Abstract
Introduction
Patients with end-stage renal disease (ESRD) requiring hemodialysis (HD) have an increased risk of thrombotic events and bleeding. Antisense reduction of factor XI (FXI) with IONIS-FXIRx is a novel strategy that may safely reduce the risk of thrombotic events.
Methods
This multicenter study enrolled 49 patients receiving HD in 2 parts. First, 6 participants (pharmacokinetics [PK] cohort) received 1 open-label 300 mg dose of IONIS-FXIRx both before and after HD. Subsequently, 43 participants were treated in a double-blind, randomized design with 200 mg or 300 mg IONIS-FXIRx or placebo for 12 weeks. The PK, pharmacodynamics (PD), and adverse events of IONIS-FXIRx were evaluated (ClinicalTrials.gov: NCT02553889).
Results
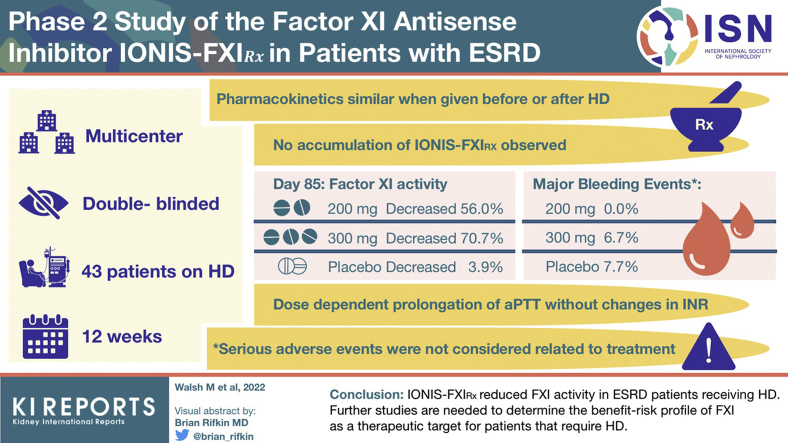
The PK of IONIS-FXIRx was consistent with previous studies and similar whether injected before or after HD. No accumulation of IONIS-FXIRx was observed after repeat administration. By day 85, mean levels of FXI activity fell 56.0% in the 200 mg group, 70.7% in the 300 mg group, and 3.9% in the placebo group compared with baseline. FXI antigen levels paralleled FXI activity. Dose-dependent prolongation of activated partial thromboplastin time (aPTT) was observed, with no changes in international normalized ratio (INR). IONIS-FXIRx was not associated with drug-related serious adverse events. In the randomized phase of the study, major bleeding events occurred in 0 (0.0%; 200 mg), 1 (6.7%; 300 mg), and 1 (7.7%; placebo) patients and were not considered related to treatment.
Conclusion
IONIS-FXIRx reduced FXI activity in patients with ESRD receiving HD. Further studies are needed to determine the benefit-risk profile of FXI as a therapeutic target for patients who require HD.
Keywords: anticoagulation, antisense oligonucleotide, FXI, hemodialysis, pharmacokinetics, randomized controlled trial
Graphical abstract
The risk of cardiovascular disease is markedly elevated in patients with ESRD compared with those without chronic kidney disease. This is likely due, at least in part, to excess thrombotic events, including myocardial infarctions, ischemic strokes, and peripheral artery disease. Although there is abundant evidence that antithrombotic therapies reduce the risk of cardiovascular disease in patients without ESRD, there is little evidence to inform their use in patients with ESRD.
Antithrombotic therapies increase the risk of bleeding. This concern is exacerbated in patients with ESRD owing to their high baseline risk and difficulties in managing current antithrombotic therapy, including maintaining the therapeutic range of heparins and vitamin K antagonists, bioaccumulation of dabigatran, and potentially, factor Xa inhibitors. In addition to the increased risk of bleeding, vitamin K antagonists, the most often used antithrombotic in ESRD, may also increase the risk of vascular calcification.1 Therefore, there is a need for safer anticoagulants for such high-risk patients.
FXI has emerged as a target for new anticoagulants that may result in a safer treatment strategy than those that are currently available. Positioned in the intrinsic pathway of coagulation, FXI can be activated by factor XIIa or thrombin and is important for the stabilization and growth of thrombi but likely not for their initiation. In contrast, factor Xa and thrombin are essential for both thrombosis and hemostasis. Studies evaluating congenital FXI deficiencies have revealed a reduced risk of venous thromboembolism and stroke in individuals with FXI deficiency and higher risk in those with elevated FXI levels.2, 3, 4, 5, 6, 7, 8 Supporting the concept that targeting FXI can dissociate thrombosis and hemostasis, a previous phase 2 study with IONIS-FXIRx (also known as BAY2306001) revealed that FXI knockdown before elective knee arthroplasty significantly reduced the risk of postoperative venous thromboembolism compared with enoxaparin without increasing the risk of bleeding.9
Given the need for safer antithrombotic therapies in ESRD, we tested the PK and PD of a novel inhibitor of FXI using an antisense oligonucleotide in patients with ESRD receiving HD.10 The primary objectives of the study were to evaluate the PK, PD, and safety and tolerability of IONIS-FXIRx in patients with ESRD receiving HD.
Methods
Clinical Study
This was a 2-part, phase 2 multicenter study (NCT02553889) in patients with ESRD receiving HD at least 3 times weekly. The primary outcome measure was safety and tolerability of IONIS-FXIRx, evaluated according to the frequency and severity of adverse events, including bleeding events. PD parameters were evaluated as secondary outcome measures, including changes over time in FXI antigen, FXI activity, aPTT, and INR. Other outcome measures included PK parameters.
The study was conducted from 23 October 2015 to 26 August 2016, enrolled 49 participants from 8 sites in Canada, was designed by the sponsor and academic partners, and conducted by the Population Health Research Institute, Hamilton Health Sciences/McMaster University Hamilton, Canada. The protocol was approved by the research ethics board at each participating center, and all participants provided informed consent. Detailed eligibility criteria are provided in the Supplementary Methods. Briefly, eligible participants were men and women aged 18 to 80 years who had been receiving chronic HD for at least 3 months. Key exclusion criteria included a recent thrombotic or bleeding event, an abnormal coagulation profile or elevated liver enzymes, concomitant use of an anticoagulant or antiplatelet agent other than HD circuit anticoagulation (e.g., heparin) or low-dose aspirin (<100 mg), or a life expectancy <1 year. The full trial protocol can be accessed at https://clinicaltrials.gov/ct2/show/NCT02553889.
The study consisted of 2 parts (Supplementary Figure S1). In the first part, patients received 1 open-label, 300-mg dose of IONIS-FXIRx subcutaneously immediately after HD and again 28 days after immediately before HD, to evaluate the effects of HD on IONIS-FXIRx PK (PK cohort). The IONIS-FXIRx drug solution was 200 mg/ml. Therefore, the 1.5-ml dose was divided into two 0.75-ml doses administered at 2 different sites. Participants in the PK cohort were then followed for an additional 42 days for safety monitoring.
In the second part of the study, participants were randomized to receive double-blind treatment with IONIS-FXIRx 200 mg or 300 mg or matching saline placebo. Participants were allocated to either a 200 mg dose group or matching saline placebo in a 2:1 ratio, or to a 300 mg dose group or matching saline placebo in a 2:1 ratio, for an overall allocation ratio of 1:1:1 to the 200 mg/300 mg/pooled placebo groups. Allocation was stratified by study center and performed using an interactive web-based randomization system designed by an independent statistician to ensure concealment. All participants, study investigators, and study health care professions performing assessments and the sponsor were blinded to group allocation.
The intervention was dosed subcutaneously in 12 weeks on days 1, 5, 8, 12, and 15 and then weekly until day 78; patients were followed-up to day 162. Comprehensive clinical laboratory testing was done, and PK samples were drawn, at each dosing day and weekly to day 162. To explore whether reducing FXI had antithrombotic potential, at each study visit, we evaluated HD circuit clotting by evaluating the degree of clotting in the dialyzer and venous chamber in a blinded manner, graded on a semiquantitative scale at every study visit (Supplementary Table S1).11 Anticoagulation during HD treatments continued for all patients according to the local standard of care for each HD unit.
Analytical Methods
Quantification of IONIS-FXIRx Concentrations in Plasma
A previously described hybridization-based enzyme-linked immunosorbent assay was used to quantify the concentrations of IONIS-FXIRx.12 Analyses were conducted at PPD Laboratories (Richmond, VA) and performed based on the principles and requirements described in Yu et al.13 CFR part 58. The assay was conducted with synthesized putative shortened oligonucleotide metabolite standards of the full-length 20-mer oligonucleotide and revealed no measurable cross-reactivity, confirming the assay’s specificity for IONIS-FXIRx. The quantitation range was 2.00 to 200 ng/ml, with the lower and higher ends of the range defining the lower and upper limit of quantitation, respectively. There were no active metabolites for IONIS-FXIRx present in plasma.
Clinical Analyses and Quantification of FXI Activity and Antigen Levels
Medpace Reference Laboratories (Cincinnati, OH) performed the routine clinical laboratory analyses. Coagulation assays, which were performed by Hemostasis Reference Laboratory Inc. (Hamilton, ON, Canada), included measurement of FXI activity levels using a 1-stage aPTT-based clotting assay on a BCS-XP coagulometer, quantification of FXI antigen levels using enzyme-linked immunosorbent assay, and determination of prothrombin time (PT) and INR using Innovin reagent (Siemens Healthcare Diagnostics, Deerfield, IL) on the BCS-XP coagulometer.
Statistical Analysis
The sample size is not based on a statistical rationale. Rather, the PK cohort sample size of 6 participants was based on previous experience and findings from most PK studies that 5 participants are typically sufficient to accurately determine single-dose PK. The randomized component was based on previous experience that 10 to 15 patients per group are typically sufficient to evaluate multidose PK and PD while identifying major harms. The protocol specified that should the minimum specified sample size of 42 randomized participants leave uncertainty owing to drop out, additional participants could be added.
Demographic and baseline characteristics were summarized for all-randomized patients and for the patients who had completed the trial per protocol. Patient age, sex, ethnicity, race, vascular access, cause of ESRD, and HD circuit anticoagulation were summarized as numbers and percentages. The PK population comprised all subjects from both parts of the study who had at least one PK sample taken after administration of IONIS-FXIRx. All PD analyses were performed based on the intent-to-treat (population and were repeated on the patients who completed the trial per protocol. The intent-to-treat population consisted of all-randomized patients who had taken at least 1 dose of randomized study medication, and for whom a baseline measurement of FXI activity and antigen, aPTT, PT, and INR and at least 1 postrandomization measurement were available. The per-protocol population excluded patients with major protocol deviations and those who did not complete treatment to week 8. The overall sample size was based on numbers of participants required to demonstrate reliable PK and PD in similar studies and not based on hypothesis testing. As an early phase study, the designers sought to gain familiarity with FXI inhibition in a fragile population rather than precisely delineate the effects of FXI inhibition on clinical outcomes.
Safety analyses were performed on the safety population, which was defined as all-randomized patients who received at least 1 dose of study medication. Treatment-emergent adverse events were defined as adverse events that occurred for the first time on or after the date of the first dose of double-blind study medication or that had been in existence earlier and worsened during the study period. The numbers and percentages of patients with treatment-emergent adverse events were summarized for each treatment group by system organ class and preferred term defined by the Medical Dictionary for Regulatory Activities version 18.1. The 95% CIs for bleeding events were calculated using the exact binomial method.
Pharmacokinetics
Noncompartmental PK analysis of IONIS-FXIRx was carried out on each individual patient data set for which full PK sampling profiles were available, using Phoenix WinNonlin version 6.3 (Pharsight Corp., Mountain View, CA). For the PK cohort in the first part of the study, IONIS-FXIRx plasma PK was evaluated on day 1, when IONIS-FXIRx was administered after completion of HD, and on day 29, when IONIS-FXIRx was administered before HD. Day 29 assessments were done to determine the effect of a 4-hour HD period on peak plasma concentration (Cmax), time to Cmax (Tmax), and partial exposure (area under the curve0–4 h and area under the curve0–24 h) parameters for IONIS-FXIRx. For patients randomized in the second part of the study, PK assessments included plasma IONIS-FXIRx apparent terminal elimination half-life (t1/2λz) and concentrations at 3 hours postadministration (near Cmax), at trough (during the treatment period), and during the post-treatment period.
Pharmacodynamics
The per-protocol population was used to compare changes and percent changes from baseline in FXI activity, FXI antigen levels, aPTT, and INR for patients receiving 200 mg or 300 mg IONIS-FXIRx or pooled placebo; analyses used analysis of variance or Wilcoxon ranked sum tests as appropriate. Normality was evaluated by applying the Kolmogorov-Smirnov test on the residuals (difference between individual values and group mean).
Exploratory Analysis of HD Circuit Clotting
The extent of clotting on the dialyzer and venous chamber was quantified using a visual scale with descriptive categories from 1 to 4 (Supplementary Table S1). Assessments of clotting were conducted by trained personnel blinded to treatment allocation. The comparison of HD circuit clotting events was analyzed for each patient between 2 intervals during the treatment period. The absolute difference in the percent of category ≥3 events (i.e., blood stripes on >5% of the surface of the dialyzer, clot formation in the venous chamber, coagulated filter, or coagulated system preventing HD) for each patient was evaluated for the intervals between week 6 to 13 and events before week 6. P values were generated based on a post hoc analysis using 1-way analysis of variance for the differences in the percent of events between the intervals, with treatment as the main effect.
Results
Baseline Characteristics
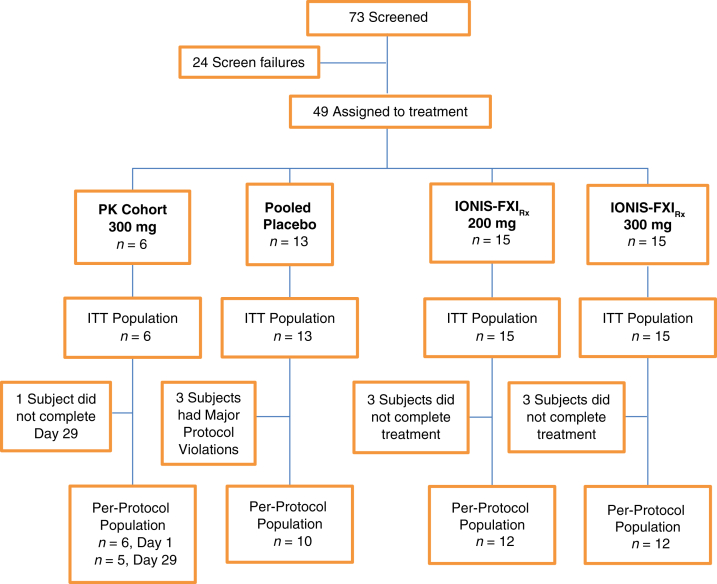
The study was conducted from 23 October 2015 to 26 August 2016 and enrolled 49 participants from 8 sites in Canada. The baseline characteristics of the study participants across the treatment cohorts are found in Table 1, and the disposition of study participants is found in Figure 1.
Table 1.
Baseline characteristics of patients included in the PK cohort and the randomized IONIS-FXIRx and placebo groups (ITT population)
| Characteristic | PK cohort IONIS-FXIRx 300 mg (n = 6) |
Randomized cohorts |
||
|---|---|---|---|---|
| Pooled placebo (n = 13) | IONIS-FXIRx 200 mg (n = 15) |
IONIS-FXIRx 300 mg (n = 15) |
||
| Female, n (%) | 0 (0.0) | 4 (30.8) | 5 (33.3) | 12 (80.0) |
| Male, n (%) | 6 (100.0) | 9 (69.2) | 10 (66.7) | 3 (20.0) |
| Median age, yr (min, max) | 63 (50, 68) | 63 (40, 80) | 56 (31, 77) | 58 (29, 76) |
| Race, n (%) | ||||
| White | 5 (83.3) | 8 (61.5) | 10 (66.7) | 6 (40.0) |
| Black or African-American | 1 (16.7) | 3 (23.1) | 4 (26.7) | 5 (33.3) |
| Other | 0 (0) | 2 (15.4) | 1 (6.7) | 4 (26.7) |
| Vascular access, n (%) | ||||
| Central venous catheter | 3 (50.0) | 8 (61.5) | 8 (53.3) | 9 (60.0) |
| Arteriovenous fistula | 1 (16.7) | 5 (38.5) | 6 (40.0) | 5 (33.3) |
| Arteriovenous graft | 2 (33.3) | 0 (0.0) | 1 (6.7) | 1 (6.7) |
| Urea reduction ratio, mean (SD) | NA | 74 (10) | 65 (14) | 73 (7) |
| Cause of ESRD, n (%) | ||||
| Diabetes | 2 (33.3) | 5 (38.5) | 5 (33.3) | 8 (53.3) |
| Hypertension | 0 (0.0) | 2 (15.4) | 0 (0.0) | 1 (6.7) |
| Glomerulonephritis | 1 (16.7) | 2 (15.4) | 4 (26.7) | 2 (13.3) |
| Polycystic kidney disease | 0 (0.0) | 0 (0.0) | 2 (13.3) | 0 (0.0) |
| Other | 3 (50.0) | 4 (30.8) | 4 (26.7) | 4 (26.7) |
| HD circuit anticoagulation n (%) | ||||
| Unfractionated heparin | 6 (100) | 11 (84.6) | 12 (80.0) | 14 (93.3) |
| Saline flushes/no anticoagulation | 0 (0.0) | 0 (0.0) | 1 (6.7) | 1 (6.7) |
| Aspirin use, n (%) | 4 (66.7) | 6 (46.2) | 5 (33.3) | 7 (46.7) |
| Pre-HD | ||||
| FXI activity (U/ml), mean (SD) | 0.99 (0.17) | 0.93 (0.19) | 1.00 (0.22) | 0.94 (0.24) |
| FXI antigen (U/ml), mean (SD) | 1.00 (0.28) | 1.08 (0.26) | 1.03 (0.26) | 1.01 (0.29) |
| aPTT (s), mean (SD) | 27.0 (2.2) | 27.4 (2.5) | 29.0 (8.8) | 27.4 (2.4) |
| INR mean (SD) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) |
| Post-HD | ||||
| FXI activity (U/ml), mean (SD) | ND | 0.98 (0.23) | 1.08 (0.21) | 0.99 (0.36) |
| FXI antigen (U/ml), mean (SD) | ND | 1.12 (0.38) | 1.22 (0.21) | 1.06 (0.40) |
aPTT, activated partial thromboplastin time; ESRD, end-stage renal disease; FXI, factor IX; HD, hemodialysis; INR, international normalized ratio; ITT, intent-to-treat; max, maximum; min, minimum; NA, not applicable; ND, not done; PK, pharmacokinetics.
Figure 1.
Disposition of study participants. Three patients in the placebo group experienced protocol deviations and were excluded from the per-protocol population owing to disallowed concomitant treatment with warfarin (n = 1) and clopidogrel (n = 2). The 200 mg and 300 mg IONIS-FXIRx groups had 3 patients in each group who were excluded from the per-protocol population owing to incomplete dosing to week 8. Adverse events that led to discontinue treatment were as follows: In the PK cohort, 1 participant discontinued owing to sepsis with hemoptysis deemed unlikely related to IONIS-FXIRx. In the 200 mg group, 1 participant discontinued treatment owing to hepatic enzyme increased during hospitalization with pneumonia deemed unlikely related to IONIS-FXIRx. In the 300 mg group, 1 participant discontinued treatment owing to arteriovenous fistula site hemorrhage judged possibly related to IONIS-FXIRx. ITT, intent-to-treat; PK, pharmacokinetics.
The PK cohort in the first part (open-label phase) of the study comprised 6 participants recruited from a single center, all of whom received the pre-HD dose of IONIS-FXIRx and 5 of whom received the post-HD dose. One patient was unable to receive the post-HD dose because of an intercurrent infectious illness unrelated to the study medication. A total of 43 participants were recruited into the randomized multiple-dosing component of the study with 15 allocated to the 200 mg group, 15 to the 300 mg group, and 13 to placebo.
Although the first part of the study (open-label phase) included only males, the second part (randomized phase) included an even distribution of females (n = 21; 48.8%) and males (n = 22; 51.2%). The randomized phase included 24 (55.8%) White and 12 (27.9%) Black or African-American participants with a mean age of 59 years (range, 29–80). Baseline disease characteristics, including FXI antigen, FXI activity, aPTT, and INR, were similar across the placebo and IONIS-FXIRx treatment groups. The FXI activity and antigen levels reported for the study population at baseline (day 1) before and after HD were within the normal reference range for healthy individuals established by the reference laboratory (FXI activity 0.73–1.45 U/ml and FXI antigen 0.72–1.63 U/ml).
PKs of Single- and Multiple-Dose IONIS-FXIRx
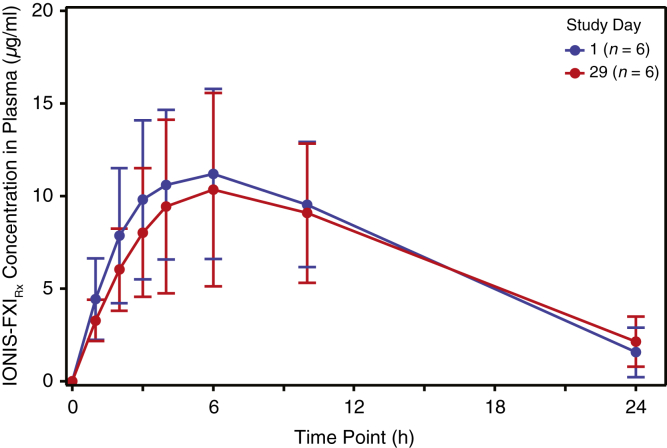
In the PK cohort, the concentration-time profile of IONIS-FXIRx was similar regardless of whether treatment was administered before or after HD (Figure 2). The extent of IONIS-FXIRx exposure in 24 hours (area under the curve0–24 h) did not differ significantly between pre-HD (geometric mean 142 [39.7% coefficient of variation] h × μg/ml) and post-HD (geometric mean 136 [45.6% coefficient of variation] h × μg/ml). Median Tmax values were 5.97 (range 2.98–10.0) hours when administered pre-HD and 6.03 (range 4.00–9.95) hours when administered post-HD (Supplementary Table S2). Neither IONIS-FXIRx nor its 10-mer metabolites were detectable in the dialysate.
Figure 2.
Mean (±SD) IONIS-FXIRx concentrations after a single 300 mg dose administered to 6 participants after HD (day 1) and 5 participants before HD (day 29). Note: 1 of the 6 participants did not receive the day 29 dose owing to an intercurrent illness unrelated to IONIS-FXIRx. HD, hemodialysis.
For participants in the PK population of the second (randomized) part of the study, repeated dosing revealed a stable Cmax between the first dose on day 1 and after the last dose on day 78 for both the 200 mg and 300 mg doses (Supplementary Table S3) and a t1/2 geometric mean of 16.9 days (39.9% coefficient of variation) for the 200 mg dose and 13.1 days (35.1% coefficient of variation) for the 300 mg dose. There was negligible urinary excretion of IONIS-FXIRx (geometric mean 0.0424% dose excreted at 78 days from the 4 participants with urine output). As found in Supplementary Table S3, Cmax was dose dependent and similar between day 1 and day 78, suggesting there was no accumulation of IONIS-FXIRx at Cmax following repeated weekly administrations. Plasma trough and post-treatment concentrations of IONIS-FXIRx were dose dependent, increased during the loading period, and approached steady state by day 36 (6 weeks). After treatment was stopped, IONIS-FXIRx concentrations decreased with time, with a t1/2λz of approximately 2 weeks.
Safety
Bleeding events were reported in 1 participant (16.7%) in the PK cohort, 1 participant (6.7%) in the 200 mg group, 7 participants (46.7%) in the 300 mg group, and 2 participants (15.4%) in the placebo group (Table 2). Of these participants, 3 had major bleeding events, including bleeding of the respiratory tract owing to hemoptysis that occurred during an episode of pneumosepsis (PK cohort, n = 1), asymptomatic bleeding at the central venous catheter insertion site requiring transfusion (300 mg group, n = 1), and recurrent hemothorax requiring transfusion (placebo group, n = 1). FXI activity levels were ≥0.55 U/ml before the major bleeding events and were not considered related to the study drug. Clinically relevant nonmajor bleeding events were not observed in this study. Minor bleeding events were primarily observed at vascular access sites, and although they were most frequent in the 300-mg IONIS-FXIRx group, they did not seem to be correlated with FXI activity or antigen levels. One participant (6.7%) in the 200 mg group, 2 (13.3%) in the 300 mg group, and 1 (7.7%) in the placebo group developed arteriovenous fistula site bleeding. No excessive postoperative bleeding was reported in the 2 participants treated with IONIS-FXIRx who underwent renal transplantation. FXI activity levels were reduced to 0.41 U/ml in 1 renal transplant recipient and were normal in the other (0.75 U/ml).
Table 2.
Bleeding events (safety population)
| Outcome | PK cohort IONIS-FXIRx 300 mg (n = 6) |
Randomized cohorts |
||
|---|---|---|---|---|
| Pooled placebo (n = 13) | IONIS-FXIRx 200 mg (n = 15) |
IONIS-FXIRx 300 mg (n = 15) |
||
| Major bleeding, n (%) | 1 (16.7) | 1 (7.7) | 0 (0.0) | 1 (6.7) |
| [95% CI]a | [0.4%–64.1%] | [0.2%,–6.0%] | [0.0%–21.8%] | [0.2%–31.9%] |
| Minor bleeding, n (%) | 0 (0.0) | 1 (7.7) | 1 (6.7) | 6 (40.0) |
| [95% CI]a | [0.0%–45.9%] | [0.2%–36.0%] | [0.2%–31.9%] | [16.3%–67.7%] |
| Any bleeding, n (%) | 1 (16.7) | 2 (15.4) | 1 (6.7) | 7 (46.7) |
| [95% CI]a | [0.4%–64.1%] | [1.9%–45.4%] | [0.2%–31.9%] | [21.3%–73.4%] |
PK, pharmacokinetics.
Exact binomial 95% CI.
Adverse events were reported in 14 participants (93.3%) in each of the IONIS-FXIRx treatment groups and in 10 participants (76.9%) in the placebo group (Table 2). More than 90% of adverse events were mild in severity, and the most common adverse events were related to the injection site. There was no detectable hepatotoxicity or effect on platelets, and no treatment-related changes were observed in hepatic function based on transaminases or severe thrombocytopenia. Serious adverse events occurred in 6 participants (20.0%) in both the 200 mg and 300 mg IONIS-FXIRx groups compared with 4 participants (30.8%) in the placebo group (Table 2). The serious adverse events were deemed by the investigators to be unrelated to the study treatment. In addition, 2 participants died during the study: 1 in the IONIS-FXIRx 200 mg group owing to aspiration pneumonia and 1 in the placebo group owing to heart failure. Neither death was considered related to the study drug.
IONIS-FXIRx Effect on FXI Antigen and Activity
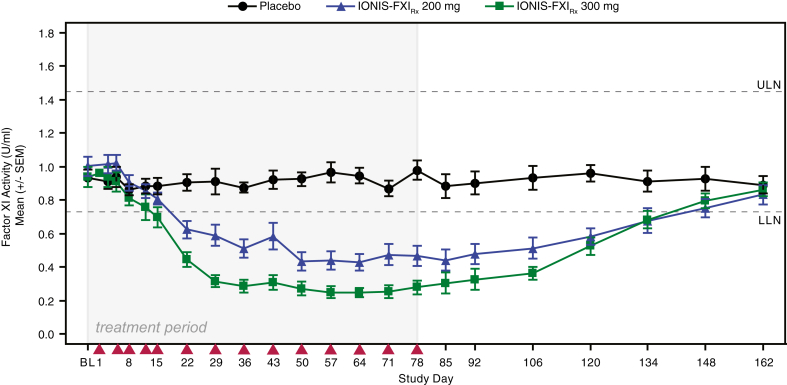
As illustrated in Figure 3, intent-to-treat population decreases in FXI activity were greatest at day 85 with significant (P ≤ 0.001) mean ± SD decreases from baseline of 56.0% ± 25.1% in the 200 mg group (n = 12) and 70.7% ± 16.7% in the 300 mg group (n = 12) compared with a 3.9% ± 24.4% decrease with placebo (n = 10). Similar reductions in FXI antigen levels were observed after IONIS-FXIRx treatment: mean ± SD levels at day 85 decreased from baseline by 65.5% ± 19.1% in the 200 mg group (n = 12) and by 78.1% ± 15.2% in the 300 mg group (n = 12) (Supplementary Figure S2). The reductions in FXI activity levels were maximal at approximately 6 weeks after treatment initiation, consistent with previous studies and reflective of the antisense mechanism (time needed to inhibit FXI mRNA and subsequently FXI protein production) coupled with the long half-life of FXI protein (∼55 hours).9 FXI activity and antigen levels determined at both pre- and post-HD time points (baseline [day 1] and on days 29, 78, and 162) had similar results for all treatment groups, suggesting HD did not alter circulating FXI activity or antigen levels.
Figure 3.
Effect of IONIS-FXIRx 200 mg and 300 mg on FXI activity compared with placebo during the 78-day treatment period and subsequent 85-day washout period. Points represent mean; whiskers represent SEM (intent-to-treat population). Red arrowheads indicate dosing day. FXI, factor XI; LLN, lower limit of normal; ULN, upper limit of normal.
A strong PK/PD relationship between IONIS-FXIRx plasma trough concentrations and FXI activity was observed after 78 days (12 weeks) of treatment. The estimated mean ± SEM half maximal inhibitory concentration was 40.4 ± 9.7 ng/ml for FXI activity, with baseline FXI activity (E0) estimated at 0.882 ± 0.057 U/ml. Similar half maximal inhibitory concentration values were obtained when FXI activity data were normalized to baseline.
Reduction in FXI activity and antigen levels with IONIS-FXIRx resulted in dose-dependent prolongation of aPTT but no clinically significant changes in PT or INR (Supplementary Figure S3). Maximum mean ± SD aPTT prolongation was observed on day 92 with 46.1 ± 30.6 seconds in the 200 mg group, 49.4 ± 37.5 seconds in the 300 mg group, and 36.0 ± 25.4 seconds in the placebo group. Thus, IONIS-FXIRx affected only the intrinsic pathway, as evidenced by the prolongation of aPTT with no effect on PT/INR.
HD Circuit Clotting
HD circuit thrombosis with IONIS-FXIRx and placebo was similar from randomization up to week 6 with 73 of 99 observations (73.7%) in the 200 mg, 41 of 103 observations (39.8%) in the 300 mg group, and 47 of 91 observations (51.6%) in the placebo group displaying a category 3 or 4 clot. Between weeks 6 and 13, category 3 or 4 clots occurred less frequently than in the previous period before week 6 in the 200 mg group (−28.9%) and the 300 mg group (−19.0%) compared with placebo (−1.6%). These differences were more pronounced in the per-protocol population (Table 3).
Table 3.
HD circuit clotting results for patients included in the randomized IONIS-FXIRx and placebo groups (per-protocol and ITT populations)
| Overall air trap and dialyzer events with a clotting category ≥3 | Pooled placebo (n = 10) | IONIS-FXIRX 200 mg (n = 12) |
IONIS-FXIRX 300 mg (n = 12) |
|---|---|---|---|
| Number of category ≥3 events/total events, (%)a | |||
| Before week 6 (baseline) | |||
| Per-protocol population | 43/70 (61.4%) | 60/83 (72.3%) | 38/85 (44.7%) |
| Intent-to-treat population | 47/91 (51.6%) | 73/99 (73.7%) | 41/103 (39.8%) |
| Between week 6 and week 13 | |||
| Per-protocol population | 49/79 (62.0%) | 38/95 (40.0%) | 18/90 (20.0%) |
| Intent-to-treat population | 52/102 (51.0%) | 41/98 (41.8%) | 19/101 (18.8%) |
| Subject difference in percent of clotting scores ≥3 between weeks 6–13 and before week 6 | |||
| Per-protocol population | |||
| Mean difference (SEM) | −1.6% (5.6) | −32.5% (7.9) | −25.9% (8.1) |
| P value vs. placebob | 0.0074 | 0.029 | |
| P value vs. 200 mg groupb | 0.54 | ||
| Intent-to-treat population | |||
| Mean difference (SEM) | −0.2% (7.7) | −28.9% (8.1) | −19.0% (7.5) |
| P value vs. placebob | 0.013 | 0.093 | |
| P value vs. 200 mg groupb | 0.33 | ||
HD, hemodialysis; ITT, intent-to-treat.
The number of category ≥3 events from the total events from all circuit clotting categories. Category 3 events included clot formation on venous chamber and blood stripes affecting ≥5% of the fibers found at the surface of the dialyzer. Category 4 events included coagulated system (treatment cannot continue without new setup) and coagulated filter.
P values are based on 1-way analysis of variance of the differences, using treatment group as the independent model variable. The comparison is between the change in the percent of category ≥3 events between the 2 periods. For each patient, the percent of scores ≥3 before week 6 was subtracted from the percent of scores ≥3 for weeks 6 to 13.
Discussion
This is the first reported use of FXI inhibition with an antisense oligonucleotide in patients with ESRD. IONIS-FXIRx had predictable PK parameters that were not significantly affected by HD. No evidence of IONIS-FXIRx accumulation was observed in ESRD participants after 12 weeks of dosing. In this small study, IONIS-FXIRx treatment produced significant, dose-dependent, sustained reductions in FXI antigen and activity and was well tolerated without major safety concerns or major bleeding.
FXI enhances both the formation and stability of clots in vitro by amplifying thrombin generation when coagulation is initiated by low levels of tissue factor or thrombin.14 FXI-dependent amplification of thrombin formation also leads to activation of thrombin-activatable fibrinolysis inhibitor, which renders clots less sensitive to fibrinolysis. Inhibition of FXI may therefore reduce clot propagation by slowing thrombin generation and, indirectly, by enhancing clot dissolution.15 This mechanism of action may improve the safety of anticoagulation by reducing pathologic clot propagation while permitting clot formation in response to tissue injury. Evidence for this comes from both epidemiologic data in subjects with FXI deficiency and from early phase clinical trials of FXI inhibition. Patients with genetically low FXI levels are at lower risk of ischemic stroke and venous thromboembolism, whereas increased FXI levels are associated with an increased risk.2 Similarly, inhibition of FXI was more effective than standard low-molecular-weight heparin therapy at preventing deep vein thrombosis in patients undergoing knee replacement, without increasing the risk of bleeding.9,16,17 Our study provides initial evidence that similarly effective anticoagulation without excessive major bleeding risk may be possible in patients undergoing HD despite their high background risk of bleeding. Future research is needed to determine whether FXI inhibition safely prevents major thrombotic events, such as myocardial infarction and ischemic stroke, particularly in light of the high number of minor bleeds we observed in the 300-mg group.
The need for safe, effective anticoagulation in ESRD is clear. Vitamin K antagonists and direct oral anticoagulants both lack clear efficacy and safety evaluations.18, 19, 20 The need to study the safety of anticoagulation by FXI inhibition is highlighted by the long duration of the effect on FXI. Although the long duration of effect may reduce variability in the effect compared with shorter lived anticoagulants or those that require frequent dose adjustments (e.g., vitamin K antagonists), this must be balanced against the high baseline risk of bleeding events that may be exacerbated by an anticoagulant. Although it is fortunate that FXI inhibition can be reversed with factor concentrates or fresh frozen plasma, these products may not be available or suitable in all settings which would make the long duration of effect potentially detrimental.
Antisense oligonucleotides are an attractive novel therapeutic platform. The binding sites for these types of hydrophilic drugs differ from the binding sites of low-molecular-weight hydrophobic drugs, and they are not substrates for cytochrome P450 enzymes; thus, no drug-drug interactions on the level of plasma protein binding are expected at clinically relevant concentrations, a potentially important property in patients with ESRD, who typically receive a large number of concomitant medications.13,21,22 In addition, similar to other antisense oligonucleotides, IONIS-FXIRx is highly bound to plasma proteins in mice, monkeys, and humans, thereby limiting glomerular filtration and urinary excretion in patients without ESRD.23,24 Furthermore, antisense oligonucleotides have an increasingly well-established safety profile as a platform and have no class effects on the liver, kidney, cardiac, central nervous system, muscle, or bone marrow function; broad therapeutic effects at doses with demonstrated acceptable safety and tolerability.25
Limitations of the study include the small sample size of exclusively White, Canadian patients with ESRD in a relatively short treatment period of 12 weeks. In addition, there were differences in the proportions of males and females between the 200 mg and 300 mg IONIS-FXIRx groups. Small studies, such as this, are suitable to precisely evaluate PK and PD parameters and can identify some major, common safety issues but are not suitable to estimate treatment benefits and harms. As such, whether the excess number of minor bleeding events observed in the 300 mg IONIS-FXIRx group is due to the drug or the play of chance is uncertain and requires further study.
FXI inhibition with an antisense oligonucleotide is a promising strategy to provide safe, effective anticoagulation to high-risk populations, such as patients with ESRD. The results of this study support the conduct of larger trials that may demonstrate whether FXI inhibition reduces clinically important thrombotic events while maintaining an acceptable safely profile.
Disclosure
MW serves on a steering committee for Bayer. MW is supported by a Clive Kearon Mid-Career Award from the Department of Medicine, McMaster University. JW is a professor of Medicine and Biochemistry and Biomedical Sciences at the McMaster University and Executive Director of the Thrombosis and Atherosclerosis Research Institute and has received honoraria from Alnylam, Bayer, Boehringer Ingelheim, Bristol Myers Squib, Daiichi-Sankyo, Ionis Pharmaceuticals, Janssen, Merck, PhaseBio, Pfizer, and Regeneron. JT is an employee of Population Health Research Institute. SWJ, RZY, YW, RSG, and SB are employees and shareholders of Ionis Pharmaceuticals Inc. CB was an employee and shareholder of Ionis Pharmaceuticals Inc at the time of the study. All the other authors declared no competing interests.
Data Sharing
Individual patient data are not available for sharing.
Acknowledgments
The authors thank the patients who participated in the CS4 study and their family members. The authors also thank all contributors to the CS4 study, including the study investigators, medical monitors, drug safety members, study coordinators, and laboratory technicians. MW is supported by a McMaster University Department of Medicine Clive Kearon Mid-Career Award. AS is supported by a Wellcome Trust Clinical Research Career Development Fellowship. Editing support was provided by Lisa Hannan of Ionis Pharmaceuticals Inc. and copy editing by Autumn Kelly MA, CMPP (AKH Communications; San Diego, CA, USA). The CS4 study and analysis was funded by Ionis Pharmaceuticals Inc. The authors had full editorial control of the manuscript and provided their final approval of all content. Graphics support was provided by Tracy Reigle of Ionis Pharmaceuticals Inc. The results presented in this paper have not been published previously, in whole or in part, except in abstract form.
Author Contributions
MW, CB, RZY, YW, SWJ, and SB designed the study. MW, AS, JT, and the CS4 investigators carried out the study. MW, CB, RZY, and SWJ analyzed the data. MW, CB, RZY, JW, and SWJ drafted and revised the paper. All authors approved the final version of the manuscript.
Footnotes
Supplementary Appendix 1. CS4 lead site investigators and collaborating authors.
Supplementary Methods. Eligibility criteria.
Table S1. Clotting assessment scale.
Table S2. PK parameters of IONIS-FXIRx.
Table S3. IONIS-FXIRx Cmax for repeated doses.
Figure S1. Study design.
Figure S2. FXI antigen.
Figure S3. Activated partial thromboplastin time international normalized ratio.
Consort Checklist.
Supplementary Material
Supplementary Appendix 1. CS4 lead site investigators and collaborating authors.
Supplemental Methods. Eligibility criteria.
Table S1. Clotting assessment scale.
Table S2. PK parameters of IONIS-FXIRx
Table S3. IONIS-FXIRx Cmax for repeated doses
Figure S1. Study design.
Figure S2. FXI antigen
Figure S3. Activated partial thromboplastin time international normalized ratio.
Consort Checklist
STROBE Statement (PDF)
References
- 1.De Vriese A.S., Caluwé R., Raggi P. The atrial fibrillation conundrum in dialysis patients. Am Heart J. 2016;174:111–119. doi: 10.1016/j.ahj.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Preis M., Hirsch J., Kotler A., et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129:1210–1215. doi: 10.1182/blood-2016-09-742262. [DOI] [PubMed] [Google Scholar]
- 3.Cushman M., O’Meara E.S., Folsom A.R., Heckbert S.R. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of thromboembolism Etiology. Blood. 2009;114:2878–2883. doi: 10.1182/blood-2009-05-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meijers J.C., Tekelenburg W.L., Bouma B.N., Bertina R.M., Rosendaal F.R. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 5.Salomon O., Steinberg D.M., Koren-Morag N., Tanne D., Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- 6.Salomon O., Steinberg D.M., Zucker M., Varon D., Zivelin A., Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105:269–273. doi: 10.1160/TH10-05-0307. [DOI] [PubMed] [Google Scholar]
- 7.Siegerink B., Rosendaal F.R., Algra A. Antigen levels of coagulation factor XII, coagulation factor XI and prekallikrein, and the risk of myocardial infarction and ischemic stroke in young women. J Thromb Haemost. 2014;12:606–613. doi: 10.1111/jth.12531. [DOI] [PubMed] [Google Scholar]
- 8.Suri M.F., Yamagishi K., Aleksic N., Hannan P.J., Folsom A.R. Novel hemostatic factor levels and risk of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Cerebrovasc Dis. 2010;29:497–502. doi: 10.1159/000297966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Büller H.R., Bethune C., Bhanot S., et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–240. doi: 10.1056/NEJMoa1405760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bethune C., Walsh M., Jung S., et al. Pharmacokinetics and pharmacodynamics of Ionis-FXIRx, an antisense inhibitor of factor XI, in patients with end-stage renal disease on hemodialysis. Blood. 2017;130(suppl 1):1116. [Google Scholar]
- 11.Sagedal S., Hartmann A., Sundstrøm K., Bjørnsen S., Brosstad F. Anticoagulation intensity sufficient for haemodialysis does not prevent activation of coagulation and platelets. Nephrol Dial Transplant. 2001;16:987–993. doi: 10.1093/ndt/16.5.987. [DOI] [PubMed] [Google Scholar]
- 12.Yu R.Z., Baker B., Chappel A., Geary R.S., Chueng E., Levin A.A. Development of an ultrasensitive noncompetitive hybridization-ligation enzyme-linked immunosorbent assay for the determination of phosphorothioate oligodeoxynucleotide in plasma. Anal Biochem. 2002;304:19–25. doi: 10.1006/abio.2002.5576. [DOI] [PubMed] [Google Scholar]
- 13.Yu R.Z., Geary R.S., Flaim D.J., et al. Lack of pharmacokinetic interaction of mipomersen sodium (ISIS 301012), a 2’-O-methoxyethyl modified antisense oligonucleotide targeting apolipoprotein B-100 messenger RNA, with simvastatin and ezetimibe. Clin Pharmacokinet. 2009;48:39–50. doi: 10.2165/0003088-200948010-00003. [DOI] [PubMed] [Google Scholar]
- 14.von dem Borne P.A., Cox L.M., Bouma B.N. Factor XI enhances fibrin generation and inhibits fibrinolysis in a coagulation model initiated by surface-coated tissue factor. Blood Coagul Fibrinolysis. 2006;17:251–257. doi: 10.1097/01.mbc.0000224843.33216.5f. [DOI] [PubMed] [Google Scholar]
- 15.Bouma B.N., Mosnier L.O., Meijers J.C., Griffin J.H. Factor XI dependent and independent activation of thrombin activatable fibrinolysis inhibitor (TAFI) in plasma associated with clot formation. J Thromb Haemost. 1999;82:1703–1708. [PubMed] [Google Scholar]
- 16.Thomas D., Thelen K., Kraff S., et al. BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: first evaluation of safety, pharmacodynamics, and pharmacokinetics. Res Pract Thromb Haemost. 2019;3:242–253. doi: 10.1002/rth2.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitz J.I., Bauersachs R., Becker B., et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA. 2020;323:130–139. doi: 10.1001/jama.2019.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu A., Niu J., Winkelmayer W.C. Oral anticoagulation in patients with end-stage kidney disease on dialysis and atrial fibrillation. Semin Nephrol. 2018;38:618–628. doi: 10.1016/j.semnephrol.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw K., Amstutz U., Kim R.B., CPNDS Clinical Recommendation Group Clinical practice recommendations on genetic testing of CYP2C9 and VKORC1 variants in warfarin therapy. Ther Drug Monit. 2015;37:428–436. doi: 10.1097/FTD.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 20.Hanni C., Petrovitch E., Ali M., et al. Outcomes associated with apixaban vs warfarin in patients with renal dysfunction. Blood Adv. 2020;4:2366–2371. doi: 10.1182/bloodadvances.2019000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geary R.S., Bradley D.J., Watanabe T., et al. Lack of pharmacokinetic interaction for ISIS 113715, a 2’-0-methoxyethyl modified antisense oligonucleotide targeting protein tyrosine phosphatase 1B messenger RNA, with oral antidiabetic compounds metformin, glipizide or rosiglitazone. Clin Pharmacokinet. 2006;45:789–801. doi: 10.2165/00003088-200645080-00003. [DOI] [PubMed] [Google Scholar]
- 22.Burnier M., Pruijm M., Wuerzner G., Santschi V. Drug adherence in chronic kidney diseases and dialysis. Nephrol Dial Transplant. 2015;30:39–44. doi: 10.1093/ndt/gfu015. [DOI] [PubMed] [Google Scholar]
- 23.Geary R.S., Norris D., Yu R., Bennett C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev. 2015;87:46–51. doi: 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe T.A., Geary R.S., Levin A.A. Plasma protein binding of an antisense oligonucleotide targeting human ICAM-1 (ISIS 2302) Oligonucleotides. 2006;16:169–180. doi: 10.1089/oli.2006.16.169. [DOI] [PubMed] [Google Scholar]
- 25.Crooke S.T., Baker B.F., Witztum J.L., et al. The effects of 2’-O-methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucleic Acid Ther. 2017;27:121–129. doi: 10.1089/nat.2016.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.