Abstract
Aims:
Insulin potentiates glucose-stimulated insulin secretion. These effects are attenuated in beta cell–specific insulin receptor knockout mice and insulin resistant humans. This investigation examines whether short duration insulin exposure regulates beta cell responsiveness to arginine, a non-glucose secretagogue.
Materials and Methods:
Arginine-stimulated insulin secretion was studied in 10 healthy humans. In each subject arginine was administered as a bolus followed by continuous infusion on two occasions one month apart, after sham/saline or hyperinsulinemic-isoglycemic clamp, respectively providing low and high insulin pre-exposure conditions. Arginine-stimulated insulin secretion was measured by C-peptide deconvolution, and by a selective immunogenic (DAKO) assay for direct measurement of endogenous but not exogenous insulin.
Results:
Pre-exposure to exogenous insulin augmented arginine-stimulated insulin secretion. The effect was seen acutely following arginine bolus (endogenous DAKO insulin incremental AUC240–255min 311.6±208.1 (post-insulin exposure) versus 120.6±42.2 μU/ml•min (sham/saline) (t-test P=0.021)), as well as in response to continuous arginine infusion (DAKO insulin incremental AUC260–290min 1095.3±592.1 (sham/saline) versus 564.8±207.1 μU/ml•min (high insulin)(P=0.009)). Findings were similar when beta cell response was assessed using C-peptide, insulin secretion rates by deconvolution, and the C-peptide to glucose ratio.
Conclusions:
We demonstrate a physiologic role of insulin in regulation of the beta cell secretory response to arginine.
Keywords: Arginine, Beta cell regulation, insulin resistance, insulin secretion
Introduction:
Type 2 diabetes is characterized by defective insulin action and secretion. Previous studies demonstrate pancreatic beta cells are responsive to insulin and insulin-like growth factor −1 (IGF-1). Insulin/IGF-1 receptors and their signaling proteins are present and participate in regulation of insulin secretion in rodent pancreatic beta cells and in humans [1–5]. In vitro insulin potentiates glucose-stimulated insulin secretion in mouse and human isolated beta cells [6]. In beta cell-specific insulin receptor knockout (βIRKO) mice, glucose-stimulated insulin secretion is defective, and animals develop progressive glucose intolerance [7]. We [8–10] and others [11, 12] have demonstrated that pre-exposure to insulin potentiates glucose-stimulated insulin secretion in healthy humans and this effect is attenuated in impaired glucose tolerance and type 2 diabetes. Together, multiple lines of evidence now show beta cells are insulin/IGF-1 responsive in rodents and humans in vivo, supporting that diminished insulin secretory response to glucose in type 2 diabetes might be related to defective beta cell insulin/IGF-1 signaling.
Arginine is another potent physiologic stimulus for insulin secretion [13, 14]. Arginine-stimulated insulin secretion provides a clinical measure of beta cell functional mass and secretory capacity [15–17]. Arginine-stimulated insulin secretion remains present in type 1 diabetes for a period of time after glucose stimulated insulin secretion is reduced [18]. Similarly, in type 2 diabetes arginine-stimulated insulin secretion is better preserved than that to glucose, with the response modulated by disease duration and anti-diabetic therapies [19]. Although glucose-stimulated insulin secretion is impaired in βIRKO islets, arginine-stimulated secretion is preserved [7], suggesting in rodents effects of arginine are independent of, or not mediated entirely by, insulin receptor signaling. In vivo interactions in humans have not previously been examined.
To evaluate the role of insulin to modulate the insulin secretory response to a non-glucose stimulus, we studied effects of pre-exposure to raised insulin concentrations on insulin secretory response to arginine in healthy humans.
Materials and Methods
Study Approval
The Joslin Committee on Human Studies approved these investigations. Written informed consent was obtained from all participants prior to study initiation.
Study Design
Subjects were recruited from newspapers, posted flyers, and web-based postings. Participants included 10 healthy persons, with no first-degree relative with diabetes, on no prescription medications other than oral contraceptives. Participants reported receiving contraception only for birth control, known menstrual disorders were exclusionary. Each participant underwent two study visits during which they underwent either a 4-hour saline infusion (sham/saline clamp providing conditions of low/physiologic insulin exposure, as a time and infusion volume control), or a hyperinsulinemic (high insulin exposure) iso-glycemic clamp (Figure 1) in a crossover study design. All paired studies were conducted approximately four weeks apart to minimize hormonal cycle effects. Participants were masked to the order of the clamps, sham/saline or insulin. Prior to study visits participants were instructed to refrain from vigorous exercise and consume 250 grams or more of carbohydrate per day for three days, and to fast overnight for 10–12 hours. Upon presentation to the clinical research center, an intravenous catheter was inserted into each arm, one for infusions and the other for blood sampling. The arm used for phlebotomy was placed into a heated box to ensure arterialization of venous blood [20, 21]. Potassium chloride (KCl) was administered at 10 mEq/h to prevent hypokalemia during both clamps.
Figure 1: Schematic of study protocol performed in healthy humans.
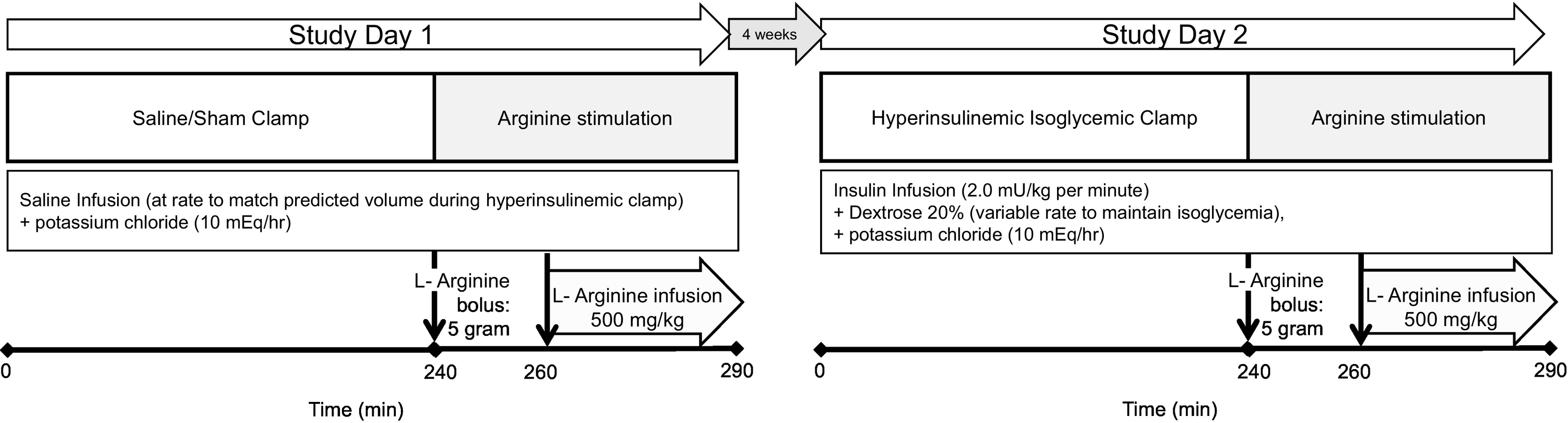
Each participant underwent two study visits during which either a 4-hour sham/saline infusion (Study Day 1, left) or a hyperinsulinemic clamp (Study Day 2, right) was performed, and then on both occasions arginine was administered to stimulate endogenous insulin secretion as both a 5g intravenous bolus (at time 240 minutes), and a 500 mg/kg continuous infusion (time 260–290 minutes).
On the day of the first visit, for the sham/saline clamp, saline was infused at the volume rate hypothetically required to maintain euglycemia during a hyperinsulinemic clamp during which insulin at 14 pmol/kg per minute (2.0 mU/kg per minute) would be administered [8, 22] in a person with similar insulin sensitivity, which corresponded to the volume calculated for a glucose utilization of 10 mg/kg/minute. Saline infusion for 240 minutes was followed by intravenous administration of arginine, first as a 5g bolus over 30 seconds (at time 240 minutes), followed by 500 mg/kg continuous infusion initiated at 260 minutes and administered over 30 minutes, to induce arginine-stimulated insulin secretion. The sham/saline clamp was performed first as there is a small but statistically significant decline in glucose concentrations with the prolonged fast and the timed glucose value from the first study was used as the glycemic target for the hyperinsulinemic clamp. The second visit (high insulin) occurred four weeks later. B28-Asp insulin (Novolog™, Novo Nordisk, Bagsvaerd, Denmark), with relative receptor binding and in vitro potency similar to regular human insulin [23] but immunologically distinguishable from endogenous insulin, was administered as a two-stepped primed (56 pmol/kg per minute followed by 28 pmol/kg per min, each for 5 minutes), continuous infusion (14 pmol/kg per minute (2.0 mU/kg per minute))[8–10]. Infusion of 20% dextrose at variable rate was used to maintain isoglycemia, to match but not exceed individual plasma glucose concentrations during the prior sham/saline condition. After 240 minutes arginine was administered as before. During arginine administration insulin was continued, as well as 20% dextrose, adjusted as needed to match glycemia to the sham/saline clamp. Subjects were masked to whether the sham or insulin clamp was being performed on a given study day.
Assays
Glycohemoglobin was assessed by high-performance liquid chromatography (HPLC)(Tosho 2.2; Tosho Bioscience), and potassium, total cholesterol, high density lipoprotein, and triglycerides were measured in the clinical laboratory of the Joslin Diabetes Center (Beckman Synchron CX9). Serum glucose was measured using the glucose oxidase method (YSI 2300 STAT). Immunoassays were performed in duplicate in Joslin’s Specialized Assay Core Facility (DERC) using commercial assay kits for total insulin, measuring both endogenous (secreted) and exogenous (administered) insulin, and C-peptide (RIA; Diagnostic Systems Laboratories, Webster, TX, USA), with endogenous serum insulin assayed using an ELISA that would not detect the administered B28-Asp insulin (DAKO Insulin ELISA; DakoCytomation, Carpinteria, CA, USA). Additional assays included serum free fatty acids (FFA; NEFA ELISA, Wako Chemicals, Richmond, VA, USA), proinsulin (total proinsulin, Mercodia, Uppsala, Sweden), cortisol (Diasorin, Saluggia, Italy), and glucagon (Millipore, Billerica, MA, USA).
Statistics and Calculations
The primary study endpoint was the difference in rate of endogenous insulin secretion in response to arginine following pre-exposure to low (saline) versus high insulin conditions. Sample size estimates were based on the change in area under the curve for C-peptide with a magnitude of change and coefficient of variation similar to changes in the studies to evaluate the effect of insulin to potentiate the beta cell response to glucose in healthy humans [8], and ten subjects per group would permit detection of a 40% change in C-peptide response to arginine, with 80% power and an alpha of 0.05. Endogenous insulin release in response to arginine in humans was assessed in four ways: 1) a direct measurement of endogenous insulin secretion using the DAKO insulin assay (DakoCytomation, Carpinteria, CA, USA) which detects endogenous insulin, but not the immunologically distinct B28-Asp insulin exogenously administered; 2) C-peptide, as a proxy for insulin secretion; 3) insulin secretion rate (ISR), calculated from plasma C-peptide by the deconvolution method, using I(nsulin-)SEC(retion) (ISEC, Version 3.4a, Hovorka, 1994) and population estimates of C-peptide kinetics [27]; and 4) to account for potential differences in glycemia between the two study conditions, which alone could account for any potentially observed difference in the insulin secretory response, C-peptide to glucose ratio was calculated at each study time point. Finally, because arginine bolus led to modest differences in glucose at the start of arginine administration, we calculated fold change in C-peptide to glucose ratios using average values between 230 to 240 minutes (prior to arginine bolus) and 260 minutes (prior to start of arginine continuous infusion).
For measures of insulin secretion, including as above direct endogenous insulin measurement (DAKO), C-peptide, ISR by deconvolution, and C-peptide to glucose ratio, we compared the response to arginine following pre-exposure to hyperinsulinemic or sham/saline conditions both in response to acute arginine bolus (at 240 minutes) and arginine infusion (260 to 290 minutes), using mixed model repeated-measures analysis, with autoregressive structure (AR(1)) as repeated covariance type, and both time and study condition (sham/saline or insulin) in the model. A post hoc analysis was performed to assess for potential heterogeneity in response between male and female subjects. Results are presented as mean ± standard error. Categorical data were analyzed using χ2-testing. P-values <0.05 were considered significant. Analysis was performed using SPSS (SPSS Inc., Version 17.0. Chicago, IL).
Results
Participant Characteristics:
Twenty subjects were evaluated for study participation. Ten were excluded, due to overweight (n=1), large weight loss in the preceding year (n=1), mother with gestational diabetes (n=1), sister with polycystic ovarian disease (n=1), diagnosed with dyslipidemia (n=1), poor intravenous access (n=1), and inability to schedule the long physiologic study visits (n=4).
Clinical and metabolic characteristics of the ten subjects in the clamp studies are shown (Table 1). Schema of the infusion protocols are summarized in Figure 1. Participants were insulin sensitive (M-value of glucose utilization during isoglycemic-hyperinsulinenic clamp at 240 minutes: 11.2±1.5 mg/kg/minute).
Table 1:
Clinical and metabolic characteristics of human study subjects.
| Age (years) | 27.4 ± 2.6 |
| Sex | 5 Male / 5 Female |
| Insulin Sensitivity (M240 mins mg/kg/minute) | 11.2 ± 1.5 |
| Systolic BP (mm Hg) | 107.0 ± 5.8 |
| Diastolic BP (mm Hg) | 68.8 ± 4.2 |
| Height (m) | 1.7 ± 0.1 |
| Weight (kg) | 68.7 ± 6.4 |
| Waist (cm) | 80.8 ± 5.8 |
| BMI (kg/m2) | 22.7 ± 1.1 |
| Hematocrit (proportion of 1.0) (%) | 0.416 ± 0.023 (41.6 ± 2.3) |
| HbA1c (mmol/mol) (%) | 36 ± 0.9 (5.4 ± 0.2) |
| Fasting insulin (μU/mL) (pmol/ml) | 4.37 ± 1.41 (30.3 ± 9.8) |
| Fasting C-peptide (ng/mL) (nmol/L) | 0.86 ± 0.35 (0.29 ± 0.12) |
| Fasting Glucose (mmol/L) (mg/dl) | 4.23 ± 0.31 (76.2 ± 5.6) |
| Cholesterol (mmol/L) (mg/dl) | 4.48 ± 0.36 (173.0 ± 13.8) |
| Triglycerides (mmol/L) (mg/dL) | 0.88 ± 0.18 78.2 ± 16.3 |
| HDL (mmol/L) (mg/dL) | 1.85 ± 0.30 (71.3 ± 11.6) |
| Direct LDL (mmol/L) (mg/dL) | 2.28 ± 0.35 (88.2 ± 13.5) |
| TSH (IU/mL) | 1.9 ± 0.5 |
Clinical and metabolic characteristics of human study subjects are presented as mean ± standard error. Conversions of Scientific International to Conventional units: glucose (mmol/L) ÷ 0.0555 for mg/dl; insulin (pmol/L) ÷ 6.945 for μU/ml; C-peptide (nmol/L) ÷ 333 for ng/ml; cholesterol, HDL, and LDL (mmol/L) ÷ 0.0259 for mg/dl; triglycerides (mmol/L) ÷ 0.0113 for mg/dl. Body Mass Index (BMI), Blood Pressure (BP), Low Density Lipoprotein (LDL), High Density Lipoprotein (HDL), Thyroid Stimulating Hormone (TSH).
Glycemia During Clamp Studies:
Overall, fasting and subsequent plasma glucose concentrations were comparable during sham/saline (low insulin) and hyperinsulinemic clamps from 0 to 240 minutes (mixed model repeated measures0–240min P=0.771) (Figure 2A), despite transiently lower levels following insulin exposure prior to achievement of steady state. On the sham/saline day, plasma glucose rose immediately following arginine bolus and infusion, consistent with prior reports of arginine effects on plasma glucose [28, 29]. On the hyperinsulinemic study day, glucose levels were not fully matched to the sham/saline day during the arginine bolus and infusion consistent with the goal to match but not exceed individual plasma glucose during the sham/saline day, resulting in plasma glucose levels modestly lower from 240 to 255 minutes (P=0.002) and 260 to 290 minutes (P=0.034) (Figures 2A and 3A). These differences were statistically significant, but it is critical to highlight that plasma glucose levels were lower after insulin pre-exposure compared to sham/saline, which would be expected to result in reduced beta cell response.
Figure 2: Increased arginine-stimulated insulin secretion after insulin pre-exposure in healthy humans.
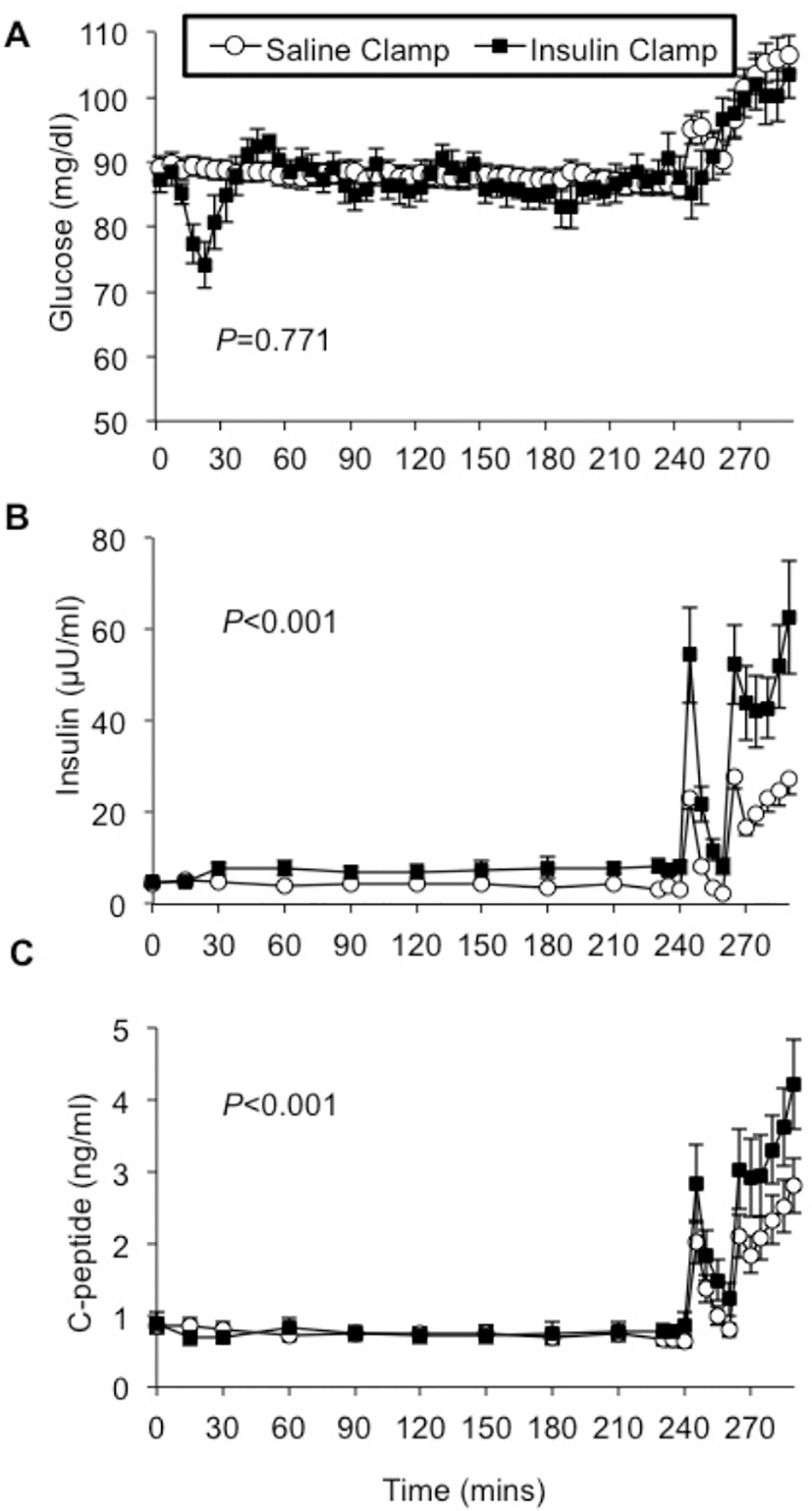
Each participant underwent two study visits during which either a 4-hour sham/saline infusion or a hyperinsulinemic clamp was performed, and then arginine was administered to stimulate endogenous insulin secretion as both a 5g intravenous bolus (at time 240 minutes), and a 500 mg/kg continuous infusion (time 260–290 minutes). Plasma glucose levels were overall well matched throughout both studies after stabilization and before arginine [A]. Arginine bolus and infusion increased endogenous insulin [B] and C-peptide [C] concentrations, and these responses were significantly augmented after insulin pre-exposure. Saline clamp (○), insulin clamp (●).
Figure 3: Increased arginine-stimulated insulin secretion corrected for glycemia after insulin pre-exposure in healthy humans.
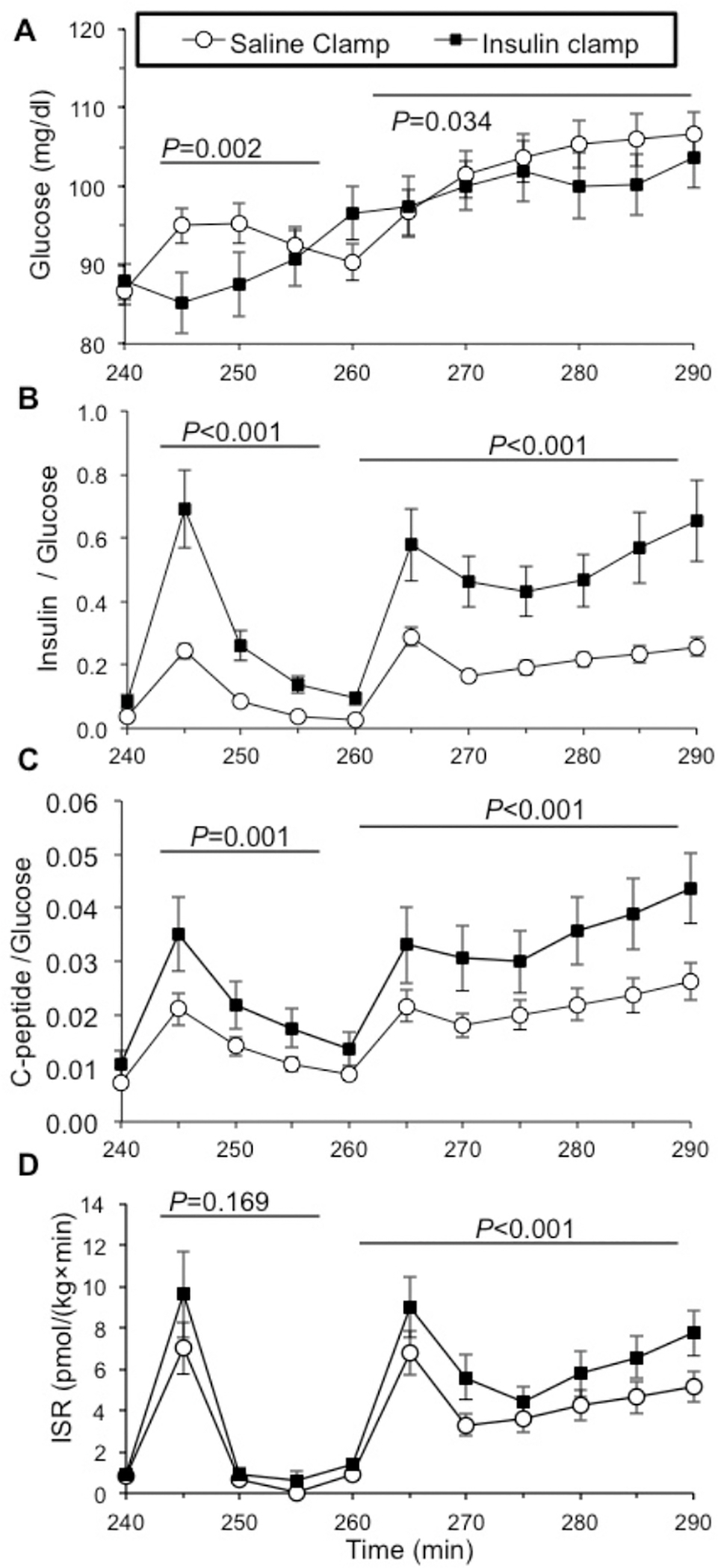
During arginine stimulation (by intravenous bolus at time 240 minutes and continuous infusion time 260–290 minutes) the mean plasma glucose concentrations were lower following hyperinsulinemic compared with saline pre-exposure [A]. To account for potential confounding of different glucose concentrations achieved during the two study conditions, the insulin to glucose [B] and C-peptide-to-glucose [C] ratios were calculated, and found higher in response to arginine stimulation with hyperinsulinemia. The insulin secretion rate (ISR) estimated using the C-peptide deconvolution method was also higher in response to arginine bolus and infusion after insulin compared to saline pre-exposure [D]. After insulin pre-exposure there was a trend toward higher ISR in response to acute bolus arginine administration (P=0.169), and a significantly higher ISR response to arginine infusion (P<0.001). Saline clamp (○), insulin clamp (●).
Arginine-Stimulated Insulin Secretion:
Beta cell function (insulin and C-peptide responses) to arginine bolus and infusion was assessed by the DAKO ELISA assay that recognizes only endogenous insulin, as well as by C-peptide secretion, and by Insulin Secretion Rate (ISR) as calculated by deconvolution [27].
Insulin response to a 5g arginine bolus was higher after pre-exposure to 4 hours of high physiologic insulin concentrations, compared to sham/saline, as assessed by direct measurement of endogenous insulin (DAKO assay) with insulin incremental AUC240–255min 120.6±42.2 (sham/saline) versus 311.6±208.1 μU/ml•min (insulin)(T-test P=0.021) and mixed model repeated measures analysis for DAKO insulin values 240–255min (sham/saline versus insulin pre-exposure P<0.001) (Figure 2B). Likewise, C-peptide concentrations after arginine bolus were higher following high insulin pre-exposure (mixed model repeated measures analysis of C-peptide values 240–255min, sham/saline versus insulin pre-exposure, P=0.005) (Figure 2C). Both insulin-to-glucose ratio (mixed model repeated measures comparing values 240–255min, P<0.001) (Figure 3B) and C-peptide-to-glucose ratio demonstrated increased response after high insulin compared with sham/saline pre-exposure (mixed model repeated measures analysis comparing values 240–255min, P=0.001) (Figure 3C). Fold change in C-peptide to glucose ratios from baseline (from minutes 230 to 240) was similarly augmented following insulin pre-exposure (mixed model repeated measures analysis comparing values 240–255 min, P=0.001, not shown), consistent with modest differences in glucose after arginine not impacting the augmenting effect of insulin pre-exposure. ISR by deconvolution in response to 5g arginine bolus was numerically higher but did not reach statistical significance (mixed model repeated measures analysis comparing values 240–255 min, P=0.169) (Figure 3D).
In response to the 30-minute continuous arginine infusion, the beta cell functional response was also higher after pre-exposure to high insulin than after sham/saline, as assessed by direct measurement of endogenous insulin (DAKO assay, with DAKO insulin incremental AUC260–290min 564.8±207.1 (sham/saline) versus 1095.3±592.1 μU/ml•min (high insulin)(P=0.009), and mixed model repeated measures comparing values 260–290 min, P<0.001) (Figure 2B). C-peptide concentrations were also increased following high insulin compared with sham/saline pre-exposure with C-peptide incremental AUC260–290min 39.4±19.6 (sham/saline) versus 55.9±28.1 ng/ml•min (high insulin)(P=0.071) and compared by mixed model repeated measures (values 260–290min, P<0.001) (Figure 2C). C-peptide to glucose ratio was also higher after insulin infusion (mixed model repeated measures comparing sham/saline versus insulin exposure values 260–290min, P<0.001) (Figure 3C), as was fold change in C-peptide to glucose ratio, compared to the baseline value of the mean C-peptide to glucose ratio at 260 minutes (P<0.001, not shown). The calculated ISR by deconvolution in response to continuous arginine infusion was greater after pre-exposure to insulin compared to saline (comparison of sham/saline vs. insulin exposure values 260–290min, P<0.001) (Figure 3D). Finally, post hoc analysis by sex suggests all beta cell responses to arginine following insulin pre-exposure are more robust in male compared to female subjects.
Discussion
Multiple lines of evidence now support altered insulin/IGF-1 signaling within the beta cell itself contributes to beta cell dysfunction and type 2 diabetes pathogenesis. In vitro and in vivo studies demonstrate insulin/IGF-1 signaling pathways regulate beta cell insulin processing [30] and modulate glucose-stimulated insulin secretion in mouse and man. Insulin exposure augments glucose-stimulated insulin secretion in rodents [7, 31], isolated human islets [3], and healthy humans in vivo [8, 10], but is impaired in βIRKO mice [7] and in humans with impaired glucose tolerance and type 2 diabetes [9, 11, 12]. These observations are further supported by the recent identification of a protein that is able to calibrate insulin action selectively in beta cells [32]. Whether insulin directly regulates beta cell secretory response to other physiologic stimuli was previously unknown. We now show pre-exposure to insulin potentiates beta cell insulin secretory response to arginine in humans with normal glucose tolerance.
Variations in glycemia strongly effect insulin secretion, so consideration of plasma glucose differences between the sham/saline and high insulin conditions is important. In our studies, plasma glucoses were well matched prior to arginine stimulation. Following arginine infusion, there was a rapid rise in plasma glucose during sham/saline clamp (Figure 3A), in contrast to modestly lower glucose concentrations during high insulin infusion; therefore this difference in glucose concentrations does not account for increased arginine-stimulated insulin secretion observed after insulin pre-exposure.
To confirm our findings we assessed in vivo insulin secretion by multiple methodologies, and found that all of them supported insulin augments arginine-stimulated insulin secretion. We previously described methodology to distinguish endogenous insulin in the presence of biologically equivalent but immunologically distinct analog insulin, using a selective immunoassay [8]. We also present data on change in C-peptide concentrations and insulin secretion rates by deconvolution, as most studies use C-peptide to estimate beta cell function, with caveats that C-peptide clearance is modestly increased during hyperinsulinemia [8, 10] and intracellular insulin processing may alter insulin to C-peptide secretion rates [30, 33], which could introduce bias. Finally, to account for potential differences in glycemia between study conditions, we calculated insulin-to-glucose and C-peptide-to-glucose ratios at each study time point. Estimates of insulin secretion using ISR by deconvolution differences are somewhat more modest than those using insulin or C-peptide measures alone, and while our methods do not specifically address an explanation for this, it could represent that the experimental conditions change hepatic insulin extraction. However, despite some differences in magnitude of effect, our findings of increased beta cell response to arginine following exposure to high insulin compared to sham/saline were consistent across all of the approaches used to quantify of beta cell response.
Interpretation of our findings must take into account several aspects of experimental design. Four to 5 grams of arginine can be found in common food portions including 4 ounces of chicken breast or one cup of soybeans, so the arginine exposure used in our study is physiologically relevant. High doses of insulin were chosen for these investigations based on doses previously shown to augment glucose-stimulated insulin secretion [8–10]; effects of lower insulin doses or shorter exposures on insulin secretion remain unknown. Study limitations include the recognition that peripheral blood sampling provides only indirect assessment of insulin secretion, and that our findings do not fully exclude the possibility that an augmented response to arginine after exogenous insulin was attributable to beta cell rest during insulin infusion, subsequently permitting a more exuberant response to the secretory stimulus. However, achieving isoglycemia, and including a volume control, prior to arginine stimulation is the best way to match the metabolic milieu that beta cells were exposed to prior to arginine stimulation. Finally, the study population intended to reflect the general population but post hoc analysis suggest the beta cell response to arginine following insulin pre-exposure is more robust in male compared to female subjects. This observation could be the result of chance, given no a priori hypothesis or underlying mechanism, and the two subsets are underpowered to confirm a difference by sex, thus additional studies would be warranted to examine this potential heterogeneity.
Various in vitro and in vivo studies have supported arginine’s beneficial effects on glucose metabolism and insulin sensitivity [3, 43, 44]. Insulin increases L-arginine transport in vitro in human umbilical vein endothelium cells (HUVEC) by increasing SLC7A1 promoter activity [37] and cationic amino acid transporter 1 activity and expression [38, 39]. Increased L-arginine transport has been proposed as the mechanism that underlies insulin-induced HUVEC relaxation [37]. In HUVEC from women with gestational diabetes or pre-eclampsia, increased L-arginine transport [40, 41] is reported to maintain effects of insulin on HUVEC vasodilation [42]. Furthermore, L-arginine supplementation improves insulin sensitivity (and insulin-mediated vasodilation) in healthy persons and in obesity, type 2 diabetes, and coronary artery disease [3, 43, 44]. L-Arginine may improve glucose transport and glycogen synthesis through enhanced signal transduction and direct activation of AKT and AMPK pathways in rat skeletal muscle. Plasma arginine levels are reduced in diabetes [45]. Our detailed human physiology studies expand our understanding to the in vivo effects of arginine on insulin secretion.
The scientific question addressed in this study is whether insulin impacts the beta cell secretory response to the non-glucose secretagogue, arginine, in healthy humans. In a future study the evaluation of insulin resistant individuals such as those with obesity or type 2 diabetes would be necessary to address whether this effect is diminished in insulin resistant states, as has been seen with diminished insulin potentiation of the beta cell response to glucose in insulin resistant compared to insulin sensitive persons [8–12]. Other studies that address differences between sexes and between species will provide further insights into the ability of insulin to regulate amino-acid effects on beta cell biology.
In summary, we demonstrate that in healthy humans insulin itself plays a role in regulation of the beta cell response to arginine as a secretory stimulus. Furthermore, mRNA expression of the SLC7A7 cation (arginine) amino acid transporter is upregulated in beta cells of humans with type 2 diabetes. Our findings provide continued support for a physiologically important role of insulin in the regulation of beta cell function for a secretagogue beyond glucose. Consequences of diminished insulin effects at the level of the beta cell in pathogenic states could be an important contributing mechanism to progressive beta cell dysfunction underlying type 2 diabetes.
Highlights.
Arginine-stimulated insulin secretion is better preserved than that to glucose
In rodents effects of arginine are independent of insulin receptor signaling
In vivo interactions in healthy humans have not previously been examined
Insulin pre-exposure augments arginine-stimulated insulin secretion in vivo
Insulin acts a physiologic role in regulation of the β-cell secretory response to arginine
Acknowledgements:
We wish to thank C. Ronald Kahn MD and Gordon Weir MD for the many hours of thoughtful discussion, and Gordon C. Weir, MD for providing array data from previously analyzed pancreas samples.
Funding:
We thank our funding sources including the American Diabetes Association 06-CD-07 (ABG), National Institute of Health R01 DK070648 (ABG), R01 DK067536 (RNK), the Joslin Diabetes and Endocrinology Research Center (DERC) P30 DK036836, the National Natural Science Foundation of China (grant number 81170779) (PL), and support for the Joslin Clinical Research Center from its philanthropic donors.
Abbreviations
- βIRKO
Beta cell-specific insulin receptor knockout
- IGF-1
insulin-like growth factor -1
- HPLC
high-performance liquid chromatography
- FFA
free fatty acids
- ISR
insulin secretion rate
- HUVEC
human umbilical vein endothelium cells
Footnotes
Conflicts of Interest: The authors have declared that no conflict of interest exists. Dr. Goldfine completed the work when employed at the Joslin Diabetes Center and is now an employee of Novartis Institutes of Biomedical Research. Dr. Halperin completed the work when employed at Brigham and Women’s Hospital and Joslin Diabetes Center and now works at Brigham and Women’s Hospital and Form Health, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability:
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Kulkarni RN, Winnay JN, Daniels M, et al. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J Clin Invest. 1999; 104: R69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lingohr MK, Dickson LM, Wrede CE, McCuaig JF, Myers MG Jr., Rhodes CJ. IRS-3 inhibits IRS-2-mediated signaling in pancreatic beta-cells. Mol Cell Endocrinol. 2003; 204: 85–99 [DOI] [PubMed] [Google Scholar]
- 3.Luciani DS, Johnson JD. Acute effects of insulin on beta-cells from transplantable human islets. Mol Cell Endocrinol. 2005; 241: 88–98 [DOI] [PubMed] [Google Scholar]
- 4.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet. 1999; 23: 32–40 [DOI] [PubMed] [Google Scholar]
- 5.Xuan S, Kitamura T, Nakae J, et al. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest. 2002; 110: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspinwall CA, Lakey JR, Kennedy RT. Insulin-stimulated insulin secretion in single pancreatic beta cells. J Biol Chem. 1999; 274: 6360–6365 [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999; 96: 329–339 [DOI] [PubMed] [Google Scholar]
- 8.Bouche C, Lopez X, Fleischman A, et al. Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc Natl Acad Sci U S A. 2010; 107: 4770–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halperin F, Lopez X, Manning R, Kahn CR, Kulkarni RN, Goldfine AB. Insulin augmentation of glucose-stimulated insulin secretion is impaired in insulin-resistant humans. Diabetes. 2012; 61: 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez X, Cypess A, Manning R, O’Shea S, Kulkarni RN, Goldfine AB. Exogenous insulin enhances glucose-stimulated insulin response in healthy humans independent of changes in free fatty acids. J Clin Endocrinol Metab. 2011; 96: 3811–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderwald C, Tura A, Grassi A, et al. Insulin infusion during normoglycemia modulates insulin secretion according to whole-body insulin sensitivity. Diabetes Care. 2011; 34: 437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mari A, Tura A, Natali A, et al. Influence of hyperinsulinemia and insulin resistance on in vivo beta-cell function: their role in human beta-cell dysfunction. Diabetes. 2011; 60: 3141–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer JP, Benson JW, Walter RM, Ensinck JW. Arginine-stimulated acute phase of insulin and glucagon secretion in diabetic subjects. J Clin Invest. 1976; 58: 565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer JP, Walter RM, Ensinck JW. Arginine-stimulated acute phase of insulin and glucagon secretion. I. in normal man. Diabetes. 1975; 24: 735–740 [DOI] [PubMed] [Google Scholar]
- 15.Hannon TS, Kahn SE, Utzschneider KM, et al. Review of methods for measuring beta-cell function: Design considerations from the Restoring Insulin Secretion (RISE) Consortium. Diabetes Obes Metab. 2018; 20: 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcelli-Tourvieille S, Hubert T, Pattou F, Vantyghem MC. Acute insulin response (AIR): review of protocols and clinical interest in islet transplantation. Diabetes Metab. 2006; 32: 295–303 [DOI] [PubMed] [Google Scholar]
- 17.Rickels MR, Naji A, Teff KL. Acute insulin responses to glucose and arginine as predictors of beta-cell secretory capacity in human islet transplantation. Transplantation. 2007; 84: 1357–1360 [DOI] [PubMed] [Google Scholar]
- 18.Chaillous L, Rohmer V, Maugendre D, et al. Differential beta-cell response to glucose, glucagon, and arginine during progression to type I (insulin-dependent) diabetes mellitus. Metabolism. 1996; 45: 306–314 [DOI] [PubMed] [Google Scholar]
- 19.Sjostrand M, Carlson K, Arnqvist HJ, et al. Assessment of beta-cell function in young patients with type 2 diabetes: arginine-stimulated insulin secretion may reflect beta-cell reserve. J Intern Med. 2014; 275: 39–48 [DOI] [PubMed] [Google Scholar]
- 20.Goldfine AB, Ebbeling CB, Ludwig DS. Antegrade intravenous catheterization for metabolic studies in man. Diabetologia. 2002; 45: 1742–1743 [DOI] [PubMed] [Google Scholar]
- 21.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976; 41: 565–573 [DOI] [PubMed] [Google Scholar]
- 22.Rizza RA, Mandarino LJ, Gerich JE. Mechanisms of insulin resistance in man. Assessment using the insulin dose-response curve in conjunction with insulin-receptor binding. Am J Med. 1981; 70: 169–176 [DOI] [PubMed] [Google Scholar]
- 23.Brange J, Owens DR, Kang S, Volund A. Monomeric insulins and their experimental and clinical implications. Diabetes Care. 1990; 13: 923–954 [DOI] [PubMed] [Google Scholar]
- 24.Marselli L, Thorne J, Dahiya S, et al. Gene expression profiles of Beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One. 2010; 5: e11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marselli L, Sgroi DC, Bonner-Weir S, Weir GC. Laser capture microdissection of human pancreatic beta-cells and RNA preparation for gene expression profiling. Methods Mol Biol. 2009; 560: 87–98 [DOI] [PubMed] [Google Scholar]
- 26.Marselli L, Thorne J, Ahn YB, et al. Gene expression of purified beta-cell tissue obtained from human pancreas with laser capture microdissection. J Clin Endocrinol Metab. 2008; 93: 1046–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992; 41: 368–377 [DOI] [PubMed] [Google Scholar]
- 28.Giugliano D, Torella R, Siniscalchi N, Improta L, D’Onofrio F. The effect of acetylsalicylic acid on insulin response to glucose and arginine in normal man. Diabetologia. 1978; 14: 359–362 [DOI] [PubMed] [Google Scholar]
- 29.Seino Y, Kurahachi H, Goto Y, Taminato T, Ikeda M, Imura H. Comparative insulinogenic effects of glucose, arginine and glucagon in patients with diabetes mellitus, endocrine disorders and liver disease. Acta Diabetol Lat. 1975; 12: 89–99 [DOI] [PubMed] [Google Scholar]
- 30.Liew CW, Assmann A, Templin AT, et al. Insulin regulates carboxypeptidase E by modulating translation initiation scaffolding protein eIF4G1 in pancreatic beta cells. Proc Natl Acad Sci U S A. 2014; 111: E2319–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol. 2009; 29: 3219–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansarullah Jain C, Far FF, et al. Inceptor counteracts insulin signalling in beta-cells to control glycaemia. Nature. 2021; 590: 326–331 [DOI] [PubMed] [Google Scholar]
- 33.Neerman-Arbez M, Halban PA. Novel, non-crinophagic, degradation of connecting peptide in transformed pancreatic beta cells. J Biol Chem. 1993; 268: 16248–16252 [PubMed] [Google Scholar]
- 34.Gunton JE, Kulkarni RN, Yim S, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005; 122: 337–349 [DOI] [PubMed] [Google Scholar]
- 35.Shirakawa J, Fernandez M, Takatani T, et al. Insulin Signaling Regulates the FoxM1/PLK1/CENP-A Pathway to Promote Adaptive Pancreatic beta Cell Proliferation. Cell Metab. 2017; 25: 868–882 e865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Jesus DF, Zhang Z, Kahraman S, et al. m(6)A mRNA Methylation Regulates Human beta-Cell Biology in Physiological States and in Type 2 Diabetes. Nat Metab. 2019; 1: 765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez M, Gallardo V, Rodriguez N, et al. Insulin-stimulated L-arginine transport requires SLC7A1 gene expression and is associated with human umbilical vein relaxation. J Cell Physiol. 2011; 226: 2916–2924 [DOI] [PubMed] [Google Scholar]
- 38.Guzman-Gutierrez E, Westermeier F, Salomon C, et al. Insulin-increased L-arginine transport requires A(2A) adenosine receptors activation in human umbilical vein endothelium. PLoS One. 2012; 7: e41705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.San Martin R, Sobrevia L. Gestational diabetes and the adenosine/L-arginine/nitric oxide (ALANO) pathway in human umbilical vein endothelium. Placenta. 2006; 27: 1–10 [DOI] [PubMed] [Google Scholar]
- 40.Salsoso R, Guzman-Gutierrez E, Saez T, et al. Insulin restores L-arginine transport requiring adenosine receptors activation in umbilical vein endothelium from late-onset preeclampsia. Placenta. 2015; 36: 287–296 [DOI] [PubMed] [Google Scholar]
- 41.Vasquez G, Sanhueza F, Vasquez R, et al. Role of adenosine transport in gestational diabetes-induced L-arginine transport and nitric oxide synthesis in human umbilical vein endothelium. J Physiol. 2004; 560: 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guzman-Gutierrez E, Arroyo P, Salsoso R, et al. Role of insulin and adenosine in the human placenta microvascular and macrovascular endothelial cell dysfunction in gestational diabetes mellitus. Microcirculation. 2014; 21: 26–37 [DOI] [PubMed] [Google Scholar]
- 43.McKnight JR, Satterfield MC, Jobgen WS, et al. Beneficial effects of L-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids. 2010; 39: 349–357 [DOI] [PubMed] [Google Scholar]
- 44.Wascher TC, Graier WF, Dittrich P, et al. Effects of low-dose L-arginine on insulin-mediated vasodilatation and insulin sensitivity. Eur J Clin Invest. 1997; 27: 690–695 [DOI] [PubMed] [Google Scholar]
- 45.Pieper GM, Siebeneich W, Dondlinger LA. Short-term oral administration of L-arginine reverses defective endothelium-dependent relaxation and cGMP generation in diabetes. Eur J Pharmacol. 1996; 317: 317–320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.


