Abstract
Plant virus nanoparticles (PVNPs) have inherent immune stimulatory ability, and have been investigated as immune adjuvants to stimulate an anti-tumor immune response. The combination of immune stimulation, nanoparticle structure and the ability to deliver other therapeutic molecules provides a flexible platform for cancer immunotherapy. Researching multifunctional PVNPs and their modification will generate novel reagents for cancer immunotherapy. Here we review the properties of PVNPs, and their potential for clinical utilization to activate anti-tumor innate and lymphoid immune responses. PVNP have potential utility for cancer immunotherapy as vaccine adjuvant, and delivery systems for other reagents as mono immunotherapy or combined with other immunotherapies. This review outlines the potential and challenges in developing PVNPs as cancer immunotherapy reagents.
Keywords: cancer immunotherapy, in situ vaccination, nanoparticles, molecular farming, combination therapy, virus-like particle
Graphical abstract:
Plant virus nanoparticles as adjuvants and antigen delivery systems to activate the immune system and to improve immunotherapy efficacy.
1. Introduction
Cancer is a major cause of human morbidity and mortality. Despite improving therapies and outcomes of treatment for some cancers, prognosis for many patients remains poor. Limitations associated with current treatment options including drug resistance, off-target effects and adverse clinical events, and tumor genetic heterogeneity, emphasize the urgency of developing more effective therapies [1].
Mammalian viruses, have been studied as oncolytic viruses, nanocarriers or vaccines to improve conventional cancer therapy [2]. Each virus interacts differently with cells in vivo but all are nanoparticles with a natural ability to deliver cargo to target cells. Most viruses can also be engineered by either genetic modification or modification post production to further generate promising options for cancer therapies [3].
Oncolytic viruses (OVs) are currently of considerable interest. Many OVs are preferentially modified for replicating in tumor cells and inducing an immune-mediated anti-tumor effect by combining tumor lysis with induction of typical antiviral responses that can reverse the tumor-mediated immune suppression [4–6]. Additionally, OVs induce release of both tumor-associated antigens (TAAs), and neoantigens, and the antiviral immune response promotes the ingestion and cross-presentation of these antigens by antigen-presenting cells (APCs) [6]. However, since OVs depend on successful invasion of tumor cells and replication, the host immune responses against the OVs can neutralize the OVs cell invasion, block tumor activity, and inactivate by neutralizing antibodies in the blood stream [1, 7, 8].
While of interest and potential clinical value, medical application of mammalian viruses are challenged by the possibility of mutation-driven reversion of attenuated versions to virulent forms, and host-genome integration of virus genome sequences [1]. Plant viruses do not infect mammalian cells, so they lack these infection-related drawbacks and provide useful tools for manipulating tumors and anti-tumor immunity [1, 3]. In contrast with OVs, plant viruses do not infect mammalian cells and so do not replicate within and kill cancer cells directly, PVNPs are a novel nanoparticle class of immunostimulatory agents [3]. However, plant viruses do carry foreign antigens that will be recognized by the immune system and responded against, so it is necessary to understand the molecular processes involved in the interaction of plant viruses and immune cells.
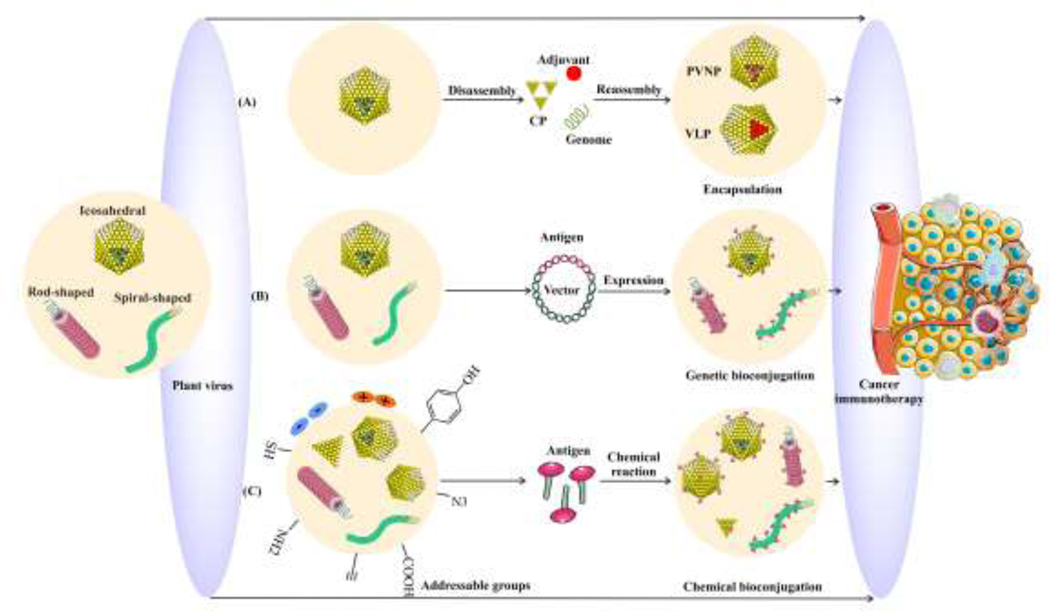
We consider two subsets of plant viruses, whole viruses, referred to in this context as viral nanoparticles (VNPs), and virus-like particles (VLPs). VLPs are unable to replicate in plants and most commonly are genome-free counterparts of their VNPs. VLPs mimic the native structure of viruses, and have both advantages and disadvantages for immunotherapy [3]. VNPs or VLPs can act as both immune adjuvant, and antigen delivery systems through inherent recognition as pathogens and can accommodate loading of either antigens or exogenous immune adjuvants via genetic expression, conjugation or encapsulation methods [9–11] (Fig. 1).
Fig. 1.
Diversity of plant virus-based modifications and formulations for cancer immunotherapy. (A) PVNPs disassembled and reassembled to encapsulate anti-tumor agents. (B) Genetic engineered plant viruses co-express selected peptides with the coat protein (CP) to produce a chimeric capsid. (C) Variable parameters including shape and size (different aspect ratio), charge, and surface addressable groups allow conjugation of functionalized immunogenic agents on PVNPs through surface chemistry reaction.
VNPs or VLPs, like most nanoparticles, are efficiently taken up by APCs and can stimulate immune responses. As with all nanoparticles, delivery to the tumor is a challenge. While systemic delivery remains a potential option, published utilization in cancer immunotherapy has focused on direct injection into tumors to stimulate anti-tumor immunity by disrupting the local immunosuppression, which supports first local and then systemic anti-tumor immunity, a process called “in situ vaccination (ISV)”. ISV utilizes the tumor antigens in the treated tumor itself as the vaccination antigen source, but could also deliver tumor specific antigens [12]. Further, the presence or absence of the “viral genome’s” in plant VNPs (PVNPs) and their derived VLPs, respectively, causes different immunostimulatory responses as described in this review. PVNPs and their VLPs are nanoparticle antigen or immunostimulatory reagent delivery platforms that include varying levels of inherent immunestimulatory properties [1, 3]. Many outstanding biologic questions remain and the potential to bring these systems into the clinic is the focus of this review.
Overall, understanding the molecular mechanisms that drive PVNP-mediated recognition and activation of the human immune system may pave the way for new reagents that support effective tumor immunotherapies. Here, we first present the properties of PVNPs and their potential for modification and delivery of antigen/adjuvant. Then we discuss the cellular and molecular mechanisms involved in PVNP- immune interaction and its potential for cancer immunotherapy as vaccine or delivery systems in monotherapy and combinatorial forms. Finally, we summarize the challenges and limitations recognized for PVNP application which must be addressed to facilitate translation of PVNPs-based immunotherapies into the clinic.
2. Properties and potentials of plant viruses
Plant viruses contain an RNA or DNA genome, capsid, and unlike mammalian viruses, rarely carry a lipid envelope derived from the cell that generated them [3]. Although plant viruses may cause phytopathology, they are not infectious for animals. Plant viruses have various morphologies, including icosahedron, rod and spiral shapes [3]. Like most viruses, their size, shape, and physico-chemical properties identify plant viruses as bionanomaterials [13] (Fig. 1). There is minimal potential for direct toxicity of PVNPs in vivo, since they are noninfectious, biocompatible, biodegradable, and non-teratogenic [3]. These characteristics enable minimal toxicity even in high doses (up to 100 mg/kg) and support safety of PVNPs for in vivo application [14–16]. Any potential toxicity is mediated by the desired biomedical function of the PVNPs themselves, such as their immunostimulatory properties.
Plant viruses can be manipulated genetically and physico-chemically for the production of modified PVNPs and VLPs [17]. Briefly, physical manipulation including conditions such as pH, temperature, or buffer formulation and concentration in association with incubation with reagents of interest enables loading of selected cargoes into or on PVNPs [3] (Fig. 1A). Genetic manipulation enables expression of selected proteins with the coat protein (CP) to produce chimeric capsids with new epitopes/cargoes [3, 13] (Fig. 1B). Chemical modifications support loading by functionalizing selected cargoes though coupling reagents or enzymatic reactions to bioconjugate cargoes to external or internal surfaces of PVNPs [3] (Fig. 1C).
As with any clinical reagent, production issues must be considered. PVNPs can be produced efficiently in large quantities by purification from virus-infected plants [18–20], or plant molecular farming (PMF), which refers to the engineering of plants for production of PVNPs or recombinant proteins by transgenic plants since they can be grown on an agricultural scale [21, 22]. Overall, these modification strategies enable production of targeted PVNPs that carry ligands for specific target cells.
3. Plant viruses induce innate and adoptive immune responses
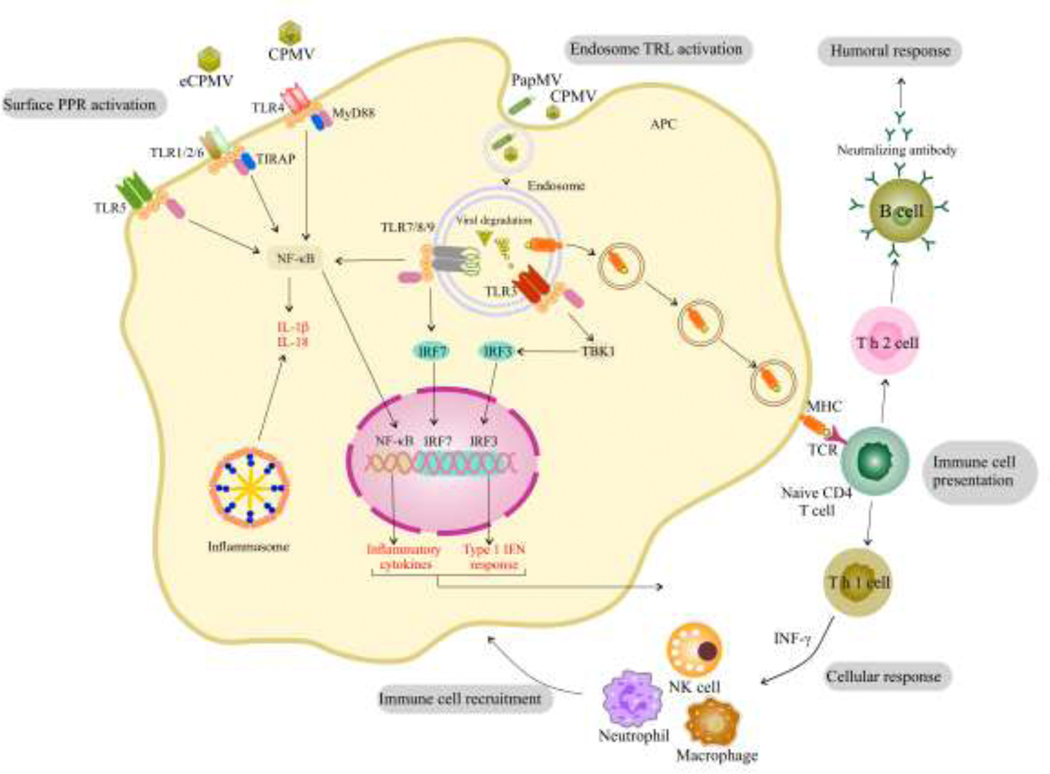
There are many mammalian pathogen pattern recognition receptors (PRRs) and their expression varies among cell types. Each cell expresses a unique array of PRRs and activation of a given PRR in each cell type produces different responses. Immune cells express a wide variety of PRRs and respond strongly to PRR signaling. Upon encountering any virus, whether it is infectious or not, the PRRs of immune cells, such as dendritic cells (DCs), macrophages, and B cells recognize pathogen-associated molecules produced by viruses. PRRs are classified into multiple types including toll-like receptors (TLRs), cytosolic DNA sensors (CDS), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene I (RIG-I) like receptors (RLRs), C-type lectin domain (CTLD) proteins, and absent in melanoma (AIM)-like receptors (ALRs) [23, 24]. Plant viruses have recently been shown to primarily engage TLRs which are expressed on the cell surface, in endocytic compartments, and in the cytoplasm [25]. Most recently, we showed that cowpea mosaic virus (CPMV) capsids are recognized by TLR2 and TLR4 on membrane surface of the phagocytes, and its encapsulated nucleic acids are recognized by cytoplasmic TLR7 to induce cytokines secretion as well anti-tumor immunity [26].
The nucleic acids carried by PVNP are potentially recognized by TLRs, giving PVNP of all sorts the potential to be immunostimulatory. Most plant viruses have RNA genomes, and viral double-stranded RNA (dsRNA) and single-stranded RNA (ssRNA) are pathogen associated molecular patterns (PAMPs) recognized by specific TLRs (e.g., TLR 3/7) expressed by many immune cells including professional APCs [27]. Papaya mosaic virus (PapMV) coat proteins (CP) self-assembled around a ssRNA promotes activation of endosomal TLR7 in plasmacytoid DCs (pDCs) which stimulates innate immunity, and induces production of type I interferons, such as interferon-α (IFN-α), which are important in the anti-tumor immune responses [28]. Other RNA-carrying PVNPs, like tobacco mosaic virus (TMV) and CPMV, stimulate an anti-tumor immune response [29]. Not surprisingly, recognition of PVNP by TLRs is likely a broad characteristic of mammals since mice, dogs and humans all respond.
TLR ligation sometimes engage SYK (spleen tyrosine kinase), as part of a cytoplasmic protein-tyrosine kinase signaling pathway in innate immune cells resulting in downstream signaling and consequent pathogen recognition, inflammasome activation, and antiviral response. Upon PVNP exposure, activation of endosomal TLRs is thought to contribute to SYK- and MyD88-coupled PRR signaling as well as cytokine production [27, 30]. For example, CPMV can activate endosomal TLR7/8 in human monocytes, that involves SYK signaling mediating endosomal acidification as well as activation of human monocytes to produce CXCL10 and type I and II IFNs [27].
TLR signaling mediates either type I interferon related pathways through transcription of interferon regulatory factors (IRF), or inflammatory pathways through activation of nuclear factor kappaB (NF-ҡB) or both [31]. While recognition of viral nucleic acids has long been recognized, recognition of the viral capsid in immune stimulation is poorly understood. There is developing data showing that at least some PVNP capsids are agonists for TLRs. CPMV induces human monocytes in vitro to secrete high levels of cytokines and chemokines such as CXCL10, MIP-1α, and MIP-1β [27]. Furthermore, bone marrow-derived dendritic cells (BMDCs) exposed to cowpea chlorotic mottle virus (CCMV), CPMV, and SeMV produced different levels of pro-inflammatory cytokines such as IL-1β, IL-6, IL-12, IFN-α, IFN-γ, and TNF-α, that shows how these viruses can regulate T cell-mediated immune responses [32–34]. CPMV, PepMV, PVX and TMV can induce anti-tumor effects via secretion of TNF, IL-12, IFN-γ, and IL-6 in animal cancer models [29, 34–38].
There is variability in the capacity for immune stimulation by different PVNP, but the basis for this variability is not clear. The definition of what constitutes a nanoparticle is not clearly established, but generally nanoparticle is defined as larger than a larger protein (10 nm) and smaller than any free living microorganism which is roughly 500 nm. PVNPs are between 20–300 nm in their longest dimension. Similar to other nanoparticles in this size range, they are efficiently ingested by phagocytic cells and any proteins they contain can be processed for antigen presentation by APCs. [39–41]. The repetitive three-dimensional structure of the non-enveloped capsid of PVNPs is essentially a protein crystal that can stimulate PRRs and thus affect the efficiency of phagocytosis and immune responses [42–44]. There is variability of PRR stimulation by different PVNPs and the basis of that variability is not yet understood. The empty capsid of CPMV (eCPMV) is an RNA-free VLP that is recognized by TLR2 and TLR4 and signals through a MyD88-dependent pathway, to induce inflammatory cytokines, while the ssRNA of CPMV is recognized by TLR7 and induces type I IFNs secretion [26](Fig. 2). Even without type I IFN induction, eCPMV also can induce immunogenicity in treated tumors, which proves that the capsid structure of this particular VLP also can stimulate anti-tumor effects [45]. Notably, the use of free coat protein and naked nucleic acid can be less immunogenic than VNPs and VLP owing in part to sensitivity to proteases and nucleases [29, 46, 47]. There is data in PVNPs showing that the assembled capsid is significantly more immunostimulatory than the dissociated coat proteins [29, 46–48], which may in part be due to the noted increased phagocytosis of nanoparticles or there could be a requirement for that structure for TLR recognition, or both could contribute. The recognition of the capsids of these non-enveloped and non-infectious viruses in mammals is poorly understood and once understood will enable more rapid and effective usage of PVNPs for cancer immunotherapy.
Fig. 2.
Mechanisms by which PVNPs can activate an innate immune response. PVNPs can act as ligands for PRRs which activate transcription factors that activate the cell and induce the production of cytokines and chemokines, which help direct a particular immune response, such as a TH1 or TH2 type response, as well as influence the immune cells that are recruited to the site of injection. Inflammasome activation is associated with activation of some TLRs stimulated by PVNPs. These pathways also influence antigen presentation by MHC.
The inherent immunogenicity of a PVNP will likely lead to the development of an anti-PVNP immune response, both by B cells and by T cells. Anti-PVNP specific antibodies are demonstrated in response to administration of CPMV [49], alfalfa mosaic virus (AMV) [50], and pepper mild mottle virus (PMMoV) [51]. Interestingly, TMV nanoparticles camouflaged using serum albumin (SA) does not inhibit anti-TMV antibody production [52]. Interestingly, unlike OVs that must infect cells and whose infective capacity would be blocked by neutralizing antibodies, antibodies against PVNPs do not appear to block their immunostimulatory properties, and in fact may enhance it [49].
4. PVNP-based cancer immunotherapies
The immune stimulating properties of PVNPs supports their use in carrying antigens for vaccines, including tumor antigens, which is discussed below. However, there is a role for PVNP to simulate anti-tumor immunity when they are introduced into tumors without exogenous antigens as ISV reagents [53]. The major block to immunotherapy is the immunosuppressive microenvironment of tumors. This local immunosuppressive TME blocks immune effector functions and any tumor immunotherapy must overcome this local immunosuppression to be successful [54]. ISV is a simple and effective immunotherapy strategy in which immunostimulatory agents or treatments are delivered directly to identified tumors [55, 56]. The immune stimulation can generate an effective response against the treated tumor and generate tumor-recognizing T cells that can find and eliminate metastatic tumors that were not directly treated [54]. The process is referred to as “in situ vaccination” because the process exploits the antigens within the tumor without the need to identify them or bring in identified antigens. ISV is a simple and quickly applied approach to rapidly provide anti-tumor vaccination for cancer patients. ISV could be effectively used for patients with potential unidentified metastatic disease in the time between pathologic diagnosis and surgery to remove the primary tumor, although it is not yet used in this manner.
Beyond the inherent immunostimulating of PVNPs, they can be nanocarriers for antigens, immunostimulatory agents, or both to activate immune responses [57]. PVNPs, owing to natural tropism for APCs as adjuvant, and due to high loading capacity of immunogenic epitopes can activate or enhance the anti-tumor immunity of the body [58]. Table 1 and Fig. 4 depict the functions and applications of PVNPs in tumor immunotherapy.
Table 1.
A summary of multifunctional PVNPs for cancer immunotherapies
| Immunotherapies functions | PVNP | Delivery agents | Tumor candidate | Function and major effect | Ref |
|---|---|---|---|---|---|
| Vaccine delivery | PVX | HPV-E7 antigen | Cervical cancers | Induce a humoral and cell immune response | [59, 60] |
| PVX, CPMV, CCMV, SeMV, TMV | HER2 epitopes | Breast cancer | Production of HER2-specific antibodies in mice | [34, 58, 61, 62] | |
| CPMV | NYESO-1157–165 | NY-ESO 1+malignancies | Uptake and activation of antigen presenting cells and stimulated a potent CD8+T cell response in transgenic human HLA-A2 expressing mice | [63] | |
| PVX | Idiotypic (Id) | B-cell lymphoma | Induction of strong antibody response | [64] | |
| TMV | Peptide p15 | Melanoma | Improve the survival in mouse model | [43] | |
| Adjuvant delivery | CCMV | CpG | Colon cancer and Melanoma | Enhances the efficacy of CpG, slowing tumor growth in mouse models | [65] |
| Monotherapy | CPMV, TMV, PVX, AMV | No antigen | Melanoma, Breast, Glioma and Ovarian cancer | Anti tumor activity by innate and adaptive immune responses | [29, 35, 38, 45, 53, 66] [29][67] [68] |
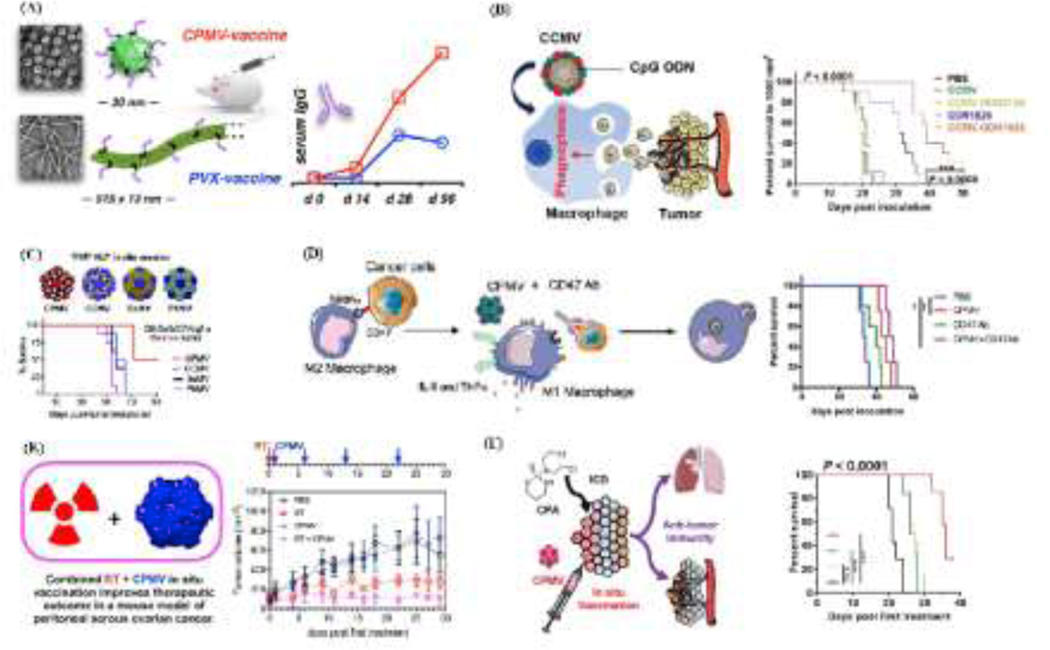
Fig. 4.
Examples of PVNP -based cancer immunotherapies. A) PVNP based vaccines; HER2 antigen conjugated to Cowpea mosaic virus (CPMV) and Potato virus x (PVX) nanoparticles resulted in higher antibody responses (image copywrite [58]). B) PVNP based stimulatory agents; CpG ODN1826 is encapsulated by the in vitro disassembly and reassembly of Cowpea chlorotic mottle virus (CCMV) and significantly enhances the efficacy of ODN1826, slowing tumor growth and prolonging survival in mouse models (image copyright [65]). C) PVNPs as monotherapy; in situ vaccination of PVNPs based CPMV, CCMV, Physalis mosaic virus (PhMV), Sesbania mosaic virus (SeMV) overcomes the local immunosuppression and stimulates a potent anti-tumor response in several mouse cancer models. PVNPs-based combination therapies leading to a potent anti-tumor immune response (image copyright [87]). D) CPMV in situ vaccination (ISV) and CD47-blocking antibodies (image copyright [89]). E) ISV of CPMV and radiation therapy (image copyright [66]). F) ISV of CPMV and chemotherapy agents (image copyright [35]).
4.1. PVNP monotherapy modulates immunosuppressive TME
TME refers to the internal environment of tumors, and is composed of tumor, stromal and immune cells, microvessels and interstitium, and is infiltrated by biological molecules [69]. Studies support a crucial role of immune–immune and immune–tumor TME interaction networks, and the tumor immune microenvironment (TIME) on tumor growth, metastasis and resistance to treatments [70, 71]. Nanoparticles, owing to their special physico-chemical properties, and the possible enhanced permeability and retention (EPR) of tumor blood vessels, can be enriched in the TME, modulate the TIME and induce anti-tumor effects [72].
In vivo, systemic, and intratumoral inoculation of PVNPs (ISV) meaningfully inhibited tumor cell growth and prevented distant metastasis of tumors without clinical toxicity [73–77]. Notably, the studies clarified that the anti-tumor mechanism of PVNPs includes the modulation of the TIME.
Myeloid-derived suppressor cells (MDSCs) originate from pluripotent hematopoietic stem cell, are classified into monocytic and granulocytic lineages, and can infiltrate tumors and differentiate to tumor-associated macrophages (TAMs) and tumor-associated neutrophils(TANs) [78, 79]. The TME can attract or differentiate MDSC differentiation into immune suppressive cells similar to M2 macrophages and N2 neutrophils [29, 74, 80]. In progressive tumors, M2 type TAMs and N2 type TANs release immunosuppressive mediators in the TME [79, 81–83]. These mediators inhibit T-cell activation, and promote tumor growth, invasion and metastasis (Fig. 3a). M1-TAMs and N1-TANs can be attracted by immune stimulation and play an anti-tumor role by releasing immunostimulatory mediators among other functions [79, 80].
Fig. 3.
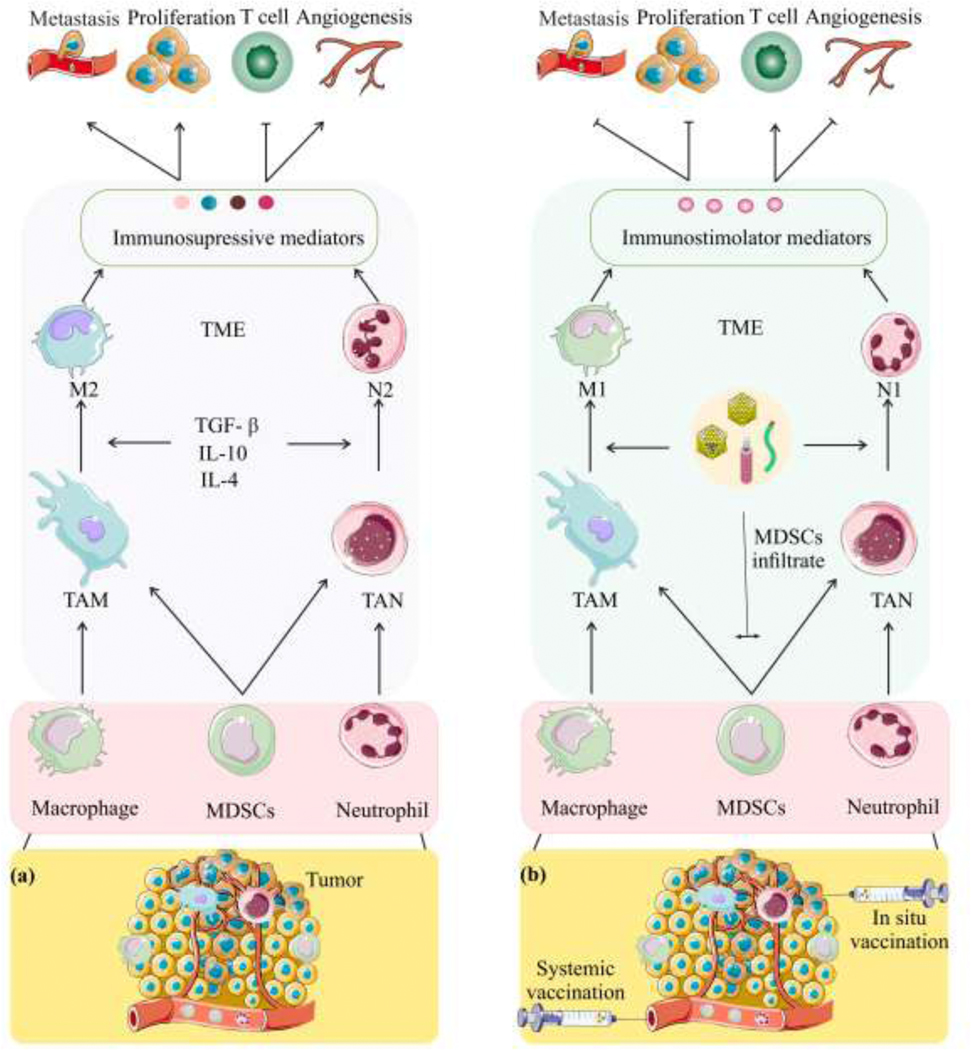
PVNPs can regulate the tumor microenvironment (TME), and inhibit tumor growth (a) TME recruit and polarizes myeloid cells to become TAMs and TANs. TAMs can polarize to pro-tumor M2 or pro-tumor N2 and inhibit T-cell activation, and promote tumor growth, invasion and metastasis (b) systemic and in situ vaccination of PVNPs can polarize TAMs and TANs into pro-inflammatory M1 macrophages/N1 neutrophils and activate T-cell, and inhibit tumor growth, invasion and metastasis Abbreviations: TAMs, tumor-associated macrophages; TANs, tumor-associated neutrophils; TGF-β, transforming growth factor β; TME, tumor microenvironment; MDSCs, myeloid-derived suppressor cells.
Administration of PVNPs for influencing the TME are done by both systemic administration and ISV (Fig. 3b, and Fig. 4C). In PVNP-ISV, the tumor itself is used as the antigen source, and PVNPs is introduced as an adjuvant. PVNP-ISV efficacy was demonstrated in a variety of mouse models of human cancers, and CPMV [74], tobacco mosaic virus (TMV) [29], potato virus X (PVX) [67] and papaya mosaic virus (PapMV) [73], have been shown to generate anti-tumor immune effects by modulating the TME. For example, in situ treatment of ovarian cancer with CPMV recruited tumor infiltrated neutrophils (TINs) with anti-tumor activity to the tumor site and induced inflammatory responses detected by elevation in IL-6 levels. Further, in situ CPMV administration modulates the tumor-inhibiting microenvironment by downregulating the production of IL-10, and TGF-β immune suppressing cytokines [84]. In another study, in situ TMV administration in a mouse model of dermal melanoma lead to production of inflammatory cytokines such as IL-6, and G-CSF and recruitment of TINs which stimulate T cells, and boost the production of IFN-γ by T cells making a more immunostimulatory TME [29]. CPMV-ISV by syringe and active microneedle (MN) delivery systems increase the influx of anti-tumor TANs and TAMs by inducing factors that promote an immunostimulatory TME in melanoma [74]. Most recently, we have shown that ISV with alfalfa in situ vaccination with mosaic virus (AMV) in a breast tumor mouse model leads to suppression of tumor progression and prolonged survival [68]. These findings show that ISV-based PVNP immunotherapy with intrinsic adjuvant properties are able to control tumor progression by coordinating innate and adaptive immune responses involving APCs, TINs, and T cells.
4. 2. PVNP-based antigen delivery
PVNPs can deliver specific antigens without the need for another immune adjuvant [34, 85]. Peptide antigens derived from tumor antigens can be genetically expressed on viral coat proteins (CP) (Fig. 1B, and Fig. 4A) or conjugated to PVNP by chemical methods (Fig. 1C). This approach creates an adjuvant-antigen platform with ordered, repetitively arrayed immunogenic peptides, and thus boosts the immunogenicity of those selected epitopes [85–87] (Table 1). The human papillomavirus (HPV) genotypes 16 and 18 cause approximately 70 % of cervical cancers. Fusion of the sequence for HPV-E7 viral oncoprotein with the DNA sequence of the PVX coat protein generated a DNA fusion vaccine which induced a humoral and cell-mediated immune response in a cervical tumor mouse model [59]. Addressing the transient expression of E7 in this study, another study developed a related vaccine through introducing the E7 HPV sequence in PVX which did not block infection of plants to generate PVX and associated with higher protein expression levels in plants [54]. They also found that E7CP fusion stabilizes the E7 protein [60]. Human epidermal growth factor receptor 2 (HER2) epitopes on PVX stimulated the production of anti-HER2 specific antibodies in mice [62]. Further, tumor antigen-PVNP conjugates on PVX [64], and HER2 on CCMV, CPMV, and SeMV-PVNPs [34] induced immune responses and reduced tumor growth in mouse models. Displaying multiple copies of human HLA-A2 restricted peptide antigen NY-ESO-1157–1165 on CPMV enhanced uptake and activation of APCs as well as stimulated a potent CD8+T cell response in transgenic human HLA-A2 expressing mice [63].
PVNP have also been studied for delivering immune related therapies beyond tumor antigens. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inducer of cancer cell apoptosis and has been tested by itself for cancer therapy [1], however, the low in vivo stability of these agents led to developing a multivalent TRAIL-displaying PVX. Besides improving the efficacy of TRAIL delivery, PVX-TRAIL promoted the crosslinking of TRAIL receptors (DR4/DR5) and thus, boosted caspase-mediated apoptosis in breast cancer xenografts [84]. Overall, genetically engineered plant viruses can carry therapeutically relevant peptides and proteins with promising safety and efficacy and potentially can be optimized and implemented for clinical usage.
4. 3. PVNP-based exogenous adjuvant delivery
Vaccines carry antigen and adjuvant, with the adjuvant needed to stimulate an immune response against the antigen (Fig. 4b). Various adjuvants have different immune stimulatory properties since they are recognized by different pathogen recognition receptors which are also variably expressed on different cell types. PVNPs have inherent adjuvant properties but can also be used to deliver other adjuvants to potentially improve immune responses. For example, encapsulation based self-assembly of CpG oligonucleotides with CCMV, (CCMV ODN1826-VLPs), can promotes activation of TAMs ex vivo, and intratumoral injection of this reagent induces robust anti-tumor responses in the TME [65]. This strategy can protect nucleic acid adjuvants from nucleases in vivo and concentrate them in potential APCs to improve their immune impact [65, 88].
4.4. PVNPs in combination therapy
As with all immunotherapies, tumor heterogeneity and tumor-mediated immunosuppression can limit the therapeutic efficacy of monotherapy. Therefore, a current focus of immunotherapy for cancer is combinations of immunotherapy strategies. PVNPs combined with other tumor immunotherapeutic options can improve both local control and systemic anti-tumor immune responses. A variety of general approaches have been explored for combining plant virus-based immunotherapies and other modalities of cancer, (Fig. 4).
Lee et al. (Fig. 4F), showed that melanoma cancer therapy by co-administration of PVX and doxorubicin (DOX) (PVX+DOX) or PVX-loaded DOX (PVX-DOX) was better than monotherapy, in part because DOX induces immunogenic cell death. Their combination, especially in PVX+DOX form (unlinked), increased anti-tumor cytokine/chemokine responses and suppressed tumor progression when administered as an intratumoral in situ vaccine [67]. Cai et al. [35], used cyclophosphamide (CPA) an alkylating agent with immunogenic activity and in situ CPMV vaccination. CPA-mediated cell death and associated exposure and release of tumor antigens which were recognized and processed by CPMV-induced immune cell infiltration into tumor site.
CD47 is an immune checkpoint biomolecule overexpressed on cancer cells and acts as a ‘do not eat me’ signal to phagocytes though binding to SIRPα on macrophages. Wang et al. [89], combined antibodies that block CD47 with CPMV as an in situ vaccine (Fig. 4D). Results revealed that CD47 blockade also primes anti-tumor T cell responses by either activating APCs or inhibiting interactions between CD47 on ovarian cancer cells and the protein thrombospondin 1 on T cells [89]. In situ CPMV vaccination activates the innate immune system and mediates activated phagocyte recruitment to tumors. Therefore, this combination therapy boosts innate cell phagocytosis of cancer cells which leads to presentation of tumor antigens and priming of the adaptive immune system leading to a potent anti-tumor immune response [89]. Like virtually any strategy that activates T cells, ISV with CPMV increases the expression of checkpoint regulators on effector T cells in the TME. Combining treatment with CPMV and selected checkpoint-targeting antibodies, specifically anti-PD-1 antibodies, or agonistic OX40-specific antibodies, reduced tumor burden, prolonged survival, and induced tumor antigen-specific immunologic memory to prevent relapse in three immunocompetent syngeneic mouse tumor models [90].
The abscopal effect of radiation has been known for many years as immune mediated, but it is infrequent and not dependable. An immunotherapeutic approach combining radiation therapy (RT) with CPMV was tested in a preclinical syngeneic mouse model of ovarian carcinoma (Fig. 4E). Utilizing CPMV particles in combination with RT can turn an immunologically “cold” tumor into an immunologically “hot” tumor through infiltration of activated myeloid cells, and CD4+ and CD8+ T lymphocytes into the TME and activating their anti-tumor effector responses [66].
5. Future perspectives of PVNPs for cancer immunotherapy
The exploitation of plant virus-based immunotherapy promises to generate clinical reagents that are reasonably inexpensive, safe for patients and caregivers, stable long term at room temperature, do not require tumor antigen identification, and can be scaled-up for production of safe/effective vaccines [91]. However, there are challenges and limitations that should be considered. Expression of some peptides genetically as part of coat proteins will likely impair capsid formation. The requirement for effective infection and PVNP generation in plants limits expression of vaccine epitopes on the surface of virus particles since the protein epitope could disrupt infection and assembly of the virus [92].
As with any developing pharmaceutical platform, there are many challenges to obtaining reagents that are acceptable to regulatory agencies. For manufacturing, additional research must consider the codon optimization between selected peptides and plant codon usage to reduce unstable protein accumulation [21, 22]. Other complementary issues, such as thylakoid localization, promoter usage, and untranslated region selection must be considered [93].
PVNPs have basic characteristics common to most types of nanoparticles that can influence their fates in vivo [94, 95]. In vivo biological barriers such as interactions with serum, and immune cells or antibodies impact the application of native/functionalized PVNPs in clinical settings [17]. In blood circulation, the PVNP surface may be covered with serum proteins, termed protein corona (PC), that increase their uptake by phagocytic cells [52, 96]. Anti-PVNP antibodies [49, 97] change the interaction of immune cells with PVNP and can eliminate them before reaching the target site [98]. The various delivery challenges can generally be avoided or reduced by ISV.
If the usage involves multiple applications of PVNP over weeks, then the immune system will likely generate anti-PVNP antibodies that can reduce their half-life in circulation and promote clearance from the body [99, 100]. However, this may not be a problem, particularly since the PVNP are not infecting cells, so the concept of “neutralizing antibodies” which implies blocking of infection by viruses is not relevant. When PVNP are used as immune adjuvants, particularly as ISV, the anti-PVNP response could have no effect or could improve the adjuvant properties. Studies demonstrated that antibodies produced against TMV, PapMV, and CPMV do not interfere with their immunostimulating properties [48, 49, 63, 101, 102].
Protein corona (PC) formation can be an obstacle to developing PVNPs for in vivo applications. The likely interactions of PVNPs and their physiological effects in the bloodstream show that PVNPs incubated in human plasma can remain stable and acquire a PC lower than what accumulates on synthetic nanoparticles [103]. For example, the PC bound to TMV nanoparticles is approximately 6-fold less than for silica nanoparticles [104, 105]. The reduced protein corona may be due to surface properties, such as patches of positive and negative charges, or heterogeneous hydrophobic and hydrophilic domains [17, 88]. Thus, further studies on biodistribution and rapid clearance of PVNPs will be required to understand the variable PC formation for each PVNP [106].
PVNPs also can interact with red blood cell (RBCs), but blood hemolysis and coagulation assays confirmed the biocompatibility of TMV, TBSV, CPMV particles [97, 98]. PVX induces a slight and dose-dependent hemolysis rate; nonetheless, according to ISO/TR 7406, it has less of this tendency than many synthetic nanoparticles [22, 98]. The lower affinity of PVX for RBCs may be because of greater flexibility of the PVX capsid compared to synthetic nanoparticles [94, 107, 108].
If non-immune cells or tissues are the target of PVNPs, clearance by the immune system should be minimized, but if the immune system is the target, then phagocytosis by immune cells in the appropriate anatomic location is usually the desired outcome, and the approach must be designed with this in mind [109]. The common strategy for reducing nanoparticles clearance and achieving long circulation times, is creating ‘stealth’ or ‘camouflage’ effects by PEGylation and self-protein decoration to support bio-inspired stealth [52, 95]. However, these modification cannot completely inhibit opsonizing proteins and the creation of a PC [110]. Furthermore, PEGylation of nanoparticles, which does increase half-life in circulation, has other associated challenges. PEGylation needs optimization in choosing specific PEG polymer and PEG density, and many people have anti-PEG antibodies in the blood, due to using PEG in commercial products, and such antibodies limit the efficacy of the PEG shield [111]. Coating of PVNPs with serum albumin can prevent antibody recognition without interfering with their uptake by macrophages [52]. However, the relationships are complex and the coating of TMV with serum albumin leads to rapid clearance of TMV in endolysosomal conditions that can have implications for cargo delivery [52, 112].
If the goal is to achieve long half-life in circulation, there are options that can be considered and further developed. For example, “don’t-eat-me” or “marker of self” signals such as CD47 could be utilized on the PVNPs [89, 113], or using membranes from immune cells or red blood cells to coat loaded nanoparticles for systemic delivery [114]. However, the modifications may impact immune stimulation and associated efficacy. This very general challenge of rapid clearance of nanoparticles delivered IV, is reduced using ISV which delivers the reagent specifically to tumors. While it is more challenging to directly inject tumors that are deeper in the body, surgeons and interventional radiologists have the skill to utilize real time imaging and accurately inject into tumors almost anywhere in a patient.
Viruses of any sort are fundamentally engineerable reagents and the technology to engineer each virus will expand, opening new therapeutic strategies. Incorporating bioactivatable aspects into rational design of novel viral reagents will provide flexibility and further expand therapeutic options [115]. Further, forward-looking researchers could employ directed evolution using mutagenesis strategies to generate virus libraries that meet a desired phenotypes for new viral variants addressing a user-defined goal. Bioinformatics analysis can be applied for alignment of different viral capsid genes/proteins sequences (through their phylogeny, genetic, and functional similarities), or protein structure which could generate viruses with novel properties, including new chimeric capsids [115]. Computer technologies such as machine learning and mathematical modeling systems could further improve the translation potential of PVNPs for clinical cancer immunotherapy.
Overall, PVNPs are highly flexible and manipulatable molecular machines that can be used to stimulate anti-tumor immune responses. Notably, they appear to stimulate a sustained immune response and created an immune memory in most mouse models.
While once they get to clinical trials, PVNP will be first tested in late stage patients that have failed prior therapy efforts. However, since the safety profile is so good, it is likely that PVNP-ISV will quickly move into utilization in early stage patients that may not have known metastatic disease. The ISV reaction that disrupts tumor-mediated immunosuppression and activates naïve or effector tumor-specific T cells occurs within a few days of intratumoral injection, so ISV with PVNP or other adjuvants could be done in the 1–2 weeks between pathologic diagnosis and surgery that most patients experience. This could safely stimulate antitumor immunity and associated expansion of tumor-recognizing T cells when the potential of metastasis exists but is not identified, which would open better treatment for many early stage cancer patients.
6. Conclusions
PVNPs are highly promising platforms for cancer immunotherapy because of unique physico-chemical and genetic properties that differentiate them from other naturally derived or synthetic nanoparticles. Many PVNPs have inherent immunostimulatory properties and preclinical studies have extensively demonstrated PVNPs as useful vehicles for delivery of tumor antigens and immune stimulatory molecules to DCs and other APCs. PVNP based immunotherapy as monotherapy can overcome tumor- immunosuppressive signals in TME. The intrinsic abilities of PVNPs to induce cellular immunity boosts the immunogenicity of poorly immunogenic tumors and thus, improves anti-tumor immune effects against previously “cold” tumors in vivo. Combination of PVNPs with chemotherapy and radiotherapy can potentiate anti-tumor responses by increasing immunogenic cell death and combination with checkpoint blockade increases the pool of tumor-recognizing T cells to improve efficacy. As interest in this field grows, the number of plant virus-based immunotherapies in pre-clinical trials will continue to expand and hopefully lead to clinical testing and approval for therapies using these reagents to improve cancer immunotherapy.
Acknowledgements
This work was supported by grants U01CA218292 and R01CA224605 from the US National Institute of Health to SF.
Footnotes
Conflict of interest
Dr. Fiering is a founder of Mosaic Immunoengineering which is developing PVNP for immune stimulation and vaccine utilization.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Keshavarz M, Ebrahimzadeh MS, Miri SM, Dianat-Moghadam H, Ghorbanhosseini SS, Mohebbi SR, Keyvani H, Ghaemi A, Oncolytic Newcastle disease virus delivered by Mesenchymal stem cells-engineered system enhances the therapeutic effects altering tumor microenvironment, Virology Journal 17(1) (2020) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Keshavarz M, Sabbaghi A, Miri SM, Rezaeyan A, Arjeini Y, Ghaemi A, Virotheranostics, a double-barreled viral gun pointed toward cancer; ready to shoot?, Cancer Cell International 20(1) (2020) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dianat-Moghadam H, Heidarifard M, Mahari A, Shahgolzari M, Keshavarz M, Nouri M, Amoozgar Z, TRAIL in oncology: From recombinant TRAIL to nano- and self-targeted TRAIL-based therapies, Pharmacological Research 155 (2020) 104716. [DOI] [PubMed] [Google Scholar]
- [4].Huang H, Liu Y, Liao W, Cao Y, Liu Q, Guo Y, Lu Y, Xie Z, Oncolytic adenovirus programmed by synthetic gene circuit for cancer immunotherapy, Nature Communications 10(1) (2019) 4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bommareddy PK, Shettigar M, Kaufman HL, Integrating oncolytic viruses in combination cancer immunotherapy, Nature Reviews Immunology 18(8) (2018) 498. [DOI] [PubMed] [Google Scholar]
- [6].Harrington K, Freeman DJ, Kelly B, Harper J, Soria J-C, Optimizing oncolytic virotherapy in cancer treatment, Nature Reviews Drug Discovery 18(9) (2019) 689–706. [DOI] [PubMed] [Google Scholar]
- [7].Jayawardena N, Poirier JT, Burga LN, Bostina M, Virus-Receptor Interactions and Virus Neutralization: Insights for Oncolytic Virus Development, Oncolytic Virother 9 (2020) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deng S, Iscaro A, Zambito G, Mijiti Y, Minicucci M, Essand M, Lowik C, Muthana M, Censi R, Mezzanotte L, Development of a New Hyaluronic Acid Based Redox-Responsive Nanohydrogel for the Encapsulation of Oncolytic Viruses for Cancer Immunotherapy, Nanomaterials 11(1) (2021) 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fusciello M, Fontana F, Tähtinen S, Capasso C, Feola S, Martins B, Chiaro J, Peltonen K, Ylösmäki L, Ylösmäki E, Artificially cloaked viral nanovaccine for cancer immunotherapy, Nature communications 10(1) (2019) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nandedkar TD, Nanovaccines: recent developments in vaccination, Journal of biosciences 34(6) (2009) 995–1003. [DOI] [PubMed] [Google Scholar]
- [11].Shahgolzari M, Pazhouhandeh M, Milani M, Yari Khosroushahi A, Fiering S, Plant viral nanoparticles for packaging and in vivo delivery of bioactive cargos, WIREs Nanomedicine and Nanobiotechnology 12(5) (2020) e1629. [DOI] [PubMed] [Google Scholar]
- [12].Saleh T, Shojaosadati SA, Multifunctional nanoparticles for cancer immunotherapy, Human vaccines & immunotherapeutics 12(7) (2016) 1863–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Röder J, Dickmeis C, Commandeur U, Small, Smaller, Nano: New Applications for Potato Virus X in Nanotechnology, Frontiers in Plant Science 10(158) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaiser CR, Flenniken ML, Gillitzer E, Harmsen AL, Harmsen AG, Jutila MA, Douglas T, Young MJ, Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo, International journal of nanomedicine 2(4) (2007) 715. [PMC free article] [PubMed] [Google Scholar]
- [15].Singh P, Prasuhn D, Yeh RM, Destito G, Rae CS, Osborn K, Finn M, Manchester M, Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo, Journal of controlled release 120(1–2) (2007) 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nikitin N, Trifonova E, Karpova O, Atabekov J, Biosafety of plant viruses for human and animals, Moscow University biological sciences bulletin 71(3) (2016) 128–134. [Google Scholar]
- [17].Balke I, Zeltins A, Use of plant viruses and virus-like particles for the creation of novel vaccines, Advanced drug delivery reviews 145 (2019) 119–129. [DOI] [PubMed] [Google Scholar]
- [18].Gergerich RC, Dolja VV, Introduction to plant viruses, the invisible foe, The plant health instructor 1 (2006) 24. [Google Scholar]
- [19].Legrand P, Biological assays for plant viruses and other graft transmissible pathogens diagnoses: a review, EPPO Bulletin 45(2) (2015) 240–251. [Google Scholar]
- [20].Zherdev A, Vinogradova S, Byzova N, Porotikova E, Kamionskaya A, Dzantiev B, Methods for the Diagnosis of Grapevine Viral Infections: A Review, Agriculture 8(12) (2018) 195. [Google Scholar]
- [21].Peyret H, Lomonossoff GP, When plant virology met Agrobacterium: the rise of the deconstructed clones, Plant biotechnology journal 13(8) (2015) 1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ibrahim A, Odon V, Kormelink R, Plant Viruses in Plant Molecular Pharming: Toward the Use of Enveloped Viruses, Frontiers in Plant Science 10(803) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hansen JD, Vojtech LN, Laing KJ, Sensing disease and danger: a survey of vertebrate PRRs and their origins, Developmental & Comparative Immunology 35(9) (2011) 886–897. [DOI] [PubMed] [Google Scholar]
- [24].Pulendran B, Arunachalam PS, O’Hagan DT, Emerging concepts in the science of vaccine adjuvants, Nature Reviews Drug Discovery 20(6) (2021) 454–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Reed SG, Orr MT, Fox CB, Key roles of adjuvants in modern vaccines, Nature medicine 19(12) (2013) 1597. [DOI] [PubMed] [Google Scholar]
- [26].Mao C, Beiss V, Fields J, Steinmetz NF, Fiering S, Cowpea mosaic virus stimulates antitumor immunity through recognition by multiple MYD88-dependent toll-like receptors, Biomaterials 275 (2021) 120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Albakri MM, Veliz FA, Fiering SN, Steinmetz NF, Sieg SF, Endosomal toll like receptors play a key role in activation of primary human monocytes by cowpea mosaic virus, Immunology 159(2) (2020) 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carignan D, Herblot S, Laliberté-Gagné M-È, Bolduc M, Duval M, Savard P, Leclerc D, Activation of innate immunity in primary human cells using a plant virus derived nanoparticle TLR7/8 agonist, Nanomedicine: Nanotechnology, Biology and Medicine 14(7) (2018) 2317–2327. [DOI] [PubMed] [Google Scholar]
- [29].Murray AA, Wang C, Fiering S, Steinmetz NF, In situ vaccination with cowpea vs tobacco mosaic virus against melanoma, Molecular pharmaceutics 15(9) (2018) 3700–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dennehy KM, Ferwerda G, Faro-Trindade I, Pyż E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, Adema GJ, Kullberg B-J, Schweighoffer E, Tybulewicz V, Mora-Montes HM, Gow NAR, Williams DL, Netea MG, Brown GD, Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors, European Journal of Immunology 38(2) (2008) 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lin Y-C, Huang D-Y, Chu C-L, Lin Y-L, Lin W-W, The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3, Science signaling 6(289) (2013) ra71–ra71. [DOI] [PubMed] [Google Scholar]
- [32].Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh C-S, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A, Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells, The Journal of Immunology 154(10) (1995) 5071–5079. [PubMed] [Google Scholar]
- [33].Ramos HJ, Davis AM, George TC, Farrar JD, IFN-α is not sufficient to drive Th1 development due to lack of stable T-bet expression, The Journal of Immunology 179(6) (2007) 3792–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cai H, Shukla S, Wang C, Masarapu H, Steinmetz NF, Heterologous Prime-Boost Enhances the Antitumor Immune Response Elicited by Plant-Virus-Based Cancer Vaccine, Journal of the American Chemical Society 141(16) (2019) 6509–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cai H, Wang C, Shukla S, Steinmetz NF, Cowpea Mosaic Virus Immunotherapy Combined with Cyclophosphamide Reduces Breast Cancer Tumor Burden and Inhibits Lung Metastasis, Advanced Science 6(16) (2019) 1802281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang C, Fiering SN, Steinmetz NF, Cowpea mosaic virus promotes anti tumor activity and immune memory in a mouse ovarian tumor model, Advanced Therapeutics 2(5) (2019) 1900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jobsri J, Allen A, Rajagopal D, Shipton M, Kanyuka K, Lomonossoff GP, Ottensmeier C, Diebold SS, Stevenson FK, Savelyeva N, Plant virus particles carrying tumour antigen activate TLR7 and Induce high levels of protective antibody, PLoS One 10(2) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kerstetter-Fogle A, Shukla S, Wang C, Beiss V, Harris PL, Sloan AE, Steinmetz NF, Plant Virus-Like Particle In Situ Vaccine for Intracranial Glioma Immunotherapy, Cancers 11(4) (2019) 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF, Nanoparticles target distinct dendritic cell populations according to their size, Eur J Immunol 38(5) (2008) 1404–13. [DOI] [PubMed] [Google Scholar]
- [40].Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M, Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors, Journal of immunology (Baltimore, Md. : 1950) 173(5) (2004) 3148–54. [DOI] [PubMed] [Google Scholar]
- [41].Thérien A, Bédard M, Carignan D, Rioux G, Gauthier-Landry L, Laliberté-Gagné M-È, Bolduc M, Savard P, Leclerc D, A versatile papaya mosaic virus (PapMV) vaccine platform based on sortase-mediated antigen coupling, Journal of Nanobiotechnology 15(1) (2017) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McCormick AA, Palmer KE, Genetically engineered Tobacco mosaic virus as nanoparticle vaccines, Expert review of vaccines 7(1) (2008) 33–41. [DOI] [PubMed] [Google Scholar]
- [43].McCormick AA, Corbo TA, Wykoff-Clary S, Nguyen LV, Smith ML, Palmer KE, Pogue GP, TMV-peptide fusion vaccines induce cell-mediated immune responses and tumor protection in two murine models, Vaccine 24(40–41) (2006) 6414–6423. [DOI] [PubMed] [Google Scholar]
- [44].Loor F, Comparative immunogenicities of tobacco mosaic virus, protein subunits, and reaggregated protein subunits, Virology 33(2) (1967) 215–220. [DOI] [PubMed] [Google Scholar]
- [45].Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, Fiering S, In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer, Nature nanotechnology 11(3) (2016) 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Marbrook J, Matthews R, The differential immunogenicity of plant viral protein and nucleoproteins, Virology 28(2) (1966) 219–228. [DOI] [PubMed] [Google Scholar]
- [47].Denis J, Acosta-Ramirez E, Zhao Y, Hamelin M-E, Koukavica I, Baz M, Abed Y, Savard C, Pare C, Macias CL, Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform, Vaccine 26(27–28) (2008) 3395–3403. [DOI] [PubMed] [Google Scholar]
- [48].Evtushenko EA, Ryabchevskaya EM, Nikitin NA, Atabekov JG, Karpova OV, Plant virus particles with various shapes as potential adjuvants, Scientific reports 10(1) (2020) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shukla S, Wang C, Beiss V, Steinmetz NF, Antibody Response against Cowpea Mosaic Viral Nanoparticles Improves In Situ Vaccine Efficacy in Ovarian Cancer, ACS Nano 14(3) (2020) 2994–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Masoumi H, Jones P, Hague N, ANTIGENIC ANALYSIS OF THE COAT PROTEIN OF ALFALFA MOSAIC VIRUS AND ITS INVOLVEMENT IN APHID TRANSMISSION, JOURNAL OF AGRICULTURAL SCIENCE AND TECHNOLOGY (JAST) 7(1–2) (2005) 31–40. [Google Scholar]
- [51].Phatsaman T, Hongprayoon R, Wasee S, Monoclonal antibody-based diagnostic assays for pepper mild mottle virus, Journal of Plant Pathology (2019) 1–7. [Google Scholar]
- [52].Gulati N, Pitek A, Czapar A, Stewart P, Steinmetz N, The in vivo fates of plant viral nanoparticles camouflaged using self-proteins: overcoming immune recognition, Journal of Materials Chemistry B 6(15) (2018) 2204–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Czapar AE, Tiu BDB, Veliz FA, Pokorski JK, Steinmetz NF, Slow release formulation of cowpea mosaic virus for in situ vaccine delivery to treat ovarian cancer, Advanced Science 5(5) (2018) 1700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sheen MR, Fiering S, In situ vaccination: Harvesting low hanging fruit on the cancer immunotherapy tree, WIREs Nanomedicine and Nanobiotechnology 11(1) (2019) e1524. [DOI] [PubMed] [Google Scholar]
- [55].Gorbet M-J, Singh A, Mao C, Fiering S, Ranjan A, Using nanoparticles for in situ vaccination against cancer: mechanisms and immunotherapy benefits, International Journal of Hyperthermia 37(3) (2020) 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mao C, Gorbet M-J, Singh A, Ranjan A, Fiering S, In situ vaccination with nanoparticles for cancer immunotherapy: understanding the immunology, International Journal of Hyperthermia 37(3) (2020) 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Laliberté-Gagné M-È, Bolduc M, Thérien A, Garneau C, Casault P, Savard P, Estaquier J, Leclerc D, Increased Immunogenicity of Full-Length Protein Antigens through Sortase-Mediated Coupling on the PapMV Vaccine Platform, Vaccines 7(2) (2019) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shukla S, Myers JT, Woods SE, Gong X, Czapar AE, Commandeur U, Huang AY, Levine AD, Steinmetz NF, Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers, Biomaterials 121 (2017) 15–27. [DOI] [PubMed] [Google Scholar]
- [59].Massa S, Simeone P, Muller A, Benvenuto E, Venuti A, Franconi R, Antitumor activity of DNA vaccines based on the human papillomavirus-16 E7 protein genetically fused to a plant virus coat protein, Human gene therapy 19(4) (2008) 354–364. [DOI] [PubMed] [Google Scholar]
- [60].Morgenfeld M, Segretin ME, Wirth S, Lentz E, Zelada A, Mentaberry A, Gissmann L, Bravo-Almonacid F, Potato Virus X Coat Protein Fusion to Human Papillomavirus 16 E7 Oncoprotein Enhance Antigen Stability and Accumulation in Tobacco Chloroplast, Molecular Biotechnology 43(3) (2009) 243. [DOI] [PubMed] [Google Scholar]
- [61].Frolova OY, Petrunia IV, Komarova TV, Kosorukov VS, Sheval EV, Gleba YY, Dorokhov YL, Trastuzumab-binding peptide display by Tobacco mosaic virus, Virology 407(1) (2010) 7–13. [DOI] [PubMed] [Google Scholar]
- [62].Shukla S, Wen AM, Commandeur U, Steinmetz NF, Presentation of HER2 epitopes using a filamentous plant virus-based vaccination platform, Journal of Materials Chemistry B 2(37) (2014) 6249–6258. [DOI] [PubMed] [Google Scholar]
- [63].Patel BK, Wang C, Lorens B, Levine AD, Steinmetz NF, Shukla S, Cowpea Mosaic Virus (CPMV)-Based Cancer Testis Antigen NY-ESO-1 Vaccine Elicits an Antigen-Specific Cytotoxic T Cell Response, ACS Applied Bio Materials 3(7) (2020) 4179–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jobsri J, Allen A, Rajagopal D, Shipton M, Kanyuka K, Lomonossoff GP, Ottensmeier C, Diebold SS, Stevenson FK, Savelyeva N, Plant virus particles carrying tumour antigen activate TLR7 and Induce high levels of protective antibody, PloS one 10(2) (2015) e0118096-e0118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cai H, Shukla S, Steinmetz NF, The Antitumor Efficacy of CpG Oligonucleotides is Improved by Encapsulation in Plant Virus Like Particles, Advanced Functional Materials 30(15) (2020) 1908743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Patel R, Czapar AE, Fiering S, Oleinick NL, Steinmetz NF, Radiation therapy combined with cowpea mosaic virus nanoparticle in situ vaccination initiates immune-mediated tumor regression, ACS omega 3(4) (2018) 3702–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee KL, Murray AA, Le DH, Sheen MR, Shukla S, Commandeur U, Fiering S, Steinmetz NF, Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response, Nano letters 17(7) (2017) 4019–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shahgolzari M, Pazhouhandeh M, Milani M, Fiering S, Khosroushahi AY, Alfalfa mosaic virus nanoparticles-based in situ vaccination induces antitumor immune responses in breast cancer model, Nanomedicine 16(2) (2021) 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fu B, Huang X, Deng J, Gu D, Mei Q, Deng M, Tang S, Lü M, Application of multifunctional nanomaterials in cancer vaccines, Oncology reports 39(3) (2018) 893–900. [DOI] [PubMed] [Google Scholar]
- [70].Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Understanding the tumor immune microenvironment (TIME) for effective therapy, Nature medicine 24(5) (2018) 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chew V, Toh HC, Abastado J-P, Immune Microenvironment in Tumor Progression: Characteristics and Challenges for Therapy, Journal of Oncology 2012 (2012) 608406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Azizi M, Dianat-Moghadam H, Salehi R, Farshbaf M, Iyengar D, Sau S, Iyer AK, Valizadeh H, Mehrmohammadi M, Hamblin MR, Interactions Between Tumor Biology and Targeted Nanoplatforms for Imaging Applications, Advanced Functional Materials 30(19) (2020) 1910402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lebel M-È, Chartrand K, Tarrab E, Savard P, Leclerc D, Lamarre A, Potentiating cancer immunotherapy using papaya mosaic virus-derived nanoparticles, Nano letters 16(3) (2016) 1826–1832. [DOI] [PubMed] [Google Scholar]
- [74].Lizotte P, Wen A, Sheen M, Fields J, Rojanasopondist P, Steinmetz N, Fiering S, In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer, Nature nanotechnology 11(3) (2016) 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Shoeb E, Hefferon K, Future of cancer immunotherapy using plant virus-based nanoparticles, Future Sci OA 5(7) (2019) FSO401-FSO401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Brennan FR, Gilleland LB, Staczek J, Bendig MM, Hamilton WD, Gilleland HE Jr, A chimaeric plant virus vaccine protects mice against a bacterial infection, Microbiology 145(8) (1999) 2061–2067. [DOI] [PubMed] [Google Scholar]
- [77].Hammond J, McGarvey P, Yusibov V, Plant biotechnology: new products and applications, Springer Science & Business Media; 2012. [Google Scholar]
- [78].Gabrilovich D, Nagaraj S, Kusmartsev S, Tumor Associated CD8+ T-Cell Tolerance Induced by Bone Marrow Derived Immature Myeloid Cells, Journal of Immunotherapy 28(6) (2005) 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM, Polarization of tumor-associated neutrophil phenotype by TGF-β:“N1” versus “N2” TAN, Cancer cell 16(3) (2009) 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Martinez FO, Sica A, Mantovani A, Locati M, Macrophage activation and polarization, Frontiers in bioscience: a journal and virtual library 13 (2008) 453. [DOI] [PubMed] [Google Scholar]
- [81].Mantovani A, Sozzani S, Locati M, Allavena P, Sica A, Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes, Trends in immunology 23(11) (2002) 549–555. [DOI] [PubMed] [Google Scholar]
- [82].Gordon S, Martinez FO, Alternative activation of macrophages: mechanism and functions, Immunity 32(5) (2010) 593–604. [DOI] [PubMed] [Google Scholar]
- [83].Murdoch C, Giannoudis A, Lewis CE, Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues, Blood 104(8) (2004) 2224–2234. [DOI] [PubMed] [Google Scholar]
- [84].Wang C, Fiering SN, Steinmetz NF, Cowpea Mosaic Virus Promotes Anti-Tumor Activity and Immune Memory in a Mouse Ovarian Tumor Model, Advanced Therapeutics 2(5) (2019) 1900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Clarke J, Harnessing plant viruses to treat autoimmune diseases, Nature Reviews Rheumatology 16(7) (2020) 352–352. [DOI] [PubMed] [Google Scholar]
- [86].Jegerlehner A, Tissot A, Lechner F, Sebbel P, Erdmann I, Kündig T, Bächi T, Storni T, Jennings G, Pumpens P, A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses, Vaccine 20(25–26) (2002) 3104–3112. [DOI] [PubMed] [Google Scholar]
- [87].Steinmetz NF, Shukla S, Vaccination using plant virus particles linked to HER2 antigens, Google Patents, 2020. [Google Scholar]
- [88].Mutwiri GK, Nichani AK, Babiuk S, Babiuk LA, Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides, Journal of controlled release 97(1) (2004) 1–17. [DOI] [PubMed] [Google Scholar]
- [89].Wang C, Steinmetz NF, CD47 blockade and cowpea mosaic virus nanoparticle in situ vaccination triggers phagocytosis and tumor killing, Advanced healthcare materials 8(8) (2019) 1801288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wang C, Steinmetz NF, A Combination of Cowpea Mosaic Virus and Immune Checkpoint Therapy Synergistically Improves Therapeutic Efficacy in Three Tumor Models, Advanced Functional Materials 30(27) (2020) 2002299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Criscuolo E, Caputo V, Diotti RA, Sautto GA, Kirchenbaum GA, Clementi N, Alternative Methods of Vaccine Delivery: An Overview of Edible and Intradermal Vaccines, Journal of Immunology Research 2019 (2019) 8303648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hefferon K, Plant Virus Expression Vectors: A Powerhouse for Global Health, Biomedicines 5(3) (2017) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Morgenfeld M, Lentz E, Segretin ME, Alfano EF, Bravo-Almonacid F, Translational fusion and redirection to thylakoid lumen as strategies to enhance accumulation of human papillomavirus E7 antigen in tobacco chloroplasts, Mol Biotechnol 56(11) (2014) 1021–31. [DOI] [PubMed] [Google Scholar]
- [94].Lico C, Giardullo P, Mancuso M, Benvenuto E, Santi L, Baschieri S, A biodistribution study of two differently shaped plant virus nanoparticles reveals new peculiar traits, Colloids and Surfaces B: Biointerfaces 148 (2016) 431–439. [DOI] [PubMed] [Google Scholar]
- [95].Bruckman MA, Randolph LN, VanMeter A, Hern S, Shoffstall AJ, Taurog RE, Steinmetz NF, Biodistribution, pharmacokinetics, and blood compatibility of native and PEGylated tobacco mosaic virus nano-rods and -spheres in mice, Virology 449 (2014) 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pitek AS, Wen AM, Shukla S, Steinmetz NF, The Protein Corona of Plant Virus Nanoparticles Influences their Dispersion Properties, Cellular Interactions, and In Vivo Fates, Small 12(13) (2016) 1758–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Liu R, Vaishnav RA, Roberts AM, Friedland RP, Humans have antibodies against a plant virus: evidence from tobacco mosaic virus, PloS one 8(4) (2013) e60621-e60621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gonzalez MJ, Plummer EM, Rae CS, Manchester M, Interaction of Cowpea mosaic virus (CPMV) nanoparticles with antigen presenting cells in vitro and in vivo, PloS one 4(11) (2009) e7981–e7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Fausther-Bovendo H, Kobinger GP, Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what’s important?, Human vaccines & immunotherapeutics 10(10) (2014) 2875–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hwang C, Sanda M, Prospects and limitations of recombinant poxviruses for prostate cancer immunotherapy, Current opinion in molecular therapeutics 1(4) (1999) 471. [PubMed] [Google Scholar]
- [101].Babin C, Majeau N, Leclerc D, Engineering of papaya mosaic virus (PapMV) nanoparticles with a CTL epitope derived from influenza NP, Journal of Nanobiotechnology 11(1) (2013) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kemnade JO, Seethammagari M, Collinson-Pautz M, Kaur H, Spencer DM, McCormick AA, Tobacco mosaic virus efficiently targets DC uptake, activation and antigen-specific T cell responses in vivo, Vaccine 32(33) (2014) 4228–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pitek AS, Wen AM, Shukla S, Steinmetz NF, Nanomedicine: The Protein Corona of Plant Virus Nanoparticles Influences their Dispersion Properties, Cellular Interactions, and In Vivo Fates (Small 13/2016), Small 12(13) (2016) 1682–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Berardi A, Bombelli FB, Thuenemann EC, Lomonossoff GP, Viral nanoparticles can elude protein barriers: exploiting rather than imitating nature, Nanoscale 11(5) (2019) 2306–2316. [DOI] [PubMed] [Google Scholar]
- [105].Berardi A, Evans DJ, Bombelli FB, Lomonossoff GP, Stability of plant virus-based nanocarriers in gastrointestinal fluids, Nanoscale 10(4) (2018) 1667–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zackova Suchanova J, Hejtmankova A, Neburkova J, Cigler P, Forstova J, Spanielova H, The Protein Corona Does Not Influence Receptor-Mediated Targeting of Virus-like Particles, Bioconjugate Chemistry 31(5) (2020) 1575–1585. [DOI] [PubMed] [Google Scholar]
- [107].Lin Y-S, Haynes CL, Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity, Journal of the American Chemical Society 132(13) (2010) 4834–4842. [DOI] [PubMed] [Google Scholar]
- [108].Zhao Y, Sun X, Zhang G, Trewyn BG, Slowing II, Lin VS-Y, Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects, ACS nano 5(2) (2011) 1366–1375. [DOI] [PubMed] [Google Scholar]
- [109].Fadeel B, Hide and Seek: Nanomaterial Interactions With the Immune System, Frontiers in Immunology 10(133) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Dai Q, Bertleff Zieschang N, Braunger JA, Björnmalm M, Cortez Jugo C, Caruso F, Particle targeting in complex biological media, Advanced healthcare materials 7(1) (2018) 1700575. [DOI] [PubMed] [Google Scholar]
- [111].Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P, Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents, Expert Opinion on Drug Delivery 9(11) (2012) 1319–1323. [DOI] [PubMed] [Google Scholar]
- [112].Wen AM, Infusino M, De Luca A, Kernan DL, Czapar AE, Strangi G, Steinmetz NF, Interface of physics and biology: engineering virus-based nanoparticles for biophotonics, Bioconjugate chemistry 26(1) (2015) 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Oldenborg P-A, Zheleznyak A, Fang Y-F, Lagenaur CF, Gresham HD, Lindberg FP, Role of CD47 as a marker of self on red blood cells, Science 288(5473) (2000) 2051–2054. [DOI] [PubMed] [Google Scholar]
- [114].Deng G, Sun Z, Li S, Peng X, Li W, Zhou L, Ma Y, Gong P, Cai L, Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth, ACS Nano 12(12) (2018) 12096–12108. [DOI] [PubMed] [Google Scholar]
- [115].Chen MY, Butler SS, Chen W, Suh J, Physical, chemical, and synthetic virology: Reprogramming viruses as controllable nanodevices, Wiley Interdiscip Rev Nanomed Nanobiotechnol 11(3) (2019) e1545. [DOI] [PMC free article] [PubMed] [Google Scholar]