Abstract
The susceptibilities to three antimicrobials of 195 Helicobacter pylori strains isolated from Mexican patients is reported; 80% of the strains were resistant to metronidazole, 24% were resistant to clarithromycin, and 18% presented a transient resistance to amoxicillin. Resistance to two or more antimicrobials increased significantly from 1995 to 1997.
Eradication of Helicobacter pylori infection is the most effective means to cure peptic ulcer diseases and prevent possible recurrent episodes. The most common treatment regimens include a proton pump inhibitor plus two of the following antimicrobial agents: amoxicillin; clarithromycin; or metronidazole or tetracycline. H. pylori resistance to metronidazole and clarithromycin has increased worldwide (5, 15). Resistance to amoxicillin has been reported (10); however, this phenomenon is rare and unstable (2).
Indications for treatment have been extended (3), and with a more extended use of antibiotics, the probability of selection of resistant strains increases.
We report here our findings on antimicrobial susceptibility patterns among H. pylori strains isolated from children and adults during a 3-year period in Mexico.
Strains.
One hundred ninety-five H. pylori strains isolated during the period from 1995 to 1997 were studied: 51 strains were isolated from children (mean age, 11 ± 4 years) with nonulcer dyspepsia (23 males and 28 females), whereas 144 strains were isolated from adults (mean age, 52 ± 17 years), 48 with nonulcer dyspepsia and 96 with peptic ulcers (93 males and 51 females). All patients were seen at the Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, in Mexico City, Mexico, and had no history of eradication treatment. Organisms were recovered from antrum and corpus gastric biopsies and identified by morphology and biochemical tests. Organisms were stored at −70°C in 20% glycerol–brain hearth infusion broth until tested.
Antimicrobial susceptibility assay.
Susceptibility to amoxicillin, clarithromycin, and metronidazole was determined by the Epsilometer test (E test; AB Biodisk, Solna, Sweden) (4). H. pylori isolates were grown for 2 days on Columbia blood agar plates; growth was suspended in Columbia broth to achieve a McFarland opacity of 3 and spread on blood agar plates. The antimicrobial drug strip was placed on the plate and incubated at 37°C for 72 h in an incubator with a 9% CO2 atmosphere (Nuare, Plymouth, Minn.). The MIC was defined by the point of intersection of the inhibitory zone with the strip (4). To confirm results, each strain was tested at least twice. The cutoff values for susceptibility were 8 μg/ml for metronidazole, 4 μg/ml for amoxicillin, and 2 μg/ml for clarithromycin (1, 10).
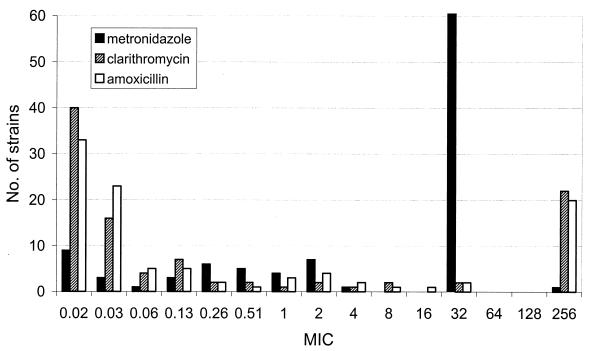
The susceptibility pattern obtained for the H. pylori isolates is described in Table 1. The rate of resistance to metronidazole was high (76.9%); resistance to clarithromycin was moderate (24%). Resistance to amoxicillin was observed in 18.5% of the isolates; however, resistance disappeared after a single passage or after freezing the strains. In contrast, resistance to clarithromycin and metronidazole remained unchanged after passage or freezing. Of interest, 30.7% of isolates were multiresistant, 17.9% to metronidazole and clarithromycin and 12.8% to metronidazole and amoxicillin; 8.7% were resistant to all three antibiotics tested. The distribution of MICs of the three antibiotics for the 110 H. pylori strains isolated during 1997 is presented in Fig. 1.
TABLE 1.
Susceptibility patterns of H. pylori strains isolated from Mexican children and adults
| Antibiotic(s) | No. (%) of resistant isolates
|
||
|---|---|---|---|
| Children (n = 51) | Adults (n = 144) | Totala(n = 195) | |
| Metronidazole | 40 (78.4) | 110 (76.3) | 150 (76.9) |
| Clarithromycin | 11 (21.6) | 36 (25) | 47 (24) |
| Amoxicillinb | 8 (15.7) | 28 (19.4) | 36 (18.5) |
| Metronidazole + clarithromycin | 9 (17.6) | 26 (18) | 35 (17.9) |
| Metronidazole + amoxicillin | 6 (11.8) | 19 (13.2) | 25 (12.8) |
| Metronidazole + clarithromycin + amoxicillin | 2 (4) | 15 (10.4) | 17 (8.7) |
Differences were not significant for any comparison between children and adults.
Resistance to amoxicillin was transient in all cases; it was lost after passage or freezing.
FIG. 1.
Distribution of metronidazole, clarithromycin, and amoxicillin MICs for 110 H. pylori strains isolated during 1997.
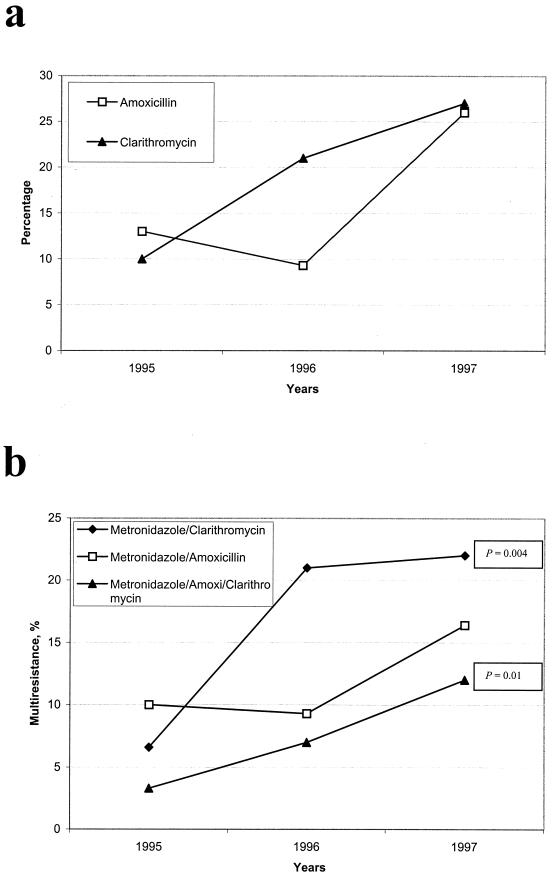
The yearly rate of resistance during the period from 1995 to 1997 was studied (Fig. 2). Resistance to metronidazole was similar throughout the 3 years; in contrast, resistance to amoxicillin and clarithromycin increased significantly during the period of study (Fig. 2a). Resistance to clarithromycin increased from 10% in 1995 to 27% in 1997, whereas resistance to amoxicillin increased from 13% in 1995 to 26% in 1997. Multidrug resistance also increased during this period (Fig. 2b); the rising trend was significant for resistance to metronidazole and clarithromycin (χ2 for linear trend = 8.073; P = 0.004) as well as for resistance to all three antibiotics tested (χ2 = 5.94; P = 0.01). There was no difference in the rate of resistance to any of the three antibiotics tested when we compared children versus adults, men versus women, or ulcer disease isolates versus nonulcer dyspepsia isolates.
FIG. 2.
Prevalence of H. pylori strains resistant to amoxicillin, clarithromycin, and metronidazole isolated in Mexico City during the period from 1995 to 1997. The numbers of strains studied were 30 in 1995, 43 in 1996, and 110 in 1997. (a) Resistance to amoxicillin or to clarithromycin; (b) resistance to two or three of the antibiotics tested. The trend was significant for metronidazole and clarithromycin (P = 0.004) and for the three antibiotics (P = 0.01).
In this study we report the susceptibility of H. pylori strains from Mexico, a country with a high prevalence of infection (13), to the most commonly used antibiotics. A high rate of resistance to metronidazole was found consistently throughout the 3 years of the study; these results are similar to frequencies reported for other developing countries (9). In Mexico, metronidazole has commonly been used to treat diarrheal diseases for many years (12); this is probably the cause of the high rate of resistance to this antibiotic. We could not demonstrate higher metronidazole resistance in females, despite the fact that gender has been suggested to be a risk factor for resistance to metronidazole, probably because of its use in the treatment of gynecologic infections (14). The value of the E test for testing susceptibility to metronidazole has been questioned recently (11); however, when assay conditions are carefully controlled, results are comparable to those of agar dilution and disk diffusion (8, 14). We found moderate resistance to clarithromycin that increased yearly. Previous reports have suggested that the rate of resistance to clarithromycin is higher in strains isolated from children than in strains from adults (7); however, we found no difference between the two groups.
Recent reports have documented the existence of strains with resistance to amoxicillin, although in most cases this resistance was demonstrated to be transitory (2). In isolates from our community, a moderate prevalence of amoxicillin resistance was observed; nevertheless, this was unstable in all cases.
Few studies have reported the rate of multidrug resistance in H. pylori isolates. In this study, we document resistance to at least two antimicrobial agents in 30% of the isolates. A high rate of resistance to both metronidazole and clarithromycin (18%) was found; resistance to either of these two antimicrobials is a risk factor for treatment failure (5). We are concerned that resistance to both clarithromycin and amoxicillin is continuously increasing in our community; in 1997 the rate of resistance to either drug was over 25%, suggesting that eventually these antimicrobials will lose their efficacy against H. pylori infections. Resistance to all three antimicrobial drugs tested rose from 4% in 1995 to 12% in 1997. These results stress the need for alternative therapies. Clinicians in our community at present are recommending the use of furazolidone and tetracycline as alternatives; the use of these antibiotics in combination with ranitidine bismuth citrate has been shown to be effective and inexpensive in other communities (6).
In conclusion, we documented a high rate of resistance to metronidazole and a rising resistance to both clarithromycin and amoxicillin in our community. It is of concern to us that multidrug resistance to these antimicrobial agents is also rising significantly.
Acknowledgments
This project was supported by CONACYT (grant 28040 M), BykGulden-México, and the Coordinación de Investigación, Instituto Mexicano del Seguro Social, México.
REFERENCES
- 1.DeLoney C R, Schiller N L. Characterization of an in vitro-selected amoxicillin-resistant strain of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:3368–3373. doi: 10.1128/aac.44.12.3368-3373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dore M P, Realdi G, Mura I, Ostato M S, Graham D Y, Sepulveda A R. Amoxicillin-resistant strains of H. pylori undergo reversible loss of resistance after storage. Gut. 1997;41(Suppl. 1):A8. [Google Scholar]
- 3.The European Helicobacter pylori Study Group. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. Gut. 1997;41:8–13. doi: 10.1136/gut.41.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glupczynski Y, Labbe M, Willy H, Yourassowsky E C. Evaluation of the E test for quantitative antimicrobial susceptibility testing of Helicobacter pylori. J Clin Microbiol. 1991;29:2072–2075. doi: 10.1128/jcm.29.9.2072-2075.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham D Y. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 6.Graham D Y, Osato M S, Hoffman J, Opekun A R, Anderson S Y, El-Zimaity H M T. Furazolidone combination therapies for Helicobacter pylori infection in the United States. Aliment Pharmacol Ther. 2000;14:211–215. doi: 10.1046/j.1365-2036.2000.00640.x. [DOI] [PubMed] [Google Scholar]
- 7.Jesch I, Kindermann A, Krauss-Etschmann S, Autenrieth I, Lehn N, Koletzko S. Prevalence of clarithromycin-resistant H. pylori-strains in children: effect of therapy. Gut. 1999;45(Suppl. III):A93. [Google Scholar]
- 8.Kalach N, Bergeret M, Benhamou P H, Dupont C, Raymond J. High levels of resistance to metronidazole and clarithromycin in Helicobacter pylori strains in children. J Clin Microbiol. 2001;39:394–397. doi: 10.1128/JCM.39.1.394-397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mégraud F. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori. Gastroenterology. 1998;115:1278–1282. doi: 10.1016/s0016-5085(98)70101-5. [DOI] [PubMed] [Google Scholar]
- 10.Mendonca S, Ecclissato C, Sartori M S, Ortiz A P, Aparecida R, Pedrazzoli M D. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxicillin, tetracycline, and furazolidone in Brazil. Helicobacter. 2000;5:79–83. doi: 10.1046/j.1523-5378.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 11.Piccolomini R, Bonaventura G D, Catamo G, Carbone F, Neri M. Comparative evaluation of the E test, agar dilution, and broth microdilution for testing susceptibilities of Helicobacter pylori strains to 20 antimicrobial agents. Ann Intern Med. 1997;109:11–17. doi: 10.1128/jcm.35.7.1842-1846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres J, Gonzalez-Arroyo S, Pérez R, Muñoz O. Inappropriate treatment in children with bloody diarrhea: clinical and microbiological studies. Arch Med Res. 1995;26:23–29. [PubMed] [Google Scholar]
- 13.Torres J, Leal-Herrera Y, Pérez-Pérez G I, Gómez A, Camorlinga-Ponce M, Cedillo-Ribera R, Tapia-Conyer R, Muñoz O. A community-based seroepidemiological study of Helicobacter pylori infection in Mexico. J Infect Dis. 1998;178:1089–1094. doi: 10.1086/515663. [DOI] [PubMed] [Google Scholar]
- 14.van der Wouden E J, Thijs J C, van Zwet A A, Kleibeuker J H. Nitroimidazole resistance in Helicobacter pylori. Aliment Pharmacol Ther. 2000;14:7–14. doi: 10.1046/j.1365-2036.2000.00675.x. [DOI] [PubMed] [Google Scholar]
- 15.van der Wouden E J, van Zwet A A, Thijs J C, Vosmaer G D C, Kleibeuker J H. Rapid increase in the prevalence of metronidazole-resistant Helicobacter pylori in The Netherlands. Emerg Infect Dis. 1997;3:385–389. doi: 10.3201/eid0303.970320. [DOI] [PMC free article] [PubMed] [Google Scholar]