Abstract
Chronic kidney disease (CKD) increasingly affects younger people, including adolescents and young adults. CKD among females is accompanied by unique reproductive and gynecologic health concerns; though to date, this area has not been well studied. Hormonal disruptions attributed to CKD may underlie the high prevalence of abnormal uterine bleeding and influence the age of menarche in adolescents. Period poverty as a socioeconomic barrier further exacerbates the female-specific burdens of CKD. Reduced fertility in CKD is likely multifactorial and may be related to a reduction in ovarian reserve, reproductive hormone disturbances, and gonadotoxic medication use in addition to low sexual function and activity. Fertility, sexual function and activity, and risk of sexually transmitted infections increase with transplantation. Pregnancy is possible at any stage of CKD, although often accompanied by high risks of maternal and fetal complications. Contraception is thus an important consideration in CKD, but use is low and the risks and benefits of different forms in the setting of CKD are not well characterized. Though patients with CKD report reproductive health as an important element of care, many nephrologists report lack of confidence and training in this area, highlighting the need for targeted research and education. The unique reproductive health care needs of the growing transgender youth population warrant attention in nephrology training with multidisciplinary input. This review will discuss female reproductive health and gynecologic considerations in adolescents and young adults with CKD while proposing clinical and research strategies to improve this understudied yet important aspect of kidney care.
Keywords: adolescent nephrology, chronic kidney disease, contraception, female reproductive health, sexual function, uterine bleeding
The prevalence of CKD in children is steadily increasing, with a higher incidence of kidney replacement therapy in adolescents compared with other age groups worldwide.1 Although the most common causes of kidney disease at a global level are hypertension and diabetes,2,3 childhood onset of kidney disease is most frequently due to congenital abnormalities and hereditary disorders.4, 5, 6, 7 The reduced rate of congenital abnormalities of the kidney and urinary tract among females may help to explain the lower incidence of CKD compared with males in the adolescent population.8 Furthermore, compared with the adult population, glomerulonephritides are a more common cause of CKD in children, particularly in the adolescent population after puberty.4,8
CKD in the female population is often accompanied by abnormal uterine bleeding, sexual dysfunction, reduced fertility, and higher risk pregnancies.9,10 Commonly used immunosuppressive medications (e.g., cyclophosphamide, mycophenolate mofetil) for autoimmune glomerular disorders, which disproportionately affect females, have important implications for uterine bleeding, fertility, and the potential for fetal malformations.11 According to the North American Pediatric Renal Trials and Collaborative Studies database, adolescents represent the largest group of pediatric kidney transplant recipients.12 Although CKD is associated with increased abnormal uterine bleeding,13, 14, 15 kidney transplantation, at least in the adult population, may restore uterine bleeding.15,16 Kidney transplantation guidelines17,18 discourage pregnancy in females for the first year post-transplant owing to risk of allograft rejection and pregnancy complications. Finally, pregnancy itself can have a detrimental and permanent impact on kidney function.19,20 Taken together, these multiple factors underscore the critical value of providing reproductive care to all females living with CKD, including adolescents who require individualized care during this phase of physiological and social transition. This narrative review will broadly summarize female reproductive and gynecologic considerations in the care of the adolescent and young adult populations with CKD.
Methods
For the purpose of providing a summary on female reproductive and gynecologic health among adolescents with CKD, the first author (DHC) searched 2 electronic sources, MEDLINE and Google Scholar. The terms “reproductive health” or “gynecology” in combination with “chronic kidney disease,” “chronic renal insufficiency,” “end-stage kidney disease,” “chronic renal failure,” “dialysis,” “transplant,” and “nephrology” and other related terms helped identify relevant literature. The terms “contraception,” “menstruation,” “sexual dysfunction,” and “adolescent” in combination with the same Medical Subject Headings were also searched in MEDLINE. These searches were completed by May 2021. Reference lists from relevant articles were hand-searched, and the search was further supplemented by key articles from nephrologists with expertise in women’s health (SBA and SMD). Priority for inclusion in this review was given to original articles reporting original data (i.e., observational studies as randomized control trials were lacking), clinical practice guidelines, and systematic reviews.
Kidney Disease and the Menstrual Cycle
The menstrual cycle encompasses the time between the first day of uterine bleeding to the next first day of uterine bleeding,21 and a healthy menstrual cycle lasts 24 to 38 days with bleeding occurring for ≤8 days (on average, 5 days).22 Details regarding the healthy menstrual cycle are outlined elsewhere.21,23
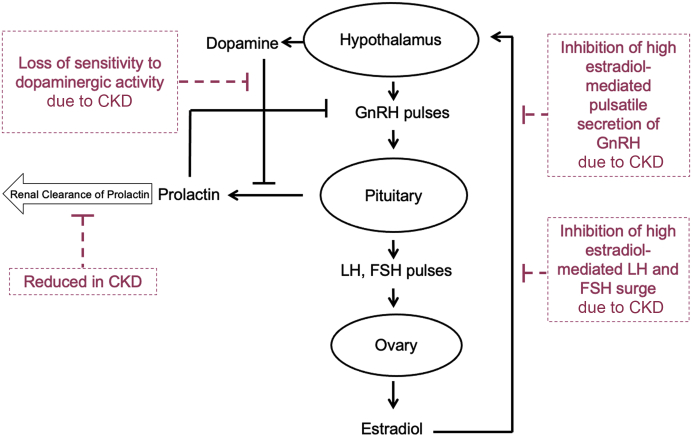
In CKD, disruption of the hypothalamic-pituitary-ovarian axis results in an abnormal reproductive hormone profile, where the degree of disruption increases with CKD progression (Figure 1).13,14,24 As such, those with kidney failure are believed to have the most severe hormonal disruptions, and most studies have been conducted in this population.13,24 In kidney failure, the pulsatile release of gonadotropin-releasing hormone is impaired, resulting in a lack of follicle-stimulating hormone and luteinizing hormone cyclicity.13 Consequently, estradiol levels stay relatively low, inhibiting the surge and ovulation of the luteinizing hormone. Elevated prolactin levels owing to reduced clearance and increased production also contribute to anovulation.13,24,25 A possible mechanism of hormonal abnormalities in kidney failure is that high prolactin levels negatively feed back into the hypothalamic-pituitary-ovarian axis and inhibit gonadotropin-releasing hormone secretion, thus preventing gonadotropin release and resulting in abnormal uterine bleeding.26, 27, 28 In a prospective study of 57 female adolescents with stage 4 CKD and kidney failure treated with hemodialysis and peritoneal dialysis, 49% had hyperprolactinemia.29 When comparing participants with and without menstrual disturbances, prolactin levels were higher in those with menstrual disturbances.29
Figure 1.
Hypothalamic-pituitary-ovarian axis in females with kidney disease. From Ahmed SB, Ramesh S. Sex hormones in women with kidney disease. Nephrology Dialysis Transplantation, 2016, volume 31, issue 11, pages 1787–1795 © The Author(s). Published by Oxford University Press on behalf of the ERA-EDTA. All rights reserved.26 CKD, chronic kidney disease; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
Kidney Disease and Age of Menarche
Menarche is the first occurrence of uterine bleeding and the beginning of the female reproductive lifespan. Among healthy adolescents, the median age of menarche is approximately 12 to 13 years.30,31 Multiple factors are associated with the onset of menarche in the general population. An inverse association between body mass index,32, 33, 34 height, and weight35 with age of menarche has been found. Earlier menarche is reported among those living with anyone other than a family consisting of 2 biological parents,33,36, 37, 38 though study results vary regarding the impact of low socioeconomic status on early36,38,39 and late33 onset of menarche. Urban residence and Black race/ethnicity have been associated with earlier menarche, although these differences may or may not be in part attributed to socioeconomic status.33,34,38,40 Increasing reports reveal associations between both early and late menarche and adverse health outcomes, including risk of cardiovascular disease, CKD, and overall mortality.41, 42, 43
Given the multiple factors associated with onset of menarche, it is challenging to elucidate the association, if any, between CKD and onset of uterine bleeding. In a prospective cohort study of 57 female adolescents with stage 4 CKD and kidney failure treated with hemodialysis and peritoneal dialysis, Serret-Montaya et al.29 reported a median age of menarche of 12 years after exclusion of participants with primary amenorrhea. The primary causes of CKD were glomerulonephritis (22.8%) and congenital abnormalities of the kidney and urinary tract (22.8%), and most participants had a healthy nutritional status. Although the median age of menarche was similar in those with and without abnormal uterine bleeding, information including estimated glomerular filtration rate, ethnicity, and socioeconomic status was not reported. In a cross-sectional study of 287 girls with CKD onset before menarche, the median age of menarche was 12 years, though 10% had delayed menarche (defined as menarche at ≥15 years), which was associated with African-American race, lower estimated glomerular filtration rate, corticosteroid use, and longer CKD duration, concluding delayed menarche may suggest a risk of short stature.44
From the perspective of nephrologists, being aware of age of menarche is an important consideration as the American Academy of Pediatrics has suggested that the menstrual cycle is a vital sign in female patients.31 Nevertheless, in a study of 75 nephrologists (95% pediatric, 5% adult) practicing in the United States and Puerto Rico, Vasylyeva et al.45 reported that 17% never/rarely documented the age of menarche of adolescent patients and more than a third never/rarely documented the date of the patient's last menstrual period. This discrepancy highlights the need for nephrologists to take comprehensive menstrual histories, including age of menarche, to consider this sex-specific factor in the care of adolescents living with kidney disease.
Kidney Disease and Abnormal Uterine Bleeding
Abnormal uterine bleeding is defined as any disruption of a healthy menstrual cycle in terms of the volume of blood loss, duration, frequency, and regularity of menses.22 Abnormal uterine bleeding, particularly irregular or long (≥40 days) menstrual cycles, has been associated with premature mortality in comparison to regular or short cycles in the general population.46 Abnormal uterine bleeding is also associated with absenteeism in school and work.47, 48, 49 In the general population of reproductive-aged women, the estimated prevalence of abnormal uterine bleeding is at least 10% to 30%,50 whereas heavy menstrual bleeding affects 30% of women throughout their reproductive lifespan.51 Heavy menstrual bleeding is defined as the loss of ≥80 ml of blood on each menstrual cycle, which is clinically indicated by 1 or more of the following factors: bleeding that lasts >7 days, bleeding that soaks through ≥1 menstrual products every hour for several hours, bleeding that requires simultaneous use of multiple menstrual products to manage flow, bleeding that requires a change of menstrual product during the night, or the presence of blood clots at least the size of a quarter.52,53 Abnormal duration of menses includes prolonged (>8 days) and shortened (<3 days) uterine bleeding, whereas abnormal frequency of menses includes infrequent (>38 days apart) and frequent (<24 days apart) uterine bleeding.22 Absent uterine bleeding is defined by an absence of menses for 90 days, and irregular uterine bleeding is defined by variation in cycle length by ≥10 days.
Among adolescents in the general population, the prevalence of heavy, infrequent, and absent menstrual bleeding is reported as 34%, 20%, and 8%, respectively.54 Nevertheless, information on abnormal uterine bleeding is sparse in the adolescent CKD population. A prospective cohort study found that >50% of adolescent girls with stage 4 CKD and kidney failure treated with dialysis reported abnormal uterine bleeding.29 Moreover, although 54% reported regular menstrual cycles at baseline, only 47% reported regular menses a year after. Nevertheless, in premenopausal adult female populations with CKD, the prevalence of abnormal uterine bleeding is high and becomes increasingly common with disease progression.13,14,24 In a small study of 17 women aged 18 to 42 years with kidney failure treated with hemodialysis, only 1 woman reported regular uterine bleeding, whereas 6 reported irregular uterine bleeding, and 10 had absent uterine bleeding.13 In a cross-sectional study of women <55 years of age with kidney failure treated with hemodialysis and peritoneal dialysis,14 58% reported absence of uterine bleeding. Furthermore, most of the menstruating women experienced irregular uterine bleeding, which was most often heavy menstrual bleeding. Abnormal uterine bleeding, especially heavy menstrual bleeding, is an important consideration in the CKD population, as potential implications include worsening anemia, increasing the need for erythropoietin-stimulating agents, and blood transfusions.24,55 This may be especially relevant for those in need of a kidney transplant, given the risk of sensitization.24In a retrospective cohort study of 129 women with kidney failure (aged 41.6 ± 14.2 years with follow-up for 9.5 ± 10.2 years) treated with dialysis or kidney transplantation and followed by a gynecologist,15 78.7% had regular uterine bleeding before dialysis, though this decreased to 30.6% after dialysis initiation. The remaining participants reported infrequent (26%) or absent (43%) uterine bleeding after dialysis initiation.
We are unaware of any specific treatment regimens for abnormal uterine bleeding that differentiate between stages of CKD. Nevertheless, possible treatment for abnormal uterine bleeding must be balanced with the risks of worsening patients’ kidney health and evaluation of their comorbidities, contraindications, preferences, and suitability for adolescents and young adults. Hormone therapy using progestin-only or combined estrogen-progestin hormonal contraception can temporarily improve uterine bleeding.56 For instance, with the progestin-only intrauterine device, injectable, and subdermal implant, some individuals experience a cessation of bleeding after months to a year of use despite initially having irregular and/or heavy bleeding.53,56, 57, 58 The combined oral contraceptive pill, transdermal patch, and vaginal ring can also regulate bleeding, and if used continuously without hormone-free weeks (i.e., long/extended-cycle use), they can prevent uterine bleeding and related symptoms.57,59 It is important to note, however, that estrogen-containing options increase thrombotic risk.59 Tranexamic acid, danazol therapy, gonadotropin-releasing hormone agonists, and nonsteroidal anti-inflammatory medications are additional treatment options, though risks and timelines of use must be assessed carefully in the context of CKD, especially with the latter.56
Though abnormal uterine bleeding is prevalent in the context of kidney disease, a study consisting of largely pediatric nephrologists from the United States and Puerto Rico reported that almost 90% were not at all confident/somewhat confident in managing abnormal uterine bleeding.45 In addition, in a study of adult nephrologists from the United States and Canada, more than 65% of the respondents reported a lack of confidence in women's health issues, including menstrual disorders,60 whereas only 15% reported discussing menstrual irregularities with their patients.61 These findings highlight a gap in knowledge with regard to the gynecologic care of female patients with CKD and underscore the need for accessible educational resources and training for nephrologists in this important area of patient care.
Kidney Disease and Period Poverty
Period poverty is defined as a lack of knowledge pertaining to uterine bleeding and an inability to access menstrual products,62 serving as a socioeconomic, cultural, and political barrier. CKD is associated with significant socioeconomic disparities,63,64 and period poverty only exacerbates the economic toll of CKD. Menarche and menstrual management are fundamental aspects in adolescent female health,65 but a lack of education and resources leads to challenges with menstrual management, leaving female adolescents to deal with stigma, shame, fear, and anxiety; for some, there are direct effects on education, health, and well-being.66 Period poverty results in some young people to miss up to a fifth of their school year.67 Coupled with missing school for medical appointments, adolescents with CKD may be at greater risk of absenteeism, leading to grade retention, academic underachievement, and interruption of studies, all compromising their psychosocial well-being and quality of life.68,69 Finally, although there are no studies focusing on period poverty among menstruating individuals with CKD, socioeconomic position and country income level and may also influence one’s access to safe menstrual products and hygiene management facilities.63,64,70, 71, 72
Kidney Disease and Sexual Activity and Function
Adolescents with CKD tend to experience later onset of puberty68 and initiate sexual intercourse at a later age compared with the general age-matched population.73 American adolescents with CKD are less likely to report ever having sex compared with age-, gender-, and race-matched high school students, and they became sexually active at a later age than controls (26.7% versus 41.6%; mean ± SD 15.1 ± 1.6 versus 14.6 ± 1.6 years, respectively). The percentage of participants having ≥2 partners and/or engaging in unprotected gender or using alcohol or illicit drugs during gender were comparable in the 2 groups.73 Nevertheless, whether these results differ by sex and gender is unknown.
Sexual dysfunction in females is defined as loss of libido, reduced vaginal lubrication, and inability to orgasm, including vaginismus, dyspareunia, and infertility.74 In the United States, almost 30% of high school students reported being sexually active,75,76 with nearly 50% of young females reporting sexual dysfunction.77 The prevalence of sexual dysfunction in the adolescent CKD population is unknown. In the adult CKD population, a systematic review found that 30% to 80% of women with CKD reported sexual dysfunction and scored lower overall and in each domain of the Female Sexual Function Index questionnaire compared with healthy women.78 In a cross-sectional study of 106 women under the age of 50 years,79 rates of female sexual dysfunction were highest in the CKD group (81%) and lowest among kidney transplant recipients (50%). In a prospective cohort study of 39 women (mean age 36 ± 5.9 years) with kidney failure treated with hemodialysis for more than 6 months,16 41% reported an active sexual life compared with 88% after kidney transplantation, in conjunction with improved reproductive hormone profiles and Female Sexual Function Index scores. Factors that may affect sexual function in the CKD population include the adverse psychosocial effects of having a chronic illness, depression, anxiety, and negative body image.78,80,81 Physical challenges, such as decreased libido and vaginal lubrication, orgasmic impairment, and dyspareunia, are common among women with CKD, whereas comorbidities and sociodemographic factors can exacerbate the risk.78,79,81,82
Kidney Disease and Sexually Transmitted Infections
Youth aged 15 to 24 years account for approximately half of new sexually transmitted infection (STI) cases in the United States,83,84 and it is estimated that 1 of 4 sexually active adolescent females have an STI, most often Chlamydia trachomatis infection and human papillomavirus (HPV) infection. Adolescents in general are particularly at risk for STIs from both behavioral and biological standpoints. Adolescents are more likely to engage in high-risk sexual behaviors, such as having concurrent partners or sex without a condom. From a biological perspective, adolescent females are particularly susceptible to STIs, such as Chlamydia trachomatis and HPV, because of lower production of cervical mucus and increased cervical ectopy.85
For many adolescents living with kidney disease, the nephrologist functions as the primary care provider and may be the only contact to perform STI screening and reproductive health counseling.86 A high index of suspicion for STIs is particularly important in transplant recipients owing to their maintenance immunosuppressant medications. In a single-center, American, retrospective medical record review study of all pediatric transplant recipients aged 13 years and older (n = 49) spanning up to 11 years of follow-up, more than half of adolescent female kidney transplant recipients reported being sexually active, 75% of those sexually active reported using hormonal contraception, and 37.5% had had at least 1 STI.87 STIs identified in this study included gonococcal and chlamydial urethritis/cervicitis, Trichomonas vaginitis, herpes simplex virus 2 genital sores, pelvic inflammatory disease, and human immunodeficiency virus. Owing to the retrospective nature of the study, assessment of condom use was not possible.
Though not specifically studied in the pediatric population, the prevalence of syphilis was found to be significantly higher in the kidney failure population treated with dialysis.88,89 The incidence of syphilis in the adult kidney failure population is >3× higher than in the general population, and many affected patients had late-stage syphilis.90 Potential reasons for increased STI diagnoses include immunosuppression and recognizing that patients with kidney failure have a tremendous burden of symptoms that may prevent STI detection at an early stage. The apparent elevated rate of STIs among patients with CKD may suggest increased sexual activity; however, this has not been well studied in the CKD population.
There are no guidelines for primary prevention of STIs specific to adolescents with CKD; the Centers for Disease Control and Prevention recommends that this important aspect of health be incorporated into all types of health care visits for adolescents and young adults.91 HPV causes most of the cervical, anal/rectal, and oropharyngeal cancers in women. A US Renal Database System study of older women (mean age 65 years) between 2005 and 2011 revealed that the incidence of HPV-associated cancers in women with kidney failure is rising annually and is overall higher than in women of the general population.92 The incidence of HPV-associated cancers in younger female populations across the stages of CKD, however, is unknown.
In the United States, HPV vaccination is recommended through the age of 26 years for those not vaccinated previously at the routine age of 11 or 12 years.93 General recommendations with respect to counseling adolescents on sexual behaviors include discussions surrounding risk-reduction behaviors (e.g., consistent and correct condom use and reduction in the number of sex partners, including concurrent partners). Unfortunately, pediatric and adult nephrologists practicing in the United States and Puerto Rico never/rarely reported documenting patient sexual activity (29.5%), number of sexual partners (74.7%), and STI history (38.1%).45 Increasing the dialogue on sexual activity and STIs among adolescents with CKD is important to providing better care, considering the immunosuppressed states of patients.
Kidney Disease and Contraception
In a retrospective cohort study of 35,732 women receiving dialysis in the United States (115,713 person-years) aged 15 to 44 years from 2005 to 2014,94 the rate of contraceptive use was low at 5.3%, with the intrauterine device and oral contraceptive pill being the most common methods of contraception. Younger age, Native American and Black race/ethnicity, kidney failure owing to glomerulonephritis, kidney failure treatment with hemodialysis, and predialysis nephrology care were associated with a higher likelihood of contraceptive use. In a national survey evaluating high-risk behaviors in American adolescents with CKD, 54.8% of sexually active adolescents reported condoms as the most common contraception method, though whether use differed by sex and gender was not reported.73 Although the oral contraceptive pill is the second most common contraceptive used by adolescents in the general population in high-income countries,75,95 oral contraceptive use by adolescents with CKD is unknown.
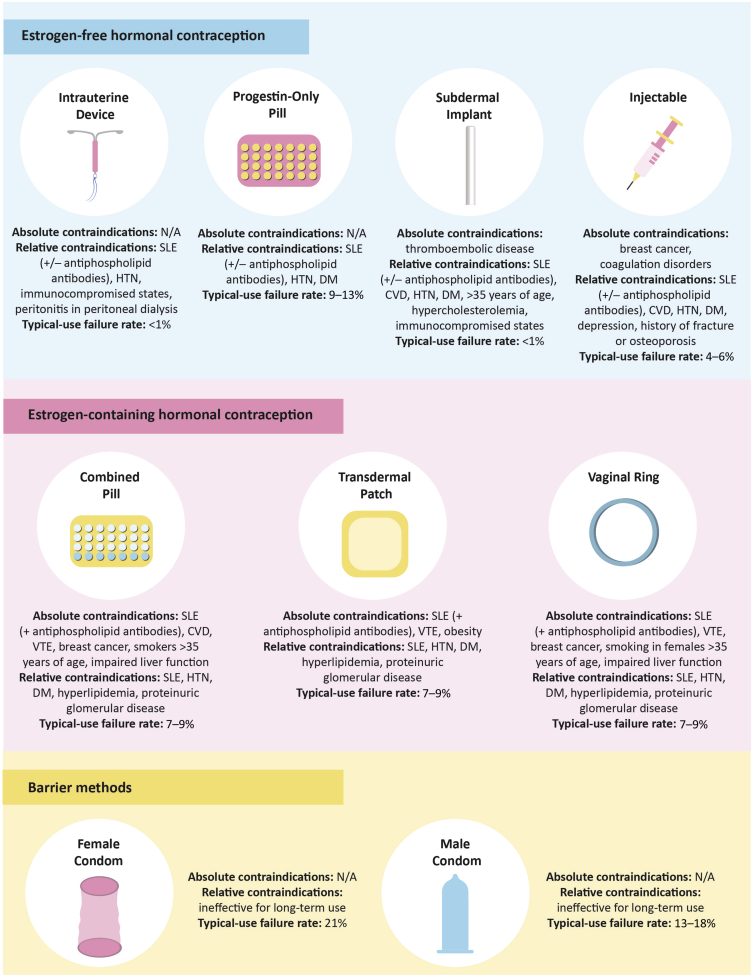
Hormonal composition of contraceptive options is an important consideration in adolescent females with CKD (Figure 2). Estrogen-containing oral contraceptive pills are associated with increased risk of proteinuria,96, 97, 98 increased blood pressure,99,100 venous thromboembolism, arterial thrombosis,101 and cervical cancer,59,102 in part owing to activation of the renin-angiotensin-aldosterone system,82,96,99,100,103 and should be used with caution in people with CKD. Similar concerns on the estrogen-containing transdermal patch and vaginal ring also exist, though this has not been studied specifically in the population with CKD.59,104 Of note, bone mass accrual continues up to approximately age 25 years, and although there are conflicting data on the effects of estrogen-containing hormonal contraception on bone mineral density, there is currently no evidence supporting increased risks of osteoporosis or fracture among users.105,106 How estrogen-containing hormonal contraception may affect bone health in adolescents with CKD is unknown.
Figure 2.
Contraceptive options and considerations for adolescent females with kidney disease. (Adapted from Ahmed et al.,82 Attini et al.,115 Sachdeva,101Watnick,104 and Wiles and Lightstone102). CVD, cardiovascular disease; DM, diabetes mellitus; HTN, hypertension; N/A, not available; SLE, systemic lupus erythematosus; VTE, venous thromboembolism.
Long-acting reversible contraceptives, and specifically intrauterine contraception, are recommended by multiple international societies as the first line of contraception for adolescents owing to their low typical-use failure rates and high 1-year continuation rates.107, 108, 109, 110, 111, 112 Use of long-acting reversible contraceptives in the adolescent CKD population is unknown, but compared with estrogen-containing contraceptives, these progestin-only alternatives confer lower risks of venous thromboembolism in the general population.113 Clinical practice guidelines for contraception in kidney disease recommend that the progestin-only pill, progestin subdermal implant, and progestin intrauterine device are safe and effective for women with CKD.114,115 In addition, the progestin-only injectable may be another contraceptive option as it confers lower thrombotic risks compared to estrogen-containing choices. Of note, there are older case reports of nonhormonal intrauterine devices being associated with peritonitis in women on peritoneal dialysis,116, 117, 118 though one study highlights this association with progestin intrauterine device use.119
As with the general adolescent population, contraception counseling in the adolescent population with CKD is of critical importance. Although most contraceptives are intended for use by females, it is imperative to highlight that contraception and the consequences of unprotected sex are important priorities to discuss with patients with CKD of all gender identities. Kidney health care providers play an important role in ensuring that adolescents with CKD have access to high-quality and safe reproductive health care services and contraceptive methods. Nevertheless, in surveys of 200 German and 196 American nephrologists, fewer than half report contraception counseling to adult women on dialysis.120,121 Nephrologists who do provide contraception or preconception counseling report counseling an average of <1 woman per month, citing lack of training and personal knowledge/confidence.60 In contrast, nearly two-thirds of nephrologists caring for adolescents with CKD report being very confident or confident providing contraceptive counseling,45 although most reported being comfortable discussing barrier methods rather than other forms of contraception, such as long-acting reversible contraceptives, which are recommended as the first line of contraception among adolescents122 and are safe in CKD.123 Although a clinical practice guideline on pregnancy and kidney disease exists,114,115 increased attention is urgently required to aid nephrologists provide patient-centered and disease-specific contraceptive care. Especially for those taking teratogenic medications, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and mycophenolate mofetil for the treatment of kidney disease, it is imperative that these patients use contraception to avoid adverse pregnancy outcomes. Reports of congenital malformations after taking angiotensin-converting enzyme inhibitors,124 neonatal and long-term complications for fetuses exposed to angiotensin receptor blockers,125 and an elevated incidence of structural malformations with mycophenolate mofetil exposure during pregnancy126 highlight the need for effective contraception when pregnancy is not desired.
Kidney Disease and Fertility
Reduced fertility has been observed in the female CKD population compared with the general population,10 postulated secondary to multiple factors, including a reduction in ovarian reserve.9,127, 128, 129 Individuals with female biology are born with a finite number of ovarian follicles,130 and anti-Müllerian hormone (AMH), produced by preantral and small antral ovarian follicles, is the gold standard measure of ovarian reserve.131,132 As a woman’s ovarian reserve naturally depletes with age, AMH levels also decline.130 AMH levels can be used to evaluate female fertility and menopausal status.9,133
We are unaware of any studies evaluating ovarian reserve in the adolescent population with CKD; however, AMH levels in women of reproductive age with CKD and kidney failure, particularly in those treated with kidney transplantation, seem to be lower compared with age-matched healthy individuals, suggesting a reduced ovarian reserve in women with CKD.127, 128, 129 Furthermore, in a prospective study of 46 females with kidney failure treated with hemodialysis, those with normal uterine bleeding had higher concentrations of AMH compared with those with abnormal uterine bleeding, and an unexpected decline in AMH level was found after kidney transplantation.128
Fertility can be negatively affected by treatment for CKD, such as cyclophosphamide.11 There is limited evidence that co-treatment with a gonadotropin-releasing hormone agonist may decrease the gonadotoxicity of this alkylating agent.134, 135, 136 Therefore, fertility preservation is an important consideration for young patients undergoing gonadotoxic treatment. For females, options include cryopreservation and banking of oocyte, embryo, and ovarian tissue; preservation of fertility in the context of kidney disease has been reviewed in detail elsewhere.9,82 Assisted reproductive technologies, such as in vitro fertilization, seem to be safe in kidney transplant recipients,137, 138, 139 although we are unaware of related studies in the non-transplant CKD population.
It is also important to note nephrologists’ communication of fertility status with their patients, especially if parenthood is considered a meaningful goal. Studies evaluating Canadian, American, and Puerto Rican pediatric and adult nephrologists found that most discussed potential teratogenicity of medication and risks of infertility with cyclophosphamide use.45,60 One study found that 95% of respondents in an international survey of pediatric and adult nephrologists agreed that kidney function affects reproductive hormone status.61 Nevertheless, only 35% reported regularly discussing fertility with their patients. Although kidney disease affects the entire spectrum of reproductive health, frequent reproductive assessment and counseling should become a common part of nephrologists’ practices.45,60,61
Transgender Individuals and Reproductive Care
The proportion of transgender individuals (i.e., gender identity does not align with sex assigned at birth) has increased over time, where youth account for a large proportion of this group.140 Transgender adolescents have unique reproductive health care needs. A transgender boy or nonbinary individual requires gynecologic and reproductive care, including contraception counseling, and most transgender and gender-diverse adolescents with female biology express desire to have children in the future.141 Despite this important consideration, information regarding the reproductive care of transgender boys and nonbinary individuals within the CKD context is lacking.
Conclusion
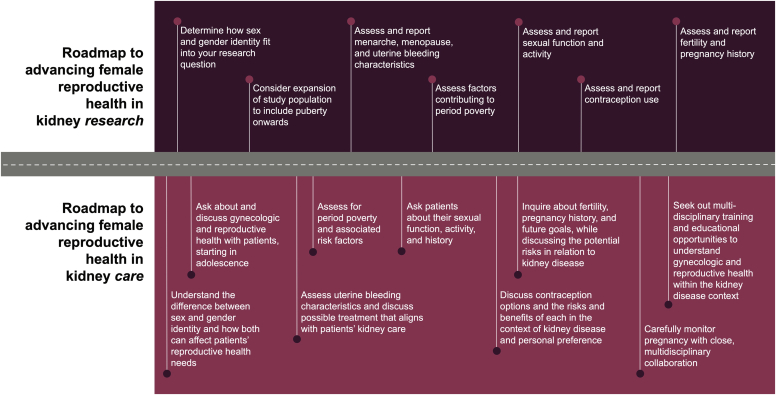
Female reproductive and gynecologic health in CKD, and particularly in adolescents, is an important yet understudied area. Kidney disease is associated with abnormal hypothalamic-pituitary-ovarian function. Abnormal uterine bleeding and low fertility are common. Although CKD is associated with high-risk pregnancy, contraceptive use is low in the setting of CKD. Despite the high prevalence of menstrual and fertility disorders, gynecologic and reproductive health is not often addressed by nephrologists with many reporting a lack of knowledge and confidence in this area. Providers should feel comfortable obtaining detailed sexual histories to properly counsel on and test for STIs, particularly given that CKD is an immunocompromised state. With special considerations to the transition from pediatric to adult nephrology and the growing transgender youth population, focused training in these important areas of female health in addition to multidisciplinary collaborations is urgently required. We propose a “roadmap” to female reproductive kidney research and care (Figure 3). Large, prospective studies in addition to dedicated educational resources are required to equip kidney health care providers with the knowledge needed to provide patient-centered and disease-specific care that includes gynecologic and reproductive health.
Figure 3.
Roadmap to advancing female reproductive health in kidney research and care.
Disclosure
All the authors declare no competing interests.
Acknowledgments
The authors gratefully acknowledge Sarah Gil and Alexa Desjarlais for the graphic design. DHC is supported by graduate scholarships from the Canadian Institutes of Health Research, University of Calgary, and the Libin Cardiovascular Institute.
References
- 1.Kaspar C.D.W., Bholah R., Bunchman T.E. A review of pediatric chronic kidney disease. Blood Purif. 2016;41:211–217. doi: 10.1159/000441737. [DOI] [PubMed] [Google Scholar]
- 2.Jha V., Garcia-Garcia G., Iseki K., et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/s0140-6736(13)60687-x. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases Kidney disease statistics for the United States. National Institute of Health—National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease Published January 18, 2021. Accessed December 4, 2021.
- 4.North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) 2008 annual report. NAPRTCS. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Fwww.naprtcs.org%2Fsystem%2Ffiles%2F2008_Annual_CKD_Report.pdf&clen=2915899. Accessed February 24, 2021.
- 5.Ardissino G., Dacco V., Testa S., et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111:e382–e387. doi: 10.1542/peds.111.4.e382. [DOI] [PubMed] [Google Scholar]
- 6.Mong Hiep T.T., Ismaili K., Collart F., et al. Clinical characteristics and outcomes of children with stage 3–5 chronic kidney disease. Pediatr Nephrol. 2010;25:935–940. doi: 10.1007/s00467-009-1424-2. [DOI] [PubMed] [Google Scholar]
- 7.Ingelfinger J.R., Kalantar-Zadeh K., Schaefer F., World Kidney Day Steering Committee Averting the legacy of kidney disease—focus on childhood. Kidney Dis. 2016;2:46–52. doi: 10.1159/000443819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harambat J., Van Stralen K.J., Kim J.J., Tizard E.J. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–373. doi: 10.1007/s00467-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumanski S.M., Ahmed S.B. Fertility and reproductive care in chronic kidney disease. J Nephrol. 2019;32:39–50. doi: 10.1007/s40620-018-00569-9. [DOI] [PubMed] [Google Scholar]
- 10.Wiles K.S., Nelson-Piercy C., Bramham K. Reproductive health and pregnancy in women with chronic kidney disease. Nat Rev Nephrol. 2018;14:165–184. doi: 10.1038/nrneph.2017.187. [DOI] [PubMed] [Google Scholar]
- 11.Leroy C., Rigot J.-M., Leroy M., et al. Immunosuppressive drugs and fertility. Orphanet J Rare Dis. 2015;10:136. doi: 10.1186/s13023-015-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro R., Sarwal M.M. Pediatric kidney transplantation. Pediatr Clin North Am. 2010;57:393–400. doi: 10.1016/j.pcl.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Lim V.S., Henriquez C., Sievertsen G., Frohman L.A. Ovarian function in chronic renal failure: evidence suggesting hypothalamic anovulation. Ann Intern Med. 1980;93:22–27. doi: 10.7326/0003-4819-93-1-21. [DOI] [PubMed] [Google Scholar]
- 14.Holley J.L., Schmidt R.J., Bender F.H., et al. Gynecologic and reproductive issues in women on dialysis. Am J Kidney Dis. 1997;29:685–690. doi: 10.1016/s0272-6386(97)90120-7. [DOI] [PubMed] [Google Scholar]
- 15.Chakhtoura Z., Meunier M., Caby J., et al. Gynecologic follow up of 129 women on dialysis and after kidney transplantation: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2015;187:1–5. doi: 10.1016/j.ejogrb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Filocamo M.T., Zanazzi M., Li Marzi V., et al. Sexual dysfunction in women during dialysis and after renal transplantation. J Sex Med. 2009;6:3125–3131. doi: 10.1111/j.1743-6109.2009.01400.x. [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 18.EBPG Expert Group on Renal Transplantation European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant. 2002;17(suppl 4):50–55. [PubMed] [Google Scholar]
- 19.Piccoli G.B., Cabiddu G., Attini R., et al. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol. 2015;26:2011–2022. doi: 10.1681/asn.2014050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hladunewich M.A., Melamed N., Bramham K. Pregnancy across the spectrum of chronic kidney disease. Kidney Int. 2016;89:995–1007. doi: 10.1016/j.kint.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 21.Welt C.K. Physiology of the normal menstrual cycle. https://www.uptodate.com/contents/physiology-of-the-normal-menstrual-cycle?search=menstrual%20cycle&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1 UptoDate. Accessed May 7, 2021.
- 22.Hoffman B.L., Schorge J.O., Halvorson L.M., et al. Williams Gynecology. 4th ed. McGraw-Hill Education LLC; 2020. Chapter 8: abnormal uterine bleeding. [Google Scholar]
- 23.The Society of Obstetricians and Gynaecologists of Canada Normal periods—menstrual cycle basics. The Society of Obstetricians and Gynaecologists of Canada. https://www.yourperiod.ca/normal-periods/menstrual-cycle-basics/ Accessed December 4, 2021.
- 24.Cochrane R., Regan L. Undetected gynaecological disorders in women with renal disease. Hum Reprod. 1997;12:667–670. doi: 10.1093/humrep/12.4.667. [DOI] [PubMed] [Google Scholar]
- 25.Sievertsen G.D., Lim V.S., Nakawatase C., Frohman L.A. Metabolic clearance and secretion rates of human prolactin in normal subjects and in patients with chronic renal failure. J Clin Endocrinol Metab. 1980;50:846–852. doi: 10.1210/jcem-50-5-846. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed S.B., Ramesh S. Sex hormones in women with kidney disease. Nephrol Dial Transplant. 2016;31:1787–1795. doi: 10.1093/ndt/gfw084. [DOI] [PubMed] [Google Scholar]
- 27.Ginsburg E.S., Owen W.F. Reproductive endocrinology and pregnancy in women on hemodialysis. Semin Dial. 2007;6:105–116. doi: 10.1111/j.1525-139x.1993.tb00273.x. [DOI] [Google Scholar]
- 28.Palmer B.F., Clegg D.J. Gonadal dysfunction in chronic kidney disease. Rev Endocr Metab Disord. 2017;18:117–130. doi: 10.1007/s11154-016-9385-9. [DOI] [PubMed] [Google Scholar]
- 29.Serret-Montaya J., Zurita-Cruz J.N., Villasís-Keever M.A., et al. Hyperprolactinemia as a prognostic factor for menstrual disorders in female adolescents with advanced chronic kidney disease. Pediatr Nephrol. 2020;35:1041–1049. doi: 10.1007/s00467-020-04494-7. [DOI] [PubMed] [Google Scholar]
- 30.Chumlea W.C., Schubert C.M., Roche A.F., et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111:110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- 31.Diaz A., Laufer M.R., Breech L.L., et al. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118:2245–2250. doi: 10.1542/peds.2006-2481. [DOI] [PubMed] [Google Scholar]
- 32.Ramezani Tehrani F., Mirmiran P., Gholami R., Moslehi N., Azizi F. Factors influencing menarcheal age: results from the cohort of Tehran lipid and glucose study. Int J Endocrinol Metab. 2014;12:e16130. doi: 10.5812/ijem.16130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yermachenko A., Dvornyk V. Nongenetic determinants of age at menarche: a systematic review. BioMed Res Int. 2014;2014:371583. doi: 10.1155/2014/371583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deardorff J., Abrams B., Ekwaru J.P., Rehkopf D.H. Socioeconomic status and age at menarche: an examination of multiple indicators in an ethnically diverse cohort. Ann Epidemiol. 2014;24:727–733. doi: 10.1016/j.annepidem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George I.M.S., Williams S., Silva P.A. Body size and the menarche: the Dunedin study. J Adolesc Health. 1994;15:573–576. doi: 10.1016/1054-139x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- 36.Culpin I., Heron J., Araya R., et al. Father absence and timing of menarche in adolescent girls from a UK cohort: the mediating role of maternal depression and major financial problems. J Adolesc. 2014;37:291–301. doi: 10.1016/j.adolescence.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Steppan M., Whitehead R., McEachran J., Currie C. Family composition and age at menarche: findings from the international Health Behaviour in School-aged Children study. Reprod Health. 2019;16:176. doi: 10.1186/s12978-019-0822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez G.M. Trends and patterns in menarche in the United States: 1995 through 2013–2017. Centers for Disease Control and Prevention. https://stacks.cdc.gov/view/cdc/93643 Published September 10, 2020. Accessed March 10, 2021.
- 39.James-Todd T., Tehranifar P., Rich-Edwards J., et al. The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Ann Epidemiol. 2010;20:836–842. doi: 10.1016/j.annepidem.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu T., Mendola P., Buck G.M. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics. 2002;110:752–757. doi: 10.1542/peds.110.4.752. [DOI] [PubMed] [Google Scholar]
- 41.Lakshman R., Forouhi N.G., Sharp S.J., et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- 42.Noh J.H., Koo H. Older menarche age and short reproductive period linked to chronic kidney disease risk. Medicine. 2019;98:e15511. doi: 10.1097/MD.0000000000015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J.J., Cook-Wiens G., Johnson B.D., et al. Age at menarche and risk of cardiovascular disease outcomes: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. J Am Heart Assoc. 2019;8 doi: 10.1161/jaha.119.012406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H.S., Ng D.K., Matheson M.B., et al. Delayed menarche in girls with chronic kidney disease and the association with short stature. Pediatr Nephrol. 2020;35:1471–1475. doi: 10.1007/s00467-020-04559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasylyeva T.L., Page-Hefley S., Almaani S., et al. Evaluation of the reproductive care provided to adolescent patients in nephrology clinics: a pediatric nephrology research consortium study. Kidney Int Rep. 2021;6:1411–1415. doi: 10.1016/j.ekir.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y.X., Arvizu M., Rich-Edwards J.W., et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ. 2020;371:m3464. doi: 10.1136/bmj.m3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma P., Malhotra C., Taneja D.K., Saha R. Problems related to menstruation amongst adolescent girls. Indian J Pediatr. 2008;75:125–129. doi: 10.1007/s12098-008-0018-5. [DOI] [PubMed] [Google Scholar]
- 48.Lee L.K., Chen P.C., Lee K.K., Kaur J. Menstruation among adolescent girls in Malaysia: a cross-sectional school survey. Singapore Med J. 2006;47:869–874. [PubMed] [Google Scholar]
- 49.Tanaka E., Momoeda M., Osuga Y., et al. Burden of menstrual symptoms in Japanese women: results from a survey-based study. J Med Econ. 2013;16:1255–1266. doi: 10.3111/13696998.2013.830974. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z., Doan Q.V., Blumenthal P., Dubois R.W. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10:183–194. doi: 10.1111/j.1524-4733.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 51.Market Opinion and Research International (MORI). Women’s Health in 1990. [Research study conducted on behalf of Parke-Davis Laboratories]. MORI. Published 1990.
- 52.The Society of Obstetricians and Gynaecologists of Canada Abnormal pain and menstrual bleeding—heavy menstrual bleeding (HMB) overview. The Society of Obstetricians and Gynaecologists of Canada. https://www.yourperiod.ca/abnormal-pain-and-menstrual-bleeding/ Published January 11, 2021 Accessed February 10, 2021.
- 53.Singh S., Best C., Dunn S., et al. No. 292-abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can. 2018;40:e391–e415. doi: 10.1016/j.jogc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Nur Azurah A.G., Sanci L., Moore E., Grover S. The quality of life of adolescents with menstrual problems. J Pediatr Adolesc Gynecol. 2013;26:102–108. doi: 10.1016/j.jpag.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Nelson A.L., Ritchie J.J. Severe anemia from heavy menstrual bleeding requires heightened attention. Am J Obstet Gynecol. 2015;213:97.e1–97.e6. doi: 10.1016/j.ajog.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt E., Pachtman S.L., Diedrich J.T., Contraception in chronic kidney disease and renal transplantation . Springer International Publishing; 2020. Obstetric and Gynecologic Nephrology; pp. 225–243. [Google Scholar]
- 57.The Society of Obstetricians and Gynaecologists of Canada Contraception. The Society of Obstetricians and Gynaecologists of Canada. https://www.sexandu.ca/contraception/ Published January 15, 2021. Accessed February 10, 2021.
- 58.Your Life. Contraception at a glance. Your Life. https://www.your-life.com/en/contraception-methods/#methods- Published 2021. Accessed February 10, 2021.
- 59.Brynhildsen J. Combined hormonal contraceptives: prescribing patterns, compliance, and benefits versus risks. Ther Adv Drug Saf. 2014;5:201–213. doi: 10.1177/2042098614548857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hendren E., Reynolds M., Mariani L., et al. Confidence in women’s health: a cross border survey of adult nephrologists. J Clin Med. 2019;8:176. doi: 10.3390/jcm8020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramesh S., James M.T., Holroyd-Leduc J.M., et al. Sex hormone status in women with chronic kidney disease: survey of nephrologists’ and renal allied health care providers’ perceptions. Can J Kidney Health Dis. 2017;4 doi: 10.1177/2054358117734534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark H., As Sy E. Menstrual hygiene and health—a call for dignity, rights and empowerment. The Partnership for Maternal, Newborn & Child Health—World Health Organization. Published 2021. https://www.who.int/pmnch/media/news/2020/menstrual_hygiene_health/en/ Accessed June 26, 2021.
- 63.Crews D.C., Gutiérrez O.M., Fedewa S.A., et al. Low income, community poverty and risk of end stage renal disease. BMC Nephrol. 2014;15:192. doi: 10.1186/1471-2369-15-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garrity B.H., Kramer H., Vellanki K., et al. Time trends in the association of ESRD incidence with area-level poverty in the US population. Hemodial Int. 2016;20:78–83. doi: 10.1111/hdi.12325. [DOI] [PubMed] [Google Scholar]
- 65.Sommer M., Caruso B.A., Sahin M., et al. A time for global action: addressing girls’ menstrual hygiene management needs in schools. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1001962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sommer M., Sahin M. Overcoming the taboo: advancing the global agenda for menstrual hygiene management for schoolgirls. Am J Public Health. 2013;103:1556–1559. doi: 10.2105/AJPH.2013.301374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cousins S. Rethinking period poverty. Lancet. 2020;395:857–858. doi: 10.1016/s0140-6736(20)30605-x. [DOI] [PubMed] [Google Scholar]
- 68.Ferris M.E., Miles J.A., Seamon M.L. Adolescents and young adults with chronic or end-stage kidney disease. Blood Purif. 2016;41:205–210. doi: 10.1159/000441317. [DOI] [PubMed] [Google Scholar]
- 69.Moreira J.M., Bouissou Morais Soares C.M., Teixeira A.L., et al. Anxiety, depression, resilience and quality of life in children and adolescents with pre-dialysis chronic kidney disease. Pediatr Nephrol. 2015;30:2153–2162. doi: 10.1007/s00467-015-3159-6. [DOI] [PubMed] [Google Scholar]
- 70.Crews D.C., Bello A.K., Saadi G. Burden, access, and disparities in kidney disease. Kidney Dis. 2019;5:126–134. doi: 10.1159/000494897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bikbov B., Purcell C.A., Levey A.S. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/s0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ojo A. Addressing the global burden of chronic kidney disease through clinical and translational research. Trans Am Clin Climatol Assoc. 2014;125:229–246. [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao N., Stolfi A., Malatesta-Muncher R., et al. Risk behaviors in teens with chronic kidney disease: a study from the Midwest Pediatric Nephrology Consortium. Int J Nephrol. 2019;2019:7828406. doi: 10.1155/2019/7828406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holley J.L., Schmidt R.J. Sexual dysfunction in CKD. Am J Kidney Dis. 2010;56:612–614. doi: 10.1053/j.ajkd.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Szucs L.E., Lowry R., Fasula A.M., et al. Condom and contraceptive use among sexually active high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Suppl. 2020;69(suppl-1):11–18. doi: 10.15585/mmwr.su6901a2external. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greydanus D.E., Matytsina L. Female sexual dysfunction and adolescents. Curr Opin Obstet Gynecol. 2010;22:375–380. doi: 10.1097/GCO.0b013e32833d9418. [DOI] [PubMed] [Google Scholar]
- 77.Moreau C., Kågesten A.E., Blum R.W. Sexual dysfunction among youth: an overlooked sexual health concern. BMC Public Health. 2016;16:1170. doi: 10.1186/s12889-016-3835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navaneethan S.D., Vecchio M., Johnson D.W., et al. Prevalence and correlates of self-reported sexual dysfunction in CKD: a meta-analysis of observational studies. Am J Kidney Dis. 2010;56:670–685. doi: 10.1053/j.ajkd.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 79.Basok E.K., Atsu N., Rifaioglu M.M., et al. Assessment of female sexual function and quality of life in predialysis, peritoneal dialysis, hemodialysis, and renal transplant patients. Int Urol Nephrol. 2009;41:473–481. doi: 10.1007/s11255-008-9475-z. [DOI] [PubMed] [Google Scholar]
- 80.Finkelstein F.O., Shirani S., Wuerth D., Finkelstein S.H. Therapy insight: sexual dysfunction in patients with chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:200–207. doi: 10.1038/ncpneph0438. [DOI] [PubMed] [Google Scholar]
- 81.Ali S., Dave N.N. Sexual dysfunction in women with kidney disease. Adv Chronic Kidney Dis. 2020;27:506–515. doi: 10.1053/j.ackd.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Ahmed S.B., Vitek W.S., Holley J.L. Fertility, contraception, and novel reproductive technologies in chronic kidney disease. Semin Nephrol. 2017;37:327–336. doi: 10.1016/j.semnephrol.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 83.Shannon C.L., Klausner J.D. The growing epidemic of sexually transmitted infections in adolescents. Curr Opin Pediatr. 2018;30:137–143. doi: 10.1097/mop.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sexually transmitted infections prevalence, incidence, and cost estimates in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/std/statistics/prevalence-incidence-cost-2020.htm Updated February 18, 2021. Accessed October 26, 2021.
- 85.Burchell A.N., Winer R.L., de Sanjosé S., Franco E.L. Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(suppl 3):52–61. doi: 10.1016/j.vaccine.2006.05.031. Chapter 6. [DOI] [PubMed] [Google Scholar]
- 86.Valderas J.M., Starfield B., Forrest C.B., et al. Routine care provided by specialists to children and adolescents in the United States (2002-2006) BMC Health Serv Res. 2009;9:221. doi: 10.1186/1472-6963-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ashoor I., Aviles D., Pasternak R., Vehaskari V.M. Sexually transmitted infections in pediatric renal transplant recipients: time to take notice! Pediatr Transplant. 2015;19:584–587. doi: 10.1111/petr.12554. [DOI] [PubMed] [Google Scholar]
- 88.Dahmani O., Belkhalfa S., Ayad K., et al. Late latent syphilis in two hemodialysis units. Saudi J Kidney Dis Transpl. 2013;24:124–127. doi: 10.4103/1319-2442.106306. [DOI] [PubMed] [Google Scholar]
- 89.Saxena A.K., Panhotra B.R., Naguib M., et al. Nosocomial transmission of syphilis during haemodialysis in a developing country. Scand J Infect Dis. 2002;34:88–92. doi: 10.1080/00365540110077353. [DOI] [PubMed] [Google Scholar]
- 90.Weathers E.N., Waller J.L., Nahman N.S., Jr., et al. Incidence, risk factors and distribution of syphilis in the end-stage renal disease population in the USA. Clin Kidney J. 2020;13:625–630. doi: 10.1093/ckj/sfz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.STI treatment guidelines Detection of STIs in Special Populations—Adolescents. Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/std/treatment-guidelines/adolescents.htm Updated October 26, 2021. Accessed October 26, 2021.
- 92.Han J., Waller J.L., Colombo R.E., et al. Incidence and risk factors for HPV-associated cancers in women with end-stage renal disease. J Investig Med. 2020;68:1002–1010. doi: 10.1136/jim-2019-001262. [DOI] [PubMed] [Google Scholar]
- 93.Human papillomavirus (HPV) ACIP vaccine recommendations. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/hpv.html Updated November 21, 2014. Accessed October 26, 2021.
- 94.Shah S., Christianson A.L., Thakar C.V., et al. Contraceptive use among women with end-stage kidney disease on dialysis in the United States. Kidney Med. 2020;2:707–715.e1. doi: 10.1016/j.xkme.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Farquhar C.M., Roberts H., Okonkwo Q.L., Stewart A.W. A pilot survey of the impact of menstrual cycles on adolescent health. Aust N Z J Obstet Gynaecol. 2009;49:531–536. doi: 10.1111/j.1479-828x.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 96.Ahmed S.B., Hovind P., Parving H.-H., et al. Oral contraceptives, angiotensin-dependent renal vasoconstriction, and risk of diabetic nephropathy. Diabetes Care. 2005;28:1988–1994. doi: 10.2337/diacare.28.8.1988. [DOI] [PubMed] [Google Scholar]
- 97.Ribstein J., Halimi J.-M., du Cailar G., Mimran A. Renal characteristics and effect of angiotensin suppression in oral contraceptive users. Hypertension. 1999;33:90–95. doi: 10.1161/01.hyp.33.1.90. [DOI] [PubMed] [Google Scholar]
- 98.Monster T.B.M., Janssen W.M.T., de Jong P.E., et al. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med. 2001;161:2000. doi: 10.1001/archinte.161.16.2000. [DOI] [PubMed] [Google Scholar]
- 99.Ahmed S.B., Kang A.K., Burns K.D., et al. Effects of oral contraceptive use on the renal and systemic vascular response to angiotensin II infusion. J Am Soc Nephrol. 2004;15:780–786. doi: 10.1097/01.asn.0000114555.16297.5a. [DOI] [PubMed] [Google Scholar]
- 100.Kang A.K., Duncan J.A., Cattran D.C., et al. Effect of oral contraceptives on the renin angiotensin system and renal function. Am J Physiol Regul Integr Comp Physiol. 2001;280:R807–R813. doi: 10.1152/ajpregu.2001.280.3.R807. [DOI] [PubMed] [Google Scholar]
- 101.Sachdeva M. Contraception in kidney disease. Adv Chronic Kidney Dis. 2020;27:499–505. doi: 10.1053/j.ackd.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 102.Wiles K., Lightstone L. Glomerular disease in women. Kidney Int Rep. 2018;3:258–270. doi: 10.1016/j.ekir.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Odutayo A., Cherney D., Miller J., et al. Transdermal contraception and the renin-angiotensin-aldosterone system in premenopausal women. Am J Physiol Ren Physiol. 2015;308:F535–F540. doi: 10.1152/ajprenal.00602.2014. [DOI] [PubMed] [Google Scholar]
- 104.Watnick S. Pregnancy and contraceptive counseling of women with chronic kidney disease and kidney transplants. Adv Chronic Kidney Dis. 2007;14:126–131. doi: 10.1053/j.ackd.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Technical consultation on the effects of contraception on bone health. World Health Organization. http://apps.who.int/iris/bitstream/handle/10665/69845/WHO_RHR_07.08_eng.pdf;jsessionid=4F06B83DC2BB1F2D0FCC1EA68E53F4F4?sequence=1 Published 2005. Accessed June 6, 2021.
- 106.Dombrowski S., Jacob L., Hadji P., Kostev K. Oral contraceptive use and fracture risk—a retrospective study of 12,970 women in the UK. Osteoporos Int. 2017;28:2349–2355. doi: 10.1007/s00198-017-4036-x. [DOI] [PubMed] [Google Scholar]
- 107.Ott M.A., Sucato G.S., Committee on Adolescence Contraception for adolescents. Pediatrics. 2014;134:e1257–e1281. doi: 10.1542/peds.2014-2300. [DOI] [PubMed] [Google Scholar]
- 108.ACOG Committee Opinion: Adolescent Pregnancy, Contraception, and Sexual Activity. The American College of Obstetricians and Gynecologists. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity Accessed June 6, 2021.
- 109.Faculty of Sexual & Reproductive Healthcare Clinical Guidance Contraceptive choices for young people. https://www.fsrh.org/standards-and-guidance/documents/cec-ceu-guidance-young-people-mar-2010/ Accessed Jun 6, 2021.
- 110.Di Meglio G., Crowther C., Simms J. Contraceptive care for Canadian youth. Paediatr Child Health. 2018;23:271–277. doi: 10.1093/pch/pxx192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Black A., Guilbert E., Costescu D., et al. Canadian contraception consensus (part 3 of 4): chapter 7—intrauterine contraception. J Obstet Gynaecol Can. 2016;38:182–222. doi: 10.1016/j.jogc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 112.ACOG committee opinion no. 392, December 2007 Intrauterine device and adolescents. Obstet Gynecol. 2007;110:1493–1495. doi: 10.1097/01.AOG.0000291575.93944.1a. [DOI] [PubMed] [Google Scholar]
- 113.Tepper N.K., Whiteman M.K., Marchbanks P.A., et al. Progestin-only contraception and thromboembolism: a systematic review. Contraception. 2016;94:678–700. doi: 10.1016/j.contraception.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wiles K., Chappell L., Clark K., et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol. 2019;20:401. doi: 10.1186/s12882-019-1560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Attini R., Cabiddu G., Montersino B., et al. Contraception in chronic kidney disease: a best practice position statement by the Kidney and Pregnancy Group of the Italian Society of Nephrology. J Nephrol. 2020;33:1343–1359. doi: 10.1007/s40620-020-00717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Plaza M.M. Intrauterine device-related peritonitis in a patient on CAPD. Perit Dial Int. 2002;22:538–539. [PubMed] [Google Scholar]
- 117.Korzets A., Chagnac A., Ori Y., et al. Pneumococcal peritonitis complicating CAPD—was the indwelling intrauterine device to blame? Clin Nephrol. 1991;35:24–25. [PubMed] [Google Scholar]
- 118.Stuck A., Seiler A., Frey R.J. Peritonitis due to an intrauterine contraceptive device in a patient on CAPD. Perit Dial Bull. 1986;6:158–159. [Google Scholar]
- 119.Bieber S.D., Jefferson J.A., Anderson A.E. Migration of an intrauterine device and peritonitis in a peritoneal dialysis patient. Clin Nephrol. 2013;80:146–150. doi: 10.5414/cn107391. [DOI] [PubMed] [Google Scholar]
- 120.Duffner J., Schulte-Kemna L., Reister B., et al. Survey among nephrologists in Germany: current practice and management of pregnant women on dialysis. Clin Nephrol. 2017;88:264–269. doi: 10.5414/CN109032. [DOI] [PubMed] [Google Scholar]
- 121.Sachdeva M., Barta V., Thakkar J., et al. Pregnancy outcomes in women on hemodialysis: a national survey. Clin Kidney J. 2017;10:276–281. doi: 10.1093/ckj/sfw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Allen S., Barlow E. Long-acting reversible contraception. Pediatr Clin North Am. 2017;64:359–369. doi: 10.1016/j.pcl.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 123.Burgner A., Hladunewich M.A. Women’s reproductive health for the nephrologist. Am J Kidney Dis. 2019;74:675–681. doi: 10.1053/j.ajkd.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 124.Cooper W.O., Hernandez-Diaz S., Arbogast P.G., et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451. doi: 10.1056/nejmoa055202. [DOI] [PubMed] [Google Scholar]
- 125.Bullo M., Tschumi S., Bucher B.S., et al. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists. Hypertension. 2012;60:444–450. doi: 10.1161/hypertensionaha.112.196352. [DOI] [PubMed] [Google Scholar]
- 126.Sifontis N.M., Coscia L.A., Constantinescu S., et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698–1702. doi: 10.1097/01.tp.0000252683.74584.29. [DOI] [PubMed] [Google Scholar]
- 127.Stoumpos S., Lees J., Welsh P., et al. The utility of anti-Müllerian hormone in women with chronic kidney disease, on haemodialysis and after kidney transplantation. Reprod Biomed Online. 2018;36:219–226. doi: 10.1016/j.rbmo.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 128.Sikora-Grabka E., Adamczak M., Kuczera P., et al. Serum anti-Müllerian hormone concentration in young women with chronic kidney disease on hemodialysis, and after successful kidney transplantation. Kidney Blood Press Res. 2016;41:552–560. doi: 10.1159/000443458. [DOI] [PubMed] [Google Scholar]
- 129.Wiles K., Anckaert E., Holden F., et al. Anti-Müllerian hormone concentrations in women with chronic kidney disease. Clin Kidney J. 2019;14:537–542. doi: 10.1093/ckj/sfz164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wilkosz P., Greggains G.D., Tanbo T.G., Fedorcsak P. Female reproductive decline is determined by remaining ovarian reserve and age. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fleming R., Seifer D.B., Frattarelli J.L., Ruman J. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod Biomed Online. 2015;31:486–496. doi: 10.1016/j.rbmo.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 132.Jirge P.R. Ovarian reserve tests. J Hum Reprod Sci. 2011;4:108–113. doi: 10.4103/0974-1208.92283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iwase A., Nakamura T., Osuka S., et al. Anti-Müllerian hormone as a marker of ovarian reserve: what have we learned, and what should we know? Reprod Med Biol. 2016;15:127–136. doi: 10.1007/s12522-015-0227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kado R., McCune W.J. Ovarian protection with gonadotropin-releasing hormone agonists during cyclophosphamide therapy in systemic lupus erythematosus. Best Pract Res Clin Obstet Gynaecol. 2020;64:97–106. doi: 10.1016/j.bpobgyn.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 135.Marder W., McCune W.J., Wang L., et al. Adjunctive GnRH—a treatment attenuates depletion of ovarian reserve associated with cyclophosphamide therapy in premenopausal SLE patients. Gynecol Endocrinol. 2012;28:624–627. doi: 10.3109/09513590.2011.650752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Somers E.C., Marder W., Christman G.M., et al. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 2005;52:2761–2767. doi: 10.1002/art.21263. [DOI] [PubMed] [Google Scholar]
- 137.Yaprak M., Doğru V., Sanhal C.Y., et al. In vitro fertilization after renal transplantation: a single-center experience. Transplant Proc. 2019;51:1089–1092. doi: 10.1016/j.transproceed.2019.01.105. [DOI] [PubMed] [Google Scholar]
- 138.Kosoku A., Uchida J., Maeda K., et al. Successful pregnancy after in vitro fertilization in an ABO-incompatible kidney transplant recipient receiving rituximab: a case report. BMC Nephrol. 2019;20:206. doi: 10.1186/s12882-019-1396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Warzecha D., Szymusik I., Grzechocińska B., et al. In vitro fertilization and pregnancy outcomes among patients after kidney transplantation: case series and single-center experience. Transplant Proc. 2018;50:1892–1895. doi: 10.1016/j.transproceed.2018.02.144. [DOI] [PubMed] [Google Scholar]
- 140.Nolan I.T., Kuhner C.J., Dy G.W. Demographic and temporal trends in transgender identities and gender confirming surgery. Transl Androl Urol. 2019;8:184–190. doi: 10.21037/tau.2019.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Langer M.D., Silver E.J., Dodson N.A., et al. Fertility desires of adolescent females: decreased desire for children in those identifying as transgender/gender diverse and in depressed adolescents. J Pediatr Adolesc Gynecol. 2020;33:703–707. doi: 10.1016/j.jpag.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]