Abstract
Purpose
To evaluate the effect of ATP-sensitive potassium channel openers cromakalim prodrug 1 (CKLP1) and diazoxide on IOP in three independent mouse models of ocular hypertension.
Methods
Baseline IOP was measured in TGFβ2 overexpression, steroid-induced, and iris dispersion (DBA/2J) ocular hypertension mouse models, followed by once daily eyedrop administration with CKLP1 (5 mM) or diazoxide (5 mM). The IOP was measured in conscious animals with a handheld rebound tonometer. Aqueous humor dynamics were assessed by a constant perfusion method. Effect of treatment on ocular tissues was evaluated by transmission electron microscopy.
Results
CKLP1 decreased the IOP by 20% in TGFβ2 overexpressing mice (n = 6; P < 0.0001), 24% in steroid-induced ocular hypertensive mice (n = 8; P < 0.0001), and 43% in DBA/2J mice (n = 15; P < 0.0001). Diazoxide decreased the IOP by 32% in mice with steroid-induced ocular hypertension (n = 13; P < 0.0001) and by 41% in DBA/2J mice (n = 4; P = 0.005). An analysis of the aqueous humor dynamics revealed that CKLP1 decreased the episcleral venous pressure by 29% in TGFβ2 overexpressing mice (n = 13; P < 0.0001) and by 72% in DBA/2J mice (n = 4 control, 3 treated; P = 0.0002). Diazoxide lowered episcleral venous pressure by 35% in steroid-induced ocular hypertensive mice (n = 3; P = 0.03). Tissue histology and cell morphology appeared normal when compared with controls. Accumulation of extracellular matrix was reduced in CKLP1- and diazoxide-treated eyes in the steroid-induced ocular hypertension model.
Conclusions
ATP-sensitive potassium channel openers CKLP1 and diazoxide effectively decreased the IOP in ocular hypertensive animal models by decreasing the episcleral venous pressure, supporting a potential therapeutic application of these agents in ocular hypertension and glaucoma.
Keywords: ocular hypertension, glaucoma, mouse models, KATP channels, diazoxide, CKLP1
Glaucoma is a progressive neurodegenerative disorder of the eye and is the primary cause of irreversible blindness worldwide. With an aging population and expanding demographics, the prevalence of the disease is expected to increase, with more than 140 million people affected by 2040.1,2 Currently, there is no cure for glaucoma, but treatments that decrease the IOP have been shown to slow down the disease progression.3,4
The most common first-line treatment for glaucoma is the use of eyedrops containing ocular hypotensive medications. Unfortunately, all classes of ocular hypotensive agents are associated with mild to severe side effects.3,4 For example, topical medications like prostaglandin analogs (e.g., latanoprost), beta blockers (e.g., timolol), and carbonic anhydrase inhibitors (e.g., brinzolamide) can cause significant conjunctival hyperemia, iris pigmentation, hypertrichosis, and even systemic cardiac and respiratory side effects in susceptible individuals.2–5 Furthermore, topical medications may require multiple daily dosing, making it difficult for elderly patients to accurately instill the drops or to remember when to apply the drops. Both the side effect profiles and the inability to appropriately maintain the prescribed medication results in up to 50% patient noncompliance.6,7 Additionally, a high proportion of patients with glaucoma can become nonresponsive to frontline therapies requiring dosing with multiple drugs, further adding to treatment challenges. As a result, the development of therapeutics with little or no side effects can address a significant need in patients with glaucoma.
ATP-sensitive potassium (KATP) channel openers are hetero-octameric transmembrane proteins, that connect the metabolic and energetic states of cells by virtue of their sensitivity to micromolar concentrations of intracellular ATP.8 Our laboratory has shown that several pharmacologic openers of KATP channels (diazoxide, cromakalim, nicorandil, etc.) lower the IOP in ex vivo human eyes and in several normotensive animal models including mice, rats, rabbits, dogs, and nonhuman primates.9–18 Because commercially available KATP channel openers have limited aqueous solubility, we developed a novel, water-soluble, direct phosphate linked prodrug (cromakalim prodrug 1 [CKLP1]) based on the KATP channel opener levcromakalim. CKLP1 exhibits similar ocular hypotensive properties as levcromakalim and was found to decrease the IOP by directly lowering episcleral venous pressure with no observable side effects in normotensive mice.15 Owing to its unique site of action, KATP channel openers were shown to work in combination with existing glaucoma medications to lower IOP more than each treatment alone.15 Thus, KATP channel openers may be a novel therapeutic option for patients with glaucoma.
The effects of KATP channel openers on IOP in ocular hypertensive eyes are unknown. Based on their effects on normotensive animals, we hypothesized that KATP channel openers would similarly lower the IOP in animal models of ocular hypertension by decreasing the episcleral venous pressure. To test this, three separate animal models of ocular hypertension (TGFβ2 overexpression, steroid-induced, and iris dispersion [DBA/2J] mice) were treated with either CKLP1 or diazoxide followed by evaluation of the IOP, various parameters of aqueous humor dynamics, and tolerability.
Methods
Reagents
CKLP1 (originally described as [3S,4R]-2) was synthesized as previously described and formulated by dissolving in PBS.12 Diazoxide was purchased from MilliporeSigma (St. Louis, MO) and dissolved in dimethyl sulfoxide (DMSO) to make a 100-mM stock solution. Stock solutions were diluted 20-fold in 10% Cremophor EL (MilliporeSigma) prepared in PBS, for a final working concentration of 5 mM.
A formulation of dexamethasone acetate as a suspension was prepared as previously described.19 All reagents required for suspension formulation were purchased from either MilliporeSigma or Spectrum Chemicals (New Brunswick, NJ). Briefly, sodium chloride, creatinine, EDTA, sodium bisulfite, polysorbate 80, benzyl alcohol, and carboxymethylcellulose were added sequentially, pH adjusted to 7.0 and brought to final volume with water (Table 1). Anhydrous, micronized dexamethasone powder (Spectrum Chemicals) was added to the suspension formulation to a final concentration of 10 mg/mL. Stainless steel beads (5 mm) were added to the dexamethasone suspension formulation and vortexed for 5 minutes at room temperature. The dexamethasone suspension formulation was placed at 4°C, protected from light, and mixed overnight on a rotator. Following overnight rotation, the dexamethasone suspension formulation was isolated, aliquoted, and stored at 4°C for up to two weeks. Suspension formulation for vehicle was stored protected from light at 4°C for up to 90 days.19
Table 1.
Reagents for Dexamethasone Acetate Formulation
| Reagent | Volume/100 mL | Source |
|---|---|---|
| Sodium chloride | 0.667 g | Millipore Sigma |
| Creatinine | 0.5 g | Spectrum Chemical |
| Edetate disodium dihydrate | 0.05 g | Millipore Sigma |
| Carboxymethylcellulose sodium | 0.5 g | Millipore Sigma |
| Polysorbate 80 | 0.1 mL | Millipore Sigma |
| Benzyl alcohol | 0.9 mL | Millipore Sigma |
| Sodium bisulfite | 0.1 g | Spectrum Chemical |
| Sodium hydroxide 1% | To adjust pH to 7 | Millipore Sigma |
| Dexamethasone acetate | 10 mg/mL | Spectrum Chemical |
Mouse Models of Ocular Hypertension
All animal experiments were preapproved by the respective Institutional Animal Care and Use Committees at Mayo Clinic, Rochester, Minnesota, or the North Texas Eye Research Institute, University of North Texas Health Science Center, Fort Worth, Texas. All animal experiments adhered strictly to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Animals were housed with no more than 5 five total mice per cage and maintained in the animal facilities with daily 12-hour light/12-hour dark cycles (lights on at 6:00 am) and unlimited access to standard rodent pellets and water.
TGFβ2 Ocular Hypertension Model
TGFβ2 cDNA (NM_003238) was obtained from Origene (Rockville, MD) in pCMV6-XL5 vector. Nucleotides 1086 and 1991 were changed from G to C to convert amino acids 226 and 228 of the human TGFβ2 protein from cysteines to serines. The cDNA was subcloned into pacAd5.CMV.KN.pA shuttle vector (Gene Transfer Vector Core, University of Iowa) using EcoRI/Xba1 restriction for making Ad.hTGFβ2226/228 as described previously.20 Before injection, C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were anesthetized with ketamine/xylazine/acepromazine (73/7.8/1.8 mg/kg), pupils were dilated with one to two drops of cyclopentolate (Mydriacyl; Alcon, Fort Worth, TX), and eyes were topically anesthetized with two drops of 0.5% tropicamide (Alcaine; Alcon). A 2-µL suspension of Ad.hTGFβ2226/228 (5 × 107 pfu) was injected intravitreally with a Hamilton 33G needle containing a 10° bevel and a glass microsyringe (Hamilton, Reno, NV).
Steroid-induced Model of Ocular Hypertension
Six-month-old C57BL/6J mice (Jackson Laboratory) were anesthetized with ketamine, xylazine, and acepromazine (90, 10, and 1 mg/kg body weight). A 20-µL volume of dexamethasone acetate (10 mg/mL) or suspension formulation (vehicle) was loaded into a 32G Hamilton glass microsyringe. For injection, the beveled end of the needle (facing up) was inserted into the conjunctival fornix and the syringe volume expelled slowly over 15 seconds. For each animal, one eye received dexamethasone acetate and the fellow eye received vehicle. Injections were performed once a week until the end of the experiment.
DBA/2J Mice
DBA/2J mice were obtained from Jackson Laboratory at approximately 4 months of age. Mice were housed in the Mayo Clinic animal facilities until they were 8 to 10 months old to ensure the development of ocular hypertension.21
IOP Measurements and Treatments
IOP was measured using a handheld rebound tonometer (Icare Tonolab, Colonial Medical Supply, Franconia, NH) using previously described methods.10,15 Briefly, animals were acclimatized to the handling through sham IOP measurements for up to 5 days. After acclimation, daily IOP measurements were taken at three separate time points corresponding with 1, 4, and 23 hours after treatment (approximately 10:00 am, 2:00 pm, and the following morning at 9:00 am), averaged, and recorded as the daily IOP. For all experiments, baseline values were determined by measuring and averaging IOPs for 3 consecutive days before treatment. For TGFβ2 overexpression and DBA/2J experiments, the IOPs were measured three times daily corresponding with 1, 4, and 23 hours after treatment until the termination of the experiment. For the steroid-induced ocular hypertension model, after the first injection, IOP was measured once weekly, 2 days after each injection (three timepoints corresponding with 1, 4, and 23 hours after treatment) until the end of the experiment. CKLP1 or diazoxide was instilled topically in a 5-µL bolus to one eye of each animal while the contralateral eye received vehicle (10% Cremophor EL in PBS for diazoxide; PBS for CKLP1). In mice injected with dexamethasone acetate in one eye and the vehicle (suspension formulation) in the contralateral eye, one group was treated with either diazoxide or CKLP1 in both eyes, whereas the other group received the respective vehicle (DMSO in 10% Cremophor for diazoxide; PBS for CKLP1). All drugs were added once daily at a final working concentration of 5 mM10,11,13 for the entire duration of the treatment period.
Evaluation of Aqueous Humor Dynamics In Vivo by Constant Flow Infusion
All parameters related to aqueous humor dynamics (outflow facility, aqueous flow rate, episcleral venous pressure, uveoscleral outflow) were determined by constant flow infusion as described previously.15,22,23 Briefly, mice were anesthetized with ketamine/xylazine (100/10 mg/kg body weight). A 32G needle connected to a calibrated variable height manometer and a BLPR-2 pressure transducer (World Precision Instruments, Sarasota, FL) was inserted into the anterior chamber. The transducer was attached to a SP101i microdialysis infusion pump (World Precision Instruments), a TBM4M Bridge Amplifier, and a Lab-Trax analog to digital converter (World Precision Instruments) via a three-way valve. Once pressure was stabilized, data were recorded and analyzed using the LabScribe4 software (World Precision Instruments). Among the various aqueous humor dynamic parameters, the outflow facility and episcleral venous pressures were directly measured, and the uveoscleral and aqueous flow rate were calculated based on a modified Goldmann equation, as previously described.22,23
Histology
After completion of the experiments, animals were euthanized, and eyes were enucleated and fixed in 10% neutral buffered formalin (Thermo Fisher Scientific, Pittsburgh, PA). Eyes were removed from 10% buffered formalin and postfixed in 2% osmium tetroxide (Electron Microscopy Sciences, Hatfield, PA). Eyes were dehydrated in ascending alcohol concentrations (70%–100%) and cleared with acetone (Fisher Chemical, Fair Lawn, NJ). Whole eyes were embedded in epoxy resin and thin sectioned with an ultramicrotome (Leica Microsystems, Buffalo Grove, IL). Sections were stained with 2% uranyl acetate (Electron Microscopy Sciences) and lead citrate (Mager Scientific, Dexter, MI) for evaluation of cell and tissue morphology using a JEOL 1400 transmission electron microscope (JEOL USA, Peabody, MA).
Statistics
All values are expressed as mean ± standard deviation. Means from treated and control eyes within the same population were compared using Student's two-tailed paired t-test and independent two-tailed t-tests were used for comparing means from separate cohorts. Differences were considered significant when P < 0.05. Statistical analyses were performed with Microsoft Excel and its data analysis add-on feature.
Results
Effect of CKLP1 on IOP in TGFβ2 Overexpressing Ocular Hypertensive Mouse Model
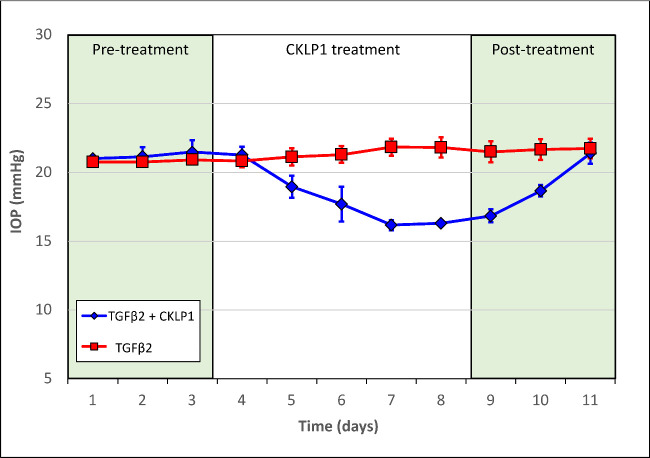
To evaluate the effect of CKLP1 during high IOP, one eye of C57BL/6J mice was injected intravitreally with adenoviral vector encoding the bioactive form of TGFβ2.20 At 5 days after the injection, when elevated IOP reached a plateau (OS 21.2 ± 0.62 mm Hg and OD 20.8 ± 0.2 mm Hg, compared with control baseline IOPs of OS 12.7 ± 0.4 mm Hg and OD 12.6 ± 0.5 mm Hg; n = 6, in a separate group of mice treated with PBS only), the left eye of each mouse was started on a once daily ocular topical regimen of 5 mM CKLP1 for 5 consecutive days and the contralateral eye received vehicle. The IOP progressively decreased to 16.4 ± 0.4 mm Hg in the TGFβ2 overexpressing eyes reaching a 23% decrease (compared with the average baseline IOP of the TGFβ2-treated eye before start of CKLP1 treatment) on day 5 of treatment (Fig. 1). When the IOP was averaged over the entire 5 days of treatment, the IOP was lowered in CKLP1-treated TGFβ2-overexpressing eyes by 20% (vehicle control, 21.4 ± 0.7 mm Hg; CKLP1 treated, 17.8 ± 2.0 mm Hg; n = 6; P < 0.0001). After the cessation of treatment, the IOP returned to levels similar to the vehicle-treated eye within 3 days (vehicle control, 21.4 ± 0.7 mm Hg; CKLP1 treated, 20.4 ± 1.3 mm Hg; n = 6; P = 0.1) (Fig. 1).
Figure 1.
Effect of CKLP1 on TGFβ2 ocular hypertensive mice. Once daily treatment with 5 mM CKLP1 significantly lowered IOP in TGFβ2 overexpressing mice by 20% (n = 6; P < 0.0001) when compared with the vehicle treated contralateral eye. IOP returned to baseline within 72 hours of cessation of treatment (n = 6; P = 0.1). Pretreatment refers to IOP measured before treating with CKLP1, but after injection of Ad.TGFβ2226/228 or control in the mice eyes.
Effect of CKLP1 and Diazoxide on IOP in Steroid-induced Ocular Hypertensive Mice
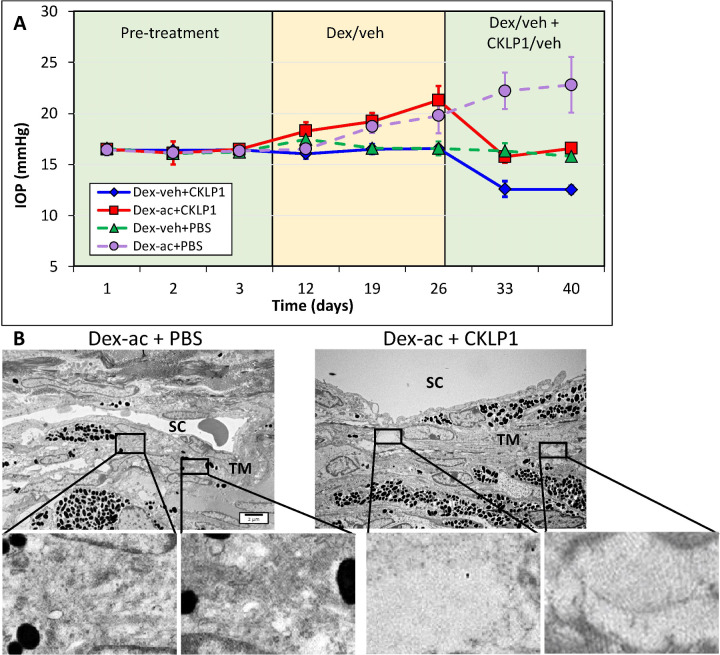
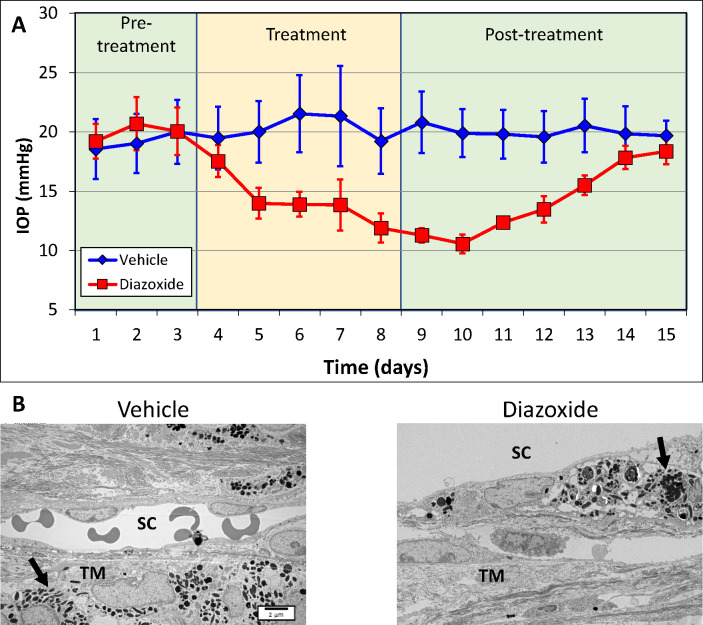
To further validate the hypotensive effects of KATP channel openers during elevated IOP, we used the steroid-induced ocular hypertensive mouse model, where the IOP is increased through weekly dexamethasone acetate injections into the conjunctival fornix of one eye, while the contralateral eye received vehicle. After IOP elevation in the dexamethasone acetate injected eye, both eyes of each animal were treated with topical drops of 5 mM CKLP1 dissolved in PBS (once daily along with continued weekly injections of dexamethasone). After three weekly injections of dexamethasone acetate, IOP increased from an average baseline value of 16.5 ± 0.2 mm Hg to 21.3 ± 1.4 mm Hg (n = 8; P < 0.0001), while no change was seen in the vehicle treated contralateral eye (baseline, 16.4 ± 0.1 mm Hg; after vehicle treatment, 16.4 ± 0.1 mm Hg; n = 8; P = 0.7) (Fig. 2A). After subsequent once daily treatment with CKLP1 (along with continued weekly injections of dexamethasone acetate), the average IOP in eyes treated with dexamethasone acetate was decreased to 16.2 ± 0.3 mm Hg, corresponding with a 24% decrease (P < 0.0001, n = 8). In conformation with our previous reports of IOP reduction in normotensive eyes,12,14–18 CKLP1 also decreased the IOP in the contralateral eyes that received vehicle (suspension formulation) by 23% (P < 0.0001) (Fig. 2A, solid lines). For controls, a separate group of mice (n = 5) was similarly injected with dexamethasone acetate in one eye and the suspension formulation in the contralateral eye. Both eyes in this group were treated with PBS (vehicle for CKLP1). In these mice, dexamethasone acetate increased the IOP from 16.0 ± 0.1 mm Hg to 20.4 ± 1.1 mm Hg (P = 0.0008), whereas no change was seen following treatment with PBS (22.8 ± 2.7 mm Hg; P = 0.12) (Fig. 2A, dotted lines).
Figure 2.
Effect of CKLP1 treatment on steroid-induced ocular hypertensive mice. (A) Once weekly injection of dexamethasone acetate (dex-ac) in the conjunctival fornix of C57BL/6J mice increased the IOP from 16.5 ± 0.2 to 21.3 ± 1.4 (n = 8; P < 0.0001). After treatment with once daily CKLP1 (5 mM), the IOP was decreased by 24% (P < 0.0001, n = 8) (solid red line). The contralateral eye, injected with only vehicle (veh) (suspension formulation without dexamethasone acetate) did not show any increase in IOP, but after CKLP1 treatment showed a 23% reduction in the IOP (P < 0.0001) (solid dark blue line). When eyes injected with dexamethasone acetate or vehicle were treated with PBS (vehicle for CKLP1), no changes in IOP were observed compared with baseline (dotted lines on graph). (B) Representative transmission electron microscopy images of the conventional outflow pathway of dexamethasone acetate/vehicle (dex-ac + PBS) and dexamethasone acetate/CKLP1 (dex-ac + CKLP1) treated eyes. In eyes treated with dexamethasone acetate and vehicle, excess ECM was found in the trabecular meshwork (TM) consistent with a previous report in this model (insets).19 However, normal ECM was observed in the trabecular meshwork of eyes treated with dexamethasone acetate and CKLP1 (insets). The remaining cell and tissue structures in the conventional outflow pathway appeared normal in both CKLP1 and vehicle treated eyes. SC, Schlemm's canal.
After termination of CKLP1 treatment, ultrastructural evaluation of the trabecular meshwork showed evidence of extracellular matrix (ECM) deposition in the dexamethasone acetate treated eyes (Fig. 2B) in the trabecular meshwork just below Schlemm's canal (i.e., juxtacanalicular region), consistent with a previous report.19 In contrast, little or no excess ECM was observed in the trabecular meshwork from eyes that were injected with dexamethasone acetate and subsequently treated with CKLP1 (Fig. 2B). No other changes were observed in eyes from the vehicle-treated groups. Overall, the trabecular meshwork showed intact beams and healthy cells, with an uninterrupted inner and outer wall of Schlemm's canal (Fig. 2B).
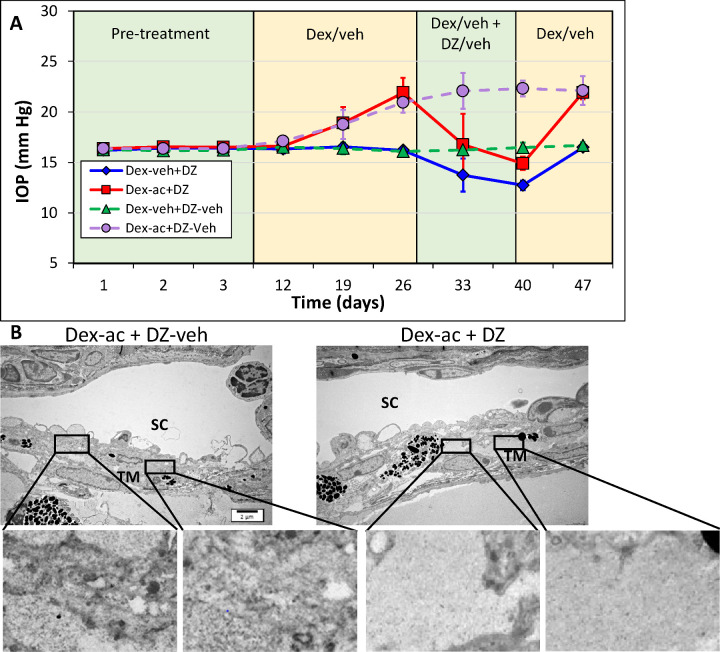
To determine if the IOP-lowering effect of CKLP1 is also true for other KATP channel openers, we treated a separate cohort of dexamethasone acetate–induced ocular hypertensive mice with 5 mM diazoxide. In this group, injection with dexamethasone acetate increased the IOP from 16.8 ± 0.7 mm Hg at baseline to 21.9 ± 1.5 mm Hg (n = 13; P < 0.0001) (Fig. 3A). Using a treatment strategy with diazoxide similar to that described above with CKLP1, IOP in the steroid-induced eye decreased to 14.9 ± 0.7 mm Hg (P < 0.0001), corresponding with a 32% decrease in the IOP. In five of the mice, diazoxide treatment was stopped but dexamethasone acetate injection and IOP measurements continued for one additional week. IOP in these animals increased to 21.9 ± 0.7, similar to prediazoxide treatment levels (P = 0.99), suggesting reversibility and specificity of diazoxide as an ocular hypotensive agent. In the eyes that were injected with the suspension formula only, no change in the IOP was noted when compared with baseline (16.3 ± 0.1 mm Hg vs. 16.2 ± 0.3 mm Hg, n = 13; P = 0.2). However, after treatment with diazoxide, the IOP was decreased by 22% (P < 0.0001) (Fig. 3, solid lines). In a separate group, when the dexamethasone acetate injected animals were treated with the vehicle for diazoxide (DMSO in 10% Cremophor), there was a slight increase in the IOP (dexamethasone acetate, 20.9 ± 1.0 mm Hg; vehicle, 22.3 ± 0.8 mm Hg; n = 9; P = 0.0001). When the ultrastructure was analyzed after the termination of treatment, eyes that received dexamethasone acetate followed by diazoxide treatment looked normal, with healthy looking trabecular meshwork cells on beams and no interruptions in Schlemm's canal inner and outer wall endothelial cell layer (Fig. 3B). In eyes that were injected with dexamethasone acetate but were not treated with diazoxide, sporadic ECM buildup could be seen in the juxtacanalicular region of the trabecular meshwork just below Schlemm's canal. No histological changes were observed in the trabecular meshworks from the vehicle-treated mice.
Figure 3.
Effect of diazoxide on steroid-induced ocular hypertensive mice. (A) Dexamethasone acetate (dex-ac)-induced elevated IOP was abrogated by once daily treatment with diazoxide (5 mM). Diazoxide decreased the IOP by 32% (n = 13; P < 0.0001) compared with baseline (solid red line). Once treatment with diazoxide was stopped, IOP returned to pretreatment levels within 1 week (P = 0.99). Eyes treated with vehicle (veh) (suspension formulation without dexamethasone acetate) alone showed no increase in IOP, but after treatment with diazoxide, showed a 22% decrease in the IOP (P < 0.0001) compared with baseline (solid dark blue line). In a separate group of mouse eyes, similarly injected with dexamethasone acetate or the suspension formulation, but treated only with the vehicle for diazoxide (DZ-veh, DMSO in 10% Cremophor), no decrease in the IOP was noted in comparison with baseline (dotted lines on graph). (B) Histological analysis of the conventional outflow pathway ultrastructure by transmission electron microscope showed healthy looking trabecular meshwork (TM) cells and an intact inner and outer wall of Schlemm's canal. In some eyes, ECM deposition could be seen in the trabecular meshwork after treatment with dexamethasone acetate (insets). However, diazoxide treated eyes showed little or no ECM deposition (insets). SC, Schlemm's canal.
Effect of CKLP1 and Diazoxide on Elevated IOP in DBA/2J Mice
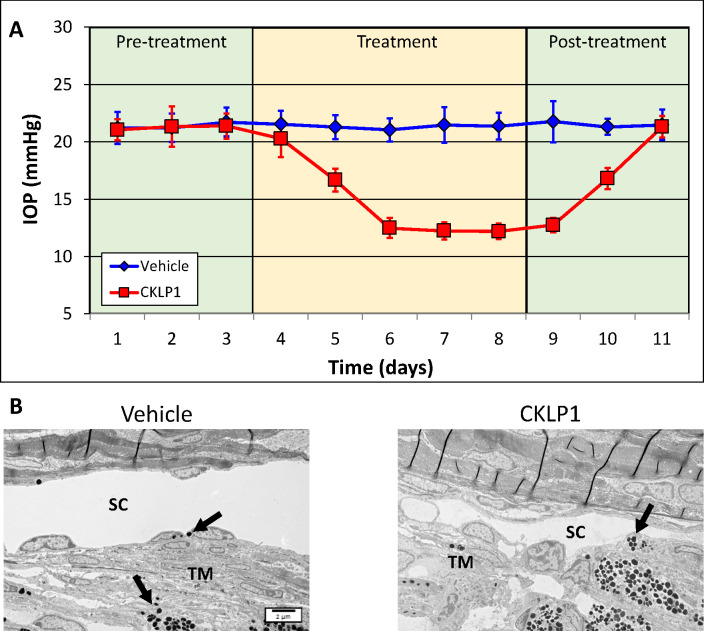
Because the TGFβ2 overexpression and steroid-induced animal models represent acute ocular hypertension models, we wanted to evaluate a model with chronic elevated IOP. We selected DBA/2J mice, because these mice develop an elevated IOP around 6 months of age owing to the resistance of aqueous humor removal from the anterior chamber. The accumulation of dispersed iris pigment in this model is caused by mutations in the tyrosine-related protein (Tyrp1b) and transmembrane glycoprotein (GpnmbR150X) genes.24–28 We selected 8 to 10-month-old mice with an average baseline IOP of 21.4 ± 0.7 mm Hg OD and 21.3 ± 0.6 mm Hg OS. CKLP1 was added to OS once daily for 5 days, and the OD received vehicle (PBS). At the end of treatment, the IOP in the treated eye was decreased to 12.2 ± 0.7 mm Hg, equivalent to a 43% decrease (P < 0.0001, n = 15), whereas no change was observed in the contralateral vehicle-treated eye (baseline, 21.4 ± 0.7 mm Hg; vehicle treatment, 21.4 ± 1.2; P = 0.9, n = 15) (Fig. 4A). Within 72 hours after termination of CKLP1 treatment, IOP in the treated eye returned to levels similar to the vehicle treated eyes (OD [vehicle treated], 21.5 ± 1.3 mm Hg; OS [previously treated with CKLP1], 21.3 ± 0.9; P = 0.8; n = 5), indicating the specific and reversible nature of the drug effects. Histological assessment of the trabecular outflow ultrastructure revealed pigment dispersion-related findings, including aberrant ECM formation as well as pigment ingesting macrophage like cells. However, no effects of CKLP1 treatment were apparent from the histology (Fig. 4B).
Figure 4.
Effect of CKLP1 treatment in DBA/2J mice. (A) DBA/2J mice (8–10 months old) treated with CKLP1 once daily for five consecutive days showed a 43% decrease in the IOP compared with baseline (P < 0.0001; n = 15). The IOP in the treated eyes returned to baseline levels within 72 hours after termination of treatment. (B) Histological analysis of the trabecular meshwork (TM) shows pigment dispersion-related anomalies, which are characteristics associated with this mouse strain (black arrows). No effects associated with CKLP1 treatment were noted. SC, Schlemm's canal.
A separate set of DBA/2J mice (n = 4) was treated similar to the regimen described above, but with diazoxide instead of CKLP1. In this set of animals, diazoxide lowered IOP from a baseline value of 19.8 ± 1.8 mm Hg to 11.7 ± 1.5 mm Hg, corresponding with a 41% decrease (P = 0.0002, n = 4). No significant changes were noted in the IOP of vehicle-treated eyes when compared with baseline (P = 0.6, n = 4) (Fig. 5A). An ultrastructure analysis showed evidence of pigment dispersion, which is the hallmark of this mouse model. Similar to DBA/2J mice treated with CKLP1, no adverse effects of diazoxide treatment were evident in the ultrastructural analysis of the trabecular meshwork region from CKLP1-treated and vehicle-treated control eyes (Fig. 5B).
Figure 5.
Effect of diazoxide treatment in DBA/2J mice. (A) Treatment with diazoxide (DZ) for 5 consecutive days in DBA/2J mice (8–10 months old) decreased the IOP by 41% (P = 0.0002; n = 4). After termination of treatment, the IOP returned to baseline levels within 6 days. (B) Histological analysis showed similar morphology and cell histology between treated and control samples indicating tissue tolerability of diazoxide treatment. Anomalous findings such as pigment dispersion and pigment filled vesicles (black arrows) are characteristics of this mouse model and were found in both treated and control eyes. SC, Schlemm's canal; TM, trabecular meshwork.
Effect of KATP Channel Openers on Aqueous Humor Dynamics in Mouse Models of Ocular Hypertension
We have shown previously that, in normotensive animal models, KATP channel openers decrease the IOP by lowering episcleral venous pressure through the modulation of the region distal to Schlemm's canal. Given that the KATP channel openers CKLP1 and diazoxide both decreased the IOP in ocular hypertensive mouse models, we reasoned that these drugs would also decrease the episcleral venous pressure in these animals. To test this hypothesis, we analyzed aqueous humor dynamics using a constant flow perfusion system15,22,23,29 in groups of TGFβ2-overexpressing, steroid-induced, and DBA/2J ocular hypertensive mice after treatment with either CKLP1 or diazoxide, because both of these drugs have similar mechanism of actions.10–13,15 In all three ocular hypertension models, treatment with CKLP1 or diazoxide significantly lowered IOP via reduction of the episcleral venous pressure (Table 2). In TGFβ2 mice, CKLP1 treatment lowered episcleral venous pressure by 29% (vehicle control, 10.22 ± 0.18 mm Hg; CKLP1, 7.15 ± 0.30 mm Hg; P < 0.001, n = 13). When mice with steroid-induced elevated IOP were treated with diazoxide, the episcleral venous pressure was decreased by 35% (vehicle control, 8.33 ± 0.42 mm Hg; diazoxide, 5.39 ± 1.53 mm Hg; P = 0.03, n = 3). Last, in DBA/2J mice, the episcleral venous pressure was lowered by 72% after CKLP1 treatment (vehicle control, 12.02 ± 1.53 mm Hg, n = 3; CKLP1, 3.40 ± 0.82 mm Hg; P < 0.001, n = 4). The aqueous flow rate was also decreased in DBA/2J mice treated with CKLP1 by 44% (vehicle, 0.34 ± 0.11 µL/min [n = 3]; CKLP1, 0.19 ± 0.04 µL/min [n = 4]; P = 0.04) (Table 2). The aqueous outflow facility and uveoscleral outflow showed no change in any of the model systems between drug- and vehicle-treated eyes. The aqueous flow rate was not affected by CKLP1 or diazoxide in TGFβ2 overexpression or steroid-induced models of ocular hypertension (Table 2).
Table 2.
Effect of KATP Channel Openers on Aqueous Humor Dynamics
| IOP (mm Hg) | |||||
|---|---|---|---|---|---|
| AHD Parameters | Vehicle Control | Treated | P Value | Baseline | After Treatment |
| TGFβ2 overexpressed mice + CKLP1 (n = 13) | |||||
| Aqueous outflow facility (µL/min/mm Hg) | 0.012 ± 0.007 | 0.016 ± 0.010 | 0.27 | 23.3 ± 0.6 | 16.2 ± 0.4 (P = 0.006) |
| Uveoscleral outflow (µL/min) | 0.018 ± 0.052 | 0.021 ± 0.047 | 0.90 | ||
| Aqueous flow rate (µL/min) | 0.127 ± 0.073 | 0.125 ± 0.063 | 0.94 | ||
| Episcleral venous pressure (mm Hg) | 10.217 ± 0.180 | 7.146 ± 0.300 | <0.001 | ||
| Steroid-induced elevated IOP + diazoxide (n = 3) | |||||
| Aqueous outflow facility (µL min/mm Hg) | 0.073 ± 0.044 | 0.040 ± 0.022 | 0.32 | 21.9 ± 1.5 | 14.9 ± 0.7 (P < 0.0001) |
| Uveoscleral outflow (µL/min) | 0.015 ± 0.007 | 0.020 ± 0.015 | 0.72 | ||
| Aqueous flow rate (µL/min) | 1.075 ± 0.969 | 1.000 ± 1.075 | 0.95 | ||
| Episcleral venous pressure (mm Hg) | 8.333 ± 0.421 | 5.393± 1.530 | 0.03 | ||
| DBA/2J+CKLP1 (control, n = 3; treated, n = 4) | |||||
| Aqueous outflow facility (µL/min/mm Hg) | 0.016 ± 0.005 | 0.015 ± 0.002 | 0.70 | 21.3 ± 0.6 | 12.2 ± 0.7 (P < 0.0001) |
| Uveoscleral outflow (µL/min) | 0.077 ± 0.015 | 0.055 ± 0.038 | 0.40 | ||
| Aqueous flow rate (µL/min) | 0.342 ± 0.110 | 0.186 ± 0.043 | 0.04 | ||
| Episcleral venous pressure (mm Hg) | 12.015 ± 1.53 | 3.402 ± 0.816 | <0.001 | ||
Discussion
The KATP channel openers CKLP1 and diazoxide were both found to effectively lower IOP during conditions of ocular hypertension as evidence from the data in three independent mouse models of elevated IOP. In all three models, the KATP channel openers lowered the IOP by decreasing the episcleral venous pressure, similar to findings in our previous studies in normotensive mice.14–16 Among existing glaucoma drugs, only the prostaglandin analog latanoprost has been reported to decrease the episcleral venous pressure in mice,22 while netarsudil (brand name Rhopressa) has been found to decrease the episcleral venous pressure in humans.30 However, both latanoprost (uveoscleral) and netarsudil (trabecular outflow) also affect other aspects of aqueous humor dynamics and IOP. The KATP channel openers are unique in this respect; they have been shown to only target the episcleral venous pressure to effectively decrease the IOP.15
All mouse ocular hypertensive models used for this study are well-established and have been characterized widely. In general, the ocular anterior segment physiology of living mice is considered similar to human eyes owing to a negligible washout rate and a linear pressure flow relationship over a wide range of IOPs, a property leveraged in the continuous flow perfusion method to accurately measure the different parameters of aqueous humor dynamics.22,31,32 The mouse eye is also anatomically similar to humans with a well-defined trabecular meshwork containing three distinct regions (uveal, corneoscleral, and juxtacanalicular) and a true Schlemm's canal with inner and outer wall endothelium.33,34
TGFβ2 has been reported widely as a key molecule involved in the development of glaucoma.35–38 When overexpressed in mouse eyes by intracameral injection of Ad.hTGFβ2226/228, TGFβ2 has been reported to cause a rapid and sustained IOP increase between days 4 and 21, along with a decreased outflow facility.20 The pathogenesis is believed to be through an increase in TGFβ2 levels in aqueous humor and subsequent upregulated expression of CTGF, PAI-1 and NOX4 in ocular anterior segment tissues.20 It has been shown in TGFβ2-overexpressing mice that the extent of the IOP elevation is correlated directly with the level of TGFβ2 mRNA in the eye, suggesting that the model is directly based on TGFβ2 expression, a potent risk factor for glaucoma.20 In light of this finding, the strong ocular hypotensive effects of CKLP1 on this model further adds confidence to its potential to decrease the IOP in glaucoma.
The steroid-induced ocular hypertension mouse model closely mimics glucocorticoid-induced glaucoma in humans and also shows similar histological changes observed in the trabecular meshwork of glucocorticoid-treated, high IOP eyes.19 Common to this model is the buildup of ECM in the trabecular meshwork, in part owing to the involvement of the TGFβ signaling pathway.39 In our studies, it is interesting to note that treatment with CKLP1 and diazoxide both seemed to decrease the buildup of excess ECM caused by dexamethasone acetate. This finding is consistent with previous reports of KATP channel activation in nonocular tissues. Studies performed in the heart and pancreas have shown that diazoxide can directly affect Erk1/2 phosphorylation and matrix metalloproteinase activity.40,41 The Erk1/2 pathway is a strong modulator of matrix metalloproteinases, which have been shown to be necessary for ECM turnover in the trabecular meshwork and uveoscleral outflow pathway in association with IOP reduction.42,43 Additionally, the KATP channel opener iptakalim was shown to reduce accumulation of collagen IV and fibronectin in the renal vasculature of a rat hypertension model by inhibiting TGFβ1 expression and normalizing matrix metalloproteinase 9 and TIMP1 function.44 We have previously shown that upregulation of Erk1/2 phosphorylation is necessary for IOP lowering properties of diazoxide.13 Given the potential association with KATP channel activation and ECM remodeling, it is interesting to note that we did not see an effect on outflow facility after treatment with CKLP1 or diazoxide. Although the result is consistent with studies we performed in normotensive mice,15 we cannot rule out the fact that evaluation of a larger sample size may show some alterations in outflow facility after treatment with KATP channel openers. Future studies designed specifically to understand the changes in ECM structure after treatment with KATP channel openers will be able to provide the required insights and possibly new target molecules for IOP regulation. Nevertheless, the ocular hypotensive effects of KATP channel openers in the steroid-induced ocular hypertensive model seem to validate the IOP-lowering abilities of CKLP1 and diazoxide during events of aberrant ECM deposition.
Mutations in the tyrosine-related protein 1 (Tyrp1b) gene and a stop codon mutation in the transmembrane glycoprotein nmb (GpnmbR150X) gene in the DBA/2J mice result in iris pigment dispersion.24,26–28 Additionally, melanosomal toxicity and abnormal immunity also contribute to increased pigment dispersion in these mice.25,45 These pigment granules enter and block the aqueous drainage systems of the anterior chamber, causing an increase in the IOP.24–27,46 Owing to the iris atrophy and concomitant anterior synechia, the DBA/2J mice develop secondary glaucoma, resulting in significant elevation of IOP starting at 6 months of age. For this reason, all animals for the current study were selected in the 8- to 10-month age range to provide sufficient time for initiation of ocular hypertension.21,47 The elevated IOP consequently causes progressive degenerative alterations of the retinal tissue and optic nerve head that are reminiscent of glaucomatous disease progression in humans.47–49 Both CKLP1 and diazoxide showed a significant decrease in the IOP in this model, decreasing the IOP by 43% and 41%, respectively. Given these findings, it will be interesting to assess the effect of long-term treatment with these KATP channel openers on retinal cell degeneration in this model.
One of the limitations with the DBA/2J model is the significant variability in the development of elevated IOP and glaucoma-like disease progression. This factor has mainly been attributed to the age of animals, differences in breeding colonies, and possible environmental factors.48,50–54 To overcome these issues, we sourced all our mice at a young age (4 months) from a specific breeding colony (Jackson Laboratory) and aged them in our animal facility until 8 to 10 months of age. The IOP of these mice were monitored weekly and animals demonstrating a similar level of elevated IOP were selected for treatment. Therefore, we used well-defined ocular hypertensive animals.
Of the various hypertensive models tested, all showed that a decrease in the episcleral venous pressure was the main aqueous humor parameter affected by treatment with CKLP1 and diazoxide. This finding is consistent with results that were previously reported in normotensive animals after treatment with CKLP1.15 We were surprised to see a significant effect on aqueous flow rate after treatment with CKLP1 in DBA/2J mice. This is the first time we have seen an effect of a KATP channel opener on an aqueous humor dynamic parameter other than episcleral venous pressure. Because this is also the first time DBA/2J mice were treated with KATP channel openers, it is possible that the aqueous suppression is unique to this particular model. Owing to the inherent ocular issues found in the DBA/2J mice, their eyes have a compromised blood aqueous barrier.45,55 According to the blood–aqueous barrier model proposed by Freddo,56 an increase in pigmentary protein deposits along with a disrupted blood aqueous barrier can cause an increased production of aqueous humor to normalize the protein content in the fluid. Reports of increased protein concentration (flares) found after treatment with timolol in patients with an intact blood–aqueous barrier but low aqueous production indirectly supports this notion. According to Freddo's model, without the blood aqueous barrier, the flares would have been normalized through increased aqueous production.56–58 Because KATP channel openers have cell protective properties,59–63 it is feasible that treatment with CKLP1 may normalize the blood–aqueous barrier in DBA/2J mice. As a consequence, the rate of aqueous humor formation would be suppressed indirectly by KATP channel openers owing to improved functioning of the relevant ocular cells. Although speculative, this hypothesis will be interesting to evaluate in DBA/2J mice before and after the onset of iris pigment dispersion and with KATP channel opener treatment in future studies. If it is found that KATP channel openers indeed have a protective effect on damaged cells of the anterior chamber, it would make KATP channel openers a promising drug class for other ocular hypertensive glaucomas like pseudoexfoliation or pigment dispersion, where in addition to lowering the IOP, the KATP channel openers can help to normalize the functions of the iris along with other cells and tissues of the anterior segment.
In summary, the KATP channel openers CKLP1 and diazoxide show a robust ocular hypotensive effect in three independent mouse models of ocular hypertension by lowering episcleral venous pressure. The fact that these KATP channel openers were able to successfully lower pressure in three rodent models of elevated IOP developed on different etiological aspects of glaucoma, provides confidence to the potential of this class of drugs being used as new ocular hypotensive agents that can lower IOP across various forms of glaucoma.
Acknowledgments
Supported by NIH grant EY21727 (to M.P.F.), EY031758 (to G.W.R.); Mayo Clinic Department of Ophthalmology grant (to U.R.C.), and Mayo Foundation (to M.P.F., and G.W.R.).
These author's interests have been reviewed and managed by the Mayo Clinic and University of Minnesota in accordance with their Conflict of Interest policies.
Disclosure: U. Roy Chowdhury, None; J.C. Millar, None; B.H. Holman, None; K.J. Anderson, None; P.I. Dosa, CKLP1 (P), Qlaris Bio. Inc (C); G.W. Roddy, None; M.P. Fautsch, CKLP1 (P), Qlaris Bio. Inc (C)
References
- 1. Quigley HA, Broman AT.. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 3. Boland MV, Ervin AM, Friedman D, et al.. Treatment for Glaucoma: Comparative Effectiveness . Rockville, MD: Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 4. Boland MV, Ervin AM, Friedman DS, et al.. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013; 158: 271–279. [DOI] [PubMed] [Google Scholar]
- 5. Lee DA, Higginbotham EJ.. Glaucoma and its treatment: a review. Am J Health Syst Pharm. 2005; 62: 691–699. [DOI] [PubMed] [Google Scholar]
- 6. Haynes RB, McDonald HP, Garg AX.. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002; 288: 2880–2883. [DOI] [PubMed] [Google Scholar]
- 7. Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM.. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005; 140: 598–606. [DOI] [PubMed] [Google Scholar]
- 8. Babenko AP, Aguilar-Bryan L, Bryan J.. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998; 60: 667–687. [DOI] [PubMed] [Google Scholar]
- 9. Chowdhury UR, Bahler CK, Hann CR, et al.. ATP-sensitive potassium (KATP) channel activation decreases intraocular pressure in the anterior chamber of the eye. Invest Ophthalmol Vis Sci. 2011; 52: 6435–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chowdhury UR, Holman BH, Fautsch MP.. ATP-sensitive potassium (KATP) channel openers diazoxide and nicorandil lower intraocular pressure in vivo. Invest Ophthalmol Vis Sci. 2013; 54: 4892–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roy Chowdhury U, Bahler CK, Holman BH, Dosa PI, Fautsch MP. Ocular hypotensive effects of the ATP-sensitive potassium channel opener cromakalim in human and murine experimental model systems. PLoS One. 2015; 10: e0141783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy Chowdhury U, Viker KB, Stoltz KL, Holman BH, Fautsch MP, Dosa PI. Analogs of the ATP-sensitive potassium (KATP) channel opener cromakalim with in vivo ocular hypotensive activity. J Med Chem. 2016; 59: 6221–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roy Chowdhury U, Bahler CK, Holman BH, Fautsch MP. ATP-sensitive potassium (KATP) channel openers diazoxide and nicorandil lower intraocular pressure by activating the Erk1/2 signaling pathway. PLoS One. 2017; 12: e0179345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roy Chowdhury U, Dosa PI, Fautsch MP. ATP sensitive potassium channel openers: a new class of ocular hypotensive agents. Exp Eye Res. 2017; 158: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roy Chowdhury U, Rinkoski TA, Bahler CK, et al.. Effect of cromakalim prodrug 1 (CKLP1) on aqueous humor dynamics and feasibility of combination therapy with existing ocular hypotensive agents. Invest Ophthalmol Vis Sci. 2017; 58: 5731–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roy Chowdhury U, Dosa PI, Fautsch MP. Modulation of intraocular pressure by ATP sensitive potassium channel openers. In: Samples JR, Knepper PA, eds. Glaucoma Research and Clinical Advances 2018 to 2020. Amsterdam: Kugler Publications; 2018: 201–219. [Google Scholar]
- 17. Roy Chowdhury U, Kudgus RA, Rinkoski TA, et al.. Pharmacological and pharmacokinetic profile of the novel ocular hypotensive prodrug CKLP1 in Dutch-belted pigmented rabbits. PLoS One. 2020; 15: e0231841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roy Chowdhury U, Kudgus RA, Holman BH, et al.. Pharmacological profile and ocular hypotensive effects of cromakalim prodrug 1, a novel ATP-sensitive potassium channel opener, in normotensive dogs and nonhuman primates. J Ocul Pharmacol Ther. 2021; 37: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel GC, Phan TN, Maddineni P, et al.. Dexamethasone-induced ocular hypertension in mice: effects of myocilin and route of administration. Am J Pathol. 2017; 187: 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF.. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010; 51: 2067–2076. [DOI] [PubMed] [Google Scholar]
- 21. Saleh M, Nagaraju M, Porciatti V.. Longitudinal evaluation of retinal ganglion cell function and IOP in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2007; 48: 4564–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Millar JC, Clark AF, Pang IH.. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011; 52: 685–694. [DOI] [PubMed] [Google Scholar]
- 23. Millar JC, Phan TN, Pang IH, Clark AF.. Strain and age effects on aqueous humor dynamics in the mouse. Invest Ophthalmol Vis Sci. 2015; 56: 5764–5776. [DOI] [PubMed] [Google Scholar]
- 24. Anderson MG, Libby RT, Mao M, et al.. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006; 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson MG, Smith RS, Hawes NL, et al.. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002; 30: 81–85. [DOI] [PubMed] [Google Scholar]
- 26. Howell GR, Libby RT, John SW.. Mouse genetic models: an ideal system for understanding glaucomatous neurodegeneration and neuroprotection. Prog Brain Res. 2008; 173: 303–321. [DOI] [PubMed] [Google Scholar]
- 27. Howell GR, Libby RT, Marchant JK, et al.. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet. 2007; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang B, Smith RS, Hawes NL, et al.. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999; 21: 405–409. [DOI] [PubMed] [Google Scholar]
- 29. Millar JC, Pang IH.. Non-continuous measurement of intraocular pressure in laboratory animals. Exp Eye Res. 2015; 141: 74–90. [DOI] [PubMed] [Google Scholar]
- 30. Kazemi A, McLaren JW, Kopczynski CC, Heah TG, Novack GD, Sit AJ.. The effects of netarsudil ophthalmic solution on aqueous humor dynamics in a randomized study in humans. J Ocul Pharmacol Ther. 2018; 34: 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lei Y, Overby DR, Boussommier-Calleja A, Stamer WD, Ethier CR.. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011; 52: 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boussommier-Calleja A, Bertrand J, Woodward DF, Ethier CR, Stamer WD, Overby DR.. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest Ophthalmol Vis Sci. 2012; 53: 5838–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith RS, Zabaleta A, Savinova OV, John SW.. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Overby DR, Bertrand J, Schicht M, Paulsen F, Stamer WD, Lutjen-Drecoll E.. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest Ophthalmol Vis Sci. 2014; 55: 3727–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wordinger RJ, Sharma T, Clark AF.. The role of TGF-beta2 and bone morphogenetic proteins in the trabecular meshwork and glaucoma. J Ocul Pharmacol Ther. 2014; 30: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prendes MA, Harris A, Wirostko BM, Gerber AL, Siesky B.. The role of transforming growth factor beta in glaucoma and the therapeutic implications. Br J Ophthalmol. 2013; 97: 680–686. [DOI] [PubMed] [Google Scholar]
- 37. Fuchshofer R, Tamm ER.. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012; 347: 279–290. [DOI] [PubMed] [Google Scholar]
- 38. Fuchshofer R. The pathogenic role of transforming growth factor-beta2 in glaucomatous damage to the optic nerve head. Exp Eye Res. 2011; 93: 165–169. [DOI] [PubMed] [Google Scholar]
- 39. Kasetti RB, Maddineni P, Patel PD, Searby C, Sheffield VC, Zode GS.. Transforming growth factor beta2 (TGFbeta2) signaling plays a key role in glucocorticoid-induced ocular hypertension. J Biol Chem. 2018; 293: 9854–9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simoncikova P, Ravingerova T, Andelova E, Tribulova N, Barancik M.. Changes in rat myocardium associated with modulation of ischemic tolerance by diazoxide. Gen Physiol Biophys. 2007; 26: 75–85. [PubMed] [Google Scholar]
- 41. Zhang B, Shi Y, Zou J, et al.. KATP channels in high glucose-induced rat mesangial cell proliferation and release of MMP-2 and fibronectin. Exp Ther Med. 2017; 14: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alexander JP, Acott TS.. Involvement of the Erk-MAP kinase pathway in TNFalpha regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci. 2003; 44: 164–169. [DOI] [PubMed] [Google Scholar]
- 43. Shearer TW, Crosson CE.. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002; 43: 3016–3020. [PubMed] [Google Scholar]
- 44. Xue H, Zhang YL, Liu GS, Wang H.. A new ATP-sensitive potassium channel opener protects the kidney from hypertensive damage in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2005; 315: 501–509. [DOI] [PubMed] [Google Scholar]
- 45. Mo JS, Anderson MG, Gregory M, et al.. By altering ocular immune privilege, bone marrow-derived cells pathogenically contribute to DBA/2J pigmentary glaucoma. J Exp Med. 2003; 197: 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. John SW. Mechanistic insights into glaucoma provided by experimental genetics the cogan lecture. Invest Ophthalmol Vis Sci. 2005; 46: 2649–2661. [DOI] [PubMed] [Google Scholar]
- 47. John SW, Smith RS, Savinova OV, et al.. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998; 39: 951–962. [PubMed] [Google Scholar]
- 48. Inman DM, Sappington RM, Horner PJ, Calkins DJ.. Quantitative correlation of optic nerve pathology with ocular pressure and corneal thickness in the DBA/2 mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2006; 47: 986–996. [DOI] [PubMed] [Google Scholar]
- 49. Libby RT, Li Y, Savinova OV, et al.. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005; 1: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW.. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006; 7: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Libby RT, Anderson MG, Pang IH, et al.. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005; 22: 637–648. [DOI] [PubMed] [Google Scholar]
- 52. Scholz M, Buder T, Seeber S, Adamek E, Becker CM, Lutjen-Drecoll E.. Dependency of intraocular pressure elevation and glaucomatous changes in DBA/2J and DBA/2J-Rj mice. Invest Ophthalmol Vis Sci. 2008; 49: 613–621. [DOI] [PubMed] [Google Scholar]
- 53. Danias J, Lee KC, Zamora MF, et al.. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest Ophthalmol Vis Sci. 2003; 44: 5151–5162. [DOI] [PubMed] [Google Scholar]
- 54. Williams RW, Strom RC, Rice DS, Goldowitz D.. Genetic and environmental control of variation in retinal ganglion cell number in mice. J Neurosci. 1996; 16: 7193–7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crosbie DE, Keaney J, Tam LCS, Daniel Stamer W, Campbell M, Humphries P. Age-related changes in eye morphology and aqueous humor dynamics in DBA/2J mice using contrast-enhanced ocular MRI. Magn Reson Imaging. 2019; 59: 10–16. [DOI] [PubMed] [Google Scholar]
- 56. Freddo TF. A contemporary concept of the blood-aqueous barrier. Prog Retin Eye Res. 2013; 32: 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stur M, Grabner G, Huber-Spitzy V, Schreiner J, Haddad R.. Effect of timolol on aqueous humor protein concentration in the human eye. Arch Ophthalmol. 1986; 104: 899–900. [DOI] [PubMed] [Google Scholar]
- 58. Tindall GT, Collins WF Jr., Kirchner JA. Unilateral septal technique for transsphenoidal microsurgical approach to the sella turcica. Technical note. J Neurosurg. 1978; 49: 138–142. [DOI] [PubMed] [Google Scholar]
- 59. Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990; 247: 1341–1344. [DOI] [PubMed] [Google Scholar]
- 60. Gao S, Long CL, Wang RH, Wang H.. K(ATP) activation prevents progression of cardiac hypertrophy to failure induced by pressure overload via protecting endothelial function. Cardiovasc Res. 2009; 83: 444–456. [DOI] [PubMed] [Google Scholar]
- 61. Kane GC, Behfar A, Dyer RB, et al.. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006; 15: 2285–2297. [DOI] [PubMed] [Google Scholar]
- 62. Kane GC, Liu XK, Yamada S, Olson TM, Terzic A.. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005; 38: 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nichols CG, Lederer WJ.. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991; 261: H1675–H1686. [DOI] [PubMed] [Google Scholar]