Abstract
Improving the quality of medication use and medication safety are important priorities for prescribers who care for older adults. The objective of this article was to identify four exemplary articles with this focus in 2020. We selected high-quality studies that moved the field of research forward and were not merely replication studies. The chosen articles cover domains related to deprescribing, medication safety, and optimizing medication use. The first study, a noninferiority randomized clinical trial in England, evaluated whether antihypertensive medication reduction is possible without significant changes in systolic blood pressure control or adverse events over the 12-week follow-up (domain: deprescribing). The second study, a prospective cohort study of women at Kaiser Permanente Southern, California, examined the association between bisphosphonate use and atypical femur fracture (domain: medication safety). The third study examined the effectiveness and safety of a multifaceted antimicrobial stewardship and quality improvement initiative in reducing unnecessary antimicrobial use for unlikely cystitis cases in noncatheterized residents in 25 nursing homes across the United States (domain: optimizing medication use). Lastly, the fourth study, a population-based cohort study in the United Kingdom, examined the association of tramadol use with risk of hip fracture (domain: medication safety). Collectively, this review succinctly highlights pertinent topics related to promoting safe use of medications and promotes awareness of optimizing older adults’ medication regimens.
Keywords: medication-related problems, aged, 80 years and over, deprescription, polypharmacy, inappropriate prescribing
1. INTRODUCTION
Medication use quality and safety has become a global public health issue and an important national health priority in many countries in line with the third World Health Organization (WHO) Global Patient Safety Challenge: Medication Without Harm. This initiative targeted three key areas of medication safety: high-risk situations, polypharmacy, and transitions of care. 1 Optimal medication use can improve health outcomes in older adults and reduce healthcare costs.
Prescribing medications in older adults is a complex process. Given this challenge, clinicians face multiple and often competing tasks, prescribing evidence-based therapy and trying to avoid unnecessary polypharmacy. These challenges might lead to concomitant overprescribing and underprescribing in the same patient. 2 Aspects of suboptimal prescribing include unnecessary polypharmacy (using medications more than what is clinically indicated), potentially inappropriate medications (medications with potential harms that outweigh their possible benefits) and underutilizing medications (i.e., potential prescribing omissions [PPOs] - failure to prescribe a medication that is clinically indicated). 2
Further complicating the ability to optimize medication use is the absence of information regarding efficacy and safety of medications in older adults, especially those that are frail. Of particular concern is the lack of evidence-based clinical practice guidelines to guide medication management decisions in older adults with multimorbidity, as most clinical recommendations focus on the management of a single disease. 3 Older adults are underrepresented in randomized controlled Phase III efficacy trials even if the medications will be used frequently in this age group. 4, 5 Furthermore, clinical trials often do not measure certain adverse drug events (ADEs) that are important to older adults, such as falls or cognitive impairment.6 In the absence of such information, clinicians are left to extrapolate available medication information from observational studies or clinical trial information from younger age groups, and to utilize guiding geriatric medication principles to achieve medication effectiveness while attempting to avoid medication risks. 7–9
Collating the relevant literature related to the quality use of medications and medication safety among older adults can be challenging due to lack of consistent search terms in computerized databases. Therefore, in this annual special article, we identify exemplary articles published in 2020 related to medication use quality and medication safety. We highlight four articles by providing a study summary, critique and implications.
2. METHODS
2.1. Data sources and search strategy
We conducted an EMBASE database search with the aid of a librarian and restricted the search to English language articles published (in print) in 2020 and including older adults (mean age ≥ 65 years). The search strategy combined terms related to aging, medication related problems and adverse drug reactions (Supplementary Appendix S1). In addition, we performed a manual search for relevant articles from select high-impact journals (Supplementary Appendix S2). The de-duplicated citations were then uploaded to Abstrackr software (abstrackr.cebm.brown.edu), a free online citation screening tool, to screen the titles and abstracts.
2.2. Eligibility criteria and study selection
We included articles related to medication use quality and medication safety with innovative objectives and those that utilized rigorous observational or experimental designs with reliable and valid measures. We excluded review articles (except systematic reviews with a meta-analysis), cross-sectional studies, case-series, case reports and small single-center epidemiological studies.
One author (TCE) reviewed article titles and abstracts for relevance and identified 170 for full text review. Three co-authors (TCE, ZAM, SG) reviewed full text articles to determine eligibility. Disagreements were resolved by discussion. Of the 56 selected articles, we individually rated the top four articles based on study methods, innovation, and clinical relevance and likelihood of moving the field forward (vs. replication studies). Through consensus we selected the four articles to feature with. For each chosen article, we provided a study summary, strengths and limitations, and interpretation and implications to the care of older adults. We categorized all articles under major domains in Supplementary Appendix S3.
3. SUMMARY OF STUDIES
3.1. Effect of Antihypertensive Medication Reduction vs Usual Care on Short-term Blood Pressure Control in Patients with Hypertension Aged 80 Years and Older: The OPTIMISE Randomized Clinical Trial (Domain: Deprescribing)
3.1.1. Study summary
Management of hypertension in older adults may be complicated because of frailty, multimorbidity, and polypharmacy, all which may increase risk of treatment adverse effects. Thus, deprescribing of antihypertensive medications is recommended for some older patients when harms of treatment may outweigh the benefits of continued treatment. The evidence base for deprescribing antihypertensives is limited. The Optimising Treatment for Mild Systolic Hypertension in the Elderly (OPTIMISE) study aimed to establish whether antihypertensive medication reduction is possible without significant changes in systolic blood pressure control or adverse events over a 12-week period (Figure 1). 10
Figure 1.
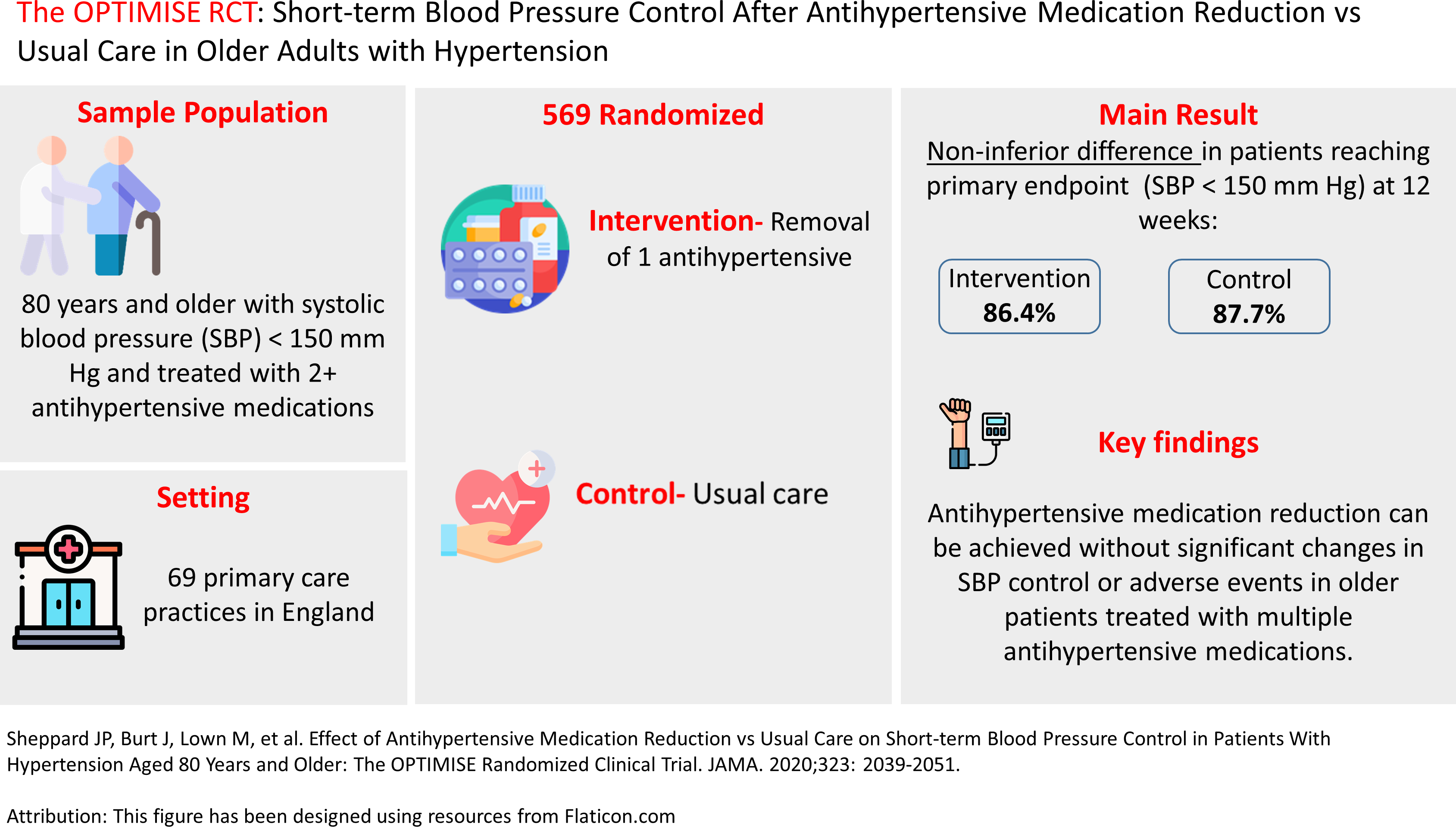
Infographic on short-term blood pressure control after antihypertensive medication reduction vs usual care. 10
This randomized, unblinded, noninferiority trial was conducted in 69 primary care sites in England. Participants aged 80 years and older were eligible if they had systolic blood pressure lower than 150 mm Hg, were receiving at least two antihypertensive medications, and their primary care physician considered them appropriate for medication reduction based on one or more specified characteristics (i.e., polypharmacy, comorbidity, nonadherence). Participants were randomized to antihypertensive medication reduction (removal of one drug [intervention], n = 282) or usual care (no medication changes mandated [control], n = 287). Primary care physicians were allowed to reinstate treatment using the safety monitoring algorithm based on level of blood pressure or presence of adverse events. Enrollment occurred between April 2017 and September 2018. The primary outcome was systolic blood pressure lower than 150 mm Hg at 12-week follow-up. The prespecified noninferiority margin was a relative risk (RR) of 0.90. Several secondary outcomes were specified.
The participants’ mean age was 84.8 years and 48.5% were women. For the primary outcome, 229 (86.4%) patients in the intervention group and 236 (87.7%) patients in the control group achieved a systolic blood pressure lower than 150 mm Hg at 12 weeks (adjusted RR, 0.98 [97.5% 1-sided CI, 0.92 to ∞]). For secondary outcomes, medication reduction was sustained in 187 (66.3%) of intervention participants at 12 weeks. No differences existed between groups in frailty, quality of life, or adverse effects, however, there was a statistically nonsignificant higher incidence of at least one serious adverse event in the intervention group (12 [4.3%] events) vs the usual care group (7 [2.4%] events; adjusted RR, 1.72 [95% CI, 0.7 to 4.3]). The mean change in systolic blood pressure was 3.4 mm Hg (95% CI, 1.1 to 5.8 mm Hg) higher in the intervention group compared with the control group, while the differences in diastolic blood pressure were not statistically different.
3.1.2. Strengths and limitations
Strengths of this study included a high completion rate (94%) and the pragmatic approach that implemented deprescribing within primary care practices with a standardized drug withdrawal guide that allowed for tailored care. However, the sample was highly selected based on primary care provider identification of people who might benefit from medication reduction; of these, only 1 in 10 were enrolled as 80% of those who were invited did not respond or declined. Nevertheless, analyses indicated that trial participants were similar to the primary care general population with regard to age, blood pressure, morbidity and frailty. The secondary outcomes addressing safety and patient-centered outcomes were likely underpowered. Longer term follow-up is needed to determine whether the small increase in systolic blood pressure observed in the intervention arm increases cardiovascular outcomes. Additionally, more than 97% of participants were White, which limits generalizability.
3.1.3. Interpretation and implications
Among older patients treated with multiple antihypertensive medications, medication reduction can be achieved without substantial change in blood pressure control. These results suggest that for every 10 patients who have their antihypertensive reduced, 9 would still have controlled blood pressure at 12-week follow-up based on the target used in this study. Given that one-third of participants in the intervention group had to have their discontinued medication restarted by 12 weeks, clinicians need to closely monitor when deprescribing. Uncertainty remains regarding the target blood pressure goals for older adults, especially those with multimorbidity and frailty and the long-term safety of deprescribing antihypertensives. In the meantime, for older adults where the risks of antihypertensive treatment may outweigh the benefits, providers are encouraged to utilize a shared decision-making approach so that patients are able to make informed decisions about their care.
3.2. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates (Domain: Medication Safety)
3.2.1. Study summary
Concerns about atypical femur fractures have led to decreased bisphosphonate use. 11 However, uncertainty remains regarding the association between bisphosphonates and atypical femur fractures. Thus, a prospective cohort study of women (50+ years) at Kaiser Permanente Southern California examined the association between bisphosphonate use and atypical femur fracture. 12 Participants included women who had received at least one prescription for oral or intravenous bisphosphonate for osteoporosis from January 1, 2007, to November 30, 2017. The primary outcome was atypical femur fracture. Fractures were independently adjudicated by two reviewers blinded to treatment. Risk factor information was derived from electronic health records, including age, patient-reported race or ethnic group, glucocorticoid use, height, weight, and smoking status. Duration of bisphosphonate use was defined from pharmacy records. In addition, the authors conducted a risk-benefit analysis, estimating the number of osteoporotic and hip fractures prevented compared with the number of bisphosphonate-related atypical femur fractures occurring for 1 to 10 years of bisphosphonate use.
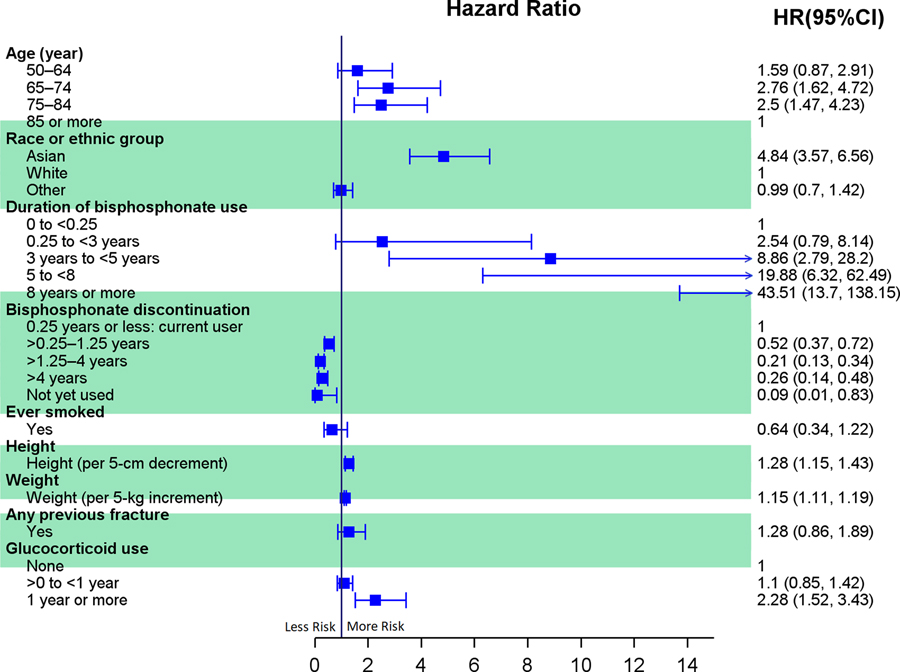
A total of 196,129 women (59.5% were 65+ years) met inclusion criteria, among whom 277 atypical femur fractures occurred (1.74 fractures per 10,000 patient-years). After multivariable adjustment, the following risk factors were associated with risk of atypical femur fracture: duration of bisphosphonate use beyond 3 years (HR, 8.86, 95% CI, 2.79 to 28.20 for 3 years to less than 5 years; HR, 43.51, 95% CI, 13.70 to 138.15 for 8 years or more; both vs. less than 3 months), Asian ancestry (HR, 4.84, 95% CI, 3.57 to 6.56, vs. White), shorter height (HR per 5-cm decrement, 1.28, 95% CI, 1.15 to 1.43), higher weight (HR per 5-kg increment, 1.15, 95% CI, 1.11 to 1.19), and glucocorticoid use for 1 year or more (HR, 2.28, 95% CI, 1.52 to 3.43, vs. no glucocorticoid use). Bisphosphonate discontinuation was associated with a decrease in risk of atypical fracture. Bisphosphonate use for 1 to 10 years was associated with greater benefit (i.e., prevention of osteoporotic and hip fractures) than risk (i.e., atypical fractures) in White women but less so among Asian women (Figure 2).
Figure 2.
The forest plot of risk factors associated with risk of atypical femur fracture. HRs and 95% CIs are from a model adjusted for these variables. The arrow shows the upper limit of the confidence interval spread further than the specified hazard ratio range (0 to 15). HR, hazard ratio; CI, confidence interval. 12
3.2.2. Strengths and limitations
This study has several strengths, including the large sample, use of blinded radiographic adjudication by two reviewers, and careful attention to potential confounding. However, there are important limitations to note. Alendronate was the most prescribed bisphosphonate, so results should not be generalized to other medications. In addition, only two atypical femur fractures occurred in Black women, which prevented analyses among them. Similarly, confidence intervals for certain estimates were wide, indicating imprecise estimates. Finally, this study was conducted at Kaiser Permanente Southern California; given the integrated healthcare structure and relatively high percentage of women of Asian race, these results may not be generalizable to other US regions.
3.2.3. Interpretation and implications
This study provides clinically useful insights, showing that the risk of atypical femur fracture was associated with longer duration of bisphosphonate treatment, Asian ancestry, shorter height, higher weight, and glucocorticoid use for 1 year or more. These results lay the groundwork for creating a risk calculator that could be used in clinical decision making. Of note, age did not increase the risk of atypical fracture, with the oldest women (85+ years) in the cohort displaying comparatively lower risk for atypical fracture than younger ages. Moreover, this study provides some of the only observational data showing that bisphosphonate discontinuation was associated with reduced risk of atypical fracture, however, this needs to be balanced with risk of other fractures in the absence of a bisphosphonate. Future research is needed to evaluate this risk-benefit balance from deprescribing bisphosphonates. Moreover, the risk-benefit analysis in this study found that the absolute risk of bisphosphonate-related atypical femur fractures was very low compared to the number of osteoporotic and hip fractures that can be prevented from bisphosphonate use. This risk-benefit finding was particularly favorable in White women but less so in Asian women. In summary, this study provides results on risk factors for atypical femur fractures that clinicians can use to inform prescribing decisions as well as to educate patients. Future research should also focus on the optimal duration of drug holidays and the best sequences of use of osteoporosis drug therapies (e.g., bisphosphonates followed by denosumab) that maximize benefits and minimize harms.
3.3. Multifaceted Antimicrobial Stewardship Program for the Treatment of Uncomplicated Cystitis in Nursing Home Residents (Domain: optimizing medication use)
3.3.1. Study summary
Inappropriate antibiotic use for nursing home residents with urinary symptoms without cystitis or for asymptomatic bacteriuria is associated with an increased risk of antimicrobial resistance, adverse drug events, and Clostridioides difficile infections. 13 This study was a quality improvement intervention to determine the association of a multifaceted antimicrobial stewardship and quality improvement program with the reduction in unnecessary antimicrobial use for unlikely cystitis among noncatheterized nursing home residents (Figure 3). 14 The primary outcome was the incidence of antibiotic treatment for unlikely cystitis cases. Unlikely cystitis was defined as asymptomatic bacteriuria, contaminated urinary specimens, or noninfectious conditions that can be confused with cystitis (e.g., nonspecific symptoms in the absence of urinary-specific symptoms). Secondary outcomes included overall antibiotic use for any urinary tract infection, C difficile infections, all-cause hospitalizations and death. The participants and setting were residents with a suspected urinary tract infection (UTI) in 25 nursing homes across the United States. The authors collected baseline data for 3 months and then conducted the intervention from May 1, 2017, to April 30, 2018. Intervention nursing homes (n = 12) were randomized to receive a 1-hour introductory webinar, pocket-sized educational cards, tools for system change, and educational clinical vignettes addressing the diagnosis and treatment of suspected uncomplicated cystitis. Monthly web-based coaching calls were held for staff of intervention nursing homes. All facilities received quarterly feedback reports regarding the management of uncomplicated cystitis. Control group nursing homes (n = 13) received usual care. Nursing home staff submitted de-identified baseline and follow-up case report forms for each suspected nursing home-acquired UTI as well as a monthly summary sheet identifying aggregate facility-level metrics. Suspected nursing home-acquired UTI was defined as any clinical suspicion of UTI as determined by the prescribing clinician.
Figure 3.
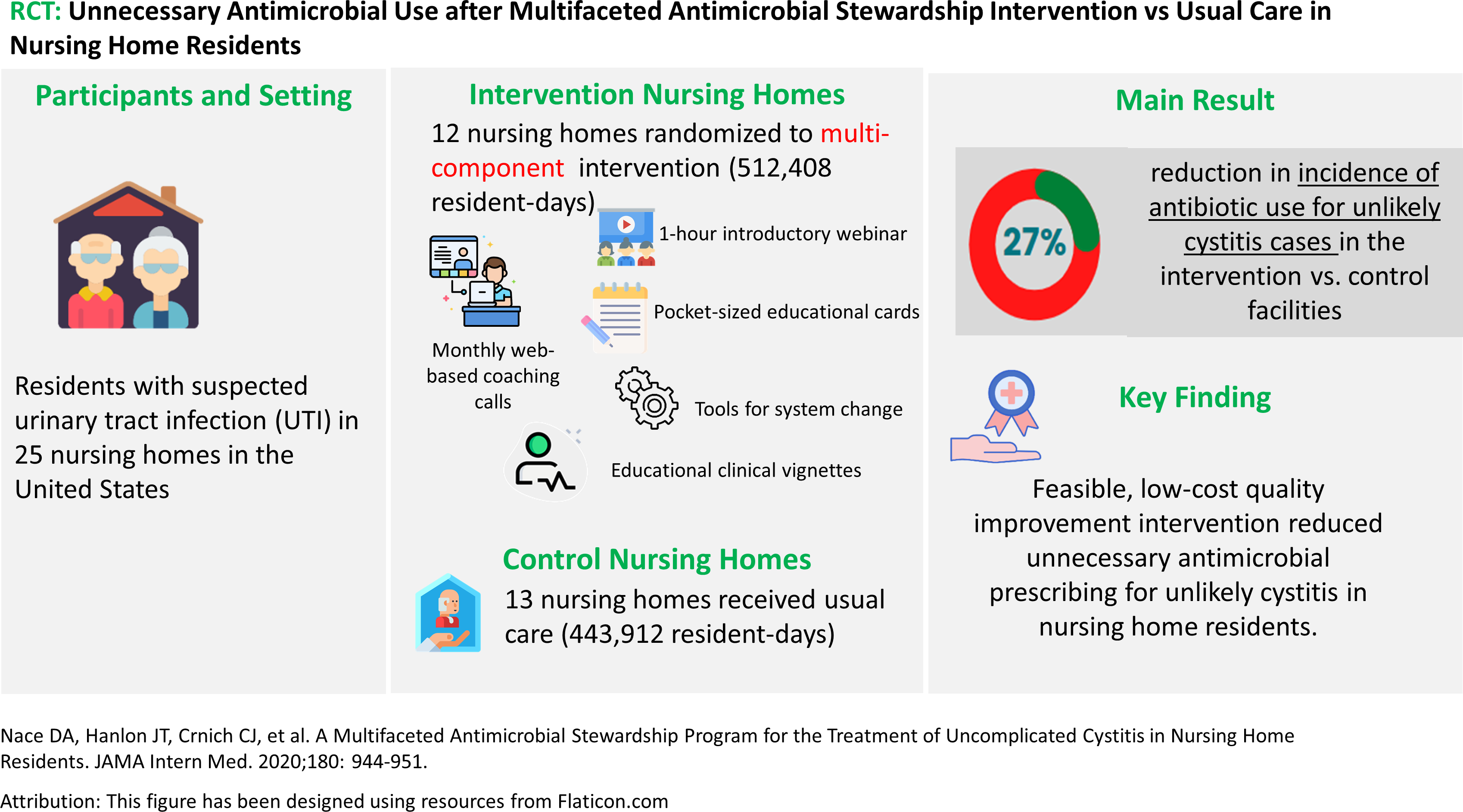
Infographic on unnecessary antimicrobial use after multifaceted antimicrobial stewardship intervention vs usual care. 14
The results showed fewer unlikely cystitis cases were treated with antibiotics in intervention facilities compared with control facilities (adjusted incidence rate ratio [AIRR], 0.73 [95%CI, 0.59–0.91]); C difficile infection rates were also lower in intervention nursing homes vs control nursing homes (AIRR, 0.35 [95%CI, 0.19–0.64]). Overall antibiotic use for any type of UTI was 17% lower in the intervention facilities than the control facilities (AIRR, 0.83 [95%CI, 0.70–0.99]; P = .04). There was no increase in all-cause hospitalizations or deaths due to the intervention (all-cause hospitalizations: AIRR, 0.95 [95%CI, 0.75–1.19]; death: AIRR, 0.92 [95%CI, 0.73–1.16]).
3.3.2. Strengths and limitations
The strengths of the study include the incorporation of a control group, the use of a national sample of nursing homes, the large number of resident-days of follow-up, the use of a pragmatic intervention, and high completion rates. An additional strength is the inclusion of both post-acute care and long-term care residents which increases generalizability of the findings compared with prior research. The main limitations were the lack of blinding of facilities, collection of data via self-report from staff on site, and that very few providers participated in the coaching calls (nearly all were nurses). Participating sites may not be representative of nursing homes with different resource levels in the US. Despite this, facilities included in this trial have similar nurse staffing levels with that of the national data across all US nursing homes in 2014. 15
3.3.3. Interpretation and implications
This study showed that a feasible, low-cost quality improvement intervention reduced inappropriate antibiotic prescribing for unlikely cystitis and reduced C difficile infection rates in nursing home residents while not increasing risk of hospitalization or death. As is often the case with multicomponent interventions in geriatric research, it will be useful to determine if any component has more impact than others. Given the time constraints for nursing home staff, monthly web-based coaching calls may be challenging for providers. Lessons learned from this study offer a strong foundation for future work that can lead to widespread implementation.
3.4. Association of Tramadol Use with Risk of Hip Fracture (Domain: Medication Safety)
3.4.1. Study summary
Despite an increasing trend in tramadol prescription rates in the US and Canada, the safety profile, such as risk of hip fracture, remains unclear. 16 Wei et al conducted a population-based cohort study to examine the association between tramadol use and the risk of hip fracture.17 Participants included individuals aged 50 years or older with no history of hip fracture, cancer, or opioid use disorder and enrolled in the Health Improvement Network (THIN) from 770 general practices in the United Kingdom. The analytic sample involved patients who had initiated treatment with tramadol or one of the following active comparator analgesics: codeine, naproxen, ibuprofen, celecoxib, or etoricoxib between 2000 and 2017. Five propensity score-matched cohorts were created by matching tramadol initiators to active comparator initiators.
The primary outcome was incident hip fracture during a 1-year follow-up period. Hip fracture was identified by using the Read classification system codes in THIN. Cause-specific Cox proportional hazard models were used to regress the hazard of incident hip fracture for tramadol initiators compared to codeine or non-steroidal anti-inflammatory drugs (NSAIDs) initiators, accounting for the competing risk of death. In addition, sex-specific analyses were conducted to examine tramadol initiation and risk of hip fracture between females and males.
From the total of 372,372 participants who met inclusion criteria, 146,956 tramadol initiators were matched 1:1 with codeine initiators (mean age of 67 years and 60% were women). The initiation of tramadol was associated with a higher risk of hip fracture than the initiation of codeine (hazard ratio [HR]= 1.28, 95% CI 1.13 – 1.46) or NSAIDs, see Supplemental Figure S1. The increased risk for fracture was observed for both men (HR= 1.60, 95% CI, 1.24–2.06) and women (HR= 1.18, 95% CI, 1.02–1.38) who initiated tramadol compared with codeine. Similarly, for all NSAIDs comparisons, the increase in fracture risk was higher in men than in women. In a sensitivity analysis restricted to individuals aged 60 years or older, tramadol initiation was still associated with a greater risk of hip fracture when compared with initiation of codeine or NSAIDs.
3.4.2. Strengths and limitations
This study has several strengths, including the large sample, propensity-matching analysis, robust sensitivity analyses, use of a new-user design and multiple active comparators which minimizes the potential selection and confounding by indication bias. However, there are some important limitations including the inability to adjust for bone density and frailty, two potential confounders, and lack of data on over-the-counter medication use.
3.4.3. Interpretation and implications
This population-based, propensity score–matched cohort study provides robust evidence that tramadol is associated with a greater risk of hip fracture compared to other analgesics. In a previous population-based study, tramadol use was associated with higher risk of fall, a strong predictor for hip fracture 18 and all-cause mortality. 19 Treating pain in older adults is challenging given the available options such as NSAIDS and opioids pose considerable risks to this population. Tramadol is often considered as an alternative to these options (due to their adverse effects) for the management of pain (e.g., fibromyalgia, osteoarthritis, and chronic low-back pain). 20–22 However, given the clinical and economic impact of hip fracture, results from this study suggest clinicians should consider the potential risk of hip fracture when initiating tramadol, particularly among the most vulnerable to hip fracture. Although not explicitly stated by the authors, the rationale for selecting codeine and NSAIDs as comparison drugs was likely to minimize potential confounding by indication. Moreover, given the emphasis on reducing the overutilization of opioids, tramadol may also be used in place of hydrocodone, oxycodone and other opioids to avoid use of these agents. 16, 23 Additional research would be helpful to determine the comparative risk of tramadol with the initiators of commonly prescribed opioids.
4. DISCUSSION
In this review, we identified and provided a critique for four studies published in 2020 that highlight important topics relating to medication use quality and safety. The first article discusses a successful deprescribing intervention for antihypertensive medication reduction without significant changes in systolic blood pressure control or adverse events in older patients with hypertension. 10 The second article found that the risk of atypical femur fracture increased with longer duration of bisphosphonate treatment. 12 The third article features a successful multifaceted antimicrobial stewardship and quality improvement intervention to reduce unnecessary antibiotic prescribing for uncomplicated cystitis in nursing home residents, 14 and the fourth calls attention to tramadol’s associated risk of fracture. 17 Together, these studies along with articles included in Supplementary Appendix S3 highlight challenges related to medication use quality and safety in older adults as well as highlight further research opportunities. While the studies summarized in this paper serve as exemplary research on optimizing medication use in older adults, research using robust methods and quality data remain critical to answering clinically important questions. By improving appropriate prescribing in clinical practice and better understanding how to achieve this through research, we stand to improve older adults’ clinical health outcomes.
Supplementary Material
Supplementary Appendix S1: Key search terms
Supplementary Appendix S2: List of journals manually searched
Supplementary Appendix S3: List of 2020 articles that addressed medication use quality and safety
Supplemental Figure S1. Forest plot of hazard ratios for the association between tramadol use with risk of hip fracture. HRs and 95% CIs are from five separate cause-specific Cox proportional hazard models. HR, hazard ratio; CI, confidence interval. 17
Key Points.
Medication optimization in older adults presents many challenges.
We identified major studies published in 2020 on medication use quality and safety in older adults to help guide care.
Why does this matter?
This review features new research that adds to the evidence base to promote optimal medication use in older adults.
ACKNOWLEDGEMENTS
Z.A. Marcum was supported by the National Institute on Aging of the NIH (K76AG059929). K.E. Schmader received support from the Duke Pepper Older Americans Independence Center, NIA P30AG028716. S.L. Gray was supported by the National Institute on Aging (1R24AG064025, U01AG006781) and Centers for Disease Control and Prevention (U01CE002967). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: The authors have non conflicts of interest to declare.
REFERENCES
- [1].Donaldson LJ, Kelley ET, Dhingra-Kumar N, Kieny M-P, Sheikh A. Medication Without Harm: WHO’s Third Global Patient Safety Challenge. The Lancet. 2017;389: 1680–1681. [DOI] [PubMed] [Google Scholar]
- [2].Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for Medication Appropriateness in Older Adults. Clin Geriatr Med. 2018;34: 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boyd C, Smith CD, Masoudi FA, et al. Decision Making for Older Adults With Multiple Chronic Conditions: Executive Summary for the American Geriatrics Society Guiding Principles on the Care of Older Adults With Multimorbidity. J Am Geriatr Soc. 2019;67: 665–673. [DOI] [PubMed] [Google Scholar]
- [4].Ruiter R, Burggraaf J, Rissmann R. Under-representation of elderly in clinical trials: An analysis of the initial approval documents in the Food and Drug Administration database. Br J Clin Pharmacol. 2019;85: 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Marum RJ. Underrepresentation of the elderly in clinical trials, time for action. Br J Clin Pharmacol. 2020;86: 2014–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the Evidence: A Systematic Review of the Inclusion and Analysis of Older Adults in Randomized Controlled Trials. J Gen Intern Med. 2011;26: 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67: 674–694. [DOI] [PubMed] [Google Scholar]
- [8].O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hanlon JT, Semla TP, Schmader KE. Alternative Medications for Medications in the Use of High-Risk Medications in the Elderly and Potentially Harmful Drug-Disease Interactions in the Elderly Quality Measures. J Am Geriatr Soc. 2015;63: e8–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sheppard JP, Burt J, Lown M, et al. Effect of Antihypertensive Medication Reduction vs Usual Care on Short-term Blood Pressure Control in Patients With Hypertension Aged 80 Years and Older: The OPTIMISE Randomized Clinical Trial. JAMA. 2020;323: 2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in Media Reports, Oral Bisphosphonate Prescriptions, and Hip Fractures 1996–2012: An Ecological Analysis. J Bone Miner Res. 2015;30: 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Black DM, Geiger EJ, Eastell R, et al. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. N Engl J Med. 2020;383: 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nace DA, Drinka PJ, Crnich CJ. Clinical Uncertainties in the Approach to Long Term Care Residents With Possible Urinary Tract Infection. J Am Med Dir Assoc. 2014;15: 133–139. [DOI] [PubMed] [Google Scholar]
- [14].Nace DA, Hanlon JT, Crnich CJ, et al. A Multifaceted Antimicrobial Stewardship Program for the Treatment of Uncomplicated Cystitis in Nursing Home Residents. JAMA Intern Med. 2020;180: 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Harrington C, Schnelle JF, McGregor M, Simmons SF. Article Commentary: The Need for Higher Minimum Staffing Standards in U.S. Nursing Homes. Health Services Insights. 2016;9: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bigal LM, Bibeau K, Dunbar S. Tramadol Prescription over a 4-Year Period in the USA. Curr Pain Headache Rep. 2019;23: 76. [DOI] [PubMed] [Google Scholar]
- [17].Wei J, Lane NE, Bolster MB, et al. Association of Tramadol Use With Risk of Hip Fracture. J Bone Miner Res. 2020;35: 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Söderberg KC, Laflamme L, Möller J. Newly Initiated Opioid Treatment and the Risk of Fall-Related Injuries. CNS Drugs. 2013;27: 155–161. [DOI] [PubMed] [Google Scholar]
- [19].Zeng C, Dubreuil M, LaRochelle MR, et al. Association of Tramadol With All-Cause Mortality Among Patients With Osteoarthritis. JAMA. 2019;321: 969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020;72: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76: 318–328. [DOI] [PubMed] [Google Scholar]
- [22].Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166: 514–530. [DOI] [PubMed] [Google Scholar]
- [23].Harrison ML, Walsh TL. The effect of a more strict 2014 DEA schedule designation for hydrocodone products on opioid prescription rates in the United States. Clinical Toxicology. 2019;57: 1064–1072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix S1: Key search terms
Supplementary Appendix S2: List of journals manually searched
Supplementary Appendix S3: List of 2020 articles that addressed medication use quality and safety
Supplemental Figure S1. Forest plot of hazard ratios for the association between tramadol use with risk of hip fracture. HRs and 95% CIs are from five separate cause-specific Cox proportional hazard models. HR, hazard ratio; CI, confidence interval. 17