Abstract
Metastasis is responsible for most of the hepatocellular carcinoma (HCC)–associated death. However, its underlying mechanism has yet to be fully elucidated. Glycolysis-derived lactate has been shown to be a powerful regulator of cancer metastasis. Heat shock protein A12A (HSPA12A) encodes a novel member of HSP70 family. We have recently demonstrated that heat shock protein A12A (HSPA12A) inhibited renal cancer cell migration by suppressing lactate output and glycolytic activity, which were mediated by unstabilizing CD147 and promoting its degradation. By striking contrast, here we demonstrated that HSPA12A promoted migration of human HCC cells. Extracellular acidification, lactate export, and glycolytic activity in HCC cells were also promoted following HSPA12A overexpression. Further analysis revealed that HSPA12A interacted with MCT4 and increased its membrane localization, thereby promoting export of lactate generated from glycolysis; this led, ultimately, to HCC cell migration. Our results revealed the opposite effect of HSPA12A on migration of renal cancer cells and that of HCC cells. Of note, in contrast to the inhibitory effect on CD147 expression in renal cancer cells, we found that HSPA12A increased CD147 expression in HCC cells, indicating that the expression of CD147 might exist heterogeneity in different cancer cell types. Taken together, we identified HSPA12A as an activator of HCC migration, a role opposite to that of renal cancer cells. Inhibiting HSPA12A might be a potential therapeutic intervention for HCC metastasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-021-01251-z.
Keywords: Hepatocellular carcinoma, Metastasis, Heat shock protein A12A (HSPA12A), Monocarboxylate transporter 4 (MCT4), Lactate export
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent and life-threatening malignancies worldwide. The poor prognosis in HCC patients is strongly associated with extrahepatic and intrahepatic metastasis, even of those with curative surgical treatment (Hsu et al. 2018; Zhou et al. 2019). Intrahepatic metastasis indicates tumor invasion of the portal vein and its branches, tumor thrombi formation, thereby causing multiple metastases in the liver after thrombi falling off. Extrahepatic metastasis represents tumor spread outside the liver, mainly through blood, lymphatic, and planting metastasis. The median survival time of HCC patients with extrahepatic metastasis is less than 7 months (Zhang et al. 2019). Therefore, a more comprehensive understanding of HCC metastasis is urgently needed to develop effective intervention approach for reducing the risk of death from HCC metastasis.
Cancer cell invasion and metastasis are complex processes that involve many genetic alterations and the subsequent metabolic transitions (Budanov et al. 2010). Unlike most normal cells that utilize glucose through mitochondrial oxidative phosphorylation, tumor cells exhibit a pronounced Warburg effect which metabolizes glucose into lactate through glycolysis pathway even in non-hypoxic environments (Koppenol et al. 2011). The glycolysis-generated lactate is exported to create a local microenvironment that confers cancer cell growth, invasion, and metastasis. First, the extracellular lactate activates the expression of genes for cancer metastasis and angiogenesis as well as forms an acid environment for weakening the tumor-killing capacity of immune cells (Fischer et al. 2007; Gottfried et al. 2006; Vegran et al. 2011). Second, lactate efflux is essential for energy metabolism in order to ensure the progression of glycolysis and for pH regulation to prevent intracellular acidification of cancer cells. Monocarboxylate transporter 4 (MCT4) is the main transporter across the plasma membrane for lactate efflux. Therefore, targeting MCT4-mediated lactate efflux holds therapeutic promises for HCC metastasis. However, the regulation of MCT4 in HCC cells has not been clearly addressed.
Heat shock proteins (HSPs) are a group of ubiquitous molecular chaperones that exert versatile effects in conditions of stress. HSP90β and HSP27 are involved in HCC cell proliferation, migration, and invasion (Ge et al. 2017; Meng et al. 2019). Heat shock protein A12A (HSPA12A) is identified as a distant member of HSP70 family (Han et al. 2003). Though HSPA12A expresses at low level in normal liver, the expression is increased in HCC and non-alcoholic fatty liver (Cheng et al. 2020; Kong et al. 2019). The classical tumor node metastasis (TNM) staging system is based on tumor size, number of positive lymph nodes, and presence of distant metastasis (Pichler et al. 2013). Particularly, we have recently demonstrated a positive correlation between HSPA12A expression and TNM stage in HCC patients (Cheng et al. 2020), suggesting a possible role of HSPA12A in HCC progression. In supporting this hypothesis, we have shown that HSPA12A promotes HCC growth (Cheng et al. 2020). However, the role of HSPA12A in HCC metastasis remains unclear.
Intriguingly, we have recently demonstrated that HSPA12A inhibits migration of human renal carcinoma cells via destabilizing CD147 (Min et al. 2020). Unexpectedly, here in this study, we found that HSPA12A promoted the migration of HCC cells in an MCT4-dependent manner. The findings revealed that HSPA12A plays opposite roles in the migration process of HCC and that of renal cancer cells, and suggested that inhibiting HSPA12A might represent a meaningful approach for limiting HCC metastasis.
Materials and methods
Reagents and antibodies
Primary antibody for E-Cadherin, focal adhesion kinase (FAK), p-FAK, MMP2, and MMP9 were purchased from Bioworld Technology (Louis Park, MN). Primary antibody for Vimentin and IgG were from SantaCruz (Dallas, TX). Primary antibody against CD147, PKM2, and MCT4 were from Proteintech Group (Rosemont, IL). Primary antibody for HSPA12A, GLUT4, and LDHA was from Abcam (Cambridge, MA). Primary antibody for Integrin-β1, GLUT1, HK2, and PFKFB3 was from Cell Signaling Technology (Beverly, MA). Anti-flag and anti-α-tubulin antibodies were from Sigma-Aldrich (St. Louis, MO). Bovine serum albumin (BSA) was from Roche (Basel, Switzerland). Normal goat serum (NGS) and CyTM3-conjugated secondary antibody were from Jackson lmmunoResearch (West Grove, PA). Trizol reagent and Lipofectamine 3000 were from Life Technology (Carlsbad, CA). Dulbecco’s Modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were from Gibco (Shelton, CT). High-sig enhanced chemiluminescence (ECL) western blotting substrate was from Tanon (Shanghai, China).
Cell culture
Human HCC cell line HepG2 was purchased from the Type Culture Collection of the Chinese Academy of Sciences. Cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin.
Overexpression of HSPA12A
For gain-of-function experiments, HepG2 cells were infected with adenovirus (20 MOI) that carry 3 flag-tagged Hspa12a expression sequence (Ad-HSPA12A) for 16 h. HepG2 cells infected with empty virus served as normal controls (NCs). The construction of adenovirus vector was described previously (Kong et al. 2019; Liu et al. 2020).
Knockdown of MCT4
Followed by HSPA12A overexpression, HepG2 cells were transfected with the siRNA targeting homo MCT4 mRNA or a scrambled control (GenePharma Inc., China) using Lipofectamine 3000. The sense and antisense sequences of the siRNA were 5′-GCAUUGAAUUUGGUGCCUUTT-3′ and 5′-AAGGCACCAAAUUCAAUGCTT-3′, respectively.
Examination of migratory ability
The migratory ability of HepG2 cells was evaluated by wound healing assay. HepG2 cells were seeded in 6-well plates. After 50% confluent, cells were transfected with adenovirus and grown until totally confluent. A scratch was created by a same size pipette tip for each well. Cells were washed twice with PBS to remove floating cells. Afterwards, cells were cultured in the DMEM medium containing 1% FBS. At 0, 24, 48, and 72 h after scratching, images were obtained using a light microscope at × 10 magnification. The wound closure areas were measured using NIH ImageJ software (National Institutes of Health, Bethesda, MD).
Evaluation of pH and lactate
After adenovirus infection for 72 h, the medium pH of cell cultures was determined by microprocessor pH Meter (HANNA, Italy). For medium pH measurement, we quickly took out six-cell plate from the incubator, collected 2 ml of culture medium to a 10-ml centrifuge tube, and then measured medium pH with a pH probe within 2 min. The medium volume for measurement was 2 ml. The medium was then collected and centrifuged at 2000 rpm for 10 min at 4 °C. After then, the lactate contents in the medium were determined using a colorimetric kit (Jiancheng bioengineering institute, Nanjing, China). For intracellular lactate measurement, cells were lysed with lysis buffer and centrifuged. After centrifugation, the supernatant was collected for lactate content analysis using a lactate assay kit according to the manufacturer’s instruction.
Immunoblotting
HepG2 cells were collected and lysed after treatment. Equal amounts of protein extract were separated by 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore Corp., Bedford, MA). After being blocked in 5% skimmed milk for 1 h at room temperature, the membranes were then probed with according primary antibodies at 4 °C overnight. After thoroughly washing, membranes were incubated with peroxidase-conjugated secondary antibodies. For loading control, the same membranes were probed with anti-α-tubulin. The signals were quantified by scanning densitometry and the results from each experimental group were expressed as relative integrated intensity compared with that of controls.
Immunofluorescence staining
Immunofluorescence staining was performed on HepG2 cells that grow on coverslips. Briefly, after being blocked with 5% normal goat serum, the fixed cells were incubated with anti-MCT4 primary antibodies overnight at 4 °C followed by incubation with the CyTM3-conjugated secondary antibody at 37 °C for 1 h to visualize the staining. The dilution used for anti-MCT4 primary antibody and secondary antibody was 1:100 and 1:200, respectively. Hoechst 33,342 was used to counterstain nuclei. The staining was photographed by a fluorescence microscopy and quantified using Cellsens Dimention 1.15 software (Olympus, Tokyo, Japan).
Immunoprecipitation
HepG2 cells were overexpressed with flag-tagged HSPA12A. Protein extracts were pretreated with protein A agarose for 2 h at 4 °C to remove non-specific binding proteins. After centrifugation at 15,000 × g for 7 min at 4 °C, aliquots of equal volume and protein content were precipitated with anti-flag or anti-IgG antibodies (1 μg antibody:1 mg protein) overnight at 4 °C, followed by incubating with protein A agarose for 6 h at 4 °C. The fractions were then analyzed by mass spectrometry. In another set of experiments, the immunoprecipitates were immunoblotted against MCT4 and HSPA12A.
Mass spectrometry
Ad-HSPA12A or Ad-NC HepG2 cells were used for mass spectrometry analysis according to previous study (Kong et al. 2019). In brief, the above-obtained immunoprecipitates were separated by SDS-PAGE followed by Coomassie blue staining, digested in gel with trypsin, and analyzed by liquid chromatography-tandem mass spectrometry (LC–MS/MS). Peptides were dissolved in solvent A (2% FA in 3% ACN) and loaded directly onto a reversed-phase Trap column (Chrom XP C18-CL-3 m 120A, Eksigent). Peptide separation was performed using a reversed-phase analytical column (3C18-CL-120, 3um, 120A, Eksigent). Eluting peptides from the column were analyzed using an AB Sciex 5600 + TripleTOF™ system. MS/MS data were processed using ProteinPilotTM Software 4.5 (AB Sciex). Tandem mass spectra were searched against UniProt_Homo sapiens (160,566 sequences, released on April 9. 2016) database concatenated with reverse decoy database. Trypsin/P was specified as cleavage enzyme allowing up to 3 missing cleavages, 4 modifications per peptide, and 5 charges.
Quantitative real-time PCR
For cDNA synthesis, total RNA was isolated from cultured cells using Trizol Reagent. The quantitative RT-PCR analysis was performed with SYBR Green Master Mix on a Step One Plus real-time PCR System (Applied Biosystems). Gene expression levels were normalized to GAPDH. The primers used for PCR are listed in Supplementary Table S1.
Statistical analysis
Data are represented as mean ± standard deviation. Comparisons between groups were assessed by unpaired two-tailed Student t-test or two-way ANOVA followed by Bonferroni’s test as a post hoc test. Statistical significance was set at P value of < 0.05.
Results
HSPA12A promotes migratory ability of HCC cells
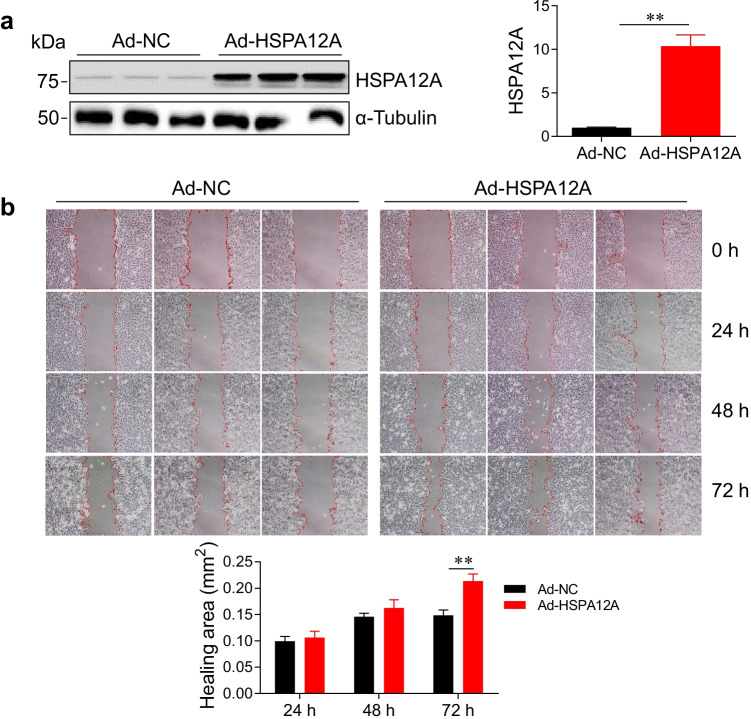
We have recently demonstrated that the levels of HSPA12A expression were positively correlated with HCC TNM stage (Cheng et al. 2020), suggesting that HSPA12A may play a role in the metastasis of HCC. To address this issue, we examined the effects of HSPA12A on HCC cell migration. To this end, HSPA12A was overexpressed (Ad-HSPA12A) in human HCC HepG2 cells by infection with Hspa12a-adenovirus, and the HepG2 cells infected with empty adenovirus served as negative controls (Ad-NC) (P < 0.01, Fig. 1a). The healing areas after wounding were used to evaluate the migratory ability of HCC cells. We found that at 72 h after wounding, the healing area was significantly increased by 43.8% in Ad-HSPA12A cells compared with the time-matched Ad-NC controls, indicating that HSPA12A promotes HCC cell migratory ability (P < 0.01, Fig. 1b).
Fig. 1.
HSPA12A promoted HCC cell migration. HepG2 cells were infected with Hspa12a-adenovirus to overexpress HSPA12A (Ad-HSPA12A). HepG2 cells infected with empty virus served as negative controls (Ad-NC). The following experiments were performed subsequently. a HSPA12A expression. Expression of HSPA12A was examined by immunoblotting. The blots against α-tubulin served as loading controls. All data were presented as mean ± SD. **P < 0.01, n = 4/group. b Wound-healing assay. Representative images show wound healing of Ad-NC and Ad-HSPA12A HepG2 cells at the indicated times after wounding. Red lines indicate the wound areas. Closure (healing) area was calculated as (wound area at 0 h) − (remaining wound area). All data were presented as mean ± SD. **P < 0.01, n = 3/group. Ad-NC, empty adenovirus-infected controls; Ad-HSPA12A, HSPA12A overexpression by infection with HSPA12A adenovirus
HSPA12A activates Integrin-β/focal adhesion kinase signaling
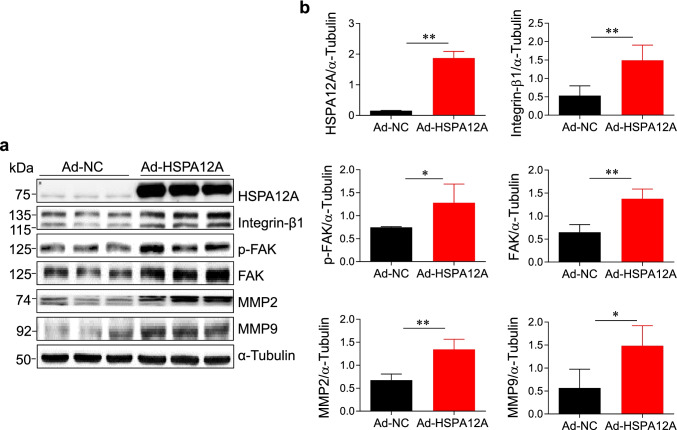
Given that Integrin-β/FAK signaling is an important regulator for tumor cell migration (Lee et al. 2015; Shen et al. 2019), effects of HSPA12A on Integrin-β/FAK/MMP signaling activation were examined by immunoblotting. We found that overexpression of HSPA12A in HepG2 cells significantly upregulated Integrin-β1 and FAK expression as well as increased FAK phosphorylation, respectively, when compared to those in NC controls (P < 0.01 or 0.05, Fig. 2). In line with it, the expression of matrix metalloproteinase-2 and -9 (MMP2 and MMP9), two downstream targets of Integrin-β/FAK signaling for cell migration, was also increased by HSPA12A overexpression (P < 0.01 or 0.05, Fig. 2).
Fig. 2.
a, b HSPA12A activated Integrin-β1/FAK signaling in HCC cells. HepG2 cells were infected with Hspa12a-adenovirus to overexpress HSPA12A (Ad-HSPA12A) and HepG2 cells infected with empty virus served as negative controls (Ad-NC). Seventy-two hours after infection, HepG2 cells were harvested and immunoblotted with the indicated primary antibodies. The blots against α-tubulin served as loading controls. All data were presented as mean ± SD. **P < 0.01 and *P < 0.05, n = 4/group. Ad-NC, empty adenovirus-infected controls; Ad-HSPA12A, HSPA12A overexpression by infection with HSPA12A adenovirus. FAK, Focal adhesion kinase; MMP, matrix metallopeptidase
HSPA12A enhances epithelial mesenchymal transition of HCC cells
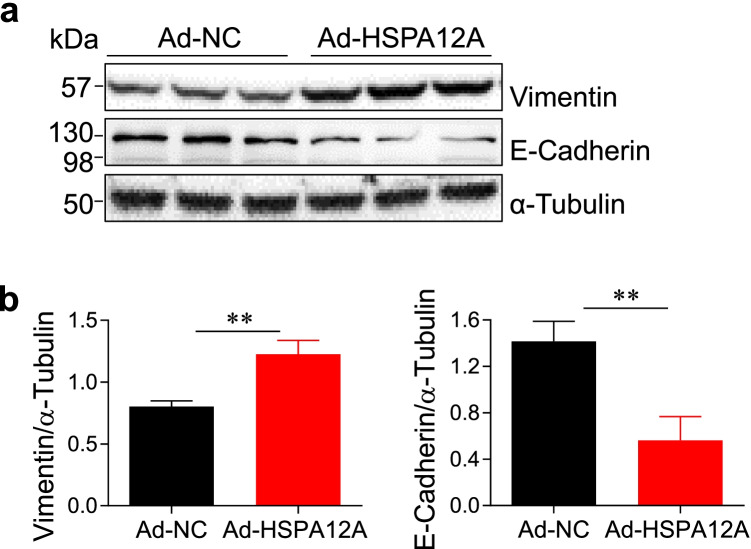
Epithelial mesenchymal transition (EMT), an evolutionarily conserved developmental program, confers metastatic properties of cancer cells by enhancing mobility and invasion (Mittal 2018). We therefore examined whether EMT is involved in the HSPA12A-induced promotion of HCC cell migration. Immunoblotting analysis revealed a significant increase of vimentin (mesenchymal maker) expression and a significant decrease of E-cadherin (epithelial marker) expression in Ad-HSPA12A HepG2 cells compared to Ad-NC controls, respectively (P < 0.01, Fig. 3). The findings indicate that HSPA12A enhances EMT of HCC cells.
Fig. 3.
a, b HSPA12A increased epithelial mesenchymal transition (EMT) of HCC cells. HepG2 cells with HSPA12A overexpression (Ad-HSPA12A) or normal expression (Ad-NC) were harvested for immunoblotting with the primary antibodies against vimentin and E-cadherin. The blots against α-tubulin served as loading controls. All data were presented as mean ± SD. **P < 0.01, n = 4/group for vimentin and n = 3/group for E-cadherin. Ad-NC, empty adenovirus-infected controls; Ad-HSPA12A, HSPA12A overexpression by infection with HSPA12A adenovirus
HSPA12A promotes lactate export and glycolytic activity in HCC cells
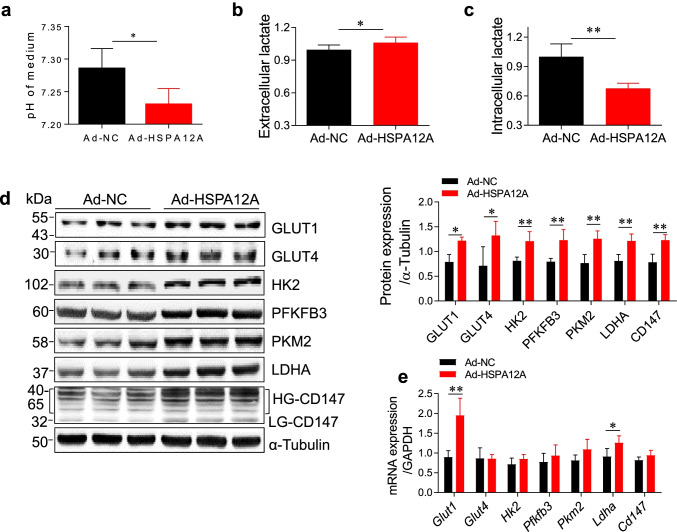
We then sought to address the underlying mechanism of HSPA12A-induced promotion of HCC cell migration. Lactate, once considered a waste product of glycolysis, has emerged as a critical regulator of cancer metastasis (Chen et al. 2017; Doherty and Cleveland 2013; Lin et al. 2018; Zhang et al. 2017). We found that the medium acidification and extracellular lactate contents of HepG2 cell cultures were increased by HSPA12A overexpression compared with those of NC controls (P < 0.05, Fig. 4a, b). By contrast, the intracellular lactate contents were decreased by HSPA12A overexpression in HepG2 cells compared with NC controls (P < 0.01, Fig. 4c). These results suggest that HSPA12A promotes lactate export of HCC cells.
Fig. 4.
HSPA12A enhanced lactate export and glycolytic activity in HCC cells. HepG2 cells were infected with Hspa12a-adenovirus to overexpress HSPA12A (Ad-HSPA12A) and HepG2 cells infected with empty virus served as negative controls (Ad-NC). Seventy-two hours after infection, the following measurements were performed. a Medium acidification. The medium pH values of HepG2 cell cultures were determined by microprocessor pH meter. All data were presented as mean ± SD. *P < 0.05, n = 4/group. b Extracellular lactate. The lactate contents in medium were determined using a colorimetric assay. All data were presented as mean ± SD. *P < 0.05, n = 6/group. c Intracellular lactate. HepG2 cells were lysed and subjected to colorimetric assay. Cell lysate protein concentration was determined for loading control. All data were presented as mean ± SD. **P < 0.01, n = 4 /group. d Protein expression of the genes linking to glycolysis. Expression of the indicated genes was determined by immunoblotting. The blots against α-tubulin served as loading controls. All data were presented as mean ± SD. **P < 0.01 and *P < 0.05, n = 4/group. e mRNA levels of the genes linking to glycolysis. mRNA expression of the indicated genes was determined by quantitative PCR experiment. The PCR results of GAPDH served as internal controls. All data were presented as mean ± SD. **P < 0.01 and *P < 0.05, n = 4/group. Ad-NC, empty adenovirus-infected controls; Ad-HSPA12A, HSPA12A overexpression by infection with HSPA12A adenovirus; GLUT1, glucose transporter 1; GLUT4, glucose transporter type 4; HK2, hexokinase 2; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; LDHA, lactate dehydrogenase A; HG-CD147, high-glycosylation CD147; LG-CD147, low-glycosylation CD147
Enhanced efflux of lactate can activate glycolytic pathway (Updegraff et al. 2018); we therefore further analyze the effect of HSPA12A on HCC glycolytic activity, and expression of the genes linking to glycolysis was examined. Overexpression of HSPA12A in HepG2 cells upregulated the protein expression of genes for glucose uptake (GLUT1 and GLUT4), glycolysis (HK2, PFKFB3, PKM2, and LDHA), and lactate efflux co-factor (CD147) (P < 0.01 or 0.05, Fig. 4d). Moreover, mRNA levels of genes for GLUT1 and LDHA were increased following HSPA12A overexpression (P < 0.01 or 0.05, Fig. 4e). The findings suggest that HSPA12A promotes glycolysis of HCC cells.
HSPA12A forms complex with MCT4 in HCC cells
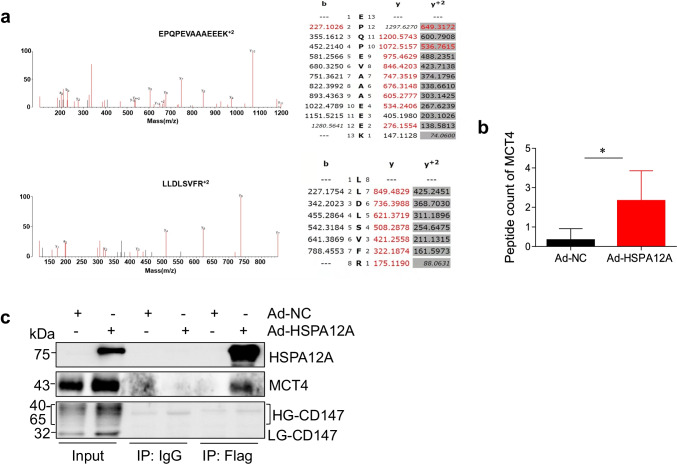
To investigate how HSPA12A promoted lactate export and glycolysis of HCC cells, an unbiased screen was performed. To this end, flag-tagged HSPA12A was immunoprecipitated from Ad-HSPA12A HepG2 cells for mass spectrometry. The results indicated an interaction between HSPA12A and MCT4, a key monocarboxylate transporter of lactate export (P < 0.05, Fig. 5a, b). To further verify the interaction between HSPA12A and MCT4, we performed immunoprecipitation-immunoblotting assay. Indeed, MCT4 protein was recovered in HSPA12A immunoprecipitates of HepG2 cells (Fig. 5c). However, no interaction between HSPA12A and lactate efflux co-factor CD147 was detected. The data indicate that HSPA12A interacts with MCT4 in HCC cells.
Fig. 5.
HSPA12A formed complex with MCT4 in HCC cells. a Mass spectrometry. HepG2 cells were overexpressed with the flag-tagged HSPA12A (Ad-HSPA12A) or control flag adenovirus (Ad-NC). Cellular protein extracts were immunoprecipitated with primary antibody for flag. The immunoprecipitates were subjected to mass spectrometric analysis. The MS/MS and main sequence ions of representative MCT4 peptide EPQPEVAAAEEEK + 2 (m/z: 713.8378) and LLDLSVFR + 2 (m/z: 401.7570) were shown. b Comparison of peptide count of MCT4 by mass spectrometry. All data were presented as mean ± SD. *P < 0.05, n = 3/group. c Immunoprecipitation-immunoblotting analysis. HepG2 cells were overexpressed with HSPA12A by infection with flag-tagged HSPA12A adenovirus (Ad-HSPA12A) or control flag adenovirus (Ad-NC) for 72 h. Immunoprecipitation with flag was performed. The immunoprecipitates were immunoblotted for HSPA12A, MCT4, and CD147. Protein extracts without immunoprecipitation (input) served as positive controls, and immunoprecipitates from IgG incubation served as negative controls. Note that MCT4 was recovered in the HSPA12A immunoprecipitates. Ad-NC, empty adenovirus-infected controls; Ad-HSPA12A, HSPA12A overexpression by infection with HSPA12A adenovirus; MCT4, monocarboxylate transporter 4; HG-CD147, high-glycosylation CD147; LG-CD147, low-glycosylation CD147
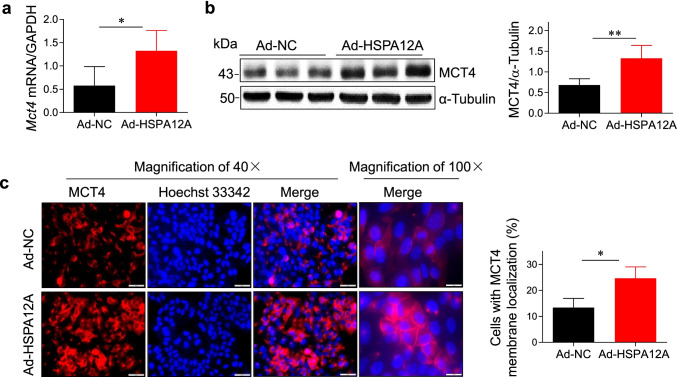
HSPA12A increases MCT4 expression and cell membrane localization of HCC cells
The interaction between HSPA12A and MCT4 motivated us to investigate whether MCT4 was involved in the HSPA12A-induced promotion of HCC cell migration. We therefore evaluated the effect of HSPA12A on MCT4 expression in HepG2 cells. As shown in Fig. 6a and b, HSPA12A upregulated MCT4 expression at both mRNA and protein levels in HepG2 cells, respectively, when compared to the Ad-NC controls (P < 0.01 or P < 0.05).
Fig. 6.
HSPA12A increased MCT4 expression and cell membrane localization of HCC cells. HepG2 cells were infected with Hspa12a-adenovirus to overexpress HSPA12A (Ad-HSPA12A) and HepG2 cells infected with empty virus served as negative controls (Ad-NC). Seventy-two hours after infection, the following measurements were performed. a MCT4 mRNA expression. The mRNA levels of MCT4 in HepG2 cells were determined by quantitative PCR. The PCR results of GAPDH served as internal controls. All data were presented as mean ± SD. *P < 0.05, n = 5/group. b MCT4 protein expression. The protein levels of MCT4 in HepG2 cells were evaluated by immunoblotting. The blots against α-tubulin served as loading controls. All data were presented as mean ± SD. **P < 0.01, n = 4/group. c Immunofluorescence. The HepG2 cells were immunofluorescence stained with MCT4 (red). Hoechst 33,342 was used to counterstain nuclei (blue). Cells with MCT4 membrane localization were quantified. Scale bar = 50 μm (magnification × 40), or 20 μm (magnification × 100). All data were presented as mean ± SD. *P < 0.05, n = 3/group. Ad-NC, empty adenovirus-infected controls; Ad-HSPA12A, HSPA12A overexpression by infection with HSPA12A adenovirus; MCT4, monocarboxylate transporter 4
Lactate is transported across the cell membrane mainly through the membrane-localized MCT4; we then examined the effect of HSPA12A on MCT4 membrane localization of HepG2 cells. The results of immunofluorescence staining revealed that membrane MCT4 was significantly increased in Ad-HSPA12A HepG2 cells compared with Ad-NC controls (P < 0.05, Fig. 6c). Altogether, the results suggest that HSPA12A increases MCT4 expression and plasma membrane localization of HCC cells.
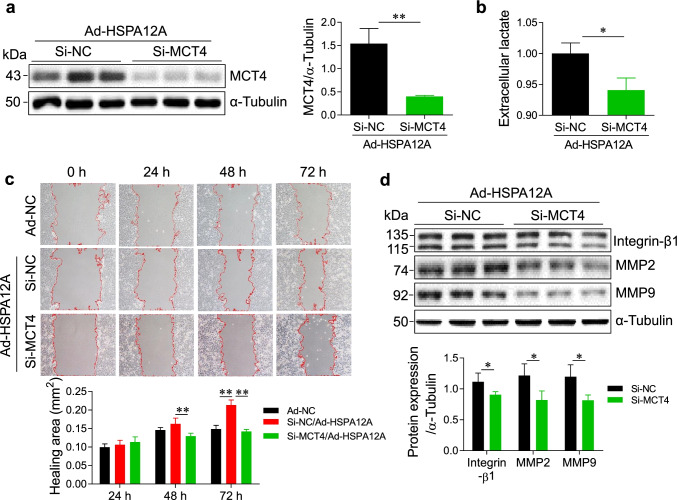
Knockdown of MCT4 inhibits the HSPA12A-induced HCC cell migration
To determine whether the HSPA12A-induced HCC cell migration is mediated by MCT4, we detected the effects of MCT4 knockdown on HSPA12A-induced HCC migration. To this end, we knocked down MCT4 in Ad-HSPA12A HepG2 cells by transfection with MCT4-targeted siRNA while the Ad-HSPA12A HepG2 cells transfected with scrambled RNA served as the negative controls (Si-NC) (P < 0.05, Fig. 7a). We found that knockdown of MCT4 reduced extracellular lactate contents in Ad-HSPA12A HepG2 cells, suggesting that MCT4 knockdown inhibits the HSPA12A-induced lactate output (P < 0.05, Fig. 7b). Importantly, the wound-healing assay showed that at 72 h after wounding, knockdown of MCT4 reversed HSPA12A-overexpressing induced promotion of wound closure (P < 0.01, Fig. 7c). Concomitantly, the protein expression levels of Integrin-β1, MMP2, and MMP9 in Ad-HSPA12A HepG2 cells were reduced following MCT4 knockdown (P < 0.05, Fig. 7d). Collectively, the findings suggest that knockdown of MCT4 inhibits the HSPA12A-enhanced lactate efflux and migration of HCC cells (Fig. 8).
Fig. 7.
Knockdown of MCT4 mitigated the HSPA12A-induced lactate output and migration of HCC cells. HepG2 cells were infected with Hspa12a-adenovirus to overexpress HSPA12A (Ad-HSPA12A). Subsequently, the Ad-HSPA12A HepG2 cells were transfected with MCT4-targeted siRNA (Si-MCT4) to knockdown MCT4 expression or transfected with scramble control siRNA (Si-NC). Forty-eight hours after Si-RNA transfection, the following measurements were performed. a MCT4 expression. Expression of MCT4 was examined by immunoblotting. The blots against α-tubulin served as loading controls. All data were presented as mean ± SD. **P < 0.01, n = 3/group. b Extracelluar lactate contents. Measurement of extracelluar lactate levels was performed. All data were presented as mean ± SD. *P < 0.05, n = 3/group. c Wound-healing assay. Representative images showed wound healing of Ad-NC HepG2 cells, Si-NC and Si-MCT4 HepG2 cells with Ad-HSPA12A overexpression at the indicated times after wounding. Red lines indicate the wound areas. Closure (healing) area was calculated as (wound area at 0 h) − (remaining wound area). All data were presented as mean ± SD. **P < 0.01, n = 3/group. d Immunoblotting. Expressions of Integrin-β1, MMP2, and MMP9 were examined by immunoblotting. The blots against α-tubulin served as loading controls. All data were presented as mean ± SD. *P < 0.05, n = 4/group. Ad-NC, empty adenovirus-infected controls; Ad-HSPA12A, HSPA12A overexpression by infection with HSPA12A adenovirus; MCT4, monocarboxylate transporter 4; Si-NC, cells transfected with scrambled siRNA; Si-MCT4, cells transfected with MCT4-targeted siRNA; MMP, Matrix metallopeptidase
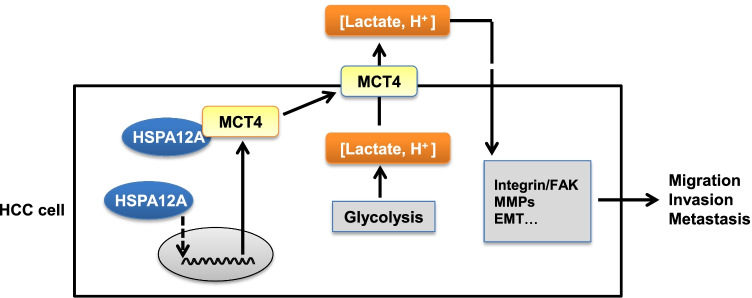
Fig. 8.
Mechanistic scheme. HSPA12A upregulates MCT4 expression, interacts with MCT4, as well as increases MCT4 localization on cell membrane, thereby facilitates the efflux of lactate that generated from glycolysis, and ultimately promotes the migration of HCC cells
Discussion
We have recently demonstrated that HSPA12A inhibits migration of renal cancer cells (Min et al. 2020). By striking contrast, we found in this study that the migration of HCC cells was promoted by HSPA12A. Our findings revealed that HSPA12A plays opposite roles in the migration process of HCC and that of renal cancer cells. The findings indicate that targeting HSPA12A expression could be a potential strategy for controlling HCC metastasis.
Owing to the tumor-associated local inflammation, ischemia, hypoxia, metabolic disorders, and other stresses, several HSPs are induced to play diverse roles in carcinogenesis (Hellman et al. 2004; Jolly and Morimoto 2000; Soderstrom et al. 2019; Tang et al. 2005; Toraih et al. 2019). For instance, HSP27, HSP70, and HSP90 promote the invasion and metastasis of liver cancer by activating Akt signaling pathway, NF-κB signaling pathway, or PKM2-mediated glucose metabolism (Gong et al. 2013; Xu et al. 2017; Zhang et al. 2016). As a distant member of HSP70 family, HSPA12A has been shown by our recent studies as a novel player for the development of nonalcoholic steatohepatitis as well as for the hepatic protection from LPS challenge (Kong et al. 2019; Liu et al. 2020). Of particular interest, we have demonstrated that HSPA12A mRNA level was higher by 3.14-fold in human HCC tissues than normal liver tissues. Also, we have shown that HSPA12A protein expression is significantly upregulated in HCC cell lines compared to normal hepatocytes and HSPA12A expression is positively correlated with tumor node metastasis (TNM) stage in HCC patients (Cheng et al. 2020), indicating a possible role of HSPA12A in HCC progression. This hypothesis is supported by the observation that HSPA12A promotes HCC cell proliferation through promoting PCNA trimerization (Cheng et al. 2020). However, the role of HSPA12A in HCC metastasis remains unclear. Here in this study, we demonstrated that HSPA12A promoted HCC cell migratory ability, suggesting that HSPA12A promotes HCC cell proliferation and migration to stimulate HCC progression.
We have previously demonstrated that HSPA12A inhibits migration of renal cancer cells through destabilizing CD147 (Min et al. 2020). CD147 is a glycoprotein that promotes MCT4 expression and localizing to cell membrane for lactate export (Eichner et al. 2016; Kirk et al. 2000; Updegraff et al. 2018). By striking contrast to that in renal cancer cells, we found that HSPA12A promoted migration as well as upregulated CD147 expression in HCC cells. Our findings are supported by previous studies which demonstrated that the same gene may play opposite roles in cancer pathogenesis. As examples, DNAJB6 promotes colorectal cancer cell invasion whereas decreases the malignancy, growth, and migration of breast cancer cells (Mitra et al. 2008; Zhang et al. 2015). Similarly, miR-939 acts as tumor suppressor in pediatric anaplastic large cell lymphoma whereas it acts as an activator in proliferation of human ovarian cancer cells (Garbin et al. 2020; Ying et al. 2015). The opposite roles of HSPA12A in the migration process of HCC and that of renal cancer cells may be related to the differential regulation of CD147 and MCT4 and the subsequent glucose metabolism alterations; however, the exact underlying controlling mechanisms is not clear and is needed to be further investigated in future studies.
The effects of tumor cell metabolism on metastasis have been outlined recently. In order to meet the needs of energy and biosynthesis, the expression and function of metabolic enzymes and transporters in tumor cells are altered, leading to increased glucose uptake, accelerated glycolysis process, and promoted lactate efflux (Shang et al. 2016). The increased lactate efflux to extracellular environment will stimulate tumor metastasis. As examples, lactate enhances motility of tumor cells in a concentration-dependent manner (Goetze et al. 2011). The lactate-induced extracellular acidification weakens the adhesion ability of tumor cells by regulating the interaction between integrin and collagen, leading to a predisposition for metastasis (Payen et al. 2016). Moreover, a 9-year follow-up survey indicated that lactate accumulation in primary cervical cancer was negatively correlated with survival of patients (Walenta et al. 2000). In this study, we found that HSPA12A increased the expression of genes linking to glucose uptake (GLUT1 and GLUT4) and glycolytic catalysis (HK2, PFKFB3, PKM2, and LDHA) in HCC cells. Importantly, HSPA12A increased extracellular lactate content and acidification, suggesting that the increased extracellular lactate and glycolysis is involved in the HSPA12A-induced HCC cell migration.
Following efflux from cells, intracellular lactate contents are decreased for ensuring the energy metabolism by glycolysis as well as for preventing intracellular acidification of cancer cells. Thus, lactate efflux is critical for both metastasis and survival of cancer cells. As a hydrophilic and a weak acid, lactate efflux across membranes is mediated by transporters that belong to the monocarboxylate transporter (MCT) family (Payen et al. 2019). Among the 14 members of this family, MCT4 presents in HCC cells at high level and has been shown promoting the migration of HCC cells. Moreover, MCT4 expression level is negatively correlated with the prognosis of HCC patients (Gao et al. 2015; Ohno et al. 2014). However, the regulation of MCT4 expression and its function in HCC has not been fully understood. Here, we demonstrated the following findings in HCC cells: (1) HSPA12A upregulated MCT4 expression at both mRNA and protein levels; (2) HSPA12A interacted with MCT4; (3) HSPA12A increased membrane localization of MCT4; (4) knockdown of MCT4 attenuated the HSPA12A-induced lactate efflux of HCC cells; (5) knockdown of MCT4 attenuated the HSPA12A-induced migration of HCC cells. Altogether, the data suggest that the HSPA12A-induced promotion of HCC migration is in an MCT4-dependent manner.
In conclusion, the present study identified HSPA12A as a novel stimulator of HCC cell migration in an MCT4-mediated mechanism. Also, our findings revealed that HSPA12A plays opposite roles in the migration process of HCC and that of renal cancer cells. The data support the potential utility of targeting HSPA12A as a therapeutic strategy for management of HCC metastasis. However, although rare, synchronous renal cell carcinoma and hepatocellular carcinoma is reported, so HSPA12A inhibition might promote renal cancer progression in those cases, although it inhibits spread of hepatocellular carcinoma cells. Further research may be deserved to solve this problem.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- HCC
Hepatocellular carcinoma
- HSPA12A
Heat shock protein A12A
- MCT4
Monocarboxylate transporter 4
- FAK
Focal adhesion kinase
- MMP
Matrix metallopeptidase
- GLUT1
Glucose transporter 1
- GLUT4
Glucose transporter type 4
- HK2
Hexokinase 2
- PFKFB3
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- LDHA
Lactate dehydrogenase A
- HG-CD147
High-glycosylation CD147
- LG-CD147
Low-glycosylation CD147
- Ad-NC
Empty adenovirus-infected controls
- Ad-HSPA12A
HSPA12A overexpression by infection with HSPA12A adenovirus
Author contribution
Z. D. conceived and supervised the study; Z. D. and L. L. designed the experiments; X. M., H. C., X. C., Z. C., X. Z., Q. M., B. X., and L. F. performed the experiments; X. M. and H. C. analyzed the data; Z. D. wrote the manuscript with contribution from all the authors.
Funding
This work was supported by the National Natural Science Foundation of China (81970692, 81870234, and 81770854), by the Jiangsu Province’s Outstanding Medical Academic Leader program (15), by a project funded by Collaborative Innovation Center for Cardiovascular Disease Translational Medicine, and by a Scientific Research Foundation for the introduction of talent, the First Affiliated Hospital with Wannan Medical College (YR202002). We also thank Translational Medicine Core Facilities, Medical School of Nanjing University, for the generous help in mass spectrum analysis.
Availability of data and material
All data presented in this study are available on request from the corresponding authors.
Code availability
Not applicable.
Declarations
Consent to participate
All authors have consented to participate.
Consent for publication
All authors have consented for publication.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinxu Min, Hao Cheng, and Xiaofei Cao contributed equally to this study.
References
- Garbin A, Lovisa F, Holmes AB, Damanti CC, Gallingani I, Carraro E, Accordi B, Veltri G, Pizzi M, d'Amore ESG, Pillon M, Biffi A, Basso K, Mussolin L. miR-939 acts as tumor suppressor by modulating JUNB transcriptional activity in pediatric anaplastic large cell lymphoma. Haematologica. 2020;2:610–613. doi: 10.3324/haematol.2019.241307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Fillmore RA, Metge BJ, Rajesh M, Xi Y, King J, Ju J, Pannell L, Shevde LA, Samant RS. Large isoform of MRJ (DNAJB6) reduces malignant activity of breast cancer. Breast Cancer Res. 2008;2:R22. doi: 10.1186/bcr1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno A, Yorita K, Haruyama Y, Kondo K, Kato A, Ohtomo T, Kawaguchi M, Marutuska K, Chijiiwa K, Kataoka H. Aberrant expression of monocarboxylate transporter 4 in tumour cells predicts an unfavourable outcome in patients with hepatocellular carcinoma. Liver Int. 2014;6:942–952. doi: 10.1111/liv.12466. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Lee JH, Karin M. Stressin’ Sestrins take an aging fight. EMBO Mol Med. 2010;10:388–400. doi: 10.1002/emmm.201000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff BL, Zhou X, Guo Y, Padanad MS, Chen PH, Yang C, Sudderth J, Rodriguez-Tirado C, Girard L, Minna JD, Mishra P, DeBerardinis RJ, O'Donnell KA. Transmembrane protease TMPRSS11B promotes lung cancer growth by enhancing lactate export and glycolytic metabolism. Cell Rep. 2018;8:2223–2233.e6. doi: 10.1016/j.celrep.2018.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther. 2015;146:132–149. doi: 10.1016/j.pharmthera.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;19:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Liu PH, Ho SY, Huang YH, Lee YH, Lee RC, Nagaria TS, Hou MC, Huo TI. Metastasis in patients with hepatocellular carcinoma: prevalence, determinants, prognostic impact and ability to improve the Barcelona Clinic Liver Cancer system. Liver Int. 2018;10:1803–1811. doi: 10.1111/liv.13748. [DOI] [PubMed] [Google Scholar]
- Tang D, Khaleque MA, Jones EL, Theriault JR, Li C, Wong WH, Stevenson MA, Calderwood SK. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;1:46–58. doi: 10.1379/csc-44r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraih EA, Alrefai HG, Hussein MH, Helal GM, Khashana MS, Fawzy MS. Overexpression of heat shock protein HSP90AA1 and translocase of the outer mitochondrial membrane TOM34 in HCV-induced hepatocellular carcinoma: A pilot study. Clin Biochem. 2019;63:10–17. doi: 10.1016/j.clinbiochem.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;5:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;7:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- Cheng H, Cao X, Min X, Zhang X, Kong Q, Mao Q, Li R, Xue B, Fang L, Liu L, Ding Z. Heat-shock protein A12A is a novel PCNA-binding protein and promotes hepatocellular carcinoma growth. FEBS J. 2020;24:5464–5477. doi: 10.1111/febs.15276. [DOI] [PubMed] [Google Scholar]
- Ge H, Du J, Xu J, Meng X, Tian J, Yang J, Liang H. SUMOylation of HSP27 by small ubiquitin-like modifier 2/3 promotes proliferation and invasion of hepatocellular carcinoma cells. Cancer Biol Ther. 2017;8:552–559. doi: 10.1080/15384047.2017.1345382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HJ, Zhao MC, Zhang YJ, Zhou DS, Xu L, Li GB, Chen MS, Liu J. Monocarboxylate transporter 4 predicts poor prognosis in hepatocellular carcinoma and is associated with cell proliferation and migration. J Cancer Res Clin Oncol. 2015;7:1151–1162. doi: 10.1007/s00432-014-1888-8. [DOI] [PubMed] [Google Scholar]
- H. K. Soderstrom, J. T. Kauppi, N. Oksala, T. Paavonen, L. Krogerus, J. Rasanen,T. Rantanen (2019) Overexpression of HSP27 and HSP70 is associated with decreased survival among patients with esophageal adenocarcinoma. World J Clin Cases 3:260–269. 10.12998/wjcc.v7.i3.260 [DOI] [PMC free article] [PubMed]
- Liu J, Du S, Kong Q, Zhang X, Jiang S, Cao X, Li Y, Li C, Chen H, Ding Z, Liu L. HSPA12A attenuates lipopolysaccharide-induced liver injury through inhibiting caspase-11-mediated hepatocyte pyroptosis via PGC-1alpha-dependent acyloxyacyl hydrolase expression. Cell Death Differ. 2020;9:2651–2667. doi: 10.1038/s41418-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Chen S, Lei YY, Han JX, Zhong WL, Wang XR, Liu YR, Gao WF, Zhang Q, Tan Q, Liu HJ, Zhou HG, Sun T, Yang C. Hsp90beta promotes aggressive vasculogenic mimicry via epithelial-mesenchymal transition in hepatocellular carcinoma. Oncogene. 2019;2:228–243. doi: 10.1038/s41388-018-0428-4. [DOI] [PubMed] [Google Scholar]
- Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;9:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang S, Jiang B, Huang L, Ji Z, Li X, Zhou H, Han A, Chen A, Wu Y, Ma H, Zhao W, Zhao Q, Xie C, Sun X, Zhou Y, Huang H, Suleman M, Lin F, Zhou L, Tian F, Jin M, Cai Y, Zhang N, Li Q. c-Src phosphorylation and activation of hexokinase promotes tumorigenesis and metastasis. Nat Commun. 2017;8:13732. doi: 10.1038/ncomms13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;9:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;2:453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- Hellman K, Alaiya AA, Schedvins K, Steinberg W, Hellstrom AC, Auer G. Protein expression patterns in primary carcinoma of the vagina. Br J Cancer. 2004;2:319–326. doi: 10.1038/sj.bjc.6601944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen J, Yong J, Qiao L, Xu L, Liu C. An essential role of RNF187 in Notch1 mediated metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;1:384. doi: 10.1186/s13046-019-1382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Groselj-Strele A, Hoefler G, Pummer K, Zigeuner R. Comparison of the 2002 and 2010 TNM classification systems regarding outcome prediction in clear cell and papillary renal cell carcinoma. Histopathology. 2013;2:237–246. doi: 10.1111/his.12001. [DOI] [PubMed] [Google Scholar]
- Shen M, Jiang YZ, Wei Y, Ell B, Sheng X, Esposito M, Kang J, Hang X, Zheng H, Rowicki M, Zhang L, Shih WJ, Celia-Terrassa T, Liu Y, Cristea I, Shao ZM, Kang Y. Tinagl1 suppresses triple-negative breast cancer progression and metastasis by simultaneously inhibiting Integrin/FAK and EGFR signaling. Cancer Cell. 2019;1:64–80.e7. doi: 10.1016/j.ccell.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, Siegwart DJ, Wan Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A. 2017;3:580–585. doi: 10.1073/pnas.1614035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;15:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Li N, Cheng H, Zhang X, Cao X, Qi T, Dai L, Zhang Z, Chen X, Li C, Li Y, Xue B, Fang L, Liu L, Ding Z. HSPA12A Is a novel player in nonalcoholic steatohepatitis via promoting nuclear PKM2-mediated M1 macrophage polarization. Diabetes. 2019;2:361–376. doi: 10.2337/db18-0035. [DOI] [PubMed] [Google Scholar]
- Xu Q, Tu J, Dou C, Zhang J, Yang L, Liu X, Lei K, Liu Z, Wang Y, Li L, Bao H, Wang J, Tu K. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol Cancer. 2017;1:178. doi: 10.1186/s12943-017-0748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner R, Heider M, Fernandez-Saiz V, van Bebber F, Garz AK, Lemeer S, Rudelius M, Targosz BS, Jacobs L, Knorn AM, Slawska J, Platzbecker U, Germing U, Langer C, Knop S, Einsele H, Peschel C, Haass C, Keller U, Schmid B, Gotze KS, Kuster B, Bassermann F. Immunomodulatory drugs disrupt the cereblon-CD147-MCT1 axis to exert antitumor activity and teratogenicity. Nat Med. 2016;7:735–743. doi: 10.1038/nm.4128. [DOI] [PubMed] [Google Scholar]
- Shang RZ, Qu SB, Wang DS. Reprogramming of glucose metabolism in hepatocellular carcinoma: progress and prospects. World J Gastroenterol. 2016;45:9933–9943. doi: 10.3748/wjg.v22.i45.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, Mueller-Klieser W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;4:916–921. [PubMed] [Google Scholar]
- Zhang TT, Jiang YY, Shang L, Shi ZZ, Liang JW, Wang Z, Zhang Y, Hao JJ, Jia XM, Xu X, Cai Y, Zhan QM, Wang MR. Overexpression of DNAJB6 promotes colorectal cancer cell invasion through an IQGAP1/ERK-dependent signaling pathway. Mol Carcinog. 2015;10:1205–1213. doi: 10.1002/mc.22194. [DOI] [PubMed] [Google Scholar]
- Payen VL, Mina E, Van Hee VF, Porporato PE, Sonveaux P. Monocarboxylate Transporters in Cancer Mol Metab. 2019;33:48–66. doi: 10.1016/j.molmet.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payen VL, Porporato PE, Baselet B, Sonveaux P. Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci. 2016;7:1333–1348. doi: 10.1007/s00018-015-2098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal V (2018) Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol 395–412. 10.1146/annurev-pathol-020117-043854 [DOI] [PubMed]
- Gong W, Wang ZY, Chen GX, Liu YQ, Gu XY, Liu WW. Invasion potential of H22 hepatocarcinoma cells is increased by HMGB1-induced tumor NF-kappaB signaling via initiation of HSP70. Oncol Rep. 2013;3:1249–1256. doi: 10.3892/or.2013.2595. [DOI] [PubMed] [Google Scholar]
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;5:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- Min X, Zhang X, Li Y, Cao X, Cheng H, Li Y, Li C, Kong Q, Mao Q, Peng P, Ni Y, Li J, Duan Y, Liu L, Ding Z. HSPA12A unstabilizes CD147 to inhibit lactate export and migration in human renal cell carcinoma. Theranostics. 2020;19:8573–8590. doi: 10.7150/thno.44321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying X, Li-ya Q, Feng Z, Yin W, Ji-hong L. MiR-939 promotes the proliferation of human ovarian cancer cells by repressing APC2 expression. Biomed Pharmacother. 2015;71:64–69. doi: 10.1016/j.biopha.2015.02.020. [DOI] [PubMed] [Google Scholar]
- Lin YH, Wu MH, Huang YH, Yeh CT, Cheng ML, Chi HC, Tsai CY, Chung IH, Chen CY, Lin KH. Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;1:188–203. doi: 10.1002/hep.29462. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tao X, Jin G, Jin H, Wang N, Hu F, Luo Q, Shu H, Zhao F, Yao M, Fang J, Cong W, Qin W, Wang C. A targetable molecular chaperone Hsp27 confers aggressiveness in hepatocellular carcinoma. Theranostics. 2016;4:558–570. doi: 10.7150/thno.14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Truong QA, Park S, Breslow JL. Two Hsp70 family members expressed in atherosclerotic lesions. Proc Natl Acad Sci U S A. 2003;3:1256–1261. doi: 10.1073/pnas.252764399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Jiang H, Tu K, Yu W, Zhang J, Hu Z, Zhang H, Hao D, Huang P, Wang J, Wang A, Xiao Z, He C. ANKHD1 is required for SMYD3 to promote tumor metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;1:18. doi: 10.1186/s13046-018-1011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this study are available on request from the corresponding authors.
Not applicable.