Abstract
Pancreatic cancer is resistant to conventional therapeutic interventions, mainly due to abundant cancer stromal cells and poor immune cell infiltration. Here, we used a targeted cancer therapy approach based on attenuated Salmonella typhimurium engineered to express cytolysin A (ClyA) to target cancer stromal cells and cancer cells and treat pancreatic cancer in mice. Nude mice bearing subcutaneous or orthotopic human pancreatic cancers were treated with engineered S. typhimurium expressing ClyA. The tumor microenvironment was monitored to analyze stromal cell numbers, stromal cell marker expression, and immune cell infiltration. The attenuated bacteria accumulated and proliferated specifically in tumor tissues after intravenous injection. The bacteria secreted ClyA into the tumor microenvironment. A single dose of ClyA-expressing Salmonella markedly inhibited growth of pancreatic cancer both in subcutaneous xenograft- and orthotopic tumor-bearing nude mice. Histological analysis revealed a marked decrease in expression of stromal cell markers and increased immune cell (neutrophils and macrophages) infiltration into tumors after colonization by ClyA-expressing bacteria. ClyA-expressing S. typhimurium destroyed cancer stromal cells and cancer cells in mouse models of human pancreatic cancer. This approach provides a novel strategy for combining anticancer and anti-stromal therapy to treat pancreatic cancer.
Keywords: bacterial cancer therapy, pancreatic cancer, cytolysin A, ClyA, cancer stromal cells, immune cell infiltration
Graphical abstract
Pancreatic cancers are rich in stromal cells and resistant to conventional therapies. Targeted cancer therapy with an attenuated bacteria-secreting oncolytic payload showed marked suppression on both subcutaneous and orthotopic pancreatic cancers through the destruction of cancer cells and stromal cells, with increased immune sensitivity characterized by immune cell infiltration in TMEs.
Introduction
Pancreatic ductal adenocarcinoma (PDAC), which covers more than 90% of pancreatic cancer cases and is known simply as pancreatic cancer,1,2 is a highly aggressive malignancy; indeed, the median survival time following diagnosis is 3–5 months, with a median 5-year survival of 8%.3,4 PDAC is the fourth leading cause of cancer-related death in Western countries, even though it is only the ninth most common malignancy.4,5 However, only 20% of patients are suitable for surgical resection when diagnosed at an early stage.6 The most common chemotherapeutic drugs approved for pancreatic cancer treatment are gemcitabine and 5-fluorouracil (5-FU); however, only modest advances have been made to improve patient outcomes.6,7 Cancer immunotherapy with checkpoint blockers has shown remarkable responses in some cancers;8, 9, 10, 11 however, they fail to show any efficacy against PDAC,10,12 even dual or triple combination therapy with a programmed cell death 1 (PD-1) inhibitor (nivolumab), a cytotoxic T lymphocyte-associated protein 4 (CTLA-4) inhibitor (ipilimumab), and a mitogen-activated protein kinase (MEK) inhibitor (cobimetinib).13 Thus, a new paradigm for therapy is needed if we are to improve the prospects of patients with pancreatic cancer.
Attenuated Salmonella typhimurium strains target various types of cancer, including colon cancer,14,15 cervical cancer,16 breast cancer,17,18 and glioma.19,20 The unique properties of tumor microenvironments (TMEs), such as low oxygen, abundant nutrients released from necrotic cancer cells, immunosuppressive conditions, and chemotaxis, promote bacterial colonization and proliferation in tumor tissues.21, 22, 23 A genetically engineered S. typhimurium strain defective in ppGpp synthesis (ΔppGpp) shows an increased 50% lethal dose (LD50) (10,000- to 1,000,000-fold) compared with the wild-type strain.24 The avirulent ΔppGpp strain (SL) specifically colonizes and proliferates in tumor tissue, thereby recruiting immune cells and inhibiting tumor growth.14,25,26 To increase the efficacy of this so-called bacterial cancer therapy (BCT), SL was engineered further to express anticancer agents in the TME. Bacterial engineering allows researchers to explore different therapeutic mechanisms. Previously, we engineered SL to express different therapeutic payloads: these include Noxa, a mitochondrial target domain, that induces cancer cell death by increasing mitochondrial permeability;27 heterologous bacterial flagellin (FlaB, Vibrio vulnificus flagellin B), which modulates anticancer immunity;15 and cytolysin A (ClyA), a native bacterial toxin from S. typhimurium that kills cancer cells and cancer stromal cells via its pore-forming activity.25,28
The TME is a complex milieu that contains tumor cells and non-malignant host stromal cells, including blood endothelial cells (BECs), lymphatic endothelial cells (LECs), mesenchymal stem cells, cancer-associated fibroblasts (CAFs), and pericytes.29,30 These cells secrete inhibitory signals that suppress immune cells and play crucial roles in cancer initiation, progression, and metastasis.29, 30, 31 In particular, PDAC is characterized by a dense fibrous stroma that inhibits drug penetration and immune cell infiltration.32 Therefore, targeted depletion of cancer stromal cells along with cancer cells would be an effective strategy to manage PDAC.33
Here, we used an avirulent SL strain engineered to express ClyA (SLClyA) to treat PDAC in various mouse models. We demonstrate anticancer effects against subcutaneous xenograft and orthotopic human PDACs, which are thought to be clinically relevant and offer site-specific pathology.34 The engineered SLClyA showed specific tumor-targeting ability, destruction of cancer stromal cells and cancer cells, and subsequent inhibition of cancer growth.
Results
Engineering ClyA-producing Salmonella
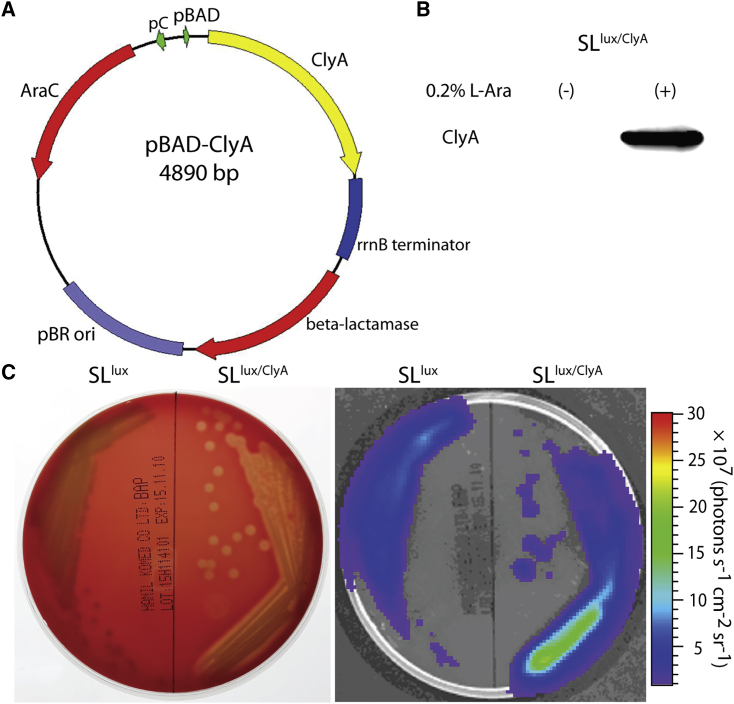
To generate an inducible vector system for bacterial expression of a therapeutic gene, we cloned the clyA gene into the pBAD plasmid vector (Figure 1A), as described previously.28 Western blot analysis revealed that SLlux/ClyA expressed the ClyA protein after l-arabinose induction, whereas no ClyA protein was detected in the absence of l-arabinose (Figure 1B). SLlux/ClyA lysed blood cells and generated a clear bioluminescence signal in areas corresponding to hemolysis (Figure 1C). Furthermore, additional experiments were performed with the AsPC-1 cell line, which showed effective cell killing in vitro as confirmed by crystal violet staining and lactate dehydrogenase release assay (Figure S1).
Figure 1.
In vitro expression of ClyA by engineered S. typhimurium carrying the pBAD-ClyA plasmid
(A) Map of the engineered plasmid pBAD-ClyA. (B) The bioluminescent ΔppGpp Salmonella (SLlux) strain was transformed with pBAD-ClyA (SLlux/ClyA). Expression of ClyA (34 kDa) in bacterial culture was analyzed by western blotting with an anti-ClyA antibody without (−) or with (+) induction with 0.2% l-arabinose. (C) After spreading 100 μL of 40% l-arabinose on the sheep blood, the plates were divided into two parts; the left side was streaked with live SLlux, whereas the right side was streaked with live SLlux/ClyA. Then, the plates were incubated at 37°C overnight. Engineered SLlux/ClyA lyses blood cells (left) and produces a clear bioluminescent signal in the corresponding area of hemolysis (right).
Bacterial distribution and ClyA expression in tumor tissue
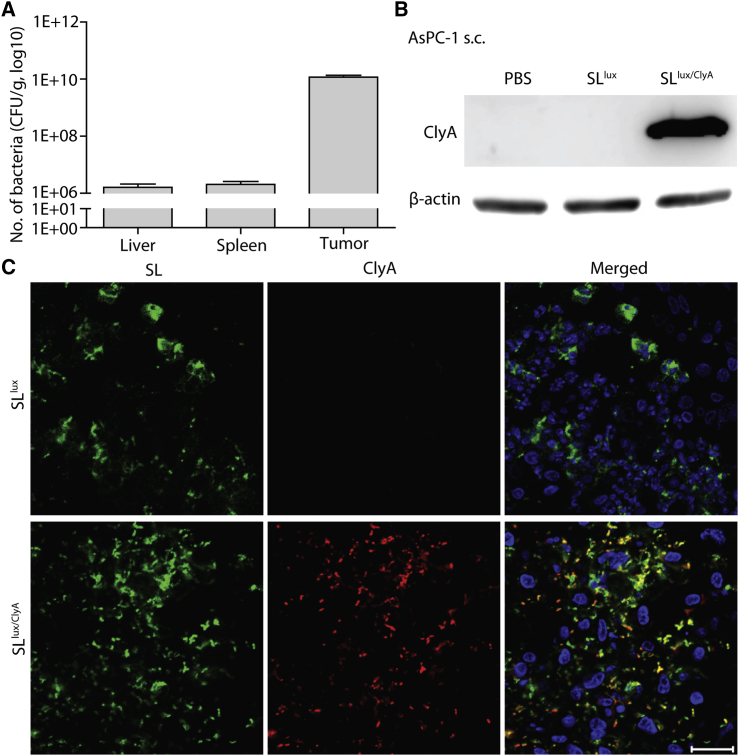
We reported previously that systemically injected SLlux/ClyA colonized the liver and spleen initially, but began to proliferate preferentially in tumors at 3 days post-inoculation (dpi) (Figure S2).15,25 To check selective accumulation and proliferation of bacteria in pancreatic cancer, we administered SLlux/ClyA (3.0 × 107 colony-forming units [CFU]) to BALB/c athymic nu−/nu− mice bearing AsPC-1 xenografts via intravenous (i.v.) injection. Normal organs (liver and spleen) and tumor tissues were extracted at 3 dpi, and viable bacteria were counted. We observed a high number of bacteria (>1010 CFU/g) in AsPC-1 xenografts; indeed, numbers were 1,000- to 10,000-fold higher than those in the liver and spleen (Figure 2A). The number of bacteria agreed with the results of in vivo and ex vivo imaging at 3 dpi, which showed specific bioluminescence signals in tumors but not in the liver and spleen (Figures S3 and S4). Therefore, expression of therapeutic genes at this time point should cause minimal, if any, toxicity to normal tissues. Thus, we decided to administer l-arabinose to tumor-bearing mice at 3 dpi.
Figure 2.
Bacterial colonization and expression of ClyA in AsPC-1 xenografts
BALB/c athymic nu−/nu− mice bearing AsPC-1 tumors were injected intravenously with engineered SLlux/ClyA (3.0 × 107 CFU), followed by intraperitoneal injection of l-arabinose (daily, starting at 3 dpi). (A) Viable bacteria in tumors were counted at 3 dpi. Quantification of bacterial numbers in the liver, spleen, and tumor (n = 11 mice) is shown. (B) Western blot analysis of ClyA expression in AsPC-1 tumor tissue from mice injected with engineered SLlux or SLlux/ClyA at 6 h after l-arabinose induction (representative images of three repetitions). (C) Immunofluorescence staining shows bacterial colonization and ClyA expression in AsPC-1 tumor tissues at 6 h after l-arabinose induction. Sections were stained with an anti-Salmonella antibody (green), an anti-ClyA antibody (red), and DAPI/antifade (blue). A merged image is shown (Merged). Data are representative of three independent experiments. Scale bar, 20 μm.
Next, we examined expression of ClyA in implanted AsPC-1 tumors treated with SLlux/ClyA in the presence of l-arabinose. Control experiments used tumors treated with SLlux carrying an empty vector. Western blot analysis of excised AsPC-1 tumor tissues demonstrated expression of ClyA in tumors colonized by SLlux/ClyA exposed to l-arabinose, but not in tumors exposed to PBS or SLlux (Figure 2B). Expression of ClyA in AsPC-1 tumor tissue was further assessed by immunofluorescence staining. Histological analysis revealed abundant bacteria in tumor tissues from Salmonella-injected mice. The ClyA protein was detected only in tumor tissues harboring SLlux/ClyA in the presence of l-arabinose (Figure 2C). Taken together, these results confirm that engineered SLlux/ClyA specifically expresses and secretes ClyA in pancreatic cancer tissue.
Anticancer effects of engineered ClyA-secreting Salmonella in subcutaneous pancreatic cancer models in nude mice
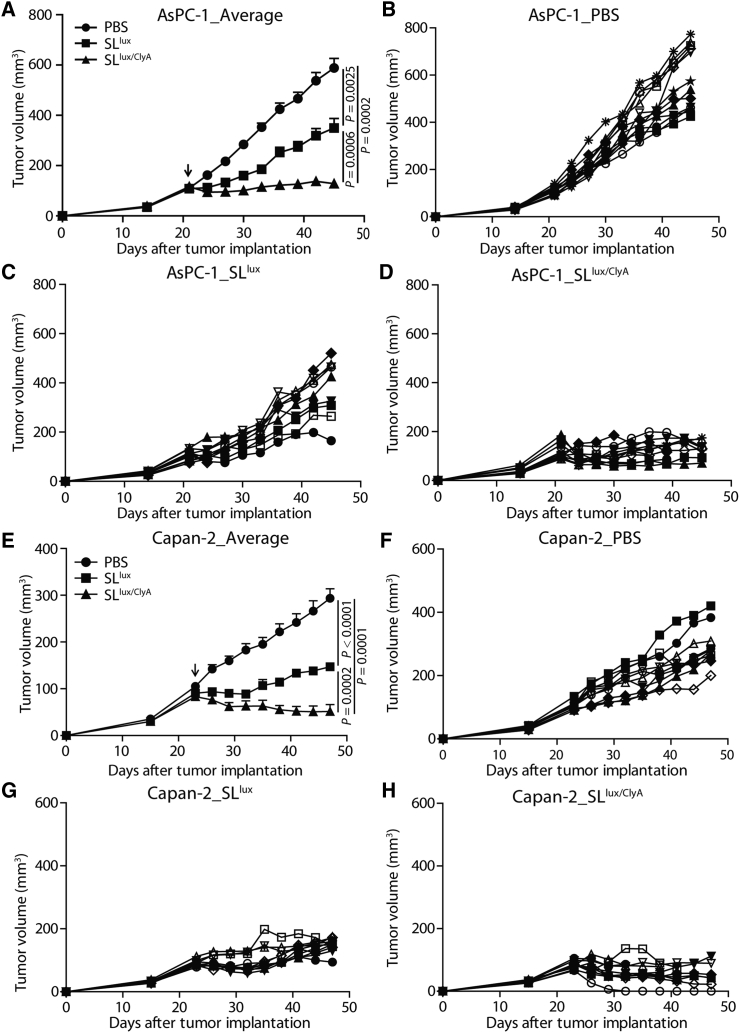
To evaluate the antitumor effects of engineered Salmonella, BALB/c athymic nu−/nu− mice were implanted subcutaneously with AsPC-1 or Capan-2 tumors and injected i.v. with PBS, SLlux, or SLlux/ClyA (+l-arabinose induction from 3 dpi). Tumor-bearing mice tolerated treatment with all types of salmonellae. In mice treated with SLlux/ClyA in the presence of l-arabinose, tumor growth was significantly lower than that in the other groups (Figure 3; Figures S5 and S6). At the end of the treatment (45 days after tumor implantation), growth of AsPC-1 treated with SLlux/ClyA was suppressed significantly, whereas the volume of tumors treated with PBS or SLlux increased by 6-fold and 3-fold, respectively (Figures 3A–3D; p (PBS versus SLlux) = 0.0025; p (PBS versus SLlux/ClyA) = 0.0002; p (SLlux versus SLlux/ClyA) = 0.0006). A similar result was observed in Capan-2-bearing mice. For example, 47 days after tumor implantation, the volume of Capan-2 tumors was 51 ± 14 mm3 in the group treated with SLlux/ClyA, whereas it was 294 ± 20 mm3 or 147 ± 7 mm3 in the groups treated with PBS or SLlux, respectively (Figures 3E–3H). These results demonstrate that ClyA-secreting Salmonella suppresses cancer growth in subcutaneous human pancreatic cancer models.
Figure 3.
Anticancer effects of engineered ClyA-expressing bacteria in subcutaneous cancer models
Nude mice were implanted subcutaneously with AsPC-1 (A–D, n = 12 mice per group) or Capan-2 (E–H, n = 10 mice per group) cancer cells. When the tumor reached 100–120 mm3, mice were treated with 3.0 × 107 CFU engineered bacteria (SLlux or SLlux/ClyA, indicated by an arrow). (A) Average tumor growth in the AsPC-1 cancer model. (B–D) AsPC-1 tumors were treated with PBS (B), engineered SLlux (C), or SLlux/ClyA (D). (E) Average tumor growth in the Capan-2 cancer model. (F–H) Capan-2 tumors were treated with PBS (F), engineered SLlux (G), or SLlux/ClyA (H).
Bacterial colonization and anticancer effects in orthotopic pancreatic cancer-bearing nude mice
To further explore the anticancer effects of engineered ClyA-secreting Salmonella, we established an orthotopic pancreatic cancer model via surgical implantation of AsPC-1 stably expressing firefly luciferase (AsPC-1/Fluc) (Figure S7A). H&E staining of pancreatic tissues obtained 11 days after surgical orthotopic implantation (SOI) clearly showed cancer development and invasion of cancer cells into normal pancreatic tissue (Figure S7B). Generation and growth of orthotopic pancreatic cancer was observed by bioluminescence imaging (Figure S7C). Changes in bioluminescence activity in an individual mouse are shown in Figure S7D. Histological analysis revealed a poorly differentiated structure of pancreatic adenocarcinoma. Abundant cancer stromal cells were also found by immunofluorescence staining with fibroblast activation protein (FAP) and platelet-derived growth factor receptor β (PDGFRβ), which suggested similar complexity to clinical PDAC patient-derived cancer samples (Figure S8).
BALB/c athymic nu−/nu− mice bearing orthotopic pancreatic cancer were injected i.v. with engineered SLlux/ClyA 15 days after SOI. Counting of viable bacteria at 3, 11, and 20 dpi revealed specific colonization and proliferation in the orthotopic cancer, resulting in >1,000- and 10,000-fold higher numbers of bacteria than in the spleen and liver, respectively (Figure S9A). The number of bacteria in the orthotopic cancers remained high at 3 and 11 dpi (p = 0.1949), followed by a gradual decrease (100-fold) until 20 dpi. This suggests that the engineered Salmonella can be used as an active vehicle for delivery and expression of anticancer agents in orthotopic pancreatic cancer.
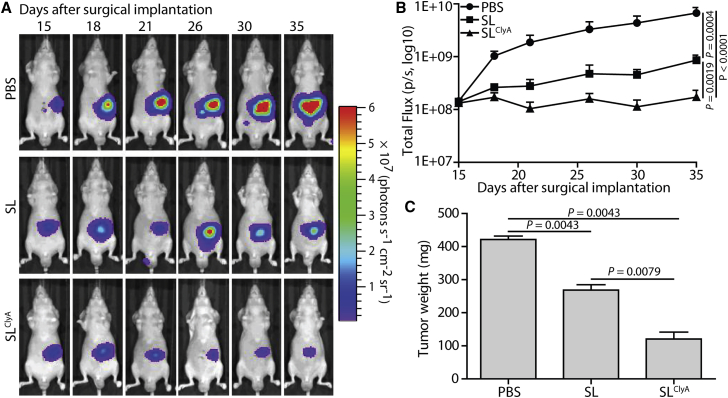
Next, we examined anticancer activity in orthotopic pancreatic cancer models after i.v. injection of PBS, SL, or SLClyA on day 15 after SOI. As shown in Figure 4A, SLClyA suppressed pancreatic cancer growth, as demonstrated by bioluminescence imaging of mice bearing orthotopic AsPC-1/Fluc tumors (p < 0.0001). Compared with that in the control PBS-treated group (negative control), orthotopic cancer growth was suppressed in mice treated with SL (p = 0.0004). The change in bioluminescence activity in orthotopic AsPC-1/Fluc cancers further indicated that SLClyA mediated robust anticancer effects (Figure 4B). At the end of the experiment (day 35 after SOI), orthotopic pancreatic tumors were excised and weighed. The average weight of primary tumors in the group treated with SLClyA was significantly lower than that in the group treated with SL (121 ± 21 mg versus 269 ± 16 mg; p = 0.0079) or PBS (121 ± 21 mg versus 422 ± 10 mg; p = 0.0043) (Figure 4C). These results indicate that SLClyA inhibits pancreatic cancer growth in an orthotopic human pancreatic cancer model (Figure S9B).
Figure 4.
Suppression of orthotopic pancreatic cancer by engineered ClyA-secreting S. typhimurium
Orthotopic human pancreatic cancer mouse models (BALB/c nude mice) were generated by surgical orthotopic implantation (SOI) of one 1-mm3 tumor fragment onto the pancreatic tail. At 15 days after orthotopic implantation, mice were treated with 1.0 × 107 CFU engineered non-light-emitting bacteria (SL or SLClyA), and tumor development was observed by IVIS imaging (n = 11 mice per group). (A) Changes in the signal generated in tumors from representative mice, as monitored by IVIS. (B) Changes in total flux in tumors, as measured from the day after treatment. (C) Tumor weight at day 35 after surgical implantation.
Changes in cancer stromal cells after treatment with ClyA-secreting Salmonella
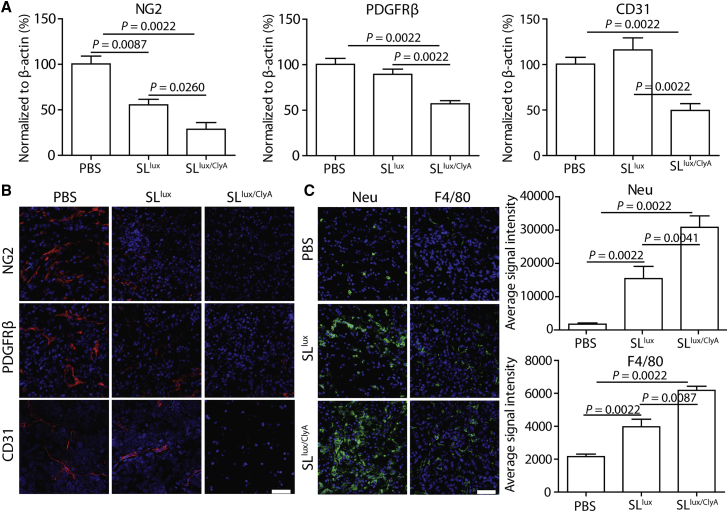
To examine changes in cancer stromal cells after treatment with ClyA-secreting Salmonella, we evaluated expression of several stromal cell markers in AsPC-1 before and after treatment with SLlux or SLlux/ClyA. Western blot analysis revealed significant reductions in expression of neural/glial antigen 2 (NG2), PDGFRβ, and cluster of differentiation 31 (CD31) after treatment with SLlux/ClyA, indicating damaged CAFs, pericytes, and BECs or LECs (Figure S10; Figure 5A; p < 0.01). These results were verified by immunofluorescence staining, which revealed a marked reduction in expression of these markers in tumors treated with SLlux/ClyA (Figure 5B). Furthermore, there was marked infiltration of tumor tissue by immune cells such as macrophages and neutrophils (p < 0.01) after treatment with SLlux/ClyA or SLlux (Figure 5C). Taken together, the data show that engineered ClyA-secreting Salmonella destroys cancer stromal cells and increases immune cell infiltration, thereby mediating robust anticancer activity.
Figure 5.
Changes in cancer stromal cells after treatment with ClyA-expressing bacteria
All samples (n = 6 mice per group) were collected from AsPC-1 subcutaneous cancer model mice at 5 days after bacterial infection (3.0 × 107 CFU; 48 h after ClyA induction). (A) Expression of stromal cell markers was analyzed by western blotting; the signal was normalized to that of β-actin (n = 6). (B) Expression of representative markers was confirmed with immunofluorescence staining. NG2, neural/glial antigen 2; PDGFRβ, platelet-derived growth factor receptor β; CD31, cluster of differentiation 31. (C) Immune cell infiltration after treatment with SLlux/ClyA (n = 6). Neu, neutrophil marker; F4/80, macrophage marker. Scale bars, 50 μm.
Anticancer activity against an immunocompetent mouse cancer model
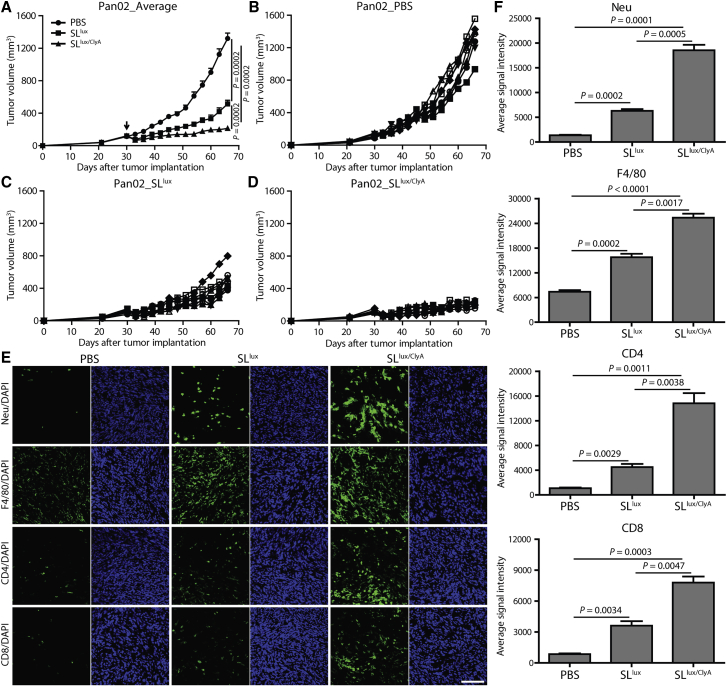
In contrast to T cell immature immunodeficient nude mice, a murine pancreatic cancer model (Pan02) was established in C57BL/6 mice to study anticancer efficacy in an immune-competent host. When the size of tumors reached approximately 120 mm3, tumor-bearing mice were i.v. injected with PBS, SLlux, or SLlux/ClyA (+l-arabinose induction from 3 dpi). Consistent findings were observed in the Pan02 cancer model compared with AsPC-1 and Capan-2 cancer models. The engineered attenuated bacteria (SLlux) significantly halted cancer growth compared with the PBS-treated control group (p = 0.0002), and induction of ClyA expression (SLlux/ClyA) further enhanced anticancer efficacy (Figures 6A–6D). By immunofluorescence staining, we monitored the immune cell infiltration and observed increased neutrophil, macrophage, CD4+, and CD8+ T lymphocyte infiltration in tumor tissues (Figures 6E and 6F). Flow cytometry analysis also showed drastically increased immune cell infiltration and activation, with conversion of M2-like macrophages into M1-like macrophages (Figure S11); there was no significant change in immune checkpoints such as PD-1 and CTLA-4 (data not shown). Moreover, ClyA-expressing bacteria significantly promoted the secretion of inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α (Figure S12). These findings indicated that bacterial therapy exhibited a high potential for TME modification via recruiting a large amount of immune cells and relieving the immunosuppressive conditions.
Figure 6.
Anticancer activity in the immunocompetent Pan02 murine cancer model
Immune intact C57BL/6 mice were implanted subcutaneously with Pan02 (A–D, n = 8 mice per group) cancer cells. When the tumor reached 100–120 mm3, mice were treated with 3.0 × 107 CFU engineered bacteria (SLlux or SLlux/ClyA, indicated by an arrow). (A) Average tumor growth in the Pan02 cancer model. (B–D) Pan02 tumors were treated with PBS (B), engineered SLlux (C), or SLlux/ClyA (D). (E) Immune cells checked with immunofluorescence staining (n = 3). The average signal intensity was calculated, and results are shown in (F). CD4/CD8, CD4+/CD8+ T cells. Scale bar, 100 μm.
Discussion
Engineered Salmonella-secreting ClyA showed tumor-selective accumulation and drug secretion in mouse models of subcutaneous and orthotopic pancreatic cancer, resulting in marked inhibition of tumor growth. Subsequent induction of ClyA expression altered the TME by destroying cancer stromal cells and cancer cells, which allowed infiltration by immune cells, which may convert non-immunogenic “cold” tumors into immunogenic “hot” tumors. This integrated strategy of microbial delivery of an oncolytic payload represents a new direction for pancreatic cancer management.
BCT has shown some remarkable achievements and has been studied extensively in colon cancer, breast cancer, prostate cancer, lung cancer, and glioma.15,20,23,35,36 However, BCT of PDAC required further investigation.37,38 In previous studies, the invasive strain A1-R was applied as an anticancer agent in patient-derived orthotopic xenograft (PDOX) models, showing strong anticancer activity in combination with an anti-angiogenesis agent (bevacizumab) in epidermal growth factor receptor (EGFR)-positive pancreatic cancer,39 which was comparable to chemotherapies (single drug with 5-FU or gemcitabine).40 In addition, the PDOX and cell line-based xenografts showed consistent results. Here, we established an orthotopic PDAC model (i.e., AsPC-1/Fluc cell line), which showed similar TME configuration with clinical specimens. We examined the cancer-targeting efficacy of a non-invasive bacterial strain using AsPC-1 xenografts; engineered S. typhimurium showed very specific accumulation in tumor tissues when compared with normal organs such as the liver and spleen (1,000- to 10,000-fold higher in tumor tissues), which agrees with our previous reports.14,15,26 Tumor-selective targeting was observed consistently in an orthotopic PDAC model, which is a more clinically relevant than subcutaneous model in mice; this targeting was maintained at high levels for a long time. The tumor-targeting efficiency of engineered Salmonella was supported by the marked regression of PDAC growth in mouse models. The ClyA-secreting Salmonella suppressed growth of AsPC-1 and Capan-2 xenografts when compared with controls (Salmonella carrying an empty vector). This antitumor activity was reproduced in an orthotopic PDAC model based on AsPC-1. Considering that these results were observed in immunocompromised mice, we speculate that the Salmonella engineered to secrete an oncolytic protein (i.e., ClyA) destroys cancer cells directly.28
Pancreatic cancer is one of the most stroma-rich cancers; indeed, up to 80% of the tumor mass is made up of stromal tissue.41 PDAC stroma is highly heterogeneous and comprises pancreatic stellate cells, CAFs, vascular cells, infiltrating immune cells, and an abundant extracellular matrix.41, 42, 43 The stromal cells suppress anticancer immunity by inhibiting immune cell infiltration and by secreting immunosuppressive molecules such as transforming growth factor β (TGF-β), IL-10, and PDGFβ, making the tumor resistant to most chemotherapeutic drugs. Thus, targeted destruction of cancer stroma would be a promising treatment for pancreatic cancer. Preclinical studies targeting PDAC stroma with hyaluronidase to deplete hyaluronic acid showed enhanced drug delivery efficacy, vascular decompression, and stromal remodeling.44, 45, 46 Several representative markers are widely used to dissect cancer stromal cells. For example, NG2 identifies pericytes, which encapsulate the endothelial cells to form blood vessels. CD31 is primarily expressed by BECs, LECs, and some myeloid cells, which can be used for blood vessel staining. PDGFRβ is a marker for CAFs and pericytes.33 These markers are frequently used for stromal cell studies and are highly expressed in pancreatic cancer tissues. In the present study, bacteria-mediated ClyA secretion in the TME destroyed both cancer stromal cells and cancer cells, thereby facilitating immune cell infiltration of PDAC such as neutrophils, macrophages, and CD4+ and CD8+ T cells as exemplified in the Pan02 cancer model. ClyA secretion further promoted inflammation and immune cell infiltration. The damaged cancer cells were recognized by the host immune system, which then activated adaptive immunity. Given the low immunogenicity and low efficacy of immune checkpoint blockers in PDAC, the present approach to modifying the tumor stroma using engineered bacteria producing ClyA would be an effective strategy for treating PDAC.
The therapeutic effect of bacteria is due mainly to their immunomodulatory activity. Salmonella infection of tumors may trigger antitumor responses by inducing migration of innate immune cells, including dendritic cells (DCs), neutrophils, macrophages, and neutrophils into colonized tumors, and by enhancing expression of TNF-α, IL-1β, and other pro-inflammatory cytokines.14,15,26 In addition to the innate immune response, Salmonella infection also induces adaptive immune responses against tumor cells by upregulating expression of connexin 43, which promotes the formation of gap junctions between tumor cells and adjacent DCs, enabling transfer of tumor antigens to DCs and cytotoxic T cells.47 The oncolytic Salmonella used in the present study might reproduce this active anticancer immunity in PDAC, an immunologically cold tumor, via destruction of the cancer stroma and by promoting immune cell infiltration. Thus, this study provides a new opportunity for combinational therapies involving immune checkpoint blockades.
In conclusion, genetically engineered bacteria displayed specific tumor colonization in both subcutaneous and orthotopic PDAC with robust anticancer activities by destruction of cancer stromal cells and recruitment of immune cells. Deliberate production of ClyA from the armed bacteria breaks down cancer stromal cells to liberate the immunosuppressed immune cells and make PDAC more exposed to immunosurveillance. Therefore, targeted cancer therapy with engineered Salmonella secreting ClyA is a promising and effective approach to PDAC treatment owing to direct cytotoxicity and immunomodulation through TME remodeling. This programmable strategy based on oncolytic bacteria is a prospective approach to PDAC management in the future.
Materials and methods
Bacterial strains
The S. typhimurium strain defective in ppGpp synthesis (relA::cat, spoT::kan, SL) has been described previously.48 The bacterial luciferase gene lux was transduced by P22HT int transduction.49 The therapeutic payload, ClyA-encoding plasmid (pBAD-ClyA), has been reported previously.28 The engineered bacteria was administered to mice via i.v. injection at a dose of 3.0 × 107 CFU for lux-transduced bacteria (SLlux/ClyA) or 1.0 × 107 CFU for non-transduced bacteria (SLClyA). Expression of ClyA was induced by daily intraperitoneal (i.p.) injection of 120 mg of l-arabinose, starting from 3 dpi of bacteria (Table S1).
Cell lines
Human PDAC cell lines AsPC-1 (CRL-1682) and Capan-2 (HTB-80) were obtained from the American Type Culture Collection and authenticated by the Waterborne Virus Bank (Seoul, Korea). The authenticated murine PDAC cell line (Pan02) was purchased from the National Infrastructure of Cell Line Resource (Beijing, China). The cells were maintained at 37°C/5% CO2 in RPMI 1640, McCoy’s 5a modified medium, and DMEM and supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin.
Western blot analysis
Protein concentration was measured using a bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL, USA). Protein samples were separated in 8%–12% sodium dodecyl sulfate-polyacrylamide gels, transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), and blocked with 5% skim milk for 2 h at room temperature. The membrane was then probed with a primary antibody (Table S2), followed by a horseradish peroxidase-conjugated secondary antibody (Table S2). Immunoreactive proteins were detected using luminol reagents (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and visualized with a Fuji Film image reader (LAS-3000; Fuji Film, Tokyo, Japan).
Animal models
Male BALB/c athymic nu−/nu− mice (6–8 weeks old [18–25 g)]) were purchased from the Orient Company (Seongnan, Korea), and female C57BL/6 mice were purchased from the Hunan SJA Laboratory Animal Company (Changsha, China). All experiments and euthanasia procedures were performed in accordance with protocols approved by the Chonnam National University Animal Research Committee (Gwangju, Korea) and Hunan University Animal Research Committee (Changsha, China). Mice were anesthetized with 2% isoflurane (for tumor assessment) or a mixture of ketamine (200 mg/kg) and xylazine (10 mg/kg) (for surgery). AsPC-1 (5 × 106), Capan-2 (5 × 106), and Pan02 (3 × 106) cells were individually implanted subcutaneously into the right flank to generate the mouse cancer models. Tumors were measured with a caliper every 3 days. Tumor volume (mm3) was calculated using the following formula: (L × H × W)/2, where L is the length, W is the width, and H is the height of the tumor in millimeters. Treatments were initiated when the tumor size reached around 120 mm3.
To establish an orthotopic human pancreatic cancer model, tumor fragments were implanted onto the pancreatic tail of BALB/c athymic nu−/nu− mice using a SOI method. First, 5 × 106 AsPC-1 cells stably expressing firefly luciferase (AsPC-1/Fluc) were injected subcutaneously into BALB/c athymic nu−/nu− mice. When tumors reached 1 cm in diameter, they were excised and the necrotic area was removed. Next, viable tumors were minced into 1-mm3 pieces prior to surgical transplantation. Recipient animals were anesthetized and a laparotomy was performed. One tumor fragment was implanted orthotopically onto the pancreatic tail of each mouse using an 8-0 surgical suture. The abdominal wall was then closed with a 7-0 surgical suture and the animals were kept in a sterile environment.
Viable bacterial counts
To quantify bacteria-specific colonization and proliferation, tumor tissues and organs (liver and spleen) of engineered SLlux/ClyA-infected mice were excised and homogenized in PBS. Samples were serially diluted (10-fold) and plated onto ampicillin/kanamycin-containing Luria-Bertani (LB) plates. After overnight incubation at 37°C, the bacterial titer (CFU/g tissue) was determined by counting the colonies and calculating with the corresponding dilution factor and tissue weight.
Immunofluorescence analysis
For immunofluorescence analysis, tumor tissues were collected from mice and fixed in 4% paraformaldehyde for 2 h at 4°C. The tissues were then washed in PBS, transferred to a 30% sucrose solution, and incubated overnight at 4°C. Fixed tissues were embedded in OCT compound and kept at −80°C. Samples were then sectioned (6 μm thick) using a cryomicrotome (Thermo Scientific, Kalamazoo, MI, USA) and mounted on glass slides. The slides were blocked with 5% BSA and then incubated with primary antibodies overnight at 4°C. The sections were then washed and incubated with fluorochrome-conjugated secondary antibodies for 1 h at room temperature. After counterstaining with DAPI (1:10,000, Invitrogen), the sections were mounted in the ProLong antifade mounting solution (Invitrogen), examined under an LSM510 fluorescence microscope (Zeiss, Germany), and processed using LSM image software.
Optical bioluminescence imaging
Bioluminescence imaging of tumors was performed using an in vivo imaging system (IVIS 100; Caliper Life Sciences). Establishment of orthotopic pancreatic cancer was assessed after i.p. injection of 750 μg d-luciferin, as described previously.28
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0. The Mann-Whitney U test was used to determine the statistical significance of differences in tumor growth, tumor weight, and tumor total flux between the control and treatment groups. A p value <0.05 was considered statistically significant. All data are expressed as the mean ± SEM.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) (grant NRF-2020M3A9G3080282), the Pioneer Research Center Program (2015M3C1A3056410), and the Bio & Medical Technology Development Program of the NRF, funded by the Korean government (MSIT) (NRF-2018M3A9H3024850). W.T. was supported by the National Natural Science Foundation of China (no. 82001753) and the Postdoctoral Science Foundation of China (no. 2020M682562). J.H.Z. was supported by the Hunan Natural Science Foundation (no. 2020JJ5094) and the Fundamental Research Funds for the Central Universities, China. Y.H. was supported by an NRF of Korea grant, funded by the MSIT (no. 2018R1A5A2024181).
Author contributions
W.T. performed the experiments, analyzed the data, drafted the figures, and co-wrote the manuscript. M.T.-Q.D. developed and performed immunofluorescence staining. C.Z., Y.Q., Y.Z., and Y.G. assisted with the animal experiments. Y.H. analyzed and discussed the data. J.H.Z. and J.-J.M. conceived the study, supervised the experiments, analyzed the data, and co-wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.08.023.
Contributor Information
Jin Hai Zheng, Email: jhzheng@hnu.edu.cn.
Jung-Joon Min, Email: jjmin@jnu.ac.kr.
Supplemental information
References
- 1.Bahrami A., Khazaei M., Bagherieh F., Ghayour-Mobarhan M., Maftouh M., Hassanian S.M., et al. Targeted stroma in pancreatic cancer: Promises and failures of target therapies. J. Cell. Physiol. 2017;232:2931–2937. doi: 10.1002/jcp.25798. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., Neale R.E., Tempero M., Tuveson D.A., Hruban R.H., Neoptolemos J.P. Pancreatic cancer. Nat. Rev. Dis. Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 3.Buscail L., Bournet B., Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020;17:153–168. doi: 10.1038/s41575-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos J.P., Kleeff J., Michl P., Costello E., Greenhalf W., Palmer D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018;15:333–348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 6.Karanikas M., Esempidis A., Chasan Z.T., Deftereou T., Antonopoulou M., Bozali F., Amarantidis K., Man Y.G. Pancreatic cancer from molecular pathways to treatment opinion. J. Cancer. 2016;7:1328–1339. doi: 10.7150/jca.15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammed A., Janakiram N.B., Pant S., Rao C.V. Molecular targeted intervention for pancreatic cancer. Cancers (Basel) 2015;7:1499–1542. doi: 10.3390/cancers7030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kvistborg P., Philips D., Kelderman S., Hageman L., Ottensmeier C., Joseph-Pietras D., Welters M.J., van der Burg S., Kapiteijn E., Michielin O., et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl. Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. [DOI] [PubMed] [Google Scholar]
- 9.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C., Bellmunt J., Burris H.A., Petrylak D.P., Teng S.L., et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 12.Royal R.E., Levy C., Turner K., Mathur A., Hughes M., Kammula U.S., Sherry R.M., Topalian S.L., Yang J.C., Lowy I., Rosenberg S.A. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leinwand J., Miller G. Regulation and modulation of antitumor immunity in pancreatic cancer. Nat. Immunol. 2020;21:1152–1159. doi: 10.1038/s41590-020-0761-y. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.E., Phan T.X., Nguyen V.H., Dinh-Vu H.V., Zheng J.H., Yun M., Park S.G., Hong Y., Choy H.E., Szardenings M., et al. Salmonella typhimurium suppresses tumor growth via the pro-inflammatory cytokine interleukin-1β. Theranostics. 2015;5:1328–1342. doi: 10.7150/thno.11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng J.H., Nguyen V.H., Jiang S.N., Park S.H., Tan W., Hong S.H., Shin M.G., Chung I.J., Hong Y., Bom H.S., et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017;9:eaak9537. doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- 16.Hiroshima Y., Zhang Y., Zhao M., Zhang N., Murakami T., Maawy A., Mii S., Uehara F., Yamamoto M., Miwa S., et al. Tumor-targeting Salmonella typhimurium A1-R in combination with trastuzumab eradicates HER-2-positive cervical cancer cells in patient-derived mouse models. PLoS ONE. 2015;10:e0120358. doi: 10.1371/journal.pone.0120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganai S., Arenas R.B., Forbes N.S. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br. J. Cancer. 2009;101:1683–1691. doi: 10.1038/sj.bjc.6605403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M., Yang M., Ma H., Li X., Tan X., Li S., Yang Z., Hoffman R.M. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 19.Momiyama M., Zhao M., Kimura H., Tran B., Chishima T., Bouvet M., Endo I., Hoffman R.M. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle. 2012;11:628–632. doi: 10.4161/cc.11.3.19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen M., Zheng J.H., Choi J.M., Pei J., Li C.H., Li S.Y., Kim I.Y., Lim S.H., Jung T.Y., Moon K.S., et al. Genetically-engineered Salmonella typhimurium expressing TIMP-2 as a therapeutic intervention in an orthotopic glioma mouse model. Cancer Lett. 2018;433:140–146. doi: 10.1016/j.canlet.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y., Chen Y., Liu X., Min J.J., Tan W., Zheng J.H. Targeted cancer immunotherapy with genetically engineered oncolytic Salmonella typhimurium. Cancer Lett. 2020;469:102–110. doi: 10.1016/j.canlet.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Kasinskas R.W., Forbes N.S. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 2007;67:3201–3209. doi: 10.1158/0008-5472.CAN-06-2618. [DOI] [PubMed] [Google Scholar]
- 23.Zhou S., Gravekamp C., Bermudes D., Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer. 2018;18:727–743. doi: 10.1038/s41568-018-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Na H.S., Kim H.J., Lee H.C., Hong Y., Rhee J.H., Choy H.E. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine. 2006;24:2027–2034. doi: 10.1016/j.vaccine.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Jiang S.N., Park S.H., Lee H.J., Zheng J.H., Kim H.S., Bom H.S., Hong Y., Szardenings M., Shin M.G., Kim S.C., et al. Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Mol. Ther. 2013;21:1985–1995. doi: 10.1038/mt.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phan T.X., Nguyen V.H., Duong M.T., Hong Y., Choy H.E., Min J.J. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol. Immunol. 2015;59:664–675. doi: 10.1111/1348-0421.12333. [DOI] [PubMed] [Google Scholar]
- 27.Jeong J.H., Kim K., Lim D., Jeong K., Hong Y., Nguyen V.H., Kim T.H., Ryu S., Lim J.A., Kim J.I., et al. Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium. PLoS ONE. 2014;9:e80050. doi: 10.1371/journal.pone.0080050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen V.H., Kim H.S., Ha J.M., Hong Y., Choy H.E., Min J.J. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010;70:18–23. doi: 10.1158/0008-5472.CAN-09-3453. [DOI] [PubMed] [Google Scholar]
- 29.Mao Y., Keller E.T., Garfield D.H., Shen K., Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303–315. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 31.Kim H.M., Jung W.H., Koo J.S. Expression of cancer-associated fibroblast related proteins in metastatic breast cancer: An immunohistochemical analysis. J. Transl. Med. 2015;13:222. doi: 10.1186/s12967-015-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waghray M., Yalamanchili M., di Magliano M.P., Simeone D.M. Deciphering the role of stroma in pancreatic cancer. Curr. Opin. Gastroenterol. 2013;29:537–543. doi: 10.1097/MOG.0b013e328363affe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lampi M.C., Reinhart-King C.A. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci. Transl. Med. 2018;10:eaao0475. doi: 10.1126/scitranslmed.aao0475. [DOI] [PubMed] [Google Scholar]
- 34.Qiu W., Su G.H. Development of orthotopic pancreatic tumor mouse models. Methods Mol. Biol. 2013;980:215–223. doi: 10.1007/978-1-62703-287-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiroshima Y., Zhang Y., Murakami T., Maawy A., Miwa S., Yamamoto M., Yano S., Sato S., Momiyama M., Mori R., et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–12357. doi: 10.18632/oncotarget.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiroshima Y., Zhao M., Maawy A., Zhang Y., Katz M.H., Fleming J.B., Uehara F., Miwa S., Yano S., Momiyama M., et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) J. Cell. Biochem. 2014;115:1254–1261. doi: 10.1002/jcb.24769. [DOI] [PubMed] [Google Scholar]
- 37.Park S.H., Zheng J.H., Nguyen V.H., Jiang S.N., Kim D.Y., Szardenings M., Min J.H., Hong Y., Choy H.E., Min J.J. RGD peptide cell-surface display enhances the targeting and therapeutic efficacy of attenuated Salmonella-mediated cancer therapy. Theranostics. 2016;6:1672–1682. doi: 10.7150/thno.16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choe E., Kazmierczak R.A., Eisenstark A. Phenotypic evolution of therapeutic Salmonella enterica serovar Typhimurium after invasion of TRAMP mouse prostate tumor. MBio. 2014;5:e01182-14. doi: 10.1128/mBio.01182-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manuel E.R., Chen J., D’Apuzzo M., Lampa M.G., Kaltcheva T.I., Thompson C.B., Ludwig T., Chung V., Diamond D.J. Salmonella-based therapy targeting indoleamine 2,3-dioxygenase coupled with enzymatic depletion of tumor hyaluronan induces complete regression of aggressive pancreatic tumors. Cancer Immunol. Res. 2015;3:1096–1107. doi: 10.1158/2326-6066.CIR-14-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiroshima Y., Zhao M., Zhang Y., Maawy A., Hassanein M.K., Uehara F., Miwa S., Yano S., Momiyama M., Suetsugu A., et al. Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle. 2013;12:2774–2780. doi: 10.4161/cc.25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui R., Yue W., Lattime E.C., Stein M.N., Xu Q., Tan X.L. Targeting tumor-associated macrophages to combat pancreatic cancer. Oncotarget. 2016;7:50735–50754. doi: 10.18632/oncotarget.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Apte M.V., Park S., Phillips P.A., Santucci N., Goldstein D., Kumar R.K., Ramm G.A., Buchler M., Friess H., McCarroll J.A., et al. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Mei L., Du W., Ma W.W. Targeting stromal microenvironment in pancreatic ductal adenocarcinoma: controversies and promises. J. Gastrointest. Oncol. 2016;7:487–494. doi: 10.21037/jgo.2016.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobetz M.A., Chan D.S., Neesse A., Bapiro T.E., Cook N., Frese K.K., Feig C., Nakagawa T., Caldwell M.E., Zecchini H.I., et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Provenzano P.P., Cuevas C., Chang A.E., Goel V.K., Von Hoff D.D., Hingorani S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson C.B., Shepard H.M., O’Connor P.M., Kadhim S., Jiang P., Osgood R.J., Bookbinder L.H., Li X., Sugarman B.J., Connor R.J., et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther. 2010;9:3052–3064. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- 47.Saccheri F., Pozzi C., Avogadri F., Barozzi S., Faretta M., Fusi P., Rescigno M. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci. Transl. Med. 2010;2:44ra57. doi: 10.1126/scitranslmed.3000739. [DOI] [PubMed] [Google Scholar]
- 48.Song M., Kim H.J., Kim E.Y., Shin M., Lee H.C., Hong Y., Rhee J.H., Yoon H., Ryu S., Lim S., Choy H.E. ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1. J. Biol. Chem. 2004;279:34183–34190. doi: 10.1074/jbc.M313491200. [DOI] [PubMed] [Google Scholar]
- 49.Davis R.W., Botstein D., Roth J.R. Cold Spring Harbor Laboratory Press; 1980. Advanced Bacterial Genetics: A Manual for Genetic Engineering. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.