Abstract
Hedgehog (Hh) signaling has been widely acknowledged to play essential roles in many developmental processes, including endochondral ossification and growth plate maintenance. Furthermore, a rising number of studies have shown that Hh signaling is necessary for tendon enthesis development. Specifically, the well-tuned regulation of Hh signaling during development drives the formation of a mineral gradient across the tendon enthesis fibrocartilage. However, aberrant Hh signaling can also lead to pathologic heterotopic ossification in tendon or osteophyte formation at the enthesis. Therefore, the therapeutic potential of Hh signaling modulation for treating tendon and enthesis disease remains uncertain. For example, increased Hh signaling may enhance tendon-to-bone healing by promoting the formation of mineralized fibrocartilage at the healing interface, but pathologic heterotopic ossification may also be triggered in the adjacent tendon. Further work is needed to elucidate the distinct functions of Hh signaling in the tendon and enthesis to support the development of therapies that target the pathway.
Keywords: Tendon enthesis, Growth plate, Mineralization, Fibrocartilage, Heterotopic ossification, Hedgehog signaling, Development, Healing
Hedgehog (Hh) signaling in tendon enthesis development and mineralization
In this review, we summarize how Hh signaling regulates the development and mineralization of the tendon enthesis, how its inactivation can impair tendon enthesis healing, and how its activation can lead to pathologic heterotopic ossification. Additionally, we discuss the gaps in our mechanistic understanding of this process in the tendon and enthesis, focusing on the contrasts between its beneficial activation during development and its pathologic activation during disease. These insights will help move the field towards development of Hh-specific cellular and molecular strategies for improving tendon-to-bone healing and for preventing tendon heterotopic calcification.
The Hh signaling pathway
Hh signaling is initiated when a Hh ligand binds to the cell surface receptor Patched 1 (PTCH1), which reverses the repression of the cell surface protein Smoothened (SMO) in the primary cilium, a subcellular protrusion emanating from the apical surface of mammalian cells [1, 2] (Figure 1). When a Hh ligand is absent, PTCH1 represses SMO activity. After the Hh ligand binds to PTCH1, SMO is released and triggers downstream signal transduction through proteins such as GLI-Kruppel family member Gli1, Gli2, and Gli3. Gli1 activates transcriptional activity whereas Gli3 plays a transcriptional repressor role. Gli2 can either activate or repress transcriptional activity, depending on the conditions. Numerous other cellular factors are involved in Hh signaling by regulating activities downstream of Gli1/2/3. For example, suppressor of fused homolog (Sufu) functions as a negative mediator of Hh signaling and kinesin protein 7 (Kif7) exerts both positive and negative regulatory roles on Hh signaling. Furthermore, the functions of many Hh-related proteins on Hh activation or inactivation are dependent on tissue type and signaling status, an area of ongoing study [3, 4].
Figure 1.
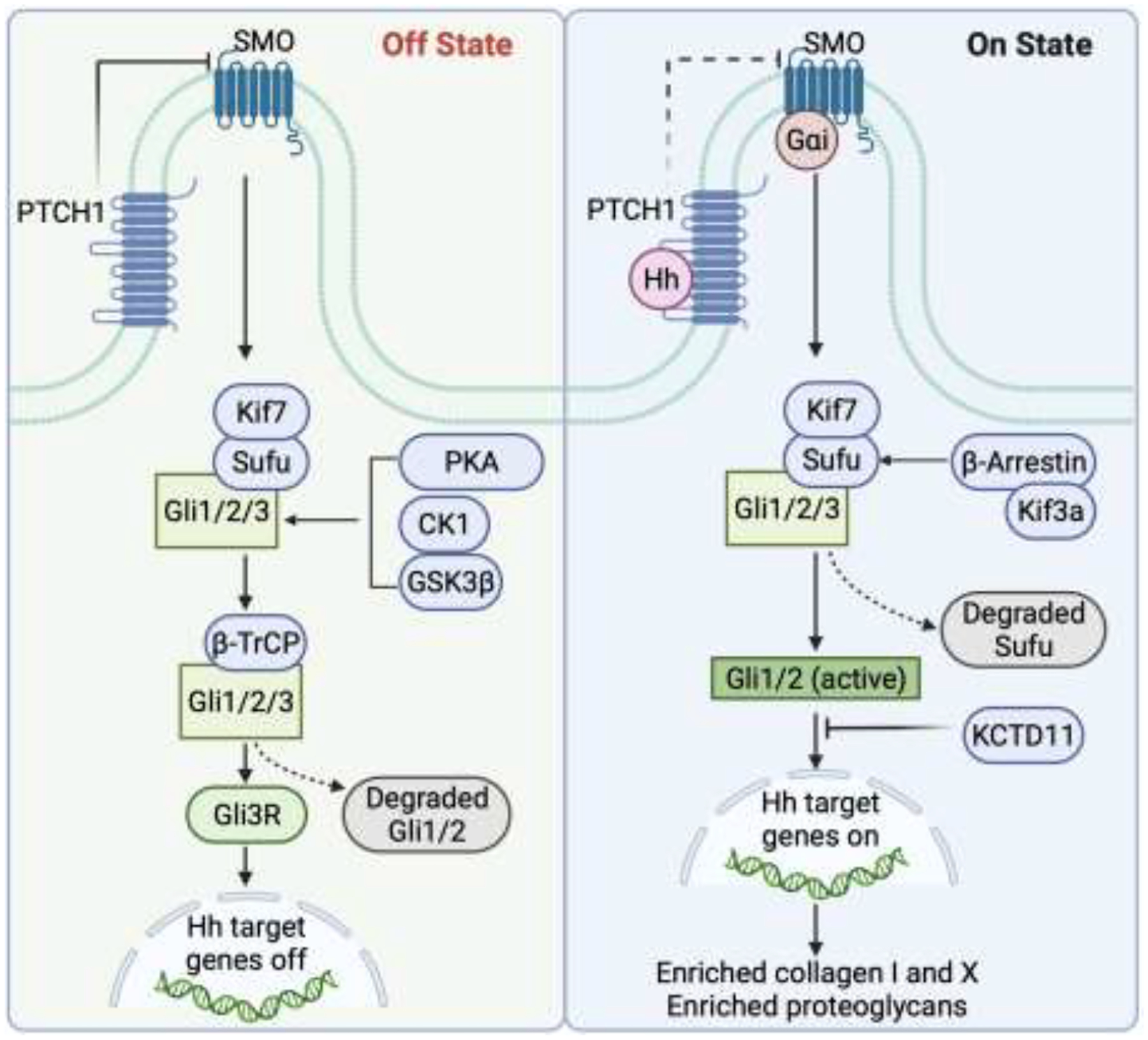
Hh signaling is initiated when a Hh ligand binds to the cell surface receptor PTCH1, which results in repression and accumulation of the cell surface protein SMO in the primary cilium. SMO then triggers downstream signal transduction through proteins such as Sufu and Gli1/2/3. Other cellular factors, such as proteinase kinase A (PKA), casein kinase 1 (CK1), glycogen synthase kinase β3 (GSK3β), β-Arrestin, the kinesin family member 3a (Kif3a), beta-transducin repeats-containing proteins (β-TrCP), and the potassium channel tetramerisation doman containing 11 (KCTD11), engage in the regulation of downstream Hh transcription.
The Hh signaling pathway is well-recognized to govern organ patterning, early embryonic development, formation of the musculoskeletal system, and tumorigenesis [5, 6]. Early in development, a gradient in sonic hedgehog (Shh), one of the three mammalian hedgehog orthologs, regulates mesoderm patterning [7]. The influence of Shh continues at later stages, regulating the polarizing activity of cells residing in the posterior distal region of the limb bud and further controlling patterning of both the anterior-posterior and proximal-distal axes [8]. Similarly, Shh is critical for craniofacial development, mediating survival and proliferation of neural crest cells [9]. During postnatal stages of development, endochondral skeletal growth is dependent on another Hh ortholog, Indian hedgehog (Ihh), as demonstrated, e.g., by observations that Ihh mutant mice have severe dwarfism [10].
The role of Hh signaling in tendon enthesis development
Hh signaling is also critical for the formation of the enthesis, a mineralized transitional tissue which bridges soft connective tissues such as tendon, ligament, and meniscus to bone [11–13]. This specialized tissue is critical for effective force transmission between unmineralized and mineralized tissues. The enthesis has classically been categorized as either fibrous or fibrocartilaginous, depending on its structural and compositional makeup and its anatomic site [14]. The fibrous enthesis is composed of perforating mineralized collagen fibers and can often be found in soft tissue-to-bone attachments into the metaphyses and diaphyses [15]. The tendon/ligament end of the fibrous enthesis is enriched in collagen type I. As it inserts into bone periosteum or cortex, mineralization of the collagen type I matrix is observed. The fibrocartilagous enthesis is displays spatial gradients in extracellular matrix composition and mineralization (Figure 2). The tendon/ligament end of the fibrocartilaginous enthesis transitions from tendon to unmineralized fibrocartilage, then to mineralized fibrocartilage, and finally to bone, with a corresponding shifts in constituents from collagen type I, to collagen type II and aggrecan, and finally to mineralized collagen type II and X [11]. Load transfer across dissimilar materials such as tendon and bone (which differ in modulus by two orders-of-magnitude) is an engineering challenge, as stresses are amplified at their interface [14, 16]. Fibrous entheses attach over relatively large surface areas, effectively distributing forces across the attachment footprint to reduce stress and achieve load transfer. In contrast, fibrocartilaginous entheses attach over much smaller surface areas and require a number of multiscale structural and compositional mechanisms to achieve effective load transfer (e.g., a spatial gradient in mineral content) [17–19].
Figure 2.
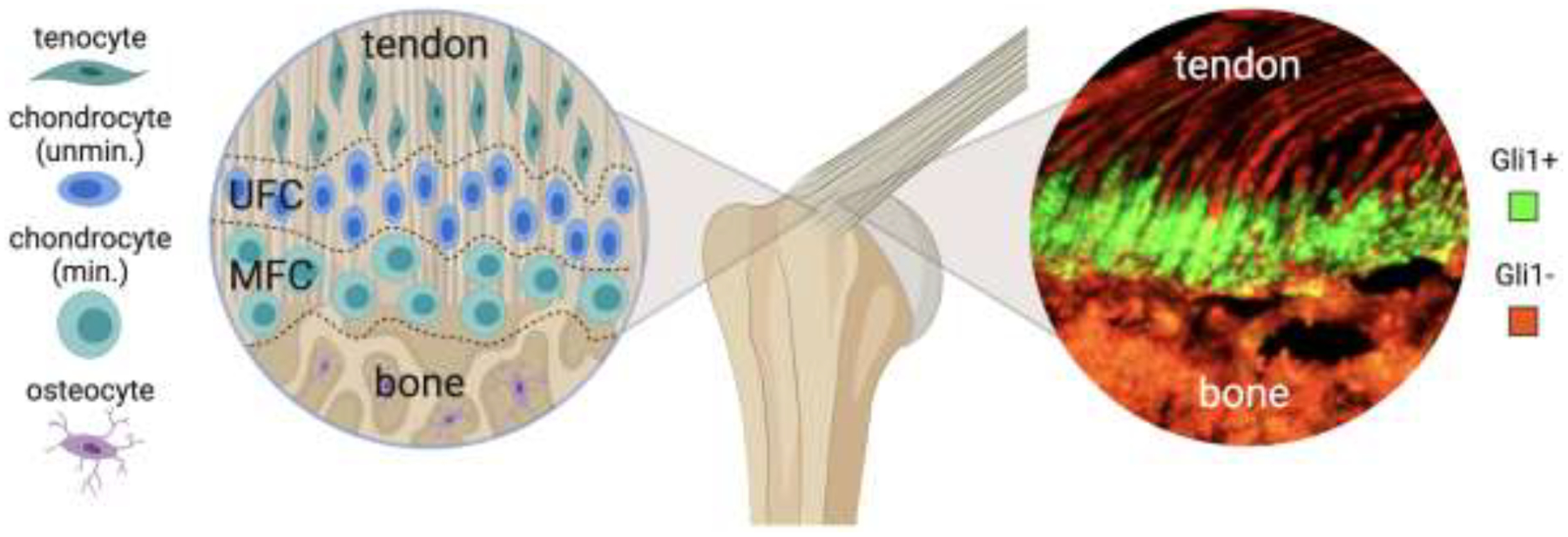
Tendon attaches to bone across the enthesis, a fibrocartilaginous attachment with spatial gradients in structure, composition, and cell phenotype. Unmin.: unmineralized, Min.: mineralized chondrocytes, UFC: unmineralized fibrocartilage, MFC: mineralized fibrocartilage, Gli1+: Gli1-lineage cells, Gli-: cells not derived from Gli1 lineage.
The formation of the enthesis begins during embryonic development, and is orchestrated by the functions of multiple progenitor cell populations [20, 21]. Cells positive for both SRY-box transcription factor 9 (Sox9) and scleraxis (Scx) distinguish enthesis-specific progenitors from cartilage and tendon progenitors, which are positive for only Sox9 or Scx, respectively [20]. Depending on the anatomical location, Sox9-lineage enthesis progenitors ultimately either differentiate into, or are replaced by, a Hh-positive cell population marked by Gli1 [22]. In the entheses that migrate along bone shaft during development, Sox9-lineage cells are replaced by Gli1-lineage (Gli1+) cells, whereas in the entheses that remain stationary throughout development, Sox9-lineage cells differentiate into Gli1+ cells [22]. During postnatal development, Gli1+ cells initially reside in the unmineralized enthesis fibrocartilage of 4 week-old mice and then fully mineralized enthesis fibrocartilage of 8-week old mice [23, 24]. Gli1+ cells and their progenies are retained in the enthesis region throughout postnatal development, eventually populating the entire fibrocartilage region between tendon and bone (Figure 2) [24]. Lineage tracing has revealed that most of these cells lose their responsiveness to Hh signaling as the skeleton matures [21]. These findings, combined with the observation that ablation of the Gli1+ cells leads to a loss of mineralized fibrocartilage, suggest that these cells play a critical role in enthesis mineralization [24]. A number of Hh-related ligands, including Shh and Ihh regulate the expression of Gli1, depending on the development stage, but the origin of these ligands inducing Gli1 expression at the enthesis is unclear.
Cells responsive to Gli1 during enthesis development are also regulated by mechanical loading, as limb paralysis during the neonatal period leads to an increased number of Gli1+ cells in the mouse entheses [24, 25]. Since primary cilia have been reported to receive and transduce mechanical loading, activation of Gli1 during enthesis mineralization in the first few weeks after birth is also concurrent with cilium incidence, further implicating Hh signaling as responsible for enthesis mechanosensing [25]. Moreover, tendon entheses with inactivated Hh signaling cannot adapt to altered biophysical loading and also have abnormal ciliogenesis. However, the underlying mechanism of how mechanical loading, Hh signaling, and primary cilia interactively regulate each other and then contribute to tendon enthesis formation and remodeling is unclear.
The role of Hh signaling on enthesis development and mineralization has been further explored by using transgenic murine models with both loss-of-function and gain-of-function [12, 24–26]. One such model that has been widely studied utilizes conditional deletion and activation of SMO, a receptor which is necessary for the transduction of hedgehog signaling in response to Hh ligands. SMO deletion at the tendon enthesis leads to substantial defects in tissue structure and composition, including decreased mineralization, abnormal collagen content, decreased proteoglycan content, and altered biomechanical properties [12, 24–26]. In contrast, SMO activation leads to increased protein expression of tenascin (Tnc), biglycan (Bgn), and collagen type 2 (Col2). Similarly, constitutive activation of SMO in tendon cells results in the ectopic expression of chondrogenic markers in the tendon midsubstance, while SMO deletion in tendon cells decreases fibrocartilage differentiation at the enthesis [12].
Besides Hh signaling, other signaling pathways, such as fibroblast growth factor (FGF), transforming growth factor beta (TGFβ), bone morphogenetic proteins (BMPs), and growth differentiation factor (GDF), also play important roles in tendon enthesis development. Using loss-of-function mouse models, these pathways have been shown to regulate differentiation and specification of enthesis progenitors and to coordinate enthesis tenogenesis, chondrogensis, and osteogenesis to form the fibrocartilagous transition [14, 16, 27, 28]. However, it remains unclear how these pathways interact with Hh signaling to regulate enthesis development and mineralization in particular, although crosstalk between Hh, TGFβ, and BMP signaling pathways has been proposed in other tissues and biologic processes (Figure 3) [29–35]. A better understanding of crosstalk between these factors during enthesis formation and function is necessary to help motivate Hh-related drug development for enthesis regeneration.
Figure 3.
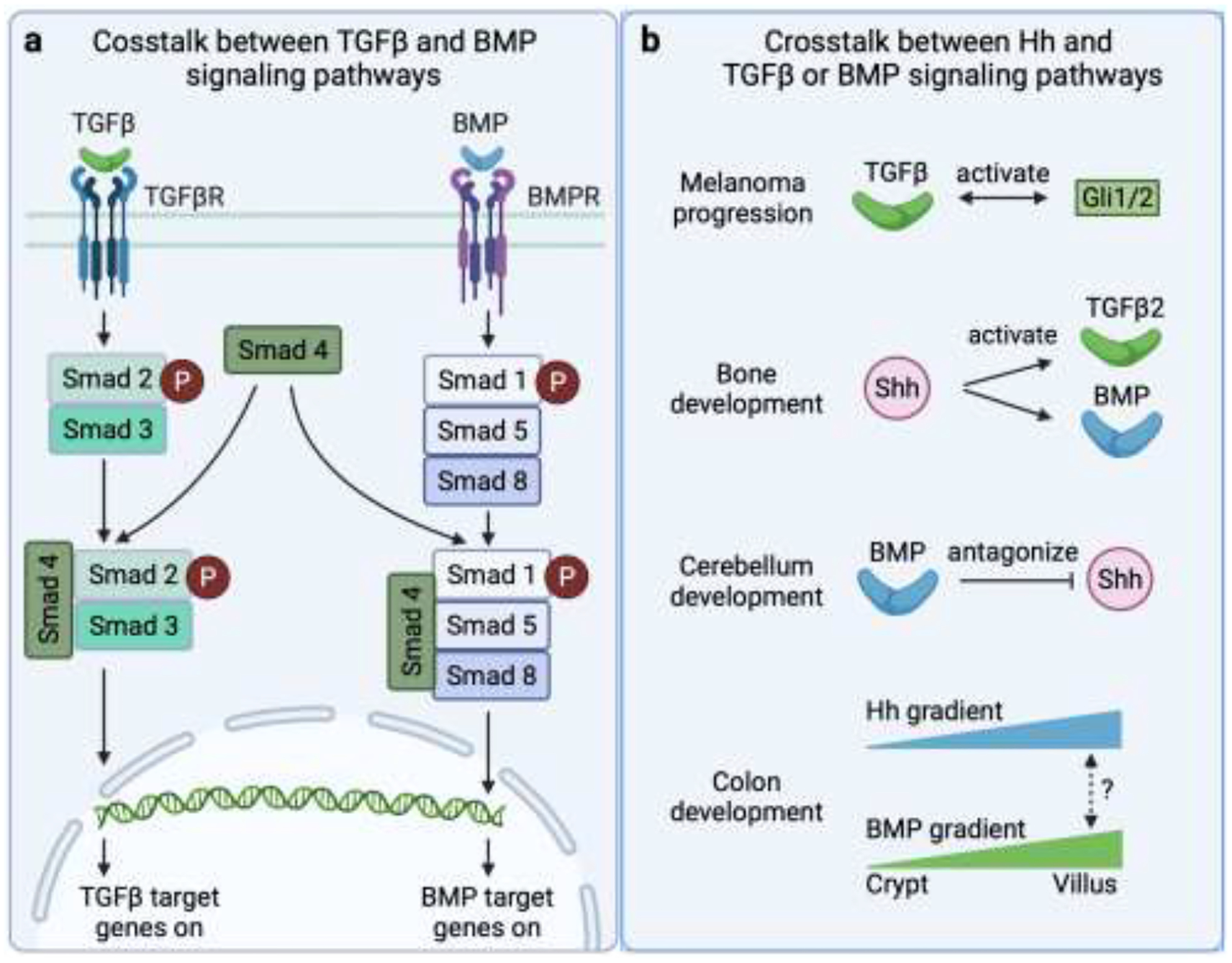
Crosstalk between Hh, TGFβ, and BMP signaling pathways. (a) Crosstalk between TGFβ and BMP signaling pathways. (b) Proposed crosstalk between components of Hh and TGFβ or BMP signaling pathways.
Similarities between tendon enthesis development and growth plate biology
Our understanding of enthesis development, particularly as it relates to mineralization, is based on the substantive literature on growth plate biology. Superficially, the enthesis and the growth plate share many similarities, e.g., there are spatially varying distributions of chondrocyte phenotypes and extracellular matrix components (Figure 4). Round “resting” chondrocytes with minimal proliferative activity reside on the unmineralized end of the growth plate [36]. A gradient of chondrocyte phenotypes is then seen, shifting from proliferating, to pre-hypertrophic, to hypertrophic (mineralizing), and finally to terminal chondrocytes (ultimately replaced by osteoblasts) [36]. At the enthesis, round quiescent chondrocytes are located on the unmineralized side and hypertrophic chondrocytes, with obvious lacuna, are seen on the mineralized end adjacent to bone [16]. Consistent with the hypertrophic phenotype, collagen X is highly expressed by the mineralizing chondrocytes in both the tendon enthesis and the growth plate [37, 38]. However, there are key differences between enthesis and growth plate biology. Most critically, in the growth plate, a combination of vascular invasion, hypertrophic chondrocyte apoptosis, and chondrocyte transdifferentiation results in the replacement of mineralized cartilage with bone [36]. These processes are not seen at the enthesis, leading to some descriptions of the enthesis as an “arrested growth plate” [27]. This difference was made clear when tracking the Gli1+ cells at the enthesis and at the adjacent secondary center of ossification: Gli1+ cells persisted through maturity at the enthesis but disappeared from the adjacent bone that was formed via endochondral ossification [24, 39].
Figure 4.
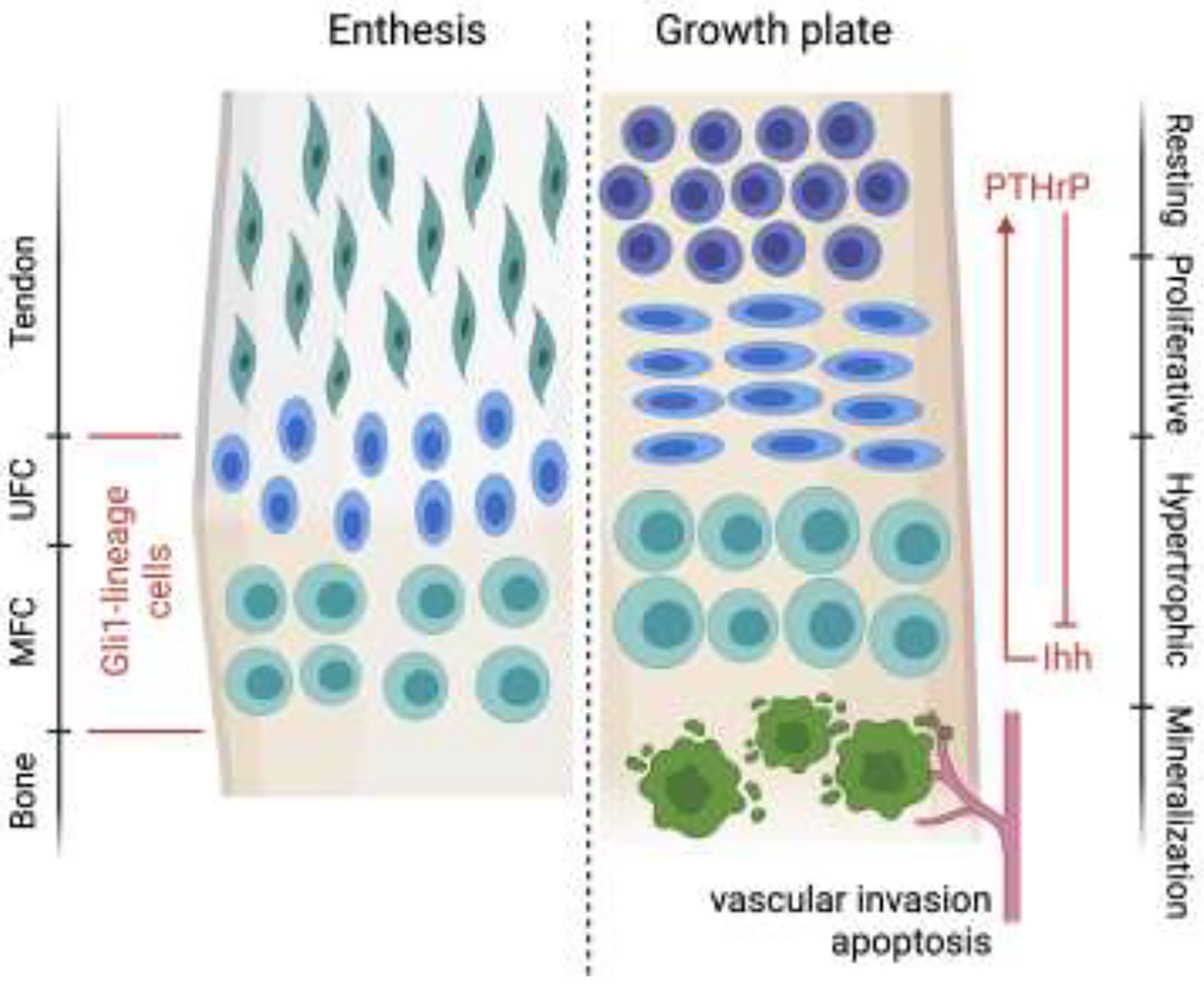
The enthesis shares similarities with the growth plate, including a spatial gradient in cell phenotypes, Hh-responsive cells, and mineralizing chondrocytes. However, unlike the growth plate, vascular invasion and apoptosis does not occur in the enthesis zone of hypertrophic chondrocytes. Mineralized fibrocartilage remains at the mature enthesis, in contrast to growth plate cartilage, which is replaced by bone. UFC: unmineralized fibrocartilage, MFC: mineralized fibrocartilage.
An abundance of previous work has determined the contribution of Ihh to endochondral skeleton development and growth plate maintenance via regulating chondrocyte proliferation and differentiation [36, 40, 41]. Furthermore, Ihh also influences osteoblast differentiation activity because of absence of osteoblasts and expression of osteoblast markers (i.e., Runx2, osteopontin) after Ihh deletion in chondrocytes [40, 41]. From the perspective of growth plate development and maintenance, Ihh and parathyroid hormone-like hormone protein (PTHrP) coordinate through a negative feedback loop to drive chondrocyte fate to leave the proliferative stage [36]. Ihh is synthesized by prehypertropic or early hypertropic chondrocytes whereas PTHrP is synthesized by prechondral cells. This creates opposing spatial gradients of the two factors. Ihh promotes chondrocyte proliferation and PTHrP production, while PTHrP prevents chondrocytes from proliferating, resulting in a negative feedback look that controls terminal chondrocyte differentiation and therefore inhibits Ihh production [36, 42]. Previous work examining enthesis mineralization has also demonstrated essential roles for Hh and PTHrP during the formation, maintenance, and healing of the enthesis [11, 24, 39, 43].
Hedgehog signaling during tendon and enthesis healing
Insufficient or aberrant Hh signaling has been associated with poor tendon-to-bone healing, enthesitis, and tendon heterotopic ossification [39, 44]. Due to the stress concentrations inherent to the attachment of mechanically dissimilar tissues, regenerating the enthesis during healing remains the goal for effective repair of connective tissues to bone. Unfortunately, fibrovascular scar tissue dominates the healing response, resulting in a persistent clinical challenge for surgically reconnecting tendon to bone, with alarmingly high failure rates [45,46]. Furthermore, conditions leading to inappropriate mineralization result in significant pain and disability at and around joints [13]. The prevalence of these conditions and the lack of effective therapeutic strategies motivate the development of novel treatment approaches, e.g., by studying molecular and cellular cues that drive enthesis development and/or developing agonists and antagonists for Hh signaling control.
As expected from the established role of Hh signaling during enthesis development, numerous studies have demonstrated the importance of Hh signaling during tendon-to-bone healing. Activation of Hh signaling has been observed at the enthesis during rotator cuff healing or after ACL reconstruction, with increased expression of Hh-related components and increased numbers of Hh-responsive cells (Table 1) [39, 47]. Specifically, expression of Ihh, PTCH1, Smo, and Gli1 was significantly increased at the tendon-bone interface during the early healing phases after rotator cuff repair or ACL reconstruction [47–50]. Additionally, lineage tracing studies showed that Gli1+ cells populated the immature enthesis after needle punch injury, in contrast to the injury response at the mature enthesis, which involved only a small number of Gli1+ cells [39]. The higher numbers of Gli1+ cells at the injured enthesis of immature mice resulted in markedly better healing compared to adult mice. These cells were positive for the stem cell marker Notch1 and are thought to retain progenitor characteristics [39]. Furthermore, following injury, this population of cells was observed to form clusters adjacent to the site of injury and express Ki67, a marker of proliferation [39]. The importance of Hh signaling for enthesis healing was also demonstrated in mice with tendon- and enthesis-specific SMO deletion, where tendon and enthesis cells had reduced cellularity and decreased ECM and mineralization, leading to impaired healing [39]. Although no study has directly assessed the effect of Hh upregulation on enthesis healing, a study investigating mesenchymal stem cell treatment on enthesis healing found that treatment was associated with increased Hh activity, increased Sox9-positive cells, and stronger and more wide-spreading staining for type II collagen [49].
Table 1.
Hh signaling involved in tendon and enthesis healing (ACL: anterior cruciate ligament).
| Tissue type | Species | Injury model | Cell population after injury | Gene expression after injury | Protein expression after injury |
|---|---|---|---|---|---|
| Supraspinatus tendon enthesis32 | Mature mouse | Enthesis needle punch | Increasing numbers of Hh-lineage cells over time | Low expression of Notch 1 | |
| Immature mouse | Enthesis needle punch | Consistently high numbers of Hh-lineage cells throughout healing period | High expression of Notch 1 | ||
| Supraspinatus tendon enthesis43 | Rat | Tendon transection, followed by transosseous repair | Increased Sox9-positive cells and Ihh-posibve cells at enthesis | Increased expression of Gli1, PTCH1, Ihh, Col2, and Sox9 | |
| Supraspinatus tendon enthesis43 | Rat | Tendon transection, followed by transosseous repair | Similar expression of Scx, Tnmd; Increased expression of Smo, Col1. Acan | ||
| ACL enthesis40 | Rat | Full-thick ness transection of ACL followed by reconstruction using flexor tendon graft | Increased expression of Gli1 and PTCH1 | ||
| ACL enthesis41 | Mouse | Full-thickness transection of ACL followed by reconstruction with autograft | Similar expression of Scx, Sox9, and matrix metalloproteinases; Increased expression of Acan | Increased expression of Ihh Wnt, and PTHrP | |
| Tibialis anterior tendon44 | Mouse | Full-thickness transection followed by suture repair | Higher expression of Mkx, Scx, Egr1, Col1a1, Col1a2, Tnmd, Thbs4, Tppp3, Bglap, Gli1 Shh |
Hh signaling also appears to be involved in the healing response of the tendon mid-substance, although the mechanisms of action remain unclear. Hh ligands, the PTCH1 receptor, and the downstream component Gli1 were significantly upregulated in sheath tissues of mouse tibialis anterior tendon after injury [51]. Furthermore, injured tibialis anterior tendons in mice with tendon-specific SMO knockout had impaired healing processes compared to wild type controls, as demonstrated by downregulated ECM-related genes, reduced cell proliferation and collagen deposition, and thinner tendon sheath tissues [51]. In contrast, Hh activation via conditional knockout of a Hh suppressor gene improved tendon healing by promoting cell proliferation and tendon sheath remodeling. Coupled with the findings that TGFβ signaling plays a critical role in tendon midsubstance healing, potential crosstalk between Hh and TGFβ signals may explain a yet-to-be determined mechanism through which tendon healing is dependent on Hh signaling [51–53].
Hh signaling in tendon/enthesis heterotopic calcification
Heterotopic ossification (HO) is the pathologic ectopic formation of bone/mineralization in a soft tissue. Records of this phenomenon date back to World War I in the context of blast and gunshot wounds [54]. Since that time, aberrant soft tissue ossification has been observed in numerous areas such as major orthopedic surgery postoperatively, traumatic brain injury, spinal cord lesions, and burns (Figure 5) [55]. Considerable research has gone into uncovering the biochemical pathways and mechanisms underlying the development of heterotopic ossification in tendon, including the contribution of hedgehog signaling. Treatment approaches to prevent HO have shown only limited efficacy.
Figure 5.
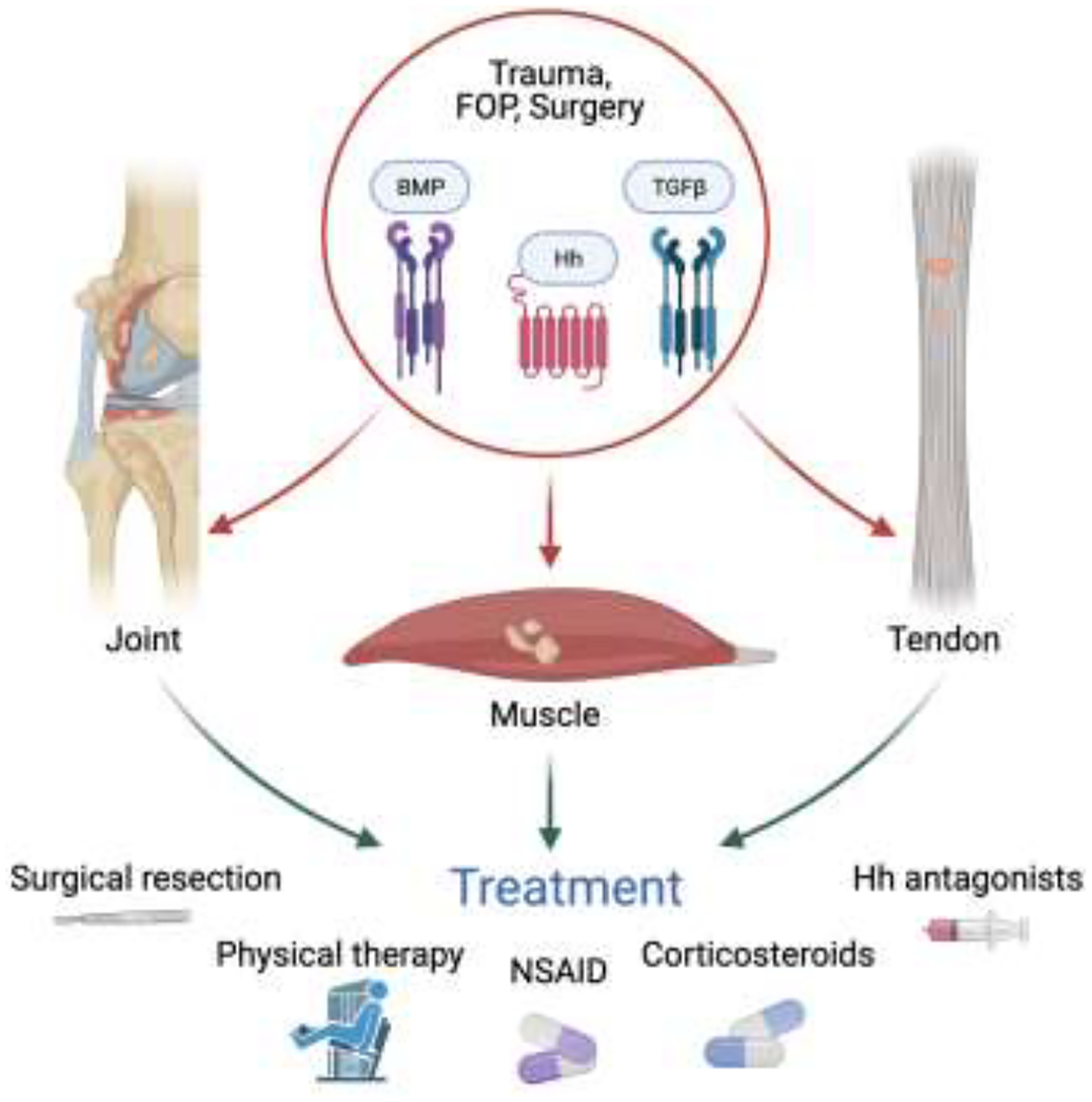
Heterotopic ossification occurs after trauma, surgery, and rare genetic conditions such as fibrodysplasia ossificans progressiva (FOP) and is driven by aberrant Hh, BMP, and/or TGFβ signaling. Treatments such as surgical resection and NSAIDs are only marginally effective.
Presumed mechanisms that drive tendon HO
BMPs have long been recognized as important signaling factors necessary for proper skeletal development and homeostasis [56]. In the canonical pathway, BMP receptor activation leads to Smad complex formation and subsequent translocation to the nucleus [57]. Type 1 BMP (BMP-1) signaling has been implicated as a mechanism for HO, particularly in studies of patients with fibrodysplasia ossificans progressiva (FOP), a rare but serious genetic disorder characterized by sporadic HO throughout the body [58]. A mutation in activin A receptor 1 (ACVR1), the gene encoding the BMP-1 receptor, was found to cause constitutive activation of the BMP signaling pathway and subsequent widespread HO [59]. The FOP phenotype has since been recreated in a mouse model via a ACVR1 knockout [59]. Additionally, conditional activation of ACVR1 driven by Scx-Cre transgene consistently caused progressive HO in ligament and tendon, further supporting this pathway’s contribution to tendon-specific HO [60]. In injury-induced tendon mineralization models, BMP receptor kinase inhibitors were found to significantly reduce tendon mineralization, thus presenting a potential pharmacologic target for future HO therapy development [61].
BMP and TGFβ pathways share multiple signaling hubs and then cooperatively or counteractively regulate cell behavior and tissue homeostasis [62]. Therefore, it is not surprising that TGFβ signaling has also been reported to contribute to tendon/enthesis HO. Evaluation of muscle from HO patients and Achilles tendon from trauma-induced HO mice showed increased numbers of cells positive for pSmad2/3, indicative of activated TGFβ signaling [63]. Monocytes/macrophages associated with HO were found to produce TGFβ1 and cause aberrant chondrogenic differentiation of progenitor cells [64]. Furthermore, treatment with neutralizing TGFβ antibodies attenuated tendon and cartilage HO of BMP-induced mouse models [63, 65]. Reduction of systemic macrophage TGFβ levels successfully ameliorated HO in Achilles tendon [52]. Knockout of TGFβ receptor II in a subpopulation of mesenchymal stem cells prevented the occurrence of cartilage and tendon HO, implicating a cellular source of TGFβ leading to HO [63]. Besides mesenchymal stem cells, tendon-derived progenitor cells positive for both Scx and cathepsin K were also found to participate in pathologic tendon HO [66].
Another studied pathway for HO development involves the Mohawk (Mkx) gene, a member of a larger class of homeobox genes, which codes a tendon/ligament associated transcription factor. Although originally discovered to mediate postnatal tendon homeostasis, the Mohawk transcription factor has recently been implicated in HO [67]. Mice deficient in the Mkx gene exhibited complete penetrance of the HO phenotype by two months of age [68]. In rats, onset of the HO phenotype in Mkx-deficient mutants occurred even sooner than the wild type mice, suggesting a disruption during tenogenesis [69]. Increased BMP pathway genes in the Mkx-deficient rats suggest a common pathway between these models and the development of HO. Interestingly, hedgehog signaling was also shown to be upregulated during tendon ossification in Mkx-deficient mice [66].
Perturbations in extracellular matrix (ECM) maintenance have also been illustrated to lead to tendon HO. Biglycan (Bgn) and fibromodulin (Fmod) are two small leucine-rich proteoglycans (SLRPs) highly involved in tendon and bone ECM formation and maintenance [70]. Double deficient Bgn−/−/Fmod−/− mice had abnormal collagen fibril structure and successive development of HO in quadriceps, patellar, and Achilles tendons [71]. The severity of the HO phenotype in these Bgn−/−/Fmod−/− mice was rescued by moderate exercise, indicating an essential role of biophysical signaling in maintaining cell phenotype and ECM remodeling [72]. Besides ECM, disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) proteinases, responsible for the construction and breakdown of various ECM components, have been documented to be involved in HO. Transgenic mice of dual knockout Adamts7−/− Adamts12−/−, two elements within the family of ADAMTS proteinases, were found to develop HO in adult hindlimb tendons [73]. These Adamts7−/− Adamts12−/− mice also exhibited reduced levels of Bgn and Fmod, further suggesting distorted ECM composition as a driver of tendon HO.
Traumatic insults in the form of injury or burn can recapitulate the tendon HO phenotype. The first reproducible animal model for tendon HO was described in 1953 after the observation of ossification following tenotomy of rat Achilles tendon [74]. Further work established mouse and rat models associated with other clinically relevant traumas such as burns, blast injury, and amputation [64, 75–78]. Further examination of HO formation in a trauma model revealed significant hypoxia inducible factor-1α (HIF-1α) expression in HO tissue [79]. Since HIF-1α is a transcription factor known to be critical for the chondrocyte survival [80], the activation of HIF-1α in this trauma-induced HO model provides evidence for its role to arrest mesenchymal cells towards a chondrogenic pathway and subsequent HO formation. Besides HIF-1α, activated nerve growth factor, accompanied with axonal invasion, and TGFβ produced by monocytes or macrophages were also shown to drive osteochondral differentiation of tendon-specific stem cells after soft tissue trauma [64, 78].
Contribution of Hh signaling to HO in tendon
Aberrant Hh signaling has been found to contribute to the development of HO in tendon. Patients with progressive osseous heteroplasia (POH), a rare disease characterized by extra-skeletal bone formation similar to FOP, lack a functional α-subunit of the stimulatory G protein (Gαs), a potent negative regulator of Hh signaling, due to a null mutation in GNAS [81]. Highly upregulated Hh signaling was seen at sites of ectopic bone derived from samples from POH patients [44]. In mice, loss of GNAS caused upregulation of Hh signaling and subsequent development of HO within the Achilles tendon akin to POH patients [44]. When analyzing a different major inhibitor of Hh signaling, Sufu, the development of spontaneous HO in tendon was again demonstrated [66]. In the aforementioned Mkx−/− mouse model of HO, tissue-specific inactivation of SMO, ameliorated the extent of tendon ossification [82]. Pharmacologic inhibition of Hh signaling via SMO antagonism or, more recently, Gli1/Gli2 inhibition, was also successful in decreasing ectopic bone formation [66, 83].
Enthesophytes/enthesis heterotopic ossification
The formation of enthesophytes, or bone spurs at ligament or tendon insertion sites, is an understudied phenomenon, and a direct link between this pathology and Hh signaling has not yet been confirmed. The involvement of pathways closely related to Hh signaling in enthesophyte formation has not been well established. BMP signaling, for example, drives the formation of enthesophytes in spondyloarthropathies, and its inhibition reduced excessive bone formation in a model of ankylosing enthesitis [84, 85]. BMP has been shown to stimulate Ihh expression in chondrocytes, and to coordinate their proliferation and differentiation [86, 87]. Additionally, both BMP and Ihh have been heavily implicated in the formation of osteophytes, bone spurs that originate at bone edges rather than enthesis regions [88–90]. Although the pathophysiologies of enthesophytes and osteophytes are not identical, they are positively associated and have several mechanistic similarities [91, 92]. Together, these observations point to a likely role of hedgehog signaling in abnormal bone formation at entheses, an area which merits further investigation. A better understanding of the mechanisms behind enthesophyte formation is essential for the development of improved therapeutics, to improve patient outcomes for a condition that can cause chronic pain and significantly reduce mobility in affected joints [93, 94].
Targeting Hh signaling for treatment of enthesopathy/tendinopathy
The Hh signaling pathway has served as an alluring target for therapeutic modulation for a wide range of pathologies. Generally, Hh inhibitors have been used to treat pathologies of unregulated growth, such as cancer, for which there are a number of approved Hh-related treatments. Hh-modulating approaches may also be of therapeutic benefit in the treatment of various tendon and enthesis disorders as well as to enhance tendon-to-bone healing. However, there are currently no FDA-approved Hh-modulating therapeutics for the treatment of these conditions. As such, repurposing Hh-modulating therapeutics from other areas to treat disorders of the tendon and its bony attachment presents an exciting new opportunity.
Therapeutic approaches for Hh signaling inhibition and agonism
Much of the development and application of Hh-modulating therapeutics has come from Hh inhibitor use in cancer research and treatment. Activation of the Hh signaling pathway results in upregulation of pro-angiogenic and pro-proliferative genes, and abnormal activation of Hh signaling has been linked to numerous malignancies [95, 96]. An extensive amount of preclinical and clinical research has been conducted on the use of Hh inhibitors in cancer treatment, which has led to FDA approval of several Hh inhibiting therapeutics [97]. There are numerous mechanisms by which the Hh signaling pathway can be inhibited. One method of inhibiting Hh signaling is by targeting the Hh ligand. Targeting of the Shh ligand has been achieved both through direct antibody targeting as well as through the targeting of a membrane-bound acyltransferase necessary for the palmitoylation of Shh during the final steps of protein synthesis [98–101]. The Hh signaling pathway can also be antagonized via inhibition of the cell surface receptor SMO. It has been demonstrated that cyclopamine, an alkaloid derived from V. californicum, can bind to SMO and inhibit Hh signaling [102, 103]. Since the discovery of the Hh-antagonizing potential of cyclopamine, numerous small molecules capable of binding to and inhibiting Smo have been developed [98]. Additionally, itraconazole, an FDA-approved antifungal drug, has been shown to target SMO and inhibit Hh signaling via the prevention of SMO accumulation in primary cilia [25, 97, 104]. Hh signaling can also be inhibited via the targeting of Gli1 transcription factors, which are downstream of the Hh ligand and its cell surface receptors. Arsenic trioxide has been shown to inhibit the activity of Gli1 and Gli2 via direct binding [105, 106]. Additionally, small molecule antagonists of GLI transcription factors have been developed [107, 108].
While numerous Hh inhibitors have been characterized, with downregulation of Hh signaling possible through several different mechanisms, comparably fewer Hh agonists have been identified. Several synthetic glucocorticoids as well as several synthetic and endogenous oxysterols are capable of upregulating Hh signaling via action on SMO [109–113]. Additionally, several small molecule SMO agonists (SAG) have been developed, including the purine purmorphamine and the benzothiophene, as well as their derivatives [114–118]. Hh signaling plays an important role in both cellular proliferation and differentiation during the development of the nervous system, as well as the maintenance of precursor cells involved in the regeneration of tissue following injury [119–121]. In the setting of damage and degeneration of peripheral nerves, subcutaneous administration of a Shh-IgG fusion protein returned nerve conduction velocities from diabetic levels to non-diabetic levels in a rat model of diabetes [122]. The benefits of the pro-angiogenic and pro-proliferative effects of Hh agonism may also extend to pathologies of ischemic origin, including those affecting the skeletal muscle, heart, and brain [96, 123]. In a mouse model of hindlimb ischemia, intraperitoneal injection of Shh recombinant protein improved blood-flow and limb salvage [124]. Upregulation of Hh signaling in cardiomyocytes via Shh gene transfer preserved cardiac function in adult animal models of myocardial ischemia [125]. However, despite promising preclinical research supporting the therapeutic potential of Hh agonism, to date, no Hh agonists have been approved for human use.
Modulation of Hh signaling as a treatment strategy for tendon and enthesis pathologies
Given the integral roles of Hh signaling in the formation of the enthesis and in pathologies such as HO, Hh signaling is a clear therapeutic target for a wide range of tendon and enthesis conditions. For tendon-to-bone repair, Hh agonism has the potential to promote fibrocartilage formation and mineralization, as seen during enthesis development, but only if an ample supply of responding progenitor cells are available. The ability of Hh agonism to increase bone volume is also of relevance, given the decrease in bone mineral density at the enthesis following rotator cuff injury and the positive effects of bone-targeted interventions for enhancing tendon-to-bone healing [50, 126, 127]. Increased Hh signaling through systemic administration of a Hh agonist has been shown to improve fracture healing in a mouse model [126], and is therefore an attractive strategy for improving tendon-to-bone healing. However, due to the region-specific activity of Hh signaling, i.e., it is both necessary for enthesis formation and pathologic in the context of HO, treatments targeting Hh signaling must be spatially and temporally controlled. For example, Hh agonists for tendon-to-bone repair should be localized to the repair site to enhance mineralization at the healing interface, without pathologic mineralization in the adjacent tendon or the formation of enthesophytes. Therefore, more appropriate, biocompatible carriers for Hh agonists or antagonists, instead of oral intake and intramuscular/intraperitoneal injection, should be developed to spatiotemporally control their release and activities [128–131]. For the treatment of HO, our current understanding of Hh signaling in the musculoskeletal system strongly supports the use of existing Hh inhibitors to be repurposed for the treatment of HO [44]. Finally, more work is needed to understand the role of Hh signaling in tendon midsubstance healing before a Hh-targeted therapy is developed. Notably, neither Hh inhibitors nor agonists are approved by the FDA for tendon and enthesis conditions, and additional pre-clinical testing is needed to evaluate efficacy and mechanisms of actions.
Cell-based therapies dependent on Hh signaling
As described earlier, cells positive for Gli1, an essential hedgehog signaling transcription factor, build the tendon enthesis and facilitate spatially graded mineralization [24, 39]. Consistent with the premise that Gli1 is a marker of enthesis stem cells, studies in other tissues have demonstrated that Gli1-expressing cells act as stem/progenitor cells to maintain tissue homeostasis and regulate repair. A critical role for Gli1+ cells in development and response to injury is reflected in multiple tissues, including teeth, bone, bone marrow, skin, colon, kidney, and heart [132–141] (Figure 6). During tooth development, Gli1+ cells located within dental mesenchyme proliferated and differentiated into various cell types (i.e. ameloblasts, odontoblasts) required for periodontal ligament and dental pulp formation [138, 139]. Furthermore, Gli1+ cells surrounding the neurovascular bundle were activated upon injury to give rise to reparative dentin, cementum, periodontal ligament, and alveolar bone [136, 137]. Consistent with the mechanosensitivity of Gli1+ cells at the tendon enthesis, physiological occlusal forces regulated sclerostin expression of alveolar bone osteocytes and diminished the multipotent capacity of Gli1+ cells in alveolar bone and periodontal ligament [136]. In growing bone, Gli1+ cells beneath the growth plate differentiated into bone marrow adipocytes, osteoblasts, and stromal cells during development and osteoblasts and chondrocytes during bone fracture healing [141]. In tissues such as muscle, skin, and colon, Gli1+ cells have been reported to serve as stem cells within tissue-specific niches and actively regenerate injured tissues [132, 133, 142]. In contrast, Gli1+ cells residing in bone marrow, kidney, lung, liver, and heart promoted unexpected ectopic differentiation and caused myofibroblast-driven fibrosis and osteoblast-induced calcification after organ injury [134, 135, 140]. Therefore, a role for Gli1-responsive cells as a pool of stem/progenitor cells and their multi-potency can be beneficial or detrimental, depending on the context of tissue type and injury. Tissue disease or injury can push Gli1-responsive cells towards pathology-related phenotypes and drive inappropriate tissue remodeling. Future studies should consider Gli1-responsive cells as a therapeutic target and decipher the molecular mechanisms governing their differentiation in different tissues.
Figure 6.
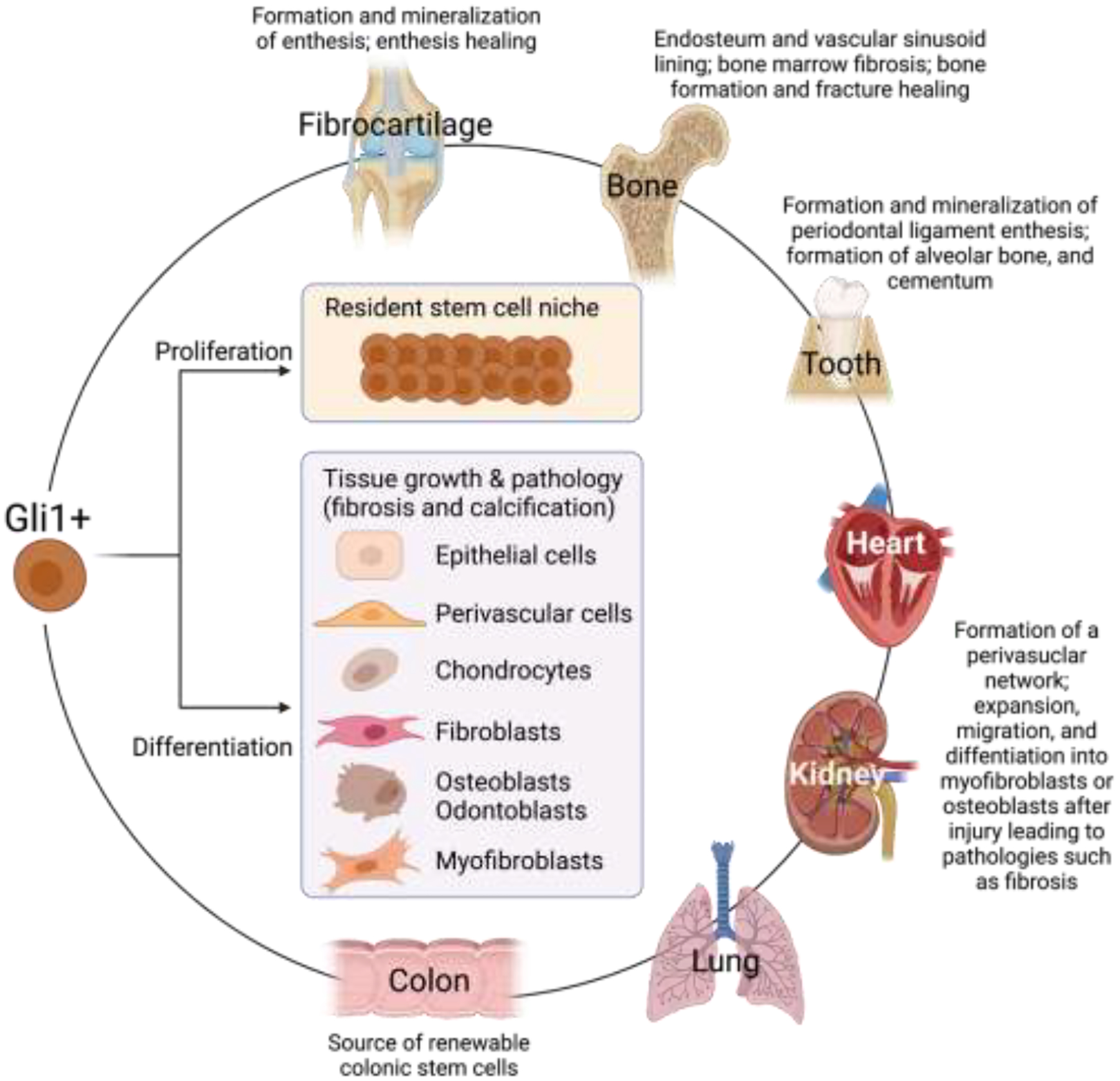
A critical role for Gli1+ or Gli1-responsive cells has been described for the development and injury response of multiple tissues. Gli1-responsive cells may proliferate to maintain a stem cell niche or differentiate to populate and build a wide range of tissues.
Perspective
The role of Hh signaling in the development of a wide range of tissues and in the progression of malignancies has been well explored. Building on this large body of literature, recent work has explored the importance of Hh signaling in tendon and enthesis development and pathology. It is clear that Hh signaling can play either a positive or a negative role on the tendon and enthesis, depending on the context. During enthesis development, Hh signaling is necessary for the formation of a functional tendon-to-bone attachment. Presumably, Hh signaling will also be necessary for regeneration of the enthesis during tendon-to-bone healing. In contrast, aberrant Hh signaling can lead to pathologic mineralization in the tendon or the formation of enthesophytes in the joint. Tendon midsubstance healing, on the other hand, presents a paradox: Hh signaling appears to promote tenogenesis after injury, in sharp contrast to Hh signaling also promoting pathologic HO in tendon under certain circumstances. Therefore, despite significant progress in our understanding of Hh signaling during tendon/enthesis development and repair, further insights into the molecular and cellular regulation of Hh signaling is necessary before we can develop thoughtful therapeutic strategies.
Cellular therapies have tremendous potential for treatment of musculoskeletal pathologies. Transplantation of progenitor cells with the capacity to proliferate and integrate with the native tissue and deposit appropriate ECM may transform the treatment of intractable clinical problems such as rotator cuff repair. Although multiple cell sources, including stem cells derived from bone marrow, adipose tissue, and tendon, have been applied in different injured animal models, the beneficial effect of cellular therapies in tendon and enthesis disorders remains elusive. As discussed in this review, Gli1-responsive cells have been appreciated to modulate development and regeneration of a wide-range of tissues and are a promising candidate for cellular therapy. Furthermore, Gli1-reponsive cells in the tendon enthesis feature more likely as progenitors instead of stem cells and they may be more inclined to progress towards a cell phenotype with characteristics of tenogenesis, chondrogenesis, and osteogenesis (rather than myogenesis or adipogenesis) after delivery to the healing enthesis. However, concerns remain about the use of these cells, which may localize to adjacent tissues and cause HO. Therefore, future studies should investigate factors driving their phenotype shift and manipulation of tissue niches to guide differentiation, and techniques should be developed to localize the delivery of these cells to the injury site.
Lastly, Hh-related pharmacologic approaches hold great potential for treatment of tendon and enthesis pathologies, including tendon-to-bone repair and HO. Some agonists, such as SAG, Hh-Ag1.5, and purmorphamine, have already been developed and used to activate Hh signaling for improving healing of fractured bone and injured meniscus [114, 126, 143]. Compared to the FDA-approved therapeutics of Hh antagonists, there is much more work to be done for translational application of Hh agonists. Any mechanistic differences between the different Hh agonists should be addressed, since inappropriate Hh stimulation can cause oncogenesis, unwanted osteogenesis, and fibrosis. Besides screening additional small molecules as Hh agonists and thoroughly characterizing their function, the application of these molecules in the laboratory and clinical settings needs to be optimized (e.g., delivery methods and dosing details). The pharmacological manipulation of Hh signaling is also associated with the precise delivery of drug molecules. Given the multifaceted molecular and cellular elements involved in tendon and enthesis healing, pharmacological treatment of Hh inhibition/agonism should be integrated and coordinated with other modalities (i.e., physical therapy) to achieve tendon regeneration.
Highlights.
The formation and mineralization of enthesis fibrocartilage is regulated by hedgehog signaling.
Hedgehog signaling can be beneficial, driving enthesis healing, or detrimental, driving tendon heterotopic ossification.
Hedgehog signaling is a promising therapeutic target for tendon and enthesis pathologies.
Acknowledgements
Funding support from NIH grant R01 AR055580. All figures were generated using BioRender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bangs F and Anderson KV, Primary cilia and mammalian hedgehog signaling. Cold Spring Harb. Perspect. Biol, 9 (2017). pp. a028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdes JM, Davis EE, and Katsanis N, The vertebrate primary cilium in development, homeostasis, and disease. Cell, 137 (2009). pp. 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahebjam S, Siu LL, and Razak AA, The utility of hedgehog signaling pathway inhibition for cancer. Oncologist, 17 (2012). pp. 1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pak E and Segal RA, Hedgehog signal transduction: key players, oncogenic drivers, and cancer therapy. Dev Cell, 38 (2016). pp. 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingham PW, Pattern formation: Hedgehog points the way. Curr. Biol, 4 (1994). pp. 347–350. [DOI] [PubMed] [Google Scholar]
- 6.Amakye D, Jagani Z, and Dorsch M.J.N.m., Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med, 19 (2013). pp. 1410. [DOI] [PubMed] [Google Scholar]
- 7.Kahane N and Kalcheim CJ, Neural tube development depends on notochord-derived Sonic hedgehog released into the sclerotome. Development, 147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlen HW, Buelens LA, and Vortkamp AJBDRPCETR, Hedgehog signaling in skeletal development. Defects Res. C. Embryo Today, 78 (2006). pp. 267–279. [DOI] [PubMed] [Google Scholar]
- 9.Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon APJG, and development, Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev, 18 (2004). pp. 937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Jacques B, Hammerschmidt M, McMahon APJG, and development, Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev, 13 (1999). pp. 2072–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomopoulos S, Genin GM, and Galatz LM, The development and morphogenesis of the tendon-to-bone insertion What development can teach us about healing. J. Musculoskelet. Neuronal Interact, 10 (2010). pp. 35. [PMC free article] [PubMed] [Google Scholar]
- 12.Liu CF, Breidenbach A, Aschbacher-Smith L, Butler D, and Wylie C, A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PloS one, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schett G, Lories RJ, D’Agostino M-A, Elewaut D, Kirkham B, Soriano ER, and McGonagle D, Enthesitis: from pathophysiology to treatment. Nat. Rev. Rheumatol, 13 (2017). pp. 731–741. [DOI] [PubMed] [Google Scholar]
- 14.Derwin KA, Galatz LM, Ratcliffe A, and Thomopoulos S, Enthesis repair: challenges and opportunities for effective tendon-to-bone healing. J. Bone Joint Surg. Am, 100 (2018). pp. e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostolakos J, Durant TJ, Dwyer CR, Russell RP, Weinreb JH, Alaee F, Beitzel K, McCarthy MB, Cote MP, and Mazzocca AD, The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J., 4 (2014). pp. 333. [PMC free article] [PubMed] [Google Scholar]
- 16.Lu HH and Thomopoulos S, Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu. Rev.Biomed. Eng, 15 (2013). pp. 201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossetti L, Kuntz L, Kunold E, Schock J, Müller K, Grabmayr H, Stolberg-Stolberg J, Pfeiffer F, Sieber S, and Burgkart R, The microstructure and micromechanics of the tendon–bone insertion. Nat. Mater, 16 (2017). pp. 664–670. [DOI] [PubMed] [Google Scholar]
- 18.Deymier AC, Schwartz AG, Cai Z, Daulton TL, Pasteris JD, Genin GM, and Thomopoulos S, The multiscale structural and mechanical effects of mouse supraspinatus muscle unloading on the mature enthesis. Acta Biomater., 83 (2019). pp. 302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golman M, Abraham AC, Kurtaliaj I, Marshall BP, Hu YJ, Schwartz AG, Guo XE, Birman V, Thurner PJ, and Genin GM, Toughening mechanisms for the attachment of architectured materials: The mechanics of the tendon enthesis. Sci. Adv, 7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blitz E, Sharir A, Akiyama H, and Zelzer E, Tendon-bone attachment unit is formed modularly by a distinct pool of Scx-and Sox9-positive progenitors. Development, 140 (2013). pp. 2680–2690. [DOI] [PubMed] [Google Scholar]
- 21.Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, and Thomopoulos S, Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J. Orthop. Res, 25 (2007). pp. 1621–1628. [DOI] [PubMed] [Google Scholar]
- 22.Felsenthal N, Rubin S, Stern T, Krief S, Pal D, Pryce BA, Schweitzer R, and Zelzer E, Development of migrating tendon-bone attachments involves replacement of progenitor populations. Development, 145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyment NA, Breidenbach AP, Schwartz AG, Russell RP, Aschbacher-Smith L, Liu H, Hagiwara Y, Jiang R, Thomopoulos S, and Butler DL, Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev. Biol, 405 (2015). pp. 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz AG, Long F, and Thomopoulos S, Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development, 142 (2015). pp. 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang F, Schwartz AG, Moore ER, Sup ME, and Thomopoulos S, Primary cilia as the nexus of biophysical and hedgehog signaling at the tendon enthesis. Sci. Adv, 6 (2020). pp. eabc1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breidenbach AP, Aschbacher-Smith L, Lu Y, Dyment NA, Liu CF, Liu H, Wylie C, Rao M, Shearn JT, and Rowe DW, Ablating hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. J. Orthop. Res, 33 (2015). pp. 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelzer E, Blitz E, Killian ML, and Thomopoulos S, Tendon-to-bone attachment: From development to maturity. B Birth. Defects Res. C Embryo Today, 102 (2014). pp. 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen PT, Lambertsen KL, and Frich LH, Assembly, maturation, and degradation of the supraspinatus enthesis. J. Shoulder Elbow Surg, 27 (2018). pp. 739–750. [DOI] [PubMed] [Google Scholar]
- 29.Faiao-Flores F, Alves-Fernandes D, Pennacchi PC, Sandri S, Vicente ALSA, Scapulatempo-Neto C,Vazquez V.d.L., Reis RM, Chauhan J, and Goding C, Targeting the hedgehog transcription factors GLI1 and GLI2 restores sensitivity to vemurafenib-resistant human melanoma cells. Oncogene, 36 (2017). pp. 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Zhao F, He X, Wang J, Zhang Y, Zhang H, Ni Y, Sun J, Wang X, and Dou J, Combining TGF-β1 knockdown and miR200c administration to optimize antitumor efficacy of B16F10/GPI-IL-21 vaccine. Oncotarget, 6 (2015). pp. 12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, and Tabin CJ, Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS genetics, 2 (2006). pp. e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez J, Sohn P, Zeng X, Doetschman T, Robbins DJ, and Serra R, TGFβ2 mediates the effects of Hedgehog on hypertrophic differentiation and PTHrP expression. Development, 129 (2002), pp. 1913–1924 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Rios I, Alvarez-Rodríguez R, Martí E, and Pons S, Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signalling. Development, 131 (2004), pp. 3159–3168. [DOI] [PubMed] [Google Scholar]
- 34.Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, and Yuen ST, Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl. Acad. Sci. USA, 104 (2007). pp. 15418–15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spit M, Koo BK, and Maurice MM, Tales from the crypt: intestinal niche signals in tissue renewal, plasticity and cancer. Open biol. 8 (2018). pp. 180120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kronenberg HM, Developmental regulation of the growth plate. Nature, 423 (2003). pp. 332–336. [DOI] [PubMed] [Google Scholar]
- 37.Álvarez J, Balbín M, Fernández M, and López JM, Collagen metabolism is markedly altered in the hypertrophic cartilage of growth plates from rats with growth impairment secondary to chronic renal failure. J. Bone Miner. Res, 16 (2001). pp. 511–524. [DOI] [PubMed] [Google Scholar]
- 38.Fujioka H, Wang GJ, Mizuno K, Balian G, and Hurwitz SR, Changes in the expression of type-X collagen in the fibrocartilage of rat Achilles tendon attachment during development. J. Orthop. Res, 15 (1997). pp. 675–681. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz AG, Galatz LM, and Thomopoulos S, Enthesis regeneration: a role for Gli1+ progenitor cells. Development, 144 (2017). pp. 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razzaque MS, Soegiarto DW, Chang D, Long F, and Lanske B, Conditional deletion of Indian hedgehog from collagen type 2α1-expressing cells results in abnormal endochondral bone formation. J Pathol, 207 (2005). pp. 453–461. [DOI] [PubMed] [Google Scholar]
- 41.Long F, Zhang XM, Karp S, Yang Y, and McMahon AP, Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 128 (2001). pp. 5099–8. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi T, Chung U, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, and Kronenberg HM, PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development, 129 (2002). pp. 2977–86. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Macica C, Nasiri A, Judex S, and Broadus AE, Mechanical regulation of PTHrP expression in entheses. Bone, 41 (2007). pp. 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore EM,Kaplan FS, and Yang Y, Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat. Med, 19 (2013). pp. 1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galatz LM, Ball CM, Teefey SA, Middleton WD, and Yamaguchi K, The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Joint Surg. Am, 86 (2004). pp. 219–224. [DOI] [PubMed] [Google Scholar]
- 46.Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, and Galatz LM, Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year followup. J. Bone Joint Surg. Am, 95 (2013). pp. 627–632. [DOI] [PubMed] [Google Scholar]
- 47.Carbone A, Carballo C, Ma R, Wang H, Deng X, Dahia C, and Rodeo S, Indian hedgehog signaling and the role of graft tension in tendon-to-bone healing: evaluation in a rat ACL reconstruction model. J. Orthop. Res, 34 (2016). pp. 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng XH, Lebaschi A, Camp CL, Carballo CB, Coleman NW, Zong J, Grawe BM, and Rodeo SA,Expression of signaling molecules involved in embryonic development of the insertion site is inadequate for reformation of the native enthesis: evaluation in a novel murine ACL reconstruction model. J. Bone Joint Surg. Am, 100 (2018). pp. e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zong JC, Mosca MJ, Degen RM, Lebaschi A, Carballo C, Carbone A, Cong GT, Ying L, Deng XH, and Rodeo SA, Involvement of Indian hedgehog signaling in mesenchymal stem cell–augmented rotator cuff tendon repair in an athymic rat model. J. Shoulder Elbow Surg, 26 (2017). pp. 580–588. [DOI] [PubMed] [Google Scholar]
- 50.Shah SA, Kormpakis I, Havlioglu N, Ominsky MS, Galatz LM, and Thomopoulos S, Sclerostin antibody treatment enhances rotator cuff tendon-to-bone healing in an animal model. J. Bone Joint Surg. Am, 99 (2017). pp. 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Zhang X, Huang H, Xia Y, Yao Y, Mak AF, Yung PS, Chan KM, Wang L, and Zhang C, Osteocalcin expressing cells from tendon sheaths in mice contribute to tendon repair by activating Hedgehog signaling. Elife, 6 (2017). pp. e30474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Zhang C, Zhu S, Lu P, Zhu T, Gong X, Zhang Z, Hu J, Yin Z, and Heng BC, Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFβ signaling pathway. Stem Cells, 33 (2015). pp. 443–455. [DOI] [PubMed] [Google Scholar]
- 53.Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T, Yokoyama S, Nishida K, Akimoto T, Takahashi M, and Miyaki S, The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA, 107 (2010). pp. 10538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards DS and Clasper JC, Heterotopic ossification: a systematic review. Genes Dev, 10 (1996). pp. 1580–94. [DOI] [PubMed] [Google Scholar]
- 55.Vanden Bossche L and Vanderstraeten G, Heterotopic ossification: a review. J. Rehabil. Med, 37 (2005). pp. 129–36. [DOI] [PubMed] [Google Scholar]
- 56.Hogan BL, Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev., 10 (1996). pp. 1580–94. [DOI] [PubMed] [Google Scholar]
- 57.Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, and Shi LL, Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis., 1 (2014). pp. 87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, and Kaplan FS, A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet, 38 (2006). pp. 525–7. [DOI] [PubMed] [Google Scholar]
- 59.Hildebrand L, Schmidt-von Kegler M, Walther M, Seemann P, and Stange K, Limb specific Acvr1-knockout during embryogenesis in mice exhibits great toe malformation as seen in Fibrodysplasia Ossificans Progressiva (FOP). Dev. Dyn, 248 (2019). pp. 396–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dey D, Bagarova J, Hatsell SJ, Armstrong KA, Huang L, Ermann J, Vonner AJ, Shen Y, Mohedas AH, Lee A, Eekhoff EM, van Schie A, Demay MB, Keller C, Wagers AJ, Economides AN, and Yu PB, Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci. Transl. Med, 8 (2016). pp. 366ra163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang K, Asai S, Hast MW, Liu M, Usami Y, Iwamoto M, Soslowsky LJ, and Enomoto-Iwamoto M, Tendon mineralization is progressive and associated with deterioration of tendon biomechanical properties, and requires BMP-Smad signaling in the mouse Achilles tendon injury model. Matrix Biol, 52–54 (2016). pp. 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ning J, Zhao Y, Ye Y, and Yu J, Opposing roles and potential antagonistic mechanism between TGF-β and BMP pathways: Implications for cancer progression. EBioMedicine, 41 (2019). pp. 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Li F, Xie L, Crane J, Zhen G, Mishina Y, Deng R, Gao B, Chen H, and Liu S, Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice. Nature communications, 9 (2018). pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sorkin M, Huber AK, Hwang C, Carson WF, Menon R, Li J, Vasquez K, Pagani C, Patel N, and Li S, Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nature communications, 11 (2020). pp. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Li F, Xie L, Crane J, Zhen G, Mishina Y, Deng R, Gao B, Chen H, and Liu S, Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice. Nat. commun, 9 (2018). pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng H, Xing W, Han Y, Sun J, Kong M, Gao B, Yang Y, Yin Z, Chen X, and Zhao Y, Tendon-derived cathepsin K-expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification. Clin. Invest, 130 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson DM, Arredondo J, Hahn K, Valente G, Martin JF, Wilson-Rawls J, and Rawls A, Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev. Dyn, 235 (2006). pp. 792–801. [DOI] [PubMed] [Google Scholar]
- 68.Kiseleva AA, Korobeynikov VA, Nikonova AS, Zhang P, Makhov P, Deneka AY, Einarson MB, Serebriiskii IG, Liu H, and Peterson JR, Unexpected activities in regulating ciliation contribute to off-target effects of targeted drugs. Clin. Cancer Res, (2019). pp. 3535.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuki H, Ito Y, Shinohara M, Yamashita S, Ichinose S, Kishida A, Oyaizu T, Kayama T, Nakamichi R, Koda N, Yagishita K, Lotz MK, Okawa A, and Asahara H, Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc. Natl. Acad. Sci. USA, 113 (2016). pp. 7840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilda M, Bachner D, Just W, Geerkens C, Kraus P, Vogel W, and Hameister H, A comparison of the expression pattern of five genes of the family of small leucine-rich proteoglycans during mouse development. J. Bone Miner. Res, 15 (2000). pp. 2187–96. [DOI] [PubMed] [Google Scholar]
- 71.Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, and Young MF, Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J, 16 (2002). pp. 673–80. [DOI] [PubMed] [Google Scholar]
- 72.Kilts T, Ameye L, Syed-Picard F, Ono M, Berendsen AD, Oldberg A, Heegaard AM, Bi Y, and Young MF, Potential roles for the small leucine-rich proteoglycans biglycan and fibromodulin in ectopic ossification of tendon induced by exercise and in modulating rotarod performance. Scand. J. Med. Sci. Sports, 19 (2009). pp. 536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mead TJ, McCulloch DR, Ho JC, Du Y, Adams SM, Birk DE, and Apte SS, The metalloproteinase-proteoglycans ADAMTS7 and ADAMTS12 provide an innate, tendon-specific protective mechanism against heterotopic ossification. JCI insight, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buck RC, Regeneration of tendon. J. Pathol. Bacteriol, 66 (1953). pp. 1–18. [PubMed] [Google Scholar]
- 75.Tannous O, Griffith C, O’Toole RV, and Pellegrini VD Jr., Heterotopic ossification after extremity blast amputation in a Sprague-Dawley rat animal model. J. Orthop. Trauma, 25 (2011). pp. 506–10. [DOI] [PubMed] [Google Scholar]
- 76.Peterson JR, Agarwal S, Brownley RC, Loder SJ, Ranganathan K, Cederna PS, Mishina Y, Wang SC, and Levi B, Direct Mouse Trauma/Burn Model of Heterotopic Ossification. J. Vis. Exp, (2015). pp. e52880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Polfer EM, Hope DN, Elster EA, Qureshi AT, Davis TA, Golden D, Potter BK, and Forsberg JA, The development of a rat model to investigate the formation of blast-related post-traumatic heterotopic ossification. Bone Joint J, 97 (2015). pp. 572–6. [DOI] [PubMed] [Google Scholar]
- 78.Lee S, Hwang C, Marini S, Tower RJ, Qin Q, Negri S, Pagani CA, Sun Y, Stepien DM, and Sorkin M, NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat. Commun, 12 (2021). pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin L, Shen Q, Xue T, and Yu C, Heterotopic ossification induced by Achilles tenotomy via endochondral bone formation: expression of bone and cartilage related genes. Bone, 46 (2010). pp. 425–31. [DOI] [PubMed] [Google Scholar]
- 80.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, and Johnson RS, Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev, 15 (2001). pp. 2865–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eddy MC, Jan De Beur SM, Yandow SM, McAlister WH, Shore EM, Kaplan FS, Whyte MP, and Levine MA, Deficiency of the alpha-subunit of the stimulatory G protein and severe extraskeletal ossification. J. Bone Miner. Res, 15 (2000). pp. 2074–83. [DOI] [PubMed] [Google Scholar]
- 82.Liu H, Xu J, and Jiang R, Mkx-Deficient Mice Exhibit Hedgehog Signaling-Dependent Ectopic Ossification in the Achilles Tendons. J. Bone Miner. Res, 34 (2019). pp. 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.AlMuraikhi N, Almasoud N, Binhamdan S, Younis G, Ali D, Manikandan M, Vishnubalaji R, Atteya M, Siyal A, and Alfayez M, Hedgehog signaling inhibition by smoothened antagonist BMS-833923 reduces osteoblast differentiation and ectopic bone formation of human skeletal (mesenchymal) stem cells. Stem cells Int, 2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lories RJ and Luyten FP, Bone morphogenetic proteins in destructive and remodeling arthritis. Arthritis Res. Ther, 9 (2007). pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lories RJ, Derese I, and Luyten FP, Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J. Clin. Invest, 115 (2005). pp. 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, and Vortkamp A, BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development, 128 (2001). pp. 4523–4534. [DOI] [PubMed] [Google Scholar]
- 87.Grimsrud CD, Romano PR, D’Souza M, Puzas JE, Schwarz EM, Reynolds PR, Roiser RN, and O’Keefe RJ, BMP signaling stimulates chondrocyte maturation and the expression of Indian hedgehog. J. Orthop. Res, 19 (2001). pp. 18–25. [DOI] [PubMed] [Google Scholar]
- 88.Zoricic S, Maric I, Bobinac D, and Vukicevic S, Expression of bone morphogenetic proteins and cartilage-derived morphogenetic proteins during osteophyte formation in humans. J. Anat, 202 (2003). pp. 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruiz-Heiland G, Horn A, Zerr P, Hofstetter W, Baum W, Stock M, Distler JH, Nimmerjahn F, Schett G, and Zwerina J, Blockade of the hedgehog pathway inhibits osteophyte formation in arthritis. Ann. Rheum. Dis, 71 (2012). pp. 400–407. [DOI] [PubMed] [Google Scholar]
- 90.Bechtold TE, Saunders C, Decker RS, Um H-B, Cottingham N, Salhab I, Kurio N, Billings PC, Pacifici M, and Nah HD, Osteophyte formation and matrix mineralization in a TMJ osteoarthritis mouse model are associated with ectopic hedgehog signaling. Matrix Biol, 52 (2016). pp. 339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rogers J, Shepstone L, and Dieppe P, Bone formers: osteophyte and enthesophyte formation are positively associated. Ann. Rheum. Dis, 56 (1997). pp. 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hardcastle SA, Dieppe P, Gregson CL, Arden NK, Spector TD, Hart DJ, Edwards MH, Dennison EM, Cooper C, and Williams M, Osteophytes, enthesophytes, and high bone mass: a bone-forming triad with potential relevance in osteoarthritis. Arthritis Rheumatol, 66 (2014). pp. 2429–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tay SH, Yeo JG, Leong JY, Albani S, and Arkachaisri T, Juvenile Spondyloarthritis: What More Do We Know About HLA-B27, Enthesitis, and New Bone Formation? Front. Med, 8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clunie G and Horwood N, Loss and gain of bone in spondyloarthritis: what drives these opposing clinical features? Ther Adv. Musculoskelet. Dis, 12 (2020). pp. 1759720X20969260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steg AD, Katre AA, Bevis KS, Ziebarth A, Dobbin ZC, Shah MM, Alvarez RD, and Landen CN, Smoothened antagonists reverse taxane resistance in ovarian cancer. Mol. Cancer Ther, 11 (2012). pp. 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ng JM and Curran T, The Hedgehog’s tale: developing strategies for targeting cancer. Nat. Rev. Cancer, 11 (2011). pp. 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clara JA, Monge C, Yang Y, and Takebe N, Targeting signalling pathways and the immune microenvironment of cancer stem cells-A clinical update. Nat. Re. Clin. Oncol, 17 (2020). pp. 204–232. [DOI] [PubMed] [Google Scholar]
- 98.Rimkus TK, Carpenter RL, Qasem S, Chan M, and Lo H-W, Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers, 8 (2016). pp. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petrova E, Rios-Esteves J, Ouerfelli O, Glickman JF, and Resh MD, Inhibitors of Hedgehog acyltransferase block Sonic Hedgehog signaling. Nat. Chem. Biol, 9 (2013). pp. 247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Coon V, Laukert T, Pedone CA, Laterra J, Kim KJ, and Fults DW, Molecular therapy targeting Sonic hedgehog and hepatocyte growth factor signaling in a mouse model of medulloblastoma. Mol. Cancer Ther, 9 (2010). pp. 2627–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen MH, Li YJ, Kawakami T, Xu SM, and Chuang PT, Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev, 18 (2004). pp. 641–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cooper MK, Porter JA, Young KE, and Beachy PA, Teratogen-mediated inhibition of target tissue response to Shh signaling. Science, 280 (1998). pp. 1603–1607. [DOI] [PubMed] [Google Scholar]
- 103.Incardona JP, Gaffield W, Kapur RP, and Roelink H, The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development, 125 (1998). pp. 3553–3562. [DOI] [PubMed] [Google Scholar]
- 104.Burgos-Ojeda D, McLean K, Bai S, Pulaski H, Gong Y, Silva I, Skorecki K, Tzukerman M, and Buckanovich RJ, A Novel Model for Evaluating Therapies Targeting Human Tumor Vasculature and Human Cancer Stem-like Cells. Cancer Res, 73 (2013). pp. 3555–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.List A, Beran M, DiPersio J, Slack J, Vey N, Rosenfeld C, and Greenberg P, Opportunities for Trisenox (arsenic trioxide) in the treatment of myelodysplastic syndromes. Leukemia, 17 (2003). pp. 1499–1507. [DOI] [PubMed] [Google Scholar]
- 106.Beauchamp EM, Ringer L, Bulut G, Sajwan KP, Hall MD, Lee YC, Peaceman D, Özdemirli M, Rodriguez O, and Macdonald TJ, Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J. Clin. Invest, 121 (2011). pp. 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lauth M, Bergström Å, Shimokawa T, and Toftgård R, Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl. Acad. Sci. USA, 104 (2007). pp. 8455–8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wickström M, Dyberg C, Shimokawa T, Milosevic J, Baryawno N, Fuskevåg OM, Larsson R, Kogner P, Zaphiropoulos PG, and Johnsen JI, Targeting the hedgehog signal transduction pathway at the level of GLI inhibits neuroblastoma cell growth in vitro and in vivo. Int. J. Cancer, 132 (2013). pp. 1516–1524. [DOI] [PubMed] [Google Scholar]
- 109.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, and Rohatgi R, Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat. Chem. Biol, 8 (2012). pp. 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Myers BR, Sever N, Chong YC, Kim J, Belani JD, Rychnovsky S, Bazan JF, and Beachy PA, Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev. Cell, 26 (2013). pp. 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nedelcu D, Liu J, Xu Y, Jao C, and Salic A, Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat. Chem. Biol, 9 (2013). pp. 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nachtergaele S, Whalen DM, Mydock LK, Zhao Z, Malinauskas T, Krishnan K, Ingham PW, Covey DF, Siebold C, and Rohatgi R, Structure and function of the Smoothened extracellular domain in vertebrate Hedgehog signaling. Elife, 2 (2013). pp. e01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J, Lu J, Bond MC, Chen M, Ren XR, Lyerly HK, Barak LS, and Chen W, Identification of select glucocorticoids as Smoothened agonists: potential utility for regenerative medicine. Pro. Natl. Acad. Sci 107 (2010). pp. 9323–9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen JK, Taipale J, Young KE, Maiti T, and Beachy PA, Small molecule modulation of Smoothened activity. Pro. Natl. Acad. Sci. U S A, 99 (2002). pp. 14071–14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, and Shulok J, Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J. Biol, 1 (2002). pp. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seifert K, Büttner A, Rigol S, Eilert N, Wandel E, and Giannis A, Potent small molecule Hedgehog agonists induce VEGF expression in vitro. Bioorg. Med. Chem, 20 (2012). pp. 6465–6481. [DOI] [PubMed] [Google Scholar]
- 117.Brunton SA, Stibbard JH, Rubin LL, Guicherit OM, Kruse LI, Price S, di Lucrezia R, MacKinnon CH, Avery A, and Park Y, Potent agonists of the Hedgehog signaling pathway. Bioorg. Med. Chem. Lett, 19 (2009). pp. 4308–4311. [DOI] [PubMed] [Google Scholar]
- 118.Wu X, Walker J, Zhang J, Ding S, and Schultz PG, Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem. Biol, 11 (2004). pp. 1229–1238. [DOI] [PubMed] [Google Scholar]
- 119.Dellovade T, Romer JT, Curran T, and Rubin LL, The hedgehog pathway and neurological disorders. Annu. Rev. Neurosci, 29 (2006). pp. 539–563. [DOI] [PubMed] [Google Scholar]
- 120.Ruat M, Roudaut H, Ferent J, and Traiffort E, Hedgehog trafficking, cilia and brain functions. Differentiation, 83 (2012). pp. S97–S104. [DOI] [PubMed] [Google Scholar]
- 121.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo J-M, Aguilar A, Schneider-Maunoury S, and Alvarez-Buylla A, Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci, 11 (2008). pp. 277–284. [DOI] [PubMed] [Google Scholar]
- 122.Kusano KF, Allendoerfer KL, Munger W, Pola R, Bosch-Marce M, Kirchmair R, Yoon Y, Curry C, Silver M, and Kearney M, Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arteriosler. Thromb. Vasc. Biol, 24 (2004). pp. 2102–2107. [DOI] [PubMed] [Google Scholar]
- 123.Hadden MK, Hedgehog Pathway Agonism: Therapeutic Potential and Small-Molecule Development. ChemMedChem, 9 (2014). pp. 27–37. [DOI] [PubMed] [Google Scholar]
- 124.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Pepinsky RB, Shapiro R, Taylor FR, Baker DP, and Asahara T, The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nature medicine, 7 (2001). pp. 706–711. [DOI] [PubMed] [Google Scholar]
- 125.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, and Tkebuchava T, Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat. Med, 11 (2005). pp. 1197–1204. [DOI] [PubMed] [Google Scholar]
- 126.McKenzie JA, Maschhoff C, Liu X, Migotsky N, Silva MJ, and Gardner MJ, Activation of hedgehog signaling by systemic agonist improves fracture healing in aged mice. J. Orthop. Res, 37 (2019). pp. 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thangarajah T, Henshaw F, Sanghani-Kerai A, Lambert SM, Pendegrass CJ, and Blunn GW, Supraspinatus detachment causes musculotendinous degeneration and a reduction in bone mineral density at the enthesis in a rat model of chronic rotator cuff degeneration. Shoulder Elbow, 9 (2017). pp. 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith BD and Grande DA, The current state of scaffolds for musculoskeletal regenerative applications. Nat. Rev. Rheumatol, 11 (2015). pp. 213–222. [DOI] [PubMed] [Google Scholar]
- 129.Pugliese E, Coentro JQ, and Zeugolis DI, Advancements and challenges in multidomain multicargo delivery vehicles. Adv. Mater, 30 (2018). pp. 1704324. [DOI] [PubMed] [Google Scholar]
- 130.Miao T, Wang J, Zeng Y, Liu G, and Chen X, Polysaccharide-based controlled release systems for therapeutics delivery and tissue engineering: from bench to bedside. Adv. Sci, 5 (2018). pp. 1700513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saltzman WM and Olbricht WL, Building drug delivery into tissue engineering design. Nat. Rev. Drug Discov, 1 (2002). pp. 177–186. [DOI] [PubMed] [Google Scholar]
- 132.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, and Basler KJ, GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature, 558 (2018). pp. 449–453. [DOI] [PubMed] [Google Scholar]
- 133.Brownell I, Guevara E, Bai CB, Loomis CA, and Joyner AL, Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell, 8 (2011). pp. 552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, and Humphreys B.D.J.C.s.c., Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell, 16 (2015). pp. 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, and Gratwohl S.J.C.s.c., Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell, 19 (2016). pp. 628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Men Y, Wang Y, Yi Y, Jing D, Luo W, Shen B, Stenberg W, Chai Y, Ge WP, and Feng JQJDC, Gli1+ periodontium stem cells are regulated by osteocytes and occlusal force. Cell Stem Cell, 54 (2020). pp. 639–654. e6. [DOI] [PubMed] [Google Scholar]
- 137.Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, and Chai Y.J.C.s.c., Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell, 14 (2014). pp. 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seidel K, Ahn CP, Lyons D, Nee A, Ting K, Brownell I, Cao T, Carano RA, Curran T, and Schober MJD, Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development, 137 (2010). pp. 3753–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Y, Feng J, Li J, Zhao H, Ho TV, and Chai YJD, An Nfic-hedgehog signaling cascade regulates tooth root development. Development, 142 (2015). pp. 3374–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schneider RK, Mullally A, Dugourd A, Peisker F, Hoogenboezem R, Van Strien PM, Bindels EM, Heckl D, Büsche G, and Fleck D.J.C.s.c., Gli1+ mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell, 20 (2017). pp. 785–800. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shi Y, He G, Lee WC, McKenzie JA, Silva MJ, and Long FJNC, Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat. Commun, 8 (2017). pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yao L, Tichy ED, Zhong L, Mohanty S, Wang L, Ai E, Yang S, Mourkioti F, Qin L.J.J.o.B., and M. Research, Gli1 Defines a Subset of Fibro-adipogenic Progenitors that Promote Skeletal Muscle Regeneration With Less Fat Accumulation. J. Bone Miner. Res 36 (2021). pp. 1159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei Y, Sun H, Gui T, Yao L, Zhong L, Yu W, Heo SJ, Han L, Dyment NA, and Liu XS, The critical role of Hedgehog-responsive mesenchymal progenitors in meniscus development and injury repair. Elife, 10 (2021). pp. e62917. [DOI] [PMC free article] [PubMed] [Google Scholar]


