incremental peritoneal dialysis: a 10 year single-centre experience. J Nephrol. 2016;29:871–879. doi: 10.1007/s40620-016-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeloka T., Sanwaria P., Chaudhari L., Periera A. “Ico-Alone” single nocturnal exchange to initiate peritoneal dialysis in patients with residual renal function—five year, single centre experience. Indian J Nephrol. 2013;23:276–279. doi: 10.4103/0971-4065.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan H, Abreu Z, Bargman JM. Incremental peritoneal dialysis in incident end-stage kidney disease patients. Perit Dial Int. Published online August 9, 2021. https://doi.org/10.1177/08968608211036796 [DOI] [PubMed]
- 16.Neri L., Viglino G., Marinangeli G., et al. Incremental start to PD as experienced in Italy: results of censuses carried out from 2005 to 2014. J Nephrol. 2017;30:593–599. doi: 10.1007/s40620-017-0403-0. [DOI] [PubMed] [Google Scholar]
- 17.Cho Y., Bello A.K., Levin A., et al. Peritoneal dialysis use and practice patterns: an international survey study. Am J Kidney Dis. 2021;77:315–325. doi: 10.1053/j.ajkd.2020.05.032. [DOI] [PubMed] [Google Scholar]
- 18.Baxter Healthcare Ltd . 2014. Dianeal Peritoneal Dialysis Solutions Product Information: Old Toongabbie. [Google Scholar]
- 19.Heimbürger O., Waniewski J., Werynski A., Lindholm B. A quantitative description of solute and fluid transport during peritoneal dialysis. Kidney Int. 1992;41:1320–1332. doi: 10.1038/ki.1992.196. [DOI] [PubMed] [Google Scholar]
- 20.Zuo X., Ye X., Sun F., et al. Glucose absorption in nephropathy patients receiving continuous ambulatory peritoneal dialysis. Asia Pac J Clin Nutr. 2015;24:394–402. doi: 10.6133/apjcn.2015.24.3.16. [DOI] [PubMed] [Google Scholar]
- 21.Grodstein G.P., Blumenkrantz M.J., Kopple J.D., Moran J.K., Coburn J.W. Glucose absorption during continuous ambulatory peritoneal dialysis. Kidney Int. 1981;19:564–567. doi: 10.1038/ki.1981.53. [DOI] [PubMed] [Google Scholar]
- 22.Law S., Davenport A. Glucose absorption from peritoneal dialysate is associated with a gain in fat mass and a reduction in lean body mass in prevalent peritoneal dialysis patients. Br J Nutr. 2020;123:1269–1276. doi: 10.1017/S0007114520000306. [DOI] [PubMed] [Google Scholar]
- 23.Jager K.J., Merkus M.P., Huisman R.M., et al. Nutritional status over time in hemodialysis and peritoneal dialysis. J Am Soc Nephrol. 2001;12:1272–1279. doi: 10.1681/ASN.V1261272. [DOI] [PubMed] [Google Scholar]
- 24.Lambie M., Chess J., Do J.Y., et al. Peritoneal dialysate glucose load and systemic glucose metabolism in non-diabetics: results from the GLOBAL fluid cohort study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P.K., Culleton B.F., Ariza A., et al. Randomized, controlled trial of glucose-sparing peritoneal dialysis in diabetic patients. J Am Soc Nephrol. 2013;24:1889–1900. doi: 10.1681/ASN.2012100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szeto C.C., Chow K.M., Kwan B.C., Chung K.Y., Leung C.B., Li P.K. New-onset hyperglycemia in nondiabetic Chinese patients started on peritoneal dialysis. Am J Kidney Dis. 2007;49:524–532. doi: 10.1053/j.ajkd.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Jiang N., Qian J., Lin A., Lindholm B., Axelsson J., Yao Q. Initiation of glucose-based peritoneal dialysis is associated with increased prevalence of metabolic syndrome in non-diabetic patients with end-stage renal disease. Blood Purif. 2008;26:423–428. doi: 10.1159/000153248. [DOI] [PubMed] [Google Scholar]
- 28.Bredie S.J., Bosch F.H., Demacker P.N., Stalenhoef A.F., van Leusen R. Effects of peritoneal dialysis with an overnight icodextrin dwell on parameters of glucose and lipid metabolism. Perit Dial Int. 2001;21:275–281. [PubMed] [Google Scholar]
- 29.Martikainen T., Teppo A.M., Gronhagen-Riska C., Ekstrand A. Benefit of glucose-free dialysis solutions on glucose and lipid metabolism in peritoneal dialysis patients. Blood Purif. 2005;23:303–310. doi: 10.1159/000086553. [DOI] [PubMed] [Google Scholar]
- 30.Babazono T., Nakamoto H., Kasai K., et al. Effects of icodextrin on glycemic and lipid profiles in diabetic patients undergoing peritoneal dialysis. Am J Nephrol. 2007;27:409–415. doi: 10.1159/000105123. [DOI] [PubMed] [Google Scholar]
- 31.Cho Y., Johnson D.W., Craig J.C., et al. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev. 2014;3 doi: 10.1002/14651858.CD007554.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Johnson D.W., Brown F.G., Clarke M., et al. The effect of low glucose degradation product, neutral pH versus standard peritoneal dialysis solutions on peritoneal membrane function: the balANZ trial. Nephrol Dial Transplant. 2012;27:4445–4453. doi: 10.1093/ndt/gfs314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu H.Y., Hung K.Y., Huang T.M., et al. Safety issues of long-term glucose load in patients on peritoneal dialysis—a 7-year cohort study. PLoS One. 2012;7:e30337. doi: 10.1371/journal.pone.0030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liberek T., Topley N., Jörres A., Coles G.A., Gahl G.M., Williams J.D. Peritoneal dialysis fluid inhibition of phagocyte function: effects of osmolality and glucose concentration. J Am Soc Nephrol. 1993;3:1508–1515. doi: 10.1681/ASN.V381508. [DOI] [PubMed] [Google Scholar]
- 35.Htay H., Cho Y., Pascoe E.M., et al. Predictors of residual renal function decline in peritoneal dialysis patients: the balANZ trial. Perit Dial Int. 2017;37:283–289. doi: 10.3747/pdi.2016.00206. [DOI] [PubMed] [Google Scholar]
- 36.Wang A.Y., Brimble K.S., Brunier G., et al. ISPD cardiovascular and metabolic guidelines in adult peritoneal dialysis patients. Part I—assessment and management of various cardiovascular risk factors. Perit Dial Int. 2015;35:379–387. doi: 10.3747/pdi.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies S.J. Longitudinal relationship between solute transport and ultrafiltration capacity in peritoneal dialysis patients. Kidney Int. 2004;66:2437–2445. doi: 10.1111/j.1523-1755.2004.66021.x. [DOI] [PubMed] [Google Scholar]
- 38.Davies S.J., Phillips L., Naish P.F., Russell G.I. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol. 2001;12:1046–1051. doi: 10.1681/ASN.V1251046. [DOI] [PubMed] [Google Scholar]
- 39.Bargman J.M., Thorpe K.E., Churchill D.N. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 40.Menon M.K., Naimark D.M., Bargman J.M., Vas S.I., Oreopoulos D.G. Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant. 2001;16:2207–2213. doi: 10.1093/ndt/16.11.2207. [DOI] [PubMed] [Google Scholar]
- 41.Wang A.Y.M., Wang M., Woo J., et al. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int. 2002;62:639–647. doi: 10.1046/j.1523-1755.2002.00471.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang A.Y.M., Wang M., Woo J., et al. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. 2004;15:2186–2194. doi: 10.1097/01.ASN.0000135053.98172.D6. [DOI] [PubMed] [Google Scholar]
- 43.Suda T., Hiroshige K., Ohta T., et al. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant. 2000;15:396–401. doi: 10.1093/ndt/15.3.396. [DOI] [PubMed] [Google Scholar]
- 44.Pagé D.E., Knoll G.A., Cheung V. The relationship between residual renal function, protein catabolic rate, and phosphate and magnesium levels in peritoneal dialysis patients. Adv Perit Dial. 2002;18:189–191. [PubMed] [Google Scholar]
- 45.Kagan A., Elimalech E., Lemer Z., Fink A., Bar-Khayim Y. Residual renal function affects lipid profile in patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int. 1997;17:243–249. [PubMed] [Google Scholar]
- 46.Pérez Fontan M., Rodríguez-Carmona A., García-Naveiro R., Rosales M., Villaverde P., Valdés F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int. 2005;25:274–284. [PubMed] [Google Scholar]
- 47.Han S.H., Lee S.C., Ahn S.V., et al. Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant. 2007;22:2653–2658. doi: 10.1093/ndt/gfm242. [DOI] [PubMed] [Google Scholar]
- 48.Wong J., Vilar E., Davenport A., Farrington K. Incremental haemodialysis. Nephrol Dial Transplant. 2015;30:1639–1648. doi: 10.1093/ndt/gfv231. [DOI] [PubMed] [Google Scholar]
- 49.Lin Y.F., Huang J.W., Wu M.S., et al. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrol (Carlton) 2009;14:59–64. doi: 10.1111/j.1440-1797.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M., Wang M., Li H., et al. Association of initial twice-weekly hemodialysis treatment with preservation of residual kidney function in ESRD patients. Am J Nephrol. 2014;40:140–150. doi: 10.1159/000365819. [DOI] [PubMed] [Google Scholar]
- 51.Obi Y., Streja E., Rhee C.M., et al. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: a cohort study. Am J Kidney Dis. 2016;68:256–265. doi: 10.1053/j.ajkd.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Zou W., Wu J., Liu L., He Q. Comparison between incremental and thrice-weekly haemodialysis: systematic review and meta-analysis. Nephrol (Carlton) 2019;24:438–444. doi: 10.1111/nep.13252. [DOI] [PubMed] [Google Scholar]
- 53.Teruel-Briones J.L., Fernández-Lucas M., Rivera-Gorrin M., et al. Progression of residual renal function with an increase in dialysis: haemodialysis versus peritoneal dialysis. Nefrol (Engl Ed) 2013;33:640–649. doi: 10.3265/Nefrologia.pre2013.May.12038. [DOI] [PubMed] [Google Scholar]
- 54.Garofalo C., Borrelli S., De Stefano T., et al. Incremental dialysis in ESRD: systematic review and meta-analysis. J Nephrol. 2019;32:823–836. doi: 10.1007/s40620-018-00577-9. [DOI] [PubMed] [Google Scholar]
- 55.Saran R., Robinson B., Abbott K.C., et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73(suppl 1):A7–A8. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho Y, Bello AK, Levin A, et al. Uptake, practice pattern and care capacity for patients receiving peritoneal dialysis across the world: an international survey study. Am J Kidney Dis. Forthcoming.
- 57.Liew A. Prescribing peritoneal dialysis and achieving good quality dialysis in low and low-middle income countries. Perit Dial Int. 2020;40:341–348. doi: 10.1177/0896860819894493. [DOI] [PubMed] [Google Scholar]
- 58.GDP per capita (current US$) - Indonesia The World Bank. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?end=2019&locations=ID&start=1967&view=chart Updated 2019. Accessed December 1, 2020.
- 59.Blake P.G., Floyd J., Spanner E., Peters K. How much extra does “adequate” peritoneal dialysis cost? Perit Dial Int. 1996;16(suppl 1):S171–S175. [PubMed] [Google Scholar]
- 60.Agar J.W.M., Barraclough K.A. Water use in dialysis: environmental considerations. Nat Rev Nephrol. 2020;16:556–557. doi: 10.1038/s41581-020-0296-3. [DOI] [PubMed] [Google Scholar]
- 61.Piccoli G.B., Cupisti A., Aucella F., et al. Green nephrology and eco-dialysis: a position statement by the Italian Society of Nephrology. J Nephrol. 2020;33:681–698. doi: 10.1007/s40620-020-00734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robert B., Bernard C., Renner S., et al. Plastic waste reduction in different peritoneal dialysis strategies: the impact of disposable choice on carbon footprint. Nephrol Dial Transplant. 2019;34(suppl 1) doi: 10.1093/ndt/gfz106.FP573. [DOI] [Google Scholar]
- 63.Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrol. 1996;7:198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 64.Tattersall J., Greenwood R., Farrington K. Urea kinetics and when to commence dialysis. Am J Nephrol. 1995;15:283–289. doi: 10.1159/000168850. [DOI] [PubMed] [Google Scholar]
- 65.Masakane I., Taniguchi M., Nakai S., et al. Annual dialysis data report 2016, JSDT renal data registry. Ren Replace Ther. 2018;4:45. doi: 10.1186/s41100-018-0183-6. [DOI] [Google Scholar]
- 66.Paniagua R., Amato D., Vonesh E., et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 67.Lo W.K., Ho Y.W., Li C.S., et al. Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int. 2003;64:64–656. doi: 10.1046/j.1523-1755.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 68.Navaratnarajah A., Clemenger M., McGrory J., et al. Flexibility in peritoneal dialysis prescription: impact on technique survival. Perit Dial Int. 2020;41:49–56. doi: 10.1177/0896860820911521. [DOI] [PubMed] [Google Scholar]
- 69.Lebeau J.P., Cadwallader J.-S., Aubin-Auger I., et al. The concept and definition of therapeutic inertia in hypertension in primary care: a qualitative systematic review. BMC Fam Pract. 2014;15:130. doi: 10.1186/1471-2296-15-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Auguste B.L., Bargman J.M. Incremental peritoneal dialysis: new ideas about an old approach. Semin Dial. 2018;31:445–448. doi: 10.1111/sdi.12712. [DOI] [PubMed] [Google Scholar]
- 71.Osataphan S., Chalermchai T., Ngaosuwan K. Clinical inertia causing new or progression of diabetic retinopathy in type 2 diabetes: a retrospective cohort study. J Diabetes. 2017;9:267–274. doi: 10.1111/1753-0407.12410. [DOI] [PubMed] [Google Scholar]
- 72.Paul S.K., Klein K., Thorsted B.L., Wolden M.L., Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. doi: 10.1186/s12933-015-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Webb D.G., Horne R., Pinching A.J. Treatment-related empowerment: preliminary evaluation of a new measure in patients with advanced HIV disease. Int J STD AIDS. 2001;12:103–107. doi: 10.1258/0956462011916875. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization Adherence to long-term therapies: evidence for action:section II—improving adherence rates: guidance for countries. World Health Organization. https://www.who.int/chp/knowledge/publications/adherence_introduction.pdf Published 2003. Accessed June 30, 2021.
- 75.Manera K.E., Johnson D.W., Craig J.C., et al. Establishing a core outcome set for peritoneal dialysis: report of the SONG-PD (Standardized Outcomes in Nephrology-Peritoneal Dialysis) consensus workshop. Am J Kidney Dis. 2020;75:404–412. doi: 10.1053/j.ajkd.2019.09.017. [DOI] [PubMed] [Google Scholar]