Abstract
Cow's milk is currently the most consumed product worldwide. However, due to various direct and indirect contamination sources, different chemical and microbiological contaminants have been found in cow's milk. This review details the main contaminants found in cow's milk, referring to the sources of contamination and their impact on human health. A comparative approach highlights the poor efficacy and effects of the pasteurization process with other methods used in the treatment of cow's milk. Despite pasteurization and related techniques being the most widely applied to date, they have not demonstrated efficacy in eliminating contaminants. New technologies have appeared as alternative treatments to pasteurization. However, in addition to causing physicochemical changes in the raw material, their efficacy is not total in eliminating chemical contaminants, suggesting the need for new research to find a solution that contributes to improving food safety.
Keywords: human health; chemical contaminant; microbiological contaminant; alternative; technology; food safety
1. Introduction
Milk is a fluid secreted by the female of the mammalian species and fulfills the nutritional requirements of the neonate, for instance: (i) the energetic part (provided by lipids, lactose, and in excess by proteins), essential amino acids, and (ii) amino groups necessary for the biosynthesis of non-essential amino acids (provided by proteins), essential fatty acids, vitamins, inorganic elements, and water. 1
Global milk production has increased by about 20% in the last decade, from 694 million tons in 2008 2 to 843 million tons in 2018. 3 As a result, bovine milk is the most consumed food product representing about 48% of the total milk consumed globally, the European Union (EU), Australia, and New Zealand being the most important producers, followed by the United States and India. 4
Collection and processing expose milk to different contaminants, mainly pesticide residues, metals, mycotoxins, hormones, and others reaching the cow through feeding or drug administration by producers. 5 Thus, milk can contain hazardous materials, of either biological or chemical origin.
Although pasteurization has been an efficient antimicrobial method and has contributed to reducing many diseases, several infectious episodes associated with pasteurized milk have continued to occur, mainly when raw milk has an exaggerated population of microorganisms that increase the margin of survival and by post pasteurization contamination. 6 The biggest problem of pathogens in pasteurized milk is that they persist without causing any organoleptic alteration, increasing sanitary risk since the consumer cannot suspect their presence, showing that pasteurization has some drawbacks in treating pathogens. 7
As population and industrial growth increased, new contaminants appeared, and with this, contamination of cow's milk also increased not only by compounds of biological origin but also by compounds of chemical origin, as mentioned above. 8 However, pasteurization has remained the only established treatment, even though it is only effective for eliminating most biological and non-chemical compounds. 9 In contrast, the literature mentions very few alternative treatments to treat chemical contaminants in cow's milk, leading to a critical analysis of their application to ensure sufficient quality in the milk consumed. Given this evidence, the bibliographic review here aims to identify the different types of contaminants in raw/pasteurized cow's milk and analyze the application of alternative processes for the elimination or degradation of contaminants.
2. Contaminants present in cow's milk
There are several hazards of contamination of cow's milk, ranging from biological to chemical compounds. The risk of biological contamination of cow's milk derives mainly from cattle milking due to the exposure of udders to the environment, equipment, storage, dirty pipes, and others. 10 Chemical contamination of cow's milk comes from several sources: application of agrochemicals, 11 use of legal or illegal veterinary products, 12 feed and forages contaminated with natural toxins, 13 or through the improper use of chemicals during milk production, processing and packaging stages. 14
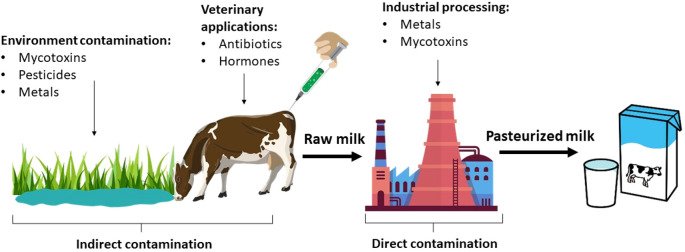
Figure 1 shows the direct and indirect pathways for contaminants entry into bovine milk.
Figure 1. Sources of contamination of bovine milk.
Indirect contamination is associated with the ingestion of contaminants both from the environment and from substances of veterinary use. The most common environmental contaminants are mycotoxins, pesticides, and metals consumed by cattle through feed, forages, and water. In addition, antibiotics and hormones are administered to the cow orally, by injection, or as intramammary infusions to treat diseases, promote animal growth and increase milk production. 5 On the other hand, direct contamination occurs during milk processing from milking, handling, storage and even pasteurization. During the industrialization process, milk comes into contact with metals, residues of cleaning products, mycotoxins, among others.
For better analysis and understanding, the classification of contaminants according to the origin is microbial contaminants and chemical contaminants ( Figure 2).
Figure 2. Distribution of literature related to contaminants in bovine milk between 2010-2021.
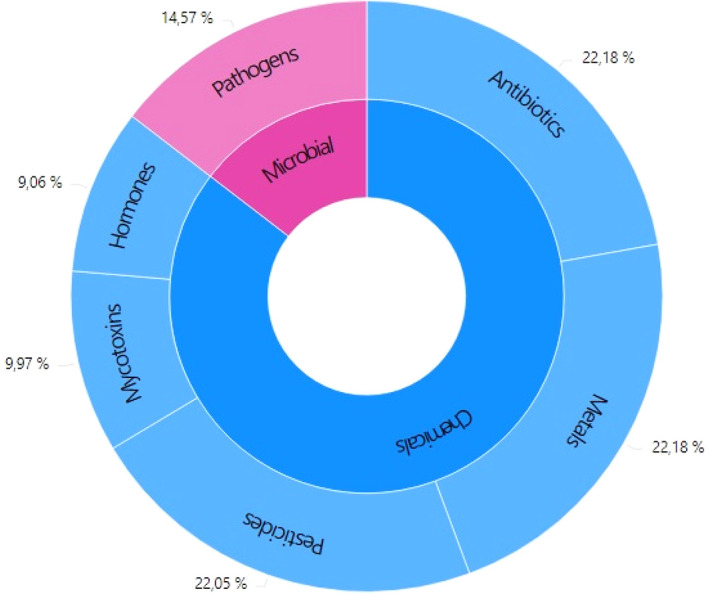
About 14.57% of the literature reports contamination of cow's milk by pathogenic microorganisms. Although the objective of the pasteurization process is the elimination of these microorganisms, there is evidence of their presence in pasteurized milk, which will be presented later. Although pathogenic microorganisms are considered the main hazard that threatens the safety of milk, they do not represent the highest percentage of reported cases. The contaminants that have been more reported in the literature are of chemical origin ( Figure 2). Among chemical contaminants, metals, pesticides, and antibiotics stand out. Among chemical contaminants, the most reported are heavy metals (22.18%), pesticides (22.05%), and antibiotics (22.18%); due to bad practices in agriculture and cattle. Although reports of mycotoxins in milk are relatively low (9.97%), they are of great importance due to the increase in reported cases of contamination with Aflatoxin M1 (AFM1). The International Agency for Research on Cancer has classified AFM1 as a carcinogenic substance. 15 This means that the food safety of milk is at risk, as any of these compounds compromise the health of the final consumer. Below is a detailed classification of the different types of contaminants present in both raw and pasteurized milk and the negative effects they have on consumer health.
2.1 Microbial contaminants
The presence of several pathogenic microorganisms has been reported in raw and pasteurized cow's milk ( Table 1). Microbial contamination of raw milk can be due to diseases such as mastitis, improper handling on production farms, milking equipment, water sources, and feeding of cattle, utensils, and equipment used for milk storage on the farm or during transport. 16 Likewise, poor hygienic practices within the dairy industry can lead to the formation of biofilms on the sprinklers of cooling systems, pipes, cooling tanks, storage, and transport tanks. The contact of pasteurized milk with these surfaces increases the risk of contamination with pathogenic microorganisms, posing a danger to the consumer and the quality of the product. 17
Table 1. Pathogens in bovine milk reported in literature.
| Pathogens | Type of milk | Reference |
|---|---|---|
| Mycobacterium | Raw | 18 , 19 |
| Pseudomonas | Raw | 20 , 21 , 22 , 23 , 24 , 25 , 16 , 26 , 27 , 28 , 29 |
| Pasteurized | 16 | |
| Hafnia | Raw | 25 , 23 , 21 |
| Serratia | Raw | 22 , 25 , 24 , 21 |
| Klebsiella | Raw | 30 |
| Pasteurized | 30 | |
| Citrobacter | Raw | 25 |
| Escherichia | Raw | 16 , 30 , 31 |
| Pasteurized | 16 , 30 | |
| Staphylococcus | Raw | 32 , 27 , 28 , 29 |
| Bacillus | Raw | 27 , 28 , 20 , 21 |
| Lactococcus | Raw | 32 , 26 , 21 , 27 , 28 , 20 |
| Corynebacterium | Raw | 28 |
| Streptococcus | Raw | 27 , 28 |
| Enterobacter | Raw | 27 , 28 , 25 |
| Mycoplasma | Raw | 27 |
| Enterococcus | Raw | 27 , 28 , 21 , 32 |
| Acinetobacter | Raw | 27 , 28 , 20 , 29 , 26 , 33 , 32 |
| Sneathia | Raw | 27 |
| Kocuria | Raw | 27 , 28 , 32 |
| Neisseria | Raw | 27 |
| Fusobacterium | Raw | 27 |
| Macrococcus | Raw | 27 |
| Trueperella | Raw | 27 |
| Halomonas | Raw | 27 |
| Micrococcus | Raw | 27 |
| Enhydrobacter | Raw | 27 |
| Psychrobacter | Raw | 27 , 28 |
| Campylobacter | Raw | 34 , 31 , 35 |
| Brachybacterium | Raw | 28 |
| Dermacoccus | Raw | 28 |
| Leucobacter | Raw | 28 |
| Microbacterium | Raw | 28 , 20 |
| Aerococcus | Raw | 28 |
| Lactobacillus | Raw | 28 , 33 |
| Ochrobactrum | Raw | 28 |
| Pantoea | Raw | 28 |
| Paracoccus | Raw | 28 |
| Sphingomonas | Raw | 28 |
| Deinococcus | Raw | 28 |
| Aspergillus | Raw | 28 |
| Cladosporium | Raw | 28 |
| Eurotium | Raw | 28 |
| Penicillium | Raw | 28 |
| Wallemia | Raw | 28 |
| Listeria | Raw | 31 , 36 |
| Yersinia | Raw | 31 , 36 |
| Salmonella | Raw | 30 , 16 |
| Pasteurized | 30 , 16 | |
| Vibrio | Raw | 30 |
| Pasteurized | 30 | |
| Stenotrophomonas | Raw | 33 , 32 |
| Chryseobacterium | Raw | 33 |
| Paenibacillus | Raw | 20 , 21 |
| Coliforms | Pasteurized | 37 |
According to Table 1, Most cases of contamination are recorded in raw milk due to inadequate milking, processing, storage, and transport conditions. On the other hand, although few studies report the presence of microorganisms in pasteurized milk, it is doubtful that it is an efficient process for their elimination. The main types of microorganisms present in milk are bacteria, yeasts, and molds, which represent the different types of microorganisms present in cow's milk. The presence of Corynebacteria, Staphylococcus, Streptococcus, Bacillus, and Micrococcus species has been evidenced in the teat of dairy cattle. 38 , 39 These microorganisms have also been identified in cow's milk, 27 , 28 , 40 , 41 demonstrating that during milking, milk can become contaminated by contact with the cow's teat under unhygienic conditions. On the other hand, as a result of mastitis, Staphylococcus and Streptococcus species have been identified in bovine milk samples, 42 , 43 with Staphylococcus aureus being the main cause of mastitis. 43 The presence of Enterobacteriaceae, Pseudomonas spp., Staphylococcus spp., and lactic acid bacteria has been identified in the equipment used for milking. 17 It is evident that the conditions under which milk is obtained on farms are not the most adequate because these different microorganisms are found in cow's milk. 33 , 42 , 44 , 45
Consumption of milk contaminated by pathogenic microorganisms such as Campylobacter, Salmonella, Yersinia, E. coli, Listeria, and S. aureus can cause muscle and stomach pain, gastrointestinal diseases with diarrhea, fever, and nausea. 31 These microorganisms are commonly found in the intestinal flora or in the udder of cows, thus facilitating milk contamination. 31 In addition, Campylobacter spp. and E. Coli O157:H7 are capable of producing Guillain-Barrés syndrome and hemolytic uremic syndrome, respectively. 46
2.2 Chemical contaminants
For a more detailed analysis, the chemical contaminants found in cow's milk have been classified into five groups: pesticides, metals, antibiotics, mycotoxins, and hormones ( Table 2).
Table 2. Chemical contaminants in bovine milk reported in literature.
| Compounds | Type of milk | MRL a (μg/kg) | MRL b (μg/kg) | Reference | |
|---|---|---|---|---|---|
| Pesticides | Hexachlorocyclohexane (HCH) | Raw | - | - | 11 , 47 , 48 , 49 , 50 , 51 |
| Pasteurized | - | - | 52 , 53 , 51 | ||
| UHT | - | - | 47 | ||
| Butachlor | Raw | - | - | 11 | |
| Pasteurized | - | - | 54 | ||
| Cyhalothrin | Raw | 30 | 50 | 11 , 55 , 56 | |
| Pasteurized | - | - | 54 | ||
| Cypermethrin | Raw | 100 | 20 | 11 , 56 , 57 | |
| Pasteurized | - | - | 54 , 58 , 59 | ||
| Fenvalerate | Raw | - | 40 | 11 | |
| Pasteurized | - | - | 59 | ||
| Deltamethrin | Raw | 30 | 20 | 11 , 55 , 56 , 57 , 55 | |
| Pasteurized | - | - | 60 | ||
| Malathion | Raw | - | - | 11 , 61 , 62 | |
| Chlorpyrifos | Raw | - | - | 11 , 55 , 56 , 61 , 55 , 60 | |
| Pasteurized | - | - | 54 , 58 , 59 , 60 | ||
| UHT | - | - | 60 | ||
| Carbofuran | Raw | - | - | 55 , 62 | |
| Permethrin | Raw | - | 50 | 56 , 57 | |
| Pasteurized | - | - | 54 , 58 , 59 | ||
| Profenophos | Raw | - | - | 11 , 60 | |
| Pasteurized | - | - | 54 , 60 | ||
| UHT | - | - | 60 | ||
| Ethion | Raw | - | - | 11 | |
| Pasteurized | - | - | 54 , 63 | ||
| Dichloro diphenyl trichloroethane (DDT) | Raw | - | - | 11 , 64 , 65 , 47 , 66 , 48 , 50 , 51 | |
| Pasteurized | - | - | 64 , 52 , 54 , 67 , 51 | ||
| UHT | - | - | 47 | ||
| Dicofol | Pasteurized | - | - | 59 | |
| Aldrin+Dieldrin | Raw | - | - | 64 , 47 , 49 | |
| Pasteurized | - | - | 64 , 53 | ||
| UHT | - | - | 47 | ||
| Endrin | Raw | - | - | 68 , 69 , 49 | |
| Pasteurized | - | - | 70 , 53 , 54 , 67 | ||
| Fipronil | Raw | - | - | 11 , 65 , 60 | |
| Pasteurized | - | - | 54 , 60 | ||
| Hexaflumuron | Raw | - | - | 65 | |
| Teflubenzuron | Raw | - | - | 65 | |
| Diflufenican | Raw | - | - | 65 | |
| Piperophos | Raw | - | - | 65 | |
| Dimethoate | Raw | - | - | 60 , 62 | |
| Pasteurized | - | - | 60 | ||
| Atrazine | Pasteurized | - | - | 58 , 59 | |
| Diazinon | Raw | - | 20 | 62 , 60 | |
| Pasteurized | - | - | 58 , 59 , 60 | ||
| UHT | - | - | 60 | ||
| Lindane | Raw | - | - | 64 , 51 | |
| Pasteurized | - | - | 64 , 51 | ||
| Endosulfane | Raw | - | - | 11 , 65 , 47 , 68 , 48 , 71 , 49 , 51 , 56 | |
| Pasteurized | - | - | 52 , 70 , 53 , 54 , 67 | ||
| UHT | - | - | 47 | ||
| Hexachlorobenzene | Raw | - | - | 72 | |
| Pasteurized | - | - | 70 , 58 , 59 | ||
| Heptachlor epoxide | Raw | - | - | 65 , 47 , 73 , 68 | |
| Pasteurized | - | - | 59 | ||
| UHT | - | - | 47 , 73 | ||
| Heptachlor | Raw | - | - | 68 , 69 , 51 | |
| Pasteurized | - | - | 73 , 52 , 70 , 51 | ||
| UHT | - | - | 73 | ||
| Chlordane | Pasteurized | - | - | 52 , 53 , 67 | |
| Methoxychlor | Raw | - | - | 47 , 69 | |
| Pasteurized | - | - | 54 | ||
| UHT | - | - | 47 | ||
| Azoxystrobin | Pasteurized | - | - | 74 | |
| Chlorantranilliprole | Pasteurized | - | - | 74 | |
| Flubendiamide | Pasteurized | - | - | 74 | |
| Imidacloprid | Raw | - | - | 55 | |
| Pasteurized | - | - | 74 | ||
| Lufenuron | Pasteurized | - | - | 74 | |
| Metalaxyl | Pasteurized | - | - | 74 | |
| Novaluron | Pasteurized | - | - | 74 | |
| Uniconazol | Pasteurized | - | - | 74 | |
| Monuron | Pasteurized | - | - | 75 | |
| Methabenzthiazuron | Pasteurized | - | - | 75 | |
| Buturon | Pasteurized | - | - | 75 | |
| Linuron | Pasteurized | - | - | 75 | |
| Aziprotryne | Pasteurized | - | - | 75 | |
| Bitertanol | Pasteurized | - | - | 75 | |
| Clofentezine | Pasteurized | - | - | 75 | |
| Methyl Parathion | Raw | - | - | 62 , 76 | |
| Metals | Cadmium | Raw | - | - | 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 |
| Pasteurized | - | - | 87 , 77 , 88 | ||
| UHT | - | - | 89 , 90 | ||
| Lead | Raw | - | - | 77 , 91 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 92 , 85 , 86 | |
| Pasteurized | - | - | 87 , 77 , 93 , 88 | ||
| UHT | - | - | 92 , 94 , 89 , 90 | ||
| Copper | Raw | - | - | 77 , 79 , 80 , 81 , 82 , 84 , 92 , 89 , 88 , 85 , 86 | |
| Pasteurized | - | - | 87 , 77 , 93 , 88 | ||
| UHT | - | - | 92 , 94 , 89 , 90 | ||
| Zinc | Raw | - | - | 77 , 95 , 80 , 81 , 82 , 88 , 85 , 96 , 86 | |
| Pasteurized | - | - | 77 , 93 , 88 , 96 | ||
| UHT | - | - | 94 , 89 , 90 | ||
| Selenium | Raw | - | - | 82 , 85 , 96 , 86 | |
| Pasteurized | - | - | 96 | ||
| UHT | - | - | 94 | ||
| Chromium | Raw | - | - | 77 , 91 , 88 , 85 , 96 , 86 | |
| Pasteurized | - | - | 77 , 93 , 88 , 96 | ||
| UHT | - | - | 90 | ||
| Nickel | Raw | - | - | 77 , 91 , 79 , 97 , 88 , 85 , 86 | |
| Pasteurized | - | - | 77 , 93 , 88 | ||
| UHT | - | - | 94 , 89 | ||
| Iron | Raw | - | - | 80 , 82 , 89 , 88 , 85 , 96 , 86 | |
| Pasteurized | - | - | 93 , 88 , 96 | ||
| UHT | - | - | 94 , 89 , 90 | ||
| Arsenic | Raw | - | - | 98 , 91 , 83 , 84 , 97 , 88 , 85 , 96 , 86 | |
| Pasteurized | - | - | 88 , 96 | ||
| Magnesium | Raw | - | - | 95 , 82 , 83 , 88 , 85 , 86 | |
| Pasteurized | - | - | 93 , 88 | ||
| UHT | - | - | 90 | ||
| Manganese | Raw | - | - | 82 , 89 , 88 , 85 , 86 | |
| Pasteurized | - | - | 93 , 88 | ||
| UHT | - | - | 89 , 90 | ||
| Aluminum | Raw | - | - | 98 , 91 , 85 , 96 , 86 | |
| Pasteurized | - | - | 96 | ||
| Molybdenum | Raw | - | - | 98 , 86 | |
| Mercury | Raw | - | - | 91 , 84 , 99 , 97 , 88 , 86 | |
| Pasteurized | - | - | 88 | ||
| UHT | - | - | 94 | ||
| Tin | Raw | - | - | 97 , 85 , 86 | |
| Cobalt | Raw | - | - | 77 , 79 , 89 , 88 , 86 | |
| Pasteurized | - | - | 77 , 88 | ||
| UHT | - | - | 94 , 89 | ||
| Antibiotics | Oxytetracycline | Raw | 100 | 100 | 100 , 101 , 102 , 103 , 104 , 105 |
| Pasteurized | - | - | 106 , 58 , 107 , 102 , 108 , 105 , 109 | ||
| UHT | - | - | 102 , 108 | ||
| Lincomycin | Raw | 150 | 150 | 100 , 101 , 110 | |
| Pasteurized | - | - | 111 , 112 | ||
| UHT | - | - | 111 | ||
| Quinolone | Raw | - | - | 104 , 113 | |
| Pasteurized | - | - | 111 , 114 , 113 | ||
| UHT | - | - | 111 , 114 | ||
| Tetracycline | Raw | 100 | 100 | 102 , 103 , 115 , 116 , 104 , 105 , 110 | |
| Pasteurized | - | - | 111 , 114 , 117 , 107 , 102 , 108 , 109 | ||
| UHT | - | - | 111 , 117 , 102 , 108 | ||
| Doxycicline | Raw | - | - | 103 , 104 | |
| Pasteurized | - | - | 106 | ||
| UHT | - | - | 108 | ||
| Penicillin G | Raw | - | - | 101 , 118 , 119 , 120 , 121 , 122 | |
| Pasteurized | - | - | 106 , 109 | ||
| Trimethoprim | Raw | - | 50 | 123 | |
| Amoxicillin | Raw | 4 | 4 | 124 , 119 , 120 , 121 , 122 | |
| Pasteurized | - | - | 58 , 109 | ||
| Cefalexin | Raw | - | 100 | 120 , 125 | |
| Cephapirin | Raw | - | 60 | 101 , 120 | |
| Fleroxacin | Raw | - | - | 126 | |
| Chlortetracycline | Raw | 100 | 100 | 102 , 104 | |
| Pasteurized | - | - | 102 | ||
| UHT | - | - | 102 , 108 | ||
| Enrofloxacin | Raw | - | 100 | 126 , 127 , 115 , 116 , 128 , 105 , 113 , 129 , 119 , 120 , 122 , 110 | |
| Pasteurized | - | - | 127 , 108 , 105 | ||
| UHT | - | - | 108 | ||
| Ciprofloxacin | Raw | - | 100 | 126 , 127 , 103 , 129 , 119 , 120 , 122 | |
| Pasteurized | - | - | 127 , 108 | ||
| UHT | - | - | 108 | ||
| Lomefloxacin | Raw | - | - | 126 | |
| Tilmicosin | Pasteurized | - | 50 | 112 , 130 | |
| Erythromycin A | Pasteurized | - | 40 | 130 | |
| Tylosin | Raw | 100 | 50 | 103 , 116 | |
| Pasteurized | - | - | 112 , 109 | ||
| Spiramycin | Pasteurized | 200 | 200 | 112 | |
| Streptomycin | Raw | 200 | 200 | 131 , 116 , 128 , 110 | |
| Pasteurized | - | - | 111 | ||
| UHT | - | - | 111 | ||
| Gentamicin | Raw | 200 | 100 | 131 , 116 , 128 | |
| Pasteurized | - | - | 109 | ||
| UHT | - | - | 108 | ||
| Gatifloxacin | Raw | - | - | 127 | |
| Pasteurized | - | - | 127 | ||
| Ofloxacin | Raw | - | - | 127 | |
| Pasteurized | - | - | 127 , 132 | ||
| Norfloxacin | Raw | - | - | 127 | |
| Pasteurized | - | - | 127 , 108 | ||
| UHT | - | - | 108 | ||
| Sulfamethoxazole | Raw | - | - | 127 , 103 , 105 , 123 | |
| Pasteurized | - | - | 127 | ||
| Sulfamethazine | Pasteurized | - | - | 127 , 58 | |
| UHT | - | - | 114 | ||
| Sulfadimethoxine | Raw | - | - | 103 | |
| Pasteurized | - | - | 58 | ||
| Sulfadiazine | Pasteurized | - | - | 133 | |
| Sulfathioazole | Pasteurized | - | - | 58 | |
| Ceftiofur | Raw | - | - | 103 | |
| Sulfonamides | Raw | - | - | 116 , 128 , 104 | |
| Pasteurized | - | - | 114 | ||
| UHT | - | - | 114 | ||
| Cefazolin | Raw | - | 50 | 101 , 125 | |
| Cephoperazone | Raw | - | 50 | 101 , 119 , 120 , 122 , 125 | |
| Dicloxacillin | Raw | - | 30 | 101 , 119 , 120 , 121 , 122 | |
| Ampicillin | Raw | - | 4 | 101 , 120 , 121 | |
| Cloxacillin | Raw | - | 30 | 101 , 134 , 120 , 121 | |
| Cefacetrile | Raw | - | 125 | 101 | |
| Chloramphenicol | Raw | - | - | 116 , 128 , 104 | |
| Rifaximin | Raw | - | - | 101 | |
| Mycotoxins | Aflatoxin M 1 | Raw | 0.5 | 0.05 | 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 |
| Pasteurized | - | - | 135 , 144 , 137 , 140 , 141 , 142 , 145 , 146 | ||
| UHT | - | - | 137 , 141 , 147 , 148 , 145 , 146 | ||
| Ochratoxin A | Raw | - | - | 135 , 136 , 137 | |
| Pasteurized | - | - | 135 , 144 , 136 , 149 | ||
| α-zearalenol | Raw | - | - | 135 , 137 , 150 | |
| Pasteurized | - | - | 135 | ||
| Fumonisin B1 | Raw | - | - | 137 | |
| Pasteurized | - | - | 144 , 137 | ||
| Fumonisin B2 | Pasteurized | - | - | 144 | |
| β-zearalenol | Raw | - | - | 150 | |
| Pasteurized | - | - | 144 | ||
| Zearalenone | Raw | - | - | 136 , 144 , 137 , 151 , 150 , 135 , 152 , 153 | |
| Pasteurized | - | - | 135 , 154 | ||
| Aflatoxin B1 | Raw | - | - | 136 , 137 , 140 | |
| Pasteurized | - | - | 144 , 136 , 137 , 140 | ||
| UHT | - | - | 137 | ||
| Aflatoxin B2 | Raw | - | - | 137 | |
| Pasteurized | - | - | 137 | ||
| Aflatoxin G1 | Raw | - | - | 137 | |
| Pasteurized | - | - | 137 | ||
| Aflatoxin G2 | Raw | - | - | 137 | |
| Pasteurized | - | - | 137 | ||
| Zearalanol | Raw | - | - | 137 | |
| α-zearalenone | Raw | - | - | 137 | |
| Cyclopiazonic acid | Pasteurized | - | - | 137 | |
| α-zearalanol | Pasteurized | - | - | 155 | |
| Deepoxy-deoxynivalenol | Raw | - | - | 137 | |
| Deoxynivalenol | Raw | - | - | 151 , 152 , 150 | |
| Aflatoxin M2 | Raw | - | - | 138 | |
| Pasteurized | - | - | 137 | ||
| UHT | - | - | 137 , 151 | ||
| Hormones | Leptin | Pasteurized | - | - | 124 , 156 |
| Triiodothyronine and Thyroxine | Pasteurized | - | - | 156 | |
| Prednisolone | Raw | - | 6 | 124 , 138 | |
| Relaxin | Pasteurized | - | - | 156 | |
| Insulin | Raw | - | - | 157 | |
| Pasteurized | - | - | 156 , 157 | ||
| Oxytocin | Pasteurized | - | - | 156 , 158 , 157 , 159 | |
| Adiponectin | Raw | - | - | 160 | |
| Pasteurized | - | - | 156 | ||
| Estriol | Raw | - | - | 161 | |
| UHT | - | - | 160 , 162 | ||
| 17α-Estradiol | Raw | - | - | 161 | |
| Pasteurized | - | - | 162 | ||
| UHT | - | - | 161 , 162 | ||
| 17β-Estradiol | Raw | - | - | 161 , 163 | |
| Pasteurized | - | - | 164 , 163 , 162 , 165 , 166 | ||
| UHT | - | - | 161 , 162 | ||
| Estrone | Raw | - | - | 161 , 167 , 163 | |
| Pasteurized | - | - | 164 , 163 , 162 | ||
| UHT | - | - | 161 , 162 | ||
| Testosterone | Raw | - | - | 161 , 167 | |
| Pasteurized | - | - | 161 | ||
| 4-Androstenediol | Raw | - | - | 161 | |
| 5-Androstenediol | Raw | - | - | 161 | |
| 4-Androstenedione | Raw | - | - | 161 | |
| Progesterone | Raw | - | - | 161 , 167 , 168 , 169 | |
| Pasteurized | - | - | 168 | ||
| UHT | - | - | 161 | ||
| 17α-Hydroxyprogesterone | Raw | - | - | 161 | |
| Cortisone | Raw | - | - | 161 | |
| Cortisol | Raw | - | - | 161 , 170 | |
| Corticosterone | Raw | - | - | 161 | |
| Hydrocortisone | Pasteurized | - | - | 171 | |
| Insulin-like Growth factor-I | Raw | - | - | 157 | |
| Pasteurized | - | - | 58 , 157 , 171 | ||
| Pregnenolone | Raw | - | - | 167 | |
| Androstenedione | Raw | - | - | 167 | |
| Pasteurized | - | - | 167 | ||
| Dehydroepiandrostenedione | Raw | - | - | 167 | |
| 5-α-Androstane-3,17-dione | Raw | - | - | 167 | |
| Prolactin | Pasteurized | - | - | 156 | |
| Growth Hormone | Pasteurized | - | - | 156 , 58 | |
MRLa: Maximum Residue Levels by Codex Alimentarius; MRLb: Maximum Residue Levels by European Union, EU. UHT: ultra-high temperature.
2.2.1 Pesticides
A variety of pesticide residues in detectable amounts in raw milk, pasteurized, and UHT (ultra-high temperature) milk has been reported by several authors. This is due, among other factors, to the lipophilic properties and resistance to biodegradation of these types of contaminants. 8 There are three possible forms in which pesticides can enter the animal's body 172 : (i) through contaminated water, (ii) through the pores of the skin when the animal is sprayed or soaked to treat ectoparasites, and (iii) through contaminated feed and forage, the latter being the main source of entry.
-
(i)
The presence of organophosphorus pesticide residues (malathion, methyl-parathion, diazinon, ethion) was identified. The average concentrations detected were 0.032-0.78, 0.13, 0.32-0.74, 0.010 μg/L for malathion, methyl-parathion, diazinon and ethion, respectively. 62 , 173 Fipronil and chlorpyrifos were other pesticides found in water samples supplied to livestock. 174 , 175 Ashoub & Azam 176 identified DDT (Dichloro diphenyl trichloroethane), aldrin, heptachlor epoxide, lindane, methoxychlor, diazinon, and deltamethrin in water samples from cattle farms. These same compounds have been identified in cattle drinking water and in cow's milk. 11 , 47 , 51 , 54 , 55 , 62 , 65 , 177 – 180 This verifies that water contaminated by pesticides and supplied to cattle is one of the main routes of contamination of raw cow's milk.
-
(ii)
According to the analysis of Table 2, Claborn et al. 181 report the presence of malathion residues in cow's milk after cattle were sprayed with this pesticide for the treatment of ectoparasites. Malathion was found to be completely secreted from the udder 24 hours after application. In contrast to malathion, lindane was reported not to be completely excreted in milk until seven days after application to the cow's skin. 182 Residues of chlorpyrifos and ethion have been found in cow milk up to 24 and 72 hours after application, respectively. 183 This confirms that skin contaminated with these pesticides is another route of contamination of raw cow's milk.
-
(iii)
In forage, concentrations of 0.02 mg kg -1 of DDT residue were reported. 184 The presence of cypermethrin, chlorpyrifos, cyhalothrin, and deltamethrin in forage was reported in a range of mean concentrations between 1.03-6.01 ng g -1. In addition to the presence of pesticides in forages, residues of lindane, DDT, fenvalerate, ethion, malathion, profenofos were also reported in feed. The mean concentrations of these varied in the range of 0.63-4.05 ng g -1. 175 The presence of deltamethrin in feed was also reported in a concentration range of 41.99-381.30 μg kg -1. 185 Another investigation revealed the presence of malathion, dimethoate, methyl-parathion, diazinon in the feed fed to cattle. The range of detected concentrations was between 0.01-80.45 μg L -1. 62 All the contaminants reported in forage and feed were also detected in cow's milk. 11 , 54 , 55 , 57 , 60 – 62 , 177 , 179 , 186 Thus, like water, pesticide-contaminated forage and feed are a route of contamination as they are directly ingested by cattle and excreted through cow's milk.
Pesticides are one of the most commonly found contaminants, not only in raw cow's milk but also after the pasteurization and UHT process. Their presence in milk, even below the maximum permitted levels, represents a health risk to the consumer. It is related to Hodgkin's disease (HD), non-Hodgkin's lymphoma (NHL), Parkinson's disease, endocrine disruption, respiratory and reproductive disorders, among others. 187
It is important to note that organochlorine pesticides such as hexachlorocyclohexane, dichloro diphenyl trichloroethane, and endosulfane are still present despite having been banned since the 1970s because of their high persistence in the environment and their harmful effects on human health, 188 are still detected in cow's milk. This indicates that they are still used in agriculture and animal husbandry. With a few exceptions (cyhalothrin, cypermethrin, fenvalerate, deltamethrin, permethrin, and diazinonella), the vast majority of pesticides found in cow's milk are not regulated by Codex and the EU. This demonstrates the low efficiency of the regulatory controls of these contaminants in the unprocessed and post-processed product, leading to an inefficient safety of this food product.
2.2.2 Metals
Although metals are found in the environment either naturally or due to industrial and/or agricultural activities, there are several routes by which they reach the milk. Namely, ingestion of contaminated food, fodder, and/or contaminated drinking water. In the soil, they are absorbed by many crop plant species, which, when ingested by animals, are transferred to the lactating glands and finally excreted in milk. 172 Equipment used in the dairy industry is another source of contamination directly to milk with metals such as chromium and nickel. 189 Heavy metals such as cadmium, lead, mercury, and arsenic reach milk by indirect contact through feed consumed by cattle. 189 Although the literature does not report the presence of metals in water or fodder destined for cattle, as well as in pesticides, these can be another of the main routes of contamination.
Several heavy metals have been reported in the literature to be found in raw cow's milk. The metals least found in studies of raw cow milk are tin and molybdenum. These elements are not abundant in nature, and their presence in fodder or water for animal consumption will depend on soil characteristics, while the most reported are lead, cadmium, copper, and zinc, due to environmental pollution produced by man mainly in industrial activities. 79 , 190 Minerals such as Fe, Cu, and Zn are necessary for various biological functions. However, high concentrations of these minerals have negative effects on human health. 96 Lead is one of the non-essential metals classified as carcinogenic to humans by the International Agency for Research on Cancer. 191 Cadmium is associated with the formation of human lung, kidney, breast, prostate, urinary tract cancer because it affects cell proliferation, differentiation, and other cellular activities. 192
None of the heavy metals reported in the literature consulted have established maximum residue limits (MRLs) by Codex 193 and the EU. 194 However, these contaminants are known to represent a high risk to human health. Stricter control measures should be adopted in the dairy industry, considering that cow's milk is one of the most consumed products by humans worldwide.
2.2.3 Antibiotics
Antibiotics are used in livestock activities in three basic ways: therapeutic, prophylactic, and growth promoters. About 80% of dairy cattle are subjected to antibiotic treatments on at least one occasion throughout their lives, mostly used as growth promoters and for the treatment of various diseases such as mastitis, arthritis, respiratory diseases, gastrointestinal diseases, and bacterial infections. 195 Cows eliminate antibiotics and their metabolites through milk, depending on the dose and route of application, level of milk production, type and degree of mammary disease, and time between treatment and milking. On the other hand, oral, intramuscular, or intravenous administration is less important from the point of view of milk hygiene than intramammary application. However, intramammary antibiotics are easy to apply and generally cheaper, so they are preferred in dairy farms.
The most common disease in dairy cows is mastitis, whose treatment includes the wide use of tetracyclines, β-lactams, oxytetracycline, difloxacin, among others, being the β-lactams of greater application. 8 Within the latter group, the most employed are penicillin, ampicillin, and amoxicillin. 196 According to the literature, the presence of antibiotics in milk has been evidenced, highlighting tetracycline, oxytetracycline, penicillin, and amoxicillin. 103 , 124 , 197 , 198 While other antibiotics less reported in milk were rifamixin, gatifloxacin, spiramycin, and lomeflaxacin, with no indication in the studies of the purpose of their application in cattle. 101 , 112 , 126 , 127
The consumption of contaminated milk with antibiotic residues is an emerging public health problem worldwide. Therefore, it is important to control the presence of antibiotic residues in food to avoid the appearance of resistance to these antibiotics in humans. The presence of antibiotics at concentrations even below the MRL in milk can cause undesirable effects on human health such as ototoxicity and nephrotoxicity, 199 endocrine disruption, 200 hypersensitivity, and especially bacterial resistance. 130 According to the literature consulted, 43 antibiotics present in cow's milk have been identified, of which 18 are not regulated by Codex 193 and EU standards. 194
Considering that the use of antibiotics in cattle generates residues in milk, their excessive use should be avoided, and the elimination times before milking should be respected in order to avoid the presence of these contaminants.
2.2.4 Mycotoxins
The quality of food products is commonly affected by toxin contamination, of which 60 to 80 % are caused by mycotoxins. 201 This means a risk for human health and great economic losses in the industrial sector.
Mycotoxins are natural contaminants produced by Aspergillus, Penicillium, and Fusarium fungi, 154 the most prominent being AFM1, which results from the metabolism of aflatoxin B1 in the liver of contaminated animals. 15 , 143 In the 1960s, the first reported case of aflatoxin contamination was reported for the first time, beginning the concern for this type of contaminant. Even during this decade, high consumption of feed contaminated by this mycotoxin was reported, which led to indirect contamination of cow's milk for consumption, compromising the safety of this product. 202 Therefore, it is considered that the main routes of entry of mycotoxins into milk are contaminated crops and feed ingested by cows. 136
It is known that approximately 0.3-6.2% of AFB1 (Aflatoxin B1) present in animal feed is converted to AFM1. 15 This mycotoxin is neither degraded nor removed by industrial food processes such as pasteurization and sterilization, nor by the cooking of feed. 203 This represents a difficult problem to deal with at the industrial level due to the stability of mycotoxins in general to thermal, physical, and chemical treatments. 204
AFM1 mycotoxin is the only regulated by Codex 193 and EU 194 and the most reported in cow's milk according to the literature. However, other abundant mycotoxins have been identified in this food product, such as ochratoxin A and zearalenone. The fungi of the genus Aspergillus and Penicillium produce Ochratoxin A, while fungi of the genus Fusarium produces zearalenone, commonly found in cattle feed. 138 On the other hand, aflatoxin G2, aflatoxin G1, aflatoxin B2, and zearalanol show a lower incidence in cow's milk. The literature on the effects on human health associated with the ingestion of mycotoxin-contaminated milk is scarce or almost non-existent, unlike AFM1. Therefore, studies on this type of contaminants should be expanded.
2.2.5 Hormones
The use of hormones in the livestock industry increases production yields and medical treatments. Their fat-soluble characteristics favor their high persistence and presence in cow's milk due to the high-fat contents. 156 Therefore, the supply of hormones to cattle represents a form of direct contamination that, like other contaminants, is excreted through milk. However, the European Union banned the use of hormones through the Directive 96/22/EC, and enforcement is regulated by Directive 96/23/EC. 165
Prednisolone in combination with amoxicillin and clavulanic acid is used to treat mastitis in cows' udders, 205 being an access route of this contaminant to milk. The 17β-estradiol and progesterone, with the highest presence in cow milk, are sex hormones widely used to induce lactation, improve fertility and synchronize the estrous cycle. 8 , 168 The hormones least found in studies in milk were testosterone, somatostatin, and cortisone. The presence of estrogens in cow's milk has been linked to diseases such as breast cancer 206 and conditions in the gastrointestinal tract. 156 Other diseases associated with the presence of hormones in cow's milk have included acne, prostate cancer, uterine cancer, and male reproductive disorders. 167
Table 2 shows that several hormones are frequently present in cow's milk, with prednisolone being the only one regulated by the EU. 194 This indicates that regulations should be established for different hormones considering that they are the chemical compounds mostly used to increase milk production yield to preserve quality and consumer safety.
3. Pasteurization process in cow's milk
The principles and name of pasteurization come from the studies of the French scientist Louis Pasteur. His interest in milk and other food products was due to their putrefaction, which he later attributed to the growth of undesirable microorganisms. 207 Several pathogenic microorganisms are found in raw milk: Pseudomonas, Enterobacter, Bacillus, Clostridium, Microbacterium, and Micrococcus. Pathogenic microorganisms in cow's milk have been linked to infectious diseases such as campylobacteriosis, salmonellosis, yersiniosis, listeriosis, tuberculosis, brucellosis, staphylococcal enterotoxin intoxication, streptococcal infections, and Escherichia coli O157: H7 infection. 208
It was not until the end of the 1880s that heat treatment began to be used to commercialize milk. This arose with the main objective of inactivating Mycobacterium tuberculosis, the cause of tuberculosis in humans associated with the consumption of raw milk. Thus, pasteurization became a process universally employed by developed countries after World War II. However, there is evidence that not all pathogenic microorganisms can be eliminated during pasteurization, such as Staphylococcus aureus, micrococci, Streptococcus spp, and Bacillus. 209 Which calls into question the efficiency of this process.
The US Food and Drug Administration (FDA) establishes a maximum limit for bacteria in raw cow's milk of 100,000 cfu ml -1 and 20,000 cfu ml -1 for pasteurized milk. 209
Pasteurization is a technology classified on the basis of operating temperatures and exposure times as follows: LTLT, HTST, and UHT. Low-temperature long-time pasteurization (LTLT) uses a minimum temperature of 62.8°C and a minimum time of 30 min. High-temperature short-time pasteurization (HTST) uses a minimum temperature of 71.1°C, a minimum time of 15 seconds, and ultra-high temperature pasteurization (UHT) works at a minimum of 135°C and during a minimum time of 1 second. 210 Pasteurized milk under UHT conditions can be stored for several months without refrigeration. 211 Whereas the shelf life of pasteurized milk ranges from 10 to 20 days when kept under refrigerated conditions below 6.1°C. 212
It has been shown that the application of pasteurization denatures proteins with bacteriostatic capacity, as is the case of lactoferrin. This is a glycoprotein that binds iron, and its complete denaturation has been evidenced losing its inhibitory capacity on Escherichia coli under UHT conditions. 213 For this reason, it is suggested that heat treatment should be applied below 75°C to avoid denaturation of proteins with bacteriostatic capacity and at the same time cause inactivation of pathogenic microorganisms. 213
On the other hand, the HTST process degrades up to 20% of the vitamins (B1, B6, B12, and C) present in milk. 214 This evidence shows that, although pasteurization and UHT have been widely used to eliminate pathogenic microorganisms, it is not entirely efficient for this purpose. There are even losses of milk mineralization, varying its nutritional composition.
The presence of microbial contaminants in different samples of pasteurized milk shows that, although pasteurization aims to eliminate microorganisms present in milk, it is not totally effective. Moreover, with the appearance of other contaminants, the quality of milk no longer depends only on the presence of microorganisms. It is, therefore, necessary to study other methods of decontamination to ensure the safety and health of consumers.
4. Alternative methods for the treatment of cow's milk
International regulations require maximum limits for microbial and chemical contaminants to ensure the quality of drinking milk. Pasteurization is a technology widely used in the dairy industry. However, it is exclusive for the elimination of microbial contaminants. The literature mentions alternatives for eliminating specific microbial and chemical contaminants ( Table 3).
Table 3. Alternative methods to pasteurization for removal of contaminants in bovine milk.
| Contaminant | Process | Reference | |
|---|---|---|---|
| Pathogens | Escherichia coli | Inactivation with supercritical carbon dioxide technology | 215 |
| Escherichia coli and Listeria innocua | Inactivation using a UV-C thin film reactor | 216 | |
| Inactivation by pulsed electric fields | 217 | ||
| Aerobic bacteria | Reduction by UV-C irradiation | 218 | |
| Listeria monocytogenes | Inactivation by ozonation | 219 | |
| Staphylococcus aureus, Listeria monocytogenes, Lactobacillus plantarum, Lactobacillus pentosus, Salmonella Typhimurium, Escherichia coli, Pseudomonas fluorescens | Inactivation by combinations of ultrasound, hydrogen peroxide, and active lactoperoxidase system | 220 | |
| Aerobic bacteria, coliforms, yeasts, and molds | Inactivation by carbon dioxide at high pressure | 221 | |
| Escherichia coli, Salmonella, Listeria monocytogenes, Enterobacteriaceae, lactic acid bacteria, and Pseudomonas spp. | Inactivation by high-pressure processing | 222 | |
| Escherichia coli, Salmonella, yeasts, and lactobacillus spp. | Inactivation by ND-YAG laser | 223 | |
| Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Listeria innocua | Inactivation by pulsed electric fields | 224 | |
| Escherichia coli, Staphylococcus aureus, and Pseudomonas fluorescens | Inactivation by manothermosonication | 225 | |
| Pesticides | Organophosphates (chlorpyrifos, diazinon, fenitrothion, malathion, methyl parathion) | Degradation by lactic acid bacteria | 226 |
| Methyl parathion | High-intensity ultrasound | 227 | |
| Dimethoate, fenthion, malathion, methyl parathion, monocrotophos, phorate, and trichlorfon | Degradation by lactobacillus spp. bacteria at 42°C | 228 | |
| Metals | Pb 2+ and Hg 2+ | Adsorption with pluronic p123 diacrylate hydrogels | 229 |
| Lead | Biosorption with Saccharomyces cerevisiae | 230 | |
| Biosorption with Lactobacillus acidophilus ATCC 4356 | 231 | ||
| Mercury | Biosorption with Lactobacillus acidophilus ATCC 4356 | 232 | |
| Biosorption with Saccharomyces cerevisiae | 233 | ||
| Copper | Adsorption using imac hp resin | 92 | |
| Cadmium | Biosorption with Saccharomyces cerevisiae | 234 , 235 | |
| Biosorption with Lactobacillus acidophilus ATCC 4356 | 231 | ||
| Antibiotics | Amoxicillin, doxycycline, ciprofloxacin, and sulfadiazine | Ozonization | 236 |
| Chlortetracycline and cefazolin | Electrochemical method | 237 | |
| Tetracycline | Electrochemical method | 238 | |
| Adsorption with molecularly imprinted polymer | 239 | ||
| Ciprofloxacin | Adsorption with BiPO 4 @ fluorescent photocatalytic graphene oxide-based magnetic molecular imprinted polymer | 240 | |
| Amoxicillin, ciprofloxacin, doxycycline | Decomposition by gamma irradiation | 241 | |
| Mycotoxins | Aflatoxin M1 | Adsorption with molecularly imprinted polymer coated on the surface of the stainless-steel plate | 242 |
| Removal using Saccharomyces cerevisiae and Lactobacillus helveticus | 243 | ||
| Adsorption with clay minerals (kaolin and bentonite) | 244 | ||
| Elimination by a combination of yeast and probiotic bacteria species | 245 , 246 | ||
| Biofilm elimination of Lactobacillus rhamnosus gg | 247 | ||
| Adsorption with clay minerals (kaolin and bentonite) | 248 |
UV-C: Ultraviolet-C (200-280 nm); Nd:YAG: neodymium-doped yttrium aluminum garnet; Pb: lead; Hg: mercury; ATCC: American Type Culture Collection; BiPO 4: Bismuth phosphate (III).
Supercritical carbon dioxide has been used as an inactivating agent for E. coli, where the greatest reduction in the content of microorganisms was observed during a residence time of 20 minutes, achieving almost complete inactivation after 70 minutes. 215 Complete inactivation of coliforms, molds, and yeasts was achieved, while a maximum reduction of aerobic bacteria of 4.96 log was obtained using high-pressure carbon dioxide. 221 Using a thin-film UV-C (Ultraviolet-C) reactor with flow-guiding elements allowed a 4.58 log and 3.19 log reduction for E. coli and L. innocua, respectively. 216 Makarapong et al. 218 employed a UV-C reactor for the inactivation of aerobic bacteria achieving a 4.60 log and 4.70 log reduction at 48W and 39W, respectively. UV-C lamp wattage did not significantly influence the fat concentration in the milk, which means that it is necessary to improve the method to guarantee an effective reduction of these microorganisms if milk transport time exceeds two hours without cooling. It was verified that L. monocytogenes was completely inactivated in milk with ozone for 15 minutes. However, nutritional values were affected. 219 Exposure of milk to Nd:YAG laser did not alter the physicochemical properties of milk, but the percentage of reduction was low for E.coli (30%), Salmonella sp (25%), yeasts (47%), and Lactobacillus sp (30%). 223 The combination of ultrasound with hydrogen peroxide and an active lactoperoxidase system was able to guarantee the microbial quality of milk as it was able to completely inactivate Staphylococcus aureus, Listeria monocytogenes, Lactobacillus plantarum, Lactobacillus pentosus, Salmonella Typhimurium, Escherichia coli, and Pseudomonas fluorescens at 10 minutes at an amplitude of 125 μm. 220 The application of ultrasound in combination with variations in temperature, time, and constant pressure (manothermosonication) achieved minimal reductions of up to 1.6 log CFU/ml for E. coli and P.fluorescens and 1.05 log CFU/ml for S. aureus. Further studies are needed to ensure effective inactivation using manothermosonication. 225 The application of high pressures (400-600MPa) effectively inactivated (5 log CFU/ml) E. coli, Salmonella and L. monocytogenes, Enterobacteriaceae, lactic acid bacteria, and Pseudomonas spp. 222 One of the most widely used methods for the inactivation of microorganisms in cow's milk is pulsed electric fields (PEF). This method was applied for the inactivation of E.coli and L. innocua, achieving a reduction of 2 log CFU/ml. 217 It was found that combining this method with preheating at 50°C achieved a 5-6 log CFU/ml reduction of Pseudomonas aeruginosa and a total reduction of E. coli, S. aureus, and L. innocua. 224
Biosorption methods employing the use of microorganisms prove to be efficient in the removal of pesticides, metals, and mycotoxins. Biosorption with lactic acid bacteria managed to eliminate organophosphate pesticides from cow's milk, being more effective for chlorpyrifos, fenitrothion, and malathion, whose degradation constants were greater than 0.018 h -1. On the other hand, diazinon and methyl parathion were more resistant when applying of the different strains of lactic acid bacteria separately and in combination. The degradation rate constants were correlated with the measurement of phosphatase activity, and it was found that the lower the phosphatase activity, the lower the degradation constant. 226 The same method was applied for this group of contaminants finding that dimethoate and methyl parathion were the most stable with the lowest degradation rate constants (0.0165-0.0184 and 0.0213 h -1, being more efficient for the removal of malathion with higher degradation rate constants (0.0218-0.0420 h -1). 228 Although the application of lactic acid bacteria was shown to be an effective method for removing diazinon, dimethoate, and methyl parathion in cow's milk it was not very selective since it cannot eliminate all the organophosphates studied.
Biosorption with Saccharomyces Cerevisiae allowed the removal of 70% of lead, mercury, and cadmium metals. 230 , 233 – 235 The removal percentage was higher when Lactobacillus Acidophilus was used, eliminating 80, 75, and 72%, respectively. 231 , 232 The use of Saccharomyces cerevisiae and Lactobacillus helveticus removed AFM1 from milk by an as yet unknown binding mechanism. 243 A combination of probiotic bacteria with yeast species managed to remove 90.88% of AFM1 within 72 hours. 245 This percentage of removal was higher than that obtained in another study (19-61%). 246 By applying a biofilm of Lactobacillus rhamnosus, an AFM1 removal of 60.74% was achieved. Despite that, the method is not a viable alternative for application because a reduction in the percentage of fat and total dry matter was observed. 247
Biosorption methods employing microorganisms ( Lactobacillus acidophilus and Saccharomyces cerevisiae) are efficient for removing heavy metals in cow's milk (lead, mercury, copper, and cadmium). However, they require a minimum fermentation period of 4 days. When using lactic acid bacteria to degrade organophosphorus pesticides, a minimum fermentation period of 24 hours is required. These times would represent economic losses for the industry, and given the existing world demand for milk, it would be almost impossible to apply them on a large scale.
Adsorption methods prove to be efficient for removing metals, antibiotics, and mycotoxins. By adsorption with diacrylate Pluronic P123 (P123-DA) hydrogels removed about 85.3% and 81.9% of Pb 2+, and Hg 2+ ions, respectively. 229 Resins have been another adsorbent used in the adsorption of heavy metals in cow's milk. IMAC HP resin was described for the removal of copper ions (76.89%). 92 Tetracycline, oxytetracycline, chlortetracycline, and doxycycline have been removed by adsorption on a molecularly imprinted polymer, achieving 81.83, 95.47, 96.44, and 93.25% removal, respectively. 239 A photocatalytic-fluorescent polymer, produced from graphene oxide and bismuth phosphate with molecular magnetic imprinting, allowed ciprofloxacin's complete degradation. 240 Bodbodak et al., 242 developed a molecularly imprinted polymer coated on the surface of a stainless-steel plate as an adsorbent material for the decontamination of AFM1 in cow's milk. This method was able to remove 87.3 to 96.2% of AFM1 without causing a change in the physicochemical properties of the milk. Adsorption with kaolin and natural calcium bentonite clay for adsorption was able to remove AFM1 by 86.1-93.3% and 93.7-97.7%, respectively. It was observed that no change in the nutritional properties of milk would occur. 244 Despite this, few studies have been reported in cow's milk. Therefore, there are not enough to consider its application at the industrial level.
Other methods less reported in the literature were also applied for the removal of pesticides and antibiotics. The ultrasonic treatment proved to be effective for the degradation of 97.10% of methyl parathion. However, this method is limited by the generation of degradation products with toxic effects. 227 For the elimination of antibiotics in cow's milk, methods such as ozonation have been applied, with about 95% degradation for amoxicillin, doxycycline, ciprofloxacin, and sulfadiazine. 236 Electrochemical oxidation applied for the removal of small concentrations of chlortetracycline, cefazolin, 237 and oxytetracycline 238 was also described. Gamma radiation was also found to be effective for the removal of amoxicillin, ciprofloxacin, and doxycycline by 90% in cow's milk samples. 241 However, of all the antibiotics detected in cow's milk, they have only been tested for the elimination of amoxicillin, doxycycline, ciprofloxacin, sulfadiazine, chlortetracycline, cefazolin, y tetracycline. More studies are needed to validate the application of these methods for the decontamination of cow's milk.
It has not been demonstrated that a single method is capable of eliminating different groups of contaminants, as is the case of pasteurization for microbial contaminants. Despite the wide use of hormones in the cattle industry and their consequent generation of traces in cow's milk, no removal methods have been reported for them. The alternative methods studied to date have been applied on an industrial scale, and many of them alter the nutritional properties of milk. The fact that most of these chemical contaminants are not regulated by standards does not oblige the dairy industry to use alternative methods to pasteurization. Nor is it economically viable to use a different method for the elimination of each contaminant present in milk. However, to guarantee the safety of milk, it is essential to study processes that complement pasteurization and can eliminate pathogenic microorganisms and chemical contaminants.
5. Conclusions and future prospects
The presence of contaminants in raw cow's milk (many of them banned) is an indication that they are currently used illegally in both agriculture and animal husbandry. Although the presence of contaminant residues in milk represents a health risk to the consumer, there are no MRLs established for all of them. In addition, pasteurization processes are not efficient for the degradation or elimination of the different contaminants addressed.
Although, the literature exposes alternative methods for removing various contaminants in milk, they are still not sufficient nor applied on an industrial scale. Instead, they have been applied individually or in very small families of contaminants. There are no evidence or results concerning the interactions between them or with intermediate products formed on cow's milk, nor changes in the organoleptic properties. A particular case is hormones, which although they are a direct source of contamination, with evidence of their presence in raw, pasteurized, and UHT milk, the literature does not report specific elimination methods for these types of contaminants.
However, alternative methods have proven to be efficient in degrading several contaminants present in milk. Based on this hypothesis, it is suggested to deepen the application of these methods, including the study of interactions between different families of contaminants, application of new materials, or modification of existing ones. Studies on toxicity or changes in organoleptic properties. In this sense, the field of nano-biotechnology, nano-fibers, nano-membranes, biochar, MOF's (metal-organic framework), among others, could play a relevant role, guaranteeing the safety of the milk consumed, and consequently, a better quality of life for consumers.
Data availability
No data are associated with this article.
Acknowledgments
The authors would like to thank the Universidad Técnica de Manabí for the support offered.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 3 approved
References
- 1. O’Mahony JA, Fox PF: Milk: An Overview. Milk Proteins. Singh H, Boland M, Thompson A, editors. San Diego: Academic Press;2014; p.19–73. 10.1016/B978-0-12-405171-3.00002-7 [DOI] [Google Scholar]
- 2. FAO: Food Outlook. Trade and Market Division of FAO. Food and Agriculture Organization of the United Nations;2010 [cited 2020 Sep, 29]. Reference Source [Google Scholar]
- 3. FAO: Food Outlook-Biannual Report on Global Food Markets. 2019 [cited 2020 Sep, 27]. Reference Source
- 4. Fox PF: Milk|Bovine Milk. Encyclopedia of Dairy Sciences. Fuquay JW, editor. San Diego: Academic Press;2011; p.478–483. 10.1016/B978-0-12-374407-4.00312-5 [DOI] [Google Scholar]
- 5. Jensen RG: Contaminants in Bovine Milk. Handbook of Milk Composition. Jensen RG, editor. San Diego: Academic Press;1995; p.887–901. 10.1016/B978-012384430-9/50040-8 [DOI] [Google Scholar]
- 6. Oliveira GB, et al. : Psychrotrophic bacteria in milk: How much do we really know?. Braz. J. Microbiol. 2015;46:313–321. 10.1590/S1517-838246220130963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alegbeleye OO, et al. : Hazards of a ‘healthy’ trend? An appraisal of the risks of raw milk consumption and the potential of novel treatment technologies to serve as alternatives to pasteurization. Trends Food Sci. Technol. 2018;82:148–166. 10.1016/j.tifs.2018.10.007 [DOI] [Google Scholar]
- 8. Fischer WJ, et al. : Contaminants of Milk and Dairy Products: Contamination Resulting from Farm and Dairy Practices. Reference Module in Food Science. Elsevier;2016. 10.1016/B978-0-08-100596-5.00698-3 [DOI] [Google Scholar]
- 9. Ryser ET: Pasteurization of Liquid Milk Products: Principles, Public Health Aspects. Reference Module in Food Science. Elsevier;2016. 10.1016/B978-0-08-100596-5.21367-X [DOI] [Google Scholar]
- 10. Tamime AY: Milk processing and quality management. John Wiley & Sons;2009. 10.1002/9781444301649 [DOI] [Google Scholar]
- 11. Bedi JS, et al. : Pesticide Residues in Bovine Milk in Punjab, India: Spatial Variation and Risk Assessment to Human Health. Arch. Environ. Contam. Toxicol. 2015;69(2):230–240. 10.1007/s00244-015-0163-6 [DOI] [PubMed] [Google Scholar]
- 12. Kantiani L, et al. : Fully automated analysis of beta-lactams in bovine milk by online solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Anal. Chem. 2009;81(11):4285–4295. 10.1021/ac9001386 [DOI] [PubMed] [Google Scholar]
- 13. Hoogenboom LA, et al. : Carry-over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011;28(3):359–372. 10.1080/19440049.2010.547521 [DOI] [PubMed] [Google Scholar]
- 14. Schettler T: Human exposure to phthalates via consumer products. Int. J. Androl. 2006;29(1):134–139. 10.1111/j.1365-2605.2005.00567.x [DOI] [PubMed] [Google Scholar]
- 15. Mollayusefian I, et al. : The concentration of aflatoxin M1 in raw and pasteurized milk: A worldwide systematic review and meta-analysis. Trends Food Sci. Technol. 2021;115:22–30. 10.1016/j.tifs.2021.06.033 [DOI] [Google Scholar]
- 16. Yadav J, et al. : Comparative evaluation of pathogenic bacterial incidence in raw and pasteurized milk. Int. J. Eng. Sci. Invention. 2014;3(5):11–20. [Google Scholar]
- 17. Weber M, et al. : Bacterial community composition of biofilms in milking machines of two dairy farms assessed by a combination of culture-dependent and–independent methods. PLoS One. 2019;14(9):1–21. 10.1371/journal.pone.0222238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franco MM, et al. : Occurrence of mycobacteria in bovine milk samples from both individual and collective bulk tanks at farms and informal markets in the southeast region of Sao Paulo. Brazil. BMC Vet. Res. 2013;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zumárraga MJ, et al. : Detection of Mycobacterium bovis–infected dairy herds using PCR in bulk tank milk samples. Foodborne Pathog. Dis. 2012;9(2):132–137. 10.1089/fpd.2011.0963 [DOI] [PubMed] [Google Scholar]
- 20. Vithanage NR, et al. : Biodiversity of culturable psychrotrophic microbiota in raw milk attributable to refrigeration conditions, seasonality and their spoilage potential. Int. Dairy J. 2016;57:80–90. 10.1016/j.idairyj.2016.02.042 [DOI] [Google Scholar]
- 21. Oliveira Pinto C, et al. : Identificação de bactérias psicrotróficas proteolíticas isoladas de leite cru refrigerado e caracterização do seu potencial deteriorador. Revista do Instituto de Laticínios Cândido Tostes. 2015;70(2):105–116. 10.14295/2238-6416.v70i2.401 [DOI] [Google Scholar]
- 22. Lo R, et al. : Culture-independent bacterial community profiling of carbon dioxide treated raw milk. Int. J. Food Microbiol. 2016;233:81–89. 10.1016/j.ijfoodmicro.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 23. Vithanage NR, et al. : Comparison of identification systems for psychrotrophic bacteria isolated from raw bovine milk. Int. J. Food Microbiol. 2014;189:26–38. 10.1016/j.ijfoodmicro.2014.07.023 [DOI] [PubMed] [Google Scholar]
- 24. Machado SG, et al. : Pseudomonas spp. and Serratia liquefaciens as Predominant Spoilers in Cold Raw Milk. J. Food Sci. 2015;80(8):M1842–M1849. 10.1111/1750-3841.12957 [DOI] [PubMed] [Google Scholar]
- 25. Decimo M, et al. : Characterization of Gram-negative psychrotrophic bacteria isolated from Italian bulk tank milk. J. Food Sci. 2014;79(10):M2081–M2090. 10.1111/1750-3841.12645 [DOI] [PubMed] [Google Scholar]
- 26. Neubeck M, et al. : Biodiversity of refrigerated raw milk microbiota and their enzymatic spoilage potential. Int. J. Food Microbiol. 2015;211:57–65. 10.1016/j.ijfoodmicro.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 27. Kable ME, et al. : The Core and Seasonal Microbiota of Raw Bovine Milk in Tanker Trucks and the Impact of Transfer to a Milk Processing Facility. MBio. 2016;7(4). 10.1128/mBio.00836-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vacheyrou M, et al. : Cultivable microbial communities in raw cow milk and potential transfers from stables of sixteen French farms. Int. J. Food Microbiol. 2011;146(3):253–262. 10.1016/j.ijfoodmicro.2011.02.033 [DOI] [PubMed] [Google Scholar]
- 29. Raats D, et al. : Molecular analysis of bacterial communities in raw cow milk and the impact of refrigeration on its structure and dynamics. Food Microbiol. 2011;28(3):465–471. 10.1016/j.fm.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 30. Marjan S, et al. : Drug-resistant bacterial pathogens in milk and some milk products. Nutr. Food Sci. 2014;44(3):241–248. 10.1108/NFS-05-2013-0061 [DOI] [Google Scholar]
- 31. Artursson K, et al. : Foodborne pathogens in unpasteurized milk in Sweden. Int. J. Food Microbiol. 2018;284:120–127. 10.1016/j.ijfoodmicro.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 32. Mallet A, et al. : Quantitative and qualitative microbial analysis of raw milk reveals substantial diversity influenced by herd management practices. Int. Dairy J. 2012;27(1-2):13–21. 10.1016/j.idairyj.2012.07.009 [DOI] [Google Scholar]
- 33. Boubendir A, et al. : Changes in bacterial populations in refrigerated raw milk collected from a semi-arid area of Algeria. Ann. Microbiol. 2015;66(2):777–783. [Google Scholar]
- 34. Christidis T, et al. : Campylobacter spp. Prevalence and Levels in Raw Milk: A Systematic Review and Meta-Analysis. J. Food Prot. 2016;79(10):1775–1783. 10.4315/0362-028X.JFP-15-480 [DOI] [PubMed] [Google Scholar]
- 35. Bianchini V, et al. : Prevalence in bulk tank milk and epidemiology of Campylobacter jejuni in dairy herds in Northern Italy. Appl. Environ. Microbiol. 2014;80(6):1832–1837. 10.1128/AEM.03784-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruusunen M, et al. : Pathogenic bacteria in Finnish bulk tank milk. Foodborne Pathog. Dis. 2013;10(2):99–106. 10.1089/fpd.2012.1284 [DOI] [PubMed] [Google Scholar]
- 37. Silva R, et al. : Pasteurized milk: efficiency of pasteurization and its microbiological conditions in Brazil. Foodborne Pathog. Dis. 2010;7(2):217–219. 10.1089/fpd.2009.0332 [DOI] [PubMed] [Google Scholar]
- 38. Braem G, et al. : Culture-independent exploration of the teat apex microbiota of dairy cows reveals a wide bacterial species diversity. Vet. Microbiol. 2012;157(3-4):383–390. 10.1016/j.vetmic.2011.12.031 [DOI] [PubMed] [Google Scholar]
- 39. White DG, et al. : Isolation and identification of coagulase-negative Staphylococcus species from bovine body sites and streak canals of nulliparous heifers. J. Dairy Sci. 1989;72(7):1886–1892. 10.3168/jds.S0022-0302(89)79307-3 [DOI] [PubMed] [Google Scholar]
- 40. Ercolini D, et al. : Molecular identification of mesophilic and psychrotrophic bacteria from raw cow's milk. Food Microbiol. 2009;26(2):228–231. 10.1016/j.fm.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 41. Desmasures N, Bazin F, Gueguen M: Microbiological composition of raw milk from selected farms in the Camembert region of Normandy. J. Appl. Microbiol. 1997;83(1):53–58. 10.1046/j.1365-2672.1997.00166.x [DOI] [PubMed] [Google Scholar]
- 42. Wilson DJ, Gonzalez RN, Das HH: Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J. Dairy Sci. 1997;80(10):2592–2598. 10.3168/jds.S0022-0302(97)76215-5 [DOI] [PubMed] [Google Scholar]
- 43. Makovec JA, Ruegg PL: Results of milk samples submitted for microbiological examination in Wisconsin from 1994 to 2001. J. Dairy Sci. 2003;86(11):3466–3472. 10.3168/jds.S0022-0302(03)73951-4 [DOI] [PubMed] [Google Scholar]
- 44. Jayarao BM, Wang L: A study on the prevalence of gram-negative bacteria in bulk tank milk. J. Dairy Sci. 1999;82(12):2620–2624. 10.3168/jds.S0022-0302(99)75518-9 [DOI] [PubMed] [Google Scholar]
- 45. Ternstrom A, Lindberg AM, Molin G: Classification of the spoilage flora of raw and pasteurized bovine milk, with special reference to Pseudomonas and Bacillus. J. Appl. Bacteriol. 1993;75(1):25–34. 10.1111/j.1365-2672.1993.tb03403.x [DOI] [PubMed] [Google Scholar]
- 46. Claeys WL, et al. : Raw or heated cow milk consumption: Review of risks and benefits. Food Control. 2013;31(1):251–262. 10.1016/j.foodcont.2012.09.035 [DOI] [Google Scholar]
- 47. Aydin S, et al. : Organohalogenated pollutants in raw and UHT cow's milk from Turkey: a risk assessment of dietary intake. Environ. Sci. Pollut. Res. Int. 2019;26(13):12788–12797. 10.1007/s11356-019-04617-0 [DOI] [PubMed] [Google Scholar]
- 48. Kaushik CP, Kaushik A, Sharma HR: Seasonal trends in organochlorine pesticide residues in raw bovine milk from rural areas of Haryana, India. Bull. Environ. Contam. Toxicol. 2014;92(1):15–22. 10.1007/s00128-013-1094-4 [DOI] [PubMed] [Google Scholar]
- 49. Gutiérrez R, et al. : Organochlorine pesticide residues in bovine milk from organic farms in Chiapas, Mexico. Bull. Environ. Contam. Toxicol. 2012;89(4):882–887. 10.1007/s00128-012-0764-y [DOI] [PubMed] [Google Scholar]
- 50. Kaushik CP, et al. : Changing patterns of organochlorine pesticide residues in raw bovine milk from Haryana, India. Environ. Monit. Assess. 2011;182(1-4):467–475. 10.1007/s10661-011-1890-4 [DOI] [PubMed] [Google Scholar]
- 51. Bošnir J, et al. : Organochlorine pesticide residues in cows’ milk from Karlovac County, Croatia. Acta Aliment. 2010;39(3):317–326. 10.1556/AAlim.39.2010.3.8 [DOI] [Google Scholar]
- 52. Avancini RM, et al. : Organochlorine compounds in bovine milk from the state of Mato Grosso do Sul–Brazil. Chemosphere. 2013;90(9):2408–2413. 10.1016/j.chemosphere.2012.10.069 [DOI] [PubMed] [Google Scholar]
- 53. Lans-Ceballos E, Lombana-Gomez M, Pinedo-Hernandez J: Organochlorine insecticide residues in pasteurized milk distributed in Monteria Colombia. Rev. Salud Publica. (Bogota). 2018;20(2):208–214. 10.15446/rsap.v20n2.51175 [DOI] [PubMed] [Google Scholar]
- 54. Gill JPS, et al. : Pesticide Residues in Peri-Urban Bovine Milk from India and Risk Assessment: A Multicenter Study. Sci. Rep. 2020;10(1):8054. 10.1038/s41598-020-65030-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shahzadi N, et al. : Identification of pesticides residues in different samples of milk. J. Agroaliment. Process. Technol. 2013;19(2):167–172. [Google Scholar]
- 56. Sajid MW, et al. : The impact of seasonal variation on organochlorine pesticide residues in buffalo and cow milk of selected dairy farms from Faisalabad region. Environ. Monit. Assess. 2016;188(10):589. 10.1007/s10661-016-5594-7 [DOI] [PubMed] [Google Scholar]
- 57. ul Hassan A, et al. : Organochlorine and pyrethroid pesticides analysis in dairy milk samples collected from cotton growing belt of Punjab, Pakistan. Pak. J. Agric. Sci. 2014;51(2):331–335. [Google Scholar]
- 58. Welsh JA, et al. : Production-related contaminants (pesticides, antibiotics and hormones) in organic and conventionally produced milk samples sold in the USA. Public Health Nutr. 2019;22(16):2972–2980. 10.1017/S136898001900106X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen X, et al. : Method for the quantification of current use and persistent pesticides in cow milk, human milk and baby formula using gas chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014;970:121–130. 10.1016/j.jchromb.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 60. Jayasinghe J, et al. : Pesticide residues in cow milk and dairy products from the major milk producing area of Sri Lanka. AGROFOR Int. J. 2019;4:83–90. 10.7251/AGRENG1903083J [DOI] [Google Scholar]
- 61. Nath A, et al. : Carcinogenic pesticides residue detection in cow milk and water samples from Patna, India. Current Trends in Biotechnology and Chemical Research. 2013;3(1):1–7. [Google Scholar]
- 62. Fagnani R, et al. : Organophosphorus and carbamates residues in milk and feedstuff supplied to dairy cattle. Pesqui. Vet. Bras. 2011;31(7):598–602. 10.1590/S0100-736X2011000700009 [DOI] [Google Scholar]
- 63. Salas JH, et al. : Organophosphorus pesticide residues in Mexican commercial pasteurized milk. J. Agric. Food Chem. 2003;51(15):4468–4471. 10.1021/jf020942i [DOI] [PubMed] [Google Scholar]
- 64. Kampire E, et al. : Organochlorine pesticide in fresh and pasteurized cow's milk from Kampala markets. Chemosphere. 2011;84(7):923–927. 10.1016/j.chemosphere.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 65. Tian H: Determination of chloramphenicol, enrofloxacin and 29 pesticides residues in bovine milk by liquid chromatography-tandem mass spectrometry. Chemosphere. 2011;83(3):349–355. 10.1016/j.chemosphere.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 66. Kuba J, et al. : Comparison of DDT and its metabolites concentrations in cow milk from agricultural and industrial areas. J. Environ. Sci. Health B. 2015;50(1):1–7. 10.1080/03601234.2015.964128 [DOI] [PubMed] [Google Scholar]
- 67. Castilla Y, Mercado I, González G: Determinación y cuantificación de los niveles de compuestos organoclorados en leche pasteurizada. Producción + Limpia. 2012;7(1):19–31. [Google Scholar]
- 68. Hernández M, Vidal JV, Marrugo JL: Organochlorine pesticides in cows' milk supplemented with cotton waste in San Pedro, Colombia. Revista de salud publica (Bogota, Colombia). 2010;12(6):982–989. [PubMed] [Google Scholar]
- 69. Bulut S, et al. : Organochlorine pesticide (OCP) residues in cow’s, buffalo’s, and sheep’s milk from Afyonkarahisar region, Turkey. Environ. Monit. Assess. 2011;181(1-4):555–562. 10.1007/s10661-010-1849-x [DOI] [PubMed] [Google Scholar]
- 70. Luzardo OP, et al. : Polychlorobiphenyls and organochlorine pesticides in conventional and organic brands of milk: occurrence and dietary intake in the population of the Canary Islands (Spain). Chemosphere. 2012;88(3):307–315. 10.1016/j.chemosphere.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 71. Karabasanavar NS, Singh SP: Occurrence of endosulphan residues in dairy milk in plains of Uttarakhand, India. Int. J. Dairy Technol. 2013;66(2):189–193. 10.1111/1471-0307.12007 [DOI] [Google Scholar]
- 72. Kim DG, et al. : Monitoring of environmental contaminants in raw bovine milk and estimates of dietary intakes of children in South Korea. Chemosphere. 2013;93(3):561–566. 10.1016/j.chemosphere.2013.06.055 [DOI] [PubMed] [Google Scholar]
- 73. Özdemir C, et al. : Determination of organochlorine pesticide residues in pasteurized and sterilized milk using QuEChERS sample preparation followed by gas chromatography–mass spectrometry. J. Food Process. Preserv. 2019;43(11):e14173. 10.1111/jfpp.14173 [DOI] [Google Scholar]
- 74. Bommuraj V, et al. : Human pharmaceutical and pesticide residues in Israeli dairy milk in association with dietary risk assessment. Food Addit. Contam. Part B Surveill. 2020;13(4):233–243. 10.1080/19393210.2020.1764114 [DOI] [PubMed] [Google Scholar]
- 75. Rejczak T, Tuzimski T: QuEChERS-based extraction with dispersive solid phase extraction clean-up using PSA and ZrO2-based sorbents for determination of pesticides in bovine milk samples by HPLC-DAD. Food Chem. 2017;217:225–233. 10.1016/j.foodchem.2016.08.095 [DOI] [PubMed] [Google Scholar]
- 76. Melgar MJ, Santaeufemia M, Garcia MA: Organophosphorus pesticide residues in raw milk and infant formulas from Spanish northwest. J. Environ. Sci. Health B. 2010;45(7):595–600. 10.1080/03601234.2010.502394 [DOI] [PubMed] [Google Scholar]
- 77. Ştefănescu L, Stezar CI, Groza IŞ: The influence of environmental contamination on heavy metals and organochlorine compounds levels in milk. Environ. Eng. Manag. J. 2011;10(1):37–42. [Google Scholar]
- 78. Norouzirad R, et al. : Lead and cadmium levels in raw bovine milk and dietary risk assessment in areas near petroleum extraction industries. Sci. Total Environ. 2018;635:308–314. 10.1016/j.scitotenv.2018.04.138 [DOI] [PubMed] [Google Scholar]
- 79. Ismail A, et al. : Estimated daily intake and health risk of heavy metals by consumption of milk. Food Addit. Contam. Part B Surveill. 2015;8(4):260–265. 10.1080/19393210.2015.1081989 [DOI] [PubMed] [Google Scholar]
- 80. Meshref AMS, Moselhy WA, Hassan NE-HY: Heavy metals and trace elements levels in milk and milk products. J. Food Meas. Charact. 2014;8(4):381–388. 10.1007/s11694-014-9203-6 [DOI] [Google Scholar]
- 81. Maas S, et al. : Trace metals in raw cows’ milk and assessment of transfer to Comté cheese. Food Chem. 2011;129(1):7–12. 10.1016/j.foodchem.2010.09.034 [DOI] [Google Scholar]
- 82. Pilarczyk R, et al. : Concentrations of toxic heavy metals and trace elements in raw milk of Simmental and Holstein-Friesian cows from organic farm. Environ. Monit. Assess. 2013;185(10):8383–8392. 10.1007/s10661-013-3180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Singh M, et al. : Assessment of contamination of milk and milk products with heavy metals. Int. J. Dairy Sci. 2020;72(6):608–615. 10.33785/IJDS.2019.v72i06.005 [DOI] [Google Scholar]
- 84. Bilandžić N, et al. : Trace element levels in raw milk from northern and southern regions of Croatia. Food Chem. 2011;127(1):63–66. 10.1016/j.foodchem.2010.12.084 [DOI] [Google Scholar]
- 85. Chen L, et al. : Analysis of 17 elements in cow, goat, buffalo, yak, and camel milk by inductively coupled plasma mass spectrometry (ICP-MS). RSC Adv. 2020;10(12):6736–6742. 10.1039/D0RA00390E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gabryszuk M, et al. : Content of mineral elements in milk and hair of cows from organic farms. J. Elem. 2010;15(2):259–267. [Google Scholar]
- 87. Suturovic Z, et al. : Determination of heavy metals in milk and fermented milk products by potentiometric stripping analysis with constant inverse current in the analytical step. Food Chem. 2014;155:120–125. 10.1016/j.foodchem.2014.01.030 [DOI] [PubMed] [Google Scholar]
- 88. Choudhury T, et al. : Evaluation of elemental, microbial and biochemical status of raw and pasteurized cow's milk. Int. Food Res. J. 2018;25(4):1682–1690. [Google Scholar]
- 89. Akhtar S, et al. : Minerals and heavy metals in raw and ultra heat treated commercial milks in Pakistan. International Journal of Food and Allied Sciences. 2015;1:18. 10.21620/ijfaas.2015118-24 [DOI] [Google Scholar]
- 90. Santos C, et al. : Determination of the concentrations of essential and toxic metals in UHT milk produced in Mato Grosso State, Brazil. Int. Food Res. J. 2015;22(3):981–986. [Google Scholar]
- 91. Qu XY, et al. : Analysis and Risk Assessment of Seven Toxic Element Residues in Raw Bovine Milk in China. Biol. Trace Elem. Res. 2018;183(1):92–101. 10.1007/s12011-017-1116-x [DOI] [PubMed] [Google Scholar]
- 92. Abdelfatah E, et al. : Heavy metal residues and health risk assessment in raw milk and dairy products with a trail for removal of copper residues. Benha Veterinary Medical Journal. 2019;36:51–64. 10.21608/bvmj.2019.79648 [DOI] [Google Scholar]
- 93. Soares VA, et al. : Determination of nutritional and toxic elements in pasteurized bovine milk from Vale do Paraiba region (Brazil). Food Control. 2010;21(1):45–49. 10.1016/j.foodcont.2009.03.010 [DOI] [Google Scholar]
- 94. Ribeiro Sant'Ana MA, Carvalho TC, Silva IF: Concentration of heavy metals in UHT dairy milk available in the markets of Sao Luis, Brazil, and potential health risk to children. Food Chem. 2021;346:128961. 10.1016/j.foodchem.2020.128961 [DOI] [PubMed] [Google Scholar]
- 95. Nascimento IR, et al. : Determination of the mineral composition of fresh bovine milk from the milk-producing areas located in the State of Sergipe in Brazil and evaluation employing exploratory analysis. Microchem. J. 2010;96(1):37–41. 10.1016/j.microc.2010.01.010 [DOI] [Google Scholar]
- 96. Totan FE, Filazi A: Determination of some element levels in various kinds of cow’s milk processed in different ways. Environ. Monit. Assess. 2020;192(2):1–10. [DOI] [PubMed] [Google Scholar]
- 97. Arianejad M, et al. : Levels of Some Heavy Metals in Raw Cow's Milk from Selected Milk Production Sites in Iran: Is There any Health Concern?. Health Promot. Perspect. 2015;5(3):176–182. 10.15171/hpp.2015.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gonzalez-Montana JR, et al. : Some toxic metals (Al, As, Mo, Hg) from cow's milk raised in a possibly contaminated area by different sources. Environ. Sci. Pollut. Res. Int. 2019;26(28):28909–28918. 10.1007/s11356-019-06036-7 [DOI] [PubMed] [Google Scholar]
- 99. Najarnezhad V, Akbarabadi M: Heavy metals in raw cow and ewe milk from north-east Iran. Food Addit. Contam. Part B Surveill. 2013;6(3):158–162. 10.1080/19393210.2013.777799 [DOI] [PubMed] [Google Scholar]
- 100. Chiesa LM, et al. : Analysis of antibiotic residues in raw bovine milk and their impact toward food safety and on milk starter cultures in cheese-making process. LWT. 2020;131:109783. 10.1016/j.lwt.2020.109783 [DOI] [Google Scholar]
- 101. Moretti S, et al. : Multiclass method for the determination of 62 antibiotics in milk. J. Mass Spectrom. 2016;51(9):792–804. 10.1002/jms.3834 [DOI] [PubMed] [Google Scholar]
- 102. Mesgari Abbasi M, et al. : Simultaneous Determination of Tetracyclines Residues in Bovine Milk Samples by Solid Phase Extraction and HPLC-FL Method. Adv. Pharm. Bull. 2011;1(1):34–39. 10.5681/apb.2011.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Alija G, Hajrulai-Musliu Z, Uzunov R: Development and validation of confirmatory LC–MS/MS method for multi-residue analysis of antibiotic drugs in bovine milk. SN Appl. Sci. 2020;2(9). 10.1007/s42452-020-03361-2 [DOI] [Google Scholar]
- 104. Dimitrieska-Stojkovic E, et al. : Screening of veterinary drug residues in milk from individual farms in Macedonia. Maced. Vet. Rev. 2011;34(1):5–13. [Google Scholar]
- 105. Moudgil P, et al. : Analysis of antibiotic residues in raw and commercial milk in Punjab, India vis-à-vis human health risk assessment. J. Food Saf. 2019;39(4):1–8. [Google Scholar]
- 106. Florez DHA, Dutra FVA, Borges KB: Magnetic solid phase extraction employing a novel restricted access material based on mesoporous polyaniline coated with hydrophilic monomers and casein for determination of antibiotics in milk samples. Microchem. J. 2019;150:104145. 10.1016/j.microc.2019.104145 [DOI] [Google Scholar]
- 107. Spisso BF, et al. : Pilot survey of commercial pasteurized milk consumed in the metropolitan area of Rio de Janeiro, Brazil, for tetracyclines residues, including the 4-epimers of oxytetracycline, tetracycline and chlortetracycline. Food Addit. Contam. Part B Surveill. 2010;3(4):220–227. 10.1080/19393210.2010.531401 [DOI] [PubMed] [Google Scholar]
- 108. Novaes SFd, et al. : Residues of veterinary drugs in milk in Brazil. Ciência Rural. 2017;47(8): p.1–7. 10.1590/0103-8478cr20170215 [DOI] [Google Scholar]
- 109. Zhang WQ, et al. : Analysis of Veterinary Drug Residues in Pasteurized Milk Samples in Chinese Milk Bars. J. Food Prot. 2020;83:204–210. 10.4315/0362-028X.JFP-19-333 [DOI] [PubMed] [Google Scholar]
- 110. Du B, et al. : Evaluation of an ELISA-based visualization microarray chip technique for the detection of veterinary antibiotics in milk. Food Control. 2019;106:106713. 10.1016/j.foodcont.2019.106713 [DOI] [Google Scholar]
- 111. Du B, et al. : Presence of tetracyclines, quinolones, lincomycin and streptomycin in milk. Food Control. 2019;100:171–175. 10.1016/j.foodcont.2019.01.005 [DOI] [Google Scholar]
- 112. Juan C, et al. : Determination of macrolide and lincosamide antibiotics by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry in meat and milk. Food Control. 2010;21(12):1703–1709. 10.1016/j.foodcont.2010.05.004 [DOI] [Google Scholar]
- 113. Mohammed HA, et al. : Rapid tests for detection of enrofloxacin residues in liquid milk. Benha Veterinary Medical Journal. 2016;30(1):97–103. 10.21608/bvmj.2016.31351 [DOI] [Google Scholar]
- 114. Zhang YD, et al. : Occurrence of tetracyclines, sulfonamides, sulfamethazine and quinolones in pasteurized milk and UHT milk in China's market. Food Control. 2014;36(1):238–242. 10.1016/j.foodcont.2013.08.012 [DOI] [Google Scholar]
- 115. Kumarswamy N, et al. : Detection of antibiotic residues in raw cow milk in Thrissur, India. Pharm. Innov. 2018;7(8):452–454. [Google Scholar]
- 116. Bilandžić N, et al. : Veterinary drug residues determination in raw milk in Croatia. Food Control. 2011;22(12):1941–1948. 10.1016/j.foodcont.2011.05.007 [DOI] [Google Scholar]
- 117. Urapen R, Masawat P: Novel method for the determination of tetracycline antibiotics in bovine milk based on digital-image-based colorimetry. Int. Dairy J. 2015;44:1–5. 10.1016/j.idairyj.2014.12.002 [DOI] [Google Scholar]
- 118. Jank L, et al. : beta-lactam antibiotics residues analysis in bovine milk by LC-ESI-MS/MS: a simple and fast liquid-liquid extraction method. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012;29(4):497–507. 10.1080/19440049.2011.604044 [DOI] [PubMed] [Google Scholar]
- 119. Dorival-García N, et al. : Simultaneous determination of quinolone and β-lactam residues in raw cow milk samples using ultrasound-assisted extraction and dispersive-SPE prior to UHPLC−MS/MS analysis. Food Control. 2016;60:382–393. 10.1016/j.foodcont.2015.08.008 [DOI] [Google Scholar]
- 120. Junza A, et al. : Comparative study of the LC-MS/MS and UPLC-MS/MS for the multi-residue analysis of quinolones, penicillins and cephalosporins in cow milk, and validation according to the regulation 2002/657/EC. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879(25):2601–2610. 10.1016/j.jchromb.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 121. Rama A, et al. : Assessment of antibacterial drug residues in milk for consumption in Kosovo. J. Food Drug Anal. 2017;25(3):525–532. 10.1016/j.jfda.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Junza A, et al. : Multiclass method for the determination of quinolones and β-lactams, in raw cow milk using dispersive liquid-liquid microextraction and ultra high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2014;1356:10–22. 10.1016/j.chroma.2014.06.034 [DOI] [PubMed] [Google Scholar]
- 123. Dinali LAF, et al. : Efficient development of a magnetic molecularly imprinted polymer for selective determination of trimethoprim and sulfamethoxazole in milk. Microchem. J. 2020;154:104648. 10.1016/j.microc.2020.104648 [DOI] [Google Scholar]
- 124. Li H, et al. : Simultaneous determination of amoxicillin and prednisolone in bovine milk using ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012;900:59–63. 10.1016/j.jchromb.2012.05.031 [DOI] [PubMed] [Google Scholar]
- 125. Liu X, et al. : Solid phase extraction using magnetic core mesoporous shell microspheres with C18-modified interior pore-walls for residue analysis of cephalosporins in milk by LC-MS/MS. Food Chem. 2014;150:206–212. 10.1016/j.foodchem.2013.10.145 [DOI] [PubMed] [Google Scholar]
- 126. Kantiani L, Farre M, Barcelo D: Rapid residue analysis of fluoroquinolones in raw bovine milk by online solid phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A. 2011;1218(50):9019–9027. 10.1016/j.chroma.2011.09.079 [DOI] [PubMed] [Google Scholar]
- 127. Meng Z, et al. : Residues investigation of fluoroquinolones and sulphonamides and their metabolites in bovine milk by quantification and confirmation using ultra-performance liquid chromatography-tandem mass spectrometry. Food Chem. 2015;174:597–605. 10.1016/j.foodchem.2014.11.067 [DOI] [PubMed] [Google Scholar]
- 128. Bilandžić N, et al. : Concentrations of veterinary drug residues in milk from individual farms in Croatia. Mljekarstvo. 2011;61(3):260–267. [Google Scholar]
- 129. Navratilova P, et al. : Fluoroquinolone residues in raw cow’s milk. Czech J. Food Sci. 2011;29(6):641–646. 10.17221/22/2011-CJFS [DOI] [Google Scholar]
- 130. Zhou W, et al. : Simultaneous determination of 16 macrolide antibiotics and 4 metabolites in milk by using Quick, Easy, Cheap, Effective, Rugged, and Safe extraction (QuEChERS) and high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1061-1062:411–420. 10.1016/j.jchromb.2017.07.046 [DOI] [PubMed] [Google Scholar]
- 131. Zeina K, Fawwak S, Abi P: Quantification of antibiotic residues and determination of antimicrobial resistance profiles of microorganisms isolated from bovine milk in Lebanon. Food Nutr. Sci. 2013;04(7):1–9. 10.4236/fns.2013.47A001 [DOI] [Google Scholar]
- 132. Hou XL, et al. : Development and validation of an ultra high performance liquid chromatography tandem mass spectrometry method for simultaneous determination of sulfonamides, quinolones and benzimidazoles in bovine milk. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014;962:20–29. 10.1016/j.jchromb.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 133. Wang Y, et al. : Rapid Determination of Trace Sulfonamides in Milk by Graphene Oxide-Based Magnetic Solid Phase Extraction Coupled with HPLC–MS/MS. Food Anal. Methods. 2016;9(9):2521–2530. 10.1007/s12161-016-0433-6 [DOI] [Google Scholar]
- 134. Jank L, et al. : High-throughput method for the determination of residues of beta-lactam antibiotics in bovine milk by LC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015;32(12):1992–2001. [DOI] [PubMed] [Google Scholar]
- 135. Huang LC, et al. : Simultaneous determination of aflatoxin M1, ochratoxin A, zearalenone and alpha-zearalenol in milk by UHPLC-MS/MS. Food Chem. 2014;146:242–249. 10.1016/j.foodchem.2013.09.047 [DOI] [PubMed] [Google Scholar]
- 136. Wang X, Li P: Rapid screening of mycotoxins in liquid milk and milk powder by automated size-exclusion SPE-UPLC-MS/MS and quantification of matrix effects over the whole chromatographic run. Food Chem. 2015;173:897–904. 10.1016/j.foodchem.2014.10.056 [DOI] [PubMed] [Google Scholar]
- 137. Benkerroum N: Mycotoxins in dairy products: A review. Int. Dairy J. 2016;62:63–75. 10.1016/j.idairyj.2016.07.002 [DOI] [Google Scholar]
- 138. Mao J, et al. : Multi-mycotoxins analysis in raw milk by ultra high performance liquid chromatography coupled to quadrupole orbitrap mass spectrometry. Food Control. 2018;84:305–311. 10.1016/j.foodcont.2017.08.009 [DOI] [Google Scholar]
- 139. Puga-Torres B, et al. : Determination of Aflatoxin M1 in Raw Milk from Different Provinces of Ecuador. Toxins (Basel). 2020;12(8):498. 10.3390/toxins12080498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Keller L, et al. : Incidence of Mycotoxins (AFB1 and AFM1) in Feeds and Dairy Farms from Rio de Janeiro State, Brazil. Vet. Med. 2016;1:29–35. [Google Scholar]
- 141. Kos J, et al. : Occurrence and estimation of aflatoxin M1 exposure in milk in Serbia. Food Control. 2014;38:41–46. 10.1016/j.foodcont.2013.09.060 [DOI] [Google Scholar]
- 142. Tomašević I, et al. : Two year survey on the occurrence and seasonal variation of aflatoxin M1 in milk and milk products in Serbia. Food Control. 2015;56:64–70. 10.1016/j.foodcont.2015.03.017 [DOI] [Google Scholar]
- 143. Ruangwises N, Ruangwises S: Aflatoxin M(1) contamination in raw milk within the central region of Thailand. Bull. Environ. Contam. Toxicol. 2010;85(2):195–198. 10.1007/s00128-010-0056-3 [DOI] [PubMed] [Google Scholar]
- 144. Zhao Z, et al. : Multi-mycotoxin analysis of animal feed and animal-derived food using LC–MS/MS system with timed and highly selective reaction monitoring. Anal. Bioanal. Chem. 2015;407(24):7359–7368. 10.1007/s00216-015-8898-5 [DOI] [PubMed] [Google Scholar]
- 145. Duarte SC, et al. : Aflatoxin M1 in marketed milk in Portugal: Assessment of human and animal exposure. Food Control. 2013;30(2):411–417. 10.1016/j.foodcont.2012.08.002 [DOI] [Google Scholar]
- 146. Fallah AA: Assessment of aflatoxin M1 contamination in pasteurized and UHT milk marketed in central part of Iran. Food Chem. Toxicol. 2010;48(3):988–991. 10.1016/j.fct.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 147. Cano-Sancho G, et al. : Occurrence of aflatoxin M(1) and exposure assessment in Catalonia (Spain). Rev. Iberoam. Micol. 2010;27(3):130–135. 10.1016/j.riam.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 148. Silva MV, et al. : Occurrence and estimative of aflatoxin M1 intake in UHT cow milk in Paraná State, Brazil. Food Control. 2015;53:222–225. 10.1016/j.foodcont.2015.01.025 [DOI] [Google Scholar]
- 149. Pattono D, Gallo PF, Civera T: Detection and quantification of ochratoxin A in milk produced in organic farms. Food Chem. 2011;127(1):374–377. 10.1016/j.foodchem.2010.12.051 [DOI] [Google Scholar]
- 150. Winkler J, et al. : Development of a multi-toxin method for investigating the carryover of zearalenone, deoxynivalenol and their metabolites into milk of dairy cows. Food additives & contaminants. Part A. 2015;32(3):371–380. [DOI] [PubMed] [Google Scholar]
- 151. Pleadin J, et al. : Presence of Fusarium mycotoxins in feedstuffs and cow milk sampled from Croatian farms during 2015. Mljekarstvo/Dairy. 2017;67(2):102–111. 10.15567/mljekarstvo.2017.0202 [DOI] [Google Scholar]
- 152. Signorini ML, et al. : Exposure assessment of mycotoxins in cow's milk in Argentina. Food Chem. Toxicol. 2012;50(2):250–257. 10.1016/j.fct.2011.09.036 [DOI] [PubMed] [Google Scholar]
- 153. Mahmoudi R: Occurrence of Zearalenone in raw animal origin food produced in North-West of Iran. J. Food Qual. Hazards Control. 2014;1(1):25–28. [Google Scholar]
- 154. Jiang K, et al. : Reduced graphene oxide and gold nanoparticle composite-based solid-phase extraction coupled with ultra-high-performance liquid chromatography-tandem mass spectrometry for the determination of 9 mycotoxins in milk. Food Chem. 2018;264:218–225. 10.1016/j.foodchem.2018.05.041 [DOI] [PubMed] [Google Scholar]
- 155. Xia X, et al. : Ultra-high-pressure liquid chromatography–tandem mass spectrometry for the analysis of six resorcylic acid lactones in bovine milk. J. Chromatogr. A. 2009;1216(12):2587–2591. 10.1016/j.chroma.2009.01.033 [DOI] [PubMed] [Google Scholar]
- 156. Baumrucker CR, Macrina AL: Hormones and Regulatory Factors in Bovine Milk. Reference Module in Food Science. Elsevier;2020. [Google Scholar]
- 157. Ollikainen P, Muuronen K: Determination of insulin-like growth factor-1 and bovine insulin in raw milk and its casein and whey fractions after microfiltration and ultrafiltration. Int. Dairy J. 2013;28(2):83–87. 10.1016/j.idairyj.2012.09.001 [DOI] [Google Scholar]
- 158. Mishra M, Ali S, Das M: A New Extraction Method for the Determination of Oxytocin in Milk by Enzyme Immune Assay or High-Performance Liquid Chromatography: Validation by Liquid Chromatography–Mass Spectrometry. Food Anal. Methods. 2012;6(5):1308–1319. [Google Scholar]
- 159. Mishra M, Ali S, Das M: Analysis of oxytocin in milk samples and intake pattern in different age groups of Indian population. Toxicol. Mech. Methods. 2014;24(5):342–346. 10.3109/15376516.2014.910851 [DOI] [PubMed] [Google Scholar]
- 160. Singh S, et al. : Circulating and milk adiponectin change differently during energy deficiency at different stages of lactation in dairy cows. J. Dairy Sci. 2014;97(3):1535–1542. 10.3168/jds.2013-7598 [DOI] [PubMed] [Google Scholar]
- 161. Goyon A, et al. : Determination of steroid hormones in bovine milk by LC-MS/MS and their levels in Swiss Holstein cow milk. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016;33(5):804–816. 10.1080/19440049.2016.1175186 [DOI] [PubMed] [Google Scholar]
- 162. Chen C, et al. : A preliminary risk assessment of potential exposure to naturally occurring estrogens from Beijing (China) market milk products. Food Chem. Toxicol. 2014;71:74–80. 10.1016/j.fct.2014.05.028 [DOI] [PubMed] [Google Scholar]
- 163. Azzouz A, et al. : Simultaneous determination of 20 pharmacologically active substances in cow's milk, goat's milk, and human breast milk by gas chromatography–mass spectrometry. J. Agric. Food Chem. 2011;59(9):5125–5132. 10.1021/jf200364w [DOI] [PubMed] [Google Scholar]
- 164. Socas-Rodríguez B, et al. : Multiclass analytical method for the determination of natural/synthetic steroid hormones, phytoestrogens, and mycoestrogens in milk and yogurt. Anal. Bioanal. Chem. 2017;409(18):4467–4477. 10.1007/s00216-017-0391-x [DOI] [PubMed] [Google Scholar]
- 165. Kaklamanos G, Theodoridis G: Rapid multi-method for the determination of growth promoters in bovine milk by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013;930:22–29. 10.1016/j.jchromb.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 166. Florez DHÂ, Oliveira HL, Borges KB: Polythiophene as highly efficient sorbent for microextraction in packed sorbent for determination of steroids from bovine milk samples. Microchem. J. 2020;153:104521. 10.1016/j.microc.2019.104521 [DOI] [Google Scholar]
- 167. Regal P, Cepeda A, Fente C: Development of an LC-MS/MS method to quantify sex hormones in bovine milk and influence of pregnancy in their levels. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012;29(5):770–779. 10.1080/19440049.2011.653989 [DOI] [PubMed] [Google Scholar]
- 168. Trapiella-Alfonso L, et al. : Development of a quantum dot-based fluorescent immunoassay for progesterone determination in bovine milk. Biosens. Bioelectron. 2011;26(12):4753–4759. 10.1016/j.bios.2011.05.044 [DOI] [PubMed] [Google Scholar]
- 169. Tan X-t, et al. : Analysis of 13 kinds of steroid hormones in raw milk using modified QuEChERS method combined with UPLC-QTOF-MS. J. Integr. Agric. 2016;15(9):2163–2174. 10.1016/S2095-3119(16)61386-2 [DOI] [Google Scholar]
- 170. Gellrich K, et al. : Cortisol levels in skimmed milk during the first 22 weeks of lactation and response to short-term metabolic stress and lameness in dairy cows. J. Anim. Sci. Biotechnol. 2015;6(1):31. 10.1186/s40104-015-0035-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ma L, et al. : Multiresidue analysis of glucocorticoids in milk by LC-MS/MS with low-temperature purification and dispersive solid-phase extraction. J. Sep. Sci. 2017;40(13):2759–2768. 10.1002/jssc.201700064 [DOI] [PubMed] [Google Scholar]
- 172. Nag SK: Contaminants in milk: routes of contamination, analytical techniques and methods of control. Improving the Safety and Quality of Milk. Griffiths MW, editor. Woodhead Publishing;2010; p.146–178. 10.1533/9781845699420.2.146 [DOI] [Google Scholar]
- 173. Ahmadi F, et al. : Determination of organophosphorus pesticides in water samples by single drop microextraction and gas chromatography-flame photometric detector. J. Chromatogr. A. 2006;1101(1-2):307–312. 10.1016/j.chroma.2005.11.017 [DOI] [PubMed] [Google Scholar]
- 174. Pathirana K, et al. : Pesticide contaminated crop residues and water usage for dairy cattle rearing in Walapane DS division, Sri Lanka. Int. J. Innov. Res. Comput. Sci. Technol. 2015;2(6). [Google Scholar]
- 175. Bedi J, et al. : Pesticide residues in milk and their relationship with pesticide contamination of feedstuffs supplied to dairy cattle in Punjab (India). J. Anim. Feed Sci. 2018;27(1):18–25. 10.22358/jafs/82623/2018 [DOI] [Google Scholar]
- 176. Ashoub M, Azam A: Seasonal variations on the levels of some pesticide residues in dairy farms. Benha Veterinary Medical Journal. 2016;30(1):312–322. 10.21608/bvmj.2016.31401 [DOI] [Google Scholar]
- 177. Srivastava S, Narvi S, Prasad S: Organochlorines and organophosphates in bovine milk samples in Allahabad region. Int. J. Environ. Res. 2008;2(2):165–168. [Google Scholar]
- 178. Waliszewski SM, et al. : Detection of some organochlorine pesticides in cow's milk. Food Addit. Contam. 1996;13(2):231–235. 10.1080/02652039609374401 [DOI] [PubMed] [Google Scholar]
- 179. Losada A, et al. : Organochlorine pesticide residues in bovine milk from León (Spain). Sci. Total Environ. 1996;181(2):133–135. 10.1016/0048-9697(96)80244-0 [DOI] [PubMed] [Google Scholar]
- 180. John PJ, Bakore N, Bhatnagar P: Assessment of organochlorine pesticide residue levels in dairy milk and buffalo milk from Jaipur City, Rajasthan, India. Environ. Int. 2001;26(4):231–236. 10.1016/S0160-4120(00)00111-2 [DOI] [PubMed] [Google Scholar]
- 181. Claborn HV, et al. : Pesticide Residues, Malathion in Milk and Fat from Sprayed Cattle. J. Agric. Food Chem. 1956;4(11):941–942. 10.1021/jf60069a004 [DOI] [Google Scholar]
- 182. Bushland RC, et al. : Contamination of meat and milk by chlorinated hydrocarbon insecticides used for livestock pest control. J. Econ. Entomol. 1950;43(5):649–652. 10.1093/jee/43.5.649 [DOI] [Google Scholar]
- 183. Rodrigues FM, et al. : Development of a headspace solid-phase microextraction/gas chromatography–mass spectrometry method for determination of organophosphorus pesticide residues in cow milk. Microchem. J. 2011;98(1):56–61. 10.1016/j.microc.2010.11.002 [DOI] [Google Scholar]
- 184. Willett LB, et al. : Mechanisms of movement of organochlorine pesticides from soils to cows via forages. J. Dairy Sci. 1993;76(6):1635–1644. 10.3168/jds.S0022-0302(93)77497-4 [DOI] [PubMed] [Google Scholar]
- 185. El Bahgy HE, Elbarbary HA, Ibrahim SS: Open Access Estimation of deltamethrin residues in cow’s and goat’s environment and trials to reduce its level in milk. Veterinary world. 2018;11(5):606–611. 10.14202/vetworld.2018.606-611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Heck MC, et al. : Estimation of children exposure to organochlorine compounds through milk in Rio Grande do Sul, Brazil. Food Chem. 2007;102(1):288–294. 10.1016/j.foodchem.2006.05.019 [DOI] [Google Scholar]
- 187. Sabarwal A, Kumar K, Singh RP: Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018;63:103–114. 10.1016/j.etap.2018.08.018 [DOI] [PubMed] [Google Scholar]
- 188. Karasali H, Maragou N: Pesticides and Herbicides: Types of Pesticide. Encyclopedia of Food and Health. 2016;319–325. 10.1016/B978-0-12-384947-2.00535-3 [DOI] [Google Scholar]
- 189. Fischer WJ, et al. : CONTAMINANTS OF MILK AND DAIRY PRODUCTS|Environmental Contaminants. Encyclopedia of Dairy Sciences. Roginski H, editor. Oxford: Elsevier;2002; p.525–533. 10.1016/B0-12-227235-8/00758-6 [DOI] [Google Scholar]
- 190. Licata P, et al. : Levels of “toxic” and “essential” metals in samples of bovine milk from various dairy farms in Calabria, Italy. Environ. Int. 2004;30(1):1–6. 10.1016/S0160-4120(03)00139-9 [DOI] [PubMed] [Google Scholar]
- 191. IARC W: World Cancer Report 2008. World Health Organization;2008; vol.29. [Google Scholar]
- 192. Zhou X, et al. : Relationships between Pb, As, Cr, and Cd in individual cows' milk and milk composition and heavy metal contents in water, silage, and soil. Environ. Pollut. 2019;255(Pt 2):113322. 10.1016/j.envpol.2019.113322 [DOI] [PubMed] [Google Scholar]
- 193. FAO: Maximum residue limits (MRLs) and risk management recommendations (RMRs) for residues of veterinary drugs in foods. CX/MRL 2–2018. London: FAO;2018. [Google Scholar]
- 194. Europea, C: Reglamentos UE N 37/2010 de la Comisión de 22 de diciembre de 2009. Diario Oficial de La Unión Europea;2010. [Google Scholar]
- 195. Bacanlı M, Başaran N: Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019;125:462–466. 10.1016/j.fct.2019.01.033 [DOI] [PubMed] [Google Scholar]
- 196. De Oliveira AP, et al. : Antimicrobial susceptibility of Staphylococcus aureus isolated from bovine mastitis in Europe and the United States. J. Dairy Sci. 2000;83(4):855–862. 10.3168/jds.S0022-0302(00)74949-6 [DOI] [PubMed] [Google Scholar]
- 197. Ghidini S, et al. : Residues of beta-lactam antibiotics in bovine milk: confirmatory analysis by liquid chromatography tandem mass spectrometry after microbial assay screening. Food Addit. Contam. 2003;20(6):528–534. 10.1080/0265203031000098696 [DOI] [PubMed] [Google Scholar]
- 198. Navratilova P, et al. : Occurrence of tetracycline, chlortetracycline, and oxytetracycline residues in raw cow’s milk. Czech J. Food Sci. 2009;27(5):379–385. 10.17221/177/2008-CJFS [DOI] [Google Scholar]
- 199. Shi Q, et al. : Utilization of a lateral flow colloidal gold immunoassay strip based on surface-enhanced Raman spectroscopy for ultrasensitive detection of antibiotics in milk. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;197:107–113. 10.1016/j.saa.2017.11.045 [DOI] [PubMed] [Google Scholar]
- 200. Marrugo-Padilla A, Méndez-Cuadro D, Rodríguez-Cavallo E: Combined tetracycline and pyrethroid residues increases protein carbonylation in bovine milk. Int. Dairy J. 2020;107:104708. 10.1016/j.idairyj.2020.104708 [DOI] [Google Scholar]
- 201. Eskola M, et al. : Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’of 25%. Crit. Rev. Food Sci. Nutr. 2020;60(16):2773–2789. 10.1080/10408398.2019.1658570 [DOI] [PubMed] [Google Scholar]
- 202. Becker-Algeri TA, et al. : Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016;81(3):R544–R552. 10.1111/1750-3841.13204 [DOI] [PubMed] [Google Scholar]
- 203. Sweeney MJ, Dobson AD: Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998;43(3):141–158. 10.1016/S0168-1605(98)00112-3 [DOI] [PubMed] [Google Scholar]
- 204. Marin S, et al. : Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013;60:218–237. 10.1016/j.fct.2013.07.047 [DOI] [PubMed] [Google Scholar]
- 205. Liu Y, et al. : Simultaneous detection and comparative pharmacokinetics of amoxicillin, clavulanic acid and prednisolone in cows' milk by UPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016;1008:74–80. 10.1016/j.jchromb.2015.11.031 [DOI] [PubMed] [Google Scholar]
- 206. Ganmaa D, Sato A: The possible role of female sex hormones in milk from pregnant cows in the development of breast, ovarian and corpus uteri cancers. Med. Hypotheses. 2005;65(6):1028–1037. 10.1016/j.mehy.2005.06.026 [DOI] [PubMed] [Google Scholar]
- 207. Wilbey RA: HEAT TREATMENT OF FOODS|Principles of Pasteurization. Encyclopedia of Food Microbiology. Batt CA, Tortorello ML, editors. Oxford: Academic Press;2014; p.169–174. 10.1016/B978-0-12-384730-0.00159-2 [DOI] [Google Scholar]
- 208. Steele JH: History, trends, and extent of pasteurization. J. Am. Vet. Med. Assoc. 2000;217(2):175–178. 10.2460/javma.2000.217.175 [DOI] [PubMed] [Google Scholar]
- 209. Özer B, Yaman H: MILK AND MILK PRODUCTS|Microbiology of Liquid Milk. Encyclopedia of Food Microbiology. Batt CA, Tortorello ML, editors. Oxford: Academic Press;2014; p.721–727. 10.1016/B978-0-12-384730-0.00219-6 [DOI] [Google Scholar]
- 210. Ryser ET: Liquid Milk Products|Pasteurization of Liquid Milk Products: Principles, Public Health Aspects. Encyclopedia of Dairy Sciences. Fuquay JW, editor. San Diego: Academic Press;2011; p.310–315. 10.1016/B978-0-12-374407-4.00287-9 [DOI] [Google Scholar]
- 211. Lorenzen PC, et al. : A survey of the quality of extended shelf life (ESL) milk in relation to HTST and UHT milk. Int. J. Dairy Technol. 2011;64(2):166–178. 10.1111/j.1471-0307.2010.00656.x [DOI] [Google Scholar]
- 212. Sarkar S: Microbiological considerations: pasteurized milk. Int. J. Dairy Sci. 2015;10(5):206–218. 10.3923/ijds.2015.206.218 [DOI] [Google Scholar]
- 213. Xiong L, et al. : Effect of heat treatment on bacteriostatic activity and protein profile of bovine whey proteins. Food Res. Int. 2020;127:108688. 10.1016/j.foodres.2019.108688 [DOI] [PubMed] [Google Scholar]
- 214. Meunier-Goddik L, Sandra S: Liquid Milk Products: Pasteurized Milk. Reference Module in Food Science. Elsevier;2016. 10.1016/B978-0-08-100596-5.00876-3 [DOI] [Google Scholar]
- 215. Ceni G, et al. : Continuous inactivation of alkaline phosphatase and Escherichia coli in milk using compressed carbon dioxide as inactivating agent. J. CO2 Util. 2016;13:24–28. 10.1016/j.jcou.2015.11.003 [DOI] [Google Scholar]
- 216. Barut Gok S, et al. : Inactivation of E. coli and L. innocua in milk by a thin film UV-C reactor modified with flow guiding elements (FGE). Int. J. Food Microbiol. 2021;343:109105. 10.1016/j.ijfoodmicro.2021.109105 [DOI] [PubMed] [Google Scholar]
- 217. Sharma P, et al. : Reduction of bacterial counts and inactivation of enzymes in bovine whole milk using pulsed electric fields. Int. Dairy J. 2014;39(1):146–156. 10.1016/j.idairyj.2014.06.003 [DOI] [Google Scholar]
- 218. Makarapong D, et al. : Development of an innovative apparatus using UV-C for controlling the number of microorganisms in raw milk after milking. Int. J. Dairy Technol. 2020;73(1):301–305. 10.1111/1471-0307.12654 [DOI] [Google Scholar]
- 219. Sheelamary M, Muthukumar M: Effectiveness of ozone in inactivating Listeria monocytogenes from milk samples. World Journal of Young Researchers. 2011;1(3):40–44. [Google Scholar]
- 220. Shamila-Syuhada AK, et al. : Inactivation of microbiota and selected spoilage and pathogenic bacteria in milk by combinations of ultrasound, hydrogen peroxide, and active lactoperoxidase system. Int. Dairy J. 2016;61:120–125. 10.1016/j.idairyj.2016.05.002 [DOI] [Google Scholar]
- 221. Hongmei L, et al. : Inactivation of microorganisms naturally present in raw bovine milk by high-pressure carbon dioxide. Int. J. Food Sci. Technol. 2014;49(3):696–702. 10.1111/ijfs.12352 [DOI] [Google Scholar]
- 222. Stratakos AC, et al. : Effect of high pressure processing on the safety, shelf life and quality of raw milk. Innovative Food Sci. Emerg. Technol. 2019;52:325–333. 10.1016/j.ifset.2019.01.009 [DOI] [Google Scholar]
- 223. Yasmin N, et al. : Inactivation of foodborne pathogens on food packaging and in cow milk by exposure to a Nd:YAG laser. Can. J. Phys. 2017;95(7):662–669. 10.1139/cjp-2016-0676 [DOI] [Google Scholar]
- 224. Sharma P, et al. : Bacterial inactivation in whole milk using pulsed electric field processing. Int. Dairy J. 2014;35(1):49–56. 10.1016/j.idairyj.2013.10.005 [DOI] [Google Scholar]
- 225. Cregenzán-Alberti O, et al. : Suitability of ccRSM as a tool to predict inactivation and its kinetics for Escherichia coli, Staphylococcus aureus and Pseudomonas fluorescens in homogenized milk treated by manothermosonication (MTS). Food Control. 2014;39:41–48. 10.1016/j.foodcont.2013.10.007 [DOI] [Google Scholar]
- 226. Zhang YH, et al. : Enhanced degradation of five organophosphorus pesticides in skimmed milk by lactic acid bacteria and its potential relationship with phosphatase production. Food Chem. 2014;164:173–178. 10.1016/j.foodchem.2014.05.059 [DOI] [PubMed] [Google Scholar]
- 227. Yuan S, et al. : Degradation of parathion methyl in bovine milk by high-intensity ultrasound: Degradation kinetics, products and their corresponding toxicity. Food Chem. 2020;327:127103. 10.1016/j.foodchem.2020.127103 [DOI] [PubMed] [Google Scholar]
- 228. Zhao X-H, Wang J: A brief study on the degradation kinetics of seven organophosphorus pesticides in skimmed milk cultured with Lactobacillus spp. at 42°C. Food Chem. 2012;131(1):300–304. 10.1016/j.foodchem.2011.08.046 [DOI] [Google Scholar]
- 229. Shen C, et al. : Pb(2+) and Hg(2+) removal from polluted milk by di-acrylated Pluronic P123 hydrogels. Food Chem. 2018;258:331–336. 10.1016/j.foodchem.2018.03.057 [DOI] [PubMed] [Google Scholar]
- 230. Massoud R, et al. : Lead bioremoval from milk by Saccharomyces cerevisiae. Biocatal. Agric. Biotechnol. 2019;22:101437. 10.1016/j.bcab.2019.101437 [DOI] [Google Scholar]
- 231. Massoud R, et al. : Lead and cadmium biosorption from milk by Lactobacillus acidophilus ATCC 4356. Food Sci. Nutr. 2020;8(10):5284–5291. 10.1002/fsn3.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Massoud R, et al. : Mercury Biodecontamination from Milk by using L. acidophilus ATCC 4356. J. Pure Appl. Microbiol. 2020;14(4):2313–2321. 10.22207/JPAM.14.4.10 [DOI] [Google Scholar]
- 233. Massoud R, et al. : Mercury biosorption process by using Saccharomyces cerevisiae in milk. J. Food Process. Preserv. 2021;45(1):1–9. [Google Scholar]
- 234. Massoud R, et al. : Cadmium Bioremoval by Saccharomyces cerevisiae in Milk. Journal of Medical Microbiology and Infectious Diseases. 2020;8(1):29–33. 10.29252/JoMMID.8.1.29 [DOI] [Google Scholar]
- 235. Massoud R, et al. : The Biosorption Capacity of Saccharomyces Cerevisiae for Cadmium in Milk. Dairy. 2020;1(2):169–176. 10.3390/dairy1020011 [DOI] [Google Scholar]
- 236. Alsager OA, et al. : Removal of antibiotics from water and waste milk by ozonation: kinetics, byproducts, and antimicrobial activity. Ecotoxicol. Environ. Saf. 2018;158:114–122. 10.1016/j.ecoenv.2018.04.024 [DOI] [PubMed] [Google Scholar]
- 237. Kitazono Y, et al. : Antibiotic removal from waste milk by electrochemical process: degradation characteristics in concentrated organic solution. J. Mater. Cycles Waste Manag. 2016;19(3):1261–1269. 10.1007/s10163-016-0517-9 [DOI] [Google Scholar]
- 238. Kitazono Y, et al. : Selective degradation of tetracycline antibiotics present in raw milk by electrochemical method. J. Hazard. Mater. 2012;243:112–116. 10.1016/j.jhazmat.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 239. Aguilar JFF, et al. : Selective removal of tetracycline residue in milk samples using a molecularly imprinted polymer. J. Polym. Res. 2020;27(7). 10.1007/s10965-020-02139-9 [DOI] [Google Scholar]
- 240. Kumar S, et al. : Photocatalytic, fluorescent BiPO4@Graphene oxide based magnetic molecularly imprinted polymer for detection, removal and degradation of ciprofloxacin. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;111:110777. 10.1016/j.msec.2020.110777 [DOI] [PubMed] [Google Scholar]
- 241. Alsager OA, Alnajrani MN, Alhazzaa O: Decomposition of antibiotics by gamma irradiation: Kinetics, antimicrobial activity, and real application in food matrices. Chem. Eng. J. 2018;338:548–556. 10.1016/j.cej.2018.01.065 [DOI] [Google Scholar]
- 242. Bodbodak S, et al. : Selective decontamination of aflatoxin M1in milk by molecularly imprinted polymer coated on the surface of stainless steel plate. Int. J. Dairy Technol. 2018;71(4):868–878. 10.1111/1471-0307.12551 [DOI] [Google Scholar]
- 243. Ismail A, et al. : Effect of different microbial concentrations on binding of aflatoxin M 1 and stability testing. Food Control. 2017;73:492–496. 10.1016/j.foodcont.2016.08.040 [DOI] [Google Scholar]
- 244. Moussa AI, et al. : Efficacy of Kaolin and Bentonite Clay to Reduce Aflatoxin M1 Content in Contaminated Milk and Effects on Milk Quality. Pak. Vet. J. 2020;40(2):181–186. 10.29261/pakvetj/2020.001 [DOI] [Google Scholar]
- 245. Abdelmotilib N, et al. : Aflatoxin M1 Reduction in Milk by a Novel Combination of Probiotic Bacterial and Yeast Strains. European J. Nutr. Food Saf. 2018;8:83–99. 10.9734/EJNFS/2018/39486 [DOI] [Google Scholar]
- 246. Martinez MP, et al. : Probiotic bacteria and yeasts adsorb aflatoxin M1 in milk and degrade it to less toxic AFM1-metabolites. Toxicon. 2019;172:1–7. 10.1016/j.toxicon.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 247. Assaf JC, et al. : A novel method for elimination of aflatoxin M1 in milk using Lactobacillus rhamnosus GG biofilm. Int. J. Dairy Technol. 2019;72(2):248–256. 10.1111/1471-0307.12578 [DOI] [Google Scholar]
- 248. Carraro A, et al. : Clay minerals as adsorbents of aflatoxin M1 from contaminated milk and effects on milk quality. Appl. Clay Sci. 2014;88-89:92–99. 10.1016/j.clay.2013.11.028 [DOI] [Google Scholar]