Abstract
Ambient particulate matter pollution has been linked to impaired cognitive performance, but the effect of ambient ozone exposure on cognitive function remains largely unknown. We examined the association of long-term ozone exposure with the risk of cognitive impairment among a national representative cohort of 9,544 Chinese older adults (aged 65 years and over) with baseline normal cognition from the Chinese Longitudinal Healthy Longevity Survey (2005-2018). The ozone exposure of each participant was measured by annual mean ozone concentrations for the county of residence. Cognitive function was assessed by the Chinese version of the Mini-Mental State Examination (MMSE). We defined cognitive impairment as an MMSE score below 18 points accompanied by an MMSE score that declined ≥4 points from baseline. Cox proportional hazard models were applied to explore the association of ozone exposure with cognitive impairment. During the mean follow-up time of 6.5 years, 2,601 older adults developed cognitive impairment. Each 10-μg/m3 increase in annual mean ozone exposure was associated with a 10.4% increased risk of cognitive impairment. The exposure-response relationship between ozone exposure and risk of cognitive impairment showed a linear trend. Sensitivity analyses revealed the association to be robust. We found that older adults from Eastern, Central, and Southern China were particularly susceptible. Our results show that ozone is a risk factor for late-life cognitive decline. Reducing ambient ozone pollution may help delay the onset of cognitive impairment among older adults.
Keywords: ozone, long-term exposure, cognitive impairment, older adults, cohort study
Graphical Abstract
1. Introduction
Cognitive impairment is a major and growing global health challenge in the era of population aging. For example, the number of people with dementia, a severe cognitive impairment (Nichols et al., 2019; Prince et al., 2013; Prince et al., 2015), is predicted to triple from 50 million to 152 million by 2050 worldwide, with much of the increase occurring in low- and middle-income countries (Nichols et al., 2019; Patterson 2018; Prince et al., 2013). As one of the major causes of disability and dependency among older adults, cognitive impairment, with its increasing prevalence, will impose a heavy burden on health care systems, especially for fast-aging developing countries such as China (Jia et al., 2020; Xu et al., 2017). China has the largest population with dementia in the world (Jia et al., 2020; Nichols et al., 2019), as well as a much faster pace of aging than many other countries (WHO, 2015). According to the National Bureau of Statistics, the number of older adults aged 65 and above in China reached 176 million in 2019 (NBS, 2020), and is predicted to surge to 240 million in 2030 and 365 million in 2050 (Peng, 2020). Given that most types of cognitive impairment are incurable to date, investigations of potential modifiable risk factors to prevent cognitive decline are urgently needed for an aging society.
Air pollution is a well-established risk factor for cardiovascular and respiratory diseases (Manisalidis et al., 2020). More recently, both epidemiological and toxicological studies have found associations between short-term or long-term exposure to particulate matter with aerodynamic diameter ≤2.5 μm (PM2.5) and cognitive impairment, including cognitive decline or incidence of dementia or Alzheimer’s disease (Gao et al., 2021b; Gene et al., 2012; Shi et al., 2020; Wang et al., 2020; Yu et al., 2020). The 2020 Lancet Commission on dementia prevention, intervention, and care added air pollution as a new modifiable risk factor for dementia, which accounts for around 2% of worldwide cases (Livingston et al., 2020). Although cognitive impairment has been linked to PM2.5 exposure, its relationship to exposure to nitrogen oxides (NOx) or ambient ozone is less clear (Weuve et al., 2021; Yu et al., 2020). The relatively few longitudinal cohort studies that have examined the association between ozone exposure and cognition have provided inconsistent evidence (Carey et al., 2018; Cerza et al., 2019; Chen et al., 2017; Cleary et al., 2018; Jung et al., 2015; Lo et al., 2019). Moreover, most of the previous work on linkages between ozone exposure and cognitive impairment was conducted in developed countries and regions; these findings may not be generalizable to developing countries with different ozone exposure and population characteristics. Older adults may be especially vulnerable to ozone (Bell et al., 2014; Medina-Ramón and Schwartz, 2008), exposure to which is modifiable at the population level, as evidenced by the reductions in the US due to the Clean Air Act (Abrams et al., 2019; Fann and Risley, 2011; Zhang et al., 2018b). Thus, an in-depth understanding of the impacts of ozone exposure on the development of cognitive impairment is of potential significance for primary prevention.
We therefore aimed to examine the association of long-term exposure to ozone with risk of cognitive impairment among older adults in China. This prospective cohort study included 9,544 Chinese older adults aged 65-110 years with normal baseline cognitive function from the Chinese Longitudinal Healthy Longevity Survey (CLHLS). Cox proportional hazards models were utilized to explore the association of ozone exposure with the incidence of cognitive impairment. Our hypothesis is that long-term ozone exposure is associated with increased risk of cognitive impairment. To our knowledge, this is the first large-scale prospective cohort study of long-term ozone exposure and cognitive impairment in China.
2. Materials and methods
2.1. Study population
The data used in this study were derived from the CLHLS (Duke University Center for the Study of Aging and Human Development). The goal of CLHLS was to better understand the determinants of healthy longevity in Chinese elderly. From 1998 to 2018, the CLHLS was conducted in half of the counties and cities (randomly selected) in 23 out of 31 provinces in China. The initial participants were the oldest-old, aged 80 years and older, but from 2002 on, the CLHLS also recruited young elders aged 65-79 years. Study assessments, conducted in the participant’s home, included an interview administered by a well-trained interviewer and a basic health examination performed by a nurse or medical student. Follow-up study assessments were performed approximately every 3 years. Details of the CLHLS have been described extensively elsewhere (Duke University Center for the Study of Aging and Human Development; Zeng, 2012). Informed consent was obtained from all participants and/or their relatives, and the study was approved by the Ethics Committee of Peking University (IRB00001052-13074).
We used longitudinal data from participants with baseline recruitment in 2005 (2005 wave), 2008/2009 (2008 wave), 2011/2012 (2011 wave), or 2014, with follow-up through the 2018 wave (2017–2019). A total of 27,585 older adults were interviewed from March 2005 to November 2014. A flowchart of the study sample inclusion is shown in Fig. 1. We excluded 556 participants younger than 65 years as the eligible respondents in the CLHLS dataset are those people aged 65 and over, 7,625 with cognitive impairment at baseline [Mini-Mental State Examination (MMSE) scores <18; see below], 991 with a history of stroke or cerebrovascular disease at baseline to control for the potential confounding or mediating effect of these diseases, 8,102 with no follow-up assessments, and 767 with unavailable information on ozone exposure. After exclusions, 9,544 participants were included for analysis, with 4,177 young elders aged 65-79 years and 5,367 oldest-old aged 80+ years (2,913 octogenarians, 1,790 nonagenarians, and 664 centenarians), as shown in Fig. S1. A comparison of descriptive characteristics of participants with no follow-up assessments and participants included in the study is presented in Table S1.
Fig. 1: Flowchart of study sample inclusion criteria.
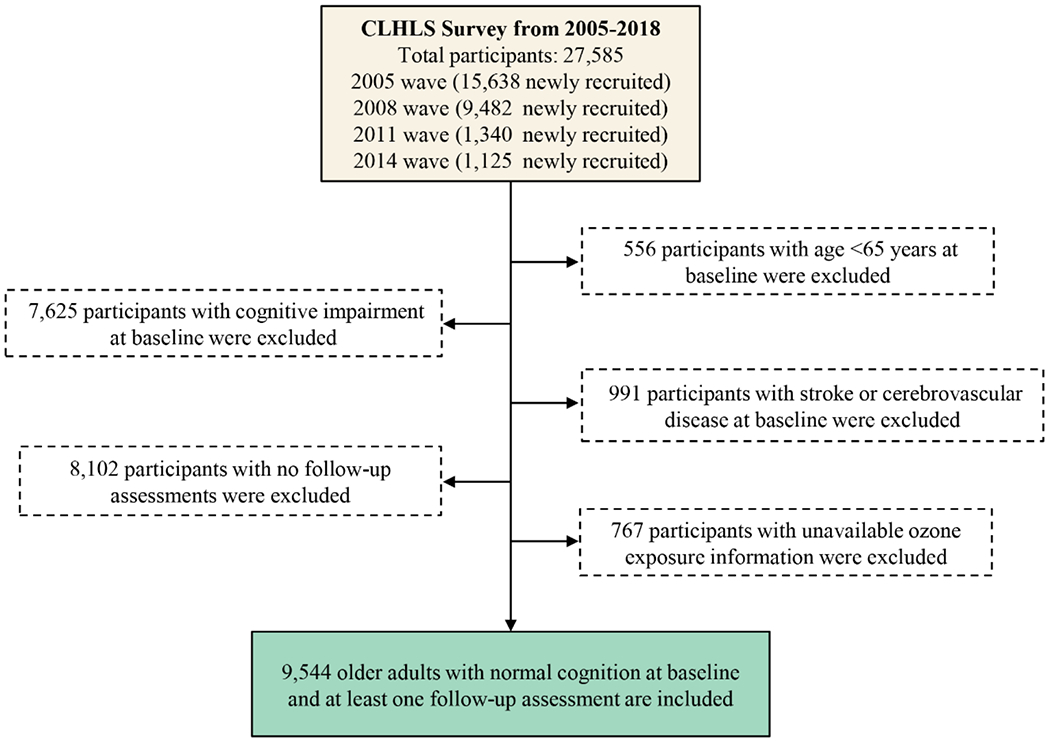
A total of 27,585 participants were recruited from March 2005 to November 2014. Participants were excluded if they were younger than 65 years at baseline (n=556), had cognitive impairment [Mini-mental Status Examination (MMSE) score < 18] at baseline (n=7,625), had a diagnosis of stroke or cerebrovascular disease at baseline (n=991), with no follow-up assessments (n=8,102), or had unavailable ozone exposure information (n=767). The total sample thus consisted of 9,544 individuals. Abbreviation: CLHLS, Chinese Longitudinal Healthy Longevity Survey.
2.2. Assessment of environmental exposure
We estimated ozone exposure based on data from a recently developed nationwide prediction model for 2005 to 2018 with a spatial resolution of 0.1° × 0.1° (approximately 10 km × 10 km) (Liu et al., 2020). These data were derived from a machine learning model based on the eXtreme Gradient Boosting (XGBoost) algorithm by using ground monitoring data (daily maximum 8-hour average concentration) combined with ozone retrievals, aerosol reanalysis, meteorological parameters, and land-use data. The model achieves high prediction accuracy at the daily level (daily maximum 8-hour ozone) between 2013-2017, with R2 values for the by-year, site-based, and sample-based CV schemes of 0.61, 0.64, and 0.78 respectively, and performs well in historical data estimation at the month level (R2 values for external testing: 0.60-0.87) and at the annual level (R2 values for external testing: 0.62-0.94) according to external testing with regional measurements from 2005 to 2012 and nationwide data in 2018 (Liu et al., 2020). Furthermore, the ozone model predictions are comparable to other studies based on machine learning approaches, even across such a broad geographic area (Liu et al., 2020). This data set is one of the first long-term and high-resolution surface ozone data sets across China, and provides basic data for future environmental health studies and policy-making for pollution control and prevention.
To estimate ozone exposure, we first computed county-level annual (according to calendar year) mean ozone concentrations by averaging values of each grid cell that fell within a particular county boundary. The sizes of study counties range from 10 to 14390 km2 [median: 1101 km2; interquartile range (IQR): 1673 km2], and on average, 14 grid cells fall within a county’s boundary. Then, we assigned ozone concentrations to each participant based on the county of residence obtained via the baseline questionnaire. There were changes in county names or codes due to administrative division changes, which could result in missing ozone exposure information. To address this issue, we double-checked the names of the counties/cities and corresponding codes for the administrative divisions in the CLHLS data, and revised the old codes in CLHLS to align with the latest codes used in the county-level ozone data set.
Given that the time between interviews in CLHLS was not exactly 3 years and our exploratory analysis showed lag 1 year exposure to have the largest effect estimate, we used the annual ozone exposure with lag 1 year as the main indicator of long-term ozone exposure level and treated ozone as a time-varying variable in the analysis. That is, we used the annual exposure during the previous calendar year. For example, if an event occurred on July 16, 2009, then the long-term ozone exposure for each participant in the risk set for that event would be the mean ozone concentration in each participant’s county of residence in 2008. The annual exposure with lag t years (t=2, 3) and the average annual exposure over two and three years, with lag 1 year (i.e., lag 1-2 years and lag 1-3 years), were calculated to explore whether different metrics of exposure yield different findings. We also estimated ozone exposure in the warm season (May-October).
We adjusted for potential confounding by PM2.5 and by air temperature. We obtained PM2.5 data for 2004-2017 from a previous study by Ma et al. (Ma et al., 2016). Monthly mean PM2.5 concentration was estimated by a two-stage spatial statistical model at 10 km × 10 km resolution, which had accurate predictions with little bias (R2 = 0.73; validation regression slope = 0.91). Like the ozone exposure assessment, county-level annual mean PM2.5 concentration was obtained by deriving the mean value within each county; PM2.5 exposure was then assigned to each participant based on county of residence. Daily mean air temperature data collected from 1,839 national meteorological stations in 23 provinces for 2005-2018 were provided by the Resource and Environment Science Data Center of the Chinese Academy of Sciences (RESDC) (http://www.resdc.cn/data.aspx?DATAID=230). There were no missing daily observations during the period for these meteorological stations. Temperature records of the station nearest to each county/city were utilized to estimate the annual mean exposure level of residents. In a given analysis, we used time-varying long-term exposure metrics for PM2.5 and temperature that were consistent with the ozone metric used (e.g., for analysis of annual ozone exposure with lag 1 year, the adjustment variables were annual PM2.5 and temperature with lag 1 year).
2.3. Quantification of cognitive function
Cognitive function was evaluated using the Chinese version of the Mini-Mental State Examination (MMSE), one of the most widely used screening tools for assessing the cognitive status of older people and documenting cognitive changes occurring over time (F.Folstein et al., 1975; Tombaugh and Mclntyre, 1992). The assessment was conducted by face-to-face interviews and all questions were answered by the respondents without a proxy. The Chinese version of MMSE was adapted from the international MMSE developed by Folstein et al. (Folstein et al., 1975) to meet the cultural and socioeconomic conditions of China (Zeng and Vaupel, 2002). The reliability of the Chinese MMSE scale is high, with Cronbach’s α coefficients ranging from 0.88 to 0.94 for the 1998-2011 waves of the CLHLS (Zhang et al., 2008; Zhong et al., 2017). The examination consists of 24 items, including the cognitive dimensions of orientation, registration, attention, memory, language, and visual construction skills. The total MMSE score can range from 0 (all answers are incorrect or “unable to answer”) to 30 (all answers are correct), with a higher score indicating better cognition. Consistent with previous studies, we defined cognitive impairment as an MMSE score below 18 points in combination with an MMSE score decline from baseline ≥4 points (Mao et al., 2020; Wang et al., 2020; Zhang et al., 2008). This definition takes into consideration the relatively low education level among Chinese older adults, as well as the reliability of cognitive decline assessed by MMSE.
2.4. Covariates
Potential confounders assessed in the baseline survey included demographic, lifestyle, health, and psychological factors (Zeng, 2012). Demographic variables included age, sex (male or female), residence (urban or rural), education level (literate or illiterate based on having received ≥1 year of schooling or not), marital status (married or not married, which includes divorced, widowed, or never married), financial support (independence or dependence based on having work or retirement income or not), economic status (self-rated rich, average, or poor), and geographic region (Northern, Eastern, Central, Southern or Western China, shown in Fig. S2). Lifestyle factors included smoking status (current smoker, former smoker or never smoker), drinking status (current drinker, former drinker or never drinker), regular exercise (yes or no), and social and leisure activity (yes or no based on engaging in any one of the following activities at present or not: outdoor activities, garden work, reading, raising poultry or pets, playing cards or Mahjong, watching TV or listening to the radio, or participating in organized social activities). Health factors included chronic disease (yes or no) and disability (yes or no). Chronic disease was defined as suffering from any of the following diseases based on self-report: hypertension, diabetes, heart disease, or respiratory disease. We also identified hypertension by systolic blood pressure of 140 mmHg or higher, or diastolic blood pressure of 90 mmHg or higher. Chronic disease was not included in the final model, but treated as a potential effect modifier in the effect modification analysis. Disability was defined as limitations in any activity of daily living: bathing, dressing, bathroom use, indoor transferring, continence or eating. Psychological factors included personality score (continuous). Seven questions were used to assess personality: looking on the bright side of things, keeping my belongings neat and clean, feeling fearful or anxious, feeling lonely and isolated, making my own decisions, feeling useless with age, and being happier when younger. The total score can range from 7 to 35, with a lower score indicating better psychological well-being (Zhang et al., 2019). All the above covariate information was obtained using a standardized and structured questionnaire. These covariates have been associated previously with cognitive impairment and may also be associated with ozone exposure through behavioral and time-activity patterns, and hence were candidate confounders to be included in models (Hersi et al., 2017; Zhang et al., 2019). We also controlled for two environmental factors: PM2.5 and air temperature.
2.5. Statistical analyses
We summarized baseline characteristics of participants according to the occurrence of cognitive impairment. Data were described as means and standard deviations (SDs) for continuous variables, and frequencies with percentages for categorical variables. Less than 1% of the data for each characteristic were missing; participants with missing covariates (2.35%) were excluded from the main analysis. We also excluded observations with ozone exposure level below the 1st percentile and above the 99th percentile to eliminate effects of very sparse data.
Using Cox proportional hazards models, we modeled long-term ozone exposure as a time-varying continuous variable with the hazard ratio and 95% CI estimated per 10-μg/m3 increase in exposure. In the primary analysis (annual ozone exposure with lag 1 year), ozone exposure was updated by calendar year, as described above. To further examine the association between ozone exposure and cognitive impairment, ozone exposure was included in models as a categorical variable (quartiles: 72.2-85.3, 85.3-92.4, 92.4-97.6, and 97.6-112.4 μg/m3, with quartile 1 as the reference group). We also explored the potential non-linear associations of ozone with cognitive impairment by including penalized splines for the ozone term in the model, and explored whether different long-term ozone exposure metrics may yield different findings.
In the Cox proportional hazards models, we used follow-up time (days) as the time metric and the Anderson-Gill formulation. Each participant was followed up from the date of baseline survey, with follow-up time ending at the date of diagnosis of cognitive impairment or censored at the date of death, loss to follow-up, or the end of follow-up (2018 wave). The final model (fully-adjusted model) in the main analysis was adjusted for age, sex, education level, geographic region, residence, marital status, financial support, economic status, smoking status, drinking status, regular exercise, social and leisure activity, personality, disability, PM2.5, and air temperature. We controlled for all confounders as time-invariant variables except for PM2.5 and air temperature, which were included in models as time-varying covariates, updated by calendar year in the same manner as ozone exposure. PM2.5 was modeled as a continuous variable, and we used penalized splines to model air temperature because of its non-linear relationship with the outcome.
To address potential non-proportionality of hazards, all models were stratified by geographic region, and included an interaction term between age (continuous) and the time interval (cut point at the follow-up time of 1,000 days). We observed no evidence of violating the proportional-hazards assumption (tested by Schoenfeld’s residuals) in the final model.
We examined whether the association between long-term ozone exposure and cognitive impairment was modified by the following baseline characteristics: age (65-79 vs. ≥80 y), sex (male vs. female), residence (urban vs. rural), educational level (literate vs. illiterate), smoking status (current vs. former or never), drinking status (current vs. former or never), regular exercise (yes vs. no), social and leisure activity (yes vs. no), disability (yes vs. no), chronic disease (yes vs. no), PM2.5 (lower tertile, middle tertile or upper tertile) and geographic region (Northern, Eastern, Central, Western or Southern China). Each potential effect modifier was assessed in a separate Cox model that included an interaction term(s) between the potential effect modifier and ozone exposure to estimate the effect of ozone in each category. In these analyses, we modeled the polytomous variables (PM2.5 and geographic region) as categorical variables. The significance of effect modification was assessed by comparing the reduction in log partial likelihood of models with and without an interaction term.
To evaluate the robustness of our primary findings, we conducted several sensitivity analyses by (1) excluding the participants lost to follow-up from the final model; (2) including participants excluded due to having no follow-up assessments in the analysis and censoring them after a specific time [participants known to have died before the first follow-up survey were censored at the date of death; other participants were censored at a fixed-point (December 31 of the year after the participant’s baseline survey) between their baseline survey and the first follow-up]; (3) adjusting for year of recruitment as a categorical variable to control for potential residual confounding by time trends in the final model; (4) including both summer (May-October) and winter (November-April) mean temperature in the the final model instead of annual mean temperature; (5) adding smoking, drinking, and exercise as time-varying variates in the final model; (6) using age as the time metric in the final model; (7) excluding some of the adjustment covariates from the final model [i.e., adjusting only for demographic characteristics (Model 1 in Table S2), only for demographic and lifestyle factors (Model 2 in Table S2), and adjusting for all covariates except PM2.5 and air temperature (Model 3 in Table S2)]; (8) performing the analysis only for ozone exposure in the warm season (May-October).
Environmental data processing and exposure matching were completed using ArcGIS (version 10.2; Environmental Systems Research Institute) and R (version 4.0.3; R Development Core Team). All statistical analyses were performed using R (version 4.0.3; R Development Core Team). Two-sided p < 0.05 was considered statistically significant, with the exception of the effect modification analysis. After applying the Bonferroni correction for multiple comparisons, we set a significance level of 0.0042 for the effect modification analysis.
3. Results
3.1. Description of the study sample
A total of 9,544 Chinese older adults with normal baseline cognitive function at recruitment from March 2005 to November 2014 and with at least one follow-up assessment were included in this study (Fig. 1). The mean age of the participants was 81.4 ± 10.8 years, ranging from 65 to 110 years; the gender composition was balanced (48.3% men) (Table 1). Most (81.3%) of the older adults lived in rural areas and more than half (54.2%) were illiterate. About 22.5% were current smokers and 22.0% were current drinkers. Nearly half of the participants (48.3%) suffered from at least one chronic condition, but fewer than one-tenth (7.6%) were disabled with regard to activities of daily living. Individuals in the sample were more likely to develop cognitive impairment if they were older, female, not married, illiterate, financially dependent, never smokers, never drinkers, or disabled; if they had poorer baseline cognitive function (i.e., a lower MMSE score) or did not have a chronic disease; or if they did not exercise or participate in social and leisure activity. During the average follow-up time of 6.5 years (62,133 person-years), 2,601 participants who had normal cognitive function at baseline (i.e., the MMSE score ≥ 18) developed cognitive impairment, 2,830 participants died, and 1,820 were lost to follow-up (Fig. S1).
Table 1:
Descriptive characteristics of study participants at baseline by the occurrence of cognitive impairment [mean±SD or n (%)].
| Total Sample | Cognitive Impairment |
||
|---|---|---|---|
| Yes | No | ||
| No. of participants | 9,544 | 2,601 | 6,943 |
| Age(y) | 81.35 ± 10.82 | 87.89 ± 9.53 | 78.90 ± 10.24 |
| Age group | |||
| 65-79 | 4,177 (43.77) | 478 (18.38) | 3,699 (53.28) |
| 80-89 | 2,913 (30.52) | 905 (34.79) | 2,008 (28.92) |
| 90-99 | 1,790 (18.76) | 826 (31.76) | 964 (13.88) |
| ≥100 | 664 (6.96) | 392 (15.07) | 272 (3.92) |
| Gender | |||
| Male | 4,605 (48.25) | 907 (34.87) | 3,698 (53.26) |
| Female | 4,939 (51.75) | 1,694 (65.13) | 3,245 (46.74) |
| Residence | |||
| Urban | 1,788 (18.73) | 447 (17.19) | 1,341 (19.31) |
| Rural | 7,756 (81.27) | 2,154 (82.81) | 5,602 (80.69) |
| Marital status a | |||
| Married | 4,161 (43.60) | 681 (26.18) | 3,480 (50.12) |
| Not married | 5,371 (56.28) | 1,919 (73.78) | 3,452 (49.72) |
| Education a | |||
| Literate | 4,331 (45.38) | 729 (28.03) | 3,602 (51.88) |
| Illiterate | 5,176 (54.23) | 1,861 (71.55) | 3,315 (47.75) |
| MMSE score | 26.95 ± 3.22 | 25.59 ± 3.55 | 27.46 ± 2.92 |
| Financial support a | |||
| Independence | 3,324 (34.83) | 502 (19.30) | 2,822 (40.65) |
| Dependence | 6,217 (65.14) | 2,099 (80.70) | 4,118 (59.31) |
| Economic status a | |||
| Rich | 1,605 (16.82) | 433 (16.65) | 1,172 (16.88) |
| Average | 6,466 (67.75) | 1,695 (65.17) | 4,771 (68.72) |
| Poor | 1,447 (15.16) | 462 (17.76) | 985 (14.19) |
| Smoking status a | |||
| Current smoker | 2,149 (22.52) | 398 (15.30) | 1,751 (25.22) |
| Former smoker | 1,281 (13.42) | 271 (10.42) | 1,010 (14.55) |
| Never smoker | 6,111 (64.03) | 1,931 (74.24) | 4,180 (60.20) |
| Drinking status a | |||
| Current drinker | 2,101 (22.01) | 462 (17.76) | 1,639 (23.61) |
| Former drinker | 977 (10.24) | 255 (9.80) | 722 (10.40) |
| Never drinker | 6,457 (67.66) | 1,882 (72.36) | 4,575 (65.89) |
| Regular exercise a | |||
| Yes | 3,191 (33.43) | 714 (27.45) | 2,477 (35.68) |
| No | 6,325 (66.27) | 1,882 (72.36) | 4,443 (63.99) |
| Social and leisure activity a | |||
| Yes | 8,994 (94.24) | 2,327 (89.47) | 6,667 (96.02) |
| No | 549 (5.75) | 274 (10.53) | 275 (3.96) |
| Personality a | 15.65 ± 3.91 | 16.38 ± 3.91 | 15.38 ± 3.87 |
| Disability a | |||
| Yes | 725 (7.60) | 367 (14.11) | 358 (5.16) |
| No | 8,815 (92.36) | 2,232 (85.81) | 6,583 (94.81) |
| Chronic disease a | |||
| Yes | 4,605 (48.25) | 1,143 (43.94) | 3,462 (49.86) |
| No | 4,886 (51.19) | 1,439 (55.32) | 3,447 (49.65) |
| Geographic region | |||
| Northern China | 1,093 (11.45) | 308 (11.84) | 785 (11.31) |
| Eastern China | 3,677 (38.53) | 964 (37.06) | 2,713 (39.08) |
| Central China | 1,742 (18.25) | 456 (17.53) | 1,286 (18.52) |
| Western China | 1,026 (10.75) | 297 (11.42) | 729 (10.50) |
| Southern China | 2,006 (21.02) | 576 (22.15) | 1,430 (20.60) |
Abbreviation: SD, standard deviation; MMSE, Mini-Mental State Examination.
Missing data: marital status (12), education (37), financial support (3), economic status (26), smoking status (3), drinking status (9), regular exercise (28), social and leisure activity (1), personality (63), disability (4), and chronic disease (53).
Our study contained a wide geographical distribution of participants with substantial variability in ozone exposure (Fig. 2). Among the 23 Chinese provinces covered by our study, high ozone pollution (monthly mean value > 100 μg/m3) was most prominent in Beijing, Hebei, Henan, Jiangsu, Shandong, Shanxi, Shanghai, and Tianjin (Fig. S3). Over the study period of 2005-2018, county-level annual mean ozone concentrations in the 23 provinces ranged from 47.9 to 126.4 μg/m3 [median: 88.8 μg/m3; interquartile range (IQR): 11.7 μg/m3]. The annual ozone exposure (lag 1 year) of participants during the follow-up period ranged from 57.3 to 122.0 μg/m3 (median: 92.4 μg/m3; IQR: 12.6 μg/m3), with an approximately normal distribution (Fig. S4).
Fig. 2: Map of ozone concentrations in China during the study period (2005-2018).
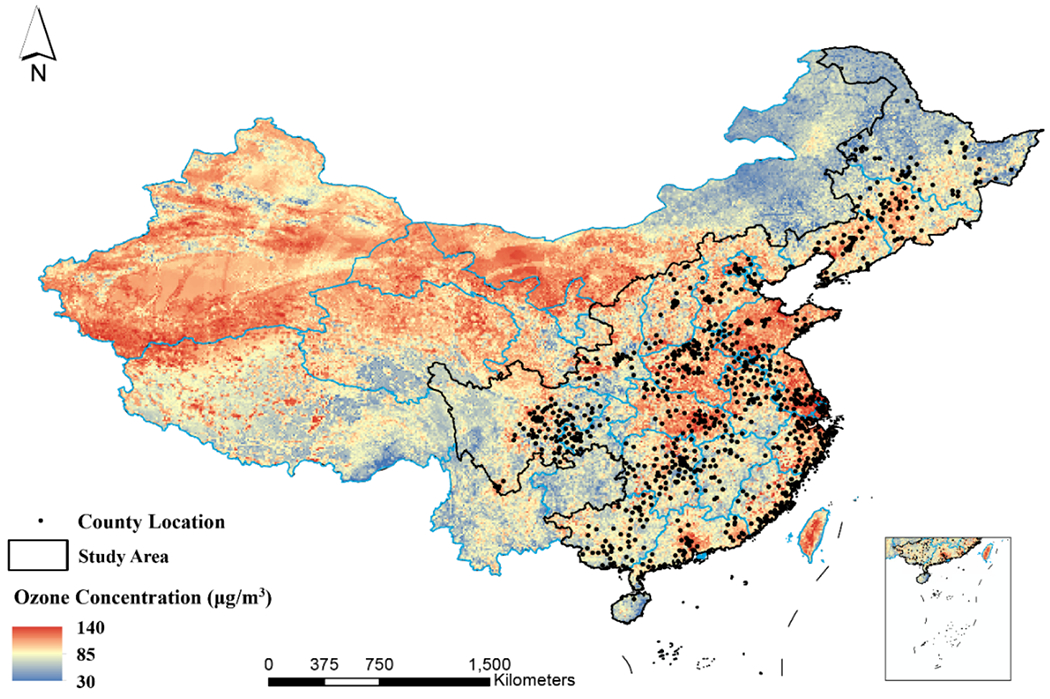
The areas within the black lines represent the geographic regions covered by the CLHLS, including 23 provinces of China. Black points represent the location of counties where participants lived. The inset in the lower right corner indicates the South China Sea. Our study included a large national representative sample with highly diverse ozone exposure levels, covering the areas of lowest and highest ozone pollution in China.
3.2. Association between long-term ozone exposure and cognitive impairment
We used Cox proportional hazards models to estimate the hazard ratios (HRs) and 95% confidence intervals (95% CIs) after controlling for potential confounders, including demographic, lifestyle, health, psychological, and environmental factors. We found that long-term ozone exposure (defined as annual exposure during the previous calendar year) was positively associated with the risk of cognitive impairment (Table 2). Based on the fully adjusted model, each 10-μg/m3 increase in annual exposure to ozone was associated with a 10.4% increase in the risk of cognitive impairment [hazard ratio (HR): 1.104; 95% confidence interval (95% CI): 1.041, 1.172]. Similar results were found for other long-term ozone exposure metrics, with HRs of 1.068 (95% CI: 1.005, 1.135) for exposure with lag 2 year, 1.044 (95% CI: 0.983, 1.109) for exposure with lag 3 year, 1.098 (95% CI: 1.032, 1.169) for exposure with lag 1-2 years, and 1.083 (95% CI: 1.017, 1.154) for exposure with lag 1-3 years (Fig. 3). The exposure-response relationship between ozone exposure and risk of cognitive impairment showed a linear trend when using penalized splines for the ozone term in the Cox model (Fig. 4). When we modeled ozone exposure as a categorical variable (quartiles; Table 2), we found that compared with the lowest quartile of ozone (72.2-85.3 μg/m3), the adjusted HR for 85.3-92.4 μg/m3, 92.4-97.6 μg/m3, and 97.6-112.4 μg/m3 was 1.182 (95% CI: 1.052, 1.328), 1.178 (95% CI: 1.034, 1.341), and 1.217 (95% CI: 1.063, 1.393), respectively. The association between ozone exposure and risk of cognitive impairment was independent of the significant association between PM2.5 exposure and risk of cognitive impairment (HR: 1.056 per 10 ug/m3 increase; 95% CI: 1.226, 1.086; Table S3).
Table 2:
Association between long-term ozone exposure and risk of cognitive impairment.
| Ozone (O3) exposurea | Observations | Participants | Cases | HR (95% CI) |
|---|---|---|---|---|
| Final model (fully-adjusted)b | ||||
| Continuous (per 10 ug/m3 increase in O3) | 63412 | 9327 | 2459 | 1.104 (1.041-1.172) |
| O3 categorized by quartiles | ||||
| 72.2 - 85.3 μg/m3 | 15853 | 3454 | 586 | 1 [Reference] |
| 85.3 - 92.4 μg/m3 | 15853 | 4399 | 693 | 1.182 (1.052-1.328) |
| 92.4 - 97.6 μg/m3 | 15853 | 4766 | 579 | 1.178 (1.034-1.341) |
| 97.6 - 112.4 μg/m3 | 15853 | 3757 | 601 | 1.217 (1.063-1.393) |
Abbreviation: HR, hazard ratio; CI, confidence interval.
Annual exposure during the previous calendar year.
Final model (fully-adjusted model) was adjusted for age, sex, education (literacy status), geographic region, residence, marital status, financial support, economic status, smoking status, drinking status, regular exercise, social and leisure activity, personality, disability, PM2.5, and air temperature.
Fig. 3: Association between long-term ozone exposure (using different metrics) and risk of cognitive impairment.
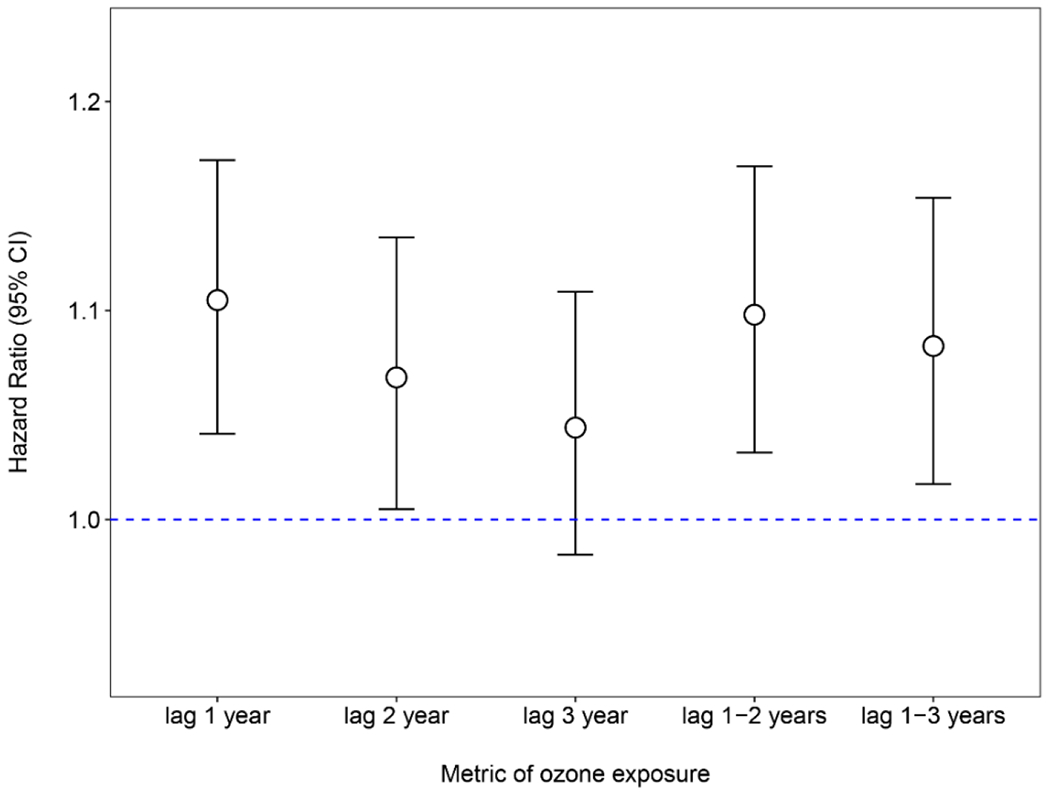
Fully-adjusted model (i.e., final model) was adjusted for age, sex, education, geographic region, residence, marital status, financial support, economic status, smoking status, drinking status, regular exercise, social and leisure activity, personality, disability, PM2.5 and air temperature. To keep the same sample size across analyses using different ozone exposure metrics, the observations with missing lag 3 year ozone exposure were removed. Abbreviations: CI, confidence interval; O3, ozone.
Fig. 4: Exposure-response curve for the association between long-term ozone exposure and risk of cognitive impairment.
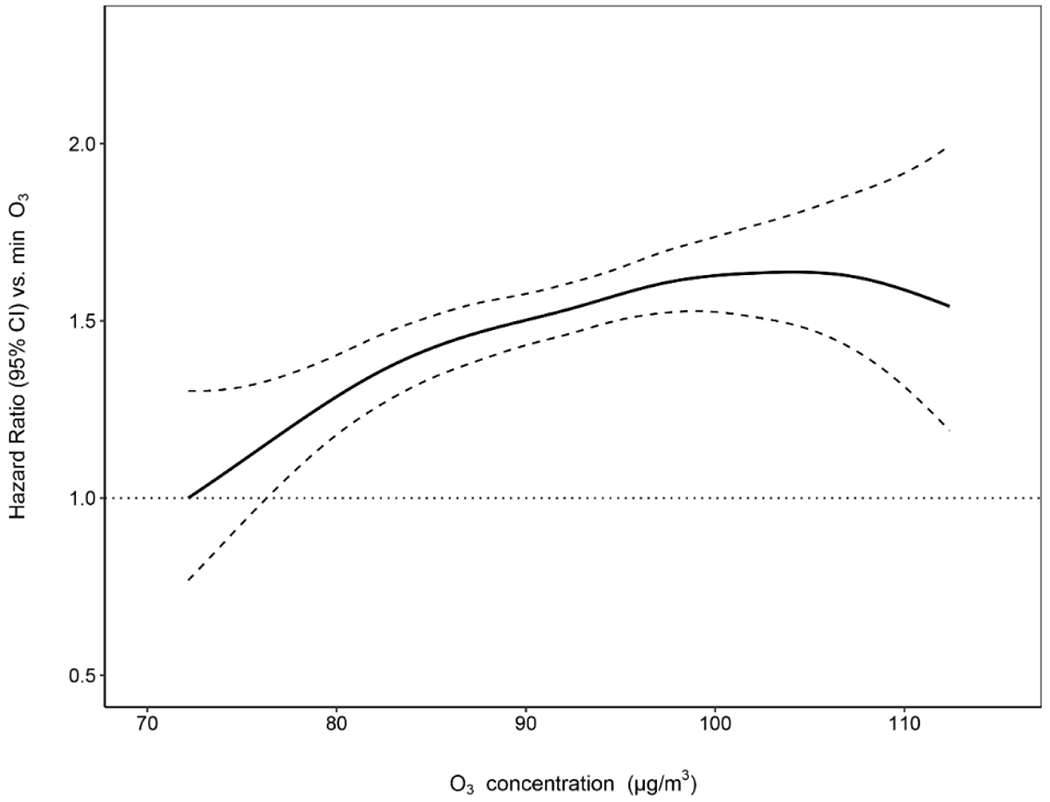
Annual ozone exposure ranged from 72.2 to 112.4 μg/m3 (median: 92.4 μg/m3; interquartile range: 12.2 μg/m3) after restricting the ozone exposure level within the 1st and 99th percentiles to eliminate the large uncertainty due to sparse data. The 1st percentile of ozone exposure was set as the reference with the HR equal to 1. The dashed lines represent 95% CIs. HRs were estimated using the Cox proportional hazards model with penalized splines among 9,327 Chinese older adults aged 65 years and over. Covariates include age, sex, education, geographic region, residence, marital status, financial support, economic status, smoking status, drinking status, regular exercise, social and leisure activity, personality, disability, PM2.5, and air temperature. Abbreviation: HR, hazard ratio; CI, confidence interval; O3, ozone.
3.3. Effect modification
To identify subpopulations that might be particularly susceptible to ozone exposure, we investigated potential effect modification by baseline characteristics and PM2.5 exposure (Fig. 5). When evaluated by geographic region (Fig. S2), associations were significantly positive for Eastern, Central, and Southern China, with HRs of 1.133 (95% CI: 1.027, 1.250), 1.318 (95% CI: 1.161, 1.496) and 1.153 (95% CI: 1.023, 1.299), respectively, but not for Northern (HR: 0.949; 95% CI: 0.799, 1.126) or Western China (HR: 0.846; 95% CI: 0.710, 1.008). Even after taking multiple comparisons into account, the difference across regions was significant (p-value < 0.001). We observed no effect modification by age, sex, residence, education level, smoking status, drinking status, regular exercise, social and leisure activity, disability, chronic disease or PM2.5 exposure.
Fig. 5: Associations between long-term ozone exposure and risk of cognitive impairment by subpopulations.
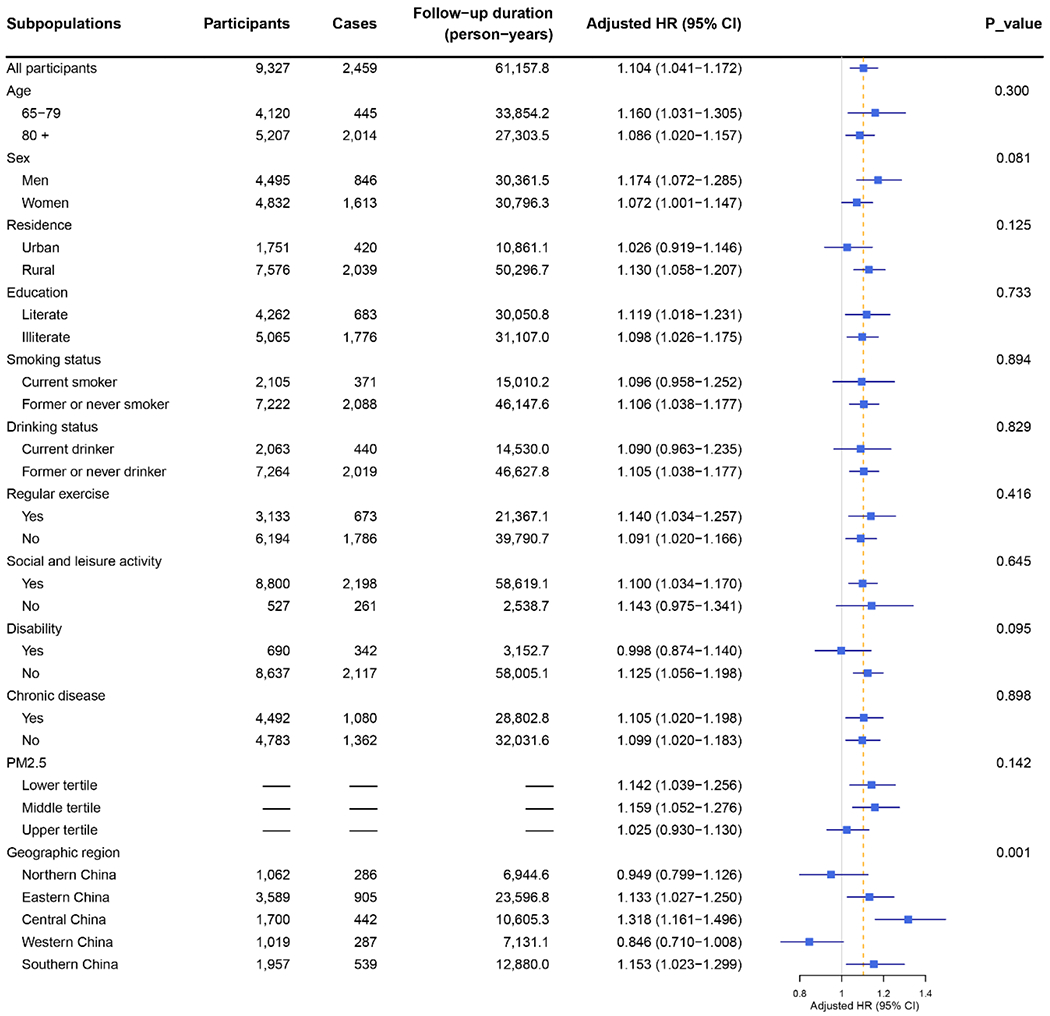
Hazard ratio (HR) equal to 1 represents no association (as indicated by the solid grey vertical line). The dashed orange vertical line indicates the fully-adjusted HR for all participants (HR=1.104). Error bars represent 95% confidence intervals (CIs). HR in each subpopulation was estimated using the Cox proportional hazards model with an interaction term between the potential effect modifier and ozone exposure. The significance of effect modification was assessed by comparing the reduction in log partial likelihood of models with and without an interaction term. Covariates include age, sex, education, geographic region, residence, marital status, financial support, economic status, smoking status, drinking status, regular exercise, social and leisure activity, personality, chronic disease, disability, PM2.5, and air temperature. Abbreviation: HR, hazard ratio; CI, confidence interval.
3.4. Sensitivity analyses
We performed several sensitivity analyses to test the robustness of our findings. Fully-adjusted (i.e., final model) results did not meaningfully change 1) after excluding participants lost to follow-up (Table S2); 2) after including participants excluded due to having no follow-up assessments in the analysis (Table S2); 3) after adjusting for year of recruitment as a categorical variable to control for time trends (Table S2); 4) after including both summer and winter temperature instead of annual mean temperature (Table S2); 5) after adding smoking, drinking, and exercise as time-varying variates (Table S2); 6) after using age as the time metric (Table S2); or 7) after excluding some of the adjustment covariates (Table S2). In addition, long-term exposure to ozone in the warm season (May-October) was significantly associated with the risk of cognitive impairment (HR: 1.049, 95% CI: 1.008, 1.093 for ozone exposure with lag 1 year) (Fig. S5), although this association was weaker compared with our main result using annual ozone exposure. However, when using penalized splines for the warm season ozone term in the Cox model (Fig. S6), the exposure-response curve was similar to the curve using annual ozone exposure. Furthermore, the associations for warm season ozone exposure lag 2 years (HR: 1.106, 95% CI: 1.061, 1.154) and lag 1-2 years (HR: 1.094, 95% CI: 1.049, 1.141) were similar in magnitude to lag 1 year and lag 1-2 years for annual ozone exposure. Overall, these various sensitivity analyses showed our findings to be robust.
4. Discussion
In this nationwide prospective cohort study of Chinese older adults aged 65-110 years, long-term exposure to ozone was associated with an elevated risk of cognitive impairment after controlling for potential confounders. Each 10-μg/m3 increment in annual ozone exposure was associated with a 10.4% increased risk of cognitive impairment. The exposure-response curve showed a continuously elevated cognitive impairment risk with increasing ozone concentrations.
Consistent with our findings, some other longitudinal cohort studies also reported a significant association between long-term exposure to ozone and increased risk of cognitive impairment. In a nationwide United States sample, prolonged exposure to ground-level ozone was found to be associated with accelerated cognitive decline (Cleary et al., 2018). Lo et al. reported a positive association of long-term exposure to ozone with the incidence of cognitive impairment among Taiwanese older adults (Lo et al., 2019). Moreover, long-term exposure to ozone was observed to be positively associated with the development of neurodegenerative diseases, such as Alzheimer’s disease and other dementias (Cerza et al., 2019; Jung et al., 2015).
There is, however, evidence of no or even a negative association between long-term ozone exposure and cognitive impairment. For example, Carey et al. found a negative association between average annual ozone exposure and dementia risk in Greater London (HR: 0.84; 95% CI: 0.75, 0.94 per 5.56 μg/m3), which Carey et al. speculated could be due to the strong negative correlation of ozone with co-pollutants (e.g., PM2.5) in their study (Carey et al., 2018). On the contrary, our results showed a significant positive effect of ozone after adjustment for PM2.5 and no evidence of interaction between long-term exposure to ozone and PM2.5, which indicates that ozone and PM2.5 may have independent detrimental effects on cognitive function among older adults. Furthermore, the median (IQR) for ozone exposure was 38.2 (35.5-41.0) μg/m3 in Carey et al., much lower than 92.4 (85.3-97.6) μg/m3 in the current study. A low concentration threshold (approximately 70 μg/m3) for the ozone-mortality relationship was reported in the American Cancer Society Cancer Prevention Study-II (Turner et al., 2016). In our study, almost all participants had annual ozone exposure greater than 70 μg/m3, perhaps allowing us, but not Carey et al., to detect a significant adverse effect of ozone pollution.
In mainland China, although emerging studies have explored associations between air pollution exposure and cognitive functioning (Wang et al., 2020; Zhang et al., 2018a), only one study has investigated ozone pollution. Gao et al., using the 2018 China Family Panel Studies (CFPS) cognitive tests, observed a significant cross-sectional negative association between long-term ozone exposure and cognitive function (Gao et al., 2021a). The mean age of participants in the CFPS study was 48.4 years, compared with a mean age of 81.4 years in our study. To our knowledge, the present work is the only longitudinal study to date to investigate the influence of ozone exposure on late-life cognitive health in mainland China. As most previous studies were conducted in developed countries and regions, our results also provide the first evidence from a heavily polluted developing country on ozone-related cognitive impairment risk in the elderly population.
Inflammation and oxidative stress have commonly been hypothesized to be the basic pathophysiologic pathways through which ozone compromises the central nervous system (CNS), thereby impairing cognitive function (Block and Calderon-Garciduenas, 2009; Carlos Martinez-Lazcano et al., 2013). Ozone inhalation may induce lung inflammation and the production and release of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6 (Carlos Martinez-Lazcano et al., 2013; Croze and Zimmer, 2018). These cytokines could alter the blood-brain barrier function and reach the brain (Croze and Zimmer, 2018), where they could induce the synthesis of additional cytokines and other pro-inflammatory molecules (Carlos Martinez-Lazcano et al., 2013). Elevated concentrations of these molecules may lead to oxidative stress and inflammation in some brain areas, contributing to CNS damage (Carlos Martinez-Lazcano et al., 2013; Croze and Zimmer, 2018). Recent studies in animal models showed that acute or chronic ozone exposure could result in brain lipid peroxidation (Guevara-Guzman et al., 2009), cerebral edema (Creťu et al., 2010), neurodegeneration in the hippocampus (Rivas-Arancibia et al., 2010) and striatum (Pereyra-Munoz et al., 2006), and memory impairment (Dorado-martinez et al., 2001). Further, the blood-brain barrier in aged mice was found to be highly penetrable in response to ozone exposure, leading to greater neuroinflammatory outcomes (Tyler et al., 2018). A recent cohort study found a diminished association between PM2.5 and cognition in men using non-steroidal anti-inflammatory drugs, supporting the role of inflammation in mediating the association between air pollution exposure and cognitive decline (Gao et al., 2021b).
It is possible that cardiovascular disease (CVD) is a mediating factor for the association between ozone exposure and cognitive impairment. Ozone has been increasingly recognized as a CVD risk factor (Khaniabadi et al., 2017; Nuvolone et al., 2013), and CVD may be a determinant of dementia (Kim et al., 2018; Wanleenuwat et al., 2019). A mediating effect of CVD has already been found in some studies linking air pollution (PM2.5 and NOx) and dementia (Grande et al., 2020; Ilango et al., 2020). To establish causality, more studies are needed to explore the possible mechanistic pathways that link ozone exposure with cognitive impairment.
Evidence generated from animal models cannot directly establish a link of causality between cognitive impairment and ozone exposure in humans because of the complex environmental exposures, genetics susceptibilities, and co-existing health conditions in human populations; however, ozone exposure can be posited as a risk factor or aggravating factor for cognitive decline, at least in combination with other factors (Croze and Zimmer, 2018). Although our examination of exposure periods was limited to up to three years, it is possible that ozone impacts on the CNS may accumulate across an individual’s lifespan.
We observed effect modification by geographic region, with significantly stronger associations in Eastern, Central, and Southern China than in Northern or Western China. Geographical differences in climate, dietary patterns, lifestyles, residential heating and cooling, or underlying health status of older adults may account for this effect modification. Further studies are warranted to confirm this effect modification and to examine the potential mechanisms of the regional difference in ozone impacts on cognitive impairment.
With the sustained increase of concentrations over the 2013-2019 period (Li et al., 2020), ground-level ozone has become a severe air pollution issue in China. In 377 cities of China in 2019, 41.7% of nonattainment days for air quality had ozone as the primary pollutant (the air pollutant with the highest individual air quality index) (MEE, 2020). Our findings suggest the urgent need to reduce ozone exposure to delay the progression of cognitive decline among older adults. Furthermore, in the sensitivity analysis restricted to warm season ozone exposure, we found long-term ozone exposure to be associated with an increased risk of cognitive impairment at concentrations above the WHO guideline (60 μg/m3 for the average of daily maximum 8-hour mean ozone concentrations in peak season) (Fig. S6), suggesting potential great benefits of reducing ozone concentrations to levels that are below the WHO guideline. In addition to air pollution controls, behavioral interventions can be applied to reduce ozone exposure at the population level, such as advising older adults not to engage in outdoor activities during warm sunny afternoons when high ozone pollution generally occurs.
Our study had the following strengths. First, we leveraged a nationwide representative longitudinal survey in 23 provinces in China, which includes about 85% of the total Chinese population. The prospective and population-based design and long follow-up period allowed us to track cognitive function changes and assess cognitive impairment in relation to long-term ozone exposure. To our knowledge, this is the first population-based cohort study linking ozone and cognitive decline among older adults conducted in mainland China. Second, benefitting from the wide geographical distribution of participants and the substantial variability of exposure level, our study examined the exposure-response relationship between long-term exposure to ozone and cognitive impairment over a wide range of concentrations. Third, we controlled for key individual risk factors, such as education level, smoking, drinking, physical activity, and personality. Fourth, our findings were robust in several sensitivity analyses. Finally, our large sample size allowed us to examine potential modifying effects of individual characteristics on the association of ozone exposure with cognitive impairment.
Several limitations of this study should be noted. First, potential exposure assessment error is unavoidable in this type of air pollution epidemiological study and may bias the estimates toward the null (Wu et al., 2019). Due to privacy concerns, information on street-level household addresses of CLHLS participants was not available. Instead, we used baseline county of residence to assign ozone exposure, which would result in exposure misclassification if there was geographic heterogeneity of ozone concentration within counties. In addition, some exposure misclassification would occur if participants moved from their baseline county of residence to another county during the follow-up period. However, relatively few older adults in China relocate, especially those living in rural areas (Dou and Liu, 2015) (81.27% participants in this study were rural residents). A previous study using the 2002-2014 CLHLS data estimated that only 356 of the 13,324 respondents moved during the follow-up period (Wang et al., 2020). Moreover, we could not take into account indoor ozone pollution due to the lack of data on indoor air quality monitoring. Because older adults spend most of the time indoors, further research with more precise personal ozone exposure assessments is warranted to validate the associations we observed in this study.
Second, except for PM2.5 and temperature, we were unable to assess the potential confounding or interactive effects of other environmental exposures (e.g., noise, NOx, and green space). Third, information on individual confounding factors was assessed via self-reports, which could introduce information bias and/or recall bias. Fourth, unmeasured confounding (e.g., body mass index) and residual confounding (most covariates were treated as time-invariant in this study) may have biased our results in an unknown direction. Fifth, the age structure of participants was not representative of the elderly population in China as CLHLS oversampled the oldest-old (aged 80 years and older). Lastly, the large proportion of participants who were excluded due to having no follow-up assessments may have led to selection bias (Table S1). However, our sensitivity analysis, including excluded participants with no follow-up assessments in the analysis, yielded results similar to the main analysis (Table S2), demonstrating the robustness of our findings.
Overall, we found that long-term exposure to ozone was associated with an increased risk of cognitive impairment among Chinese older adults. In addition to preventing respiratory disease and premature mortality, controlling ambient ozone pollution may help delay the progression of cognitive decline at the population level. The projected worsening ozone pollution due to climate change (Chen et al., 2018) makes undertaking this control even more urgent. With the rapidly aging global population (United Nations, 2019), the burden of dementia is projected to substantially increase (Jia et al., 2020; Prince et al., 2015). Our findings, if confirmed, point to the need for effective interventions to reduce ambient ozone pollution, which may bring significant public health benefits to an aging society.
Supplementary Material
Highlights.
This study examined ozone impacts on cognitive function in Chinese older adults.
Long-term ozone exposure was associated with increased cognitive impairment risk.
The association was observed at concentrations above the WHO guideline (60 μg/m3).
Older adults from Eastern, Central, and Southern China were especially susceptible.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (71921003). The Chinese Longitudinal Healthy Longevity Survey (CLHLS), which provided the data analyzed in this paper, is jointly supported by funds from the U.S. National Institute of Aging (NIA), the United Nations Fund for Population Activities (UNFPA), the National Social Science Fund of China, the National Natural Science Foundation of China, and Hong Kong Research Grant Council. E.Z. received support from the U.S. National Institute of Aging (1R21AG074238-01), the Research Education Core of the Claude D. Pepper Older Americans Independence Center at Yale School of Medicine (P30AG021342), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5-R25-HD083146). R.D. received funding support from the High Tide Foundation. S.L. is supported by an Early-Career Research Fellowship from the Gulf Research Program of the National Academies of Sciences, Engineering, and Medicine. L.S. received support from the HERCULES Center (P30 ES019776) and the Goizueta Alzheimer’s Disease Research Center (ADRC) at Emory University (P50 AG025688). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit authorship statement
Qi Gao: Data curation, Formal analysis, Visualization, Writing - Original Draft. Emma Zang: Writing - Review & Editing. Jun Bi: Funding acquisition. Robert Dubrow: Methodology, Writing - Review & Editing. Sarah Lowe: Writing - Review & Editing; Huashuai Chen: Investigation, Data Curation, Resources. Yi Zeng: Investigation, Resources. Liuhua Shi: Writing - Review & Editing. Kai Chen: Conceptualization, Methodology, Project administration, Supervision, Writing - Review & Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abrams JY; Klein M; Henneman LRF; Sarnat SE; Chang HH; Strickland MJ; Mulholland JA; Russell AG; Tolbert PE Impact of air pollution control policies on cardiorespiratory emergency department visits, Atlanta, GA, 1999-2013. Environ Int 2019;126:627–634 [DOI] [PubMed] [Google Scholar]
- Bell ML; Zanobetti A; Dominici F Who is more affected by ozone pollution? A systematic review and meta-analysis. Am J Epidemiol 2014;180:15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML; Calderon-Garciduenas L Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 2009;32:506–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey IM; Anderson HR; Atkinson RW; Beevers SD; Cook DG; Strachan DP; Dajnak D; Gulliver J; Kelly FJ Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 2018;8:e022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos Martinez-Lazcano J; Gonzalez-Guevara E; del Carmen Rubio M; Franco-Perez J; Custodio V; Hernandez-Ceron M; Livera C; Paz C The effects of ozone exposure and associated injury mechanisms on the central nervous system. Rev Neurosci 2013;24:337–352 [DOI] [PubMed] [Google Scholar]
- Cerza F; Renzi M; Gariazzo C; Davoli M; Michelozzi P; Forastiere F; Cesaroni G Long-term exposure to air pollution and hospitalization for dementia in the Rome longitudinal study. Environ Health 2019;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H; Kwong JC; Copes R; Hystad P; Donkelaar A.v.; Tu K; Brook JR; Goldberg MS; Martin RV; Murray BJ; Wilton AS; Kopp A; Burnett RT Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int 2017;108:271–277 [DOI] [PubMed] [Google Scholar]
- Chen K; Fiore AM; Chen R; Jiang L; Jones B; Schneider A; Peters A; Bi J; Kan H; Kinney PL Future ozone-related acute excess mortality under climate and population change scenarios in China: a modeling study. PLoS Med 2018;15:e1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary EG; Cifuentes M; Grinstein G; Brugge D; Shea TB Association of low-level ozone with cognitive decline in older adults. J Alzheimers Dis 2018;61:67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creťu D-I; Şovrea A; Ignat RM; Filip A; Bidian C; Creťu A Morpho-pathological and physiological changes of the brain and liver after ozone exposure. Rom J Morphol Embryol 2010;51:701–706 [PubMed] [Google Scholar]
- Croze ML; Zimmer L Ozone atmospheric pollution and Alzheimer’s disease: from epidemiological facts to molecular mechanisms. J Alzheimers Dis 2018;62:503–522 [DOI] [PubMed] [Google Scholar]
- Dorado-martinez C; Paredes-carbajal C; Mascher D; Borgonio-perez G; Rivas-arancibia S Effects of different ozone doses on memory, motor activity and lipid peroxidation levels, in rats. Int J Neurosci 2001;108:149–161 [DOI] [PubMed] [Google Scholar]
- Dou X; Liu Y Elderly migration in China: types, patterns, and determinants. J Appl Gerontol 2015;36:751–771 [DOI] [PubMed] [Google Scholar]
- Duke University Center for the Study of Aging and Human Development. Chinese Longitudinal Healthy Longevity Survey (CLHLS). https://sites.duke.edu/centerforaging/programs/chinese-longitudinal-healthy-longevity-survey-clhls/
- Folstein MF; Folstein SE; McHugh PR “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198 [DOI] [PubMed] [Google Scholar]
- Fann N; Risley D The public health context for PM2.5 and ozone air quality trends. Air Qual Atmos Health 2011;6:1–11 [Google Scholar]
- Gao H; Shi J; Cheng H; Zhang Y; Zhang Y The impact of long- and short-term exposure to different ambient air pollutants on cognitive function in China. Environ Int 2021a;151:106416. [DOI] [PubMed] [Google Scholar]
- Gao X; Coull B; Lin X; Vokonas P; Spiro A; Hou L; Schwartz J; Baccarelli AA Short-term air pollution, cognitive performance and nonsteroidal anti-inflammatory drug use in the Veterans Affairs Normative Aging Study. Nat Aging 2021b; 1:430–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S; Zadeoglulari Z; Fuss SH; Genc K The adverse effects of air pollution on the nervous system. J Toxicol 2012;2012:782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande G; Ljungman PLS; Eneroth K; Bellander T; Rizzuto D Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol 2020;77:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara-Guzman R; Arriaga V; Kendrick KM; Bernal C; Vega X; Mercado-Gomez OF; Rivas-Arancibia S Estradiol prevents ozone-induced increases in brain lipid peroxidation and impaired social recognition memory in female rats. Neuroscience 2009;159:940–950 [DOI] [PubMed] [Google Scholar]
- Hersi M; Irvine B; Gupta P; Gomes J; Birkett N; Krewski D Risk factors associated with the onset and progression of Alzheimer’s disease: a systematic review of the evidence. Neurotoxicology 2017;61:143–187 [DOI] [PubMed] [Google Scholar]
- Ilango SD; Chen H; Hystad P; van Donkelaar A; Kwong JC; Tu K; Martin RV; Benmarhnia T The role of cardiovascular disease in the relationship between air pollution and incident dementia: a population-based cohort study. Int J Epidemiol 2020;49:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L; Quan M; Fu Y; Zhao T; Li Y; Wei C; Tang Y; Qin Q; Wang F; Qiao Y; Shi S; Wang Y-J; Du Y; Zhang J; Zhang J; Luo B; Qu Q; Zhou C; Gauthier S; Jia J Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol 2020;19:81–92 [DOI] [PubMed] [Google Scholar]
- Jung C-R; Lin Y-T; Hwang B-F Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J Alzheimers Dis 2015;44:573–584 [DOI] [PubMed] [Google Scholar]
- Khaniabadi YO; Hopke PK; Goudarzi G; Daryanoosh SM; Jourvand M; Basiri H Cardiopulmonary mortality and COPD attributed to ambient ozone. Environ Res 2017;152:336–341 [DOI] [PubMed] [Google Scholar]
- Kim MY; Kim K; Hong CH; Lee SY; Jung YS Sex differences in cardiovascular risk factors for dementia. Biomol Ther (Seoul) 2018;26:521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K; Jacob DJ; Shen L; Lu X; De Smedt I; Liao H Increases in surface ozone pollution in China from 2013 to 2019: anthropogenic and meteorological influences. Atmos Chem Phys 2020;20:11423–11433 [Google Scholar]
- Liu R; Ma Z; Liu Y; Shao Y; Zhao W; Bi J Spatiotemporal distributions of surface ozone levels in China from 2005 to 2017: a machine learning approach. Environ Int 2020;142:105823. [DOI] [PubMed] [Google Scholar]
- Livingston G; Huntley J; Sommerlad A; Ames D; Ballard C; Banerjee S; Brayne C; Burns A; Cohen-Mansfield J; Cooper C; Costafreda SG; Dias A; Fox N; Gitlin LN; Howard R; Kales HC; Kivimäki M; Larson EB; Ogunniyi A; Orgeta V; Ritchie K; Rockwood K; Sampson EL; Samus Q; Schneider LS; Selbæk G; Teri L; Mukadam N Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y-TC; Lu Y-C; Chang Y-H; Kao S; Huang H-B Air pollution exposure and cognitive function in Taiwanese older adults: a repeated measurement study. Int J Environ Res Public Health 2019;16:2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z; Hu X; Sayer AM; Levy R; Zhang Q; Xue Y; Tong S; Bi J; Huang L; Liu Y Satellite-based spatiotemporal trends in PM2.5 concentrations: China, 2004-2013. Environ Health Perspect 2016;124:184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisalidis I; Stavropoulou E; Stavropoulos A; Bezirtzoglou E Environmental and health impacts of air pollution: a review. Front Public Health 2020;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C; Li ZH; Lv YB; Gao X; Kraus VB; Zhou JH; Wu XB; Shi WY; Li FR; Liu SM; Yin ZX; Zeng Y; Shi XM Specific leisure activities and cognitive functions among the oldest-old: the Chinese Longitudinal Healthy Longevity Survey. J Gerontol B Psychol Sci Soc Sci 2020;75:739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEE. Report on the State of the Ecology and Environment in China 2019. Beijing: Ministry of Ecology and Environment of China; 2020 [Google Scholar]
- Medina-Ramón M; Schwartz J Who is more vulnerable to die from ozone air pollution? Epidemiology 2008;19:672–679 [DOI] [PubMed] [Google Scholar]
- NBS. Statistical Bulletin of the People’s Republic of China on National Economic and Social Development 2019. Beijing: National Bureau of Statistics; 2020 [Google Scholar]
- Nichols E; Szoeke CEI; Vollset SE; Abbasi N; Abd-Allah F; Abdela J; Aichour MTE; Akinyemi RO; Alahdab F; Asgedom SW; Awasthi A; Barker-Collo SL; Baune BT; Béjot Y; Belachew AB; Bennett DA; Biadgo B; Bijani A; Bin Sayeed MS; Brayne C; Carpenter DO; Carvalho F; Catalá-López F; Cerin E; Choi J-YJ; Dang AK; Degefa MG; Djalalinia S; Dubey M; Duken EE; Edvardsson D; Endres M; Eskandarieh S; Faro A; Farzadfar F; Fereshtehnejad S-M; Fernandes E; Filip I; Fischer F; Gebre AK; Geremew D; Ghasemi-Kasman M; Gnedovskaya EV; Gupta R; Hachinski V; Hagos TB; Hamidi S; Hankey GJ; Haro JM; Hay SI; Irvani SSN; Jha RP; Jonas JB; Kalani R; Karch A; Kasaeian A; Khader YS; Khalil IA; Khan EA; Khanna T; Khoja TAM; Khubchandani J; Kisa A; Kissimova-Skarbek K; Kivimäki M; Koyanagi A; Krohn KJ; Logroscino G; Lorkowski S; Majdan M; Malekzadeh R; März W; Massano J; Mengistu G; Meretoja A; Mohammadi M; Mohammadi-Khanaposhtani M; Mokdad AH; Mondello S; Moradi G; Nagel G; Naghavi M; Naik G; Nguyen LH; Nguyen TH; Nirayo YL; Nixon MR; Ofori-Asenso R; Ogbo FA; Olagunju AT; Owolabi MO; Panda-Jonas S; Passos V.M.d.A.; Pereira DM; Pinilla-Monsalve GD; Piradov MA; Pond CD; Poustchi H; Qorbani M; Radfar A; Reiner RC; Robinson SR; Roshandel G; Rostami A; Russ TC; Sachdev PS; Safari H; Safiri S; Sahathevan R; Salimi Y; Satpathy M; Sawhney M; Saylan M; Sepanlou SG; Shafieesabet A; Shaikh MA; Sahraian MA; Shigematsu M; Shiri R; Shiue I; Silva JP; Smith M; Sobhani S; Stein DJ; Tabarés-Seisdedos R; Tovani-Palone MR; Tran BX; Tran TT; Tsegay AT; Ullah I; Venketasubramanian N; Vlassov V; Wang Y-P; Weiss J; Westerman R; Wijeratne T; Wyper GMA; Yano Y; Yimer EM; Yonemoto N; Yousefifard M; Zaidi Z; Zare Z; Vos T; Feigin VL; Murray CJL Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:88–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuvolone D; Balzi D; Pepe P; Chini M; Scala D; Giovannini F; Cipriani F; Barchielli A Ozone short-term exposure and acute coronary events: a multicities study in Tuscany (Italy). Environ Res 2013;126:17–23 [DOI] [PubMed] [Google Scholar]
- Patterson C World Alzheimer Report 2018 - The state of the art of dementia research: new frontiers. London: Alzheimer’s Disease International (ADI); 2018 [Google Scholar]
- Peng X Coping with population ageing in mainland China. Asian Popul Stud 2020;17:1–6 [Google Scholar]
- Pereyra-Munoz N; Rugerio-Vargas C; Angoa-Perez M; Borgonio-Perez G; Rivas-Arancibia S Oxidative damage in substantia nigra and striatum of rats chronically exposed to ozone. J Chem Neuroanat 2006;31:114–123 [DOI] [PubMed] [Google Scholar]
- Prince M; Bryce R; Albanese E; Wimo A; Ribeiro W; Ferri CP The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75 [DOI] [PubMed] [Google Scholar]
- Prince M; Wimo A; Guerchet M; Ali G-C; Wu Y-T; Prina M; International A.s.D. World Alzheimer Report 2015 - The global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International (ADI); 2015 [Google Scholar]
- Rivas-Arancibia S; Guevara-Guzman R; Lopez-Vidal Y; Rodriguez-Martinez E; Zanardo-Gomes M; Angoa-Perez M; Raisman-Vozari R Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol Sci 2010;113:187–197 [DOI] [PubMed] [Google Scholar]
- Shi L; Wu X; Danesh Yazdi M; Braun D; Abu Awad Y; Wei Y; Liu P; Di Q; Wang Y; Schwartz J; Dominici F; Kioumourtzoglou M-A; Zanobetti A Long-term effects of PM2.5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health 2020;4:e557–e565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN; Mclntyre NJ The Mini-mental State Examination: a comprehensive review. J Am Geriatr Soc 1992;40:922–935 [DOI] [PubMed] [Google Scholar]
- Turner MC; Jerrett M; Pope CA 3rd; Krewski D; Gapstur SM; Diver WR; Beckerman BS; Marshall JD; Su J; Crouse DL; Burnett RT Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med 2016;193:1134–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler CR; Noor S; Young TL; Rivero V; Sanchez B; Lucas S; Caldwell KK; Milligan ED; Campen MJ Aging exacerbates neuroinflammatory outcomes induced by acute ozone exposure. Toxicol Sci 2018;163:123–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations. World Population Ageing 2019: Highlights. New York: United Nations; 2019 [Google Scholar]
- Wang J; Li T; Lv Y; Kraus VB; Zhang Y; Mao C; Yin Z; Shi W; Zhou J; Zheng T; Kinney PL; Ji J; Tang S; Shi X Fine particulate matter and poor cognitive function among Chinese older adults: evidence from a community-based, 12-year prospective cohort study. Environ Health Perspect 2020;128:67013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanleenuwat P; Iwanowski P; Kozubski W Alzheimer’s dementia: pathogenesis and impact of cardiovascular risk factors on cognitive decline. Postgrad Med 2019;131:415–422 [DOI] [PubMed] [Google Scholar]
- Weuve J; Bennett EE; Ranker L; Gianattasio KZ; Pedde M; Adar SD; Yanosky JD; Power MC Exposure to air pollution in relation to risk of dementia and related outcomes: an updated systematic review of the epidemiological literature. Environ Health Perspect 2021;129:96001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. China country assessment report on ageing and health. Geneva: World Health Organization; 2015 [Google Scholar]
- Wu X; Braun D; Kioumourtzoglou M-A; Choirat C; Di Q; Dominici F Causal inference in the context of an error prone exposure: air pollution and mortality. Ann Appl Stat 2019;13:520–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J; Wang J; Wimo A; Fratiglioni L; Qiu C The economic burden of dementia in China, 1990-2030: implications for health policy. Bull W H O 2017;95:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X; Zheng L; Jiang W; Zhang D Exposure to air pollution and cognitive impairment risk: a meta-analysis of longitudinal cohort studies with dose-response analysis. J Glob Health 2020;10:010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y Towards deeper research and better policy for healthy aging - using the unique data of Chinese Longitudinal Healthy Longevity Survey. China Econ J 2012;5:131–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y; Vaupel JW Functional capacity and self-evaluation of health and life of oldest old in China. J Soc Issues 2002;58:733–748 [Google Scholar]
- Zhang Q; Wu Y; Han T; Liu E Changes in cognitive function and risk factors for cognitive impairment of the elderly in China: 2005-2014. Int J Environ Res Public Health 2019;16:2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X; Chen X; Zhang X The impact of exposure to air pollution on cognitive performance. Proc Natl Acad Sci U S A 2018a;115:9193–9197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y; West JJ; Mathur R; Xing J; Hogrefe C; Roselle SJ; Bash JO; Pleim JE; Gan CM; Wong DC Long-term trends in the ambient PM2.5- and O3-related mortality burdens in the United States under emission reductions from 1990 to 2010. Atmos Chem Phys 2018b;18:15003–15016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z; Gu D; Hayward MD Early life influences on cognitive impairment among oldest old Chinese. J Gerontol Ser B 2008;63:S25–S33 [DOI] [PubMed] [Google Scholar]
- Zhong B; Chen S; Tu X; Conwell Y Loneliness and cognitive function in older adults: findings from the Chinese Longitudinal Healthy Longevity Survey. J Gerontol B Psychol Sci Soc Sci 2017;72:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


