Abstract
Background
Anosmia and ageusia are symptoms commonly associated with COVID-19, but the relationship with disease severity, onset and recovery are unclear.
Objective
To examine factors associated with anosmia and ageusia and the recovery from these symptoms in an ethnically diverse cohort.
Methods
Individuals tested for SARS-CoV-2 between March and April 2020 were eligible for the study. Randomly selected participants answered a telephone questionnaire on COVID-19 symptoms with a focus on anosmia and ageusia. Additionally, relevant past medical history and data on the COVID-19 clinical course were obtained from electronic medical records. 486 patients were in the COVID-19 group and 103 were COVID-19-negative.
Results
Patients who were younger were more likely to report anosmia and/or ageusia (odds ratio (OR) for anosmia per 1-year increase in age: 0·98, 95%CI:0–97-0·99, p = 0·003; for ageusia: 0·98, 95%CI:0·97-0·99, p = 0·005) as were patients with lower eosinophil counts (OR for anosmia per 0.1-K/μL increase in eosinophils: 0·02, 95%CI:0·001-0·46, p = 0·01, for ageusia 0·10, 95%CI:0·01-0·97, p = 0·047). Male gender was independently associated with a lower probability of ageusia (OR:0·56, 95%CI:0·38-0·82, p = 0·003) and earlier sense of taste recovery (HR:1·44, 95%CI:1·05-1·98, p = 0·02). Latinos showed earlier sense of taste recovery than white patients (HR:1·82, 95%CI:1·05-3·18, p = 0·03).
Conclusion
Anosmia and ageusia were more common among younger patients and those with lower blood eosinophil counts. Ageusia was less commonly reported among men, and time to taste recovery was earlier among both men and Latinos.
Keywords: COVID-19, anosmia, ageusia, eosinophil, age, olfactory and gustatory dysfunction
Introduction
In New York City, the first documented case of COVID-19 was confirmed in March 2020. For months, New York City was the epicenter of the global pandemic, accounting for a third of the total cases in the United States. 1 Montefiore Medical Center in the Bronx borough of New York City was at the center of this outbreak, evidenced by the fact that a third of the Bronx population have tested positive for serum antibodies to SARS-CoV-2 as of August 2020. 2
In addition to the most common symptoms of cough, fever, and dyspnea, COVID-19 also causes frequent olfactory and gustatory dysfunction. Loss of smell (anosmia) has been linked to a wide range of viral infections leading to anosmia. 3 Multiple reports have emerged indicating anosmia and loss of taste (ageusia) among other symptoms of COVID-19.4–7 In fact, anosmia and ageusia were also shown to occur among otherwise asymptomatic people or in individuals with mild to moderate manifestations of SARS-Cov-2 infection.6,8–13
Even though anosmia and ageusia could be potential predictors of COVID-19, not much is known about the factors associated with the onset of these symptoms. Pre-existing conditions such as hypertension and diabetes are associated with the severity of COVID-19. 14 Limited data is available on association of pre-existing hypertension, diabetes, or hypercholesterolemia with anosmia and ageusia. Anosmia and ageusia are thought to be related to a milder form of the disease. 15 Studies also show a possible association of peripheral blood eosinophil counts with the severity of COVID-19, 16 but to our knowledge the associations of anosmia and ageusia with blood cell counts or inflammatory markers have not been established. Even less is known about the factors associated with recovery from anosmia and ageusia in COVID-19 patients.7,17 Lastly, most studies conducted on loss of smell and taste associated with COVID-19 have largely been carried out in populations that were predominantly Caucasian. Limited information is available regarding the prevalence of either symptom in an ethnically diverse population.
Therefore, in this study, we utilized the large number of confirmed cases at Montefiore Medical Center in the Bronx, NY, to determine the prevalence of anosmia and ageusia in an ethnically diverse cohort tested for COVID-19. Our goal was to examine any association of anosmia and ageusia with demographic characteristics, pre-existing conditions, and laboratory markers. We also evaluated whether severity of COVID-19 infection was associated with anosmia or ageusia. Finally, we aimed to characterize the rate of recovery from anosmia and ageusia and examine factors associated with recovery versus persistence of symptoms.
Materials and Methods
Patient Cohort
The bioinformatics team queried electronic medical records (EMR) of patients who were presented to Montefiore Medical Center, Bronx, NY and tested between March 1st and April 29th, 2020. Information was extracted from the cohort of individuals who were tested for SARS-CoV-2 by nasopharyngeal swabbing using real time PCR assay during this time. A subset of patients was randomly selected from this list using a random number generator in SAS 9·4 (SAS Institute Inc., Cary, NC). The study cohort was then contacted to request participation in a phone interview to collect data. (Refer to Supplementary Methods for patient cohort classification and exclusion criteria.)
Data Collection
Patients who agreed to participate provided a verbal informed consent. A structured phone interview was administered to collect information regarding the course and characteristics of their COVID-19 illness, specifically addressing anosmia and ageusia and recovery from both symptoms. (Refer to Supplementary Methods for details on data collection and questionnaires.) Research Electronic Data Capture (REDCap) database was used for data recording. 18 The study was approved by the Institutional Review Board of Albert Einstein College of Medicine.
Statistical Analysis
All summary statistics were expressed as means ± standard error (SE), medians ± interquartile ranges (IQR), or as frequencies and percentages where applicable. Continuous variables were compared between groups using the two sample T-test or Wilcoxon rank-sum test (for non-normally distributed data). Categorical data were analyzed by chi-square or Fisher’s exact tests, as appropriate. Multivariable logistic regression models were also fit to the data to identify factors that were independently associated with anosmia and ageusia in COVID-19 patients. Time to recovery from anosmia and ageusia was analyzed using the Kaplan-Meier method and Cox proportional hazards model. (Refer to Supplementary Methods for details on statistical analysis tools that were used.)
Results
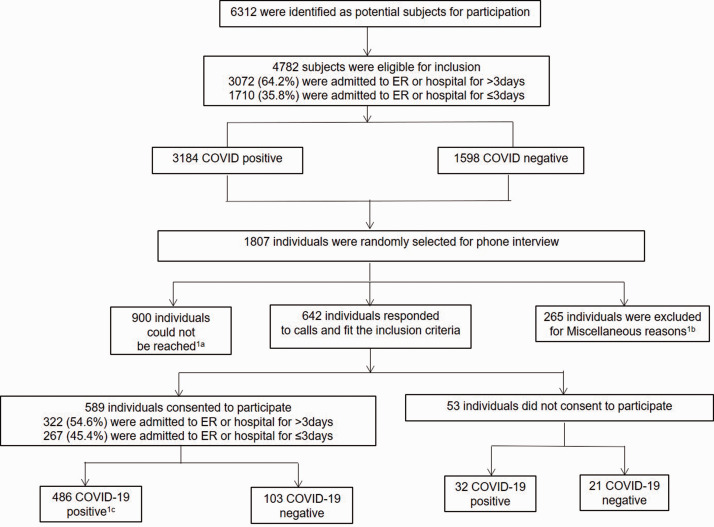
Figure 1 shows the schematic representation of the study design and enrollment of participants. From the entire cohort of individuals who were presented to Montefiore and were tested for SARS-CoV-2 between March – April 2020, 6312 potential participants were initially identified. After excluding deceased patients during this time and those who were less than 18 years old, 4782 remained. Based on the exclusion criteria listed in Supplementary Methods, among COVID-19 negative individuals, we were unable to reach 400 subjects in the COVID-19 negative group as initially intended. A total of 642 individuals were ultimately contacted. The participation rate among eligible subjects was 92%. Among those who agreed to participate in the study, 435 tested positive for COVID-19. Upon individual chart review of 154 SARS-CoV-2-negative patients, 51 of these subjects were identified as COVID-19-probable. Therefore, 103 and 486 patients were in the SARS-CoV-2 negative and positive groups, respectively.
Figure 1.
Schematic representation of the Method.1a An individual was considered as “could not be reached” if the individual with a working number was called at least 3 times and was unreachable after a minimum of three attempts or if the person was unable to respond because of persistent healthcare issues and was receiving ongoing care in a medical facility.1b Miscellaneous reasons for exclusion were: Patients who were deceased during the call period of May 6th–June 30th 2020, patients who were screened for SARS-CoV-2 prior to procedures (childbirth, surgeries, etc.), had no symptoms indicative for COVID-19 and tested negative, and those who could not remember getting tested were excluded from the study.1c COVID- 19 positive patients were defined as either patients who tested SARS-CoV-2-positive (N = 435), or as patients who tested negative but presented with COVID-19-like symptoms and met clinical criteria for probable COVID-19 illness as determined by the treating physicians (N = 51).
Clinical Characteristics in Symptomatic Patients Presented for COVID-19 Evaluation
Supplementary Table S1 characterizes symptomatic patients who had COVID-19 or were COVID-19 negative. The overall study cohort was representative of the Bronx population served by Montefiore Medical Center, with a majority represented by both African American and Latino individuals. The prevalence of COVID-19 did not differ significantly by age, ethnicity, or race.
COVID-19 positive patients were more likely to present with fever, cough, pneumonia, diarrhea, fatigue, body aches, sore throat, headache, and loss of appetite than COVID-19-negative patients (p < 0.01 for all, Supplementary Table S1). Anosmia and ageusia rates were 4-5-fold higher in the COVID-19 positive versus the negative group. Specifically, anosmia was observed in 33% (95% CI: 29%–38%) in COVID-19 patients compared to only 8% (95% CI: 3%–15%) in the COVID-19 negative group (p < 0.001). Ageusia was observed in 50% (95% CI: 45%–54%) of COVID-19 patients compared to 10% (95% CI: 5%–17%) in the COVID-negative group (p < 0·001). An overview of the diagnoses for COVID-19 negative patients is shown in Supplementary Figure S1 (Supplementary Materials).
Symptoms Associated With Anosmia and Ageusia in COVID-19 Positive Patients
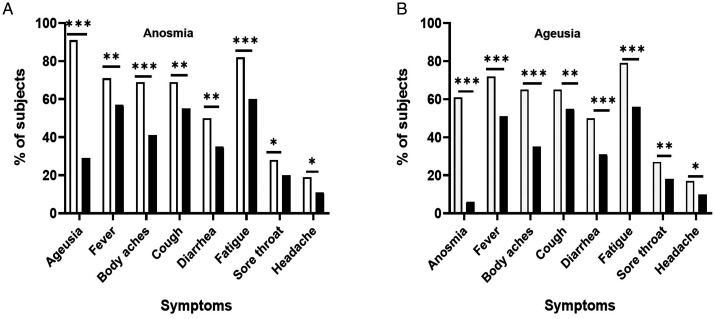
COVID-19 positive patient characteristics summarized by anosmia and ageusia status are presented in Tables 1 and 2, respectively. The median time from the patient-reported onset of COVID-19 symptoms to the reported onset of anosmia and ageusia was 2 days (IQR 0-5·0 for both symptoms). COVID-19 patients with anosmia and and/or ageusia were significantly younger and more likely to have systemic symptoms (fever, body aches, fatigue), respiratory symptoms (cough, sore throat), and diarrhea, than those without anosmia and/or ageusia ( p < 0·05 for all) (Tables 1 and 2, Figure 2). Fifty-eight percent of COVID-19 patients reported both anosmia and ageusia whereas 5·5% reported only anosmia and 36·5% only ageusia as shown in Supplementary Figure S2 (Supplementary Materials). Among those who reported anosmia, 32% had a partial loss of smell and 68% had a complete loss of smell. Among those who reported ageusia, 43% had a partial loss of taste whereas 57% lost the taste completely.
Table 1.
Characteristics of Patients With COVID-19 Illness by the Lack of Sense of Smell.
| Characteristics | Anosmia*,N = 162 | No Anosmia*,N = 324 | P-Value |
|---|---|---|---|
| Demographics | |||
| Age, years (±SE) | 53 (±1.2) | 59 (±1.0) | <0.001 |
| Gender, N (%) women | 81 (50) | 144 (46) | 0.4 |
| Race/ethnicity, N (%) | 0.06 | ||
| White | 23 (14) | 22 (7) | |
| Black | 55 (34) | 133 (42) | |
| Latino | 56 (34) | 102 (32) | |
| Asian | 5 (3) | 5 (2) | |
| Unknown | 24 (15) | 52(17) | |
| Reported symptoms | |||
| Ageusia*, N (%) | 148 (91) | 92 (29) | <0.001 |
| Fever*, N (%) | 116 (71) | 178 (57) | <0.01 |
| Body aches*, N (%) | 111 (69) | 128 (41) | <0.001 |
| Cough*, N (%) | 112 (69) | 173 (55) | <0.01 |
| Sore throat*, N (%) | 46 (28) | 61 (20) | 0.03 |
| Belly ache*, N (%) | 38 (24) | 50 (16) | 0.06 |
| Shortness of breath*, N (%) | 50 (31) | 105 (34) | 0.6 |
| Diarrhea*, N (%) | 82 (50) | 110 (35) | <0.01 |
| Fatigue or weakness*, N (%) | 135 (82) | 183 (60) | <0.001 |
| Headache*, N (%) | 30 (19) | 35 (11) | 0.03 |
| BMI▲, kg/m2 (IQR) | 31 (27–38) | 30 (26–35) | 0.1 |
| Number of days from SARS-CoV-2 test to the phone interview (±SE) | 46·4 (±1.4) | 49·6 (±1.0) | 0.09 |
| Patient past medical history | |||
| History of hypertension▲, N (%) | 64 (39) | 164 (52) | 0.006 |
| History of hypercholesterolemia▲, N (%) | 44 (27) | 119 (38) | 0.02 |
| Laboratory markers at the time of SARS-CoV-2 test | |||
| Eosinophil counts▲, K/μL (±SE) | 0.02 (±0.004) | 0·05 (±0.006) | 0.01 |
| Fibrinogen▲, mg/dL (±SE) | 687 (±20.0) | 640 (±13.0) | 0.049 |
| Lactic dehydrogenase▲, U/L (±SE) | 426 (±13.7) | 411 (±14.2) | 0.4 |
| Outcomes | |||
| Length of emergency department or hospital stay▲, days (IQR) | 4 (2–8) | 6 (3–10) | 0.002 |
| Admission to ICU▲, N (%) | 18 (11) | 32 (10) | 0.8 |
| Endotracheal intubations▲, N (%) | 10 (6) | 21 (7) | 0.8 |
*Patient reported, ▲ Extracted and verified through medical record data extraction and review.
Table 2.
Characteristics of Patients With COVID-19 Illness by the Lack of Sense of Taste.
| Characteristics | Ageusia*,N = 242 | No Ageusia*,N = 244 | P-Value |
|---|---|---|---|
| Demographics | |||
| Age, years (±SE) | 54·6 (±1.0) | 59 (±1.0) | 0.001 |
| Gender, N (%) women | 130 (54) | 94 (40) | 0.003 |
| Race/ethnicity, N (%) | 0.8 | ||
| White | 24 (10) | 21 (9) | |
| Black | 92 (38) | 95 (41) | |
| Latino | 81 (33) | 78 (33) | |
| Asian | 7 (3) | 3 (1) | |
| Other race, or unknown | 39 (16) | 37 (16) | |
| Reported symptoms | |||
| Anosmia*, N (%) | 148 (61) | 13 (6) | <0.001 |
| Fever*, N (%) | 175 (72) | 119 (51) | <0.001 |
| Body aches*, N (%) | 157 (65) | 83 (35) | <0.001 |
| Cough*, N (%) | 157 (65) | 127 (55) | <0.01 |
| Sore throat*, N (%) | 67 (27) | 41 (18) | <0.01 |
| Belly ache*, N (%) | 53 (22) | 36 (16) | 0.07 |
| Shortness of breath*, N (%) | 76 (32) | 77 (33) | 0.8 |
| Diarrhea*, N (%) | 122 (50) | 72 (31) | <0.001 |
| Fatigue or weakness*, N (%) | 191 (79) | 128 (56) | <0.001 |
| Headache*, N (%) | 41 (17) | 24 (10) | 0.03 |
| BMI▲, kg/m2 (IQR) | 31 (28–38) | 30 (25–35) | 0.3 |
| Number of days from SARS-CoV-2 test to the phone interview (±SE) | 46·7 (±1.1) | 50 (±1·2) | 0.04 |
| Patient past medical history | |||
| History of hypertension▲, N (%) | 110 (45) | 117 (50) | 0.3 |
| History of hypercholesterolemia▲, N (%) | 79 (32) | 85 (36) | 0.4 |
| Laboratory markers at the time of SARS-CoV-2 test | |||
| Eosinophil counts▲, K/μL (±SE) | 0.03 (±0.005) | 0.05 (±0.007) | 0.02 |
| Fibrinogen▲, mg/dL (±SE) | 680 (±15.0) | 629 (±15.0) | 0.02 |
| Lactic dehydrogenase▲, U/L (±SE) | 442 (±16.4) | 399 (±12.0) | 0.04 |
| Outcomes | |||
| Length of emergency department or hospital stay▲, days (IQR) | 5 (3–10) | 5 (3–9) | 0.2 |
| Admission to ICU▲, N (%) | 25 (10) | 25 (11) | 0.8 |
| Endotracheal intubations▲, N (%) | 17 (7) | 14 (6) | 0.7 |
* Patient reported. ▲ Extracted and verified through medical record data extraction and review.p<0.05 are shown in bold and italic format.
Figure 2.
Symptoms associated with the presence of anosmia (A) and ageusia (B). A, Patients with anosmia (n = 162) (□) and without anosmia (n = 314) (■). B, Patients with ageusia (n = 242) (□) and without ageusia (n = 244) (■), * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
Laboratory markers
Individuals with COVID-19 who reported anosmia and ageusia demonstrated significantly lower peripheral blood eosinophil counts and higher serum fibrinogen levels (Tables 1 and 2). Other inflammatory markers measured at the time of SARS-CoV-2 test, such as absolute neutrophil counts, CRP, ESR, and ferritin, were not significantly associated with either patient-reported anosmia or ageusia (data not shown). (Refer to Supplementary Results for results on pre-existing conditions and severity.)
Multivariable logistic regression to determine association with anosmia and ageusia
Multivariable logistic regression indicated that age and peripheral blood eosinophil counts were independently associated with both anosmia and ageusia after adjusting for other factors (Tables 3 and 4). Younger age was independently associated with higher likelihood of both anosmia (OR: 0·98 per 1-year increase, 95% CI: 0·97-0·99, p = 0·003) and ageusia (OR: 0·98 per 1-year increase, 95% CI: 0·97-0·99, p = 0·005). Lower peripheral blood eosinophil counts at the time of the SARS-CoV-2 test were also associated with a higher likelihood of anosmia (OR: 0·021 per 0.1 K/μL increase in eosinophils, 95% CI: 0·001-0·46, p = 0·01) and ageusia (OR:0·10 per 0.1 K/μL increase, 95% CI: 0·01-0·97, p = 0·047). In case of ageusia, male gender was independently associated with lower likelihood of ageusia (OR:0·56, 95% CI: 0·38–0·82, p = 0·003). Ethnicity/race was not significantly associated with anosmia or ageusia. Association of hypertension and high cholesterol with anosmia in unadjusted analysis was no longer significant after adjusting for other factors. Fibrinogen and lactate dehydrogenase (LDH) were not included in the multivariable models since there were insufficient data for these variables across the cohort.
Table 3.
Multivariable Logistic Regression Models of Outcome Anosmia (N = 438, Events = 141)*.
|
Full Model |
Final Model |
|||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value | |
| Age (per 1-year increase) | 0.98 (0.97, 1.00) | 0.013 | 0.98 (0.97, 0.99) | 0.003 |
| Male (reference = female) | 0.80 (0.53, 1.21) | 0.29 | ||
| Black (reference = white) | 0.58 (0.26, 1.32) | 0.20 | ||
| Latino (reference = white) | 0.80 (0.35, 1.82) | 0.59 | ||
| Other/unknown race (reference = white) | 0.61 (0.25, 1.51) | 0.29 | ||
| Eosinophil count at time of SARS-CoV-2 test (per 0.1 K/μL increase in eosinophils) | 0.017 (0.001, 0.42) | 0.013 | 0.021 (0.001, 0.46) | 0.014 |
| History of hypertension (reference = no) | 0.96 (0.57, 1.64) | 0.89 | ||
| History of hypercholesterolemia (reference = no) | 0.87 (0.50, 1.51) | 0.62 | ||
*Hosmer-Lemeshow goodness of fit test: p = 0.58 for full model; p = 0.86 for final model.
Full model includes past medical history and laboratory variables with sufficient available data and p < .05 for association with anosmia or ageusia at the bivariate level, as well as demographic characteristics. Final model includes variables with p-value<.05 after applying stepwise backward selection.p<0.05 are shown in bold and italic format.
Table 4.
Multivariable Logistic Regression Models of Outcome Ageusia (N = 438, Events = 219)*.
|
Full Model |
Final Model |
|||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value | |
| Age (per 1-year increase) | 0.98 (0.97, 0.99) | 0.004 | 0.98 (0.97, 0.99) | 0.005 |
| Male (reference = female) | 0.56 (0.38, 0.83) | 0.004 | 0.56 (0.38, 0.82) | 0.003 |
| Black (reference = white) | 1.00 (0.46, 2.18) | 1.00 | ||
| Latino (reference = white) | 1.06 (0.48, 2.34) | 0.88 | ||
| Other/unknown race (reference = white) | 1.19 (0.50, 2.81) | 0.69 | ||
| Eosinophil count at time of SARS-CoV-2 test (per 0.1 K/μL increase in eosinophils) | 0.09 (0.01, 0.95) | 0.046 | 0.10 (0.01, 0.97) | 0.047 |
| History of hypertension (reference = no) | 1.08 (0.66, 1.77) | |||
| History of hypercholesterolemia (reference = no) | 1.12 (0.68, 1.85) | |||
*Hosmer-Lemeshow goodness of fit test: p = 0.42 for full model; p = 0.09 for final model.
Full model includes past medical history and laboratory variables with sufficient available data and p < .05 for association with anosmia or ageusia at the bivariate level, as well as demographic characteristics. Final model includes variables with p-value < .05 after applying stepwise backward selection.p<0.05 are shown in bold and italic format.
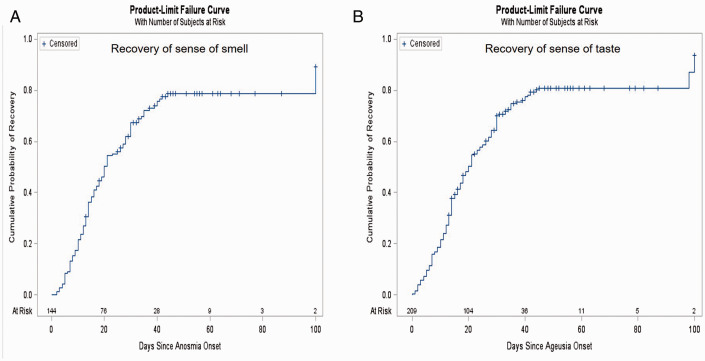
Factors Associated With Recovery From Anosmia and Ageusia
Tables 5 and 6 summarize factors associated with recovery from anosmia and ageusia, respectively. For each symptom, the median recovery time was 20 days and approximately 80% had recovered by 45 days (recovery of sense of smell and sense of taste, Figure 3(A) and (B) respectively). Men showed earlier recovery of the sense of taste compared to women in multivariable analyses (HR: 1·44, 95% CI: 1·05-1·98, p = 0·02, Table 6). Additionally, Latino patients exhibited earlier recovery of the sense of taste compared to white individuals (HR:1·82, 95% CI: 1·05-3·18, p = 0·03). No significant predictors for recovery of anosmia were identified (Table 5).
Table 5.
Cox Proportional Hazards Regression Models for Time to Anosmia Recovery (N = 144, Events = 109).
|
Unadjusted |
Multivariable Model |
|||
|---|---|---|---|---|
| HR (95% CI) | P-Value | Adjusted HR (95% CI) | P-Value | |
| Age (per 1-year increase) | 1.00 (0.98, 1.01) | 0.55 | 1.00 (0.98, 1.01) | 0.66 |
| Male (reference = female) | 1.40 (0.96, 2.05) | 0.084 | 1.37 (0.93, 2.01) | 0.11 |
| Black (reference = white) | 0.95 (0.53, 1.70) | 0.85 | 0.99 (0.53, 1.85) | 0.97 |
| Latino (reference = white) | 1.30 (0.74, 2.29) | 0.37 | 1.26 (0.71, 2.26) | 0.43 |
| Other/unknown race (reference = white) | 0.98 (0.50, 1.92) | 0.95 | 1.01 (0.51, 2.01) | 0.98 |
Table 6.
Cox Proportional Hazards Regression Models for Time to Ageusia Recovery (N = 209, Events = 162).
|
Unadjusted |
Multivariable Model |
|||
|---|---|---|---|---|
| HR (95% CI) | P-Value | Adjusted HR (95% CI) | P-Value | |
| Age (per 1-year increase) | 1.00 (0.99, 1.01) | 0.36 | 1.00 (0.99, 1.01) | 0.67 |
| Male (reference = female) | 1.48 (1.08, 2.02) | 0.014 | 1.44 (1.05, 1.98) | 0.024 |
| Black (reference = white) | 1.29 (0.74, 2.26) | 0.37 | 1.31 (0.74, 2.32) | 0.36 |
| Latino (reference = white) | 1.94 (1.12, 3.37) | 0.019 | 1.82 (1.05, 3.18) | 0.034 |
| Other/unknown race (reference = white) | 1.34 (0.72, 2.50) | 0.35 | 1.37 (0.73, 2.57) | 0.32 |
p<0.05 are shown in bold and italic format
Figure 3.
Kaplan Meier plot of time to recovery of (A) anosmia and (B) ageusia. A, Time to recovery of the sense of smell (N = 144, Events = 109). B, Time to recovery of the sense of taste (N = 209, Events = 162).
Discussion
In this ethnically diverse cohort who sought testing for SARS-CoV-2 during the 2020 pandemic, we show that COVID-19-positive patients are more likely to report anosmia and ageusia than COVID-19-negative patients. Among COVID-19-positive patients, individuals with anosmia and/or ageusia were more likely to report other COVID-19-related symptoms compared to patients without anosmia and/or ageusia.
We observed that younger age was independently associated with a higher likelihood of reporting anosmia and ageusia. Our cohort reported a lower frequency of the loss of smell compared to other studies on self-reported anosmia, which has ranged from 34 to 68%.12,19–21 Notably, our COVID-19 positive study cohort was comparatively older than other reported cohorts. This could be one of the reasons for the lower frequency of reported smell and taste loss. Studies show that underreporting of olfactory dysfunction increases with age.22,23 Moein et al. showed that 98% of COVID-19 inpatients, in a cohort of patients with a mean age of 46 years, showed some olfactory dysfunction based on objective testing, but only 35% self-reported subjective loss of smell. 24 We also observed a higher rate of self-reported ageusia (51%) in our cohort of COVID-19 patients. Studies have shown that people often misconstrue the loss of smell as loss of taste and complaints of taste loss frequently represent the loss of smell.25,26 It is therefore possible that the proportion of patients who reported loss of smell was lower than the actual occurrence of anosmia. Therefore, in our cohort, reported ageusia could potentially represent both olfactory and gustatory dysfunction in a substantial number of subjects.
We further observed that lower peripheral blood eosinophil counts at the time of the SARS-CoV-2 test were independently associated with a higher likelihood of self-reported loss of taste and smell in COVID-19-positive patients. Low blood eosinophil counts in patients with COVID-19 have also been reported to be associated with fever, fatigue, and shortness of breath. 27 While investigating the mechanism of this association is beyond the scope of this study, it is worth mentioning that expression levels of angiotensin-converting enzyme 2 (ACE2) used by SARS-CoV-2 as a receptor for entry into target cells 28 are inversely associated with the T-helper type 2 immune response biomarkers such as serum immunoglobulin E levels, interleukin-13 expression in nasal epithelium, and fractioned exhaled nitric oxide. 29 ACE2 expression in airway cells has shown borderline significant inverse association with peripheral blood eosinophil counts, possibly suggesting that COVID-19 severity may be reduced in patients with respiratory allergies. 29
Other reports suggest that anosmia is associated with a milder presentation of the disease. 15 We could not establish this association in our study, using ICU admission or endotracheal intubation frequency as outcome measures of disease severity. It should be noted, however that the rate of hospitalization overall was unusually high in our cohort of patients, both among the entire cohort and those selected for phone interviews (Figure 1). The population of the Bronx borough of New York City was hit the hardest with the highest mortality and admission rates among all five boroughs. 1 Our institution had strict criteria for admission during the pandemic, as resources and hospital bed availability became scarce over a short amount of time. During this difficult time, people with less severe COVID-19 infection were advised to stay home during the study period and may not have presented to the ER. The fact that a high number of our study subjects were admitted for hospital care does create a selection bias for our study cohort, suggesting our sample may be weighted to subjects with more severe COVID-19 infection compared to all individuals who contract SARS-CoV-2 in an endemic population.
Existent literature provides limited information regarding the recovery from both anosmia and ageusia. Most studies show an average recovery time of 1–21 days for anosmia for COVID-19 patients with about 98% recovering after 28 days.17,20,21 One study shows that around two-thirds of adults with non-severe COVID-19 still had anosmia and ageusia, two months after their symptom onset. 30 For our cohort, the median recovery time for both anosmia and ageusia was 20 days. Twenty percent of patients who had lost their smell and taste had persistent anosmia and ageusia at approximately 44-45 days from the time of the onset of these symptoms. This indicates that a substantial number of COVID-19 patients require a longer time for recovery of the loss of smell and taste. The exact reason for the variability in time for recovery of these symptoms is not yet known. We were unable to identify any significant predictors for the recovery from anosmia, however male gender and Latino ethnicity were significantly associated with earlier recovery from ageusia.
The limitations of this study are its retrospective nature and symptom recording based on patient self-report. We were also unable to determine the degree of the loss of sense of smell and taste. Another limitation of our study was our inability to query patients with the most severe outcomes of COVID-19 (i.e., prolonged hospital stays, prolonged ventilator dependence, or death). Similarly, we did not capture those who were asymptomatic or had minor symptoms who did not get tested during the pandemic outbreak of our study period. Further prospective studies need to be conducted to determine if there is a relationship between olfactory and gustatory dysfunction and COVID-19 severity.
Conclusion
In conclusion, this study summarizes the prevalence of anosmia and ageusia in a large ethnically diverse cohort of patients during a pandemic outbreak of COVID-19. This study not only provides insight into the factors that are associated with anosmia and ageusia, but it also examines factors that are associated with the recovery of these symptoms. We further report that a substantial number of COVID-19 positive patients suffer from a prolonged loss of smell and taste even when the other symptoms subside. Patients with these symptoms are more likely to have systemic disease and thus may need closer follow up and treatment. Our findings suggest that eosinophils may play a critical role in the pathophysiology of the disease and could serve as a predictor of anosmia and ageusia. Our study leverages a large cohort of patients sampled from our patient population to better understand the natural history of this new disease. Prospective studies on the natural history of anosmia and ageusia and the incidence of long-term sequelae are needed to fully characterize these unique symptoms among COVID-19 patients.
Supplemental Material
Supplemental material, sj-pdf-1-ajr-10.1177_19458924211004800 for COVID-19-Induced Anosmia and Ageusia Are Associated With Younger Age and Lower Blood Eosinophil Counts by Esha Sehanobish, Mali Barbi, Valerie Fong, Meryl Kravitz, Denise Sanchez Tejera, Mohammad Asad, Cynthia Matsumura, Denisa Ferastraoaru, Meaghan O’Neill, Merhunisa Karagic, Nadeem Akbar, Danielle M. Bottalico, Viraj Patel, Alexandre Peshansky, Mahendra Rangareddy, Golda Hudes, Mimi Kim, Ruth Eisenberg, Avindra Nath, Bryan R. Smith, Thomas J. Ow and Elina Jerschow in American Journal of Rhinology & Allergy
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Elina Jerschow served on the Advisory Board for GSK, Sanofi/Regeneron, and Novartis/Genentech; she is a consultant for GSK and has research support from AstraZeneca and from Cumberland Pharmaceuticals. Golda Hudes served on the Advisory Board for Regeneron and GSK. She has research support from AstraZeneca, Merck, Genentech, and Regeneron. Other authors report no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported in part by National Institute of Neurological Disorders and Stroke, National Institutes of Health (NS003130).
ORCID iD: Esha Sehanobish https://orcid.org/0000-0003-1090-2991
Supplemental Material
Supplemental material for this article is available online.
References
- 1.COVID-19: Data NYC Health: City of New York; 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
- 2.COVID-19: Data NYC Health: City of New York; 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data-testing.page
- 3.Suzuki M, Saito K, Min WP, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007; 117(2):272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haehner A, Draf J, Drager S, de With K, Hummel T. Predictive value of sudden olfactory loss in the diagnosis of COVID-19. ORL J Otorhinolaryngol Relat Spec. 2020; 82(4):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benezit F, Le Turnier P, Declerck C, et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis. 2020; 20(9):1014–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliezer M, Hautefort C, Hamel AL, et al. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020; 146(7):674. [DOI] [PubMed] [Google Scholar]
- 7.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. [DOI] [PMC free article] [PubMed]
- 8.Zhang Q, Shan KS, Abdollahi S, Nace T. Anosmia and ageusia as the only indicators of coronavirus disease 2019 (COVID-19). Cureus. 2020; 12(5):e7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; 277(8):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020; 58(3):295–298. [DOI] [PubMed] [Google Scholar]
- 11.Mercante G, Ferreli F, De Virgilio A, et al. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020; 146(8):723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020; 26(7):1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roland LT, Gurrola JG, 2nd, Loftus PA, Cheung SW, Chang JL. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int Forum Allergy Rhinol. 2020; 10(7):832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020; 94:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020; 10(7):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanni F, Akker E, Zaman MM, Figueroa N, Tharian B, Hupart KH. Eosinopenia and COVID-19. J Am Osteopath Assoc. 2020; 120(8):504. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID-19 pandemic—an observational cohort study. J Otolaryngol Head Neck Surg. 2020; 49(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020; 10(7):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020; 71(15):889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boscolo-Rizzo P, Borsetto D, Fabbris C, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2020; 146(8):729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wehling E, Nordin S, Espeseth T, Reinvang I, Lundervold AJ. Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch Clin Neuropsychol. 2011; 26(3):260–269. [DOI] [PubMed] [Google Scholar]
- 23.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002; 288(18):2307–2312. [DOI] [PubMed] [Google Scholar]
- 24.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020; 10(8):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deems DA, Doty RL, Settle RG, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania smell and taste center. Arch Otolaryngol Head Neck Surg. 1991; 117(5):519–528. [DOI] [PubMed] [Google Scholar]
- 26.Soter A, Kim J, Jackman A, Tourbier I, Kaul A, Doty RL. Accuracy of self-report in detecting taste dysfunction. Laryngoscope. 2008; 118(4):611–617. [DOI] [PubMed] [Google Scholar]
- 27.Xie G, Ding F, Han L, et al. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy. 2021; 76(2):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020; 146(1):203–206.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2020; 27(2):258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ajr-10.1177_19458924211004800 for COVID-19-Induced Anosmia and Ageusia Are Associated With Younger Age and Lower Blood Eosinophil Counts by Esha Sehanobish, Mali Barbi, Valerie Fong, Meryl Kravitz, Denise Sanchez Tejera, Mohammad Asad, Cynthia Matsumura, Denisa Ferastraoaru, Meaghan O’Neill, Merhunisa Karagic, Nadeem Akbar, Danielle M. Bottalico, Viraj Patel, Alexandre Peshansky, Mahendra Rangareddy, Golda Hudes, Mimi Kim, Ruth Eisenberg, Avindra Nath, Bryan R. Smith, Thomas J. Ow and Elina Jerschow in American Journal of Rhinology & Allergy