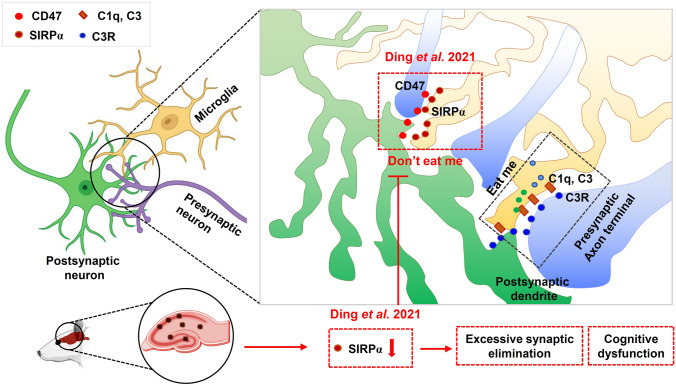
Microglia, the principal immune cells in the brain, play a vital role in the development and homeostasis of the central nervous system [1, 2]. During early brain development, microglia-mediated synapse pruning contributes to eliminating excess synapses that are unnecessary in adulthood [3]. Excessive microglia-mediated pruning in the adult brain is implicated in neurodegeneration-associated behavioral deficits [4, 5]. In a previous study, “eat me” and “don’t eat me” signals were described as positive and negative signals in modulating synaptic pruning [3, 6]. The complement cascade components C1q and C3 are essential components of the “eat me” signal and promote microglial engulfment of inappropriate and/or extraneous synapses by activating the microglial C3 receptor [7].
In contrast to the “eat me” signal, the ‘‘don’t eat me’’ signal serves as a negative regulator of synaptic pruning to avoid excessive elimination of synapses [7, 8]. Although a previous study published in Neuron has demonstrated that the “don’t eat me’’ signal CD47 and its receptor SIRPα protect synapses from excessive pruning during development in the dorsal lateral geniculate nucleus (dLGN), their findings mainly focused on the role of only the CD47 signal in synaptic pruning [7]. More work is still needed to investigate the effects of SIRPα. Although systemic knockout of SIRPα induces increased microglial pruning in the dLGN during neurodevelopment, the exact effect of the microglial SIRPα and SIRPα-CD47 signal axis in the brain and neurodegenerative diseases remains less clear. In a recent study that used a microglia-specific SIRPα conditional knockout mouse model, Ding et al. elucidated the role of microglial SIRPα in synaptic remodeling during neurodevelopment and demonstrated the important function of microglial SIRPα in neurodegenerative diseases [9].
A previous study has confirmed that CD47 is synaptically localized and enriched in the dLGN to prevent excessive synaptic elimination during postnatal development [7]. However, the expression and localization of SIRPα during developmental stages was unclear. Therefore, Ding et al. first analyzed the level of SIRPα expression in different brain cells [9]. Immunostaining results showed that SIRPα is mainly expressed in neurons and microglia. Microglial SIRPα expression levels were high at the early stage of neurodevelopment but decreased later. The changes in microglial SIRPα expression indicate that it helps to moderate synapse pruning during neurodevelopment (Fig. 1).
Fig. 1.
The “eat me” signal is the key to recognizing unnecessary or inappropriate synapses during neurodevelopment. In contrast, the ‘‘don’t eat me’’ signal serves as a negative regulator of synaptic pruning to avoid excessive elimination of synapses [4]. Using a microglial SIRPα conditional knockout mouse model, Ding et al. [9] provided evidence that SIRPα deletion induces significant synapse loss during early neurodevelopment. In addition, they found that the expression of microglial SIRPα significantly decreased in Alzheimer’s disease (AD) pathology and demonstrated that the decreased expression of microglial SIRPα resulted in excessive microglial engulfment of synapses and exacerbated the cognitive impairment in AD.
To further evaluate the role of microglial SIRPα in synapse pruning, brain slices from microglial SIRPα knockout mice were double-stained for pre- and post-synaptic proteins [9]. They found that loss of microglial SIRPα decreased the synaptic density without affecting the number and morphology of neurons at the early stage of neurodevelopment, indicating that this SIRPα-deficient decrease in synaptic density is not due to neuron growth defects at an early stage. Furthermore, they detected more synaptic puncta inside the microglia of SIRPα-knockout mice than control animals. This indicated elevated microglial phagocytosis of synaptic structures in SIRPα-knockout mice, and this was confirmed in a neuron-microglia in vitro cell co-culture system. Using a synaptosome-microglia co-culture system, they found that microglia in the control group preferred to engulf synaptosomes with a CD47 signal deficiency. In contrast, SIRPα-knockout microglia did not recognize CD47 deficiency and subsequently induced microglial phagocytosis, indicating that microglial SIRPα plays a crucial role in attenuating synaptic elimination. CD47-knockout mice further confirmed this conclusion by displaying a significantly decreased synaptic density.
The authors also analyzed the expression of CD47 in neurons at different time points and asked how synaptic CD47 expression was regulated [9]. Double staining of synaptic markers and CD47 demonstrated that CD47 is differentially expressed in synapses in an age-dependent pattern: the number of CD47-positive synapses was increased at postnatal day 30 compared with postnatal day 5. Because microglia prefer to engulf synapses from less active neurons [7], the authors hypothesized that the regulation of CD47 expression might be associated with neuronal activity. CD47 expression was measured following the inhibition of neuronal activity using the channel blocker clozapine-N-oxide (i.e., isoflurane). As expected, the expression of CD47 was markedly decreased in less active neurons, which facilitated their elimination.
Although the ''eat me'' signal C1q is significantly elevated and contributes to synaptic loss in neurodegenerative disease [10], the role of the “don’t eat me signal”, especially microglial SIRPα-CD47, in synapto-pathological diseases was unclear. Therefore, Ding et al. [9] next analyzed the role of microglial SIRPα in both human Alzheimer’s disease (AD) patients and AD mice. Their results showed that SIRPα expression was decreased in both AD patients and AD mice. To better understand the alteration of microglial SIRPα in AD, Aβ oligomer was incubated with primary microglia or injected into the brain, and this significantly decreased the expression of microglial SIRPα. Moreover, consistent with the alteration of microglial SIRPα, Aβ oligomer also inhibited CD47 expression in primary neurons, suggesting that the SIRPα-CD47 signal contributes to the loss of synapses in AD.
To further elucidate the role of SIRPα-CD47 signaling in AD pathology, cognitive function and typical AD pathology were measured in microglial SIRPα-knockout AD mice [9]. The authors found that loss of microglial SIRPα significantly augmented the cognitive deficits and synaptic loss at 5 and 8 months of age. No significant differences were found in the deposition of Aβ plaques between AD and microglial SIRPα-knockout AD mice, indicating that loss of SIRPα contributed to the cognitive decline and synaptic loss without affecting Aβ deposition. These results were further confirmed in an intracerebroventricular Aβ injection-induced AD mouse model with SIRPα deficiency [9]. Taken together, these findings suggest that dysfunction of the SIRPα-CD47-mediated “don’t eat me” signal contributes to the excessive synaptic elimination in AD.
Using a microglial SIRPα conditional knockout mouse model, Ding et al. [9] provided evidence that microglial-specific SIRPα deletion induced significant synapse loss during early neurodevelopment, which furthers our understanding of the SIRPα-CD47 complex. In addition, the authors found that the expression of microglial SIRPα significantly declined in AD pathology and demonstrated that this decline resulted in excessive microglial engulfment of synapses and exacerbated the cognitive impairment in AD. This fills a critical gap in our understanding of microglial SIRPα signaling in regulating synaptic pruning in neurodegeneration. Nevertheless, there remain several intriguing questions worth further investigation. First, another protein named SIRPβ1 also regulates macrophage phagocytosis and is expressed by microglia [11]. Different from SIRPα, microglial expression of SIRPβ1 increases in AD [11]. However, it is unclear how microglial-specific SIRPβ1 works during early neurodevelopment and AD. Second, SIRPα has been shown to negatively regulate macrophage phagocytosis, wherein activation of SIRPα usually reduces phagocytosis [11]. Therefore, another remaining question is why changes in microglial-specific SIRPα only affect the phagocytosis of synapses without affecting Aβ deposition. Third, a recent study found that astrocytic interleukin-3 (IL-3) programs microglia through targeting CD123, a specific receptor for IL-3, to induce microglial Aβ clustering and phagocytosis. Therefore, it is worth investigating whether astrocytes also regulate microglial SIRPα. Finally, studying the expression of SIRPα in different microglial subtypes (e.g., M1 and M2) is another possible direction in understanding the regulation of SIRPα-CD47-mediated signaling. Unraveling these mechanisms underlying microglial SIRPα regulation of synaptic loss and phagocytosis may help identify promising therapeutic options for synapto-pathological and neurodegenerative disease [12].
Acknowledgements
This research highlight was supported by the National Key Research and Development Program of China (2017YFB0403801), the National Natural Science Foundation of China (31971096 and 31771256) and the Sigma Xi Grants in Aid of Research (GIAR) program (G03152021115804390). We would like to acknowledge Dr. Lorelei Tucker from Augusta University for helping edit our manuscript.
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Chongyun Wu and Luodan Yang have contributed equally to this work.
References
- 1.Grassivaro F, Menon R, Acquaviva M, Ottoboni L, Ruffini F, Bergamaschi A, et al. Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. J Neurosci. 2020;40:784–795. doi: 10.1523/JNEUROSCI.1523-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duveau A, Bertin E, Boué-Grabot E. Implication of neuronal versus microglial P2X4 receptors in central nervous system disorders. Neurosci Bull. 2020;36:1327–1343. doi: 10.1007/s12264-020-00570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakai J. Core Concept: How synaptic pruning shapes neural wiring during development and possibly, in disease. Proc Natl Acad Sci U S A. 2020;117:16096–16099. doi: 10.1073/pnas.2010281117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S, Dissing-Olesen L, Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol. 2016;36:128–134. doi: 10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartels T, de Schepper S, Hong S. Microglia modulate neurodegeneration in Alzheimer's and Parkinson's diseases. Science. 2020;370:66–69. doi: 10.1126/science.abb8587. [DOI] [PubMed] [Google Scholar]
- 6.Scott-Hewitt N, Perrucci F, Morini R, Erreni M, Mahoney M, Witkowska A, et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 2020;39:e105380. doi: 10.15252/embj.2020105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron. 2018;100:120–134.e6. doi: 10.1016/j.neuron.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivest S. A 'don't eat me' immune signal protects neuronal connections. Nature. 2018;563:42–43. doi: 10.1038/d41586-018-07165-8. [DOI] [PubMed] [Google Scholar]
- 9.Ding X, Wang J, Huang MX, Chen ZP, Liu J, Zhang QP, et al. Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration. Nat Commun. 2021;12:2030. doi: 10.1038/s41467-021-22301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li SM, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang HY, Li FW, Yang YY, Chen J, Hu XM. SIRP/CD47 signaling in neurological disorders. Brain Res. 2015;1623:74–80. doi: 10.1016/j.brainres.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22:374–385. doi: 10.1038/s41593-018-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]