Abstract
Purpose:
No approved therapies directly target retinal ganglion cells (RGCs) for neuroprotection or neuroenhancement in glaucoma. Recombinant human nerve growth factor (rhNGF) has been shown to promote RGC survival and function in animal models of optic neuropathy. Here we evaluate safety, tolerability, and efficacy of short-term, high-dose rhNGF eye drops versus placebo in a cohort of glaucoma patients.
Methods:
This study is a single-center, randomized, double-masked, vehicle-controlled, parallel group study designed to assess safety and tolerability as well as short-term neuroenhancement of structure and function (Clinicaltrials.gov NCT02855450). Sixty open-angle glaucoma patients were randomized 40:20 to receive either 180 μg/ml rhNGF or vehicle control eye drops in both eyes, three times daily for 8 weeks, with a 24-week post-treatment follow-up. One eye was officially selected as the study eye, although both eyes were studied and dosed. Primary endpoints were safety, as assessed through adverse events, and tolerability, as assessed through patient reported outcomes. Secondary outcome measures included best corrected visual acuity (BCVA), Humphrey visual field (HVF), electroretinogram (ERG), and optical coherence tomography (OCT) of retinal nerve fiber layer (RNFL) thickness at baseline, after 8 weeks of treatment, and at 4 and 24 weeks after treatment (12- and 32-weeks total).
Results:
Of the 60 randomized subjects, 23 were female (38%) and the average age was 66.1 years. Through week 32, there were no treatment-related serious adverse events, including no unexpectedly severe progression of optic neuropathy, no adverse events affecting ocular function or pressure, and no drug-related systemic toxicity. Topical high-dose rhNGF was tolerated well, with low level of symptom burden mainly eliciting periocular ache (in 52% of treated, 5% of placebo) and only 3 patients (7.5%) discontinuing treatment due to discomfort, out of whom 1 patient (2.5%) prematurely withdrawing from the study. There were no statistically significant differences in global indices of HVF, and no meaningful differences in total, quadrant, or clock-hour mean RNFL thickness between the groups, although both of these function and structure measures showed non-significant trends towards significance in favor of rhNGF. Real-world participant data was used to generate an estimate of cohort size needed to power subsequent studies.
Conclusions:
rhNGF is safe and tolerable in a topical 180 μg/ml formulation. Although no statistically significant short-term neuroenhancement was detected in this trial, given the strong effects of NGF in preclinical models and trends detected in this study, analysis for efficacy in a neuroprotection trial is warranted.
Introduction
Glaucoma, the leading cause of irreversible blindness worldwide, is an optic neuropathy characterized by progressive degeneration of retinal ganglion cells (RGCs) and their optic nerve axons, with typical defects in structural and functional measures. Reducing intraocular pressure (IOP), one of the major risk factors for glaucoma, is the mainstay of glaucoma treatment, although in many cases disease continues to progress even with significant IOP reduction. Therefore, additional approaches are still greatly needed for vision preservation or restoration in glaucoma patients, in particular therapeutic approaches independent of IOP reduction that are effective in protecting RGCs from degeneration (neuroprotection) or boosting the function of sick RGCs (neuroenhancement).
Nerve growth factor (NGF) is a naturally occurring polypeptide member of the neurotrophin family. Discovered in 1952 by Rita Levi-Montalcini and Stanley Cohen, NGF strongly promotes differentiation, survival, and axon and dendrite growth of neurons throughout the nervous system1. A recent study demonstrated reduced serum level of NGF in early and moderate glaucoma subjects compared to healthy controls2 and previous preclinical studies demonstrated reduced axonal transport of neurotrophic factors in RGCs induced by IOP3,4. For degenerating RGCs, NGF is neuroprotective in multiple pre-clinical models of glaucoma5. For example, topical ocular administration of NGF protects RGCs and optic nerve axons from degeneration6, and similarly inhibits apoptosis in ocular hypertension7 and after partial optic nerve transection (pONT)8. These and other data discussed below situate NGF as a strong candidate for RGC neuroprotection or neuroenhancement.
An ophthalmic solution of 20 μg/ml recombinant human NGF (rhNGF) was found safe and effective in promoting healing of persistent epithelial defects in patients with neurotrophic keratopathy9, and is now FDA- and EU-approved at this lower topical concentration. Limited human testing has suggested that higher-concentration topical NGF treatment may improve parameters of visual function such as visual field and visual acuity7, but studies have generally been small and nonrandomized. Furthermore limited real-world experience with patients in neuroprotection or neuroenhancement trials has been challenging for clinical trial design. The primary objective of this study was to formally assess safety and tolerability of topical rhNGF180 μg/ml versus vehicle in patients with chronic primary open angle glaucoma. Additional secondary objectives included evaluating measures of efficacy towards short-term neuroenhancement effects on RGC structure and function, and to gathering “real-world” data towards designing subsequent neuroenhancement or neuroprotection trials in glaucoma.
Methods
Study oversight:
This study was performed after approval by the institutional review board at Stanford University, complied with the ethical standards defined by the Declaration of Helsinki and Good Clinical Practice, and was registered at ClinicalTrials.gov (NCT02855450). Written informed consent was obtained from all patients before enrollment and randomization.
Clinical trial design:
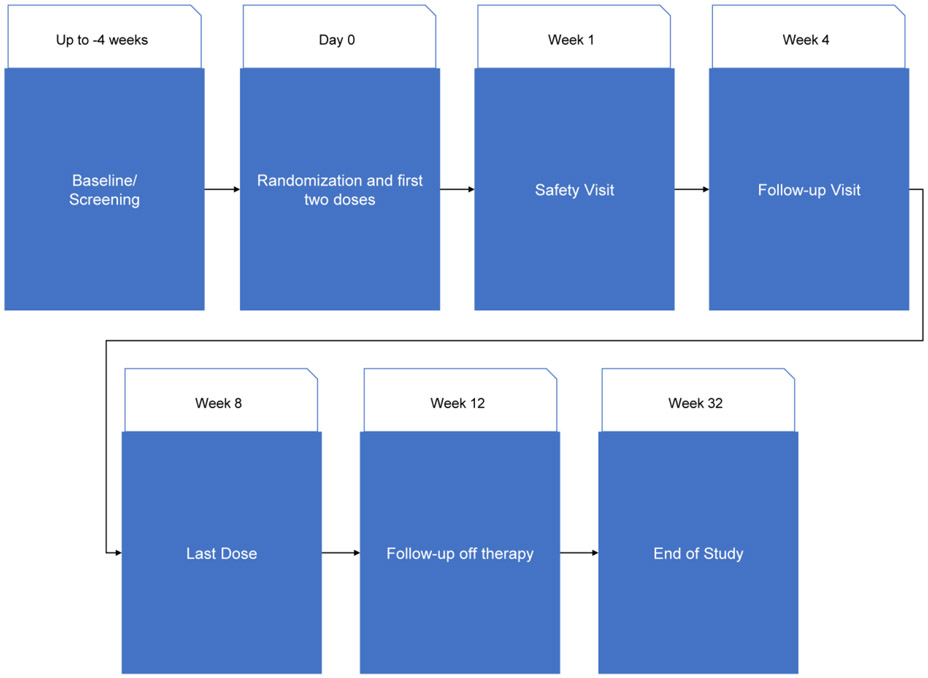
This was a phase Ib, single-center, randomized, double-masked, vehicle-controlled, parallel group study to evaluate the safety and efficacy of a 180 μg/ml rhNGF topical solution versus vehicle in 60 study participants with primary open angle glaucoma (POAG), over 8 weeks of treatment and a 24-week post-treatment follow-up period (Figure 1). One eye was officially selected as the study eye although both eyes underwent treatment and data collection.
Figure 1.
Study Visit Schedule. Multiple visits (Screening, Day 0, Week 8, Week 12, and Week 32) required patients to return two or three days in the same week for testing, including repeat visual fields.
Study participants and eligibility criteria:
Entry criteria included adult patients diagnosed with POAG characterized by clinical evidence of progressive RGC dysfunction and degeneration demonstrated on three repeated reliable visual fields and/or a structural modality within 14 months prior to entering into the study, and residual visual field preservation in at least one quadrant. Only one eye per patient was entered as the study eye, although both eyes were treated; if both eyes were eligible, the study eye was chosen randomly. Females of childbearing potential were included if not intending to become pregnant during the study, and consented to a urine pregnancy test upon entering and exiting the study treatment period.
Exclusion criteria included other optic nerve or retinal degenerative disease or co-morbidity causing significant vision loss, blindness in one eye, history of ocular herpes zoster, requirement for acyclovir or related products treatment during study duration, evidence of corneal opacification or lack of optical clarity, removal of lens or intraocular lens replacement within 3 months, any other ocular surgery within 9 months prior to initiation of study drug, uveitis or other ocular inflammatory disease, use of systemic steroids or other immunosuppressive medications, participation in any other clinical trial of a non-clinically approved drug by ocular or systemic administration within the last 3 months, diabetic macular edema, current chemotherapy treatment, history of malignancy not counting basal cell carcinomas unless treated successfully two years prior to inclusion in the trial, known hypersensitivity to one of the components of the study or procedural medications, or history of drug, medication or alcohol abuse or addiction.
Treatment protocol:
Participants were randomized in a 2:1 ratio to receive 180 μg/ml rhNGF or vehicle eye drops three times a day (TID) for 8 weeks in both eyes. The schedule of study visits and evaluations is summarized in Table 1.
Table 1:
Study Plan.
| Baseline Visit (Screening)1 |
Day 0 Therapy Initiation |
Week 1 (± 2days) |
Week 4 (± 4days) |
Week 8 (± 7days) |
Week 12 (± 7days) |
Week 32 (± 7days) |
|
|---|---|---|---|---|---|---|---|
| GENERAL ASSESSMENTS | |||||||
| Informed consent | X | ||||||
| Randomization | X | ||||||
| Inclusion / Exclusion criteria | X | X | |||||
| Pregnancy Test (if applicable) | X | X | |||||
| Demographics, Medical History, Medications2 | X | X | X | X | X | X | X |
| AE assessment | X | X | X | X | X | X | X |
| VISUAL SYSTEM EXAMS | |||||||
| BCVA (ETDRS letters) | X | X | X | X | X | ||
| IOP3 (mm Hg) | X | X | X | X | X | X | X |
| Slit lamp examination | X | X | X | X | X | X | X |
| External ocular examination | X | X | X | X | X | X | X |
| Humphrey 24-2 or 10-2 Visual Field4 | X5 | X | X6 | X7 | |||
| Dilated fundus ophthalmoscopy | X | X | X | X | X | ||
| OCT (retinal thickness) | X | X | X | X | |||
| Mitochondrial redox potential | X | X | X | X | X | ||
| ERG8 | X | X | X | X | |||
| STUDY THERAPY | |||||||
| VAS for ocular tolerability | X | X | X | X | X | ||
| Study Drug Dispensing9 | X | X | |||||
| Assess Medication Dosing Compliance10 | X | X | |||||
Schedule of study visits surrounding the 8 weeks of investigational product dosing.
Baseline Screening Visit could occur within 4 weeks prior to initiating therapy.
Demographic information collected at screening including medications taken within the preceding 30 days.
Intraocular pressure (IOP) testing was performed using Goldmann Tonometer.
Visual field testing was performed using SITA (Swedish Interactive Testing Algorithm) Standard.
Two fields on different days for a total of 3 before initiating treatment.
Three fields within 4 weeks until the end of treatment.
Three fields within 4 weeks until the end of follow-up.
Electroretinography (ERG) performed once at Therapy Initiation (Day 0) visit, at Week 8, at Week 12 and at Week 32.
At Day 0, patient was instructed on self-administering the drug at home.
At Week 4 and 8, subject was instructed to return all used and unused investigational product.
AE = Adverse event
BCVA = Best corrected visual acuity
ERG = Electroretinography
IOP = Intraocular pressure
OCT = Optical coherence tomography
VAS = Visual Analogue Scale
Safety Assessments:
Adverse events (AEs) reported by MedDRA preferred term were recorded at the scheduled study visits and at any unscheduled visit during the study. A Visual Analogue Scale (VAS) was used to determine ocular tolerability to the study medication, assessed by the patient using a self-administered scale. Additional pre-specified safety parameters included unexpectedly severe progression of glaucoma as measured by central vision loss, by visual field testing, or by examination of the optic nerve; intolerance or allergy to the drug; local or systemic toxicities; or changes in IOP that differed from those expected in the course of glaucoma that were identified to be potentially related to the drug.
Efficacy Assessments:
Exploratory secondary outcomes included functional assessments including as mean, median, and distribution of change in best corrected visual acuity (BCVA) (measured in Early Treatment Diabetic Retinopathy Study [ETDRS] charts), changes in visual field using Swedish Interactive Threshold Algorithm-Standard test Humphrey Field Analyzer 24-2 (Carl Zeiss Meditec, San Leandro, CA), changes in photopic negative response (PhNR) on the electroretinography (ERG; details described elsewhere10); and structural assessments, including changes in ganglion cell complex (GCC) and retinal nerve fiber layer (RNFL) thickness measured by optical coherence tomography (OCT [Carl Zeiss Meditec, San Leandro, CA]).
Masking:
Patients, investigators, study site and contract research organization (CRO) staff were masked during the entire trial. The study was unmasked after the last enrolled patient completed their week 32 visit (end of the masked follow-up), and the database was locked. The vials of rhNGF and vehicle control were identical in appearance and the contents were indistinguishable. A list of sequential kit numbers was generated by a member of the CRO programming group, who was not involved in the conduct of the study. Each kit was randomly assigned into a numbered treatment order. Patients were assigned to treatment in numerical order. A tear-off label from the kit box, with the kit number, was attached to the investigational product-dispensing log.
Statistical Analysis:
The safety population included all patients who received at least one dose of study medication, and was used in the analysis of all safety endpoints. The intent-to-treat population included all randomized patients, and was used for all exploratory efficacy analyses. All exploratory efficacy variables were tabulated with descriptive statistics at the 0-, 4-, 8-, 12- and 32-week time points, and inferential tests with 95% confidence limits of the difference between treatments were calculated. Time points for VF testing were clustered and averaged by patient eye, with baseline exams from day −185 to day 1, Week 4 from study day 24 through 32, Week 8 from study day 49 through 70, and Week 32 from study day 210 through 280. For analysis purposes, all ocular assessments were analyzed separately by enrolled (primary) versus secondary eye. All hypotheses were tested at a 0.05 level of significance using a two-sided test. Generally, the analysis of the primary eye was considered as the primary result, whereas analyses in secondary eyes were considered as supportive. No statistical inference was foreseen for the analyses of primary safety outcomes. Differences between categorical variables were tested using Fisher exact test for binary variables, at 0.05 significance level. For continuous variables, means and standard deviations were calculated in each treatment group and differences were tested using unpaired 2-sided t-tests, at 0.05 significance level. For the VAS Tolerability Scores, the change from baseline was compared between treatment groups. An analysis of covariance (ANCOVA) with treatment and respective baseline value in the model was used. The least-squares means estimate of the treatment effect and the treatment group difference (both with 95% confidence intervals) were reported.
Results
Randomization and retention:
A total of 60 subjects were randomized to a study treatment and all were treated as randomized, with at least 1 dose of study medication: 40 to treatment with rhNGF and 20 to vehicle (Figure 2). All but one subject randomized to the rhNGF group completed the full treatment period, and 98% and 92% of participants completed to the week 12 and week 32 (final) visits in the rhNGF and vehicle groups, respectively.
Figure 2.
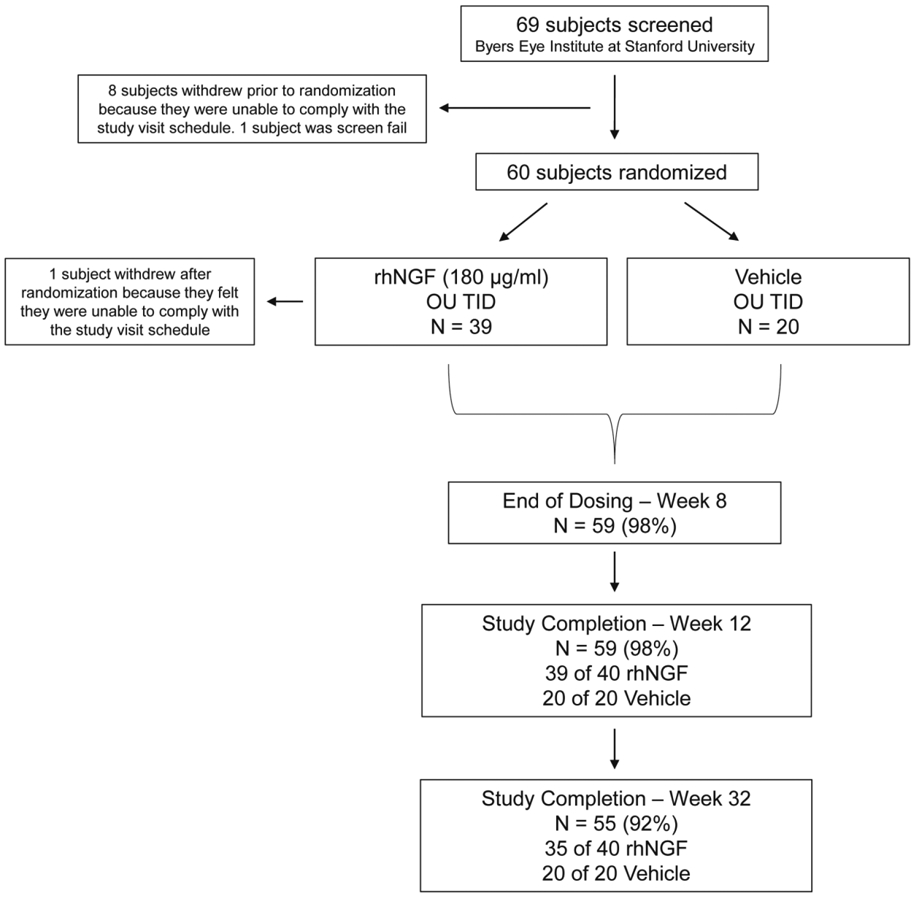
Study design and patient disposition. In sixty (60) open-angle glaucoma patients, one eye was officially selected as the study eye although both eyes underwent dosing and data collection. Primary endpoints were safety, as assessed through adverse events, and tolerability, as assessed through Visual Analogue Scale (VAS). Secondary objectives were to measure changes in best corrected visual acuity (BCVA), Humphrey visual field (HVF), electroretinography (ERG), and optical coherence tomography (OCT) at baseline and at the week 8, 12, and 32 visits. Exploratory objectives included flavoprotein fluorescence and adaptive optics imaging and OCT angio. Results through week 32 for all subjects are reported.
Patient demographics are shown in Table 2. In general, the two groups were balanced for age, gender, ethnicity and race. There were no significant differences between initial IOP, number of glaucoma drops, and BCVA between groups. Glaucoma severity was similar in both groups, with baseline mean deviation (MD) of −13.42 ± 9.20 dB (mean ± standard deviation (SD)) in the treatment group and −16.22 ± 9.29 dB (mean ± SD) in vehicle group (p = 0.3478). During the study, patients continued their clinical standard of care for glaucoma.
Table 2.
Baseline Demographics and Characteristics
| Characteristics | rhNGF 180 μg/ml | Vehicle | Overall | P value |
|---|---|---|---|---|
| Number of subjects | 40 | 20 | 60 | |
| Age (years) | ||||
| Mean ± SD | 68.0 (13.98) | 62.2 (13.28) | 66.1 (13.92) |
0.1260a |
| Median (range) | 69.5 (22-89) | 59 (31-87) | 66 (22-89) | |
| <= 65 n (%) | 15 (37.5) | 12 (60.0) | 27 (45.0) | 0.0986b |
| > 65 n (%) | 25 (62.5) | 8 (40.0) | 33 (55.0) | |
| Gender, no. (%) | ||||
| Male n (%) | 26 (65.0) | 11 (55.0) | 37 (61.7) | 0.4526b |
| Female n (%) | 14 (35.0) | 9 (45.0) | 23 (38.3) | |
| Ethnicity, n (%) | ||||
| Hispanic, Latino, or Spanish | 2 (5.0) | 1 (5.0) | 3 (5.0) | 1b |
| Not Hispanic, Latino or Spanish | 38 (95.0) | 19 (95.0) | 57 (95.0) | |
| Race, n (%) | ||||
| Asian | 11 (27.5) | 5 (25.0) | 16 (26.7) | 0.5019c |
| Black or African American | 4 (10.0) | 4 (20.0) | 8 (13.3) | |
| White | 25 (62.5) | 10 (50.0) | 35 (58.3) | |
| American Indian or Alaska Native | - | - | - | |
| Native Hawaiian or Other Pacific Islander | - | - | - | |
| other | - | 1 (5.0) | 1 (1.7) | |
| Hypertension, no. (%) | 18 (45.0) | 9 (45.0) | 27 (45.0) | 1b |
| Diabetes mellitus, no. (%) | 4 (10.0) | 4 (20.0) | 8 (13.3) | 0.2827b |
| Study eye, n (%) | ||||
| Right | 22 (55.0) | 12 (60.0) | 34 (56.7) | 0.7125b |
| Left | 18 (45.0) | 8 (40.0) | 26 (43.3) | |
| IOP (mm Hg) | ||||
| Mean ± SD | 13.18 (4.7) | 15.15 (8.70) | 13.83 (6.31) | 0.2569a |
| Median (range) | 13 (6-48) | 13 (6-48) | 14 (6-48) | |
| Number of glaucoma medications | ||||
| Mean ± SD | 1.93 (0.86) | 2.25 (0.91) | 0.1808a | |
| Median (range) | 2 (1-4) | 2 (0-4) | ||
| Lens status | ||||
| Phakic | 18 (45.0) | 11 (55.0) | 0.465b | |
| Pseudophakic | 22 (55.0) | 9 (45.0) | ||
| BCVA ETDRS letters, mean ± SD | 73.23 (16.62) | 71.4 (13.06) | 0.6445a | |
BCVA = Best corrected visual acuity; IOP = Intraocular pressure; rhNGF = recombinant human nerve growth factor; SD = standard deviation
Student t-test
Fisher’s exact test
Exact permutation chi-square test
Primary Safety and Tolerability Outcomes:
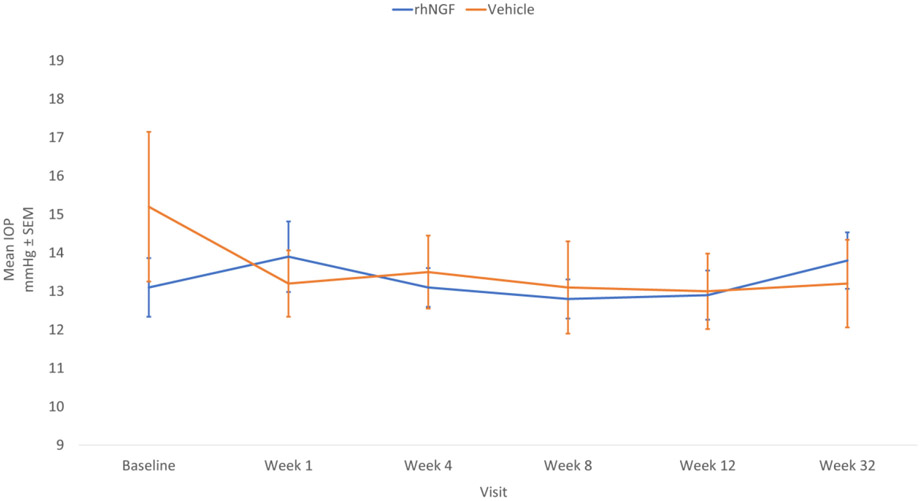
No (0.0%) subjects in either group reported local or systemic treatment-emergent serious adverse events. During the 8-week treatment period, there were no (0.0%) reports of unexpectedly severe progression of optic neuropathy in either treatment group. Intolerance or allergy to the drug was reported in the rhNGF group only, with four (10.0%) subjects reporting a total of six events. A total of 7.5% (3) of subjects in the rhNGF group subjects reported three Treatment Emergent AEs (TEAEs) that led to premature withdrawal from treatment, with one AE in one subject leading to study discontinuation. In contrast, no (0.0%) subjects in the vehicle group reported AEs that led to premature withdrawal of the treatment or to study discontinuation. There was no significant change from baseline and no difference in IOP between the rhNGF and the vehicle groups during treatment and follow-up periods (Figure 3). The most common AEs detected in the rhNGF and vehicle treatment groups are listed in Table 3. Notable AEs included eye pain, eye irritation and photophobia. Major protocol deviations for lack of patient compliance with using prescribed drops were reported for 2.6% and 5.0% of subjects in the rhNGF group and vehicle group, respectively.
Figure 3.
Mean intraocular pressure (IOP) at baseline, treatment and follow-up periods in study eyes. Standard error of the mean (SEM) indicated by vertical bars. Note that follow-up time is not on a linear scale.
Table 3:
Summary of Treatment-Related Adverse Events by System Organ Class and Preferred Term
| System order class preferred term | rhNGF n (%) |
Vehicle n (%) |
Overall n (%) |
|---|---|---|---|
| Number of Subjects | 40 | 20 | 60 |
| Number of treatment related TEATs | 28 (70.0) | 5 (25.0) | 33 (55.0) |
| Eye Disorders | 28 (70.0) | 5 (25.0) | 33 (55.0) |
| Eye pain | 21 (52.5) | 1 (5.0) | 22 (36.7) |
| Eye irritation | 6 (15.0) | 3 (15.0) | 9 (15.0) |
| Photophobia | 7 (17.5) | - | 7 (11.7) |
| Conjunctival Hyperemia | 3 (7.5) | 1 (5.0) | 4 (6.7) |
| Blepharitis | 2 (5.0) | 1 (5.0) | 3 (5.0) |
| Vision Blurred | 3 (7.5) | 1 (5.0) | 4 (6.7) |
| Dry Eye | 2 (5.0) | 1 (5.0) | 3 (5.0) |
| Foreign Body Sensation | 2 (5.0) | 1 (5.0) | 3 (5.0) |
| Lacrimation Increased | 2 (5.0) | 1 (5.0) | 3 (5.0) |
| Uveitis | 2 (5.0) | - | 2 (3.3) |
| Abnormal Sensation In Eye | - | 1 (5.0) | 1 (1.7) |
| Blepharochalasis | 1 (2.5) | - | 1 (1.7) |
| Chalazion | 1 (2.5) | - | 1 (1.7) |
| Dry eye | - | 1 (5.0) | 1 (1.7) |
| Eye Inflammation | 1 (2.5) | - | 1 (1.7) |
| Eye Swelling | 1 (2.5) | - | 1 (1.7) |
| Eyelid Edema | 1 (2.5) | - | 1 (1.7) |
| Eyelid Ptosis | 1 (2.5) | - | 1 (1.7) |
| Keratitis | 1 (2.5) | - | 1 (1.7) |
| Ocular Discomfort | 1 (2.5) | - | 1 (1.7) |
| Ocular Hyperemia | 1 (2.5) | - | 1 (1.7) |
| Punctate Keratitis | - | 1 (5.0) | 1 (1.7) |
| Nervous System Disorders | 3 (7.5) | - | 3 (5.0) |
| Headache | 3 (7.5) | - | 3 (5.0) |
TEATs = Treatment emergent adverse events; rhNGF = recombinant human nerve growth factor
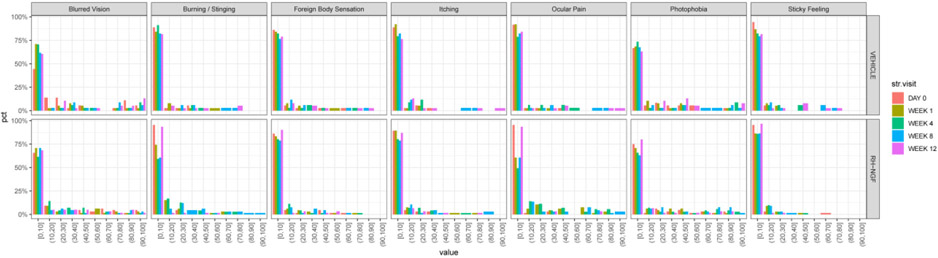
Tolerability was also assessed using a VAS Ocular Tolerability Score (Figure 4.). At baseline, there was a low level of symptom burden in both treatment groups (rhNGF group: median 0.7 mm, range 0 to 54; vehicle group: median 2.9 mm, range 0 to 44). Symptom burden remained low at Week 8 for both eyes in both treatment groups, with a mean (SD) change from baseline at Week 8 of 3.6 (16.08) mm in the rhNGF group and −0.9 (6.51) mm in the vehicle group, and no statistically significant effect of treatment with rhNGF at Week 8 (p=0.283). There was no statistically significant change in symptoms at any visit, with the exception of the ocular pain subscale, which showed a statistically significant (p=0.002) worsening in the rhNGF group of 20.3 mm (95% CI: 7.9 to 32.7) relative to the vehicle group.
Figure 4.
Ocular tolerability by a visual analog scale (VAS) from 0 to 100 mm (0= “no symptoms”; 100= “worst possible discomfort”) for each of 7 different symptoms: foreign body sensation, burning or stinging, itching, ocular pain, sticky feeling, blurred vision, and photophobia. Percentage of patients by 10mm categories by study arm, by visit, plotted.
Secondary Exploratory Efficacy Outcomes:
A number of outcome measures were explored to assess for any biological signal of short-term efficacy, looking for neuroenhancement (improvement) in structure or function related to glaucoma between the rhNGF versus vehicle groups.
BCVA scores:
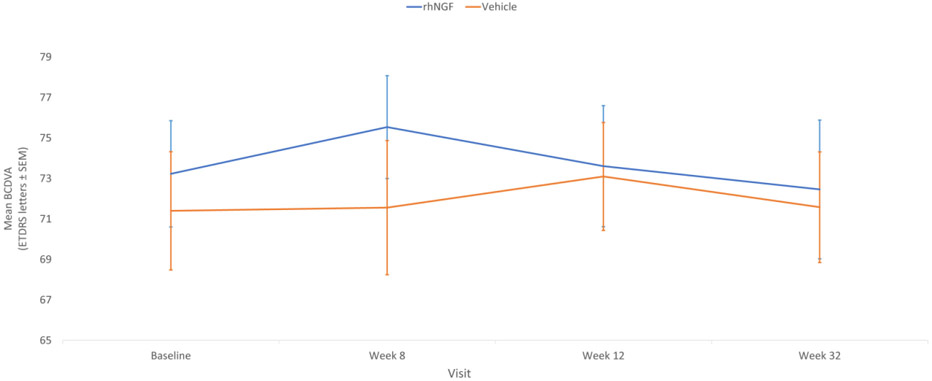
There was no statistically significant effect of treatment with rhNGF at Week 8 in either eye. At baseline, BCVA scores were comparable between treatment groups (mean (SD): rhNGF 74.7 (14.08); vehicle 71.4 (13.06)) and remained stable during the course of the study, with no statistically significant changes in either group (mean (SD) change from baseline at Week 8: rhNGF 0.9 (5.19); vehicle −0.1 (6.87)), and no statistically significant difference between the two groups (p=0.410). Findings in the secondary eye supported those in the primary eye, showing no statistical significance (Figure 5).
Figure 5.
Best-corrected distance visual acuity (BCVA) as ETDRS letters at baseline, treatment and follow-up periods in study eyes. Standard error of the mean (SEM) indicated by vertical bars. Note that follow-up time is not on a linear scale.
PhNR:
Mean (SD) was slightly higher at baseline in the rhNGF group [21.1 (9.86)] than in vehicle group [17.5 (8.29)]. There was no statistically significant effect of treatment with rhNGF at week 8 in either eye (mean (SD) change at week 8: rhNGF −2.5 (9.53); vehicle 3.2 (11.29)). There was no statistically significant difference between the two treatments (p=0.192). Findings in the secondary eye supported those in the primary eye.
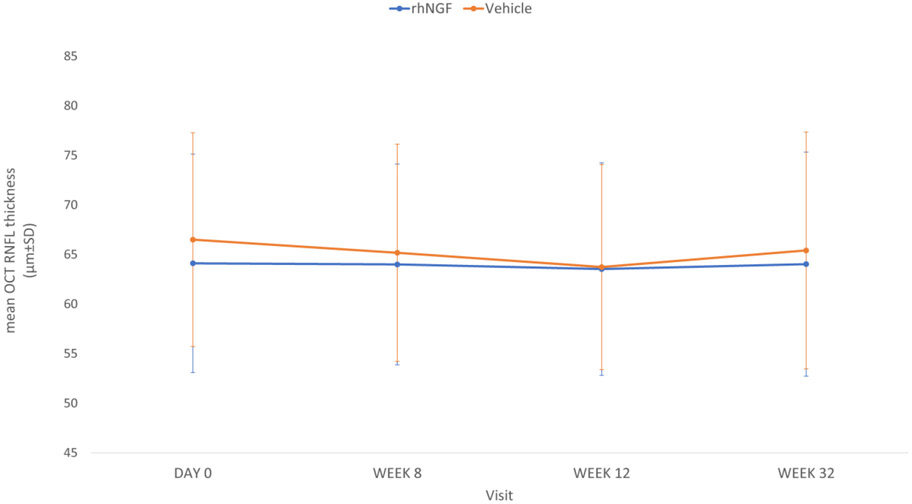
OCT RNFL:
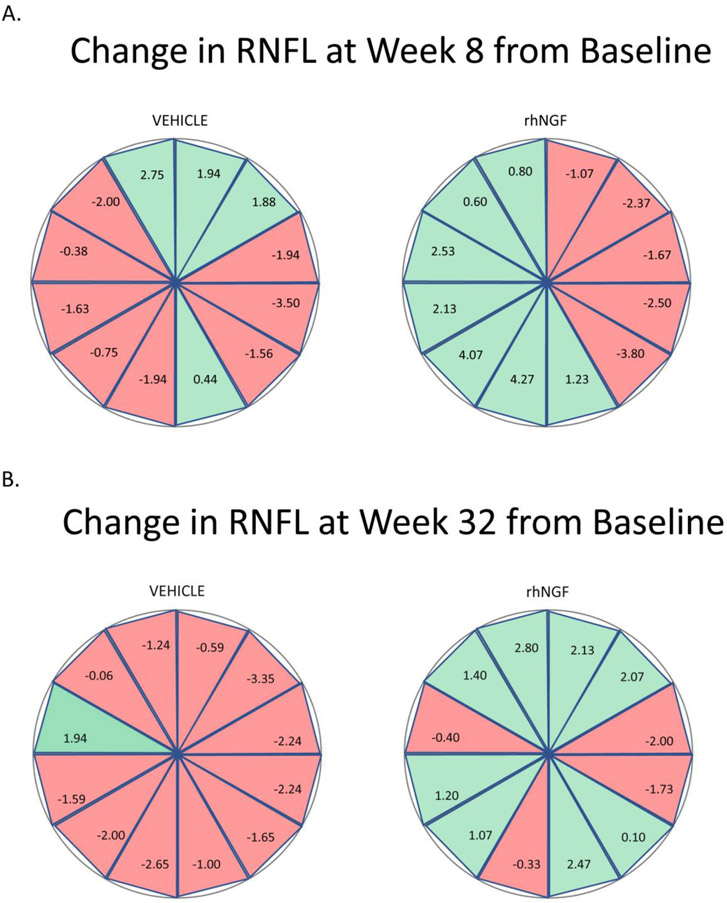
There was no statistically significant effect of treatment with rhNGF at week 8 in either eye At baseline the average RNFL thickness in the primary eye was similar in the rhNGF group [64.8 (11.07)] as the vehicle group [65.2 (10.96)]. Mean (SD) change at week 8 in average RNFL thickness inthe rhNGF group (−0.7 (4.88)) did not differ significantly from the vehicle group (−1.0 (5.62)) at week 8. There was no statistically significant difference between the two treatments (p=0.846). Findings in the secondary eye supported those in the primary eye (Figure 6). Analysis of change in RNFL thickness by clock hours (Figure 7), revealed that more sectors in the vehicle group progressed to be thinner from baseline to 8 weeks with additional progression from 8 weeks to 32 weeks, compared to rhNGF group, however these results were not statistically significant with the current sample size.
Figure 6.
Mean optical coherence tomography (OCT) retinal nerve fiber layer (RNFL) thickness at baseline, treatment and follow-up visits in study eyes. Standard deviation (SD) indicated by vertical bars. Note that follow-up time is not on a linear scale.
Figure 7.
Change in sectoral RNFL thickness by clock hour at (A) baseline to 8 weeks, and at (B) baseline to 32 weeks.
Humphrey visual field (HVF):
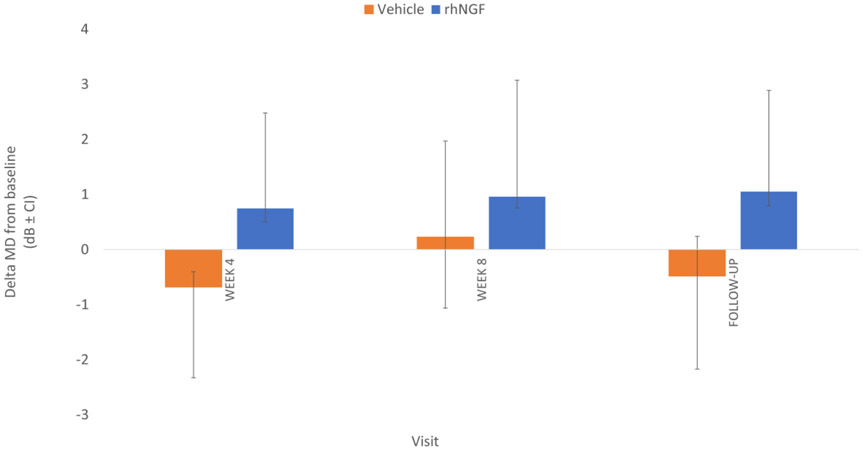
For visual field analysis, all reliable 24-2 VFs were included. Visual field parameters analysis demonstrated a non-statistically significant trend towards improvement of mean MD in the rhNGF group from −13.42 (SD +/− 9.20) at baseline to −11.95 (SD +/− 9.26) at follow-up time points (Figure 8; unadjusted p-value of 0.011).
Figure 8.
Change in mean deviation (MD) of the visual fields by treatment arm and follow-up periods from baseline, in study eyes. Standard deviation (SD) indicated by vertical bars. Note that follow-up time is not on a linear scale.
Discussion
Here we present data from a double-masked, randomized, vehicle-controlled, trial of topical, high dose rhNGF (180 μg/ml, TID for 8 weeks) in patients with progressive open angle glaucoma. The primary goal of this study was to evaluate the safety and tolerability of NGF versus vehicle. The safety profile was excellent with no short- or long-term treatment-emergent serious adverse events. The most common adverse events in our study were mild, transient and self-limited, including eye pain, eye irritation and photophobia that resolved upon treatment cessation. These surface and periocular symptoms may be expected based on NGF-induced sensory neuron sensitization and hyperalgesia via activation of their trkA receptor expression11. Despite the prevalence of these symptoms, only three patients discontinuing therapy due to lack of tolerability, out of whom only one patient prematurely withdrew from the study.
These safety data and symptom profiles are in line with the current available preclinical and clinical data showing the lack of significant systemic or serious ocular safety concerns associated with rhNGF. Specifically, in the REPARO and NGF0214 neurotrophic keratitis clinical trials in which lower concentrations up to 20 μg/ml of rhNGF were administered topically six times per day for 8 weeks, adverse events were ocular, mild, self-limited, reversible and did not require discontinuation of treatment or medication addition9,12. Higher concentrations (up to 180 μg/ml) have also been studied previuosly in limited, non-controlled trials. In a phase I, randomized, double-masked, placebo–controlled, combined single and multiple ascending dose study to evaluate the safety, tolerability and pharmacokinetics of rhNGF (using a different, E. coli-derived product) in healthy male and female volunteers, study subjects were treated in one eye with rhNGF (0.5 to 180 μg/ml) ranging from one drop daily to one drop three times a day over five days with the fellow eye receiving an identical placebo regimen; no serious adverse events, no systemic or ocular safety concerns, no systemic absorption or immunogenicity, and no clinically significant changes in ocular examinations were reported13. Reported ocular adverse events likely attributable to rhNGF in these healthy subjects included ocular pain, tenderness, soreness, pressure, burning, warmth and itchiness following installation of the study medication, similar to the adverse event profile noted in our treated population, suggesting that these symptoms are independent of ocular disease status. In another multicenter masked randomized clinical trial of rhNGF at 180 μg/ml administered for 24 weeks in patients with retinitis pigmentosa, no evidence of systemic absorption or immunogenicity and no treatment-emergent serious adverse events were reported, and adverse events were mild in intensity, transitory, and consisted mostly of ocular pain, irritation, itching and photophobia following instillation (clinicaltrials.gov NCT#02110225).
The current trial was also designed to explore structural and functional measures of neuroenhancement. Neuroprotection in glaucoma refers to interventions that can prevent or delay glaucomatous neurodegeneration, independently of IOP reduction, which represents a great unmet need in glaucoma treatment14. Neurotrophic factors including NGF regulate the differentiation, survival and function of RGCs in animal models of glaucoma15. Evidence obtained in animals with experimentally induced retinal and/or optic nerve degenerations indicate a number of soluble neurotrophic factor candidates in addition to NGF, including brain derived neurotrophic factor (BDNF), b-fibroblast growth factor (b-FGF), transforming growth factor-b (TGF-b), tumor necrosis factor-a (TNF-a), vascular endothelial growth factor (VEGF), neuropeptide-Y (NPY), and ciliary neurotrophic factor (CNTF), and others, can protect against retinal cell degeneration alone or in combination with other growth factors16. NGF and both its receptors, TrkA and p75, are widely expressed in the central visual pathways including in the optic nerve and retina17. In addition, intravitreal NGF injection enhances expression of BDNF, β-FGF, TGF-β, VEGF and NP-Y, suggesting that the neuroprotective effects of NGF may also be amplified through the stimulation of other biological mediators18. Other than this study on topical rhNGF, CNTF is under investigation for neuroprotection in the visual system, in macular telangiectasia19 (clinicaltrials.gov NCT#01327911) and in glaucoma (clinicaltrials.gov NCT#01408472 and NCT#04577300).
A first question when considering such a clinical candidate is what NGF protein preparation to use. Sourcing of NGF for clinical use has been varied. The NGF applied in many preclinical and clinical studies was a murine protein extracted and purified from the male mouse submaxillary glands20,21. NGF is highly conserved across different species and is 90% homologous at the amino-acid sequence between human and mouse22. In contrast, rhNGF developed in compliance with current Good Manufacturing Practice to ensure quality and sterility was used in this study. It is expressed in E.coli as a pro-peptide, isolated and solubilized with a strong denaturing agent, and subsequently refolded into the natural conformation. The pro-sequence is then digested off with trypsin, and rhNGF is further purified into a topical formulation23. Clinical data are also available from rhNGF expressed in and subsequently purified from Chinese hamster ovary (CHO) cells. In vitro preclinical studies using both E. coli- and CHO-expressed rhNGF have been demonstrated to have similar levels of activity to murine NGF, including similar biological activity24-26. The CHO-derived rhNGF was investigated using subcutaneous systemic delivery in several hundreds of patients for the treatment of HIV- or diabetes-related peripheral neuropathy, with favorable tolerability and local pain at the injection site being the most common side effect27-29. These data closely match the tolerability and side effect profile of high-concentration topical therapy studied here, and again suggest these are treatment-emergent effects likely of NGF acting directly on peripheral nerve nociceptive neurons, wherever administered.
A second question in considering rhNGF for therapy is whether topical delivery can reach the retina or optic nerve. Of note, in a pharmacokinetic study evaluating the ocular biodistribution of topical murine NGF in rats tested at three concentrations (10, 200 and 500 μg/ml). After one dose of topical instillation, a significant increase in NGF protein was detected in the serum and in all ocular tissues, except lens. Twofold increase in NGF levels in both the retina and optic nerve was demonstrated 6 hours after topical conjunctival administration of 200 μg/mL NGF.30. In another study, evaluating topical rhNGF of 180 μg/ml and 540 μg/ml concentrations in a rat model of pONT, rhNGF was detected in the optic nerve, consistent with reduced RGC apoptosis seen in vivo at both doses, but the 180 μg/ml dosing appeared to be most effective in preserving cell survival8. Additional support for bioavailability of topical NGF to the retina and optic nerve comes from detection of high levels in ocular tissues, including the retina and optic nerve after topical administration of (125)I-labeled NGF at a concentration of 200 μg/mL30. Other studies in rodents reported that topical NGF could affect brain cholinergic neurons. In a rat model of glaucoma, a reduced concentration of NGF in the cerebrospinal fluid, lateral geniculate nucleus and visual cortex was restored after 35 days of ocular topical NGF31. Other studies on topical NGF application have similarly shown biochemical and structural effects on nucleus basalis and septum neurons in a rat model32, and recovery of chemically injured cholinergic neurons in adult mouse forebrain33. Detection of topically applied NGF in the posterior segment of non-human primates would be supportive as smaller animal studies do not provide significant basis that topical NGF will reach therapeutically effective dosage in the retina or optic nerve of humans. However, to our knowledge, thus far no study testing ocular biodistribution of topical NGF in large mammals has been published. Pharmacokinetics, pharmacodynamics and biodistribution data in rodent must be interpreted with caution due to extreme differences in ocular tissue biology and total body size.
Evidence for pharmacodynamic effect on the retina has also been suggested in several preliminary reports of human patients treated with topically applied murine NGF for posterior segment ocular diseases5. For example, in 6 patients (5 children and 1 adult) with severe visual impairment due to compressive optic neuropathy associated with optic nerve glioma, treatment with a 10-day course of 200 μg/ml topical NGF resulted in improved vision and optic nerve function as measured by distance visual acuity, visual field and visual evoked potential (VEP); improvement persisted at least 1 to 3 months before returning to baseline after the discontinuation of the topical treatment34,35. In another study, 3 patients with advanced glaucoma progressing despite maximum medical therapy were treated with 200 μg/ml topical NGF 4 times daily over 3 months and reported improved HVF, VEP and pattern ERG (PERG) that persisted upon discontinuation of treatment up to 3 months7. Together these data provide objective human evidence that topically applied NGF can achieve pharmacologically active concentrations in the back of the eye and suggest a delayed or continued biologic effect of the topical NGF on RGC function5,7, although these studies were all small, non-randomized case series.
The premise for rhNGF to increase RGC survival (neuroprotection) or shorter-term RGC function (neuroenhancement) in the face of optic nerve insults is well supported by preclinical data using intravitreal or topical delivery36. Most such studies have focused on postmortem histologic measures of RGC apoptosis rather than shorter-term functional assays. In our study, we did not have access to direct quantification of RGCs apoptosis although candidate technologies for such detection are now entering clinical trials in human subjects37. With only 8 weeks on treatment, our study was too short to detect a neuroprotective effect of rhNGF, and did not detect any statistically significant, shorter-term neuroenhancement effects, measured by RGC function (i.e. visual field testing) or structure (i.e. OCT). Limitations in our trial other than short duration, small sample size, and regional patient demographics that may not adequately represent United States POAG population may have included topical delivery as discussed above, or other factors such as variability in receptor expression, not yet studied in human glaucoma populations. Nevertheless, given the strong effect previously demonstrated in animal models, and the safety profile of topical 180 μg/ml rhNGF in this current and previous studies, rhNGF remains a very promising candidate for neuroprotection in glaucoma and worthy of additional investigation.
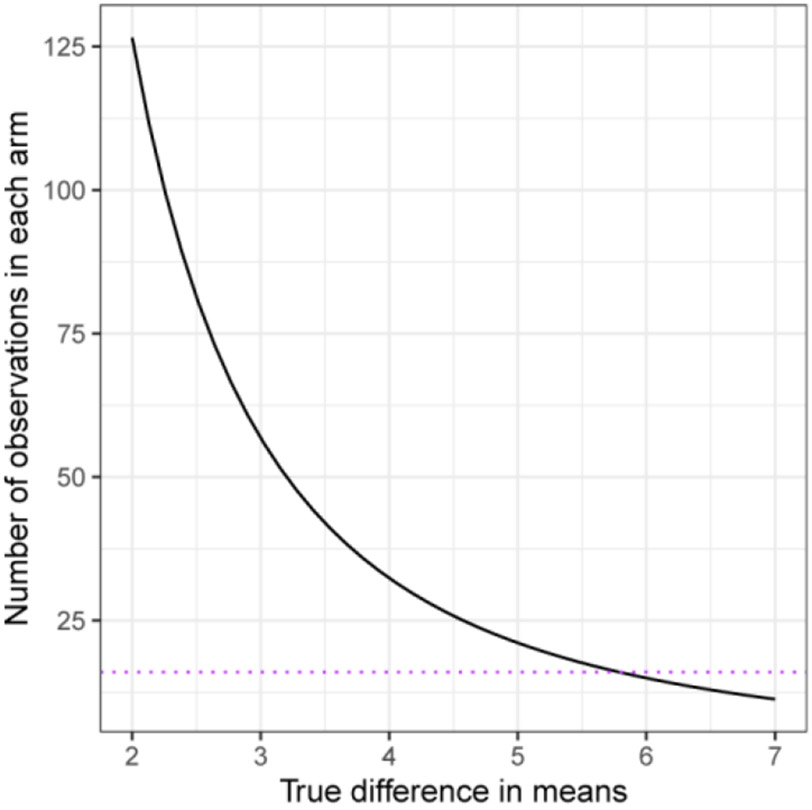
Towards that goal, this study also contributes to our experience with trial design and may be used to inform the design of future shorter-term neuroenhancement and longer-term neuroprotection trials. First, this trial recruited a wide diversity of patients in the interest of exploring efficacy measures at different ends of the glaucoma disease spectrum and demographics. In the absence of formal data to model cohort sizes for power analyses at the beginning of this study, no formal sample size calculation was performed, and the sample sizes were set based on typical phase 2 safety trial cohorts, and to make appropriate post-hoc power estimates. Our data suggested that selecting patients with more uniform characteristics including certain severity of disease, recent rapid progression, and reliable indices of testing quality all may enhance power to detect a neuroprotection or neuroenhancement effect. Recent modeling for neuroprotection trials similarly support this premise38-40. Our modeling from these real-world trial data similarly suggest, for example, that one may need about 125 patients per arm, if an effect size of 2 micrometers in the OCT RNFL measurements is expected by an intervention (Figure 9); of course, one would still need to take into account the test-retest variability of OCT measurements. Potentially combining OCT with VF data and using a trend-based analysis instead of event-based analysis may reduce required sample sizes and/or length of follow-up required for evaluating new treatments for vision preservation in a slowly progressive disease such as glaucoma39,40. RGC regeneration may require treatment longer than 8 weeks for detectable neuroprotective structural or functional effect. Now that we demonstrated good safety and tolerability profiles at 8 weeks of treatment in glaucoma patients, a longer treatment duration trial that could potentially translate the trends observed in this study further towards significance, as well as evaluate for potential long-term side effects, is warranted. In addition, a larger study population will enable sub-group analysis, which was not possible here due to small sample. Another major contributor to accelerating clinical trials for neuroprotection maybe derived from implementation of advanced or exploratory biomarkers (reviewed extensively here41).
Figure 9.
Required sample sizes for 80 percent power in t-test of retinal nerve fiber layer (RNFL). Curve for 80% power and significance level 0.05 for a t-test between two groups assuming that the within-group variability in changes is completely due to measurement error that has standard deviation (SD) 4.0.
These advances in trial design will be timely, as a number of candidate therapeutic approaches are being explored. As noted above, another neurotrophic factor CNTF is in phase 2 trials for glaucoma neuroprotection. Nicotinamide (vitamin B3) has demonstrated strong neuroprotective effects in rodent glaucoma models42,43, and has a strong safety record in human trials44-46 as well as one recent study on glaucoma patients47 with another pending report (clinicaltrials.gov NCT#03797469). Electrical stimulation, previously shown to strongly enhance neurotrophic factors’ effects on promoting RGC survival14,48,49, has also been explored in glaucoma patients50,51 and deserves further study of dosing and delivery optimization. A number of other strong candidates such as anti-complement C1q antibodies (see clinicaltrials.gov NCT#03488550) are in various stages of preclinical and early clinical testing for neuroprotection for glaucoma or other diseases. Broad adoption of clinical trials in glaucoma can now adequately test candidate neuroprotective treatments with a reasonable time and cost profile, and will further facilitate improvements in trial design and biomarker development towards novel therapeutics.
Acknowledgments:
The authors gratefully acknowledge clinical trial support from Dompe Pharma, the National Eye Institute P30-EY026877, and Research to Prevent Blindness, Inc.
Footnotes
Commercial Relationships Disclosure: Clinical trial grant to Stanford University from Dompe Pharma.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Levi-Montalcini R, Booker B. Destruction of the Sympathetic Ganglia in Mammals by an Antiserum to a Nerve-Growth Protein. Proc Natl Acad Sci U S A. 1960;46(3):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oddone F, Roberti G, Micera A, et al. Exploring Serum Levels of Brain Derived Neurotrophic Factor and Nerve Growth Factor Across Glaucoma Stages. PLoS One. 2017;12(1):e0168565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, McKinnon SJ, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41(11):3460–3466. [PubMed] [Google Scholar]
- 4.Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41(3):764–774. [PubMed] [Google Scholar]
- 5.Rocco ML, Soligo M, Manni L, Aloe L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr Neuropharmacol. 2018;16(10):1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colafrancesco V, Parisi V, Sposato V, et al. Ocular application of nerve growth factor protects degenerating retinal ganglion cells in a rat model of glaucoma. J Glaucoma. 2011;20(2):100–108. [DOI] [PubMed] [Google Scholar]
- 7.Lambiase A, Aloe L, Centofanti M, et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc Natl Acad Sci U S A. 2009;106(32):13469–13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo L, Davis BM, Ravindran N, et al. Topical recombinant human Nerve growth factor (rhNGF) is neuroprotective to retinal ganglion cells by targeting secondary degeneration. Sci Rep. 2020;10(1):3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology. 2020;127(1):14–26. [DOI] [PubMed] [Google Scholar]
- 10.Pham BH, Goldberg JL, Marmor MF. The rapid N-wave as a potentially useful measure of the photopic negative response. Doc Ophthalmol. 2020;141(3):253–257. [DOI] [PubMed] [Google Scholar]
- 11.Jankowski MP, Koerber HR. Neurotrophic Factors and Nociceptor Sensitization. In: Kruger L, Light AR, eds. Translational Pain Research: From Mouse to Man. Boca Raton (FL) 2010. [Google Scholar]
- 12.Bonini S, Lambiase A, Rama P, et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018;125(9):1332–1343. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari MP, Mantelli F, Sacchetti M, et al. Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. Bio Drugs. 2014;28(3):275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119(5):979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberti G, Mantelli F, Macchi I, Massaro-Giordano M, Centofanti M. Nerve growth factor modulation of retinal ganglion cell physiology. J Cell Physiol. 2014;229(9):1130–1133. [DOI] [PubMed] [Google Scholar]
- 16.Lavail MM. Survival factors for treatment of retinal degenerative disorders: preclinical gains and issues for translation into clinical studies. Retina. 2005;25(8 Suppl):S25–S26. [DOI] [PubMed] [Google Scholar]
- 17.Lambiase A, Mantelli F, Bonini S. Nerve growth factor eye drops to treat glaucoma. Drug News Perspect. 2010;23(6):361–367. [DOI] [PubMed] [Google Scholar]
- 18.Lenzi L, Coassin M, Lambiase A, Bonini S, Amendola T, Aloe L. Effect of exogenous administration of nerve growth factor in the retina of rats with inherited retinitis pigmentosa. Vision Res. 2005;45(12):1491–1500. [DOI] [PubMed] [Google Scholar]
- 19.Chew EY, Clemons TE, Jaffe GJ, et al. Effect of Ciliary Neurotrophic Factor on Retinal Neurodegeneration in Patients with Macular Telangiectasia Type 2: A Randomized Clinical Trial. Ophthalmology. 2019;126(4):540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338(17):1174–1180. [DOI] [PubMed] [Google Scholar]
- 21.Bocchini V, Angeletti PU. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969;64(2):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullrich A, Gray A, Berman C, Dull TJ. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983;303(5920):821–825. [DOI] [PubMed] [Google Scholar]
- 23.Rattenholl A, Lilie H, Grossmann A, Stern A, Schwarz E, Rudolph R. The pro-sequence facilitates folding of human nerve growth factor from Escherichia coli inclusion bodies. Eur J Biochem. 2001;268(11):3296–3303. [DOI] [PubMed] [Google Scholar]
- 24.Trabjerg E, Kartberg F, Christensen S, Rand KD. Conformational characterization of nerve growth factor-beta reveals that its regulatory pro-part domain stabilizes three loop regions in its mature part. J Biol Chem. 2017;292(40):16665–16676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Rodriguez A, Abad P, Arias-Alvarez M, et al. Recombinant rabbit beta nerve growth factor production and its biological effects on sperm and ovulation in rabbits. PLoS One. 2019;14(7):e0219780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colangelo AM, Finotti N, Ceriani M, et al. Recombinant human nerve growth factor with a marked activity in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102(51):18658–18663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apfel SC, Kessler JA, Adornato BT, Litchy WJ, Sanders C, Rask CA. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. NGF Study Group. Neurology. 1998;51(3):695–702. [DOI] [PubMed] [Google Scholar]
- 28.Apfel SC, Schwartz S, Adornato BT, et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: A randomized controlled trial. rhNGF Clinical Investigator Group. JAMA. 2000;284(17):2215–2221. [DOI] [PubMed] [Google Scholar]
- 29.Schifitto G, Yiannoutsos C, Simpson DM, et al. Long-term treatment with recombinant nerve growth factor for HIV-associated sensory neuropathy. Neurology. 2001;57(7):1313–1316. [DOI] [PubMed] [Google Scholar]
- 30.Lambiase A, Tirassa P, Micera A, Aloe L, Bonini S. Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Invest Ophthalmol Vis Sci. 2005;46(10):3800–3806. [DOI] [PubMed] [Google Scholar]
- 31.Sposato V, Parisi V, Manni L, et al. Glaucoma alters the expression of NGF and NGF receptors in visual cortex and geniculate nucleus of rats: effect of eye NGF application. Vision Res. 2009;49(1):54–63. [DOI] [PubMed] [Google Scholar]
- 32.Lambiase A, Pagani L, Di Fausto V, et al. Nerve growth factor eye drop administrated on the ocular surface of rodents affects the nucleus basalis and septum: biochemical and structural evidence. Brain Res. 2007;1127(1):45–51. [DOI] [PubMed] [Google Scholar]
- 33.Di Fausto V, Fiore M, Tirassa P, Lambiase A, Aloe L. Eye drop NGF administration promotes the recovery of chemically injured cholinergic neurons of adult mouse forebrain. Eur J Neurosci. 2007;26(9):2473–2480. [DOI] [PubMed] [Google Scholar]
- 34.Falsini B, Chiaretti A, Barone G, et al. Topical nerve growth factor as a visual rescue strategy in pediatric optic gliomas: a pilot study including electrophysiology. Neurorehabil Neural Repair. 2011;25(6):512–520. [DOI] [PubMed] [Google Scholar]
- 35.Chiaretti A, Falsini B, Servidei S, Marangoni D, Pierri F, Riccardi R. Nerve growth factor eye drop administration improves visual function in a patient with optic glioma. Neurorehabil Neural Repair. 2011;25(4):386–390. [DOI] [PubMed] [Google Scholar]
- 36.Mesentier-Louro LA, Rosso P, Carito V, et al. Nerve Growth Factor Role on Retinal Ganglion Cell Survival and Axon Regrowth: Effects of Ocular Administration in Experimental Model of Optic Nerve Injury. Mol Neurobiol. 2019;56(2):1056–1069. [DOI] [PubMed] [Google Scholar]
- 37.Cordeiro MF, Migdal C, Bloom P, Fitzke FW, Moss SE. Imaging apoptosis in the eye. Eye (Lond). 2011;25(5):545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quigley HA. Clinical trials for glaucoma neuroprotection are not impossible. Current opinion in ophthalmology. 2012;23(2):144–154. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z, Crabb DP, Chauhan BC, Crowston JG, Medeiros FA. Improving the Feasibility of Glaucoma Clinical Trials Using Trend-Based Visual Field Progression Endpoints. Ophthalmol Glaucoma. 2019;2(2):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Z, Medeiros FA. Sample Size Requirements of Glaucoma Clinical Trials When Using Combined Optical Coherence Tomography and Visual Field Endpoints. Sci Rep. 2019;9(1):18886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beykin G, Norcia AM, Srinivasan VJ, Dubra A, Goldberg JL. Discovery and clinical translation of novel glaucoma biomarkers. Prog Retin Eye Res. 2021;80:100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams PA, Harder JM, Foxworth NE, et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355(6326):756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cimaglia G, Votruba M, Morgan JE, Andre H, Williams PA. Potential Therapeutic Benefit of NAD(+) Supplementation for Glaucoma and Age-Related Macular Degeneration. Nutrients. 2020;12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen AC, Martin AJ, Choy B, et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N Engl J Med. 2015;373(17):1618–1626. [DOI] [PubMed] [Google Scholar]
- 45.Knip M, Douek IF, Moore WP, et al. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43(11):1337–1345. [DOI] [PubMed] [Google Scholar]
- 46.Kamal M, Abbasy AJ, Muslemani AA, Bener A. Effect of nicotinamide on newly diagnosed type 1 diabetic children. Acta Pharmacol Sin. 2006;27(6):724–727. [DOI] [PubMed] [Google Scholar]
- 47.Hui F, Tang J, Williams PA, et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clinical & experimental ophthalmology. 2020;48(7):903–914. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg JL. Role of electrical activity in promoting neural repair. Neurosci Lett. 2012;519(2):134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corredor RG, Goldberg JL. Electrical activity enhances neuronal survival and regeneration. J Neural Eng. 2009;6(5):055001. [DOI] [PubMed] [Google Scholar]
- 50.Gall C, Schmidt S, Schittkowski MP, et al. Alternating Current Stimulation for Vision Restoration after Optic Nerve Damage: A Randomized Clinical Trial. PLoS One. 2016;11(6):e0156134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabel BA, Gudlin J. Vision restoration training for glaucoma: a randomized clinical trial. JAMA Ophthalmol. 2014;132(4):381–389. [DOI] [PubMed] [Google Scholar]