Abstract
Asthma is classically described as either a T2 eosinophilic phenotype or a non-T2 neutrophilic phenotype. T2 asthma usually responds to classical bronchodilation therapy and corticosteroid treatment. Non-T2 neutrophilic asthma is often more severe. Patients with non-T2 asthma or late-onset T2 asthma show poor response to the currently available anti-inflammatory therapies. These therapeutic failures result in increased morbidity and cost associated with asthma and pose a major healthcare problem. Recent evidence suggests that some non-T2 asthma is associated with elevated Th17 cell immune responses. Th17 cells producing interleukin 17A and 17F are involved in the neutrophilic inflammation and airway remodeling processes in severe asthma and have been suggested to contribute to the development of subsets of corticosteroid-insensitive asthma. This review explores the pathological role of Th17 cells in corticosteroid insensitivity of severe asthma and potential targets to treat this endotype of asthma.
Keywords: T helper 17 cells, corticosteroid insensitivity, severe asthma, T2 asthma, non-T2 asthma, airway neutrophilia, interleukin 17, interleukin 6, RhoA, Rho-associated kinase
Asthma is a common and chronic obstructive airway disease with a high healthcare burden. It is defined by clinical symptoms of recurrent wheezing, coughing, and shortness of breath varying with time and intensity, as well as variable expiratory airflow limitation1. Inflammation is viewed as the key factor in asthma, with anti-inflammatory corticosteroids as the mainstay of treatment. Severe asthma is defined as those patients who require high-dose inhaled corticosteroids plus a second drug and/or systemic corticosteroids to maintain control and whose symptoms worsen when treatment is decreased, or asthma where patients remain uncontrolled despite adherence to optimized maximal therapy2. Severe asthma is a costly public health burden, encompassing up to 10% of all asthma patients but contributing to most of the healthcare cost.
An asthma subset characterized by eosinophilic airway inflammation and abundant T helper 2 (Th2) cells is defined as type 2 (T2) asthma which is further defined by a sputum eosinophil count of ≥ 2%, a blood eosinophil count of ≥150 cells/µL, a fractional exhaled nitric oxide ≥ 20 ppb, and/or clinically allergy-driven asthma3. However, these biomarker numbers are arbitrary cutoffs within continuously distributed values and T2 inflammation exists on a continuum in asthma. Allergic asthma is characterized by asthma symptoms that occur with exposure to an aeroallergen with confirmatory allergen specific IgE and a total IgE of at least 30 IU/mL. There is considerable overlap between allergic and T2 asthma. Patients with allergic asthma are much more likely to have high eosinophil counts, and asthma patients with high eosinophil counts, especially those that develop asthma during childhood, often have concomitant allergies4,5. T2 asthma usually responds to classical bronchodilation therapy and corticosteroid treatment6 and/or can be controlled with newly developed T2-targeted biologic therapies7–11. However, in some patients with severe asthma, especially late-onset T2 asthma, airway eosinophilic inflammation persists despite corticosteroid treatment (Figure 1)12,13. In addition, almost half of patients with severe asthma have non-eosinophilic airway inflammation or a lack of eosinophilic and neutrophilic airway inflammation. This group is defined as having non-T2 or T2-low asthma14–17. The non-T2 asthma is usually characterized by neutrophilic rather than eosinophilic airway inflammation and associated with a number of clinical features including obesity, later onset of disease, poor responsive to gcocorticoids and higher risks of exacerbation18–24. Paucigranulocytic asthma (PGA) is another subset of non-T2 asthma with persistent asthma symptoms but absence of both eosinophilic and neutrophilic airway inflammation25. This endotype may be due to changes in airway smooth muscle (ASM)16,26 or airway inflammation not reflected in the lumen or detected by sputum cytometry27. Non-T2 asthma has a poor response to the currently available anti-inflammatory therapies. It is a problem urgently needing a solution, particularly for patients with late-onset and more severe asthma12,28,29, which is characterized by a high rate of severe exacerbations that may require hospitalization and lead to further morbidities. Unfortunately, the disease mechanisms driving non-T2 asthma are poorly understood, and there is a lack of point of care biomarkers, both of which greatly hinders the development of new therapeutic strategies for this subset of asthma26,30. Bronchial Thermoplasty (BT) is an endoscopic procedure that uses temperature-controlled radiofrequency energy to impact airway remodeling31. BT is well tolerated and reduces asthma symptoms and improves the quality of life of patients32. Since BT ablates ASM mass and airway nerve fibers, both of which may reduce airway hyperresponsiveness (AHR)33–35, it is a potential effective treatment in patients with severe asthma, including some non-T2 asthma patients with ASM remodeling and AHR36. It should be noted that although BT was approved by the US Food and Drug Administration in 2010, the National Asthma Education and Prevention Program currently suggests that BT treatment is limited for selected patients in a clinical trial or registry37. More clinical trials are needed to determine the potential application of BT for non-T2 asthma.
FIG 1.
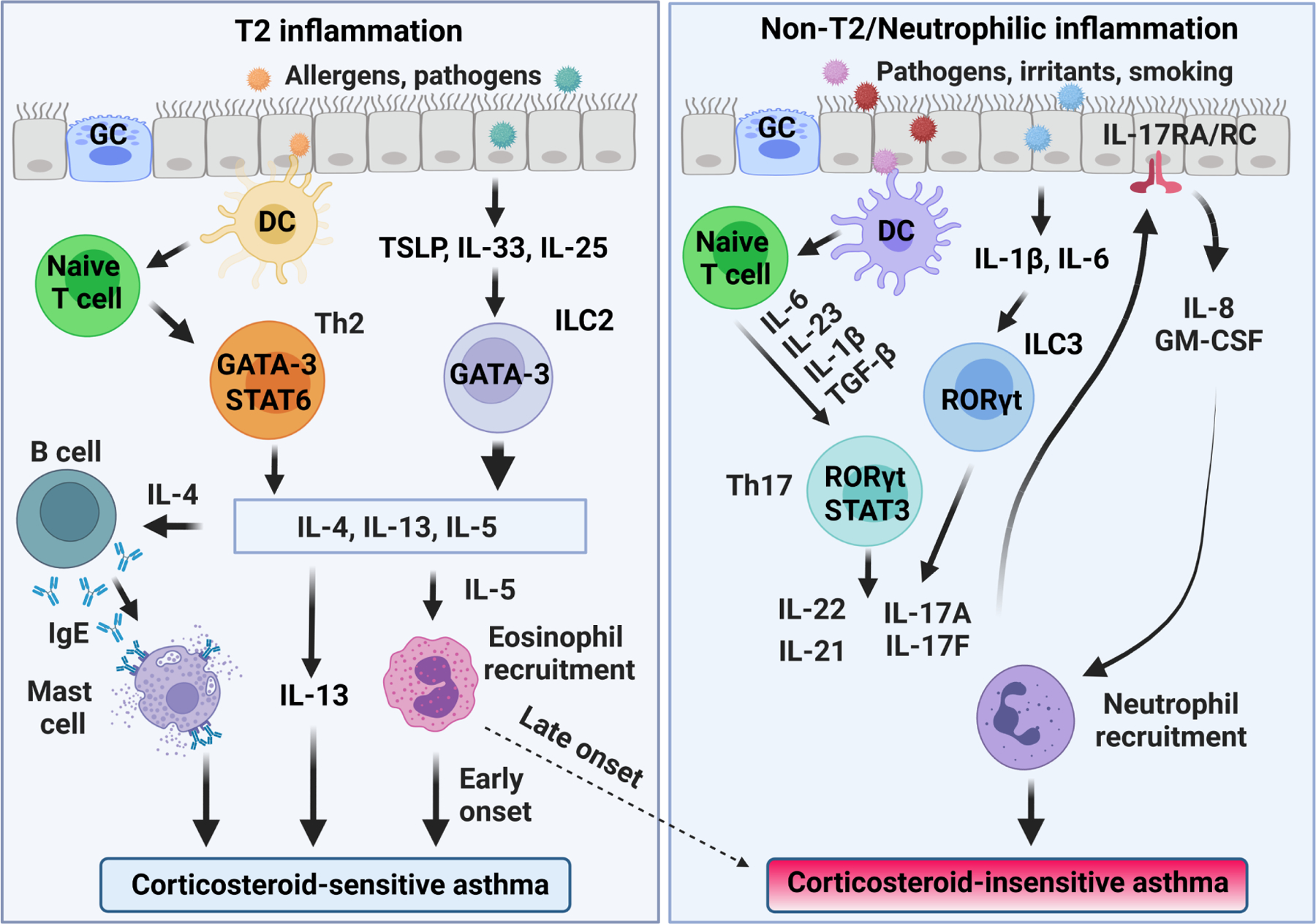
T2 and non-T2 inflammation in corticosteroid-sensitive and -insensitive asthma. Airway epithelia stimuli result in the production of alarmins, thymic stromal lymphopoietin (TSLP), IL-33, and IL-25 that stimulate differentiation of innate lymphoid type 2 cells (ILC2). Dendritic cells (DC) induce the differentiation of Th2 cells. ILC2 and Th2 cells produce the T2 cytokines IL-4, IL-5, and IL-13 via GATA binding protein 3 (GATA3), contributing to the development of corticosteroid-sensitive asthma. Late-onset eosinophilic asthmatics can have persistent airway eosinophilia despite corticosteroid therapy (dashed line). Corticosteroid-insensitive asthma results from exposure to pathogens, irritants, and smoking triggering release of TGF-β, IL-6, IL-1β, and IL-23 that stimulate differentiation of Th17 cells via transcription factors RORγt and STAT3. Th17 cells produce cytokines IL-17A, IL-17F, IL-21, and IL-22 that stimulate the production of neutrophilic chemokines (e.g., IL-8 and GM-CSF). Innate lymphoid type 3 cells (ILC3) also produce IL-17 and play roles in obesity-associated, corticosteroid-insensitive asthma. GC, goblet cells; GM-CSF, granulocyte-macrophage colony-stimulating factor. Created with BioRender.com.
Recent evidence suggests that some, but not all non-T2 asthma is associated with elevated T helper 17 (Th17) cell immune responses and this tends to be more prominent in adult patients with severe and corticosteroid-insensitive asthma (Figure 1)14,16. Th17 cells producing the interleukin 17 (IL-17) family of cytokines are involved in the neutrophilic inflammation and airway remodeling processes in severe asthma38,39, and these cells have been suggested to contribute to the development of at least some subsets of corticosteroid-insensitive asthma40,41. Here, we review corticosteroid-insensitive severe asthma by focusing especially on the pathological role of Th17 cells in non-T2 asthma and potential targets to treat this endotype of asthma.
NEUTROPHILIA IN SEVERE ASTHMA
Neutrophilia in severe asthma was first described in bronchial biopsy studies that aimed to distinguish eosinophilic asthma from non-eosinophilic asthma42. The Severe Asthma Research Program has identified neutrophilic inflammation as an important hallmark of a distinct cluster of patients with moderate to severe asthma43. A more recent study using biopsy samples also demonstrated that bronchial neutrophilia was present in 54% of mild-to-severe asthma patients, and this percentage rose to 68% when only severe asthma patients were considered44. In addition, bronchial neutrophilia is frequently present in sudden-onset fatal asthma in the absence of eosinophils and is associated with lung function alterations of increased airflow limitation, airway closure/air trapping, and altered reversibility patterns44–46. Importantly, these patients respond poorly to corticosteroid treatment, indicating the need for new therapies for this type of asthma47.
Inflammatory phenotypes in asthma are best defined based on sputum cell counts. Thus, neutrophil percentages in sputum exceeding the numbers in healthy individuals were initially used to define ‘pathologic’ neutrophil percentages, leading to a cut-off of 61% to define ‘neutrophilic asthma’ 47,48. This cut-off was later adjusted to 76% because of higher ‘normal’ values in healthy individuals49,50. However, sputum neutrophilia does not always predict neutrophilic bronchial inflammation51. In addition, although neutrophilic inflammation predominates in this cluster, neutrophilia can also coexist with eosinophilia42,51,52, illustrating the complexity of severe asthma. Therefore, how to define neutrophilic asthma in relation to severe asthma remains an open question and there is a debate whether neutrophilic asthma represents a true endotype of disease53 because so many factors can influence the presence and function of neutrophils in airways. Thus, it may be more useful to think of neutrophils as a manifestation of an inflammatory process that is contributing to airway pathology in multiple ways. For example, Th17 cells and innate lymphoid type 3 cells (ILC3) have been linked with neutrophilic airway inflammation (Figure 1). These cells secrete IL-17 cytokines (IL- 17A and IL- 17F) that stimulate the production and release of neutrophilic chemokines in airway epithelial cells and fibroblasts, leading to neutrophil recruitment to the airway54,55. Hence tissue neutrophilia may be a biomarker of elevated IL-17 activity. Several other factors may also contribute to neutrophilic inflammation56. Treatment with high dose inhaled or oral corticosteroids has been shown to contribute to the high number of airway neutrophils in asthmatics57,58. More neutrophilic airway inflammation was found in obese versus nonobese asthmatics21. Smoking worsens asthma symptoms and morbidity, and promotes neutrophilic asthma59,60. In fact, smoking cessation decreased airway neutrophil number, alleviated clinical symptoms, and reduced total mortality in asthmatics61–63. The presence of airway bacteria has been suggested as contributing to airway neutrophilia64. There are significant differences in airway bacteria species in patients with neutrophilic versus eosinophilic asthma, which may alter the corticosteroid sensitivity in these patients65. Importantly, macrolide antibiotics that have antibacterial and anti-inflammatory effects are of some benefit for both T2 and non-T2 asthma66–68. A large randomized, double-blind, placebo-controlled clinical study demonstrated that add-on azithromycin significantly reduced asthma exacerbations with an improvement in quality of life in patients with persistent uncontrolled asthma69,70. Further studies showed that azithromycin treatment reduces key sputum cytokines associated with non-T2 asthma such as IL-6 and IL-1β71.
How neutrophils may contribute to altered airway pathophysiology in asthma is largely based on circumstantial evidence56. For example, chemokines released by neutrophils attract monocytes/macrophages to the airway, thus altering airway inflammation72. Neutrophils can cause ASM hyperresponsiveness73 and exosomes secreted from neutrophils also regulate ASM remodeling74. It was found that neutrophils in asthma patients secrete a higher level of transforming growth factor β (TGF-β) and matrix metalloprotease-9 (MMP-9) to promote airway remodeling, leading to poor lung function75–77. Increased neutrophil elastase in asthmatic patients can cause airway narrowing via induction of airway mucus gland hyperplasia, mucus secretion, and ASM cell proliferation78,79. In addition, increased neutrophils also reduced epithelial barrier function in the airways80. However, while targeting neutrophils inhibits airway inflammation and alleviates airway hyperresponsiveness in some animal models of asthma81–83, this strategy failed to show benefit in asthma patients84, leading to question the exact role of neutrophils in asthma85. Furthermore, neutrophils are heterogenous with proinflammatory and anti-inflammatory subsets86. For example, neutrophils in the airways of asthmatic patients consist of distinct subsets with different/increased activation states87,88. Thus, more precise characterization of neutrophil subsets and the delineating mechanisms for the phenotypic changes in the airways of asthmatics will be essential for the future development of neutrophil-targeting therapies. In addition, one also must take into account the importance of neutrophils in host defense mechanisms and the consequences that could develop as a result of their inhibition in the airways.
TH17 CELL DIFFERENTIATION
Th17 cells are a distinct CD4+ T helper cell subset that is characterized by the expression of the transcription factor retinoic acid–related orphan receptor-γt (RORγt)89. They are derived from naïve CD4+ T cells and play a key role in the pathogenesis of non-T2 neutrophilic asthma. Th17 cell differentiation relies on the coordination of several well-characterized cytokines and transcription factors, with TGF-β1, IL-6 and IL-23 as the most prominent drivers of Th17 cell differentiation. They induce specific transcription factors responsible for the expression of Th17 cell specific cytokines such as IL-17A and IL-17F. Multiple transcription factors have been shown to be important for the development of Th17 cells, including RORγt, signal transducer and activator of transcription 3 (STAT3), interferon regulatory factor 4 (IRF4), basic leucine zipper ATF-like transcription factor (BATF), and runt-related transcription factor 1 (RUNX1). Of these, RORγt appears to be the master transcription factor that regulates the differentiation of Th17 cells90.
TGF-β is a regulatory cytokine that has multiple effects on T cell development, homeostasis, and tolerance91. Interestingly, TGF-β is required for the development of both Th17 cells and regulatory T-cells (Tregs) by triggering the expression of their differentiating transcription factors, RORγt and forkhead box P3 (FOXP3), respectively92. In fact, both transcription factors are initially up-regulated after naïve CD4+ T cells encounter TGF-β93. Whether subsequent differentiation of the cells is skewed towards a Treg phenotype or a proinflammatory Th17 cell phenotype depends mainly on the cytokine milieu. TGF-β alone induces differentiation of FOXP3-dependent Treg cells94,95, whereas the presence of IL-6 inhibits Treg development and induces Th17 cell differentiation94. IL-6 also directly activates STAT3, whereas TGF-β both inhibits suppressor of cytokine signaling 3 (SOCS3), a negative regulator of STAT3 signaling, and activates SMAD2 to promote RORγt and IL-17A expression96–98. Additional cytokines can further drive Th17 cell differentiation. For example, IL-1β signaling was reported to enhance the phosphorylation of STAT3 by repressing SOCS3 to favor human Th17 cell differentiation99. Interestingly, FOXP3 is present in several isoforms due to alternative splicing. In the absence of a second signal from a proinflammatory cytokine, full length FOXP3 directly binds and inhibits RORγt function, thus driving Treg differentiation100, whereas FOXP3 isoforms lacking exon 7 inhibit the function of full length FOXP3 in a dominant-negative manner101,102. A recent study showed that IL-1β can promote Th17 cell development through induction of FOXP3 isoforms lacking exon 7102.
IL-23 is a proinflammatory cytokine that plays an important role in the regulation of numerous inflammatory diseases by integrating the innate and adaptive immune systems103. IL-23 is essential for the maintenance, expansion, and proper function of Th17 cells through a positive feedback loop104. During chronic inflammation, activated dendritic cells and macrophages produce IL-23 that promotes the development and differentiation of Th17 cells105. Importantly, IL-23 is required for full function of Th17 cells in vivo. In the absence of IL-23, Th17 cells activated with TGF-β1 plus IL-6 exhibit impaired pathogenic function in vivo despite increased IL-17 production106. In addition, IL-23 also enhances Th2 cytokine production and eosinophilic airway inflammation107. Serum IL-23 is elevated in asthmatic patients and is associated with airflow obstruction108. Deletion of IL-23 gene or treatment with an anti-IL-23 antibody reduced airway inflammation and decreased airway resistance in mice109,110. However, in a recent phase 2a trial, the monoclonal anti-IL-23 antibody risankizumab reduced IL-23 target genes but had no clinical benefit in asthmatic patients111. Thus, further basic and clinical studies are needed to determine the pathological roles of IL-23 signaling pathways in asthma.
IL-17 CYTOKINES AND NEUTROPHILIA IN SEVERE ASTHMA
Th17 cells secrete Th17-associated cytokines IL-17A, IL-17F, IL-21, and IL-22. Among these cytokines, IL-21 acts in an autocrine manner to promote IL-17A production112 whereas IL-22 enhances the proliferation and migration of human ASM cells113,114, leading to airway remodeling and hyperresponsiveness115. IL-17A and IL-17F are particularly important in immune responses against bacterial and fungal infections116. They belong to the IL-17 family (including IL-17A, IL-17B, IL-17C, IL-17D, IL-17E [known as IL-25], and IL-17F), and they share common receptor subunits, IL-17 receptor A (IL-17RA), and IL-17 receptor C (IL-17RC).116 IL-17A and IL-17F can form homodimers and heterodimers, and may have similar functions to induce neutrophil recruitment to the airway117,118. IL-17C, mainly released by epithelial cells119, enhances IL-17A and IL-17F release from Th17 cells120 whereas IL-25 promotes T2 inflammation through induction of IL-4, IL-5 and IL-13121. Little is known about IL-17D. A recent study found that IL-17D exerts anti-inflammatory effects via regulation of ILC3 function122.
IL-17A and IL-17F are pro-inflammatory cytokines known to stimulate neutrophil maturation, migration, and function55,123. Overexpression of IL-17A in mice results in significant peripheral neutrophilia124. The number of cells positive for IL-17A was initially found to be increased significantly in sputum and bronchoalveolar lavage fluids of subjects with asthma in comparison with control subjects125. Subsequent studies demonstrated that IL-17A was elevated in bronchial tissues, peripheral blood mononuclear cells (PBMCs), and serum from asthmatic patients126–132. Importantly, IL-17A production in asthma patients positively correlates with AHR and clinical severity of asthma38,127,131–135. Similarly, IL-17F was also increased in asthma patients126,136, correlated with both airway neutrophils and more severe disease137,138. A loss-of-function IL-17F mutant antagonizes wildtype IL-17F and is inversely related to asthma risk.139,140
Another study found a correlative increase in IL-17A and IL-17F in bronchial biopsies in patients with increasing asthma severity39. In addition, expression of specific IL-17 receptor subunits, IL-17RA and IL-17RC118, were also increased in the bronchial tissues and PBMCs of asthmatic patients128,129. These findings indicate that IL-17A and IL-17F are likely important cytokines in the pathogenesis of neutrophilic asthma. It should be noted that in addition to Th17 cells, other cell types including ICL3, bone-marrow-derived neutrophils, B cells, IL-17-producing CD8+ T cells, natural killer T cells, mucosal-associated invariant T cells, etc. also release IL-17A and IL-17F in response to different cytokines (reviewed by Hynes and Hinks)123. It is not yet clear which are the main sources of IL-17A and IL-17F secretion and what are their contributions to the pathogenesis of neutrophilic asthma.
CONTRIBUTION OF TH17 CELLS AND IL-17 CYTOKINES TO CORTICOSTEROID INSENSITIVITY IN SEVERE ASTHMA
Glucocorticoids, a class of corticosteroids, are currently the most effective treatment for asthma. The anti-inflammatory effects of glucocorticoids are mediated by their intracellular receptors (GRα) while the GRβ variant acts as a dominant negative inhibitor of GRα141. Glucocorticoids bind to GRα in the cytoplasm and the glucocorticoid/GRα complex translocates into the nucleus to repress pro-inflammatory genes and transactivate anti-inflammatory genes, thus inhibiting activation, infiltration, and survival of inflammatory and epithelial cells, as well as the pro-inflammatory function of ASM cells142–145. Corticosteroid insensitivity can be inherited or acquired. GRα mutations were associated with insensitivity or hypersensitivity to glucocorticoids146,147. Reduced GRα expression148, defective GRα nuclear translocation149, increased phosphorylation of GRα with impaired activity150 and increased expression of the dominant negative GRβ151 also play roles in the induction of corticosteroid insensitivity.
Corticosteroid-based drugs can effectively manage T2 inflammation via inducing apoptosis of Th2 cells and eosinophils and inhibiting T2 cytokine production. Thus, patients with allergic asthma generally respond well to corticosteroids, with improved lung function and reduced exacerbations. However, up to 10% of asthmatics respond poorly to corticosteroid-based therapies, called corticosteroid-insensitive, -refractory or -resistant asthma. Patients with corticosteroid-insensitive asthma account for a large percentage of the overall costs for asthma worldwide. Their asthma is less stable and more difficult to control, and they are subject to higher morbidity and mortality152–155. There are many reasons why asthma patients fail to benefit from corticosteroid-based therapies155, including lack of adherence to prescribed therapy156,157. PGA manifests with no sputum eosinophilia or neutrophilia25 and inhaled corticosteroids have limited effects in patients with PGA27,158. Airway neutrophils also play an important role in mediating severe and corticosteroid-insensitive asthma159–161. In fact, corticosteroids promote the apoptosis of eosinophils but inhibits neutrophil apoptosis, which may explain why increased neutrophils are associated with inhaled corticosteroid-treated severe asthma162–164.
Mounting experimental and clinical evidence supports a role of Th17 and IL-17 cytokines in corticosteroid-insensitive asthma. McKinley et al.165 first linked Th17 cells with corticosteroid-insensitive allergic airway disease in an animal model characterized by elevated neutrophil chemokines and growth factors as well as neutrophilic inflammation in the lung. Importantly, Th17-driven allergic airway disease was not abrogated by the corticosteroid treatments that were effective in inhibiting Th2-driven airway disease. Treatment with the corticosteroid drug dexamethasone significantly inhibited T2 cytokines but not IL-17 production in vitro165. The transfer of primed ovalbumin-specific Th2 cells into mice induces a corticosteroid-sensitive allergic asthma, whereas the transfer of ovalbumin-specific Th17 cells induces a severe corticosteroid-insensitive asthma165. Furthermore, after exposure to antigen, mice overexpressing the transcription factor RORγt exhibited predominantly neutrophilic airway inflammation with enhanced lung expression of IL-17 and IL-22. The neutrophilic airway inflammation in RORγt-overexpressing mice was effectively suppressed by anti-IL-17 antibody, but not by dexamethasone166. In fact, dexamethasone was reported to enhance Th17 cell differentiation in vitro167 and IL-17 can synergize with dexamethasone to induce neutrophil-promoting cytokine colony-stimulating factor 3 (CSF3) in both ASM cells and fibroblasts, leading to corticosteroid insensitivity168. Other mechanisms for Th17 cell-mediated corticosteroid insensitivity in severe asthma have been proposed, including up-regulation of the expression of GRβ in peripheral mononuclear cells151 and increased expression of mitogen-activated protein kinase 1 (MEK1) in CD4+ T cells that inhibits GRα activity169,170. Human studies also suggest the involvement of Th17 cells and IL-17 cytokines in patients with severe corticosteroid-insensitive asthma38,128,129. These findings demonstrated that Th17 cells and IL-17 are sufficient to promote many of the hallmark characteristics of neutrophilic asthma in vivo and that these responses are corticosteroid-insensitive. It should be noted that IL-17 produced by ILC3 may play a role in corticosteroid insensitivity associated with the obesity phenotype of asthma171.
Interestingly, dual positive Th2/Th17 cells were found in the blood, tissue, and bronchoalveolar lavage fluid of subjects with the most severe form of asthma and who manifest corticosteroid insensitivity169,172,173. In fact, there has been a shift from viewing asthma as T2 vs non-T2 as a binary situation. For example, some patients with severe asthma have a mixed neutrophilic and eosinophilic inflammation in their sputum174. These patients typically have the most severe asthma symptoms and poor response to inhaled corticosteroids43,175–177. Israel and Reddel have postulated that IL-6 and IL-17 may stimulate Th2 and Th17 cell responses in the airway, thus promoting both T2 and non-T2 inflammation12. Upon stimulation with IL-21, IL-1β, IL-6, and anti-IFN-γ, native T cells differentiate into dual-positive Th2-Th17 cells, leading to more severe asthma subtypes178. Deletion of IL-17 and RORγt genes or treatment with an RORγt inhibitor blocked both Th2 and Th17 cell responses, leading to a reduction of neutrophilic and eosinophilic inflammation in mice with allergic asthma179. Interestingly, inhibition of Th2 cell cytokines augments Th17-dependent neutrophilia, whereas blockade of IL-17 augments Th2-stimulated eosinophilia in experimental allergic asthma180, suggesting that Th2 and Th17 cell responses co-exist in airways and are reciprocally regulated. Hence combined blockade of both T2 and non-T2 inflammation might be able to achieve better therapeutic benefits in controlling severe corticosteroid-insensitive asthma.
THERAPEUTIC IMPLICATIONS OF TARGETING TH17 CELL RESPONSES IN CORTICOSTEROID-INSENSITIVE ASTHMA
Because corticosteroid-insensitive neutrophilic asthma is associated with excessive Th17 responses, inhibiting Th17 signaling might offer effective therapeutic options for corticosteroid-insensitive asthma. Potential therapeutic approaches include directly targeting Th17-related cytokines, cytokine receptors, and intracellular signaling pathways, as well as inhibiting Th17-specific transcription factors (Figure 2 and Table 1).
FIG 2.
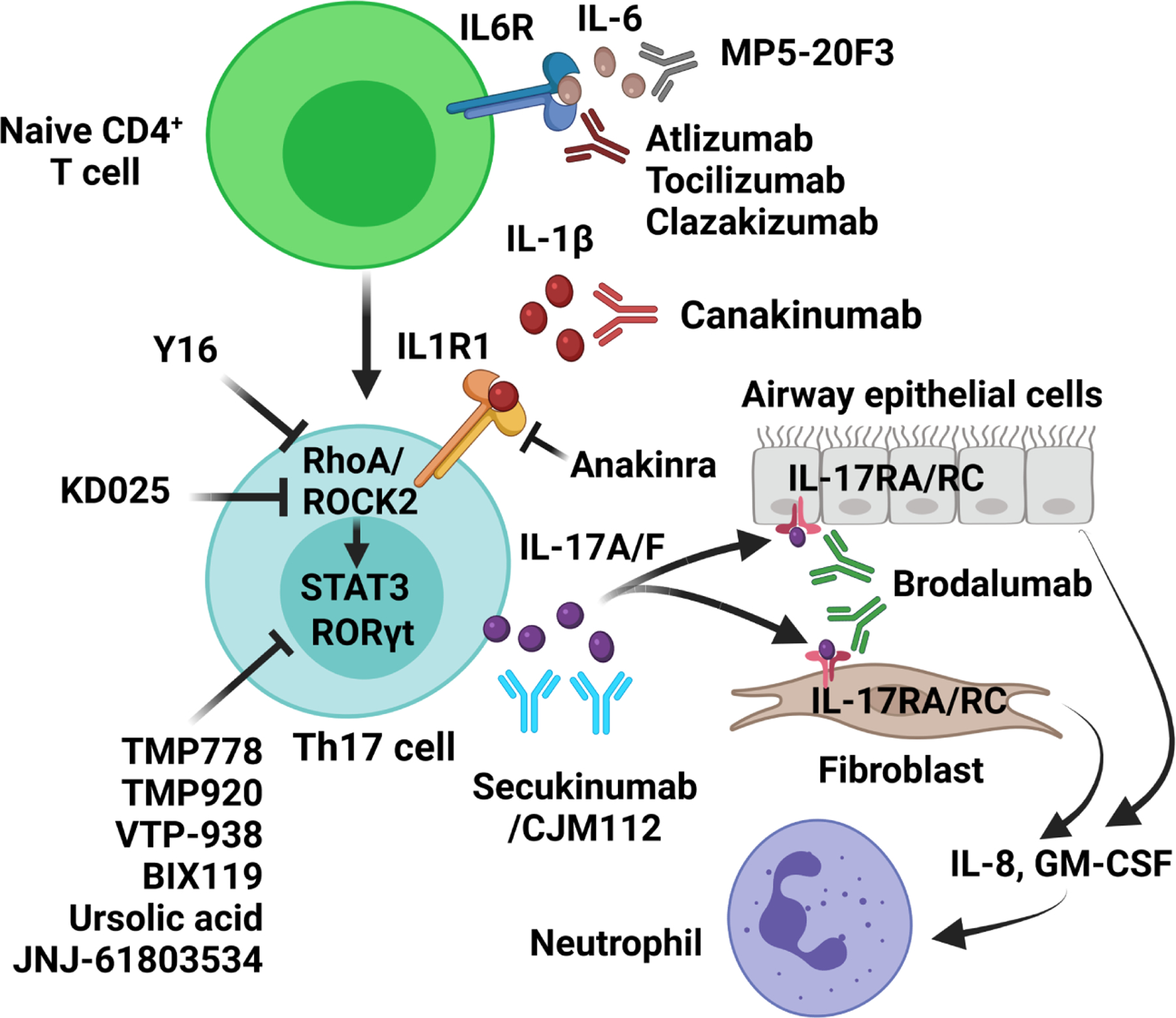
Therapeutic agents targeting Th17 cell responses. TGF-β, IL-1β and IL-6 stimulate differentiation of naïve T cells into the Th17 lineage. Full differentiation of Th17 cells requires the cooperative action of RORγt and STAT3. Th17 cells produce the IL-17 family of cytokines including IL-17A and IL-17F, which stimulate the production and release of neutrophilic chemokines (e.g., IL-8 and GM-CSF) in airway epithelial cells and fibroblasts via its receptor IL-17RA and IL-1RC, leading to recruitment of neutrophils into the airways. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL6R, IL-6 receptor; IL1R1, IL-1 receptor; IL-17RA, IL-17 receptor A; IL-17RC, IL-17 receptor C; RORγt, retinoic acid receptor-related orphan receptor-γt; ROCK2, Rho-associated kinase 2. Created with BioRender.com.
TABLE 1.
Emerging therapies targeting Th17 cell responses in corticosteroid-insensitive asthma
| Target | Reagent | Type of drug | System | Reference |
|---|---|---|---|---|
| IL-17A | Anti-IL17A | mAb | Mice | 183 |
| Secukinumab/CJM112 | mAb | Human | 184, 185 | |
| IL-17RA | Brodalumab | mAb | Human | 186 |
| IL-6 | MP5-20F3 | mAb | Mice | 193 |
| IL-6R | Atlizumab | mAb | Mice | 166 |
| Tocilizumab | mAb | Human | 201 | |
| Clazakizumab | mAb | Human | 202 | |
| IL-1β | Canakinumab | mAb | Human | 214 |
| IL-1R1 | Anakinra | Antagonist | Human | 210, 215 |
| RORγt | TMP778/920 | Inverse agonists | Cells | 218–220 |
| Ursolic acid | Inhibitor | Mice | 179 | |
| VTP-938 | Inverse agonist | Mice | 225 | |
| BIX119 | Inhibitor | Mice | 115 | |
| JNJ-61803534 | Inhibitor | Mice and human | 226 | |
| RhoA | Y16 | Inhibitor | Mice | 232 |
| ROCK2 | KD025 | Inhibitor | Mice | 234 |
Blocking IL-17A and IL-17RA:
Blocking IL-17A by its monoclonal antibody was demonstrated to improve lung function in several experimental murine asthma models. Kudo et al.181 found that IL-17A blocking antibodies can attenuate the contractile response of smooth muscle cells in the airways. Manni et al.182 demonstrated that IL-17A contributes to AHR in an experimental model of corticosteroid-insensitive Th2/Th17 asthma. Camargo et al.183 showed that treatment with IL-17A antibody alleviated pulmonary inflammation, remodeling, and oxidative stress in an experimental model of lipopolysaccharide (LPS)-exacerbated asthma. However, two clinical trials of humanized anti-IL-17A monoclonal antibodies, secukinumab (AIN457) and CJM112 failed to improve asthmatic symptoms in patients with severe asthma184,185. IL-17 binds to receptor complexes that have IL-17RA as the common subunit. Brodalumab, also known as AMG-827, is a humanized monoclonal antibody that binds to IL-17RA, thereby blocking the receptor and the downstream signal pathways of IL-17A, IL-17F and other IL-17 isoforms. The efficacy and safety of brodalumab was evaluated in patients on inhaled corticosteroids with inadequately controlled moderate to severe asthma186. Although a nominally positive response was seen in a subgroup with bronchodilator reversibility, no difference was observed in Asthma Control Questionnaire (ACQ) scores between subjects treated with brodalumab compared to placebo in the overall study population. Since the patients in this clinical trial were not selected for non-T2 asthma and the trial design may have precluded detection of benefit for these agents in patients with heterogeneous phenotypes, it is possible that subgroups of patients, particularly those with high numbers of sputum neutrophils or with a high degree of lung function reversibility would respond more favorably186–188. It should be noted that the US Food and Drug Administration issued a black box warning after six patients treated with brodalumab across four clinical trials committed suicide, but no causal relationship was identified. Nonetheless, the relative lack of efficacy in the initial asthma clinical trial and a questionable safety issue has resulted in discontinuation of brodalumab studies for the treatment of asthma. Thus, further clinical studies are needed to determine the efficacy of targeting IL-17/IL-17RA signaling with other IL-17/IL-17RA drugs in more precisely defined patient subsets and/or endpoint selection. Furthermore, therapy with IL17A/IL17RA antibodies may have failed because other Th17-associated cytokines such as IL-22 also contribute to severe asthma independently from IL-17. Thus, a broader upstream approach that targets Th17 cells might be more effective than anti-IL-17/IL-17 receptor therapies115. In addition, it was reported that anti-IL-17A augmented Th2 cell responses and eosinophilia in experimental allergic asthma180. Thus, it may require combination therapies blocking both Th17 and Th2 cell responses to effectively control severe asthma179,180.
Blocking IL-6 and IL6R:
IL-6 is an important cytokine for the induction of Th17 cell differentiation, as well as a downstream target of IL-17A. In the presence of TGF-β, IL-6 drives naive T cells to differentiate into Th17 cells92,189,190. Th17 cells release more IL-6 to further promote Th17 cell differentiation191. Studies also showed that IL-6 induced expression of IL-21 that amplified an autocrine loop to induce more IL-21 and IL-23 receptor in naïve CD4+ T cells. Both IL-21 and IL-23 can induce IL-17 expression192. Chu et al.193 observed that increased sputum IL-6 was associated with mixed eosinophilic-neutrophilic bronchitis and impaired lung function in patients. An anti-IL-6 antibody reduced neutrophilic and eosinophilic cytokines/chemokines and alleviated airway inflammation in mice193. In addition, IL-6 production was increased in IL-17A-induced corticosteroid-insensitive airway inflammation in an animal study of allergic airway inflammation, and both airway neutrophilia and AHR were effectively attenuated by treatment with an anti-IL6R antibody166. A more recent study showed that blockade of IL-6 signaling attenuates toluene diisocyanate-induced Th2/Th17 responses and ameliorates corticosteroid-insensitive asthma in mice194. IL-6 may also play a significant role in subtypes of obese non-T2 asthma. Peters et al.195 found that patients with plasma IL-6-high asthma had worse lung function and asthma control. Blood neutrophils were increased in the plasma IL-6-high group, suggesting a role for systemic IL-6-mediated neutrophilic inflammation in mediating an “outside-in mechanism of lung dysfunction.” Conventional asthma research generally focuses on pathogenic factors that arise inside the lung (“inside out”) such as increases in airway inflammation. Peters’ work suggests that lung dysfunction can occur from pathogenesis outside the lung (“outside in”), such as the low-grade systemic inflammation that occurs during obesity. Indeed, a severe asthma research program 3 (SARP3) study of severe asthmatic patients demonstrated that obesity was associated with elevated plasma IL-6 levels and that plasma IL-6 levels predicted asthma exacerbation risk independently of T2 biomarkers196. Furthermore, single nucleotide polymorphism (SNP) rs4129267 in the IL6R gene has been associated with an increased risk of asthma197 whereas the SNP rs2228145 is linked with reduced lung function in severe asthma198. In addition to acting on Th17 cells and neutrophils, IL-6 can also bind to soluble IL6R and causes IL-6 trans-signaling (IL-6TS) on airway epithelial cells199 and ASM cells200, leading to impaired epithelial barrier function, airway inflammation and remodeling. These studies prompt consideration of treatment approaches for severe asthma via inhibiting cytokines associated with systemic inflammation (e.g., IL-6). In a preliminary report, two patients with severe persistent, non-atopic asthma were treated with tocilizumab, a humanized anti-IL6R monoclonal antibody, and both patients exhibited decreased Th2 and Th17 cell responses and clinical improvement201. In the PrecISE clinical study sponsored by the U.S. National Heart, Lung, and Blood Institute to investigate several treatments for severe asthma, the efficacy of the anti-IL-6 monoclonal antibody clazakizumab will be examined202.
Blocking IL-1β and its receptor (IL1R1):
IL-1β plays an important role in the pathogenesis of asthma203. Increased levels of IL-1β were detected in the airways or the sputum of patients with asthma204,205 and was associated with increased neutrophil counts in elder patients with more severe asthma206. IL-1β signaling was reported to enhance the phosphorylation of STAT3 by repressing SOCS3 to favor human Th17 cell differentiation99. In the presence of IL-2 and TGF-β, IL-1β can also induce transdifferentiation of ILC2s into IL-17-secreting cells207. IL-1β is generated by the nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing protein 3 (NLRP3) inflammasome, mainly in monocytes and macrophages. Neutrophilic airway inflammation, disease severity, and corticosteroid insensitivity in human asthma all correlate with NLRP3 and IL-1β expression208. Treatment with anti–IL-1β neutralizing antibody, caspase-1 inhibitor Ac-YVAD-cho, and NLRP3 inhibitor MCC950 each suppressed IL-1β responses and corticosteroid insensitivity in experimental murine models of neutrophilic asthma208. Furthermore, the expression level of IL1R1 strongly correlates with increased neutrophils in sputum and airflow limitation of asthmatic patients209 and LPS-induced neutrophilic airway inflammation in healthy volunteers was attenuated by the IL1R1 antagonist anakinra210. In addition, IL-1β can modulate AHR in asthma via regulation of ASM contraction and relaxation211,212. IL-1β signaling is also involved in airway remodeling through hypersecretion of mucus in asthma213. These findings highlight the important role of IL-1β in the pathogenesis of asthma via both airway inflammation and remodeling. In a randomized double-blind placebo-controlled trial, IL-1β blocking antibody canakinumab significantly reduced the late asthmatic response in patients with mild asthma214. In addition, targeting IL-1β for airway inflammation in asthma by anakinra was to be tested in clinical trials, but was suspended due to the COVID-19 pandemic215.
Blocking the transcription factor RORγt:
RORs are members of the nuclear receptors, a superfamily of structurally conserved, ligand-regulated transcription factors216. Two isoforms of RORγ, RORγ1 and RORγ2 (or RORγt), have been identified. RORγ1 is ubiquitously expressed, whereas RORγt is highly expressed in specific sub-populations of immune cells217. RORγt is the key transcription factor required for Th17 cell differentiation and for production of Th17 cell cytokines by innate and adaptive immune cells. TMP778 and TMP920, two inverse agonists of RORγt, were shown to potently suppress Th17-cell generation and IL-17 secretion by differentiated Th17 cells in vitro218. TMP778 also inhibited human Th17 signature gene expression in vitro as well as murine Th17-cell differentiation in vivo219,220. In addition, mice with deletion of RORγt gene or treatment with the RORγt inhibitor ursolic acid had diminished Th17 and Th2 cell responses, leading to reduced neutrophil and eosinophil numbers in the airway179. Interestingly, RORγt-deficient T cells were defective in differentiating into Th2 cells but express a higher level of B-cell lymphoma 6 (BCL6) than wild-type T cells under Th2 cell differentiation conditions. BCL6 knockdown restored Th2 cell differentiation in RORγt-deficient T cells. BCL6 is known to suppress the differentiation of naive T cells into Th2 cells221,222 via inhibition of GATA-3 expression223 and BCL6-deficient mice exhibited a marked increase in Th2 cell responses224. Thus, RORγt blockade diminishes Th2 cell responses, at least in part, via upregulation of BCL6 in T cells179. Whitehead et al.225 recently reported that the orally available selective RORγt inverse agonist VTP-938 not only attenuates Th17 cell development and neutrophilic inflammation of the airway, but also diminishes AHR in an environmentally relevant house dust mite extract–mediated model of asthma. Interestingly, a recent animal study suggests that RORγt inhibitors can block both Th17-associated IL-17 and IL-22, which might be more effective than anti-IL-17 alone to treat severe asthma115. Furthermore, JNJ-61803534, a potent and selective RORγt inhibitor, exhibited an acceptable biosafety profile in both preclinical and clinical trials226. However, genetic loss of RORγt contributes to chronic fungal infections in humans227 and RORγt blockade with multiple agents has led to thymic lymphomas in mice228. Thus, although blockade of RORγt with small molecule agents might provide a novel strategy in the management of Th17-dependent neutrophilic asthma, further studies are needed to evaluate the potential on-target toxicities associated with chronic usage of these agents before considering therapeutic use for severe asthma in human subjects.
Blocking RhoA/ROCK signaling pathways:
Increased activation of Rho-associated kinase (ROCK) was observed in asthmatic patients, and this has been suggested as a potential therapeutic target for asthma229,230. This signaling pathway is the key regulator of T-cell maturation, activation and differentiation231. Ablation of RhoA or treatment with Y16, a specific RhoA inhibitor impaired Th17 cell differentiation via downregulation of STAT3 and RORγt, and alleviated house dust mite-triggered allergic airway inflammation in mice232. ROCK, a serine/threonine kinase, is one of the main downstream signaling molecules of RhoA. There are two highly homologous isoforms: ROCK1 and ROCK2233. Zanin-Zhorov et al.234 reported that ROCK2 controls IL-17A secretion in human T-cells via the regulation of STAT3 and RORγt. KD025, a selective ROCK2 inhibitor, modulates inflammation by decreasing STAT3 activation and increasing the suppressive function of Tregs. Treatment of human T cells with KD025 induced a beneficial shift in the Th17/Treg balance234. It should be noted that the RhoA/ROCK signaling pathways also play important roles in ASM contraction, AHR, and airway remodeling235–237. Thus RhoA/ROCK antagonists with pleiotropic effects on numerous inflammatory and airway cell signaling pathways could provide novel therapeutic benefit in corticosteroid-insensitive asthma.
CONCLUSION
Severe, corticosteroid-insensitive asthma is a significant clinical problem, adversely affecting quality of life, increasing healthcare costs, and lacking good therapeutic options. Both human and animal studies have demonstrated that excessive Th17 responses are likely a key factor in this type of corticosteroid-insensitive, neutrophilic asthma. Therefore, targeting Th17-associated cytokines may provide therapeutic approaches to reduce excessive Th17 signaling that could offer advantages over classic therapies, such as corticosteroids, for patients with severe asthma. However, Th17 cells are not homogenous, but rather consist of both non-pathogenic and pathogenic cell populations106,238–241. Therefore, the consequences of long-term inhibition of the Th17 pathway must be carefully evaluated to determine the risk versus benefit in blocking this pathway. Although a few trials have reported varying data targeting Th17 cell responses and Th17 related cytokines, there remain multiple challenges to identify, develop and implement the “ideal” Th17-targeted interventional strategy with respect to safety and the treatment of corticosteroid-insensitive neutrophilic asthma.
Sources of funding:
This work was supported, in part, by the National Institutes of Health (NIH) grants R01HL116849 to Y. Tu and T.B. Casale; 5R21ES029566 to Y. Tu and P.W. Abel; Nebraska State LB595 Research Program to Y. Tu and P.W. Abel.
Abbreviations used:
- AHR
airway hyperresponsiveness
- AQC
Asthma Quality Control
- ASM
airway smooth muscle
- BATF
basic leucine zipper ATF-like transcription factor
- BCL6
B-cell lymphoma 6
- BT
bronchial thermoplasty
- CSF3
colony-stimulating factor 3
- FOXP3
forkhead box P3
- GATA3
GATA binding protein 3
- GC
goblet cells
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GR
glucocorticoid receptor
- IL1R1
Interleukin 1 receptor, type I
- IL6R
interleukin 6 receptor
- IL-6TS
IL-6 trans-signaling
- IL-17
interleukin 17
- IL-17RA
IL-17 receptor A
- IL-17RC
IL-17 receptor C
- ILC2
innate lymphoid type 2 cells
- ILC3
innate lymphoid type 3 cells
- IRF4
interferon regulatory factor 4
- MMP-9
matrix metalloprotease-9
- NLRP3
the nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing protein 3
- PBMCs
peripheral blood mononuclear cells
- PGA
paucigranulocytic asthma
- ROCK
Rho-associated kinase
- RORγt
retinoic acid–related orphan receptor-γt
- RUNX1
runt-related transcription factor 1
- SARP3
severe asthma research program 3
- SNP
single nucleotide polymorphism
- SOCS3
suppressor of cytokine signaling 3
- STAT3
signal transducer and activator of transcription 3
- TGF-β
transforming growth factor β
- Th2
T helper 2 cells
- Th17
T helper 17 cells
- Tregs
regulatory T-cells
- T2
type 2
- TSLP
thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have declared that no conflict of interest exists.
REFERENCES
- 1.2021 Global Initiative for Asthma (GINA) Report, Global Strategy for Asthma Management and Prevention 2021. Available from https://ginasthma.org/gina-reports/
- 2.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–373 [DOI] [PubMed] [Google Scholar]
- 3.The Global Initiative for Asthma (GINA): Diagnosis and Management of Difficult-to-treat and Severe Asthma in Adolescent and Adult Patients 2021. Available from: https://ginasthma.org/severeasthma/
- 4.Hansbro PM, Kim RY, Starkey MR, Donovan C, Dua K, Mayall JR, et al. Mechanisms and treatments for severe, steroid-resistant allergic airway disease and asthma. Immunol Rev 2017; 278: 41–62. [DOI] [PubMed] [Google Scholar]
- 5.Foster PS, Maltby S, Rosenberg HF, Tay HL, Hogan SP, Collison AM, et al. Modeling TH2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol Rev 2017; 278:20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol 2015; 15:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corren J, Lemanske RF Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med 2011;365:1088–1098 [DOI] [PubMed] [Google Scholar]
- 9.Nair P, Pizzichini MMM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 2009; 360:985–993. [DOI] [PubMed] [Google Scholar]
- 10.Ortega H, Chupp G, Bardin P, Bourdin A, Garcia G, Hartley B, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J 2014; 44:239–241. [DOI] [PubMed] [Google Scholar]
- 11.Llanos JP, Ortega H, Bogart M, Packnett ER, Manjelievskaia J, Bell CF, et al. Real-World Effectiveness of Mepolizumab in Patients with Severe Asthma: An Examination of Exacerbations and Costs. J Asthma Allergy 2020; 13:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israel E, and Reddel HK. Severe and Difficult-to-Treat Asthma in Adults. N Engl J Med 2017; 377:965–976. [DOI] [PubMed] [Google Scholar]
- 13., Coverstone AM, Seibold MA, Peters MC. Diagnosis and Management of T2-High Asthma. J Allergy Clin Immunol Pract 2020; 8:442–450. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18:716–725. [DOI] [PubMed] [Google Scholar]
- 15.Peters MC, Ringel L, Dyjack N, Herrin R, Woodruff PG, Rios C, et al. A Transcriptomic Method to Determine Airway Immune Dysfunction in T2-High and T2-Low Asthma. Am J Respir Crit Care Med 2019; 199:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy 2020; 75:311–325. [DOI] [PubMed] [Google Scholar]
- 17.Murphy RC, Pavord ID, Alam R, Altman MC. Management Strategies to Reduce Exacerbations in non-T2 Asthma. J Allergy Clin Immunol Pract 2021; 9:2588–2597. [DOI] [PubMed] [Google Scholar]
- 18.Thomas RA, Green RH, Brightling CE, Birring SS, Parker D, Wardlaw AJ, et al. The influence of age on induced sputum differential cell counts in normal subjects. Chest 2004; 126:1811–1814. [DOI] [PubMed] [Google Scholar]
- 19.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non- eosinophilic asthma. Thorax 2007; 62:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ducharme ME, Prince P, Hassan N, Nair P, Boulet LP. Expiratory flows and airway inflammation in elderly asthmatic patients. Respir Med 2011; 105:1284–1289. [DOI] [PubMed] [Google Scholar]
- 21.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A large subgroup of mild- to- moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med 2012;185:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telenga ED, Tideman SW, Kerstjens HA, Hacken NH, Timens W, Postma DS, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy 2012; 67:1060–1068. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FD, Vercelli D. Asthma. Lancet 2013; 382:1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokes JR, Casale TB. Characterization of asthma endotypes: implications for therapy. Ann Allergy Asthma Immunol 2016; 117:121–125. [DOI] [PubMed] [Google Scholar]
- 25.Svenningsen S, Nair P. Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Front Med (Lausanne) 2017; 4:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tliba O, Panettieri RA Jr. Paucigranulocytic asthma: uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol 2019; 143:1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demarche S, Schleich F, Henket M, Paulus V, Van Hees T, Louis R. Detailed analysis of sputum and systemic inflammation in asthma phenotypes: are paucigranulocytic asthmatics really non-inflammatory? BMC Pulm Med 2016; 16:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corren J, Castro M, O’Riordan T, Hanania NA, Pavord ID, Quirce S, et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J Allergy Clin Immunol Pract 2020; 8:516–526. [DOI] [PubMed] [Google Scholar]
- 29.Ntontsi P, Samitas K, Zervas E, Gaga M. Severe asthma: what is new in the new millennium. Curr Opin Allergy Clin Immunol 2020; 20:202–207. [DOI] [PubMed] [Google Scholar]
- 30.Mersha TB, Afanador Y, Johansson E, Proper SP, Bernstein JA, Rothenberg ME, et al. Resolving clinical phenotypes into endotypes in allergy: molecular and omics approaches. Clin Rev Allergy Immunol 2021; 60:200–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonta PI, Chanez P, Annema JT, Shah PL, Niven R. Bronchial thermoplasty in severe asthma: best practice recommendations from an Expert Panel. Respiration 2018; 95:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G, AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pretolani M, Dombret MC, Thabut G, Knap D, Hamidi F, Debray MP, et al. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med 2014; 190:1452–1454. [DOI] [PubMed] [Google Scholar]
- 34.Facciolongo N, Di Stefano A, Pietrini V, Galeone C, Bellanova F, Menzella F, et al. Nerve ablation after bronchial thermoplasty and sustained improvement in severe asthma. BMC Pulm Med 2018; 18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao SY, Linderholm AL, Yoneda KY, Kenyon NJ, Harper RW. Airway transcriptomic profiling after bronchial thermoplasty. ERJ Open Res 2019; 5:00123–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trivedi A, Pavord ID, Castro M. Bronchial thermoplasty and biological therapy as targeted treatments for severe uncontrolled asthma. Lancet Respir Med 2016; 4:585–592. [DOI] [PubMed] [Google Scholar]
- 37.Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, et al. 2020 Focused Updates to the Asthma Management Guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol 2020; 146:1217–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 2006; 7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 2009; 123:1185–1187. [DOI] [PubMed] [Google Scholar]
- 40.Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med 2014; 190:1094–1101. [DOI] [PubMed] [Google Scholar]
- 41.Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, et al. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-γ(high) immunophenotypes: potential benefits of calcitriol. J Allergy Clin Immunol 2015; 136:628–637.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999; 160:1001–1008. [DOI] [PubMed] [Google Scholar]
- 43.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 2014; 133:1557–1563.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bullone M, Carriero V, Bertolini F, Folino A, Mannelli A, Di Stefano A, et al. Elevated serum IgE, oral corticosteroid dependence and IL-17/22 expression in highly neutrophilic asthma. Eur Respir J 2019; 54: 1900068. [DOI] [PubMed] [Google Scholar]
- 45.Lamblin C, Gosset P, Tillie-Leblond I, Saulnier F, Marquette CH, Wallaert B, et al. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med 1998; 157:394–402. [DOI] [PubMed] [Google Scholar]
- 46.Tsokos M, Paulsen F. Expression of pulmonary lactoferrin in sudden-onset and slow-onset asthma with fatal outcome. Virchows Arch 2002; 441:494–499 [DOI] [PubMed] [Google Scholar]
- 47.Green RH, Brightling CE, Woltmann G, D Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002; 57:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson JL, Scott R, Boyle WJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 2006; 11:54–61. [DOI] [PubMed] [Google Scholar]
- 49.Schleich F, Brusselle G, Louis R, Vandenplas O, Michils A, Pilette C, et al. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Resp Med 2014; 108:1723–1732. [DOI] [PubMed] [Google Scholar]
- 50.Chung KF. Asthma phenotyping: a necessity for improved therapeutic precision and new targeted therapies. J Intern Med 2016; 279:192–204 [DOI] [PubMed] [Google Scholar]
- 51.Arron JR, Choy DF, Laviolette M, Kelsen SG, Hatab A, Leigh R, et al. Disconnect between sputum neutrophils and other measures of airway inflammation in asthma. Eur Respir J 2014; 43:627–629. [DOI] [PubMed] [Google Scholar]
- 52.Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Kita H, et al. Integrating longitudinal information on pulmonary function and inflammation using asthma phenotypes. J Allergy Clin Immunol 2014; 133:1474–1477. [DOI] [PubMed] [Google Scholar]
- 53.Nair P, Surette MG, Virchow JC. Neutrophilic asthma: misconception or misnomer? Lancet Respir Med 2021; 9:441–443. [DOI] [PubMed] [Google Scholar]
- 54.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol 2009; 123:986–994; quiz 995–996. [DOI] [PubMed] [Google Scholar]
- 55.Newcomb DC, Peebles RS Jr. Th17-mediated inflammation in asthma. Curr Opin Immunol 2013; 25:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ray A, Kolls JK. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol 2017; 38:942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanović R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med 2000; 161:9–16. [DOI] [PubMed] [Google Scholar]
- 58.Fukakusa M, Bergeron C, Tulic MK, Fiset PO, Al Dewachi O, Laviolette M, et al. Oral corticosteroids decrease eosinophil and CC chemokine expression but increase neutrophil, IL-8, and IFN-gamma-inducible protein 10 expression in asthmatic airway mucosa. J Allergy Clin Immunol 2005; 115:280–286. [DOI] [PubMed] [Google Scholar]
- 59.Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J 2004; 24:822–833. [DOI] [PubMed] [Google Scholar]
- 60.Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med 2007; 175:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaudhuri R, Livingston E, McMahon AD, Lafferty J, Fraser I, Spears M, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med 2006; 174:127–133. [DOI] [PubMed] [Google Scholar]
- 62.Westergaard CG, Porsbjerg C, Backer V. The effect of smoking cessation on airway inflammation in young asthma patients. Clin Exp Allergy 2014; 44:353–361. [DOI] [PubMed] [Google Scholar]
- 63.Perret JL, Bonevski B, McDonald CF, Abramson MJ. Smoking cessation strategies for patients with asthma: improving patient outcomes. J Asthma Allergy 2016; 9:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One 2014; 9:e100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor SL, Leong LEX, Choo JM, Wesselingh S, Yang IA, Upham JW, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol 2018: 141:94–103.e15. [DOI] [PubMed] [Google Scholar]
- 66.Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immunomodulatory effects of macrolide antibiotics-part 2: advantages and disadvantages of long-term, low-dose macrolide therapy. Respiration 2011; 81:75–87. [DOI] [PubMed] [Google Scholar]
- 67.Simpson JL, Powell H, Boyle M, Scott R, Gibson P. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med 2008; 177:148–155. [DOI] [PubMed] [Google Scholar]
- 68.Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomized double-blind placebo-controlled trial. Thorax 2013; 68:322–329. [DOI] [PubMed] [Google Scholar]
- 69.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomized, double-blind, placebo-controlled trial. Lancet 2017; 390:659–668. [DOI] [PubMed] [Google Scholar]
- 70.Al-Hajjaj MS, Al Moamary MS. Role of long-term azithromycin therapy for severe bronchial asthma. Ann Thorac Med 2020; 15:47–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shukla SD, Taylor SL, Gibson PG, Barker D, Upham JW, Yang IA, et al. Add-on azithromycin reduces sputum cytokines in non-eosinophilic asthma: an AMAZES substudy. Thorax 2021; 76:733–736. [DOI] [PubMed] [Google Scholar]
- 72.Soehnlein O, Weber C, Lindbom L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol 2009; 30:538–546. [DOI] [PubMed] [Google Scholar]
- 73.Anticevich SZ, Hughes JM, Black JL, Armour CL. Induction of hyperresponsiveness in human airway tissue by neutrophils – mechanism of action. Clin Exp Allergy 1996; 26:549–556. [PubMed] [Google Scholar]
- 74.Vargas A, Roux-Dalvai F, Droit A, Lavoie JP. Neutrophil-Derived Exosomes: A New Mechanism Contributing to Airway Smooth Muscle Remodeling. Am J Respir Cell Mol Biol 2016; 55:450–461. [DOI] [PubMed] [Google Scholar]
- 75.Chu HW, Trudeau JB, Balzar S, Wenzel SE. Peripheral blood and airway tissue expression of transforming growth factor beta by neutrophils in asthmatic subjects and normal control subjects. J Allergy Clin Immunol 2000; 106:1115–1123 [DOI] [PubMed] [Google Scholar]
- 76.Cundall M, Sun Y, Miranda C, Trudeau JB, Barnes S, Wenzel SE. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J Allergy Clin Immunol 2003; 112:1064–1071. [DOI] [PubMed] [Google Scholar]
- 77.Stănescu D, Sanna A, Veriter C, Kostianev S, Calcagni PG, Fabbri LM, et al. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax 1996; 51:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vignola AM, Bonanno A, Mirabella A, Riccobono L, Mirabella F, Profita M, et al. Increased levels of elastase and alpha1-antitrypsin in sputum of asthmatic patients Am J Respir Crit Care Med, 1998; 157:505–511. [DOI] [PubMed] [Google Scholar]
- 79.Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Systemic upregulation of neutrophil α-defensins and serine proteases in neutrophilic asthma. Thorax 2011; 66:942–947. [DOI] [PubMed] [Google Scholar]
- 80.Pothoven KL, Norton JE, Suh LA, Carter RG, Harris KE, Biyasheva A, et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J Allergy Clin Immunol 2017; 139:1966–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nabe T, Hosokawa F, Matsuya K, Morishita T, Ikedo A, Fujii M, et al. Important role of neutrophils in the late asthmatic response in mice. Life Sci 2011; 88:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Byrne PM, Walters EH, Gold BD, Aizawa HA, Fabbri LM, Alpert SE, et al. Neutrophil depletion inhibits airway hyperresponsiveness induced by ozone exposure. Am Rev Respir Dis 1984; 130:214–219. [DOI] [PubMed] [Google Scholar]
- 83.Mattos MS, Ferrero MR, Kraemer L, Lopes GAO, Reis DC, Cassali GD, et al. CXCR1 and CXCR2 Inhibition by Ladarixin Improves Neutrophil-Dependent Airway Inflammation in Mice. Front Immunol 2020; 11:566953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Byrne PM, Metev H, Puu M, Richter K, Keen C, Uddin M, et al. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomized, double-blind, placebo-controlled trial. Lancet Respir Med 2016; 4:797–806. [DOI] [PubMed] [Google Scholar]
- 85.Crisford H, Sapey E, Rogers GB, Taylor S, Nagakumar P, Lokwani R, et al. Neutrophils in asthma: the good, the bad and the bacteria. Thorax 2021; 76:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mincham KT, Bruno N, Singanayagam A, Snelgrove RJ. Our evolving view of neutrophils in defining the pathology of chronic lung disease. Immunology 2021; 164:701–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Monteseirin J. Neutrophils and asthma. J Investig Allergol Clin Immunol 2009; 19:340–354. [PubMed] [Google Scholar]
- 88.Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev 2016; 273:48–60. [DOI] [PubMed] [Google Scholar]
- 89.Steinman L A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 2007; 13:139–145. [DOI] [PubMed] [Google Scholar]
- 90.Hirahara K, Ghoreschi K, Laurence A, Yang X-P, Kanno Y, O’Shea JJ. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine and Growth Factor Rev 2010; 21:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol 2006; 24:99–146. [DOI] [PubMed] [Google Scholar]
- 92.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 93.Manel N, Unutmaz D, Littman DR. The differentiation of human Th-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORT. Nat Immunol 2008; 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nature Immunol 2007; 8: 345–350. [DOI] [PubMed] [Google Scholar]
- 95.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORct function. Nature 2008; 453: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006; 126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 97.Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol 2009; 183:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoon JH, Sudo K, Kuroda M, Kato M, Lee IK, Han JS, et al. Phosphorylation status determines the opposing functions of Smad2/Smad3 as STAT3 cofactors in TH17 differentiation. Nat Commun 2015; 6:7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Basu R, Whitley SK, Bhaumik S, Zindl CL, Schoeb TR, Benveniste EN, et al. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance. Nat Immunol 2015; 16: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, et al. The role of 2 foxp3 isoforms in the generation of human cd4+ tregs. J Clin Invest 2005; 115:3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mailer RK, Falk K, Rotzschke O. Absence of leucine zipper in the natural foxp3delta2delta7 isoform does not affect dimerization but abrogates suppressive capacity. PloS One 2009; 4:e6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mailer RK, Joly AL, Liu S, Elias S, Tegner J, Andersson J. IL-1β promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci Rep 2015; 5:14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Floss DM, Schröder J, Franke M, Scheller J. Insights into IL-23 biology: from structure to function. Cytokine & Growth Factor Reviews 2015; 26:569–578. [DOI] [PubMed] [Google Scholar]
- 104.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol 2007; 19:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain Th-17 cell-mediated pathology. Nat Immunol 2007; 8:1390–1397. [DOI] [PubMed] [Google Scholar]
- 107.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 2008; 178:1023–1032. [DOI] [PubMed] [Google Scholar]
- 108.Ciprandi G, Cuppari C, Salpietro AM, Tosca MA, Rigoli L, Grasso L, et al. Serum IL-23 strongly and inversely correlates with FEV1 in asthmatic children. Int Arch Allergy Immunol 2012; 159:183–186. [DOI] [PubMed] [Google Scholar]
- 109.Halwani R, Sultana A, Vazquez-Tello A, Jamhawi A, Al-Masri AA, Al-Muhsen S. Th-17 regulatory cytokines IL-21, IL-23, and IL-6 enhance neutrophil production of IL-17 cytokines during asthma. J Asthma, 2017; 54:893–904. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Liang R, Xie A, Shi W, Huang H, Zhong Y. Antagonistic Peptides That Specifically Bind to the First and Second Extracellular Loops of CCR5 and Anti-IL-23p19 Antibody Reduce Airway Inflammation by Suppressing the IL-23/Th17 Signaling Pathway. Mediators Inflamm 2020; 2020:1719467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brightling CE, Nair P, Cousins DJ, Louis R, Singh D. Risankizumab in severe asthma -a phase 2a, placebo-controlled trial. N Engl J Med 2021; 385:1669–1679. [DOI] [PubMed] [Google Scholar]
- 112.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007; 448:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang Y, Al-Alwan L, Risse PA, Roussel L, Rousseau S, Halayko AJ, et al. TH17 cytokines induce human airway smooth muscle cell migration. J Allergy Clin Immunol 2011; 127:1046–1053.e2. [DOI] [PubMed] [Google Scholar]
- 114.Chang Y, Al-Alwan L, Risse PA, Halayko AJ, Martin JG, Baglole CJ, et al. Th17-associated cytokines promote human airway smooth muscle cell proliferation. FASEB J 2012; 26:5152–5160. [DOI] [PubMed] [Google Scholar]
- 115.Lamb D, De Sousa D, Quast K, Fundel-Clemens K, Erjefält JS, Sandén C, et al. RORgammat inhibitors block both IL-17 and IL-22 conferring a potential advantage over anti-IL-17 alone to treat severe asthma. Respir Res 2021; 22:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annual Review of Immunology 2009; 27:485–517. [DOI] [PubMed] [Google Scholar]
- 117.Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem 2007; 282:13447–13455. [DOI] [PubMed] [Google Scholar]
- 118.Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol 2008; 181:2799–2805. [DOI] [PubMed] [Google Scholar]
- 119.Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol 2013; 190:2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 2011; 12:1159–1166. [DOI] [PubMed] [Google Scholar]
- 121.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 Induces IL-4, IL-5, and IL-13 and Th2-Associated Pathologies In Vivo. Immunity 2001; 15:985–995. [DOI] [PubMed] [Google Scholar]
- 122.Huang J, Lee HY, Zhao X, Han J, Su Y, Sun Q, et al. Interleukin-17D Regulates Group 3 Innate Lymphoid Cell Function Through Its Receptor CD93. Immunity 2021: 54:673–686.e4. [DOI] [PubMed] [Google Scholar]
- 123.Hynes GM, Hinks TSC. The role of interleukin-17 in asthma: a protective response? ERJ Open Res 2020; 6:00364–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol 1998; 161:6383–6389. [PubMed] [Google Scholar]
- 125.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 2001; 108:430–438. [DOI] [PubMed] [Google Scholar]
- 126.Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest, 2010; 138: 1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 2003, 111:1293–1298. [DOI] [PubMed] [Google Scholar]
- 128.Zhao Y, Yang J, Gao Y, and Guo W. Th17 immunity in patients with allergic asthma. International Archives of Allergy and Immunology, 2010; 151:297–307. [DOI] [PubMed] [Google Scholar]
- 129.Hamzaoui A, Maalmi H, Berraıes A, Abid H, Ammar H, and Hamzaoui K. Transcriptional characteristics of CD4+ T cells in young asthmatic children: RORC and FOXP3 axis. J Inflamm Res 2011; 4:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Agache I, Ciobanu C, Agache C, and Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respiratory Medicine, 2010; 104:1131–1137. [DOI] [PubMed] [Google Scholar]
- 131.Albano GD, Di Sano C, Bonanno A, Riccobono L, Gagliardo R, Chanez P, et al. Th17 immunity in children with allergic asthma and rhinitis: a pharmacological approach. PLoS ONE, 2013, 8:e58892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, and Tsai YG. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy, 2013; 43:1018–1026. [DOI] [PubMed] [Google Scholar]
- 133.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol 2010; 11:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barczyk A, Pierzchala W, Sozañska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med 2003; 97:726–733. [DOI] [PubMed] [Google Scholar]
- 135.Sun Y-C, Zhou Q-T, Yao W-Z. Sputum interleukin-17 is increased and associated with airway neutrophilia in patients with severe asthma. Chin Med J (Engl) 2005; 118:953–956. [PubMed] [Google Scholar]
- 136.Kawaguchi M, Onuchic LF, Li XD, Essayan DM, Schroeder J, Xiao HQ, et al. Identification of a novel cytokine, ML −1, and its expression in subjects with asthma. J Immunol 2011; 167:4430–4435. [DOI] [PubMed] [Google Scholar]
- 137.Ricciardolo FLM, Sorbello V, Folino A, Gallo F, Massaglia GM, et al. Identification of IL-17F/frequent exacerbator endotype in asthma. J Allergy Clin Immunol 2017; 140:395–406. [DOI] [PubMed] [Google Scholar]
- 138.Sorbello V, Ciprandi G, Di Stefano A, Massaglia GM, FavatàG, Conticello S et al. Nasal IL-17F is related to bronchial IL-17F/neutrophilia and exacerbations in stable atopic severe asthma. Allergy 2015; 70:236–240. [DOI] [PubMed] [Google Scholar]
- 139.Kawaguchi M, Takahashi D, Hizawa N, Suzuki S, Matsukura S, Kokubu F, et al. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J Allergy Clin Immunol 2006;117: 795–801. [DOI] [PubMed] [Google Scholar]
- 140.Hizawa N, Kawaguchi M, Huang SK, Nishimura M. Role of interleukin-17F in chronic inflammatory and allergic lung disease. Clin Exp Allergy 2006; 36:1109–1114. [DOI] [PubMed] [Google Scholar]
- 141.Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor β isoform. Specificity and mechanisms of action. J Biol Chem 1999; 274:27857–27866. [DOI] [PubMed] [Google Scholar]
- 142.Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 2002; 16:61–71. [DOI] [PubMed] [Google Scholar]
- 143.Dennis M, Itkin IH. Effectiveness and complications of Aerosol DEXAMETHASONE phosphate in severe asthma. J Allergy 1964; 35:70–76. [DOI] [PubMed] [Google Scholar]
- 144.Schwiebert LM, Beck LA, Stellato C, Bickel CA, Bochner BS, Schleimer RP. Glucocorticosteroid inhibition of cytokine production: relevance to antiallergic actions. J Allergy Clin Immunol 1996; 97:143–152. [DOI] [PubMed] [Google Scholar]
- 145.Hirst SJ, Lee TH. Airway smooth muscle as a target of glucocorticoid action in the treatment of asthma. Am J Respir Crit Care Med 1998; 158:S201–S206. [DOI] [PubMed] [Google Scholar]
- 146.van Rossum EF, Koper JW, van den Beld AW, Uitterlinden AG, Arp P, Ester W, et al. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf) 2003; 59:585–592. [DOI] [PubMed] [Google Scholar]
- 147.Charmandari E Primary generalized glucocorticoid resistance and hypersensitivity. Horm Res Paediatr 2011; 76:145–155. [DOI] [PubMed] [Google Scholar]
- 148.Ramamoorthy S, Cidlowski JA. Ligand-induced repression of the glucocorticoid receptor gene is mediated by an NCoR1 repression complex formed by long-range chromatin interactions with intragenic glucocorticoid response elements. Mol Cell Biol 2013; 33:1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ito K, Chung KF, Adcock IM. Update on glucocorticoid action and resistance. J Allergy Clin Immunol 2006; 117:522–543. [DOI] [PubMed] [Google Scholar]
- 150.Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol 2007; 21:2311–2319. [DOI] [PubMed] [Google Scholar]
- 151.Vazquez-Tello A, Halwani R, Hamid Q, Al-Muhsen S. Glucocorticoid receptor-beta up-regulation and steroid resistance induction by IL-17 and IL-23 cytokine stimulation in peripheral mononuclear cells. J Clin Immunol 2013; 33:466–478. [DOI] [PubMed] [Google Scholar]
- 152.Chan MT, Leung DY, Szefler SJ, Spahn JD. Difficult-to-control asthma: clinical characteristics of steroid-insensitive asthma. J Allergy Clin Immunol 1998;101:594–601. [DOI] [PubMed] [Google Scholar]
- 153.Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, et al. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. Eur Respir J 1999; 13:1198–1208. [DOI] [PubMed] [Google Scholar]
- 154.Bush A, Zar HJ. WHO universal definition of severe asthma. Curr Opin Allergy Clin Immunol 2011; 11:115–121. [DOI] [PubMed] [Google Scholar]
- 155.Henderson I, Caiazzo E, McSharry C, Guzik TJ, Maffia P. Why do some asthma patients respond poorly to glucocorticoid therapy? Pharmacol Res 2020; 160:105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Irwin RS, Curley FJ, French CL. Difficult-to-control asthma. Contributing factors and outcome of a systematic management protocol. Chest 1993; 103:1662–1669. [DOI] [PubMed] [Google Scholar]
- 157.Heaney LG, Conway E, Kelly C, Johnston BT, English C, Stevenson M, et al. Predictors of therapy resistant asthma: outcome of a systematic evaluation protocol. Thorax 2003; 58:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010: 65:384–390. [DOI] [PubMed] [Google Scholar]
- 159.Louis R, Djukanovic R. Is the neutrophil a worthy target in severe asthma and chronic obstructive pulmonary disease? Clin Exp Allergy 2006; 36:563–567. [DOI] [PubMed] [Google Scholar]
- 160.Kamath AV, Pavord ID, Ruparelia PR, Chilvers ER. Is the neutrophil the key effector cell in severe asthma? Thorax 2005; 60:529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Foley SC, Hamid Q. Images in allergy and immunology: neutrophils in asthma. J Allergy Clin Immunol 2007; 119:1282–1286. [DOI] [PubMed] [Google Scholar]
- 162.Cox G Glucocorticoid treatment inhibits apoptosis in human neutrophils: separation of survival and activation outcomes. J Immunol 1995; 154: 4719–4725. [PubMed] [Google Scholar]
- 163.Saffar AS, Ashdown H, Gounni AS. The molecular mechanisms of glucocorticoids-mediated neutrophil survival. Current Drug Targets, 2011; 12: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zijlstra GJ, Ten Hacken NH, Hoffmann RF, van Oosterhout AJ, Heijink IH. Interleukin-17A induces glucocorticoid insensitivity in human bronchial epithelial cells. Eur Respir J 2012; 39:439–445. [DOI] [PubMed] [Google Scholar]
- 165.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008; 181:4089–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ano S, Morishima Y, Ishii Y, Yoh K, Yageta Y, Ohtsuka S, et al. Transcription factors GATA-3 and RORϒt are important for determining the phenotype of allergic airway inflammation in a murine model of asthma. J Immunol 2013; 190:1056–1065. [DOI] [PubMed] [Google Scholar]
- 167.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol 2013; 6:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ouyang S, Liu C, Xiao J, Chen X, Lui AC, Li X. Targeting IL-17A/glucocorticoid synergy to CSF3 expression in neutrophilic airway diseases. JCI Insight 2020; 5:e132836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 2014; 134:1175–1186.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tsitoura DC, Rothman PB. Enhancement of MEK/ERK signaling promotes glucocorticoid resistance in CD4+ T cells. J Clin Invest 2004; 113:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med 2014; 20:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med 2010; 207:2479–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Malmhall C, Bossios A, Radinger M, Sjostrand M, Lu Y, Lundback B, et al. Immunophenotyping of circulating T helper cells argues for multiple functions and plasticity of T cells in vivo in humans--possible role in asthma. PLoS One 2012; 7:e40012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu W, Liu S, Verma M, Zafar I, Good JT, Rollins D, et al. Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J Allergy Clin Immunol 2017; 139:1548.e4–1558.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chiarella SE, Budinger GRS, Mutlu GM. β2-agonist therapy may contribute to the air pollution and IL-6-associated risk of developing severe asthma with dual-positive TH2/TH17 cells. J Allergy Clin Immunol 2015; 135:290–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Loza MJ, Djukanovic R, Chung KF, Horowitz D, Ma K, Branigan P, et al. Validated and longitudinally stable asthma phenotypes based on cluster analysis of the ADEPT study. Respir Res 2016; 17:165–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Domvri K, Porpodis K, Tzimagiorgis G, Chatzopoulou F, Kontakiotis T, Kyriazis G, et al. Th2/Th17 cytokine profile in phenotyped Greek asthmatics and relationship to biomarkers of inflammation. Respir Med 2019; 151:102–110. [DOI] [PubMed] [Google Scholar]
- 178.Sun W, Yuan Y, Qiu L, Zeng Q, Jia J, Xiang X, et al. Increased Proportion of Dual-Positive Th2-Th17 Cells Promotes a More Severe Subtype of Asthma. Can Respir J 2021; 9999122. doi: 10.1155/2021/9999122. eCollection 2021. [DOI] [PMC free article] [PubMed]
- 179.Na H, Lim H, Choi G, Kim BK, Kim SH, Chang YS, Nurieva R et al. Concomitant suppression of TH2 and TH17 cell responses in allergic asthma by targeting retinoic acid receptor-related orphan receptor γt. J Allergy Clin Immunol 2018; 41:2061–2073.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 2015; 7:301ra129. [DOI] [PubMed] [Google Scholar]
- 181.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 2012; 18:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Manni ML, Mandalapu S, McHugh KJ, Elloso MM, Dudas PL, Alcorn JF. Molecular mechanisms of airway hyperresponsiveness in a murine model of steroid-resistant airway inflammation. J Immunol 2016; 196: 963–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Camargo LDN, Righetti RF, Aristóteles LRCRB, Dos Santos TM, de Souza FCR, Fukuzaki S, et al. Effects of Anti-IL-17 on Inflammation, Remodeling, and Oxidative Stress in an Experimental Model of Asthma Exacerbated by LPS. Front Immunol 2018; 8:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Safety, Tolerability, and Efficacy of AIN457 in Patients With Uncontrolled Asthma. https://www.clinicaltrials.gov/ct2/show/NCT01478360.
- 185.Study to Assess the Efficacy and Safety of CJM112 in Patients With Inadequately Controlled Severe Asthma. https://clinicaltrials.gov/ct2/show/results/NCT03299686?term=NCT03299686&draw=2&rank=1.
- 186.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 2013; 188:1294–1302. [DOI] [PubMed] [Google Scholar]
- 187.Krishnamoorthy N, Douda DN, Bruggemann TR, Ricklefs I, Duvall MG, Abdulnour RE, et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol 2018; 3:eaao4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Manni ML, Trudeau JB, Scheller EV, Mandalapu S, Elloso MM, Kolls JK, et al. The complex relationship between inflammation and lung function in severe asthma. Mucosal Immunol 2014; 7:1186–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010; 40: 1830–1835. [DOI] [PubMed] [Google Scholar]
- 190.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA 2008; 105:18460–18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 2008; 29:628–636. [DOI] [PubMed] [Google Scholar]
- 192.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007; 8:967–974. [DOI] [PubMed] [Google Scholar]
- 193.Chu DK, Al-Garawi A, Llop-Guevara A, Pillai RA, Radford K, Shen P, et al. Therapeutic potential of anti-IL-6 therapies for granulocytic airway inflammation in asthma. Allergy Asthma Clin Immunol 2015; 11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen S, Chen Z, Deng Y, Zha S, Yu L, Li D, et al. Prevention of IL-6 signaling ameliorates toluene diisocyanate-induced steroid-resistant asthma. Allergol Int 2021; S1323–8930(21)00080–0. doi: 10.1016/j.alit.2021.07.004. Online ahead of print. [DOI] [PubMed]
- 195.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med 2016; 4:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Peters MC, Mauger D, Ross KR, Phillips B, Gaston B, Cardet JC, et al. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med 2020, 202:973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ferreira MA, Matheson MC, Duffy DL, Marks GB, Hui J, Le Souef P, et al. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet 2011; 378:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hawkins GA, Robinson MB, Hastie AT, Li X, Li H, Moore WC, et al. The IL6R variation Asp(358)Ala is a potential modifier of lung function in subjects with asthma. J Allergy Clin Immunol 2012;130:510–515 e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jevnikar Z, Ostling J, Ax E, Calven J, Thorn K, Israelsson E, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol 2019; 143:577–590. [DOI] [PubMed] [Google Scholar]
- 200.Robinson MB, Deshpande DA, Chou J, Cui W, Smith S, Langefeld C, IL-6 trans-signaling increases expression of airways disease genes in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2015; 309:L129–L138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Esty B, Harb H, Bartnikas LM, Charbonnier LM, Massoud AH, Leon-Astudillo C, et al. Treatment of severe persistent asthma with IL-6 receptor blockade. J Allergy Clin Immunol Pract 2019; 7:1639–1642.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.PrecISE (Precision Interventions for Severe and/or Exacerbation-Prone Asthma) Network Study. Clinicaltrials.gov [accessed 2021 July 20]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT04129931
- 203.Osei ET, Brandsma CA, Timens W, Heijink IH, Hackett TL. Current perspectives on the role of interleukin-1 signaling in the pathogenesis of asthma and COPD. Eur Respir J 2020; 55:1900563. [DOI] [PubMed] [Google Scholar]
- 204.Pujol JL, Cosso B, Daures JP, Clot J, Michel FB, Godard P. Interleukin-1 release by alveolar macrophages in asthmatic patients and healthy subjects. Int Arch Allergy Appl Immunol 1990; 91:207–210. [DOI] [PubMed] [Google Scholar]
- 205.Borish L, Mascali JJ, Dishuck J, Beam WR, Martin RJ, Rosenwasser LJ. Detection of alveolar macrophage-derived IL-1 beta in asthma. Inhibition with corticosteroids. J Immunol 1992; 149:3078–3082. [PubMed] [Google Scholar]
- 206.Busse PJ, Birmingham JM, Calatroni A,Manzi J, Goryachokovsky A, Fontela G, Federman AD, Wisnivesky JP. et al. Effect of aging on sputum inflammation and asthma control. J Allergy Clin Immunol 2017; 139:1808–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Golebski K, Ros XR, Nagasawa M, van Tol, Heesters BA, Aglmous H, et al. Sticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nat Commun 2019; 10:2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim RY, Pinkerton JW, Essilfie AT, Robertson AAB, Baines KJ, Brown AC, et al. Role for NLRP3 Inflammasome–mediated, IL-1β–Dependent Responses in Severe, Steroid-Resistant Asthma. Am J Respir Crit Care Med 2017; 196:283–297. [DOI] [PubMed] [Google Scholar]
- 209.Evans MD, Esnault S, Denlinger LC, Jarjour NN. Sputum cell IL-1 receptor expression level is a marker of airway neutrophilia and airflow obstruction in asthmatic patients. J Allergy Clin Immunol 2018; 142:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hernandez ML, Mills K, Almond M, Todoric K, Aleman MM, Zhang H, et al. IL-1 receptor antagonist reduces endotoxin-induced airway inflammation in healthy volunteers. J Allergy Clin Immunol 2015; 135:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hakonarson H, Herrick DJ, Serrano PG, Grunstein MM. Autocrine role of interleukin 1beta in altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J Clin Invest 1997; 99:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hakonarson H, Whelan R, Leiter J, Kim C, Chen M, Campbell D, et al. T lymphocyte-mediated changes in airway smooth muscle responsiveness are attributed to induced autocrine release and actions of IL-5 and IL-1beta. J Allergy Clin Immunol 2002; 110:624–633. [DOI] [PubMed] [Google Scholar]
- 213.Wu S, Li H, Yu L, Wang N, Li X, Chen W. IL-1β upregulates Muc5ac expression via NF-κB-induced HIF-1α in asthma. Immunol Lett 2017; 192:20–26. [DOI] [PubMed] [Google Scholar]
- 214.Pascoe S, Kanniess F, Bonner J. A monoclonal antibody to IL-1β attenuates the late asthmatic response to antigen challenge in patients with mild asthma. Oral presentation 752 at: Annual ERS International Congress; September 3–7, 2016; London, UK. [Google Scholar]
- 215.National Institutes of Health Clinical Center. National Institutes of Health; Bethesda, MD: 2018. Late phase administration anakinra as a rescue treatment for inhaled allergen challenge-induced airway inflammation (LateAna). NCT03513458. ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT03513458. [Google Scholar]
- 216.Cook DN, Kang HS, Jetten AM. Retinoid-related orphan receptors (RORs): Regulatory Functions in Immunity, Development, Circadian Rhythm, and Metabolism. Nucl Receptor Res 2015; 2:101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jetten AM, Kang HS, Takeda Y. Retinoic acid-related orphan receptors α and γ: key regulators of lipid/glucose metabolism, inflammation, and insulin sensitivity, Front Endocrinol (Lausanne) 2013; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xiao S, Yosef N, Yang J, Wang Y, Zhou L, Zhu C, et al. Small-molecule RORt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 2014; 40:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Skepner J, Ramesh R, Trocha M, Schmidt D, Baloglu E, Lobera M, et al. Pharmacologic Inhibition of RORt Regulates Th17 Signature Gene Expression and Suppresses Cutaneous Inflammation In Vivo. J Immunol 2014; 192:2564–2575. [DOI] [PubMed] [Google Scholar]
- 220.Skepner J, Trocha M, Ramesh R, Qu XA, Schmidt D, Baloglu E, et al. In vivo regulation of gene expression and T helper type 17 differentiation by RORt inverse agonists. Immunology 2015; 145:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 2009; 31:457–468. [DOI] [PubMed] [Google Scholar]
- 222.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science 2009; 325:1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kusam S, Toney LM, Sato H, Dent AL. Inhibition of Th2 differentiation and GATA-3 expression by BCL-6. J Immunol 2003; 170:2435–2441. [DOI] [PubMed] [Google Scholar]
- 224.Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet 1997; 16:161–170. [DOI] [PubMed] [Google Scholar]
- 225.Whitehead GS, Kang HS, Thomas SY, Medvedev A, Karcz TP, Izumi G, et al. Therapeutic suppression of pulmonary neutrophilia and allergic airway hyperresponsiveness by an RORγt inverse agonist. JCI Insight 2019; 5:e125528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xue X, De Leon-Tabaldo A, Luna-Roman R, Castro G, Albers M, Schoetens F, et al. Preclinical and clinical characterization of the RORgammat inhibitor JNJ-61803534. Sci Rep 2021; 11:11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, et al. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 2015; 349:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Guntermann C, Piaia A, Hamel ML, Theil D, Rubic-Schneider T, Del Rio-Espinola A, et al. Retinoic-acid-orphan-receptor-C inhibition suppresses Th17 cells and induces thymic aberrations. JCI Insight 2017; 2:e91127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Schaafsma D, Gosens R, Zaagsma J, Halayko AJ, Meurs H. Rho kinase inhibitors: a novel therapeutical intervention in asthma? Eur J Pharmacol 2008; 585:398–406. [DOI] [PubMed] [Google Scholar]
- 230.Zhang Y, Saradna A, Ratan R, Ke X, Tu W, Do DC, et al. RhoA/Rho-kinases in asthma: from pathogenesis to therapeutic targets. Clin Transl Immunology 2020; 9:e01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Saoudi A, Kassem S, Dejean A, Gaud G. Rho-GTPases as key regulators of T lymphocyte biology, Small GTPases 2014; 5:e28208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang JQ, Kalim KW, Li Y, Zheng Y, Guo F. Ablation of RhoA impairs Th17 cell differentiation and alleviates house dust mite-triggered allergic airway inflammation. J Leukoc Biol 2019; 106:1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell Mol Life Sci 2010; 67:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, Chen W, Scher JU, Mo R, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci U S A 2014; 111:16814–16819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chiba Y, Ueno A, Shinozaki K, Takeyama H, Nakazawa S, Sakai H, et al. Involvement of RhoA-mediated Ca2+ sensitization in antigen-induced bronchial smooth muscle hyperresponsiveness in mice. Respir Res 2005; 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wei B, Shang YX, Li M, Jiang J, Zhang H. Cytoskeleton changes of airway smooth muscle cells in juvenile rats with airway remodeling in asthma and the RhoA/ROCK signaling pathway mechanism. Genet Mol Res 2014; 13:559–569. [DOI] [PubMed] [Google Scholar]
- 237.Ke X, Do DC, Li C, Zhao Y, Kollarik M, Fu Q, et al. Ras homolog family member A/Rho- associated protein kinase 1 signaling modulates lineage commitment of mesenchymal stem cells in asthmatic patients through lymphoid enhancer-binding factor 1. J Allergy Clin Immunol 2019; 143:1560–1574.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chung Y, Chang SH, Martinez GJ, Yang XO, Kang HS, Ma L, et al. Critical regulation of early Th17 cell differentiation by IL-1 signaling. Immunity 2009; 30:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ghoreschi K, Laurence A, Yang X, Tato CM, Mandy J, Konkel J, et al. Generation of Pathogenic Th17 Cells in the Absence of TGF-β Signaling. Nature 2010; 467: 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 2012; 13:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang C, Yosef N, Gaublomme J, Wu C, Lee Y, Clish CB, et al. CD5L/AIM Regulates Lipid Biosynthesis and Restrains Th17 Cell Pathogenicity. Cell 2015; 163:1413–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]


