Abstract
Mutations of the X-linked methyl-CpG-binding protein 2 (MECP2) gene in humans are responsible for most cases of Rett syndrome (RTT), an X-linked progressive neurological disorder. While genome-wide screens in clinical trials have revealed several putative RTT-associated mutations in MECP2, their causal relevance regarding the functional regulation of MeCP2 at the etiologic sites at the protein level requires more evidence. In this study, we demonstrated that MeCP2 was dynamically modified by O-linked-β-N-acetylglucosamine (O-GlcNAc) at threonine 203 (T203), an etiologic site in RTT patients. Disruption of the O-GlcNAcylation of MeCP2 specifically at T203 impaired dendrite development and spine maturation in cultured hippocampal neurons, and disrupted neuronal migration, dendritic spine morphogenesis, and caused dysfunction of synaptic transmission in the developing and juvenile mouse cerebral cortex. Mechanistically, genetic disruption of O-GlcNAcylation at T203 on MeCP2 decreased the neuronal activity-induced induction of Bdnf transcription. Our study highlights the critical role of MeCP2 T203 O-GlcNAcylation in neural development and synaptic transmission potentially via brain-derived neurotrophic factor.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-021-00784-8.
Keywords: MeCP2, O-GlcNAcylation, Dendrite development, Synaptic transmission, Brain-derived neurotrophic factor
Introduction
Rett syndrome (RTT) is a severe X-linked neurodevelopmental disorder that preferentially occurs in females, with an incidence of ~1:10,000 [1, 2]. Almost 90% of RTT cases are caused by methyl-CpG-binding protein 2 (MECP2) gene mutations such as missense, nonsense, insertion, deletion, and splice-site variations [3–5], and loss of MeCP2 is closely related to the occurrence of RTT [1, 6]. MeCP2 activity in normal central nervous system development and function is controlled by both precise expression levels [7, 8] and post-translational modifications (PTMs). For example, neurons from Mecp2-null mutant mice have smaller somas [9, 10], decreased dendritic complexity [11], and dysfunction of synaptic plasticity [12–14]. Gain-of-function MeCP2 by overexpression in transgenic mice and monkeys results in progressive neurological and psychiatric dysfunctions [15, 16]. These genetic studies suggest that precise and dynamic expression of MeCP2 is critical to maintain normal brain development and function.
In addition to the expression level, PTMs of MeCP2 such as phosphorylation have been demonstrated to be critical regulators of its role in dendritic growth, spine maturation, and the activation of Ca2+-dependent brain-derived neurotrophic factor (Bdnf) gene expression, suggesting that MeCP2 PTMs particularly impact neurodevelopmental processes and activity-dependent gene expression [17–21]. Recently, the novel PTM O-GlcNAcylation has emerged as a potent regulator of neurogenesis and synaptic plasticity [22, 23]. O-GlcNAcylation is a highly dynamic process [24–26]. O-GlcNAc transferase (OGT) catalyzes the addition of O-GlcNAc to serine (S) and threonine (T) residues on intracellular proteins, whereas O-GlcNAcase (OGA) results in the removal of O-GlcNAc modifications [24, 26, 27]. Previous studies have identified O-GlcNAcylation as a potent modulator of neuronal differentiation [22, 28] and synaptic plasticity [29]. Interestingly, MeCP2 has been found to be O-GlcNAcylated and phosphorylated simultaneously in rat cortical neurons [30, 31]. However, despite the critical roles of both O-GlcNAcylation and MeCP2 in neural development and synaptic transmission, the physiological function and molecular mechanisms of human MeCP2 O-GlcNAcylation remain elusive.
In this report, we first used mass spectrometry (MS) to systematically identify the O-GlcNAcylation sites on mouse, rat, and human MeCP2. We found that human MeCP2 T203, a site previously implicated in the pathogenesis of RTT [32–34], was O-GlcNAcylated at relatively high levels at baseline compared to rodent species. Furthermore, we demonstrated the critical role of MeCP2 T203 O-GlcNAcylation in the regulation of dendrite outgrowth, dendritic spine morphogenesis, and synaptic transmission both in vitro and in vivo. Mechanistic studies suggested that this may be due to the regulation of the induction of Bdnf transcription induced by neuronal activity. Together, our results identified a previously unknown function of O-GlcNAcylated MeCP2 T203, which may be essential for understanding the molecular mechanisms behind the neuropathology of RTT caused by MECP2 mutation.
Materials and Methods
Animals
Pregnant ICR (Institute for Cancer Research) mice were purchased from SiBeiFu Biotechnology Co., Ltd (Beijing, China) and housed in the animal breeding facility of the Beijing Institute of Basic Medical Sciences. All procedures were approved by the Animal Care and Use Committee of the Beijing Institute of Basic Medical Sciences and were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. MECP2 transgenic (Tg) mice (JAX lab, #008679, Bar Harbor, USA) [15] were kindly provided by Dr. Zilong Qiu at the Institute of Neuroscience, Chinese Academy of Sciences. The genotypes of MECP2 Tg mice were determined by PCR assays using murine tail DNA, with primers as follows: 5’-CGCTCCGCCCTATCTCTGA-3’ (forward) and 5’-ACAGATCGGATAGAAGACTC-3’ (reverse).
Plasmids
The rat Mecp2 gene expression plasmid was a gift from Dr. Zilong Qiu at the Institution of Neuroscience, Chinese Academy of Sciences. The human MECP2-e1 gene expression plasmid was a gift from Dr. Keping Hu at the Chinese Academy of Medical Sciences and Peking Union Medical College. N-terminal hemagglutinin (HA)-tagged MECP2 and GFP-tagged MECP2 coding sequence (CDSs) were subcloned into the pXJ40-HA and pEGFP-C1 vectors, respectively. Other constructs of various MECP2 mutations and indicated truncations were all generated based on the recombinant pXJ40-HA-hMeCP2 or pEGFP-hMeCP2 constructs. N-terminal histidine (His)-tagged MECP2 and glutathione S-transferase (GST)-tagged MECP2 CDSs were subcloned into the pET-28a and pGEX-6P-1 vectors, respectively. GST-tagged full-length OGT was subcloned into the pET-28a plasmid. The GST-OGT (323–1041) and GST-OGA (31–624) plasmids were gifts from Dr. Huadong Pei at the National Center for Protein Sciences (Beijing). The PCR primers used for subcloning of truncated or mutant MeCP2 and OGT are listed in Table S3.
The MeCP2 LEMPRA (lentivirus-mediated protein-replacement assay) plasmid (named pLenti-FUGW-shMeCP2-GFP-IRES-Flag-MECP2) is a lentiviral vector with dual promoters. It was constructed by inserting the H1 promoter-driven mouse Mecp2-specific shRNA cassette against the sequence of 5′-GTCAGAAGACCAGGATCTC-3′ into the indicated site [35], and the Flag-tagged shRNA-resistant human MECP2 coding sequence was inserted under the control of the Ubiquitin-C promoter. The shRNA-resistant Flag-MECP2 was generated by introducing five silent nucleotide mutations indicated in the following by lower-case letters within the coding sequence of MECP2: 5′-GagcGAAGACCAaGAcCTC-3′ [20].
Cell Culture and DNA Transfection
HEK293T were cultured in Dulbecco's Modified Eagle Medium (DMEM) basic (Gibco, C11995500BT, Thermo Fisher Scientific, Waltham, USA) and Neuro2A cells in Minimum Essential Media (MEM) basic (Gibco, C11095500BT), supplemented with 10% fetal bovine serum (FBS) (Gibco, 10099-141C) and 100 U/mL penicillin-streptomycin (Gibco, 15140122), in a 37 °C incubator with a humidified, 5% CO2 atmosphere. Lipofectamine 2000 (Invitrogen, 11668019, Thermo Fisher Scientific, Waltham, USA) was used for transfection following the manufacturer’s protocol.
Mass Spectrometry
HA-MeCP2 was ectopically expressed in HEK293T cells. Proteins were isolated by co-immunoprecipitation (co-IP), and eluted using 200 μg/mL of HA peptide. The endogenous mouse MeCP2 was enriched by co-IP with anti-MeCP2 antibody (Cell Signaling Technology, 3456, Beverly, USA) and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gel was stained with Coomassie brilliant blue. Visualized bands were excised, de-stained with ammonium bicarbonate buffer, and dehydrated in 75% acetonitrile. Following rehydration with 50 mmol/L ammonium bicarbonate, the gel slices were crushed and subjected to overnight digestion with trypsin or chymotrypsin. The peptides were extracted with acetonitrile containing 0.1% formic acid and vacuum dried. Proteolytic peptides were reconstituted with mobile phase A (2% acetonitrile containing 0.1% formic acid) and then separated on an on-line C18 column (75 μm inner diameter, 360 μm outer diameter, 10 cm, 3 μm C18). Mobile phase A consisted of 0.1% formic acid in 2% acetonitrile and mobile phase B was 0.1% formic acid in 84% acetonitrile. A linear gradient from 3% to 100% B over 75 min at a flow rate of 350 nL/min was applied. Mass spectrometric analysis was carried out on a Q-Exactive mass spectrometer (Thermo Fisher Scientific) operated in a data-dependent scan mode. A survey scan [mass-to-charge ratio (m/z) 375–1300] was performed at a resolution of 60,000 full width at half maximum followed by product ion scans (MS2) to fragment the 50 most abundant precursors with collision-induced dissociation. The activation time was set at 30 ms, the isolation width was 1.5 amu, the normalized activation energy was 35%, and the activation q was 0.25.
The raw files of mass spectrometry were scanned and analyzed with Proteome Discoverer software (version 2.1, Thermo Fisher Scientific) using the MASCOT search engine with percolator against the human or rodent ref-sequence protein database. The mass tolerance was set to 20 parts per million for precursor and 0.5 Da for product ion. Missed cleavages were no more than two for each peptide. O-GlcNAc of serine/threonine (S/T) were used as variable modifications.
In Vitro O-GlcNAcylation Assay by Chemoenzymatic Labelling
Chemoenzymatic labelling and biotinylation of proteins in cell lysates were carried out following the manufacturer’s instructions. Briefly, wild-type (WT) and MECP2 Tg mouse brain lysates (200 μg) were labelled using the Click-iT O-GlcNAc Enzymatic Labelling System protocol (Molecular Probes, C33368, Thermo Fisher Scientific, Waltham, USA), then conjugated with an alkyne-biotin compound according to the Click-iT Protein Analysis Detection Kit protocol (Molecular Probes, C33372). A parallel negative control experiment was performed in the absence of the labelling enzyme GalT or UDP-GalNAz. Methanol and chloroform were used to precipitate the biotinylated lysates. The biotinylated products were solubilized using 1% SDS solution, and neutralized with the neutralization buffer (6% Nonidet P40, 100 mmol/L Na2HPO4, 150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5, and protease inhibitor cocktail). The lysates were then incubated with streptavidin resin with end-to-end rotation at 4 °C overnight. The resin was then washed five times with low-salt buffer (100 mmol/L Na2HPO4, 150 mmol/L NaCl, 0.1% SDS, 1% Triton X-100, 0.5% sodium deoxycholate) and five times with high-salt buffer (100 mmol/L Na2HPO4, 500 mmol/L NaCl, 0.2% Triton X-100). Biotinylated proteins were boiled with SDS loading buffer and then resolved with SDS-PAGE and subjected to Western blotting using anti-MeCP2 antibody (Cell Signaling Technology, 3456). To quantify the level of O-GlcNAcylation, the intensity of the total MeCP2 protein band (Input) and the O-GlcNAc MeCP2 protein band (Elution) were measured, and the ratio of the intensity of the O-GlcNAc protein versus the intensity of the total protein was taken as the level of O-GlcNAcylation [36].
Primary Culture of Cortical and Hippocampal Neurons and Pharmacological Analysis
Cortical and hippocampal neurons were dissected and cultured from embryonic day 15.5 (E15.5) and E17.5 mouse brain respectively, and were maintained in Neurobasal medium (Gibco, 21103-049) supplemented with 2% B27 (Gibco, 17504044), 1 mmol/L GlutaMAX (Gibco, 35050061), and 100 U/mL penicillin-streptomycin (Gibco, 15140122). Cells were typically seeded at a density of 1–3 × 105 cells/cm2 on dishes coated with poly-L-lysine (Sigma-Aldrich, P1524, St. Louis, USA). Neuronal cultures were treated overnight with 1 μmol/L tetrodotoxin (TTX) (Kangte Biotech, purity > 99%; 121206, Shaoxing, China) to reduce endogenous neuronal activity prior to stimulation. Neurons were depolarized with 55 mmol/L extracellular KCl as previously described [17].
For the pharmacological inhibition or activation of O-GlcNAcylation assay, primary hippocampal neurons were treated with the OGT inhibitor OSMI-1 (Sigma-Aldrich, SML1621), or the OGA inhibitor PUGNAC (Sigma-Aldrich, A7229) and 2 μL DMSO as a control. In the dose-dependent inhibition assay, neurons were treated with 5, 10, and 20 μmol/L OSMI-1 or 10, 20, and 40 μmol/L PUGNAC at day 9 in vitro (DIV 9). In the time-dependent inhibition assay, neurons were treated with 20 μmol/L OSMI-1 or 40 μmol/L PUGNAC at DIV 7, DIV 9, and DIV 11. The neurons were collected for subsequent analysis at DIV 14.
Lentivirus Package, Purification, and Infection
Lentiviruses were produced by co-transfection of HEK293T cells with the MeCP2 LEMPRA plasmid and the helper plasmids psPAX2 and VSV-G. Lentiviruses were concentrated by ultra-centrifugation 48 h–72 h after transfection, and viral titers were determined by infection of HEK293T cells and determined by qPCR. The primary hippocampal neurons were isolated from E17.5 mouse embryos for in vitro culture. The neurons at DIV 7 were infected with the indicated purified LEMPRA lentivirus at 1 × 106 transduction units per mL (TU/mL) for 7 days, and then fixed at DIV 14 for immunofluorescent staining and confocal imaging.
Real Time Quantitative RT-PCR
Total RNA was extracted using TRIzol (Invitrogen, 15596018), and 0.5 μg of RNA was used for reverse transcription using the Reverse Transcription System (TaKaRa, RR036, Kusatsu, Japan) according to the manufacturer’s protocol. Real-time PCR was conducted in triplicate using SYBR Green PCR master mix (CWBIO, CW2601, Beijing, China) with the appropriate forward and reverse primers. For quantitative analysis of gene expression, results were averaged from three replicates in three independent experiments. Values were normalized to Actb levels. All PCR reactions were performed in triplicate with the following primers. Bdnf total: 5′-TGCCTAGATCAAATGGAGCTTCTC-3′ (forward) and 5′-CCGATATGTACTCCTGTTCTTCAGC-3′ (reverse); Bdnf exon IV: 5′-CAGAGCAGCTGCCTTGATGTT-3′ (forward) and 5′-GCCTTGTCCGTGGACGTTTA-3′ (reverse); Bdnf exon VI: 5′-GGGATCCGAGAGCTTTGTGTGGA-3′ (forward) and 5′-GTAGGCCAAGTTGCCTTGTCCGT-3′ (reverse); Acta2: 5′-GAGCTACGAACTGCCTGACG-3′ (forward) and 5′-TACCCCCTGACAGGACGTTG-3′ (reverse); Actb: 5′-GGCTGTATTCCCCTCCATCG-3′ (forward) and 5′-CCAGTTGGTAACAATGCCATGT-3′ (reverse).
Expression and Purification of Recombinant Proteins
Both His-tagged and GST-tagged proteins were expressed in Escherichia coli BL21. Bacteria were treated with 0.1 mmol/L isopropyl β-D-thiogalactoside (Thermo Fisher Scientific, AM9462) at 16 °C for 16 h to induce protein expression. To purify the His-tagged recombinant protein, the induced bacteria were harvested and suspended in 10 mmol/L phosphate-buffered saline (PBS) (pH 7.4) containing 20 mmol/L imidazole (Sigma-Aldrich, I2399) and 1 mmol/L phenylmethanesulfonyl fluoride (PMSF) (Sigma-Aldrich, 10837091001), followed by ultrasonication. The recombinant protein in the supernatant was incubated with Ni magnetic beads for 2 h at 4 °C, washed three times in 10 mmol/L PBS (pH 7.4), eluted with 10 mmol/L PBS (pH 7.4) containing 200 mmol/L iminazole, and dialyzed with 10 mmol/L PBS (pH 7.4). The protein level was assessed by Coomassie brilliant blue staining.
To purify GST-tagged recombinant proteins, induced bacteria were harvested and suspended in 10 mmol/L PBS (pH 7.4) containing 1 mmol/L PMSF, followed by ultrasonication. Recombinant GST-tagged protein in the supernatant was purified using glutathione-Sepharose 4B beads (GE Healthcare, 17-0756-01, Atlanta, USA), washed three times in 10 mmol/L PBS (pH 7.4), eluted with 10 mmol/L PBS (pH 7.4) containing 20 mmol/L reduced glutathione, and dialyzed with 10 mmol/L PBS (pH 7.4). The protein level was assessed by Coomassie brilliant blue staining.
In Vitro O-GlcNAcylation Assay
Purified recombinant GST-OGT fusion protein (323–1041) was incubated with WT His-tagged recombinant hMeCP2 or various His-tagged hMeCP2 mutants in 50 μL reactions (50 mmol/L Tris-HCl, 12.5 mmol/L MgCl2, 2 mmol/L UDP-GlcNAc, 1 mmol/L dithiothreitol, pH 7.5) overnight at 37 °C, and Western blot analysis was carried out with anti-O-GlcNAc (RL2) antibody (Abcam, ab2739, Boston, USA). For O-GlcNAc cleavage assay, O-GlcNAcylated proteins were treated with purified recombinant GST-OGA (31–624) fusion protein for 2 h at 37 °C in a volume of 50 μL, and Western blot analysis was carried out with anti-O-GlcNAc (RL2) antibody (Abcam, ab2739). Recombinant protein levels were assessed by Coomassie brilliant blue staining [37].
GST Pull-Down Assay
Bacteria-expressed GST, GST-hMeCP2, or GST-OGT fusion proteins were immobilized on glutathione Sepharose 4B beads (GE Healthcare, 17-0756-01) and washed three times with GST binding buffer (50 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, 50 mmol/L NaF, 2 mmol/L EDTA, 1% Nonidet P40, and protease inhibitor cocktail). The beads were next incubated with His-hMeCP2 or His-OGT recombinant protein lysates at 4 °C for 4 h under rotation. Beads were washed with GST binding buffer (50 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, 50 mmol/L NaF, 2 mmol/L EDTA, 1% Nonidet P40, and protease inhibitor cocktail) and proteins were eluted, followed by Western blotting with the indicated antibodies.
Co-Immunoprecipitation (Co-IP) Assay
For endogenous co-IP assays, mouse brain lysates or cell lysates (1–2 mg) with protease inhibitor cocktail were incubated with anti-MeCP2 (Cell Signaling Technology, 3456) or anti-OGT (Sigma-Aldrich, HPA030751) antibody overnight at 4 °C. After incubation, protein A/G agarose beads (Thermo Fisher Scientific, 78610) were used for precipitation for 2 h. The precipitates were then washed 5 times with the lysis buffer and eluted by boiling in SDS sample buffer for Western blotting. The gel was transferred to polyvinylidene fluoride (PVDF) membrane (GE Healthcare, 10600023), which was blocked with 5% milk in Tris-buffered saline with 0.1% Tween 20 (TBST) buffer for 1 h at room temperature. It was then incubated overnight at 4 °C with antibody, washed three times in TBST, and the signals were revealed by horseradish peroxidase (HRP) reaction using the SuperSignal Chemiluminescent Substrate (Beyotime, P0018AS, Shanghai, China).
For co-IP in HEK293T cells, the cells were plated on 60-mm plates, and transfected using Lipofectamine 2000 when they reached 50% confluence. A total of 5 μg of DNA was used in 60 mm plates at a molar ratio of 1:1 for GFP-tagged and HA-tagged constructs. Cells were harvested 36 h later, rinsed with cold PBS, harvested, and lysed for 20 min at 4 °C in a modified radio-immunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher Scientific, 89900). 10% of the supernatant was saved for the input control, and the rest was incubated with 2 μg anti-HA antibody (Cell Signaling Technology, 3724) or anti-GFP (Cell Signaling Technology, 2955) overnight at 4 °C. The immune complex was isolated by addition of 30 mL of a 50% slurry of mixed protein A/G agarose for 2 h, washed three times with the lysis buffer, then eluted by boiling-SDS lysis, and resolved by 8% SDS-PAGE. The gel was transferred to a PVDF membrane, which was blocked with 5% milk in TBST buffer for 1 h at room temperature. It was then incubated overnight at 4 °C with the antibody, washed three times in TBST, and the signals were revealed by HRP reaction using the SuperSignal Chemiluminescent Substrate (Beyotime, P0018AS).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP was prepared using the Upstate Biotechnology and Abcam kit following the manufacturer’s protocol. Briefly, Neuro2A cells were cross-linked at room temperature by the addition of 1% formaldehyde for 15 min. Cross-linking was stopped by 0.2 mol/L glycine for 5 min at room temperature. Cells were washed three times in 10 mL ice-cold 10 mmol/L PBS (pH 7.4), re-suspended in lysis buffer (50 mmol/L Tris-HCl at pH 8.0, 1% SDS, 5 mmol/L EDTA, and protease inhibitors), and directly sheared by sonication and processed for ChIP assay. Lysates were pre-cleared by incubation in 1 mL diluted chromatin with a salmon sperm DNA/protein A-Sepharose slurry (50 μL 50% slurry in 10 mmol/L Tris-HCl, pH 8.0, 1 mmol/L EDTA) for 2 h at 4 °C with agitation. Beads were pelleted using brief centrifugation and the supernatant fraction collected. The rest of the supernatant was divided into two fractions: one for an equivalent amount of normal rabbit IgG control and the second incubated with ChIP-grade anti-MeCP2 (Abcam, ab2828) at 4 °C with agitation overnight. Salmon sperm DNA/protein A-Sepharose slurry was added to the immune complexes and incubated at 4 °C for 2–4 h. Sepharose beads were collected and washed sequentially for 10 min each in the following buffers: once in low-salt buffer (0.1% SDS, 1% Triton X-100, 2 mmol/L EDTA, 20 mmol/L Tris-HCl pH 8.0, 150 mmol/L NaCl), three times in high-salt buffer (0.1% SDS, 1% Triton X-100, 2 mmol/L EDTA, 20 mmol/L Tris-HCl, pH 8.0, 500 mmol/L NaCl), twice in LiCl buffer (250 mmol/L LiCl, 1% Nonidet P40, 1% deoxycholate, 10 mmol/L Tris-HCl pH 8.0, 1 mmol/L EDTA), and twice in TE buffer (10 mmol/L Tris-HCl, 1 mmol/L EDTA, pH 8.0). Complexes were eluted using elution buffer (250 μL of 1% SDS and 100 mmol/L NaHCO3), formaldehyde cross-links were reversed, and DNA was precipitated and re-suspended. Both input and immunoprecipitated samples were analyzed by quantitative RT-PCR with the Bdnf promotor IV primers: 5′-GGTCTTTAAGGTGGCCCAAG-3′ (forward) and 5′-TGGAGCATGTGATCAAAACAA-3′ (reverse).
In Utero Electroporation
In utero electroporation of E13.5 pregnant mice (ICR; SiBeiFu Co., Beijing, China) was performed as previously described [38]. Specifically, pregnant mice were lightly anesthetized using 4% isoflurane (Yuyanbio, Shanghai, China) for induction, and 1.5%–2% isoflurane with 1 L/min oxygen for maintenance. The uterine horns were exposed by Cesarean section and sterile, pre-warmed saline was repeatedly applied during the operation to keep the intestines moist. The mice were kept on a heating pad during the entire operation. For electroporation, 1 μL of DNA plasmids (2 μg/μL) was injected into the lateral ventricle through a pulled glass capillary tube. DNA was electroporated into the neocortex. After electroporation, the uterine horns were carefully repositioned in the abdominal cavity, which was then filled with pre-warmed saline. The mice were left to recover in a clean cage and embryos were allowed to continue their development. At E17.5 or postnatal day 15 (P15), pregnant mice or pups were sacrificed. The brains were dissected and post-fixed with 4% paraformaldehyde (PFA) overnight at 4 °C. Coronal brain slices from mice with in utero electroporation were cut on a cryostat (Thermo Fisher Scientific, FSE) for immunofluorescent staining, or cut on an automated vibrating-blade microtome (Leica, VT1200 S, Mannheim, Germany) for electrophysiological recording.
Immunofluorescent Staining
The immunofluorescent staining of frozen brain sections and cultured neurons was performed using standard techniques as previously described in our lab [39, 40]. Briefly, frozen sections (30 μm) or cultured neurons were washed three times for 10 min each with 0.5% Triton X-100/PBS (PBS-T) and then blocked with 5% goat serum in PBST for 1 h. The sections or cultured neurons were then incubated overnight at 4 °C with primary antibodies, washed three times for 10 min each with 0.5% PBS-T and subsequently treated with Alexa Fluor 568- or Alexa Fluor 488-conjugated fluorescent secondary antibody (1:500; Biotium, 20103, 20012. Fremont, USA) for 1 h at room temperature. The nucleus was counterstained with DAPI (ZSGB-BIO, ZLI-9556, Beijing, China) during mounting onto glass slides with anti-fade solution. All images were processed and analyzed using an Olympus FV-1200 (Tokyo, Japan) and ImageJ software (NIH, USA).
Electrophysiology
Preparation of brain slices was performed as previously described [41]. A single slice was then transferred to the recording chamber and submerged in continuously-flowing oxygenated NaHCO3-buffered saline (1.0–1.5 mL/min) at 32 °C. The recording electrodes had a resistance of 3–5 MΩ when filled with the internal solution consisting of (in mmol/L): 135 cesium methanesulfonate, 10 HEPES, 0.2 EGTA, 8 NaCl, 4 Mg-ATP, and 0.3 Na3GTP (pH 7.2 with CsOH, osmolality adjusted to 280–290 mOsm). The slice was visualized with a 40 × water-immersion objective LUMPLFLN 40XW, Olympus) using standard infrared and differential interference contrast microscopy, and a Retiga ELECTRO CCD camera (QImaging, Surrey, BC, Canada). Cells in the cortex up to ~ 60 μm beneath the slice surface were patched and monitored. Recording in normal voltage-clamp mode was performed with an Axon 700B amplifier (Molecular Devices, Sunnyvale, USA) and Clampex 10.5 software (Molecular Devices). After formation of a tight seal (> 1 GΩ), fast and slow capacitance compensation was automatically performed. Neurons were excluded from analysis when their series resistance was >25 MΩ and changed by > 25% during the experiment. Data were filtered at 2 kHz and acquired at a sampling rate of 10 kHz. For miniature excitatory postsynaptic current (mEPSC) recording, the target neurons were held at − 70 mV in the presence of SR95531 (25 μmol/L; Sigma-Aldrich, S106) and TTX (1 μmol/L, purity > 99%; Kangte Biotech, 121206). For miniature inhibitory postsynaptic current (mIPSC) recording, the target neurons were held at 0 mV in the presence of 6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX) (25 μmol/L; Sigma-Aldrich, C239), DL-2-Amino-5-phosphonopentanoic acid (DL-AP5) (50 μmol/L; Abcam, ab120271), and TTX (1 μmol/L). Miniature events were collected for 5 consecutive min and analyzed. All mEPSCs or mIPSCs above a threshold value (5 pA) were included in the data analysis and each event was verified visually. Experiments were carried out in a genotype-blinded manner. No statistical analysis was used to predetermine the sample sizes used for experiments; however, our sample sizes are similar to those reported previously.
All drugs were from Sigma-Aldrich (St. Louis, USA) unless otherwise noted. Drugs were dissolved as concentrated stocks and stored at − 20 °C. Working solutions of different drugs were prepared immediately before use. During experiments, drugs were applied in the flowing bath solution. Total replacement of the medium in the recording chamber occurred within 1 min. Data were analyzed with software including Clampfit (Version 10.5, Molecular Devices), MiniAnalysis (version 6.0.3, Synaptosoft, Fort Lee, USA), Prism (version 8.0, GraphPad software, San Diego, USA), and Origin (version 9.0, OriginLab Corporation, Northampton, USA).
Dendritic Length and Spine Density Analysis
The length of dendritic branches in primary cultured hippocampal neurons was determined as follows: EGFP-positive neurons were randomly selected from each condition, and the dendritic lengths of all protrusions were analyzed using the Fiji software (a distribution of the software ImageJ, NIH, USA). At least three independent experiments were performed, and > 50 neurons per condition were analyzed. For spine density analysis, confocal Z-stacks of neurons in the hippocampus were acquired with a confocal microscope (Olympus, FV-1200) using an oil-immersion 60 × objective lens. Images were analyzed with Fiji software. Protrusions in direct contact with the dendrites were counted as spines, and the average spine density was calculated as the number of spines per μm dendritic length. At least 500 μm of dendrites from seven or more neurons were analyzed for each group (> 3 mice per group). All data were analyzed with one-way analysis of variance (ANOVA).
Statistical Analysis
Data are presented as the mean ± SEM and were analyzed by two-tailed Student’s t-test, or one-way ANOVA followed by the Bonferroni test. Kruskal–Wallis ANOVA followed by Dunn’s post hoc test was applied to analyze the data from electrophysiological experiments. A χ2-test was applied to analyze the distribution of cells in the layers of the neocortex, as well as the distribution of different types of dendritic spine. Unless otherwise indicated, in the figures, *P <0.05, **P <0.01, and ***P <0.001. Changes were considered significant if the P-value was <0.05.
Results
Identification of O-GlcNAcylation Sites on MeCP2
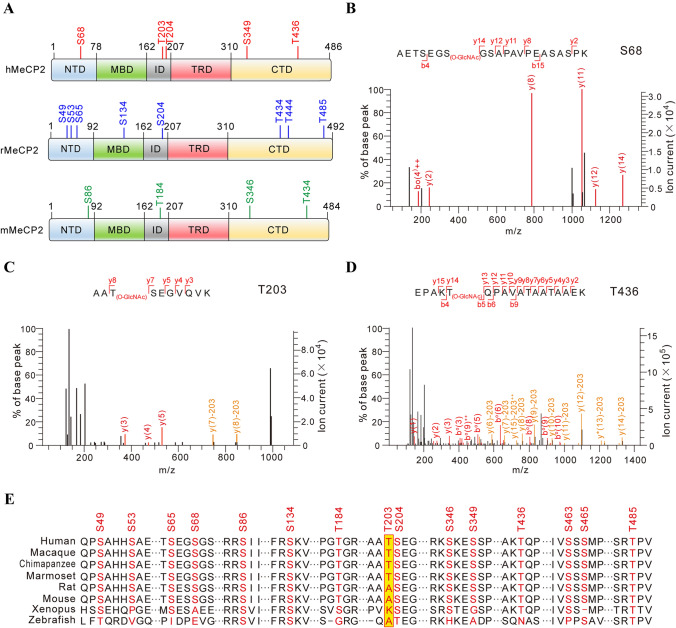
O-GlcNAcylation is highly dynamic and reversible in cellular systems. In order to better capture and concentrate O-GlcNAcylated MeCP2 for MS, HA-tagged MeCP2 in different species were co-expressed with OGT in HEK293T cells, and co-immunoprecipitated with anti-HA antibody-conjugated beads followed by in-gel trypsin digestion (Fig. S1A). The digested MeCP2 peptides were subjected to high-resolution MS (nanoLC-LTQ-CID/ETD-Orbitrap) analysis (Table S1; Fig. S1B–F). In addition to the O-GlcNAcylated sites at T434 and T444, which have been previously identified on rat MeCP2 [30, 31], we mapped 11 novel O-GlcNAcylation sites in mouse (mMeCP2), rat (rMeCP2), and human (hMeCP2) (Fig. 1A). To examine the potential relevance of these novel MeCP2 O-GlcNAc sites for neurodevelopmental disorders including RTT, we next compared them with the MECP2 mutation sites related to RTT disease reported in RettBASE [32–34], a database that catalogues clinically-relevant MECP2 mutations. Interestingly, we found that three O-GlcNAcylated sites (S68, T203, and S204) were mutated in some RTT clinical cases [42] (Table S2). In addition, hMeCP2 T436 (homologue of T434 in mMeCP2 and rMeCP2) O-GlcNAcylation may also be implicated, although no identified clinical cases have been associated with the mutation in the RettBASE database and previous reports [30, 32, 33, 43]. Therefore, we mainly focused on the three O-GlcNAcylated sites of hMeCP2 S68, T203, and T436 in the subsequent assays (Fig. 1B–D). Of these sites, additional analysis pointed to T203 as a particularly interesting target. We compared the MeCP2 O-GlcNAcylation sites identified in this study from eight species to assess their conservation (Fig. 1E). While the majority of these sites were conserved among vertebrates, T203 O-GlcNAcylation was selectively conserved in primates (human, macaque, chimpanzee, and marmoset), but not in rodents. Given that MeCP2 T203 is an etiologic site in RTT patients, this site may be of particular evolutionary functional significance in the brains of higher primates.
Fig. 1.
O-GlcNAcylation sites in rodent and human MeCP2. A MS identifies O-GlcNAcylation sites in rodent and human MeCP2 protein. hMeCP2, human MeCP2; rMeCP2, rat MeCP2; mMeCP2, mouse MeCP2; NTD, N-terminal domain; MBD, methyl-CpG binding domain; ID, intervening domain; TRD, transcriptional repression domain; CTD, C-terminal domain. B–D Three representative mass spectra of O-GlcNAcylation sites in hMeCP2 (S68, T203, and T436). hMeCP2 was purified from HEK293T cells and analyzed by MS to identify the O-GlcNAcylation sites. E Multiple-sequence alignment of MeCP2 protein from eight representative species reveals the conservation of O-GlcNAcylation sites (red, identified O-GlcNAcylation sites; yellow, T203 site).
MeCP2 is O-GlcNAcylated at T203 by Direct Interaction with OGT
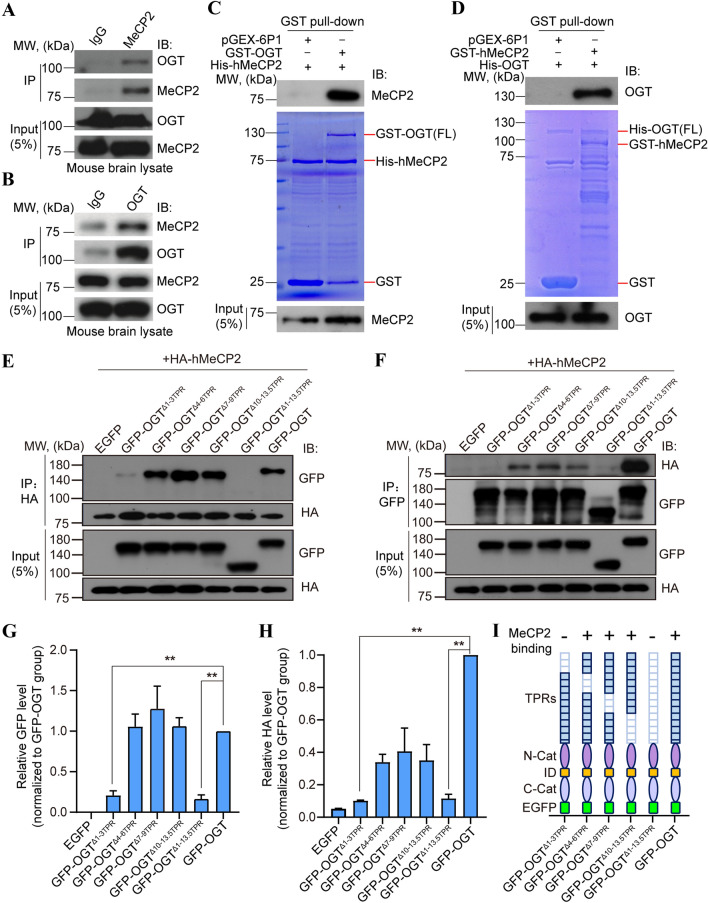
The above MS results indicated that MeCP2 may serve as a new protein substrate of OGT (Fig. S1G). To test this hypothesis, we first investigated the interaction between MeCP2 and OGT, and identified the MeCP2-binding domain within OGT. Endogenous MeCP2 from mouse whole-brain lysates were co-immunoprecipitated with native OGT (Fig. 2A). Reciprocal assays in mouse brain lysates showed that OGT also co-immunoprecipitated with MeCP2 (Fig. 2B), strongly suggesting physiological interaction in vivo, which is also in agreement with previous report [44]. To further explore if MeCP2 and OGT bind via direct protein–protein interaction, we performed an in vitro GST protein pull-down assay using purified GST-fused OGT and hMeCP2 protein. GST-fused OGT, but not GST alone, was able to pull down MeCP2 and vice versa, suggesting direct binding with each other (Fig. 2C, D). To reveal the reciprocal binding domains supporting the interaction of OGT with MeCP2, we first generated various human OGT deletion mutants. Co-IP assays showed that deletion of the entire N-terminal tetratricopeptide repeat (TPR) domain of OGT (aa 2–465) completely disrupted the interaction between OGT and MeCP2, suggesting the TPR domain is necessary (Fig. 2E, F). Next, domain mapping analysis showed that deletion of 4–6 TPR (aa 114–214), 7–9 TPR (aa 215–316), or 10–13.5 TPR (aa 317–465), had no effect on the interaction between OGT and MeCP2 (Fig. 2E, F). In contrast, deletion of the 1–3 TPR domain (aa 2–113) of OGT abolished the binding (Fig. 2E–H), suggesting that OGT directly interacts with MeCP2 via its N-terminal 1–3 TPR domain. Moreover, in order to identify critical OGT-binding domains on MeCP2, we used MeCP2 deletion mutants in co-IP binding experiments with OGT, and found that both the N-terminal domain (NTD) and C-terminal domain (CTD) of MeCP2 are necessary (Fig. S2).
Fig. 2.
OGT directly interacts with and O-GlcNAcylates MeCP2. A Western blots of mouse brain lysates immunoprecipitated with anti-MeCP2 antibody, followed by Western blots with an anti-OGT antibody. IB, immunoblotting. MW, molecular weight. B Reciprocal co-IP assay of mouse brain lysates with an anti-OGT antibody, followed by Western blots with an anti-MeCP2 antibody. C, D GST pull-down assays for His-hMeCP2 and GST-OGT, or His-OGT and GST-hMeCP2. Input and pull-down samples were analyzed with anti-MeCP2 and anti-OGT antibodies. Input represents 5% of the amount used for pull-down assay. E, F Lysates of HEK293T cells transfected with HA-tagged hMeCP2 and GFP-tagged full-length and indicated deletion mutants of OGT immunoprecipitated with anti-HA or anti-GFP antibodies, followed by Western blots with anti-GFP and anti-HA antibodies, respectively. Input represents 5% of the amount used for pull-down assay. G, H Representative quantification of Western blot results followed by co-IP assay in E and F, respectively. Histograms show the mean ± SEM. One-way ANOVA followed by the Bonferroni test, **P < 0.01. I Summary diagram of the serial deletion mutants of OGT and their binding capacities to MeCP2. Deletion of either the entire TPRs or TPRs 1–3 within OGT dramatically disrupts its binding to MeCP2.
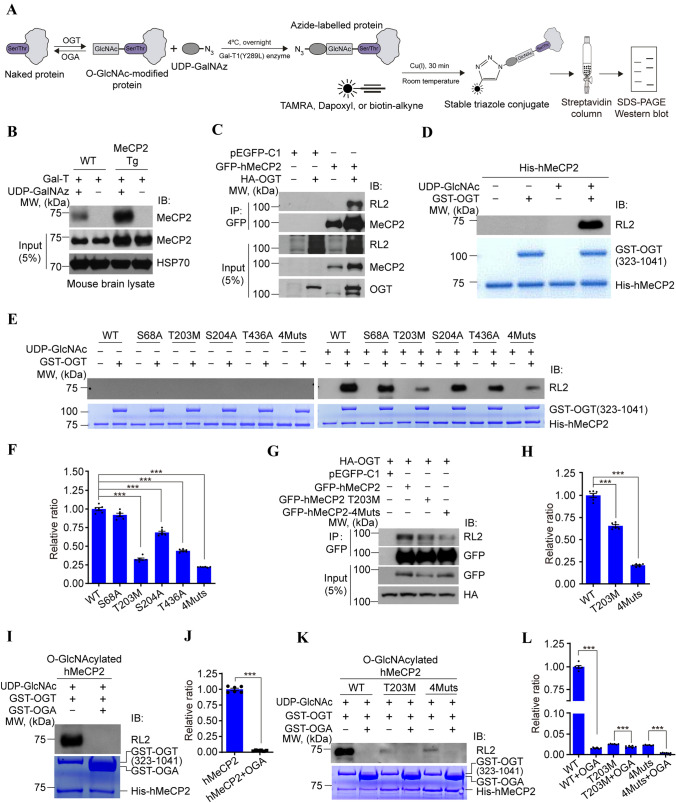
To further study the mechanisms of MeCP2 O-GlcNAcylation by OGT in vivo, we set up a well-established chemo-enzymatic labelling approach [45] to detect MeCP2 O-GlcNAcylation in whole brain lysates (Fig. 3A). O-GlcNAc-modified proteins from mouse brain lysates were enzymatically labelled with an azido-N-acetylgalactosamine sugar and biotinylated via Cu(I)-mediated [3 + 2] azide-alkyne cycloaddition chemistry, which were captured with streptavidin agarose beads, and subsequently immunoblotted with an antibody against MeCP2 (Fig. 3A). As shown in Fig. 3B, O-GlcNAcylation occurred in endogenous MeCP2 in WT mice in vivo, and in comparison, much stronger O-GlcNAcylation modification of MeCP2 was detected in transgenic mice overexpressing MECP2 [15]. The above results suggest direct protein–protein binding between MeCP2 and OGT that results in the O-GlcNAcylation of MeCP2 (Fig. 2). To further confirm that OGT catalyzes MeCP2 upon direct protein binding, we used co-IP assays to demonstrate that ectopic GFP-tagged hMeCP2 was O-GlcNAcylated in the presence of OGT in transfected HEK293T cells (Fig. 3C). In addition, in vitro O-GlcNAcylation assays further confirmed that His-tagged hMeCP2 was directly O-GlcNAcylated by the GST-tagged enzyme domain of OGT (323–1041) [37] (Fig. 3D).
Fig. 3.
T203 residue of HMeCP2 is dynamically O-GlcNAcylated by OGT. A Schematic of the chemoenzymatic labelling approach for biotinylation, capture, and detection of O-GlcNAcylated protein from brain or cell lysates. B Detection of O-GlcNAcylated MeCP2 protein in brain lysates from wild-type (WT) and MECP2 transgenic (Tg) mice using chemoenzymatic labelling. Level of O-GlcNAcylated MeCP2 is higher in MECP2 Tg mice than in WT controls. In the absence of GalT or UDP-GalNAz, no O-GlcNAcylated MeCP2 is detected. HSP70 is the loading control. C OGT elevates the O-GlcNAcylation level of MeCP2. Exogenous GFP-tagged hMeCP2 was co-expressed with or without HA-tagged OGT in HEK293T cells for co-IP assay followed by Western blots with anti-MeCP2 and anti-RL2 antibodies, respectively. Input represents 5% of the amount used for co-IP assay. pEGFP-C1 mock vector is the negative control. D OGT directly O-GlcNAcylates MeCP2 by in vitro glycosylation assay. Recombinant His-tagged hMeCP2 protein was incubated with or without purified GST-OGT (323–1041) and UDP-GlcNAc, followed by Western blots with an anti-RL2 antibody. The loading of recombinant proteins used for the in vitro assay is confirmed by Coomassie blue staining. E, F Identification of the O-GlcNAc modified sites on MeCP2 by in vitro glycosylation assays. Purified WT or mutant His-hMeCP2 was used as the substrate of GST-OGT (323–1041) in the presence of UDP-GlcNAc. The loading of GST-OGT and His-hMeCP2 is confirmed by Coomassie blue staining. Quantification shows the significantly decreased O-GlcNAc level in the hMeCP2 mutations T203M, S204A, T436A, and 4Muts. Histograms show the mean ± SEM. One-way ANOVA followed by the Bonferroni test, ***P < 0.001. G, H Co-IP assays showing dramatically reduced O-GlcNAc modified level in T203M or 4Muts of hMeCP2 compared with WT in the presence of OGT in transfected HEK293T cells. Cell lysates were immunoprecipitated with anti-GFP beads and immunoblotted as indicated in G. Input represents 5% of the total amount used for co-IP assay. pEGFP-C1 mock vector is the negative control. Quantification showing the significantly decreased RL2 level in T203M and 4Muts mutants compared with WT control. Histograms show the mean ± SEM. One-way ANOVA followed by the Bonferroni test, ***P < 0.001. I, J In vitro glycosylation assays showing OGA inversely regulates the O-GlcNAcylation of hMeCP2. Quantification shows a significantly decreased RL2 level of hMeCP2 in the presence of GST-OGA. Histograms show the mean ± SEM. t-test, ***P < 0.001. K, L In vitro glycosylation assays showing the O-GlcNAcylation of the T203M mutant or 4Muts mutant of hMeCP2 is also reversed by OGA. Quantification showing the significantly decreased RL2 level of WT and hMeCP2 mutants in the presence of GST-OGA. Histograms show the mean ± SEM. One-way ANOVA followed by the Bonferroni test, ***P < 0.001.
Since our MS results indicated that several O-GlcNAcylation sites in hMeCP2 were potentially relevant to RTT (Figs. 1 and S1), we set out to further identify major functional O-GlcNAcylation activity among these sites. To test this, MeCP2 S68A, T203M, S204A, T436A, and quadruple site mutations to alanine (4 Muts) were generated. It should be noted that to better mimic the clinical mutation site of T203, we generated the T203M mutation instead of T203A. We found that the level of O-GlcNAcylation was significantly reduced in the T203M and 4 Muts variations in vitro (Fig. 3E, F) and in vivo (Fig. 3G, H). However, changes in O-GlcNAcylation were insignificant for the other MeCP2 mutants, suggesting that the T203 site is the predominant O-GlcNAcylation site on hMeCP2. Of note, the T203 site has previously been implicated in the pathogenesis of RTT. Interestingly, MeCP2-4Muts still showed a weak level of O-GlcNAcylation, which further supports our MS results showing multiple O-GlcNAcylation sites on MeCP2 (Figs. 1 and S1).
To interrogate the reversibility of MeCP2 O-GlcNAcylation, we introduced the GST-tagged OGA (31–624) recombinant protein, which removes O-GlcNAc modifications. OGA almost completely removed the O-GlcNAcylation on MeCP2 in vitro (Fig. 3I–L), implying that MeCP2 O-GlcNAcylation is a reversible and dynamic process.
T203 O-GlcNAcylation is Required for Dendrite Development in Cultured Neurons
To understand the functional importance of MeCP2 O-GlcNAcylation, we next examined its impact on neuronal development. Overexpression of MeCP2 inhibits the dendritic growth of hippocampal neurons and disrupts excitatory synapse formation and pruning [46, 47], and MECP2 transgenic mice also have progressive neurological and neurobehavioral disorders [15, 48, 49]. If MeCP2 O-GlcNAcylation is involved in this aberrant neural developmental physiology, we reasoned that mutating O-GlcNAc sites in an MeCP2-overexpression system may rescue the defects. Thus, for our next experiments, we used MeCP2 overexpression as an experimental model to test the importance of MeCP2 T203 O-GlcNAcylation for neuronal development.
First, we compared the morphological differences in the dendritic branches of cultured mouse hippocampal neurons using anti-MAP2 immunofluorescent staining after infection with the indicated lentivirus (LV)-GFP control or LV-hMeCP2 ectopic expression lentivirus (Fig. S3A). The total length of dendrites was significantly inhibited by overexpressing ectopic hMeCP2 compared with cells infected with the LV-GFP control virus (207.4 ± 7.85 μm in LV-hMeCP2 and 294.2 ± 10.17 μm in LV-GFP control, P < 0.001, n = 62 and 67 neurons, respectively). However, overexpression of the hMeCP2 T203M or hMeCP2-4Muts mutant had no significant effect on the length of dendrites compared with LV-GFP control neurons (269.0 ± 9.58 μm in LV-hMeCP2 T203M and 263.6 ± 8.81 μm in LV-hMeCP2-4Muts; P = 0.1348 and 0.0829, n = 63 and 56 neurons, respectively) (Fig. S3B, C). In stark contrast, overexpression of the hMeCP2 O-GlcNAc-deficient mutant hMeCP2 S68A, S204A, or T436A all had inhibitory effects on dendrite development similar to WT hMeCP2 (P < 0.001, n = 70, 60, and 63 neurons, respectively). These results suggest that T203M, but not S68A, S204A, or T436A, specifically rescues the aberrant dendritic morphology seen with hMeCP2 overexpression.
In addition, we measured the length of primary and secondary dendritic branches after overexpression of the indicated ectopic hMeCP2. As shown in Fig. S3D and E, overexpression of WT hMeCP2 significantly reduced the length of both primary and secondary branches compared with LV-GFP controls (162.0 μm ± 6.02 μm in LV-hMeCP2 and 214.4 μm ± 6.56 μm in LV-GFP control for primary branches, P < 0.001, n = 62 neurons; 31.3 μm ± 3.70 μm in LV-hMeCP2 and 71.4 μm ± 5.87 μm in LV-GFP control for secondary branches, P < 0.001, n = 27 neurons). Overexpression of hMeCP2 S68A, S204A, and T436A also significantly decreased the length of primary branches (P < 0.001, n = 58, 50, and 63 neurons, respectively), but overexpression of the hMeCP2 T203M or hMeCP2-4Muts mutant had no effect on the length of both primary and secondary branches (Fig. S3D, E). The rescue effect seen with MeCP2 T203M and MeCP2-4Muts were relatively specific, because overexpression of either the S68A, S204A, or T436A mutant inhibited neuronal dendrite development similar to overexpressed WT hMeCP2 (Fig. S3B–E). Together, these results indicate that O-GlcNAcylation of hMeCP2 T203 critically underlies the dendritic deficits with overexpression of ectopic hMeCP2 in cultured hippocampal neurons, and mutating T203 rescues these deficits in dendritic length and branching.
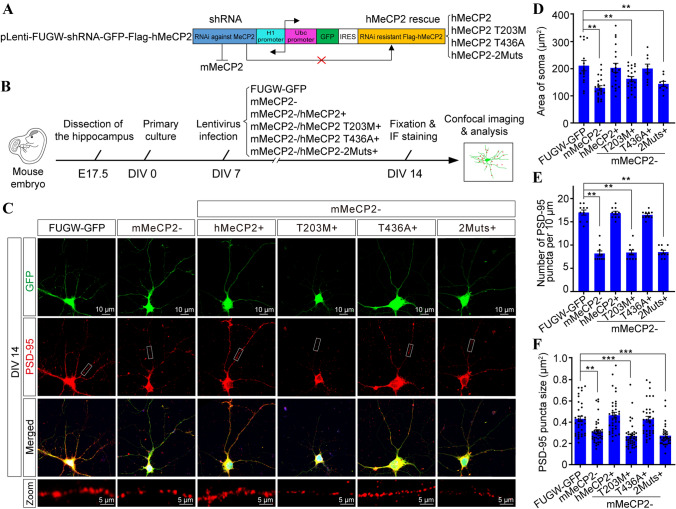
Previous studies have shown that loss of MeCP2 induces abnormalities in neuronal differentiation and migration in the developing cortex [50]. To further illustrate the importance of MeCP2 O-GlcNAcylation in neuronal development, we tested whether T203 O-GlcNAcylation had any effect on dendritic spine morphogenesis in cultured hippocampal neurons. A lentiviral-based rescue construct (LEMPRA) was used to exogenously introduce Flag-tagged shRNA-resistant human MECP2 to replace endogenous mouse Mecp2, the expression of which was specifically knocked down by shRNA [20] (Fig. 4A). Cultured hippocampal neurons from E17.5 mouse embryos were infected with the indicated lentivirus at DIV 7, then fixed at DIV 14 for immunofluorescent staining with the postsynaptic marker postsynaptic density (PSD)-95 antibody (Fig. 4B) to evaluate the formation of dendritic spines (Fig. 4C). Efficient knockdown of endogenous mMeCP2 and expression of equivalent levels of exogenous hMeCP2 were confirmed by Western blot analysis (Fig. S5). Consistent with previous reports showing smaller somas in Mecp2-null neurons [9, 10], quantification revealed that the somatic area in shMeCP2 (mMeCP2−) neurons was dramatically reduced compared with FUGW-GFP control neurons (129.1 μm2 ± 8.18 μm2 in mMeCP2−, and 211.5 μm2 ± 18.7 μm2 in FUGW-GFP control, P < 0.01, n = 22 and 15 neurons, respectively). Importantly, exogenous expression of WT hMeCP2 or hMeCP2 T436A (203.4 μm2 ± 16.71 μm2 in mMeCP2−/hMeCP2+ and 200.9 μm2 ± 16.04 μm2 in mMeCP2−/hMeCP2 T436A+, P = 0.9904 and 0.9875, respectively versus FUGW-GFP control, n = 20 and 9 neurons, respectively), but not hMeCP2 T203M or hMeCP2-2Muts mutants (162.9 μm2 ± 8.43 μm2 in mMeCP2−/hMeCP2 T203M+ and 143.9 μm2 ± 9.49 μm2 in mMeCP2−/hMeCP2-2Muts+, P = 0.0348 and 0.0174, respectively vs FUGW-GFP control, n = 23 and 9 neurons, respectively), efficiently rescued the smaller soma deficiency in mMeCP2− neurons (Fig. 4D).
Fig. 4.
T203 O-GlcNAcylation is required for dendritic spine formation and soma size maintenance in cultured hippocampal neurons. A The recombinant lentivirus-mediated protein-replacement assay construct, pLEMPRA-MeCP2, was generated to knock down endogenous mMeCP2 and express Flag-tagged ectopic hMeCP2. B Schematic of the experimental design. C Representative images of mouse primary hippocampal neurons infected with indicated lentivirus at DIV 7; lentivirus FUGW-GFP is the negative control. Neurons were collected and fixed at DIV 14 for immunofluorescent staining with anti-PSD-95 antibody for measurement of dendritic spines. Green (GFP), soma and dendrites of LEMPRA lentivirus-positive neurons; red, PSD-95-positive dendritic spines were shown in; boxed areas, higher magnification of PSD-95 positive puncta (red) along secondary dendritic branches; scale bars 10 μm and 5 μm). D Area of soma of indicated lentivirus-positive neurons (mean ± SEM, one-way ANOVA followed by the Bonferroni test, **P < 0.01). E, F Linear density and area of PSD-95 puncta along the secondary dendritic branches in indicated lentivirus-positive neurons (mean ± SEM, one-way ANOVA followed by the Bonferroni test, **P < 0.01, ***P < 0.001).
In agreement with previous reports in Mecp2-null mice [7, 51, 52], we found that the density of PSD-95 puncta was significantly decreased after knocking down endogenous MeCP2 (8.2 ± 0.53 per 10 μm in mMeCP2− and 17.0 ± 0.56 per 10 μm in FUGW-GFP control, P < 0.001, n = 10 neurons). Both exogenously-expressed Flag-tagged WT hMeCP2 and hMeCP2 T436A mutants efficiently rescued the decrease in PSD-95 puncta after MeCP2 knockdown (16.9 ± 0.39 per 10 μm in mMeCP2−/hMeCP2+ and 16.5 ± 0.27 per 10 μm in mMeCP2−/hMeCP2 T436A+, P = 0.9997 and 0.8970, respectively vs FUGW-GFP control, n = 10). Interestingly, in stark contrast, exogenously-expressed hMeCP2 T203M and hMeCP2-2Muts both showed significantly decreased numbers of PSD-95 puncta vs FUGW-GFP control neurons (8.4 ± 0.52 per 10 μm in mMeCP2−/hMeCP2 T203M+ and 8.5 ± 0.37 per 10 μm in mMeCP2−/hMeCP2-2Muts+, P < 0.01 and P < 0.01, respectively, n = 10 neurons) (Fig. 4E), demonstrating an inability to rescue the decrease in PSD-95 induced by MeCP2 knockdown. In addition, we calculated the average size of PSD-95 puncta within the dendritic spines, and found that it was lower in mMeCP2− neurons than in FUGW-GFP controls (0.312 μm2 ± 0.018 μm2 in mMeCP2−, n = 35; 0.432 μm2 ± 0.026 μm2 in FUGW-GFP control, n = 38; P = 0.001). Ectopic expression of both WT hMeCP2 and hMeCP2 T436A mutants rescued the decreased PSD-95-positive puncta size in mMeCP2− neurons to the normal level vs FUGW-GFP controls (0.466 μm2 ± 0.027 μm2 in mMeCP2−/hMeCP2+, n = 7, P = 0.738; 0.428 μm2 ± 0.027 μm2 in mMeCP2−/hMeCP2 T436A+, n = 34 P = 0.999). However, exogenously-expressed hMeCP2 T203M and hMeCP2-2Muts in mMeCP2− neurons both showed smaller PSD-95-positive puncta than FUGW-GFP controls (0.270 μm2 ± 0.019 μm2 in mMeCP2−/hMeCP2 T203M+, n = 37, P < 0.001; 0.273 μm2 ± 0.018 μm2 in mMeCP2−/hMeCP2-2Muts, n = 33, P < 0.001) (Fig. 4F). Together, these results suggest a requirement of T203 O-GlcNAcylation for dendritic spine morphogenesis.
It should be noted that pharmacological inhibition of OGT activity by OSMI-1 also significantly suppressed the arborization and dendritic spine morphogenesis in cultured neurons (Fig. S4), the phenotypes of which were similar to those of mMeCP2− and mMeCP2−/hMeCP2 T203M+ mutant neurons shown in Fig. 4C. In contrast, pharmacological inhibition of OGA activity by PUGNAC significantly promoted the arborization and dendritic spine formation in cultured neurons (Fig. S4). Taken together, our results indicate that O-GlcNAcylation and MeCP2 are both essential for neurodevelopment, and hMeCP2 T203 O-GlcNAcylation may be involved in the regulation of neurite outgrowth and dendritic spine morphogenesis in cultured hippocampal neurons. Moreover, T203 O-GlcNAcylation is also sufficient for the maintenance of the soma during neuronal differentiation in vitro.
T203 O-GlcNAcylation Promotes Cortical Neuron Migration and Maturation In Vivo
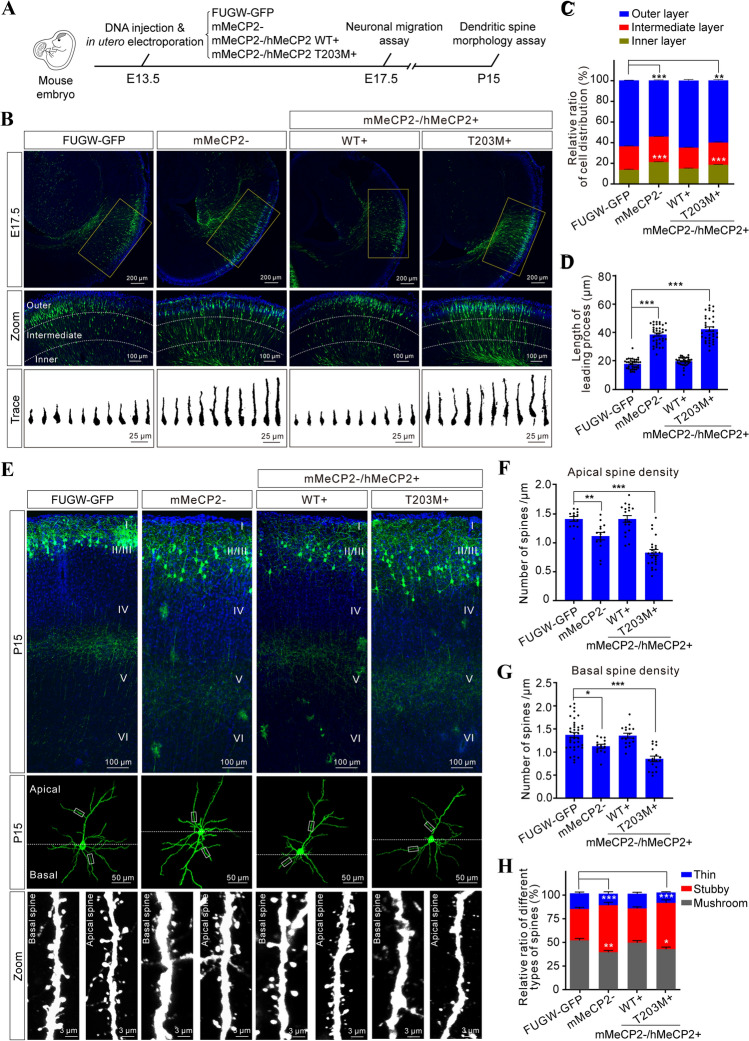
To further understand the role of T203 O-GlcNAcylation in cortical development in vivo, we manipulated the expression of exogenous hMeCP2 in the developing mouse neocortex using the above-described LEMPRA plasmid by in utero electroporation [20, 38]. Neuronal migration and dendritic differentiation are critical events in cortical construction. Therefore, we analyzed the effects of T203 O-GlcNAcylation on neuronal migration and dendritic spine morphogenesis at E17.5 and P15, respectively (Fig. 5A). First, we found that loss-of-function of MeCP2 by shRNA-mediated knockdown resulted in significant migration defects at E17.5 when compared with electroporation of GFP alone (Fig. 5B, C), in agreement with previous studies [50]. To exclude possible off-target effects of the shRNA system, we showed that the migration defects in MeCP2 knockdown neurons were rescued by exogenous expression of WT hMeCP2. However, exogenous expression of the hMeCP2 T203M mutant did not rescue the migration defects in mMeCP2 knockdown neurons (Fig. 5B, C), indicating that T203 O-GlcNAcylation is required for proper neuronal migration in the embryonic neocortex.
Fig. 5.
T203 O-GlcNAcylation is required for dendritic spine morphogenesis in vivo. A Schematic of the experimental design. B Upper panels, distribution of GFP+ pyramidal neurons in the indicated plasmid-electroporated neocortex at E17.5. Middle panels (zoom), higher magnification to illustrate the detailed distribution within the neocortex, showing the inner, intermediate, and outer layers. Lower panels (trace), Fiji tracings of representative single GFP+ neurons from the indicated groups. Scale bars, 200 μm, 100 μm, and 25 μm. C Relative ratios of GFP+ neuron distribution (%) in distinct neocortical layers (mean ± SEM, χ2-test, **P < 0.01, ***P < 0.001). D Length of the leading process (LP) in GFP+ neurons electroporated with the indicated plasmid (mean ± SEM, one-way ANOVA followed by the Bonferroni test, ***P < 0.001). E Representative images of dendritic spines on apical and basal dendrites of GFP+ neurons at P15 after electroporation with the indicated plasmid (Zoom panels, higher magnification to illustrate the detailed dendritic spine morphology; scale bars, 100 μm, 50 m, and 3 μm). F, G Spine density on apical and basal dendrites in GFP+ neuron (mean ± SEM, one-way ANOVA followed by the Bonferroni test, *P < 0.05, **P < 0.01, ***P < 0.001). H Distribution of three subtypes of dendritic spine in GFP+ neurons at P15 (mean ± SEM, χ2-test, *P < 0.05, **P < 0.01, ***P < 0.001).
Previous studies have shown that the leading process (LP) branch is a critical determinant for nuclear translocation during neuronal migration [53]. Therefore, we analyzed the morphology and projection of the LP in MeCP2 knockdown (mMeCP2−) and rescued neurons (mMeCP2−/hMeCP2+). Interestingly, we found that the length of the LP in confirmed mMeCP2− neurons was much longer than in FUGW-GFP controls (38.94 μm ± 1.07 μm in 35 mMeCP2− neurons and 18.18 μm ± 0.62 μm in 37 FUGW-GFP control neurons, P < 0.001). Moreover, the abnormal LP branches in confirmed mMeCP2− neurons were rescued by exogenous expression of WT hMeCP2, but not the hMeCP2 T203M mutant (19.98 μm ± 0.49 μm in mMeCP2−/hMeCP2+ 36 neurons, P = 0.4528, and 42.76 μm ± 1.53 μm in 35 mMeCP2−/hMeCP2 T203M+ neurons, P < 0.001 vs FUGW-GFP controls) (Fig. 5D). Collectively, these results indicate that the abnormally developed LP branches may be responsible for the migration defects in mMeCP2− neurons, and that hMeCP2 T203 O-GlcNAcylation is critical for maintaining normal LP branch morphology and the navigation of migrating neuronal precursors in vivo.
Dendritic spines have been shown to be abnormal in the cerebral cortex in RTT patients and Mecp2-null mice [9, 11, 52, 54]. Thus, we next asked whether MeCP2 T203 O-GlcNAcylation is essential for postnatal dendritic spine morphogenesis in vivo. First, we analyzed both dendrite and dendritic spine morphology in layer II/III cortical projection neurons in P15 mice that had been manipulated by in utero electroporation on E13.5 as described above (Fig. 5A). GFP fluorescence was used to trace the morphology of entire neurons, including the apical and basal dendritic arbors. Initial analysis did not find striking differences based on gross morphology of the dendrites including polarity and dendritic orientation in individual groups of electroporated neurons (Fig. 5E). Next, we imaged apical and basal dendritic segments at high magnification for each group of electroporated neurons (Fig. 5E), and found that the density of both apical and basal spines were significantly decreased in mMeCP2− neurons compared with FUGW-GFP controls (1.118 ± 0.065 per μm and 1.13 ± 0.041 per μm in 13 mMeCP2− neurons, P = 0.0045; 1.414 ± 0.042 per μm and 1.37 ± 0.054 per μm in 13 FUGW-GFP control neurons, P = 0.013) (Fig. 5F, G). Meanwhile, the spine formation defects in mMeCP2− neurons were rescued by the ectopic expression of WT hMeCP2, but not by the hMeCP2 T203M mutant (1.415 ± 0.057 per μm and 1.36 ± 0.052 per μm in 17 mMeCP2−/hMeCP2+ neurons, P = 0.99, and 0.831 ± 0.052 per μm and 0.85 ± 0.059 per μm in 17 mMeCP2−/hMeCP2 T203M+ neurons, P < 0.001 vs FUGW-GFP controls) (Fig. 5F, G).
Dendritic spines are morphologically heterogeneous within the neocortex, and these differences between spines reflect functional viability and pathological states [55]. To interrogate the effect of hMeCP2 T203 O-GlcNacylation on spine morphology, we next classified spine shapes into thin, mushroom, and stubby categories as previously defined [56]. We found significantly fewer mushroom spines in mMeCP2− neurons on P15, compared with FUGW-GFP control neurons (38.79% ± 1.89% in 17 mMeCP2− neurons and 51.18% ± 2.03% in 38 FUGW-GFP control neurons, P = 0.001) (Fig. 5H). In contrast, no changes in spine morphology were observed in mMeCP2−/hMeCP2+ rescued neurons compared with FUGW-GFP control neurons (48.76% ± 2.27% in 18 mMeCP2−/hMeCP2+ neurons and 51.18% ± 2.03% in 38 FUGW-GFP controls, P = 0.9993). However, mMeCP2−/hMeCP2 T203M+ neurons had significantly fewer mushroom spines with relatively increased numbers of stubby spines (41.96% ± 2.31% mushroom type and 48.50% ± 3.11% stubby type in 18 mMeCP2−/hMeCP2 T203M+ neurons; 51.18% ± 2.03% mushroom type and 32.90% ± 1.58% stubby type in 38 FUGW-GFP control neurons, P = 0.0465 and P < 0.001, respectively) (Fig. 5H). Therefore, our results indicate that hMeCP2 T203 O-GlcNAcylation plays a critical role in the regulation of dendritic spine morphogenesis. Notably, the features displayed in mMeCP2−/hMeCP2 T203M+ cortical neurons nicely mimic the defects in dendrite and spine maturation and synaptogenesis in RTT patients [9, 11, 54, 57].
T203 O-GlcNAcylation Regulates Excitatory Synaptic Transmission
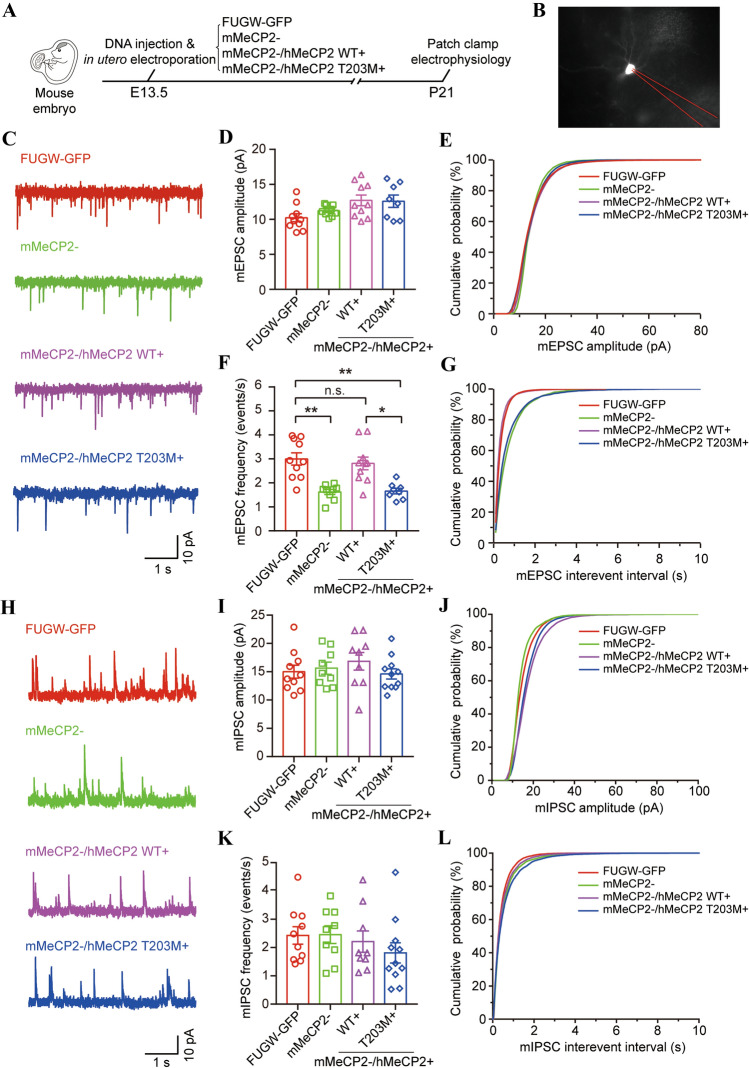
Previous studies have shown that shRNA-mediated MeCP2 knockdown affects homeostatic synaptic plasticity in hippocampal neurons [58, 59]. Long-term potentiation is also impaired at excitatory synapses of layers II/III and V in the primary somatosensory cortex of Mecp2-null mutant mice [60, 61]. However, the bases of these deficits remain unclear. To investigate whether synaptic transmission is regulated by hMeCP2 T203 O-GlcNAcylation, we treated neurons in layers II/III of the developing cortex with various LEMPRA constructs using in utero electroporation, and recorded from GFP-positive neurons on P21 (Fig. 6A). First, we recorded miniature excitatory postsynaptic currents (mEPSCs) in FUGW-GFP control and mMeCP2− projection neurons (Fig. 6B, C). The mEPSC amplitudes remained similar in mMeCP2− neurons and FUGW-GFP controls (10.23 pA ± 0.57 pA in 10 FUGW-GFP controls, and 11.24 pA ± 0.26 pA in 9 mMeCP2− neurons, P = 0.7859). Exogenous expression of WT or T203M mutant hMeCP2 in mMeCP2− neurons had no effect on the mEPSC amplitudes (12.71 pA ± 0.77 pA in 10 mMeCP2−/hMeCP2+ neurons, P = 0.0939, and 12.59 pA ± 0.88 pA in 8 mMeCP2−/hMeCP2 T203M+ neurons, P = 0.1793 vs FUGW-GFP controls) (Fig. 6D, E). In contrast, in agreement with the decreased dendritic spine density in mMeCP2− neurons (Figs. 4E and 5F, G) and a previous report [52], the mEPSC frequency decreased from 3.00 Hz ± 0.26 Hz in 10 FUGW-GFP controls to 1.62 Hz ± 0.11 Hz in 9 mMeCP2− neurons (P = 0.0048) (Fig. 6F, G). In addition, the reduced frequency of mEPSCs in mMeCP2− neurons was efficiently rescued by the exogenous expression of WT hMeCP2, but not the hMeCP2 T203M mutant (2.81 Hz ± 0.26 Hz in 10 mMeCP2−/hMeCP2+ neurons, compared with 9 FUGW-GFP control neurons, P > 0.9999; 1.66 Hz ± 0.12 Hz in 8 mMeCP2−/hMeCP2 T203M+ neurons, compared with FUGW-GFP controls and mMeCP2−/hMeCP2+ neurons, P = 0.0093 and 0.0259, respectively) (Fig. 6F, G), further indicating that T203 O-GlcNAcylation is involved in the regulation of postsynaptic spine density and excitatory synaptic transmission in vivo.
Fig. 6.
T203 O-GlcNAcylation is essential for excitatory synaptic transmission in the neocortex. A Schematic of the experimental design. B Representative image of a recorded GFP+ cell electroporated with plasmid shown in A. C Representative traces of mEPSC recorded in layer II/III GFP+ neurons electroporated with indicated plasmids. D, E Amplitude and cumulative distributions of mEPSCs in recorded GFP+ neurons electroporated with indicated plasmid (mean ± SEM, Kruskal–Wallis ANOVA followed by Dunn’s post hoc test, P > 0.05 for all comparisons). F, G Frequency and cumulative distributions of mEPSCs in recorded GFP+ neurons electroporated with indicated plasmid (mean ± SEM, Kruskal–Wallis ANOVA followed by Dunn’s post hoc test, *P < 0.05, **P < 0.01, n.s., not significantly different). H Representative traces of mIPSC recorded in layer II/III GFP+ neurons electroporated with indicated plasmid. I, J Amplitude and cumulative distributions of mIPSCs in recorded GFP+ neurons electroporated with indicated plasmid (mean ± SEM, Kruskal–Wallis ANOVA followed by Dunn’s post hoc test, P > 0.9999 for all comparisons). K, L Frequency and cumulative distributions of mIPSC in recorded GFP+ neurons electroporated with indicated plasmid (mean ± SEM, Kruskal–Wallis ANOVA followed by Dunn’s post hoc test, P > 0.9999 for all comparisons).
Interestingly, both the amplitude and the frequency of miniature inhibitory postsynaptic currents (mIPSCs) did not significantly differ between mMeCP2− neurons and FUGW-GFP controls (amplitude: 15.63 pA ± 1.07 pA in 9 mMeCP2− neurons, and 15.01 pA ± 1.15 pA in 10 FUGW-GFP controls, P > 0.9999; frequency: 2.455 Hz ± 0.31 Hz in 9 mMeCP2− neurons, and 2.426 Hz ± 0.31 Hz in 10 FUGW-GFP controls, P > 0.9999) (Fig. 6H–L). Moreover, neither amplitude nor frequency of mIPSCs was changed in mMeCP2−/hMeCP2+ or mMeCP2−/hMeCP2 T203M+ neurons compared with FUGW-GFP controls (amplitude: 16.85 pA ± 1.56 pA in 9 mMeCP2−/hMeCP2+ neurons, P > 0.9999, and 14.61 pA ± 0.89 pA in 11 mMeCP2−/hMeCP2 T203M+ neurons, P > 0.9999; frequency: 2.209 ± 0.38 Hz in 9 mMeCP2−/hMeCP2+ neurons, P[0.9999, and 1.811 ± 0.36 Hz in 11 mMeCP2−/hMeCP2 T203M+ neurons, P = 0.8159) (Fig. 6H–L). Together, these results indicate that T203M O-GlcNAcylation may be essential for the establishment of excitatory, but not inhibitory, synaptic transmission during neurodevelopment in the cortex.
Neuronal Activity-Induced Bdnf Transcription is Dependent on T203 O-GlcNAcylation
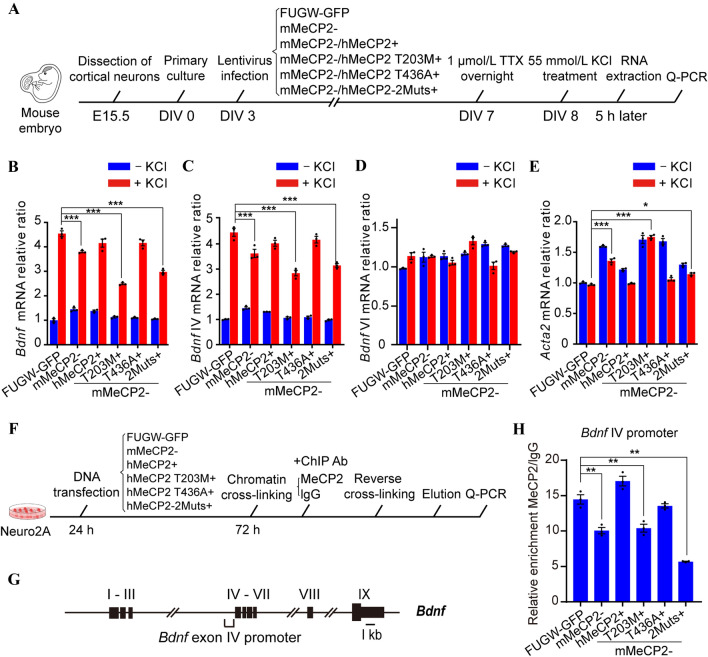
MeCP2 regulates the expression of thousands of genes during neural development. One of the most important target genes is brain-derived neurotrophic factor (BDNF) [17, 18, 20]. Numerous studies have shown that BDNF is critical for dendritic spine morphogenesis, synaptic maturation, and synaptic plasticity [62–65]. Moreover, activity-induced release of BDNF modulates spine morphology in conjunction with spontaneous neurotransmitter release [63]. Therefore, we asked whether the impaired dendritic branching, spine morphogenesis, and synaptic transmission in mMeCP2−/hMeCP2 T203M+ neurons were due to impaired Bdnf transcription. To evaluate the effect of T203 O-GlcNAcylation on neuronal activity-induced Bdnf transcription, primary mouse cortical neurons were dissected from E15.5 embryos and infected with various LEMPRA lentivirus constructions as described above (Fig. 4A) at DIV 3 for 4 days. We then added TTX, a selective Na+ channel blocker, into the culture medium at a final concentration of 1 μmol/L to block the production of action potentials for 12 h. On DIV 8, the cultured cortical neurons were treated with KCl at a final concentration of 55 mmol/L for 5 h to trigger synchronous membrane depolarization [18, 66, 67]. The neurons were then harvested for RNA extraction and real time quantitative RT-PCR assays to investigate the transcription of MeCP2 target genes (Fig. 7A).
Fig. 7.
T203 O-GlcNAcylation activates Bdnf exon IV promoter-dependent transcription. A Schematic of the experimental design. B–E Transcription of total Bdnf, Bdnf exon IV, Bdnf exon VI, and Acta2 at the mRNA level in response to KCl treatment (mean ± SEM, one-way ANOVA followed by the Bonferroni test, *P < 0.05, ***P < 0.001). F Schematic of the experimental design. G Schematic of all nine exons and the exon IV promoter within the Bdnf locus. H Quantification of the ChIP assay. The binding activity of Bdnf exon IV promoter to MeCP2 in indicated transfected cells measured by Q-PCR amplification (mean ± SEM, one-way ANOVA followed by the Bonferroni test, **P < 0.01).
The transcription of total mouse Bdnf and its exon IV, but not exon VI, was dramatically enhanced by > 4-fold in FUGW-GFP cortical neurons after KCl treatment (P < 0.001, n = 3) (Fig. 7B–D). The up-regulation of total Bdnf and exon IV mRNA transcription was significantly lower in mMeCP2− neurons than in FUGW-GFP controls after treatment with KCl (P < 0.001 and P < 0.001, respectively, n = 3) (Fig. 7B, C). Interestingly, the compromised up-regulation of Bdnf transcription in mMeCP2− neurons was significantly rescued by exogenous expression of WT hMeCP2 and the hMeCP2 T436A mutant (P = 0.1015 and 0.1158, respectively, n = 3), but not by the hMeCP2 T203M or hMeCP2-2Muts mutants (P < 0.001 and P < 0.001, respectively, n = 3) (Fig. 7B, C). These results suggest that T203 O-GlcNAcylation of hMeCP2 is essential for neuronal activity-induced enhanced transcription of Bdnf, especially for exon IV, but not exon VI. In addition, we examined the transcription of Acta2, a downstream gene normally repressed by MeCP2 in cortical neurons [46]. We found that the Acta2 mRNA level was up-regulated in mMeCP2− neurons in both absence and presence of KCl (P < 0.001, n = 3) (Fig. 7E). Exogenous expression of WT hMeCP2 suppressed the up-regulated transcription of Acta2 in mMeCP2− neurons, but hMeCP2 T203M and hMeCP2-2Muts failed to reverse the transcriptional activation effect in mMeCP2− neurons (P > 0.9999 and P < 0.001, respectively, n = 3) (Fig. 7E), indicating that T203 O-GlcNAcylation of hMeCP2 is also required to suppress Acta2 transcription. These results suggest that hMeCP2 T203 O-GlcNAcylation is particularly important for activity-induced Bdnf expression in cortical neurons.
Finally, we applied ChIP analysis to quantify and compare the binding affinity of WT hMeCP2 and various hMeCP2 mutants to the mouse Bdnf exon IV promoter in mouse Neuro2A cells (Fig. 7F, G). As expected, binding of MeCP2 to the Bdnf exon IV promoter was significantly decreased in mMeCP2− cells (Fig. 7H). Exogenous expression of either WT hMeCP2 or the hMeCP2 T436A mutant dramatically rescued the binding deficiency in mMeCP2− cells (P = 0.013 and 0.5981, respectively, vs FUGW-GFP controls, n = 3). In contrast, both hMeCP2 T203M and hMeCP2-2Muts failed to rescue the decreased binding of the Bdnf exon IV promoter in mMeCP2− cells (P = 0.0004 and P < 0,001, respectively vs FUGW-GFP controls, n = 3) (Fig. 7H), suggesting decreased binding capacity of hMeCP2 T203M, but not hMeCP2 T436A, to the Bdnf exon IV promoter. Taken together, these results indicate that MeCP2 directly controls mouse Bdnf transcription by binding to the exon IV promoter. T203 O-GlcNAcylation of hMeCP2 is required to maintain Bdnf exon IV promoter binding, and therefore plays a critical role in the up-regulation of Bdnf transcription following neural activation.
Discussion
Collectively, our results indicate that O-GlcNAcylation of hMeCP2 at T203 is critical for dendritic growth, spine formation, baseline and induced synaptic transmission, and the regulation of activity-induced Bdnf transcription. Using a LEMPRA-based shRNA system with exogenous expression of various hMeCP2 O-GlcNAc site mutants, we demonstrated that O-GlcNAcylation regulates MeCP2 activity during neurodevelopment, and pinpointed T203 as a major novel PTM site for O-GlcNAc modification of hMeCP2. Given that T203 mutations have been implicated in clinical cases of RTT [32–34], these results suggest that hMeCP2 T203 O-GlcNAcylation not only supports normal cortical neurodevelopment, but its deregulation may contribute to the pathogenesis of RTT.
Mutations of the MECP2 gene are the most prevalent cause of RTT, and some mutated sites are substrates for PTM [33, 68–70]. This suggests that PTMs are a potentially crucial component for maintaining normal MeCP2 function, and its deregulation may result in neurodevelopmental pathologies. Extensive studies have previously shown that phosphorylation [17–20], acetylation [71, 72], SUMOylation, [68] and other types of PTM [33] can control MeCP2 function, suggesting diverse forms of regulation.
Of particular interest is the finding that rat MeCP2 protein is O-GlcNAcylated [30, 31], but the functional significance and mechanism were unclear. Previous results have implicated O-GlcNAcylation in the pathogenesis of neurodegenerative diseases [73]. For example, the imbalance of O-GlcNAcylation relative to phosphorylation of microtubule-associated Tau protein may be closely involved in the pathogenesis of Alzheimer’s disease [74]. In addition, OGT-mediated O-GlcNAcylation modulates both the maturity and function of excitatory synapses in the developing brain [23], and changes in OGT levels may contribute to deficits in synaptic plasticity during brain aging [29]. Here, our results further illustrate a novel role for O-GlcNAcylation as a regulator of MeCP2 function during neurodevelopment. In particular, we identified T203 as a critical site for the establishment and maintenance of normal dendrite and spine development and synaptic transmission, in that exogenous expression of its mutant form T203M was not able to rescue the deficits induced by knockdown of endogenous mouse MeCP2, whereas rescue effects were found with other putative O-GlcNAcylation sites including S68, S204, and T436. The unique role of T203 may be due to its location on the hMeCP2 protein. MeCP2 can be subdivided into five domains corresponding to the NTD, the methyl-CpG binding domain (MBD), the intervening domain (ID), the transcriptional repression domain (TRD), and the CTD. By analyzing the MECP2 mutation database, Bellini et al. have reported that ~ 25% of residues in the CTD and ID have been associated with pathogenic missense mutations in RTT [33,75]. In addition, the ID domain has been shown to be involved in MeCP2-mediated multiple protein–protein interactions as well as diverse phosphorylation events [75]. Interestingly, T203 is located within the ID domain (Fig. 1B). Therefore, it is possible that the O-GlcNAcylation of T203 may affect its binding to DNA or its protein partners, resulting in subsequent regulation of neurodevelopment. However, the precise biological partners of T203 remain to be further identified. Our results showed that the T203M mutation had no effect on its binding to histone deacetylase 1 (HDAC1) (Fig. S6), but did result in reduced binding to the Bdnf exon IV promoter (Fig. 7). This suggests that an HDAC1-independent transcriptional activation mechanism may underlie T203 O-GlcNAcylation in cortical neurons.
Although increasing evidence points to O-GlcNAcylation as a mediator of neurodegeneration and a modulator of neuronal signaling pathways in the brain [23, 27, 74, 76–79], the underlying molecular mechanisms that support these crucial functions are not yet fully understood. Previously, the O-GlcNAcylation of the cAMP response element binding protein (CREB), a common upstream regulator of Bdnf, was found to critically regulate neuronal gene expression, axonal and dendritic growth, and long-term memory [78]. MeCP2 associates with CREB1 at the promoter of an activated target but not a repressed target [80]. In addition, CREB signaling has also recently been shown to be involved in RTT pathogenesis [81]. Interestingly, WT hMeCP2, but not its T203M mutant, was able to rescue activity-dependent Bdnf transcription (Fig. 7B). The finding that T203M dramatically reduces O-GlcNAcylation of hMeCP2 suggests that O-GlcNAcyated T203 is necessary to support Bdnf transcription following neural depolarization. Interestingly, T203M did not notably impact Creb expression levels following KCl treatment (data not shown). This suggests that neuronal activation stimulates a variety of responses at both the O-GlcNAcylation PTM level and the gene expression level to control neuronal development. First, both MeCP2 and CREB proteins are dynamically regulated via O-GlcNAcylation to stimulate normal dendrite and spine growth [78]. Second, neural activity may result in increased association of MeCP2 with CREB1 [80], and the coupling between T203 O-GlcNAcylated MeCP2 and Bdnf exon IV promoter, as well as the dissociation between phosphorylated MeCP2 and the Bdnf exon III promoter to promote Bdnf transcription [17]. It should be noted that although the T203 residue is O-GlcNAcylated on hMeCP2 protein and T203M mutation shows abnormal phenotypes both in vivo and in vitro, given the complexity and diversity of protein PTM, so far, we cannot totally rule out a non-O-GlcNAcylation mechanism underlying the phenotypes and decreased neuronal activity-induced induction of Bdnf transcription in hMeCP2 T203M mutant neurons. Together, our data and previous results indicate that, upon neuronal activation, O-GlcNAcylation of MeCP2 may up-regulate the CREB–BDNF signaling pathway via multiple mechanisms to promote neuronal development and synaptic plasticity, with T203 as a direct binding partner of the Bdnf promoter.
As previously noted, given the prevalence of PTMs on MeCP2, it is interesting to further consider the role of O-GlcNAc among other forms of PTM, especially in the context of RTT. Thus far, the best characterized PTM of MeCP2 protein is its phosphorylation [17–20], and deregulation of MeCP2 phosphorylation may be involved in the pathogenesis of RTT [69, 70]. Interestingly, O-GlcNAcylation has been closely linked to phosphorylation as both modifications can occur at the same or adjacent sites [82], and functional interaction between both modifications has also been previously characterized [83]. However, in this study, our MS assay did not identify the O-GlcNAc site T203 also as a phosphorylation site (Figs. 1 and S1), suggesting that the neurodevelopmental regulatory effects are likely due to O-GlcNAcylation at T203, rather than phosphorylation. In addition, we analyzed the effects of T203M deglycosylation on the phosphorylation of MeCP2 S421 and S80 [18, 19], but did not find any significant changes in the phosphorylation level on either site (Fig. S7). Thus, it is possible that T203 O-GlcNAcylation affects neuronal development and activity-dependent transcription by independently recruiting a specific set of molecular coactivators to target gene promoters. Given the complexity of various genetic mutations in the pathogenesis of RTT [33], and that some mutations impact PTM sites [33], delineating the effects of disease-related PTM sites and their interactions may lead to novel therapeutic targets for reversing the neural deficiencies in RTT.
In this study, we identified several previously unknown O-GlcNAcylation sites on hMeCP2 and revealed novel functions of T203 O-GlcNAc modification in the regulation of neuronal development and synaptic transmission. Furthermore, we provided mechanistic insight into the downstream signaling of MeCP2 T203 O-GlcNAcylation, adding to our understanding of the complex signaling network following MeCP2 PTMs in mediating neuronal activity-dependent transcription. Future work will need to further map out major relevant molecular pathways affected by MeCP2 T203 O-GlcNAcylation and their possible involvement in the pathogenesis of RTT disorders.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Zilong Qiu at the Institute of Neuroscience, the Chinese Academy of Sciences, Dr. Huadong Pei at the National Center for Protein Sciences (Beijing), and Dr. Ke-Ping Hu at the Chinese Academy of Medical Sciences and Peking Union Medical College for providing the valuable reagents for this work. We also thank all members of the Wu laboratory for discussion. This work was supported by the National Natural Science Foundation of China (31770929 and 31522029), and the Beijing Municipal Science and Technology Commission (Z181100001518001 and Z161100000216154) of China.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Footnotes
Juanxian Cheng, Zhe Zhao contributed equally to this work.
Contributor Information
Xiaotao Duan, Email: xduan@ncba.ac.cn.
Haitao Wu, Email: wuht@bmi.ac.cn.
References
- 1.Laurvick CL, Msall ME, Silburn S, Bower C, de Klerk N, Leonard H. Physical and mental health of mothers caring for a child with Rett syndrome. Pediatrics 2006, 118: e1152–e1164. [DOI] [PubMed]
- 2.Zoghbi HY. Rett syndrome and the ongoing legacy of close clinical observation. Cell 2016, 167: 293–297. [DOI] [PubMed]
- 3.Amir RE, van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 1999, 23: 185–188. [DOI] [PubMed]
- 4.Neul JL, Fang P, Barrish J, Lane J, Caeg EB, Smith EO, et al. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology 2008, 70: 1313–1321. [DOI] [PMC free article] [PubMed]
- 5.Bienvenu T, Carrié A, de Roux N, Vinet MC, Jonveaux P, Couvert P, et al. MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum Mol Genet 2000, 9: 1377–1384. [DOI] [PubMed]
- 6.van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet 2005, 77: 442–453. [DOI] [PMC free article] [PubMed]
- 7.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 2001, 27: 327–331. [DOI] [PubMed]
- 8.Tate P, Skarnes W, Bird A. The methyl-CpG binding protein MeCP2 is essential for embryonic development in the mouse. Nat Genet 1996, 12: 205–208. [DOI] [PubMed]
- 9.Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 2010, 143: 527–539. [DOI] [PMC free article] [PubMed]
- 10.Li Y, Wang H, Muffat J, Cheng AW, Orlando DA, Lovén J, et al. Global transcriptional and translational repression in human-embryonic-stem-cell-derived Rett syndrome neurons. Cell Stem Cell 2013, 13: 446–458. [DOI] [PMC free article] [PubMed]
- 11.Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, et al. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol Dis 2009, 35: 219–233. [DOI] [PMC free article] [PubMed]
- 12.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science 2007, 315: 1143–1147. [DOI] [PMC free article] [PubMed]
- 13.Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci 2006, 26: 319–327. [DOI] [PMC free article] [PubMed]
- 14.Na ES, Nelson ED, Kavalali ET, Monteggia LM. The impact of MeCP2 loss- or gain-of-function on synaptic plasticity. Neuropsychopharmacology 2013, 38: 212–219. [DOI] [PMC free article] [PubMed]
- 15.Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet 2004, 13: 2679–2689. [DOI] [PubMed]
- 16.Liu Z, Li X, Zhang JT, Cai YJ, Cheng TL, Cheng C, et al. Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature 2016, 530: 98–102. [DOI] [PubMed]
- 17.Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 2003, 302: 885–889. [DOI] [PubMed]
- 18.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 2003, 302: 890–893. [DOI] [PubMed]
- 19.Tao J, Hu K, Chang Q, Wu H, Sherman NE, Martinowich K, et al. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc Natl Acad Sci U S A 2009, 106: 4882–4887. [DOI] [PMC free article] [PubMed]
- 20.Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 2006, 52: 255–269. [DOI] [PMC free article] [PubMed]
- 21.Rutlin M, Nelson SB. MeCP2: phosphorylated locally, acting globally. Neuron 2011, 72: 3–5. [DOI] [PubMed]
- 22.Olivier-van Stichelen S, Wang P, Comly M, Love DC, Hanover JA. Nutrient-driven O-linked N-acetylglucosamine (O-GlcNAc) cycling impacts neurodevelopmental timing and metabolism. J Biol Chem 2017, 292: 6076–6085. [DOI] [PMC free article] [PubMed]
- 23.Lagerlöf O, Hart GW, Huganir RL. O-GlcNAc transferase regulates excitatory synapse maturity. Proc Natl Acad Sci U S A 2017, 114: 1684–1689. [DOI] [PMC free article] [PubMed]
- 24.Hart GW. Three decades of research on O-GlcNAcylation - A major nutrient sensor that regulates signaling, transcription and cellular metabolism. Front Endocrinol (Lausanne) 2014, 5: 183. [DOI] [PMC free article] [PubMed]
- 25.Bond MR, Hanover JA. A little sugar goes a long way: The cell biology of O-GlcNAc. J Cell Biol 2015, 208: 869–880. [DOI] [PMC free article] [PubMed]
- 26.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007, 446: 1017–1022. [DOI] [PubMed]
- 27.Rexach JE, Clark PM, Hsieh-Wilson LC. Chemical approaches to understanding O-GlcNAc glycosylation in the brain. Nat Chem Biol 2008, 4: 97–106. [DOI] [PMC free article] [PubMed]
- 28.Parween S, Varghese DS, Ardah MT, Prabakaran AD, Mensah-Brown E, Emerald BS, et al. Higher O-GlcNAc levels are associated with defects in progenitor proliferation and premature neuronal differentiation during in-vitro human embryonic cortical neurogenesis. Front Cell Neurosci 2017, 11: 415. [DOI] [PMC free article] [PubMed]
- 29.Wheatley EG, Albarran E, White CW 3rd, Bieri G, Sanchez-Diaz C, Pratt K, et al. Neuronal O-GlcNAcylation improves cognitive function in the aged mouse brain. Curr Biol 2019, 29: 3359–3369.e4. [DOI] [PMC free article] [PubMed]
- 30.Wang ZH, Udeshi ND, O'Malley M, Shabanowitz J, Hunt DF, Hart GW. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteomics 2010, 9: 153–160. [DOI] [PMC free article] [PubMed]
- 31.Rexach JE, Rogers CJ, Yu SH, Tao JF, Sun YE, Hsieh-Wilson LC. Quantification of O-glycosylation stoichiometry and dynamics using resolvable mass tags. Nat Chem Biol 2010, 6: 645–651. [DOI] [PMC free article] [PubMed]
- 32.Krishnaraj R, Ho G, Christodoulou J. RettBASE: Rett syndrome database update. Hum Mutat 2017, 38: 922–931. [DOI] [PubMed]
- 33.Bellini E, Pavesi G, Barbiero I, Bergo A, Chandola C, Nawaz MS, et al. MeCP2 post-translational modifications: A mechanism to control its involvement in synaptic plasticity and homeostasis? Front Cell Neurosci 2014, 8: 236. [DOI] [PMC free article] [PubMed]
- 34.Kharrat M, Triki C, Maalej M, Ncir S, Ammar M, Kammoun F, et al. First description of an unusual novel double mutation in MECP2 co-occurring with the m.827A>G mutation in the MT-RNR1 gene associated with angelman-like syndrome. Int J Dev Neurosci 2019, 79: 37–44. [DOI] [PubMed]
- 35.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002, 295: 868–872. [DOI] [PubMed]
- 36.Rao XJ, Duan XT, Mao WM, Li XX, Li ZH, Li Q, et al. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat Commun 2015, 6: 8468. [DOI] [PMC free article] [PubMed]
- 37.Peng CM, Zhu Y, Zhang WJ, Liao QC, Chen YL, Zhao XY, et al. Regulation of the hippo-YAP pathway by glucose sensor O-GlcNAcylation. Mol Cell 2017, 68: 591–604.e5. [DOI] [PubMed]
- 38.Navarro-Quiroga I, Chittajallu R, Gallo V, Haydar TF. Long-term, selective gene expression in developing and adult hippocampal pyramidal neurons using focal in utero electroporation. J Neurosci 2007, 27: 5007–5011. [DOI] [PMC free article] [PubMed]
- 39.Wu HT, Barik A, Lu YS, Shen CY, Bowman A, Li L, et al. Slit2 as a β-catenin/Ctnnb1-dependent retrograde signal for presynaptic differentiation. Elife 2015, 4. DOI:10.7554/eLife.07266. [DOI] [PMC free article] [PubMed]
- 40.Yang HH, Zhu Q, Cheng JX, Wu Y, Fan M, Zhang JY, et al. Opposite regulation of Wnt/β-catenin and Shh signaling pathways by Rack1 controls mammalian cerebellar development. Proc Natl Acad Sci U S A 2019, 116: 4661–4670. [DOI] [PMC free article] [PubMed]
- 41.Yang HH, Yang CJ, Zhu Q, Wei MP, Li Y, Cheng JX, et al. Rack1 controls parallel fiber-Purkinje cell synaptogenesis and synaptic transmission. Front Cell Neurosci 2019, 13: 539. [DOI] [PMC free article] [PubMed]
- 42.Lombardi LM, Baker SA, Zoghbi HY. MECP2 disorders: From the clinic to mice and back. J Clin Invest 2015, 125: 2914–2923. [DOI] [PMC free article] [PubMed]
- 43.Trinidad JC, Barkan DT, Gulledge BF, Thalhammer A, Sali A, Schoepfer R, et al. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol Cell Proteomics 2012, 11: 215–229. [DOI] [PMC free article] [PubMed]
- 44.Gao J, Yang Y, Qiu RF, Zhang K, Teng X, Liu RQ, et al. Proteomic analysis of the OGT interactome: Novel links to epithelial-mesenchymal transition and metastasis of cervical cancer. Carcinogenesis 2018, 39: 1222–1234. [DOI] [PMC free article] [PubMed]
- 45.Thompson JW, Griffin ME, Hsieh-Wilson LC. Methods for the detection, study, and dynamic profiling of O-GlcNAc glycosylation. Methods Enzymol 2018, 598: 101–135. [DOI] [PMC free article] [PubMed]
- 46.Cheng TL, Wang ZZ, Liao QM, Zhu Y, Zhou WH, Xu WQ, et al. MeCP2 suppresses nuclear microRNA processing and dendritic growth by regulating the DGCR8/Drosha complex. Dev Cell 2014, 28: 547–560. [DOI] [PubMed]
- 47.Wang M, Li HP, Takumi T, Qiu ZL, Xu X, Yu X, et al. Distinct defects in spine formation or pruning in two gene duplication mouse models of autism. Neurosci Bull 2017, 33: 143–152. [DOI] [PMC free article] [PubMed]
- 48.Jiang MH, Ash RT, Baker SA, Suter B, Ferguson A, Park J, et al. Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. J Neurosci 2013, 33: 19518–19533. [DOI] [PMC free article] [PubMed]
- 49.Yu B, Yuan B, Dai JK, Cheng TL, Xia SN, He LJ, et al. Reversal of social recognition deficit in adult mice with MECP2 duplication via normalization of MeCP2 in the medial prefrontal cortex. Neurosci Bull 2020, 36: 570–584. [DOI] [PMC free article] [PubMed]
- 50.Nakashima H, Tsujimura K, Irie K, Imamura T, Trujillo CA, Ishizu M, et al. MeCP2 controls neural stem cell fate specification through miR-199a-mediated inhibition of BMP-Smad signaling. Cell Rep 2021, 35: 109124. [DOI] [PubMed]
- 51.Wood L, Gray NW, Zhou ZL, Greenberg ME, Shepherd GM. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in an RNA interference model of methyl-CpG-binding protein 2 deficiency. J Neurosci 2009, 29: 12440–12448. [DOI] [PMC free article] [PubMed]
- 52.Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 2007, 56: 58–65. [DOI] [PMC free article] [PubMed]
- 53.Yanagida M, Miyoshi R, Toyokuni R, Zhu Y, Murakami F. Dynamics of the leading process, nucleus, and Golgi apparatus of migrating cortical interneurons in living mouse embryos. Proc Natl Acad Sci U S A 2012, 109: 16737–16742. [DOI] [PMC free article] [PubMed]
- 54.Belichenko PV, Oldfors A, Hagberg B, Dahlström A. Rett syndrome: 3-D confocal microscopy of cortical pyramidal dendrites and afferents. Neuroreport 1994, 5: 1509–1513. [PubMed]
- 55.Sala C, Segal M. Dendritic spines: The locus of structural and functional plasticity. Physiol Rev 2014, 94: 141–188. [DOI] [PubMed]
- 56.Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z Zellforsch Mikrosk Anat 1969, 100: 487–506. [DOI] [PubMed]
- 57.Neul JL, Zoghbi HY. Rett syndrome: A prototypical neurodevelopmental disorder. Neuroscientist 2004, 10: 118–128. [DOI] [PubMed]
- 58.Blackman MP, Djukic B, Nelson SB, Turrigiano GG. A critical and cell-autonomous role for MeCP2 in synaptic scaling up. J Neurosci 2012, 32: 13529–13536. [DOI] [PMC free article] [PubMed]
- 59.Qiu Z, Sylwestrak EL, Lieberman DN, Zhang Y, Liu XY, Ghosh A. The Rett syndrome protein MeCP2 regulates synaptic scaling. J Neurosci 2012, 32: 989–994. [DOI] [PMC free article] [PubMed]
- 60.Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J Neurosci 2009, 29: 11263–11270. [DOI] [PMC free article] [PubMed]
- 61.Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol Psychiatry 2010, 67: 657–665. [DOI] [PubMed]
- 62.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci 2001, 2: 24–32. [DOI] [PubMed]
- 63.Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 2008, 319: 1683–1687. [DOI] [PMC free article] [PubMed]
- 64.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the Hippocampus. Nature 1996, 381: 706–709. [DOI] [PubMed]
- 65.Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience 2013, 239: 34–45. [DOI] [PMC free article] [PubMed]
- 66.Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 1998, 20: 727–740. [DOI] [PubMed]
- 67.Tao X, West AE, Chen W, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron 2002, 33: 383–395. [DOI] [PubMed]
- 68.Tai DJ, Liu YC, Hsu WL, Ma YL, Cheng SJ, Liu SY, et al. MeCP2 SUMOylation rescues Mecp2-mutant-induced behavioural deficits in a mouse model of Rett syndrome. Nat Commun 2016, 7: 10552. [DOI] [PMC free article] [PubMed]
- 69.Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, et al. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron 2011, 72: 72–85. [DOI] [PMC free article] [PubMed]
- 70.Ebert DH, Gabel HW, Robinson ND, Kastan NR, Hu LS, Cohen S, et al. Activity-dependent phosphorylation of MeCP2 threonine 308 regulates interaction with NCoR. Nature 2013, 499: 341–345. [DOI] [PMC free article] [PubMed]
- 71.Pandey S, Simmons GE Jr, Malyarchuk S, Calhoun TN, Pruitt K. A novel MeCP2 acetylation site regulates interaction with ATRX and HDAC1. Genes Cancer 2015, 6: 408–421. [DOI] [PMC free article] [PubMed]
- 72.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325: 834–840. [DOI] [PubMed]
- 73.Lefebvre T, Guinez C, Dehennaut V, Beseme-Dekeyser O, Morelle W, Michalski JC. Does O-GlcNAc play a role in neurodegenerative diseases? Expert Rev Proteomics 2005, 2: 265–275. [DOI] [PubMed]
- 74.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: A mechanism involved in Alzheimer's disease. Proc Natl Acad Sci U S A 2004, 101: 10804–10809. [DOI] [PMC free article] [PubMed]
- 75.Bedogni F, Rossi RL, Galli F, Cobolli Gigli C, Gandaglia A, Kilstrup-Nielsen C, et al. Rett syndrome and the urge of novel approaches to study MeCP2 functions and mechanisms of action. Neurosci Biobehav Rev 2014, 46 Pt 2: 187–201. [DOI] [PubMed]
- 76.Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Exploring the O-GlcNAc proteome: Direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci U S A 2004, 101: 13132–13137. [DOI] [PMC free article] [PubMed]
- 77.Tallent MK, Varghis N, Skorobogatko Y, Hernandez-Cuebas L, Whelan K, Vocadlo DJ, et al. In vivo modulation of O-GlcNAc levels regulates hippocampal synaptic plasticity through interplay with phosphorylation. J Biol Chem 2009, 284: 174–181. [DOI] [PubMed]
- 78.Rexach JE, Clark PM, Mason DE, Neve RL, Peters EC, Hsieh-Wilson LC. Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation. Nat Chem Biol 2012, 8: 253–261. [DOI] [PMC free article] [PubMed]
- 79.Khidekel N, Ficarro SB, Clark PM, Bryan MC, Swaney DL, Rexach JE, et al. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol 2007, 3: 339–348. [DOI] [PubMed]
- 80.Chahrour M, Jung SY, Shaw C, Zhou XB, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320: 1224–1229. [DOI] [PMC free article] [PubMed]
- 81.Bu Q, Wang AX, Hamzah H, Waldman A, Jiang KE, Dong QP, et al. CREB signaling is involved in rett syndrome pathogenesis. J Neurosci 2017, 37: 3671–3685. [DOI] [PMC free article] [PubMed]
- 82.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu Rev Biochem 2011, 80: 825–858. [DOI] [PMC free article] [PubMed]
- 83.Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett 2010, 584: 2526–2538. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.