Abstract
Context:
Phthalates may disrupt maternal-fetal-placental endocrine pathways, affecting pregnancy outcomes and child development. Placental corticotropin releasing hormone(pCRH) is critical for healthy pregnancy and child development, but understudied as a target of endocrine disruption.
Objective:
To examine phthalate metabolite concentrations (as mixtures and individually) in relation to pCRH.
Design:
Secondary data analysis from a prospective cohort study
Setting:
Prenatal clinics in Tennessee, USA.
Patients:
1018 pregnant women (61.4% non-Hispanic Black, 32% non-Hispanic White, 6.6% other) participated in the CANDLE study and provided data. Inclusion criteria included: low-medical-risk singleton pregnancy, age 16–40, and gestational weeks 16–29.
Intervention:
none.
Main outcome measures:
Plasma pCRH at two visits (mean gestational ages 23.0 and 31.8 weeks) and change in pCRH between visits (ΔpCRH).
Results:
In weighted quantile sums (WQS) regression models, phthalate mixtures were associated with higher pCRH at Visit 1 (β=0.07, 95%CI: 0.02, 0.11) but lower pCRH at Visit 2 (β=−0.08, 95%CI: −0.14, −0.02). In stratified analyses, among women with gestational diabetes(n=59), phthalate mixtures were associated with lower pCRH at Visit 1 (β=−0.17, 95%CI: −0.35, 0.0006) and Visit 2 (β=−0.35, 95%CI: −0.50, −0.19), as well as greater ΔpCRH (β=0.16, 95%CI: 0.07, 0.25). Among women with gestational hypertension (n=102), phthalate mixtures were associated with higher pCRH at Visit 1 (β=0.20, 95%CI: 0.03, 0.36) and Visit 2 (β=0.42; 95%CI: 0.19, 0.64) and lower ΔpCRH (β=−0.17, 95%CI: −0.29, −0.06). Significant interactions between individual phthalate metabolites and pregnancy complications were observed.
Conclusions:
Phthalates may impact placental CRH secretion, with differing effects across pregnancy. Differences in results between women with and without gestational diabetes and gestational hypertension suggest a need for further research examining whether women with pregnancy complications may be more vulnerable to endocrine-disrupting effects of phthalates.
Keywords: phthalates, endocrine disrupting chemicals, pregnancy complications, corticotropin releasing hormone, placenta
Introduction
Phthalates are synthetic chemicals that are commonly used in consumer products, resulting in widespread human exposure through ingestion, inhalation, and dermal routes (1). Many studies including the National Health and Nutrition Examination Survey (NHANES), have demonstrated that nearly 100% of individuals sampled- including pregnant women- have measurable levels of one or more phthalate metabolites in their urine (2–5). Urinary phthalate metabolite measurement is the preferred method of phthalate exposure assessment in humans and concentrations reflect recent exposures, given phthalates’ short-half life in the body (several hours)(6). Extensive evidence in animal models indicates that phthalates are endocrine disruptors and reproductive toxicants and, increasingly, epidemiological studies suggest similar developmental and reproductive impacts in humans (7,8). For example, a number of studies have examined the potential impact of phthalates on pregnancy complications (including gestational diabetes (9–12)) and hypertensive disorders of pregnancy (13–15) as well as preterm birth (16–22) and subsequent offspring developmental outcomes (2,23–25). More subtle, subclinical impacts on pregnancy physiology have also been noted including disruption of placental hormone production and activity (reviewed in 26).
Largely overlooked in the literature on placental impacts of phthalate exposure is placental corticotropin releasing hormone (pCRH), a 41-amino acid neuropeptide which is produced by the placenta and rises exponentially across gestation (reviewed in 27). The identical molecule is produced by the paraventricular nucleus of the hypothalamus in both pregnant and non-pregnant individuals and plays a critical regulatory role in the hypothalamic-pituitary-adrenal (HPA) axis, the body’s major stress pathway (28). During pregnancy, maternal pCRH concentrations are 10,000 times higher than the CRH levels observed in non-pregnant individuals, thus pCRH measured in circulation during pregnancy is assumed to be almost exclusively of placental origin. In animal studies, pCRH regulates pathways involved in myometrial contraction, promoting labor (29,30), and in epidemiological studies higher and/or more steeply increasing pCRH in mid-pregnancy is linked to increased odds of subsequent preterm birth (31–35). pCRH has been additionally linked to maternal hypertensive disorders of pregnancy (36,37), maternal postpartum depression (38,39), and offspring developmental outcomes (40,41) . Consistent with its role in the HPA axis, some studies have found pCRH to be responsive to socioenvironmental factors, such as experiences of trauma. For example, prior work has demonstrated that pCRH production was higher and rose more steeply among women who reported childhood trauma (42,43).
Despite increasing interest in psychosocial predictors of pCRH, until recently, other types of stressors, such as environmental exposures, have been largely ignored. In the Puerto Rican PROTECT cohort, Cathey et al (2019) reported that concentrations of several maternal urinary phthalates (including monocarboxyisononyl phthalate [MCNP], mono-(3-carboxypropyl) phthalate [MCPP], mono-2-ethyl 5-carboxypentyl phthalate [MECPP], mono (2-ethyl-5-hydroxyhexyl) phthalate [MEHHP], and mono-(2-ethyl-5-oxohexyl) phthalate [MEOHP], but not mono(2-ethylhexyl)phthalate [MEHP]) were inversely associated with pCRH concentrations (44). By contrast, in an in vitro model, MEHP treatment increased pCRH protein and mRNA levels and promoted pro-labor gene pathways (45). To our knowledge, these associations have not been examined in other epidemiological studies. Our objective in this study was to build upon this limited literature to examine maternal phthalate concentrations in relation to pCRH in mid and late pregnancy, additional examining potential moderators including pregnancy complications, fetal sex, and maternal history of childhood trauma. Pregnancy complications and history of childhood trauma were selected for consideration due to their associations with CRH in prior work (36,42,43,46), whereas fetal sex was considered in light of the extensive evidence that phthalates (like many endocrine disruptors) can have differential effects on male and female fetuses (and by extension their placentas) (26).
Methods
Study population and overview of study activities.
The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study recruited pregnant women receiving care at selected prenatal clinics in Shelby County, Tennessee from 2006–2011 and methods have been described elsewhere in detail (47). Inclusion criteria for participation included: low-medical-risk singleton pregnancy, 16–40 years of age, weeks 16–29 of pregnancy, and intending to deliver at a participating medical center. Low-medical-risk was defined as lacking major medical conditions including (but not limited to) chronic hypertension requiring therapy, endocrine disease, and insulin-dependent diabetes. Participants completed two prenatal study visits. Visit 1 (V1) occurred at 16–29 weeks gestation and Visit 2 (V2) occurred at 22–39 weeks gestation, roughly corresponding to the 2nd and 3rd trimesters, respectively. This secondary analysis was conducted as part of the Environmental influences on Child Health Outcomes (ECHO) PATHWAYS consortium.
Ethical approval.
Institutional Review Board approval was obtained from the University of Tennessee Health Sciences Center (the primary site of data collection) and other participating institutions. All participants provided written informed consent prior to engaging in study activities.
Urine collection and phthalate metabolite measurement.
Spot urine samples were collected in sterile, phthalate-free polypropylene containers at both prenatal visits. At the time of sample collection, specific gravity (SpG), a measure of urine dilution, was measured using a handheld refractometer. Samples were then frozen at −80°C until shipment (on dry ice) for phthalate metabolite analysis using previously described methods (48,49). Enzymatic deconjugation, solid phase extraction, and high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) were used to assay a panel of 21 phthalate metabolites. Blanks were included for several quality control samples including standard reference materials, procedural blanks, and matrix spikes. The analytical laboratory is part of the CDC Proficiency Testing Program (Biomonitoring Quality Assurance for State Program; BQASP) and German-External Quality Assurance Scheme (G-EQUAS) designed to optimize comparability and reliability of analyses across labs and between batches (50).
The limits of detection (LODs) ranged from 0.012 to 0.304 ng/mL and only metabolites with ≥80% of samples above the LOD were included in models for the analysis, resulting in 15 metabolites: mono-isobutyl phthalate (MiBP), monoethyl phthalate (MEP), mono-methyl phthalate (MMP), mono-n-butyl phthalate (MBP), mono-benzyl phthalate (MBzP), MCNP, mono-carboxy isooctyl phthalate (MCIOP), monocarboxyisononyl phthalate (MCINP), MCPP, MEHP, MEHHP, MEOHP, MECPP and mono(2-carboxymethylhexyl) phthalate (MCMHP), and phthalic acid (PA). Values below the LOD were imputed as LOD/sqrt(2) following convention (51). Given the extensive evidence of di(2-ethylhexyl) phthalate (DEHP)’s endocrine-disrupting properties (reviewed in 52), following convention, we additionally calculated the molar sum of five di(2-ethylhexyl) phthalate (DEHP) metabolites (ΣDEHP: MEHP, MEHHP, MEOHP, MECPP, MCMHP) (2). Phthalate metabolite values were adjusted for urine dilution using the following formula: [phthalate metabolite]_adj= [phthalate metabolite]_raw*[(SpG_median-1)/(SpG-1)] where SpG_median refers to the median SG for the cohort (53). Phthalate metabolite concentrations were log-transformed in all analyses.
Blood collection and pCRH measurement.
At both prenatal study visits maternal blood was collected using EDTA plasma separator tubes, and after processing, plasma was frozen at −80°C. Samples were shipped on dry ice to University of Newcastle, Australia. Using standard protocols, pCRH was measured (in pg/mL) by radioimmunoassay with an extraction recovery of 87% (54). The assay sensitivity was high compared to prior work with inter- and intra-assay coefficients of variation of 8.7% and 7.3%. pCRH values were log-transformed for analyses due to non-normality. In addition to considering V1 and V2 values independently, we calculated change in pCRH (ΔpCRH) as (log)V2 pCRH minus (log)V1 pCRH.
Collection of covariate data.
Pregnant women reported on demographic characteristics, health, and lifestyle behaviors during pregnancy. Variables of interest (selected a priori) included maternal age at enrollment, race and ethnicity (non-Hispanic White/non-Hispanic Black/Hispanic), pre-pregnancy body mass index (BMI; continuous), maternal education (<high school/high school, GED, or technical school/college or greater), marital status (married or partnered/single), parity (parous/nulliparous), fetal sex, and gestational weeks at biospecimen collection. Gestational age was calculated based on last menstrual period and subsequently confirmed with ultrasound dating; in cases of discrepancies, the latter was used for final determination of gestational age. At each study visit, maternal active smoking was defined as cotinine levels at or above 200 ng/mL as measured in single spot urine samples. Based on prior analyses in this cohort, we additionally included childhood trauma (which was previously related to pCRH) in models, but did not include maternal psychosocial measures in pregnancy (which were not associated with pCRH) (43). Maternal history of childhood trauma was assessed using three items from the Traumatic Life Events Questionnaire (TLEQ): (1) physical abuse; (2) witnessing family violence while growing up; and (3) sexual abuse before age 13. A total childhood traumatic exposure types count was created based on these measures (0–3 range) (55,56). Finally, maternal gestational diabetes and gestational hypertension were determined by participant report and confirmed by medical record abstraction. Both were included in the current analysis based on prior work in this cohort suggesting associations with pCRH (43).
Statistical Analysis.
CANDLE participants with phthalate metabolite and pCRH measurements at V1 and/or V2 were eligible for inclusion in the current analysis. Descriptive statistics (geometric mean, standard deviation, median, mix, max, %<LOD, percentages, frequency) were conducted to examine the distribution of phthalate metabolite and pCRH concentrations (at each time point) as well as the covariates of interest. Bivariate analyses were used to examine the relationship between the variables of interest and histograms and scatterplots were created to visualize these associations.
We assessed three outcome measures: log (V1 pCRH), log (V2 pCRH), and ΔpCRH, equivalent to log(pCRH) at V2 minus log(pCRH) at V1. Our exposure was phthalate metabolite concentrations. Models predicting log(V1 pCRH) included V1 phthalate metabolite concentrations, whereas models predicting log(V2 pCRH) included V2 phthalate metabolite concentrations. For models predicting ΔpCRH between the two visits as the outcome, we included V1 values for gestational age, phthalate metabolites, and cotinine as well as the change in gestational age between the two study visits (weeks). In general, for time varying covariates (gestational weeks and cotinine) we included the values measured at the same visit as the outcome measures (e.g., V1 cotinine and V1 gestational age in models predicting V1 pCRH). In all multivariable models, we adjusted for a set of covariates selected a priori based on the prior literature, particularly our recent work on determinants on pCRH in this cohort (43). These included: maternal age, race/ethnicity, marital status, education, parity, fetal sex, maternal urinary cotinine, maternal history of childhood trauma, gestational diabetes, gestational hypertension, and gestational age at sample collection.
Our primary models utilized weighted quantile sum (WQS) regressions to model the joint effect of all specific gravity-adjusted phthalate metabolites on the pCRH outcomes and their relative contributions to that mixture effect (57). In WQS regression, each SG-adjusted phthalate metabolite is divided into quintiles, multiplied with a model-derived simplex vector of weights, and summed to generate a single weighted mixture index called the WQS which is then used as a predictor variable in a linear regression (57). WQS regression is constrained to evaluate mixture effects in either a positive or negative direction, and both directions were evaluated for each outcome. In total, 1000 bootstraps were used to obtain stable mixture weight estimates for each WQS regression (57). When fewer than 100 of the 1000 bootstrapped weights were associated with sum mixture coefficients in a given direction, we made note of this by lightening the plot colors for those estimates, and we recommend interpreting those results with caution. There may be no detectable mixture association in a given direction for some models, and in those cases the models will not return any estimates. The “HC0” Huber-White heteroskedasticity-consistent standard error sandwich estimator was used to calculate confidence intervals (58). WQS regressions are commonly performed using separate training and validation datasets to avoid high Type I error, though our own simulations show this also leads to a substantial loss of power [under review], which we avoided by using the full dataset for both training and validation. However, this can generate anti-conservative confidence intervals and p-values, and so we additionally applied a permutation test (59) with 200 iterations to generate proper “confirmatory” p-values with a nominal Type I error rate that more accurately estimate WQS coefficient uncertainty [(23,59), under review]. As permutation test p-values will always be higher than the original p-values, permutation tests were only applied to models with original p-values < 0.05.
In addition to fitting mixtures models, secondarily, we employed the more conventional approach of considering the log-transformed, specific gravity-adjusted phthalate metabolites (and ΣDEHP) in individual linear regression models (one metabolite per model as well as a model with ΣDEHP as the main exposure measure). The same three outcomes (V1 pCRH, V2 pCRH, and ΔpCRH) were considered and we included the same set of covariates described above. We elected to fit individual linear regression models rather than hierarchical longitudinal models as we were concerned that the associations between phthalates and CRH might differ across the two visits, given the exponential rise in CRH that occurs across pregnancy (42,54). In the individual models, we additional assessed the possibility of non-linearity by creating smooth plots of component plus residuals versus log-transformed, SG-adjusted phthalate metabolite concentrations by Visit.
Based on our observations regarding pCRH in prior work in this cohort (43), we additionally considered effect modification by fetal sex, gestational hypertension, gestational diabetes, and childhood trauma. To evaluate effect modification, we refit the WQS regression models while stratifying for each of the pregnancy complications individually, employing an approach in which random subsets of predictor variables are selected instead of bootstrapping observations for the weight estimation stage of the model (59). This procedure, random subset WQS regression (WQSRS regression), has an advantage over the bootstrap method when categorical covariates have few subjects in one or more strata. In this situation the random subset procedure is unaffected, but bootstrapping can result in some bootstrapped samples with no subjects in the rare stratum, causing an unidentified model. The random subset approach was selected here because there were small numbers of observations in some strata for the ethnicity covariate for observations having either pregnancy complication. As in the mixtures models based on the full cohort, we again did not split the data into training and validation datasets and then implemented the permutation test to maintain power while generating accurate p-values. For models considering interaction by fetal sex or childhood trauma, few significant interaction terms were observed, thus those analyses were not pursued further (Supplemental Table 1).
In our secondary models, we evaluated effect modification in the models with individual phthalate metabolites by including interaction terms (e.g., phthalate*gestational diabetes). We reparameterized the individual phthalate metabolite models to obtain effect estimates and 95% confidence intervals (CIs) for all women with and without each of these pregnancy complications. All analyses were conducted using R statistical software. WQS models were constructed using the gWQS package (version 3.0.4) (60).
Results
Descriptive statistics.
In total, 1483 women were enrolled in the study at V2 and had a live birth. pCRH concentrations were measured in samples from 1303 women and phthalate metabolite concentrations were measured in 1173, resulting in a total of 1018 women with complete data to contribute to the current analyses at V1 and 1014 at V2. On average, women were 26.4±5.5 years old with a pre-pregnancy BMI of 27.9±7.7 kg/m2 (Table 1). Most participants identified as non-Hispanic Black (61.4%), with the remainder identifying as Non-Hispanic White (32.0%) or other races/ethnicities (6.6%). The majority (56.2%) had a high school, GED, or technical school education. Less than 10% of women were smokers (6.4% and 9.9% in V1 and V2, respectively) and 39.9% of women were nulliparous. During study participation, 59 (5.8%) and 102 (10.0%) women developed gestational diabetes and gestational hypertension, respectively.
Table 1.
Characteristics of CANDLE mother-child dyads (n=1018).
| Characteristics (continuous) | Mean±SD | N (%) |
|---|---|---|
| Maternal age (years) | 26.4±5.5 | |
| Pre-pregnancy BMI (kg/m2) | 27.9±7.7 | |
| Gestational age at Visit 1 (V1; weeks) | 23.0±3.0 | |
| Gestational age at Visit 2 (V2; weeks) | 31.8±1.7 | |
| Change in gestational age (V2-V1; weeks) | 8.9±7.3 | |
| Sexual abuse | 184 (18.1) | |
| Characteristics (categorical) | ||
| Hispanic | 67 (6.6) | |
| College or Higher | 339 (33.3) | |
| Nulliparous | 436 (39.9) | |
| Cotinine detected in V1 urine | 65 (6.4) | |
| Cotinine detected in V2 urine | 101 (9.9) | |
| Gestational diabetes | 59 (5.8) | |
| Gestational hypertension | 102 (10.0) | |
| Fetal sex- female | 510 (49.9) |
The phthalate metabolites of interest were detectable in the vast majority of women, with 12 of 15 metabolites detectable in >95% of participants (not shown). At both visits, median MEP concentrations were highest out of all metabolites measured (V1: 114 ng/mL, V2: 103 ng/mL) and median MHPP concentrations were the lowest (V1: 1.02 ng/mL, V2: 0.35 ng/mL) (Table 2). Comparing women with and without gestational diabetes, levels of phthalate metabolites were similar at Visit 1, but tended to be lower (for 13 of 15 metabolites) among women with gestational diabetes at Visit 2. By contrast, women with gestational hypertension tended to have higher concentrations of phthalate metabolites than women without gestational hypertension, particularly at Visit 2 (Table 2). pCRH levels were considerably higher at V2 compared to V1 (median V1 pCRH: 37.6 pg/mL; V2 pCRH: 235.2 pg/mL) (Table 2). pCRH concentrations were higher among women with gestational diabetes and gestational hypertension compared to women without those complications, with the differences increasing dramatically by Visit 2.
Table 2.
Median specific gravity adjusted phthalate metabolite1 and pCRH concentrations by study visit, gestational hypertension (GHTN; n=102), and gestational diabetes (GDM; n=59).
| VISIT 1 (16–29 weeks; n=1018) | VISIT 2 (22–39 weeks; n=1014) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total cohort | No GHTN | GHTN | No GDM | GDM | Total cohort | No GHTN | GHTN | No GDM | GDM | |
| Phthalate metabolites (ng/mL) | ||||||||||
| MBP | 29.80 | 29.66 | 32.13 | 29.87 | 26.22 | 16.06 | 15.67 | 18.29 | 16.04 | 13.55 |
| MBzP | 19.35 | 18.68 | 21.20 | 18.98 | 18.67 | 10.40 | 10.20 | 12.79 | 10.48 | 9.17 |
| MCINP | 3.06 | 3.12 | 2.85 | 3.06 | 3.89 | 0.48 | 0.47 | 0.59 | 0.48 | 0.38 |
| MCIOP | 11.84 | 12.21 | 11.44 | 11.84 | 12.81 | 2.21 | 2.16 | 2.59 | 2.22 | 2.01 |
| MCPP | 1.95 | 1.96 | 1.94 | 1.96 | 1.78 | 1.43 | 1.40 | 1.77 | 1.43 | 1.28 |
| MCMHP | 16.32 | 16.32 | 16.65 | 16.31 | 16.68 | 6.24 | 6.13 | 6.47 | 6.26 | 4.77 |
| MECPP | 17.21 | 16.89 | 19.69 | 17.28 | 17.96 | 11.90 | 11.65 | 13.37 | 11.90 | 9.19 |
| MEHHP | 26.18 | 26.12 | 27.33 | 26.22 | 26.40 | 8.27 | 8.12 | 9.60 | 8.38 | 5.99 |
| MEHP | 7.01 | 6.97 | 7.92 | 7.09 | 5.27 | 2.31 | 2.30 | 2.11 | 2.33 | 1.65 |
| MEOHP | 12.50 | 12.46 | 13.59 | 12.56 | 12.60 | 6.28 | 6.19 | 6.94 | 6.33 | 4.43 |
| MEP | 122.08 | 119.00 | 155.15 | 122.60 | 99.52 | 111.84 | 110.72 | 116.17 | 113.76 | 61.14 |
| MHPP | 1.10 | 1.12 | 1.01 | 1.10 | 1.12 | 0.37 | 0.36 | 0.41 | 0.37 | 0.36 |
| MiBP | 11.96 | 11.83 | 11.70 | 11.96 | 10.57 | 7.74 | 7.59 | 8.73 | 7.76 | 5.89 |
| MMP | 4.95 | 5.05 | 4.92 | 5.14 | 2.38 | 2.39 | 2.36 | 2.82 | 2.40 | 2.11 |
| PA | 59.53 | 58.53 | 72.75 | 59.46 | 65.27 | 70.12 | 68.06 | 81.06 | 70.41 | 58.86 |
| pCRH (pg/mL) | 37.60 | 37.97 | 40.28 | 37.81 | 41.81 | 235.20 | 229.90 | 334.88 | 230.96 | 313.85 |
LODs for phthalate metabolites range from 0.012 to 0.137.
Multivariable models: full cohort.
In covariate-adjusted WQS mixtures models using data from the full cohort, we observed a positive association between the phthalate mixture and pCRH at Visit 1 (β=0.07; 95% CI: 0.02, 0.11; permutation test p (PTp)=0.14). By contrast, at Visit 2, there was an inverse association between the phthalate mixture and pCRH (β=−0.08; 95% CI: −0.14, −0.02; PTp=0.06) with high weights for MEP and MCINP. Neither association was statistically significant after the permutation test. In individual metabolite models, at V1, MBP was associated with significantly higher pCRH (β=0.07; 95% CI: 0.004, 0.13). While no other significant associations were observed at that timepoint, consistent with the results of the mixtures models, estimates were mostly positive (Supplemental Table 2). At V2, we observed a positive association between MMP and pCRH concentrations (β=0.04; 95% CI: 0.01, 0.08) and a trend towards an inverse association between MEP and pCRH (β=−0.03; 95% CI: −0.07, 0.004); no associations with other metabolites were noted, however most estimates were in the negative direction. At both visits the estimate for the phthalate mixture was as strong or stronger than the results of any individual metabolite alone. Analyses examining the change in pCRH from V1 to V2 showed no associations with individual phthalate metabolite concentrations. Assessment of non-linearity (to examine potentially non-monotonic effects of phthalates on pCRH) suggested predominantly linear patterns with some evidence of change in slope at the highest concentrations (≥95th percentile) for the DEHP metabolites at V1 and for MCINP and MIBP at V2 (not shown).
Stratified analyses: gestational diabetes.
Stratified analyses indicated differing patterns of association in relation to gestational diabetes status. In WQS regression models, among women who developed gestational diabetes in pregnancy, a significant inverse mixture association with pCRH was observed at V2 (β=−0.35; 95% CI: −0.50, −0.19; PTp<0.005) with similarly high weights for MBP, MEOHP, MEHHP, and MCIOP (Figure 2). By contrast, among women without gestational diabetes, we observed a positive mixture association at V1 (β=0.07; 95%CI: 0.02, 0.11; PTp=0.05) with high weights for MBP, MCIOP, and MCPP, although this was nonsignificant after the permutation test (Figure 2). When we considered the change in pCRH between the two visits, we observed a strong positive association among women with gestational diabetes (β=0.16; 95% CI: 0.07, 0.25; PTp=0.02) that was driven by the DEHP metabolites MEHHP, MECPP, and MEHP. By contrast, among women without gestational diabetes we observed a weak inverse association (β=−0.02; 95%CI: −0.05, −0.002; PTp=0.46) with MCMHP most heavily weighted (Figure 2).
Figure 2.
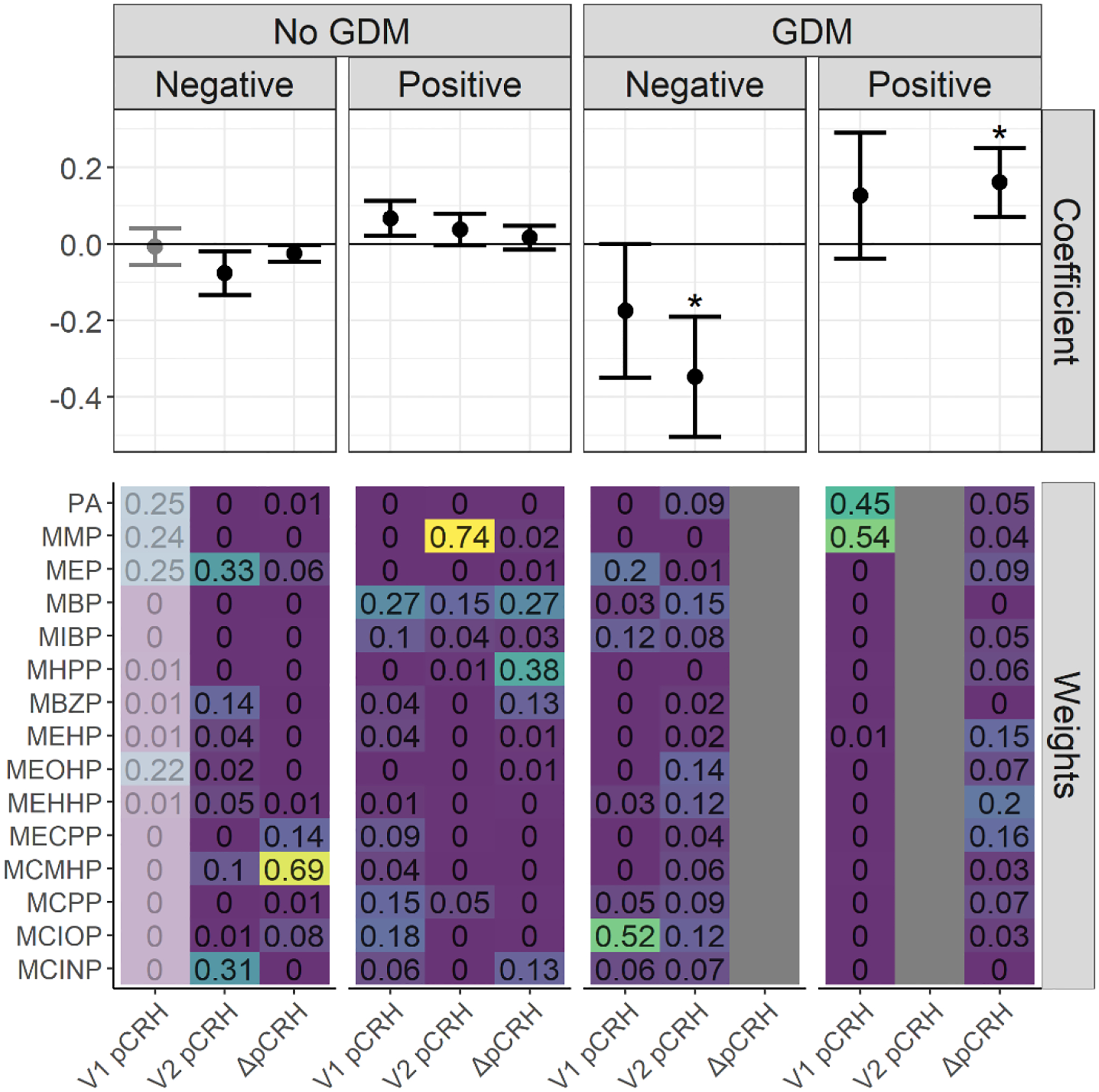
WQS regression coefficients and weights for associations between phthalate mixtures and log(pCRH) in models stratified by gestational diabetes (GDM) status (in pg/mL)1,2. The forest plots show WQS regression means and 95% CIs in the negative and positive directions. Asterisks denote permutation test p-values < 0.05. WQS weights for all phthalate metabolites are indicated on the heat maps3.
1 Models adjusted for gestational age at sample collection, cotinine, maternal age, maternal race and ethnicity, marital status, fetal sex, maternal education, pre-pregnancy BMI, parity, gestational diabetes, gestational hypertension, and maternal childhood traumatic life events. Models examining the outcome ΔpCRH are additionally adjusted for the change in gestational age between the visits.
2 Exposure is Visit 1 phthalate metabolite concentrations for the outcome Visit 1 log(pCRH). Exposure is Visit 2 phthalate metabolite concentrations for the outcomes Visit 2 log(pCRH) and ΔpCRH.
3 For participants with diabetes, the WQS regression for ΔpCRH in the negative direction and for V2 pCRH in the positive direction found no bootstrapped mixture coefficients in the desired direction and therefore returned no estimate for those respective directions and outcomes, and therefore no estimates are presented for those models on the plots. Some WQS regression estimates (e.g., the negative mixture association with V1 pCRH for participants without diabetes) are de-emphasized since these model results were derived from <100 (<10%) of the total bootstrap iterations within the WQS regression. These bootstrap iterations in a given direction are used to derive mixture weights and to compile to WQS mixture index, and so estimates based on only a few iterations in the desired direction may be unstable and should be interpreted with caution.
Results of secondary models examining interactions between individual phthalate metabolite concentrations and gestational diabetes were similar (but not identical) to WQS results (Supplemental Figure 1). In individual metabolite models, we observed no evidence of interactions at V1 (not shown). At V2, interactions with gestational diabetes were observed for a number of phthalate metabolites including MBP, MEHHP, MEOHP, MIBP, and phthalate acid as well as ΣDEHP. Among women with gestational diabetes, all associations between phthalate metabolites and pCRH at V2 were inverse and tended to be stronger than those observed in women without gestational diabetes. Among women with gestational diabetes, significant inverse associations were also noted for other metabolites at V2 including MEHHP, MEOHP, MIBP, and ΣDEHP. Among women without gestational diabetes, the only associations observed were a negative association between MBzP and pCRH (β=−0.04, 95% CI: −0.08, −0.01) and a positive relationship between MMP and pCRH (β=0.05, 95% CI: 0.01, 0.09). When we examined ΔpCRH from V1 to V2, results were similar, with phthalate*gestational diabetes interactions noted for a number of metabolites, including MCPP, MECPP, MEHHP, MEHP, MEOHP, MCMHP, and MMP, as well as ΣDEHP. With the exception of MBP, among women with gestational diabetes, all metabolites were positively associated with ΔpCRH indicating higher phthalate metabolite concentrations were associated with a greater rise in pCRH between the two visits. These associations were significant for ΣDEHP (β=0.139, 95% CI: 0.003, 0.276) as well as the DEHP metabolites, MECHP, MEHP, and MEOHP. Among women without gestational diabetes, the direction of association between phthalate metabolites and ΔpCRH was variable and associations were weaker and non-significant for all metabolites.
Stratified analyses: gestational hypertension.
Stratified analyses also suggested different patterns of association in relation to gestational hypertension. In WQS regression models, among women who developed gestational hypertension in pregnancy, we observed positive associations with pCRH at both V1 (β=0.20; 95% CI: 0.03, 0.36; PTp=0.16) with high weights for MCPP, MCIOP and MBzP; as well as V2 (β=0.42; 95% CI: 0.19, 0.64; PTp=0.005) with high weights for MMP, MCIOP, MIBP, and MECPP (Figure 3). However, we observed an inverse association with ΔpCRH (β=−0.17; 95% CI: −0.29, −0.06; PTp=0.06) with high weights for MBP, MCMHP, and MEP. Only the positive association at V2 remained statistically significant after the permutation test. By contrast, among women without gestational hypertension, associations were weakly positive at V1 (β=0.06; 95% CI: 0.01, 0.11; PTp=0.09), negative at V2 (β=−0.09; 95% CI: −0.14, −0.04; PTp=0.01) (Figure 3). Only the estimate for the negative direction at V2 was statistically significant after the permutation test. MBP was the strongest contributor to the positive associations observed at V1 whereas MCINP, MEP, and MEHHP were most heavily weighted at V2.
Figure 3.
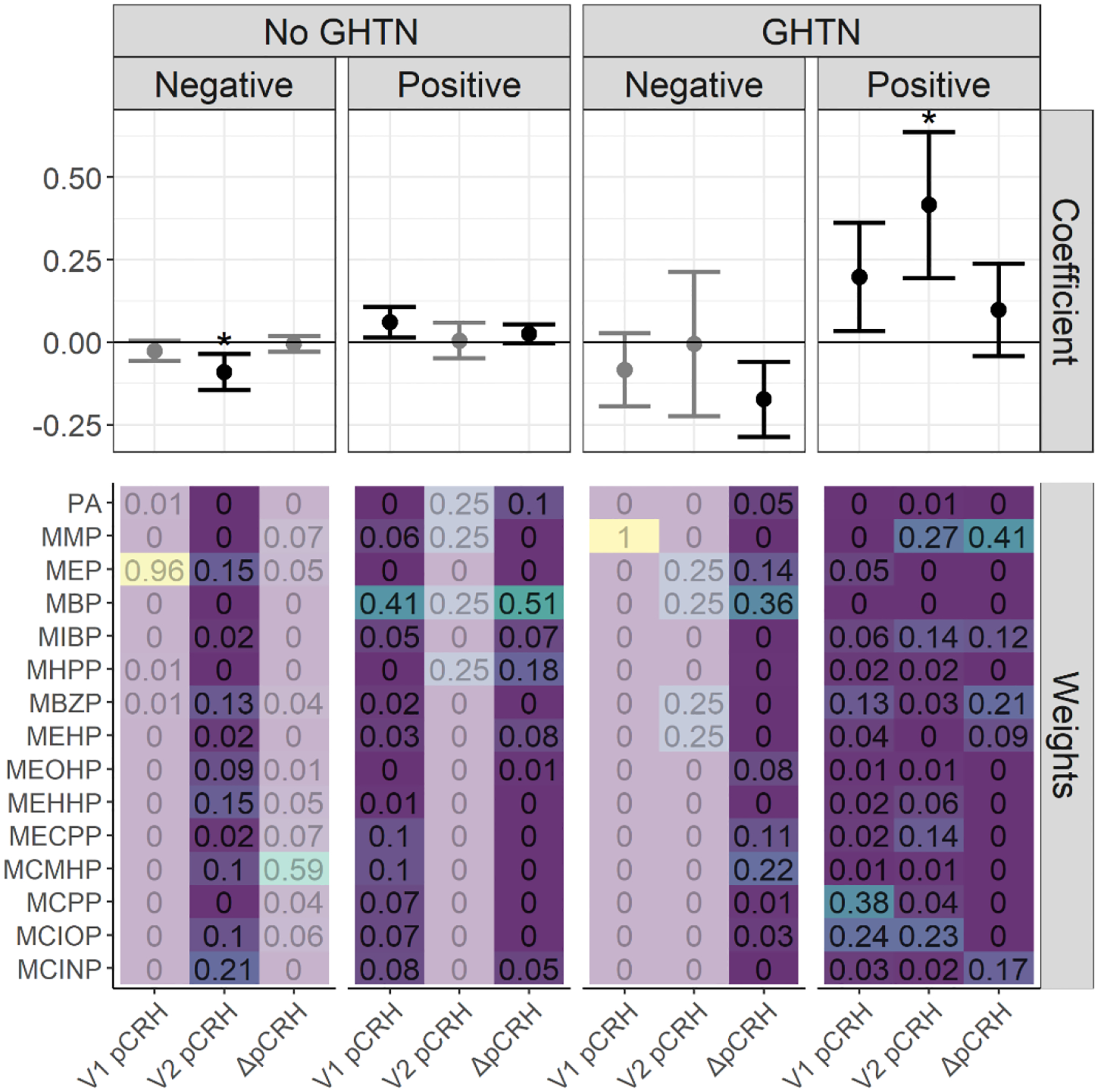
WQS regression coefficients and weights for associations between phthalate mixtures and log(pCRH) in models stratified by gestational hypertension (GHTN) status (in pg/mL)1,2. The forest plots show WQS regression means and 95% CIs in the negative and positive directions. Asterisks denote permutation test p-values < 0.05. WQS weights for all phthalate metabolites are indicated on the heat maps3.
1 Models adjusted for gestational age at sample collection, cotinine, maternal age, maternal race and ethnicity, marital status, fetal sex, maternal education, pre-pregnancy BMI, parity, gestational diabetes, gestational hypertension, and maternal childhood traumatic life events. Models examining the outcome ΔpCRH are additionally adjusted for the change in gestational age between the visits.
2 Exposure is Visit 1 phthalate metabolite concentrations for the outcome Visit 1 log(pCRH). Exposure is Visit 2 phthalate metabolite concentrations for the outcomes Visit 2 log(pCRH) and ΔpCRH.
3 Some WQS regression estimates (e.g., the negative mixture association with V1 pCRH for participants without GHTN) are de-emphasized since these model results were derived from <100 (<10%) of the total bootstrap iterations within the WQS regression. These bootstrap iterations in a given direction are used to derive mixture weights and to compile to WQS mixture index, and so estimates based on only a few iterations in the desired direction may be unstable and should be interpreted with caution.
In linear regression models examining interactions between individual phthalate metabolites and gestational hypertension, we again observed no associations at V1 (not shown), with associations apparent at V2 (Supplemental Figure 2). Interactions between gestational hypertension and phthalates were observed for most metabolites at V2, particularly MBzP, MCINP, MCIOP, ΣDEHP, MECPP, MEHHP, MEOHP, MIBP, and MMP. Among women with gestational hypertension, all phthalate metabolites were positively associated with pCRH levels at V2, whereas most associations were inverse and non-significant among women without gestational hypertension. Among women with gestational hypertension, significant positive associations were also noted for a number of other metabolites at V2, including MCIOP, MECPP, MEHHP, MIBP, and MMP, and as well as ΣDEHP. Among women without gestational hypertension, V2 pCRH was significantly and inversely associated with several metabolites including MBzP, MEHHP, and MEP. Results from models examining the interaction between ΔpCRH and gestational hypertension showed few significant interactions, with the exception phthalic acid. Among women with gestational hypertension, most (but not all) phthalate metabolites showed negative associations with ΔpCRH, suggesting a lower rise between the two visits, though results were only significant for phthalic acid. Among women without gestational hypertension, the direction of association differed by metabolite and no significant associations were observed.
Discussion
In this analysis of 1018 participants from the CANDLE pregnancy cohort, we evaluated associations between gestational phthalate exposures and pCRH concentrations at two timepoints, mid and late pregnancy. Overall, we observed that phthalate mixtures were associated with higher pCRH in mid-pregnancy, but lower pCRH later in pregnancy. Although few associations were observed when we considered the individual phthalate metabolites in separate models, MBP appeared to drive associations at the first visit, while MEP drove associations in the second visit. When we subsequently considered pregnancy complications in our models, effect modification was evident. Among women with gestational diabetes, phthalate mixtures were associated with lower pCRH concentrations at each timepoint (particularly in late pregnancy), but a steeper rise in pCRH. Among women with gestational hypertension, phthalate mixtures were associated with higher pCRH at both timepoints (again, particularly in late pregnancy), but also a lower rise in pCRH between the visits. Analyses considering individual metabolites further corroborated these results. Among women who did not have pregnancy complications, phthalates were associated with lower pCRH in late pregnancy (V2), though the associations were weaker than those observed among women with complications. In general, associations between phthalates and pCRH among women with pregnancy complications tended to be more pronounced in late pregnancy (V2) than in mid-pregnancy (V1), which may reflect increasing production of pCRH and/or greater severity of pregnancy complications in late pregnancy.
Many epidemiological studies have examined associations between phthalates and preterm birth with most, but not all, reporting that DBP, diisobutyl phthalate (DiBP), butyl benzyl phthalate (BBzP), and DEHP may be associated with adverse outcomes (4,14,16–19,21,22,34). Our results suggest that phthalate exposure may impact pCRH production and that those impacts may vary across pregnancy, but the mixed directionality of these associations does not clearly support the hypothesis that phthalates contribute to preterm birth through the pCRH pathway. Other pathways of interest include oxidative stress, inflammation, and other hormones; research on these mechanisms is underway (reviewed in 61). To our knowledge, no study of phthalates and preterm birth has specifically considered women with pregnancy complications, whose risks and vulnerability to phthalates may differ from the general population.
Previous epidemiological work on phthalates and pCRH is extremely limited. In PROTECT, a study of 676 pregnant women in Puerto Rico, phthalate metabolites and pCRH were measured at two visits at 16–20 and 24–28 weeks gestation (44). In linear mixed models examining phthalate metabolites individually, multiple metabolites (including MCNP, MCCP, MECPP, MEHHP, and MEOHP) were associated with lower pCRH concentrations, with the strongest associations observed for the DEHP metabolites. For example, an interquartile (IQR) increase in MECPP was associated with 18% lower pCRH levels; associations were significant, but slightly weaker, for other DEHP metabolites such as MEHHP and MEOHP. In general, associations were stronger later in gestation. In our study, results from the second visit (at 22–39 weeks) were generally consistent with the PROTECT findings, although MEP appeared to drive our association rather than the DEHP metabolites. However, our results earlier in pregnancy suggesting positive associations diverged from their findings.
Of note, pCRH concentrations differed considerably across the two studies, with median pCRH concentrations of 82.4 and 86.6 pg/mL at the two PROTECT visits as compared to 37.6 and 235.2 pg/mL at the two CANDLE visits. This difference may partially reflect differences in timing of the visits, however the lack of a rise in pCRH between the two visits in PROTECT is surprising given the documented exponential rise in pCRH during mid-late pregnancy (62–64). Notably, PROTECT pCRH values were based on serum samples assayed using enzyme linked immunoassay (ELISA), whereas CANDLE analyses were performed on plasma samples assayed using radioimmunoassay (RIA). Plasma has been proposed as the preferred matrix and RIA, which is more widely used in the pCRH literature, may provide greater sensitivity than ELISA (65). To date, evidence directly comparing the two analytic approaches is limited. However consistent with the differences observed between our results and those of PROTECT, in a recent comparison of assay types, we observed a clear increase in pCRH across gestation when RIA was used, while concentrations were flat over time when ELISA was used (66). Overall, the numerous methodological differences may contribute to differences in results in these two studies. Unfortunately, no information was provided on pregnancy complications (including gestational diabetes and gestational hypertension) among PROTECT participants, precluding that comparison.
To our knowledge, no other epidemiological study has examined this association, though there is at least one complementary in vitro study on this topic (45). Wang et al. administered MEHP at doses from 1 to 150 μM to purified primary cytotrophoblasts from healthy term human placentas and measured pCRH expression. No changes were observed at doses below 100 μM, however at 100 and 150 μM, pCRH was upregulated in a dose-dependent manner. Knockdown experiments additionally identified NF-kB inducing kinase (NIK), a signaling component of the NF-kB pathway, as central to MEHP’s upregulation of pCRH. These results contrast to those reported in the PROTECT study and to some extent, the current results, both of which suggested lower pCRH in relation to DEHP metabolite exposure in healthy pregnant women. However, the doses used by Wang et al were higher than those observed in human studies (including CANDLE) and reflected acute exposures (24 hours) in term placental tissue, making them less directly applicable to human studies based on chronic exposures starting well before parturition. At present, we know of no in vitro or animal studies examining chronic, low-dose exposure to phthalates (similar to that experienced by humans) in relation to pCRH.
That pCRH may be vulnerable to endocrine disruption is further supported by several recent epidemiological studies looking at other environmental chemical exposures. Additional work in the PROTECT cohort examined 12 phenols and parabens in relation to pCRH concentrations in pregnancy (67). An IQR increase in Bisphenol S (a Bisphenol A substitute increasingly used in plastics manufacture) was associated with an 11.35% decrease in pCRH (95% CI: −18.71, −3.33) with stronger associations in early versus late pregnancy. By contrast, a IQR increase in triclosan, an antibacterial and antifungal chemical widely used in cleansers, was associated with a 9.20% increase in pCRH (95% CI: −0.97, 20.42). Similarly, in a California pregnancy cohort, second trimester pCRH was examined in relation to exposure to per- and poly-fluoroalkyl substances, a class of persistent synthetic chemicals found in consumer products such as cookware, food, drinking water, and clothing (68,69). In that study, an IQR increase in perfluorononanoic acid was associated with higher pCRH (β=5.17, 95% CI: 1.79, 8.55) and weaker associations were also observed for perfluorooctanoic acid (β=3.62, 95% CI: −0.42, 7.66). Interestingly, in stratified analyses, associations were stronger among women who also reported higher levels of non-chemical stressors (e.g., depression, food insecurity, and financial strain) compared to women who did not report such stressors. By contrast, in our study, we did not observe an interaction between phthalate exposures and stressors (in the form of childhood trauma history) on CRH. Notably, the recall of childhood trauma was limited to items on threatening experiences (e.g. sexual and physical violence), but we did not assess deprivation adversity (as the California study did). It is possible that phthalate*stressor interactions would have been observed with the use of a more extensive childhood trauma scale. Another notable difference was that the California study examined current stressors (but not childhood trauma) whereas we did not include current stressors in models given their lack of association with CRH in our prior work (43).
Among CANDLE participants, concentrations of many phthalate metabolites were somewhat higher than those reported in other pregnancy cohorts recruited contemporaneously. For instance, median levels of MBzP were 18.81 and 9.65 ng/mL (at Visits 1 and 2, respectively) in CANDLE, compared to 3.10 ng/mL in the multi-center U.S. pregnancy cohort TIDES (recruitment from 2010–2012) and 7.0 ng/mL in the Boston-based LIFECODES study (among participants recruited 2006–2008) (2,70). Such differences in exposure may be geographic and may also reflect higher phthalate exposures among non-White women, as have been reported elsewhere (9,71). Consistent with those previously reported disparities, in CANDLE, concentrations of some phthalate metabolites (most notably MBP and MEP, found in personal care products) were higher among Black participants compared to White participants (not shown). Some evidence suggests that products marketed towards women of color may contain high concentrations of toxic chemicals including endocrine disruptors, contributing to disproportionate burden of chemical exposures (72). Racial/ethnic, geographic, and socioeconomic differences in diet, particularly consumption of processed and fast foods, may also contribute to disparities in phthalate exposures (73–75).
A novel finding emerging from our analyses was the notable difference in associations between women with and without pregnancy complications in both mixtures and individual metabolite models. A growing number of studies have examined prenatal phthalate exposure as a potential contributor to hypertensive disorders of pregnancy and gestational diabetes. While evidence implicating phthalates in the etiology of gestational diabetes is fairly consistent and compelling (9–12,70), results of studies on associations with gestational hypertensive disorders have been more mixed (13–15,76). Largely ignored in the literature thus far, however, the extent to which women with pregnancies complicated by these disorders may by more vulnerable to further physiologic dysregulation by environmental chemical exposures. Theoretically, pregnancy complications represent physiologic stressors, and the additional burden of chemical exposures may result in greater dysregulation (for instance, of pCRH production). In this study, we were unable to examine the timing of urine collection relative to the diagnosis of pregnancy complications. Given that GDM is typically diagnosed through glucose challenge tests administered between 24–28 weeks gestation, it is probable that the second urine collection (at mean GA 31.8±1.7 weeks) occurred after GDM diagnosis (if any), and notably, among women with GDM, associations were stronger at the second visit relative to the first. Diagnosis of GHTN, which is more temporally variable, could have occurred before or after Visit 2. As was the case with GDM, among women with GHTN, associations between phthalate metabolites and pCRH were again stronger at the second visit, this time in the positive direction. An alternative explanation for these results is that dysregulation of pCRH contributes to pregnancy complications (as some studies suggest) and that environmental exposures may further exacerbate those risks.
In our study, among women with gestational hypertension, phthalate mixtures were associated with higher pCRH levels across pregnancy but a lower pCRH rise between the two visits. By contrast, in women without gestational hypertension, associations were weaker and in late pregnancy, the phthalate mixture was associated with lower pCRH. Women with gestational hypertension also tended to have higher median concentrations of most phthalates and higher pCRH at both timepoints. Indeed, multiple studies have indicated that women with hypertensive disorders of pregnancy may have higher pCRH levels, but lower CRH binding protein levels than compared to women with uncomplicated pregnancies (46,77–81). High pCRH levels have also been associated with increased placental resistance as assessed via uterine artery pulsatility and arterial resistance (82). More recent work has found increased methylation of the gene encoding CRH binding protein in women with early (but not late) onset pre-eclampsia, compared to women with uncomplicated pregnancies (83). Elevated pCRH may contribute to preeclampsia risk, furthermore, through dysregulation of the placental nitric oxide/cGMP pathway (84–86).
We also observed associations in women with gestational diabetes. Phthalate mixtures were associated with lower pCRH levels at both visits (with stronger associations observed at the second visit, which would be after the time of a typical gestational diabetes diagnosis), as well as a larger rise in pCRH between visits. In this sample, women with gestational diabetes tended to have lower median phthalate metabolite concentrations compared to women without gestational diabetes, particularly in late pregnancy. In contrast to the literature on hypertensive disorders and pCRH, little epidemiological work has examined relationships between CRH and gestational diabetes, though in this CANDLE sample, pCRH levels were higher among women with gestational diabetes compared to those without gestational diabetes. Interestingly, in vitro work in trophoblast cells showed that pCRH modulates the expression of glucose transporters, GLUT1 and GLUT3, suggesting a role of pCRH in glucose activity during pregnancy (87). Work in mouse models additionally suggests that blocking CRH receptor signaling in pregnant dams results in impaired glucose tolerance, without changes in insulin sensitivity or β-cell proliferation (88). It has also been proposed that by stimulating cortisol production, pCRH may mobilize glucose in the maternal bloodstream, promoting fetal growth (89). This is supported by epidemiological work indicating positive associations between pCRH and cord blood glucose (90). With limited understanding of the associations between pCRH and pregnancy complications, it is difficult to evaluate how phthalates may fit in. Given the very limited work in this area, there is a need for additional work specifically designed to evaluate the impact of environmental exposures in women with complicated pregnancies.
Our study has several notable strengths. The CANDLE cohort is large and this analysis of 1018 women included 625 Black women, a group that is typically under-represented in pregnancy cohort studies, yet who may experience higher phthalate exposures due to lifestyle and consumer goods (91,92). The cohort is also socioeconomically diverse and resides in the Southern U.S., a geographic area that has been underrepresented in environmental epidemiology cohorts. We assessed mixtures of phthalates at two time points through WQS regression, allowing us to simultaneously quantify numerous correlated metabolites. This increasingly popular mixtures approach may better approximate real-life exposure than the traditional approach of considering each metabolite individually and in general, the associations observed were stronger in the mixtures models compared to the individual phthalate models. In addition, we present novel results suggesting effect modification in relation to common pregnancy complications (gestational diabetes and gestational hypertension). While many studies have examined phthalates as potential contributors to these disorders (e.g. 9,10,12,15), we know little about whether these complications may also enhance vulnerability to endocrine disruption, as our results may suggest. Finally, very little is known about pCRH as a target of endocrine disruption and this research adds to that small literature. We used highly precise and reliable gold standard assays to measure pCRH in mid- and late-pregnancy and our results suggest a need for further research on this important, understudied hormone.
At the same time, we note several limitations. Phthalates are non-persistent chemicals and their half-lives in the human body are several hours (6). Thus, a single spot urine collection represents only recent exposures and may not accurately capture exposure levels across longer time spans, such as a trimester of pregnancy, resulting in the potential for exposure misclassification and bias towards the null. While some metabolites like MEP and MBP are relatively stable over time, others show greater variability (93). In addition, we unexpectedly observed that median concentrations of all phthalate metabolites decreased from Visit 1 to Visit 2. While it is possible that this difference could reflect pharmacokinetic changes across pregnancy, other recent studies (including several with phthalate analyses conducted at the same lab as in this analysis) have not reported systematic decreases in phthalate metabolites across pregnancy. In the GAPPS cohort, 10 of 16 metabolites measured decreased from the second to the third trimester (94), while in Generation R, only 4 of 11 metabolites decreased (95), and in the PROGRESS study, nearly all phthalate metabolite concentrations non-significantly increased over that period (96). We considered laboratory batch effects as a possible explanation, however extensive quality control and assurance (QA/QC) procedures were conducted at the time of assay (and revisited at the time of this analysis); despite the unexpected difference in median values, no QA/QC concerns were noted, nor were there any systematic differences in the way the samples were collected and processed across visits. An additional complication resulting from the difference in phthalate metabolite concentrations across the two visits is that it confounds our ability to evaluate the possibility that phthalates exert non-monotonic effects on CRH; that is, the impacts of phthalates on CRH may differ at high and low concentrations, a possibility suggested by some prior work on endocrine disruptors (97). That said, our within-visit analyses indicated predominantly linear associations, with the exception of a small set of metabolites for which slopes changed at the highest concentrations (≥95th percentile). In light of the unexpected and unexplained decrease in metabolite concentrations across pregnancy, additional work to replicate these findings will be important.
An additional limitation is that we measured pCRH at only two timepoints, limiting our ability to characterize the rise with more sophisticated modelling approaches. While the CANDLE cohort was representative of Shelby County, TN, our results may not be generalizable to the U.S. population as a whole. In addition, while our findings on phthalates and pregnancy complications are thought-provoking, the study was not specifically designed to examine pregnancy complications, and in fact excluded women with several major medical issues at enrollment, thus the numbers of women who developed gestational diabetes and gestational hypertension were relatively small (59 and 102, respectively). That said, our study provides insight into environmental exposures, pCRH, and pregnancy complications among women who were healthy at baseline. Future work focused on women with these complications is needed to examine the extent to which they may be particularly susceptible to endocrine disruption. Finally, given the large number of metabolites considered in the secondary individual linear regression models, results from those models should be interpreted with caution.
In summary, in this large, diverse U.S. cohort, associations between phthalate concentrations and pCRH varied across gestation and in relation to pregnancy complications. Alterations in pCRH concentrations in pregnancy may have profound implications for the course of pregnancy as well as subsequent child health and development. In addition to risks related to fetal growth restriction and preterm birth (35,98), gestational pCRH exposures may impact maternal postpartum psychiatric health as well as infant and child biology, temperament, behavior (38–40,99,100). With ours being the largest study on this topic to date, more work is needed to examine the extent to which pCRH may be vulnerable to dysregulation by phthalates as well as the many endocrine disrupting chemicals in the modern environment and to explore the possibility that women with pregnancy complications may have heightened vulnerability to endocrine disruptors.
Supplementary Material
Figure 1.
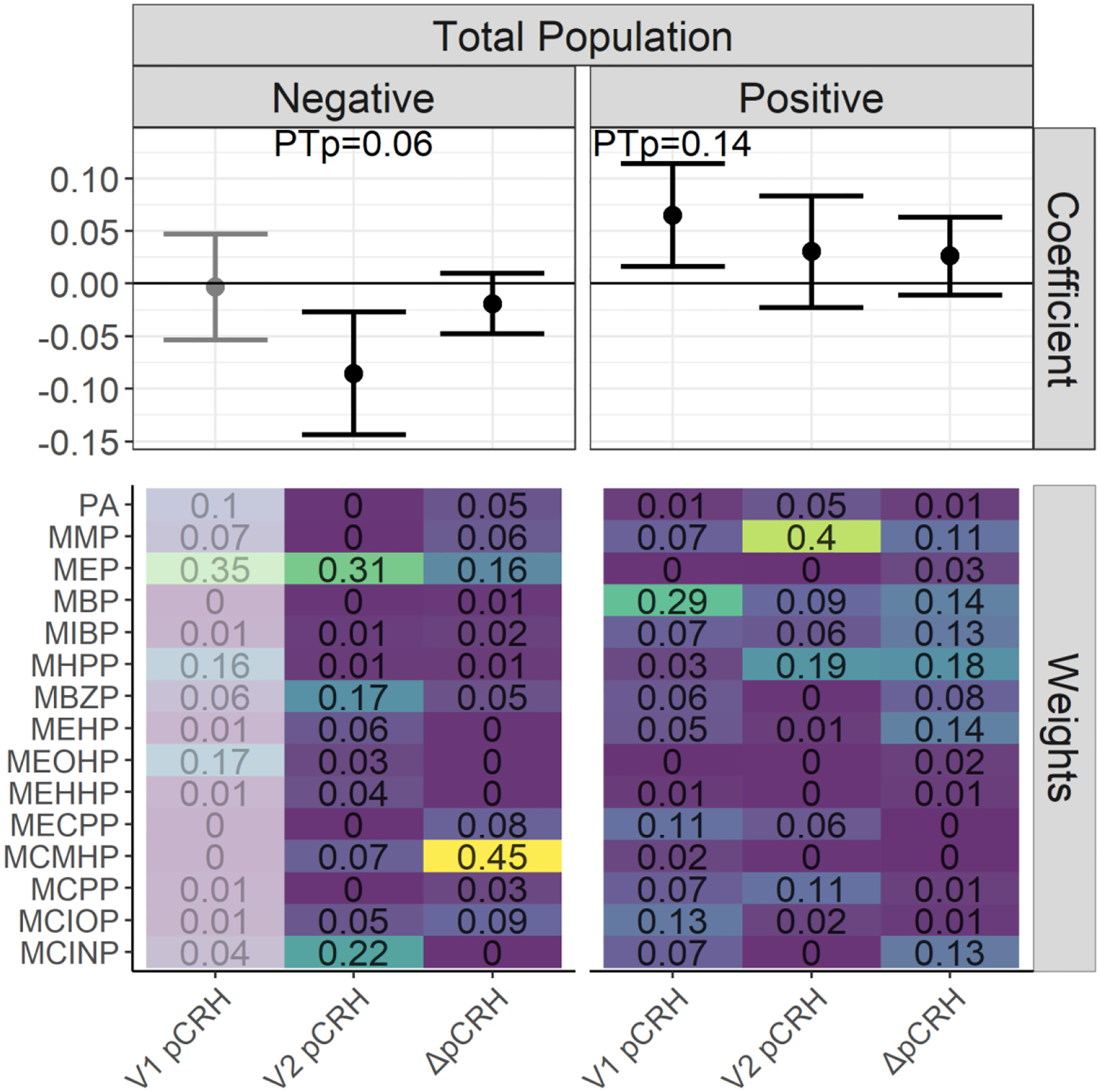
WQS regression coefficients and weights for associations between phthalate mixtures and log(pCRH) in pg/mL1,2. The forest plots show WQS regression means and 95% CIs in the negative and positive directions. Permutation test p-value (PTp) indicates p-values after the application of the permutation test. WQS weights for all phthalate metabolites are indicated on the heat maps3.
1 Models adjusted for gestational age at sample collection, cotinine, maternal age, maternal race and ethnicity, marital status, fetal sex, maternal education, pre-pregnancy BMI, parity, gestational diabetes, gestational hypertension, and maternal childhood traumatic life events. Models examining the outcome ΔpCRH are additionally adjusted for the change in gestational age between the visits.
2 Exposure is Visit 1 phthalate metabolite concentrations for the outcome Visit 1 log(pCRH). Exposure is Visit 2 phthalate metabolite concentrations for the outcomes Visit 2 log(pCRH) and ΔpCRH.
3 The WQS regression coefficient and weight estimates in the negative direction for the V1 pCRH outcome are de-emphasized since these model results were derived from <100 (<10%) of the total bootstrap iterations within the WQS regression. These bootstrap iterations in a given direction are used to derive mixture weights and to compile to WQS mixture index, and so estimates based on only a few iterations in the desired direction may be unstable and should be interpreted with caution
Acknowledgements:
We thank the CANDLE participants as well as the staff of CANDLE and ECHO PATHWAYS.
Study funding/competing interest(s):
The ECHO PATHWAYS Consortium is funded by NIH UG3OD023271 and UH3OD023271. The CANDLE study is funded by the Urban Child Institute as well as CIHR award number MWG-146331. Additional support for this analysis was provided by NIH P30ES005022, P30ES001247, and T32ES007271.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. The authors report no conflicts of interest.
Disclosure Statement: The authors have nothing to disclose.
Data availability.
The data utilized for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement. Contact the corresponding author for more information.
References
- 1.Wang Y, Zhu H, Kannan K. A Review of Biomonitoring of Phthalate Exposures. Toxics. 2019;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, Redmon JB. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 2015;30(4):963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, Vélez Vega C, Cordero JF, Alshawabkeh A, Meeker JD. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ Int. 2019;132:105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, Gaudreau E, Foster WG, Choeurng V, Fraser WD. Phthalate and bisphenol A exposure among pregnant women in Canada--results from the MIREC study. Environ Int. 2014;68:55–65. [DOI] [PubMed] [Google Scholar]
- 6.Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure-- an update and latest results. Int J Androl. 2006;29(1):155–165; discussion 181–155. [DOI] [PubMed] [Google Scholar]
- 7.Dorman DC, Chiu W, Hales BF, Hauser R, Johnson KJ, Mantus E, Martel S, Robinson KA, Rooney AA, Rudel R, Sathyanarayana S, Schantz SL, Waters KM. Systematic reviews and meta-analyses of human and animal evidence of prenatal diethylhexyl phthalate exposure and changes in male anogenital distance. J Toxicol Environ Health B Crit Rev. 2018;21(4):207–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez-Razo LD, Martínez-Ibarra A, Vázquez-Martínez ER, Cerbón M. The impact of Di-(2-ethylhexyl) Phthalate and Mono(2-ethylhexyl) Phthalate in placental development, function, and pathophysiology. Environ Int. 2021;146:106228. [DOI] [PubMed] [Google Scholar]
- 9.James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, McElrath TF, Seely EW. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ Int. 2016;96:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaffer RM, Ferguson KK, Sheppard L, James-Todd T, Butts S, Chandrasekaran S, Swan SH, Barrett ES, Nguyen R, Bush N, McElrath TF, Sathyanarayana S. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ Int. 2019;123:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zukin H, Eskenazi B, Holland N, Harley KG. Prenatal exposure to phthalates and maternal metabolic outcomes in a high-risk pregnant Latina population. Environ Res. 2021;194:110712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher BG, Frederiksen H, Andersson AM, Juul A, Thankamony A, Ong KK, Dunger DB, Hughes IA, Acerini CL. Serum Phthalate and Triclosan Levels Have Opposing Associations With Risk Factors for Gestational Diabetes Mellitus. Front Endocrinol (Lausanne). 2018;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The HOME Study. Environ Health. 2015;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environ Health Perspect. 2016;124(10):1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philips EM, Trasande L, Kahn LG, Gaillard R, Steegers EAP, Jaddoe VWV. Early pregnancy bisphenol and phthalate metabolite levels, maternal hemodynamics and gestational hypertensive disorders. Hum Reprod. 2019;34(2):365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014;70:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson KK, Rosen EM, Barrett ES, Nguyen RHN, Bush N, McElrath TF, Swan SH, Sathyanarayana S. Joint impact of phthalate exposure and stressful life events in pregnancy on preterm birth. Environ Int. 2019;133(Pt B):105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao H, Wang YF, Huang K, Han Y, Zhu YD, Zhang QF, Xiang HY, Qi J, Feng LL, Zhu P, Hao JH, Tao XG, Tao FB. Prenatal phthalate exposure in relation to gestational age and preterm birth in a prospective cohort study. Environ Res. 2019;176:108530. [DOI] [PubMed] [Google Scholar]
- 20.Hu JMY, Arbuckle TE, Janssen P, Lanphear BP, Braun JM, Platt RW, Chen A, Fraser WD, McCandless LC. Associations of prenatal urinary phthalate exposure with preterm birth: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Can J Public Health. 2020;111(3):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Téllez-Rojo MM. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 2009;117(10):1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong Q, Liu HL, Fu H, Niu QS, Wu HB, Huang F. Prenatal exposure to phthalates with preterm birth and gestational age: A systematic review and meta-analysis. Chemosphere. 2021;282:130991. [DOI] [PubMed] [Google Scholar]
- 23.Day DB, Collett BR, Barrett ES, Bush NR, Swan SH, Nguyen RHN, Szpiro AA, Sathyanarayana S. Phthalate mixtures in pregnancy, autistic traits, and adverse childhood behavioral outcomes. Environ Int. 2021;147:106330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harley KG, Berger KP, Kogut K, Parra K, Lustig RH, Greenspan LC, Calafat AM, Ye X, Eskenazi B. Association of phthalates, parabens and phenols found in personal care products with pubertal timing in girls and boys. Hum Reprod. 2019;34(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoaff J, Papandonatos GD, Calafat AM, Ye X, Chen A, Lanphear BP, Yolton K, Braun JM. Early-Life Phthalate Exposure and Adiposity at 8 Years of Age. Environ Health Perspect. 2017;125(9):097008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warner GR, Dettogni RS, Bagchi IC, Flaws JA, Graceli JB. Placental outcomes of phthalate exposure. Reprod Toxicol. 2021;103:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson M The physiological roles of placental corticotropin releasing hormone in pregnancy and childbirth. J Physiol Biochem. 2013;69(3):559–573. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AL, Fishman LM. Corticotropin-releasing hormone. N Engl J Med. 1988;319(4):213–222. [DOI] [PubMed] [Google Scholar]
- 29.Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept. 2002;108(2–3):159–164. [DOI] [PubMed] [Google Scholar]
- 30.Tyson EK, Smith R, Read M. Evidence that corticotropin-releasing hormone modulates myometrial contractility during human pregnancy. Endocrinology. 2009;150(12):5617–5625. [DOI] [PubMed] [Google Scholar]
- 31.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180(1 Pt 3):S257–263. [DOI] [PubMed] [Google Scholar]
- 32.Makrigiannakis A, Semmler M, Briese V, Eckerle H, Minas V, Mylonas I, Friese K, Jeschke U. Maternal serum corticotropin-releasing hormone and ACTH levels as predictive markers of premature labor. Int J Gynaecol Obstet. 2007;97(2):115–119. [DOI] [PubMed] [Google Scholar]
- 33.Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–1463. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz RJ, Gennaro S, O’Connor C, Dwivedi A, Gibeau A, Keshinover T, Welsh T. CRH as a Predictor of Preterm Birth in Minority Women. Biol Res Nurs. 2016;18(3):316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191(4):1063–1069. [DOI] [PubMed] [Google Scholar]
- 36.Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM. Stress questionnaires and stress biomarkers during pregnancy. J Womens Health (Larchmt). 2009;18(9):1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laatikainen TJ. Corticotropin-releasing hormone and opioid peptides in reproduction and stress. Ann Med. 1991;23(5):489–496. [DOI] [PubMed] [Google Scholar]
- 38.Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66(2):162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glynn LM, Sandman CA. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med. 2014;76(5):355–362. [DOI] [PubMed] [Google Scholar]
- 40.Howland MA, Sandman CA, Glynn LM, Crippen C, Davis EP. Fetal exposure to placental corticotropin-releasing hormone is associated with child self-reported internalizing symptoms. Psychoneuroendocrinology. 2016;67:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandman CA. Prenatal CRH: An integrating signal of fetal distress. Dev Psychopathol. 2018;30(3):941–952. [DOI] [PubMed] [Google Scholar]
- 42.Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, Wadhwa PD. Maternal Exposure to Childhood Trauma Is Associated During Pregnancy With Placental-Fetal Stress Physiology. Biol Psychiatry. 2016;79(10):831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steine IM, LeWinn KZ, Lisha N, Tylavsky F, Smith R, Bowman M, Sathyanarayana S, Karr CJ, Smith AK, Kobor M, Bush NR. Maternal exposure to childhood traumatic events, but not multi-domain psychosocial stressors, predict placental corticotrophin releasing hormone across pregnancy. Soc Sci Med. 2020;266:113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cathey AL, Watkins D, Rosario ZY, Vélez C, Alshawabkeh AN, Cordero JF, Meeker JD. Associations of Phthalates and Phthalate Replacements With CRH and Other Hormones Among Pregnant Women in Puerto Rico. J Endocr Soc. 2019;3(6):1127–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang XK, Agarwal M, Parobchak N, Rosen A, Vetrano AM, Srinivasan A, Wang B, Rosen T. Mono-(2-Ethylhexyl) Phthalate Promotes Pro-Labor Gene Expression in the Human Placenta. PLoS One. 2016;11(1):e0147013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laatikainen T, Virtanen T, Kaaja R, Salminen-Lappalainen K. Corticotropin-releasing hormone in maternal and cord plasma in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1991;39(1):19–24. [DOI] [PubMed] [Google Scholar]
- 47.Sontag-Padilla L, Burnms R, Shih R, Griffin B, Martin L, Chandra A, Tylavsky F. The Urban Child Institute CANDLE Study: Methodological Overview and Baseline Sample description. RAND Corporation; 2015. [Google Scholar]
- 48.Guo Y, Weck J, Sundaram R, Goldstone AE, Louis GB, Kannan K. Urinary concentrations of phthalates in couples planning pregnancy and its association with 8-hydroxy-2’-deoxyguanosine, a biomarker of oxidative stress: longitudinal investigation of fertility and the environment study. Environ Sci Technol. 2014;48(16):9804–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocha BA, Asimakopoulos AG, Barbosa F Jr., Kannan K. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci Total Environ. 2017;586:152–162. [DOI] [PubMed] [Google Scholar]
- 50.Kannan K, Stathis A, Mazzella MJ, Andra SS, Barr DB, Hecht SS, Merrill LS, Galusha AL, Parsons PJ. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the Children’s Health Exposure Analysis Resource laboratory network. Int J Hyg Environ Health. 2021;234:113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hornung R, Reed L. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1989;5(1):46–51. [Google Scholar]
- 52.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36(6):E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54(10):615–627. [DOI] [PubMed] [Google Scholar]
- 54.Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, Bisits AM, McElduff P, Giles WB, Smith DW. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab. 2009;94(6):2066–2074. [DOI] [PubMed] [Google Scholar]
- 55.Kubany ES, Leisen MB, Kaplan AS, Kelly MP. Validation of a brief measure of posttraumatic stress disorder: the Distressing Event Questionnaire (DEQ). Psychol Assess. 2000;12(2):197–209. [DOI] [PubMed] [Google Scholar]
- 56.Slopen N, Roberts AL, LeWinn KZ, Bush NR, Rovnaghi CR, Tylavsky F, Anand KJS. Maternal experiences of trauma and hair cortisol in early childhood in a prospective cohort. Psychoneuroendocrinology. 2018;98:168–176. [DOI] [PubMed] [Google Scholar]
- 57.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat. 2015;20(1):100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White H A heteroskdasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 59.Curtin P, Kellogg J, Cech N, Gennings C. A random subset implementation of weighted quantile sum (WQSRS) regression for analysis of high dimensional mixtures. Communications in Statistics – Simulation and Computation 2019;50:1119–1134. [Google Scholar]
- 60.Renzetti S, Curtin P, Just A, Bello G, Gennings C. gWQS. R package version 3.0.4. 2021.
- 61.Ferguson KK, Chin HB. Environmental chemicals and preterm birth: Biological mechanisms and the state of the science. Curr Epidemiol Rep. 2017;4(1):56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frim DM, Emanuel RL, Robinson BG, Smas CM, Adler GK, Majzoub JA. Characterization and gestational regulation of corticotropin-releasing hormone messenger RNA in human placenta. J Clin Invest. 1988;82(1):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. [DOI] [PubMed] [Google Scholar]
- 64.Campbell EA, Linton EA, Wolfe CD, Scraggs PR, Jones MT, Lowry PJ. Plasma corticotropin-releasing hormone concentrations during pregnancy and parturition. J Clin Endocrinol Metab. 1987;64(5):1054–1059. [DOI] [PubMed] [Google Scholar]
- 65.Latendresse G, Ruiz RJ. Bioassay research methodology: measuring CRH in pregnancy. Biol Res Nurs. 2008;10(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrera CL, Bowman ME, McIntire DD, Nelson DB, Smith R. Revisiting the placental clock: Early corticotrophin-releasing hormone rise in recurrent preterm birth. PLoS One. 2021;16(9):e0257422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Calafat AM, Cordero JF, Meeker JD. A repeated measures study of phenol, paraben and Triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environ Health. 2019;18(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eick SM, Goin DE, Cushing L, DeMicco E, Smith S, Park JS, Padula AM, Woodruff TJ, Morello-Frosch R. Joint effects of prenatal exposure to per- and poly-fluoroalkyl substances and psychosocial stressors on corticotropin-releasing hormone during pregnancy. J Expo Sci Environ Epidemiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. 2019;29(2):131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bellavia A, Hauser R, Seely EW, Meeker JD, Ferguson KK, McElrath TF, James-Todd T. Urinary phthalate metabolite concentrations and maternal weight during early pregnancy. Int J Hyg Environ Health. 2017;220(8):1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bloom MS, Wenzel AG, Brock JW, Kucklick JR, Wineland RJ, Cruze L, Unal ER, Yucel RM, Jiyessova A, Newman RB. Racial disparity in maternal phthalates exposure; Association with racial disparity in fetal growth and birth outcomes. Environ Int. 2019;127:473–486. [DOI] [PubMed] [Google Scholar]
- 72.Zota AR, Shamasunder B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol. 2017;217(4):418.e411–418.e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ Int. 2019;131:105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zota AR, Phillips CA, Mitro SD. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003–2010. Environ Health Perspect. 2016;124(10):1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martínez Steele E, Khandpur N, da Costa Louzada ML, Monteiro CA. Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS One. 2020;15(7):e0236738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warembourg C, Basagaña X, Seminati C, de Bont J, Granum B, Lyon-Caen S, Manzano-Salgado CB, Pin I, Sakhi AK, Siroux V, Slama R, Urquiza J, Vrijheid M, Thomsen C, Casas M. Exposure to phthalate metabolites, phenols and organophosphate pesticide metabolites and blood pressure during pregnancy. Int J Hyg Environ Health. 2019;222(3):446–454. [DOI] [PubMed] [Google Scholar]
- 77.Perkins AV, Eben F, Wolfe CD, Schulte HM, Linton EA. Plasma measurements of corticotrophin-releasing hormone-binding protein in normal and abnormal human pregnancy. J Endocrinol. 1993;138(1):149–157. [DOI] [PubMed] [Google Scholar]
- 78.Perkins AV, Linton EA, Eben F, Simpson J, Wolfe CD, Redman CW. Corticotrophin-releasing hormone and corticotrophin-releasing hormone binding protein in normal and pre-eclamptic human pregnancies. Br J Obstet Gynaecol. 1995;102(2):118–122. [DOI] [PubMed] [Google Scholar]
- 79.Karteris E, Goumenou A, Koumantakis E, Hillhouse EW, Grammatopoulos DK. Reduced expression of corticotropin-releasing hormone receptor type-1 alpha in human preeclamptic and growth-restricted placentas. J Clin Endocrinol Metab. 2003;88(1):363–370. [DOI] [PubMed] [Google Scholar]
- 80.Purwosunu Y, Sekizawa A, Farina A, Wibowo N, Okazaki S, Nakamura M, Samura O, Fujito N, Okai T. Cell-free mRNA concentrations of CRH, PLAC1, and selectin-P are increased in the plasma of pregnant women with preeclampsia. Prenat Diagn. 2007;27(8):772–777. [DOI] [PubMed] [Google Scholar]
- 81.Liapi CA, Tsakalia DE, Panitsa-Faflia CC, Antsaklis AI, Aravantinos DI, Batrinos ML. Corticotropin-releasing-hormone levels in pregnancy-induced hypertension. Eur J Obstet Gynecol Reprod Biol. 1996;68(1–2):109–114. [DOI] [PubMed] [Google Scholar]
- 82.Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM, Light KC. Stress and placental resistance measured by Doppler ultrasound in early and mid-pregnancy. Ultrasound Obstet Gynecol. 2008;32(1):23–30. [DOI] [PubMed] [Google Scholar]
- 83.Hogg K, Blair JD, McFadden DE, von Dadelszen P, Robinson WP. Early onset pre-eclampsia is associated with altered DNA methylation of cortisol-signalling and steroidogenic genes in the placenta. PLoS One. 2013;8(5):e62969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clifton VL, Read MA, Leitch IM, Giles WB, Boura AL, Robinson PJ, Smith R. Corticotropin-releasing hormone-induced vasodilatation in the human fetal-placental circulation: involvement of the nitric oxide-cyclic guanosine 3’,5’-monophosphate-mediated pathway. J Clin Endocrinol Metab. 1995;80(10):2888–2893. [DOI] [PubMed] [Google Scholar]
- 85.Karteris E, Vatish M, Hillhouse EW, Grammatopoulos DK. Preeclampsia is associated with impaired regulation of the placental nitric oxide-cyclic guanosine monophosphate pathway by corticotropin-releasing hormone (CRH) and CRH-related peptides. J Clin Endocrinol Metab. 2005;90(6):3680–3687. [DOI] [PubMed] [Google Scholar]
- 86.Makrigiannakis A, Vrekoussis T, Zoumakis E, Navrozoglou I, Kalantaridou SN. CRH Receptors in Human Reproduction. Curr Mol Pharmacol. 2018;11(1):81–87. [DOI] [PubMed] [Google Scholar]
- 87.Gao L, Lv C, Xu C, Li Y, Cui X, Gu H, Ni X. Differential regulation of glucose transporters mediated by CRH receptor type 1 and type 2 in human placental trophoblasts. Endocrinology. 2012;153(3):1464–1471. [DOI] [PubMed] [Google Scholar]
- 88.Simpson SJS, Smith LIF, Jones PM, Bowe JE. UCN2: a new candidate influencing pancreatic β-cell adaptations in pregnancy. J Endocrinol. 2020;245(2):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gangestad SW, Caldwell Hooper AE, Eaton MA. On the function of placental corticotropin-releasing hormone: a role in maternal-fetal conflicts over blood glucose concentrations. Biol Rev Camb Philos Soc. 2012;87(4):856–873. [DOI] [PubMed] [Google Scholar]
- 90.Valsamakis G, Papatheodorou D, Chalarakis N, Manolikaki M, Margeli A, Papassotiriou I, Barber TM, Kumar S, Kalantaridou S, Mastorakos G. Maternal chronic stress correlates with serum levels of cortisol, glucose and C-peptide in the fetus, and maternal non chronic stress with fetal growth. Psychoneuroendocrinology. 2020;114:104591. [DOI] [PubMed] [Google Scholar]
- 91.James-Todd TM, Chiu YH, Zota AR. Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep. 2016;3(2):161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chan M, Mita C, Bellavia A, Parker M, James-Todd T. Racial/Ethnic Disparities in Pregnancy and Prenatal Exposure to Endocrine-Disrupting Chemicals Commonly Used in Personal Care Products. Curr Environ Health Rep. 2021;8(2):98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120(5):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paquette AG, MacDonald J, Lapehn S, Bammler T, Kruger L, Day DB, Price ND, Loftus C, Kannan K, Marsit C, Mason WA, Bush NR, LeWinn KZ, Enquobahrie DA, Prasad B, Karr CJ, Sathyanarayana S. A Comprehensive Assessment of Associations between Prenatal Phthalate Exposure and the Placental Transcriptomic Landscape. Environ Health Perspect. 2021;129(9):97003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos S, Sol CM, van Zwol-Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral MP, Kannan K, Jaddoe VWV, Trasande L. Maternal phthalate urine concentrations, fetal growth and adverse birth outcomes. A population-based prospective cohort study. Environ Int. 2021;151:106443. [DOI] [PubMed] [Google Scholar]
- 96.Wu H, Kupsco AJ, Deierlein AL, Just AC, Calafat AM, Oken E, Braun JM, Mercado-Garcia A, Cantoral A, Téllez-Rojo MM, Wright RO, Baccarelli AA. Trends and Patterns of Phthalates and Phthalate Alternatives Exposure in Pregnant Women from Mexico City during 2007–2010. Environ Sci Technol. 2020;54(3):1740–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zoeller RT, Vandenberg LN. Assessing dose-response relationships for endocrine disrupting chemicals (EDCs): a focus on non-monotonicity. Environ Health. 2015;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee C Intergenerational health consequences of in utero exposure to maternal stress: evidence from the 1980 Kwangju uprising. Soc Sci Med. 2014;119:284–291. [DOI] [PubMed] [Google Scholar]
- 99.Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005;27(5):299–305. [DOI] [PubMed] [Google Scholar]
- 100.Sandman CA, Curran MM, Davis EP, Glynn LM, Head K, Baram TZ. Cortical Thinning and Neuropsychiatric Outcomes in Children Exposed to Prenatal Adversity: A Role for Placental CRH? Am J Psychiatry. 2018;175(5):471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement. Contact the corresponding author for more information.


