Abstract
Traumatic brain injury (TBI) continues to be a significant public healthcare concern. Neuroinflammation that occurs in the secondary phase of TBI leads to cognitive and physical dysfunction. A number of therapeutic modalities have been evaluated in an attempt to find a suitable treatment. The only drug approved for the treatment of idiopathic pulmonary fibrosis, pirfenidone, has been evaluated for its antifibrotic, anti-inflammatory, and anti-oxidant properties for various disorders, but this is the first study to examine its effects in an experimental TBI model. Twenty-four Wistar rats were randomly divided into three groups: control, trauma, and pirfenidone. The two latter groups underwent experimental diffuse cortical injury mimicking TBI. Neurological assessment was performed using the Garcia test, histological analysis was performed to examine neuroprotective and anti-inflammatory effects, and biochemical analyses of neuron-specific enolase (NSE), S-100B, caspase-3, and thiobarbituric acid reactive substances were performed. The pirfenidone group had a better Garcia test score (P=0.001), an increased anti-inflammatory effect (P<0.001), and an enhanced neuroprotective effect (P=0.007) along with decreased NSE, S100B, and TBARS levels compared to the trauma group. However, pirfenidone did not show a beneficial effect on caspase-3 levels. Pirfenidone may help decrease mortality and morbidity rates after TBI through its anti-inflammatory and antioxidant effects.
Keywords: Pirfenidone, traumatic brain injury, neuroprotective, anti-inflammatory, anti-oxidant
Introduction
Traumatic brain injury (TBI) is defined as acute biomechanical injury to the brain caused by sudden trauma, either by a penetrating force or when the head hits an object. Symptoms may range from mild findings such as headache, confusion, and loss of consciousness, to severe findings such as neurological deficit, coma, and even death. TBI is a multistep ongoing cascade of pathologies composed of white matter degradation, neuronal loss, protein misfolding, changes in neurotransmitters, and persistent neuroinflammation [1]. It continues to be a substantial public healthcare concern, affecting people of all ages. Despite advances in diagnosis and clinical management, many unanswered questions remain regarding its physiopathology, which makes it difficult to devise a definitive treatment protocol. Traumatic force to the brain is usually accompanied by extracranial injuries, which makes it difficult to assess the true outcome of TBI. Strategies for dealing with the pathology are mainly focused on surgical interventions to either stop an ongoing hemorrhage or craniectomy to allow for adequate decompression of the cerebrum, the volume of which is increased due to edema. Following surgical intervention, palliative treatment is applied, including controlled hypothermia, hypocapnia, induced coma, hyperosmolar therapy, and diuretics [2]. Although the mortality rate due to TBI has decreased over the last several decades, a large proportion of survivors require prolonged rehabilitation due to physical, cognitive, and psychological disorders, creating a burden for the survivor and their family [1]. The continued neurological and physical disabilities add to the burden on the health care system. Due to the enormous burden of TBI, there has been a great deal of research effort to understand and treat this complex entity. To find a suitable treatment, experimental studies have evaluated the effects of numerous drugs focusing on ameliorating the effects of the secondary phase of TBI, where an uncontrolled cascade of inflammation adds to the tissue damage from the primary phase. Although the primary phase is inevitable, ameliorating neurological deficits caused by the neuroinflammation during the secondary phase by suppressing detrimental immune responses has proven to be effective.
The secondary phase involves apoptosis where cells are actively eliminated by a programmed cell death mechanism during morphogenesis, tissue remodeling, and resolution of the immune response and is increased in TBI. The increased immune response can be evaluated via various markers and methods. Neuron-specific enolase (NSE), S-100B, caspase-3 (CASP3), and thiobarbituric acid-reactive substances (TBARS) are markers that are increased in serum and cerebrospinal (CSF) after TBI [3-6].
Pirfenidone (5-methyl-1-phenyl-2-[1H]-pyridone) (PIR) is a pyridine that has orphan designation for the treatment of idiopathic pulmonary fibrosis (IPF). The mechanism of action is not fully understood, but anti-fibrotic, anti-inflammatory and anti-oxidant properties have been shown. It is believed to exert these effects by regulating transforming growth factor (TGF)-β and tumor necrosis factor (TNF)-α, inhibiting fibroblast proliferation and collagen synthesis [7]. Pirfenidone has shown promising results in the treatment of cardiac remodeling, diabetic nephropathy, leiomyoma, liver fibrosis, multiple sclerosis, hippocampal neuron loss, dementia, and pancreatic cancer [8-15]. However, to the best of our knowledge, no previous studies have evaluated the effects of PIR in an experimental TBI model. This study examined whether the anti-fibrotic, anti-inflammatory and anti-oxidant properties of pirfenidone could have beneficial effects in experimental TBI through neurological, histological and biochemical analyses.
Materials and methods
Ethics & animals
The study was approved by Ankara Research and Training Hospital Ethics Committee (no: 0032/240516/p9) and all procedures were carried out in compliance with the “Principles of Laboratory Animal Care” (NIH publication 82-23, revised in 1985 and further implemented in 1996). The ARRIVE Essential 10 checklist (https://arriveguidelines.org/resources/author-checklists) was used as the reporting guideline. Twenty-four (n=24) healthy adult male Wistar rats weighing 250-300 g, maintained in a temperature-controlled room (24 ± 2°C) under a 12-h light/12-h dark cycle with free access to standard chow and water, were randomly divided into the control (n=8), trauma (n=8) and PIR (n=8) groups. We investigated the effects of PIR after TBI by examining the neurological status of the rats, and conducting histological analyses of the cellular structure and biochemical analyses of inflammatory markers. These analyses were end-stage examinations to determine the effects of PIR on prognosis and not the precise mechanisms underlying these effects.
Procedure & drug administration
Marmarou et al. [16] had previously described a diffuse cortical injury model but this was modified with an additional steel plate to decrease the rate of post-traumatic seizures. In order to induce anesthesia, the rats were given 60 mg/kg of ketamine hydrochloride IP (Alfamine 10%, Egevet Veterinary Services) and 5 mg/kg of xylazin IP (Alfazyne 2%, Egevet Veterinary Services). A midline scalp incision was made to expose the coronal and lambdoid sutures. A round aluminum plate roughly 10 mm in diameter with a thickness of 3 mm was fixed onto the cranium using bone wax. A 450 g cylindrical lead weight was dropped onto the cranium via a tube 70 cm in height. The control group received only anesthesia and scalp incision but no further intervention. The trauma group received 2 mL of 0.9% NaCl immediately after trauma and on the following day, while the animals in the PIR group were administered Pirfenex (Cipla, India) at a dose of 500 mg/kg/day immediately after trauma and on the day after by orogastric gavage.
Neurological assessment
In order to evaluate the neurological status of the rats, an 18-point scale proposed by Garcia [17] was used, totaling three to 18 points (Table 1). Spontaneous activity, symmetry in four limb movement, forepaw outstretching, climbing, body proprioception, and response to vibrissae touch were used as test parameters.
Table 1.
Garcia neurological assessment [17]
| 0 | 1 | 2 | 3 | ||
|---|---|---|---|---|---|
| Spontaneous Activity | Cage observation (5 minutes) | No movement | Did not rise up and barely moved | Slightly affected rat, hesitated to move, reached only 1 wall | Moved freely, approached at least 3 walls |
| Symmetry in 4 limbs | Rat held in the air by the tail | One limb did not move at all | Minimal movement on one side | Limbs on one side moved slowly | All four limbs extended symmetrically |
| Forepaw outstretching | Hindlimbs held in the air by the tail | One limb did not move at all | One side moved minimally | One side stretched less than the other, forepaw walking impaired | Both forelimbs outstretched and walked symmetrically on forepaws |
| Climbing | Wire cage | - | Failed to climb | One side was impaired while climbing | Climbed easily and gripped tightly |
| Body proprioception | Touching the rat on the sides with a blunt stick | - | No response to one side | Reacted slowly on one side | Equally startled on both sides and turned head |
| Response to vibrissae touch | Blunt stick brushed on each side towards the whiskers | - | No response to one side | Reacted slowly on one side | Equally startled on both sides and turned head |
The brain tissue was extracted en bloc 1 week after trauma under anesthesia with careful attention to avoid inducing any additional trauma. Samples for histological and biochemical analyses were obtained from the right frontal lobe adjacent to the interhemispheric fissure.
Histological analysis
The samples were fixed for 24 h in 10% phosphate-buffered formaldehyde. Then the specimens were sliced in the vertical axis and placed into cassettes 4 mm in thickness. Tissue was fixed in an ethanol bath for 24 h. Then the specimens were infiltrated with paraffin wax and clamped into a microtome for sectioning in the horizontal plane at a thickness of 5 µm.
All samples were kept in 10% buffered formaldehyde for 24 h. Then the specimens were stained with hematoxylin & eosin (H&E) and evaluated under a light microscope (Nikon Eclipse 80i) by a pathologist (GG) blinded to the study for neuron loss (Figure 1), inflammation, congestion, (Figure 2) and gliosis (Figure 3). Table 2 shows the evaluation used for scoring along with interpretation of the findings. The histopathological scoring system was based on two main categories; anti-inflammatory and neuroprotective effects. The amount of congestion was measured by quantifying the number of congested vascular structures per high-power field (hpf) and inflammation was determined by the inflammatory cell count. These two parameters were used for scoring the anti-inflammatory effect as they are easy to reproduce and not prone to bias. The same parameters were applied for evaluation of the neuroprotective effect. The amount of neuron loss and gliosis are suitable parameters to measure the neuroprotective effects of an agent. Neuron loss was measured in quartiles and the number in the healthiest animals in the control group evaluated under 5 hpf was considered 100%. The same approach was used when evaluating gliosis with the controls considered to have no gliosis. The healthy controls had a maximum score of 12, while the minimum score of 0 indicated the most damage. Congestion and inflammation were used to estimate the anti-inflammatory effects of treatment, while neuron loss and gliosis were used to estimate the neuroprotective effect.
Figure 1.
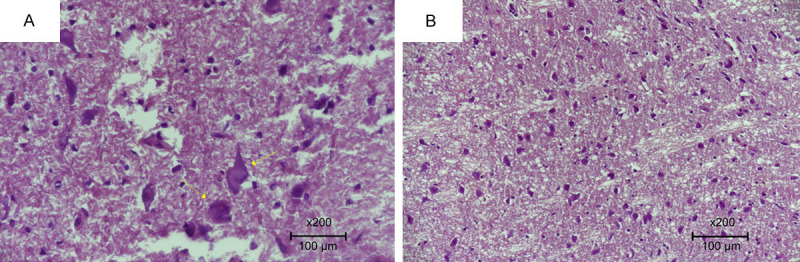
Specimens were evaluated under a light microscope for neuron loss. A. Significant neuronal degeneration in the trauma group indicated by arrows (Score 0). B. Decreased neuron loss and degeneration in the PIR group (Score 3) (H&E ×200).
Figure 2.
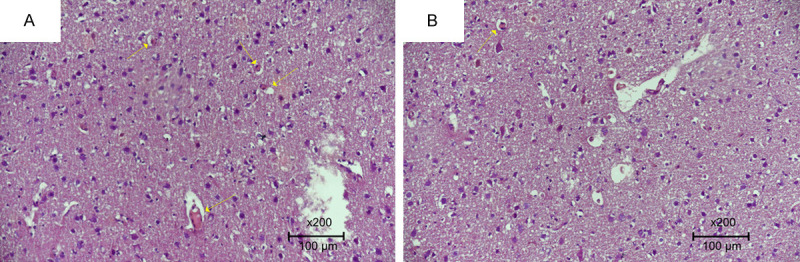
Specimens were evaluated under a light microscope for inflammation and congestion. A. 4 congested vascular structures at 1 hpf in the trauma group (Score 0). B. 1 congested vascular structure per 1 hpf in the PIR group (Score 2) (H&E ×200).
Figure 3.
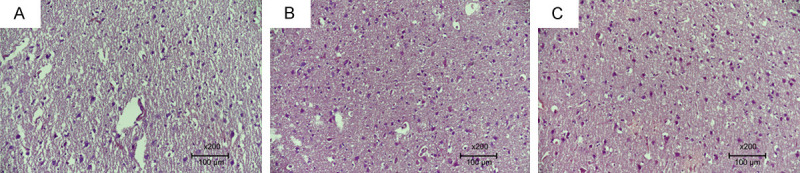
Specimens were evaluated under a light microscope for gliosis. A. Absence of gliosis in the control group (Score 3). B. Significant gliosis along with increased cellularity in the trauma group (Score 0). C. Mild gliosis observed in the PIR group (Score 2) (H&E ×200).
Table 2.
Histopathologic analysis
| Anti-inflammatory | Neuroprotective | ||||
|---|---|---|---|---|---|
|
|
|
||||
| congestion1 | inflammation | neuron loss2 | gliosis3 | ||
| points | 0 | >3 | Small groups of inflammatory cells within the parenchyma | >75% | Extended |
| 1 | 2-3 | Few inflammatory cells within the parenchyma | 50-75% | Limited | |
| 2 | 1 | Perivascular inflammatory cells | 25-50% | Mild | |
| 3 | none | No inflammation | <25% | None | |
Congested vascular structures per 1 high power field (hpf).
The specimens obtained from the control group was evaluated under 5 hpf and average number of neurons was taken into account as %100 (60 neurons) when comparing with other groups.
The specimens from the control group were evaluated as normal at 3 points and the case where the maximum gliosis was observed was scored a 0 point. 1-2 points were scored in between these cases.
Biochemical analysis
The tissue samples were subjected to biochemical analysis by a blinded biochemistry consultant in order to evaluate for NSE, S100B, CASP3, and TBARS. The tissue samples were centrifuged after a homogeneous aqueous mixture was obtained with a solution of 0.9% sodium chloride. The supernature of these mixtures was subjected to a commercial solid-phase enzyme immunoassay kit (SHANGHAI YEHUA Biological Technology Co., Ltd).
Statistical analysis
All statistical analyses were performed using SPSS 21.0 (SPSS, Inc., Chicago). The results were tested for normality and where there was no normal distribution Mann-Whitney U test was used and the minimum, maximum, and median values were derived. Where normal distribution was present, Student’s T test was used for statistical evaluation of the data. Statistical significance was accepted as P<0.05 (C: Control, T: Trauma, P: PIR).
Results
One animal in the PIR group died 4 days after the intervention and therefore biochemical analyses and Garcia test were not performed. However, neural tissue was excised from this animal and used for histological analysis. One animal in the trauma group died on the night of the operation so all analyses were omitted.
The minimum, maximum, and median scores on the Garcia Test, anti-inflammatory and neuroprotective effects along with total histopathological score are listed in Table 3. Table 4 summarizes the values compared between groups. The trauma group had the worst Garcia test score and histopathological parameters, and the PIR group had significantly better Garcia test scores and histopathological parameters that were statistically significant.
Table 3.
Distribution of anti-inflammatory effect, neuroprotective effect, total histopathological score and Garcia Test among groups
| Anti-Inflammatory | Neuroprotective | Histopathological Score | Garcia Test | |
|---|---|---|---|---|
| Control | Min:5.80 | Min:5.80 | Min:11.80 | Min:17.80 |
| Max:6.00 | Max:6.00 | Max:12.00 | Max:18.00 | |
| Median:6.00 | Median:6.00 | Median:12.00 | Median:18.00 | |
| Trauma | Min:1.00 | Min:1.00 | Min:4.00 | Min:14.80 |
| Max:2.00 | Max:4.00 | Max:6.00 | Max:15.00 | |
| Median:2.00 | Median:2.50 | Median:5.00 | Median:15.00 | |
| Pirfenidone | Min:3.00 | Min:3.00 | Min:6.00 | Min:17.90 |
| Max:4.00 | Max:4.00 | Max:8.00 | Max:18.00 | |
| Median:4.00 | Median:4.00 | Median:8.00 | Median:18.00 |
Table 4.
Comparison of Evaluated Measures among groups
| Anti-Inflammatory | Neuroprotective | Histopathological Value | Garcia Test (7th Day) | |
|---|---|---|---|---|
| Control vs. Trauma | <0.001 | <0.001 | <0.001 | <0.001 |
| Pirfenidone vs. Trauma | <0.001 | =0.007 | <0.001 | =0.001 |
P values, Mann Whitney U Test.
The mean values and standard deviation of the biochemical analyses are shown in Graphs 1 and 2. The mean values of the groups were compared and are summarized in Table 5. The trauma group had a significantly higher NSE, S100B, CASP3 and TBARS levels than the control group.
Graph 1.
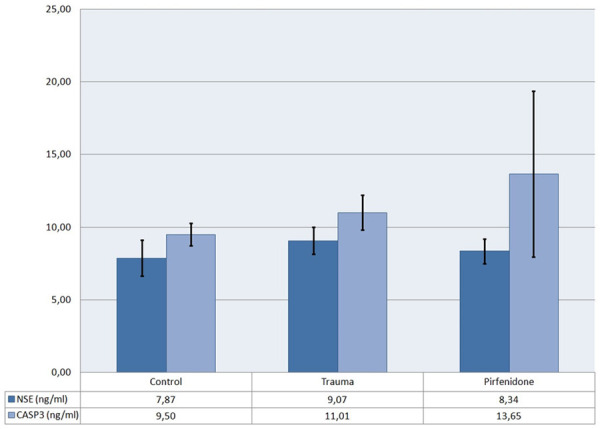
Mean values along with standard deviation of NSE and CASP3 levels.
Graph 2.
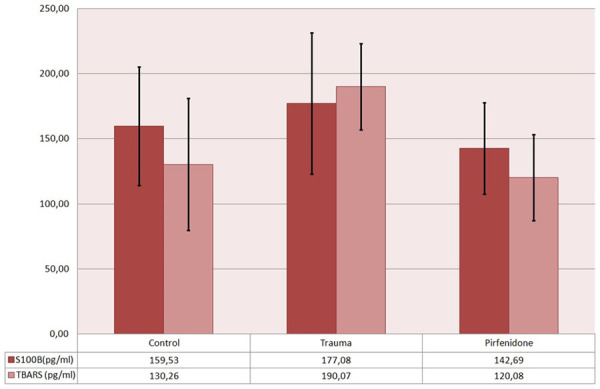
Mean values along with standard deviation of S100B and TBARS levels.
Table 5.
Comparison of biochemical values
| NSE | S100B | CASP3 | TBARS | |
|---|---|---|---|---|
| Control vs. Trauma | P<0.05 (7.86 vs. 9) | P<0.05 (159 vs. 177) | P<0.05 (9.5 vs. 11) | P<0.05 (130 vs. 190) |
| Pirfenidone vs. Control | P=0.20 (8.3 vs. 7.86) | P=0.22 (142 vs. 159) | P<0.05 (13 vs. 9.5) | P=0.329 (120 vs. 130) |
| Pirfenidone vs. Trauma | P=0.07 (8.3 vs. 9) | P=0.09 (142 vs. 177) | P=0.126 (13 vs. 11) | P<0.05 (120 vs. 190) |
Independent Sample T-Test.
PIR treatment did not show a beneficial effect on CASP3 level compared to the trauma group. NSE and S100B levels were lower in the PIR group than in the trauma group but the differences were not statistically significant. However, the PIR group showed a significant decrease in TBARS Level compared to the trauma group.
Discussion
This study evaluated the effects of pirfenidone after experimental TBI. The animals were subjected to closed head trauma as previously described by Marmarou et al. [16] with the addition of a steel plate to avoid any cranial fractures that would yield higher incidences of mortality and seizure. The treatment group received PIR, while the other groups were observed without treatment. Neurological, histological, and biochemical analyses were performed in all animals. The treatment improved neurological outcomes, had anti-inflammatory and neuroprotective effects, and reduced the levels of biochemical markers of inflammation.
TBI is the main cause of neurological impairment globally. At present, no definitive treatments are available that can either reverse or ameliorate the neurological deficits or improve long term neurological impairments in TBI patients. Neuroinflammation is a vital determinant of TBI outcome. The secondary phase is closely associated with worsening of neurological outcomes. It consists mainly of inflammation which may have both beneficial and detrimental effects. The rapid activation and migration of microglia to the trauma site where they secrete proinflammatory cytokines and neurotoxic products results in the enhanced production of reactive oxygen species (ROS) ultimately resulting in oxidative stress leading to oxidative injury to lipids, DNA, proteins, and finally neurons [18]. PIR is thought to exert its anti-oxidant effects by ameliorating increases in malondialdehyde (MDA), superoxide dismutase, and myeloperoxidase levels and anti-inflammatory effects by inhibition of the release of the proinflammatory cytokines, TNF-α, interleukin (IL)-1β, IL-6, and IL-12, and enhancing the production of anti-inflammatory cytokines such as IL-10 [6]. These properties suggest that it has potential benefits during the secondary phase of TBI.
NSE, an enzyme released from neurons after injury, plays a pivotal role in cerebral glycolytic energy metabolism in the brain, thus making it a suitable marker of TBI [19]. NSE concentrations are high in neurons and neuroendocrine cells, and increased levels are detected after stroke and subarachnoid hemorrhage [3]. S100B, a small Ca+2-binding protein, is expressed in astrocytes, Schwann cells, and oligodendrocytes. It promotes neuroproliferation, differentiation, and cytoskeleton assembly to enhance neuronal recovery by inflammation. However, its extracellular concentration determines whether it will show a neurotrophic or neurotoxic effect [20]. S100B levels are elevated in injured patients and in various neurological conditions, which makes it a significant marker of TBI.
Increased S100B levels are correlated with severity of injury and predict unfavorable outcomes after TBI along with elevated CSF concentrations in patients with brain death due to TBI [21]. Both NSE and S100B are correlated with better GCS scores in pediatric patients after TBI [22]. TBARS, the most widely used marker for indexing the lipid peroxidation end-product MDA, is important for assessing oxidative damage to lipids and proteins. Previous studies have reported increased TBARS levels in patients after TBI with lower GCS scores and higher mortality rates [23].
In the present study the trauma group had significantly higher levels of NSE, S-100B, CASP3, and TBARS than the control group. On the other hand, the levels of NSE, S100B, and TBARS in the PIR group were lower than those of the trauma group and similar to those of the control group. The decrease in levels of the proinflammatory markers NSE, S100-B, and CASP-3 in the PIR group support its anti-inflammatory effect. In addition, the PIR group also showed an anti-oxidant effect, as demonstrated by the decreased TBARS levels compared to the trauma group. The decreases in NSE and S-100B levels were not statistically significant due to the small sample size and a large standard deviation and further studies in larger numbers of animals are required to confirm our findings. In addition, further studies are required to determine the detailed mechanisms by which PIR has these effects. These findings were strongly correlated with the higher anti-inflammatory and neuroprotective scores in the PIR group determined by histological analysis.
To the best of our knowledge, no previous studies have evaluated the effects of PIR on NSE, S-100B, and CASP3 levels although there is strong evidence of decreased levels of inflammatory and oxidative stress markers in experimental models [24]. Our results are in line with a previous study that focused on the effects of PIR on cognitive impairment and oxidative stress by evaluating TBARS level, a sensitive marker of lipid peroxidation [14]. Another study showed that neuron loss in the rat hippocampus induced by the excitotoxicity kainic acid is attenuated by PIR due to its anti-inflammatory and anti-oxidant effects [13].
Histopathological evaluation provides insight into the cellular structure of the tissue along with the extent of tissue deterioration. To the best of our knowledge there have been no previous reports of a scoring system for evaluating the traumatic effects of TBI and effects of potential therapeutic agents. We devised a scoring system involving the evaluation of four measurements to confirm the biochemical and neurological findings. The results supported the assumption that lower levels of inflammatory markers lead to better neurological outcomes and improved histological findings. In this study, the PIR group had significantly higher anti-inflammatory and neuroprotective scores than the trauma group. These higher scores may have been due to the ability of PIR to attenuate the inflammatory process by decreasing the levels of pro-inflammatory markers and inflammatory cells [11]. The scores may also be attributable to the anti-oxidant effects of PIR due to the decreased nitric oxide metabolite levels via down regulation of iNOS gene expression and MDA production [25]. This scoring system may be useful for future studies as it reflects both the anti-inflammatory and neuroprotective effects.
Although biochemical and histological analyses have suggested that PIR can reduce neuroinflammatory processes and have neuroprotective effects, these findings would only be beneficial if correlated with neurological status and therefore with outcome. The clinical significance of these findings was evidenced by neurological assessment using the Garcia test, which is a well-established neurological assessment tool used in evaluation of rats. The PIR group showed a better neurological outcome, as indicated by the significantly higher score on day 7 compared to the trauma group. Rats that received PIR showed increased spontaneous activity along with improved locomotor activity and response to touch. PIR decreased inflammatory and oxidative stress, allowing the survival of more neurons and thus showing a higher incidence of functional corticospinal tracts. Therefore, the Garcia test is useful for assessing the effects of therapeutic agents, and the score correlates the histological and biochemical findings with clinical outcome.
This preliminary study evaluating the effects of PIR after TBI does have limitations. The low number of subjects is a handicap of this study, limiting the significance of statistical results. Due to the low number of subjects, dose-response was also not evaluated. A vehicle group may also be employed to distinguish the net effect of PIR. Additionally, this study aimed to evaluate the outcome of PIR treatment, and the net mechanism in which PIR is able to exert these effects should be the target of further studies.
Conclusion
In conclusion, although myriad agents have been analyzed for their effect after TBI, very few have made it to clinical use. Due to its burden on the health system and patients, a dire need for effective treatment is evident. The physiological response of the brain to trauma is inflammation, angiogenesis, neurogenesis, brain plasticity, and spontaneous regenerative mechanisms which fall short in counteracting the damage progression. If these mechanisms could be modulated or enhanced, new therapeutic options could be enlightened. This experimental study evaluated the effect of Pirfenidone, a novel drug approved for IPF with known anti-fibrotic, anti-inflammatory, and anti-oxidative properties after experimental traumatic brain injury. The study with limitations has revealed anti-inflammatory and neuroprotective effectiveness along with better neurological outcome. Pirfenidone may prove to be valuable in decreasing brain injury and functional deficits after TBI.
Acknowledgements
The authors report no conflict of interest. We acknowledge that there was no financial interest or benefit that has arisen from this research. No funding was used. The authors thank the personnel of Ankara Research and Training Hospital Experimental Animal Laboratory.
Disclosure of conflict of interest
None.
References
- 1.Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20:148. doi: 10.1186/s13054-016-1318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vella MA, Crandall ML, Patel MB. Acute management of traumatic brain injury. Surg Clin North Am. 2017;97:1015–1030. doi: 10.1016/j.suc.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross SA, Cunningham RT, Johnston CF, Rowlands BJ. Neuron-specific enolase as an aid to outcome prediction in head injury. Br J Neurosurg. 1996;10:471–6. doi: 10.1080/02688699647104. [DOI] [PubMed] [Google Scholar]
- 4.Hårdemark HG, Ericsson N, Kotwica Z, Rundström G, Mendel-Hartvig I, Olsson Y, Påhlman S, Persson L. S-100 protein and neuron-specific enolase in CSF after experimental traumatic or focal ischemic brain damage. J Neurosurg. 1989;71:727–31. doi: 10.3171/jns.1989.71.5.0727. [DOI] [PubMed] [Google Scholar]
- 5.Lorente L, Martín MM, Argueso M, Ramos L, Solé-Violán J, Riaño-Ruiz M, Jiménez A, Borreguero-León JM. Serum caspase-3 levels and mortality are associated in patients with severe traumatic brain injury. BMC Neurol. 2015;15:228. doi: 10.1186/s12883-015-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohl A, Gullo Jda S, Silva CC, Bertotti MM, Felisberto F, Nunes JC, de Souza B, Petronilho F, Soares FM, Prediger RD, Dal-Pizzol F, Linhares MN, Walz R. Plasma levels of oxidative stress biomarkers and hospital mortality in severe head injury: a multivariate analysis. J Crit Care. 2012;27:523, e11–9. doi: 10.1016/j.jcrc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Carter NJ. Pirfenidone: in idiopathic pulmonary fibrosis. Drugs. 2011;71:1721–32. doi: 10.2165/11207710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, Liu X, Bai Y, Cui C, Li J, Li Y, Hu S, Wei Y. In vitro effects of pirfenidone on cardiac fibroblasts: proliferation, myofibroblast differentiation, migration and cytokine secretion. PLoS One. 2011;6:e28134. doi: 10.1371/journal.pone.0028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, Donohue M, Ramachandrarao S, Xu R, Fervenza FC, Kopp JB. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol. 2011;22:1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BS, Margolin SB, Nowak RA. Pirfenidone: a novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J Clin Endocrinol Metab. 1998;83:219–23. doi: 10.1210/jcem.83.1.4503. [DOI] [PubMed] [Google Scholar]
- 11.García L, Hernández I, Sandoval A, Salazar A, Garcia J, Vera J, Grijalva G, Muriel P, Margolin S, Armendariz-Borunda J. Pirfenidone effectively reverses experimental liver fibrosis. J Hepatol. 2002;37:797–805. doi: 10.1016/s0168-8278(02)00272-6. [DOI] [PubMed] [Google Scholar]
- 12.Walker JE, Margolin SB. Pirfenidone for chronic progressive multiple sclerosis. Mult Scler. 2001;7:305–12. doi: 10.1177/135245850100700506. [DOI] [PubMed] [Google Scholar]
- 13.Castro-Torres RD, Chaparro-Huerta V, Flores-Soto ME, Bañuelos-Pineda J, Camins A, Orozco-Suárez SA, Armendáriz-Borunda J, Beas-Zárate C. A single dose of pirfenidone attenuates neuronal loss and reduces lipid peroxidation after kainic acid-induced excitotoxicity in the pubescent rat hippocampus. J Mol Neurosci. 2014;52:193–201. doi: 10.1007/s12031-013-0121-6. [DOI] [PubMed] [Google Scholar]
- 14.Dutta D, Hari Kumar SL, Grewal AK. Neuroprotective effect of pirfenidone on scopolamine induced cognitive impairment and oxidative stress. Indian J Physiol Pharmacol. 2017;1:416–429. [Google Scholar]
- 15.Kozono S, Ohuchida K, Eguchi D, Ikenaga N, Fujiwara K, Cui L, Mizumoto K, Tanaka M. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013;73:2345–56. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- 16.Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–35. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 18.Mckee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. doi: 10.1016/B978-0-444-52892-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman ST. Chapter 10 - acute seizures and status epilepticus. In: Skolnick BE, Alves WM, editors. Handbook of neuroemergency clinical trials (Second Edition) San Diego, USA: Academic Press; 2018. pp. 189–230. [Google Scholar]
- 20.Blanch CM, Montalban X, Comabella M. Handbook of Clinical Neurology Vol 146. 2018. Chapter 5 - multiple sclerosis, and other demyelinating and autoimmune inflammatory diseases of the central nervous system; pp. 67–84. [DOI] [PubMed] [Google Scholar]
- 21.Helbok R, Beer R. Handbook of Clinical Neurology Vol 146. 2018. Chapter 14 - cerebrospinal fluid and brain extracellular fluid in severe brain trauma; pp. 237–58. [DOI] [PubMed] [Google Scholar]
- 22.Park SH, Hwang SK. Prognostic value of serum levels of S100 calcium-binding protein B, neuron-specific enolase, and interleukin-6 in pediatric patients with traumatic brain injury. World Neurosurg. 2018;118:e534–e542. doi: 10.1016/j.wneu.2018.06.234. [DOI] [PubMed] [Google Scholar]
- 23.Hohl A, Gullo Jda S, Silva CC, Bertotti MM, Felisberto F, Nunes JC, de Souza B, Petronilho F, Soares FM, Prediger RD, Dal-Pizzol F, Linhares MN, Walz R. Plasma levels of oxidative stress biomarkers and hospital mortality in severe head injury: a multivariate analysis. J Crit Care. 2012;27:523, e11–9. doi: 10.1016/j.jcrc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salazar-Montes A, Ruiz-Corro L, López-Reyes A, Castrejón-Gómez E, Armendáriz-Borunda J. Potent antioxidant role of pirfenidone in experimental cirrhosis. Eur J Pharmacol. 2008;595:69–77. doi: 10.1016/j.ejphar.2008.06.110. [DOI] [PubMed] [Google Scholar]


