Abstract
Freezing during the flowering of Prunus sibirica is detrimental to fruit production. The late flowering (LF) type, which is delayed by 7–15 days compared with the normal flowering (NF) type, avoids damages at low temperature, but the molecular mechanism of LF remains unclear. Therefore, this study was conducted to comprehensively characterize floral bud differentiation. A histological analysis showed that initial floral bud differentiation was delayed in the LF type compared to the NF type. Genome-wide associated studies (GWAS) showed that a candidate gene (PaF106G0600023738.01) was significantly associated with LF type. It was identified as trehalose-6-phosphate phosphatase (PsTPPF), which is involved in trehalose-6-phosphate (Tre6P) signaling pathway and acts on floral transition. A whole-transcriptome RNA sequencing analysis was conducted, and a total of 6,110 differential expression (DE) mRNAs, 1,351 DE lncRNAs, and 148 DE miRNAs were identified. In addition, 24 DE mRNAs related with floral transition were predicted, and these involved the following: three interactions between DE lncRNAs and DE mRNAs of photoperiod pathway with two mRNAs (COP1, PaF106G0400018289.01 and CO3, MXLOC_025744) and three lncRNAs (CCLR, LTCONS_00031803, COCLR1, LTCONS_00046726, and COCLR2, LTCONS_00046731); one interaction between DE miRNAs and DE mRNAs with one mRNA, encoding trehalose-6-phosphate synthase (PsTPS1, PaF106G0100001132.01), and one miRNA (miRNA167h). Combined with the expression profiles and Tre6P levels, functions of PsTPPF and PsTPS1 in Tre6P regulation were considered to be associated with flowering time. A new network of ceRNAs correlated with LF was constructed, and it consisted of one mRNA (PsTPS1), one lncRNA (TCLR, LTCONS_00034157), and one miRNA (miR167h). This study provided insight into the molecular regulatory mechanism of LF in Prunus sibirica.
Keywords: TPP, Tre6P, TPS, floral transition, GWAS, ceRNA
Introduction
Prunus sibirica is a stone fruit and woody oil plant that has excellent resistance to extreme environment conditions and is both economically and ecologically valuable (Zhang et al., 2006; Wang et al., 2017). It is an early flowering phenotype species, and its yield is susceptible to low-temperature weather during flowering. When P. sibirica flowers late, damage is avoided, and apricot yield is ensured; however, only few studies have investigated the associated mechanism (Wang et al., 2019).
Floral transition time is an important factor affecting the flowering of plants, and it further affects their life cycle. It is controlled by a complex network of numerous genes involved in genetic pathways, and several floral transition genes have been reported, including CONSTAN (CO) in photoperiod pathway (Suarez-Lopez et al., 2001), DELLA proteins in gibberellin (GA) pathway (Hou et al., 2008), VRNs in vernalization pathway (Levy et al., 2002), and SQUAMOSA promoter binding protein-like (SPLs) in age pathway (Wu and Poethig, 2006), and trehalose-6-phosphate synthase (TPS1) in trehalose-6-phosphate (Tre6P) signaling pathway (Wahl et al., 2013).
Using the sequencing technique process, genome-wide associate studies (GWASs) have been conducted to identify candidate genes associated with important traits in perennial fruit trees. For example, studies have investigated Prunus persica and Pyrus pyrifolia to identify fruit quality and phonological traits (Cao et al., 2016; Zhang et al., 2021), Prunus armeniaca to analyze pathogen resistance (Mariette et al., 2016), Prunus mume to determine floral traits (Zhang et al., 2018), and Malus domestica for fruit quality traits (Duan et al., 2017). Recently, several candidate genes associated with flowering time in P. persica (Li et al., 2019), Helianthus annuus (Bonnafous et al., 2018), and Brassica napus (Huang et al., 2021) have been identified by GWASs.
Whole transcriptome RNA sequencing analyses have been performed to investigate non-coding RNAs (ncRNAs), which participate in multiple biological and abiotic functions. NcRNAs are divided into two categories by length, small RNAs and long non-coding RNAs (lncRNAs). Notably, ncRNAs played important roles in the control of flowering; for example, two well-known lncRNAs, COLD-INDUCED LONG ANTISENSE INTRAGENIC RNA (COOLAIR) and COLD-ASSISTED INTRONIC NON-CODING RNA (COLDAIR) acted on vernalization process associated with controlling flowering time by silencing the transcription of FLC (Liu et al., 2010; Heo and Sung, 2011). The studies of Wu and Poethig (2006) and Wu et al. (2009) first reported that miR156 and miR172 controlled floral transition in Arabidopsis thaliana, with opposite regulatory effects. Several micro RNAs (miRNAs) involved in floral transition have also been studied, such as miR159 (Li et al., 2013), miR168 (Xian et al., 2014), milR169d (Xu et al., 2014), and miR390 (Fahlgren et al., 2006; Garcia, 2008). Competing endogenous RNAs (ceRNAs) have been found to be an RNA molecule class that possesses at least one common miRNA response element (MRE) that is accessible to miRNA binding (Salmena et al., 2011). Furthermore, protein-coding RNAs and ncRNAs, such as pseudogene transcripts, lncRNAs, and circular RNAs (circRNAs), have been found to communicate and co-regulate with each other by competitively combining same miRNAs; these are considered to be ceRNAs, and they possibly participate in biology processes (Tay et al., 2014). ceRNAs have been investigated in studies focusing on human disease in relation to their functions of competitive expression with a disease-causing gene (Lü et al., 2016; Liang et al., 2018). However, only a few studies have focused on the ceRNA network and association between ceRNA networks and flower traits in plants; for example, studies have investigated ceRNA networks in Solanum lycopersicum (Yang et al., 2019) and Brassica campestris (Liang et al., 2019).
To elucidate the molecular mechanism of late flowering (LF) in P. sibirica, we conducted a GWAS to identify the loci and candidate genes associated with LF. We also conducted an integrated analysis of mRNAs, lncRNAs, and miRNAs to construct ceRNAs networks by whole transcriptome RNA-sequencing. This study provides new insight into the genetic regulation of LF mechanisms in P. sibirica.
Materials and Methods
Plant Materials
A total of 66 P. sibirica accessions were used in this study, which grown in the Inner Mongol Forest Seed Breeding Center in Horinger County within the Inner Mongolia Autonomous Region. Full bloom date (FBD) was defined as when up to 50% of the flowers opened (Pérez-Pastor et al., 2004), and the FBD of all the accessions were measured from 2015 to 2017 (Supplementary Table 1). Fresh young leaves of P. sibirica accessions were collected, immediately frozen in liquid nitrogen, and stored in a refrigerator at −80°C prior to conducting the GWAS. A minimum of 5 g (fresh weight) of floral buds collected from LF and normal flowering (NF) accessions were respectively used to conduct histological analyses and whole-transcriptome RNA sequencing.
Sampling and Observations of Tissue Morphology in Paraffin Sections
Floral buds (a minimum of 20 floral buds from each sample) from the LF and NF types were sampled every 10 days from June 20 to July 10, 2017, every 3 days from July 10 to October 11, and every 7 days from October 11, 2017 to April 3, 2018. After fixation in an FAA fixative solution for 24 h, the buds were kept in a 70% alcohol solution under 4°C. The materials were then subjected to alcohol dehydration, xylene treatment, waxed treatment, embedding, and slicing at a thickness of 6–10 μm. Samples were subsequently dyed with Fast Green and counterstained with safranin. Tissues were observed, and images were obtained under an optical microscope, Olympus BX-51 (Olympus Optical, Tokyo, Japan).
DNA Preparation, Sequencing, Sequence Alignment, and SNP Calling
A total of 66 P. sibirica accessions were used in this study, of which 41 have been used in our previous study (Genome Sequence Archive, PRJCA001987) and 25 were newly sequenced. The leaf DNA of 25 P. sibirica accessions was extracted with CTAB methods, and sequence libraries were constructed with fragment sizes of up to 300 bp. The libraries were then sequenced on an Illumina HiSeq X Ten platform. After sequencing, clean reads were obtained using fastp (version 1.12.6, default parameters) by removing low-quality reads with adapters at both or either end, and with the number of N bases accounting for more than 5% (Chen et al., 2018b). The genome sequence data of 25 P. sibirica accessions were submitted to Genome Sequence Archive (PRJCA006925).
Whole-genome sequences for each accession were mapped to the P. sibirica “F106” reference genome (Genome Database for Rosaceae, tfGDR1049) using BWA-MEM (version 0.7.17, -K 100000000 -v 3 -Y) (Li, 2013). Duplicates were then marked using the genome analysis toolkit (GATK) MarkDuplicates (version 4.1.2.0, –VALIDATION_STRINGENCY SILENT –OPTICAL_DUPLICATE_PIXEL_DISTANCE 2500 –ASSUME_SORT_ORDER “queryname”) (McKenna et al., 2010). A GATK Haplotype Caller was used to detected SNP variant calls (version 4.1.2.0, -ERC GVCF). The GATK (version 4.1.2.0) was used for hard filtering with the following parameters: -filter “QD < 2.0” –filter-name “QD2” -filter “QUAL < 30.0” –filter-name “QUAL30” -filter “SOR > 3.0” –filter-name “SOR3” -filter “FS > 60.0” –filter-name “FS60” -filter “MQ < 40.0” –filter-name “MQ40”-filter “MQRankSum < -12.5” –filter-name “MQRankSum-12.5” -filter “ReadPosRankSum < -8.0” –filter-name “ReadPosRankSum-8.” All the SNPs were annotated for potential coding effects using ANNOVAR (version 2018-04-16, default parameters) (Yang and Wang, 2015).
Genome-Wide Association Study
To improve the statistical power of the analysis, a set of bi-allelic SNPs with missing rates of less than0.2 and minor allele frequency (MAF) of >0.05 were obtained to conduct subsequent analyses. Principal component analysis was performed using Smartpca (Zhang, 2009). A distance matrix was generated with VCF2D (version 1.0), and a neighbor-joining tree was constructed with 1,000 bootstraps using TreeBeST (version 1.9.2). A mixed linear model (MLM) program in Efficient Mixed-Model Association Expedited (EMMAX, version beta-07Mar2010, default parameters) (Kang et al., 2010) software was used for the GWAS. The results were visualized as Manhattan plots and Q–Q plots using the R package “qqman” (Turner, 2014). We defined the cutoff of associated signals as the Bonferroni test threshold (Gao et al., 2010), which was set as0.05/total SNPs [−log10 (0.05/2598398) = 7.71]. Candidate regions were defined as ±10 kilobase (kb) on either side of significant association peaks. Exons and introns of the candidate genes were drawn with Illustrator for Biological Sequences (IBS, version 1.0.3) (Liu et al., 2015) using genomic DNA sequences.
Total RNA Extraction, lncRNA and small RNA Library Construction, and Sequencing
The total RNA was extracted from floral buds of the LF and NF types obtained on July 10, 2017 using a ethanol precipitation protocol and a CTAB-PBIOZOL reagent. The results were qualified and then quantified using a NanoDrop and an Agilent 2100 (Thermo Fisher Scientific, Waltham, MA, United States) bioanalyzer. Two biological replicates were analyzed for each RNA sample.
For the long non-coding RNA library, total RNA was treated with Ribo-Zero™ Magnetic Kit (plant leaf) (epicenter) to deplete rRNA. The transcription was reversed, and the adapters were ligated. The qualified libraries were sequenced pair end on the Hiseq X-Ten (BGI-Shenzhen, Shenzhen, China) platform. After sequencing, clean reads were obtained by removing low-quality reads and reads, with the number of N bases accounting for more than 10%. The clean reads generated by high-throughput sequencing were mapped on the P. sibirica genome using the HISAT2 software (version 2.0.4) (Kim et al., 2015), and the reads mapped on the genome were assembled into transcripts using the StringTie software (version 1.0.4) (Pertea et al., 2015). The raw data of mRNA sequencing were submitted to the Genome Sequence Archive (PRJCA001987).
Small RNA library was prepared with 1 μg total RNA for each sample. Total RNA was purified by electrophoretic separation on a 15% urea denaturing polyacrylamide gel electrophoresis (PAGE) gel, and small RNA regions corresponding to the 18–30 nt bands in the marker lane (14–30 ssRNA Ladder Marker, TAKARA, Dalian, China) were excised and recovered. The adaptors were then ligated, transcribed into cDNA, and purified. Final ligation PCR products were sequenced using the BGISEQ-500 platform (BGI-Shenzhen, Shenzhen, China). The small RNA sequencing raw data were submitted to the Genome Sequence Archive (PRJCA006925).
Functional Annotation and Expression Calculation
The CPC (Kong et al., 2007), txCdsPredict, and CNCI (Sun et al., 2013) software, and Pfam database (Finn et al., 2016) were used to predict the coding ability of the transcript and distinguish between mRNA and lncRNA. The transcripts were then aligned to the reference sequence with Bowtie2 (Langmead and Salzberg, 2012), and expression levels were calculated using fragments per kilobase per million (FPKM) with RSEM (Li and Dewey, 2011). The transcripts were subsequently aligned against the Kyoto Encyclopedia of Genes (KEGG) database (Kanehisa et al., 2007) and Gene ontology (GO) database (Ye et al., 2006). miRA (Evers et al., 2015) was used to predict novel miRNAs by exploring the characteristic hairpin structure of miRNA precursor. The small RNA expression levels were calculated by transcripts per kilobase million (TPM; ’t Hoen et al., 2008).
Significant differentially expressed genes, including mRNAs and lncRNAs, were selected with | Log2Ratio| ≥ 1.00 and adjusted p-value ≤ 0.001 by DEG-seq (Storey, 2003; Wang et al., 2010). The P values were corrected using the Benjamini–Hochberg method. DESeq2 (Love et al., 2014) with the default threshold “| Log2Ratio| ≥ 1.00 and adjusted p-value ≤ 0.1” was used to detected significant differentially expressed miRNAs. Heatmaps were visualized with TBtools (version 1.098661) (Chen et al., 2018a).
Target Gene Prediction of lncRNAs and miRNAs
The function of lncRNAs is mainly realized by their action on target genes in a cis or trans relationship, and cis regulation of lncRNAs and their target mRNAs are based on a location relationship. Trans-regulation was predicted here by calculating the binding energy. In addition, the two correlation coefficients of lncRNA and mRNA were calculated using Spearman and Pearson (Spearman_COR ≥ 0.6 and Pearson_COR ≥ 0.6). A cis relationship was defined as lncRNA within 10 K upstream of mRNA, or within 20 kb downstream of mRNA. If the lncRNA and mRNA binding energy was beyond this range, RNAPlex (Tafer and Hofacker, 2008) was used to analyze the binding energy, and if it was found to be less than −30, it was defined as a trans relationship.
psRobot (Wu et al., 2012), TAPIR (Bonnet et al., 2010), and TargetFinder (Fahlgren and Carrington, 2010) were used to predict the plant targets of the miRNA. Furthermore, GO and KEGG pathway enrichment analyses of the target genes were annotated based on the GO database (Ye et al., 2006) and KEGG database (Kanehisa et al., 2007).
Identification of ceRNAs Involved in Tre6P Signaling Pathway
A hypergeometric distribution model was used to test whether the DE Tre6P signaling pathway genes shared a significant number of miRNA binding sites with DE lncRNAs. The LF-related ceRNAs were selected according to the following criteria: (1) the DE floral transition pathway coding-genes, lncRNAs, and miRNAs were significantly differentially expressed; (2) the DE floral transition pathway coding-genes shared the same DE miRNA with ceRNA (mRNA/lncRNA) and with the same MRE; and (3) the expression levels of the floral transition pathway coding-genes and predicted ceRNAs were opposite to those of the shared miRNA. The ceRNA network was presented using Cytoscape (version 3.8.0) (Shannon et al., 2003).
Determining Tre6P Content
Trehalose-6-phosphate (Tre6P) was extracted from the frozen pulverized floral bud tissues of eight randomly selected accessions (four NF and four LF types, 0.1–0.5 g fresh weight), and three biological replicates were performed. Tissue homogenates were extracted for 30 min of ice-cold PBS (pH 7.4) and centrifuged for 15 min at 3,000 × g and 4°C. Supernatants were then used for detection of Tre6P content using Plant Trehalose-6-Phosphate (T6P) ELISA Kit (Yan Qi Biological Technology, Shanghai, China). The optical density (OD) of the samples and six standard products was measured at the wavelength of 450 nm with BioTek’s Gen5™ Microplate Readers (BioTek, Winooski, VT, United States) and calculated based on the standard curve (with a correlation coefficient R2 ≥ 0.99), which was established using the concentrations and ODs of six standard products (0, 7.5, 15, 30, 60, and 120 pg/ml). The Non-linear Curve Fit was established to build the standard curve using the Origin Pro 2021 software (Supplementary Figure 1). Student’s t-test was performed in analysis and conducted using the Origin Pro 2021 software. Data are given as the means ± standard deviation (SD) of three independent biological replicates.
Quantitative Real-Time PCR Validation of lncRNAs and mRNAs
Total RNA was extracted using GenePure Plus Plantpoly RNA Kit (rich in polysaccharides and polyphenols) (CodonX Biotechnology, Beijing, China). One microgram of DNA-free RNA was transcribed into first-strand cDNA using All-in-One First-Strand Synthesis MasterMix (with dsDNase) (CodonX Biotechnology, Beijing, China). qRT-PCR was conducted with a Roche LightCyler 480 instrument using 2 × SYBR Green qPCR Premix (Universal) (CodonX Biotechnology, Beijing, China). Primers for target genes were designed using Primer 3.0.1 The actin gene was used as an internal reference to normalize the qRT-PCR data. All the primers are listed in Supplementary Table 2. Each reaction was performed in triplicate, and the data from real-time PCR amplification were analyzed using the 2–ΔΔCt method. Student’s t-test was performed in the analysis and conducted using the Origin Pro 2021 software. The data are presented as the means ± standard deviation (SD) of three independent biological replicates.
Results
Morphological Comparisons Between NF and LF Types
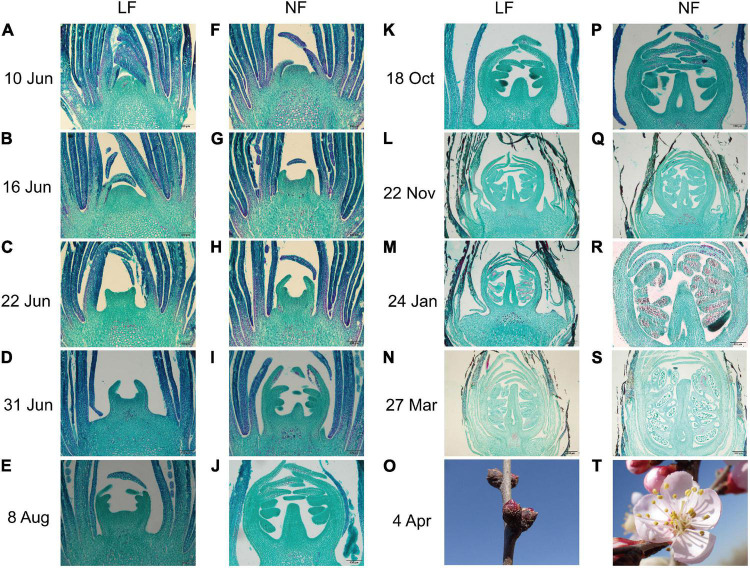
Both NF and LF type accessions were selected to explore the process of floral bud differentiation. The FBD of LF types was found to be delay by 7–15 days compared to that of NF types (Supplementary Table 1). Histological analysis showed that floral bud differentiation was divided into six floral bud differentiation stages, namely, the undifferentiated (Figure 1A), initial differentiation (Figures 1B,F), sepal differentiation (Figures 1C,G), petal differentiation (Figures 1D,H), stamen differentiation (Figures 1E,I), and pistil differentiation (Figures 1J,K) stages, and four reproductive differentiation stages (Figures 1L–N,P–S). Compared with the NF types, the initial differentiation stage was exhibited at least 6 days later in the LF types (Figures 1B,F). All of the floral bud differentiation stages were delayed in the LF types, and this resulted in the LF types remaining in the bud stage while the NF group was blooming (Figures 1O,T).
FIGURE 1.
Comparison of the flower bud differentiation process between (A–E,K–O) LF and (F–J,P–T) NF in P. sibirica. (A) Undifferentiated stage with buds in the leaf bud state; the growth point is small and tightly wrapped in layers of bud scales. (B,F) Initial differentiation stage with the top of the growth point bulges gradually and the bud scales become loose. (C,G) Calyx differentiation stage with calyx primordia gradually becoming elongate and bent inward. (D,H) Petal differentiation stage with petal primordium as a small second lobe at the bottom of the calyx primordium, wrapped in the calyx primordium and elongate with the calyx primordium. (E,I) Stamen differentiation stage with stamen primordium as the third lobe inside, forming a number of protuberances. (J,K) Pistil differentiation stage with pistil primordium bulges in the middle of the bottom and continued elongation. (L,P) Initiation of ovary and differentiation of sporogenous tissue. (M,Q) Initiation of ovule and formation of microspore mother cell. (N,R) Differentiation of ovule. (S) Formation of pollen grain. (O) Flower bud of LF. (T) Bloom of NF.
Genome-Wide Association Study of Flowering Time
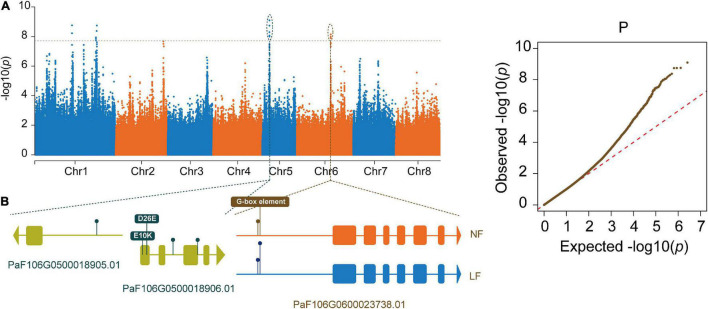
To better identify the candidate genes associated with flowering time, we conducted a GWAS for this trait in P. sibirica. The phenotypes of 66 accessions were classified into NF (43) and LF (23) types base on the FBD from the 3 years in succession. A total of 2,598,398 high-quality SNPs were identified by mapping against the P. sibirica reference genome, and these were used in the subsequent analyses. Principal component analysis (PCA) showed two differentiated clusters, which were consistent with the classifications from the FBD (Supplementary Figure 2). The GWAS on flowering time was conducted using an MLM, and this identified 16 SNPs with 12 predicted genes for the flowering time (Figure 2A), which were located on chromosomes 1, 5, and 6 (Supplementary Table 3). Among the SNPs, seven with three predicted genes (PaF106G0500018905.01, PaF106G0500018906.01, and PaF106G0600023738.01) were located in the gene region (Figure 2 and Supplementary Table 3), including five SNPs on chromosome 5 (Chr. 5: 3872427 bp,3874628 bp, 3874678 bp, 3874988 bp, and 3875280 bp) and two SNPs on chromosome 6 (Chr. 6: 18107193 bp and 18107219 bp). All the seven SNPs were highly associated with the phenotype of flowering time, which could be explained by 86.36–93.94% of the phenotypic variance (Supplementary Table 4). The predicted functions of PaF106G0500018906.01 and PaF106G0600023738.01 were associated with ubiquitin-conjugating enzyme E2 (UBC) and trehalose-6-phosphate phosphatase (TPP), respectively, whereas the predicted function of PaF106G0500018905.01 was unknown. In particular, an SNP mutation (Chr. 6: 18107219 bp) was found to be located on the promoter of PsTPPF (PaF106G0600023738.01), which resulted in a point mutation in the third nucleotide of a G-box core sequence (TACGTG), suggesting that the mutated site might hinder the binding of G-box interaction proteins, thus affecting the transcriptional expression PsTPPF. These results will be valuable for the development of molecular markers for late flower breeding in P. sibirica.
FIGURE 2.
GWAS of flowering time in Prunus sibirica. (A) Manhattan and quantile–quantile (QQ) plots for GWAS. (B) Gene structures of candidate genes. Brown box indicated G-box element in the promoter of PsTPPF. Rounded rectangle and triangle represented exons and the last exons, respectively.
Analysis of lncRNA and mRNA in NF and LF Types
To comprehensively understand the regulatory mechanism associated with flowering time, whole-transcriptome RNA sequencing of P. sibirica floral buds was conducted, and 515,976,068 raw reads were obtained. After filtering of adaptor sequences and low-quality reads, over 97% clean reads remained (Supplementary Table 5). Approximately 61% of these clean data were mapped to the P. sibirica genome (Supplementary Table 5). A total of 28,545 mRNAs, including 24,518 known and 4,027 novel ones, and 3,423 novel lncRNAs were identified (Supplementary Table 6). We found that most of the lncRNAs contained only one exon, and that most of the mRNAs have two exons (Supplementary Figure 3A). The length of the lncRNAs was concentrated at 500 bp, while the mRNAs were 500–1,000 bp in length (Supplementary Figure 3B). We also found that most of the lncRNAs and mRNAs contained only one transcript (Supplementary Figure 3C).
We analyzed the expression profiles of the differential expression (DE) mRNAs and lncRNAs. A total of 6,110 DEmRNAs and 1,351 DE lncRNAs were identified. Of these, 3,017 known mRNAs, 586 novel mRNAs, and 731 lncRNAs were upregulated. In contrast, 2,038 known mRNAs, 469 novel mRNAs, and 620 lncRNAs were downregulated (Supplementary Figure 4). Notably, PsTPPF was highly expressed in LF than NF types; it was a candidate gene identified by GWAS (Figure 3).
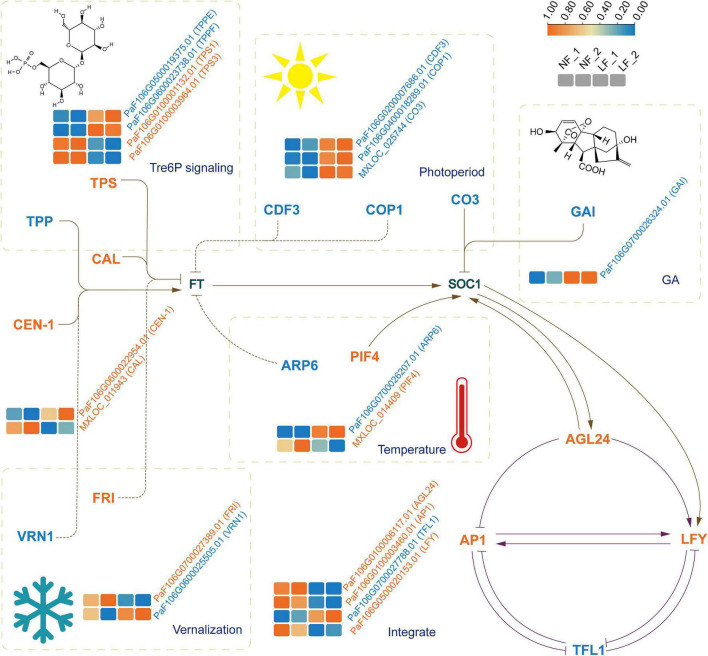
FIGURE 3.
The expression profiles of genes implicated in floral transition from six pathways. The four boxes (left to right) in one row of each heat map correspond to the expression levels in NF_1, NF_2, LF_1, and LF_2, respectively. TPS, trehalose-6-phosphate synthase, TPP, trehalose-6-phosphate phosphatase, CDF3, cycling dof factor 3; COP1, constitutively photomorphogenic 1; CO3, CONSTANS like 3; GAI, GA insensitive; ARP6, actin related protein 6; PIF4, phytochrome interacting factor 4; FRI, FRIGIDA; VRN1, vernalization 1; AGL24, agamous like24; AP1, apetala1, LFY, LEAFY; TFL1, terminal flower 1. Full and dashed lines indicated the directly and indirectly acting, respectively. Arrows meant positive relationships and stubs meant the opposite relationships.
A DE lncRNA-DE mRNA network was constructed according to location relationship and binding sequence. A total of 9,345 pairs of cis-regulatory (Supplementary Table 7) and 1,871 pairs of trans-regulatory genes were identified; of which, 2,723 pairs (1,829 mRNAs and 1,054 lncRNAs) in cis-regulatory relationship and 633 pairs (38 mRNAs and 280 lncRNAs) in trans-regulatory were found to be DE mRNAs–DE lncRNA interactions. GO and KEGG pathway analyses of the putative target DE mRNAs were performed to better understand the potential function of the DE lncRNAs. The GO analysis showed that 852, 1,119, and 871 genes enriched the biological process (BP), cellular component (CC), and molecular function (MF) categories, respectively. Cellular process (GO: 0009987) in the BP category, membrane (GO:0016020) in the CC category, and binding (GO:0005488) in the MFs category were the top subcategories of each category (Supplementary Figure 5A). The most enriched 20 KEGG pathways of the target mRNAs were analyzed, and the most significant enriched pathways were the biosynthesis of secondary metabolites (ko:01110), plant hormone signal transduction (ko: 04075), and starch and sucrose metabolism (ko: 00500) (Supplementary Figure 5B).
Analysis of sRNA in NF and LF Types
A total of 116,046,350 raw reads were generated from the small RNA libraries (Supplementary Table 8). After filtering for adaptor sequences and low-quality reads, 111,180,013 clean reads remained (Supplementary Table 8), and over 81% of the clean tags were mapped to the reference genome (Supplementary Table 8). We identified 241 conserved miRNAs (Supplementary Figure 6A) and 317 predicted miRNAs (Supplementary Figure 6B). The lengths of the conserved miRNAs ranged from 18 to 24 nucleotides (nt), and 21 nt miRNAs (142) were the most abundant (Supplementary Figure 7A). The lengths of the predicted miRNAs ranged from 19 to 30 nt, and 30 nt miRNAs (95) were the most abundant (Supplementary Figure 7B).
We identified 148 DE miRNAs in LF vs. NF types, including 80 upregulated miRNAs and 68 downregulated miRNAs (Supplementary Figure 8). Among these miRNAs, 352 DE miRNA–DE mRNA interactions, including 37 DE miRNA and 310 DE mRNAs were identified according to the negative regulation between the DE miRNAs and the corresponding DE mRNAs. miR482d-5p_4 was the most abundant miRNA, followed by miR167d, miR395a-3p, and miR396a (Supplementary Figure 9). To understand the potential functions of the DE miRNAs, we conducted GO and KEGG pathway analyses of the putative target DE mRNAs. The GO analysis showed that 187, 404, and 304 genes could be categorized as BP, CC, and MF categories, respectively. The most enriched subcategories of each category were as follows: cellular process (GO:0009987) in the BP category, membrane part (GO:0044425), membrane (GO:0016020) in the CC category, and catalytic activity (GO:0003824) in the MF category (Supplementary Figure 10A). We analyzed the enriched 20 KEGG pathways of the target mRNAs, and the top two significant enriched pathways were found to be metabolic pathways (ko:01100) and the biosynthesis of secondary metabolites (ko:01110) (Supplementary Figure 10B).
Identification of DE mRNAs and Their Corresponding lncRNAs and miRNAs Involved in Floral Transition Pathways
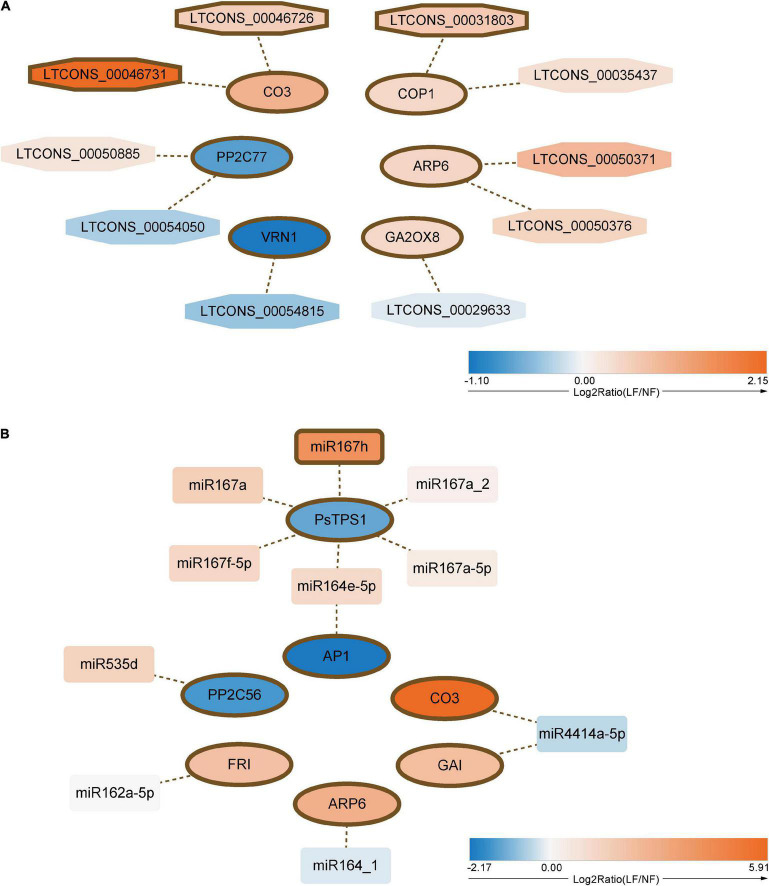
To understand the relationship between lncRNAs and miRNAs, and flowering time, we further selected 24 DE mRNAs by blasting with known floral transition genes (Figure 3 and Supplementary Table 9), which involved in the photoperiod, vernalization, GA, temperature, and Tre6P signaling pathways. We analyzed the 24 mRNAs and their corresponding miRNAs and lncRNAs. Ten lncRNA–DE mRNA pairs, containing ten lncRNAs and six DE mRNAs, were selected to construct the lncRNA–mRNA network (Figure 4A). Specially and specifically, three DE lncRNA-DE mRNA pairs, COP1 (PaF106G0400018289.01), CCLR (LTCONS_00031803), CO3 (MXLOC_025744), COCLR1 (LTCONS_00046726), CO3 (MXLOC_025744), and COCLR2 (LTCONS_00046731) were positively regulated. In addition, we identified 12 miRNA-mRNA interactions of 24 DE mRNAs (Figure 4B). Notably, miR167h and its target mRNA, PsTPS1 (PaF106G0100001132.01), encoding trehalose-6-phosphate synthase, were predicted, and these have been previously identified as being involved in the Tre6P signaling pathway of floral transition (Schluepmann et al., 2003).
FIGURE 4.
Interaction networks of differential expression (DE) floral transition genes with (A) lncRNAs and (B) miRNAs. Ellipse, hexagon, and round rectangle indicated the mRNA, lncRNA, and miRNA, respectively. Arbitrary shape with thick border represented differentially expressed.
Regulation of LF by the Tre6P Signaling Pathway
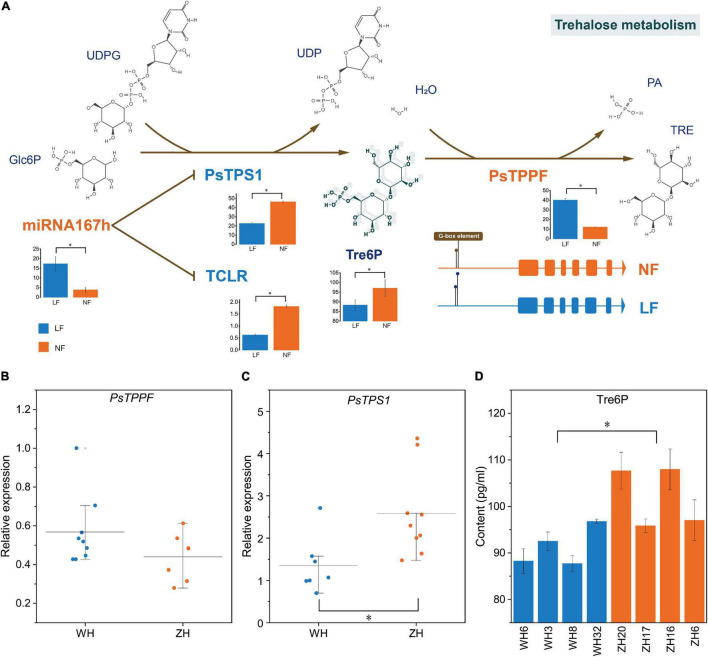
There are three genes associated with the LF types that were obtained from GWAS: PaF106G0500018905.01 and PaF106G0500018906.01 were not expressed in either LF or NF types, but PsTPPF was differentially expressed in LF vs. NF types. TPS and TPP can affect floral transition by controlling Tre6P content (Wahl et al., 2013), which is the only product of the enzymatic synthesis reaction of TPS and the only substrate of the enzymatic degradation reaction by TPP. In RNA-seq, PsTPS1 was expressed at low levels in the LF types, whereas PsTPPF was highly expressed in the LF types(Figure 5A). Furthermore, we analyzed the expression profiles of PsTPS1 and PsTPPF in the two types of groups to confirm the result by qRT-PCR, and the expression patterns were found to be consistent with the sequencing results. This suggested that the expression levels of PsTPS1 and PsTPPF may be associated with flowering time in P. sibirica (Figures 5B,C). We further found that the Tre6P content in most of the LF-type floral buds was lower than that in the NF types, which indicates that the decrease in Tre6P content in LF was caused by the expression levels of PsTPS1 and PsTPPF (Figure 5D).
FIGURE 5.
Trehalose-6-phosphate (Tre6P) signaling pathways regulation mechanism. Relative transcript levels of (A) PsTPPF and (B) PsTPS1, and content of (C) Tre6P in eight randomly selected accessions. (D) CeRNA of Tre6P signaling pathway. Trehalose-6-phosphate synthase (TPS) catalyzes glucose-6-phosphate (Glc6P) and UDP-glucose (UDPG) into Tre6P, and then Tre6P is dephosphorylated into trehalose by trehalose-6-phosphate phosphatase (TPP). PsTPS1 was down-regulated by competitively binding miR167h with TCLR. The histograms were visualized using FPKM of mRNA and lncRNA and TPM of miRNA. H2O: Water. PA, Phosphoric acid; TRE, Trehalose; *, significant differences p < 0.05.
Construction of ceRNA Networks of Tre6P Signaling Pathway
Two candidate DE mRNAs involved in the Tre6P signaling pathway, PsTPS1 and PsTPPF, were identified with an integrative strategy of combining GWAS and whole-transcriptome RNA sequencing analysis. To better understand the gene regulatory network of the LF types, putative DE lncRNA–DE miRNA–DE mRNAs in ceRNA networks were identified. According to the ceRNA hypothesis, the expression levels of lncRNAs and miRNAs, and mRNAs and miRNAs should show negative correlations, and the lncRNAs and mRNAs should show a positive correlation (Tay et al., 2014). We identified a ceRNA network consisting of one mRNA (PsTPS1), one miRNA (miR167h, a miR167 family member), and one lncRNA (TCLR) (Figure 5A). In the constructed ceRNA network, the expression profiles of PsTPS1 and TCLR were negatively correlated with miR167h. In addition, there was not a ceRNA network of PsTPPF.
Discussion
When flowering in early spring, the yield of P. sibirica is reduced when temperatures are low (Dennis, 1979), and promoting the LF trait has become an important breeding objective. In this study, we comprehensive investigated the characterization of floral bud differentiation and found that compared with the NF types, the delay in the initial differentiation stage resulted in delay flowering time in the LF types. Many floral transition genes of A. thaliana, Vitis vinifera, and M. domestica have been found to affect the flowering time of transgenic plants at the time of floral transition (Boss et al., 2006; Tränkner et al., 2010; Wahl et al., 2013).
Floral transition time is one of the factors affecting the flowering time of plants (Blázquez et al., 2001), and it is regulated by a complex network composed of multiple genes that affect flowering time in response to exogenous environmental and endogenous influences (Blázquez et al., 2001). The results of this study show that 24 DE mRNAs of a regulatory network from both environmental and endogenous signals relate to floral transition and likely control the LF trait. Our results show that COP1 and CO3 act on photoperiod pathway, and these have been previously reported to affect the flowering time in A. thaliana and Oryza sativa (Jang et al., 2008; Kim et al., 2008). We also found that COP1 and CO3 interacted with three new lncRNAs (CCLR, COCLR1, and COCLR2). The expression patterns of PIF4 and ARP6 in regulating LF are in accordance with those of earlier studies conducted on A. thaliana, which found PIF4 and ARP6 controlling flowering time by activating the expression of FT and FLC, respectively, in response to warm temperatures (Deal et al., 2005; Kumar et al., 2012). Our results indicate that environmental signals, such as photoperiod and temperature, may be associated with LF in P. sibirica. Integration genes, such as TFL1, in Arabis alpina (Wang et al., 2011), Populus (Mohamed et al., 2010), M. domestica (Kotoda et al., 2006), Rosa, and Fragaria vesca (Iwata et al., 2012) have been found to act as a photoperiod-regulated floral repressor. In this study, TFL1 was found to be differentially expressed in NF vs. LF types, and this result is supported by those of previous studies. Furthermore, phytohormone metabolic and signaling pathways have previously been proposed as flowering regulators (Ohto et al., 2001; Davis, 2009). For example, GA2OXs and GAI have been reported to control the flowering time of Kalanchoë blossfeldiana, Petunia hybrida, and A. thaliana (Wilson and Somerville, 1995; Gargul et al., 2013) through GA metabolic and signal pathways, and FsPP2Cs negatively regulated the flowering time in A. thaliana in the ABA signal pathway (Reyes et al., 2006). These genes were also differentially expressed in NF vs. LF types in our study, and they likely play important roles in controlling the LF trait of P. sibirica. In summary, the genes identified here provide information about the regulatory pathways of floral transition, and can be used as genetic resources for developing LF varieties of P. sibirica.
Previous studies have shown a correlation between sugar content and floral transition in A. thaliana (Ohto et al., 2001; Ortiz-Marchena et al., 2015), M. domestica (Li et al., 2018), and R. chinensis (Guo et al., 2017). Tre6P is an intermediate product of starch and sucrose metabolism, and it acts both as energy source and signal molecule of flowering time (Schluepmann et al., 2003; Fichtner and Lunn, 2021). Moreover, Tre6P content is regulated by the expression of TPS and TPP in A. thaliana (Schluepmann et al., 2003; Wahl et al., 2013). Two candidate genes (PsTPPF and PsTPS1) involved in Tre6P signaling pathway were found by GWAS and the whole-transcriptome RNA sequencing analysis in our study, and their expression levels showed inhibitory effects to the content level of Tre6P. However, as floral transition is controlled by an intricate genetic network, it is considered that the other 22 floral transition genes may also regulate the LF trait together with PsTPPF and PsTPS1 in the LF types of P. sibirica.
The ceRNA regulatory network has not been widely constructed in plants, and the first ceRNA interaction of pho2-induced phosphate starvation1 (IPS1)-miR399 was reported in A. thaliana (Franco-Zorrilla et al., 2007). Therefore, to explore the ceRNA network and functions of the important candidate genes associated with LF in P. sibirica, we conducted an integrated analysis of the ceRNAs of PsTPPF and PsTPS1 in the Tre6P signaling pathway. We identified a ceRNA network composed of one interaction ceRNA, one lncRNA (TCLR), and one mRNA (PsTPS1), which shared with one miRNA (miR167h). We hypothesize that the low expression of PsTPS1 is negatively regulated by TCLR competitively binding to miR167h, and that the high expression of PsTPPF is caused by a single SNP located in its promoter, which might result in the LF trait in P. sibirica. Studies have shown that miR167a-d played important roles in floral organ development in A. thaliana (Wu et al., 2006). However. miR167h may be related with LF through the Tre6P signaling pathway in P. sibirica.
We proposed that a new regulatory flowering gene (PsTPPF) is related to LF in P. sibirica, and we constructed a new ceRNA network (PsTPS1, TCLR, and miR167h) for the LF types of P. sibirica. However, it is also considered that the other 22 differentially expressed floral transition genes with their interactional lncRNAs and miRNAs may also be involved in the regulation of LF of P. sibirica, and further studies are, thus, required. This study identified the molecular regulation mechanism associated with LF in P. sibirica, and the results can be used as a valuable genetic resource for breeding LF varieties of P. sibirica.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
TW and LW designed and supervised the experiments. WX conducted the experiments, analyzed the data, and wrote the manuscript. WB, HL, CC, and HB helped analyzed the sequencing data and experiments. WB, MH, GZ, HZ, NG, and YC performed the sample collection. TW, LW, and WX revised the manuscript. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Grandomics for assisting on the bioinformation analysis.
Footnotes
Funding
This study was supported by the National Key R&D Program of China (2018YFD1000606), key R&D and Promotion of He’nan Province of China (192102110063), and Fundamental Research Funds for the Central Non-profit Research Institution of Chinese Academy of Forestry (CAFYBB2020ZY003).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.802827/full#supplementary-material
References
- Blázquez M., Koornneef M., Putterill J. (2001). Flowering on time: genes that regulate the floral transition. EMBO Rep. 2 1078–1082. 10.1093/embo-reports/kve254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnafous F., Fievet G., Blanchet N., Boniface M.-C., Carrère S., Gouzy J., et al. (2018). Comparison of GWAS models to identify non-additive genetic control of flowering time in sunflower hybrids. Theor. Appl. Genet. 131 319–332. 10.1007/s00122-017-3003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet E., He Y., Billiau K., Van De Peer Y. (2010). TAPIR, a web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics 26 1566–1568. 10.1093/bioinformatics/btq233 [DOI] [PubMed] [Google Scholar]
- Boss P. K., Sreekantan L., Thomas M. R. (2006). A grapevine TFL1 homologue can delay flowering and alter floral development when overexpressed in heterologous species. Funct. Plant Biol. 33 31–41. 10.1071/FP05191 [DOI] [PubMed] [Google Scholar]
- Cao K., Zhou Z., Wang Q., Guo J., Zhao P., Zhu G., et al. (2016). Genome-wide association study of 12 agronomic traits in peach. Nat. Commun. 7:13246. 10.1038/ncomms13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Chen H., He Y., Xia R. (2018a). TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv [Preprint] 10.1101/289660 [DOI] [Google Scholar]
- Chen S., Zhou Y., Chen Y., Gu J. (2018b). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34 i884–i890. 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. J. (2009). Integrating hormones into the floral-transition pathway of Arabidopsis thaliana. Plant Cell Environ. 32 1201–1210. 10.1111/j.1365-3040.2009.01968.x [DOI] [PubMed] [Google Scholar]
- Deal R. B., Kandasamy M. K., Mckinney E. C., Meagher R. B. (2005). The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of flowering locus C expression and repression of flowering in Arabidopsis. Plant Cell 17 2633–2646. 10.1105/tpc.105.035196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis F. G., Jr. (1979). Factors affecting yield in apple, with emphasis on ‘Delicious’. Hortic. Rev. 1 395–422. [Google Scholar]
- Duan N., Bai Y., Sun H., Wang N., Ma Y., Li M., et al. (2017). Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat. Commun. 8:249. 10.1038/s41467-017-00336-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers M., Huttner M., Dueck A., Meister G., Engelmann J. C. (2015). miRA: adaptable novel miRNA identification in plants using small RNA sequencing data. BMC Bioinformatics 16:370. 10.1186/s12859-015-0798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlgren N., Carrington J. C. (2010). “miRNA target prediction in plants,” in Plant MicroRNAs, eds Meyers B., Green P. (Clifton, NJ: Springer; ), 51–57. 10.1007/978-1-60327-005-2_4 [DOI] [PubMed] [Google Scholar]
- Fahlgren N., Montgomery T. A., Howell M. D., Allen E., Dvorak S. K., Alexander A. L., et al. (2006). Regulation of auxin response factor3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr. Biol. 16 939–944. 10.1016/j.cub.2006.03.065 [DOI] [PubMed] [Google Scholar]
- Fichtner F., Lunn J. E. (2021). The role of trehalose 6-phosphate (Tre6P) in plant metabolism and development. Annu. Rev. Plant Biol. 72 737–760. 10.1146/annurev-arplant-050718-095929 [DOI] [PubMed] [Google Scholar]
- Finn R. D., Coggill P., Eberhardt R. Y., Eddy S. R., Mistry J., Mitchell A. L., et al. (2016). The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44 D279–D285. 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla J. M., Valli A., Todesco M., Mateos I., Puga M. I., Rubio-Somoza I., et al. (2007). Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 39 1033–1037. 10.1038/ng2079 [DOI] [PubMed] [Google Scholar]
- Gao X., Becker L. C., Becker D. M., Starmer J. D., Province M. A. (2010). Avoiding the high Bonferroni penalty in genome-wide association studies. Genet. Epidemiol. 34 100–105. 10.1002/gepi.20430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D. (2008). A miRacle in plant development: role of microRNAs in cell differentiation and patterning. Sem. Cell Dev. Biol. 19 586–595. 10.1016/j.semcdb.2008.07.013 [DOI] [PubMed] [Google Scholar]
- Gargul J., Mibus H., Serek M. (2013). Constitutive overexpression of Nicotiana GA 2 ox leads to compact phenotypes and delayed flowering in Kalanchoë blossfeldiana and Petunia hybrida. Plant Cell Tissue Org. Cult. 115 407–418. 10.1007/s11240-013-0372-5 [DOI] [Google Scholar]
- Guo X., Yu C., Luo L., Wan H., Zhen N., Xu T., et al. (2017). Transcriptome of the floral transition in Rosa chinensis ‘Old Blush’. BMC Genomics 18:199. 10.1186/s12864-017-3584-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J. B., Sung S. (2011). Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 331 76–79. 10.1126/science.1197349 [DOI] [PubMed] [Google Scholar]
- Hou X., Hu W.-W., Shen L., Lee L. Y. C., Tao Z., Han J.-H., et al. (2008). Global identification of DELLA target genes during Arabidopsis flower development. Plant Physiol. 147 1126–1142. 10.1104/pp.108.121301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Min Y., Schiessl S., Xion X., Jan H. U., He X., et al. (2021). Integrative analysis of GWAS and transcriptome to reveal novel loci regulation flowering time in semi-winter rapeseed. Plant Sci. 310:110980. 10.1016/j.plantsci.2021.110980 [DOI] [PubMed] [Google Scholar]
- Iwata H., Gaston A., Remay A., Thouroude T., Jeauffre J., Kawamura K., et al. (2012). The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. Plant J. 69 116–125. 10.1111/j.1365-313X.2011.04776.x [DOI] [PubMed] [Google Scholar]
- Jang S., Marchal V., Panigrahi K. C., Wenkel S., Soppe W., Deng X. W., et al. (2008). Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J. 27 1277–1288. 10.1038/emboj.2008.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., et al. (2007). KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36 D480–D484. 10.1093/nar/gkm882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. M., Sul J. H., Service S. K., Zaitlen N. A., Kong S.-Y., Freimer N. B., et al. (2010). Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 42 348–354. 10.1038/ng.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Langmead B., Salzberg S. L. (2015). HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12 357–360. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-K., Yun C.-H., Lee J. H., Jang Y. H., Park H.-Y., Kim J.-K. (2008). OsCO3, a CONSTANS-LIKE gene, controls flowering by negatively regulating the expression of FT-like genes under SD conditions in rice. Planta 228 355–365. 10.1007/s00425-008-0742-0 [DOI] [PubMed] [Google Scholar]
- Kong L., Zhang Y., Ye Z.-Q., Liu X.-Q., Zhao S.-Q., Wei L., et al. (2007). CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 35 W345–W349. 10.1093/nar/gkm391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoda N., Iwanami H., Takahashi S., Abe K. (2006). Antisense expression of MdTFL1, a TFL1-like gene, reduces the juvenile phase in apple. J. Am. Soc. Hortic. Sci. 131 74–81. 10.21273/jashs.131.1.74 [DOI] [Google Scholar]
- Kumar S. V., Lucyshyn D., Jaeger K. E., Alós E., Alvey E., Harberd N. P., et al. (2012). Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484 242–245. 10.1038/nature10928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9:357. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y. Y., Mesnage S., Mylne J. S., Gendall A. R., Dean C. (2002). Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science 297 243–246. 10.1126/science.1072147 [DOI] [PubMed] [Google Scholar]
- Li B., Dewey C. N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv [Preprint] arXiv: 1303.3997, [Google Scholar]
- Li X., Bian H., Song D., Ma S., Han N., Wang J., et al. (2013). Flowering time control in ornamental gloxinia (Sinningia speciosa) by manipulation of miR159 expression. Ann. Bot. 111 791–799. 10.1093/aob/mct034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Cao K., Zhu G., Fang W., Chen C., Wang X., et al. (2019). Genomic analyses of an extensive collection of wild and cultivated accessions provide new insights into peach breeding history. Genome Biol. 20:36. 10.1186/s13059-019-1648-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang D., Zhang X., Xing L., Fan S., Ma J., et al. (2018). A transcriptome analysis of two apple (Malus× domestica) cultivars with different flowering abilities reveals a gene network module associated with floral transitions. Sci. Hortic. 239 269–281. 10.1016/j.scienta.2018.04.048 [DOI] [Google Scholar]
- Liang H., Yu T., Han Y., Jiang H., Wang C., You T., et al. (2018). LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol. Can. 17:119. 10.1186/s12943-018-0870-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Zhang Y., Xu L., Zhou D., Jin Z., Zhou H., et al. (2019). CircRNA expression pattern and ceRNA and miRNA–mRNA networks involved in anther development in the CMS line of Brassica campestris. Int. J. Mol. Sci. 20:4808. 10.3390/ijms20194808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Marquardt S., Lister C., Swiezewski S., Dean C. (2010). Targeted 3’ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 327 94–97. 10.1126/science.1180278 [DOI] [PubMed] [Google Scholar]
- Liu W., Xie Y., Ma J., Luo X., Nie P., Zuo Z., et al. (2015). IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics 31 3359–3361. 10.1093/bioinformatics/btv362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü M., Tang B., Zeng S., Hu C., Xie R., Wu Y., et al. (2016). Long noncoding RNA BC032469, a novel competing endogenous RNA, upregulates hTERT expression by sponging miR-1207-5p and promotes proliferation in gastric cancer. Oncogene 35 3524–3534. 10.1038/onc.2015.413 [DOI] [PubMed] [Google Scholar]
- Mariette S., Wong Jun Tai F., Roch G., Barre A., Chague A., Decroocq S., et al. (2016). Genome-wide association links candidate genes to resistance to Plum Pox Virus in apricot (Prunus armeniaca). New Phytol. 209 773–784. 10.1111/nph.13627 [DOI] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., et al. (2010). The Genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20 1297–1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed R., Wang C. T., Ma C., Shevchenko O., Dye S. J., Puzey J. R., et al. (2010). Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J. 62 674–688. 10.1111/j.1365-313X.2010.04185.x [DOI] [PubMed] [Google Scholar]
- Ohto M.-A., Onai K., Furukawa Y., Aoki E., Araki T., Nakamura K. (2001). Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol. 127 252–261. 10.1104/pp.127.1.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Marchena M. I., Romero J. M., Valverde F. (2015). Photoperiodic control of sugar release during the floral transition: what is the role of sugars in the florigenic signal? Plant Signal. Behav. 10:e1017168. 10.1080/15592324.2015.1017168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pastor A., Ruiz-Sánchez M., Domingo R., Torrecillas A. (2004). Growth and phenological stages of Búlida apricot trees in south-east Spain. Agronomie 24 93–100. 10.1051/agro:2004004 [DOI] [Google Scholar]
- Pertea M., Pertea G. M., Antonescu C. M., Chang T.-C., Mendell J. T., Salzberg S. L. (2015). StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33 290–295. 10.1038/nbt.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes D., Rodríguez D., González-García M. P., Lorenzo O., Nicolás G., García-Martínez J. L., et al. (2006). Overexpression of a protein phosphatase 2C from beech seeds in Arabidopsis shows phenotypes related to abscisic acid responses and gibberellin biosynthesis. Plant Physiol. 141 1414–1424. 10.1104/pp.106.084681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P. P. (2011). A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146 353–358. 10.1016/j.cell.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H., Pellny T., Van Dijken A., Smeekens S., Paul M. (2003). Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 100 6849–6854. 10.1073/pnas.1132018100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13 2498–2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey J. D. (2003). The positive false discovery rate: a Bayesian interpretation and the q-value. Ann. Stat. 31 2013–2035. [Google Scholar]
- Suarez-Lopez P., Wheatley K., Robson F., Onouchi H., Valverde F., Coupland G. (2001). CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410 1116–1120. 10.1038/35074138 [DOI] [PubMed] [Google Scholar]
- Sun L., Luo H., Bu D., Zhao G., Yu K., Zhang C., et al. (2013). Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 41:e166. 10.1093/nar/gkt646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ’t Hoen P. A., Ariyurek Y., Thygesen H. H., Vreugdenhil E., Vossen R. H., De Menezes R. X., et al. (2008). Deep sequencing-based expression analysis shows major advances in robustness, resolution and inter-lab portability over five microarray platforms. Nucleic Acids Res. 36:e141. 10.1093/nar/gkn705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafer H., Hofacker I. L. (2008). RNAplex: a fast tool for RNA–RNA interaction search. Bioinformatics 24 2657–2663. 10.1093/bioinformatics/btn193 [DOI] [PubMed] [Google Scholar]
- Tay Y., Rinn J., Pandolfi P. P. (2014). The multilayered complexity of ceRNA crosstalk and competition. Nature 505 344–352. 10.1038/nature12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tränkner C., Lehmann S., Hoenicka H., Hanke M.-V., Fladung M., Lenhardt D., et al. (2010). Over-expression of an FT-homologous gene of apple induces early flowering in annual and perennial plants. Planta 232 1309–1324. 10.1007/s00425-010-1254-2 [DOI] [PubMed] [Google Scholar]
- Turner S. D. (2014). qqman: an R package for visualizing GWAS results using QQ and manhattan plots. bioRxiv [Preprint] 10.1101/005165 [DOI] [Google Scholar]
- Wahl V., Ponnu J., Schlereth A., Arrivault S., Langenecker T., Franke A., et al. (2013). Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339 704–707. 10.1126/science.1230406 [DOI] [PubMed] [Google Scholar]
- Wang L., Feng Z., Wang X., Wang X., Zhang X. (2010). DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26 136–138. 10.1093/bioinformatics/btp612 [DOI] [PubMed] [Google Scholar]
- Wang R., Albani M. C., Vincent C., Bergonzi S., Luan M., Bai Y., et al. (2011). Aa TFL1 confers an age-dependent response to vernalization in perennial Arabis alpina. Plant Cell 23 1307–1321. 10.1105/tpc.111.083451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Song S., Sheng S., Tian J., Wu R., Pang X. (2019). Comparative transcriptome analysis identifies differentially expressed genes between normal and late-blooming Siberian apricot. J. For. Res. 30 2277–2288. [Google Scholar]
- Wang Z., Zeng Y., Zhang Z., Sheng S., Tian J., Wu R., et al. (2017). Phylogeography study of the siberian apricot (Prunus sibirica L.) in Northern China assessed by chloroplast microsatellite and DNA makers. Front. Plant Sci. 8:1989. 10.3389/fpls.2017.01989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. N., Somerville C. R. (1995). Phenotypic suppression of the gibberellin-insensitive mutant (gai) of Arabidopsis. Plant Physiol. 108 495–502. 10.1104/pp.108.2.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Poethig R. S. (2006). Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133 3539–3547. 10.1242/dev.02521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Park M. Y., Conway S. R., Wang J.-W., Weigel D., Poethig R. S. (2009). The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138 750–759. 10.1016/j.cell.2009.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-J., Ma Y.-K., Chen T., Wang M., Wang X.-J. (2012). PsRobot: a web-based plant small RNA meta-analysis toolbox. Nucleic Acids Res. 40 W22–W28. 10.1093/nar/gks554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.-F., Tian Q., Reed J. W. (2006). Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 133 4211–4218. 10.1242/dev.02602 [DOI] [PubMed] [Google Scholar]
- Xian Z., Huang W., Yang Y., Tang N., Zhang C., Ren M., et al. (2014). miR168 influences phase transition, leaf epinasty, and fruit development via SlAGO1s in tomato. J. Exp. Bot. 65 6655–6666. 10.1093/jxb/eru387 [DOI] [PubMed] [Google Scholar]
- Xu M. Y., Zhang L., Li W. W., Hu X. L., Wang M.-B., Fan Y. L., et al. (2014). Stress-induced early flowering is mediated by miR169 in Arabidopsis thaliana. J. Exp. Bot. 65 89–101. 10.1093/jxb/ert353 [DOI] [PubMed] [Google Scholar]
- Yang H., Wang K. (2015). Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat. Protoc. 10 1556–1566. 10.1038/nprot.2015.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Yang C., Wang Z., Yang Z., Chen D., Wu Y. (2019). LncRNA expression profile and ceRNA analysis in tomato during flowering. PLoS One 14:e0210650. 10.1371/journal.pone.0210650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Fang L., Zheng H., Zhang Y., Chen J., Zhang Z., et al. (2006). WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 34 W293–W297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wei Z., Zhao H. (2006). Determination of ability of several arbor and shrub species to endure and survive extreme aridity with limited-areas methods under field conditions in Horqin Sandy Land. Acta Ecol. Sin. 26 467–474. [Google Scholar]
- Zhang M.-Y., Xue C., Hu H., Li J., Xue Y., Wang R., et al. (2021). Genome-wide association studies provide insights into the genetic determination of fruit traits of pear. Nat. Commun. 12:1144. 10.1038/s41467-021-21378-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhang H., Sun L., Fan G., Ye M., Jiang L., et al. (2018). The genetic architecture of floral traits in the woody plant Prunus mume. Nat. Commun. 9:1702. 10.1038/s41467-018-04093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. (2009). “Smart pca,” in Proceedings of the 21st International Joint Conference on Artificial Intelligence, Pasadena, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.