Abstract
The atezolizumab (Tecentriq), a humanized antibody against human programmed death ligand 1 (PD-L1), combined with nab-paclitaxel was granted with accelerated approval to treat unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) due to the encouraging positive results of the phase 3 IMpassion130 trial using PD-L1 biomarker from immune cells to stratify patients. However, the post-market study IMpassion131 did not support the original observation, resulting in the voluntary withdrawal of atezolizumab from the indication in breast cancer by Genentech in 2021. Emerging evidence has revealed a high frequency of false negative result using the standard immunohistochemical (IHC) staining due to heavy glycosylation of PD-L1. The removal of glycosylation prevents from the false negative staining, enabling more accurate assessment of PD-L1 levels and improving prediction for response to immune checkpoint therapy. In the present study, the natural and de-glycosylated PD-L1 expression in tumor and immune cells from nine TNBC patients were analyzed by using clone 28-8 monoclonal antibody to correlate with treatment outcome. Our results demonstrate that: (1) Removal of the glycosylation indeed enhances the detection of PD-L1 by IHC staining, (2) The PD-L1 levels on tumor cell surface after removal of the glycosylation correlates well with clinical responses for atezolizumab treatment; (3) The criteria used in the IMpassion130 and IMpassion131 trials which scored the natural PD-L1 in the immune cells failed to correlate with the clinical response. Taken together, tumor cell surface staining of PD-L1 with de-glycosylation has a significant correlation with the clinical response for atezolizumab treatment, suggesting that treatment of atezolizumab may be worthy of further consideration with de-glycosylation procedure as a patient stratification strategy. A larger cohort to validate this important issue is warranted to ensure right patient population who could benefit from the existing FDA-approved drugs.
Keywords: Immune checkpoint, PD-L1, glycosylation, atezolizumab, Tecentriq, triple-negative breast cancer
Introduction
Immune checkpoint blockade (ICB) has become the mainstay of therapeutic strategy to treat advanced cancer [1]. Clinical observations across multiple cancer types demonstrate that inhibition of immune checkpoints results in unprecedentedly sustained responses in a sub-population of patients. Functioning as immune checkpoint inhibitors (ICIs), the anti-PD-1/PD-L1 monoclonal antibodies (Mabs) block the interaction between PD-1 and PD-L1 and protect T cells from immune suppression in the tumor microenvironment in multiple cancer types. In spite of the promise, however, most tumors in humans do not respond to PD-1/PD-L1-targeted therapies. For breast cancer, it was not until recently were the anti-PD-L1/PD-1 Mabs, atezolizumab (Tecentriq) and pembrolizumab (Keytruda) approved with the therapeutic indications by the US Food and Drug Administration (FDA) as ICIs.
Atezolizumab was the first immunotherapy medicine approved to treat breast cancer. It was granted with accelerated approval in 2019 by FDA to be used in combination with nab-paclitaxel (Abraxane) to treat unresectable locally advanced or metastatic triple-negative, PD-L1-positive breast cancer. This approval was based on the IMpassion130 trial (NCT02425891) [2], which demonstrated that atezolizumab plus nab-paclitaxel prolonged progression-free survival (PFS) among patients in both the intention-to-treat (ITT) population and the subgroup of patients with PD-L1-positive tumors [3]. Significant benefit in overall survival (OS) was observed in the PD-L1-positive subgroup but not in the ITT population as a whole. In the study, PD-L1 positivity was defined by ≥1% of the proportion of tumor area being occupied by PD-L1 expressing tumor-infiltrating immune cells (% IC) of any intensity (SP142 PD-L1 immunohistochemical assay, Ventana Medical Systems, Roach) [4]. Continued accelerated approval would depend on the results of the post-market study IMpassion131, a double-blind placebo-controlled randomized phase III trial of first-line taxol/paclitaxel with and without atezolizumab for unresectable locally advanced or metastatic triple-negative breast cancer (NCT03125902). Unfortunately, IMpassion131 did not find improvement in PFS or OS by the treatment of atezolizumab plus paclitaxel compared to the placebo plus paclitaxel arm [5]. This result led to the voluntary withdrawal of atezolizumab from the indication in breast cancer by Genentech in 2021. The reasons for not observing the same benefit of atezolizumab plus nab-paclitaxel in the IMpassion130 study are not yet clear [5]. Atezolizumab has been tested and shown effective in other cancer types. Its early withdrawal from the indication of breast cancer treatment constitutes an unmet medical need warranting further investigation to understand the underlying cause in a timely manner.
Expression of tumoral PD-L1 is an important prerequisite for the effectiveness of PD-L1-targeting ICI treatments [6]. We have reported that the cell surface PD-L1 in tumor cells is heavily modified by N-glycosylation in the extracellular domain [7]. The glycosylation increases PD-L1 protein stability, hence its immune suppression function; and targeting the glycosylated PD-L1 (gPD-L1) results in exceptional anti-tumor activity in immune competent mouse models [7-11]. Importantly, glycosylated PD-L1 eludes the standard detection method of PD-L1 by immunohistochemical (IHC) staining with anti-PD-L1 antibodies, resulting in underestimation of PD-L1 expression in the tumor tissues. It was estimated that about 50% of cancer patients across the board have their tumor PD-L1 underestimated [10]. These “PD-L1 false-negative” tumors, which actually express relatively high levels of PD-L1 and, therefore, are likely responsive to anti-PD-L1 therapies, would be unfortunately excluded from the therapy based on the current protocol. Similar conclusion was further supported by later reports [12,13].
To overcome this obstacle, we have employed a de-glycosylation protocol in IHC staining which removes the glycosylation in tissue sections with the de-glycosylation enzyme before staining with the anti-PD-L1 antibodies [10]. As demonstrated in multiple cancer types, de-glycosylation facilitates PD-L1 detection to better assess the actual levels of cell surface PD-L1, which better correlates with the response to ICI therapies than the level of natural PD-L1, which was underestimated by the standard IHC staining without de-glycosylation procedure [8,12,13]. In the current study, primary tumor tissues were collected from nine Taiwanese patients with advanced breast cancer including triple-negative breast cancer (TNBC) whose tumors have progressed on prior targeted therapies or chemotherapies and subject to atezolizumab treatment. The tumor tissues prior to atezolizumab treatment were assessed by IHC for the natural and de-glycosylated PD-L1. The expression of PD-L1 in the tumor-infiltrating immune cells (% IC protocol) and the tumor cells (H score and tumor proportion score/TPS) was scored and their correlations with treatment response were compared.
Materials and methods
Patients and tumor specimens
Nine TNBC patients for whom the PD-L1 immune cell (IC) score <1% by SP142 staining were enrolled in this study. In eight metastatic patients, all of the cases received at least first line chemotherapy. The other case was given the neoadjuvant chemotherapy (NACT) with immunotherapy which is based on IMpassion031 trial. We followed up the computed tomography (CT) imaging at time of before the treatment and 3 months after the first treatment for metastasis patients. CT image for the NACT case was before treatment and before operation. Evaluation of treatment response was based on the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1). All paraffin sections were selected from the Department of Pathology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan. Institutional Review Board (IRB) approval for using these tissues in this study was given by the Research Ethics Committee of the Kaohsiung Medical University Hospital (IRB: KMUHIRB-E(I)-20180237, KMUHIRB-E(I)-20200107, and KMUHIRB-E(I)-20210245).
De-glycosylation and immunohistochemical staining
Staining for PD-L1 was conducted by following the previous report with minor modifications [10]. Briefly, formalin-fixed paraffin-embedded tissue sections (5 μm) were heated by incubating at 65°C for 1 h. The sections were then deparaffinized and dehydrated by xylene (incubated for 5 min, twice), then washed in pure ethanol twice (with the second time soaked for 5 min), followed by washing in 95% ethanol twice (with the second time soaked for 5 min), followed by soaking in 75% ethanol for 5 min, then soaking in 50% ethanol for 5 min. Finally, the slides were washed in water twice (with the second time soaked for 5 min). Antigen retrieval was conducted by heating in a steamer for 10 min in 1× trilogy buffer (Merck, Cat. 920P). The slides were then cooled down and washed three times with PBS, followed by incubation in glycoprotein denaturing buffer at room temperature for 3 h and then washed three times with PBS. The tissues were then treated with 5% PNGase F dissolved in glycosylation buffer containing 20% 10× GlycoBuffer 2 (NEB, Cat. P0704) and 10% NP-40 in double-distilled water at 37°C for 4~8 h and then washed five times with PBS. The tissues were quenched with 3% H2O2 in double-distilled water for 10 min, followed by washing three times in PBS. The tissues were blocked with 5% goat serum in PBS at room temperature for 1 h, followed by application of the primary antibody of anti-PD-L1 (28-8, abcam205921) at 1:100 in the blocking buffer at 4°C for overnight. The slides were washed three times in PBS and the secondary antibody of biotinylated goat-anti-rabbit IgG (1:1000) was applied at room temperature for 1 h. After washing for three times in PBS, the immune complexes were amplified with the ABC kit for 1 h, then washed three times in PBS, followed by adding the DAB (3,3’-Diaminobenzidine) chromogen and incubated for 5 min. Hematoxylin was then added for counterstaining and incubated for 1 min. The slides were washed in running water for 10 min, followed by dehydration of serial incubations in ethanol of 75% (3 min), 95% (1 min), 95% (3 min), 100% (1 min), 100% (3 min), twice in xylene (10 min each). The slides were then mounted for examination under microscope.
Scoring
TPS (tumor proportion score), H-score-M (H-score of counting membranous staining only), H-score-M+C (H-score of counting both membranous and cytoplasmic staining), and IC score (immune cell score) were evaluated by an experienced pathologist according to the current published guidelines without the information of patients’ clinical course and treatment response. The TPS by definition is the percentage of PD-L1-positive tumor cells with partial or complete membranous staining pattern from all the tumor cells in the section, which was widely used in the lung cancer studies and clinical trials [14,15]. The IC score stands for the percentage of tumor area (composed of intra-tumoral component and peritumoral stroma) occupied by PD-L1-positive immune cells with any intensity [3]. In other words, the space infiltrated by immune cells including lymphocytes, macrophages, dendritic cells, and granulocytes stained with punctate, linear, or circumferential patterns is the numerator, and the total tumor area is the denominator. If the IC score is more than 1, the result is defined as positive and the patient is eligible for anti-PD-L1 (atezolizumab) in the clinical practice. The assessment of H-score used in this study was similar to the H-score in previous studies [10,16], but we further separated the H-score into H-score-M and H-score-M+C. The H-score was the sum of products of the average percentage of PD-L1-positive cells with different staining intensity and the intensity score by using the following formula: H-score = [1× (% of PD-L1 positive cells with intensity category 1)] + [2× (% of PD-L1 positive cells with intensity category 2)] + [3× (% of PD-L1 positive cells with intensity category 3)]. The average percentage of tumor cells with different intensity was obtained by randomly choosing ten different filed at 400× magnification, then counting the total number of cells in each field and the number of cells belonged to each intensity, and finally calculating the average percentage of these fields. The final H-score will be ranged from 0 to 300. The representative examples of different intensity category were shown in Figure 1. The evaluation of the tumor infiltrating lymphocytes (TILs) followed the standardized protocol established by International Immuno-Oncology Biomarkers Working Group in 2017 [17]. In brief, we evaluated the average percentage of the stromal component occupied by mononuclear cells (including lymphocytes and plasma cells) within the borders of the invasive tumor at 200× or 400× magnification. The TILs in the areas of intra-tumoral region, necrosis, crush artifacts, hyalinization are excluded. Then we categorized these cases into three groups with the TILs of <10%, 10-50%, and >50%.
Figure 1.
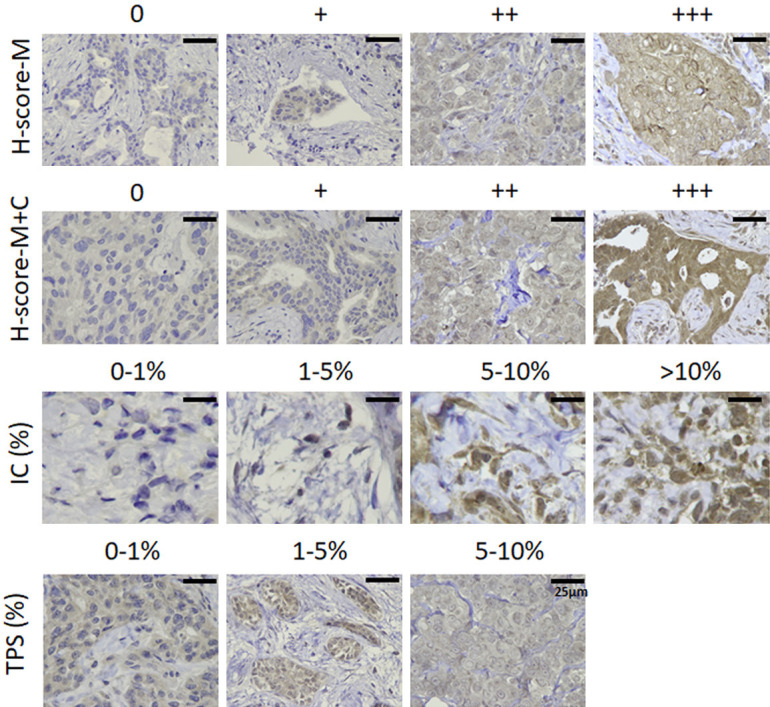
The representative case examples with different scores and percentages of H-score (M), H-score (M+C), IC score, and TPS stained with PD-L1 28-8 monoclonal antibody.
Statistical analysis
The variation between de-glycosylated and natural value of H-score and TPS were illustrated using line plot, and the fold change (FC) between de-glycosylated and natural score is computed by subtracting the mean score between de-glycosylated and natural value of H-score and TPS. Wilcoxon signed rank test was used to estimate the difference of de-glycosylated and natural value of H-score and TPS (Figure 3A-D). The exact value of de-glycosylated and natural value of H-score and TPS in each patient were illustrated in bar diagrams (Figure 3I). In Table 2, the receiving operating characteristics (ROC) analysis was used to derive optimal cut-off point in predicting the best response for atezolizumab. The performance of the median and ROC derived optimal cut-off point were compared using the area under curve (AUC), sensitivity (true positive rate), specificity (false negative rate), and accuracy (Table 2). In addition, the correlation of the ROC-determined treatment response subgroup and best treatment response for atezolizumab in each measurement were estimated using Fisher’s exact test (Table 3). The progression-free survival (PFS) was considered as the primary endpoint, and the PFS interval is tracked from the initial date of atezolizumab treatment until the first disease-progress date or the end date of study (Figure 4B-E). The PFS difference between subgroup dichotomous by ROC derived optimal cut-off were estimated using Kaplan-Meier estimator, and tested using log-rank test. The median follow-up interval since initial drug used for disease-progress was reported. A P-value less than 0.05 was considered statistically significance, and all P-values were two-sided. All analyses were performed using R software 4.0.2 (R core team, 2021).
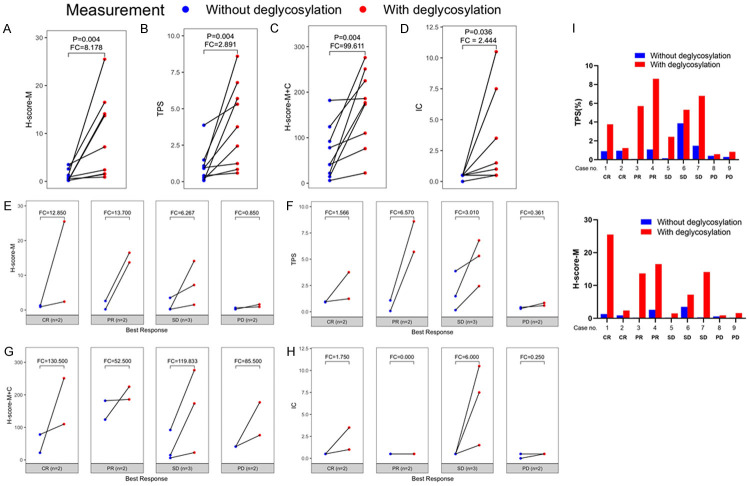
Figure 3.
PD-L1 detection was significantly enhanced after deglycosylation. (A) H-score-M of PD-L1, (B) TPS of PD-L1, (C) H-score of PD-L1-M+C, and (D) IC of PD-L1 with or without deglycosylation in all samples. (E) H-score of PD-L1-M, (F) TPS of PD-L1, (G) H-score-M+C of PD-L1, and (H) IC of PD-L1 with or without deglycosylation in subgroup according to the best response for atezolizumab. Fold changes (FC) is the subtraction of the mean score between deglycosylated and natural value of each measurements. (I) Quantification of TPS (upper) and H-score-M (lower) of IHC staining for nine TNBC patients processed with or without PNGase F pretreatment. P-value was estimated using Wilcoxon signed rank test.
Table 2.
The comparison of the performance of the median and receiving operating characteristics (ROC) derived optimal cut-off point in predicting the best response for atezolizumab
| Score | Cut-off point | AUC | Sensitivity (Sen) | Specificity (Spe) | Accuracy | |
|---|---|---|---|---|---|---|
| Medium | H-score | ≤0.6 | 0.714 | 1.000 | 0.571 | 0.67 |
| Deglycosylated H-score | ≤7.2 | 0.929 | 1.000 | 0.571 | 0.67 | |
| TPS | ≤0.91 | 0.714 | 1.000 | 0.571 | 0.67 | |
| Deglycosylated TPS | ≤3.76 | 1.000 | 1.000 | 0.571 | 0.67 | |
| Optimal | H-score | ≤0.6 | 0.714 | 1.000 | 0.571 | 0.67 |
| Deglycosylated H-score | ≤1.6 | 0.929 | 1.000 | 0.857 | 0.89 | |
| TPS | ≤0.414 | 0.714 | 1.000 | 0.714 | 0.78 | |
| Deglycosylated TPS | ≤0.839 | 1.000 | 1.000 | 1.000 | 1.00 |
Sensitivity indicates true positive rate, specificity indicates true negative rate. AUC, area under curve. Bold fonts indicates the better performance in optimal cutpoint were found in predicting the best response for Atezolizumab comparing to the median cutpoint.
Table 3.
Comparison of the treatment response by ROC derived subgroup in each measurement
| Measurement | Type | ROC cutoff comparison | p |
|---|---|---|---|
| w/o DG | H-score-M | ≤0.6 vs >0.6 | 0.444 |
| DG | H-score-M | ≤1.6 vs >1.6 | 0.083 |
| w/o DG | TPS | ≤0.414 vs >0.414 | 0.167 |
| DG | TPS | ≤0.839 vs >0.839 | 0.028 |
| w/o DG | H-score-M+C | ≤41 vs >41 | 0.444 |
| DG | H-score-M+C | ≤177 vs >177 | 0.444 |
| w/o DG | IC | ≤0 vs >0.5 | 0.222 |
| DG | IC | ≤0.5 vs >0.5 | 0.167 |
P-value is estimated using Fisher’s exact test.
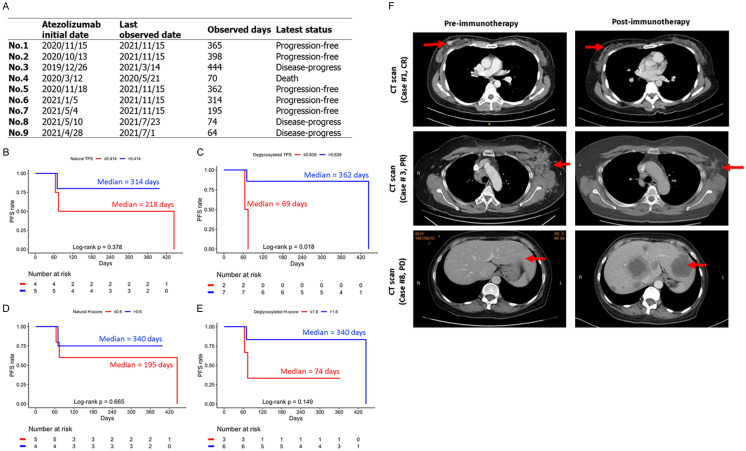
Figure 4.
Lower PD-L1 levels by TPS scoring were associated with worse PFS. (A) The sustained response to atezolizumab of the present nine cases. The PFS estimation for the ROC-derived (B) natural TPS, (C) deglycosylated TPS subgroups, (D) natural and (E) deglycosylated H-score-M subgroup, P-value was estimated using log-rank test. (F) Representative images of computed tomography (CT) scan from patients #1, 3 and 8 from pre- and post-anti-PD-L1 (atezolizumab) immunotherapy.
Results
Patient characteristics and IHC methods
Nine TNBC patients who had PD-L1 immune cell (IC) <1% by SP142 staining were included in the study (Table 1). Eight metastatic patients had received at least first line chemotherapy and yet still had progressive disease. The remaining case was given the neoadjuvant chemotherapy (NACT) with immunotherapy which is based on IMpassion031 trial.
Table 1.
The value change of TPS, H-score-M, an IC before and after deglycosylation of the present nine cases
| TPS w/o DG* | TPS with DG* | H-score-M w/o DG* | H-score-M with DG* | IC w/o DG** | IC with DG* | Best response | |
|---|---|---|---|---|---|---|---|
| No.1 | 0.910 | 3.760 | 1.3 | 25.5 | IC<1 | IC≥1, <5 | CR |
| No.2 | 0.957 | 1.240 | 0.9 | 2.4 | IC<1 | IC≥1, <5 | CR |
| No.3 | 0.076 | 5.700 | 0.2 | 13.7 | IC<1 | IC<1 | PR |
| No.4 | 1.085 | 8.600 | 2.6 | 16.5 | IC<1 | IC<1 | PR |
| No.5 | 0.157 | 2.440 | 0.2 | 1.5 | IC<1 | IC≥1, <5 | SD |
| No.6 | 3.868 | 5.310 | 3.5 | 7.2 | IC<1 | IC≥10 | SD |
| No.7 | 1.485 | 6.791 | 0.3 | 14.1 | IC<1 | IC≥5, <10 | SD |
| No.8 | 0.414 | 0.589 | 0.6 | 0.9 | IC<1 | IC<1 | PD |
| No.9 | 0.293 | 0.839 | 0.2 | 1.6 | IC<1 | IC<1 | PD |
Abbreviation: M: membranous staining; IC: immune cells; TPS: tumor proportion score; DG: deglycosylation; w/o: without; CR: complete response; SD: stable disease; PD: progressive disease.
Stained with PD-L1 28-8 clonal antibody.
Stained with PD-L1 28-8 and SP142 clonal antibody.
IC (immune cells) scoring was stained by SP142 following the IMpassion130 and 131 trials. Four scoring systems were used to analyze the IHC staining of PD-L1: TPS, H-score-M (H score of membranous staining, M), H-score-M+C (H score of membranous and cytoplasmic staining, M+C) and IC scoring were stained by Mab 28-8 in this present study. The intensities of PD-L1 were defined in four categories (Figure 1). First, membrane staining intensity (0, 1+, 2+, or 3+) of H-score-M and H-score-M+C was determined for each tissue in 10 fields. The IC score (0-1%, 1-5%, 5-10% and >10%) was calculated by the percentage of area infiltrated by the membranous PD-L1-positive immune cells in the total tumor area regardless of the staining intensity. The TPS (0-1%, 1-5% and 5-10%) was defined by the percentage of membranous PD-L1 expressing tumor cells in all tumor cells of the section regardless of the staining intensity.
Initial assessment of treatment response with de-glycosylated PD-L1 levels in tumor cell surface
As mentioned above, the 1% cut-off of PD-L1 level in IC was assessed by SP142 in predicting response to atezolizumab for TNBC based on the criteria of IMpassion130 and 131. In order to validate whether removal of N-linked glycosylation improved the prediction of response to atezolizumab, de-glycosylation (DG) of the tissue sections was performed by PNGase F treatment before detecting PD-L1 by IHC with Mab 28-8 as reported previously [10]. A few examples were shown in Figure 2 and summarized in Table 1. “Best response” was defined and evaluated 3 months after the treatment by CT scan. The results showed that two out of the nine patients (#8 and #9) had progressed disease (PD), while the best response in the other 7 patients either had their tumors completely responded (CR) (#1 and #2), partially responded (PR) (#3 and #4), or with a stable disease (SD) (#5, #6, and #7). Interestingly, based on the 1% cut-off of PD-L1 level in IC from the criteria in IMoassion130 and 131, none of the 9 patients were eligible for treatment by atezolizumab, yet 7 of them were responsive to the treatment, ranging from 2 CR, 2 PR and 3 SD (Table 1). Of note, PD-L1 detection as determined by four scoring systems was significantly enhanced after de-glycosylation (Figure 3A-D). Importantly, after de-glycosylation by PNGase F treatment, the tumor cell surface PD-L1 levels from both TPS and H-score-M seemed to correlate well with clinical responses, i.e. the two PD patients showed the lowest PD-L1 levels and the 7 patients who responded to treatment including CR, PR and SD all (except for case 2, see later) showed significantly higher PD-L1 levels after de-glycosylation (red vs blue; Figure 3E-H; Table 1). It was worth noting that CR, PR and SD all displayed an enhanced PD-L1 signal after de-glycosylation by the treatment of PNGase F, except for case 2 (Figure 3I), The results seem to suggest that tumor cell surface PD-L1 levels after de-glycosylation may be able to predict the clinical outcome.
Figure 2.
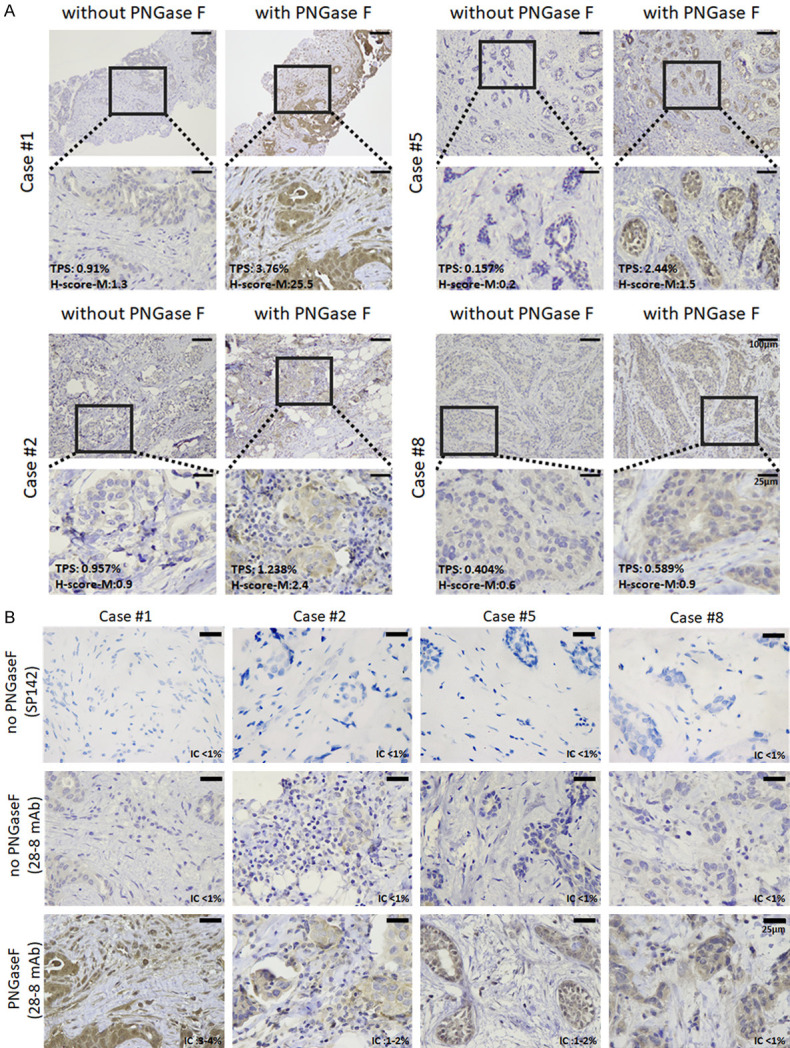
Representative photos of PD-L1 immunohistochemical staining with and without deglycosylation by PNGase F (5%) of patients #1, 2, 5 and 8. A. TPS score/H-score (membranous) of the cases (stained with PD-L1 28-8 monoclonal antibody, 100× and 400×). B. IC score of the cases (stained with PD-L1 28-8 and SP142 clonal antibody, 400×).
ROC analysis and performance analysis for the best scoring
Although the cohort is relatively small, the straightforward correlation (Figure 3; Table 1) prompted us to further verify the correlation between treatment response and de-glycosylated PD-L1. To this end, we used receiver operating characteristic (ROC) curves to evaluate different scoring systems in the accuracy of treatment prediction in patients who received atezolizumab. The results using the median and the optimal cut-off derived from the ROC analyses in predicting the best response for atezolizumab were summarized in Table 2. The ROC-derived optimal cut-off points were found superior to the median cut-off points, especially with de-glycosylation as supported by the close to 1 accuracy and specificity in predicting the response for atezolizumab (Bold in Table 2). Therefore, the optimal cut-off points from the ROC analysis was used to analyze correlation with PFS.
The latest response to atezolizumab of the study population is summarized in Figure 4A. Different from the “best response”, the “latest response” was defined as the status of the patients at the end of the observation duration (up to 11/15/2021) since the initial date of atezolizumab treatment. The status of “disease-progress” indicates the presence of any clinical evidence of disease progression in the patients, and the status of “progression-free” indicates that the patients are maintaining a progression-free status within the observation interval. Of note, one of the PR patients (#4) had a short observation time of 70 days. This was because that although the patient’s liver metastatic lesion shrank in response to atezolizumab treatment, the patient opted out of the treatment and instead received hospice care due to liver cirrhosis. The other PR patient, patient #3, received immunotherapy combined with chemotherapy for 4 months, but the tumor progressed 19 months after the treatment.
Figure 4B and 4C showed the PFS estimation according to the natural and de-glycosylated TPS subgroup dichotomized by the ROC-derived optimal cut-off points and patients who had lower PD-L1 levels by de-glycosylated TPS (red) were significantly associated with poor PFS (P=0.018<0.05). The P value of natural TPS staining, 0.378 (>0.05) indicated insignificant correlation and supports that de-glycosylation is critical for correlation with PFS. Similar trend was also observed for the H-score-M staining (Figure 4D, 4E). Patients with lower PD-L1 levels by H-score-M (red) were associated with worse PFS. Although statistically less robust than the TPS scoring system, the P values from the H-score-M scoring of de-glycosylation (0.149) vs natural staining (0.665) also supported the notion that de-glycosylation correlated better with PFS than natural staining. Patients #1 and #3 with higher de-glycosylated TPS and H-score-M staining were shown as representative cases to exhibit apparent shrinkage after receiving atezolizumab treatment (Figure 4F).
Taken together, de-glycosylated H-score-M and TPS not only contribute in predicting the better response for atezolizumab by PFS, the de-glycosylated subgroup dichotomized by using the ROC-derived optimal cut-off points also better predicted the responsiveness to the treatment in the cohort. Although the cohort is relatively small and the P value is not as significant (e.g., H-score-M), the prediction tendency is encouraging. Moreover, de-glycosylated H-score and TPS subgroup dichotomized using the ROC-derived optimal cut-off points also showed better prediction performance compared to scoring the natural PD-L1 (Table 2). Thus, the ROC analysis seems to derive the same conclusion as that from Table 1 and Figure 1.
Correlation of de-glycosylation of PD-L1 levels in tumor cells surface with response to atezolizumab treatment
We next used Fisher Exact Test to assess the statistical significance of the ROC-derived optimal cut-off points with clinical response, using as criteria to include patients with CR, PR and SD in the responsive group and patients with PD as non-responsive group. Interestingly, all criteria used by the Impassion130 and 131 trials including H-Score-M+C and IC have no correlation with treatment response at all (P-value for w/o DG, 0.444 and 0.222) (Figure 3G-H; Table 3), consistent to the conclusion of the Impassion131 trial. Even the results from de-glycosylation of H-Score-M+C (P=0.444 for DG) and IC (P=0.167 for DG) categories (Table 3) have no any correlation with the treatment response, although the reading for the PD-L1 level is generally increased in the de-glycosylation category (DG vs w/o DG) as expected (Figure 3; Table 1) [10-13].
Importantly, the PD-L1 level based on tumor membrane staining after de-glycosylation (H-Score-M with DG) showed a marginal significance (P-value =0.083) as the two PD non-responding patients had the lowest reading (0.9 and 1.6) and the rest responding patients had PD-L1 levels higher than 2.4 (2.4-25.5) except for patient #5 (Table 1). However, the correlation disappeared without de-glycosylation (H-Score-M w/o DG) (P-value =0.444). The two non-responding PD patients had very low PD-L1 levels (0.2 and 0.6 w/o DG vs 1.6 and 0.9, with DG, respectively). However, in patients No. 3, 5 and 7 who had similarly low reading of PD-L1 (0.2, 0.2 and 0.3, respectively) prior to de-glycosylation, the staining intensities significantly increased to 13.7, 1.5 and 14.1, respectively, after de-glycosylation, supporting the false negativity of PD-L1 staining by the standard protocol and consistently these patients showed favorable response to the treatment by atezolizumab. With similar analysis, the TPS with DG group also showed excellent correlation (P-value =0.028) as that of H-Score-M DG group (Table 3). Together, these results suggest that removal of N-linked glycosylation of PD-L1 in the membrane of tumor cells (H-Score-M, DG) and TPS (DG) seem to best correlate with therapeutic response to atezolizumab treatment compared to scoring the natural PD-L1 (w/o DG) using the clone 28-8 Mab, even the analysis was from a relatively small cohort.
Tumor-infiltrating lymphocytes (TILs) may contribute to anti-PD-L1 therapy
It has been shown that elevated TIL counts can serve as a good prognostic factor in responding to pembrolizumab (Keytruda) treatment in metastatic TNBC [18]. One of the patients, patient #2, had relatively low de-glycosylated H-score-M and TPS (H-score-M=2.4; TPS=1.24) but still experienced a CR response (Figure 3I). Relatively more lymphocytes were found in these patient’s tumor tissues, supporting the previous report [18] that the elevated TIL may play a role in the treatment response (Figure 5).
Figure 5.
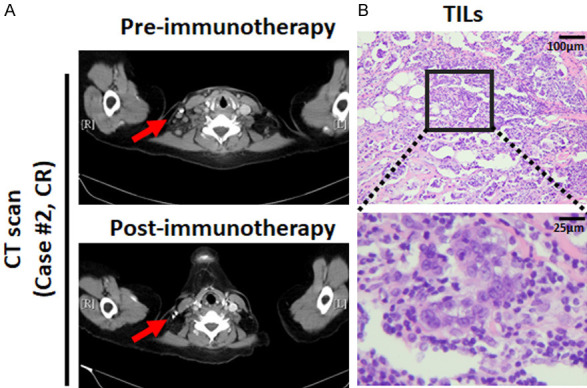
The presence of TILs was associated with response to atezolizumab. A. Representative images of computed tomography (CT) scan from patient #2 from pre- and post-anti-PD-L1 (atezolizumab) immunotherapy. B. The representative case examples with different percentages of tumor infiltrating lymphocytes (TILs) (Hematoxylin and eosin stain, 100× and 400×).
Discussion
The cut-off values of PD-L1 can be dependent on different tumor types, including tumor cells, tumor-infiltrating immune cells, or both, and the different assays [3,10,14-17]. IC scoring by SP142 staining was the FDA-approved screening criteria for TNBC patient recruitment to receive treatment by atezolizumab [3,19]. However, this predictive biomarker for treatment response is not accurate and has many limitations, which might be a contributing factor to the concerning response rate of TNBC patients with PD-L1 positive expression which is as low as 18.5% [20]. Therefore, the evaluation of PD-L1 expression in patient receiving immune checkpoint inhibitors must be carefully implemented for clinical decision-making.
We have shown a novel procedure to prevent the interference of PD-L1 detection by removing the N-linked glycosylation from the unstained slides with the recombinant glycosidase (PNGase F) before IHC staining, which markedly enhances the detection of PD-L1 in cancer cells and tumor tissue by the 28-8 Mab [10,11]. In another independent study, four clones of anti-PD-L1 Mab, namely 28-2, CAL10, 73-10, and SP142, were compared for PD-L1 detection in lung cancer tumor tissues before and after de-glycosylation [12]. The study concluded that after de-glycosylation, PD-L1 expression was markedly enhanced when stained with the 28-8, CAL10, and SP142 antibodies, and a slight decrease of PD-L1 expression was found when the 73-10 antibody was used. Consistently, our data indicated that H-score-M, H-score-M+C-, IC and TPS also have significant greater fold change compared to those without PNGase F treatment.
We have observed a close correlation between the de-glycosylated membrane PD-L1 in the tumor tissues and patient response. Patient #1 showed complete response (pCR) after NACT as judged by CT scan and post-operation pathology. This patient received eight courses of neoadjuvant chemotherapy, including four courses of epirubicin and cyclophosphamide with atezolizumab, followed by four courses of taxotere with atezolizumab. Of note, the results showed significantly higher de-glycosylated PD-L1 levels in TPS and H-score. Similarly, the two cases (patients #3 and #4) who have partial response (PR) also associated with significantly higher de-glycosylated PD-L1 levels. Patient #3 is a patient with lung metastasis after a second line chemotherapy and progression of the left axillary lymph node was noted. After 6 courses of paclitaxel plus atezolizumab, CT showed regression of the left axillary metastasis. Patient #4 is a breast cancer patient with liver and bone metastasis at first. She received four courses of epirubicin and cyclophosphamide, followed by four courses of taxotere. After the first line treatment, she still suffered liver metastasis. She received 5 courses gemcitabine + paclitaxel plus atezolizumab. We followed the CT scan and showed shrinkage of liver metastasis. Both patient #8, and #9 whose tissues showed lowest levels of de-glycosylated PD-L1 in the TPS and H-score-M, belong to PD, ie no response to the treatment. Patient #8 developed bone metastasis after the first line chemotherapy. Therefore, six courses of paclitaxel plus atezolizumab were arranged. After 3 months, CT examination found multiple new lesions in the liver. Patient #9 was diagnosed bilateral breast cancer with bone metastasis. After the four courses of epirubicin and cyclophosphamide, followed by four courses of taxotere, the patient continued to have oral vinorelbine. After half year medication, we found new liver metastasis and discussed with patient for immunotherapy. The patient then received six courses of paclitaxel plus atezolizumab. We followed up with the CT scan and found progression of liver metastasis It should be emphasized that all nine TNBC patients have the PD-L1 IC<1% under SP142 staining, and therefore would not be recommended to receive atezolizumab therapy. However, seven out of nine patients were actually responsive to atezolizumab treatment. Thus, our data demonstrate that improved PD-L1 detection after de-glycosylation is highly associated with response to atezolizumab, suggesting that increased PD-L1 signal after de-glycosylation is a favorable method for identifying patients who can benefit from the treatment.
It has been reported that tumor immune microenvironment including the infiltration of immune cells, immune inhibitory soluble factors, and cytokines provides important clues in predicting outcomes and potential treatment guideline [21-23]. TIL is the major type of immune cells present in the tumor microenvironment and has been used as prognostic biomarkers for response to immune therapy [24,25]. It is noteworthy that the tumor of patient #2, showed a moderate increase in PD-L1 after de-glycosylation, accompanied by the significant presentation of TILs, raising an interest point that TIL level might also be a favorable factor in addition to the de-glycosylated tumor membrane PD-L1 level to stratify patients for anti-PD-L1 therapy. This possibility is worthy of exploring further in the near future.
Membrane expression of PD-L1 in cancer cells is the key characteristic to achieve immune suppression and promote tumor progression [6]. The clinical data derived from this study, albeit at a small scale, in a group of intention-to-treat patients with advanced unresectable breast cancer suggest that the % IC scoring system of PD-L1 is unlikely an efficient criterion to identify breast cancer patients for atezolizumab treatment. In contrast, scoring of membrane expression of PD-L1 in cancer cells closely correlates with patient outcome, with the tumors of relatively higher PD-L1 expression responding more favorably to atezolizumab treatment. Our finding supports that patients of advanced breast cancer whose tumors, but not the tumor-infiltrating immune cells, express high levels of membrane PD-L1 as revealed by the de-glycosylation-IHC staining protocol are likely more responding to atezolizumab treatment. Our data suggest that TPS or H-score-M of the breast cancer tissue after de-glycosylation may be a more efficient tool to select appropriate patients who may benefit from the anit-PD-L1 immunotherapy with atezolizumab.
Pembrolizumab (Keytruda; Merck) is the other PD-1/PD-L1 inhibitor approved by FDA for breast cancer treatment [26]. In November 2020, FDA first granted accelerated approval to pembrolizumab for the indication in combination with chemotherapy for patients with locally recurrent unresectable or metastatic triple-negative breast cancer, whose tumors express PD-L1 based on the results of the phase III trial KEYNOTE-522 (NCT03036488) [27]. In this trial, PD-L1 expression was assessed by IHC using the 22C3 pharmDx assay (Agilent Technologies), and defined by the combined positive score (CPS), defined as the proportion (%) of PD-L1-positive cells including both tumor and immune cells in the total number of tumor cells. Patients whose tumors express PD-L1 with a combined positive score of 1% or greater are considered PD-L1-positive and eligible for pembrolizumab treatment [26]. The successful throughput of the KEYNOTE-522 trial led to the FDA approval of pembrolizumab for high-risk early-stage TNBC with a combined positive score of PD-L1 10% or greater in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery in July 2021. At the same time, FDA also confirmed the prior accelerated approval by granting regular approval to pembrolizumab for the indication in metastatic TNBC. It is noteworthy that the scoring systems of PD-L1 positivity in the IMpassion130-Impassion131 trials and the KEYNOTE-522 trial are different. It remains to be determined whether explicitly including the tumor tissues in scoring de-glycosylated PD-L1 in patient stratification as in the KEYNOTE-522 trial would help contribute to the outcome of the pembrolizumab study.
In conclusion, the current study suggests that, consistent with the observation from the IMpassion131 trial, the PD-L1 level in the tumor-associated immune cells could not predict the response to atezolizumab treatment and that the de-glycosylated PD-L1 level in cancer cell membrane outperforms the natural PD-L1 level as a biomarker for identifying patients of advanced breast cancer who are most likely responding to anti-PD1/PD-L1 therapies. These results warrant multi-institution studies to further validate the efficacy of the protocol in ICB treatments for advanced breast cancer.
Acknowledgements
We acknowledge the support from the following grants: (1) MOHW109-TDU-B-212-010001 and MOHW107-TDU-B-212-112015 from the Ministry of Health and Welfare, Taiwan; (2) KMUH-DK(C)110005, KMUH-DK-109001, KMUH108-8R36, KMUH-DK-109003-2, and KMUH-DK-109003-3 and KMUH109-9R37 from the Kaohsiung Medical University Hospital; (3) KMU-DK-109003-1 from KMU-KMUH Co-Project of Key Research; (4) 110-2320-B-037-006-, 110-2628-B-037-011-, 110-2321-B-037-002-, 109-2314-B-037-019, 109-2314-B-037-132-, and 109-2314-B-037-036-MY3 from the Ministry of Science and Technology, Taiwan; (5) KMU-KI110001, KMU-TC109A03, KMU-TC108A03-6, and KMU-TC109B05 from Kaohsiung Medical University Research Center Grant; (6) MOST 110-2639-B-039-001-ASP and the Breast Cancer Research Fund (BRCF-21-070) (to M.C.-H). This work was also financially supported in part by the Drug Development Center of the China Medical University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan. Experiments and data analysis were performed in part through the use of the Medical Research Core Facilities Center, Office of Research & Development at China Medical University, Taichung, Taiwan.
Disclosure of conflict of interest
Mien-Chie Hung holds a patent on the methodology for de-glycosylation staining.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emens LA, Adams S, Barrios CH, Dieras VC, Iwata H, Loi S, Rugo HS, Schneeweiss A, Winer EP, Patel S, Henschel V, Swat A, Kaul M, Molinero L, Chui SY, Schmid P. LBA16 IMpassion130: final OS analysis from the pivotal phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann Oncol. 2020;31:S1148–S1148. [Google Scholar]
- 3.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 4.Vennapusa B, Baker B, Kowanetz M, Boone J, Menzl I, Bruey JM, Fine G, Mariathasan S, McCaffery I, Mocci S, Rost S, Smith D, Dennis E, Tang SY, Damadzadeh B, Walker E, Hegde PS, Williams JA, Koeppen H, Boyd Z. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl Immunohistochem Mol Morphol. 2019;27:92–100. doi: 10.1097/PAI.0000000000000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miles DW, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios CH, Xu B, Wardley AM, Kaen D, Andrade L, Semiglazov V, Reinisch M, Patre M, Morales L, Russell K, Donica M, O’Shaughnessy J. LBA15 primary results from IMpassion131, a double-blind placebo-controlled randomised phase III trial of first-line paclitaxel (PAC); atezolizumab (atezo) for unresectable locally advanced/metastatic triple-negative breast cancer (mTNBC) Ann Oncol. 2020;31:S1147–S1148. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 7.Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, Khoo KH, Chang SS, Cha JH, Kim T, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Ding Q, Wang Y, Yao J, Lee CC, Wu HJ, Sahin AA, Allison JP, Yu D, Hortobagyi GN, Hung MC. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, Cha JH, Xia W, Chan LC, Kim T, Chang SS, Lee HH, Chou CK, Liu YL, Yeh HC, Perillo EP, Dunn AK, Kuo CW, Khoo KH, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Huang TH, Sahin AA, Hortobagyi GN, Yoo SS, Hung MC. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33:187–201. e110. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu JM, Li CW, Lai YJ, Hung MC. Posttranslational modifications of PD-L1 and their applications in cancer therapy. Cancer Res. 2018;78:6349–6353. doi: 10.1158/0008-5472.CAN-18-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HH, Wang YN, Xia W, Chen CH, Rau KM, Ye L, Wei Y, Chou CK, Wang SC, Yan M, Tu CY, Hsia TC, Chiang SF, Chao KSC, Wistuba II, Hsu JL, Hortobagyi GN, Hung MC. Removal of N-linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell. 2019;36:168–178. e164. doi: 10.1016/j.ccell.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YN, Lee HH, Hsu JL, Yu D, Hung MC. The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis. J Biomed Sci. 2020;27:77. doi: 10.1186/s12929-020-00670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mei J, Xu J, Yang X, Gu D, Zhou W, Wang H, Liu C. A comparability study of natural and deglycosylated PD-L1 levels in lung cancer: evidence from immunohistochemical analysis. Mol Cancer. 2021;20:11. doi: 10.1186/s12943-020-01304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Yang X, Mao Y, Mei J, Wang H, Ding J, Hua D. Removal of N-linked glycosylation enhances PD-L1 detection in colon cancer: validation research based on immunohistochemistry analysis. Technol Cancer Res Treat. 2021;20:15330338211019442. doi: 10.1177/15330338211019442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 15.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 16.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, Van den Eynden G, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SR, Schnitt S, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O’Toole S, Larsimont D, de Wind R, Curigliano G, André F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: part 1: assessing the host immune response, tils in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24:235–251. doi: 10.1097/PAP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loi S, Adams S, Schmid P, Cortés J. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): results from KEYNOTE-086. Ann Oncol. 2017;28:V608. [Google Scholar]
- 19.Lee SE, Park HY, Lim SD, Han HS, Yoo YB, Kim WS. Concordance of programmed death-ligand 1 expression between SP142 and 22C3/SP263 assays in triple-negative breast cancer. J Breast Cancer. 2020;23:303–313. doi: 10.4048/jbc.2020.23.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J. Clin. Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng W, Lira V, Hudson TE, Lemmens EE, Hanson WG, Flores R, Barajas G, Katibah GE, Desbien AL, Lauer P, Leong ML, Portnoy DA, Dubensky TW Jr. Recombinant Listeria promotes tumor rejection by CD8(+) T cell-dependent remodeling of the tumor microenvironment. Proc Natl Acad Sci U S A. 2018;115:8179–8184. doi: 10.1073/pnas.1801910115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruosso T, Gigoux M, Manem VSK, Bertos N, Zuo D, Perlitch I, Saleh SMI, Zhao H, Souleimanova M, Johnson RM, Monette A, Ramos VM, Hallett MT, Stagg J, Lapointe R, Omeroglu A, Meterissian S, Buisseret L, Van den Eynden G, Salgado R, Guiot MC, Haibe-Kains B, Park M. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J Clin Invest. 2019;129:1785–1800. doi: 10.1172/JCI96313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med. 2019;17:90. doi: 10.1186/s12916-019-1326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee N, Zakka LR, Mihm MC Jr, Schatton T. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology. 2016;48:177–187. doi: 10.1016/j.pathol.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Maibach F, Sadozai H, Seyed Jafari SM, Hunger RE, Schenk M. Tumor-infiltrating lymphocytes and their prognostic value in cutaneous melanoma. Front Immunol. 2020;11:2105. doi: 10.3389/fimmu.2020.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 27.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Untch M, Fasching PA, Cardoso F, Ding Y, Tryfonidis K, Aktan G, Karantza V, O’Shaughnessy J. VP7-2021: KEYNOTE-522: phase III study of neoadjuvant pembrolizumab + chemotherapy vs. placebo + chemotherapy, followed by adjuvant pembrolizumab vs. placebo for early-stage TNBC. Ann Oncol. 2021;32:1198–1200. [Google Scholar]