Abstract
We report on 318 patients with acute leukemia, receiving donor lymphocyte infusion (DLI) in complete hematologic remission (CHR) after allogeneic stem cell transplantation (alloSCT). DLI were applied preemptively (preDLI) for minimal residual disease (MRD, n = 23) or mixed chimerism (MC, n = 169), or as prophylaxis in high-risk patients with complete chimerism and molecular remission (proDLI, n = 126). Median interval from alloSCT to DLI1 was 176 days, median follow-up was 7.0 years. Five-year cumulative relapse incidence (CRI), non-relapse mortality (NRM), leukemia-free and overall survival (LFS/OS) of the entire cohort were 29.1%, 12.7%, 58.2%, and 64.3%. Cumulative incidences of acute graft-versus-host disease (aGvHD) grade II–IV°/chronic GvHD were 11.9%/31%. Nineteen patients (6%) died from DLI-induced GvHD. Age ≥60 years (p = 0.046), advanced stage at transplantation (p = 0.003), shorter interval from transplantation (p = 0.018), and prior aGvHD ≥II° (p = 0.036) were risk factors for DLI-induced GvHD. GvHD did not influence CRI, but was associated with NRM and lower LFS/OS. Efficacy of preDLI was demonstrated by decreasing MRD/increasing blood counts in 71%, and increasing chimerism in 70%. Five-year OS after preDLI for MRD/MC was 51%/68% among responders, and 37% among non-responders. The study describes response and outcome of DLI in CHR and helps to identify candidates without increased risk of severe GvHD.
Subject terms: Cancer immunotherapy, Immunotherapy, Cancer
Introduction
The graft-versus-leukemia (GvL) effect is the therapeutic cornerstone of allogeneic stem cell transplantation (alloSCT) in acute leukemia (AL) [1]. The infusion of donor lymphocytes (DLI) to patients with established donor chimerism was initially applied in patients with hematological relapse to reinforce GvL reaction [2, 3]. However, even in chronic myelogenous leukemia, results depended on the status of the disease at time of DLI, with patients in molecular relapse showing superior results to those in hematological relapse or blast crisis [4]. Furthermore, DLI was of limited value in the treatment of overt hematological relapse of AL [1, 5, 6]. These experiences prompted clinicians to exploit the GvL reaction in a less proliferative stage, i.e., by giving DLI to patients in complete hematological remission (CHR). These patients were either at high risk of relapse because of T-cell depletion (TCD) of the graft, unfavorable genetics of the leukemia, or advanced disease at SCT; this approach has been referred to as adjuvant or prophylactic DLI (proDLI). Similarly, DLI was given to patients who showed early signs of relapse such as persisting minimal residual disease (MRD), reappearance of molecular markers of the leukemia, or mixed chimerism (MC), which has been referred to as preemptive DLI (preDLI) [7, 8]. Data from various studies suggest that DLI in CHR may have a role in the prevention of AL relapse; however, no systematic analysis of this strategy is available so far, neither concerning the optimal schedule, nor with respect to safety and clinical efficacy [9]. Hence, the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT) performed a registry-based survey on 318 patients with AL, who received DLI in CHR after alloSCT.
Patients and methods
Patients were selected from the EBMT registry. The EBMT is a non-profit, scientific society representing >600 transplant centers that are required to report all consecutive stem cell transplantations including annual follow-up. Data are managed in a central database with internet access. Annual audits are performed to verify data accuracy. Patients provide informed consent authorizing the use of their personal information for research purposes before transplantation.
The study was approved by the general assembly of the ALWP. Eligibility criteria were: (1) age ≥18 years, (2) alloSCT from either a matched sibling donor (MSD) or matched unrelated donor (MUD), (3) documented CHR post transplant, (4) ≥1 DLI applied in CHR, i.e., before date of leukemia relapse or last follow-up (LFU), and (5) available information on the reason for application of DLI. Patients receiving any additional antileukemic treatment between SCT and date of relapse or LFU, such as tyrosine kinase inhibitors (TKI), hypomethylating agents (HMA), or conventional chemotherapy, were excluded, as were patients who had received their first DLI after the date of documented relapse, i.e., in a therapeutic setting. A specific questionnaire was distributed among contributing centers to collect information on DLI and graft-versus-host disease (GvHD) post DLI.
Definitions
PreDLI and proDLI were defined as described [7]. The preDLI cohort could include patients reported to have MC in addition to MRD. Cytogenetics [10, 11], GvHD [12, 13], CHR before SCT [14], relapse, and intensity of conditioning [15, 16] were defined as published. After SCT, complete reconstitution of hematopoiesis was not required for the diagnosis of CHR. Full donor chimerism was defined by the absence of any detectable recipient signal in blood or bone marrow, as indicated by the respective center report. Considering the genetic heterogeneity of AL, MRD measurement was performed according to local standards, using cytogenetics, molecular genetics, or flow cytometry [7, 17, 18]. Following relapse, all deaths were regarded as disease-related, whereas non-relapse mortality (NRM) was defined as death without evidence of relapse or progression of the leukemia. Overall survival (OS) was defined as interval from date of first DLI (DLI1) to date of LFU or date of death, regardless of cause. Leukemia-free survival (LFS) was calculated between the date of DLI1 and relapse, death, or LFU. Response after preemptive DLI was defined by increasing donor chimerism, decreasing MRD load, or improvement of peripheral blood counts, as indicated by the reporting centers.
Statistics
Outcome variables of interest were response, cumulative relapse incidence (CRI), NRM, acute and chronic GvHD (aGvHD and cGvHD), and OS/LFS. Probabilities of OS and LFS were estimated by the Kaplan–Meier method [19]. Cumulative incidence functions were used to estimate RI and NRM in a competing risk setting. Death and relapse were considered as competing events for GvHD. Results were expressed as the hazard ratio (HR) with a 95% confidence interval (95% CI). All tests were two-sided with the type I error rate fixed at 0.05. For a risk factor analysis of GvHD after DLI, we selected patients free of immunosuppressive treatment and without acute GvHD at DLI. All factors associated with GvHD in the univariate analysis with a p value <0.20 or considered as potentially relevant were included in the multivariate model. Then a backward stepwise selection procedure was used with a cutoff significance level of 0.05 for deleting factors. A separate analysis was performed for patients who received DLI as prophylaxis or for MC. All tests were two-sided. The type I error rate was fixed at 0.05 for determination of factors associated with time-to-event outcomes. R statistical software version 4.0.3 (R Core Team (2020) was used (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org).
Results
Patients
Three-hundred and eighteen patients suffering from acute myeloid leukemia (AML, 78%) or acute lymphoblastic leukemia (ALL, 22%) with a median age of 47.5 years (range: 18.2–70.6, 49 were older than 60) were identified. They had received DLI in CHR for MC (n = 169, 53%), persisting or recurrent MRD (n = 23, 7%), or as prophylaxis (n = 126, 40%). From the latter subgroup, 89 patients had been reported earlier in another context [20] and were updated for the present analysis. Disease status at alloSCT was complete remission in 83% (first CR [CR1], 69%, second CR [CR2], 14%) and advanced disease in 17%. Before alloSCT, 58% had received in vivo TCD, 19% ex vivo TCD, 7% in vivo plus ex vivo, and 16% no TCD. Donors were MSD (64%) or MUD (36%). Further details are provided in Table 1.
Table 1.
Characteristics of 318 patients receiving DLI in complete hematologic remission after allogeneic stem cell transplantation for acute leukemia.
| Variable | Entire cohort | PreDLI for mixed chimerism | PreDLI for MRD/molecular relapse | ProDLI | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number | n (% of the entire cohort) | 318 (100%) | 169 (53.1%) | 23 (7.2%) | 126 (39.6%) | ||||
| Follow-up after DLI (months) | Median [IQR] | 84.0 [49.1–110.9] | 81.1 (43.6–106.7) | 72.7 (46.3–90.6) | 93.8 (58.0–130.1) | ||||
| Patient age (years) | Median (min–max) [IQR] | 47.5 (18.2–70.6) [37.5–56.3] | 49.8 (18.5–69) [39.8–58.1] | 37.8 (20.5–60.1) [26.5–48.3] | 46.1 (18.2–70.6) [37.6–54.3] | ||||
| Year of alloSCT | Median (range) | 2007 (2001–2010) | 2007 (2001–2010) | 2007 (2001–2010) | 2005 (2001–2010) | ||||
| Patient sex | Male | 178 (56%) | 98 (58%) | 13 (56.5%) | 67 (53.2%) | ||||
| Female | 140 (44%) | 71 (42%) | 10 (43.5%) | 59 (46.8%) | |||||
| Diagnosis | AML | 249 (78.3%) | 137 (81.1%) | 16 (69.6%) | 96 (76.2%) | ||||
| ALL | 69 (21.7%) | 32 (18.9%) | 7 (30.4%) | 30 (23.8%) | |||||
| AML: cytogenetic subgroups [11] | Favorable | 18 (7.8%) | 10 (8.0%) | 1 (6.7%) | 7 (7.6%) | ||||
| Intermediate | 173 (74.6%) | 99 (79.2%) | 7 (46.7%) | 67 (72.8%) | |||||
| Adverse | 41 (17.7%) | 16 (12.8%) | 7 (46.7%) | 18 (19.6%) | |||||
| Missing | 17 | 12 | 1 | 4 | |||||
| ALL: subtypes | Philadelphia negative B ALL | 30 (52.6%) | 14 (56.0%) | 2 (28.6%) | 14 (56.0%) | ||||
| Philadelphia positive B ALL | 19 (33.3%) | 8 (32.0%) | 5 (71.4%) | 6 (24.0%) | |||||
| T ALL | 8 (14%) | 3 (12.0%) | 0 | 5 (20.0%) | |||||
| Missing | 12 | 7 | 0 | 5 | |||||
| Time diagnosis to alloSCT (months) | Median (min–max) [IQR] | 5.5 (1–185.1) [4.2–9.7] | 5.6 (1.4–88.2) [4.4–10.1] | 5.7 (1–64.7) [3.5–8.5] | 5.1 (1.4–185.1) [4–8.6] | ||||
| Donor | Matched sibling donor | 203 (63.8%) | 113 (66.9%) | 16 (69.6%) | 74 (58.7%) | ||||
| Unrelated donor | 115 (36.2%) | 56 (33.1%) | 7 (30.4%) | 52 (41.3%) | |||||
| Donor sex | Male | 205 (64.9%) | 108 (63.9%) | 15 (65.2%) | 82 (66.1%) | ||||
| Female | 111 (35.1%) | 61 (36.1%) | 8 (34.8%) | 42 (33.9%) | |||||
| Missing | 2 | 0 | 0 | 2 | |||||
| Female donor for male patient | Other | 263 (83.2%) | 140 (82.8%) | 18 (78.3%) | 105 (84.7%) | ||||
| Female to male | 53 (16.8%) | 29 (17.2%) | 5 (21.7%) | 19 (15.3%) | |||||
| Missing | 2 | 0 | 0 | 2 | |||||
| Myeloablative | Reduced | Myeloablative | Reduced | Myeloablative | Reduced | Myeloablative | Reduced | ||
| Conditioning | Chemotherapy based | 56 (33.7%) | 107 (70.4%) | 25 (33.8%) | 82 (86.3%) | 5 (35.7%) | 6 (66.7%) | 26 (33.3%) | 19 (39.6%) |
| TBI based | 110 (66.3%) | 45 (29.6%) | 49 (66.2%) | 13 (13.7%) | 9 (64.3%) | 3 (33.3%) | 52 (66.7%) | 29 (60.4%) | |
| Status at alloSCT | CR1 | 219 (69.3%) | 125 (74%) | 11 (47.8%) | 83 (66.9%) | ||||
| CR2+ | 45 (14.2%) | 29 (17.2%) | 4 (17.4%) | 12 (9.7%) | |||||
| Advanced | 52 (16.5%) | 15 (8.9%) | 8 (34.8%) | 29 (23.4%) | |||||
| Missing | 2 | 0 | 0 | 2 | |||||
| Stem cell source | Bone marrow | 48 (15.1%) | 19 (11.2%) | 4 (17.4%) | 25 (19.8%) | ||||
| Peripheral blood | 270 (84.9%) | 150 (88.8%) | 19 (82.6%) | 101 (80.2%) | |||||
| T-cell depletion | No | 51 (16.2%) | 31 (18.5%) | 11 (47.8%) | 9 (7.3%) | ||||
| In vivo TCD | 182 (57.8%) | 104 (61.9%) | 11 (47.8%) | 67 (54%) | |||||
| Ex vivo TCD | 61 (19.4%) | 21 (12.5%) | 1 (4.3%) | 39 (31.5%) | |||||
| Both | 21 (6.7%) | 12 (7.1%) | 0 (0%) | 9 (7.3%) | |||||
| Missing | 3 | 1 | 0 | 2 | |||||
| History of graft-versus-host disease after alloSCT (before DLI) | |||||||||
| Acute GVHD grade II–IV | No | 265 (83.9%) | 137 (81.5%) | 17 (73.9%) | 111 (88.8%) | ||||
| Yes | 51 (16.1%) | 31 (18.5%) | 6 (26.1%) | 14 (11.2%) | |||||
| Missing | 2 | 1 | 0 | 1 | |||||
| Chronic GVHD | No | 285 (90.2%) | 145 (86.3%) | 19 (82.6%) | 121 (96.8%) | ||||
| Yes | 31 (9.8%) | 23 (13.7%) | 4 (17.4%) | 4 (3.2%) | |||||
| Missing | 2 | 1 | 0 | 1 | |||||
DLI donor lymphocyte infusion, IQR interquartile range, AML acute myeloid leukemia, ALL acute lymphoblastic leukemia, alloSCT allogeneic stem cell transplantation, TBI total body irradiation, CR complete remission, MRD minimal residual disease, TCD T-cell depletion.
DLI
The median interval between alloSCT and DLI1 was 176 days (interquartile range [IQR]: 132–260), it was slightly longer in preDLI (206 days in molecular relapse/persisting MRD, 190 in MC) than in proDLI (169 days). The T-cell dose at the first infusion (DLI1) showed considerable variability (IQR: 1 × 106–1 × 107 CD3+ cells/kg). Regardless of indication for DLI, the median dose was 1 × 106/kg CD3+ cells/kg, 75% received less than 1 × 107 CD3+ cells/kg. Patients received a median of 2 DLI (IQR: 1–3). Reasons to desist from further infusions were reaching the pre-planned number of infusions (57%), GvHD (18%), leukemia relapse (12%), reaching complete donor chimerism (5%), infection (1%), and other reasons (5%). Table 2 provides further details.
Table 2.
Details of DLI given to 318 patients in complete hematologic remission.
| Entire cohort | PreDLI for mixed chimerism | PreDLI for MRD/molecular relapse | ProDLI | ||
|---|---|---|---|---|---|
| Number | n (% of the entire cohort) | 318 (100%) | 169 (53.1%) | 23 (7.2%) | 126 (39.6%) |
| Time alloSCT to first DLI | Median (min–max) [IQR] | 176 (15–1145) [132–260] | 190 (15–1145) [126–314] | 206 (42–589) [122.5–397] | 169 (30–606) [139.2–210.2] |
| CD3+ cell/kg at first DLI (×106/kg) | Median (min–max) [IQR] | 1 (0.1–70) [1–10] | 1 (0.1–70) [1–3] | 1 (0.1-70) [1–10] | 1 (0.1–70) [0.5-10] |
| Missing | 33 | 18 | 1 | 14 | |
| Groups according to CD3+ dose at first DLI (×106/kg) | <1 | 65 (22.8%) | 29 (19.2%) | 5 (22.7%) | 31 (27.7%) |
| 1–5 | 128 (44.9%) | 86 (57%) | 8 (36.4%) | 34 (30.4%) | |
| ≥5 | 92 (32.3%) | 36 (23.8%) | 9 (40.9%) | 47 (42%) | |
| Missing | 33 | 18 | 1 | 14 | |
| Number of DLI infusion given | Median [IQR] | 2 [1–3] | 2 [1–3] | 3 [2–3.5] | 1 [1–3] |
| Time DLI1–2 if 2 DLI (days) | Median (min–max) [IQR] | 36 (6–193) [32–48] | 71.5 (7–521) [34–126.2] | 42 (6–294) [33–62.5] | 43.5 (12–2422) [32.2–73.5] |
| Time DLI2–3 if 3 DLI (days) | Median (min–max) [IQR] | 42 (14–326) [34–63.5] | 43.5 (8–516) [28–99.2] | 47 (14–326) [38–89.5] | 43 (29–459) [41–64] |
| Stopped of immunosuppressive medication before DLI | No | 42 (14.2%) | 29 (19.3%) | 5 (22.7%) | 8 (6.5%) |
| Yes | 253 (85.8%) | 121 (80.7%) | 17 (77.3%) | 115 (93.5%) | |
| Missing | 23 | 19 | 1 | 3 | |
| Use of immunosuppressive prophylaxis after DLI | No | 280 (88.9%) | 143 (89.4%) | 22 (95.7%) | 115 (92.7%) |
| Yes | 27 (8.6%) | 17 (10.6%) | 1 (4.3%) | 9 (7.3%) | |
| Missing | 11 | 9 | 0 | 2 |
DLI donor lymphocyte infusion, MRD minimal residual disease, IQR interquartile range, alloSCT allogeneic stem cell transplantation, CR complete remission.
Response and outcome
Clinical response after preDLI was reported for 16 out of 21 informative patients (71%) with MRD/molecular relapse, based on decreasing MRD (n = 15), and improving peripheral blood counts without measured MRD (n = 1). Although responses were observed in both AML and ALL, no comparison could be performed due to low numbers in the different subgroups (Supplementary Table 1). Among recipients of preDLI for MC, improved donor chimerism was observed in 110/158 (70%) informative patients.
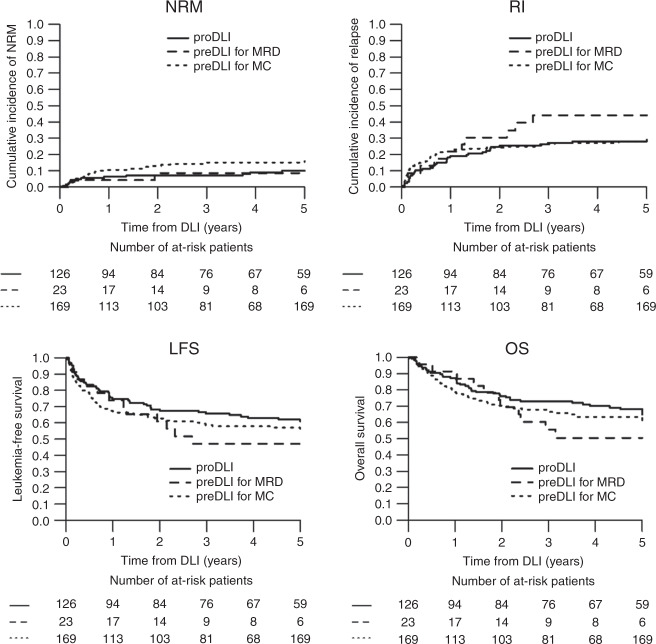
The median follow-up from DLI1 was 7.0 (IQR: 4.1–9.2) years. At 5 years, the rates of NRM, CRI, LFS, and OS of the entire cohort were 12.7% [9.2–16.7], 29.0% [24.2–34.3], 58.2% [52.7–63.7], and 64.3% [58.9–69.7], respectively. For the proDLI cohort, the 5-year NRM, CRI, LFS, and OS rates were 10%, 28%, 62%, and 68%. The respective results after preDLI for MRD were, 9%, 44%, 47%, and 51%; and for preDLI for MC they were 15%, 28%, 57%, and 63%. Overall, no relapses occurred beyond 3 years from DLI1 (Fig. 1). Among responders, the 5-year LFS and OS were 55% and 63% after preDLI for MRD/molecular relapse, and 68% and 76% after preDLI for MC, respectively. In contrast, the 5-year OS in non-responders was 37%.
Fig. 1. Outcome of 318 patients receiving donor lymphocyte infusion in complete hematological remission.
NRM non-relapse mortality, CRI cumulative relapse incidence, LFS leukemia-free survival, OS overall survival. DLI was given as prophylaxis (red curves), as preemptive therapy for minimal residual disease (MRD) or molecular relapse (blue curves), or as preemptive therapy for mixed donor chimerism (green curves).
Leukemia relapse was the most frequent cause of death, occurring in 62 patients (55% of all deaths, 19% of the entire cohort). Nineteen patients (17 % of all deaths, 6% of the entire cohort) died from GvHD (Supplementary Table 2). In a risk factor analysis for outcome after proDLI, no factor evaluable at time of DLI could be identified as being prognostic for outcome; there was a trend for better OS among patients with AML as compared to ALL. In contrast, prior transplantation in CR1 and a longer interval between SCT and first DLI were associated with better LFS and OS after preemptive DLI for MC (Supplementary Tables 3 and 4).
GvHD induced by DLI
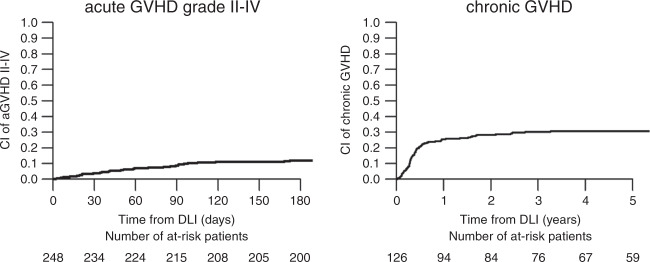
For the analysis of risk factors for DLI-induced GvHD and its influence on outcome, 70 patients had to be excluded due to clinical signs of GvHD at time of DLI (n = 12), prophylactic immunosuppression given after DLI (n = 42) or missing information on GvHD (n = 16). Accordingly, we selected 248 (47.7% receiving proDLI, 52.3%) receiving preDLI; patients who had received DLI in the absence of active GvHD, were off immunosuppressive medication by the day of DLI, and who also did not receive prophylactic immunosuppression after DLI. Outcome was comparable among patients included and excluded from this analysis (data not shown). Within the selected cohort, aGvHD grade I, II, III, and IV were reported in 25, 21, 9, and 9 patients. The cumulative incidence of aGvHD grade II–IV after DLI was 11.9% (95% CI: 8.2–16.3%), median day of onset was day +51 from DLI1 (IQR: 21–91). Five-year cumulative incidence of cGvHD was 30.7% (95% CI: 24.9–36.6%), with a median onset at day +135 (IQR: 89–237; Fig. 2). Taken together, the cumulative incidence of clinically relevant aGvHD or cGvHD was 33.7% (95% CI: 27.8–39.6%) at 5 years. In detail, 43.5% of GvHD events were observed after DLI1, 25.9%, 21.2%, and 9.4% after DLI2, DLI3, and DLI4, respectively. No differences were observed among patients receiving DLI preemptively or as pure prophylaxis.
Fig. 2. Cumulative incidence of acute graft-versus-host disease (aGvHD) grade II–IV and chronic GVHD after prophylactic or preemptive DLI.
Only patients who had received DLI in the absence of active GvHD, were off immunosuppressive medication by the day of DLI, and did not receive prophylactic immunosuppression after DLI (n = 248) were selected.
With respect to outcome, neither aGvHD nor cGvHD was associated with decreased CRI. In contrast, NRM was 29% (25/85) and 2% (3/163) among patients who did or did not develop aGvHD grade II–IV or cGvHD after DLI, suggesting a clear association between GvHD and NRM. Accordingly, LFS (HR 2.20, 95% CI: 1.38–3.51, p = 0.001) and OS (HR 2.08, 95% CI: 1.34–3.22, p = 0.001) were significantly inferior among those patients who developed GvHD.
A detailed risk factor analysis for developing either aGvHD grade II–IV or cGvHD after DLI was performed. Due to increased risk of GvHD with >1 DLI, and since the reason to give >1 DLI could not be evaluated retrospectively, only patients receiving 1 single DLI were included in the model (n = 101). Age > 60 years (p = 0.046), transplantation beyond CR1 (p = 0.003), a shorter interval from SCT to DLI (p = 0.018), and a history of aGvHD grade II–IV after SCT, but before DLI (p = 0.036) were associated with an increased risk for GvHD in multivariate analysis. In contrast, neither an unrelated donor, gender relationship between patient and donor, graft source or conditioning for SCT, or CD3+ cell count at DLI1, significantly influenced the occurrence of GvHD. See Table 3 for details.
Table 3.
Risk factors for graft-versus-host disease after DLI in complete hematologic remissiona.
| Univariate analysis | n | GVHD after DLI [95% CI] | |
| Diagnosis | AML | 77 | 30.2% [20.2–40.8] |
| ALL | 24 | 29.2% [12.4–48.3] | |
| p value | 0.84 | ||
| Patient age | <Median | 50 | 30.1% [17.9–43.2] |
| ≥Median | 51 | 29.4% [17.5–42.3] | |
| p value | 0.9 | ||
| 18–35 years | 19 | 47.4% [23.3–68.1] | |
| 36–45 years | 26 | 19.5% [6.8–37] | |
| 46–60 years | 41 | 24.4% [12.5–38.4] | |
| >60 years | 15 | 40% [15.2–64] | |
| p value | 0.17 | ||
| Time alloSCT– DLI1 | <Median | 50 | 36.1% [22.9–49.5] |
| ≥Median | 51 | 23.5% [12.9–36] | |
| p value | 0.19 | ||
| Status at alloSCT | CR1 | 72 | 23.6% [14.5–34] |
| not CR1 | 27 | 50% [28.2–68.4] | |
| p value | 0.015 | ||
| Female donor for male patient | Yes | 14 | 35.7% [12.2–60.4] |
| No | 86 | 29.2% [19.9–39.1] | |
| p value | 0.49 | ||
| Conditioning | Myeloablative | 62 | 27.4% [16.9–39] |
| Reduced intensity | 39 | 33.3% [19–48.3] | |
| p value | 0.27 | ||
| Donor | Matched sibling | 75 | 30.9% [20.7–41.6] |
| Unrelated | 26 | 26.9% [11.5–45] | |
| p value | 0.95 | ||
| Stem cell source at alloSCT | Bone marrow | 18 | 44.4% [20.6–65.9] |
| Peripheral blood | 83 | 26.8% [17.6–36.7] | |
| p value | 0.16 | ||
| In vivo T-cell depletion | No in vivo TCD | 49 | 26.6% [15.1–39.6] |
| In vivo TCD | 51 | 33.3% [20.8–46.4] | |
| p value | 0.25 | ||
| Ex vivo T-cell depletion | No ex vivo TCD | 53 | 38.8% [25.2–52.2] |
| Ex vivo TCD | 48 | 20.8% [10.6–33.3] | |
| p value | 0.027 | ||
| aGVHD grade II–V before DLI | No aGVHD II–IV before DLI | 88 | 27.5% [18.5–37.2] |
| aGVHD II–IV before DLI | 13 | 50% [18.9–74.9] | |
| Grade II | 9 | ||
| Grade III | 4 | ||
| p value | 0.07 | ||
| CD3 first DLI | CD3/Kg <median | 46 | 40.4% [25.5–54.9] |
| ≥Median | 47 | 23.4% [12.4–36.4] | |
| 0.052 | |||
| Mulitvariate model | GVHD after DLI | ||
| HR (95% CI) | p value | ||
| CR1 at HSCT | 0.32 (0.15–0.68) | 0.003 | |
| Time Tx-DLI1 > 184 days (median) | 0.38 (0.17–0.84) | 0.018 | |
| aGVHD grade II–IV before DLI | 3.42 (1.09–10.75) | 0.036 | |
| Age >60 years | 2.55 (1.02–6.38) | 0.046 | |
GVHD graft-versus-host disease, DLI donor lymphocyte infusion, CI confidence interval, CRI cumulative relapse incidence, CR complete remission, AML acute myeloid leukemia, ALL acute lymphoblastic leukemia, TCD T-cell depletion.
aOnly patients receiving 1 DLI (n = 101, 53% receiving prophylactic 47% receiving therapeutic DLI) were considered for the risk factor analysis.
Discussion
This large retrospective registry study on more than 300 patients with a median follow-up of 7 years provides mature outcome data after prophylactic and preemptive infusion of unmodified DLI after alloSCT for AL. To evaluate the pure effect of donor cells, patients from the registry who had received any additional antileukemic therapy after SCT before or at time of DLI, such as TKI, HMA, or chemotherapy had been excluded from the analysis. Response was reported after preDLI given both for MRD/molecular relapse and for MC. Improved long-term outcome was observed among responders.
Concerning patients receiving proDLI, the lack of a control group precluded a firm statement on antileukemic efficiency. This question has been addressed in a matched-pair analysis including a subgroup of the patients reported and updated here, which had shown a significantly improved OS and LFS after proDLI in patients with high-risk AML, but not in standard-risk AML and ALL [20]. Nevertheless, 5-year LFS/OS rates of 62%/68% in the larger series analyzed here were encouraging, given the high-risk characteristics of the cohort, which included 1/3 of patients transplanted beyond CR1, and 1/3 having received ex vivo T-cell-depleted grafts. These data confirm results from earlier studies using proDLI after TCD for SCT [21], and in T-cell replete SCT in high-risk AML and MDS [22, 23]. Nevertheless, prospective trials in well-defined cohorts are warranted to define the role of proDLI.
With respect to efficacy of preDLI, earlier studies had reported improved chimerism and promising outcome in children and adults with AML, receiving preDLI for mixed donor chimerism [24–26]. Similarly, antileukemic effects of unstimulated [27, 28] or modified [29] preDLI triggered by MRD or molecular relapse have been suggested. In our study, clinical response to preDLI was observed in around 70% of patients both treated for MC and MRD/molecular relapse, although the data in the latter subgroup must be interpreted with caution due to low numbers and heterogenous measurement techniques [30]. Long-term OS from DLI was achieved among responders of both cohorts (55% after MRD triggered, 76% after MC-triggered preDLI), whereas OS was 37% only among non-responders. Unfortunately, no formal comparison among responders and non-responders could be performed due to missing information on the exact date of response. However, the differences at least suggest a clinical relevance of preDLI, supporting in a large series prior data from smaller studies. In a risk factor analysis, we identified SCT in CR1 and a longer interval from the date of transplant as favorable factor for both OS and LFS after preDLI for MC. While CR1 at transplant is a favorable factor for outcome in general, the longer interval might reflect a later occurrence of MC, possibly indicating less dynamic disease. Time between SCT and cellular intervention is a well-known risk factor also for therapeutic DLI and second SCT for hematological relapse.
GvHD was the most devastating complication of DLI in CHR, leading to an increase of NRM and inferior LFS/OS without influencing the risk of relapse. In fact, GvHD was the cause of death in 19, representing 6% of the entire cohort and 17% of all deaths. Hence, identification of risk factors for DLI-induced GvHD after DLI was of great interest. To avoid bias by sequential DLI, pre-existing GvHD, and concomitant immunosuppressive medication, we limited the risk factor analysis to patients receiving exactly one unmanipulated DLI in the absence of active GvHD and off immunosuppressive medication, and who also did not receive prophylactic immunosuppression after DLI. Not unexpectedly, a history of aGvHD grade II–IV after SCT was an independent risk factor for DLI-induced GvHD. Hence, both preDLI and proDLI should probably be used carefully or even avoided in patients who had suffered from severe aGvHD before. The same is true for patients above the age of 60, who also had an increased risk of DLI-induced GvHD and might be more vulnerable to organ damage caused by GvHD and consecutive immunosuppressive treatment. Furthermore, the time interval between SCT and DLI1 is critical for the safety of DLI. Early studies had revealed excessive rates of GvHD with application during the first 30–60 days after SCT [31], which is why a delay at least until day +120 after SCT was a prerequisite for proDLI application in another study [32]. Our data confirm the outstanding role of a shorter time interval between SCT and DLI1 as risk factor for the development of clinically relevant GvHD. This also represents a practical problem at least for using proDLI, since early relapse cannot be prevented by delayed DLI. Either combination with cytoreductive or immune-modulating drugs [33, 34] or modifications of classical DLI that can be applied earlier after transplantation [35, 36] might help to overcome this limitation. Later on, DLI can be repeated using escalating cell doses, based on the development of GvHD and clinical response [22].
Several limitations of our study need to be considered. First, as a typical drawback of a registry analysis, the reason why the patients described here had received DLI in CHR, whereas others did not, could not be evaluated retrospectively, implicating the risk of a selection bias. Second, both MRD and MC were measured locally by the reporting centers, using different methods and cutoffs [30]. This precluded the quantification of MC or MRD and reproducible cutoff values, e.g., for a level of chimerism justifying the use of DLI cannot be provided. Similarly, estimates of the extent and quality and hence the clinical relevance of the response to preDLI cannot be given. However, response data were based on the same method at each individual center, which is why the overall message in terms of response rates can be regarded as reliable. Third, although collecting data from one of the largest registries available, numbers were too small to perform meaningful subgroup analyses, e.g., between AML and ALL, or based on the method of TCD used for SCT. In particular, the role of prior ex vivo TCD might have been mitigated by low numbers and an association with patient’s age. Finally, CD3+ cell counts at the various DLIs varied considerably among patients and were missing in a considerable number, which is why the influence of cell dose on efficacy and the development of GvHD might be underestimated, whereas it was reported to be relevant in earlier studies [37, 38].
In summary, our data provide long-term data on outcome and safety of preemptive and proDLI after SCT for AL, and help to identify candidates for DLI in CHR without increased risk of severe GvHD. Nevertheless, due to high relapse rates, in particular among patients receiving preDLI for MRD, the data also underscore that unmanipulated DLI alone may be insufficient to reliably prevent post-transplant relapse in AL in many cases. Hence, the combined use of targeted or immune-modulating drugs and DLI+/– short-term immunosuppression (summarized in [39]) or the application of specifically educated T cells [40] might represent more effective ways to improve maintenance and preemptive therapy after SCT for high-risk AL.
Supplementary information
Acknowledgements
The study has been performed on behalf of the Acute Leukemia Working Party of EBMT. The authors would like to acknowledge the invaluable contribution of the ALWP data managers to this work. According to EBMT rules, co-authorship has been offered to the centers contributing most patients to the analysis. Nevertheless, the authors wish to further acknowledge the contribution of all centers who reported their data on DLI to the ALWP registry (cf. Supplementary Table 5), hereby making this analysis possible.
Author contributions
CS, ML, AN, and MM designed the study, interpreted the data, and wrote the manuscript draft and final version. MM performed the statistical analysis. CS, NS, HV, A Brecht, MS, JF, FB, MC, GB, PL, DB, JT, A Bloor, AK, SG, NCG, JE, FC, and BS provided patient data. NS, HV, A Brecht, MS, JF, FB, MC, GB, PL, DB, JT, A Bloor, AK, SG, NCG, JE, FC, and BS contributed to the manuscript and critically reviewed the final version.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Arnon Nagler, Mohamad Mohty.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-021-01515-3.
References
- 1.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. doi: 10.1182/blood.V75.3.555.555. [DOI] [PubMed] [Google Scholar]
- 2.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–5. doi: 10.1182/blood.V76.12.2462.2462. [DOI] [PubMed] [Google Scholar]
- 3.Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103:767–76. doi: 10.1182/blood-2003-02-0342. [DOI] [PubMed] [Google Scholar]
- 4.Munker R, Schmid C, Madrigal JA, Kolb HJ. An update on graft-versus-host and graft-versus-leukemia reactions: a summary of the sixth International Symposium held in Schloss Ellmau, Germany, January 22-24, 2004. Bone Marrow Transplant. 2004;34:767–80. doi: 10.1038/sj.bmt.1704667. [DOI] [PubMed] [Google Scholar]
- 5.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–50. doi: 10.1182/blood.V86.5.2041.bloodjournal8652041. [DOI] [PubMed] [Google Scholar]
- 6.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–45. doi: 10.1200/jco.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 7.Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016;51:1431–8. doi: 10.1038/bmt.2016.167. [DOI] [PubMed] [Google Scholar]
- 8.Zeiser R, Beelen DW, Bethge W, Bornhauser M, Bug G, Burchert A, et al. Biology-driven approaches to prevent and treat relapse of myeloid neoplasia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:e128–e140. doi: 10.1016/j.bbmt.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Lee CJ, Savani BN, Mohty M, Gorin NC, Labopin M, Ruggeri A, et al. Post-remission strategies for the prevention of relapse following allogeneic hematopoietic cell transplantation for high-risk acute myeloid leukemia: expert review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2019;54:519–30. doi: 10.1038/s41409-018-0286-2. [DOI] [PubMed] [Google Scholar]
- 10.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83. doi: 10.1182/blood.V96.13.4075. [DOI] [PubMed] [Google Scholar]
- 11.Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–97. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 12.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/jco.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.ebmt.org/Contents/Data-Management/Registrystructure/MED-ABdatacollectionforms/Pages/MED-AB-data-collection-forms.aspx#HSCTManuals. 2017. (accessed date 10 March 2021).
- 17.Bacher U, Talano JA, Bishop MR. Monitoring and prevention of relapse after allogeneic hematopoietic cell transplantation for myeloid malignancies. Biol Blood Marrow Transplant. 2012;18(1 Suppl):S62–S73. doi: 10.1016/j.bbmt.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Bruggemann M, Kotrova M. Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation. Blood Adv. 2017;1:2456–66. doi: 10.1182/bloodadvances.2017009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 20.Schmid C, Labopin M, Schaap N, Veelken H, Schleuning M, Stadler M, et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia—a matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br J Haematol. 2019;184:782–7. doi: 10.1111/bjh.15691. [DOI] [PubMed] [Google Scholar]
- 21.Eefting M, Halkes CJ, de Wreede LC, van Pelt CM, Kersting S, Marijt EW, et al. Myeloablative T cell-depleted alloSCT with early sequential prophylactic donor lymphocyte infusion is an efficient and safe post-remission treatment for adult ALL. Bone Marrow Transplant. 2014;49:287–91. doi: 10.1038/bmt.2013.111. [DOI] [PubMed] [Google Scholar]
- 22.Jedlickova Z, Schmid C, Koenecke C, Hertenstein B, Baurmann H, Schwerdtfeger R, et al. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:663–7. doi: 10.1038/bmt.2015.234. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Liu DH, Fan ZP, Sun J, Wu XJ, Ma X, et al. Prevention of relapse using DLI can increase survival following HLA-identical transplantation in patients with advanced-stage acute leukemia: a multi-center study. Clin Transplant. 2012;26:635–43. doi: 10.1111/j.1399-0012.2012.01626.x. [DOI] [PubMed] [Google Scholar]
- 24.Rujkijyanont P, Morris C, Kang G, Gan K, Hartford C, Triplett B, et al. Risk-adapted donor lymphocyte infusion based on chimerism and donor source in pediatric leukemia. Blood Cancer J. 2013;3:e137. doi: 10.1038/bcj.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B, et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood. 2011;118:5681–8. doi: 10.1182/blood-2011-04-348805. [DOI] [PubMed] [Google Scholar]
- 26.Liga M, Triantafyllou E, Tiniakou M, Lambropoulou P, Karakantza M, Zoumbos NC, et al. High alloreactivity of low-dose prophylactic donor lymphocyte infusion in patients with acute leukemia undergoing allogeneic hematopoietic cell transplantation with an alemtuzumab-containing conditioning regimen. Biol Blood Marrow Transplant. 2013;19:75–81. doi: 10.1016/j.bbmt.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Dominietto A, Pozzi S, Miglino M, Albarracin F, Piaggio G, Bertolotti F, et al. Donor lymphocyte infusions for the treatment of minimal residual disease in acute leukemia. Blood. 2007;109:5063–4. doi: 10.1182/blood-2007-02-072470. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Wu DP, Liu QF, Qin YZ, Wang JB, Xu LP, et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood. 2014;124:1880–6. doi: 10.1182/blood-2014-03-563403. [DOI] [PubMed] [Google Scholar]
- 29.Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119:3256–62. doi: 10.1182/blood-2011-09-380386. [DOI] [PubMed] [Google Scholar]
- 30.Nagler A, Baron F, Labopin M, Polge E, Esteve J, Bazarbachi A, et al. Measurable residual disease (MRD) testing for acute leukemia in EBMT transplant centers: a survey on behalf of the ALWP of the EBMT. Bone Marrow Transplant. 2021;56:218–24. doi: 10.1038/s41409-020-01005-y. [DOI] [PubMed] [Google Scholar]
- 31.de Lima M, Bonamino M, Vasconcelos Z, Colares M, Diamond H, Zalcberg I, et al. Prophylactic donor lymphocyte infusions after moderately ablative chemotherapy and stem cell transplantation for hematological malignancies: high remission rate among poor prognosis patients at the expense of graft-versus-host disease. Bone Marrow Transplant. 2001;27:73–78. doi: 10.1038/sj.bmt.1702726. [DOI] [PubMed] [Google Scholar]
- 32.Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5675–87. doi: 10.1200/jco.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 33.Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24:282–91. doi: 10.1038/nm.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillaume T, Malard F, Magro L, Labopin M, Tabrizi R, Borel C, et al. Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2019;54:1815–26. doi: 10.1038/s41409-019-0536-y. [DOI] [PubMed] [Google Scholar]
- 35.Huang XJ, Wang Y, Liu DH, Xu LP, Chen H, Chen YH, et al. Modified donor lymphocyte infusion (DLI) for the prophylaxis of leukemia relapse after hematopoietic stem cell transplantation in patients with advanced leukemia-feasibility and safety study. J Clin Immunol. 2008;28:390–7. doi: 10.1007/s10875-008-9193-4. [DOI] [PubMed] [Google Scholar]
- 36.Jaiswal SR, Zaman S, Chakrabarti A, Sen S, Mukherjee S, Bhargava S, et al. Improved outcome of refractory/relapsed acute myeloid leukemia after post-transplantation cyclophosphamide-based haploidentical transplantation with myeloablative conditioning and early prophylactic granulocyte colony-stimulating factor-mobilized donor lymphocyte infusions. Biol Blood Marrow Transplant. 2016;22:1867–73. doi: 10.1016/j.bbmt.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Guglielmi C, Arcese W, Hermans J, Bacigalupo A, Bandini G, Bunjes D, et al. Risk assessment in patients with Ph+ chronic myelogenous leukemia at first relapse after allogeneic stem cell transplant: an EBMT retrospective analysis. The Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2000;95:3328–34. [PubMed] [Google Scholar]
- 38.Mackinnon S, Papadopoulos EB, Carabasi MH, Reich L, Collins NH, Boulad F, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86:1261–8. doi: 10.1182/blood.V86.4.1261.bloodjournal8641261. [DOI] [PubMed] [Google Scholar]
- 39.Schmid C, Kuball J, Bug G. Defining the role of donor lymphocyte infusion in high-risk hematologic malignancies. J Clin Oncol. 2021;39:397–418. 10.1200/jco.20.01719. [DOI] [PubMed]
- 40.Lulla P, Naik S, Vasileiou S, Tzannou I, Watanabe A, Kuvalekar M, et al. Clinical effects of administering leukemia-specific donor T cells to patients with AML/MDS post-allogeneic transplant. Blood. 2020 doi: 10.1182/blood.2020009471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.