Over the COVID-19 pandemic, there have been several case reports and studies describing acute pancreatitis as a presenting symptom or manifestation of COVID-19 disease.1, 2, 3, 4 The mechanisms behind these outcomes are still being investigated, but it has been hypothesized that the binding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus to angiotension-converting enzyme 2 in the pancreas can lead to pancreatic injury5 and the development of pancreatitis.
Furthermore, there is evidence that individuals with concurrent pancreatitis and COVID-19 are at elevated risk of poor outcomes. Only 2 small studies from Europe have compared outcomes between patients with COVID-19 with and without acute pancreatitis at the time of infection.6 , 7 Although both studies found that patients with COVID-19 with acute pancreatitis were at higher risk of hospitalization and mortality, the analyses were rather limited due to the small sample sizes (N = 300–400).
As previous studies have only investigated outcomes among individuals with pancreatitis and COVID-19 at the same time, it is currently unknown whether an existing history of pancreatitis before SARS-CoV-2 infection is also associated with COVID-19 severity. Thus, we performed the first study examining the relationship between pre-existing pancreatitis and COVID-19 outcomes in a large and racially/ethnically diverse retrospective cohort of patients with COVID-19 from Kaiser Permanente Southern California.
All patients with COVID-19 from Kaiser Permanente Southern California who received a positive SARS-CoV-2 polymerase chain reaction laboratory test or COVID-19 diagnosis code from March 1, 2020 to February 28, 2021 were included in the study. Pancreatitis history was ascertained using International Classification of Diseases (ICD)-9/10 codes for pancreatitis at any time before the COVID-19 diagnosis. Severe outcomes included COVID-related hospitalization, intensive respiratory support (IRS), and intensive care unit (ICU) admission within 30 days, and mortality within 60 days.
We used logistic and Cox regression to assess the associations between pancreatitis and COVID-19 outcomes. For each outcome, we ran models for pancreatitis history (any vs none), pancreatitis type (single episode acute, recurrent acute, chronic vs none), and timing since last acute pancreatitis episode (>5 years, ≤5 years vs none). For pancreatitis history, we performed subgroup analyses to assess effect modification by age group, gender, race/ethnicity, obesity, smoking, and alcohol use. Further details of the methods can be found in the Supplementary Materials.
Of 326,993 patients with COVID-19 (mean age, 45.2 years; 54.4% female), 4706 had pre-existing pancreatitis (3299 single episode acute, 783 recurrent acute, and 624 chronic). Within 30 days after COVID-19 infection, 18,230 were hospitalized, 6942 received intensive respiratory support, and 3251 were admitted to the ICU. Furthermore, 5255 patients died within 60 days. Rates of all outcomes were 2–3 times higher for those with pre-existing pancreatitis compared with those without pancreatitis (Supplementary Table 1).
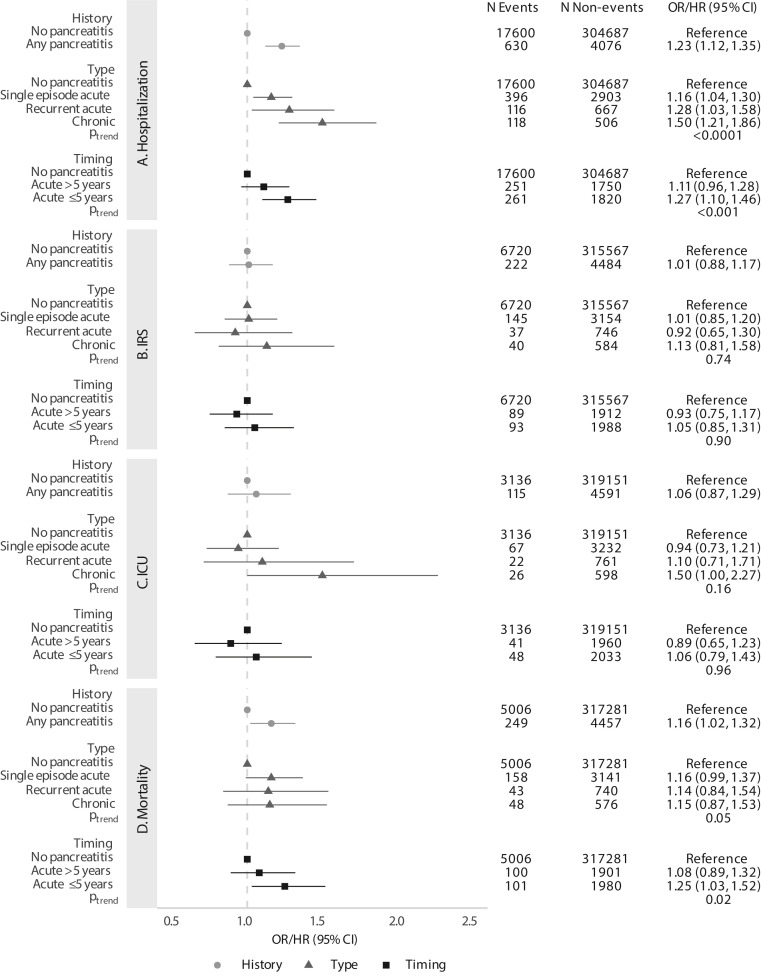
Compared with those without pancreatitis, individuals with pancreatitis had an increased risk of hospitalization (odds ratio [OR], 1.23; 95% confidence interval [CI], 1.12–1.35) and mortality (hazards ratio [HR], 1.16; 95% CI, 1.02–1.32; Figure 1 ). The risk of hospitalization increased with the progression of pancreatitis (OR, 1.16 for single episode acute, 1.28 for recurrent acute, and 1.50 for chronic, P trend < .0001). For patients with acute pancreatitis, only those who had an episode within 5 years had elevated risks of hospitalization (OR, 1.27; 95% CI, 1.10–1.46) and mortality (HR, 1.25; 95% CI, 1.03–1.52). There were no associations for intensive respiratory support and ICU, except for a borderline significant 50% increased risk of ICU admission (OR, 1.50; 95% CI, 1.00–2.27) for chronic pancreatitis (Figure 1).
Figure 1.
Associations between pre-existing pancreatitis history, type, and timing and (A) hospitalization, (B) intensive respiratory support (IRS), (C) intensive care unit (ICU) admission, and (D) mortality following COVID-19 diagnosis. All models are adjusted for age group, gender, race/ethnicity, income, college education, Medicaid insurance status, body mass index (BMI) category, diabetes, smoking, alcohol use, Charlson comorbidity score, and month of COVID-19 infection. Measure of association is OR for hospitalization, IRS, and ICU, and HR for mortality.
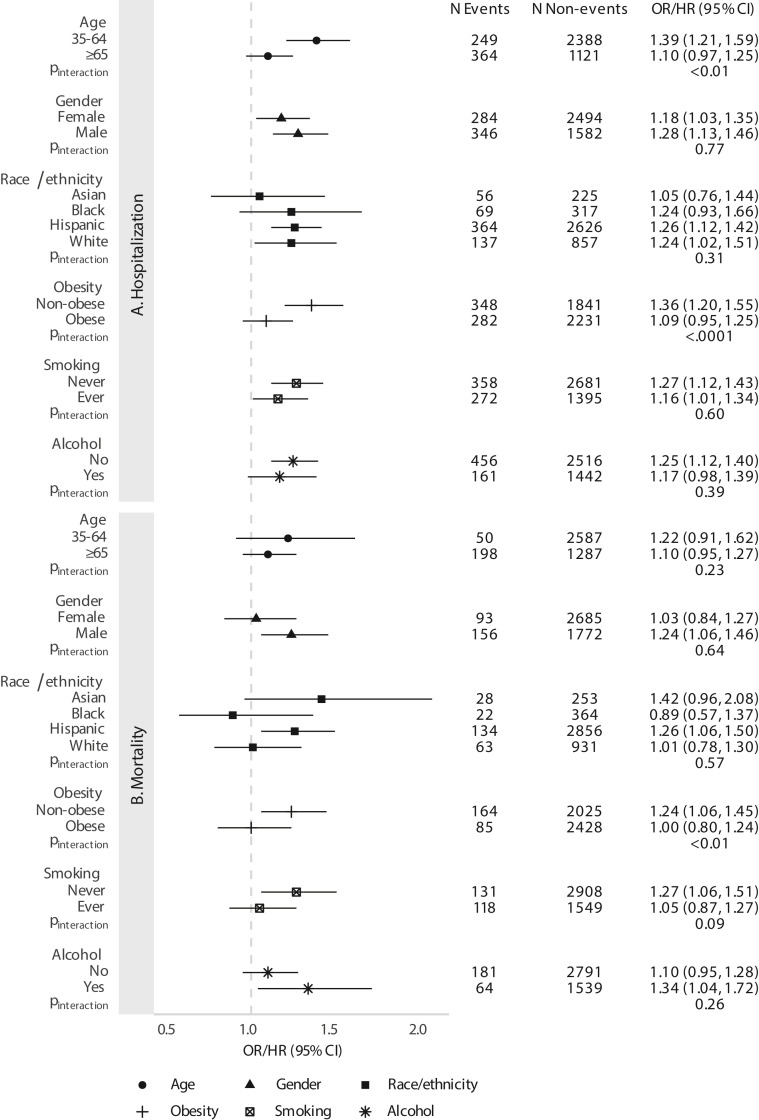
Furthermore, we observed stronger associations between pancreatitis history and hospitalization for those aged 35–64 (OR, 1.39; 95% CI, 1.21–1.59) than those aged ≥65 (OR, 1.10; 95% CI, 0.97–1.25; P interaction < .01). The associations for hospitalization were also more pronounced for nonobese (OR, 1.36; 95% CI, 1.20–1.55) compared with obese patients (OR, 1.09; 95% CI, 0.95–1.25; P interaction < .0001). Moreover, the association between pancreatitis and mortality was significant among nonobese patients (HR, 1.24; 95% CI, 1.06–1.45), but not for obese patients (HR, 1.00; 95% CI, 0.80–1.24, P interaction < .01). We did not observe significant effect modification across any of the other subgroups (Supplementary Figure 1).
Supplemental Figure 1.
Associations between any prior history of pancreatitis and (A) hospitalization and (B) mortality after COVID-19 diagnosis, stratified by subgroups. All models are adjusted for age group, gender, race/ethnicity, income, college education, Medicaid insurance status, body mass index (BMI) category, diabetes, smoking, alcohol use, Charlson comorbidity score, and month of COVID-19 infection. Measure of association is OR for hospitalization and HR for mortality. Heterogeneity was assessed using models with an interaction term for the given subgroup and pancreatitis history.
In this study of nearly 327,000 patients with COVID-19, we observed that pre-existing pancreatitis was associated with an increased risk of COVID-19–related hospitalization and mortality. Notably, the risk of hospitalization exhibited a dose-effect corresponding with more advanced forms of pancreatitis, whereas the positive associations for hospitalization and mortality were higher for those with acute pancreatitis episodes within the past 5 years. Taken together, these results suggest that individuals with more sustained or recent injuries to the pancreas could be at greater risk of developing severe COVID-19.
The poorer outcomes among patients with COVID-19 with pancreatitis history could possibly be related to a pre-existing aggravated inflammatory condition. Past studies have shown that pancreatic acinar cells release several cytokines during acute pancreatitis, including tumor necrosis factor alpha, interleukin (IL)6, and IL10, whereas T cells and macrophages are heavily involved in the inflammatory processes of chronic pancreatitis.8 There is also evidence that patterns of cytokine elevations are similar during severe acute pancreatitis and severe COVID-19 disease.9 Because tumor necrosis factor alpha, IL6, and IL10 are involved in the cytokine storm induced by SARS-CoV-2 virus,10 the prior activation of immune cells in the pancreatic microenvironment could perhaps increase the risk of a more severe inflammatory response during COVID-19 illness.
The key strengths of this study are the large, racially/ethnically heterogeneous cohort of patients with COVID-19 and the comprehensive data from the electronic medical record. This provided us with sufficient power to evaluate pancreatitis type and timing while adjusting for several important confounders. However, we did not have information on pancreatitis etiology, nor did we have laboratory data on pancreatic enzymes to evaluate the severity of pancreatitis. As our cohort only included patients up to February 2021, we also could not examine the impact of COVID-19 vaccines on the association between pancreatitis and severe COVID-19.
In this first study investigating pre-existing pancreatitis and COVID-19 severity, our findings suggest that individuals with prior inflammatory insults to the pancreas could be at greater risk of hospitalization and mortality. Given the inflammation-related similarities between pancreatitis and COVID-19, future studies should evaluate the serum levels of inflammatory markers between patients with COVID-19 with and without pre-existing pancreatitis to further elucidate this relationship.
Acknowledgments
CRediT Authorship Contributions
Brian Z. Huang, PhD, MPH (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Funding acquisition: Equal; Writing – original draft: Lead; Writing – review & editing: Lead). Margo A. Sidell, ScD (Data curation: Equal; Writing – review & editing: Equal). Bechien U. Wu, MD, MPH (Writing – review & editing: Equal). V. Wendy Setiawan, PhD (Writing – review & editing: Equal). Zhanghua Chen, PhD (Funding acquisition: Equal; Writing – review & editing: Equal). Anny H. Xiang, PhD (Funding acquisition: Equal; Supervision: Lead; Writing – review & editing: Equal).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding The study sponsors had no involvement in the study design, collection, analysis, or interpretation of the data. This study was supported by the National Cancer Institute (T32CA229110 and K99CA256525 to B.Z. Huang) and the National Institute of Environmental Health Sciences (3R01ES029963-01 to A.H. Xiang and Z. Chen) at the National Institutes of Health, and the Keck School of Medicine Department of Preventive Medicine COVID-19 Pandemic Research Center (CPRC) at the University of Southern California (USC).
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2022.02.005.
Supplementary Methods
Study Population
We performed a retrospective cohort study of all patients from Kaiser Permanente Southern California (KPSC) who were diagnosed with COVID-19 from March 1, 2020 to February 28, 2021. KPSC is a large integrated health system with comprehensive electronic medical records that serves more than 4.6 million members across Southern California. The KPSC member population is representative of the geographic Southern California region. For this study, patients who received a positive SARS-CoV-2 polymerase chain reaction (PCR) laboratory test or a diagnosis code (internal KPSC codes and International Classification of Diseases [ICD-10] codes U07.1, J80 & U07.1, J12.89 & U07.1, J20.8 & U07.1, and J22 & U07.1) for COVID-19 were included. We defined the index date as the date of the earliest laboratory order for those who received a SARS-CoV-2 PCR test and the date of the earliest COVID-19 code for those who had diagnosis codes only.
Patients who had a diagnosis code for asymptomatic COVID-19 disease and a negative PCR test within 2 weeks after the diagnosis (N = 2158) were excluded to remove potential false-positive cases. We also excluded recurrent COVID-19 cases (N = 178), patients <18 years old (N = 59,838), nonmembers and patients with <12 months of membership who had incomplete medical data (N = 87,733), individuals with other/unknown gender (N = 24), and those with invalid death data (N = 43).
This study was approved by the KPSC Institutional Review Board.
Pancreatitis Exposures
Pancreatitis history was ascertained using ICD-9 and ICD-10 codes for acute (577.0, K85x) and chronic (577.1, K86.0, K86.1) pancreatitis at any time before the COVID-19 diagnosis. Individuals were evaluated based on pancreatitis history (any vs none) and type of pancreatitis (single episode acute, recurrent acute, chronic vs none). Patients with codes for acute pancreatitis were categorized as recurrent acute if they had more than 1 acute pancreatitis code at least 30 days apart, and as single episode acute otherwise. Patients with codes for both acute and chronic pancreatitis were categorized as chronic pancreatitis patients. For acute pancreatitis, we also assessed timing from the most recent episode before COVID-19 diagnosis (split by median time ≤5 years vs >5 years).
Outcomes
We defined severe COVID-19 disease using 4 outcomes of interest: COVID-19–related hospitalization, intensive respiratory support (IRS; eg, invasive mechanical ventilation, noninvasive ventilation, high-flow mask, high-flow nasal cannula), and ICU admission within 30 days, and all-cause mortality within 60 days. Hospitalization, IRS, and ICU admissions were considered COVID-19–related if there was an associated COVID-19 diagnosis code during the hospital encounter. Information on hospitalizations, IRS, and ICU admissions were obtained from inpatient records and out-of-network claims. Mortality information was ascertained from inpatient and death records.
Covariates
Information on demographics (age, gender, race/ethnicity), insurance type, and lifestyle factors (body mass index, diabetes, smoking history, alcohol use) were obtained from the electronic medical records. Neighborhood-level education and income were attained from the geocoding database. For each patient, all diagnosis codes in the past year before COVID-19 were used to calculate the Charlson comorbidity index.
Statistical Analysis
We used logistic regression to estimate the ORs and 95% CIs for the relationship between pancreatitis and COVID-19–related hospitalization, IRS, and ICU admission, and Cox regression to estimate the HRs for all-cause mortality. For each outcome, we ran models for any pancreatitis history (any vs none), pancreatitis type (single episode acute, recurrent acute, chronic vs none), and timing since last acute pancreatitis episode (>5 years, ≤5 years vs none). To test for trends in the associations for pancreatitis type and timing, we also ran models with the exposures as continuous variables coded as consecutive numbers (eg, 1, 2, 3).
All models included age group (18–34, 35–64, ≥65), gender, race/ethnicity (Asian, black, Hispanic, white, other), body mass index category (<25 kg/m2 for underweight/normal, 25–30 kg/m2 for overweight, 30–40 kg/m2 for obese, ≥40 kg/m2 for severely obese), diabetes (yes vs no), smoking history (current, former, never), alcohol use (yes vs no), income (<$40,000, $40,000–$79,999, ≥$80,000), college education (yes vs no), Medicaid status (yes vs no), and Charlson comorbidity index (0, 1, ≥2) as covariates. We also included month of COVID-19 infection as an additional covariate to account for temporal changes in testing availability and medical care for COVID-19 during the pandemic.
For pancreatitis history, we performed subgroup analyses to assess effect measure modification by age group, gender, race/ethnicity, obesity, smoking, and alcohol use. The analyses by age group were only conducted among those aged 35 and older due to the small number of hospitalizations and deaths in those aged <35. Heterogeneity was assessed using models with an interaction term for pancreatitis history and the subgroup of interest.
To address potential reverse causation, we re-ran all models excluding individuals who had their first pancreatitis code within 5 days before COVID-19 (N = 44). Results from the sensitivity analyses were similar; therefore, we only present results from the original analyses.
All analyses were performed using SAS 9.4 (Cary, NC).
Supplementary Table 1.
Cohort Characteristics of 326,993 Adult Patients With a Confirmed COVID-19 Diagnosis From March 1, 2020 to February 28, 2021, Stratified by Prior History of Pancreatitis
| No pancreatitis (N = 322,287) | Pancreatitisa (N = 4706) | Total (N = 326,993) | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 45.1 (16.57) | 55.8 (16.89) | 45.2 (16.62) |
| Age category, N (%) | |||
| 18–34 | 100,030 (31%) | 584 (12.4%) | 100,614 (30.8%) |
| 35–64 | 179,924 (55.8%) | 2637 (56%) | 182,561 (55.8%) |
| ≥65 | 42,333 (13.1%) | 1485 (31.6%) | 43,818 (13.4%) |
| Gender, N (%) | |||
| Female | 175,052 (54.3%) | 2778 (59%) | 177,830 (54.4%) |
| Male | 147,235 (45.7%) | 1928 (41%) | 149,163 (45.6%) |
| Race/ethnicity, N (%) | |||
| Asian | 23,318 (7.2%) | 281 (6%) | 23,599 (7.2%) |
| Black | 19,231 (6%) | 386 (8.2%) | 19,617 (6%) |
| Hispanic | 197,262 (61.2%) | 2990 (63.5%) | 200,252 (61.2%) |
| Other | 16,829 (5.2%) | 55 (1.2%) | 16,884 (5.2%) |
| White | 65,647 (20.4%) | 994 (21.1%) | 66,641 (20.4%) |
| BMI category, N (%) | |||
| Underweight/normal (<25 kg/m2) | 60,784 (18.9%) | 774 (16.4%) | 61,558 (18.8%) |
| Overweight (25–30 kg/m2) | 101,202 (31.4%) | 1415 (30.1%) | 102,617 (31.4%) |
| Obese (30–40 kg/m2) | 122,871 (38.1%) | 1990 (42.3%) | 124,861 (38.2%) |
| Severely obese (≥40 kg/m2) | 30,796 (9.5%) | 523 (11.1%) | 31,319 (9.6%) |
| Missing | 6634 (2.1%) | 4 (0.1%) | 6638 (2%) |
| Diabetes, N (%) | |||
| No | 264,708 (82.1%) | 2541 (54.0%) | 267,249 (81.7%) |
| Yes | 57,579 (17.9%) | 2165 (46.0%) | 59,744 (18.3%) |
| Smoking status, N (%) | |||
| Current | 16,483 (5.1%) | 269 (5.7%) | 16,752 (5.1%) |
| Former | 59,363 (18.4%) | 1398 (29.7%) | 60,761 (18.6%) |
| Never | 239,563 (74.3%) | 3031 (64.4%) | 242,594 (74.2%) |
| Missing | 6878 (2.2%) | 8 (0.2%) | 6886 (2.1%) |
| Alcohol use, N (%) | |||
| No | 159,086 (49.4%) | 2972 (63.1%) | 162,058 (49.6%) |
| Yes | 124,467 (38.6%) | 1603 (34.1%) | 126,070 (38.5%) |
| Missing | 38,734 (12%) | 131 (2.8%) | 38,865 (11.9%) |
| Household median income, N (%) | |||
| <$40,000 | 20,421 (6.3%) | 355 (7.6%) | 20,776 (6.3%) |
| $40,000–$79,999 | 173,208 (53.8%) | 2613 (55.5%) | 175,821 (53.8%) |
| ≥$80,000 | 128,643 (39.9%) | 1738 (36.9%) | 130,381 (39.9%) |
| Missing | 15 (0%) | 0 (0%) | 15 (0%) |
| College education, N (%) | |||
| No | 184,297 (57.2%) | 2810 (59.7%) | 187,107 (57.2%) |
| Yes | 137,975 (42.8%) | 1896 (40.3%) | 139,871 (42.8%) |
| Missing | 15 (0%) | 0 (0%) | 15 (0%) |
| Medicaid, N (%) | |||
| No | 320,838 (99.6%) | 4663 (99.1%) | 325,501 (99.5%) |
| Yes | 1449 (0.4%) | 43 (0.9%) | 1492 (0.5%) |
| Charlson comorbidity score, N (%) | |||
| 0 | 223,971 (69.5%) | 1669 (35.5%) | 225,640 (69%) |
| 1 | 64,193 (19.9%) | 1260 (26.8%) | 65,453 (20%) |
| ≥2 | 34,123 (10.6%) | 1777 (37.7%) | 35,900 (11%) |
| Outcomes, N (%)b | |||
| COVID-19–related hospitalization | 17,600 (5.5%) | 630 (13.4%) | 18,230 (5.6%) |
| COVID-19–related IRSc | 6720 (2.1%) | 222 (4.7%) | 6942 (2.1%) |
| COVID-19–related ICU admission | 3136 (1%) | 115 (2.4%) | 3251 (1%) |
| Death | 5006 (1.6%) | 249 (5.3%) | 5255 (1.6%) |
All patient characteristics and outcome event rates were significantly different across individuals with and without a prior history of pancreatitis (P < .0001).
Any prior history of acute or chronic pancreatitis before COVID-19 diagnosis identified using ICD-9/10 codes (acute: 577.0, K85x; chronic: 577.1, K86.0, K86.1)
Within 30 days for hospitalization, IRS, and ICU; within 60 days for death.
Need for invasive mechanical ventilation, noninvasive ventilation, high-flow mask, or high-flow nasal cannula.
References
- 1.Correia de Sá T., et al. World J Gastrointest Surg. 2021;13:574–584. doi: 10.4240/wjgs.v13.i6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eldaly A.S., et al. J Med Case Rep. 2021;15:461. doi: 10.1186/s13256-021-03026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabłońska B., et al. World J Gastrointest Surg. 2021;13:548–562. doi: 10.4240/wjgs.v13.i6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troncone E., et al. Pancreas. 2021;50:393–398. doi: 10.1097/MPA.0000000000001770. [DOI] [PubMed] [Google Scholar]
- 5.Liu F., et al. Clin Gastroenterol Hepatol. 2020;18:2128–2130.e2. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akarsu C., et al. J Invest Surg. 2022;35:119–125. doi: 10.1080/08941939.2020.1833263. [DOI] [PubMed] [Google Scholar]
- 7.Miró Ò, et al. J Hepatobiliary Pancreat Sci. 2021;28:953–966. doi: 10.1002/jhbp.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habtezion A. Curr Opin Gastroenterol. 2015;31:395–399. doi: 10.1097/MOG.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegyi P., et al. Gastroenterology. 2020;159:824–827. doi: 10.1053/j.gastro.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragab D., et al. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]