Abstract
CD70 – a ligand protein of CD27 on lymphocytes – is expressed in a large spectrum of malignancies. It is an attractive target for antibody-based therapy and several clinical trials are currently being conducted. However, there is no evidence regarding the expression of CD70 and its relationship with expression of programmed death ligand-1 (PD-L1) and CD27+ tumor-infiltrating lymphocytes (TIL) in formalin-fixed paraffin-embedded (FFPE) tissues of thymic tumors. FFPE tissues of thymic squamous cell carcinoma (TSCC) (operative specimens, n = 31; biopsy specimens, n = 11), thymoma (n = 60), thymic carcinoid (n = 3), and lung squamous cell carcinoma (LSCC) (n = 30) were analyzed immunohistochemically. Immunoreactivity for CD70 was semi-quantitatively scored according to the proportion of positive tumor cells. Moreover, the densities of CD27-positive intratumoral TIL (iTIL) and stromal TIL of TSCC were assessed and survival was compared. Most TSCC cases (87%; 27/31) were CD70-positive. In contrast, all thymoma and thymic carcinoid cases were CD70-negative. In LSCC cases, CD70-positivity was significantly lower than TSCC cases (20%; 6/30). Biopsy and resected specimens obtained from the same patients demonstrated a consistent staining pattern (6/6 patients). The proportion of CD70-positive TSCC was comparable with those of CD5 (87%) and CD117 (90%). Correlation between CD70 and PD-L1 expression score was observed. There was no significant difference in survival between the CD70-high and CD70-low expression groups. Meanwhile, patients with CD27-positive iTIL-high tumors exhibited better survival than those with iTIL-low tumors. This tendency was weaker in the CD70-high subset. CD70 immunohistochemistry is useful in diagnosing TSCC. CD70 may prevent anti-tumor immunity via CD27. Immunotherapy targeting the CD70–CD27 axis may be a promising option for the treatment of TSCC.
Keywords: thymic carcinoma, CD70, CD27, immunohistochemistry, tumor-infiltrating lymphocyte
Introduction
CD70 belongs to the tumor necrosis factor family of proteins and acts as a ligand of CD27. Thymic epithelium and activated T-cells and B-cells are known to express CD70 (1), which promotes differentiation to effector or memory T-cells by expressing CD27 on lymphocytes (2). CD70–CD27 pathway is demonstrated to promote survival of FOXP3-positive regulatory T-cells (3). Hematological malignancies and solid tumors have also been shown to express CD70, leading to the development of antibodies against CD70 as therapeutic agents (4–6).
Thymic carcinoma is a rare disease, accounting for 14% of thymic epithelial tumors according to the International Thymic Malignancy Interest Group database (7). Thymic squamous cell carcinoma (TSCC), which accounts for the majority of thymic carcinoma cases, has some histological mimickers, such as type B3 thymoma and lung squamous cell carcinoma (LSCC). Immunohistochemical (IHC) analysis of CD5, CD117 (KIT), GLUT-1, and MUC1 is useful in differentiating TSCC from type B3 thymoma (8–19). In addition, CD5 and CD117 are helpful in distinguishing TSCC from LSCC (13, 14, 18).
Using IHC analysis in a limited number of frozen TSCC sections, we previously demonstrated that positive staining for CD70 was observed frequently in neoplastic epithelial cells of TSCC, unlike in those of thymoma and LSCC (20). Recently, an anti-CD70 antibody for formalin-fixed, paraffin-embedded (FFPE) tissues became commercially available; however, the diagnostic value of this antibody in TSCC is currently not well documented.
Programmed death ligand-1 (PD-L1) interacts programmed death-1 (PD-1) and inhibits antitumor immunity by T-cells. PD-L1 and PD-1 have become established targets of immunotherapy for various types of cancer. Several clinical trials of immune checkpoint inhibitors targeting PD-1/PD-L1 axis for thymic epithelial tumors have been conducted (21–23). These reagents seemed effective especially in thymic tumor with high-PD-L1 expression (21, 22).
Tumor-infiltrating lymphocytes (TIL) have been associated with favorable prognosis in various types of solid tumors (24–30). CD27-positive TIL are indicated to correlate with activated T-cell response in non-small cell lung cancer and renal cell carcinoma, both of which are known as CD70-expressing tumors (6, 31–33). However, currently, no studies examine the CD27+ TIL status in thymic carcinoma.
In the present study, we performed an IHC analysis for CD70 in TSCC and compared the results with those obtained in thymoma and LSCC. We also assessed the correlation between CD70 and PD-L1 expression in TSCC tissue. In addition, the correlation of the CD70-positivity of tumor cells and CD27-positive TIL on survival was analyzed.
Materials and Methods
Tissue Preparation and Patient Characteristics
TSCC (n = 31), thymoma (type A: n = 5, type AB: n = 20, type B1: n = 9, type B2: n = 9, type B3: n = 17), thymic carcinoid (n = 3), and LSCC (n = 30) resected at a single institution between 1987 and 2017 were analyzed retrospectively. Metastatic squamous cell carcinoma is excluded with clinical information. Biopsy specimens of TSCC (n = 11) and LSCC (n=12) were also analyzed. The diagnosis was based on hematoxylin and eosin staining of FFPE tissues. In addition, the diagnosis was reviewed and confirmed by two pathologists (J.K. and T.H.) according to the 4th Edition of the World Health Organization Classification of Tumours of the Lung, Pleura, Thymus & Heart. The clinical characteristics of the patients were retrospectively obtained from the electronic medical records. Written informed consent was acquired from the patients. This study was approved by the Institutional Review Board of the Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital (approval number: 2120).
IHC Evaluation
FFPE tissues were subjected to IHC analysis. Sections (3.5 μm in thickness) were cut and stained with the avidin–biotin–peroxidase complex method using the ABC Elite Kit (Vector, Burlingame, CA, USA). The antibodies used in this study were CD70 (clone 301731, R&D Systems, Minneapolis, MN, USA; 1:50 dilution), CD5 (4C7, Leica Biosystems, Wetzlar, Germany; 1:70 dilution), CD117 (YR145, Epitomics, Burlingame, CA, USA; 1:2,000 dilution), PD-L1 (E1L3N, Cell Signaling, Danvers, MA, USA; 1:100 dilution), CD8(C8/144B, Nichirei, Tokyo, Japan; 1:100 dilution), FOXP3 (236A/E7, Abcam, Cambridge, UK; 1:150 dilution), and CD27 (HPA038936, Sigma-Aldrich, St. Louis, MO, USA; 1:50 dilution). To evaluate the positivity of each marker, the proportion of positive cells in random spots was estimated and graded as follows: CD70: score 0 (<1% positivity), score 1 (1–25% positivity), score 2 (26–50% positivity), score 3 (51–75% positivity), or score 4 (76–100% positivity); PD-L1: score 0 (<1% positivity), score 1 (1–49% positivity), or score 2 (50–100% positivity). Intratumoral TIL (iTIL) and stromal TIL (sTIL) were defined as infiltrating lymphocytes within tumor nests and tumor stroma, respectively. The average counts (cells per mm2) of CD8-, FOXP3-, and CD27-positive iTIL and stromal sTIL in triplicate high-magnification images obtained with Nikon DS-Ri2 (Nikon, Tokyo, Japan) and counted with NIS-Elements D Imaging Software (Nikon, Tokyo, Japan).
Gene Expression Analysis
Analysis of gene expression was performed with real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR) using first-strand cDNA derived from RNA isolated from frozen tissues of TSCC (n = 12) and thymoma with few non-neoplastic lymphocytes (type A: n = 4, type B3: n = 5), as described previously (30). SYBR green sequence detection reagents (Applied Biosystems, Foster City, CA, USA) and sense and anti-sense primers were used. All reactions were performed in duplicate. The CD70 primers used were as follows: F, gctgctttggtcccattggtcg (exon 1); R, gaggtcctgtgtgattcagctg (exon 2/3 junction; 141-bp product). Reaction products were assayed on a LightCycler 480 Real-Time PCR Instrument (Roche Diagnostics, Rotkreuz, Switzerland) and the PCR product was measured in real time as the increase in SYBR green fluorescence. Data were analyzed using the LightCycler 480 Software version 1.5 (Roche Diagnostics). The CD70 copy number was standardized against the β-actin copy number in each sample.
Survival Analysis
Survival was defined as the period from tissue acquisition of TSCC to death by any cause. We adopted only overall survival to see the prognostic value of CD70 and other immune-related factors in limited number of patients for two reasons. First, more than 40% of the patients enrolled in this study had stage IVa or IVb diseases, and many of them received palliative-intent surgery. Moreover, our study also enrolled patients with thymic carcinoma resected almost 30 years ago, when the following-up frequency after surgery and available images were heterogenous, which might have biased progression free survival. One case was excluded from survival analysis due to the lack of data. We compared the survival of patients with TSCC between two groups defined according to the CD70 IHC score (CD70-low: IHC score 0 or 1; CD70-high: score 2, 3, or 4), the number of CD27-positive iTIL (iTIL-low: iTIL<median; iTIL-high: iTIL≥median), and the number of CD27-positive sTIL (sTIL-low: sTIL<median; sTIL-high: sTIL≥median).
Statistical Analysis
Tumor positivity, revealed by IHC (i.e., the proportion of tumors with a CD70 IHC score ≥1), was compared between TSCC and LSCC using Fisher’s exact test. Relation between CD70 and PD-L1 positivity score, and between CD8, FOXP3 and CD27-positive TIL were assessed using Spearman’s rank correlation test. Quantitative comparison of CD70 expression determined by RT-PCR between thymoma and TSCC, and comparison of TIL levels between CD70-high and low TSCC were performed using the Mann–Whitney U-test. Survival curves were generated using the Kaplan–Meier method. The univariate Cox proportional hazards regression model was employed to assess the hazards ratio according to age, while the log-rank test was used for categorical values (Masaoka–Koga staging, CD70-low/high, CD27-positive iTIL-low/high, and sTIL-low/high). Factors with p < 0.10 were included in the multivariate analysis. Subsequently, a multivariate Cox proportional hazards regression model was employed to estimate the relationship between variables and survival. A p-value < 0.05 denoted statistical significance. All calculations were performed using the R version 3.3.3 (Vienna, Austria).
Results
Patient Characteristics
The characteristics of patients with TSCC and thymoma are summarized in Table 1 . A total of 31 patients with TSCC underwent surgical tumor resection. Of those, six patients also underwent biopsies prior to surgery. Notably, five patients with advanced disease underwent only biopsy. The cases with notable lymphocyte infiltration underwent Epstein-Barr virus encoded small RNA in situ hybridization to confirm the diagnosis of TSCC. None of the patients received immune checkpoint blockade after surgery.
Table 1.
Patient characteristics.
| TSCC | Thymoma | |
|---|---|---|
| Male/female | 17/14 | 21/39 |
| Age, years (median, range) | 65.5, 41–83 | 58, 34–84 |
| Masaoka–Koga staging | n (%) | n (%) |
| I | 1 (3) | 15 (25) |
| II | 7 (23) | 33 (55) |
| III | 10 (32) | 4 (7) |
| IVa | 3 (10) | 3 (5) |
| IVb | 10 (32) | 4 (7) |
| Histological subtype | (%) | |
| A | 5 (8) | |
| AB | 20 (33) | |
| B1 | 9 (15) | |
| B2 | 9 (15) | |
| B3 | 17 (28) | |
| Neoadjuvant chemotherapy and/or radiotherapy | ||
| Yes | 6 (19) | 2 (3) |
| No | 25 (81) | 58 (97) |
TSCC, thymic squamous cell carcinoma.
IHC Analysis of CD70, CD5 and CD117
CD70 in the Healthy Thymus
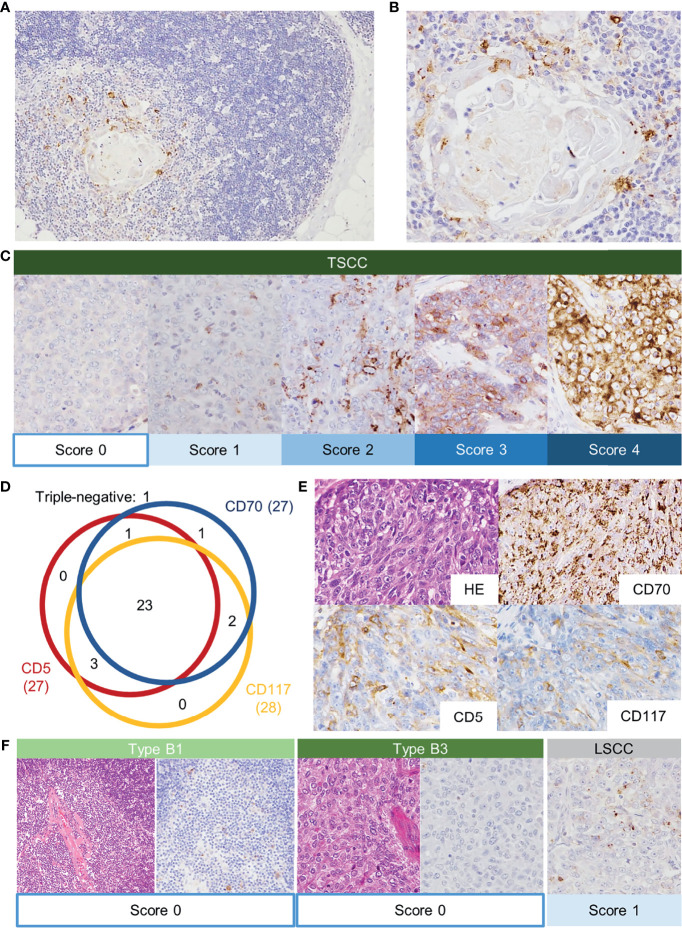
The immunoactivity of CD70 was detected as coarse-granular staining in scattered medullary epithelial cells or dendritic cells, which are indistinguishable from each other morphologically, of the adult thymus ( Figures 1A, B ). In contrast, thymocyte and epithelial cells in the cortex or subcortex did not show CD70 staining.
Figure 1.
Normal adult thymus tissue stained with anti-CD70 antibody. Thymic medullary epithelial cells or dendritic cells were positive for CD70 (A, B). Immunostaining of CD70 in thymic squamous cell carcinoma (TSCC) (C). CD70-positivity was scored according to the proportion of positive tumor cells (score 0: <1%; score 1: 1–24%; score 2: 25–49%; score 3: 50–74%; score 4: 75–100%). Schematic summary of immunohistochemical analysis (D).The numbers indicate the positive cases of each staining. The numbers in the parentheses are the total number of the positive cases. Immunostaining of CD70 (score 4), CD5 and CD117 in TSCC (E). In contrast, thymoma showed the absence of CD70 expression (F). The CD70 score in lung squamous cell carcinoma was relatively low.
IHC Analysis in TSCC, Thymoma, and LSCC
The immunostaining of CD70 in TSCC is shown in Figure 1C . Most TSCC (87%, 27/31) showed a score ≥1 for positivity: score 1: 12 cases (39%); score 2: four cases (13%); score 3: eight cases (26%); and score 4: three cases (10%) ( Table 2 and Figure 1C ). The majority of CD70-positive TSCC revealed a coarse-granular pattern similar to that observed in the normal thymus. A minority of TSCC showed both granular and membranous staining patterns. CD5- and CD117-positive cases accounted for 87% (27/31 patients) and 90% (28/31 patients) of TSCC, respectively ( Figures 1D, E ). Twenty-nine cases (94%) were positive for at least two of the markers.
Table 2.
Immunohistochemical analysis of CD70 in surgical specimens.
| TSCC | Thymoma | Thymic carcinoid | LSCC | |
|---|---|---|---|---|
| (n = 31) | (n = 60) | (n = 3) | (n = 30) | |
| CD70-positivity | 27 (87%) | 0/60 (0%) | 0/3 (0%) | 6/30 (20%)* |
| Score 0 | 4 (13%) | 60 (100%) | 3 (100%) | 24 (80%) |
| Score 1 | 12 (39%) | 0 (0%) | 0 (0%) | 4 (13%) |
| Score 2 | 4 (13%) | 0 (0%) | 0 (0%) | 2 (7%) |
| Score 3 | 8 (26%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Score 4 | 3 (10%) | 0 (0%) | 0 (0%) | 0 (0%) |
TSCC, thymic squamous cell carcinoma; LSCC, lung squamous cell carcinoma.
*The proportion of CD70-positive cases in LSCC was significantly lower than that observed in TSCC (p<0.0001).
On the other hand, all cases of thymoma and thymic carcinoid were negative for CD70. Six of 30 LSCC were CD70-positive; however, the score was ≤2 and the staining pattern showed intratumoral heterogeneity ( Figure 1F ).
In addition, the diagnostic utility of CD70 IHC analysis in small biopsy specimens were assessed, because score 1 positivity was observed in as many as 39% of operative specimens. A large proportion of TSCC was immunoreactive for CD70 (81%), CD5 (91%), and CD117 (100%). Six patients underwent both biopsy and resection of TSCC, and all specimens (biopsy and resection) were CD70-positive ( Table 3 ). On the other hand, only one biopsy specimen of LSCC was positive for CD70 (8.3%), and the positivity was significantly lower compared to TSCC (p < 0.001).
Table 3.
CD70, CD5, and CD117 immunostaining concordance between surgical and biopsy specimens.
| CD70 (%) | CD5 (%) | CD117 (%) | |
|---|---|---|---|
| Positivity in biopsy specimens of TSCC | 9/11 (81) | 10/11 (91) | 11/11 (100) |
| Concordance between surgical and biopsy specimens from the same patient | 6/6 (100) | 6/6 (100) | 6/6 (100) |
TSCC, thymic squamous cell carcinoma.
Gene Expression Analysis of CD70 by RT-PCR
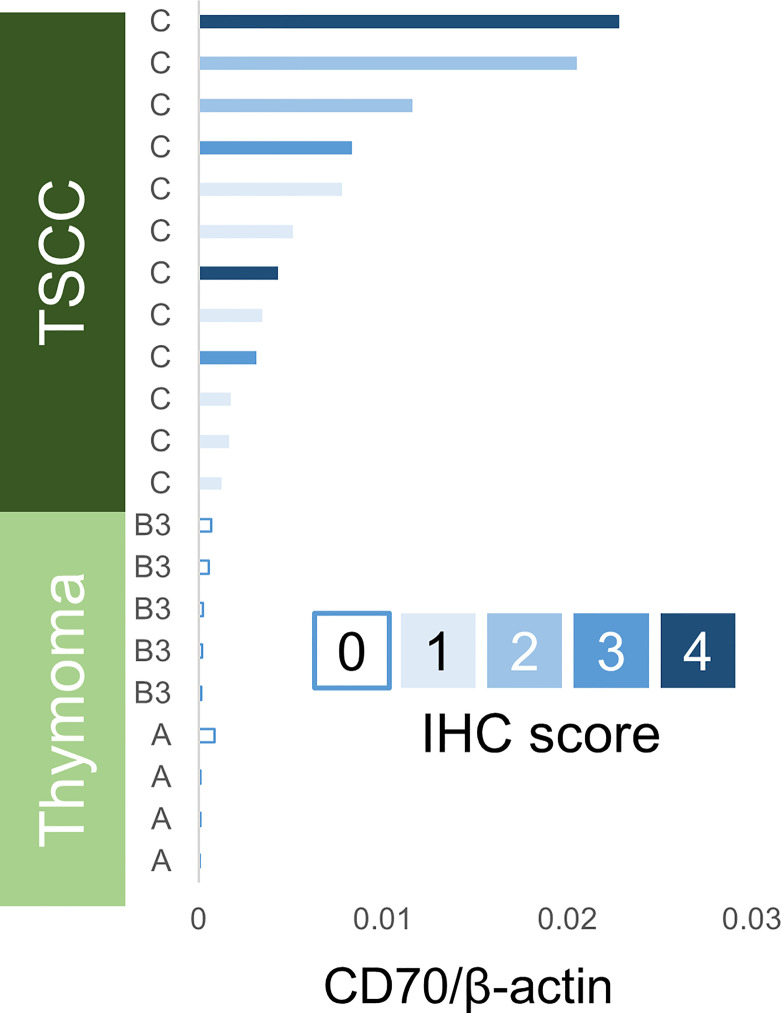
The mRNA extracted from frozen tissues of TSCC (n = 12) and thymoma (type A: n = 4; type B3: n = 5) was quantified using RT-PCR for CD70 ( Figure 2 ). The expression levels of CD70 in TSCC were significantly higher than those observed in thymoma (p < 0.001). This finding was consistent with the IHC score.
Figure 2.
CD70 expression analysis using real-time reverse transcriptase polymerase chain reaction (RT-PCR). A, type A thymoma; B3, type B3 thymoma; C, thymic squamous cell carcinoma; TSCC, thymic squamous cell carcinoma.
Association Between Tumor CD70/PD-L1 Expression and FOXP3/CD27-Positive Lymphocytes
PD-L1 was positive (≥1%) in 74% of TSCC ( Supplementary Table 1 ). Tumors with high PD-L1 score tended to have high CD70 score (p = 0.013). Some tumors were diffusely positive for both PD-L1 and CD70; however, distribution of PD-L1 positive cells and that of CD70 positive cells did not seem correlated.
CD27 was scantly stained in a small proportion of lymphocytes infiltrating the thymoma. In contrast, in TSCC, CD27-positive lymphocytes were observed in tumor nests and rather prominent in the fibrous stroma ( Figure 3A ). Correlation between the number of CD8-positive TIL and that of CD27-positive TIL, and correlation between the number of FOXP3-positive TIL and that of CD27-positive TIL were observed ( Supplementary Table 2 ). Neither the number of CD8, FOXP3, nor CD27-positive TIL showed difference between CD70-high and low tumor, whereas the number of FOXP3-positive iTIL in CD70-high TSCC was slightly higher than CD70-low (p = 0.07, Supplementary Table 3 ). On the other hand, significant correlation was observed between PD-L1 score and the level of CD27-positive iTIL ( Table 4 ).
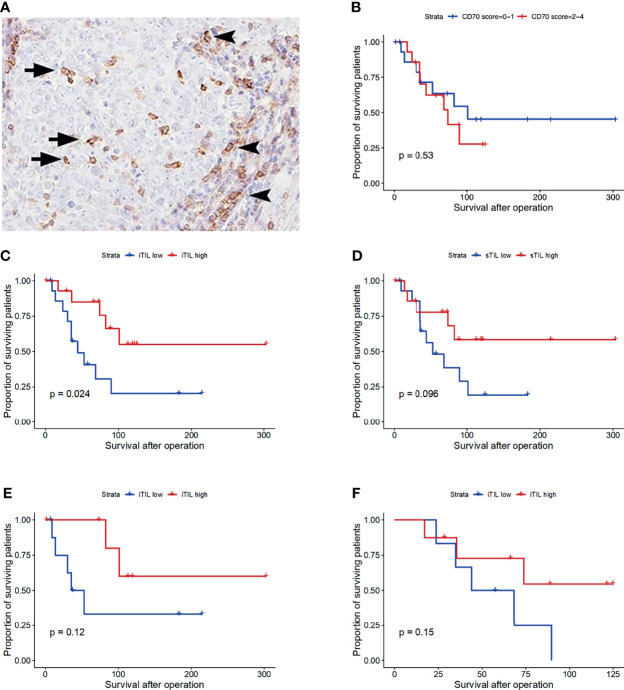
Figure 3.
CD27 staining of thymic squamous cell carcinoma (A). CD27-positive intratumoral tumor-infiltrating lymphocytes (iTIL, arrow) and stromal TIL (sTIL, arrowhead). Overall survival of patients with thymic carcinoma according to the CD70 expression status (B), the number of CD27-positive iTIL (C) and sTIL (D). Stratified survival curve in the CD70-low group (E) and CD70-high group (F). x-axis: days.
Table 4.
Correlation between CD27-positive TIL and PD-L1 expression.
| CD27+ iTIL | CD27+ sTIL | |||||
|---|---|---|---|---|---|---|
| low | high | p-value | low | high | p-value | |
| PD-L1 | 0.033 | 0.719 | ||||
| Score 0 | 7 | 1 | 4 | 4 | ||
| Score 1 | 7 | 9 | 9 | 7 | ||
| Score 2 | 1 | 5 | 2 | 4 | ||
iTIL, intratumoral tumor-infiltrating lymphocytes; sTIL, stromal tumor-infiltrating lymphocytes; PD-L1, programmed death ligand-1.
Survival Analysis of Patients With TSCC
We compared the postoperative survival of patients with TSCC between the CD70-high and CD70-low groups. Patient characteristics are shown in Supplementary Table 4 . The median survival time in the CD70-high and CD70-low groups was 74 and 101 months, respectively. There was no significant difference in survival observed between the two groups (p = 0.53, Figure 3B ).
On the other hand, patients with CD27-positive iTIL-high tumors were associated with longer survival than those with iTIL-low tumors (p = 0.024, Figure 3C ). Moreover, the CD27+ sTIL-high and -low groups showed a slight, non-statistically significant difference in the survival curve (p = 0.096, Figure 3D ).
PD-L1 expressional score neither showed potential for stratification of survival in the cohort (median overall survival: 74.0 months in score 0, 68.5 months in score 1, and 89.7 months in score 2, p=0.97).
The univariate Cox proportional hazard model for all clinicopathological variables revealed that the Masaoka–Koga stage IV, FOXP3-positive sTIL and CD27-positive iTIL status showed p-value <0.10 (p = 0.022, p = 0.008 and p = 0.033, respectively) ( Supplementary Table 5 ), while high PD-L1 expression (≥50%) did not show significant correlation with survival (p = 0.88). Neither Masaoka–Koga stage IV, FOXP3-positive sTIL or CD27-positive iTIL status was shown to be statistically significant in the multivariate analysis (p = 0.24, p = 0.36 and p = 0.73, respectively) ( Supplementary Table 6 ).
Preferable survival of iTIL-high tumor seemed more obvious in the CD70-low group than in the CD70-high group. However, the difference observed between these two subsets was not statistically significant (p = 0.12 and p = 0.15, respectively) ( Figures 3E, F ).
Discussion
In this study, using IHC analysis of FFPE tissues, we showed that CD70 was expressed in 87% of TSCC. The percentage of TSCC with >50% (score 3 and 4, 36%) was consistent with a recent study by Flieswasser et al., whereas the sample size of our study is six times as large (34). In contrast, all cases of thymoma were negative for CD70. We also demonstrated good agreement with IHC score and mRNA expression levels of CD70 by quantitative RT-PCR in TSCC and thymoma. These results indicate that CD70 can be utilized as a specific marker discriminating TSCC from thymoma.
The IHC analysis revealed the characteristic staining pattern (coarse-granular or membranous) observed in CD70 ( Figure 1 ). Notably, CD70 has been shown to be expressed in the thymic medulla (3, 20). The current study showed a consistent expression of CD70 in the normal thymic medulla adjacent to the tumor, which was also retained in <1% of tumor cells observed in the medullary island of type B1 thymoma. Thus, CD70 is one of the specific markers of medullary differentiation, along with CD40 and claudin-4 (35).
Diagnostic Potential of CD70 IHC
Positivity for CD5 and CD117 was similar to that observed for CD70. The present findings are consistent with those reported in our previous study involving IHC analysis of CD70 in frozen sections (20). All cases of thymoma were negative for CD70. Previous studies showed that 0–7% and 0–18% of thymoma were positive for CD5 and CD117, respectively (9, 10, 12, 13, 16, 18, 36). Although the percentage of CD70 positive cells in each case was rather small, the sensitivity and specificity of CD70 in distinguishing TSCC from thymoma are comparable with those of CD5 and CD117.
In the present study, 20% of LSCC cases were positive for CD70. This percentage is similar to that reported in a previous study of LSCC (27%) (6). CD5 and CD117 are utilized as diagnostic markers of TSCC; however, LSCC occasionally expresses CD5 (0–15%) or CD117 (6.2–20%) (18, 37). In the present study, we observed one CD70-positive and CD5/CD117-negative case. These findings indicate that each marker does not independently enable complete discrimination between TSCC and LSCC. Combining IHC analyses of CD70, CD5, and CD117 might enhance the diagnostic ability, while it needs larger scale studies to confirm.
Morphological analysis using biopsy specimens is sometimes limited due to the amount of tissue. In this study, all six biopsy specimens from the patients whose surgical specimens was CD70-positive were also positive for CD70. In addition, the CD70-positivity in biopsy specimens of LSCC was significantly lower than that of TSCC. Although the sample size was small, these results may imply that adding CD70 to the IHC panel has a potential to improve the specificity and sensitivity of TSCC diagnosis, especially when biopsy is the only source of tumor tissue.
Biological Meaning of CD70 and CD27 Expression in TSCC Tissue
The CD27-positive iTIL-high group exhibited significantly longer overall survival than the CD27-positive iTIL-low group. The sTIL-high and -low groups also showed a similar difference. These results are consistent with those of previous studies showing a favorable prognosis in solid tumors, including thymic carcinoma, with high numbers of TIL (24–30, 38). The number of CD8-positive TIL, on the other hand, was not significant prognostic factor in the univariate analysis. The effector T-cell response associated with CD27-positive TIL might partially explain the phenomenon as previously discussed (31). We also showed that the survival curves of the CD27-positive iTIL-high and -low groups became closer in the CD70-high subset. This trend suggests that CD70 expressed in tumor tissue represses anti-tumor immunity via CD27. In the meantime, CD27 is known to be cleaved off when interacted by CD70 (32). Therefore, another possible explanation for the trend and for the irrelevance between CD70 score and CD27+TIL is that CD27-positive iTIL-low subset of CD70-high cases may be a mixture of those with TIL-low cases and with activated CD27-CD70 axis.
Previous studies revealed the aberrant expression of CD70 in several hematological malignancies and solid malignant tumors, including lung cancer, renal cell carcinoma, glioblastoma osteosarcoma, nasopharyngeal carcinoma and ovarian carcinoma (6, 39–44),. The role of the CD70–CD27 axis in anti-tumor immunity is controversial. In physiological conditions, CD70 is expressed transiently in lymphocytes and dendritic cells and affects its receptor, CD27, leading to differentiation into effector or memory T-cells (2). On the other hand, the constitutive expression of CD70 observed in chronic viral infection and lymphoma leads to immune exhaustion (45, 46). Moreover, the expression of CD70 in renal carcinoma cell lines induces apoptosis of lymphocytes (47). We could not detect the significant difference between CD70-high and -low tumor in the levels of CD8-positive TIL or FOXP3-positive TIL; however, the number of FOXP3-positive iTIL in CD70-high TSCC was marginally larger than CD70-low TSCC. This result may reflect activation of CD70–CD27 axis and subsequent maintenance of regulatory T-cells. The impact of the amount of such TILs in TSCC to patient survival is still unclear. We suspect that CD70-expressing tumor induces immune suppressed state via CD70–CD27 pathway, which warrants further studies to confirm.
CD70 as Immunotherapeutic Target
Some anti-CD70 reagents have been developed for cancer. For example, SGN-CD70A underwent phase I trial in CD70-positive renal cell carcinoma patients (48). In addition, CD70 expression on malignant pleural mesothelioma and lower CD27–positive TIL accumulation are reported to correlate with poor prognosis (49). The present study showed that the survival curves of the CD27-positive iTIL-high and -low groups became closer in the CD70-high subset. This implies that CD70-targeted reagent may be beneficial for TSCC patients.
PD-L1 expression levels in thymic epithelial tumors and the correlation between survival have been examined in some studies but the results varied (50–53). Our study demonstrated no evident correlation between PD-L1 score and survival. It may be derived from small sample size, while PD-L1 expression alone might not be prognostic factor in thymic carcinoma patients who did not receive immunotherapy.
Patients with thymoma/thymic carcinoma have been shown that those with high-PD-L1 expression (>50% of tumor cells) appeared to have responded better to pembrolizumab (an anti-PD-L1 reagent), compared to those with low-PD-L1 expression (21, 22). Our result showed that CD70 and PD-L1 expression is correlated in TSCC. Therefore, combination of anti-CD70 and anti-PD-L1 reagents may be beneficial for many of the TSCC patients with high-PD-L1 expression. In addition, low-PD-L1 expression TSCC tended to have lower CD70 score and lower number of CD27-positive iTIL. In such cases, CD27-agonsitic treatment which is expected to facilitate immunity may be beneficial.
Chimeric antigen receptor (CAR) T cell therapy has achieved great success in the treatment of several hematologic malignancies. Recent evidence revealed that CAR T cells targeting CD70 could inhibit the growth of glioma (54), head and neck squamous cell carcinoma (55), and multiple solid tumors (56) as well as hematologic malignancies (57) in vitro and in vivo. CD70-targeting CAR T cell therapy may also be a new candidate for immunotherapy in thymic carcinoma.
Limitations
There were some limitations to our study. First, TSCC is a rare type of cancer; thus, the number of analyzed patients was small. Additional replication and collaboration studies are required. The multivariate analysis failed to show an independent survival effect of TIL or Masaoka–Koga staging, which also might partially be due to the small number of patients. Studies involving larger numbers of cases are warranted to assess the prognostic value of TIL. Second, sampling bias could not be excluded because we performed only IHC analysis in the representative specimens of each tumor. We confirmed the concordance between the staining pattern of CD70 in TSCC and expression using quantitative RT-PCR. Therefore, we suppose that a small amount of TSCC tissue may be sufficient to evaluate the expression of CD70.
Conclusion
CD70 was specifically positive in TSCC versus thymoma. In addition, in TSCC, a significantly higher positivity was observed than in LSCC. The CD70–CD27 axis is a potentially useful diagnostic marker and a promising therapeutic target.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Institutional Review Board of the Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital (approval number: 2120). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JK and TH drafted the manuscript. JK, TH, MO, YH, SH, TM, TU, and MF contributed to histopathological and immunohistochemical analysis. JK, YO, and HH contributed to the analysis of clinicopathological features. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.808396/full#supplementary-material
Abbreviations
FFPE, formalin-fixed, paraffin-embedded; RT-PCR, reverse transcriptase polymerase chain reaction; IHC, immunohistochemical; ITMIG, International Thymic Malignancy Interest Group; TIL, tumor-infiltrating lymphocytes; iTIL, intratumoral tumor-infiltrating lymphocytes; sTIL, stromal tumor-infiltrating lymphocytes, PD-L1, Programmed death ligand-1; HE, hematoxylin and eosin.
References
- 1. Hintzen RQ, Lens SMA, Koopman G, Pals ST, Spits H, van Lier RA. CD70 Represents the Human Ligand for CD27. Int Immunol (1994) 6:477–80. doi: 10.1093/intimm/6.3.477 [DOI] [PubMed] [Google Scholar]
- 2. van de Ven K, Borst J. Targeting the T-Cell Co-Stimulatory CD27/CD70 Pathway in Cancer Immunotherapy: Rationale and Potential. Immunotherapy (2015) 7:655–67. doi: 10.2217/imt.15.32 [DOI] [PubMed] [Google Scholar]
- 3. Coquet JM, Ribot JC, Bąbała N, Middendorp S, Van der Horst G, Xiao Y, et al. Epithelial and Dendritic Cells in the Thymic Medulla Promote CD4+Foxp3+ Regulatory T Cell Development via the CD27-CD70 Pathway. J Exp Med (2013) 210:715–28. doi: 10.1084/jem.20112061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McEarchern JA, Oflazoglu E, Francisco L, McDonagh CF, Gordon KA, Stone I, et al. Engineered Anti-CD70 Antibody With Multiple Effector Functions Exhibits In Vitro and In Vivo Antitumor Activities. Blood (2007) 109:1185–92. doi: 10.1182/blood-2006-07-034017 [DOI] [PubMed] [Google Scholar]
- 5. McEarchern JA, Smith LM, McDonagh CF, Klussman K, Gordon KA, Morris-Tilden CA, et al. Preclinical Characterization of SGN-70, a Humanized Antibody Directed Against CD70. Clin Cancer Res (2008) 14:7763–72. doi: 10.1158/1078-0432.CCR-08-0493 [DOI] [PubMed] [Google Scholar]
- 6. Jacobs J, Zwaenepoel K, Rolfo C, Van den Bossche J, Deben C, Silence K, et al. Unlocking the Potential of CD70 as a Novel Immunotherapeutic Target for non-Small Cell Lung Cancer. Oncotarget (2015) 6:13462–75. doi: 10.18632/oncotarget.3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang J, Ahmad U, Antonicelli A, Catlin AC, Fang W, Gomez D, et al. International Thymic Malignancy Interest Group International Database Committee and Contributors. Development of the International Thymic Malignancy Interest Group International Database: An Unprecedented Resource for the Study of a Rare Group of Tumors. J Thorac Oncol (2014) 9:1573–8. doi: 10.1097/JTO.0000000000000269 [DOI] [PubMed] [Google Scholar]
- 8. Dorfman DM, Shahsafaei A, Chan JKC. Thymic Carcinomas, But Not Thymomas and Carcinomas of Other Sites, Show CD5 Immunoreactivity. Am J Surg Pathol (1997) 21:936–40. doi: 10.1097/00000478-199708000-00008 [DOI] [PubMed] [Google Scholar]
- 9. Pan CC, Chen PC-H, Chiang H. KIT (CD117) is Frequently Overexpressed in Thymic Carcinomas But is Absent in Thymomas. J Pathol (2004) 202:375–81. doi: 10.1002/path.1514 [DOI] [PubMed] [Google Scholar]
- 10. Kojika M, Ishii G, Yoshida J, Nishimura M, Hishida T, Ota S, et al. Immunohistochemical Differential Diagnosis Between Thymic Carcinoma and Type B3 Thymoma: Diagnostic Utility of Hypoxic Marker, GLUT-1, in Thymic Epithelial Neoplasms. Mod Pathol (2009) 22:1341–50. doi: 10.1038/modpathol.2009.105 [DOI] [PubMed] [Google Scholar]
- 11. Kaira K, Murakami H, Serizawa M, Koh Y, Abe M, Ohde Y, et al. MUC1 Expression in Thymic Epithelial Tumors: MUC1 may be Useful Marker as Differential Diagnosis Between Type B3 Thymoma and Thymic Carcinoma. Virchows Arch (2011) 458:615–20. doi: 10.1007/s00428-011-1041-x [DOI] [PubMed] [Google Scholar]
- 12. Khoury T, Chandrasekhar R, Wilding G, Tan D, Cheney RT. Tumour Eosinophilia Combined With an Immunohistochemistry Panel is Useful in the Differentiation of Type B3 Thymoma From Thymic Carcinoma. Int J Exp Pathol (2011) 92:87–96. doi: 10.1111/j.1365-2613.2010.00745.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pomplun S, Wotherspoon AC, Shah G, Goldstraw P, Ladas G, Nicholson AG. Immunohistochemical Markers in the Differentiation of Thymic and Pulmonary Neoplasms. Histopathology (2002) 40:152–8. doi: 10.1046/j.1365-2559.2002.01328.x [DOI] [PubMed] [Google Scholar]
- 14. Hishima T, Fukayama M, Fujisawa M, Hayashi Y, Arai K, Funata N, et al. CD5 Expression in Thymic Carcinoma. Am J Pathol (1994) 145:268–75. [PMC free article] [PubMed] [Google Scholar]
- 15. Kornstein MJ, Rosai J. CD5 Labeling of Thymic Carcinomas and Other Nonlymphoid Neoplasms. Am J Clin Pathol (1998) 109:722–6. doi: 10.1093/ajcp/109.6.722 [DOI] [PubMed] [Google Scholar]
- 16. Song N, Chen G, Zhang P, Liu M, He WX, Jiang GN. Diagnostic and Clinical Significance of KIT(CD117) Expression in Thymic Epithelial Tumors in China. Asian Pac J Cancer Prev (2012) 13:2745–8. doi: 10.7314/APJCP.2012.13.6.2745 [DOI] [PubMed] [Google Scholar]
- 17. Thomas de Montpréville V, Ghigna MR, Lacroix L, Besse B, Broet P, Dartevelle P, et al. Thymic Carcinomas: Clinicopathologic Study of 37 Cases From a Single Institution. Virchows Arch (2013) 462:307–13. doi: 10.1007/s00428-013-1371-y [DOI] [PubMed] [Google Scholar]
- 18. Nakagawa K, Matsuno Y, Kunitoh H, Maeshima A, Asamura H, Tsuchiya R. Immunohistochemical KIT (CD117) Expression in Thymic Epithelial Tumors. Chest (2005) 128:140–4. doi: 10.1378/chest.128.1.140 [DOI] [PubMed] [Google Scholar]
- 19. Petrini I, Zucali PA, Lee HS, Pineda MA, Meltzer PS, Walter-Rodriguez B, et al. Expression and Mutational Status of C-Kit in Thymic Epithelial Tumors. J Thorac Oncol (2010) 5:1447–53. doi: 10.1097/JTO.0b013e3181e96e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hishima T, Fukayama M, Hayashi Y, Fujii T, Ooba T, Funata N, et al. CD70 Expression in Thymic Carcinoma. Am J Surg Pathol (2000) 24:742–6. doi: 10.1097/00000478-200005000-00014 [DOI] [PubMed] [Google Scholar]
- 21. Cho J, Kim H, Ku B, Choi YL, Cristescu R, Han J, et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol (2019) 37:2162–70. doi: 10.1200/JCO.2017.77.3184 [DOI] [PubMed] [Google Scholar]
- 22. Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, et al. Pembrolizumab in Patients With Thymic Carcinoma: A Single-Arm, Single-Centre, Phase 2 Study. Lancet Oncol (2018) 19:347–55. doi: 10.1016/S1470-2045(18)30062-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katsuya Y, Horinouchi H, Seto T, Umemura S, Hosomi Y, Satouchi M, et al. Single-Arm, Multicentre, Phase II Trial of Nivolumab for Unresectable or Recurrent Thymic Carcinoma: PRIMER Study. Eur J Cancer (2019) 113:78–86. doi: 10.1016/j.ejca.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 24. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science (2006) 313:1960–4. doi: 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 25. Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, et al. CD8 Tumor-Infiltrating Lymphocytes are Predictive of Survival in Muscle-Invasive Urothelial Carcinoma. Proc Natl Acad Sci U S A (2007) 104:3967–72. doi: 10.1073/pnas.0611618104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-Infiltrating Lymphocyte Grade is an Independent Predictor of Sentinel Lymph Node Status and Survival in Patients With Cutaneous Melanoma. J Clin Oncol (2012) 30:2678–83. doi: 10.1200/JCO.2011.37.8539 [DOI] [PubMed] [Google Scholar]
- 27. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N Engl J Med (2003) 348:203–13. doi: 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 28. Clemente CG, Mihm MC, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic Value of Tumor Infiltrating Lymphocytes in the Vertical Growth Phase of Primary Cutaneous Melanoma. Cancer (1996) 77:1303–10. doi: [DOI] [PubMed] [Google Scholar]
- 29. Mahmoud SMA, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AHS, et al. Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer. J Clin Oncol (2011) 29:1949–55. doi: 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 30. Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic Effect of Epithelial and Stromal Lymphocyte Infiltration in non-Small Cell Lung Cancer. Clin Cancer Res (2008) 14:5220–7. doi: 10.1158/1078-0432.CCR-08-0133 [DOI] [PubMed] [Google Scholar]
- 31. Bruno TC, Ebner PJ, Moore BL, Squalls OG, Waugh KA, Eruslanov EB, et al. Antigen-Presenting Intratumoral B Cells Affect CD4+ TIL Phenotypes in non-Small Cell Lung Cancer Patients. Cancer Immunol Res (2017) 5:898–907. doi: 10.1158/2326-6066.CIR-17-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruf M, Mittmann C, Nowicka AM, Hartmann A, Hermanns T, Poyet C, et al. pVHL/HIF-Regulated CD70 Expression is Associated With Infiltration of CD27+ Lymphocytes and Increased Serum Levels of Soluble CD27 in Clear Cell Renal Cell Carcinoma. Clin Cancer Res (2015) 21:889–98. doi: 10.1158/1078-0432.CCR-14-1425 [DOI] [PubMed] [Google Scholar]
- 33. Adam PJ, Terrett JA, Steers G, Stockwin L, Loader JA, Fletcher GC, et al. CD70 (TNFSF7) is Expressed at High Prevalence in Renal Cell Carcinomas and is Rapidly Internalised on Antibody Binding. Br J Cancer (2006) 95:298–306. doi: 10.1038/sj.bjc.6603222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flieswasser T, Camara-Clayette V, Danu A, Bosq J, Ribrag V, Zabrocki P, et al. Screening a Broad Range of Solid and Haematological Tumour Types for CD70 Expression Using a Uniform IHC Methodology as Potential Patient Stratification Method. Cancers (2019) 11:1611. doi: 10.3390/cancers11101611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ströbel P, Hartmann E, Rosenwald A, Kalla J, Ott G, Friedel G, et al. Corticomedullary Differentiation and Maturational Arrest in Thymomas. Histopathology (2014) 64:557–66. doi: 10.1111/his.12279 [DOI] [PubMed] [Google Scholar]
- 36. Hayashi A, Fumon T, Miki Y, Sato H, Yoshino T, Takahashi K. The Evaluation of Immunohistochemical Markers and Thymic Cortical Microenvironmental Cells in Distinguishing Thymic Carcinoma From Type B3 Thymoma or Lung Squamous Cell Carcinoma. J Clin Exp Hematop (2013) 53:9–19. doi: 10.3960/jslrt.53.9 [DOI] [PubMed] [Google Scholar]
- 37. Kriegsmann M, Muley T, Harms A, Tavernar L, Goldmann T, Dienemann H, et al. Differential Diagnostic Value of CD5 and CD117 Expression in Thoracic Tumors: A Large Scale Study of 1465 non-Small Cell Lung Cancer Cases. Diagn Pathol (2015) 10:210. doi: 10.1186/s13000-015-0441-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shim HS, Byun CS, Bae MK, Lee CY, Park IK, Kim DJ, et al. Prognostic Effect of Stromal Lymphocyte Infiltration in Thrymic Carcinoma. Lung Cancer (2011) 74:338–43. doi: 10.1016/j.lungcan.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 39. Law CL, Gordon KA, Toki BE, Yamane AK, Hering MA, Cerveny CG, et al. Lymphocyte Activation Antigen CD70 Expressed by Renal Cell Carcinoma is a Potential Therapeutic Target for Anti-CD70 Antibody-Drug Conjugates. Cancer Res (2006) 66:2328–37. doi: 10.1158/0008-5472.CAN-05-2883 [DOI] [PubMed] [Google Scholar]
- 40. Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. CD70(+) non-Hodgkin Lymphoma B Cells Induce Foxp3 Expression and Regulatory Function in Intratumoral CD4(+)CD25(–) T Cells. Blood (2007) 110:2537–44. doi: 10.1182/blood-2007-03-082578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wischhusen J, Jung G, Radovanovic I, Beier C, Steinbach JP, Rimner A, et al. Identification of CD70-Mediated Apoptosis of Immune Effector Cells as a Novel Immune Escape Pathway of Human Glioblastoma. Cancer Res (2002) 62:2592–9. [PubMed] [Google Scholar]
- 42. Pahl JHW, Santos SJ, Kuijjer ML, Boerman GH, Sand LGL, Szuhai K, et al. Expression of the Immune Regulation Antigen CD70 in Osteosarcoma. Cancer Cell Int (2015) 15:31. doi: 10.1186/s12935-015-0181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Agathanggelou A, Niedobitek G, Chen R, Nicholls J, Yin W, Young LS. Expression of Immune Regulatory Molecules in Epstein-Barr Virus-Associated Nasopharyngeal Carcinomas With Prominent Lymphoid Stroma. Evidence for a Functional Interaction Between Epithelial Tumor Cells and Infiltrating Lymphoid Cells. Am J Pathol (1995) 147:1152–60. [PMC free article] [PubMed] [Google Scholar]
- 44. Liu N, Sheng X, Liu Y, Zhang X, Yu J. Increased CD70 Expression is Associated With Clinical Resistance to Cisplatin-Based Chemotherapy and Poor Survival in Advanced Ovarian Carcinomas. Onco Targets Ther (2013) 6:615–9. doi: 10.2147/OTT.S44445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Penaloza-MacMaster P, Ur Rasheed A, Iyer SS, Yagita H, Blazar BR, Ahmed R. Opposing Effects of CD70 Costimulation During Acute and Chronic Lymphocytic Choriomeningitis Virus Infection of Mice. J Virol (2011) 85:6168–74. doi: 10.1128/JVI.02205-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang ZZ, Grote DM, Xiu B, Ziesmer SC, Price-Troska TL, Hodge LS, et al. TGF-β Upregulates CD70 Expression and Induces Exhaustion of Effector Memory T Cells in B-Cell non-Hodgkin’s Lymphoma. Leukemia (2014) 28:1872–84. doi: 10.1038/leu.2014.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Diegmann J, Junker K, Loncarevic IF, Michel S, Schimmel B, von Eggeling F. Immune Escape for Renal Cell Carcinoma: CD70 Mediates Apoptosis in Lymphocytes. Neoplasia (2006) 8:933–8. doi: 10.1593/neo.06451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pal SK, Forero-Torres A, Thompson JA, Morris JC, Chhabra S, Hoimes CJ, et al. A Phase 1 Trial of SGN-CD70A in Patients With CD70-Positive, Metastatic Renal Cell Carcinoma. Cancer (2019) 125:1124–32. doi: 10.1002/cncr.31912 [DOI] [PubMed] [Google Scholar]
- 49. Inaguma S, Lasota J, Czapiewski P, Langfort R, Rys J, Szpor J, et al. CD70 Expression Correlates With a Worse Prognosis in Malignant Pleural Mesothelioma Patients via Immune Evasion and Enhanced Invasiveness. J Pathol (2019) 250:205–16. doi: 10.1002/path.5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Katsuya Y, Fujita Y, Horinouchi H, Ohe Y, Watanabe SI, Tsuta K, et al. Immunohistochemical Status of PD-L1 in Thymoma and Thymic Carcinoma. Lung Cancer (2015) 88:154–9. doi: 10.1016/j.lungcan.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 51. Yokoyama S, Miyoshi H, Nakashima K, Shimono J, Hashiguchi T, Mitsuoka M, et al. Prognostic Value of Pro-Grammed Death Ligand 1 and Programmed Death 1 Expression in Thymic Carcinoma. Clin Cancer Res (2016) 22:4727–34. doi: 10.1158/1078-0432.CCR-16-0434 [DOI] [PubMed] [Google Scholar]
- 52. Weissferdt A, Fujimoto J, Kalhor N, Rodriguez J, Bassett R, Wistuba II, et al. Expression of PD-1 and PD-L1 in Thymic Epithelial Neoplasms. Mod Pathol (2017) 30:826–33. doi: 10.1038/modpathol.2017.6 [DOI] [PubMed] [Google Scholar]
- 53. Arbour KC, Naidoo J, Steele KE, Ni A, Moreira AL, Rekhtman N, et al. Expression of PD-L1 and Other Immunotherapeutic Targets in Thymic Epithelial Tumors. PloS One (2017) 12:e0182665. doi: 10.1371/journal.pone.0182665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jin L, Ge H, Long Y, Yang C, Chang YE, Mu L, et al. CD70, a Novel Target of CAR T-Cell Therapy for Gliomas. J Neuro Oncol (2018) 20:55–65. doi: 10.1093/neuonc/nox116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park YP, Jin L, Bennett KB, Wang D, Fredenburg KM, Tseng JE, et al. CD70 as a Target for Chimeric Antigen Receptor T Cells in Head and Neck Squamous Cell Carcinoma. Oral Oncol (2018) 78:145–50. doi: 10.1016/j.oraloncology.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang M, Tang X, Zhang Z, Gu L, et al. Tandem CAR-T Cells Targeting CD70 and B7-H3 Exhibit Potent Preclinical Activity Against Multiple Solid Tumors. Theranostics (2020) 10:7622–34. doi: 10.7150/thno.43991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sauer T, Parikh K, Sharma S, et al. CD70-Specific CAR T Cells Have Potent Activity Against Acute Myeloid Leukemia Without HSC Toxicity. Blood (2021) 138:318–30. doi: 10.1182/blood.2020008221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.