Abstract
Background:
Currently available infant body composition measurement methods are impractical for routine clinical use. The study developed anthropometric equations (AE) to estimate fat mass (FM, kg) during the first year using air displacement plethysmography (PEA POD® Infant Body Composition System) and Infant Quantitative Magnetic Resonance (Infant-QMR) as criterion methods.
Methods:
Multiethnic full-term infants (n=191) were measured at 3 days, 15 weeks, and 54 weeks. Sex, race/ethnicity, gestational age, age (days), weight-kg (W), length-cm (L), head circumferences-cm (HC), skinfold thicknesses mm [triceps (TRI), thigh (THI), subscapular (SCP), and iliac (IL)], and FM by PEA POD® and Infant-QMR were collected. Stepwise linear regression determined the model that best predicted FM.
Results:
Weight, length, head circumference and skinfolds of triceps, thigh and subscapular, but not iliac, significantly predicted fat mass throughout infancy in both the Infant QMR and PEA POD models. Sex had an interaction effect at 3 days and 15 weeks for both the models.
The coefficient of determination [R2] and root mean square error were 0.87 (66g) at 3 days, 0.92 (153g) at 15 weeks and 0.82 (278g) at 54 weeks for the Infant-QMR models; 0.77 (80g) at 3 days and 0.82 (195g) at 15 weeks for the PEA POD models respectively.
Conclusions:
Both PEAPOD and Infant-QMR derived models predict FM using skinfolds, weight, head circumference, and length with acceptable R2 and residual patterns.
Keywords: Body Composition, Infant-QMR, PEAPOD, Pediatrics, Infancy
Introduction
Body composition during infancy and early childhood has relevance in health care settings, clinical research, and national surveys and surveillance.1 The information has potential to screen for current and future health risks, provide anticipatory guidance, monitor therapeutic progress, and tailor treatment as in precision medicine.2 Longitudinal follow-up of fat mass (FM) through infancy and early childhood can aid in understanding its influence on general growth and development, and the risk for development of diseases in later life.3, 4 Currently validated and routinely used methods to measure FM such as air displacement plethysmography (ADP), dual energy X-ray absorptiometry (DXA), and Infant Quantitative Magnetic Resonance (Infant-QMR) are expensive, often unavailable and therefore impractical for use in community and clinical settings, and in large scale epidemiological field studies. Anthropometric measurements including weight, stature, skinfold thicknesses, and body circumferences can inform on elements of growth related to body composition.5, 6 They provide an easy, inexpensive, portable proxy for infant fat assessments in resource-limited settings, and a combination of these measures can be used to assess subcutaneous fat distribution variation in infants at the group level.3, 7
Fat mass (FM) can be estimated from anthropometric prediction equations developed using accurate body composition methods such as ADP, DXA and QMR. Several anthropometric prediction equations are available for estimating FM, % fat mass (%FM) or fat free mass (FFM) in children ages 3–18 years as described in a systematic review8 with fewer equations during the first year of life and some specific to individual ethnic groups.9, 10 In infants, anthropometric equations have been developed against total body electrical conductivity (TOBEC),11, 12 ADP9, 13–15 and DXA16, 17 with ADP being the most widely used for body fat prediction equations in the first week of life.
Validation studies by Cauble et al18 showed poor agreement, accuracy and precision for four9, 11, 13, 19 neonatal anthropometric equations using the criterion of ADP at birth and 3 months. Two FM prediction models with ADP as the criterion were developed in 349 infants, aged 1–3 days using weight, length, flank skinfold and head circumference and compared to existing equations.15 They reported reasonable accuracy for FM prediction by the Catalano (Lin’s Concordance Correlation Coefficient (CCC) 0.84) and Aris equation (Lin’s CCC 0.81) at birth. FM prediction models with ADP as the criterion were developed in 278 white European Australian infants and reported poor agreement for %Fat with a) Lingwood at birth (mean bias −0.32 ); b) Aris at 3 (mean bias −3.45) and 6 months (mean bias 0.25 with wide limits of agreement); c) Lingwood at 3 (mean bias −3.9) and 6 months (mean bias −3.7) against the ADP.14 These results indicate the lack of a consistent anthropometric equation within the age range (0–3 days) in which the equation(s) were derived, and the lack of applicability of these 0–3 day equations to older aged infants at 3 and 6 months. Such inconsistencies are likely attributable to the rapid changes in FM and hydration during the early months of life, ethnic specific differences in FM and FFM, and differences in the outcome variable across the anthropometric equations (FM, %FM, FFM). Furthermore, body composition data from multi-ethnic cohorts throughout infancy are lacking in the literature. The reader is referred to Table 1 for a list of published anthropometric equations frequently referenced in this manuscript.
Table 1:
Select published Anthropometric Equations based on TOBEC, ADP and DXA
| Reference, year and N | Population | BC method and Dependent variable | Equation | R2 And Error |
|---|---|---|---|---|
| Catalano12 N= 194 (D) 65 (V) |
2/3rd White, USA Day 0–72 |
TOBEC FM |
FM=0·54657 + 0·39055 × birth W (kg) – 0·03237 × birth L (cm) + 0·0453 × flank skinfold (mm). | R2 = 0.78 |
| De Bruin13 N=435 (D) 110 (V) |
White, Netherlands Day 21–365 |
TOBEC FM and FFM |
lnFM=−0.358+1.499[ln(W*calf circumference)/L] lnFM=−2.219+1.176[lnW*calf circumference* sq.root (sum of 3skinfolds/L)] lnFFM = 0.433+0.056[sq.root(W*L)] |
R2 = 0.87 SE 0.148 kg R2 = 0.89 SE 0.138 kg R2 = 0.95 SE 0.044 kg |
| Schmelzle18 N=185 (D) |
White, Germany Day 10–4 months |
DXA FM |
FM (newborn)= 68.2 x SigmaSFT((0.0162) x l) - 172.8 | R2 = 0.94 |
| Dung17 N=118 (D) |
Preterm infants, at hospital discharge White, Germany |
DXA and BIA FFM |
Female FFM=0.04Ht2/I+0.71W+0.29 Male FFM=0.05Ht2/I+0.68W+0.40 |
R2 = 0.919 R2 = 0.957 |
| Deierlein14
N= 128 (D) |
Multiethnic, USA, New York Term, Day 1–3 |
ADP FM |
FM=−0.012 −0.064*sex + 0.024*day of measurement post-delivery −0.150*w (kg) + 0.055*w(kg)2 + 0.046*ethnicity + 0.020*sum of three skin-fold thicknesses (triceps, sub scapular, and thigh); | R2 = 0.81, MSE = 0.08 kg. |
| Aris10
N= 88 (D) 62 (V) |
Singaporean Term infants | ADP FM |
FM =–0·022 + 0·307 × weight (kg) – 0·077 × (sex) + 0·028 × subscapular skinfold (mm) – 0·019 × gestational age (weeks) Sex: 1 male, 0 female | R2 = 0.81 |
| Lingwood20 N= 77 (birth) N=54 (6wks) N=55 (3mon) N=53 (4–5mon) |
White, Australia Term, Day 0–4 |
ADP FFM |
FFM (birth)=0.507+0.646*W-0.089*sex+0.009L FFM (6wks)=0.0260+0.528*W-0.125*sex+0.022*L FFM (3mon)=−0.338+0.434*W-0.177*sex+0.041*L FFM (4–5mon)=−0.044+0.397*W-0.427*sex+0.045*L |
R2 (birth) = 94 R2 (6wks) = 89 R2 (3mon) = 89 R2 (4–5 mon) = 87 |
| Josefson16 N=349 (D) 119 (V) |
Multiethnic, USA 12 hrs to 72 hrs of life |
ADP FM |
M0 FM= 0.54657+0.39055*weight-0.03237*L M1 FM=0.24087+0.28396*W(kg)-0.00968*L+0.06669*flank skinfold M2 FM=0.40367+0.34824*W-0.01163*L+0.0625*flank skinfold-0.02168*head circumference (cm) |
|
| Jayasinghe15 N, Birth =188 (D) N, 3 mon=92 (D) N,6 mon=51 (D) |
White, Australia Birth (0–2 days, 3 months and 6 months |
ADP %Fat |
Birth (%Fat)= 1.287 + 0.439*W − 0.026*L − 0.030*GA + 0.060*G 3months (%Fat)= 1.053 + 0.045*SS + 0.488*W−0.052*L + 0.137*G 6 month (%Fat) = 2.537 + 0.679*W − 0.090*L + 0.195*G |
R2 =72 SEE 0.093 R2 = 0.64 SEE 0.249 R2 = 0.63 SEE 0.246 |
TOBEC- Total body electrical conductivity, ADP –Air Displacement Plethysmography, DXA – Dual energy Xray Absorptiometry, D- derivation group, V – validation group, W-weight, L-length, GA-gestational age, M-model, G-Gender, SS- Subscapular skinfold, SFT- skinfold thickness, wks- weeks, mon – months, BIA - Bioelectrical Impedance, Ht2/I-impedance index, ln- log transformed
The purpose of the current study was to develop anthropometric models to estimate FM from ADP (PEA POD®) and Infant-QMR (EchoMRI-Infant: Echo Medical Systems, Houston, Texas) in the same cohort with longitudinal assessments at ages 3 days, 15 weeks, and 54 weeks. These models, if validated aim to serve as a research tool for FM assessments in similarly aged populations when more sophisticated body composition measurement methods are unavailable.
Methods
Participants
Participants were offspring of mothers enrolled in the LIFT (Lifestyle Intervention for Two) trial as previously reported20–22 on whom measures were acquired at three time points (Figure 1). LIFT is part of the LIFE-Moms consortium23 and was a parallel group, randomized controlled trial investigating the effects of a behavioral lifestyle intervention in pregnant women. Women were recruited from hospital affiliated private and clinic practices from February 2013 to October 2015. Eligibility criteria included age ≥18 years, a BMI ≥25 at baseline measurement, singleton pregnancy and gestational age between 9,0 (week, day) and 15,6 confirmed by dating ultrasound. Women diagnosed with seizures, hypertension, or pre-eclampsia were excluded in the LIFT study. The study was approved by the IRBs of St. Luke’s-Roosevelt Hospital and Columbia University and registered with ClinicalTrials.gov (NCT01616147). A written consent was obtained from a parent prior to participation in the study. Infant race/ethnicity was based on maternal self-report of maternal race/ethnicity. The study was approved by and conducted in accordance with the Institutional Review Boards at St Luke’s-Roosevelt Hospital and Columbia University Irving Medical Center. Race included the following categories: White, Black, Asian, Other and Multiracial. Ethnicity was classified as Hispanic and Non- Hispanic.
Figure 1:
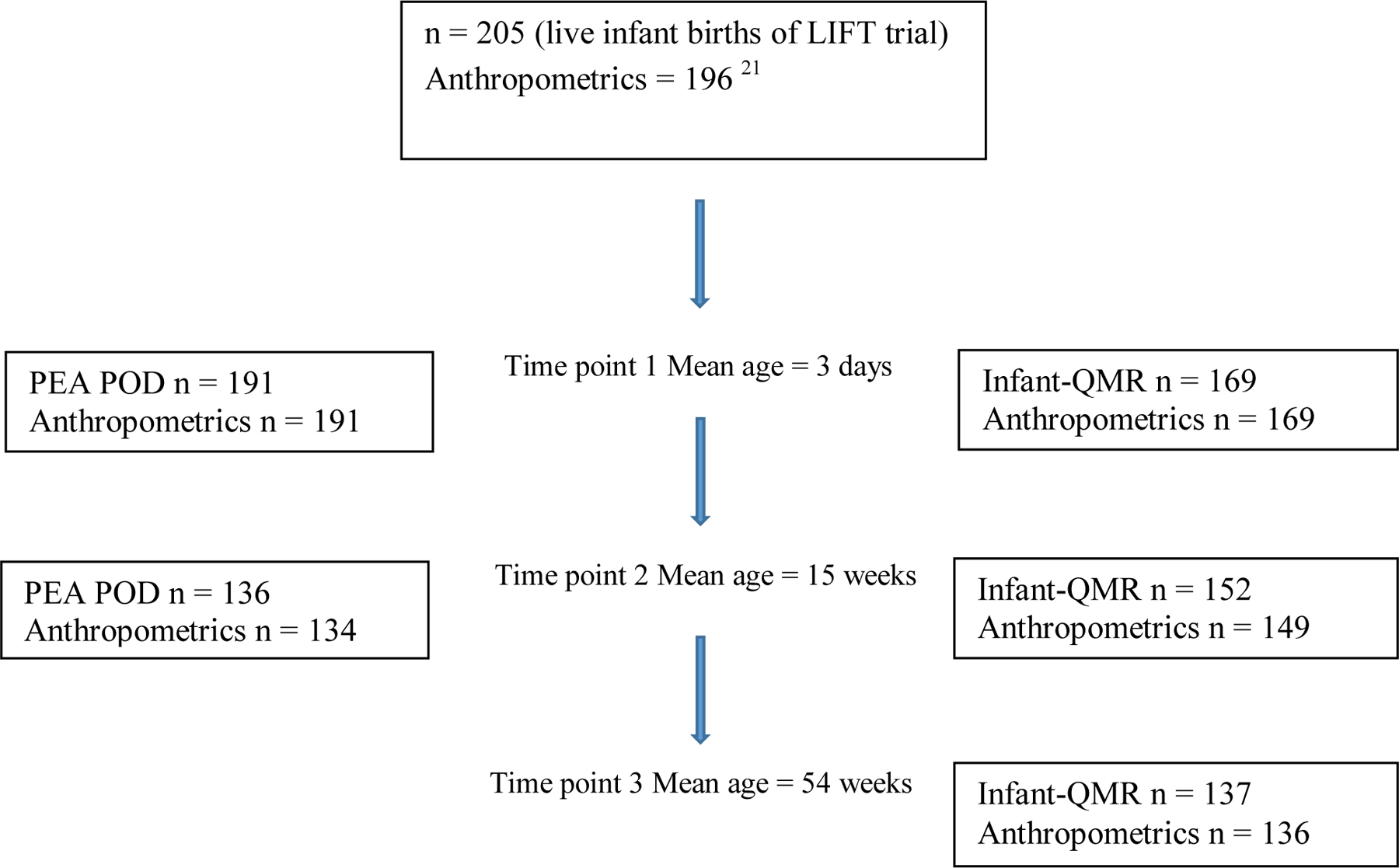
Flow diagram of LIFT infants at each follow up visit
Assessment visits
The first visit was performed prior to hospital discharge, between 1 and 4 days after birth for term infants, or, at 37 weeks post-last menstrual period for preterm infants. If preterm infants were discharged prior to 37 weeks, they were measured as close to discharge as possible. The follow-up visits were scheduled to occur between 13,0–15,0 and 48,0–56,6 weeks.
Anthropometric Measurements
Measurements were not collected on day 0 (<24 hours post-delivery) as data from our laboratory suggest that there is an initial weight loss in infants during this period.24 Trained and certified study personnel (up to 3) obtained neonatal and infant study weight, length, head circumference (HC) and skinfold thicknesses measures prior to hospital discharge between 1 and 4 days after birth for term infants (or at 36 weeks post-last menstrual period of preterm infants) and at the two follow-up visits. All measures throughout the study were conducted using the same calibrated instruments. Weight was measured on a calibrated scale (PEA POD, Cosmed), lengths on a length board (Ellard Instrumentation Ltd) and HC using a tape measure with tensiometer (Gulick II, model 67020). Small for gestational age (SGA) and large for gestational age (LGA) were determined using WHO guidelines for reference population and ultrasound for gestational age.15 Birth weight and length were extracted from medical records. Weight, length, and HC were each measured twice and when >100g difference in weight or 0.5cm difference in length and HC were detected, a third measurement was taken and the average of the 2 closest measurements was used in the analyses. Skinfold thicknesses of the triceps, sub-scapula, iliac crest, and mid-thigh were measured in duplicate using skinfold calipers (Harpenden, model HSB-BI) on the left side of the body. When the results of the duplicate skinfold measures differed by more than 0.5mm, a third measurement was acquired. The two measurements in closest agreement were averaged and used in analyses. Coefficient of variation (CV) of repeated skinfold measures for triceps (TRI), subscapular (SCP), iliac (I), and thigh (THI) in 23 infants at 3 days were 2.5%, 3.1%, 3.3% and 2.8% respectively; in 20 infants at 15 weeks were 2.5%, 2.4%, 3.6% and 1.4% respectively; and in 45 infants at 54 weeks were 2.0%, 2.5%, 2.2% and 0.8% respectively.22
Body Composition Assessment
Body Composition data for this study were obtained from the LIFT trial (Gallagher 2018) for which the infants were measured by the PEA POD (Cosmed USA, Inc) and the EchoMRI-Infant (Infant-QMR). A detailed description of the measurement protocol has been published previously.20, 22 PEA POD and Infant-QMR require short measurements times of under 4 minutes and no sedation. PEA POD is among the most widely used research method for FM and FFM in infants with an upper weight limit of 8 kg that corresponds to approximately 6 months. The PEA POD employs a 2-compartment model that estimates body fat from body weight and volume using assumed densities for FM and FFM. Specifically, a constant FM density of 0.9007g/ml25, 26 and varying age and sex-specific densities of FFM from Fomon27 (birth to 10 years) or Butte28 (0.5 to 24 months). QMR is a non – imaging technique that uses the differences in relaxation times of hydrogen protons in response to radiofrequency pulses of the electromagnetic field to provide independent measures of FM, lean mass, free water and TBW. Infant-QMR demonstrated high accuracy for TBW against deuterium dilution method in infants at age 3 days, and for small changes in FM when simulated fat phantoms using canola oil were added to newborns.29 In our laboratory, repeated PEA POD tests performed twice on the same day on 29 infants gave CV’s of 6.6% for %fat, 6.5% for FM, and 1.1% for FFM; the CV’s in 14 newborns measured 3 times by Infant-QMR with repositioning between scans were 3.27%, 1.83% and 1.34% for fat mass, lean mass, and total body water (TBW), respectively.29
Statistical analysis
Stata 15.1 was used for statistical analyses. Descriptive statistics were calculated for all variables. Means and standard deviations were calculated for continuous variables and percentages for discrete variables. Stepwise regression methods were used to identify a minimal subset of independent variables necessary to predict fat mass with significance level for removal of variables at pr (0.20) and entry of variables at pe (0.1). Dummy variables were created for the categorical variables, biological sex, and race/ethnicity and were used as independent variables in the regression equations. Interaction effects of sex with its significant skinfolds were confirmed in a series of models and subsequently retained as multiplicative terms to ascertain the most parsimonious model. The initial set of independent variables used to model FM included weight, length, triceps, iliac, subscapular, thigh, head circumference, sex, and the multiplicative terms of sex interactions. The final models included all variables with a p-value <0.05. R-squared and root mean squared error were used to evaluate the models. A residual analysis of the model obtained was performed and studentized residuals were tested for assumption of normal distribution using the Shapiro-Wilk’s test. The main effect of ethnicity was tested in each model using the F-statistic. Separate models were derived for 3 days, 15 week and 54 weeks. Significance was set at two-tailed, p<0.05.
Results
Infant characteristics
Births occurred between August 2013 and April 2015. Infant birth characteristics have been previously described20. Descriptive characteristics, anthropometric and body composition data (mean+/−SD) obtained at 3 days, 15 weeks and 54 weeks are presented in Table 2. Infant-QMR measures were obtained at 3 days (n=169), 15 weeks (n=152) and 54 weeks (n=137). PEAPOD measures were obtained at 3 days (n=191) and again at 15 weeks (n=136), in 71.2% of the baseline sample. Variations in sample size across visits was due to attrition.
Table 2:
Descriptive characteristics, anthropometric and body composition at 3 days, 15 weeks and 54 weeks.
| 3 days | 15 weeks | 54 weeks | |||
|---|---|---|---|---|---|
|
| |||||
| Infant-QMR N=169 |
PEA POD N=191 |
Infant-QMR N=152 |
PEA POD N=136 |
Infant-QMR N=137 |
|
| Demographics | Percentage (%) | ||||
|
| |||||
| Sex | |||||
| Female | 77 (46%) | 87 (46%) | 67 (44%) | 63 (46%) | 63 (45%) |
| Male | 92 (54%) | 104 (54%) | 85 (56%) | 73 (54%) | 76 (55%) |
|
| |||||
| Race/Ethnicity | |||||
| White | 43% | 44% | 47% | 45% | 45% |
| Black | 20% | 19% | 20% | 21% | 22% |
| Hispanic | 16% | 17% | 16% | 15% | 15% |
| Asian | 1% | 2% | 1% | 1% | 0% |
| Multiracial/Other | 20% | 18% | 16% | 18% | 18% |
|
| |||||
| Mean (SD) | |||||
|
| |||||
| Gestational age (wks) | |||||
| Term (n=179) | 37.5 (1.08) | ||||
| Preterm (n=12) | 34.13 (2.1) | ||||
| SGA (<10th %ile) N | 15 (8%) | ||||
| LGA (>10th %ile) N | 20 (10.5%) | ||||
|
| |||||
| Age (wks) | 0.45 (0.84) | 0.44 (0.83) | 15.0 (3.05) | 14.79 (2.53) | 54.64 (5.27) |
|
| |||||
| Anthropometrics | |||||
|
| |||||
| Weight (kg) | 3.15 (0.52) | 3.17 (0.51) | 6.16 (0.96) | 6.14 (0.91) | 9.68 (1.18) |
|
| |||||
| Length (cm) | 49.46 (2.38) | 49.55 (2.33) | 61.34 (2.98) | 61.28 (2.99) | 75.36 (2.78) |
|
| |||||
| Head Circumference (cm) | 34.12 (1.50) | 34.16 (1.49) | 40.84 (1.54) | 40.76 (1.48) | 46.35 (1.48) |
|
| |||||
| Triceps (mm) | 4.66 (1.19) | 4.66 (1.24) | 9.45 (1.92) | 9.58 (1.84) | 9.84 (2.49) |
|
| |||||
| Subscapular (mm) | 4.38 (1.19) | 4.46 (1.22) | 7.30 (1.79) | 7.3 (1.8) | 6.80 (1.69) |
|
| |||||
| Iliac (mm) | 3.91 (0.97) | 3.95 (0.96) | 9.31 (2.53) | 9.38 (2.66) | 9.06 (2.97) |
|
| |||||
| Thigh (mm) | 5.74 (1.70) | 5.81 (1.73) | 17.85 (4.25) | 17.97 (4.29) | 16.96 (4.12) |
|
| |||||
|
Body Composition
| |||||
| Fat Mass (kg) | 0.53 (0.18) | 0.34 (0.17) | 1.77 (0.52) | 1.51 (0.45) | 2.77 (0.64) |
|
| |||||
| Fat (%) | 10.5 (4.1) | 24.3 (4.8) | |||
|
| |||||
| Lean (kg) | 2.27 (0.32) | 3.70 (0.47) | 5.35 (0.57) | ||
|
| |||||
| Fat-Free Mass (kg) | 2.83 (0.4) | 4.62 (0.61) | |||
N= number, SD= standard deviation, Infant-QMR = quantitative magnetic resonance output provides fat mass, lean mass and total body water. PEA POD output provides fat mass, fat % and fat free mass
Correlations of anthropometric measures with FM
Significant moderate to strong positive correlations (r=0.5–0.8) between FM (Infant-QMR and PEA POD) with weight and skinfolds were observed at 3 days, 15 and 54 weeks (Table 3). Length and HC showed weak to moderate positive correlations (r=0.3–0.6) with Infant-QMR-FM and PEA POD-FM at all 3 time points.
Table 3:
Correlations (r) of fat mass with infant anthropometric variables
| Fat Mass (kg) | |||||
|---|---|---|---|---|---|
| 3 days | 15 weeks | 54 weeks | |||
| Infant-QMR N= 169 |
PEA POD N= 191 |
Infant-QMR N= 152 |
PEA POD N= 136 |
Infant-QMR N= 137 |
|
| Weight (kg) | 0.84* | 0.71* | 0.88* | 0.80* | 0.76* |
| Length (cm) | 0.67* | 0.50* | 0.64* | 0.5* | 0.35* |
| Head Circumference (cm) | 0.60* | 0.48* | 0.51* | 0.38* | 0.27* |
| Skinfold thickness (mm) | |||||
| Triceps | 0.72* | 0.72* | 0.58* | 0.61* | 0.62* |
| Thigh | 0.78* | 0.76* | 0.58* | 0.58* | 0.73* |
| Sub-scapular | 0.71* | 0.77* | 0.54* | 0.50* | 0.66* |
| Iliac | 0.72* | 0.70* | 0.61* | 0.62* | 0.67* |
| Sum of Skinfolds | 0.81* | 0.81* | 0.64* | 0.70* | 0.79* |
Significance p <0.05
Fat Mass prediction models
Regression models were derived for FM by Infant-QMR at 3 days, 15 weeks, and 54 weeks and by PEA POD at 3 days and 15 weeks as shown in Table 4. There was a significant sex interaction at 3 days and 15 weeks for both Infant-QMR and PEA POD (p<0.001), and multiplicative terms were used in the analysis to derive the models. As the multiplicative terms are treated as independent variables in the current models, model coefficients for male and female are presented separately for clarity in Table 4. No significant sex effect was noted at 54 weeks (Infant-QMR p=0.3). No significant effect for ethnicity was found at 3 days (Infant-QMR p=0.5; PEA POD p=0.3), 15 weeks (Infant-QMR p=0.5, PEA POD p=0.2) or at 54 weeks (Infant-QMR p=0.8). The studentized residuals were consistent with a normal distribution across all models (3 days Infant-QMR p=0.3 and PEA POD p=0.8; 15 week Infant-QMR p=0.8 and PEA POD p=0.3; 52 weeks Infant-QMR p=0.2). Weight was a consistent predictor of FM and was positively associated with FM across all models throughout infancy. Each individual skinfold when present in the model was positively associated with FM, whereas length and HC were negatively associated with FM. Infant-QMR FM and PEA POD FM were strongly correlated at 3 days (r=0.87) and 15 weeks (r=0.94). Moreover, linear regression of Infant-QMR FM on PEA POD FM were consistent with a unity slope with a non-constant intercept suggesting that Infant-QMR FM was consistently higher than the PEA POD FM.
Table 4:
Fat mass prediction models for females (F) and males (M)
| Age | Equation | R2 | RMSE | |
|---|---|---|---|---|
| 3 days | Infant-QMR | F: Fat (kg)= −0.027+0.273*W-0.016*HC+0.027*TRI+0.026*THI M: Fat (kg)= −0.027+0.273*W-0.016*HC+0.046*SCP |
0.87 | 0.066 |
| PEA POD | F: Fat (kg)= −0.028+0.237*W-0.013*L+0.014*THI+0.049*SCP M: Fat (kg)= 0.795+0.237*W-0.013*L+0.014*THI+0.049*SCP-0.026*HC |
0.77 | 0.080 | |
|
| ||||
| 15 weeks | Infant-QMR | F: Fat (kg)= 0.431+0.637*W-0.067*HC+0.038*SCP M: Fat (kg)=1.196+0.467*W-0.067*HC+0.036*TRI |
0.92 | 0.153 |
| PEA POD | F: Fat (kg)= 2.600+0.586*W-0.041*L-0.063*HC+0.015*THI+0.026*TRI M: Fat (kg)=2.917+0.586*W-0.041*L-0.063*HC-0.013*THI+0.026*TRI |
0.82 | 0.195 | |
|
| ||||
| 54 weeks | Infant-QMR | Fat (kg)=3.728+0.413*W-0.036*L-0.076*HC+0.063*SCP+0.051*THI | 0.82 | 0.278 |
Fat = fat mass (kg), W = weight (kg), L = length (cm), HC = head circumference (cm), TRI = triceps skinfolds (mm), THI = thigh skinfolds (mm), and SCP skinfolds = subscapular (mm)
N (Infant-QMR) =169 at 3 days, 152 at 15 weeks and 137 at 54 weeks
N (PEA POD) =191 at 3 days and 136 at 54 weeks.
3 days
The model that best explained QMR-FM contained weight, HC, TRI, and THI skinfolds in females (n=77); weight, HC, and SCP skinfold in males (n=92). This model explained 87% of the variance in QMR-FM with a root mean square error (RMSE) of 66 grams (g). The PEA POD-FM model contained weight, length, HC, THI, and SCP skinfolds in females (n=87); and weight, length, THI, and SCP skinfolds in males (n=104). This model explained 77% of the variance in PEAPOD-FM with a RMSE of 80g.
15 weeks
The model that best explained QMR-FM contained weight, HC, and SCP in females (n=67); and weight, HC, and TRI skinfolds in males (n=85). This model explained 92% of the variance in QMR-FM with a RMSE of 153g. The PEA POD-FM model contained weight, length, HC, THI, and TRI skinfolds in both females (n=63) and males (n=73). This model explained 82% of the variance in PEA POD-FM with a RMSE of 195g.
54 weeks
The model that best explained QMR-FM contained weight, length, HC, SCP, and THI skinfolds (n=137). This model explained 82% of the variance in QMR-FM with a RMSE of 278g. There was no effect of sex in this model.
Discussion
Fat mass prediction models using weight, head circumference, length, and skinfolds were developed in a cohort of infants measured longitudinally at 3 days, 15 and 54 weeks. The Infant-QMR derived models explained 87% (3 days), 92% (15 wks) and 82% (54 wks) of the variation in FM, and the PEAPOD derived models explained 77% (3 days) and 82% (15 wks) of the variation in FM. The Infant-QMR models explained a larger percentage of the variability in FM than the PEA POD models. The Infant-QMR models had an estimated FM standard deviation of 66 gm (12%) at 3 days, 153 gm (8%) at 15 weeks, and 278 gm (10%) at 54 weeks. The PEA POD models had an estimated FM standard deviation of 80g (23%) at 3 days and 195 gm (13%) at 15 weeks. The root mean square error expressed as a percentage of the mean fat mass was lower for Infant-QMR models (8–12%) compared to PEA POD models (13–23%) suggesting greater accuracy for Infant-QMR models. To our knowledge, these are the first FM prediction equations developed in the same cohort across the first year of life using Infant-QMR as a criterion. This effort serves to narrow a gap in the literature related to the paucity of anthropometric FM prediction models at 54 weeks of age.
Due to its high cost, Infant-QMR is available globally in just a few specialized research centers and the Infant-QMR system allows for measurements up to 12 kg. Infant-QMR presents a unique opportunity to estimate FM longitudinally across infancy to at least one-year of age, whilst the PEA POD can accommodate only up to 8kg (~6 months of age). Furthermore, the PEA POD and Infant-QMR measure different components using distinct approaches. In our study, we applied the Fomon density for FFM, which depends on hydration. The Foman27 birth constants were derived from infants of several pooled studies but placed in a single category which may not accurately represent the body composition of 3 day old infants, due to rapid changes in hydration following birth.2 Fomon27 reported lower TBW at birth (~68.6 – 69.6%) compared to other studies (74%−78%)29, 30 that are lower than our study (76.5%) which may reflect the assumptions made in methods and calculations as described by Fomon.31 Lipsmeyer et al32 reported high accuracy for both PEA POD and QMR (EchoMRI-AH Small version) against a 4C model and a strong correlation between PEA POD and QMR (r=0.96) in infants <8kg. However, the QMR EchoMRI-AH Small with adjusted FM estimation equation 33 were used and the individual components of the 4C model were obtained from the methods being compared, which could introduce bias. In our study, there was a significant mean FM difference of 190 gm (6.3% Fat) at 3 days between Infant-QMR and PEA POD and a difference of 260 gm (3.5% Fat) at 15 weeks. The between method differences in measured FM are likely due to differences in the underlying assumptions and algorithms incorporated2, 29, 33, 34 and hydration changes with age.
Body composition changes progressively from birth throughout infancy as children grow and mature, reflecting dynamic differences in FM and FFM1. It is to be expected that equations developed in one age group would not perform well in other age groups given the rapid changes in body composition throughout infancy.14, 15, 18 The newborn has higher hydration of FFM (i.e., TBW/FFM, expressed in percent) than a child at 1 year, approximately 83% versus 78–79%.27, 28 In our study population, TBW at 3 days was 76.5%22which is higher than previously reported by Fomon27 (~68%) and Butte28 (~73%), and TBW decreased to 54% at 54 weeks which is lower than Fomon (~60%) and Butte (~58%). In our study, FM increased from 341 gm (10.5% Fat) by PEAPOD and 530 gm (16%) by Infant-QMR at 3 days to 1510 gm (24.3% Fat) by PEAPOD and 1770 gm (28.7%) by Infant-QMR to 2790 gm (28%) by Infant-QMR at 54 weeks. Additionally, factors affecting fat prediction include differences in body composition methods and variables in the derived models. Hence, models based on a single center longitudinal cohort at 3 time points is a strength of this study.
Consistent with other studies that estimated FM by ADP,9, 13, 15TOBEC,11 or DXA,17, 35, 36 the current study found weight, length and skinfolds to be predictors of FM. In general, weight and length are known to explain a significant portion of the variance in infant FM3, 9, 35 (R2 0.62 −0.84) but the literature investigating the magnitude of the role of skinfolds in FM prediction is inconclusive. While one study found the sum of skinfolds were correlated more strongly with FM (r=0.62) than FFM(r=0.42) by PEAPOD in a cohort of neonates (n=251, mean age 10 days),37 other studies found skinfolds to be poor predictors of FM determined by body water dilution technique at varying ages between 4 days to 14 months.38–40 As shown in Table 1, 1-3 skinfolds have been included in some of the existing models for FM in the published literature. Our study included 3 skinfolds (Tri, SCP and Thi), where each alone or in combinations of 2 skinfolds, were significant predictors of FM across infancy. The inclusion of skinfolds to a base model of weight, length and sex in our study improved the R2 on average by 9% for 3 days and 15 weeks and by 23% for the 54 week models. Interestingly, HC was a predictor in all models except in the PEA POD female model at 3 days. Only one other study involving a PEA POD based model15 included HC as a FM predictor variable with flank (ie, iliac) skinfold. Small head circumference at birth predicts an early adiposity rebound, which predicts obesity and type 2 diabetes and coronary heart disease later in life.41 There is a paucity of data exploring the relationship between head circumference and FM.
Sex differences in FM and FFM are evident from birth throughout childhood, with girls having higher FM values.27, 28, 42–44 Up to a 3% difference in %Fat between sexes from birth through 12 months was reported by Fomon27 (1982) and Butte28. A systematic review of body composition by ADP in infants born at term45 reported a 2% higher %Fat in girls compared to boys within the first 4 days of birth. It is therefore appropriate for sex to be included as a covariate in body composition prediction models beginning at birth.9, 13, 14 These sex differences are postulated to be related to the effect of androgenic steroids present in males.46, 47 A sex interaction was observed in the current study at 3 days indicating the need for development of sex specific models at later time points. However, no sex interaction was found at 54 weeks likely due to a smaller sample size. Others have found neonatal fat mass differences based on ethnicity.13, 45, 47, 48 While ethnicity was not a predictor of FM in this study, it is acknowledged that maternal self-reported race/ethnicity was applied to the infant without consideration for the father’s ethnicity. In such multiracial and multiethnic cohorts typical of New York City, it is probable that the role of ethnicity cannot be truly determined.
Higher birth weight and accelerated infant weight gain are consistently associated with later childhood overweight.49 The first 1000 days refers to the period from conception to age 2 years that presents as a unique window of opportunity when intervention could help shape a child’s health trajectory. In the era of precision nutrition medicine, the first 1000 days are gaining recognition for a potential role in chronic disease prevention, and in particular, childhood obesity prevention.49 The simple FM prediction models presented here may aid towards this goal. Furthermore, this study adds to the literature with models based on Infant-QMR, to further knowledge in this field. Such models, after validation in different cohorts, have the potential to serve as a valuable tool both at the field/population level to collect large group data in studies assessing links between FM in infancy and development of morbidities in the future. The time points of 3 days, 15 weeks and 54 weeks correspond closely to the Bright futures/American Academy of Pediatrics recommendations for preventative health care visits of 3–5 days, 16 weeks and 52 weeks, which could provide an opportunity for application of these models after validation. While weight, height and HC are part of routine growth monitoring, the skinfold measures may be collected with additional training.50 Future application of appropriately validated FM prediction models include incorporation into electronic medical records that could inform clinicians /pediatricians to initiate discussions at these early life child wellness visits, as it is well established that a large percentage of children with early onset obesity continue to have obesity in later childhood and adulthood.
While many published equations presented in Table 1 do not provide maternal data, maternal data and factors affecting neonatal body composition for this cohort was previously discussed20. Small significant differences were reported in mean fat mass and body fat percent in the first months of life between children born to mothers with overweight or with obesity and those from mothers with normal maternal pre-pregnancy weight, based on a meta-analysis.51 It is important to note that our cohort involved infants of mothers with overweight or obesity (average BMI 30 kg/m2), which may affect the generalizability of the models. Feeding practices affect hydration in the first 2 weeks of infancy, and differences in FM and FFM exist between breast and formula fed infants.52 This dataset did not include details of feeding practices or physical activity throughout infancy. Internal and external validation studies are needed to assess the accuracy and agreement of these equations in broader and similar populations. It is acknowledged that skinfolds are prone to measurement errors12 which in this study, were minimized by using trained personnel with annual certification by a master trainer.
Conclusions
This study presents FM anthropometric prediction models developed using robust criterion methods in early infancy and childhood, that with future validation studies could have potential application in population studies. These could be valuable for assessing disease development and risk on a larger scale in longitudinal studies from birth and into adulthood with considerations for preventative strategies at a very early stage.
What is already known about this subject?
Infancy is the period of most rapid postnatal growth that is accompanied by major changes in proportion of body weight that is fat and fat free mass including changes in hydration.
Anthropometric fat prediction models maybe useful in field studies for longitudinal follow-up to inform later health outcomes.
Few ADP derived infant anthropometric models are available and no models have been reported using Infant-QMR as the criterion.
What this study adds
Anthropometric fat prediction models were developed from the same multi-ethnic cohort at 3 days, 15 weeks and 54 weeks.
This is the first study to report on anthropometric equations based on Infant-QMR as a criterion at 3 ages or time points in infancy.
Both PEAPOD and Infant-QMR derived prediction models estimate fat mass with acceptable coefficients of determination.
Acknowledgements
Funding information:
National Institutes of Health Grants T32-DK065522 (PI: S. Oberfield) supported Gopalakrishnamoorthy. U01-DK094463; U01-DK094463-Supplement (Supplement to promote diversity, T. Toro-Ramos, PhD); P30-DK026687; T32-DK007559 (Toro-Ramos, Widen; Whyte); T32DK091227 (Widen); K99/R00HD086304 to Dr. Widen.
Research reported in this publication was supported by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, U01 DK094418, U01 DK094463, U01 DK094416, 5U01 DK094466 (RCU)), the National Heart, Lung, and Blood Institute (NHLBI, U01 HL114344, U01 HL114377), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, U01 HD072834), the National Center for Complementary and Integrative Health (NCCIH), the NIH Office of Research in Women’s Health (ORWH), the Office of Behavioral and Social Science Research (OBSSR), the NIH Office of Disease Prevention (ODP), the Indian Health Service, the Intramural Research Program of the NIDDK, and the Office of the Director, National Institutes of Health (OD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the LIFE-Moms consortium members for their contributions to the development and oversight of the common measures and procedures shared across the trials.
Footnotes
Disclosure: The authors declare no conflict of interest.
Clinical Trial Registry NCT01616147; www.ClinicalTrials.gov
References
- 1.Gallagher D, Andres A, Fields DA, et al. Body Composition Measurements from Birth through 5 Years: Challenges, Gaps, and Existing & Emerging Technologies-A National Institutes of Health workshop. Obes Rev Aug 2020;21(8):e13033. doi: 10.1111/obr.13033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toro-Ramos T, Paley C, Pi-Sunyer FX, Gallagher D. Body composition during fetal development and infancy through the age of 5 years. Eur J Clin Nutr Dec 2015;69(12):1279–89. doi: 10.1038/ejcn.2015.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demerath EW, Fields DA. Body composition assessment in the infant. Am J Hum Biol May-Jun 2014;26(3):291–304. doi: 10.1002/ajhb.22500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res 2006;65 Suppl 3:65–9. doi: 10.1159/000091508 [DOI] [PubMed] [Google Scholar]
- 5.Fryar CD, Gu Q, Ogden CL, Flegal KM. Anthropometric Reference Data for Children and Adults: United States, 2011–2014. Vital Health Stat 3 Aug 2016;(39):1–46. [PubMed] [Google Scholar]
- 6.Wang J, Thornton JC, Kolesnik S, Pierson RN Jr. Anthropometry in body composition. An overview. Ann N Y Acad Sci May 2000;904:317–26. doi: 10.1111/j.1749-6632.2000.tb06474.x [DOI] [PubMed] [Google Scholar]
- 7.Yajnik CS, Fall CH, Coyaji KJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord Feb 2003;27(2):173–80. doi: 10.1038/sj.ijo.802219 [DOI] [PubMed] [Google Scholar]
- 8.Silva AM, Fields DA, Sardinha LB. A PRISMA-driven systematic review of predictive equations for assessing fat and fat-free mass in healthy children and adolescents using multicomponent molecular models as the reference method. J Obes 2013;2013:148696. doi: 10.1155/2013/148696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aris IM, Soh SE, Tint MT, et al. Body fat in Singaporean infants: development of body fat prediction equations in Asian newborns. Eur J Clin Nutr Sep 2013;67(9):922–7. doi: 10.1038/ejcn.2013.69 [DOI] [PubMed] [Google Scholar]
- 10.Sen B, Bose K, Shaikh S, Mahalanabis D. Prediction equations for body-fat percentage in Indian infants and young children using skinfold thickness and mid-arm circumference. J Health Popul Nutr Jun 2010;28(3):221–9. doi: 10.3329/jhpn.v28i3.5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol Oct 1995;173(4):1176–81. doi: 10.1016/0002-9378(95)91348-3 [DOI] [PubMed] [Google Scholar]
- 12.de Bruin NC, van Velthoven KA, Stijnen T, Juttmann RE, Degenhart HJ, Visser HK. Body fat and fat-free mass in infants: new and classic anthropometric indexes and prediction equations compared with total-body electrical conductivity. Am J Clin Nutr Jun 1995;61(6):1195–205. doi: 10.1093/ajcn/61.6.1195 [DOI] [PubMed] [Google Scholar]
- 13.Deierlein AL, Thornton J, Hull H, Paley C, Gallagher D. An anthropometric model to estimate neonatal fat mass using air displacement plethysmography. Nutr Metab (Lond) Mar 21 2012;9:21. doi: 10.1186/1743-7075-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayasinghe S, Herath MP, Beckett JM, Ahuja KDK, Byrne NM, Hills AP. Anthropometry-based prediction of body fat in infants from birth to 6 months: the Baby-bod study. Eur J Clin Nutr Oct 14 2020;doi: 10.1038/s41430-020-00768-3 [DOI] [PubMed]
- 15.Josefson JL, Nodzenski M, Talbot O, Scholtens DM, Catalano P. Fat mass estimation in neonates: anthropometric models compared with air displacement plethysmography. Br J Nutr Feb 2019;121(3):285–290. doi: 10.1017/s0007114518003355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dung NQ, Fusch G, Armbrust S, Jochum F, Fusch C. Body composition of preterm infants measured during the first months of life: bioelectrical impedance provides insignificant additional information compared to anthropometry alone. Eur J Pediatr Mar 2007;166(3):215–22. doi: 10.1007/s00431-006-0232-y [DOI] [PubMed] [Google Scholar]
- 17.Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin Nutr Nov 2002;76(5):1096–100. doi: 10.1093/ajcn/76.5.1096 [DOI] [PubMed] [Google Scholar]
- 18.Cauble JS, Dewi M, Hull HR. Validity of anthropometric equations to estimate infant fat mass at birth and in early infancy. BMC Pediatr Mar 27 2017;17(1):88. doi: 10.1186/s12887-017-0844-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingwood BE, Storm van Leeuwen AM, Carberry AE, et al. Prediction of fat-free mass and percentage of body fat in neonates using bioelectrical impedance analysis and anthropometric measures: validation against the PEA POD. Br J Nutr May 2012;107(10):1545–52. doi: 10.1017/s0007114511004624 [DOI] [PubMed] [Google Scholar]
- 20.Gallagher D, Rosenn B, Toro-Ramos T, et al. Greater Neonatal Fat-Free Mass and Similar Fat Mass Following a Randomized Trial to Control Excess Gestational Weight Gain. Obesity (Silver Spring) Mar 2018;26(3):578–587. doi: 10.1002/oby.22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janumala I, Toro-Ramos T, Widen E, et al. Increased Visceral Adipose Tissue Without Weight Retention at 59 Weeks Postpartum. Obesity (Silver Spring) Mar 2020;28(3):552–562. doi: 10.1002/oby.22736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whyte K, Johnson J, Kelly K, et al. No sustained effects of an intervention to prevent excessive GWG on offspring fat and lean mass at 54 weeks: Yet a greater head circumference persists. Pediatr Obes Jan 4 2021:e12767. doi: 10.1111/ijpo.12767 [DOI] [PMC free article] [PubMed]
- 23.Clifton RG, Evans M, Cahill AG, et al. Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity. Obesity (Silver Spring) Feb 2016;24(2):305–13. doi: 10.1002/oby.21330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hull HR, Thornton JC, Ji Y, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol Sep 2011;205(3):211.e1–7. doi: 10.1016/j.ajog.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fidanza F, Keys A, Anderson JT. Density of body fat in man and other mammals. J Appl Physiol Oct 1953;6(4):252–6. doi: 10.1152/jappl.1953.6.4.252 [DOI] [PubMed] [Google Scholar]
- 26.Wang ZM, Pierson RN Jr., Heymsfield SB. The five-level model: a new approach to organizing body-composition research. Am J Clin Nutr Jul 1992;56(1):19–28. doi: 10.1093/ajcn/56.1.19 [DOI] [PubMed] [Google Scholar]
- 27.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr May 1982;35(5 Suppl):1169–75. doi: 10.1093/ajcn/35.5.1169 [DOI] [PubMed] [Google Scholar]
- 28.Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res May 2000;47(5):578–85. doi: 10.1203/00006450-200005000-00004 [DOI] [PubMed] [Google Scholar]
- 29.Toro-Ramos T, Paley C, Wong WW, et al. Reliability of the EchoMRI Infants System for Water and Fat Measurements in Newborns. Obesity (Silver Spring) Sep 2017;25(9):1577–1583. doi: 10.1002/oby.21918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friis-Hansen B Body water compartments in children: changes during growth and related changes in body composition. Pediatrics Aug 1961;28:169–81. [PubMed] [Google Scholar]
- 31.Fomon SJ, Nelson SE. Body composition of the male and female reference infants. Annu Rev Nutr 2002;22:1–17. doi: 10.1146/annurev.nutr.22.111401.145049 [DOI] [PubMed] [Google Scholar]
- 32.Heard-Lipsmeyer ME, Hull H, Sims CR, Cleves MA, Andres A. Evaluating body composition in infancy and childhood: A comparison between 4C, QMR, DXA, and ADP. Pediatr Obes June 2020;15(6):e12617. doi: 10.1111/ijpo.12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andres A, Gomez-Acevedo H, Badger TM. Quantitative nuclear magnetic resonance to measure fat mass in infants and children. Obesity (Silver Spring) Oct 2011;19(10):2089–95. doi: 10.1038/oby.2011.215 [DOI] [PubMed] [Google Scholar]
- 34.Mitchell AD. Validation of quantitative magnetic resonance body composition analysis for infants using piglet model. Pediatr Res Apr 2011;69(4):330–5. doi: 10.1203/PDR.0b013e31820a5b9c [DOI] [PubMed] [Google Scholar]
- 35.Koo WW, Walters JC, Hockman EM. Body composition in neonates: relationship between measured and derived anthropometry with dual-energy X-ray absorptiometry measurements. Pediatr Res Nov 2004;56(5):694–700. doi: 10.1203/01.Pdr.0000142587.59238.Bd [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez G, Samper MP, Ventura P, Moreno LA, Olivares JL, Pérez-González JM. Gender differences in newborn subcutaneous fat distribution. Eur J Pediatr Aug 2004;163(8):457–61. doi: 10.1007/s00431-004-1468-z [DOI] [PubMed] [Google Scholar]
- 37.Chen LW, Tint MT, Fortier MV, et al. Which anthropometric measures best reflect neonatal adiposity? Int J Obes (Lond) Mar 2018;42(3):501–506. doi: 10.1038/ijo.2017.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabir N, Forsum E. Estimation of total body fat and subcutaneous adipose tissue in full-term infants less than 3 months old. Pediatr Res Oct 1993;34(4):448–54. doi: 10.1203/00006450-199310000-00013 [DOI] [PubMed] [Google Scholar]
- 39.Olhager E, Forsum E. Assessment of total body fat using the skinfold technique in full-term and preterm infants. Acta Paediatr Jan 2006;95(1):21–8. doi: 10.1080/08035250500323731 [DOI] [PubMed] [Google Scholar]
- 40.Tennefors C, Forsum E. Assessment of body fatness in young children using the skinfold technique and BMI vs body water dilution. Eur J Clin Nutr Mar 2004;58(3):541–7. doi: 10.1038/sj.ejcn.1601842 [DOI] [PubMed] [Google Scholar]
- 41.Eriksson B, Löf M, Eriksson O, Hannestad U, Forsum E. Fat-free mass hydration in newborns: assessment and implications for body composition studies. Acta Paediatr May 2011;100(5):680–6. doi: 10.1111/j.1651-2227.2011.02147.x [DOI] [PubMed] [Google Scholar]
- 42.Fields DA, Gilchrist JM, Catalano PM, Giannì ML, Roggero PM, Mosca F. Longitudinal body composition data in exclusively breast-fed infants: a multicenter study. Obesity (Silver Spring) Sep 2011;19(9):1887–91. doi: 10.1038/oby.2011.11 [DOI] [PubMed] [Google Scholar]
- 43.Koo WW, Walters JC, Hockman EM. Body composition in human infants at birth and postnatally. J Nutr Sep 2000;130(9):2188–94. doi: 10.1093/jn/130.9.2188 [DOI] [PubMed] [Google Scholar]
- 44.Shaw NJ, Crabtree NJ, Kibirige MS, Fordham JN. Ethnic and gender differences in body fat in British schoolchildren as measured by DXA. Arch Dis Child Oct 2007;92(10):872–5. doi: 10.1136/adc.2007.117911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiechers C, Kirchhof S, Maas C, Poets CF, Franz AR. Neonatal body composition by air displacement plethysmography in healthy term singletons: a systematic review. BMC Pediatr Dec 12 2019;19(1):489. doi: 10.1186/s12887-019-1867-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis SM, Kaar JL, Ringham BM, Hockett CW, Glueck DH, Dabelea D. Sex differences in infant body composition emerge in the first 5 months of life. J Pediatr Endocrinol Metab Nov 26 2019;32(11):1235–1239. doi: 10.1515/jpem-2019-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanfield KM, Wells JC, Fewtrell MS, Frost C, Leon DA. Differences in body composition between infants of South Asian and European ancestry: the London Mother and Baby Study. Int J Epidemiol Oct 2012;41(5):1409–18. doi: 10.1093/ije/dys139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paley C, Hull H, Ji Y, et al. Body fat differences by self-reported race/ethnicity in healthy term newborns. Pediatr Obes Oct 2016;11(5):361–8. doi: 10.1111/ijpo.12072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med Jun 2016;50(6):761–779. doi: 10.1016/j.amepre.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 50.AAP. Periodicity Schedule 2021, Bright futures American Academy of Pediatrics. [Google Scholar]
- 51.Castillo-Laura H, Santos IS, Quadros LCM, Matijasevich A. Maternal obesity and offspring body composition by indirect methods: a systematic review and meta-analysis. Cadernos De Saude Publica Nov 2015;31(10):2073–2092. doi: [DOI] [PubMed] [Google Scholar]
- 52.Gale C, Logan KM, Santhakumaran S, Parkinson JR, Hyde MJ, Modi N. Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am J Clin Nutr Mar 2012;95(3):656–69. doi: 10.3945/ajcn.111.027284 [DOI] [PubMed] [Google Scholar]


