Abstract
A comparative evaluation of the reference National Committee for Clinical Laboratory Standards (NCCLS) broth microdilution method with a novel fluorescent carboxyfluorescein diacetate (CFDA)-modified microdilution method for the susceptibility testing of fluconazole was conducted with 68 Candida strains, including 53 Candida albicans, 5 Candida tropicalis, 5 Candida glabrata, and 5 Candida parapsilosis strains. We found trailing endpoints and discordant fluconazole MICs of <8 μg/ml at 24 h and of ≥64 μg/ml at 48 h for 12 of the C. albicans strains. These strains satisfy the definition of the low-high MIC phenotype. All 12 low-high phenotype strains were correctly shown to be susceptible at 48 h with the CFDA-modified microdilution method. For the 41 non-low-high phenotype C. albicans strains, the CFDA-modified microdilution method yielded 97.6% (40 of 41 strains) agreement within ±1 dilution at 24 h compared with the reference method and 92.7% (38 of 41 strains) agreement within ±1 dilution at 48 h compared with the reference method. The five strains each from C. tropicalis, C. glabrata, and C. parapsilosis that were tested showed 100% agreement within ±2 dilutions for the two methods being evaluated.
Candida is the fourth most common cause of nosocomial bloodstream infection in the United States (2, 4), and the rate of primary bloodstream infections continue to increase (3, 9). In the Americas, Candida albicans is the species most frequently isolated from the bloodstream (16). Candidemia is often difficult to diagnose and refractory to therapy. The attributable mortality rate is 38% in the United States (28) and between 19 and 23% in Canada (9, 25, 29).
Use of the broad-spectrum antifungal fluconazole for the treatment of serious systemic Candida infections has increased. Fluconazole is a less toxic alternative to amphotericin B and has been recommended as primary therapy for candidemia in nonneutropenic and stable neutropenic patients who have not received prior fluconazole treatment and in whom Candida krusei is unlikely (5, 24). In vitro susceptibility testing for fluconazole is of clinical importance, as therapeutic success depends substantially on achieving plasma fluconazole levels that are sufficiently higher than MICs (22).
Despite great advances in the standardization of antifungal susceptibility testing, azole endpoint determination continues to be problematic and subjective and a major source of interlaboratory variability (6, 18, 23). The trailing-growth phenomenon is often responsible for these difficulties, whereby partial inhibition of fungal growth occurs over the range of azole concentrations (6, 13, 14, 23).
Previous work has demonstrated the utility of using fluorescent dyes to assess the viability of C. albicans exposed to amphotericin B (10). We have investigated the use of the vitality-specific dye carboxyfluorescein diacetate (CFDA) in the standardized NCCLS M27-A broth microdilution method (12) to assess the susceptibility of Candida spp. to fluconazole. In this study we compared the NCCLS microdilution method with a CFDA modification in determining fluconazole susceptibility for common clinical yeast isolates (C. albicans, Candida tropicalis, Candida glabrata, and Candida parapsilosis).
Antifungal drug.
Fluconazole powder (Pfizer-Roerig, Inc.) was dissolved in sterile distilled water to make a stock concentration of 10,000 μg/ml and frozen at −70°C. The stock solution was thawed once, and fresh dilutions were used.
Yeast isolates.
Yeast isolates were obtained from the National Centre for Mycology, Division of Microbiology and Public Health, Edmonton, Alberta, Canada, and two low-high phenotype C. albicans strains were kindly supplied by John H. Rex from the Center for the Study of Emerging Pathogens and Reemerging Pathogens, Laboratory of Mycology Research, University of Texas Medical School, Houston, Tex. The identity of the isolates was confirmed by standard methods (27); isolates were stored in skim milk at −70°C and were then subcultured twice on Sabouraud dextrose agar (Difco, Sparks, Md.) before use. These strains included 48 clinical isolates of C. albicans and homologous control strains ATCC 90028, ATCC 90029, ATCC 24433, ATCC 10231, and ATCC 20408; 4 clinical isolates of C. tropicalis and ATCC 750; 4 clinical isolates of C. glabrata and ATCC 90030; and 4 clinical isolates of C. parapsilosis and ATCC 22019.
Antifungal susceptibility testing.
The reference NCCLS broth microdilution method was performed as described in the M27-A document (12). Fluconazole concentrations were diluted in RPMI 1640 medium with l-glutamine. Morpholinepropanesulfonic acid (MOPS) buffer at 165 mM and pH 7 (Angus Buffers & Biochemicals, Niagara Falls, N.Y.) and 100-μl aliquots were placed into the wells of 96-well microtiter Linbro plates (Flow Laboratories Inc., McLean, Va.) with clear, U-shaped well bottoms. The final concentrations of fluconazole ranged from 0 to 64 μg/ml. Six C. albicans strains were tested per 96-well plate, which allowed for the empty outermost wells to be filled with sterile water to minimize evaporation.
Five C. albicans colonies with a diameter of ≥1 mm were suspended in sterile 0.85% saline and adjusted to a final concentration (after inoculation) of 0.5 × 103 to 2.5 × 103 cells per ml in RPMI 1640-MOPS medium. The inoculum was added to the fluconazole trays, incubated at 35°C, and evaluated at 24 and 48 h.
(i) Reference MIC endpoint.
The reference broth microdilution was scored by comparing the growth in each well with that in the growth control (drug-free) well. The MIC was defined as the minimum drug concentration at which visual growth was determined to be 80% relative to that of the growth control.
(ii) Fluorescent MIC endpoint.
The CFDA-modified microdilution method was identical to the method described for broth microdilution susceptibility testing, except that after determination of visual endpoints at 24 and 48 h of incubation at 35°C, a fluorescence assay was also performed. The supernatants were first removed from the tray wells by using a multichannel pipettor, and the remaining yeasts were resuspended to 200 μl per well in 35°C-warmed 0.1 M MOPS buffer at pH 3.5 with 50 mM citric acid. Into each well, 5 μl of a stock of 5 mg of 5(6)-CFDA (Molecular Probes, Eugene, Oreg.)/ml in dimethyl sulfoxide was added for a final concentration of 122 μg/ml. 5(6)-CFDA is a lipophilic, nonpolar substrate that traverses the cell membrane and is hydrolyzed by nonspecific intracellular esterases to the fluorescent anion carboxyfluorescein (10). A multichannel pipettor was used to resuspend well contents by pipetting, alternately filling and emptying the wells 20 times. The tray contents were then incubated in the dark at 35°C for 1 h. The well contents were resuspended again as before, and the trays were assayed for fluorescence with an FL500 microplate fluorescence reader (Bio-Tek Instruments Inc., Winooski, Vt.). 5(6)-CFDA was evaluated using excitation and emission wavelengths of 485 and 530 nm, respectively. The fluorescence of the drug-free control was defined as 100%, and the fluorescence of the fluconazole-exposed wells was reported proportionally to this value (see Fig. 2). The MIC endpoint was defined as the lowest fluconazole concentration at which the fluorescence was reduced to 80% of that of the drug-free growth control. The CFDA microdilution method was performed in triplicate for each yeast strain assayed.
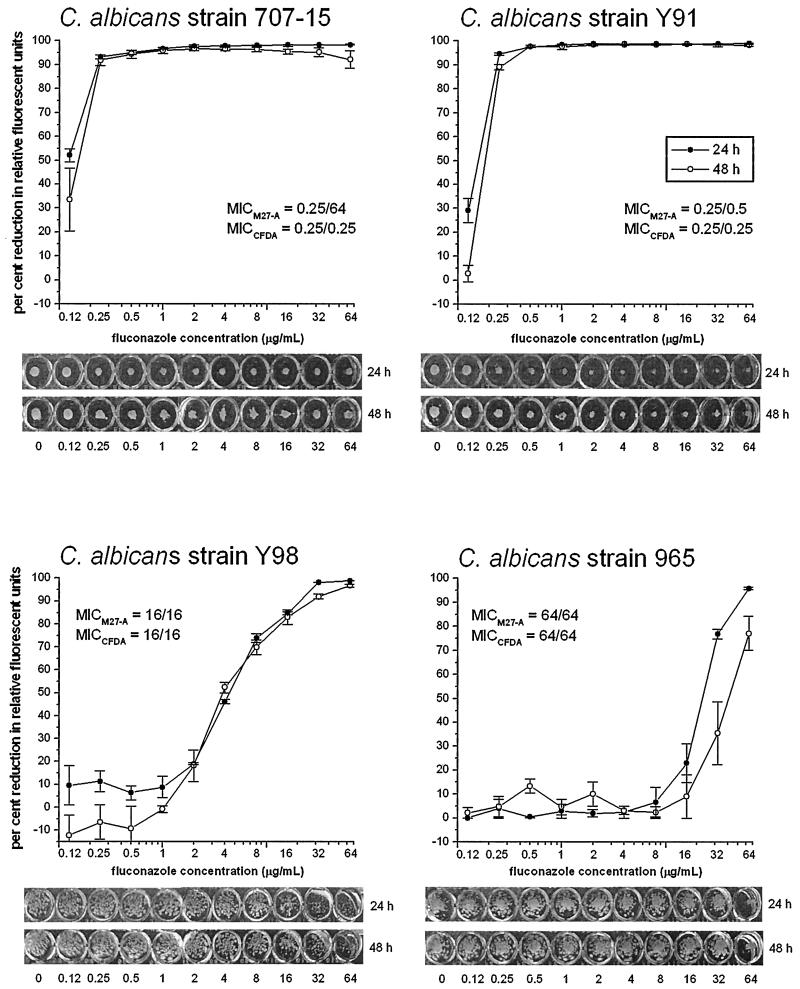
FIG. 2.
Effect of fluconazole on representative strains of C. albicans, which are low-high phenotype (707-15), susceptible (Y91), susceptible-dose dependent (Y98), and resistant (965). Each isolate was tested for susceptibility with the M27-A microdilution method (MICM27-A) and the CFDA-modified microdilution method (MICCFDA) at both 24 and 48 h. Results for the CFDA-modified method are shown graphically as relative fluorescence units. The fluorescence of the growth in the drug-free control was defined as 100%, and the fluconazole-exposed wells were scaled to this value. Error bars indicate standard error. The results of the reference M27-A method are shown below as a digital image of the unagitated growth in the 96-well plate at 24 and 48 h.
The technical time required to include the CFDA modification to the standard microdilution susceptibility test is primarily dependent on the 1-h incubation time and on the time required using a multichannel pipettor to add the CFDA and resuspend the yeasts in the wells.
The comparative evaluation of the reference NCCLS broth microdilution method and the CFDA-modified microdilution method for susceptibility to fluconazole was conducted with 68 Candida strains, including 53 C. albicans, 5 C. tropicalis, 5 C. glabrata, and 5 C. parapsilosis strains. The C. albicans isolates chosen covered a broad range of susceptibility to fluconazole (Fig. 1). The fluconazole MICs for control strains were within accepted limits, providing an internal control for the NCCLS reference method. Evaluation by the reference microdilution method determined that of the Candida strains tested; 12 strains of C. albicans manifested extreme trailing endpoints producing discordant MICs at 24 h (<8 μg/ml) and 48 h (≥ 64 μg/ml). They were thus considered to have the low-high MIC phenotype (20). All C. albicans strains with the low-high phenotype tested by the CFDA-modified microdilution method were not discordant between 24 and 48 h. These C. albicans strains were all shown to be susceptible at both 24 and 48 h. In fact, the MICs were identical for 11 of the 12 strains.
FIG. 1.
Range of fluoconazole MICs for 53 isolates of C. albicans at 24 and 48 h determined with the reference M27-A broth microdilution method. The fluconazole breakpoints for Candida species are as follows: susceptible, ≤8 μg/ml; susceptible-dose dependent, 16 to 32 μg/ml; and resistant, ≥64 μg/ml (12).
The CFDA-modified microdilution method allows for the quantification of fluconazole inhibition and the production of dose-response curves (Fig. 2). MICs were determined from these dose-response curves after plotting the 80% inhibition and rounding up to the standardized M27-A endpoint.
Table 1 summarizes the distributions of the differences in MICs and the percent agreement using the reference and CFDA-modified broth microdilution methods for non-low-high phenotype strains of C. albicans. Considering the non-low-high phenotype C. albicans strains only, the CFDA-modified broth microdilution method yielded 97.6% (40 of 41 strains) agreement within ±1 dilution compared with the NCCLS reference method. At 48 h the two methods yielded 92.7% (38 of 41) agreement within ±1 dilution and 97.6% (40 of 41) agreement within ±2 dilutions. Where MIC endpoints differed between the two methods, the interpretive susceptibility category changed for only one strain. The most common discrepancies between the different susceptibilities of C. albicans strains to fluconazole were due to the CFDA-modified microdilution MICs being 1 dilution lower than those obtained by the standard method. The five strains each of C. tropicalis, C. glabrata, and C. parapsilosis showed 100% agreement within ±2 dilutions for the two methods being evaluated.
TABLE 1.
Distribution of difference between the MICs of fluconazole for 41 strains of C. albicansa determined by comparison of the CFDA-modified and reference NCCLS M27-A broth microdilution methods
| Incubation time (h) | % Discrepancies between methodsb
|
% Agreement after no. of dilution
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| <−2 | −2 | −1 | 0 | 1 | 2 | >2 | 1 | 2 | |
| 24 | 2.4 | 0 | 31.7 | 63.4 | 2.4 | 0 | 0 | 97.6 | 97.6 |
| 48 | 2.4 | 4.9 | 34.1 | 51.2 | 7.3 | 0 | 0 | 92.7 | 97.6 |
Includes only strains of C. albicans which do not have the low-high phenotype.
The numbers −2 to 2 indicate the fold dilution difference (log2).
Endpoint determination for fluconazole susceptibility testing is recognized to be problematic and a significant source of interlaboratory variability (6, 18, 23). The trailing-growth phenomenon, which describes the partial inhibition of fungal growth over most or all of the concentration range (6, 13, 14, 23), is largely responsible for the difficulties attributed to endpoint determination, especially with Candida spp. (14). The impediment that this so-called trailing endpoint represents for azole susceptibility testing can with some isolates be exacerbated over time, producing discordant MICs of <8 μg/ml at 24 h and of ≥64 μg/ml at 48 h (1, 11, 20, 21). Such discordant MICs place these isolates into susceptible and resistant MIC interpretive categories at 24 and 48 h, respectively, if one uses the NCCLS M27-A guidelines (12) and are conveniently referred to as having a low-high MIC phenotype (20). The present evidence suggests that the lower MIC obtained at 24 h using the reference microdilution method correlates most closely with the in vivo outcome (11, 20, 21).
The M27-A method establishes the endpoint for susceptibility testing of Candida spp. to azoles at 48 h with a criterion of 80% reduction in growth (12). Modifications of the M27-A reference method are acceptable and expected (6, 21), and it has been suggested that correction can be made for trailing MIC endpoints by shortening the incubation time to 24 h and lowering the MIC endpoint to the lowest drug concentration producing a 50% reduction in growth (15, 21, 22). The requirement of a 48-h incubation for optimal testing conditions may represent a barrier to this change (7, 21). In one study (17) the results for three microdilution susceptibility test formats were shown to be reproducible and in agreement with one another after 24 h but required 48 h to achieve acceptable agreement with the macrodilution reference MICs.
Additional studies have proposed the use of a colorimetric endpoint in a microdilution format by including an oxidation-reduction indicator with the yeast and drug prior to incubation (15). However, the colorimetric method also presents trailing azole endpoints at 48 h (15, 26), and some species-specific discrepancies have been observed (15).
The CFDA-modified microdilution format does not alter the M27-A microdilution protocol but instead can be employed at 24 or 48 h to clarify MIC endpoints. The fluorescent dye CFDA is applied postincubation to the microdilution tray and thus does not interfere with the complex interaction between the yeast and antifungal drug.
The fluorescent dye CFDA is a lipophilic, nonpolar substrate that diffuses across the cell membrane and is hydrolyzed by nonspecific intracellular esterases to the fluorescent anion carboxyfluorescein (19). Cells with compromised membranes rapidly leak carboxyfluorescein, even when residual esterase activity is retained intracellularly (8). The utility of CFDA for assessing the vitality of C. albicans exposed to amphotericin B has been previously demonstrated (10).
The CFDA-modified microdilution method allowed for the stringent 80% growth inhibition endpoint to be maintained with fluconazole susceptibility testing. Furthermore, this method permitted the evaluation of MICs at 24 or 48 h with clearly demarcated endpoints despite the trailing-growth phenomenon. The CFDA-modified microdilution method determined that all 12 low-high phenotype strains of C. albicans were susceptible to fluconazole and had identical endpoints at 24 and 48 h. This result supports the in vivo evidence suggesting that the strains of C. albicans with the low-high phenotype are actually susceptible to fluconazole (1, 20, 21). In fact, one of the low-high phenotype strains, 707-15, demonstrated to be susceptible with the CFDA-modified microdilution method, has previously been shown to be susceptible in vivo (21).
There was excellent agreement between the NCCLS M27-A broth microdilution method and the CFDA-modified microdilution method using an 80% inhibition-of-growth endpoint for strains of C. albicans without the low-high phenotype. These results demonstrate that the CFDA-modified microdilution method for testing fluconazole is entirely comparable to the NCCLS reference microdilution method. The one strain of C. albicans for which the fluconazole MIC differed in the two methods being compared was shown to be very resistant using the M27-A microdilution method and susceptible using the CFDA-modified method. This strain has unusual fluorescent staining properties, and investigations are under way to determine the nature of its resistance mechanism.
A small survey of the other three major bloodstream fungal pathogens (16) (C. tropicalis, C. glabrata, and C. parapsilosis) showed excellent agreement between the M27-A microdilution and CFDA-modified microdilution methods. Further studies to evaluate the applicability of the CFDA-modified method with other antifungal agents and other species of yeast are ongoing.
In summary, the CFDA-modified microdilution method provides objective and quantifiable endpoints for fluconazole susceptibility testing at 24 and 48 h which are reproducible and easy to interpret. It eliminates the ambiguity of the low-high phenotype while maintaining the integrity of the NCCLS protocol. This method is simple to perform and provides the opportunity for automation and widespread use.
REFERENCES
- 1.Arthington-Skaggs B A, Warnock D W, Morrison C J. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 2000;44:2081–2085. doi: 10.1128/aac.44.8.2081-2085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee S N, Emori T G, Culber D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Tolson J, Henderson T, Marton W J the National Nosocomial Infections Surveillance System. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. Am J Med. 1991;91(Suppl. 3B):86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 3.Beck-Sagué C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National nosocomial infections surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) System. Am J Infect Control. 1996;24:380–388. [PubMed] [Google Scholar]
- 5.Edwards J E, Jr, Bodey G P, Bowden R A, Büchner T, de Pauw B E, Filler S G, Ghannoum M A, Glauser M, Herbrecht R, Kauffman C A, et al. International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin Infect Dis. 1997;25:43–59. doi: 10.1086/514504. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, White T, Pfaller M A. Antifungal agents and susceptibility tests. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 1640–1652. [Google Scholar]
- 7.Fromtling R A, Galgiani J N, Pfaller M A, Espinel-Ingroff A, Bartizal K F, Bartlett M S, Body B A, Frey C, Hall G, Roberts G D, Nolte F B, Odds F C, Rinaldi M G, Sugar A M, Villareal K. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob Agents Chemother. 1993;37:39–45. doi: 10.1128/aac.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jepras R I, Carter J, Pearson S C, Paul F E, Wilkinson M J. Development of a robust flow cytometric assay for determining numbers of viable bacteria. Appl Environ Microbiol. 1995;61:2696–2701. doi: 10.1128/aem.61.7.2696-2701.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlowsky J A, Zhanel G G, Klym K A, Hoban D J, Kabani A M. Candidemia in a Canadian tertiary care hospital from 1976 to 1996. Diagn Microbiol Infect Dis. 1997;28:5–9. doi: 10.1016/s0732-8893(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 10.Liao R S, Rennie R P, Talbot J A. Assessment of the effect of amphotericin B on the vitality of Candida albicans. Antimicrob Agents Chemother. 1999;43:1034–1041. doi: 10.1128/aac.43.5.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marr K A, Rustad T R, Rex J H, White T C. The trailing endpoint phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother. 1999;43:1383–1386. doi: 10.1128/aac.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Odds F C. Laboratory evaluation of antifungal agents: a comparative study of five imidazole derivatives of clinical importance. J Antimicrob Chemother. 1980;6:749–761. doi: 10.1093/jac/6.6.749. [DOI] [PubMed] [Google Scholar]
- 14.Odds F C. Laboratory tests for the activity of imidazole and triazole antifungal agents in vitro. Semin Dermatol. 1985;4:260–279. [Google Scholar]
- 15.Pfaller M A, Barry A L. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1992–1996. doi: 10.1128/jcm.32.8.1992-1996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller M A, Jones R N, Doern G V, Sader H S, Messer S A, Houston A, Coffman S, Hollis R J The Sentry Participant Group. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998. Antimicrob Agents Chemother. 2000;44:747–751. doi: 10.1128/aac.44.3.747-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller M A, Messer S A, Coffmann S. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to test five antifungal agents, including the new triazole D0870. J Clin Microbiol. 1995;33:1094–1097. doi: 10.1128/jcm.33.5.1094-1097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaller M A, Rinaldi M G. Antifungal susceptibility testing: current state of technology, limitations, and standardization. Infect Dis Clin N Am. 1993;7:435–444. [PubMed] [Google Scholar]
- 19.Pringle J, Preston R, Adams A, Stearns T, Drubin D, Haarer B, Jones E. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- 20.Revankar S G, Kirkpatrick W R, McAtee R K, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J Clin Microbiol. 1998;36:153–156. doi: 10.1128/jcm.36.1.153-156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rex J H, Nelson P W, Paetznick V L, Lozano-Chiu M, Espinel-Ingroff A. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing clinical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L for the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 23.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehan D J, Hitchcock C A, Sibley C M. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor G D, Buchanan-Chell M, Kirkland T, McKenzie M, Wiens R. Trends and sources of nosocomial fungaemia. Mycoses. 1994;37:187–190. doi: 10.1111/j.1439-0507.1994.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 26.To W, Fothergill A W, Rinaldi M G. Comparative evaluation of macrodilution and Alamar colorimetric microdilution broth methods for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1995;33:2660–2664. doi: 10.1128/jcm.33.10.2660-2664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren N G, Hazen K C. Candida, Cryptococcus, and other yeasts of medical importance. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 1184–1199. [Google Scholar]
- 28.Wey S B, Mori M, Pfaller M A, Woolson R F, Wenzel R P. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 29.Yamamura D L R, Rotstein C, Nicolle L E, Ioannou S the Fungal Disease Registry of the Canadian Infectious Disease Society. Candidemia at selected Canadian sites: results from the Fungal Disease Registry, 1992–1994. Can Med Assoc J. 1999;160:493–499. [PMC free article] [PubMed] [Google Scholar]