Abstract
Introduction
Nephrologists have recently recognized the heterogeneity of kidney diseases among patients with diabetes and begun to actively perform percutaneous renal biopsies (PRBs). Nevertheless, the association between diabetes and major bleeding complications of PRB remains unclear.
Methods
In this retrospective cohort study using the Diagnosis Procedure Combination database in Japan, we identified patients who underwent an elective PRB from July 2010 to March 2018. The primary outcome was the occurrence of major bleeding complications, defined as red blood cell transfusion within 7 days after PRB or invasive hemostasis after PRB. Multivariable regression analysis was performed to analyze the association between diabetes and major bleeding complications with adjustment for patient and hospital characteristics.
Results
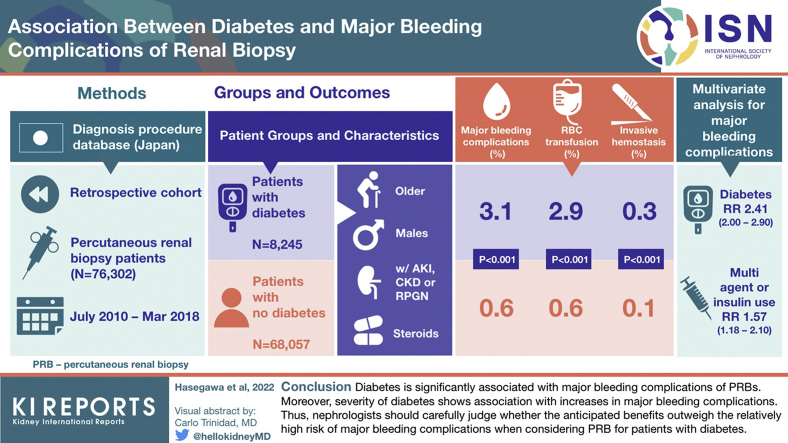
We identified 76,302 patients, including 8245 with diabetes. The proportion of PRBs performed for patients with diabetes continuously increased over time. Major bleeding complications occurred in 678 patients (0.9%), including 622 (0.8%) with red blood cell (RBC) transfusion and 109 (0.1%) with invasive hemostasis. Diabetes was significantly associated with major bleeding complications (relative risk [RR] = 2.41; 95% CI 2.00–2.90). Among patients with diabetes, multiagent or insulin treatment had significant association with major bleeding complications (RR = 1.57; 95% CI 1.18–2.10), compared with single-agent diabetes treatment.
Conclusion
Diabetes is significantly associated with major bleeding complications of PRBs. Moreover, severity of diabetes has association with increases in major bleeding complications. Thus, nephrologists should carefully judge whether the anticipated benefits outweigh the relatively high risk of major bleeding complications when considering PRB for patients with diabetes.
Keywords: bleeding complications, diabetes, kidney biopsy, renal biopsy
Graphical abstract
See Commentary on Page 149
PRBs are critical for the diagnosis and management of kidney diseases.1 In the past, patients with diabetes were considered to present typical diabetic nephropathy, and PRB was not indicated.1 Nevertheless, the situation regarding PRB for patients with diabetes has recently changed because of a paradigm shift from “diabetic nephropathy” to “diabetic kidney disease.” The clinical courses of kidney diseases in patients with diabetes have become heterogeneous and different from the typical course of diabetic nephropathy, possibly because of the widespread use of renin–angiotensin–aldosterone system inhibitors and the presence of multiple comorbid conditions.2, 3, 4 Renal complications of diabetes are now referred to as “diabetic kidney disease,” which includes various disease phenotypes, in contrast to the concept of typical diabetic nephropathy.5 Since this paradigm shift around 2014, many nephrologists have actively performed PRBs for patients with diabetes and pointed out its effectiveness in the diagnosis and treatment of renal complications of diabetes.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Nevertheless, despite this shift, the risk of PRB for patients with diabetes has not been fully evaluated. Although biopsy techniques have advanced, it is still difficult to perfectly prevent major bleeding complications, which sometimes lead to RBC transfusion or invasive hemostasis (transcatheter arterial embolization or nephrectomy).17 Most previous studies investigating risk factors for bleeding complications after PRB did not include diabetes mellitus as a covariate because of a lack of information on diabetes status in their databases18,19 or because the researchers did not focus on diabetes.20 Although diabetes was found not to be significantly associated with RBC transfusion after PRB in a recent analysis of 644 patients in the Boston Kidney Biopsy Cohort,21 the sample size of this previous study was not large enough to make a solid conclusion. Hence, in this study, we aimed to evaluate the association between diabetes mellitus and major bleeding complications after PRB, using a national inpatient database in Japan.
Methods
Data Source
For this study, we used the Diagnosis Procedure Combination database, a national inpatient database in Japan. The database is a nationwide inpatient database. This database collects data on approximately 7 million inpatients per year from >1000 hospitals, which covers approximately 90% of all tertiary care hospitals in Japan.22,23 The database contains the following information: patient baseline characteristics (age, sex, body height, bodyweight, activities of daily living [ADL] at admission assessed by the Barthel Index, and level of consciousness at admission assessed by the Japan Coma Scale); main diagnoses, comorbidities at admission, and in-hospital complications recorded using International Classification of Diseases, 10th Revision (ICD-10) codes; surgical and nonsurgical procedures; in-hospital prescriptions and devices used; and a unique hospital identifier.22
Study approval was obtained from the institutional review board of The University of Tokyo. Because of the anonymous character of the data, the need for informed consent was waived.
Patient Selection and Characteristics
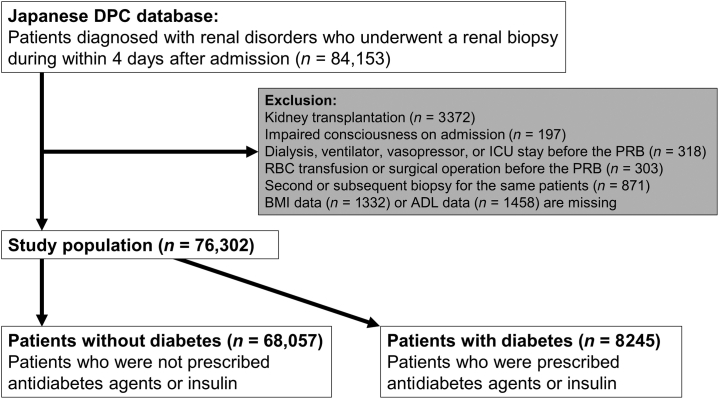
Data were obtained for patients aged ≥16 years diagnosed with having or suspected to have a renal disorder (ICD-10 codes of main diagnosis: C900, D690, E102, E107, E112, E117, E122, E127, E132, E137, E142, E147, I120, I129, N00, N01-08, N10-12, N14, N158, N159, N16-19, N25, N26, N289, N391, M30-36, R31, or R80) who underwent an elective PRB within 4 days after admission from July 2010 to March 2018. The exclusion criteria were as follows: patients with a history of kidney transplantation (ICD-10 code T861 or Z940) or receiving kidney transplant during hospitalization; patients with impaired consciousness at admission; patients undergoing hemodialysis, peritoneal dialysis, continuous renal replacement therapy, mechanical ventilation, vasopressor administration, or an intensive care unit stay before the PRB; patients receiving RBC transfusion or surgical operation under general anesthesia before the PRB; second or subsequent PRBs for the same patient during the study period; patients with no body mass index data; and patients with no ADL data.
Diabetes mellitus was defined by the in-hospital prescription of antidiabetes agents or insulin during hospitalization. In a subgroup analysis, patients with diabetes were divided into the following 2 groups by the complexity of their diabetes treatment: (i) patients receiving single-agent diabetes treatment without insulin during hospitalization (single-agent treatment) and (ii) patients receiving multiagent diabetes treatment or insulin treatment during hospitalization (multiagent or insulin treatment).
Outcome Measurement
The primary outcome was major bleeding complications, defined as (i) RBC transfusion within 7 days after the PRB or (ii) invasive hemostasis (transcatheter arterial embolization or nephrectomy) after the PRB during hospitalization. The occurrences of (i) and (ii) were separately evaluated as the secondary outcomes. We also performed 3 sensitivity analyses. First, we analyzed the association between diabetes and massive RBC transfusion (>1000 ml) after the PRB during hospitalization because RBC transfusion might be influenced by each patient’s baseline hemoglobin level.21,24 Second, we excluded patients with anemia defined using the ICD-10 codes (D5, D60–64) on admission and analyzed the association between diabetes and major bleeding complications. Finally, we changed the definition of diabetes and analyzed the association between diabetes and major bleeding complications, where diabetes was defined using the ICD-10 codes E10 to E14.
Covariates
We obtained data on the following patient characteristics and used them as covariates: age, sex, body mass index, ADL recorded using the Barthel Index, corticosteroid use, immunosuppressant use, antithrombotic use, presence of chronic kidney disease, and clinical renal syndromes. Presence of chronic kidney disease was defined using the ICD-10 code N18 or N19. Clinical renal syndromes were classified into the following 3 categories: (i) acute kidney injury (AKI) or rapidly progressive glomerulonephritis (RPGN), defined using the ICD-10 code N00, N01, N10, or N17; (ii) nephrosis, defined using the ICD-10 code N04; and (iii) others.
We also used the following hospital characteristics as covariates: hospital volume (the number of patients undergoing a PRB per year in each hospital), whether the institution was an academic hospital, and timing of the PRB. Timing of the PRB was classified into the following 2 categories: earlier years (Japanese fiscal years 2010–2015 [July 2010–March 2016]) or later years (Japanese fiscal years 2016–2017 [April 2016–March 2018]). The reason for this classification is that the proportion of PRBs that are performed for patients with diabetes has rapidly increased since Japanese fiscal year 2015, and we wanted to analyze whether the expanded indication of PRB for patients with diabetes was associated with major bleeding complications.
Statistical Analysis
Variables were expressed as median and interquartile range (25th–75th percentiles) for continuous data or as number and percentage for categorical data. Patient and hospital characteristics were compared between patients with and without diabetes using the Mann–Whitney U test (for continuous variables) or the χ2 test (for categorical variables). Multivariable regression analysis was performed to analyze the associations between diabetes mellitus and the primary or secondary outcomes, with adjustment for patient and hospital characteristics. We estimated RRs with 95% CIs using robust Poisson regression models25,26 fitted with generalized estimating equations27,28 to account for the clustering of patients within hospitals. In sensitivity analyses, we applied the same models. In the subgroup analysis, multivariable regression analysis was also performed to investigate the relationship between the complexity of diabetes treatment and major bleeding complications among patients with diabetes, again adjusting for patient and hospital characteristics. The threshold for significance was set at P < 0.05. All statistical analyses were performed using Stata, Version 16 software (StataCorp, College Station, TX).
Results
Patient Characteristics
We identified 84,153 patients aged ≥16 years who were diagnosed with or suspected of having renal disorders and who underwent an elective PRB within 4 days after admission during the study period. Of these, 3372 patients who had undergone kidney transplantation; 197 patients with impaired consciousness on admission; 318 patients who underwent hemodialysis, peritoneal dialysis, continuous renal replacement therapy, mechanical ventilation, vasopressor administration, or an intensive care unit stay before the PRB; 303 patients who had received RBC transfusion or surgical operation under general anesthesia before the PRB; 871 patients of second or subsequent PRBs for the same patient during the study period; 1332 patients without body mass index data; and 1458 cases without ADL data were excluded (Figure 1).
Figure 1.
Flowchart of patient selection. ADL, activities of daily living; BMI, body mass index; DPC, Diagnosis Procedure Combination; ICU, intensive care unit; PRB, percutaneous renal biopsy; RBC, red blood cell.
The remaining 76,302 eligible patients were classified as patients without diabetes (n = 68,057) or patients with diabetes (n = 8245). We found that the proportion of PRBs that were performed for patients with diabetes continuously increased over time. This increasing trend especially accelerated beginning in Japanese fiscal year 2015 (Supplementary Figure S1). The baseline characteristics of both groups are summarized in Table 1. Overall, the patients with diabetes were more likely to be older adults, male, and obese; more likely to have AKI or RPGN, nephrosis, and ADL-dependent status; and more likely to use corticosteroids, compared with the patients without diabetes. The median duration of inpatient care was 6 (interquartile range 5–9) days, compatible with the typical hospitalization period for PRBs in Japan.
Table 1.
Baseline characteristics of patients with and without diabetes
| Characteristic | Total patients (N = 76,302) | Patients without diabetes (n = 68,057) | Patients with diabetes (n = 8245) | P value |
|---|---|---|---|---|
| Age, yr | 50 (35–65) | 48 (33–63) | 65 (55–72) | <0.001 |
| Male, n (%) | 39,555 (51.8) | 34,561 (50.8) | 4994 (60.6) | <0.001 |
| BMI (kg/m2) | <0.001 | |||
| BMI < 18.5, n (%) | 6409 (8.4) | 5980 (8.8) | 429 (5.2) | |
| 18.5 ≤ BMI <23.0, n (%) | 32,871 (43.1) | 30,121 (44.3) | 2750 (33.4) | |
| 23.0 ≤ BMI <25.0, n (%) | 14,330 (18.8) | 12,643 (18.6) | 1687 (20.5) | |
| 25.0 ≤ BMI <30.0, n (%) | 17,608 (23.1) | 15,079 (22.2) | 2529 (30.7) | |
| BMI ≥30.0, n (%) | 5084 (6.7) | 4234 (6.2) | 850 (10.3) | |
| Main diagnosis | <0.001 | |||
| AKI or RPGN, n (%) | 3425 (4.5) | 2393 (3.5) | 1032 (12.5) | |
| Nephrosis, n (%) | 13,708 (18.0) | 10,816 (15.9) | 2892 (35.1) | |
| Others, n (%) | 59,169 (77.5) | 54,848 (80.6) | 4321 (52.4) | |
| Presence of CKD, n (%) | 8837 (11.6) | 7278 (10.7) | 1559 (18.9) | <0.001 |
| Hospital volume per yr | 0.27 | |||
| 1–24 cases, n (%) | 28,808 (37.8) | 25,714 (37.8) | 3094 (37.5) | |
| 25–44 cases, n (%) | 25,197 (33.0) | 22,412 (32.9) | 2785 (33.8) | |
| ≥45 cases, n (%) | 22,297 (29.2) | 19,931 (29.3) | 2366 (28.7) | |
| Academic hospital, n (%) | 19,831 (26.0) | 17,657 (25.9) | 2174 (26.4) | 0.41 |
| Fiscal year period | <0.001 | |||
| 2010–2015, n (%) | 53,405 (70.0) | 48,402 (71.1) | 5003 (60.7) | |
| 2016–2017, n (%) | 22,897 (30.0) | 19,655 (28.9) | 3242 (39.3) | |
| ADL | <0.001 | |||
| Independent, n (%) | 75,145 (98.5) | 67,238 (98.8) | 7907 (95.9) | |
| Dependent, n (%) | 1157 (1.5) | 819 (1.2) | 338 (4.1) | |
| Corticosteroid use, n (%) | 2365 (3.1) | 1744 (2.6) | 621 (7.5) | <0.001 |
| Immunosuppressant use, n (%) | 802 (1.1) | 691 (1.0) | 111 (1.3) | 0.005 |
| Antithrombotic use, n (%) | 1016 (1.3) | 708 (1.0) | 308 (3.7) | <0.001 |
ADL, activities of daily living; AKI, acute kidney injury; BMI, body mass index; CKD, chronic kidney disease; RPGN, rapidly progressive glomerulonephritis.
BMI calculated as weight in kilograms divided by the square of height in meters.
Association of Diabetes Mellitus With Major Bleeding Complications
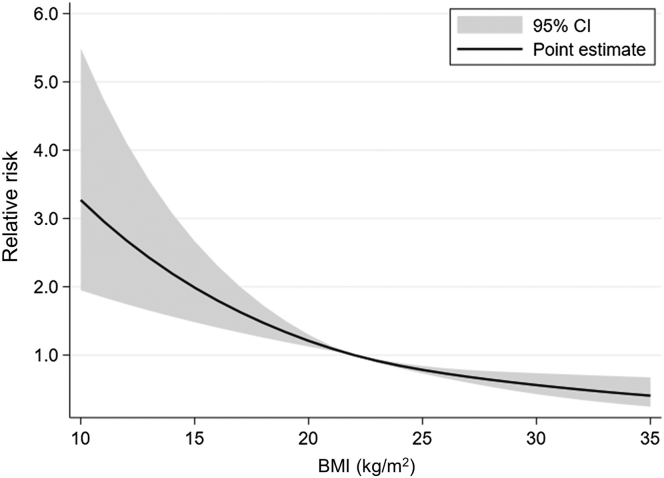
A total of 678 patients (0.9% of all patients) had major bleeding complications (Table 2). Diabetes mellitus was significantly associated with major bleeding complications (RR = 2.41; 95% CI 2.00–2.90). In addition, older age, female sex, lower body mass index, AKI or RPGN, presence of chronic kidney disease, academic hospital, ADL-dependent status, and corticosteroid use were statistically associated with major bleeding complications (Table 3 and Figure 2). As the number of PRBs for patients with diabetes drastically increased beginning in Japanese fiscal year 2015, we first included the interaction term of diabetes mellitus and the dichotomized fiscal year variable (fiscal years 2016–2017 vs. fiscal years 2010–2015) in the model. Nevertheless, the interaction term was not significant. Thus, we removed the interaction term from our model.
Table 2.
Major bleeding complications of patients with and without diabetes
| Event | Total patients (N = 76,302) | Patients without diabetes (n = 68,057) | Patients with diabetes (n = 8245) | P value |
|---|---|---|---|---|
| Major bleeding complications, n (%) | 678 (0.9) | 424 (0.6) | 254 (3.1) | <0.001 |
| RBC transfusion, n (%) | 622 (0.8) | 381 (0.6) | 241 (2.9) | <0.001 |
| Invasive hemostasis, n (%) | 109 (0.1) | 82 (0.1) | 27 (0.3) | <0.001 |
| Massive RBC transfusion, n (%) | 201 (0.3) | 70 (0.1) | 131 (1.6) | <0.001 |
RBC, red blood cell.
Some patients received both RBC transfusion and invasive hemostasis. Thus, they were not mutually exclusive.
Table 3.
Multivariable regression analysis for major bleeding complications
| Generalized estimating equations (group variable: hospital code) | Multivariable analysis |
|
|---|---|---|
| RR (95% CI) | P value | |
| Diabetes mellitus | 2.41 (2.00–2.90) | <0.001 |
| Age, yr | 1.04 (1.03–1.04) | <0.001 |
| Female, sex | 1.49 (1.27–1.76) | <0.001 |
| BMI (kg/m2) | See Figure 2 | |
| Main diagnosis | ||
| AKI or RPGN | 3.86 (3.17–4.71) | <0.001 |
| Nephrosis | 0.89 (0.70–1.14) | 0.35 |
| Others | 1 (base) | |
| Presence of CKD | 2.50 (2.11–2.97) | <0.001 |
| Hospital volume | ||
| 1–24/yr | 1 (base) | |
| 25–44/yr | 0.86 (0.71–1.05) | 0.15 |
| ≥45/yr | 0.90 (0.73–1.10) | 0.29 |
| Academic hospital | 1.41 (1.16–1.71) | 0.001 |
| Fiscal year period | 0.21 | |
| 2010–2015 | 1 (base) | |
| 2016–2017 | 0.90 (0.76–1.06) | |
| ADL | <0.001 | |
| Dependent | 2.57 (2.00–3.29) | |
| Independent | 1 (base) | |
| Corticosteroid use | 1.41 (1.05–1.89) | 0.022 |
| Immunosuppressant use | 1.13 (0.52–2.45) | 0.75 |
| Antithrombotic use | 1.14 (0.75–1.74) | 0.53 |
ADL, activities of daily living; AKI, acute kidney injury; BMI, body mass index; CKD, chronic kidney disease; RPGN, rapidly progressive glomerulonephritis; RR, relative risk.
BMI calculated as weight in kilograms divided by the square of height in meters.
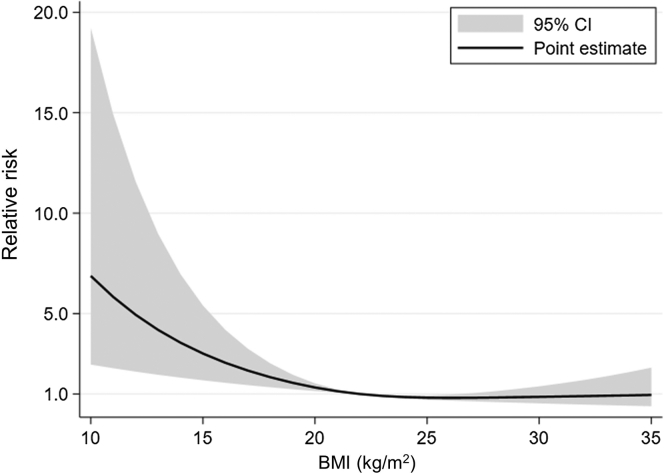
Figure 2.
Cubic spline estimation of BMI and relative risk of major bleeding complications. The horizontal axis denotes the BMI (kg/m2), and the vertical axis denotes the relative risk of major bleeding complications. BMI, body mass index.
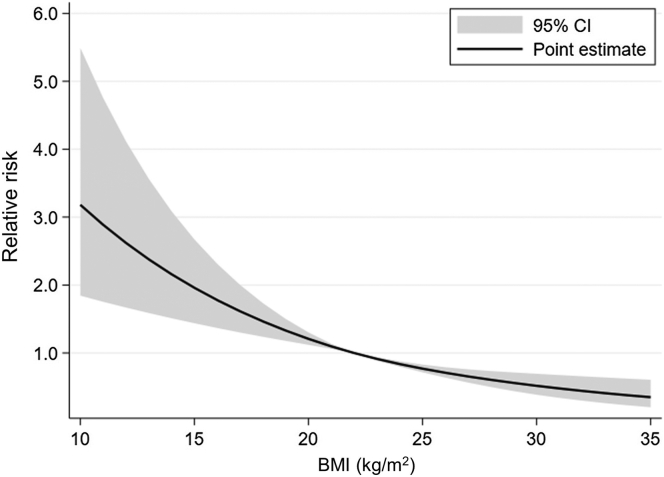
Regarding the secondary outcomes, 622 patients (0.8% of all patients) required RBC transfusion within 7 days after the PRB and 109 patients (0.1% of all patients) required invasive hemostasis after the PRB during hospitalization (Table 2). Multivariable regression analysis has revealed that diabetes mellitus was significantly associated with RBC transfusion (RR = 2.44; 95% CI 2.01–2.96) (Table 4 and Figure 3) and invasive hemostasis (RR = 1.87; 95% CI 1.16–3.02) (Table 5 and Figure 4).
Table 4.
Multivariable regression analysis for red blood cell transfusion
| Generalized estimating equations (group variable: hospital code) | Multivariable analysis |
|
|---|---|---|
| RR (95% CI) | P value | |
| Diabetes mellitus | 2.44 (2.01–2.96) | <0.001 |
| Age, yr | 1.04 (1.03–1.05) | <0.001 |
| Female, sex | 1.63 (1.38–1.93) | <0.001 |
| BMI (kg/m2) | See Figure 3 | |
| Main diagnosis | ||
| AKI or RPGN | 3.92 (3.20–4.80) | <0.001 |
| Nephrosis | 0.86 (0.66–1.11) | 0.23 |
| Others | 1 (base) | |
| Presence of CKD | 2.70 (2.26–3.22) | <0.001 |
| Hospital volume | ||
| 1–24/yr | 1 (base) | |
| 25–44/yr | 0.82 (0.66–1.00) | 0.055 |
| ≥45/yr | 0.83 (0.67–1.03) | 0.09 |
| Academic hospital | 1.43 (1.16–1.76) | 0.001 |
| Fiscal year period | 0.17 | |
| 2010–2015 | 1 (base) | |
| 2016–2017 | 0.88 (0.74–1.05) | |
| ADL | <0.001 | |
| Dependent | 2.64 (2.06-3.37) | |
| Independent | 1 (base) | |
| Corticosteroid use | 1.41 (1.03–1.92) | 0.032 |
| Immunosuppressant use | 0.93 (0.37–2.35) | 0.88 |
| Antithrombotic use | 1.03 (0.66–1.62) | 0.89 |
ADL, activities of daily living; AKI, acute kidney injury; BMI, body mass index; CKD, chronic kidney disease; RPGN, rapidly progressive glomerulonephritis; RR, relative risk.
BMI calculated as weight in kilograms divided by the square of height in meters.
Figure 3.
Cubic spline estimation of BMI and relative risk of red blood cell transfusion. The horizontal axis denotes the BMI (kg/m2), and the vertical axis denotes the relative risk of red blood cell transfusion. BMI, body mass index.
Table 5.
Multivariable regression analysis for invasive hemostasis
| Generalized estimating equations (group variable: hospital code) | Multivariable analysis |
|
|---|---|---|
| RR (95% CI) | P value | |
| Diabetes mellitus | 1.87 (1.16–3.02) | 0.01 |
| Age, yr | 1.01 (1.00–1.03) | 0.13 |
| Female, sex | 0.77 (0.52–1.15) | 0.20 |
| BMI (kg/m2) | See Figure 4 | |
| Main diagnosis | ||
| AKI or RPGN | 3.43 (1.96–6.01) | <0.001 |
| Nephrosis | 1.04 (0.64–1.67) | 0.88 |
| Others | 1 (base) | |
| Presence of CKD | 1.10 (0.68–1.78) | 0.71 |
| Hospital volume | ||
| 1–24/yr | 1 (base) | |
| 25–44/yr | 1.59 (0.90–2.83) | 0.11 |
| ≥45/yr | 1.74 (0.98–3.08) | 0.06 |
| Academic hospital | 1.50 (0.90–2.51) | 0.12 |
| Fiscal year period | 0.80 | |
| 2010–2015 | 1 (base) | |
| 2016–2017 | 0.95 (0.62–1.45) | |
| ADL | 0.025 | |
| Dependent | 2.43 (1.12–5.29) | |
| Independent | 1 (base) | |
| Corticosteroid use | 1.11 (0.46–2.70) | 0.82 |
| Immunosuppressant use | 2.52 (0.61–10.41) | 0.20 |
| Antithrombotic use | 1.87 (0.58–6.04) | 0.29 |
ADL, activities of daily living; AKI, acute kidney injury; BMI, body mass index; CKD, chronic kidney disease; RPGN, rapidly progressive glomerulonephritis; RR, relative risk.
BMI calculated as weight in kilograms divided by the square of height in meters.
Figure 4.
Cubic spline estimation of BMI and relative risk of invasive hemostasis. The horizontal axis denotes the BMI (kg/m2), and the vertical axis denotes the relative risk of invasive hemostasis. BMI, body mass index.
Sensitivity Analyses
We performed 3 sensitivity analyses to confirm the association between diabetes and major bleeding complications. First, we analyzed the association between diabetes and massive RBC transfusion (>1000 ml) after the PRB during hospitalization. A total of 201 patients (0.3% of all patients) received massive RBC transfusion after the PRB during hospitalization (Table 2). Diabetes mellitus was significantly associated with massive RBC transfusion (RR = 7.47; 95% CI 5.25–10.63) (Supplementary Table S1 and Supplementary Figure S2). Second, we excluded patients with anemia defined using the ICD-10 codes on admission and performed the same analysis. Even when patients with anemia on admission were excluded, diabetes is associated with major bleeding complications (RR = 2.63; 95% CI 2.12–3.28) (Supplementary Tables S2–S4 and Supplementary Figure S3). Finally, we changed the definition of diabetes and performed the same analysis. Even when diabetes was defined using the ICD-10 codes, diabetes had statistical association with major bleeding complications (RR = 1.34; 95% CI 1.11–1.62) (Supplementary Tables S5–S7 and Supplementary Figure S4).
Subgroup Analysis in Patients With Diabetes
In the subgroup analysis, patients with diabetes were classified as patients receiving single-agent treatment (n = 2562) or patients receiving multiagent or insulin treatment (n = 5683). The baseline characteristics of these subgroups of patients are summarized in Supplementary Table S8. Major bleeding complications occurred in 50 patients (2.0%) of the single-agent group and 204 patients (3.6%) of the multiagent or insulin treatment group (Table 6). Multiagent or insulin treatment was significantly associated with major bleeding complications compared with single-agent treatment (RR = 1.57; 95% CI 1.18–2.10) (Supplementary Table S9 and Supplementary Figure S5).
Table 6.
Major bleeding complications of the subgroups of patients with diabetes
| Event | Single-agent (n = 2562) | Multiagent or insulin (n = 5683) | P value |
|---|---|---|---|
| Major bleeding complications, n (%) | 50 (2.0%) | 204 (3.6%) | <0.001 |
| RBC transfusion, n (%) | 49 (1.9%) | 192 (3.4%) | <0.001 |
| Invasive hemostasis, n (%) | 2 (0.1%) | 25 (0.4%) | 0.008 |
| Massive RBC transfusion, n (%) | 7 (0.3%) | 124 (2.2%) | <0.001 |
RBC, red blood cell.
Some patients received both RBC transfusion and invasive hemostasis. Thus, they were not mutually exclusive.
Discussion
In this retrospective cohort study using a large-scale data set from a nationwide inpatient database in Japan, patients with diabetes were found to be more likely to have major bleeding complications after PRB compared with patients without diabetes. Moreover, a subgroup analysis revealed that, in patients with diabetes, multiagent or insulin treatment was significantly associated with higher risk of major bleeding complications compared with single-agent treatment. To the best of our knowledge, this is the first study to reveal that diabetes mellitus is a risk factor for major bleeding complications after PRB.
In our study, 0.8% of all patients received RBC transfusion and 0.1% received invasive hemostasis (transcatheter arterial embolization or nephrectomy), as major bleeding complications after PRBs (Table 2), which is compatible with the findings of previous reports.18, 19, 20,29 Nevertheless, in a previous study using a US National Inpatient Database, the major bleeding complication rate was very high compared with that in the present study.30 This may be due to differences in the health care systems in the 2 countries. In Japan, all PRBs are conducted during hospitalization. Patients in Japan usually receive a PRB soon after admission and stay in-hospital for 5 to 7 days as an observation period even if they do not have any complications.31 In contrast, outpatient PRBs are often performed in the United States. A previous study revealed that complication rates were higher in hospitalized patients undergoing PRBs than in outpatients undergoing PRBs.32 Thus, patients who receive a PRB during hospitalization in the United States may have some kind of health problems other than renal diseases.
Although previous studies identified risk factors for major bleeding complications, such as female sex, use of large-gauge needles, high serum creatinine levels, clinical renal syndrome (AKI or RPGN), and low hospital volume, their analyses did not include diabetes mellitus as a covariate because of a lack of information regarding diabetes in their databases18,19 or because the researchers did not focus on diabetes.20 In the present study, we included 76,302 patients and precisely identified patients with diabetes using in-hospital prescription and procedure data. Diabetes mellitus was a risk factor after adjustment for patient and hospital characteristics including most previously reported risk factors (Table 3 and Figure 2). Because recent cohort studies have reported that patient baseline low hemoglobin level is a risk factor for RBC transfusion,21,24 we conducted sensitivity analyses, finding that diabetes mellitus was associated with massive RBC transfusion (>1000 ml) during hospitalization (Supplementary Table S1 and Supplementary Figure S2) and that diabetes was also associated with major bleeding complications even when patients with anemia defined using ICD-10 codes on admission were excluded (Supplementary Tables S2–S4, and Supplementary Figure S3). Moreover, a subgroup analysis of patients with diabetes suggested that the severity of diabetes was associated with major bleeding complications (Supplementary Table S9 and Supplementary Figure S5).
We also observed a continuous increase throughout the study period in the proportion of PRBs that were performed for patients with diabetes, with a steep increase in the last 2 years of the study period (Supplementary Figure S1). This change is probably explained by the paradigm shift from diabetic nephropathy to diabetic kidney disease. Nevertheless, the interaction term of diabetes mellitus and the binary variable for fiscal year period (fiscal years 2016–2017 vs. fiscal years 2010–2015) was not significant in our analysis. Thus, the extended indication of PRB for patients with diabetes may not be clearly associated with major bleeding complications.
Although the high risk of major bleeding complications observed in patients with diabetes cannot be completely explained from biological perspectives, it may be associated with the fact that patients with diabetes often have impaired wound healing in clinical settings.33 Some basic studies using animal models have suggested that high glucose levels impair wound healing because of aberrant inflammatory cell infiltration and chemokine expression,34,35 which may be related to the high proportion of patients with diabetes who had major bleeding complications after PRB in our study.
This study had several limitations. First, we could not include each patient’s serum creatinine level as a covariate because of a lack of corresponding data in the database. In a previous study, patients with high serum creatinine levels were found to be susceptible to major bleeding complications after PRBs.18 Nevertheless, the effect of kidney function on bleeding risk was adjusted to some extent by the inclusion of covariates for clinical renal syndromes (AKI or RPGN) and presence of chronic kidney disease in our study. Second, we could not include needle gauge as a covariate in this study. In a previous study, use of 14-gauge needles was an independent risk factor for major bleeding complications.19 Nevertheless, in a 2018 survey administered by the Japanese Society of Nephrology,29 95% of hospitals used 16-gauge or 18-gauge needles and <5% of hospitals used 14-gauge needles. Thus, needle gauge may not have a large effect on major bleeding complications in Japanese clinical settings. Third, we could not precisely grasp patients’ blood glucose control because of a lack of laboratory data, such as hemoglobin A1c and glycoalbumin, in the database. Instead, we divided the patients with diabetes into 2 groups by the complexity of their diabetes treatment in the subgroup analysis. Although the results suggest that the severity of diabetes is associated with major bleeding complications, further studies using other databases that include laboratory data are needed to elucidate the relationship between blood glucose control and major bleeding complications after PRB. Fourth, the adjustment for antithrombotic agents in our study was not perfect because the use of these agents is generally discontinued before hospitalization for an elective PRB in Japan.31 Because the discontinuation and resumption timing of these agents may depend on each patient’s thrombosis risk, further studies are needed to determine the best management of these agents before and after PRBs. Finally, this study was conducted in Japan, where all patients undergoing PRBs need to stay in-hospital for 5 to 7 days as an observation period. Thus, our result cannot be simply applied to outpatient PRBs in other countries because a possible bias toward more RBC transfusions in patients with diabetes might exist during hospitalization because of their comorbidities, such as coronary artery disease.
In conclusion, diabetes is significantly associated with major bleeding complications of PRBs. Moreover, the severity of diabetes has association with major bleeding complications. These findings suggest that nephrologists should carefully judge whether the anticipated benefits outweigh the relatively high risk of major bleeding complications when considering PRB for patients with diabetes.
Disclosure
The Division of Chronic Kidney Disease Pathophysiology and Department of Prevention of Diabetes and Lifestyle-Related Diseases, The University of Tokyo Graduate School of Medicine, is financially supported by Kyowa Kirin Co., Ltd., or Asahi Mutual Life Insurance Company, respectively, which is not directly related to this work. MN has received honoraria, advisory fees, or research funding from Kyowa Kirin, Akebia, Astellas, Chugai, GlaxoSmithKline, JT, Torii, Tanabe-Mitsubishi, Daiichi Sankyo, Takeda, Ono, Bayer, Boehringer Ingelheim, and Alexion that are not directly related to this work. All the other authors declared no competing interests.
Acknowledgments
SH analyzed the data and wrote the original draft. AO aided in the data analysis and revised the manuscript. SA aided in the statistical analysis. RK, HM, and KF extracted data from the database. HY and MN supervised this study and revised the manuscript. All authors approved the final version of the manuscript. This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007 and 20AA2005 to HY) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907 to HY).
Footnotes
Figure S1. Trend in the proportion of renal biopsies for patients with diabetes.
Figure S2. Cubic spline estimation of body mass index and relative risk of massive red blood cell transfusion.
Figure S3. Cubic spline estimation of body mass index and relative risk of major bleeding complications (when patients with anemia defined using ICD-10 codes on admission were excluded).
Figure S4. Cubic spline estimation of body mass index and relative risk of major bleeding complications (when diabetes was defined using ICD-10 codes).
Figure S5. Cubic spline estimation of body mass index and relative risk of major bleeding complications in patients with diabetes.
Table S1. Multivariable regression analysis for massive red blood cell transfusion.
Table S2. Baseline characteristics of patients with and without diabetes (when patients with anemia defined using ICD-10 codes on admission were excluded).
Table S3. Major bleeding complications of patients with and without diabetes (when patients with anemia defined using ICD-10 codes on admission were excluded).
Table S4. Multivariable regression analysis for major bleeding complications (when patients with anemia defined using ICD-10 codes on admission were excluded).
Table S5. Baseline characteristics of patients with and without diabetes (when diabetes was defined using ICD-10 codes).
Table S6. Major bleeding complications of patients with and without diabetes (when diabetes was defined using ICD-10 codes).
Table S7. Multivariable regression analysis for major bleeding complications (when diabetes was defined using ICD-10 codes).
Table S8. Baseline characteristics of the subgroups of patients with diabetes.
Table S9. Multivariable regression analysis for major bleeding complications in the subgroup of patients with diabetes.
Supplementary Material
Figure S1. Trend in the proportion of renal biopsies for patients with diabetes.
Figure S2. Cubic spline estimation of body mass index and relative risk of massive red blood cell transfusion.
Figure S3. Cubic spline estimation of body mass index and relative risk of major bleeding complications (when patients with anemia defined using ICD-10 codes on admission were excluded).
Figure S4. Cubic spline estimation of body mass index and relative risk of major bleeding complications (when diabetes was defined using ICD-10 codes).
Figure S5. Cubic spline estimation of body mass index and relative risk of major bleeding complications in patients with diabetes.
Table S1. Multivariable regression analysis for massive red blood cell transfusion.
Table S2. Baseline characteristics of patients with and without diabetes (when patients with anemia defined using ICD-10 codes on admission were excluded).
Table S3. Major bleeding complications of patients with and without diabetes (when patients with anemia defined using ICD-10 codes on admission were excluded).
Table S4. Multivariable regression analysis for major bleeding complications (when patients with anemia defined using ICD-10 codes on admission were excluded).
Table S5. Baseline characteristics of patients with and without diabetes (when diabetes was defined using ICD-10 codes).
Table S6. Major bleeding complications of patients with and without diabetes (when diabetes was defined using ICD-10 codes).
Table S7. Multivariable regression analysis for major bleeding complications (when diabetes was defined using ICD-10 codes).
Table S8. Baseline characteristics of the subgroups of patients with diabetes.
Table S9. Multivariable regression analysis for major bleeding complications in the subgroup of patients with diabetes.
References
- 1.Dhaun N., Bellamy C.O., Cattran D.C., Kluth D.C. Utility of renal biopsy in the clinical management of renal disease. Kidney Int. 2014;85:1039–1048. doi: 10.1038/ki.2013.512. [DOI] [PubMed] [Google Scholar]
- 2.Perkins B.A., Ficociello L.H., Ostrander B.E., et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 3.Pavkov M.E., Knowler W.C., Lemley K.V., Mason C.C., Myers B.D., Nelson R.G. Early renal function decline in type 2 diabetes. Clin J Am Soc Nephrol. 2012;7:78–84. doi: 10.2215/CJN.07610711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriya T., Omura K., Matsubara M., Yoshida Y., Hayama K., Ouchi M. Arteriolar hyalinosis predicts increase in albuminuria and GFR decline in normo- and microalbuminuric Japanese patients with Type 2 diabetes. Diabetes Care. 2017;40:1373–1378. doi: 10.2337/dc17-0209. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida Y., Kashiwabara K., Hirakawa Y., et al. Conditions, pathogenesis, and progression of diabetic kidney disease and early decliner in Japan. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2019-000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mise K., Hoshino J., Ueno T., et al. Prognostic value of tubulointerstitial lesions, urinary N-acetyl-β-d-Glucosaminidase, and urinary β2-microglobulin in patients with Type 2 diabetes and biopsy-proven diabetic nephropathy. Clin J Am Soc Nephrol. 2016;11:593–601. doi: 10.2215/CJN.04980515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuichi K., Yuzawa Y., Shimizu M., et al. Nationwide multicentre kidney biopsy study of Japanese patients with type 2 diabetes. Nephrol Dial Transplant. 2018;33:138–148. doi: 10.1093/ndt/gfw417. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino J., Furuichi K., Yamanouchi M., et al. A new pathological scoring system by the Japanese classification to predict renal outcome in diabetic nephropathy. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamanouchi M., Hoshino J., Ubara Y., et al. Value of adding the renal pathological score to the kidney failure risk equation in advanced diabetic nephropathy. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuichi K., Shimizu M., Yuzawa Y., et al. Clinicopathological analysis of biopsy-proven diabetic nephropathy based on the Japanese classification of diabetic nephropathy. Clin Exp Nephrol. 2018;22:570–582. doi: 10.1007/s10157-017-1485-7. [DOI] [PubMed] [Google Scholar]
- 11.Mise K., Ueno T., Hoshino J., et al. Nodular lesions in diabetic nephropathy: collagen staining and renal prognosis. Diabetes Res Clin Pract. 2017;127:187–197. doi: 10.1016/j.diabres.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino J., Mise K., Ueno T., et al. A pathological scoring system to predict renal outcome in diabetic nephropathy. Am J Nephrol. 2015;41:337–344. doi: 10.1159/000431333. [DOI] [PubMed] [Google Scholar]
- 13.Mise K., Hoshino J., Ueno T., et al. Clinical implications of linear immunofluorescent staining for immunoglobulin G in patients with diabetic nephropathy. Diabetes Res Clin Pract. 2014;106:522–530. doi: 10.1016/j.diabres.2014.09.051. [DOI] [PubMed] [Google Scholar]
- 14.Mise K., Hoshino J., Ubara Y., et al. Renal prognosis a long time after renal biopsy on patients with diabetic nephropathy. Nephrol Dial Transplant. 2014;29:109–118. doi: 10.1093/ndt/gft349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mise K., Yamaguchi Y., Hoshino J., et al. Paratubular basement membrane insudative lesions predict renal prognosis in patients with type 2 diabetes and biopsy-proven diabetic nephropathy. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mise K., Hoshino J., Ueno T., et al. Impact of tubulointerstitial lesions on anaemia in patients with biopsy-proven diabetic nephropathy. Diabet Med. 2015;32:546–555. doi: 10.1111/dme.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan J.J., Mocanu M., Berns J.S. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11:354–362. doi: 10.2215/CJN.05750515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corapi K.M., Chen J.L., Balk E.M., Gordon C.E. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60:62–73. doi: 10.1053/j.ajkd.2012.02.330. [DOI] [PubMed] [Google Scholar]
- 19.Tondel C., Vikse B.E., Bostad L., Svarstad E. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol. 2012;7:1591–1597. doi: 10.2215/CJN.02150212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto H., Hashimoto H., Nakamura M., Horiguchi H., Yasunaga H. Relationship between hospital volume and hemorrhagic complication after percutaneous renal biopsy: results from the Japanese diagnosis procedure combination database. Clin Exp Nephrol. 2015;19:271–277. doi: 10.1007/s10157-014-0986-x. [DOI] [PubMed] [Google Scholar]
- 21.Palsson R., Short S.A.P., Kibbelaar Z.A., et al. Bleeding complications after percutaneous native kidney biopsy: results from the Boston kidney biopsy cohort. Kidney Int Rep. 2020;5:511–518. doi: 10.1016/j.ekir.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasunaga H. Real world data in Japan: chapter II the diagnosis procedure combination database. Ann Clin Epidemiol. 2019;1:76–79. [Google Scholar]
- 23.Yamana H., Moriwaki M., Horiguchi H., Kodan M., Fushimi K., Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27:476–482. doi: 10.1016/j.je.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mejia-Vilet J.M., Marquez-Martinez M.A., Cordova-Sanchez B.M., et al. Simple risk score for prediction of haemorrhagic complications after a percutaneous renal biopsy. Nephrology (Carlton) 2018;23:523–529. doi: 10.1111/nep.13055. [DOI] [PubMed] [Google Scholar]
- 25.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 26.Chen W., Qian L., Shi J., Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18:63. doi: 10.1186/s12874-018-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubbard A.E., Ahern J., Fleischer N.L., et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010;21:467–474. doi: 10.1097/EDE.0b013e3181caeb90. [DOI] [PubMed] [Google Scholar]
- 28.Hanley J.A., Negassa A., Edwardes M.D., Forrester J.E. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi T., Nagasawsa T., Tsuruya K., et al. A nationwide survey on clinical practice patterns and bleeding complications of percutaneous native kidney biopsy in Japan. Clin Exp Nephrol. 2020;24:389–401. doi: 10.1007/s10157-020-01869-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Turk A.A., Estiverne C., Agrawal P.R., Michaud J.M. Trends and outcomes of the use of percutaneous native kidney biopsy in the United States: 5-year data analysis of the Nationwide Inpatient Sample. Clin Kidney J. 2018;11:330–336. doi: 10.1093/ckj/sfx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ubara Y., Kawaguchi T., Nagasawa T., et al. Kidney biopsy guidebook 2020 in Japan. Clin Exp Nephrol. 2021;25:325–364. doi: 10.1007/s10157-020-01986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poggio E.D., McClelland R.L., Blank K.N., et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol. 2020;15:1595–1602. doi: 10.2215/CJN.04710420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura Y., Ii M., Qin G., et al. CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Invest Dermatol. 2012;132:711–720. doi: 10.1038/jid.2011.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishida Y., Kuninaka Y., Nosaka M., et al. CCL2-mediated reversal of impaired skin wound healing in diabetic mice by normalization of neovascularization and collagen accumulation. J Invest Dermatol. 2019;139:2517–2527.e5. doi: 10.1016/j.jid.2019.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.