Abstract
A new, inexpensive filtration device for the diagnosis of urinary schistosomiasis was tested against the commonly used Millipore device. The experimental protocol was performed with 25 urine samples known to be positive for Schistosoma haematobium. The results suggest that the new device is as effective as the Millipore device for the diagnosis of urinary schistosomiasis. Its low cost will be attractive to schistosomiasis control programs.
Urine filtration is one of the methods recommended by the World Health Organization for the detection of Schistosoma haematobium (4). The filtration device is composed of a plastic filter holder that contains a nylon filter (pore size, 12 to 20 μm). Complete filtration of the urine sample is ensured by a rubber O-ring that prevents urine from bypassing the filter. For diagnostic purposes, a standard 10-ml quantity of the urine to be tested is forced through the device with a syringe. If eggs of S. haematobium are present (size, 150 by 60 μm), they are unable to pass through the filter and can be observed and counted under a microscope fitted with a 10× objective.
One of the problems which has limited the use of this technique is the cost of the filtration equipment used to date (from Millipore Intertech, Bedford, Mass.). Each device costs over $2 ($1,100 for a box of 500 devices). Similar filtration equipment has recently been designed and developed (by Vestergaard-Frandesen, Kolding, Denmark) and is available at a much lower price of $0.08 per device ($40 for 500 devices). If this device is found to be equally effective for schistosome recovery, the savings in cost would be of great interest to health authorities in regions where schistosomiasis is endemic.
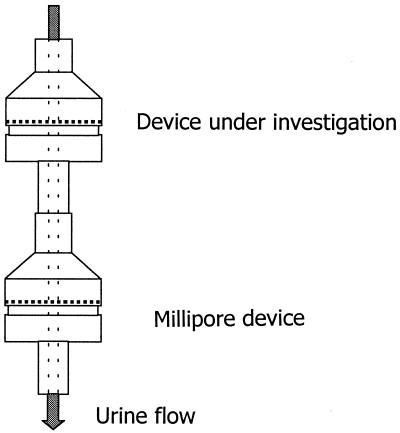
Our objective was to compare the parasite recovery performance, practicality, and ease of use of the low-cost device with those of the original device. The experiment took place on Unguja Island, Zanzibar, United Republic of Tanzania, one of two Zanzibari islands where urinary schistosomiasis is highly endemic (3). Trained technical personnel from the Unguja Helminth Control Program collected urine specimens and performed filtration tests. Single urine specimens positive for S. haematobium eggs were collected from 25 children attending the Kinyasini School in North Unguja. To evaluate the low-cost filtration device, we linked two filtration systems in series so that eggs bypassing the first filtration device would be captured by the second filtration device (Fig. 1). Only new filters were used in both devices. The absence of S. haematobium eggs on the second filter was considered an indication of the capacity of the first device to recover all of the eggs from the urine. Testing was conducted with urine samples from 25 S. haematobium-positive patients.
FIG. 1.
Concatenation of two filtration devices in series in order to test parasite recovery from the first device.
A chi-square test to determine statistical significance was performed. All patients providing urine with S. haematobium eggs were treated with praziquantel at 40 mg/kg.
The “gold standard” evaluation test was the linking of two Millipore filtration devices in series. All 25 cases were correctly identified by the first Millipore filter. In 4 of 25 cases, eggs were found on the filter in the second Millipore device (Table 1). In all of the cases in which eggs were recovered from the second filter, the urine contained a high intensity of schistosome eggs (>50 eggs/10 ml). In situations of high intensity, it has repeatedly been observed that eggs can bypass the filter (A. Montresor, personal communication). This does not change the diagnosis or the classification of the intensity of the infection of the patient.
TABLE 1.
Comparison of the performance of the new, low-cost filtration device with that of the Millipore filtration device
| Order of devicesa | No. of filters with eggs/total no. of filters (% positive)
|
|
|---|---|---|
| First device | Second device | |
| Millipore device, Millipore device | 25/25 (100) | 4/25 (16) |
| New device, Millipore device | 25/25 (100) | 1/25 (4) |
The Millipore device is the gold standard.
In our test evaluation, the low-cost filtration device was linked to a Millipore filtration device. All 25 cases were correctly identified by the first, low-cost, filter (Table 1). In only 1 of the 25 cases were eggs found on the second (Millipore) device. The performance of the low-cost prototype is therefore considered satisfactory. The single positive result obtained with the second filter also occurred with a urine sample having a high-intensity infection of >50 eggs/10 ml. In fact, the rate at which the low-cost device was bypassed was lower than that of the Millipore device, although the difference was not statistically significant (P = 0.346).
There is no agreement concerning the possibility of filter reuse (1, 2). This aspect was not investigated in the present study. However, for practical purposes, we have reused filter holders and also filters from negative tests after thorough washing. Filters from positive tests should be discarded.
Our results suggest that the low-cost filtration device can be used as an alternative to the Millipore filter device. The next step is to confirm its test properties in population-based field studies in areas where schistosomiasis is endemic.
Acknowledgments
The support of the Ivo de Carneri Foundation is gratefully acknowledged.
REFERENCES
- 1.Mshinda H, Lengeler C, Hatz C, de Savigny D. Field diagnosis of urinary schistosomiasis by multiple use of Nuclepore urine filters. J Parasitol. 1989;75:476–478. [PubMed] [Google Scholar]
- 2.Rohde R, Braun-Munzinger R A, Rasoloarison C. Potential false positive egg-counts through the reuse of polyamide filters in the diagnosis of urinary schistosomiasis. Trop Med Parasitol. 1985;36:143–144. [PubMed] [Google Scholar]
- 3.Savioli L, Dixon H, Kisumku U K, Mott K E. Control of morbidity due to S. haematobium on Pemba Island: programme organization and management. Trop Med Parasitol. 1989;40:189–194. [PubMed] [Google Scholar]
- 4.World Health Organization. The control of schistosomiasis. Second report of the W. H. O. Expert Committee. Geneva, Switzerland: World Health Organization; 1993. [PubMed] [Google Scholar]