Abstract
Introduction
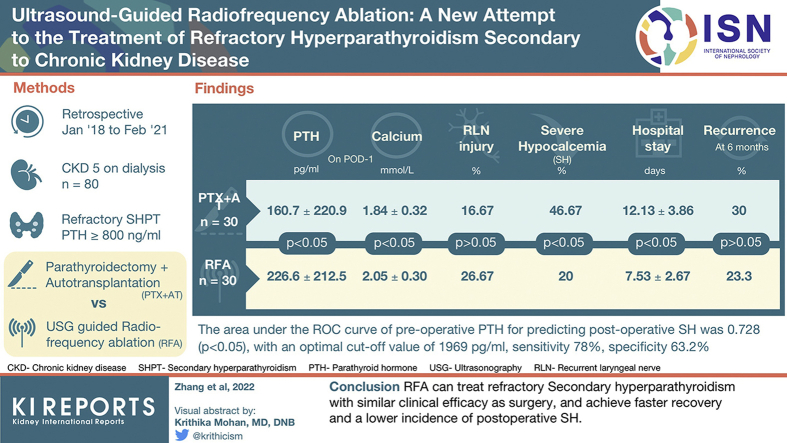
To evaluate clinical application value of radiofrequency ablation (RFA) in refractory hyperparathyroidism secondary to chronic kidney disease (CKD) by comparing the safety and effectiveness of RFA with parathyroidectomy with autotransplantation (PTX + AT).
Methods
A retrospective study was conducted on 80 patients with CKD with refractory hyperparathyroidism who underwent RFA or PTX + AT between January 2018 and February 2021. Serum parathyroid hormone (PTH), calcium, and phosphorus levels, complications, clinical symptoms, visual analogue scale (VAS) scores, hospital stay duration, and postoperative recurrence rate were compared between the 2 groups.
Results
Serum PTH, phosphorous, and calcium levels and the VAS scores improved significantly after RFA and PTX + AT (P < 0.05). Significant differences were observed between the 2 groups in postoperative (day 1 and week 1) levels of serum PTH and postoperative day 1 of serum calcium and phosphorus levels (P < 0.05), with more pronounced reduction after PTX + AT. Although the incidence of recurrent laryngeal nerve (RLN) injury was slightly higher in the RFA group compared with the PTX + AT group (26.67% vs. 16.67%; P > 0.05), RFA markedly decreased the risk of severe hypocalcemia (SH) (20% vs. 46.67%; P < 0.05) and shortened hospital stay (7.53 ± 2.67 days vs. 12.13 ± 3.86 days; P < 0.05). The 6-month recurrence rate was 23.3% (7 of 30) in the RFA group and 30% (9 of 30) in the PTX + AT group (P > 0.05).
Conclusion
RFA can treat refractory secondary hyperparathyroidism (SHPT) with similar clinical efficacy as surgery and achieve faster recovery and a lower incidence of postoperative SH.
Keywords: chronic kidney disease (CKD), parathyroidectomy with autotransplantation (PTX + AT), radiofrequency ablation (RFA), secondary hyperparathyroidism (SHPT), severe hypocalcemia (SH)
Graphical abstract
SHPT is one of the most common serious complications of CKD, especially uremia.1 Early onset SHPT is mainly treated using calcium supplements and drugs that promote calcium absorption. Nevertheless, drugs are ineffective in the advanced stage of the disease, which eventually progresses to refractory SHPT with the continuous increase in PTH levels.2 Guidelines of the Improving Global Outcomes of Kidney Disease recommend PTX for refractory SHPT.3 PTX can significantly improve the quality of life of the patients and reduce the incidence and mortality of cardiovascular events.4, 5, 6 Nevertheless, most patients with CKD have severe complications, such as irreversible cardiovascular calcification and thoracic and skeletal deformities, which increase the risk of anesthesia- and surgery-related injuries. Furthermore, some patients cannot tolerate the surgery.2 Therefore, a less invasive therapeutic intervention with greater efficacy and shorter recovery period is required for patients with refractory SHPT. RFA is a minimally invasive, nonsurgical procedure that is increasingly being used to treat refractory hyperparathyroidism and achieve clinical affirmation.7,8 Nevertheless, no study so far has compared the clinical efficacy and safety of surgery and RFA for the treatment of refractory SHPT. The aim of this study is to evaluate the clinical application of RFA against refractory SHPT in terms of clinical safety and efficacy with that of PTX + AT to provide the basis for selecting and formulating the best treatment plan for refractory SHPT.
Methods
General Clinical Data
This study was approved by the ethical and scientific review board of Fujian Provincial Hospital. The medical records of 80 patients with stage 5 CKD complicated with refractory SHPT who underwent RFA (n = 38) or PTX + AT (n = 42) between January 2018 and February 2021 were reviewed. Lost to follow-up was defined as failure to obtain the complete follow-up data. Patients who followed the inclusion and exclusion criteria were included in the study, and patients lost to follow-up were excluded. A total of 60 patients (75%, 60 of 80) met the inclusion criteria, of which 30 (78.9%, 30 of 38) underwent RFA (RFA group) and 30 (71.4%, 30 of 42) underwent PTX + AT (PTX + AT group). There were 8 patients who had a history of peritoneal dialysis and 52 who underwent hemodialysis.
The inclusion criteria were as follows: (i) CKD stage 5 complicated with refractory SHPT; (ii) history of dialysis; (iii) PTH ≥ 800 ng/ml; (iv) only presence of 4 hyperplastic parathyroid glands as indicated by technetium-99m methoxyisobutylisonitrile and neck ultrasound; and (v) no severe bleeding disorders, cardiac insufficiency, or uncontrollable hypertension.
The exclusion criteria were as follows: (i) abnormal vocal cord movement indicated by laryngoscopy; (ii) abnormal coagulation function tests (prothrombin time > 25 seconds, prothrombin activity < 40%, and platelet count < 100 × 109/l); (iv) hoarseness after the first RFA session; (v) primary hyperparathyroidism; and (vi) presence of ectopic parathyroid glands indicated by technetium-99m methoxyisobutylisonitrile imaging.
The exclusion criteria for RFA were as follows: (i) a single parathyroid gland > 3 cm in diameter or the depth of the nodule from the body surface > 3.5 cm and (ii) refusal to undergo RFA.
The exclusion criteria for surgery were as follows: (i) severe skeletal deformities or cardiovascular events that preclude surgery or anesthesia and (ii) refusal to undergo surgery.
US-Guided RFA
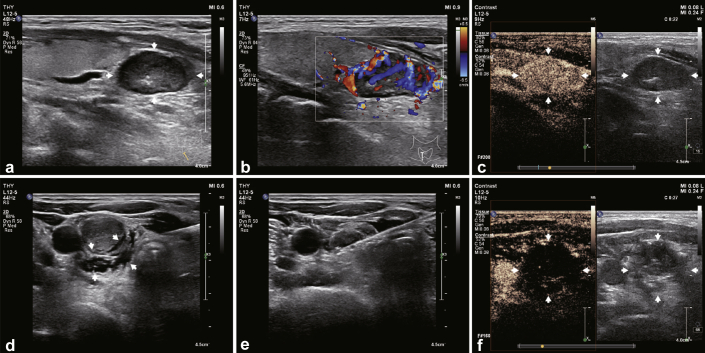
RFA was performed using a Viva RFA system (host mode VRS01, STARMed, South Korea) and a disposable monopolar RF ablation needle electrode (model 18-07s07F; working electrode length 7 mm). Ultrasonography and contrast-enhanced ultrasonography were performed using the Philips IU22 with L12–5 high-frequency line transducer to determine the specific location, number, and size of bilateral parathyroid glands, and an RFA pathway was designed. The patient was placed in a supine position with neck fully exposed, and the skin was disinfected and then covered with a sterilized towel. After anesthetizing locally with 2% lidocaine, 20 ml 0.9% sterile saline solution was injected around the hyperplastic parathyroid under ultrasound guidance to establish a buffering zone of at least 5 mm between the parathyroid gland and adjacent structures to avoid thermal injury to the latter. The RFA needle was inserted into the hyperplastic parathyroid gland from the center of the neck. The ablation power was set at 35 W and started from the deep surface of the parathyroid gland and gradually continued along the shallow side (near the probe). All 4 hyperplastic parathyroid glands were sequentially ablated from the left to the right. Intraoperative bleeding was detected by real-time ultrasound, and the patient was tested for hoarseness. The ablation needle was slowly withdrawn, and the needle track was ablated to stop any bleeding. Finally, color Doppler ultrasonography and contrast-enhanced ultrasonography were performed to confirm that the paraproliferative gland tissue was completely inactivated. All the RFA procedures were performed by the same experienced treatment group. The ablation process of the RFA is illustrated in Figure 1.
Figure 1.
The ablation process of RFA in the treatment of refractory SHPT. RFA, radiofrequency ablation; SHPT, secondary hyperparathyroidism.
PTX + AT Procedure
The patients were placed in a supine position with necks elevated by placing a sandbag under the shoulders and were anesthetized by tracheal intubation. After regular disinfection, a 6- to 8-cm incision was made in the anterior muscles in the middle of the neck to fully expose the thyroid and parathyroid glands. The RLN located in the tracheoesophageal groove was identified and exposed to prevent injury, followed by removal of all parathyroid glands around the thyroid gland and breast bone. After confirming diffuse hyperplasia by pathologic examination, the affected tissues were snap frozen and sliced into 1- to 2-mm3 sections. Approximately 20 to 30 slices (40–50 mg) of the parathyroid tissue were transplanted into the forearm muscles without an arteriovenous fistula for hemodialysis. A negative pressure drainage tube was placed in the neck. After confirming lack of active bleeding, the neck incision was sutured layer by layer. All procedures were completed by the same experienced surgical team.
Laboratory Tests and Follow-Up
The demographic data, primary disease, dialysis history, and preoperative and postoperative clinical symptoms were recorded. Serum PTH, calcium, and phosphorus levels were measured preoperatively and 1 day, 3 days, 1 week, 1 month, 3 months, and 6 months postoperation. SH was defined as the minimum value of serum calcium level lower than 1.875 mmol/l or 7.5 mg/dl, which was selected as the cutoff because the symptoms of hypocalcemia manifest below this threshold.4 The lowest calcium level on the first day postoperation was used to establish hypocalcemia9,10 to avoid the effect of active calcium supplementation given after. In addition, PTH level > 300 pg/ml 6 months after the operation was defined as recurrence.11
Statistical Analysis
The statistical analysis was performed using SPSS 26.0 version (SPSS Inc., Chicago, IL). Continuous data were presented as mean ± SD and categorical variables as numbers or percentages. Student t test was used to compare continuous variables, and categorical variables were compared by χ2 test. The non-normally distributed continuous variables were presented as median and interquartile range and compared using the Mann–Whitney U test. P < 0.05 was considered statistically significant.
Results
Baseline Characteristics of the RFA and PTX + AT Group Patients
The baseline characteristics of each group are summarized in Table 1. The sex and age distribution were similar in both groups (both P > 0.05). There were no significant differences between the RFA and PRX + AT groups in terms of dialysis type, dialysis age, symptoms, and preoperative serum biochemical indicators (PTH, calcium, phosphorus, and alkaline phosphatase) (all P > 0.05).
Table 1.
Baseline characteristics of the RFA and PTX + AT groups
| Characteristics | RFA groups | PTX + AT groups | P |
|---|---|---|---|
| Sex | |||
| Male | 16 | 18 | 0.602 |
| Female | 14 | 12 | |
| Age (yr) | 45.8 ± 13.3 | 49.9 ± 12.0 | 0.215 |
| Dialysis style | |||
| Membrane | 6 | 2 | 0.129 |
| Blood | 24 | 28 | |
| Dialysis age | 8.8 ± 4.4 | 6.8 ± 3.6 | 0.062 |
| Clinical symptoms | |||
| Bone pain | 9 | 7 | 0.559 |
| Skin pruritus | 11 | 5 | 0.081 |
| Muscle weakness | 3 | 3 | 1.001 |
| PTH (pg/ml) | 1718.5 ± 1034.36 | 2036.8 ± 878.9 | 0.207 |
| Serum calcium (mmol/l) | 2.49 ± 0.17 | 2.55 ± 0.18 | 0.143 |
| Serum phosphorus (mmol/l) | 2.47 ± 0.49 | 2.23 ± 0.42 | 0.072 |
| ALP (μg/l) | 386.9 ± 517.9 | 403.6 ± 347.4 | 0.884 |
ALP, alkaline phosphatase; PTH, parathyroid hormone; PTX + AT, parathyroidectomy with autotransplantation; RFA, radiofrequency ablation.
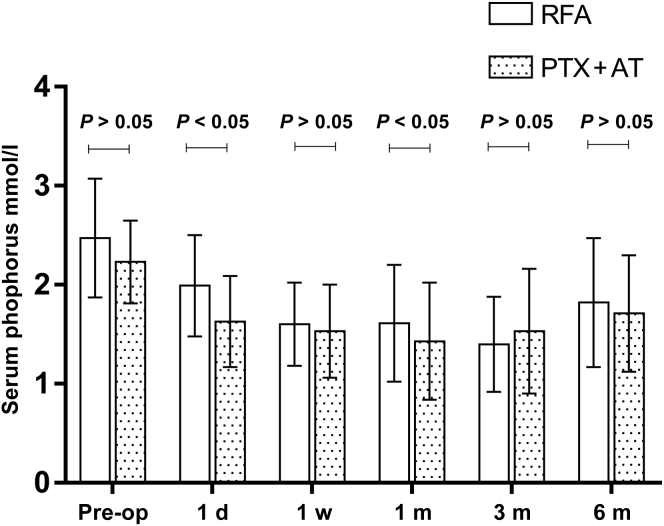
Serum Biochemical Indicators
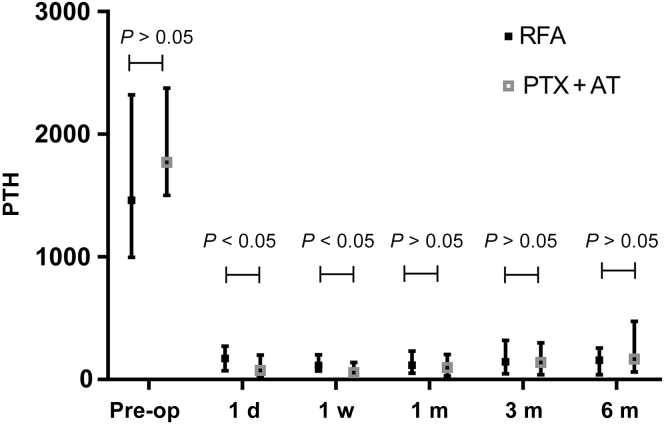
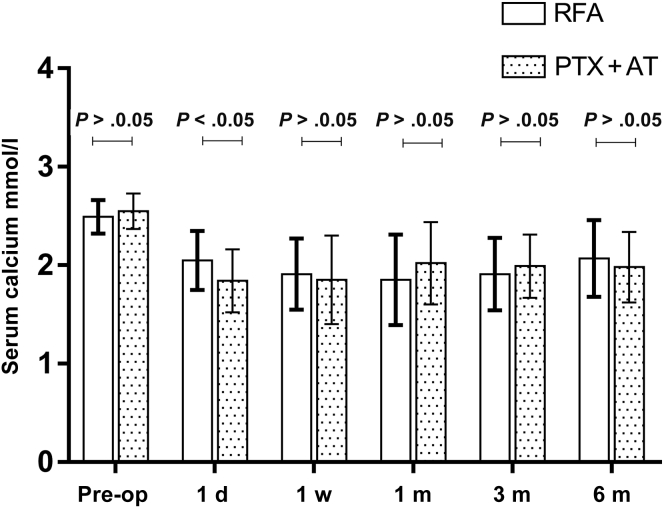
As found in Table 2 and Figure 2, Figure 3, Figure 4 to 4, the postoperative levels of PTH, calcium, and phosphorus were significantly lower compared with the respective preoperative values in both treatment groups (P < 0.05 for all). The changes in PTH, serum calcium, and serum phosphorus were small at each postoperative time point in both groups (P > 0.05). The mean PTH levels decreased linearly on day 1 postoperation in the RFA and PTX + AT groups to 226.6 ± 212.5 pg/ml and 160.7 ± 220.9 pg/ml and dropped further to 184.6 ± 210.1 pg/ml and 122.3 ± 188.7 pg/ml, respectively, on day 7 before stabilizing at the later time points. Thus, the reduction in PTH levels was more pronounced in patients who underwent PTX + AT compared with that in the RFA group (P < 0.05). The mean serum calcium levels in the RFA and PTX + AT groups on day 1 postoperation were 2.05 ± 0.30 mmol/l and 1.84 ± 0.32 mmol/l, respectively. The reduction in serum calcium levels was more pronounced in patients who underwent PTX + AT compared with that in the RFA group (P < 0.05).
Table 2.
Comparison of iPTH, serum calcium, and serum phosphorus levels between the RFA and PTX + AT groups
| Symptoms and signs | Group | Before operation | 1 d | 1 w | 1 m | 3 m | 6 m |
|---|---|---|---|---|---|---|---|
| PTH | RFA | 1827.8 ± 1179.8 | 226.6 ± 212.5a,b | 184.6 ± 210.1a,b | 196.5 ± 229.3a | 260.7 ± 311.8a | 272.1 ± 393.9a |
| PTX + AT | 2036.9 ± 878.9 | 160.7 ± 220.9a | 122.3 ± 188.7a | 254.1 ± 490.7a | 297.7 ± 466.3a | 488.2 ± 979.3a | |
| Serum calcium | RFA | 2.49 ± 0.17 | 2.05 ± 0.30a,b | 1.91 ± 0.36a | 1.85 ± 0.46a | 1.91 ± 0.37a | 2.07 ± 0.39a |
| PTX + AT | 2.55 ± 0.18 | 1.84 ± 0.32a | 1.85 ± 0.45a | 2.02 ± 0.42a | 1.99 ± 0.32a | 1.98 ± 0.36a | |
| Serum phosphorus | RFA | 2.47 ± 0.60 | 1.99 ± 0.51a,b | 1.60 ± 0.42a+ | 1.61 ± 0.59a | 1.40 ± 0.48a | 1.82 ± 0.65a |
| PTX + AT | 2.23 ± 0.42 | 1.63 ± 0.46a | 1.53 ± 0.47a | 1.43 ± 0.59a | 1.53 ± 0.63a | 1.71 ± 0.59a |
d, day; m, month; Pre-op, preoperation; PTH, parathyroid hormone; PTX + AT, parathyroidectomy with autotransplantation; RFA, radiofrequency ablation; w, week.
P < 0.05 compared with those before operation.
P < 0.05 compared with the PTX + AT group.
Figure 2.
PTH levels in the RFA and PTX + AT groups at all time periods. d, day; m, month; Pre-op, preoperation; PTH, parathyroid hormone; PTX + AT, parathyroidectomy with autotransplantation; RFA, radiofrequency ablation; w, week.
Figure 3.
Serum calcium levels in the RFA and PTX + AT groups at all time periods. d, day; m, month; Pre-op, preoperation; PTH, parathyroid hormone; PTX + AT, parathyroidectomy with autotransplantation; RFA, radiofrequency ablation; w, week.
Figure 4.
Serum phosphorus levels in the RFA and PTX + AT groups at all time periods. d, day; m, month; Pre-op, preoperation; PTH, parathyroid hormone; PTX + AT, parathyroidectomy with autotransplantation; RFA, radiofrequency ablation; w, week.
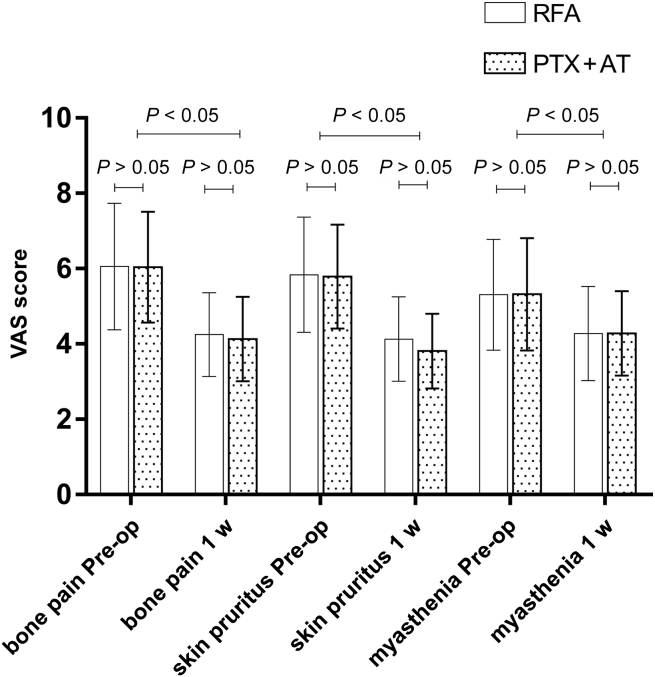
Postoperative Symptoms and Signs
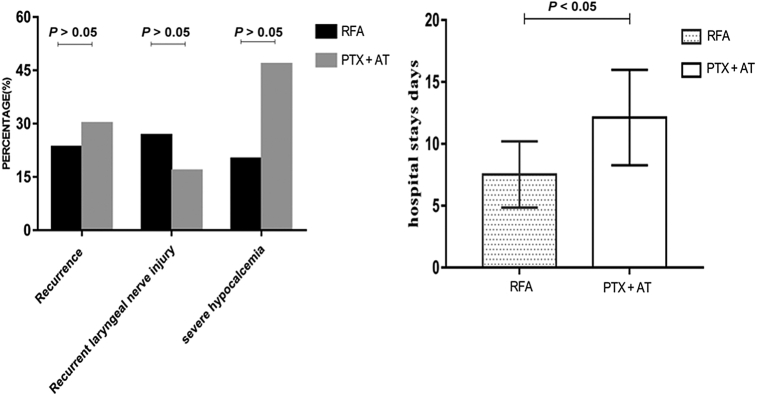
As found in Table 3 and Figure 5, VAS scores, bone pain, muscle weakness, and skin pruritus decreased significantly 1 week after RFA or PTX + AT compared with the respective preoperative scores (P < 0.05); the improvement in VAS scores and other indices were also similar in both groups at this time point (all P > 0.05). The length of hospitalization in the RFA group was 6 to 13 days (7.53 ± 2.67 days) compared with 6 to 20 days (12.13 ± 3.86 days) in the PTX + AT group, and the difference was statistically significant (t = 5.351, P = 0.001).
Table 3.
Comparisons of clinical symptoms and VAS scores between the PTX and PTX + AT groups
| Symptoms and signs | Group | Preoperation | Postoperative 1 w |
|---|---|---|---|
| Bone pain | RFA | 6.06 ± 1.68 | 4.25 ± 1.11a |
| PTX + AT | 6.04 ± 1.47 | 4.13 ± 1.12a | |
| Skin pruritus | RFA | 5.84 ± 1.53 | 4.13 ± 1.12a |
| PTX + AT | 5.79 ± 1.38 | 3.81 ± 0.99a | |
| Muscle weakness | RFA | 5.31 ± 1.47 | 4.28 ± 1.25a |
| PTX + AT | 5.32 ± 1.49 | 4.28 ± 1.21a |
PTX + AT, parathyroidectomy with autotransplantation; RFA, radiofrequency ablation; VAS, visual analogue scale; w, week.
P < 0.05 compared with preoperative.
Figure 5.
Comparison of clinical symptoms VAS scores in the RFA and PTX + AT groups. Pre-op, preoperation; PTX + AT, parathyroidectomy with autotransplantation; RFA, radiofrequency ablation; VAS, visual analogue scale; w, week.
Recurrence and Postoperative Complications
As found in Figure 6, on the basis of PTH > 300 pg/ml at 6 months postoperation as the cutoff for recurrence, there were 7 recurrent cases in the RFA group (23.33%, 7 of 30) and 9 in the PTX + AT group (30%, 9 of 30). There was no significant difference between the 2 groups (χ = 0.801, P = 0.371).
Figure 6.
Comparison of recurrence, recurrent laryngeal nerve injury, severe hypocalcemia, recurrence, and hospital stay duration in the RFA and PTX + AT groups. PTX + AT, parathyroidectomy with autotransplantation; RFA, radiofrequency ablation.
The incidence of postoperative RLN injury was similar in both groups (P > 0.05). Furthermore, 8 patients in the RFA group (26.67%, 8 of 30) had RLN injury and recovered within 2 to 30 days. There were also 5 cases (16.67%, 5 of 30) with RLN injury in the PTX + AT group, and recovery occurred within 7 to 30 days. Nevertheless, only 6 patients who underwent RFA (20%, 6 of 30) had SH compared with 14 patients in the PTX + AT group (46.67%, 14 of 30) on day 1 postoperation. The incidence of SH was significantly different in the 2 groups (P < 0.05).
Discussion
In this study, the preoperative and postoperative changes in the serum biochemical indicators between the RFA and PTX + AT groups were compared. The PTH levels in both groups decreased linearly at 1-week postoperation relative to the preoperative levels and increased slightly before gradually stabilizing 3 months after. Significant differences were observed between the 2 groups in early postoperative (day 1 and week 1) levels of serum PTH, but not at 1, 3, and 6 months postoperation. Furthermore, postoperative reduction in the PTH levels was more pronounced in the PTX + AT group. The reasons may be as follows: (i) when the hyperplastic parathyroid gland is removed or ablated, PTH secretion is rebalanced and (ii) the effect of radiofrequency thermal ablation on the tissue is diffuses. In addition to directly causing tumor cell death, thermal stimulation can also exert long-term indirect effects on tumor cells.12,13 Nikfarjam et al.14,15 found that the peak hyperthermic ablation of tumor cells occurred 4 to 5 days after. Nada-diaphorase staining further revealed that the indirect damage was not related to the initial thermal effect. Therefore, it takes a period of time for some parathyroid cells to completely lose their function until they undergo necrosis and apoptosis. Furthermore, surgical excision of the parathyroid gland may be more thorough compared with RFA, which only ablates part of the parathyroid that is visible on ultrasound. The mean serum phosphorus and calcium levels declined to 1 week after treatment in both groups and remained stable for 1 month, before increasing steadily and peaking at 6 months. The decrease in serum calcium level was more significant in the PTX + AT group compared with that in the RFA group, and the incidence of postoperative SH was significantly higher in the PTX + AT group 1-day postoperation (P < 0.05). There could be several plausible reasons for this observation. The rapid decline in PTH levels in the PTX+AT group, along with drastic changes in the internal environment and rapid calcium intake to the “hungry” bones, led to a decrease in serum calcium levels and increased the risk of SH.16,17 Compared with PTX + AT, RFA can moderately and effectively improve the levels of serum calcium, serum phosphorus, and PTH and reduce the incidence of SH.
In addition to the biochemical indices, the VAS scores of the clinical symptoms declined considerably after either intervention and were similar in both groups (P > 0.05). Nevertheless, the duration of hospital stay was significantly shorter in the RFA group compared with the PTX + AT group, indicating faster recovery with the former. Furthermore, the incidence of RLN injury was slightly higher in patients who underwent RFA (P > 0.05), whereas SH was significantly more prevalent in the PTX + AT group (P < 0.05). The recurrence rate 6 months after operation was 23.3% (7 of 30) in the RFA group and 30% (9 of 30) in the PTX + AT group (P > 0.05). The recurrence rate of SHPT was also similar in both groups (P > 0.05). Taken together, RFA is associated with less injury, faster recovery, fewer complications, and a significantly lower risk of postoperative SH compared with PTX + AT, while achieving similar clinical efficacy, improvement in clinical symptoms, and long-term recurrence rate.
In this study, the clinical safety and efficacy of ultrasound-guided percutaneous RFA and PTX + AT in the treatment of refractory SHPT to chronic renal failure were compared to verify the clinical value of RFA. Nevertheless, there are several limitations in our study that ought to be considered. In this retrospective study, because of the small sample size and the limitations of clinical practice, 5 cases had PTH levels higher than 100 ng/l on the first day after surgery (approximately 300 ng/l), most likely because of incomplete surgical resection. Therefore, the PTH levels on the first day after PTX were slightly higher than those in published articles. Subsequent studies with larger sample size and longer follow-up duration will have to be conducted to validate our findings.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The study was supported by Medical Elite Cultivation Program of Fujian, People’s Republic of China (number 2018-ZQN-12).
Footnotes
STROBE Statement
Supplementary Material
STROBE Statement (PDF)
References
- 1.Brück K., Stel V.S., Gambaro G., et al. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27:2135–2147. doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brück K., Jager K.J., Dounousi E., et al. Methodology used in studies reporting chronic kidney disease prevalence: a systematic literature review. Nephrol Dial Transplant. 2016;31:680. doi: 10.1093/ndt/gfw024. [DOI] [PubMed] [Google Scholar]
- 3.Apetrii M., Goldsmith D., Nistor I., et al. Impact of surgical parathyroidectomy on chronic kidney disease-mineral and bone disorder (CKD-MBD)—a systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moldovan D., Racasan S., Kacso I.M., et al. Survival after parathyroidectomy in chronic hemodialysis patients with severe secondary hyperparathyroidism. Int Urol Nephrol. 2015;47:1871–1877. doi: 10.1007/s11255-015-1106-x. [DOI] [PubMed] [Google Scholar]
- 5.Ma T.L., Hung P.H., Jong I.C., et al. Parathyroidectomy is associated with reduced mortality in hemodialysis patients with secondary hyperparathyroidism. BioMed Res Int. 2015;2015:639587. doi: 10.1155/2015/639587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwamoto N., Sato N., Nishida M., et al. Total parathyroidectomy improves survival of hemodialysis patients with secondary hyperparathyroidism. J Nephrol. 2012;25:755–763. doi: 10.5301/jn.5000056. [DOI] [PubMed] [Google Scholar]
- 7.Peng C., Zhang Z., Liu J., et al. Efficacy and safety of ultrasound-guided radiofrequency ablation of hyperplastic parathyroid gland for secondary hyperparathyroidism associated with chronic kidney disease. Head Neck. 2017;39:564–571. doi: 10.1002/hed.24657. [DOI] [PubMed] [Google Scholar]
- 8.Zeng Z., Peng C.Z., Liu J.B., et al. Efficacy of ultrasound-guided radiofrequency ablation of parathyroid hyperplasia: single session vs. two-session for effect on hypocalcemia. Sci Rep. 2020;10:6206. doi: 10.1038/s41598-020-63299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo C.Y., Holland P.A., Jackson B.F., et al. Immediate changes in biochemical markers of bone turnover and circulating interleukin-6 after parathyroidectomy for primary hyperparathyroidism. Eur J Endocrinol. 2000;142:451–459. doi: 10.1530/eje.0.1420451. [DOI] [PubMed] [Google Scholar]
- 10.Grey A., Mitnick M.A., Shapses S., et al. Circulating levels of interleukin-6 and tumor necrosis factor-alpha are elevated in primary hyperparathyroidism and correlate with markers of bone resorption—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3450–3454. doi: 10.1210/jcem.81.10.8855783. [DOI] [PubMed] [Google Scholar]
- 11.Tsukamoto Y. CKD-MBD (chronic kidney disease-mineral and bone disorder). KDIGO CKD-MBD Clinical practice guideline. Article in Japanese. Clin Calcium. 2010;20:1021–1027. [PubMed] [Google Scholar]
- 12.Hildebrandt B., Wust P., Ahlers O., et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 13.Wiersinga W.J., Jansen M.C., Straatsburg I.H., et al. Lesion progression with time and the effect of vascular occlusion following radiofrequency ablation of the liver. Br J Surg. 2003;90:306–312. doi: 10.1002/bjs.4040. [DOI] [PubMed] [Google Scholar]
- 14.Nikfarjam M., Muralidharan V., Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res. 2005;127:208–223. doi: 10.1016/j.jss.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Nikfarjam M., Malcontenti-Wilson C., Christophi C. Focal hyperthermia produces progressive tumor necrosis independent of the initial thermal effects. J Gastrointest Surg. 2005;9:410–417. doi: 10.1016/j.gassur.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Wang M., Chen B., Zou X., et al. A nomogram to predict hungry bone syndrome after parathyroidectomy in patients with secondary hyperparathyroidism. J Surg Res. 2020;255:33–41. doi: 10.1016/j.jss.2020.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Witteveen J.E., van Thiel S., Romijn J.A., Hamdy N.A. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol. 2013;168:R45–R53. doi: 10.1530/EJE-12-0528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.