Abstract
An outbreak of serogroup W-135 meningococcal disease was reported among pilgrims returning from the annual hajj (pilgrimage to Mecca) in mid-March 2000. Molecular characterization was used to investigate the similarity of the hajj-associated W-135 strains with those isolated in Sweden during a 23-year period (1978 to 2000). The same hajj-associated genosubtype, genosubtype P1.5,2,36b, has been documented in Sweden since 1979, while pulsed-field gel electrophoresis and the sulfadiazine resistance of the W-135 isolates indicated that the outbreak was probably due to a new clone of W-135 meningococci.
Neisseria meningitidis is a major cause of meningitis and septicemia in areas throughout the world where they are endemic and epidemic, with high rates of morbidity and mortality. On the basis of differences in their capsular polysaccharides, the epidemiology of meningococcal disease (MCD) is usually investigated by serogrouping N. meningitidis strains that have been isolated (1). Among the 13 known serogroups, serogroups A, B, and C account for about 90% of all cases (14), and serogroups Y and W-135 account for the majority of the remaining 10% of the cases. Individual N. meningitidis isolates can be further characterized, e.g., by serosubtyping of the PorA outer membrane protein (6) and, more recently, by genosubtyping of the porA gene (9, 19). This subtyping system primarily offers a useful epidemiological marker. For a more complete means of strain identification, fingerprinting of the whole chromosome, e.g., by pulsed-field gel electrophoresis (PFGE), is useful for investigation of the clonal relationships of isolates within meningococcal disease outbreaks (2, 11, 16).
Even though meningococci of serogroups B and C have caused several epidemics, serogroup A is the leading cause of outbreaks, particularly in the so-called meningitis belt (14). The annual hajj pilgrimage to Mecca, which attracts more than a million Muslim pilgrims from around the world, has played a role in the global spread of MCD. In 1987, an N. meningitidis serogroup A clone, previously isolated in East Asia and Europe, occurred among the pilgrims and spread globally when the pilgrims returned to their home countries (10, 12, 17). Saudi authorities now demand evidence that the pilgrims have been immunized with a serogroup A meningococcal vaccine.
An outbreak of serogroup W-135 MCD occurred in mid-March 2000 among Muslim pilgrims returning from the pilgrimage to Mecca and their contacts, with more than 340 cases being reported from around the world (World Health Organization, http://www.who.int/emc/outbreak-news/n2000/may/12may2000.html). All the serosubtyped strains reported to the World Health Organization were serosubtype P1.5,2. A few of them were also genosubtyped as genosubtype P1.5,2 by sequencing of variable regions 1 and 2 (VR1 and VR2, respectively) of the porA gene (5). Two individuals with invasive MCD caused by W-135, as well as two healthy carriers colonized with the same serogroup, all of whom were contacts of returning Muslim pilgrims, were also reported from Sweden in April 2000. The isolates were genosubtyped as genosubtype P1.5,2,36b by sequencing of VR1, VR2, and VR3 of the porA gene (9).
N. meningitidis serogroup W-135, previously known to cause MCD sporadically, particularly in those with complement deficiencies (4), has not been described to cause epidemics until the hajj-associated outbreak. The aim of the present study was to gain more insight into the evolving epidemiology of W-135 strains. By porA gene sequencing and PFGE, we have characterized invasive and carrier isolates of N. meningitidis W-135 collected in Sweden during a 23-year period (1978 to 2000) and compared the findings with those for reference isolates from pilgrims to the hajj.
In all, 46 N. meningitidis W-135 invasive and carrier strains isolated in Sweden from 1978 to 2000 and kept at the Swedish Reference Laboratory for Pathogenic Neisseria were included in the study along with two representative W-135 reference strains from pilgrims who attended the 2000 hajj (strains M7089B and M7034, kindly provided by T. Popovic, Centers for Disease Control and Prevention, Atlanta, Ga.) (Table 1). The isolates were cultured on GCSPP agar (3% GC medium base [Difco Laboratories, Detroit, Mich.], with 0.4% d-glucose, 0.01% l-glutamine, 0.0001% cocarboxylase, 0.0005% ferric nitrate, and 0.5% IsoVitaleX enrichment [BBL, Becton Dickinson Europe, Meylan, France]) for 18 to 20 h at 37°C in 5% CO2.
TABLE 1.
Genosubtypes, PFGE patterns, and sulfadiazine susceptibilities of W-135 meningococci isolated in Sweden between 1978 and 2000 in comparison with those of two 2000 hajj-associated reference strainsa
| Strain designation | Yr of isolation | Site of isolation | Genosubtype (VR1, VR2, VR3)b | PFGE type | Sulfadiazine susceptibility |
|---|---|---|---|---|---|
| 1 (608-9901) | 1979 | CSF | P1.5,2,36b | A | S |
| 2 (600-101) | 1980 | Blood | P1.5,2,36b | A | S |
| 3 (900-112) | 1987 | Nasopharynx | P1.5,2,36b | Unique | S |
| 4 (90-137) | 1994 | Blood | P1.5,2,36b | Unique | S |
| 5 (90-170) | 1995 | Joint fluid | P1.5,2,36b | Unique | S |
| 6 (90-66) | 1997 | Nasopharynx | P1.5,2,36b | B | S |
| 7 (90-68) | 1997 | Nasopharynx | P1.5,2,36b | B1 | S |
| 8 (90-71) | 1997 | Throat | P1.5,2,36b | B | S |
| 9 (90-7) | 1998 | Blood | P1.5,2,36b | Unique | R |
| 10 (90-188) | 1998 | Blood | P1.5,2,36b | Unique | R |
| 11 (90-410) | 1998 | Joint fluid | P1.5,2,36b | Unique | R |
| 12 (590-26) | 2000 | CSF | P1.5,2,36b | Unique | S |
| 13 (590-66)c | 2000 | CSF | P1.5,2,36b | C | R |
| 14 (590-75)c | 2000 | CSF | P1.5,2,36b | C | R |
| 15 (590-87)c | 2000 | Throat | P1.5,2,36b | C | R |
| 16 (590-95)c | 2000 | Throat | P1.5,2,36b | C | R |
| 17 (590-121) | 2000 | Blood | P1.5,2,36b | C | R |
| Ref 1 (M7089B)c | 2000 | CSF | P1.5,2,36b | C | R |
| Ref 2 (M7034)c | 2000 | Blood | P1.5,2,36b | C | R |
| 18 (600-773) | 1978 | Urogenital tract | P1.18a,3,38 | Unique | S |
| 19 (606-549) | 1978 | CSF | P1.18a,3,38 | Unique | S |
| 20 (606-396) | 1985 | Blood | P1.18a,3,38 | Unique | R |
| 21 (90-95) | 1990 | Bronchial | P1.18a,3,38 | Unique | S |
| 22 (90-193) | 1991 | Blood | P1.18a,3,38 | Unique | R |
| 23 (90-34) | 1993 | Blood | P1.18a,3,38 | Unique | R |
| 24 (90-142) | 1993 | Nasopharynx | P1.18a,3,38 | Unique | R |
| 25 (90-149) | 1995 | Nasopharynx | P1.18a,3,38 | D | R |
| 26 (90-122) | 1996 | Blood | P1.18a,3,38 | D1 | R |
| 27 (90-192) | 1998 | Throat | P1.18a,3,38 | E | R |
| 28 (90-193) | 1998 | Throat | P1.18a,3,38 | E | R |
| 29 (90-184) | 1998 | Blood | P1.18a,3,38 | E1 | R |
| 30 (90-272) | 1998 | Unknown origin | P1.18a,3,38 | F | S |
| 31 (90-274) | 1998 | Unknown origin | P1.18a,3,38 | F | S |
| 32 (590-194) | 1999 | Blood | P1.18a,3,38 | G | S |
| 33 (590-200) | 1999 | Throat | P1.18a,3,38 | G1 | S |
| 34 (590-49) | 2000 | Nasopharynx | P1.18a,3,38 | Unique | R |
| 35 (900-193) | 1987 | CSF | P1.5c,10a,36b | H1 | R |
| 36 (90-65) | 1990 | CSF | P1.5c,10a,36b | H | R |
| 37 (90-6) | 1996 | Blood | P1.5c,10a,36b | H | R |
| 38 (590-133) | 2000 | Blood | P1.5c,10a,36b | Unique | R |
| 39 (NK 793) | 1990 | Urogenital | P1.5a,2c,36b | I | S |
| 40 (90-76) | 1993 | Blood | P1.5a,2c,36b | I | S |
| 41 (90-105)d | 1996 | CSF | P1.5a,2c,36b | J | R |
| 42 (90-121)d | 1996 | CSF | P1.5a,2c,36b | J | R |
| 43 (90-55) | 1997 | Nasopharynx | P1.5a,10d,36b | Unique | R |
| 44 (90-106)e | 1995 | Throat | P1.7,16,35 | Unique | R |
| 45 (590-177) | 1999 | CSF | P1.7a,4a,37a | Unique | R |
| 46 (90-15)e | 1991 | Nasopharynx | P1.21,26-2,36bf | Unique | S |
CSF, cerebrospinal fluid; Ref, reference strain; R, resistant; S, sensitive.
Amino acid sequences of VR3: 35 (LIGSGSDQ), 36b (LLGSGSDE), 37a (LIGSATSDE), and 38 (LLGRIGDDDE).
Strains isolated from patients or carriers in Sweden; all were associated with returning Muslim pilgrims returning from the 2000 hajj as well as two reference strains from the 2000 hajj.
Serosubtyped as serosubtype P1.2.
Serosubtyped as serosubtype P1.16.
New VR2 variant found in the present study.
The strains were serogrouped and serosubtyped (with the available monoclonal antibodies, P1.2, P1.7, P1.15, and P1.16) by coagglutination (13). Antibiograms for the strains were established by the E-test (Biodisc, Solna, Sweden). Sulfadiazine was tested on ISOSP agar (3% Iso-Sensitest agar [Oxoid, Basingstoke, England], 5% defibrinated horse blood [Statens Veterinärmedicinska Anstalt, Stockholm, Sweden]), with MIC breakpoints of ≥12 mg/liter for resistance and ≤8 mg/liter for sensitivity. Susceptibility to penicillin G was tested on chocolate Mueller-Hinton agar (8). The strains were also tested for β-lactamase production by the chromogenic cephalosporin method (18) with nitrocefin disks (Biodisc).
Genosubtyping was performed as described previously (9), with DNA isolated with the Dynabeads DNA DIRECT system I (Dynal, Oslo, Norway). The VR1, VR2, and VR3 DNAs within the porA gene were sequenced. The deduced amino acid sequences of VR1 and VR2 were assigned genosubtype names according to the Neisseria meningitidis PorA Variable Region Database (http://outbreak.ceid.ox.ac.uk/porA-vr/). VR3 was assigned names according to the same system.
PFGE was performed with the GenePath Group 3 Reagent kit by using SpeI (Bio-Rad Laboratories, Hercules, Calif.). One colony of each N. meningitidis W-135 strain, grown overnight on GCSPP agar, was inoculated into 3 ml of Mueller-Hinton broth (0.3% beef extract [Difco Laboratories], 1.75% casein hydrolysate [Oxoid], and 0.15% starch [Merck, Darmstadt, Germany]) and grown on a shaker for 18 to 20 h at 37°C in 5% CO2. A total of 300 μl from each culture was used for preparation of agarose-embedded DNA according to the instructions of the manufacturer. Gel electrophoresis was run on a Gene Path System (Enb program; Bio-Rad Laboratories), photographed with a Gel Doc 2000 camera, and normalized with Molecular Analyst Fingerprinting software (version 1.6; Bio-Rad Laboratories). Comparative analysis of the DNA fingerprinting patterns between isolates divided the strains into different categories. Clusters of particular PFGE patterns are strains with identical band patterns with the same designation, e.g., type A, B, and C; and strains of, e.g., types B and D showing differences of one to three bands were designated types B1 and D1, respectively. PFGE patterns with more than three band differences compared with the patterns for types A, B, and C, etc., were designated unique for the present study.
The phenotypic characterization showed that only two strains each were serosubtypeable, and these were serosubtyped as serosubtypes P1.16 and P1.2, respectively. All other strains were nonserosubtypeable with the available monoclonal antibodies. The strains were sensitive to penicillin G (MICs, ≤0.125 mg/liter), and none were β-lactamase positive. Sulfadiazine resistance was found in 27 of the 46 strains (Table 1).
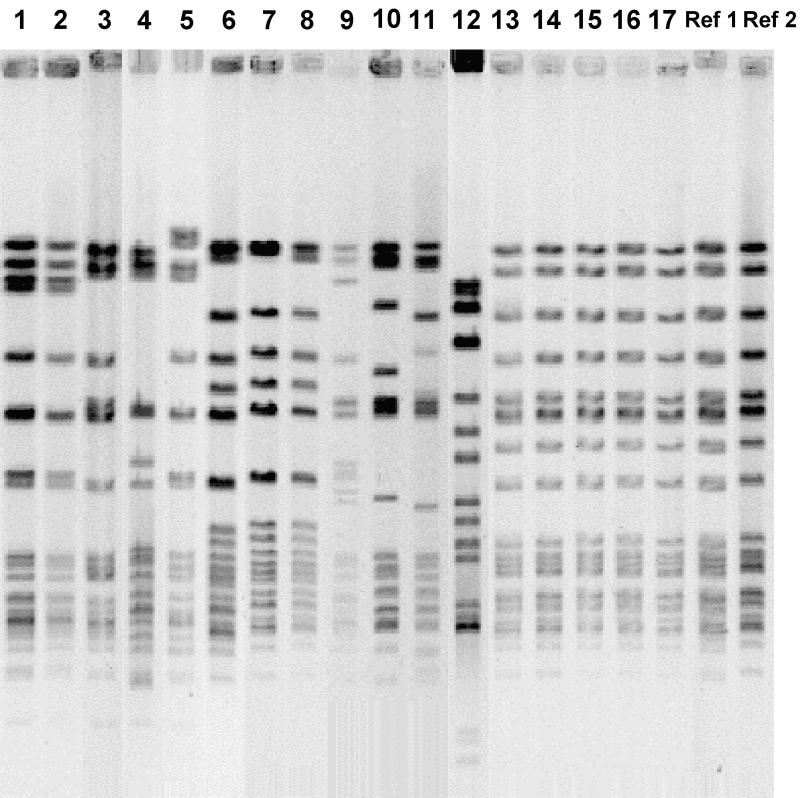
By porA gene sequencing all the strains were classified by subtype, with genosubtype P1.5,2,36b being the most prevalent one. It was demonstrated in 11 invasive and 6 carrier strains and in the 2 reference strains from participants in the 2000 hajj. As can be seen from Table 1, the four strains isolated in Sweden in April 2000 from contacts of returning Muslim pilgrims were of this genosubtype, and they made up one cluster with the same PFGE pattern as the reference strains; all six strains were resistant to sulfadiazine. This was also true for another invasive W-135 isolate from May 2000 (strain 17, Table 1); however, no information on contact with pilgrims was available. The remaining 12 strains of the same genosubtype, including the 2 earliest ones from 1979 and 1980 (both invasive isolates), were different from the hajj-associated isolates with regard to their PFGE patterns (Fig. 1). Two clusters, together with seven unique PFGE patterns, were seen for those 12 strains. Moreover, the strains of this genosubtype isolated before 1998, eight in all, were sensitive to sulfadiazine, whereas eight of nine strains isolated in the last 3 years (1998 to 2000) were resistant (Table 1).
FIG. 1.
PFGE fingerprints obtained with the SpeI endonuclease for all W-135 meningococci of genosubtype P1.5,2,36b found in Sweden since 1979 (strains 1 to 17) as well as two reference strains (Ref) from the 2000 hajj-associated outbreak (Table 1). Strains 13 to 16 are known to be hajj associated.
Another seven different genosubtypes were demonstrated, with P1.18a,3,38 being the second most frequent genosubtype found among both invasive (n = 7) and carrier (n = 8) strains (Table 1). Only a few strains of a particular genosubtype had similar PFGE patterns; however, all strains of a PFGE type had the same genosubtype. Within these subtypes, the PFGE patterns revealed seven clusters, and 13 unique strains were found. One genosubtype, genosubtype P1.21,26–2,36b, represented a new VR2 variant not described previously, as indicated in Table 1, footnote f.
The present study demonstrated eight different subtypes of the porA gene among 46 isolates of W-135 meningococci collected in Sweden during a 23-year period (1978 to 2000). The four W-135 isolates from the patients and the carriers with contact with Muslim pilgrims returning from the 2000 hajj were identical to the hajj-associated reference strains of this W-135 outbreak with regard to genosubtype, PFGE pattern, and sulfadiazine resistance. We also showed that the same genosubtype as that of the hajj-associated isolates had already occurred among W-135 strains in the late 1970s and early 1980s. In fact, it was the most prevalent genosubtype. However, PFGE distinguished them from the hajj-associated isolates, and no tendency for the secondary spread of W-135-related disease has been noted in Sweden. It therefore seems most likely that the hajj-associated outbreak in 2000 was due to a particular clone of this W-135 genosubtype. A recent report by Popovic et al. (T. Popovic, C. T. Sacchi, M. W. Reeves, A. M. Whitney, L. W. Mayer, C. A. Noble, G. W. Ajello, F. Mostashari, N. Bendana, J. Lingappa, R. Hajjeh, and N. E. Rosenstein, Letter, Emerg. Infect. Dis. 6:428–429, 2000), who used multilocus enzyme electrophoresis, showed that the hajj-associated isolates were clonally related to the disease-associated electropheretic type 37 complex, which gives further support for the assumption that there is a new and particular clone of W-135 that has invasive characteristics.
The sulfadiazine resistance of meningococci is sometimes used as a marker for the epidemiology of N. meningitidis outbreaks (7). It was therefore of interest to note that our hajj-associated isolates, as well as the reference strains, were resistant to sulfadiazine. In contrast, all W-135 isolates recovered before 1998 were sensitive, whereas 89% of the isolates recovered in the last 3 years (1998 to 2000) were resistant.
The use of phenotypic markers such as the serosubtype of the PorA protein has proved to be efficient and informative in epidemiological investigations, both of sporadic cases and of outbreaks, particularly those of serogroup B MCD. In the present study, less than 10% of the W-135 isolates could be serosubtyped. In this respect, genosubtyping of the porA gene seems to be the method of choice. The stability within this gene was obvious when sequencing of VR3 was also included with sequencing of VR1 and VR2. With the use of PFGE the hajj-associated strains were discriminated from the previous W-135 isolates in Sweden with the same genosubtype.
The reasons for the spread of the hajj-associated W-135 strains remain unclear and cannot be explained by the findings of the present study. It is well known that a high rate of carriage of particular clones of serogroup A, B, and C meningococci may precede an outbreak (3). However, no data are available from the hajj on the rate of carriage of W-135. After the N. meningitidis group A outbreak in 1987, Saudi authorities requested that pilgrims be vaccinated. The use of the bivalent group A and C vaccine might have favored the prevalence of W-135, a corollary to the increased incidence of serogroup B meningococcal disease recently reported from England among first-year university students immunized with the group C vaccine (15). Pilgrims from the United States can be vaccinated with the quadrivalent A/C/Y/W-135 polysaccharide vaccine, which is licensed in the United States. Even though the protection that this vaccine offers against MCD caused by serogroups A and C is well documented, it has not been shown for disease caused by serogroups Y and W-135, and in fact, pilgrims vaccinated with the quadrivalent vaccine also had W-135-associated disease (Popovic et al., Letter).
In conclusion, the genosubtype of the strains associated with the 2000 hajj outbreak of W-135-related MCD was common among N. meningitidis strains isolated in Sweden over a 23-year period (1978 to 2000). PFGE indicated that the 2000 hajj meningococcal W-135 P1.5,2,36b genosubtype was newly introduced in Sweden after the pilgrimage. The origin of this clone and the reasons for its spread are unclear; therefore, further investigations are warranted.
Acknowledgments
We gratefully thank Tanja Popovic for providing the two epidemiologically related reference strains from the 2000 hajj. We also thank Dan Danielsson for constructive discussions.
This study was supported by grants from the Örebro County Council Research Committee, the Foundations for Medical Research at Örebro Medical Centre Hospital, and the Adolf Lindgrens Foundation, Örebro, Sweden.
REFERENCES
- 1.Branham S E. Milestones in the history of the meningococcus. Can J Microbiol. 1956;2:175–188. doi: 10.1139/m56-023. [DOI] [PubMed] [Google Scholar]
- 2.Bygraves J A, Maiden M C. Analysis of the clonal relationships between strains of Neisseria meningitidis by pulsed field gel electrophoresis. J Gen Microbiol. 1992;138:523–531. doi: 10.1099/00221287-138-3-523. [DOI] [PubMed] [Google Scholar]
- 3.Edwards E A, Devine L F, Sengbusch G H, Ward H W. Immunological investigations of meningococcal disease. III. Brevity of group C acquisition prior to disease occurrence. Scand J Infect Dis. 1977;9:105–110. doi: 10.3109/inf.1977.9.issue-2.09. [DOI] [PubMed] [Google Scholar]
- 4.Figueroa J E, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fine A, Layton M. Serogroup W-135 meningococcal disease among travelers returning from Saudi Arabia—United States. Morb Mortal Wkly Rep. 2000;49:345–346. [PubMed] [Google Scholar]
- 6.Frasch C E, Zollinger W D, Poolman J T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 7.Holten E, Froholm L O, Gaustad P. Virulence markers in patient and carrier strains of Neisseria meningitidis. Scand J Infect Dis. 1984;16:267–270. doi: 10.3109/00365548409070399. [DOI] [PubMed] [Google Scholar]
- 8.Hughes J H, Biedenbach D J, Erwin M E, Jones R N. E test as susceptibility test and epidemiologic tool for evaluation of Neisseria meningitidis isolates. J Clin Microbiol. 1993;31:3255–3259. doi: 10.1128/jcm.31.12.3255-3259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mölling P, Unemo M, Bäckman A, Olcén P. Genosubtyping by sequencing group A, B and C meningococci; a tool for epidemiological studies of epidemics, clusters and sporadic cases. APMIS. 2000;108:509–516. [PubMed] [Google Scholar]
- 10.Moore P S, Reeves M W, Schwartz B, Gellin B G, Broome C V. Intercontinental spread of an epidemic group A Neisseria meningitidis strain. Lancet. 1989;ii:260–263. doi: 10.1016/s0140-6736(89)90439-x. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas P, Parzy D, Martet G. Pulsed-field electrophoresis analysis of clonal relationships among Neisseria meningitidis A strains from different outbreaks. Eur J Clin Microbiol Infect Dis. 1997;16:541–544. doi: 10.1007/BF01708241. [DOI] [PubMed] [Google Scholar]
- 12.Novelli V M, Lewis R G, Dawood S T. Epidemic group A meningococcal disease in Haj pilgrims. Lancet. 1987;ii:863. doi: 10.1016/s0140-6736(87)91056-7. [DOI] [PubMed] [Google Scholar]
- 13.Olcén P, Danielsson D, Kjellander J. The use of protein A-containing staphylococci sensitized with antimeningococcal antibodies for grouping Neisseria meningitidis and demonstration of meningococcal antigen in cerebrospinal fluid. Acta Pathol Microbiol Scand. 1975;83:387–396. doi: 10.1111/j.1699-0463.1975.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 14.Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983;5:71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- 15.Ramsay M E, Andrews N, Kaczmarski E B, Miller E. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet. 2001;357:195–196. doi: 10.1016/S0140-6736(00)03594-7. [DOI] [PubMed] [Google Scholar]
- 16.Riesbeck K, Orvelid-Mölling P, Fredlund H, Olcén P. Long-term persistence of a discotheque-associated invasive Neisseria meningitidis group C strain as proven by pulsed-field gel electrophoresis and porA gene sequencing. J Clin Microbiol. 2000;38:1638–1640. doi: 10.1128/jcm.38.4.1638-1640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salih M A, Ahmed H S, Karrar Z A, Kamil I, Osman K A, Palmgren H, Hofvander Y, Olcén P. Features of a large epidemic of group A meningococcal meningitis in Khartoum, Sudan in 1988. Scand J Infect Dis. 1990;22:161–170. doi: 10.3109/00365549009037897. [DOI] [PubMed] [Google Scholar]
- 18.Schoenknecht F D, Sabath L D, Thornsberry C. Susceptibility tests: special tests. In: Lennette E H, Balows A, Hausler W J, Shadomy H J Jr, editors. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. p. 1006. [Google Scholar]
- 19.Suker J, Feavers I M, Achtman M, Morelli G, Wang J F, Maiden M C. The porA gene in serogroup A meningococci: evolutionary stability and mechanism of genetic variation. Mol Microbiol. 1994;12:253–265. doi: 10.1111/j.1365-2958.1994.tb01014.x. [DOI] [PubMed] [Google Scholar]