Abstract
The present study describes a heminested multiplex reverse transcription (RT)-PCR assay which enables simultaneous detection and differentiation of Norwalk-like virus (NLV) genogroups from clinical fecal samples without the need to perform sequencing or hybridization. The assay developed was able to detect concentrations of fewer than 100 viral particles per 5 μl of clarified fecal extract and could differentiate the two genogroups with a specificity of 100%. Although the multiplex RT-PCR assay failed to detect NLV in about 3% of the fecal samples which were NLV positive by electron microscopy (EM), the assay was approximately six times more sensitive than EM for NLV detection.
The Norwalk-like viruses (NLVs), which form a genus within the family Caliciviridae, are a major cause of acute gastroenteritis worldwide (16). These viruses are highly infectious and are frequently responsible for outbreaks of gastroenteritis in closed communities such as nursing homes and hospitals (20). Although illnesses associated with NLVs are usually mild and self-limiting, with symptoms such as vomiting, diarrhea, and abdominal cramps, NLV infections have been associated with mortality on rare occasions (5, 17, 18).
In recent years, the entire genomic sequence and organization of several strains of NLV, including Norwalk virus (9), Southampton virus (10), Lordsdale virus (4), and Camberwell virus (19), have been determined. NLVs have a positive-sense single-stranded RNA genome that is approximately 7.6 kb in length and three open reading frames (ORFs) (9). ORF1 is predicted to encode a polyprotein with a molecular mass of 193 kDa and sequence similarity to the picornavirus 2C helicase, 3C protease, and 3D RNA-dependent RNA polymerase. ORF2 partially overlaps ORF1 and is predicted to encode the 56.6-kDa viral capsid protein. ORF3 is predicted to encode a 23-kDa minor structural protein (6).
Based on the genomic sequences published, numerous reverse transcription (RT)-PCR assays have been established for rapid detection and characterization of NLVs (1, 8, 14, 20). The majority of these assays target the putative RNA polymerase gene within ORF1, and nucleotide variations found within this putative gene have enabled NLVs to be classified into two main genetic groups, which have been designated genogroups 1 and 2 (13).
Diagnostic and epidemiological studies require the classification of NLVs into their appropriate genogroups. However, current methods used to determine NLV genogroups are based on RT-PCR, followed by nucleotide sequencing or Southern blot hybridization, which requires genogroup-specific oligonucleotide probes. Both of these methods can be relatively time consuming and would not be practical in a diagnostic laboratory. Hence, it was the aim of this study to develop a sensitive heminested multiplex RT-PCR assay that would enable simultaneous detection and differentiation of genogroup 1 and 2 NLVs in clinical samples.
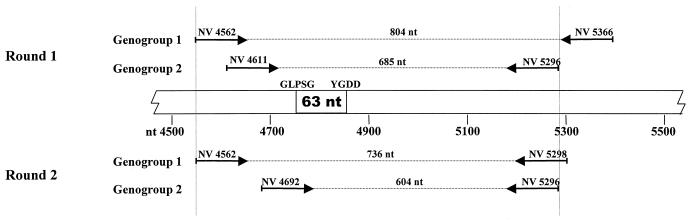
To design NLV genogroup-specific primers, published nucleotide sequences of the putative RNA-dependent RNA polymerase gene within ORF1 of NLV strains were aligned according to genogroups and examined for conserved regions. The GenBank accession numbers (strain identification/year/country of origin) of the published sequences used for genogroup 1 viruses were L07418 (Southampton/1991/United Kingdom), L23832 (Sa-1283/1984/Japan), AF093797 (unknown/Germany), L23828 (KY-89/1989/Japan), and U04469 (Desert Shield/1990/Saudi Arabia), and those used for genogroup 2 viruses were L23826 (925/1992/United Kingdom), L23830 (OTH-25/1989/Japan), U02030 (MV24/1991/Canada), U22498 (Mexico 34/1989/Mexico), L23831 (Snow Mt Agent/1976/United States), X81879 (Melksham/1989/United Kingdom), X86557 (Lordsdale/1993/United Kingdom), U07611 (Hawaii/1971/United States), and U46500 (Camberwell/1994/Australia). From these sequences, two sets of primers were designed for a heminested multiplex PCR that would produce amplicons of different molecular weights for each of the NLV genogroups. Details of these primers, such as name, nucleotide sequence, and corresponding nucleotide position in prototype Norwalk strain NV/8FIIa/68/US (9), are listed in Table 1. The putative RNA polymerase gene segments targeted in the multiplex PCR also include the 63-nucleotide (nt) region between the GLPSG and YGDD motifs (2) that is commonly amplified in the majority of the existing RT-PCR assays (Fig. 1). All of the primers used in this study were synthesized and purified by GeneWorks Pty. Ltd., Adelaide, South Australia, Australia.
TABLE 1.
NLV primers used in this study
| Primer namea (size [nt]) | Primer sequence | Genogroup | Polarity | Locationb |
|---|---|---|---|---|
| NV 4562 (22) | 5′-GATGC(A/G/T)GATTACACAGC(A/C/T)TGGG-3′ | 1 | Positive | 4562–4583 |
| NV 4611 (21) | 5′-C(A/T)GCAGC(A/C)CT(A/G/T)GAAATCATGG-3′ | 2 | Positive | 4611–4631 |
| NV 4692 (23) | 5′-GTGTG(A/G)T(G/T)GATGTGGGTGACTTC-3′ | 2 | Positive | 4692–4714 |
| NV 5296 (21) | 5′-CCA(C/T)CTGAACATTG(A/G)CTCTTG-3′ | 2 | Negative | 5276–5296 |
| NV 5298 (22) | 5′-ATCCAGCGGAACATGGCCTGCC-3′ | 1 | Negative | 5277–5298 |
| NV 5366 (21) | 5′-CATCATCATTTAC(A/G)AATTCGG-3′ | 1 | Negative | 5346–5366 |
Primers NV4562, NV5366, NV4611, and NV5296 were used in the first round. Primers NV4562, NV5298, NV4692, and NV5296 were used in the second round.
Annealing site relative to Norwalk virus (NV/8FIIa/68/US) (GenBank accession no. M87661).
FIG. 1.
Diagrammatic representation of segments within the putative RNA polymerase gene of NLV genogroup 1 and 2 that are targeted in the multiplex RT-PCR assay. The central bar represents the Norwalk virus (NV/F8IIa/68/US) genome (GenBank accession number M87661) beginning at nucleotide 4500. Arrows above and below the bar indicate the relative locations of primers during first- and second-round PCRs, respectively. Numbers above dotted lines indicate the predicted sizes of PCR products. The 63-nt region between the GLPSG and YGDD motifs that has been commonly used by most diagnostic laboratories to detect NLV strains (2) by use of RT-PCR is also shown.
Fecal samples were prepared as 20% (wt/vol) fecal suspensions in Hanks' complete balanced salt solution. The mixtures were then shaken vigorously and centrifuged at 3,500 × g for 15 min at room temperature, and the supernatant was further centrifuged at 7,000 × g for 30 min at 4°C. Clarified fluid collected from the second centrifugation was stored at the temperatures described below prior to testing with the RT-PCR assay or ultracentrifugation for electron microscopy (EM).
Viral RNA was extracted from 5 μl of the clarified extract (diluted to 140 μl with nuclease-free water) with the QIAamp Viral RNA kit (Qiagen GmbH, Hilden, Germany) in accordance with the manufacturer's instructions. RT and first-round PCR were performed by using the Superscript One-step RT-PCR System (Gibco BRL, Gaithersburg, Md.) and a 40-μl reaction mixture containing each deoxynucleoside triphosphate (i.e., dATP, dCTP, dGTP, and dTTP) at 0.2 mM, 1.2 mM MgSO4, each primer (NV4562, NV5366, NV4611, and NV5296) at 0.2 μM, 16 U of RNasin (Promega Corporation, Madison, Wis.), 0.7 μl of the Superscript II RT/Taq mix, and 10 μl of extracted RNA. The sizes of the amplicons produced with primers NV4562 and NV5366 (genogroup 1 NLV) and NV4611 and NV5296 (genogroup 2 NLV) were 805 and 686 bp, respectively. With the GeneAmp 2400 thermal cycler (The Perkin-Elmer Corporation, Norwalk, Conn.), the RT step was carried out at 50°C for 30 min, followed by PCR with initial denaturation at 94°C for 2 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 30 s; and an additional extension step of 72°C for 5 min.
Second-round PCR was performed with the Qiagen Taq DNA polymerase kit (Qiagen GmbH) in a 40-μl reaction mixture containing 0.2 mM each deoxynucleoside triphosphate, 0.2 μM each primer (NV4562, NV5298, NV4692, and NV5296), 4 μl of 10× PCR buffer, 8 μl of Q buffer, 2 U of Taq polymerase, and 2 μl of the first-round PCR mixture. The sizes of the amplicons produced with primers NV4562 and NV5298 (genogroup 1 NLV) and NV4692 and NV5296 (genogroup 2 NLV) were 737 and 605 bp, respectively. Amplification was carried out with the same thermal cycler with initial denaturation at 94°C for 5 min; 25 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 30 s; and a final extension step of 72°C for 5 min.
Agarose gel electrophoresis was performed to visualize the amplified products. Eight microliters of the PCR samples following the second-round amplification were loaded into the wells of a 2% agarose gel (Promega Corporation) containing 25 pg of ethidium bromide per ml and electrophoresed at 100 V for approximately 45 min. DNA Molecular Weight Marker VIII (Roche Diagnostics GmbH, Mannheim, Germany), which contains a mixture of pUCBM21 DNA cleaved with either HpaII or DraI and HindIII, was also electrophoresed on each gel. Bands were visualized on a UV transilluminator, and gel images were digitally recorded with the Gel.Doc 2000 system (Bio-Rad Laboratories, Richmond, Calif.).
DNA sequencing and analysis of amplified products obtained from NLV-positive fecal samples were performed as previously described (12).
The RT-PCR assay was initially tested by using a panel of eight fecal samples which were positive for genogroup 1 (n = 3) and genogroup 2 (n = 5) NLVs. These samples were obtained from either sporadic cases or separate gastroenteritis outbreaks that occurred in Victoria, Australia, in 1995 and 1996 and were identified and genotyped in a previous study in our laboratories (20). All eight fecal samples were processed during 1995 and 1996, and the clarified fluid collected from the second centrifugation was stored either at −70 or −20°C.
The limit of detection for the RT-PCR assay was assessed with 10-fold serial dilutions of two samples selected from the original panel of eight fecal samples, of which one was positive for genogroup 1 NLV and the other was positive for genogroup 2 NLV. The numbers of NLV particles present in the clarified extracts of these two fecal samples were estimated by EM. Approximately 10 ml of the clarified extract was ultracentrifuged through a 1-ml cushion of 45% (wt/vol) sucrose in 2 mM Tris-HCl buffer (pH 7.0) for 2 h at 150,000 × g by using a Beckman SW41 rotor (Beckman Instruments, Inc., Fullerton, Calif.) at 4°C. The supernatant was then discarded, and the pellet was resuspended in 200 μl of Tris-HCl buffer. For each resuspended pellet, 10 μl was negatively stained with 3% phosphotungstic acid (pH 7.0) on 400-mesh Formvar carbon-coated grids (3 mm in diameter, Agar Scientific, Standsted, United Kingdom) and examined with a Philips CM12 electron microscope (Philips, Eindhoven, The Netherlands). At least two grid squares were examined for each sample, and the number of viral particles observed per grid square was recorded. To convert this value into the number of viral particles per 200 μl of ultracentrifuged preparation, the following formula was used: [(area of grid ÷ area per grid square) × (number of viral particles observed per grid square)] × 20, where the area per grid square is 0.0016 mm2. Each 200 μl of ultracentrifuged preparation is equivalent to 10 ml of clarified extract; hence, the number of viral particles per milliliter of clarified extract is 1/10 of the number of viral particles estimated in 200 μl of ultracentrifuged preparation.
The sensitivity of the RT-PCR assay was assessed by using fecal samples collected between 1997 and 1999 from 500 individuals (aged from less than 1 to 56 years) with gastroenteritis. The fecal samples were stored at −70°C and thawed for processing as described above when required. Clarified fluid collected from the second centrifugation was used for RT-PCR testing immediately.
The specificity of the RT-PCR assay was assessed in two ways. Firstly, the assay was performed on three additional fecal samples that were positive by EM for astrovirus, rotavirus, and Sapporo-like virus, respectively. These fecal samples were processed during 1998 and 1999 and stored at −70, −20, or 4°C. Secondly, DNA sequencing was performed on the amplified products of the RT-PCR-positive specimens found among the panel of 500 fecal samples used for sensitivity testing. This was to determine whether the samples contained NLV of the genotype given by the RT-PCR assay.
A comparison of the RT-PCR assay and EM was performed with 95 randomly selected fecal samples that had been tested for NLVs with both methods. All of the samples were positive for the virus with either one or both of the methods, and these results were reviewed.
Multiplex RT-PCR assay.
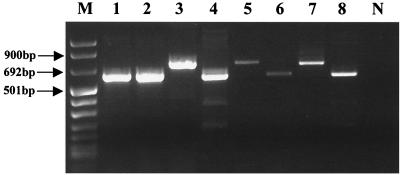
A typical ethidium-stained agarose gel with amplified products following multiplex RT-PCR is shown in Fig. 2. The products were amplified from eight different fecal samples which had been determined previously to contain either genogroup 1 or 2 NLV (20). In relation to the molecular weight standard, the sizes of the amplicons produced from the two NLV genogroups are in accordance with their predicted molecular sizes, which are 737 and 605 bp for genogroups 1 and 2, respectively. The difference in size between these amplicons is obvious when they are separated by agarose gel electrophoresis.
FIG. 2.
Typical agarose gel containing a mixture of genogroup 1 and 2 NLV PCR products. Lanes: 3, 5, and 7, genogroup 1 viruses; 1, 2, 4, 6, and 8, genogroup 2 viruses; M and N, DNA molecular size markers and a negative control, respectively.
Limit of detection.
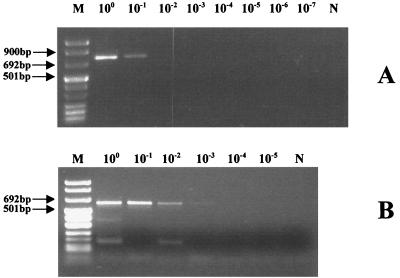
Quantification of NLV is difficult because they cannot be cultured by in vitro methods. Therefore, the limit of detection for RT-PCR assays that are designed for NLV detection is not usually determined. However, this problem was overcome in this study with the use of EM. To determine the limit of detection for the RT-PCR assay, 10-fold serial dilutions of fecal samples known to contain either genogroup 1 or 2 NLV (20) were used. Based on the quantification method described above, the concentrations of viruses in the clarified extracts used for analysis were approximately 2 × 105 viral particles/ml for genogroup 1 and 9 × 104 viral particles/ml for genogroup 2. The highest dilutions at which the multiplex PCR assay was able to exhibit a positive result were 10−1 and 10−2 for genogroups 1 and 2, respectively (Fig. 3). Thus, the limit of detection for the assay ranged from 5 (genogroup 2) to 100 (genogroup 1) viral particles per 5 μl of clarified fecal extract.
FIG. 3.
Sensitivity testing of the multiplex PCR assay with 10-fold serial dilutions of clarified fecal extracts containing either genogroup 1 or 2 NLV. (A) Fecal sample with genogroup 1 virus at a concentration of approximately 2 × 105 viral particles/ml of clarified extract. (B) Fecal sample with genogroup 2 virus at a concentration of approximately 9 × 104 viral particles/ml of clarified extract. Lanes M and N, DNA molecular size markers and a negative control, respectively.
Sensitivity testing.
Of the 500 fecal samples collected from individuals with gastroenteritis, 63 (12.6%) yielded positive results with the multiplex RT-PCR assay. The sensitivity of the assay could not be determined in absolute terms, but it may be noted that the proportion of fecal samples found to be positive by RT-PCR (12.6%) is comparable to those of previous studies using RT-PCR or enzyme immunoassay. These studies found that NLVs accounted for approximately 20% of children with gastroenteritis between 2 months and 2 years of age (15) and approximately 10% of adults with gastroenteritis within a specific setting (3).
Specificity testing.
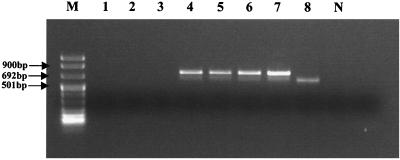
The specificity of the primers used to amplify ORF1 segments within the genome of NLVs was assessed by demonstrating the absence of amplification from fecal samples that contained other major RNA gastroenteritis viruses (astrovirus, rotavirus, and Sapporo-like virus). To ensure that the negative results were not due to inhibition, total RNA extracts from these fecal samples were also spiked with RNA extracted from a fecal sample which contained genogroup 1 virus. The results obtained were as expected, in that only those fecal samples that contained either NLV or were spiked with RNA extracted from NLV produced PCR bands (Fig. 4).
FIG. 4.
Specificity testing of multiplex PCR assay. Lanes: 1 to 3, samples containing astrovirus, rotavirus, and Sapporo-like virus, respectively; 4 to 6, the same samples spiked with 5 μl of RNA extracted from a genogroup 1-positive control; 7 and 8, NLV genogroup 1 and 2 positive controls, respectively; N, negative control; M, DNA molecular size markers.
The specificity of the RT-PCR assay was further assessed by DNA sequencing of amplicons produced from the 63 fecal samples that gave positive amplification results during sensitivity testing. Based on the sizes of the amplicons produced, viruses detected in 9 of the fecal samples belonged to genogroup 1 while those detected in the remaining 54 fecal samples belonged to genogroup 2. To confirm these genotype results, DNA sequencing was successfully performed on 62 of these amplicons. Comparison of their nucleotide sequences with equivalent published NLV nucleotide sequences (12) demonstrated a specificity level of 100% for the multiplex RT-PCR assay. All amplicons with a size of 737 bp (corresponding to that for genogroup 1 virus) had nucleotide sequences very similar to those published for genogroup 1 NLVs, whereas amplicons with a size of 605 bp (corresponding to that for genogroup 2 virus) had nucleotide sequences similar to those published for genogroup 2 NLVs.
Comparison of RT-PCR assay and EM.
To assess the sensitivity level of the multiplex RT-PCR assay in relation to that of EM, 95 fecal samples that were positive for NLV by either EM or RT-PCR were chosen for analysis. The RT-PCR assay was able to detect NLV in 92 (96.8%) of the samples tested but failed to detect NLV in 3 that were positive by EM. However, only 16 (16.8%) of the fecal samples tested were found to be positive by EM. Based on these results, the multiplex RT-PCR assay developed was approximately sixfold more sensitive than EM. This ratio of sensitivities is significantly higher than those reported in recent studies in which RT-PCR was found to be 3.2-fold (11) and 1.37-fold (7) more sensitive than EM for NLV detection. However, it should be noted that the multiplex RT-PCR assay was developed for high levels of sensitivity and specificity. Hence, primers were designed to target the most conserved regions of the putative RNA polymerase gene for the two NLV genogroups separately. These conserved regions were determined with numerous published nucleotide sequences of NLV strains that were identified in different regions of the world, thus increasing the range of NLV strains that could be detected by the multiplex RT-PCR assay.
In summary, this paper describes a multiplex RT-PCR assay that enables simultaneous detection and differentiation of genogroup 1 and 2 NLVs. The results obtained showed that the assay is highly sensitive for NLV. The method is sufficiently specific for differentiation of the two genogroups, and a broad range of NLV strains were identified in a survey using the technique. The assay is rapid, with a turnaround time of approximately 2 days, and the methods are simple and, hence, suitable for a diagnostic laboratory that is routinely performing some molecular testing. The greatly enhanced sensitivity of the assay compared to that of EM should facilitate the identification of NLV and provide valuable information on the incidence and transmission of the virus in public health settings.
Acknowledgments
We thank Anna Dimitriadis for excellent technical assistance.
REFERENCES
- 1.Ando T, Jin Q, Gentsch J R, Monroe S S, Noel J S, Dowell S F, Cicirello H G, Kohn M A, Glass R I. Epidemiologic applications of novel molecular methods to detect and differentiate small round structured viruses (Norwalk-like viruses) J Med Virol. 1995;47:145–152. doi: 10.1002/jmv.1890470207. [DOI] [PubMed] [Google Scholar]
- 2.Ando T, Noel J S, Fankhauser R L. Genetic classification of “Norwalk-like viruses.”. J Infect Dis. 2000;181(Suppl. 2):S336–S348. doi: 10.1086/315589. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeois A L, Gardiner C H, Thornton S A, Batchelor R A, Burr D H, Escamilla J, Echeverria P, Blacklow N R, Herrmann J E, Hyams K C. Etiology of acute diarrhea among United States military personnel deployed to South America and west Africa. Am J Trop Med Hyg. 1993;48:243–248. doi: 10.4269/ajtmh.1993.48.243. [DOI] [PubMed] [Google Scholar]
- 4.Dingle K E, Lambden P R, Caul E O, Clarke I N. Human enteric Caliciviridae: the complete genome sequence and expression of virus-like particles from a genetic group II small round structured virus. J Gen Virol. 1995;76:2349–2355. doi: 10.1099/0022-1317-76-9-2349. [DOI] [PubMed] [Google Scholar]
- 5.Gellert G A, Waterman S H, Ewert D, Oshiro L, Giles M P, Monroe S S, Gorelkin L, Glass R I. An outbreak of acute gastroenteritis caused by a small round structured virus in a geriatric convalescent facility. Infect Control Hosp Epidemiol. 1990;11:459–464. doi: 10.1086/646212. [DOI] [PubMed] [Google Scholar]
- 6.Glass P J, White L J, Ball J M, Leparc-Goffart I, Hardy M E, Estes M K. Norwalk virus open reading frame 3 encodes a minor structural protein. J Virol. 2000;74:6581–6591. doi: 10.1128/jvi.74.14.6581-6591.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonin P, Couillard M, d'Halewyn M A. Genetic diversity and molecular epidemiology of Norwalk-like viruses. J Infect Dis. 2000;182:691–697. doi: 10.1086/315780. [DOI] [PubMed] [Google Scholar]
- 8.Green J, Norcott J P, Lewis D, Arnold C, Brown D W G. Norwalk-like viruses: demonstration of genomic diversity by polymerase chain reaction. J Clin Microbiol. 1993;31:3007–3012. doi: 10.1128/jcm.31.11.3007-3012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Wang M, Wang K, Estes M K. Sequence and genomic organization of Norwalk virus. Virology. 1993;195:51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 10.Lambden P R, Caul E O, Ashley C R, Clarke I N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science. 1993;259:516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- 11.Maguire A J, Green J, Brown D W G, Desselberger U, Gray J J. Molecular epidemiology of outbreaks of gastroenteritis associated with small round-structured viruses in East Anglia, United Kingdom, during the 1996–1997 season. J Clin Microbiol. 1999;37:81–89. doi: 10.1128/jcm.37.1.81-89.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall J A, Yuen L K, Catton M G, Gunesekere I C, Wright P J, Bettelheim K A, Griffith J M, Lightfoot D, Hogg G G, Gregory J, Wilby R, Gaston J. Multiple outbreaks of Norwalk-like virus gastro-enteritis associated with a Mediterrean-style restaurant. J Med Microbiol. 2001;50:143–151. doi: 10.1099/0022-1317-50-2-143. [DOI] [PubMed] [Google Scholar]
- 13.Noel J S, Ando T, Leite J P, Green K Y, Dingle K E, Estes M K, Seto Y, Monroe S S, Glass R I. Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J Med Virol. 1997;53:372–383. doi: 10.1002/(sici)1096-9071(199712)53:4<372::aid-jmv10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Norcott J P, Green J, Lewis D, Estes M K, Barlow K L, Brown D W G. Genomic diversity of small round structured viruses in the United Kingdom. J Med Virol. 1994;44:280–286. doi: 10.1002/jmv.1890440312. [DOI] [PubMed] [Google Scholar]
- 15.Pang X L, Honma S, Nakata S, Vesikari T. Human caliciviruses in acute gastroenteritis of young children in the community. J Infect Dis. 2000;181(Suppl. 2):S288–S294. doi: 10.1086/315590. [DOI] [PubMed] [Google Scholar]
- 16.Riordan T. Norwalk-like viruses and winter vomiting disease. In: Morgan-Capner P, editor. Current topics in clinical virology. London, England: Public Health Laboratory Service; 1991. pp. 61–94. [Google Scholar]
- 17.Russo P L, Spelman D W, Harrington G A, Jenney A W J, Gunesekere I C, Wright P J, Doultree J C, Marshall J A. Hospital outbreak of Norwalk-like virus. Infect Control Hosp Epidemiol. 1997;18:576–579. doi: 10.1086/647676. [DOI] [PubMed] [Google Scholar]
- 18.Ryan M J, Wall P G, Adak G K, Evans H S, Cowden J M. Outbreaks of infectious intestinal disease in residential institutions in England and Wales 1992–1994. J Infect. 1997;34:49–54. doi: 10.1016/s0163-4453(97)80009-6. [DOI] [PubMed] [Google Scholar]
- 19.Seah E L, Marshall J A, Wright P J. Open reading frame 1 of the Norwalk-like virus Camberwell: completion of sequence and expression in mammalian cells. J Virol. 1999;73:10531–10535. doi: 10.1128/jvi.73.12.10531-10535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright P J, Gunesekere I C, Doultree J C, Marshall J A. Small round-structured (Norwalk-like) viruses and classical human caliciviruses in southeastern Australia, 1980–1996. J Med Virol. 1998;55:312–320. [PubMed] [Google Scholar]