Abstract
The European Commission requested the EFSA Panel on Plant Health to prepare and deliver a scientific opinion on the risk posed by bonsai plants from China consisting of Pinus parviflora grafted on Pinus thunbergii taking into account the available scientific information, including the technical information provided by China. All pests associated with P. parviflora and/or P. thunbergii were evaluated against specific criteria for their relevance for this Scientific Opinion. Forty‐three pests that fulfilled all relevant criteria were selected for further evaluation. For 24 pests that are not quarantine in the EU, the risk mitigation measures described in the technical dossier from China were evaluated taking into account the possible limiting factors. For these pests, an expert judgement is given on the likelihood of pest freedom taking into consideration the risk mitigation measures acting on the pest, including uncertainties associated with the assessment. While the estimated degree of pest freedom varied among pests, Setoptus parviflorae was the pest most frequently expected on the commodity. The Expert Knowledge Elicitation indicated, with 95% certainty, that 9,114 or more bonsai plants per 10,000 will be free from Setoptus parviflorae. For 19 pests that are quarantine in the EU, the implementation of specific measures defined in point 30 and 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 was evaluated. The requirements of point 31 are met, whereas those of point 30 are not completely fulfilled.
Keywords: European Union, commodity risk assessment, plant health, plant pest, quarantine, dwarfed plants
1. Introduction
1.1. Background and Terms of Reference as provided by European Commission
1.1.1. Background
From 14 December 2019 onwards, the new Plant Health law, Regulation (EU) 2016/2031 1 , replaced Council Directive 2000/29/EC, increasing the level of phytosanitary protection of the EU. Commission Implementing Regulation (EU) 2019/2072 2 establishes uniform condition for implementation of Regulation (EU) 2016/2031 and prohibits the introduction of Pinus plants from third countries.
In May 2019, the European Food Safety Authority (EFSA) delivered a scientific opinion evaluating the plant health risks from black pine bonsai imported from Japan 3 , following their request for derogation from importing ban. Based on this opinion, the scope and the duration of the existing derogation 4 was extended (Commission Implementing Regulation (EU) 2020/1217 5 ).
China has made a request for lifting the import ban in Annex VI of Regulation (EU) 2019/2072 for artificially dwarfed Japanese white pine (Pinus parviflora (Sieb. et Zucc.)) and has provided supplementary technical information to support this request annexed to this mandate. Following a request for additional information from EFSA in July 2018, China provided supplementary technical information in December 2020.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29 of Regulation (EC) No 178/2002, to provide a scientific opinion.
Taking into account the available scientific information, including the technical information provided by China, EFSA is requested to consider how far the existing requirements for the bonsai pine species subject to derogation in Commission Implementing Regulation (EU) 2020/1217 would cover all plant health risks from Japanese white pine bonsai (Pinus parviflora (Sieb. et Zucc.)) imported in the EU from China.
1.2. Interpretation of the Terms of Reference
The EFSA Panel on Plant Health (hereafter referred to as ‘the Panel’) will conduct a commodity risk assessment of bonsai of Pinus parviflora from China based on the Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019a), taking into account the available scientific information, including the technical information provided by China. After assessing the Dossier, the commodity turned out to be produced by grafting P. parviflora on P. thunbergii rootstock. Therefore, the assessment was extended to P. thunbergii.
The interpretation of Terms of Reference and the methodology used are fully consistent with those employed for the previous EFSA Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan (EFSA PLH Panel, 2019b). The specific question to be replied in this Opinion when assessing the pest freedom of the commodity is: ‘Taking into account (i) the risk mitigation measures in place in the nursery, and (ii) other relevant information, how many of 10,000 bonsai plants will be infested with the relevant pest/pathogen when arriving in the EU.’
Annex II of Commission Implementing Regulation (EU) 2019/2072 lists certain pests as non‐European populations or isolates or species. These pests are regulated quarantine pests. Consequently, the respective European populations or isolates or species are non‐regulated pests.
Annex VII of the same Regulation, in certain cases (e.g. point 32) makes reference to the following countries that are excluded from the obligation to comply with specific import requirements for those non‐European populations or isolates or species: Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (SeveroZapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug), San Marino, Serbia, Switzerland, Turkey, Ukraine and United Kingdom (except Northern Ireland 6 ). Those countries are historically linked to the reference to ‘non‐European countries’ existing in the previous legal framework, Directive 2000/29/EC.
Consequently, for those countries,
any pests identified, which are listed as non‐European species in Annex II of Commission Implementing Regulation (EU) 2019/2072 should be investigated as any other non‐regulated pest.
any pest found in a European country that belongs to the same denomination as the pests listed as non‐European populations or isolates in Annex II of Commission Implementing Regulation (EU) 2019/2072, should be considered as European populations or isolates and should not be considered in the assessment of those countries.
Pests listed as ‘Regulated Non‐Quarantine Pest' (RNQP) in Annex IV of the Commission Implementing Regulation (EU) 2019/2072, and deregulated pests (i.e. pest which were listed as quarantine pests in the Council Directive 2000/29/EC and were deregulated by Commission Implementing Regulation (EU) 2019/2072) were not considered for further evaluation. In case a pest is at the same time regulated as an RNQP and as a Protected Zone Quarantine pest, in the Opinion it is evaluated as Quarantine pest.
In its evaluation the Panel:
Checked whether the information in the technical dossier (hereafter referred to as ‘the Dossier’) provided by the Chinese National Plant Protection Organisation (NPPO) was sufficient to conduct a commodity risk assessment. When necessary, additional information was requested to the applicant.
Selected the relevant EU‐regulated pests and protected zone quarantine pests (as specified in Commission Implementing Regulation (EU) 2019/2072 and if applicable, in Commission Implementing Regulation (EU) 2020/1217, hereafter referred to as ‘EU‐regulated pests’) and other relevant pests present in China and associated with the commodity.
Did not assess the effectiveness of measures for Union quarantine pests for which specific measures are specified in points 30 and 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 and/or in the relevant legislative texts for emergency measures and provided that the specific country is in the scope of those emergency measures. The assessment was restricted to whether or not the applicant country implements those measures.
Assessed the effectiveness of the measures described in the dossier for those Union quarantine pests for which no specific measures are in place for the import of the commodity from China and other relevant pests present in China and associated with the commodity.
Risk management decisions are not within EFSA’s remit. Therefore, the Panel provided a rating based on expert judgement regarding the likelihood of pest freedom for each relevant pest given the risk mitigation measures proposed by the NPPO of China.
2. Data and methodologies
2.1. Data provided by the NPPO of China
The Panel considered all the data and information (hereafter called ‘the Dossier’) provided by the NPPO of China to support a derogation request from the EU import requirements for Japanese white pine bonsai. Dossier Sections 1.0, 2.0 and 3.0 were provided to EFSA in March 2018, and Dossier Sections 4.0 and 5.0 in February 2021 and June 2021, respectively, after EFSA request. The Dossier is managed by EFSA.
The overview of the Dossier is shown in Table 1. The number of the relevant section is indicated in the Opinion when referring to a specific part of the Dossier.
Table 1.
Structure and overview of the Dossier
| Dossier Section | Overview of content | Filename |
|---|---|---|
| 1.0 | Supplementary information on the issues of concern in the EU inspection report on the quarantine and supervision of plant seedlings from 19 April 2017 | COM to EFSA ARES (2018)1373425–General Admin.of quality Supervision Annex I..pdf |
| 2.0 | Provision of technical documents for the EU to lift the ban on the export of Pinus parviflora bonsai from China to the EU from 12 July 2017 | COM to EFSA ARES (2018)1373425‐ General Admin.of quality Supervision Annex II..pdf |
| 3.0 | Feedback on the report on the EU audit of the quarantine supervision system for seedlings from 22 March 2016 | COM to EFSA ARES (2018)1373425‐ General Admin.of quality Supervision Annex III..pdf |
| 4.0 | Answers to additional questions of EFSA on lifting the export ban for Pinus parviflora bonsai from China to EU received by EFSA on 11 February 2021 | COM‐21‐02‐11‐ARES 1185975‐ Annex_EN_Additional questions from EFSA.pdf |
| 5.0 | Answers to request of EFSA for clarification received by EFSA on 28 June 2021 | 2021‐6‐26 reply to questions from EU.pdf |
The specific Dossier Sections comprised:
-
1
Supplementary information on the issues of concern in the EU inspection report on the quarantine and supervision of plant seedlings from 19 April 2017
The supplementary information refers to EU recommendations in the 2015 EU Audit report and the EU evaluation of the Chinese competent authority response and EU request for further information (not including bonsai of P. parviflora), and consists of:
Tables of supplementary information from China,
Annex 1: Guangdong CIQ Requirements for the Pesticide Treatment and the Quarantine and Supervision of Growing Media for Bonsais Intended for Export (Pilot Version),
Annex 2: China’s feedback on investigations of non‐compliance notifications from the EU,
Annex 3: Administrative Measures for Reporting and Notification of Agricultural Plant Infections,
Annex 4: Notice from the Gansu CIQ on Consolidating the Inspection and Quarantine of Consignments Entering and Leaving the Province,
Annex 5: Guangdong CIQ List of Registered Businesses of Bonsais for Export to the EU.
-
2
Provision of technical documents for the EU to lift the ban on the export of Pinus parviflora bonsai from China to the EU from 12 July 2017
-
Attachment 1: Investigation Report on Organisms Harmful to Pinus parviflora including:
-
▪
Description of the investigation,
-
▪
EU’s list of harmful organisms on Pinus parviflora not found in China (28 organisms),
-
▪
EU’s List of harmful organisms on Pinus parviflora found in China (17 organisms/organisms groups), including short characterisation (e.g. geographical distribution in China, life cycle, prevention and remedy).
-
▪
Attachment 2: Comprehensive Pest Prevention and Control System for Pinus parviflora Bonsai Intended for Export to the European Union.
-
3
Feedback on the report on the EU audit of the quarantine supervision system for seedlings from 22 March 2016
The above feedback is on the draft report from Audit DG (SANTE)/2015 – 7645 of 01–11 December 2015 to China in order to evaluate the system of official controls for the export of seeds and plants for planting to the European Union (DG SANTE, 2016) (not including bonsai of P. parviflora) and consists of:
Annex 1: China's observations on the contents of the draft EU report,
Annex 2: Comments by Chinese authorities on the EU recommendations,
Annex 3: Corrections concerning the presence of pests in China.
-
4
Answers to additional questions of EFSA on lifting the export ban for Pinus parviflora bonsai from China to EU received by EFSA on 11 February 2021
The additional questions and replies focused mainly on questions on the commodity, on the compilation of the pest lists and on the pest management measures and consist of:
Appendix A – Questions to Chinese Authority referring to Annex II received by EFSA from the European Commission under ReF: Ares(2018)1373425 – 13/3/2018,
Appendix B – Instructions on how to create the initial list of pests,
Appendix C – Evidence to support the assessment of the efficacy of phytosanitary treatments,
Appendix D – Information on potentially actionable pests.
-
5
Answers to request of EFSA for clarification received by EFSA on 28 June 2021
The request for clarification and the respective answers focused mainly on specific features of the commodity, composition of the growing media, insecticide and acaricide treatments, presence/absence of specific plants in the vicinity of the nursery and presence/absence of specific pests and pathogens in Zhenjiang province.
The data and supporting information provided by China formed the basis of the commodity risk assessment. Table 2 reports the main data sources used by the NPPO of China to compile the Dossier including the references relevant for harmful organisms present in China as listed in Dossier Sections 2.0, 4.0 and 5.0.
Table 2.
Databases and literature sources used by NPPO of China for the compilation of the Dossier
| 1. Databases: |
| https://cabi.org/cpc |
| https://www.pestchina.com |
| https://scalenet.info/ |
| https://www.eppo.int/ |
| https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list |
| https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list |
| https://www.cnki.net |
| https://www.wanfangdata.com.cn |
| 2. National Plant Protection Organisations, and Other National and Local Government Agencies: |
| The No. 4 Announcement of the State Forestry Bureau on the Disease Epidemic Area of Bursaphelenchus xylophilus (2017).(a) |
| 3. Journals and Magazines of Research Institutes, Colleges and Universities, Scientific Circles, Science and Trades, etc.: |
| Beiying H, Hongtao P, Fu L, 2005. Pest Risk analysis of Lecanosticta acicola and Dothistroma pini in China. Protection Forest Science and Technology, 3, 72–74. a |
| Chao Y and Chen Y, 1980. Economic Insect Fauna of China Fasc. 20, Coleoptera: Curculionidae (I). Science Press, Beijing, China. 184 pp. |
| Chunyan L and Haibin Y, 2015. Risk analysis report of Ips typographus in China. Guangdong Forestry Science and Technology, 2, 125–129. a |
| Dai YC, 2004. First report of laminated root rot on Sabina przewalskii caused by Phellinus weirii sensu stricto in China. Plant Disease, 88, 573–573. |
| Dai YC and Qin GF, 1998. Phellinidium sulphurascens – a forest pathogen in China. Fungal Science, 13, 101–107. |
| Hua LZ, 1982. A check list of the longicorn beetles of China (Coleoptera: Cerambycidae). Guangzhou, Zhongshan University, 159. |
| Ji L, Z Wang, Wang X and An L, 2011. Forest insect pest management and forest management in China: An Overview. Environmental Management, 48, 1107–1121. https://doi.org/10.1007/s00267‐011‐9697‐1 |
| Li LY, Gao L, Wen YL and Shen YQ, 2006. Advances in research on Bursaphelenchus xylophilus. Journal of Zhejiang Forestry Science and Technology, 5, 74–80. |
| Liping P and Guijun Z, 2012. Occurrence rule and comprehensive management of common pine needle rust in North‐eastern forestry areas. China Science and Technology Panorama Magazine, 8, 244. a |
| Liu YC and Shih CH, 1957. Preliminary study on the life history of the larch caterpillar Dendrolimus sibiricus Tschetw (Lepidoptera, Lasiocampidae). Acta Entomologica Sinica, 3, 3–12. |
| Lu Q, Liu HM, Zeng FY, Chen WP, Li CX and Zhang XY, 2015. Effect of tree‐inhabitant fungi on the life cycle of the dispersal forms of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) carried by Monochamus alternatus (Coleoptera: Cerambycidae) in vitro. Acta Phytopathologica Sinica, 45, 121–129. |
| Nickle WR, 1991. Manual of Agricultural Nematology. Marcel Dekker, INC., New York, USA. 1064 pp. |
| Notice of the State Forestry Administration of the People's Republic of China on Bursaphelenchus xylophilus Infected Areas (No. 4, 2017).(a) |
| Shen YA, 1999. Study on the Bionomics and control of Thecodiplosis japonensis Uchida & Inouye. Journal of Fujian College of Forestry, 19, 50–53. |
| Shengrong S, Kesheng F and Lejin F, 2013. Studies on the pathogen and the occurrence regularity of Huangshan pine gall rust. Journal of Huangshan University, 3. |
| Teng SC, 1996. Fungi of China. Mycotaxon, Ltd Ithaca, NY USA. 586 pp. |
| Wang B, 2009. A study on the semiochemicals of Pissodes yunnanensis and Pissodes punctatus. China Academy of Forestry, 57–64. |
| Wang Q, Bi M, Ma S and Shi L, 2015. Research on spatial pattern of Monochamus alternatus's occurrence rate based on meteorological factors. Forest Research, 28, 61–66. |
| Xiaoxia S, Jizeng C and Xiaoyan L, 2014. Risk analysis of forest pests — Dendroctonus micans Kugelan. Gansu Science and Technology, 30, 143–145.(a) |
| Xueren P and Yu X, 1991. Study on the problems and current situation of Chinese pine needle‐rusts (Coleosporium). Journal of Northeast Forestry University, 5, 84–94. |
| Yin HF, Huang FS and Li ZL, 1984. Economic Insect Fauna of China, Fasc. 29, Coleoptera: Scolytidae. Science Press, Beijing, China. 205 pp. |
| Yinchu T, 2002. Occurrence and key points for control of Cercoseptoria pini‐densiflorae (Hori & Nambu) Ddghton. Plant Doctor, 15, 36. a |
| Ke Y and Guoxing D, 2007. Identification atlas of the Ips paraconfusus carried in imported timber species. Shanghai, Shanghai Scientific and Technical Publishers. a |
| Zhang B, 2013. Concerning the prevention and control of pine wood nematode disease. Inner Mongolia Forestry Investigation and Design, 36, 94–96. |
| Zhang BC and Huang YC, 1990. A list of important plant diseases in China. Review of Plant Pathology, 69, 97–118. |
| Zhongying G and Xinhua W, 2009. Quarantine and identification of harmful organisms in wooden packages. Shanghai, Shanghai Scientific and Technical Publishers. a |
| Qi Z, 2014. Biological characteristics and control of Ips typographus Heer in forest areas. Heilongjiang Science and Technology Information, 28, 250–250. a |
| Zinno Y and Endo A, 1964. Needle rust of Pinus pentaphylla MAYR caused by Coleosporium eupatorii. Journal of the Japanese Forestry Society, 46, 178–180. |
| Zinno Y and Chiba O, 1967. On a needle rust of Pinus strobus LINN caused by Coleosporium paederiae DITEL ex HIRATSUKA: Life history and morphology of the causal fungus. Journal of the Japanese Forestry Society, 49, 321–327. |
The source was not accessible/available to the Panel.
2.2. Literature searches performed by EFSA
Literature searches in different databases were undertaken by EFSA to complete a list of pests potentially associated with Pinus parviflora and P. thunbergii. The searches were run between 23 February 2021 and 15 March 2021 using the databases indicated in Table 3. No language, date or document type restrictions were applied in the search strategy.
Table 3.
Databases used by EFSA for the compilation of the pest list associated with Pinus parviflora and Pinus thunbergii
The search strategy and search syntax were adapted to each of the databases listed in Table 3, according to the options and functionalities of the different databases and CABI keyword thesaurus.
As for Web of Science, the literature search was performed using a specific, ad hoc established search string (see Appendix B). The string was run in ‘All Databases’ with no range limits for time or language filters.
Additional searches, limited to retrieve documents, were run when developing the Opinion. The available scientific information, including previous EFSA opinions on the relevant pests and diseases, and the relevant literature and legislation (e.g. Regulation (EU) 2016/2031; Commission Implementing Regulations (EU) 2018/2019; (EU) 2018/2018 7 , (EU) 2019/2072, (EU) 2020/1217) were taken into account.
2.3. Methodology
When developing the Opinion, the Panel followed the EFSA Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019a).
In the first step, pests potentially associated with the commodity in the country of origin (EU‐regulated pests and other pests) that may require risk mitigation measures are identified. Pests not regulated in the EU and not known to occur in the EU were selected based on evidence of their potential impact in the EU. After the first step, all the relevant pests that may need risk mitigation measures were identified.
In the second step, the implemented risk mitigation measures for each relevant pest were evaluated in terms of efficacy or compliance with EU requirements as explained in Section 1.2.
The assessment of Union quarantine pests was restricted to whether or not the applicant country implements specific measures specified in points 30 and 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072. For all remaining pests, the effectiveness of the risk mitigation measures applied to the commodity was evaluated and an EKE was performed.
A conclusion on the likelihood of the commodity being free from each of the relevant pest was determined and uncertainties identified using expert judgements.
Pest freedom was assessed by estimating the number of infested/infected plants out of 10,000 exported plants. Further details on the methodology used to estimate the likelihood of pest freedom are provided in Section 2.3.4.
The information provided in some sections of the Opinion are results of the Panel interpretation of the text of the applicant Dossier.
2.3.1. Commodity data
Based on the information provided by the NPPO of China, the characteristics of the commodity were summarised.
2.3.2. Identification of pests potentially associated with the commodity
To evaluate the pest risk associated with the importation of P. parviflora and P. thunbergii from China, a pest list was compiled. The pest list is a compilation of all identified plant pests associated with P. parviflora and P. thunbergii based on information provided in Dossier Sections 2.0, 4.0 and 5.0 and on searches performed by the Panel. Pests associated with Pinus mentioned in the Commission Implementing Regulation 2020/1217 and in Commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan (EFSA PLH Panel, 2019b) were also considered.
The scientific names of the host plants (i.e. P. parviflora and P. thunbergii) were used when searching in the EPPO Global database and CABI Crop Protection Compendium. In the EPPO Global database, the pest list of P. parviflora and P. thunbergii includes also some pests associated with Pinus spp. or Pinaceae, which were also taken into consideration.
The scientific names of the host plants (i.e. P. parviflora and P. thunbergii) were used when searching in the other databases excluding EUROPHYT, TRACES‐NT and Web of Science.
EUROHYT was investigated by searching for the interceptions associated with Pinus sp., P. parviflora and P. thunbergii commodities imported from China from 1995 to May 2020 and TRACES‐NT from May 2020 to 9 June 2021, respectively. For the pests selected for further evaluation, a search in the EUROPHYT and/or TRACES was performed for the years between 1995 and May 2021 for the interceptions from the whole world, at species level.
The search strategy used for Web of Science Databases was designed combining English common names for pests and diseases, terms describing symptoms of plant diseases and the scientific and common names of the commodity species (i.e. P. parviflora and P. thunbergii) and excluding pests which were identified using searches in other databases. The search strings are detailed in Appendix B. The searches for P. parviflora and P. thunbergii in Web of Science Databases were run on 23 February 2021 and 15 March 2021, respectively.
The titles and abstracts of the scientific papers retrieved were screened and the pests associated with P. parviflora and P. thunbergii were included in the pest list.
The compiled pest list (see Microsoft Excel® file in Appendix E) includes all identified agents associated with P. parviflora and/or P. thunbergii, potentially including predators and parasitoids of insects and not harmful microorganisms. The pest list was eventually further compiled with other relevant information (e.g. EPPO Codes, taxonomic information, categorisation, distribution) useful for the selection of the pests relevant for the purposes of this Opinion.
The evaluation of the compiled pest list was done in two steps: first, the relevance of the EU‐regulated pests was evaluated (Section 4.1); second, the relevance of any other plant pest was evaluated (Section 4.2).
Pests for which limited information was available on one or more criteria used to identify them as relevant for this Opinion, e.g. on potential impact, are listed in Appendix D (List of pests that can potentially cause an effect not further assessed).
2.3.3. Listing and evaluation of risk mitigation measures
The proposed risk mitigation measures were listed and evaluated. When evaluating the potential pest freedom of the commodity, the following types of potential infestation/infection sources for P. parviflora and P. thunbergii plants in export nursery and relevant risk mitigation measures were considered (see also Figure 1):
pest entry from surrounding areas,
pest entry with new plants/seeds,
pest spread within the nursery.
Figure 1.
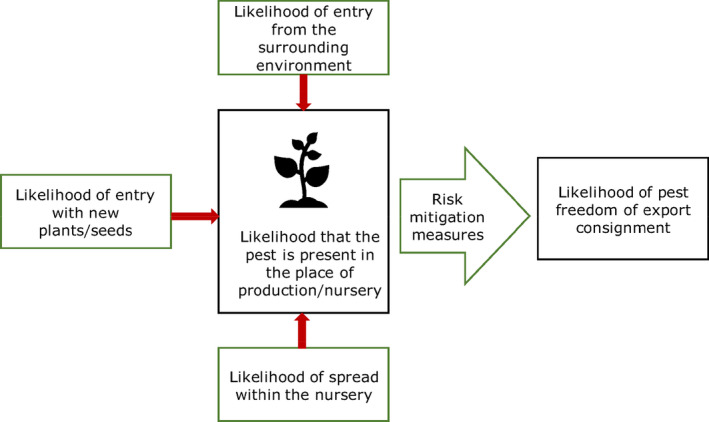
Conceptual framework to assess likelihood that plants are exported free from relevant pests (Source: EFSA PLH Panel, 2019b)
The risk mitigation measures proposed by NPPO of China were evaluated with Expert Knowledge Elicitation (EKE) according to the Guidance on uncertainty analysis in scientific assessment (EFSA Scientific Committee, 2018).
Information on the biology, likelihood of entry of the pest to the export nursery, of its spread inside the nursery and the effect of measures on the specific pests were summarised in data sheets of pests selected for further evaluation (see Appendix A).
2.3.4. Expert Knowledge Elicitation
To estimate the pest freedom of the commodity, an Expert Knowledge Elicitation (EKE) was performed following EFSA guidance (Annex B.8 of EFSA Scientific Committee, 2018). The specific question for EKE was defined as follows: ‘Taking into account (i) the risk mitigation measures in place in the nursery, and (ii) other relevant information, how many of 10,000 bonsai plants will be infested with the relevant pest/pathogen when arriving in the EU?’. The EKE question was common to all pests for which the pest freedom of the commodity was estimated.
The risk assessment uses individual plants as most suitable granularity. The following reasoning is given:
The inspections before export are targeted on individual plants.
Transportation is assumed to be performed in boxes of few plants to protect the individual plants.
The product will be distributed in the EU as individual plants to the consumer.
Before the elicitation, the list of pests was screened for pests with similar characteristics, risks, host–pest interactions, management practices in the production system. Similar pests were grouped for a common assessment.
-
–
Pests, which are likely to be confused or misclassified by typical identification standards, are not distinguished. The assessment is done on the total infestation of this group of pests. As example for this approach, a total evaluation is performed for Matsucoccus massonianae and Matsucoccus matsumurae together.
-
–
Pests which have similar risks of entry into the EU are evaluated together. The combined assessment is valid for each pest individually. Existing differences between the pests are covered by the reported uncertainties. Considering the given information, separate evaluations of these pests are unlikely to give different results. As example for this approach, the evaluation of Dendrolimus spectabilis, Dendrolimus superans and Dendrolimus tabulaeformis was performed with a common description of the risk of entry.
-
–
Pests, which share common characteristics, but may have different risks of entry into the EU, are evaluated with a simplified procedure. From this group, one pest with stronger evidence is selected for a complete evaluation (reference pest). The remaining pests are evaluated in comparison to the reference. The evaluation is focusing on the differences between the pests and their impact on the risk of entry. The elicitation discusses and reasons changes in comparison to the reference pest. As example for this approach, Coleosporium asterum was evaluated completely and used as reference for Coleosporium eupatorii and Coleosporium phellodendri.
The uncertainties associated with the EKE were taken into account and quantified in the probability distribution applying the semi‐formal method described in Section 3.5.2 of the EFSA‐PLH Guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Finally, the results were reported in terms of the likelihood of pest freedom. The lower 5% percentile of the uncertainty distribution reflects the opinion that pest freedom is with 95% certainty above this limit.
3. Commodity data
3.1. Description of the commodity
The commodity to be imported are dwarfed plants (bonsai) of Pinus parviflora (common name: Japanese white pine, five‐needle pine; family: Pinaceae) grafted on P. thunbergii (common name: Japanese black pine, two‐needle pine; family: Pinaceae) rootstocks in pots. The age of bonsai plants at the time of export is 4–5 years (Dossier Section 4.0).
The size of the commodity at the time of export is specified in Table 4 and shown in Figure 2 (Dossier Section 5.0).
Table 4.
Range of diameter and height of the plants at the time of export (according to Dossier Section 5.0)
| Size of the plants | Minimum (cm) | Range of average (cm) | Maximum (cm) |
|---|---|---|---|
| Diameter of the plants at the base | 1.0 | 2.0–2.5 | 3.5 |
| Diameter of twigs | 0.5 | 1.5–2.0 | 3.0 |
| Height of plants | 30.0 | 35.0–40.0 | 50.0 |
Figure 2.

Pictures of Pinus bonsai intended to export (Source: Dossier Section 5.0; provided by the Chinese National Plant Protection Organisation)
According to ISPM 36 (FAO, 2019 ), the commodity can be classified as ‘rooted plants in pots’. The EU legislation [e.g. Commission Implementing Regulation (EU) 2019/2072] defines the commodity as ‘naturally or artificially dwarfed plants for planting’.
3.2. Description of the production areas
The nursery ready to export the commodity is Hangzhou Newton Gardening Co., Ltd. (hereafter referred to as Newtone). The nursery is located in the Hangzhou Yuhang District at the High‐tech Agricultural Demonstration Center (hereinafter referred to as the Agricultural Center). The geographical coordinates of the nursery are 120°7' E 30°25' N (see Figure 3) (Dossier Section 4.0). The Newtone's nursery is located at the northeast corner of the Agricultural Center. The nursery is surrounded by the river on three sides and by a road on one side (Dossier Section 4.0).
Figure 3.
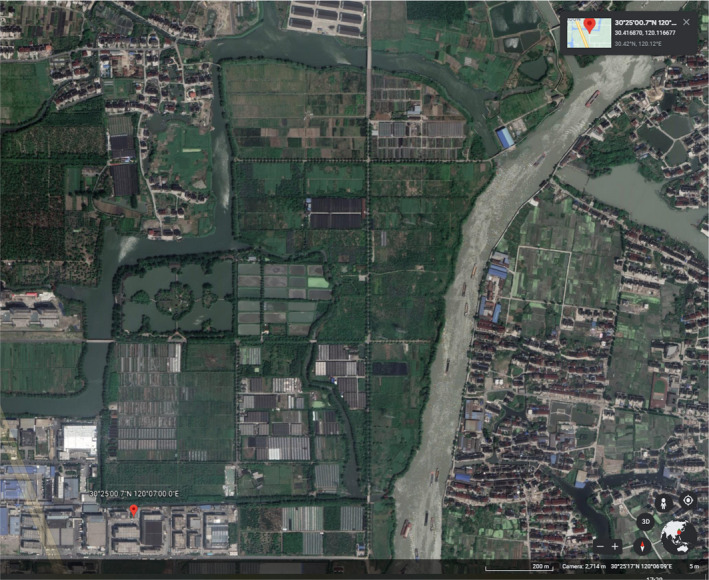
Image of the nursery and production site taken from Google Earth (online) on 5 August 2021 (Imagery date 4/18/18, geographical coordinates of the nursery: 120°7' E 30°25' N) © 2018 Google
Based on the description in Dossier Section 4.0, the production site was located by the Panel (see Figure 3).
The nursery also grows ornamental plants including abelia, bamboo, camphor, loropetalum, photinia, pyracantha, wisteria and other plants (Dossier Section 4.0).
The surrounding of the nursery mostly includes factories, villages, residential areas and rivers. Plants present in the surrounding are green plants, such as camphor, crape myrtle, ilex, loquat, loropetalum, oleander, Osmanthus fragrans, pomegranate, Prunus, rhododendron, yew, some aquatic plants and lawns. Those plant species are widely used as landscape plants (Dossier Section 4.0).
Within 15 km distance from the nursery, 183 plant taxa are reported to be present (Dossier Section 4.0), see Appendix C.
According to Dossier Section 5.0, the following plant species (hosts of rusts) are present within 1.5 km from the production site: Ainsliaea fragrans, Ainsliaea macroclinidioides, Aster ageratoides, Aster sublatus, Castanea mollissima, Castanopsis carlesii, Castanopsis sclerophylla, Castanopsis eyrei, Cyclobalanopsis glauca, Doellingeria scabra, Erigeron annuus, Eupatorium fortune, Eupatorium japonicum, Inula japonica, Kalimeris indica, Lithocarpus glaber, Paederia cavaleriei, Paederia scandens, Pinus spp., Quercus aliena and Solidago canadensis.
According to Dossier Section 2.0, a specific monitoring survey was conducted in 2012 on Pinus parviflora at the Liutong Gardening and Planting Base in Hangzhou and in the West Lake scenic spot (including Zhusu Garden, Hangzhou Flower Nursery, Botanical Garden and Viewing Fish at Flower Pond), which are located in the area of the nursery. In this monitoring survey, pests belonging to 15 taxa were found, namely, Anomala multistriata, Aphis gossypii, Callopistria albomacula, Colletotrichum gloeosporioides, Diplodia pinea, Eophileurus chinensis, Helicotylenchus dihystera, Lachnosterna kiotonensis, Maladera orientalis, Maladera spissigrada, Myzus persicae, Noctuidae, Pestalotiopsis microspora, Pyralidae and Xiphinema hunaniense.
By checking Google maps (on 17 June 2021), the Panel noticed that there are at least four large patches of forest in the radius of 10 km from the nursery.
According to Dossier Section 2.0: ‘During the whole production stage, pots with the commodity are either kept on racks at 50 cm above the floor or on the ground, …. They are kept separately, not mixed with seedlings of other tree species’.
Based on the global Köppen–Geiger climate zone classification (Kottek et al., 2006), the climate of the production area is classified as Cfa, main climate (C): warm temperate; precipitation (f): fully humid; temperature (a): hot summer.
3.3. Production and handling processes
3.3.1. Growing conditions
The plants are grown outdoor protected by 40 mesh (0.4 mm) insect‐proof net. The net is removed in winter to prevent snow damage (Dossier Section 4.0).
Irrigation is conducted using tap water or deep well water. Rainwater must be disinfected before it can be used (Dossier Section 2.0). However, no further details are provided in the Dossier on the disinfection of rainwater.
3.3.2. Source of planting material
According to Dossier Section 4.0, the seeds of P. thunbergii are purchased from companies specialising in seeds production, which have obtained the ‘Seed and Seeding Production and Management License’ issued by the local forestry authorities. The seeds have passed the quarantine inspection by the local forestry department before being transferred, and Quarantine Certificate of Seed Transportation and Quarantine be issued accompanying with seeds transportation.
Mother plants of P. parviflora used to obtain scions are grown in open fields located in the nursery since 2006. About 50 mother plants are currently present. The average number of scions per mother plant is 1,500 (Dossier Section 4.0).
3.3.3. Production cycle
According to Dossier Section 4.0, the specific cultivation process comprises in the first spring in February P. thunbergii seed sowing, in the second spring transplanting the seedlings into the pot, in the third spring the P. thunbergii is used as rootstock for grafting with P. parviflora scion, in the fourth year P. thunbergii branches are removed and P. parviflora twigs are given a shape. Some plants may grow for an additional year during which they are shaped for export.
According to Dossier Section 2.0, the cultivation medium is fresh and unused. Before use, hot steam or pesticide treatment is performed. During planting process, measures are taken to ensure that the medium will not be infested by pests.
According to Dossier Section 5.0, during production and for export coconut coir is used as a growing medium. Coconut coir is fibre powder of coconut shell, which is a kind of pure natural organic matter medium. It does not contain any soil. The processed coconut coir is very suitable for plant cultivation and is a popular horticultural medium at present.
According to Dossier Section 5.0, the repotting procedure is as follows:
Clean the root soil thoroughly before planting. Prevention of root‐parasite nematodes such as Paratrichodorus porosus and Trichodorus cedarus by using broad‐spectrum chemicals on whole plants. For example, soaking the roots by using 2,000‐fold dilution of Avermectin.
Use clean coconut coir which is imported from abroad.
Soak the roots with chemicals, such as 2,000‐fold dilution of Avermectin (Abamectin) and 2,000‐fold dilution of tea saponin, in September.
Two weeks before export, remove 2 cm of surface growing medium which is replaced by new coconut coir, and then soak the root with 2,000‐fold dilution of Avermectin for 1.5 h.
The Panel understands that sowing occurs in soil and transplanting of seedlings occurs in the second spring in pots containing coconut coir.
According to Dossier Section 2.0, the production company should identify the plants to be used for export at least half a year in advance. The production company should strictly inspect 100% of the plants intended for export. If diseases and pests are found on plants, these are treated in time and a record is kept. Roots are washed and the growing medium is inspected. The plants are repotted (the cultivation medium is fresh and unused) and taken to the export registered plantation base.
3.3.4. Pest monitoring during production
According to Dossier Section 2.0, according to actual conditions, the inspection and quarantine organisation will conduct official inspection on the export registered plantation base no less than twice every year. The inspection focuses on surrounding environment and conditions of the plantation base, cultivated varieties and their quantities, diseases and pests occurrence and prevention and control records, purchase and delivery logbook, production cancelling after verification, as well as other items that should be supervised. Supervision records are properly kept.
According to Dossier Section 2.0, for pests of concern for the EU, the inspection and quarantine organisation are expected to conduct surveillance at least six times a year at reasonable time intervals. The infested/infected plants are removed from the nursery.
According to Dossier Section 4.0, the nursery and its immediate vicinity areas (at least 2 km) are surveyed as follows: at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of 10% of the plants.
3.3.5. Export procedure
According to Dossier Section 5.0, the export is carried out in winter, from December to March.
According to Dossier Section 2.0, pruning is stopped 2 months prior to export in case the young needles are too tender.
According to Dossier Section 2.0, each plant to be exported is inspected. Plants showing injuries are discarded.
According to Dossier Section 2.0, if damage is found caused by pests about which EU is concerned, the plants are allowed to be exported only after quarantine treatments are performed and the plants meet the export requirements. In the treatment column of the phytosanitary certificate, the details on how the treatment has been performed are provided along with the date of treatment. In the absence of effective treatments, the whole batch of plants will be prohibited from being exported.
Two weeks before export 2 cm of surface growing medium is removed and replaced by new coconut coir (Dossier Section 5.0), and then, the pots are soaked for 1.5 h in 2% Avermectin in order to treat comprehensively the cultivation medium (Dossier Section 4.0 and 5.0).
According to Dossier Section 2.0, packaging materials for loading the commodity to be exported is new and clean. Labels should be placed on the packaging boxes, specifying information such product name, quantity, export batch number and nursery registration number. If wooden packaging materials are to be used, they will qualify after first being treated according to ISPM‐15 (FAO, 2018). The packaging materials are stored in pest‐proof, mould‐proof and moisture‐proof locations. The packaging materials for loading the plants to be exported should be clean and not reused. The containers should be sealed and signed by the authority. Registration number of the plantation base should be specified in the additional statement of the plant quarantine certificate.
According to Dossier Section 2.0, the commodity is packed during the daytime on hardened ground at the storage and packaging place of the export nursery. Before loading the boxes, the container is sprayed with pesticides.
3.4. Pest prevention and control implemented for the commodity
According to Dossier Section 2.0, the planting area of the production base is tidy and free of sundries; the ground of the operation area is clean and free of soil, weeds and plant residues; complete irrigation equipment is available, as well as equipment and pesticide application devices that meet quarantine requirements for cleaning, processing, pests and diseases prevention and necessary quarantine treatment, and there are fixed places for storage as well.
Before use, the soil employed for seeding is treated by using steam high temperature disinfection at the level of 100°C (Dossier Section 4.0).
Seeds of P. thunbergii are soaked in mixture of Potassium Permanganate and Triadimefon before seeding (Dossier Section 4.0).
Details of pesticide treatments (see Tables 5–8) are shown as provided in Tables 4–6 of Dossier Section 4.0 and in Table 2 of Dossier Section 5.0. The Panel assumes that the dosage and dilution of pesticides for the different pests are consistent with the indication provided by the manufacturer. However, there is uncertainty on dosages used as the way they were presented in the Dossier.
Table 5.
Details of nematicide treatment as provided in Dossier Section 4.0
| Pesticide used (active substances) | Name(s) of the target pest species | Target life stage(s) of the pest(s) | Timing and location of treatment | Dilution as provided in the Dossier | Notes |
|---|---|---|---|---|---|
| Avermectin | Root knot nematodes, such as Paratrichodorus porosus, Trichodorus cedarus | Preventive treatment | February, March or September, October before potting. | 3,000 times | Soak the whole plant for 2 h. |
| 2% Avermectin | Harmful organisms in the medium such as nematodes | Finished product | 2 weeks before export, export nursery. | 600 times | Soaking for 1.5 h. |
Table 8.
Details of insecticide and acaricide treatment as provided in Dossier Sections 4.0 and 5.0
| Pesticide used (active substances) | Name(s) of the target pest species | Target life stage(s) of the pest(s) | Timing and location of treatment | Dilution as provided in the Dossier | Notes |
|---|---|---|---|---|---|
| Cypermethrin | Monochamus spp. | Adult emergence stage | May, June, July, export nursery. | 500 times |
3 times Periodicity of treatment every 20–25 days. |
| 50% Fenitrothion | Within 1 week of the peak period of eclosion/moulting | 200 times | Periodicity of treatment every 20–25 days. | ||
| 40% Folimat | Larvae hatching | July–September, export nursery. | 1,000 times | ||
| Trichlorfon | Lepidoptera larvae | Peak hatching period larvae | June, July, export nursery. | 1,000 times | |
| 50% Fenitrothion | 1,000–1,500 times | Once every 15 days. | |||
| 40% Folimat | Moth | Adult occurrence season | July, August, export nursery. | 800 times | |
| 1.8% Avermectin | Mite | July, August, export nursery. | 5,000 times | Periodicity of treatment once a month. | |
| Pyridaben | 2,500 times | Periodicity of treatment once a month. | |||
| Propargite | 2,000 times | Alternate use 3–4 times. | |||
| 40% Omethoate a | July–September | 800 times | Periodicity of treatment once a month. |
The target organisms are not mentioned in the Dossier.
Table 7.
Details of herbicide treatment as provided in Dossier Section 4.0
| Pesticide used (active substances) | Name(s) of the target pest species | Target life stage(s) of the pest(s) | Timing and location of treatment | Dose in g/m2 used for each treatment | Notes |
|---|---|---|---|---|---|
| Sodium pentachlorophenol, two methyl four chloro, atrazine, non‐herbicide, herbicide etc. | Cronartium spp. (non‐European) | Growth stage, the alternate host a removing | March, April, export nursery. | 1~5g/m2 | Elimination of tea b and tea seeds and horse's stem within 500 m in the nursery and surrounding areas. |
Telial host.
The Panel notes that tea is not a known host for Cronartium spp.
4. Identification of pests potentially associated with the commodity
The search for potential pests associated with Pinus parviflora and/or P. thunbergii rendered 465 species (see Microsoft Excel® file in Appendix E). This list also included nine RNQPs and two deregulated pests that were subsequently excluded from the evaluation as indicated in Section 1.2.
4.1. Selection of relevant EU‐regulated pests associated with the commodity
The EU listing of Union quarantine pests and protected zone quarantine pests (Commission Implementing Regulation (EU) 2019/2072) is based on assessments concluding that the pests can enter, establish, spread and have potential impact in the EU.
The Panel selected the relevant EU‐regulated pests and protected zone quarantine pests as specified in Commission Implementing Regulation (EU) 2019/2072 and if applicable, in Commission Implementing Regulation (EU) 2020/1217.
Eighty‐eight EU‐quarantine species and 20 pests of EU concern (mentioned in Commission Implementing Regulation (EU) 2020/1217) for Pinus parviflora and P. thunbergii that are reported as associated with P. parviflora and/or P. thunbergii were evaluated (Tables 9 and 10) for their relevance of being included in this Opinion.
Table 9.
Overview of the evaluation of 88 EU‐quarantine pest species known to use Pinus parviflora and/or Pinus thunbergii as a host plant for their relevance for this Opinion
| No. | Pest name according to EU legislation a | EPPO Code | Group | Pest present in China | Pinus confirmed as a host (reference) | Pinus parviflora confirmed as a host (reference) | Pinus thunbergii confirmed as a host (reference) | Pest can be associated with the commodity | Pest relevant for the Opinion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Arceuthobium americanum as Arceuthobium spp. | AREAM | Plants | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 2 | Arceuthobium campylopodum as Arceuthobium spp. | ARECP | Plants | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 3 | Arceuthobium laricis as Arceuthobium spp. | ARELA | Plants | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 4 | Arceuthobium occidentale as Arceuthobium spp. | AREOC | Plants | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 5 | Arceuthobium pusillum as Arceuthobium spp. | AREPU | Plants | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 6 | Arceuthobium tsugense as Arceuthobium spp. | ARETS | Plants | No | Yes (EPPO, online) | Yes (EPPO, online) | No | Not evaluated | No |
| 7 | Arceuthobium vaginatum as Arceuthobium spp. | AREVA | Plants | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 8 |
Atropellis pinicola as Atropellis spp. Current name and name used in the Opinion: Godronia zelleri |
ATRPPC | Fungi | Yes (one report) | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Yes | Yes |
| 9 | Atropellis piniphila as Atropellis spp. | ATRPPP | Fungi | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 10 | Bursaphelenchus xylophilus | BURSXY | Nematodes | Yes | Yes (EPPO, online) | Yes (Koo et al., 2013; EFSA PLH Panel, 2019b) | Yes (Koo et al., 2013; EFSA PLH Panel, 2019b; CABI, online; EPPO, online) | Yes | Yes |
| 11 | Choristoneura fumiferana as Choristoneura spp. (non‐European) | CHONFU | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 12 | Choristoneura lambertiana as Choristoneura spp. (non‐European) | TORTLA | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 13 | Coniferiporia weirii (synonym: Inonotus weirii) | INONWE | Fungi | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | No | No |
| 14 | Cronartium coleosporioides as Cronartium spp. | CRONCL | Fungi | Yes (one report) | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Yes | Yes |
| 15 | Cronartium comandrae as Cronartium spp. | CRONCO | Fungi | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 16 | Cronartium comptoniae as Cronartium spp. | CRONCP | Fungi | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 17 | Cronartium harknessii as Cronartium spp. (synonym: Endocronartium harknessii) | ENDCHA | Fungi | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 18 | Cronartium himalayense as Cronartium spp. | CRONHI | Fungi | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 19 | Cronartium kamtschaticum as Cronartium spp. | CRONKA | Fungi | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 20 | Cronartium kurilense as Cronartium spp. (synonyms: Endocronartium sahoanum var. hokkaidoense, Peridermium kurilense) | CRONKU | Fungi | No | Yes (EFSA PLH Panel, 2019b; Farr and Rossman, online) | No | No | Not evaluated | No |
| 21 | Cronartium orientale as Cronartium spp. | CRONOR | Fungi | Yes | Yes (EFSA PLH Panel, 2019b; Farr and Rossman, online) | No | Yes (EFSA PLH Panel, 2019b; Farr and Rossman, online) | Yes | Yes |
| 22 | Cronartium quercuum as Cronartium spp. (synonym: Cronartium fusiforme) | CRONFU | Fungi | Yes | Yes (EPPO, online) | No | Yes (CABI, online; EPPO, online; Farr and Rossman, online) | Yes | Yes |
| 23 | Cronartium sahoanum as Cronartium spp. (synonym: Endocronartium sahoanum) | CRONSA | Fungi | No | Yes (EFSA PLH Panel, 2019b; Farr and Rossman, online) | No | No | Not evaluated | No |
| 24 | Cronartium yamabense as Cronartium spp. (synonym: Endocronartium yamabense) | CRONYA | Fungi | No | Yes (EFSA PLH Panel, 2019b; Farr and Rossman, online) | No | No | Not evaluated | No |
| 25 | Cryphalus fulvus as Scolytidae spp. (non‐European) | CRYHFU | Insects | No | Yes (CABI, online; EFSA PLH Panel, 2019b) | No | Yes (EFSA PLH Panel, 2019b) | Not evaluated | No |
| 26 | Cryphalus laricis as Scolytidae spp. (non‐European) | CRYHLR | Insects | No | Yes (EFSA PLH Panel, 2019b) | No | Yes (EFSA PLH Panel, 2019b) | Not evaluated | No |
| 27 | Cyrtogenius luteus as Scolytidae spp. (non‐European) (synonym: Dryocoetes luteus) | CYRGLU | Insects | Yes | Yes (Gómez et al., 2012) | Yes (Dossier Section 2.0) | No | No | No |
| 28 | Dendroctonus adjunctus as Scolytidae spp. (non‐European) | DENCAD | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 29 | Dendroctonus brevicomis as Scolytidae spp. (non‐European) | DENCBR | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 30 | Dendroctonus frontalis as Scolytidae spp. (non‐European) | DENCFR | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 31 | Dendroctonus micans | DENCMI | Insects | Yes | Yes (Wood and Bright, 1992; EFSA PLH Panel, 2019b) | Yes (Dossier Section 2.0) | No | No | No |
| 32 | Dendroctonus ponderosae as Scolytidae spp. (non‐European) | DENCPO | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 33 | Dendroctonus terebrans as Scolytidae spp. (non‐European) | DENCTE | Insects | No | Yes (CABI, online) | No | Yes (CABI, online) | Not evaluated | No |
| 34 | Dendroctonus valens as Scolytidae spp. (non‐European) | DENCVA | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | No | No |
| 35 | Dendrolimus sibiricus | DENDSI | Insects | Yes | Yes (EPPO, online) | Yes (EFSA PLH Panel, 2019b; EPPO, online) | Yes (EFSA PLH Panel, 2019b; EPPO, online) | Yes | Yes |
| 36 | Dryocoetes baicalicus as Scolytidae spp. (non‐European) | DRYOBA | Insects | Yes | No | Yes (Dossier Section 2.0) | No | No | No |
| 37 | Euwallacea interjectus as Scolytidae spp. (non‐European) (synonym: Xyleborus interjectus) | XYLBIN | Insects | Yes | Yes (EPPO, 2020) | Yes (EPPO, 2020; Dossier Section 2.0) | Yes (EPPO, 2020) | Yes | Yes |
| 38 | Euwallacea validus as Scolytidae spp. (non‐European) (synonym: Xyleborus validus) | XYLBVA | Insects | Yes | Yes (EPPO, 2020) | Yes (Dossier Section 2.0) | No | Yes | Yes |
| 39 | Fusarium circinatum (synonym: Gibberella circinata) | GIBBCI | Fungi | No | Yes (CABI, online; Farr and Rossman, online) | No | Yes (EFSA PLH Panel, 2019b; CABI, online; Farr and Rossman, online) | Not evaluated | No |
| 40 | Gnathotrichus sulcatus as Scolytidae spp. (non‐European) | GNAHSU | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 41 | Gremmeniella abietina | GREMAB | Fungi | No | Yes (EFSA PLH Panel, 2019b; EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 42 | Heteroborips seriatus as Scolytidae spp. (non‐European) (synonym: Xyleborus seriatus) | XYLBSE | Insects | Yes | Yes (Hoebeke and Rabaglia, 2008) | Yes (Hoebeke and Rabaglia, 2008) | Yes (Hoebeke and Rabaglia, 2008) | Yes | Yes |
| 43 | Ips amitinus | IPSXAM | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 44 | Ips calligraphus as Scolytidae spp. (non‐European) | IPSXCA | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | No | No |
| 45 | Ips confuses as Scolytidae spp. (non‐European) | IPSXCO | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 46 | Ips duplicatus | IPSXDU | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | No | No |
| 47 | Ips grandicollis as Scolytidae spp. (non‐European) | IPSXGR | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | No | No |
| 48 | Ips hauseri as Scolytidae spp. (non‐European) | IPSXHA | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | No | No |
| 49 | Ips lecontei as Scolytidae spp. (non‐European) | IPSXLE | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 50 | Ips pini as Scolytidae spp. (non‐European) | IPSXPI | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 51 | Ips plastographus as Scolytidae spp. (non‐European) | IPSXPL | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 52 | Ips sexdentatus | IPSXSE | Insects | Yes | Yes (Wood and Bright, 1992) | Yes (Dossier Section 2.0) | No | No | No |
| 53 | Ips subelongatus as Scolytidae spp. (non‐European) | IPSXFA | Insects | Yes | Yes (Wang et al., 2020) | No | No | No | No |
| 54 | Ips typographus | IPSXTY | Insects | Yes | Yes (Wood and Bright, 1992; EFSA PLH Panel, 2019b) | Yes (Dossier Section 2.0) | No | No | No |
| 55 | Monochamus alternatus as Monochamus spp. (non‐European populations) | MONCAL | Insects | Yes | Yes (EPPO, online) | Yes (EFSA PLH Panel, 2019b; CABI, online) | Yes (EFSA PLH Panel, 2019b; CABI, online; EPPO, online) | Yes | Yes |
| 56 | Monochamus carolinensis as Monochamus spp. (non‐European populations) | MONCCA | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 57 | Monochamus galloprovincialis as Monochamus spp. (non‐European populations) | MONCGA | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Yes | Yes |
| 58 | Monochamus grandis as Monochamus spp. (non‐European populations) | MONCGR | Insects | No | Yes (Esaki, 1996) | Yes (Esaki, 1996) | No | Not evaluated | No |
| 59 | Monochamus mutator as Monochamus spp. (non‐European populations) | MONCMC | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 60 | Monochamus nitens as Monochamus spp. (non‐European populations) | MONCNI | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 61 | Monochamus obtusus as Monochamus spp. (non‐European populations) | MONCOB | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 62 | Monochamus saltuarius as Monochamus spp. (non‐European populations) | MONCSL | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Yes | Yes |
| 63 | Monochamus scutellatus as Monochamus spp. (non‐European populations) | MONCST | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 64 | Monochamus sutor as Monochamus spp. (non‐European populations) | MONCSU | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Yes | Yes |
| 65 | Monochamus titillator as Monochamus spp. (non‐European populations) | MONCTI | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 66 | Monochamus urussovi as Monochamus spp. (non‐European populations) | MONCUR | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Uncertain | Yes |
| 67 | Oemona hirta | OEMOHI | Insects | No | Yes (EPPO, 2014) | No | No | Not evaluated | No |
| 68 | Orthotomicus angulatus as Scolytidae spp. (non‐European) | ORTCAN | Insects | Yes | Yes (Choo et al., 1989; EFSA PLH Panel, 2019b) | No | Yes (EFSA PLH Panel, 2019b) | No | No |
| 69 | Orthotomicus tosaensis as Scolytidae spp. (non‐European) | ORTCTO | Insects | No | Yes (Wood and Bright, 1992; EFSA PLH Panel, 2019b) | No | Yes (Wood and Bright, 1992; EFSA PLH Panel, 2019b) | Not evaluated | No |
| 70 | Phloeosinus camphoratus as Scolytidae spp. (non‐European) | PHLSCM | Insects | Yes | No | Yes (Dossier Section 2.0) | No | No | No |
| 71 | Phloeosinus perlatus as Scolytidae spp. (non‐European) | PHLSPE | Insects | Yes | No | Yes (Dossier Section 2.0) | No | No | No |
| 72 | Phloeosinus shensi as Scolytidae spp. (non‐European) | PHLSSH | Insects | Yes | No | Yes (Dossier Section 2.0) | No | No | No |
| 73 | Phloeosinus sinensis as Scolytidae spp. (non‐European) | PHLSSI | Insects | Yes | No | Yes (Dossier Section 2.0) | No | No | No |
| 74 | Pissodes nemorensis | PISONE | Insects | No | Yes (EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 75 | Pissodes nitidus | PISONI | Insects | Yes | Yes (Jin, 1989; EFSA, 2020) | Yes (EFSA, 2020) | Yes (EFSA PLH Panel, 2019b; EFSA, 2020) | Yes | Yes |
| 76 | Pissodes punctatus | PISOPU | Insects | Yes | Yes (Lei et al., 2003; EFSA, 2020) | Yes (Dossier Section 2.0) | No | Yes | Yes |
| 77 | Pissodes strobi | PISOST | Insects | No | Yes (EPPO, online) | Yes (CABI, online; EPPO, online) | Yes (EPPO, online) | Not evaluated | No |
| 78 | Pissodes yunnanensis | PISOYU | Insects | Yes | Yes (Zhang et al., 2004; EFSA, 2020) | Yes (Dossier Section 2.0) | No | Yes | Yes |
| 79 | Pityophthorus jucundus as Scolytidae spp. (non‐European) | PITOJC | Insects | Yes | Yes (EFSA PLH Panel, 2019b) | No | Yes (EFSA PLH Panel, 2019b) | No | No |
| 80 | Polygraphus proximus | POLGPR | Insects | Yes | Yes (Wood and Bright, 1992; EFSA PLH Panel, 2019b) | No | Yes (EFSA PLH Panel, 2019b) | No | No |
| 81 | Popillia japonica | POPIJA | Insects | No | Yes (USDA, 2016; EFSA PLH Panel, 2019b) | No | No | Not evaluated | No |
| 82 | Pseudocercospora pini‐densiflorae Current name and name used in the Opinion: Mycosphaerella gibsonii (synonyms: Cercoseptoria pini‐densiflorae, Cercospora pini‐densiflorae) | CERSPD | Fungi | Yes | Yes (EPPO, online) | Yes (EFSA PLH Panel, 2019b; CABI, online; Farr and Rossman, online) | Yes (Quintero, 2015; EFSA PLH Panel, 2019b; CABI, online; EPPO, online; Farr and Rossman, online) | Yes | Yes |
| 83 | Thaumetopoea pityocampa | THAUPI | Insects | No | Yes (Stastny et al., 2006) | Yes (Devkota and Schmidt, 1990) | No | Not evaluated | No |
| 84 | Tomicus brevipilosus as Scolytidae spp. (non‐European) | TOMSBR | Insects | Yes | Yes (Wood and Bright, 1992; EFSA PLH Panel, 2019b) | Yes (Wood and Bright, 1992) | Yes (EFSA PLH Panel, 2019b) | No | No |
| 85 | Xiphinema americanum sensu stricto | XIPHAA | Nematodes | No | Yes (Xu and Zhao, 2019) | No | No | Not evaluated | No |
| 86 | Xiphinema rivesi | XIPHRI | Nematodes | No | Yes (Xu and Zhao, 2019) | No | No | Not evaluated | No |
| 87 | Xyleborus aquilus as Scolytidae spp. (non‐European) | XYLBAQ | Insects | No | Yes (Wood and Bright, 1992) | Yes (Dossier Section 2.0) | No | Not evaluated | No |
| 88 | Xylosandrus compactus as Scolytidae spp. (non‐European) | XYLSCO | Insects | Yes | Yes (EPPO, 2020; EPPO, online) | Yes (EPPO, online) | Yes (EPPO, online) | Yes | Yes |
Commission Implementing Regulation (EU) 2019/2072.
Table 10.
Overview of the evaluation of pests of EU concern for Pinus parviflora and/or Pinus thunbergii mentioned in Commission Implementing Regulation (EU) 2020/1217 not regulated as quarantine pests in Commission Implementing Regulation (EU) 2019/2072
| No. | Pest name according to EU legislation a | EPPO Code | Group | Pest present in China | Pinus parviflora confirmed as a host (reference) | Pinus thunbergii confirmed as a host (reference) | Pest can be associated with the commodity | EU Quarantine pest | Pest relevant for the Opinion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Coleosporium asterum | COLSAS | Fungi | Yes | Yes (Dossier Section 2.0) | Yes (CABI, online; Farr and Rossman, online) | Yes | No | Yes |
| 2 | Coleosporium paederiae | COLSPA | Fungi | Yes | No | No | No | No | No |
| 3 | Coleosporium phellodendri | COLSPH | Fungi | Yes | Yes (Dossier Section 2.0) | No | Yes | No | Yes |
| 4 | Crisicoccus pini | DACLPI | Insects | Yes | Yes (García Morales et al., online) | Yes (García Morales et al., online) | Yes | No | Yes |
| 5 | Dendrolimus spectabilis | DENDSC | Insects | Yes | Yes (Dossier Section 2.0; EPPO, online) | Yes (CABI, online; EPPO, online) | Yes | No | Yes |
| 6 | Dendrolimus superans | DENDSU | Insects | Yes | Yes (EPPO, online) | Yes (EPPO, online) | Yes | No | Yes |
| 7 | Dothistroma septosporum | SCIRPI | Fungi | Yes | Yes (EPPO, online) | Yes (EPPO, online; Farr and Rossman, online) | Yes | No | No b |
| 8 | Sirex nitobei | SIRXNI | Insects | Yes | Yes (EFSA PLH Panel, 2019b) | Yes (EFSA PLH Panel, 2019b) | No c | No | No |
| 9 | Thecodiplosis japonensis | THEOJA | Insects | Yes | No | Yes (EFSA PLH Panel, 2019b; CABI, online; EPPO, online | No | No | No |
| 10 | Urocerus japonicus | URCEJA | Insects | No data | Yes (EFSA PLH Panel, 2019b) | Yes (EFSA PLH Panel, 2019b) | Noc | No | No |
Commission Implementing Regulation (EU) 2020/1217.
The pathogen was discarded, because it is RNQP in the EU.
The pest is not associated with small plants.
The relevance of EU‐quarantine pest for this Opinion was based on evidence that:
the pest is present in China;
Pinus parviflora and/or P. thunbergii is host of the pest;
one or more life stages of the pest can be associated with the specified commodity.
Pests that fulfilled all criteria were selected for further evaluation.
Tables 9 and 10 present an overview of the evaluation of 88 EU‐quarantine pest species and 20 pests of EU concern for P. parviflora and P. thunbergii that are reported as associated with P. parviflora and P. thunbergii.
Of these 88 EU‐quarantine pest species evaluated, 39 species are present in China and 19 of these pests [Atropellis pinicola (current name and name used in the Opinion: Godronia zelleri), Bursaphelenchus xylophilus, Cronartium coleosporioides, Cronartium orientale, Cronartium quercuum, Dendrolimus sibiricus, Euwallacea interjectus, Euwallacea validus, Heteroborips seriatus, Monochamus alternatus, Monochamus galloprovincialis, Monochamus saltuarius, Monochamus sutor, Monochamus urussovi, Pissodes nitidus, Pissodes punctatus, Pissodes yunnanensis, Pseudocercospora pini‐densiflorae (current name and name used in the Opinion: Mycosphaerella gibsonii) and Xylosandrus compactus] are known to use P. parviflora and/or P. thunbergii as hosts, and to be associated with the commodity. Hence, they were selected for further evaluation to assess whether the applicant country fulfils the specific measures described in points 30 and 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072.
4.2. Selection of other relevant pests (non‐regulated in the EU) associated with the commodity
The information provided by NPPO of China, integrated with the search EFSA performed, was evaluated in order to assess whether there are other potentially relevant pests of Pinus parviflora and/or P. thunbergii present in the country of export. For these potential pests that are non‐regulated in the EU, pest risk assessment information on the probability of entry, establishment, spread and impact is usually lacking. Therefore, these pests were also evaluated to determine their relevance for this Opinion based on evidence that:
the pest is present in China;
the pest is (i) absent or (ii) has a limited distribution in the EU;
Pinus parviflora and/or P. thunbergii is a host of the pest;
one or more life stages of the pest can be associated with the specified commodity;
the pest may have an impact in the EU.
For non‐regulated species with a limited distribution (i.e. present in one or a few EU MSs) and fulfilling the other criteria (i.e. c, d and e), either one of the following conditions should be additionally fulfilled for the pest to be further evaluated:
official phytosanitary measures have been adopted in at least one EU MS;
any other reason justified by the WG (e.g. recent evidence of presence).
Pests that fulfilled all the above criteria were selected for further evaluation.
Based on the information collected, 377 potential pests known to be associated with P. parviflora and/or P. thunbergii were evaluated for their relevance to this Opinion. Species were excluded from further evaluation when at least one of the conditions listed above (a‐e) was not met. Details can be found in Appendix E (Microsoft Excel® file). Of the evaluated pests not regulated in the EU, 24 pests (Anomala testaceipes, Ceroplastes rubens, Coleosporium asterum, Coleosporium eupatorii, Coleosporium phellodendri, Crisicoccus pini, Dendrolimus punctatus, Dendrolimus spectabilis, Dendrolimus superans, Dendrolimus tabulaeformis, Fiorinia japonica, Hemiberlesia pitysophila, Lepidosaphes pineti, Lepidosaphes pini, Lepidosaphes piniphila, Matsucoccus massonianae, Matsucoccus matsumurae, Parlatoria pinicola, Pestalotiopsis disseminata, Pestalotiopsis microspora, Popillia quadriguttata, Pyrrhoderma noxium, Setoptus parviflorae and Shirahoshizo patruelis) were selected for further evaluation because they met all of the selection criteria. These 24 pests were assessed quantitatively by means of EKE. More information on these 24 species can be found in the pest data sheets (Appendix A).
4.3. Overview of interceptions
Data on the interception of harmful organisms on plants of Pinus, Pinus sp., P. parviflora and P. thunbergii can provide information on some of the organisms that can be present on P. parviflora grafted bonsai plants on rootstock of P. thunbergii despite the current measures taken. According to EUROPHYT online (accessed on 9.6.2021) and TRACES online (accessed on 9.6.2021), there were 35 interceptions of plants for planting of Pinus sp. from Japan, Bhutan and Moldova; 22 interceptions of plants for planting of P. parviflora from Japan; and two interceptions of plants for planting of P. thunbergii from Japan destinated to the EU Member States due to the presence of harmful organisms (see Tables 11, 12 and 13), between 1995 and 9 June 2021. Two intercepted organisms are EU quarantine pests, including Dendrolimus sibiricus and few species in Xiphinema americanum group.
Table 11.
Overview of harmful organisms intercepted on Pinus sp. from all over the world based on notifications of interceptions by EU Member States (based on EUROPHYT (online) and TRACES (online), accessed on 9 June 2021) a
| Name of harmful organism | Group | Intercepted on commodity | Country of origin | Total | Year of interception |
|---|---|---|---|---|---|
| Adelges: Adelgidae pineus | Insects | Intended for planting: already planted | Bhutan | 1 | 1998 |
| Criconematidae | Nematodes | Intended for planting: bonsai | Japan | 4 | 2005 |
| Ditylenchus sp. | Nematodes | Intended for planting: bonsai | Japan | 3 | 1997 |
| Helicotylenchus dihystera | Nematodes | Intended for planting: bonsai | Unknown | 1 | 2000 |
| Heteroderidae | Nematodes | Intended for planting: bonsai | Japan | 6 | 2005, 2011 |
| Mycoshaerella dearnessii and Mycoshaerella pini | Fungi | Intended for planting: not yet planted | Moldova | 1 | 2011 |
| Pratylenchidae: Hoplotylus sp. | Nematodes | Intended for planting: already planted | Bhutan | 1 | 1998 |
| Pratylenchus sp. | Nematodes | Intended for planting: bonsai | Japan | 3 | 1997 |
| Tylenchorhynchus | Nematodes | Intended for planting: bonsai | Japan | 6 | 1996 |
| Xiphinema americanum | Nematodes | Intended for planting: bonsai | Japan | 2 | 2011 |
| Xiphinema sp. | Nematodes | Intended for planting: bonsai | Japan | 8 | 1997, 2016 |
| Xiphinema sp. | Nematodes | Intended for planting: already planted | Japan | 1 | 2009 |
Search in EUROPHYT database also resulted in a report of interception of Anoplophora chinensis and Cerambycidae on Pinus spp. from Japan in 2008, however in the interception report itself it was stated that the pest was associated with Acer palmatum instead of Pinus spp. Therefore, this report was not taken into account in this Opinion.
Table 12.
Overview of harmful organisms intercepted on Pinus parviflora from all over the world based on notifications of interceptions by EU Member States (based on EUROPHYT (online) and TRACES (online), accessed on 9 June 2021)
| Name of harmful organism | Group | Intercepted on commodity | Country of origin | Total | Year of interception |
|---|---|---|---|---|---|
| Criconematidae | Nematodes | Intended for planting: bonsai | Japan | 3 | 2011 |
| Heteroderidae | Nematodes | Intended for planting: bonsai | Japan | 2 | 2011 |
| Pratylenchus penetrans | Nematodes | Intended for planting: bonsai | Japan | 3 | 2005 |
| Tylenchorhynchus sp. | Nematodes | Intended for planting: bonsai | Japan | 3 | 2011 |
| Xiphinema americanum | Nematodes | Intended for planting: bonsai | Japan | 9 | 2010, 2011 |
| Xiphinema sp. | Nematodes | Intended for planting: bonsai | Japan | 2 | 2011 |
Table 13.
Overview of harmful organisms intercepted on Pinus thunbergii from all over the world based on notifications of interceptions by EU Member States (based on EUROPHYT (online) and TRACES (online), accessed on 9 June 2021)
| Name of harmful organism | Group | Intercepted on commodity | Country of origin | Total | Year of interception |
|---|---|---|---|---|---|
| Dendrolimus spectabilis | Insects | Intended for planting: bonsai | Japan | 2 | 2018 |
4.4. List of potential pests not further assessed
From the list of pests not selected for further evaluation, the Panel highlighted 10 species (see Appendix D) for which the currently available evidence provides no reason to select these species for further evaluation in this Opinion. A specific justification of the inclusion in this list is provided for each species in Appendix D.
4.5. Summary of pests selected for further evaluation
The 43 pests identified to be present in China while having potential for association with P. parviflora grafted bonsai plants on rootstock of P. thunbergii destined for export are listed in Table 14.
Table 14.
List of relevant pests selected for further evaluation
| Number | Current scientific name | EPPO code | Name used in the EU legislation | Taxonomic information | Group | Regulatory status |
|---|---|---|---|---|---|---|
| 1 | Anomala testaceipes | ANMLTE | – |
Coleoptera Scarabaeidae |
Insects | Not regulated in the EU. |
| 2 | Bursaphelenchus xylophilus | BURSXY | Bursaphelenchus xylophilus (Steiner and Bührer) Nickle et al. [BURSXY] |
Rhabditida Parasitaphelenchidae |
Nematodes | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 3 | Ceroplastes rubens | CERPRB | – |
Hemiptera Coccidae |
Insects | Not regulated in the EU. |
| 4 | Coleosporium asterum | COLSAS | – |
Pucciniales Coleosporiaceae |
Fungi | Not regulated in the EU. |
| 5 | Coleosporium eupatorii | COLSEU | – |
Pucciniales Coleosporiaceae |
Fungi | Not regulated in the EU. |
| 6 | Coleosporium phellodendri | COLSPH | – |
Pucciniales Coleosporiaceae |
Fungi | Not regulated in the EU. |
| 7 | Crisicoccus pini | DACLPI | – |
Hemiptera Pseudococcidae |
Insects | Not regulated in the EU. |
| 8 | Cronartium coleosporioides | CRONCL | Cronartium spp. [1CRONG] |
Pucciniales Cronartiaceae |
Fungi | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 9 | Cronartium orientale | CRONOR | Cronartium spp. [1CRONG] |
Pucciniales Cronartiaceae |
Fungi | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 10 | Cronartium quercuum | CRONQU | Cronartium spp. [1CRONG] |
Pucciniales Cronartiaceae |
Fungi | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 11 | Dendrolimus punctatus | DENDPU | – |
Lepidoptera Lasiocampidae |
Insects | Not regulated in the EU. |
| 12 | Dendrolimus sibiricus | DENDSI | Dendrolimus sibiricus Chetverikov [DENDSI] |
Lepidoptera Lasiocampidae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 13 | Dendrolimus spectabilis | DENDSC | – |
Lepidoptera Lasiocampidae |
Insects | Not regulated in the EU. |
| 14 | Dendrolimus superans | DENDSU | – |
Lepidoptera Lasiocampidae |
Insects | Not regulated in the EU. |
| 15 | Dendrolimus tabulaeformis | DENDTA | – |
Lepidoptera Lasiocampidae |
Insects | Not regulated in the EU. |
| 16 | Euwallacea interjectus | XYLBIN | Scolytidae spp. (non‐European) [1SCOLF] |
Coleoptera Curculionidae Scolytinae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 17 | Euwallacea validus | XYLBVA | Scolytidae spp. (non‐European) [1SCOLF] |
Coleoptera Curculionidae Scolytinae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 18 | Fiorinia japonica | FIORJA | – |
Hemiptera Diaspididae |
Insects | Not regulated in the EU. |
| 19 |
Godronia zelleri (synonym: Atropellis pinicola) |
ATRPPC | Atropellis spp. [1ATRPG] |
Helotiales Dermateaceae |
Fungi | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 20 | Hemiberlesia pitysophila | HEBEPI | – |
Hemiptera Diaspididae |
Insects | Not regulated in the EU. |
| 21 | Heteroborips seriatus | XYLBSE | Scolytidae spp. (non‐European) [1SCOLF] |
Coleoptera Curculionidae Scolytinae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 22 | Lepidosaphes pineti | LEPSPT | – |
Hemiptera Diaspididae |
Insects | Not regulated in the EU. |
| 23 | Lepidosaphes pini | LEPSPN | – |
Hemiptera Diaspididae |
Insects | Not regulated in the EU. |
| 24 | Lepidosaphes piniphila | LEPSPH | – |
Hemiptera Diaspididae |
Insects | Not regulated in the EU. |
| 25 | Matsucoccus massonianae | MATSMS | – |
Hemiptera Matsucoccidae |
Insects | Not regulated in the EU. |
| 26 | Matsucoccus matsumurae | MATSRE | – |
Hemiptera Matsucoccidae |
Insects | Not regulated in the EU. |
| 27 | Monochamus alternatus | MONCAL | Monochamus spp. (non‐European populations) [1MONCG] |
Coleoptera Cerambycidae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 28 | Monochamus galloprovincialis | MONCGA | Monochamus spp. (non‐European populations) [1MONCG] |
Coleoptera Cerambycidae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 29 | Monochamus saltuarius | MONCSL | Monochamus spp. (non‐European populations) [1MONCG] |
Coleoptera Cerambycidae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 30 | Monochamus sutor | MONCSU | Monochamus spp. (non‐European populations) [1MONCG] |
Coleoptera Cerambycidae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 31 | Monochamus urussovi | MONCUR | Monochamus spp. (non‐European populations) [1MONCG] |
Coleoptera Cerambycidae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 32 | Mycosphaerella gibsoni | CERSPD | Pseudocercospora pini‐densiflorae (Hori & Nambu) Deighton [CERSPD] |
Mycosphaerellales Mycosphaerellaceae |
Fungi | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 33 | Parlatoria pinicola | PARLPC | – |
Hemiptera Diaspididae |
Insects | Not regulated in the EU. |
| 34 | Pestalotiopsis disseminata | PESTDI | – |
Amphisphaeriales Amphisphaeriaceae |
Fungi | Not regulated in the EU. |
| 35 | Pestalotiopsis microspora | PESTDC | – |
Amphisphaeriales Amphisphaeriaceae |
Fungi | Not regulated in the EU. |
| 36 | Pissodes nitidus | PISONI | Pissodes nitidus Roelofs [PISONI] |
Coleoptera Curculionidae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 37 | Pissodes punctatus | PISOPU | Pissodes punctatus Langor & Zhang [PISOPU] |
Coleoptera Curculionidae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 38 | Pissodes yunnanensis | PISOYU | Pissodes yunnanensis Langor & Zhang [PISOYU] |
Coleoptera Curculionidae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
| 39 | Popillia quadriguttata | POPIQU | – |
Coleoptera Scarabaeidae |
Insects | Not regulated in the EU. |
| 40 | Pyrrhoderma noxium | PHELNO | – |
Hymenochaetales Hymenochaetaceae |
Fungi | Not regulated in the EU. |
| 41 | Setoptus parviflorae | SETPPA | – |
Acarida Phytoptidae |
Mites | Not regulated in the EU. |
| 42 | Shirahoshizo patruelis | SHIRPA | – |
Coleoptera Curculionidae |
Insects | Not regulated in the EU. |
| 43 | Xylosandrus compactus | XYLSCO | Scolytidae spp. (non‐European) [1SCOLF] |
Coleoptera Curculionidae Scolytinae |
Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072. |
The assessment of Union quarantine pests was restricted to whether or not the applicant country implements specific measures specified in points 30 and 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 (see Section 6). For all remaining pests, the effectiveness of the risk mitigation measures applied to the commodity was evaluated and an EKE was performed (see Section 5).
5. Risk mitigation measures
For all selected pests, with the exception of Union quarantine pests for which specific measures are specified in points 30 and 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072, the Panel assessed the possibility that they could be present in the export nursery and assessed the probability that pest freedom of a consignment is achieved by the proposed risk mitigation measures.
The Panel evaluated the likelihood that the pest could be present in an export nursery by evaluating the possibility that P. parviflora and/or P. thunbergii plants in the export nursery are infested either by:
introduction of the pest from the environment surrounding the nursery;
introduction of the pest with new plants/seeds;
spread of the pest within the nursery.
The information used in the evaluation of the effectiveness of the risk mitigation measures is summarised in pest data sheets (see Appendix A).
5.1. Risk mitigation measures applied in China
With the information provided by the NPPO of China (Dossier Sections 2.0 and 4.0), the Panel summarised the risk mitigation measures (see Table 15) that are proposed in the production nursery.
Table 15.
Overview of proposed risk mitigation measures for Pinus parviflora bonsai plants grafted on Pinus thunbergii designated for export to the EU from China
| Number | Risk mitigation measure | Implementation in China |
|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export |
The bonsai plants are grown outdoor under protected by 40 mesh (0.4 mm) insect‐proof net. The net is removed in winter to prevent snow damage. There is no information on whether plants are protected with the net in the storage and packaging place of the export nursery before export. During the whole production stage, pots with the commodity are either kept on racks at 50 cm above the floor or on the ground. They are kept separately, not mixed with seedlings of other tree species. Mother plants of P. parviflora are grown in open field in the nursery. Before export the commodity is packed on hardened ground at the storage and packaging place of the export nursery. |
| 2 | Growing medium and its treatment |
Before use, the soil employed for seeding P. thunbergii to obtain rootstock is treated by using steam high temperature disinfection at the level of 100°C (Dossier Section 4.0). After transplanting P. thunbergii rootstock and grafting P. parviflora, only coconut coir, which is imported from abroad, is used as growing medium, which is also true for export (Dossier Section 5.0). Coconut coir is fibre powder of coconut shell, which is a kind of pure natural organic matter medium. It does not contain any soil. According to Dossier Section 5.0, the roots including the growing medium are soaked with chemicals, such as 2,000‐fold dilution of Avermectin (Abamectin) and 2,000‐fold dilution of tea saponin, in September. |
| 3 | Treatment of seeds | Seeds of P. thunbergii are soaked in mixture of Potassium Permanganate and Triadimefon before seeding. |
| 4 | Insecticide and acaricide treatments | Details on treatments with Cypermethrin, Fenitrothion, Folimat, trichlorfon, Avermectin, Pyridaben, Propargite are provided in Table 8. For soaking during repotting, 2,000‐fold dilution of tea saponin is used in addition to Avermectin. |
| 5 | Fungicide treatments | Details on treatments with Potassium Permanganate and Triadimefon mixture, Carbendazim, Dyson zinc wettable powder, Bordeaux mixture, Thiodiazole‐copper suspension, Triadimefon wettable powder are provided in Table 6 |
| 6 | Nematicide treatments | Details on treatments with Avermectin are provided in Table 5. |
| 7 | Herbicide treatments and weed management |
To control Cronartium spp. (non‐European), the elimination of tea and tea seeds and horse's stem within 500 m in the nursery and surrounding areas is performed by using sodium pentachlorophenol, two methyl four chloro, atrazine, non‐herbicide, herbicide, etc. (see Tabel 7). There is no information on whether weeding of pots is performed. |
| 8 | Official inspections during production |
The inspection and quarantine organisation will conduct official inspection on the export registered plantation base no less than twice every year. The inspection focuses on: surrounding environment and conditions of the plantation base, cultivated varieties and their quantities, diseases and pests occurrence and prevention and control records, purchase and delivery logbook, production cancelling after verification, as well as other items that should be supervised. Supervision records are properly kept. According to Dossier Section 2.0, for pests of concern for the EU, the inspection and quarantine organisation are expected to conduct surveillance at least six times a year at reasonable time intervals. The infested/infected plants are removed from the nursery. The nursery and its immediate vicinity areas (at least 2 km) are surveyed as follows: at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of 10% of the plants. |
| 9 | Official inspections and treatments before export |
Plants to be exported are identified at least half a year in advance. The production company should strictly inspect 100% of the plants intended for export. If diseases and pests are found on plants, these are treated in time and a record is kept. Roots are washed and the soil is inspected. The plants are repotted (the cultivation medium is fresh and unused) and taken to the export registered plantation base (Dossier Section 2.0). According to Dossier Section 5.0, 2 weeks before export, 2 cm of surface growing medium is removed from the pots and replaced by new coconut coir. Then the pots are soaked with 2,000‐fold dilution of Avermectin for 1.5 h. According to Dossier Section 2.0, each plant to be exported is inspected. Plants showing injury are discarded. If damage is found caused by pests about which EU is concerned, the plants are allowed to be exported only after quarantine treatments are performed and the plants meet the export requirements. In the absence of effective treatments, the whole batch of plants will be prohibited from being exported. Packaging materials for loading the commodity to be exported is new and clean. Labels should be placed on the packaging boxes, specifying information such product name, quantity, export batch number and nursery registration number. If wooden packaging materials are to be used, they will qualify after first being treated according to ISPM‐15 (FAO, 2018). The packaging materials for loading the plants to be exported should be clean and sanitary and should not be reused. The containers should be sealed and signed by the authority. Registration No. of the plantation base should be specified. This registration No. should be specified in the additional statement of the plant quarantine certificate. The packaging materials are stored in pest‐proof, mould‐proof and moisture‐proof locations. The commodity is packed during the daytime at the storage and packaging place of the export nursery. Before loading the boxes, the container is sprayed with pesticides. |
5.2. Evaluation of the proposed measures for the selected relevant pests including uncertainties
For each evaluated pest, the relevant risk mitigation measures acting on the pest were identified. Any limiting factors on the effectiveness of the measures were documented.
All the relevant information including the related uncertainties deriving from the limiting factors used in the evaluation are summarised in pest data sheets provided in Appendix A. Based on this information, for each selected relevant pest, an expert judgement is given for the likelihood of pest freedom taking into consideration the risk mitigation measures and their combination acting on the pest.
An overview of the evaluation of each relevant pest is given in the sections below (Sections 5.2.1–5.2.24). The outcome of the EKE regarding pest freedom after the evaluation of the proposed risk mitigation measures is summarised in Section 5.2.25.
5.2.1. Overview of the evaluation of Anomala testaceipes (Coleoptera, Scarabaeidae)
| Rating of the likelihood of pest freedom | Pest free with few exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,967 out of 10,000 plants |
9,986 out of 10,000 plants |
9,994 out of 10,000 plants |
9,998 out of 10,000 plants |
9,999.9 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
0.1 out of 10,000 plants |
2 out of 10,000 plants |
6 out of 10,000 plants |
14 out of 10,000 plants |
33 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest can be present in the province and in the surroundings of the nursery since some hosts are present. Adults can fly and they feed on needles of conifers. Infestation of the commodity could occur if the protecting net is broken or not completely sealed. Measures taken against the pest and their efficacy The protecting net is expected to have an effect on reducing risk of infestation with the pest, especially in the production base. The treatment of soaking of Avermectin is expected to kill the larvae that are present on the plants. Treatment consisting in removing of the upper part of the soil is expected to remove the larvae present in the soil. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Anomala testaceipes between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures No relevant shortcomings.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Grubs (Section A.9 in Appendix A).
5.2.2. Overview of the evaluation of Ceroplastes rubens (Hemiptera; Coccidae)
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,520 out of 10,000 plants |
9,733 out of 10,000 plants |
9,860 out of 10,000 plants |
9,938 out of 10,000 plants |
9,976 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
24 out of 10,000 plants |
62 out of 10,000 plants |
140 out of 10,000 plants |
267 out of 10,000 plants |
480 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity This scale insect can be present on host plants in the area where the nursery is located. Due to the biology, Ceroplastes rubens is a good candidate to be transported with the commodity because final stages (overwintering females) can go undetected when they are hidden in the lower parts of twigs and branches. The lack of obvious symptoms at low insect density makes the detection more difficult. Measures taken against the pest and their efficacy Measures taken against the pest are good but not enough to warrant the pest‐free status for the commodity. First, the net does not have a mesh that stops the first instars to go through. Second, the insecticide applications do not completely reach the scales as they are protected by the wax shell. Third, the inspections may not be successful when the insect density is very low and the signs of presence such as honeydew are scarce. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Ceroplastes rubens between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Ceroplastes rubens has been intercepted on bonsai plants of Ilex from China in 2018 (EUROPHYT, online) and on other tropical plants destined to the UK (Malumphy, 2010), the Netherlands (Jansen, 1995), Hungary (Fetykó and Kozár, 2012) and Germany (Schönfeld, 2015). Shortcomings of current measures/procedures Net protection is not fully effective, because crawlers can go through. Pesticides treatments are not targeted to the most sensitive stage (crawlers), so that the efficacy is limited as the other stages are protected by thick wax layer.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Ceroplastes rubens (Section A.3 in Appendix A).
5.2.3. Overview of the evaluation of Coleosporium asterum (Pucciniales; Coleosporiaceae)
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,550 out of 10,000 plants |
9,714 out of 10,000 plants |
9,848 out of 10,000 plants |
9,937 out of 10,000 plants |
9,977 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants |
23 out of 10,000 plants |
63 out of 10,000 plants |
152 out of 10,000 plants |
286 out of 10,000 plants |
450 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pathogen is known to be present in the Zhenjiang province. The presence of suitable telial hosts in the immediate surroundings of the nursery (within 1.5 km) makes the infection of the commodity by means of basidiospores possible. The pathogen may go undetected during inspection before export because an asymptomatic period of at least 6 months has been reported. Measures taken against the pest and their efficacy Fungicide treatments (most of active ingredients are systemic) are expected to reduce the likelihood of infection of the pathogen and the rate of colonisation of needles. The official inspections during production and before export are expected to have some effects in detecting the pathogen. However, an asymptomatic period of at least 6 months is reported during which infection cannot be detected visually. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii bonsai plants neither from China nor from other countries due to the presence of Coleosporium asterum between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Coleosporium asterum was intercepted on cut flowers and foliage of Solidago sp. from Kenya to United Kingdom in 2016 (EUROPHYT, online). Shortcomings of current measures/procedures No relevant shortcomings.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Coleosporium group (Section A.4 in Appendix A).
5.2.4. Overview of the evaluation of Coleosporium eupatorii (Pucciniales; Coleosporiaceae)
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,815 out of 10,000 plants |
9,902 out of 10,000 plants |
9,948 out of 10,000 plants |
9,976 out of 10,000 plants |
9,992 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants |
8 out of 10,000 plants |
24 out of 10,000 plants |
52 out of 10,000 plants |
98 out of 10,000 plants |
185 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pathogen is not known to be present in the Zhenjiang province, but it is reported from a neighbouring province. However, the presence of suitable telial hosts in the immediate surroundings of the nursery (within 1.5 km) combined with the long dispersal range of aeciospores and uredospores makes the infection of the commodity by means of basidiospores possible. The pathogen may go undetected during inspection before export because an asymptomatic period of at least 6 months has been reported. Measures taken against the pest and their efficacy Fungicide treatments (most of active ingredients are systemic) are expected to reduce the likelihood of infection of the pathogen and the rate of colonisation of needles. The official inspections during production and before export are expected to have some effects in detecting the pathogen. However, an asymptomatic period of at least 6 months is reported during which infection cannot be detected visually. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii bonsai plants neither from China nor from other countries due to the presence of Coleosporium eupatorii between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures No relevant shortcomings.
Main uncertainties
|
||||
This pest was evaluated in comparison to Coleosporium asterum as reference pest, as the pests share common characteristics (see Section 2.3.4 for the methodology).
For more details, see relevant pest data sheet on Coleosporium group (Section A.4 in Appendix A).
5.2.5. Overview of the evaluation of Coleosporium phellodendri (Pucciniales; Coleosporiaceae)
| Rating of the likelihood of pest freedom | Pest free with few exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,967 out of 10,000 plants |
9,986 out of 10,000 plants |
9,994 out of 10,000 plants |
9,998 out of 10,000 plants |
9,999.9 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants |
0.1 out of 10,000 plants |
2 out of 10,000 plants |
6 out of 10,000 plants |
14 out of 10,000 plants |
33 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pathogen is not known to be present in the Zhenjiang province. In addition, no suitable telial hosts are known to be present in the surroundings of the nursery, making infection of the commodity by means of basidiospores unlikely. The pathogen may go undetected during inspection before export because an asymptomatic period of 18 months has been reported. Measures taken against the pest and their efficacy Fungicide treatments (most of active ingredients are systemic) are expected to reduce the likelihood of infection of the pathogen and the rate of colonisation of needles. The official inspections during production and before export are expected to have some effects in detecting the pathogen. However, an asymptomatic period of at least 18 months is reported during which infection cannot be detected visually. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii bonsai plants neither from China nor from other countries due to the presence of Coleosporium phellodendri between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures No relevant shortcomings.
Main uncertainties
|
||||
This pest was evaluated in comparison to Coleosporium asterum as reference pest, as the pests share common characteristics (see Section 2.3.4 for the methodology).
For more details, see relevant pest data sheet on Coleosporium group (Section A.4 in Appendix A).
5.2.6. Overview of the evaluation of Crisicoccus pini (Hemiptera; Pseudococcidae)
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,516 out of 10,000 plants |
9,704 out of 10,000 plants |
9,820 out of 10,000 plants |
9,903 out of 10,000 plants |
9,959 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
41 out of 10,000 plants |
97 out of 10,000 plants |
180 out of 10,000 plants |
296 out of 10,000 plants |
484 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Due to the biology, Crisicoccus pini is a good candidate to be transported with the commodity because all stages of development can go undetected when they are hidden in the lower parts of twigs and branches. The lack of obvious symptoms at low insect density makes the detection difficult. Measures taken against the pest and their efficacy Measures taken against the pest are good but not enough to warrant the pest‐free status for the commodity. First, the net does not have a mesh that stops the first instars to go through. Second, the insecticide applications do not completely reach the hidden parts of the tree where the insects can be found. Third, the inspections are not successful when the insect density is very low and the signs of presence such as wax and honeydew are scarce. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii bonsai plants neither from China nor from other countries due to the presence of Crisicoccus pini between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). However, mealybugs (Pseudococcidae) were intercepted on bonsai plants from Japan in 2013 (EUROPHYT, online). From the description it seems likely they were C. pini. In addition, it has been hypothesised that the introductions in North America and Europe are associated with the trade of pine bonsai plants. Shortcomings of current measures/procedures Net protection is not fully effective, because crawlers can go through. Inspections may not be effective without destructive analysis of the trees.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Crisicoccus pini (Section A.5 in Appendix A).
5.2.7. Overview of the evaluation of Dendrolimus punctatus (Lepidoptera; Lasiocampidae)
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,835 out of 10,000 plants |
9,905 out of 10,000 plants |
9,951 out of 10,000 plants |
9,980 out of 10,000 plants |
9,994 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
6 out of 10,000 plants |
20 out of 10,000 plants |
49 out of 10,000 plants |
95 out of 10,000 plants |
165 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is known to be present in the Zhenjiang province, where it is an important pest causing outbreaks. Different hosts are present in the surroundings of the nursery. Adults of the pest are able to fly long distances, and young larvae can be dispersed by the wind. Infestation of the commodity could occur if the protecting net is broken, or during the winter time when it is removed. In addition, infestation could also occur by means of scions taken from infested mother plants. Measures taken against the pest and their efficacy The protecting net is expected to have an effect on reducing risk of infestation with Dendrolimus species, especially in the production base. Insecticide treatments (especially contact and ingestion pesticides) are expected to kill the larvae that are present on the plants. The official inspections during production and before export are expected to have some effects in detecting the presence of the pest. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii bonsai plants neither from China nor from other countries due to the presence of Dendrolimus punctatus between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures No relevant shortcomings.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Dendrolimus group (Section A.7 in Appendix A).
5.2.8. Overview of the evaluation of Dendrolimus spectabilis (Lepidoptera; Lasiocampidae)
This pest was evaluated in comparison to Dendrolimus punctatus as reference pest, as the pests share common characteristics, e.g. as the same genus. Dendrolimus spectabilis, Dendrolimus superans and Dendrolimus tabulaeformis are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,983 out of 10,000 plants |
9,991 out of 10,000 plants |
9,995 out of 10,000 plants |
9,998 out of 10,000 plants |
9,999.8 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
0.2 out of 10,000 plants |
2 out of 10,000 plants |
5 out of 10,000 plants |
9 out of 10,000 plants |
17 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is not known to be present in the Zhenjiang province, but human assisted spread cannot be excluded and the different Dendrolimus species could establish in the region of the nursery. Different hosts are present in the surroundings. Adults of the pests are able to fly long distances, and young larvae can be dispersed by the wind. Infestation of the commodity could occur if the protecting net is broken, or during the time when it is removed during the winter. In addition, infestation could also occur by means of scions taken from infested mother plants. Measures taken against the pest and their efficacy The protecting net is expected to have an effect on reducing risk of infestation with Dendrolimus species, especially in the production base. Insecticide treatments (especially contact and ingestion pesticides) are expected to kill the larvae that are present on the plants. The official inspections during production and before export are expected to have some effects in detecting the presence of the pest. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii bonsai plants neither from China nor from other countries due to the presence of Dendrolimus superans and D. tabulaeformis between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). However, two interceptions of D. spectabilis on P. thunbergii bonsais from Japan occurred in Germany in 2018 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures No relevant shortcomings.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Dendrolimus group (Section A.7 in Appendix A).
5.2.9. Overview of the evaluation of Dendrolimus superans (Lepidoptera; Lasiocampidae)
This pest was evaluated in comparison to Dendrolimus punctatus as reference pest, as the pests share common characteristics, e.g. as the same genus. Dendrolimus spectabilis, Dendrolimus superans and Dendrolimus tabulaeformis are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (See Section 2.3.4 for the methodology).
The overview of the evaluation can be found in Section 5.2.8.
For more details, see relevant pest data sheet on Dendrolimus group (Section A.7 in Appendix A).
5.2.10. Overview of the evaluation of Dendrolimus tabulaeformis (Lepidoptera; Lasiocampidae)
This pest was evaluated in comparison to Dendrolimus punctatus as reference pest, as the pests share common characteristics, e.g. as the same genus. Dendrolimus spectabilis, Dendrolimus superans and Dendrolimus tabulaeformis are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (See Section 2.3.4 for the methodology).
The overview of the evaluation can be found in Section 5.2.8.
For more details, see relevant pest data sheet on Dendrolimus group (Section A.7 in Appendix A).
5.2.11. Overview of the evaluation of Fiorinia japonica (Hemiptera; Diaspididae)
This pest was evaluated in comparison to Ceroplastes Ceroplastes rubens as reference pest, as the pests share common characteristics. Fiorinia japonica and Lepidosaphes pineti are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,638 out of 10,000 plants |
9,807 out of 10,000 plants |
9,893 out of 10,000 plants |
9,949 out of 10,000 plants |
9,983 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
17 out of 10,000 plants |
51 out of 10,000 plants |
107 out of 10,000 plants |
193 out of 10,000 plants |
362 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Fiorinia japonica is an invasive species with high reproductive potential. Although not known to be present in the area, it can be introduced. Pinus parviflora bonsais are potentially good hosts. Lepidosaphes pineti is a native species known being aggressive to pines potentially including P. parviflora. For both species, at low density the inspection may not be successful because scales are hidden between the needles and difficult to see. Measures taken against the pest and their efficacy Measures taken against the pest are good but not enough to warrant the pest‐free status for the commodity. First, the net does not have a mesh that stops the first instars to go through. Second, the insecticide applications do not completely reach the scales as they are protected by the wax shell. Third, the inspections are not successful when the insect density is very low. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Fiorinia japonica and Lepidosaphes pineti between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). However, Fiorinia japonica was intercepted on dwarf pine from Japan in USA (USDA, 1939). Shortcomings of current measures/procedures Net protection is not fully effective, because crawlers can go through. Pesticide treatments are not targeted to the most sensitive stage (crawlers), so that the efficacy is limited as the other stages are protected by thick wax layer. The inspections may not be successful when the insect density is very low.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Pine needle scales (Section A.13 in Appendix A).
5.2.12. Overview of the evaluation of Hemiberlesia pitysophila (Hemiptera; Diaspididae)
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,784 out of 10,000 plants |
9,882 out of 10,000 plants |
9,933 out of 10,000 plants |
9,966 out of 10,000 plants |
9,990 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
10 out of 10,000 plants |
34 out of 10,000 plants |
67 out of 10,000 plants |
118 out of 10,000 plants |
216 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The scale insect Hemiberlesia pitysophila is an invasive species with high reproductive potential. Although not known to be present in the area, it can be introduced. Pinus parviflora bonsais are potentially good hosts. At low density the inspection may not be successful because scales are hidden between the needles and difficult to see. Measures taken against the pest and their efficacy Measures taken against the pest are good but not enough to warrant the pest‐free status for the commodity. First, the net does not have a mesh that stops the first instars to go through. Second, the insecticide applications do not completely reach the scales as they are protected by the wax shell. Third, the inspections are not successful when the insect density is very low. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Hemiberlesia pitysophila between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures Net protection is not fully effective, because crawlers can go through. Pesticide treatments are not targeted to the most sensitive stage (crawlers), so that the efficacy is limited as the other stages are protected by thick wax layer. The inspections are not successful when the insect density is very low.
Main uncertainties
|
||||
This pest was evaluated in comparison to Ceroplastes rubens as reference pest, as the pests share common characteristics (see Section 2.3.4 for the methodology).
For more details, see relevant pest data sheet on Pine needle scales (Section A.13 in Appendix A).
5.2.13. Overview of the evaluation of Lepidosaphes pineti (Hemiptera; Diaspididae)
This pest was evaluated in comparison to Ceroplastes rubens as reference pest, as the pests share common characteristics. Fiorinia japonica and Lepidosaphes pineti are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overview of the evaluation can be found in Section 5.2.11.
For more details, see relevant pest data sheet on Pine needle scales (Section A.13 in Appendix A).
5.2.14. Overview of the evaluation of Lepidosaphes pini (Hemiptera; Diaspididae)
This pest was evaluated in comparison to Lepidosaphes pineti as reference pest, as the pests share common characteristics. Lepidosaphes pini, Lepidosaphes piniphila and Parlatoria pinicola are evaluated in a combined assessment, as these scale insects have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,624 out of 10,000 plants |
9,810 out of 10,000 plants |
9,909 out of 10,000 plants |
9,966 out of 10,000 plants |
9,992 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
8 out of 10,000 plants |
34 out of 10,000 plants |
91 out of 10,000 plants |
190 out of 10,000 plants |
376 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Lepidosaphes pini and L. piniphila are native species, not known to be present in the area, but well known to be associated with Pinus parviflora. Parlatoria pinicola is a native species, present in the area and putatively associated with P. parviflora. For all three species, at low density the inspection may not be successful because scales are hidden between the needles and difficult to see. Measures taken against the pest and their efficacy Measures taken against the pest are good but not enough to warrant the pest‐free status for the commodity. First, the net does not have a mesh that stops the first instars to go through. Second, the insecticide applications do not completely reach the scales as they are protected by the wax shell. Third, the inspections are not successful when the insect density is very low. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Lepidosaphes piniphila and Parlatoria pinicola between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Lepidosaphes pini was intercepted in the UK on bonsai plants of P. parviflora var. pentaphylla from Japan (Malumphy et al., 2012) and adult females of L. pini were intercepted in Taiwan on P. thunbergii from Japan (Chen et al., 2014). Shortcomings of current measures/procedures Net protection is not fully effective, because crawlers can go through. Pesticide treatments are not targeted to the most sensitive stage (crawlers), so that the efficacy is limited as the other stages are protected by thick wax layer. The inspections may not be successful when the insect density is very low.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Pine needle scales (Section A.13 in Appendix A).
5.2.15. Overview of the evaluation of Lepidosaphes piniphila (Hemiptera; Diaspididae)
This pest was evaluated in comparison to Lepidosaphes pineti as reference pest, as the pests share common characteristics. Lepidosaphes pini, Lepidosaphes piniphila and Parlatoria pinicola are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overview of the evaluation can be found in Section 5.2.14.
For more details, see relevant pest data sheet on Pine needle scales (Section A.13 in Appendix A).
5.2.16. Overview of the evaluation of Matsucoccus massonianae (Hemiptera; Matsucoccidae)
The evaluation describes the total risk of entry by Matsucoccus massonianae and Matsucoccus matsumurae, due to likely confusions or misclassification of these pests by typical identification standards (see Section 2.3.4 for the methodology).
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,556 out of 10,000 plants |
9,710 out of 10,000 plants |
9,835 out of 10,000 plants |
9,928 out of 10,000 plants |
9,983 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
17 out of 10,000 plants |
72 out of 10,000 plants |
165 out of 10,000 plants |
290 out of 10,000 plants |
444 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is known to be present in the Zhenjiang province. Different hosts are present in the surroundings of the nursery. Adult males of the pest can fly, crawlers are small enough to pass through the net, and the infection on the commodity can also occur by means of infected scions taken from mother plants. Measures taken against the pest and their efficacy Insecticide treatments (especially contact pesticides) are expected to reduce the likelihood of infection of the pest and the rate of infestation of plants, although only mobile stages will be reached by the products (systemic insecticides are not used). The official inspections during production and before export are expected to have some effects in detecting the presence of the pest, although only mobile stages will be easy to be detected. If the infestation is low, it will be very difficult to detect visually the presence of the pest on the commodity. Finally, the protecting net is expected to have some effect in reducing the flow of crawlers blowed by the wind. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Matsucoccus massonianae, M. matsumurae or Matsucoccus sp. between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures Net protection is not 100% effective, because crawlers can go through.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Pine bast scales (Section A.12 in Appendix A).
5.2.17. Overview of the evaluation of Matsucoccus matsumurae (Hemiptera; Matsucoccidae)
The evaluation is done together with Matsucoccus massonianae on the total risk of entry, due to likely confusions or misclassification of these pests by typical identification standards (see Section 2.3.4 for the methodology).
The overview of the evaluation can be found in Section 5.2.16.
For more details, see relevant pest data sheet on Pine bast scales (Section A.12 in Appendix A).
5.2.18. Overview of the evaluation of Parlatoria pinicola (Hemiptera; Diaspididae)
This pest was evaluated in comparison to Lepidosaphes pineti as reference pest, as the pests share common characteristics, e.g. as the same genus. Lepidosaphes pini, Lepidosaphes piniphila and Parlatoria pinicola are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overview of the evaluation can be found in Section 5.2.14.
For more details, see relevant pest data sheet on Pine needle scales (Section A.13 in Appendix A).
5.2.19. Overview of the evaluation of Pestalotiopsis disseminata (Amphisphaeriales; Amphisphaeriaceae)
Pestalotiopsis disseminata and Pestalotiopsis microspora are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,478 out of 10,000 plants |
9,657 out of 10,000 plants |
9,791 out of 10,000 plants |
9,895 out of 10,000 plants |
9,967 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants |
33 out of 10,000 plants |
105 out of 10,000 plants |
209 out of 10,000 plants |
343 out of 10,000 plants |
522 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Pinus parviflora is a confirmed host of the pathogen. The pathogen is present in the province and suitable hosts, including P. parviflora, are present in the surrounding of the nursery. The pathogen could enter the nursery and spread within the nursery mainly by spores disseminated by rain/wind and by contaminated pruning tools. Infected plants may remain asymptomatic hampering a prompt detection of the pathogen during inspections. Measures taken against the pest and their efficacy The fungicide treatments are expected to reduce the rates of infection. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Pestalotiopsis disseminata and P. microspora between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures Inspections are mainly devoted to plants displaying symptoms. As plants infected by the pathogen could remain asymptomatic for a long period of time, in order to detect the presence of the pathogen laboratory analysis would be necessary.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Pestalotiopsis group (Section A.11 in Appendix A).
5.2.20. Overview of the evaluation of Pestalotiopsis microspora (Amphisphaeriales; Amphisphaeriaceae)
Pestalotiopsis disseminata and Pestalotiopsis microspora are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overview of the evaluation can be found in Section 5.2.19.
For more details, see relevant pest data sheet on Pestalotiopsis group (Section A.11 in Appendix A).
5.2.21. Overview of the evaluation of Popillia quadriguttata (Coleoptera; Scarabaeidae)
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,988 out of 10,000 plants |
9,994 out of 10,000 plants |
9,997 out of 10,000 plants |
9,999 out of 10,000 plants |
9,999.9 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
0.1 out of 10,000 plants |
1 out of 10,000 plants |
3 out of 10,000 plants |
6 out of 10,000 plants |
12 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is reported to be widely distributed throughout China. The pest can be present in the surroundings of the nursery since many hosts are present. Adults can fly and some hosts can be found in the nursery. Infestation of the soil of bonsais could occur if the protecting net is broken or not completely sealed. Measures taken against the pest and their efficacy The protecting net is expected to have an effect on reducing risk of infestation with the pest, especially in the production base. The treatment of soaking of Avermectin is expected to kill the larvae that are present on the plants. Treatment consisting in removing of the upper part of the soil is expected to remove the larvae present in the soil. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Popillia quadriguttata between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Popillia sp. was intercepted in Germany on Cycas revoluta plants for planting already planted from Costa Rica in 1999 (EUROPHYT, online). Shortcomings of current measures/procedures No relevant shortcomings.
Main uncertainties
|
||||
This pest was evaluated in comparison to Anomala testaceipes as reference pest, as the pests share common characteristics, e.g. as being grubs (see Section 2.3.4 for the methodology).
For more details, see relevant pest data sheet on Grubs (Section A.9 in Appendix A).
5.2.22. Overview of the evaluation of Pyrrhoderma noxium (Hymenochaetales; Hymenochaetaceae)
| Rating of the likelihood of pest freedom | Pest free with few exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,960 out of 10,000 plants |
9,981 out of 10,000 plants |
9,990 out of 10,000 plants |
9,996 out of 10,000 plants |
9,999.3 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants |
0.7 out of 10,000 plants |
4 out of 10,000 plants |
10 out of 10,000 plants |
19 out of 10,000 plants |
40 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Based on the similarities of climate between the province where the nursery is located and south east Queensland (Australia), it cannot be excluded that the pathogen is present or could establish in the nursery or in the surrounding. Infection of plants through pruning and grafting could be possible should the pathogen colonise suitable hosts or substrates (stumps) either in the nursery or outside the nursery. Fungicide treatments may not be fully effective against the pathogen. Plants may remain asymptomatic hampering the prompt detection of the pathogen. Measures taken against the pest and their efficacy Specific measures against the pathogen are not taken. Fungicide treatments are not expected to be fully effective in preventing infections and reducing the colonisation of plants by the pathogen. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Pyrrhoderma noxium between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures There is no information whether they apply wound dressing to prevent infections.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Pyrrhoderma noxium (Section A.15 in Appendix A).
5.2.23. Overview of the evaluation of Setoptus parviflorae (Acarida; Phytoptidae)
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,114 out of 10,000 plants |
9,415 out of 10,000 plants |
9,654 out of 10,000 plants |
9,831 out of 10,000 plants |
9,936 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
64 out of 10,000 plants |
169 out of 10,000 plants |
346 out of 10,000 plants |
585 out of 10,000 plants |
886 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Setoptus parviflorae is an eriophyoid mite found for the first time on Pinus parviflora bonsai plants from Japan. It is also present naturally in China. A closely related species, S. semiornatum, was described and reported in the UK from P. parviflora bonsai. It is likely that S. parviflorae and S. semiornatum are the same species, but in order to clarify this a taxonomic revision is needed. Measures taken against the pest and their efficacy Measures taken against the pest are good but not enough to warrant the pest‐free status for the commodity. First, the net does not have a mesh that stops the mite to go through. Second, the acaricide applications do not completely reach the mites as they are protected in needle sheaths. Third, the inspections are not successful if microscope is not used. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Setoptus parviflorae between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Repeated interceptions and outbreaks in Europe on bonsai plants of P. parviflora from Japan are reported by Pye (2011) and NVWA (2020). Shortcomings of current measures/procedures Net protection is not effective, because mites can go through. Pesticides treatments are only partially effective as mites are most of the time hidden in needle sheaths. Microscope is not used during the inspections.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Setoptus parviflorae (Section A.16 in Appendix A).
5.2.24. Overview of the evaluation of Shirahoshizo patruelis (Coleoptera; Curculionidae)
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants |
9,933 out of 10,000 plants |
9,962 out of 10,000 plants |
9,981 out of 10,000 plants |
9,992 out of 10,000 plants |
9,997.5 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants |
2.5 out of 10,000 plants |
8 out of 10,000 plants |
19 out of 10,000 plants |
38 out of 10,000 plants |
67 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is known to be present in the Zhenjiang province. Different hosts are present in the surroundings of the nursery. Adults of the pest are able to fly short distances. Infestation of the commodity could occur if the protecting net is broken, or during the time when it is removed during the winter. All stages can be associated with the commodity as long as the bark is thick enough, either under the bark (eggs, larvae, pupae) or externally on the bark (adults). Measures taken against the pest and their efficacy The protecting net is expected to have an effect on reducing risk of infestation with Shirahoshizo patruelis. Insecticide treatments (especially contact and ingestion pesticides) are expected to kill the adults that are present on the plants. The official inspections during production and before export are expected to have some effects in detecting the presence of the pest. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Shirahoshizo patruelis between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online). Shortcomings of current measures/procedures The net is not strong enough against the adult mandibles. Adults can sneak under the net by walking on the ground. The detection is difficult because adults are hardly spotted on the bark.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Pine weevils (Section A.14 in Appendix A).
5.2.25. Outcome of Expert Knowledge Elicitation
Table 16 and Figure 4 show the outcome of the EKE regarding pest freedom after the evaluation of the proposed risk mitigation measures for all the evaluated pests.
Table 16.
Assessment of the likelihood of pest freedom following evaluation of proposed risk mitigation measures against selected relevant pests on Pinus parviflora bonsai plants grafted on Pinus thunbergii designated for export to the EU. In panel A, the median value for the assessed level of pest freedom for each pest is indicated by ‘M’, the 5% percentile is indicated by ‘L’ and the 95% percentile is indicated by ‘U’. The percentiles together span the 90% uncertainty range regarding pest freedom. The pest freedom categories are defined in panel B of the table
| Number | Group | Pest species | Sometimes pest free | More often than not pest free | Frequently pest free | Very frequently pest free | Extremely frequently pest free | Pest free with some exceptional cases | Pest free with few exceptional cases | Almost always pest free |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Insects | Anomala testaceipes | L | M | U | |||||
| 2 | Insects | Ceroplastes rubens | LM | U | ||||||
| 3 | Fungi | Coleosporium asterum | LM | U | ||||||
| 4 | Fungi | Coleosporium eupatori | L | M | U | |||||
| 5 | Fungi | Coleosporium phellodendri | L | M | L | |||||
| 6 | Insects | Crisicoccus pini | LM | U | ||||||
| 7 | Insects | Dendrolimus punctatus | L | M | U | |||||
| 8 | Insects | Dendrolimus spectabilis | L | MU | ||||||
| 9 | Insects | Dendrolimus superans | L | MU | ||||||
| 10 | Insects | Dendrolimus tabulaeformis | L | MU | ||||||
| 11 | Insects | Fiorinia japonica | LM | U | ||||||
| 12 | Insects | Hemiberlesia pitysophila | L | M | U | |||||
| 13 | Insects | Lepidosaphes pineti | LM | U | ||||||
| 14 | Insects | Lepidosaphes pini | L | M | U | |||||
| 15 | Insects | Lepidosaphes piniphila | L | M | U | |||||
| 16 | Insects | Matsucoccus massonianae | LM | U | ||||||
| 17 | Insects | Matsucoccus matsumurae | LM | U | ||||||
| 18 | Insects | Parlatoria pinicola | L | M | U | |||||
| 19 | Fungi | Pestalotiopsis disseminata | L | M | U | |||||
| 20 | Fungi | Pestalotiopsis microspora | L | M | U | |||||
| 21 | Insects | Popillia quadriguttata | L | MU | ||||||
| 22 | Fungi | Pyrrhoderma noxium | L | M | U | |||||
| 23 | Mites | Setoptus parviflorae | L | M | U | |||||
| 24 | Insects | Shirahoshizo patruelis | L | M | U | |||||
| PANEL A | ||||||||||
| Pest freedom category | Pest fee plants out of 10,000 | Legend of pest freedom categories | ||
|---|---|---|---|---|
| Sometimes pest free | ≤ 5,000 | L | Pest freedom category includes the elicited lower bound of the 90% uncertainty range | |
| More often than not pest free | 5,000–≤ 9,000 | M | Pest freedom category includes the elicited median | |
| Frequently pest free | 9,000–≤ 9,500 | U | Pest freedom category includes the elicited upper bound of the 90% uncertainty range | |
| Very frequently pest free | 9,500–≤ 9,900 | |||
| Extremely frequently pest free | 9,900–≤ 9,950 | |||
| Pest free with some exceptional cases | 9,950–≤ 9,990 | |||
| Pest free with few exceptional cases | 9,990–≤ 9,995 | |||
| Almost always pest free | 9,995–≤ 10,000 | |||
| PANEL B | ||||
Figure 4.
Elicited certainty (y‐axis) of the number of pest‐free Pinus parviflora bonsai plants grafted on Pinus thunbergii (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU from China for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
Figure 5 provides an explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the proposed risk mitigation measures for Pinus parviflora bonsai plants grafted on P. thunbergii designated for export to the EU based on the example of Setoptus parviflorae.
Figure 5.
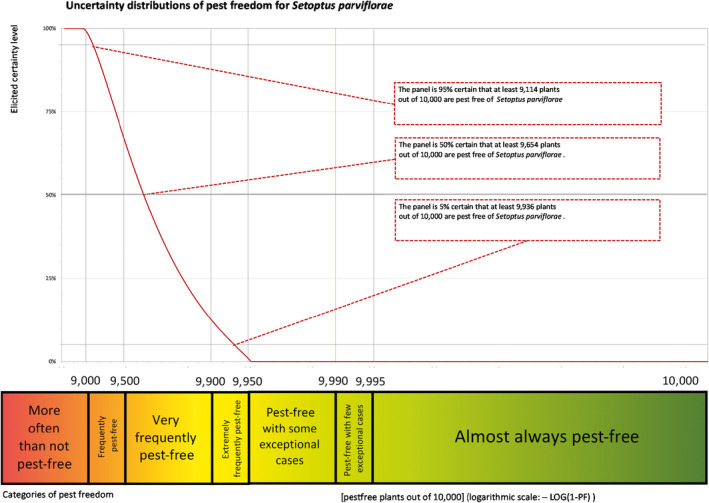
Explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the proposed risk mitigation measures for plants designated for export to the EU based on the example of Setoptus parviflorae
The Panel is 95% sure that:
-
–
9,114 or more bonsai plants per 10,000 will be free from Setoptus parviflorae,
-
–
9,478 or more bonsai plants per 10,000 will be free from Pestalotiopsis disseminata,
-
–
9,478 or more bonsai plants per 10,000 will be free from Pestalotiopsis microspora,
-
–
9,516 or more bonsai plants per 10,000 will be free from Crisicoccus pini,
-
–
9,520 or more bonsai plants per 10,000 will be free from Ceroplastes rubens,
-
–
9,550 or more bonsai plants per 10,000 will be free from Coleosporium asterum,
-
–
9,556 or more bonsai plants per 10,000 will be free from Matsucoccus massonianae,
-
–
9,556 or more bonsai plants per 10,000 will be free from Matsucoccus matsumurae,
-
–
9,624 or more bonsai plants per 10,000 will be free from Lepidosaphes pini,
-
–
9,624 or more bonsai plants per 10,000 will be free from Lepidosaphes piniphila,
-
–
9,624 or more bonsai plants per 10,000 will be free from Parlatoria pinicola,
-
–
9,638 or more bonsai plants per 10,000 will be free from Fiorinia japonica,
-
–
9,638 or more bonsai plants per 10,000 will be free from Lepidosaphes pineti,
-
–
9,784 or more bonsai plants per 10,000 will be free from Hemiberlesia pitysophila,
-
–
9,815 or more bonsai plants per 10,000 will be free from Coleosporium eupatorii,
-
–
9,835 or more bonsai plants per 10,000 will be free from Dendrolimus punctatus,
-
–
9,933 or more bonsai plants per 10,000 will be free from Shirahoshizo patruelis,
-
–
9,960 or more bonsai plants per 10,000 will be free from Pyrrhoderma noxium,
-
–
9,967 or more bonsai plants per 10,000 will be free from Anomala testaceipes,
-
–
9,967 or more bonsai plants per 10,000 will be free from Coleosporium phelodendri,
-
–
9,983 or more bonsai plants per 10,000 will be free from Dendrolimus spectabilis,
-
–
9,983 or more bonsai plants per 10,000 will be free from Dendrolimus superans,
-
–
9,983 or more bonsai plants per 10,000 will be free from Dendrolimus tabulaeformis,
-
–
9,988 or more bonsai plants per 10,000 will be free from Popillia quadriguttata.
6. Evaluation of the application of specific measures in China
Commission Implementing Regulation (EU) 2019/2072 specifies in points 30 and 31 of Annex VII measures which are required for the import of the commodity from China.
Table 17 provides special requirements for naturally or artificially dwarfed plants for planting other than seeds according to Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 including an assessment of whether or not the applicant country implements those measures. The information used in the assessment of 19 EU quarantine pests for which point 30 applies (see Section 4.1) is summarised in relevant pest data sheets (see Appendix A).
Table 17.
Special requirements for naturally or artificially dwarfed plants for planting other than seeds as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 including an assessment of whether or not the applicant country implements those measures. The Panel assumes that information on treatments required to be included in the phytosanitary certificate is provided by the applicant country according to the Article 71 of Regulation (EU) 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’
| Special requirements as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 | Implementation of the special requirements in China according to information provided in the Dossier | Relevance of special requirements for the assessed pests |
|---|---|---|
| ‘Official statement that: | – | – |
| a) the plants, including those collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime, | Yes | Relevant for all assessed pests. |
| b) the plants in the nurseries referred to in point (a) of this entry: | – | – |
| i) at least during the period referred to in point (a) of this entry: | – | – |
| — were potted, in pots which are placed on shelves at least 50 cm above ground, |
Yes, partially. Pots are also reported to be kept on the ground. |
Relevant only for Dendrolimus sibiricus |
| — have been subjected to appropriate treatments to ensure freedom from non‐European rusts, and the active ingredient, concentration and date of application of these treatments has been mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’. |
Yes Treatments are appropriate. They are expected to reduce the likelihood of infection of the pathogens and the rate of colonisation of plant tissues, although it is uncertain if freedom from non‐European rusts could be reachable. Treatments used are listed in Table 6.
Uncertainties:
|
Relevant only for Cronartium spp. (non‐European) |
| — have been officially inspected at least six times a year at appropriate intervals for the presence of Union quarantine pests of concern in accordance with Regulation (EU) No 2016/2031, and these inspections have also been carried out on plants in the immediate vicinity of the nurseries referred to in point (a) of this entry, at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus, | Yes |
Relevant for all assessed pests. However, uncertainties were identified for following pests (see the relevant pest data sheets in Appendix A):
|
| — have been found free, in these inspections, from the relevant Union quarantine pests of concern as specified in the previous indent, infested plants have been removed and the remaining plants, where appropriate, have been effectively treated, and have been held for an appropriate period and inspected to ensure freedom from such pests, | Yes |
Relevant for all assessed pests. However, uncertainties were identified for following pests (see the relevant pest data sheets in Appendix A):
|
| — have been planted in either an unused artificial growing medium or in a natural growing medium, which has been treated by fumigation or by appropriate heat treatment and has been of any Union quarantine pests, | Yes | Relevant only for Dendrolimus sibiricus. |
| — have been kept under conditions which ensure that the growing medium has been maintained free from Union quarantine pests and within 2 weeks prior to dispatch, have been: | Yes |
Relevant only for Dendrolimus sibiricus. However, uncertainties were identified (see the relevant pest data sheet in Appendix A) |
| — shaken and washed with clean water to remove the original growing medium and kept bare rooted, or | – | – |
| — shaken and washed with clean water to remove the original growing medium and replanted in growing medium which meets the conditions laid down in (i) fifth indent, or | – | – |
| — subjected to appropriate treatments to ensure that the growing medium is free from Union quarantine pests, and the active ingredient, concentration and date of application of these treatments have been indicated on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/ 2031 under the rubric ‘Disinfestation and/or disinfection treatment’. | Yes | Relevant only for Dendrolimus sibiricus. |
| ii) were packed in closed containers which have been officially sealed and bear the registration number of the registered nursery, and this number has been indicated under the rubric ‘Additional declaration’ on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, enabling the consignments to be identified.’ | Yes | Relevant for all assessed pests. |
Based on the information provided by the NPPO of China, the Panel considers that China applies the relevant measures as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 with the exception of requirement specified in letter (b) (i) second indent. That requirement is not completely fulfilled because, as an alternative, the placement of pots on the ground is also mentioned.
Point 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 specifies special requirements for Plants of Pinales, other than fruit and seeds as follows:
‘Official statement that the plants have been produced in a place of production free from Pissodes cibriani O'Brien, Pissodes fasciatus Leconte, Pissodes nemorensis Germar, Pissodes nitidus Roelofs, Pissodes punctatus Langor & Zhang, Pissodes strobi (Peck), Pissodes terminalis Hopping, Pissodes yunnanensis Langor & Zhang and Pissodes zitacuarense Sleeper.’
With respect to the point 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072, the Panel noted that from the above listed Pissodes species only Pissodes nemorensis, Pissodes nitidus, Pissodes punctatus, Pissodes strobi and Pissodes yunnanensis are associated with Pinus parviflora and/or Pinus thunbergii, and that Pissodes nemorensis and Pissodes strobi are not known to be present in China (see Appendix E).
According to information in Dossier Section 5.0, Pissodes nitidus, Pissodes punctatus and Pissodes yunnanensis are absent in the production area and in the Zhenjiang province. The information sources for this statement are (i) China National pest quarantine information system (https://www.pestchina.com/SitePages/Home.aspx), (ii) Research institutions, universities, scientific societies, scientific and trade Journals and (iii) Crop Protection Compendium (https://cabi.org/cpc) (Dossier Section 5.0). In addition, in Dossier Section 4.0, Pissodes spp. (non‐European) are indicated as target pests for which a targeted survey was performed to demonstrate pest freedom of the area.
Based on the information provided by the NPPO of China, the Panel considers that China applies the relevant measures for Pissodes cibriani, Pissodes fasciatus, Pissodes nemorensis, Pissodes nitidus, Pissodes punctatus, Pissodes strobi, Pissodes terminalis, Pissodes yunnanensis and Pissodes zitacuarense as specified in Point 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072.
7. Conclusions
There are 43 pests identified to be present in China and considered to be potentially associated with bonsai plants of Pinus parviflora grafted on Pinus thunbergii imported from China and relevant for the EU.
For 24 of these pests (Anomala testaceipes, Ceroplastes rubens, Coleosporium asterum, Coleosporium eupatorii, Coleosporium phellodendri, Crisicoccus pini, Dendrolimus punctatus, Dendrolimus spectabilis, Dendrolimus superans, Dendrolimus tabulaeformis, Fiorinia japonica, Hemiberlesia pitysophila, Lepidosaphes pineti, Lepidosaphes pini, Lepidosaphes piniphila, Matsucoccus massonianae, Matsucoccus matsumurae, Parlatoria pinicola, Pestalotiopsis disseminata, Pestalotiopsis microspora, Popillia quadriguttata, Pyrrhoderma noxium, Setoptus parviflorae and Shirahoshizo patruelis), the likelihood of the pest freedom after the evaluation of the proposed risk mitigation measures for bonsai plants of Pinus parviflora grafted on Pinus thunbergii designated for export to the EU was estimated.
For Anomala testaceipes, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘pest free with few exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,967 and 10,000 plants per 10,000 will be free from A. testaceipes.
For Ceroplastes rubens, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,520 and 10,000 plants per 10,000 will be free from C. rubens.
For Coleosporium asterum, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,550 and 10,000 plants per 10,000 will be free from C. asterum.
For Coleosporium eupatorii, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,815 and 10,000 plants per 10,000 will be free from C. eupatorii.
For Coleosporium phelodendri, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘pest free with few exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,967 and 10,000 plants per 10,000 will be free from C. phelodendri.
For Crisicoccus pini, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,516 and 10,000 plants per 10,000 will be free from C. pini.
For Dendrolimus punctatus, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,835 and 10,000 plants per 10,000 will be free from D. punctatus.
For Dendrolimus spectabilis, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,983 and 10,000 plants per 10,000 will be free from D. spectabilis.
For Dendrolimus superans, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,983 and 10,000 plants per 10,000 will be free from D. superans.
For Dendrolimus tabulaeformis, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,983 and 10,000 plants per 10,000 will be free from D. tabulaeformis.
For Fiorinia japonica, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,638 and 10,000 plants per 10,000 will be free from F. japonica.
For Hemiberlesia pitysophila, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,784 and 10,000 plants per 10,000 will be free from H. pitysophila.
For Lepidosaphes pineti, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,638 and 10,000 plants per 10,000 will be free from L. pineti.
For Lepidosaphes pini, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,624 and 10,000 plants per 10,000 will be free from L. pini.
For Lepidosaphes piniphila, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,624 and 10,000 plants per 10,000 will be free from L. piniphila.
For Matsucoccus massonianae and Matsucoccus matsumurae in total, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,556 and 10,000 plants per 10,000 will be free from M. massonianae and M. matsumurae in total.
For Parlatoria pinicola, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,624 and 10,000 plants per 10,000 will be free from P. pinicola.
For Pestalotiopsis disseminata, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,478 and 10,000 plants per 10,000 will be free from P. disseminata.
For Pestalotiopsis microspora, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,478 and 10,000 plants per 10,000 will be free from P. microspora.
For Popillia quadriguttata, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,988 and 10,000 plants per 10,000 will be free from P. quadriguttata.
For Pyrrhoderma noxium, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘pest free with few exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,960 and 10,000 plants per 10,000 will be free from P. noxium.
For Setoptus parviflorae, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘frequently pest free’ to ‘extremely frequently pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,114 and 10,000 plants per 10,000 will be free from S. parviflorae.
For Shirahoshizo patruelis, the likelihood of pest freedom for bonsai plants following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,933 and 10,000 plants per 10,000 will be free from S. patruelis.
For 19 pests which are quarantine in the EU (Bursaphelenchus xylophilus, Cronartium coleosporioides, Cronartium orientale, Cronartium quercuum, Dendrolimus sibiricus, Euwallacea interjectus, Euwallacea validus, Godronia zelleri. Heteroborips seriatus, Monochamus alternatus, Monochamus galloprovincialis, Monochamus saltuarius, Monochamus sutor, Monochamus urussovi, Mycosphaerella gibsonii, Pissodes nitidus, Pissodes punctatus, Pissodes yunnanensis and Xylosandrus compactus), the implementation of specific measures defined in point 30 and 31 of the Commission Implementing Regulation (EU) 2019/2072 was evaluated.
Based on the information provided by the NPPO of China, the Panel considers that China applies the relevant measures as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 with the exception of requirement specified in letter (b) (i) second indent. That requirement is not completely fulfilled because, as an alternative, the placement of pots on the ground is also mentioned.
Based on the information provided by the NPPO of China, the Panel considers that China applies the relevant measures for Pissodes cibriani, Pissodes fasciatus, Pissodes nemorensis, Pissodes nitidus, Pissodes punctatus, Pissodes strobi, Pissodes terminalis, Pissodes yunnanensis and Pissodes zitacuarense as specified in Point 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072.
Abbreviations
- CABI
Centre for Agriculture and Bioscience International
- EKE
Expert Knowledge Elicitation
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- ISPM
International Standards for Phytosanitary Measures
- NPPO
National Plant Protection Organisation
- PLH
Plant Health
- PRA
Pest Risk Assessment
- RNQPs
Regulated Non‐Quarantine Pests
Glossary
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017).
- Measures
Control (of a pest) is defined in ISPM 5 (FAO, 2017) as ‘Suppression, containment or eradication of a pest population’ (FAO, 1995).Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk mitigation measures that do not directly affect pest abundance.
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017).
- Protected zone
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017).
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017).
- Risk mitigation measure
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A risk mitigation measure may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017).
Appendix A – Data sheets of pests selected for further evaluation
A.1. Ambrosia and bark beetles (Euwallacea interjectus, E. validus, Heteroborips seriatus and Xylosandrus compactus)
A.1.1. Organism information
A.1.1.1. Euwallacea interjectus and E. validus
| Taxonomic information |
1. Euwallacea interjectus Current valid scientific name: Euwallacea interjectus Synonyms: Xyleborus interjectus, Xyleborus pseudovalidus Name used in the EU legislation: Listed as EU‐quarantine pest as Scolytidae spp. (non‐European) [1SCOLF] Order: Coleoptera Family: Curculionidae Subfamily: Scolytinae Common name: – Name used in the Dossier: Xyleborus interjectus 2. Euwallacea validus Current valid scientific name: Euwallacea validus Synonyms: Xyleborus validus Name used in the EU legislation: Listed as EU‐quarantine pest as Scolytidae spp. (non‐European) [1SCOLF] Order: Coleoptera Family: Curculionidae Subfamily: Scolytinae Common name: – Name used in the Dossier: Xyleborus validus |
|
| Group | Insects | |
| EPPO code |
XYLBIN: Euwallacea interjectus XYLBVA: Euwallacea validus |
|
| Regulated status |
Euwallacea interjectus and E. validus are listed in Annex II/A of Commission Implementing Regulation (EU) 2019/2072 as Scolytidae spp. (non‐European) [1SCOLF]. The pests are not regulated anywhere in the world neither listed by EPPO. |
|
| Pest status in China |
Euwallacea interjectus and E. validus are present in China. Euwallacea interjectus is present in Guangdong, Hunan, Sichuan, Yunnan (EPPO, 2020; Dossier Section 2.0), Shanghai (Wang et al., 2021), Tibet (Xizang), Zhejiang (EPPO, 2020), Anhui, Fujian, Gansu, Guizhou, Hainan, Hubei and Sichuan (Bright, 2021). Euwallacea validus is present in Anhui, Fujian (EPPO, 2020; Dossier Section 2.0), Yunnan (EPPO, 2020), Jiangsu (Chang et al., 2013) and Hunan (Bright, 2021). |
|
| Pest status in the EU | Euwallacea interjectus and E. validus are absent in the EU (EFSA PLH Panel, 2020a; EPPO, 2020). | |
| Host status on Pinus parviflora and P. thunbergii |
Euwallacea interjectus is reported on Pinus massoniana (EPPO, 2020; Atkinson, online). There is no information on whether E. interjectus can attack also Pinus parviflora and P. thunbergii. Euwallacea validus is reported on Pinus parviflora and P. thunbergii (EPPO, 2020; Atkinson, online). |
|
| PRA information |
Available Pest Risk Assessment:
–EPPO Study on the risk of bark and ambrosia beetles associated with imported non‐coniferous wood (EPPO, 2020). |
|
| Other relevant information for the assessment | ||
| Biology |
Euwallacea interjectus and E. validus are ambrosia beetles native to Asia. They were introduced into North America in 1970s and they are widely distributed in many US States (Cognato et al., 2015; EPPO, 2020). In 2019 Euwallacea interjectus was introduced also in Argentina (Landi et al., 2019). Euwallacea interjectus is associated with three fungal species: Ceratocystis ficicola, Verticillium nonalfalfae and AF‐3 of ambrosia Fusarium clade (EPPO, 2020). Females of Euwallacea interjectus are approximately 3.65–4.00 mm long (Maiti and Nivedita, 2004). They usually mate with their male siblings inside the mother galleries before emergence (Kajii et al., 2013), but are also able to lay non‐fertilised eggs producing only males (arrhenotokous parthenogenesis) (EPPO, 2020). Males cannot fly and die usually soon after the copulation with siblings. Only females fly, disperse and invade tree trunks near the ground (Kajii et al., 2013). Euwallacea interjectus infests mostly dead and dying trees, but it was also reported to attack healthy trees of Ficus carica in Japan (Kajii et al., 2013). Colonised trunks are occupied by several and subsequent generations of the beetle for few years until they are no longer suitable for insect reproduction (Kajii et al., 2013). No general information on the size of attacked stems and branches can be found. Only information was reported in the study by Kajii et al. (2013), where basal stems of Ficus carica with diameters of 14 and 29 cm were attacked by E. interjectus. There is no information on the number of generations per year. Euwallacea validus is associated with fungal species: AF‐4 of the ambrosia Fusarium clade (Fusarium oligoseptatum), Raffaelea subfusca, Graphium sp., Ceratocystis ficicola and Verticillium nonalfalfae (Aoki et al., 2018; EPPO, 2020). There is no information on precise size of E. validus. But as Euwallacea spp., it can be assumed that it will measure approximately between 2.8 and 5.7 mm (Smith and Hulcr, 2015). Euwallacea validus attacks stressed and dying trees, or trees that recently died (Berger, 2017). Euwallacea validus usually has one generation per year (Berger, 2017) and it is attracted to ethanol and conophthorin (Ranger et al., 2010). No specific information on biological cycle of E. validus was found, but a general ambrosia beetle biology is expected. Being Xyleborini, the species is inbreeder and haplodiploid. Females bore into branches or trunks of susceptible hosts. They excavate galleries in the wood, introduce ambrosia fungus and lay eggs to produce a brood. Beetle larvae feed on the growing fungus, not the wood. As a result of an oviposition staggered over time, all the pre‐adult’s development stages can be found together (eggs, larvae and pupae). Females remain with their offspring until larvae reach maturity. Males are small and flightless. Males remain within the gallery, where die after mating with siblings. New females usually mate with their brothers before emerging to attack a new host. Females are able to lay eggs and produce a brood even if they have not copulated and are not fertilised (parthenogenesis) (EPPO, 2020). There are no specific data on the flight distance and on the size of attacked stems and branches for both E. interjectus and E. validus. According to EPPO (2020), the main pathways of entry are: wood, wood packaging material, wood chips, hogwood, processing wood residues and possibly plants for planting (except seeds) and cut branches. Euwallacea interjectus and E. validus are frequently intercepted in logs, timber and wooden packaging worldwide (EPPO, 2020). There is limited evidence of damage, Euwallacea interjectus massively attacks water stressed poplar trees in Argentina (Landi et al., 2019), and it contributed to fig wilt as a vector of fungi Ceratocystis ficicola in Japan (Kajii et al., 2013). Kasson et al. (2015) indicated that E. validus could be linked to the transmission of Verticillium nonalfalfae, which is causing severe vascular wilt disease to several plants and crops. |
|
| Symptoms | Main type of symptoms |
Main symptoms caused by E. interjectus and the associated fungi on the fig trees in Japan were small and rounded entry holes, galleries in wood (but not under bark), white cylinders of frass emitted from the penetration holes, dark brown discoloration of the sapwood, poor shoot elongation, wilting of leaves and shoots, defoliation and death of trees (Kajii et al., 2013). In Shanghai, on poplars, the symptoms of infestation were wilting and decline of trees, noodle‐like frass extrusions, gallery entrances and wood‐boring dust on the base of the stem (Wang et al., 2021). No specific information on symptoms caused by E. validus was found but they are probably similar to other ambrosia beetles. |
| Presence of asymptomatic plants | No specific information on the presence of asymptomatic plants was found. Similarly, like other ambrosia beetles, such as E. fornicatus, initial phases of infestation are associated with few external symptoms. While there is no visible injury in the bark at early stage of colonisation, frass is produced and examination of the wood under the infested spot bored by the beetle, reveals the brownish staining of the xylem and necrosis caused by the fungus (Mendel et al., 2012). | |
| Confusion with other pests | Misidentification can occur between E. validus and E. interjectus due to their very similar morphology (EPPO, 2020). Morphological description and molecular identification are used in order to distinguish these species between each other. | |
| Host plant range |
Hosts of E. interjectus are Acer negundo, Artocarpus integrifolia, Bombax ceiba, Bombax insigne, Castanopsis indica, Delonix elata, Erythrina sp., Euphorbia royleana, Ficus sp., Garuga pinnata, Gmelina arborea, Hevea brasiliensis, Hymenodictyon orixense, Koelreuteria paniculata, Kydia calycina, Macaranga denticulata, Machilus sp., Maclura cochinchinensis, Mangifera indica, Nauclea orientalis, Neolamarckia cadambae, Odina wodier, Pinus massoniana, Populus sp., Pterocarpus marsupium, Pterocymbium tinctorium, Pterygota alata, Shorea assamica, S. robusta, Spondias mangifera, Sterculia villosa, Tectona grandis, Terminalia bellirica, T. myriocarpa, T. nudiflora, Theobroma cacao, Wisteria sp. and Xylia xylocarpa (Samuelson, 1981; EPPO, 2020). Hosts of E. validus are Abies firma, Ailanthus altissima, Aphananthe aspera, Carpinus tschonoskii, Castanea crenata, Celtis sinensis, Chamaecyparis obtusa, Cleyera japonica, Cryptomeria japonica, Cunninghamia lanceolata, Dalbergia hupeana, Fagus japonica var. multinervis, Fagus sp., Ficus carica, Juglans sp., Machilus sp., Magnolia obovata, Mallotus japonicus, Phellodendron amurense, Pinus densiflora, P. massoniana, P. parvifolia, P. sylvestris, P. taiwanensis, P. thunbergii, Populus deltoides, P. glandulosa, Prunus serrulata, Quercus grosseserrata, Q. velutina, Tilia amurensis, Tsuga sieboldii, Ulmus pumila and Zelkova serrata (EPPO, 2020). |
|
| Reported evidence of impact | Euwallacea interjectus and E. validus are EU quarantine pests. | |
| Evidence that the commodity is a pathway | According to EPPO (2020), Euwallacea species can possibly travel with plants for planting. Therefore, the commodity (i.e., bonsai plants) is expected to be a pathway for Euwallacea species. | |
| Surveillance information |
No surveillance information for this pest is currently available from China. Nevertheless, non‐European scolytids are included in a list of target pests (Dossier Section 4.0) for which monitoring activities (pheromone traps, light traps, etc.) are performed, together with three times a year inspections and samplings to collect insects on host plants in the survey area. There is no information on whether the pests have ever been found in the nursery or its surrounding environment. |
|
A.1.1.2. Heteroborips seriatus
| Taxonomic information |
Current valid scientific name: Heteroborips seriatus Synonyms: Xyleborus seriatus, Xyleborus orientalis, Xyleborus orientalis aceris, Xyleborus orientalis kalopanacis, Xyleborus perorientalis, Xyleborus septentrionalis, Xyleborus todo Name used in the EU legislation: Listed as EU‐quarantine pest as Scolytidae spp. (non‐European) [1SCOLF] Order: Coleoptera Family: Curculionidae Subfamily: Scolytinae Common name: – Name used in the Dossier: Xyleborus seriatus |
| Group | Insects |
| EPPO code | XYLBSE |
| Regulated status |
Heteroborips seriatus is listed in Annex II/A of Commission Implementing Regulation (EU) 2019/2072 as Scolytidae spp. (non‐European) [1SCOLF]. The pest is not regulated anywhere in the world neither listed by EPPO. |
| Pest status in China | Heteroborips seriatus is present in China in Shaanxi (Mandelshtam et al., 2019), Shanxi and Sichuan (Hoebeke and Rabaglia, 2008; Mandelshtam et al., 2019). |
| Pest status in the EU | Heteroborips seriatus is absent in the EU (Hoebeke and Rabaglia, 2008; Mandelshtam et al., 2019; EFSA PLH Panel, 2020a; Atkinson, online). |
| Host status on Pinus parviflora and P. thunbergii | Pinus parviflora and P. thunbergii are hosts of H. seriatus (Hoebeke and Rabaglia, 2008). |
| PRA information |
Available Pest Risk Assessment: – Scientific opinion on the pest categorisation of non‐EU Scolytinae of coniferous hosts (EFSA PLH Panel, 2020b). |
| Other relevant information for the assessment | |
| Biology |
Heteroborips seriatus is a bark beetle native to Asia. It is present in China, Japan, North and South Korea, Taiwan and Russian Far East. It was introduced to US (Massachusetts) in 2005 (Hoebeke and Rabaglia, 2008). It is now present in Maine, Massachusetts, Pennsylvania, Vermont (Atkinson, online), New Hampshire (Dodds and DiGirolomo, 2020), Canada (Nova Scotia) and Alaska (Webster et al., 2020). It is associated with unknown fungus, photographed and described by Nakashima et al. (1992) as oval or sausage‐shaped fungi. Based on DNA sequencing and morphological diagnostic characters, Xyleborus seriatus was renamed to H. seriatus, because the species is not related to genus Xyleborus (Mandelshtam et al., 2019). Females are reddish brown, 1.9–2.5 mm long (Hoebeke and Rabaglia, 2008). Males occur very rarely, they are flat, yellow and very hairy (Mandelshtam et al., 2018). According to Nakashima et al. (1992), females bore galleries under the bark, which are approximately 1 mm wide and 20 mm long. The place of the gallery (communal galleries and rooms) is under the bark; it does not reach sapwood. Only females take care of their brood (Nakashima et al., 1992). Females, larvae and pupae are found together in communal chambers/rooms (Hoebeke and Rabaglia, 2008). Gallery systems and boring behaviour of H. seriatus are similar to that of true bark beetles. Heteroborips seriatus occurs symbiotically with fungi that are closely related to those associated with typical bark beetles (Hoebeke and Rabaglia, 2008). There are no specific data on number of generations, the flight distance and on the size of attacked stems and branches for H. seriatus. According to EPPO (2020), the main pathways of entry for scolytides are: wood (including roundwood with or without bark, sawn wood, wood chips, hogwood, processing wood residues and wood packaging material) and possibly plants for planting (except seeds) and cut branches. |
| Symptoms | Main type of symptoms | No specific information on symptoms caused by H. seriatus was found but they are probably similar to other bark beetles. Visible symptoms include wilting of foliage on terminal ends of branches and twig, terminal dieback, entry holes on the affected trunk and branches. |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. Similarly, like other scolytids initial phases of infestation are associated with few external symptoms. While there is no visible injury in the bark at early stage of colonisation, frass is produced (Mendel et al., 2012). | |
| Confusion with other pests |
Infestation symptoms recorded in trees are not specific to H. seriatus and may be due to infestation by other bark beetles of similar size and biology. Heteroborips seriatus can be confused with other Heteroborips and Xyleborus species. It is very similar to Heteroborips cryptographus and H. indicus (Smith et al., 2020). A morphological (Hoebeke and Rabaglia, 2008) or molecular analysis is needed in order to distinguish them. |
|
| Host plant range |
Conifer hosts of H. seriatus are Abies sachalinensis, Chamaecyparis spp., Cryptomeria japonica, Larix kaempferi, Picea jezoensis, Picea ajanensis, Pinus armandii, P. parviflora, P. pentaphylla var. himekomaisu, P. tabuliformis, P. thunbergii, Thuja standishii, Tsuga spp. and Taxus (Wood and Bright, 1992; Hoebeke and Rabaglia, 2008). Broadleaves hosts are Acer rufinerve, Aesculus turbinata, Alnus incana, Alnus incana var. glauca, Betula spp., Carpinus tschonoskii, Castanopsis sp., Cleyera japonica, Fagus crenata, Kalopanax septemlobus, Mallotus japonicus, Prunus sp., Quercus spp., Rhus orientalis, Schima sp. and Tilia japonica (Wood and Bright, 1992; Hoebeke and Rabaglia, 2008). |
|
| Reported evidence of impact | Heteroborips seriatus is EU quarantine pest. | |
| Evidence that the commodity is a pathway | According to EPPO (2020), bark beetles can possibly travel with plants for planting. Therefore, the commodity (i.e., bonsai plants) is expected to be a pathway for H. seriatus. | |
| Surveillance information |
No surveillance information for this pest is currently available from China. Nevertheless, non‐European scolytids are included in a list of target pests (Dossier Section 4.0) for which monitoring activities (pheromone traps, light traps, etc.) are performed, together with three times a year inspections and samplings to collect insects on host plants in the survey area. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. |
|
A.1.1.3. Xylosandrus compactus
| Taxonomic information |
Current valid scientific name: Xylosandrus compactus Synonyms: Xyleborus compactus, Xyleborus morstatti, Xylosandrus morstatti Name used in the EU legislation: Listed as EU‐quarantine pest as Scolytidae spp. (non‐European) [1SCOLF] Order: Coleoptera Family: Curculionidae Subfamily: Scolytinae Common name: black coffee twig borer, black twig borer Name used in the Dossier: – |
| Group | Insects |
| EPPO code | XYLSCO |
| Regulated status |
Xylosandrus compactus is listed in Annex II/A of Regulation (EU) 2019/2072 as Scolytidae spp. (non‐European) [1SCOLF]. The pest is quarantine in Israel and Morocco. It is on A1 list of Chile and OIRSA (Organismo Internacional Regional de Sanidad Agropecuaria – countries: Belize, Costa Rica, Dominican Republic, El Salvador, Guatemala, Honduras, Mexico, Nicaragua and Panama) (EPPO, online_a). |
| Pest status in China | Xylosandrus compactus is present in China in Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hubei, Hunan, Jiangsu, Jiangxi, Sichuan, Xianggang, Yunnan and Zhejiang (Smith et al., 2020; EPPO, online_b). |
| Pest status in the EU | Xylosandrus compactus is present in France (Alpes‐Maritimes, Corsica, Provence‐Alpes‐Côte‐d’Azu and Var), Greece, Italy (Campania, Emilia Romagna, Lazio, Liguria, Lombardy, Sicily, Toscany and Veneto) and transient under eradication in Spain (Catalonia, Mallorca) (Faccoli, 2021; EPPO, online_b). In May 2021, it has been found in Malta and Gozo on Ceratonia siliqua during pest official survey (EUROPHYT Outbreaks database, online). |
| Host status on Pinus parviflora and P. thunbergii |
Pinus spp. is reported as a host of X. compactus (ANSES, 2017; EPPO, 2020; CABI, online; EPPO, online_c). The original source is most probably the one by Chong et al. (2009). In that study, X. compactus was reported on Pinus sp. in Beaufort of South Carolina in 2000. However, Chong et al. (2009) states later that: ‘Pines are not known to be a common host of X. compactus, but because insect specimens associated with these records were not preserved, we cannot confirm the identification.’ There is no information on whether X. compactus can also attack Pinus parviflora and P. thunbergii. However, in EPPO X. compactus is on a list of pests for Pinus parviflora (EPPO, online_d) and P. thunbergii (EPPO, online_e). The Panel considers the association of the pest with Pinus, Pinus parviflora and P. thunbergii very unlikely, yet it cannot be excluded. |
| PRA information |
Available Pest Risk Assessment:
|
| Other relevant information for the assessment | |
| Biology |
Xylosandrus compactus is an ambrosia beetle, native to Southeast Asia (Ngoan et al., 1976; Pennacchio et al., 2012). It is present in Africa, Asia, Europe, Pacific Islands, South America and the USA (EPPO, online_b). In 2011 it was first recorded in Europe, the pest was found in two Italian parks of Portici and Naples (Garonna et al., 2012). Xylosandrus compactus is associated with many fungal species, which are introduced into the galleries and become a food source for developing larvae and adult beetles. In the recent study of Morales‐Rodríguez et al. (2020), 206 OTUs (operational taxonomic units) composed the fungal community associated with X. compactus. Out of 206 OTUs, 69 were identified on a species level and the full list can be found in Morales‐Rodríguez et al. (2020). Some of the associated fungal species are plant pathogens, such as Acremonium sp., Alternaria infectoria, Arthrinium arundinis, Botrytis cinerea, Diaporthe foeniculina, Epicoccum nigrum, Eutypa leptoplaca, Fusarium spp., F. lateritium, F. solani, Fusicolla violacea, Geosmithia pallida, Neocucurbitaria cava, Neofusicoccum luteum, Nigrospora sphaerica, Penicillium brevicompactum, Pestalotiopsis biciliata, Phaeoacremonium fraxinopennsylvanicum, Phaeoacremonium prunicola, Ramularia eucalypti, R. hydrangea‐macrophyllae, Sarocladium strictum, Taphrina sadebeckii and Verticillium. Other most common fungal species are Ambrosiella xylebori, A. macrospora, Aureobasidion sp., Bionectria sp., Candida sp., Candida germanica, Cladosporium sp., C. austrohemisphaericum, C. domenicanum, Cryptococcus sp., Devriesia sp., Geosmithia lavendula, Phialemonium sp., Recurvomyces sp. and Vishniacozyma carnescens (Muthappa and Venkatasubbaiah, 1981; Hayato, 2007; Pennacchio et al., 2012; Bateman et al., 2016; Vannini et al., 2017; Morales‐Rodríguez et al., 2020). The beetle has four stages of development: egg, larva (two or three instars), pupa and adult (EPPO, 2020). According to Hara and Beardsey (1979), the beetle has only two larval instars and additional prepupa stage. On the contrary, Brader (1964) observed three larval instars. Females are brown or black, 1.4–1.9 mm long and 0.7–0.8 mm wide. Males are reddish brown, rare, flightless, 0.8–1.3 mm long and 0.42–0.46 mm wide (Pennacchio et al., 2012; Greco and Wright, 2015). Mating occurs mainly between siblings in the maternal gallery before emergence from the infested host. The ambrosia beetle has facultative arrhenotokous parthenogenesis, which means that males derive from unfertilised eggs and females from fertilised ones (Entwistle, 1964). The male to female sex ration is 1:9 (Hara and Beardsley, 1979). After mating males remain in the maternal galleries. Females on the contrary leave and colonise new hosts. They bore entrance holes into live twigs and branches of healthy and/or stressed plants (e.g. caused by drought, transplanting and pruning) (Hara and Beardsley, 1979). These entrance holes measure between 0.71 and 0.89 mm in diameter (Ngoen et al., 1976). The most frequently affected are 1–3 years old twigs (Faccoli, 2021) and the diameter of attacked twigs and branches was observed to be from 0.1 cm in dogwood (Ngoen et al., 1976) up to usually 6 cm (EPPO, 2020). However, in Sicily on carob trees (Ceratonia siliqua) the beetle also attacked branches of up to 36 cm and trunks of up to 85 cm in diameter (Gugliuzzo et al., 2019a). Females bore gallery, where they introduce and cultivate fungus and lay between 2 and 16 eggs in clusters (Hara and Beardsley, 1979). In laboratory conditions at temperature of 25 ± 2°C eggs hatched in 4–6 days after deposition, duration of larval stage was 7–8 days and of pupal stage 8–9 days. Complete cycle from an egg to mature adult took between 28.5 and 30.5 days (Ngoen et al., 1976). In Italy the adults are usually active from mid‐March until the end of September and the development from an egg to an adult takes from 4 to 6 weeks (Faccoli, 2021). It was observed that the beetle can have in different geographic conditions two (Kaneko et al., 1965; Ngoan et al., 1976; Pennacchio et al., 2012), three (Faccoli, 2021) or up to five generations annually (Gugliuzzo et al., 2020). Xylosandrus compactus overwinters as an adult in twigs and branches of its host plants in Florida, Italy and Japan (Kaneko et al., 1965; Ngoen et al., 1976; Gugliuzzo et al., 2020). In Uganda all life stages were observed all year around (Egonyu et al., 2016). Ambrosia and bark beetles (including X. compactus) orient their flight in order to choose suitable host plants by plant emitted volatiles (Byers, 1995). The main attractant is ethanol (Miller and Rabaglia, 2009; Burbano et al., 2012), which is released together with other chemicals by stressed or dying plants (Kimmere and Kozlowski, 1982). In Sicily, Gugliuzzo et al. (2019b) observed that the flight peak of X. compactus starts when the maximum temperature exceeds 20°C; the pest was able to spread more than 8 km from an infested site to a new one. Xylosandrus compactus is a serious pest of coffee in Hawaii (Greco and Wright, 2015), in India (Muthappa and Venkatasubbaiah, 1981; Ramesh, 1987) and in Uganda (Kagezi et al., 2014). It also caused economic damage to cacao in Uganda (Kagezi et al., 2014), tea in Japan (Kaneko et al., 1965), chestnut in China (Yan et al., 2001) and many other crops (EPPO, 2020). In Italy the pest severely affected Ceratonia siliqua, Laurus nobilis, Pistacia lentiscus, Quercus ilex, Ruscus aculeatus and Viburnum tinus (Garonna et al., 2012; Vannini et al., 2017; Gugliuzzo et al., 2020), Tilia platyphyllos (Faccoli, 2021) and occasionally found also on Cupressus sempervirens (Servizio Fitosanitario Regione Lazio, 2014). According to EPPO (2020), the main pathways of entry for X. compactus are plants for planting (except seeds), cut branches, bark, wood, woodchips, hogwood, processing wood residues and wood packaging material. Xylosandrus compactus was intercepted on fruits of Mangifera indica from Kenya in 2014 (EUROPHYT, online). There were six outbreaks of X. compactus in the EU, one in France (2016), one in Italy (2016), three in Spain (2019, 2020, 2020) and one in Malta (2021) (EUROPHYT Outbreaks database, online). |
| Symptoms | Main type of symptoms |
Main symptoms caused by X. compactus are leaf and stem necrosis, flagging of branches, wilting of twigs and branches, dieback, branch breakage, cankers on larger twigs and branches, saw dust in a form of frass from the entrance holes, exuding sap from entrance holes of some host plants and blackish colouration of entrance hole (Kaneko et al., 1965; Hara and Beardsey, 1979; Pennacchio et al., 2012; Greco and Wright, 2015; EPPO 2020). There is no information on the symptoms caused to Pinus plants. |
| Presence of asymptomatic plants | No specific information on the presence of asymptomatic plants is found. Similarly, like other ambrosia beetles, initial phases of infestation are associated with few external symptoms. While there is no visible injury in the bark at early stage of colonisation, frass is produced and examination of the wood under the infested spot bored by the beetle, reveals the brownish staining of the xylem and necrosis caused by the fungus (Mendel et al., 2012). | |
| Confusion with other pests |
Infestation symptoms recorded in shrubs and trees are not specific to X. compactus and may be due to infestation by other ambrosia beetles of similar size and biology. Xylosandrus compactus can be morphologically confused with other Xylosadrus species. It is very similar to Xylosandrus adherescens, X. derupteterminatus, X. mesuae and X. morigerus (Smith et al., 2020). A morphological or molecular analysis is needed in order to distinguish among them. |
| Host plant range |
Xylosandrus compactus is polyphagous pest, it has more than 200 known hosts. Conifer hosts are Araucaria heterophylla, Pinus spp. (ANSES, 2017; EPPO, 2020; CABI, online; EPPO, online_c) and Cupressus sempervirens (Servizio Fitosanitario Regione Lazio, 2014). Non‐conifer hosts are Abutilon grandifolium, Acacia auriculiformis, A. farnesiana, A. koa, A. mangium, A. melanoxylon, Acalypha wilkesiana, Acer spp., Acer barbatum, A. negundo, A. rubrum, Albizzia lebbeck, Alectryon spp., Aleurites moluccana, Alnus spp., Alpinia purpurata, Anacardium occidentale, Andira inermis, Annona cherimola, A. glabra, A. montana, A. muricata, A. reticulata, A. squamosa, Anthurium andraeanum, Antidesma pulvinatum, Asparagus myriocladus, Azalea spp., Bixa orellana, Buddleja asiatica, Buxus sempervirens, Byrsonima crassifolia, Caesalpinia kavaiensis, Callicarpa americana, C. pendunculata, Camellia spp., Camellia sinensis, Carapa guianensis, Carya glabra, C. illinoensis, Casimiroa edulis, Cassia spp., Cassia glauca, Castanea spp., Casuarina equisetifolia, Cattleya spp., Cedrela odorata, Celtis spp., Celtis laevigata, Cercis canadensis, Charpentiera spp., Cinnamomum camphora, C. verum, Citharexylum caudatutn, Citrus reticulata, Claoxylon sandwicense, Clidemia hirta, Coffea arabica, C. canephora, Colubrina oppositifolia, Coprosma spp., Cordia alliadora, Cornus florida, Corylus spp., Crotalaria spp., Croton reflexifolius, Cryptocarya oahuensis, Dalbergia spp., Dendrobium spp., Dendrobium spp., Diospyros spp., Drypetes phyllanthoides, Entandrophragma utile, Epidendrum spp., Erythrina abyssinica, Eucalyptus spp., Eucalyptus pilularis, E. robusta, E. sideroxylon, Eugenia cuminii, E. malaccensis, E. uniflora, Euphoria longana, Eusideroxylon zwageri, Euterpe oleracea, Fagus spp., Ficus spp., Ficus carica, Flacourtia indica, Flindersia brayleyana, Fraxinus ornus, F. uhdei, Gardenia spp., Gardenia jasminoides, Gouldia spp., Graptophyllum pictum, Hevea brasiliensis, Hibiscus spp., Hibiscus elatus, H. rosa‐sinensis, H. tiliaceus, Hydrangea macrophylla, Ilex anomala, Indigofera suffruticosa, Inga paterno, Jasminum multiflorum, J. sambac, Khaya grandifoliola, K. ivorensis, K. nyasica, K. senegalensis, Koelreuteria elegans, Lantana camara, Leucaena leucocephala, Liquidambar spp., Liquidambar formosana, L. styraciflua, Liriodendron spp., Litchi chinensis, lnocarpus fagifer, Macadamia integrifolia, M. ternifolia var. integrifolia, Magnolia spp., Magnolia grandiflora, Malus spp., Malvastrum, Malvastrum coromandelianum, Mangifera indica, Matisia cordata, Melaleuca leucadendra, Melastoma malabathricum, Melia azedarach, Melicoccus bijugatus, Melochia umbellata, Morella cerifera, Murraya paniculata, Myrciaria dubia, Myrsine lessertiana, Nephelim lappaceum, Olmediella betschleriana, Ostrya spp., Passiflora edulis, Pelea spp., Perrottetia sandwicensis, Persea americana, P. borbonia, Pipturus albidus, Pithecellobiutn dulce, Pittosporum tobira, Platanus spp., Platanus occidentalis, Pometia pinnata, Prosopis pallida, Prunus laurocerasus, Pseudomorus sandwicensis, Punica granatum, Quercus laurifolia, Q. nigra, Q. robur, Rhododendron spp., Rollinia emarginata, Rubus rosaefolius, Salix, Samanea saman, Sambucus simpsonii, Santalum freycitzetianum, Sapindus oahuensis, Schinus terebinthifolius, Shorea spp., Solanum sodomeum, Spondias purpurea, Stachytarpheta australis, Swietenia macrophylla, Swietenia mahogoni, Swietenia spp., Symplocos tinctoria, Syncarpia glomulifera, Tabebuia pentaphylla, Taona ciliata var. australis, Tapeinochilos ananassae, Theobroma cacao, T. grandiflorum, Tilia spp., Toona ciliata, Tristania conferta, Ulmus spp., Vinca spp., Vitex trifolia, Vitis spp., Vitis labruscana and Wikstroetnia spp. (ANSES, 2017; EPPO, 2020), Ceratonia siliqua, Laurus nobilis, Pistacia lentiscus, Quercus ilex, Ruscus aculeatus, Viburnum tinus (Garonna et al., 2012; Vannini et al., 2017; Gugliuzzo et al., 2020) and Tilia platyphyllos (Faccoli, 2021). |
| Reported evidence of impact | Xylosandrus compactus is EU quarantine pest. |
| Evidence that the commodity is a pathway | According to EPPO (2020), X. compactus can travel with plants for planting. Therefore, the commodity (i.e., bonsai plants) is expected to be a pathway. |
| Surveillance information |
No surveillance information for this pest is currently available from China. Nevertheless, non‐European scolytids are included in a list of target pests (Dossier Section 4.0) for which monitoring activities (pheromone traps, light traps, etc.) are performed, together with three times a year inspections and samplings to collect insects on host plants in the survey area. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. |
A.1.2. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of ambrosia and bark beetles (Euwallacea interjectus, E. validus, Heteroborips seriatus and Xylosandrus compactus) between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.1.3. Evaluation of the implementation and relevance of specific measures in China
Commission Implementing Regulation (EU) 2019/2072 specifies in point 30 of Annex VII measures which are required for the import of the commodity from China.
The below overview provides special requirements for naturally or artificially dwarfed plants for planting other than seeds according to Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 including an assessment of whether or not the applicant country implements those measures with respect to ambrosia and bark beetles [regulated as Scolytidae spp. (non‐European)] identified in this Opinion. The Panel assumes that information on treatments required to be included in the phytosanitary certificate is provided by the applicant country according to the Article 71 of Regulation (EU) 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’.
| Special requirements as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 | Implementation of the special requirements in China according to information provided in the Dossier | Relevance of special requirements for the pest including uncertainties |
|---|---|---|
| ‘Official statement that: | – | – |
| a) the plants, including those collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime, | Yes | Yes |
| b) the plants in the nurseries referred to in point (a) of this entry: | – | – |
| i) at least during the period referred to in point (a) of this entry: | – | – |
| — were potted, in pots which are placed on shelves at least 50 cm above ground, |
Yes, partially. Pots are also reported to be kept on the ground. |
No |
| — have been subjected to appropriate treatments to ensure freedom from non‐European rusts, and the active ingredient, concentration and date of application of these treatments has been mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’. |
Yes. Treatments are appropriate. They are expected to reduce the likelihood of infection of the non‐European rusts and the rate of colonisation of plant tissues by rust fungi, although it is uncertain if freedom from non‐European rusts could be reachable. Treatments used are listed in Table 6.
Uncertainties:
|
No |
| — have been officially inspected at least six times a year at appropriate intervals for the presence of Union quarantine pests of concern in accordance with Regulation (EU) No 2016/2031, and these inspections have also been carried out on plants in the immediate vicinity of the nurseries referred to in point (a) of this entry, at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus, | Yes |
Yes. It is not specified how the visual inspection is done and the exact timing of these inspections. The sampling and laboratory inspection of plant material may allow to identify infested plants by the beetles through sawdust detection. Uncertainties: – Sawdust can be removed by watering or insecticide application. – Sawdust can be difficult to see in the bark crevices. |
| — have been found free, in these inspections, from the relevant Union quarantine pests of concern as specified in the previous indent, infested plants have been removed and the remaining plants, where appropriate, have been effectively treated and have been held for an appropriate period and inspected to ensure freedom from such pests, | Yes |
Yes. Spray of contact insecticides can kill the adult beetles that are present on the plants at the time of spraying. All stages hidden under the bark are not expected to be affected by the insecticides. Uncertainties: – The period of ambrosia and bark beetles’ activity is not fully covered by insecticide protection. In addition, the insects are not killed when they are hidden under the bark. |
| — have been planted in either an unused artificial growing medium or in a natural growing medium, which has been treated by fumigation or by appropriate heat treatment and has been of any Union quarantine pests, | Yes | No |
| — have been kept under conditions which ensure that the growing medium has been maintained free from Union quarantine pests and within 2 weeks prior to dispatch, have been: | Yes | No |
| — shaken and washed with clean water to remove the original growing medium and kept bare rooted, or | – | – |
| — shaken and washed with clean water to remove the original growing medium and replanted in growing medium which meets the conditions laid down in (i) fifth indent, or | – | – |
| — subjected to appropriate treatments to ensure that the growing medium is free from Union quarantine pests, and the active ingredient, concentration and date of application of these treatments have been indicated on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031 under the rubric ‘Disinfestation and/or disinfection treatment’. | Yes | No |
| ii) were packed in closed containers which have been officially sealed and bear the registration number of the registered nursery, and this number has been indicated under the rubric ‘Additional declaration’ on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, enabling the consignments to be identified.’ | Yes | Yes |
Table 6.
Details of fungicide treatment as provided in Dossier Section 4.0
| Pesticide used (active substances) | Name(s) of the target pest species | Target life stage(s) of the pest(s) | Timing and location of treatment | Dilution as provided in the Dossier | Notes |
|---|---|---|---|---|---|
| Potassium, Permanganate and Triadimefon mixture | Cronartium quercuum, Scirrhia acicola, Diplodia pinea etc. | Preventive treatment | February, March or September, October before five needles pine grafting. | Potassium Permanganate 5,000 times and 2,000 times of Triadimefon mixture | Soaked for 2 h. |
| Carbendazim | Cronartium quercuum, Scirrhia acicola, Diplodia pinea, etc. | Preventive treatment | February, March or September, October after five needles pine grafting. | 800 times | Whole plant spray. Periodicity of treatment once a month. |
| 50% Dyson zinc wettable powder | Scirrhia acicula | Sprouting new leaves | April, export nursery. | 200–800 times | Every 10–15 days, 3–4 times. |
| 100% Bordeaux mixture | Cercoseptoria pini‐densiflorae | Growth period | July, export nursery. | 600 times | Periodicity of treatment once a month. |
| 20% Thiodiazole‐copper suspension | 500 times | ||||
| 25% Triadimefon wettable powder | Cronartium quercuum | Occurrence of rust spores | April, export nursery. | 1,000–1,500 times | Spray, every 10–15 days, 3 times. |
| 25% Triadimefon wettable powder | Cronartium quercuum | Maintenance period | August, September, export nursery. | 1,000–1,500 times | Spray, once every 10–15 days, 3 times. |
A.1.4. Reference List
ANSES (L’Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail), 2017. Évaluation du risque simplifiée sur Xylosandrus compactus (Eichhoff) identifiéen France métropolitaine. Avis de l’Anses. Rapport d’expertise collective. Available online: https://www.anses.fr
Aoki T, Kasson MT, Berger MC, Freeman S, Geiser DM and O’Donnell K, 2018. Fusarium oligoseptatum sp. nov., a mycosymbiont of the ambrosia beetle Euwallacea validus in the Eastern US and typification of F. ambrosium. Fungal Systematics and Evolution, 1, 23–39. https://doi.org/10.3114/fuse.2018.01.03
Atkinson TH, online. Bark and ambrosia beetles. Online database. Available online: https://www.barkbeetles.info/index.php [Accessed online: 10 May 2021].
Bateman C, Šigut M, Skelton J, Smith KE and Hulcr J, 2016. Fungal associates of the Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) are spatially segregated on the insect body. Environmental Entomology, 45, 883–890. https://doi.org/10.1093/ee/nvw070
Berger MC, 2017. Interactions between Euwallacea ambrosia beetles, their fungal symbionts and the native trees they attack in the Eastern United States. Matthew C. Berger Thesis submitted to the Davis College of Agriculture, Natural Resources and Design at West Virginia. 116 pp. https://doi.org/10.33915/etd.5186
Brader L, 1964. Etude de la relation entre le scolyte des rameaux du caféier, Xylosandrus compactus Eichh. (X. morstatti Hag.) et sa plante‐hôte. Mededlingen Landdbouwhogeschool, Wageningen, 64–7, 109 pp.
Bright DE, 2021. A Catalog of Scolytidae (Coleoptera), supplement 4 (2011–2019) with an annotated checklist of the world fauna (Coleoptera: Curculionoidea: Scolytidae). C.P. Gillette Museum of Arthropod Diversity, Department of Agricultural Biology Colorado State University. 661 pp.
Burbano EG, Wright MG, Gillette NE, Mori S, Dudley N, Jones T and Kaufmann M, 2012. Efficacy of traps, lures, and repellents for Xylosandrus compactus (Coleoptera: Curculionidae) and other ambrosia beetles on Coffea arabica plantations and Acacia koa nurseries in Hawaii. Environmental Entomology, 41, 133–140. https://doi.org/10.1603/en11112
Byers JA, 1995. Host tree chemistry affecting colonization in bark beetles, In Cardé RT and Bell WJ (eds.), Chemical ecology of insects 2. Chapman and Hall, New York, pp. 154–213.
CABI (Centre for Agriculture and Bioscience International), online. Xylosandrus compactus (shot‐hole borer). Available online: https://www.cabi.org/cpc/datasheet/57234 [Accessed: 8 September 2021].
Chang H, Liu Q, Hao D, Liu Y, A Y, Qian L and Yang X, 2013. DNA barcodes and molecular diagnostics for distinguishing introduced Xyleborus (Coleoptera: Scolytinae) species in China. Mitochondrial DNA, 25, 63–69. https://doi.org/10.3109/19401736.2013.779260
Chong JH, Reid L and Williamson M, 2009. Distribution, host plants, and damage of the black twig borer, Xylosandrus compactus (Eichhoff), in South Carolina. Journal of Agricultural and Urban Entomology, 26, 199–208.
Cognato AI, Hoebeke ER, Kajimura H and Smith SM, 2015. History of the exotic ambrosia beetles Euwallacea interjectus and Euwallacea validus (Coleoptera: Curculionidae: Xyleborini) in the United States. Journal of Economic Entomology, 108, 1129–1135. https://doi.org/10.1093/jee/tov073
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Xylosandrus compactus. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=22322 [Accessed: 11 August 2021].
Dodds KJ and DiGirolomo MF, 2020. Effect of cleaning multiple‐funnel traps on captures of bark and woodboring beetles in Northeastern United States. Insects, 11, 1–11. https://doi.org/10.3390/insects11100702
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Grégoire J‐C, Streissl F, Kertész V and Milonas P, 2020a. Scientific opinion on the list of non‐EU Scolytinae of coniferous hosts. EFSA Journal 2020;18(1):5933, 56 pp. https://doi.org/10.2903/j.efsa.2020.5933
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Grégoire J‐C, Kertész V, Streissl F and Milonas P, 2020. Scientific opinion on the pest categorisation of non‐EU Scolytinae of coniferous hosts. EFSA Journal 2020;18(1):5934, 39 pp. https://doi.org/10.2903/j.efsa.2020.5934
Egonyu JP, Ahumuza G and Ogari I, 2016. Population dynamics of Xylosandrus compactus (Coleoptera: Curculionidae: Scolytinae) on Coffea canephora in the Lake Victoria Crescent agroecological zone of Uganda. African Zoology, 51, 121–126.
Entwhistle PF, 1964. Inbreeding and arrhenotoky in the ambrosia beetle Xyleborus compactus (Eichh.) (Coleoptera: Scolytidae). Proceedings of the Royal Entomological Society of London. Series A, General Entomology 39, 83–88. https://doi.org/10.1111/j.1365‐3032.1964.tb00792.x
EPPO (European and Mediterranean Plant Protection Organization), 2017. Report of a pest risk analysis for Euwallacea fornicatus sensu lato and Fusarium euwallaceae. Available online: https://gd.eppo.int/taxon/FUSAEW/documents
EPPO (European and Mediterranean Plant Protection Organization), 2020. EPPO Technical Document No. 1081, EPPO Study on the risk of bark and ambrosia beetles associated with imported non‐coniferous wood. EPPO Paris. Available online: https://www.eppo.int/RESOURCES/eppo_publications
EPPO (European and Mediterranean Plant Protection Organization), online_a. Xylosandrus compactus (XYLSCO), Categorization. Available online: https://gd.eppo.int/taxon/XYLSCO/categorization [Accessed: 9 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Xylosandrus compactus (XYLSCO), Distribution. Available online: https://gd.eppo.int/taxon/XYLSCO/distribution [Accessed: 9 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Xylosandrus compactus (XYLSCO), Hosts. Available online: https://gd.eppo.int/taxon/XYLSCO/hosts [Accessed: 31 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Pinus parviflora (PIUPF), Pests. Available online: https://gd.eppo.int/taxon/PIUPF/pests [Accessed: 31 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_e. Pinus thunbergii (PIUTH), Pests. Available online: https://gd.eppo.int/taxon/PIUTH/pests [Accessed: 31 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), 2017. Xylosandrus compactus. EPPO Alert List. Available online: https://www.eppo.int
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Europhyt Oubreaks database, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Faccoli M, 2021. Xylosandrus compactus, un nuovo parassita forestale invade l’Italia. Forest@‐Journal of Silviculture and Forest Ecology, 18, 8–14. https://doi.org/10.3832/efor3711‐018
Garonna AP, Dole SA, Saracino A, Mazzoleni S and Cristinzio G, 2012. First record of the black twig borer Xylosandrus compactus (Eichhoff) (Coleoptera: Curculionidae, Scolytinae) from Europe. Zootaxa, 3251, 64–68. https://doi.org/10.11646/zootaxa.3251.1.5
Greco EB and Wright MG, 2015. Ecology, biology, and management of Xylosandrus compactus (Coleoptera: Curculionidae: Scolytinae) with emphasis on coffee in Hawaii. Journal of Integrated Pest Management, 6, 1–7. https://doi.org/10.1093/jipm/pmv007
Gugliuzzo A, Criscione G and Tropea Garzia G, 2019a. Unusual behavior of Xylosandrus compactus (Coleoptera: Scolytinae) on carob trees in a Mediterranean environment. Insects, 10, 1–7. https://doi.org/10.3390/insects10030082
Gugliuzzo A, Criscione G, Siscaro G, Russo A and Tropea Garzia G, 2019b. First data on the flight activity and distribution of the ambrosia beetle Xylosandrus compactus (Eichhoff) on carob trees in Sicily. EPPO Bulletin, 49, 1–12. https://doi.org/10.1111/epp.12564
Gugliuzzo A, Criscione G, Biondi A, Aiello D, Vitale A, Polizzi G and Tropea Garzia G, 2020. Seasonal changes in population structure of the ambrosia beetle Xylosandrus compactus and its associated fungi in a southern Mediterranean environment. PLoS One, 15, 13. https://doi.org/10.1371/journal.pone.0239011
Hara AH and Beardsley JW, 1979. Biology of the black twig Borer, Xylosandrus compactus (Eichhoff), in Hawaii. Proceedings of the Hawaiian Entomological Society, 13, 55–70.
Hayato M, 2007. Note on the dieback of Cornus florida caused by Xylosandrus compactus. Bulletin of the Forestry and Forest Products Research Institute (Japan), 6, 59–63.
Hoebeke ER and Rabaglia RJ, 2008. Xyleborus seriatus Blandford (Coleoptera: Curculionidae: Scolytinae), an Asian ambrosia beetle new to North America. Proceedings of the Entomological Society of Washington, 110, 470–476. https://doi.org/10.4289/07‐048.1
Kagezi GH, Kucel P, Egonyu JP, Ahumuza G, Nakibuule I, Kobusinge J and Wagoire WW, 2014. Implications of black coffee twig borer on cocoa in Uganda. Uganda Journal of Agricultural Sciences, 15, 179–189.
Kajii C, Morita T, Jikumaru S, Kajimura H, Yamaoka Y and Kuroda K, 2013. Xylem dysfunction in Ficus carica infected with wilt fungus Ceratocystis ficicola and the role of the vector beetle Euwallacea interjectus. IAWA Journal, 34, 301–312. https://doi.org/10.1163/22941932‐00000025
Kaneko T, Tamaki Y and Takagi K, 1965. Preliminary report on the biology of some scolytid beetles, the tea root borer, Xyleborus germanus Blandford, attacking tea roots, and the tea stem borer, Xyleborus compactus Eichhoff attacking tea twigs. Japanese Journal of Applied Entomology and Zoology, 9, 23–28.
Kasson MT, O’Neal ES and Davis DD, 2015. Expanded host range testing for Verticillium nonalfalfae: potential biocontrol agent against the invasive Ailanthus altissima. Plant Disease, 99, 823–835. https://doi.org/10.1094/pdis‐04‐14‐0391‐re
Kimmerer TW and Kozlowski TT, 1982. Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiology, 69, 840–847. https://doi.org/10.1104/pp.69.4.840
Landi L, Braccini CL, Knížek M, Pereyra VA and Marvaldi AE, 2019. A newly detected exotic ambrosia beetle in Argentina: Euwallacea interjectus (Coleoptera: Curculionidae: Scolytinae). Florida Entomologist, 102, 240–242. https://doi.org/10.1653/024.102.0141
Maiti PK and Nivedita S, 2004. The fauna of India and the adjacent countries. Scolytidae: Coleoptera (bark and ambrosia beetles): Volume I (part 1) introduction and tribe Xleborini. Zoological Survey of India, Kolkata, India. 268 pp.
Mandelshtam MY, Yakushkin EA and Petrov AV, 2018. Oriental ambrosia beetles (Coleoptera: Curculionidae: Scolytinae): new inhabitants of Primorsky krai in Russia. Russian Journal of Biological Invasions, 9, 355–365. https://doi.org/10.1134/s2075111718040082
Mandelshtam MY, Petrov AV, Smith SM and Cognato AI, 2019. Resurrection of Heteroborips Reitter, 1913 (Coleoptera: Curculionidae: Scolytinae) from synonymy with Xyleborus Eichhoff, 1864. The Coleopterists Bulletin, 73, 387–394. https://doi.org/10.1649/0010‐065x‐73.2.387
Mendel Z, Protasov A, Sharon M, Zveibil A, Ben Yehuda S, O’Donnell K, Rabaglia R, Wysoki M and Freeman S, 2012. An Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus Fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica, 40, 235–238. https://doi.org/10.1007/s12600‐012‐0223‐7
Miller DR and Rabaglia RJ, 2009. Ethanol and (‐)‐α‐pinene: attractant kairomones for bark and ambrosia beetles in the Southeastern US. Journal of Chemical Ecology, 35, 435–448. https://doi.org/10.1007/s10886‐009‐9613‐9
Morales‐Rodríguez C, Sferrazza I, Aleandri MP, Dalla Valle M, Speranza S, Contarini M and Vannini A, 2020. The fungal community associated with the ambrosia beetle Xylosandrus compactus invading the Mediterranean maquis in central Italy reveals high biodiversity and suggests environmental acquisitions. Fungal Biology, 125, 12–24. https://doi.org/10.1016/j.funbio.2020.09.008
Muthappa BN and Venkatasubbaiah P, 1981. Association of Ambrosiella macrospora with Xylosandrus compactus, the shot‐hole borer of robusta coffee in India. Journal of Coffee Research, 11, 54.
Nakashima T, Otomo T, Owada Y and Iizuka T, 1992. SEM observations on growing conditions of the fungi in the galleries of several ambrosia beetles:(Coleoptera Scolytidea and Platypodidae). Journal of the Faculty of Agriculture, Hokkaido University, 65, 239–273.
Ngoan ND, Wilkinson RC, Short DE, Moses CS and Mangold JR, 1976. Biology of an introduced ambrosia beetle, Xylosandrus compactus, in Florida. Annals of the Entomological society of America, 69, 872–876.
Pennacchio F, Santini L and Francardi V, 2012. Bioecological notes on Xylosandrus compactus (Eichhoff) (Coleoptera Curculionidae Scolytinae), a species recently recorded into Italy. Redia, 95, 67–77.
Ramesh PK, 1987. Observations on crop loss in robusta coffee due to mealybug and shot‐hole borer. Journal of Coffee Research, 17, 94–95.
Ranger CM, Reding ME, Persad AB and Herms DA, 2010. Ability of stress‐related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles. Agricultural and Forest Entomology, 12, 177–185. https://doi.org/10.1111/j.1461‐9563.2009.00469.x
Samuelson GA, 1981. A synopsis of Hawaiian Xyleborini (Coleoptera: Scolytidae). Pacific Insects, 23, 50–92.
Servizio Fitosanitario Regione Lazio, 2014. Xylosandrus compactus. Regione Lazio, Opuscolo informativo, 6 pp.
Smith SM and Hulcr J, 2015. Scolytus and other economically important bark and ambrosia beetles. In Vega FE and Hofstetter RW (eds.). Bark Beetles, Biology and Ecology of Native and Invasive Species. Elsevier, Academic Press. pp. 495–531. https://doi.org/10.1016/b978‐0‐12‐417156‐5.00012‐5
Smith SM, Beaver RA and Cognato AI, 2020. A monograph of the Xyleborini (Coleoptera, Curculionidae, Scolytinae) of the Indochinese Peninsula (except Malaysia) and China. ZooKeys, 983, 1–442. https://doi.org/10.3897/zookeys.983.52630
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
Vannini A, Contarini M, Faccoli M, Valle MD, Rodriguez CM, Mazzetto T, Guarneri D, Vettraino AM and Speranza S, 2017. First report of the ambrosia beetle Xylosandrus compactus and associated fungi in the Mediterranean maquis in Italy, and new host–pest associations. EPPO Bulletin, 47, 100–103. https://doi.org/10.1111/epp.12358
Wang Z, Li Y, Ernstsons AS, Sun R, Hulcr J and Gao L, 2021. The infestation and habitat of the ambrosia beetle Euwallacea interjectus (Coleoptera: Curculionidae: Scolytinae) in the riparian zone of Shanghai, China. Agricultural and Forest Entomology, 23, 104–109. https://doi.org/10.1111/afe.12405
Webster RP, de Tonnancour P, Sweeney JD, Webster VL, Kostanowicz CA, Hughes C, Anderson RS, Klymko J, Chantal C and Vigneault R, 2020. New Coleoptera records from eastern Canada, with additions to the fauna of Manitoba, British Columbia, and Yukon Territory. ZooKeys, 946, 53–112. https://doi.org/10.3897/zookeys.946.52489
Wood SL and Bright DE, 1992. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic Index. Volume A. Great Basin Naturalist Memoirs, 13. 833 pp.
Yan S, Huang H and Wang J, 2001. The occurrence of chestnut beetle and its control. South China Fruits, 30, 48.
A.2. Bursaphelenchus xylophilus and Monochamus group (M. alternatus, M. galloprovincialis, M. saltuarius, M. sutor and M. urussovi)
A.2.1. Organism information
| Taxonomic information |
1. Bursaphelenchus xylophilus Current valid scientific name: Bursaphelenchus xylophilus Synonyms: Bursaphelenchus lignicolus, Aphelenchoides xylophilus Name used in the EU legislation: Bursaphelenchus xylophilus (Steiner & Bührer) Nickle et al. [BURSXY] Order: Rhabditida (formerly: Tylenchida) Family: Parasitaphelenchidae (formerly: Aphelenchoididae) Common name: Pine wood nematode (PWN) Name used in the Dossier: Bursaphelenchus xylophilus 2. Monochamus alternatus Current valid scientific name: Monochamus alternatus Synonyms: Monochamus tesserula Name used in the EU legislation: Monochamus spp. (non‐European populations) [1MONCG]. Order: Coleoptera Family: Cerambycidae Common name: Japanese pine sawyer Name used in the Dossier: Monochamus alternatus 3. Monochamus galloprovincialis Current valid scientific name: Monochamus galloprovincialis Synonyms: – Name used in the EU legislation: Monochamus spp. (non‐European populations) [1MONCG]. Order: Coleoptera Family: Cerambycidae Common name: pine sawyer beetle or black pine sawyer beetle Name used in the Dossier: – 4. Monochamus saltuarius Current valid scientific name: Monochamus saltuarius Synonyms: – Name used in the EU legislation: Monochamus spp. (non‐European populations) [1MONCG]. Order: Coleoptera Family: Cerambycidae Common name: Japanese pine sawyer Name used in the Dossier: – 5. Monochamus sutor Current valid scientific name: Monochamus sutor Synonyms: – Name used in the EU legislation: Monochamus spp. (non‐European populations) [1MONCG]. Order: Coleoptera Family: CerambycidaeNone Common name: small white‐marmorated longhorn beetle Name used in the Dossier: – 6. Monochamus urussovi Current valid scientific name: Monochamus urussovi Synonyms: Monochamus rosenmuelleri, Monochamus sartor rosenmuelleri, Monochamus sartor urussovi Name used in the EU legislation: Monochamus spp. (non‐European populations) [1MONCG]. Order: Coleoptera Family: Cerambycidae Common name: White mottled sawyer Name used in the Dossier: – |
| Group |
Bursaphelenchus xylophilus: Nematodes Monochamus alternatus, M. galloprovincialis, M. saltuarius, M. sutor and M. urussovi: Insects |
| EPPO code |
BURSXY: Bursaphelenchus xylophilus MONCAL: Monochamus alternatus MONCGA: Monochamus galloprovincialis MONCSL: Monochamus saltuarius MONCSU: Monochamus sutor MONCUR: Monochamus urussovi |
| Regulated status |
Bursaphelenchus xylophilus is listed in Annex II/B of Commission Implementing Regulation (EU) 2019/2072 as Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle et al. [BURSXY]. Commission Delegated Regulation (EU) 2019/1702 has listed B. xylophilus as priority pest; measures to prevent its spread within the Union were set by Commission Implementing Decision 2018/618/EU. Monochamus alternatus, M. galloprovincialis, M. saltuarius, M. sutor and M. urussovi are all listed in Annex II/A of Regulation (EU) 2019/2072 as Monochamus spp. (non‐European populations) [1MONCG]. Bursaphelenchus xylophilus and Monochamus spp. (non‐European populations) are listed in the Commission Implementing Regulation (EU) 2020/1217 as pests of concern for Pinus parviflora and P. thunbergii. EPPO Categorisation: Bursaphelenchus xylophilus is included in A2 EPPO list (EPPO, online_a) and in A1 EPPO list for Azerbaijan, Georgia, Israel, Kazakhstan, Moldova, Morocco, Russia, Tunisia, Turkey and Ukraine. It is also listed as quarantine pest in Israel, Morocco, Norway and Tunisia (EPPO, online_b). Outside EPPO region, the pest is listed as follows (EPPO, online_b):
Monochamus alternatus is included in A1 EPPO list and listed as quarantine pest for Norway. Monochamus alternatus is included in A1 list in Bahrain, Paraguay, Uruguay, and it is also listed as quarantine pest for Canada (EPPO, online_c). Monochamus galloprovincialis, M. saltuarius, M. sutor and M. urussovi are included in A1 list in Kazakhstan and in A2 list of EAEU (Eurasian Economic Union ‐ Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) (EPPO, online_d,o,p,q). |
| Pest status in China |
Bursaphelenchus xylophilus is present in China in the provinces of Anhui, Fujian, Gansu, Guangdong, Guangxi, Guizhou, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Jilin, Liaoning, Shaanxi, Shandong, Sichuan, Yunnan and Zhejiang (CABI, online_a; EPPO, online_e; State Forestry and Grassland Administration Government, online_a; State Forestry and Grassland Administration Government, online_b). It is also present in the municipalities of Chongqing and Shanghai and in Xianggang (territory of Hong Kong Special Administrative Region) (CABI, online_a; EPPO, online_e). Monochamus alternatus, the main vector of Bursaphelechus xylophilus in Asia (EFSA, 2020_b), is present in Chinese provinces and municipalities reported above, except Shanghai; furthermore, it is also present in the provinces of Hebei, Jilin and Xinjiang (CABI, online_b; EPPO, online_f) where B. xylophilus is not recorded. Monochamus galloprovincialis is present in the northern provinces of Jilin, Heilongjiang and Neimenggu (Danilevsky, 2019; EPPO, online_r). Monochamus saltuarius is present in Jiangxi, Jilin, Heilongjiang, Hebei, Liaoning, Neimenggu, Shaanxi, Shandong, Shanxi, Xinjiang and Zhejiang (Danilevsky, 2019; EPPO, online_s). Monochamus sutor is present in Jilin, Heilongjiang, Henan, Liaoning, Neimenggu, Qinghai, Shandong, Xinjiang and Zhejiang (Danilevsky, 2019; EPPO, online_t). Monochamus urussovi is present in Hebei, Heilongjiang, Henan, Jilin, Liaoning, Niemenggu, Shaanxi and Xinjiang (Danilevsky, 2019; CABI, online_c; EPPO, online_g). |
| Pest status in the EU |
Bursaphelenchus xylophilus is currently present only in Portugal, the island of Madeira included and in Spain (EFSA, 2020_a). In Portugal, the status of the pest has been declared as ‘present, several outbreaks reported in mainland Portugal (Centro, Lisboa e Vale do Tejo, Alentejo regions) and Madeira, under official control’ (EPPO, online_h). In Spain, B. xylophilus has been detected from 2008 onwards in Extremadura, Galicia and Castilla y Leon; its status is currently declared as ‘present, only in some parts of the Member State concerned, under eradication’ (EPPO, online_i). Monochamus alternatus is absent in the EU (EFSA PLH Panel, 2018; EFSA, 2020_b; EPPO, online_f). Monochamus galloprovincialis is present in all EU member states except Bulgaria (Danilevsky, 2019; EPPO, online_r). Monochamus saltuarius is present in Austria, Croatia, Czech Republic, Finland, Germany, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia and Slovenia (Cherepanov, 1990; Danilevsky, 2019; EPPO, online_s). Monochamus sutor is present in Austria, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Lithuania, Netherlands, Poland, Romania, Slovakia, Slovenia, Spain and Sweden (Danilevsky, 2019; EPPO, online_t). Monochamus urussovi is present in Estonia, Finland, Latvia, Poland and Sweden (Danilevsky, 2019; EPPO, online_g). |
| Host status on Pinus parviflora and P. thunbergii |
Pinus parviflora is susceptible to B. xylophilus (Koo et al., 2013; EFSA PLH Panel, 2019) as well as P. thunbergii (Koo et al., 2013; EFSA PLH Panel, 2019; CABI, online_d; EPPO, online_j). Pinus parviflora and P. thunbergii are known as hosts for both M. alternatus and M. saltuarius (EFSA PLH Panel, 2019; CABI, online_e,f; EPPO, online_m,u). It is uncertain whether P. parviflora and P. thunbergii are hosts of M. galloprovincialis, M. sutor and M. urussovi. |
| PRA information |
Pest Risk Assessment currently available:
|
| Other relevant information for the assessment | |
| Biology |
Bursaphelenchus xylophilus is a nematode of the family Parasitaphelenchidae and it is the causal agent of the Pine Wilt Disease (PWD) in North America (where it is believed to be native), Asia and Europe. The pest has six life stages: egg, four juvenile stages and adult. The life cycle of B. xylophilus is closely related to that of its vector insects, the cerambycid beetles of the genus Monochamus, developing in the wood of dying and dead pine trees. The infection caused to host tree by the nematode occurs in summer following two possible ways: a) primary transmission by maturation feeding of Monochamus adults (both sexes) on twigs and shoots of healthy pines; b) secondary transmission by oviposition of Monochamus females on dying pines (EPPO, online_k). The development of the nematode takes place starting from 9.5°C; beyond this threshold the completion of a generation in laboratory conditions takes from 3 to 12 days with temperatures of 30 and 15°C, respectively (CABI, online_a). In the propagative life cycle, nematode densities increase rapidly in wood, where they feed on plant cells and on the hyphae of blue stain fungi (saprophytic life cycle). In spring, when the insects pupate, the third dispersal juvenile stage (JIII) colonises the wood surrounding the pupal chambers (EFSA, 2019). Here, they quickly develop into the fourth dispersal stage (JIV), which invades the chambers and enter into the tracheal system of the immature adults of Monochamus. After emergence, the vector beetles fly to healthy pines for a maturation feeding carried out on twigs and shoots (CABI, online_a). During maturation feeding, the JIV stage nematodes leave the tracheal system of the vector and penetrate into healthy pines through the feeding scars; once in the host pine, the JIV stage moults into the adult stage. Adult nematodes multiply and spread very quickly in the vascular system of the plant (up to 150 cm/day) (EFSA, 2019; EPPO, online_k), mainly moving through resin ducts (pathogenic life cycle). However, the mechanism by which the nematode affects the resin canals and the circulation of water in the tracheids, leading to rapid death of the host, is still not fully understood (Koo et al., 2013). Needles of trees infected by B. xylophilus rapidly wilt and dying pines become suitable for oviposition by Monochamus females. The larvae of the beetles will develop inside the wood along with the developing nematode population; upon completion of the insect life cycle, the newly emerged immature adults infected by the dispersal stage JIV will spread the nematode to other healthy pines. The natural spread of B. xylophilus is ensured solely by its insect vectors, and it has been estimated to be 4.5–6 km/year (Togashi and Shigesada, 2006; EFSA, 2019; EFSA 2020b). Monochamus species living on conifers in Europe and Asia are medium sized (15–35 mm) longhorn beetles that attack both healthy and dying trees, or freshly cut trunks (Hellrigl, 1970). Monochamus attacking pine trees are the main vectors of B. xylophilus (13 species currently known worldwide); other Monochamus hosts belong mainly to the genera of Abies and Picea (EFSA, 2020b). Monochamus spp. have four stages of development: egg, larva (4–9 instars), pupa and adult. They usually complete their life cycle in one or more years; only in warmer southern areas they are able to have two generations per year (Hellrigl, 1970; EFSA, 2020b). Adult beetles feed on conifer needles and thin bark of healthy tree twigs; this food source is necessary for sexual maturation after the emergence of new adults. Larvae develop under the bark, in the phloem, cambium and wood of stems or branches of weakened or dying trees, after fire, windthrows, defoliation by insects and drought. Different species of Monochamus have preferences for host plants (ex. Pinus or Picea) and parts of the tree (stem or branches) (EFSA, 2020b). Mature larva or pupa is usually the overwintering stage. After mating, females lay their eggs singly in the bark of the stems or branches of trees, generally gnawing small oviposition cavities with their robust mandibles. Larvae initially develop under the bark, where they excavate galleries feeding on the phloem and cambium; later they penetrate the sapwood by boring deep tunnels oval shaped. Mature larvae burrow a pupal chamber in the outer sapwood close to the bark. Pupal stage usually lasts 2–3 weeks, and immature adults emerge through circular exit holes. Adult beetles can live from 1 to 5 months and are able to fly actively from a few hundred metres up to 2–3.5 km (Akbulut and Stamps, 2012; EFSA, 2020b). However, long flight distances (10 km or more) are flown by adult beetles searching for suitable hosts when these are scarce or absent (EFSA, 2020b). Human assisted spread easily occurs mainly through the transport of infested commodities, particularly wood and wood packaging material containing immature stages (larvae, pupae, immature adults) (EFSA PLH Panel, 2019), as confirmed by the frequency of interceptions (EUROPHYT/TRACES‐NT, online). Monochamus alternatus is native to Asia (China, Japan, Laos, South Korea, Taiwan and Vietnam) where is known as the main vector of B. xylophilus (EFSA PLH Panel, 2018). Monochamus alternatus is usually a univoltine species but becomes multivoltine (2–3 generations a year) in southern subtropical areas, whereas it can take 2 years to complete its cycle in colder climate (CABI, online_b). The beetle overwinters as mature larva. The emergence period of adult beetles varies from late March to early November. Newly metamorphosed adults emerge through a round hole in the bark, 9 mm in diameter (CABI, online_b). A sexual maturation feeding on the bark of pine twigs is needed before mating; 1‐year‐old twigs are generally preferred (CABI, online_b). Female lifetime fecundity largely varies from few eggs up to 343, and increases with beetle longevity, body size, warmer climate conditions and a longer oviposition period. In China (Jiangsu), it is on average 87.6 eggs (CABI, online_b). Adults are nocturnal, poor or medium flyers, usually moving 10–12 m; longer distances are flown before sexual maturity, with the maximum flight distance reported is of 1–2 km (Togashi and Shigesada, 2006; CABI, online_b). Monochamus urussovi is a Palearctic species, present in Europe and northern Asia. Monochamus urussovi is a uni‐ or semivoltine species, completing its life cycle in 1 year in southern Alps (Hellrigl, 1970) and in 2–3 years in northern Asia (Cherepanov, 1990); its biology is apparently very similar to that of M. sartor, of which it is considered a vicariant species for Russia, northern Asia and Japan (Hellrigl, 1970). As ‘the general biology of all European Monochamus spp. is similar’ (Putz et al., 2016), some information on the biology of M. sartor can be used as proxy for M. urussovi. Monochamus urussovi is an important pest for Picea and Abies species (mainly Abies sibirica) and it is only rarely found on Pinus sp. of pine stands in Siberia, where is replaced by M. galloprovincialis (Cherepanov, 1990). Monochamus urussovi is considered a potential vector of B. xylophilus (Evans et al., 1996) and is also known as a vector of the closely related nematode species B. mucronatus (Togashi et al., 2008). Newly metamorphosed adults usually emerge in late May/early June from circular exit holes, 6–12 mm diameter, located in the lower part of the trunk of dead mature trees (Cherepanov, 1990). Adult longevity is up to two months during which they incessantly feed, causing shoot damage (Cherepanov, 1990). After mating, females lay 9–20 eggs singly in the bark, in scars bored with the mandibles. Stems of 16–40 cm diameter or more are preferred for oviposition (Cherepanov, 1990). No specific data is available on flight distance covered by M. urussovi, but M. sartor is known to fly mean distances ranging from 695 to 873 m per flight, with a maximum of 3.1 km and 7.5 km throughout the insect lifespan (Putz et al., 2016). Monochamus galloprovincialis is considered ecologically associated with Pinus sylvestris in all its wide distribution area in Eurasia, ranging from Portugal to east Siberia (Cherepanov 1990; Akbulut and Stamps, 2012); it can also attack other Pinus species, and only occasionally Picea (EFSA PLH Panel, 2018). Monochamus galloprovincialis is currently the sole known vector of B. xylophilus in Europe (Portugal and Spain); it is unknown whether M. galloprovincialis is a vector of B. xylophilus also in Asia. In Europe, the beetle has one generation per year, overwinters as mature larva (Hellrigl, 1970; Akbulut and Stamps, 2012); in northern Asia it requires 2 years to complete its life cycle, and the larvae hibernate under the bark the first year and in the wood the second year (Cherepanov, 1990). After pupation in April–May, adults usually emerge from 4–8 mm wide exit holes and fly for maturation feeding from June to August in Europe (Hellrigl, 1970; Akbulut and Stamps, 2012) and from July to September in Northern Asia (Cherepanov, 1990). Monochamus galloprovincialis attacks preferably branches of 3–8 cm in diameter (Hellrigl, 1970) or thin barked parts of the stem. Females lay eggs singly in cavities made by mandibles (Cherepanov, 1990). Adult lifespan is about 2–3 months; fecundity varies from 37–87 eggs/female in Europe (Akbulut and Stamps, 2012) and about 20 eggs/female in Northern Asia (Cherepanov, 1990). Monochamus galloprovincialis is known to be a good flyer, able to cover distances of 2.3–3.5 km; occasionally 8–13 km in fragmented forests with lower availability of host trees (EFSA, 2020b). Monochamus saltuarius has a wide distribution range in the northern part of Eurasia, from Finland to east Siberia, Sakhalin, Korea and Japan. It is a species mainly associated with Picea and Abies, on which attacks dying branches (3–8 cm in diameter) in the lower part of the stem, or trunks no more than 10 cm in diameter (Hellrigl, 1970). In China, Korea and Japan, however, M. saltuarius is also known as a pest for several Pinus species, and it has been also reported as a vector of B. xylophilus (Koo et al., 2013; EFSA PLH Panel, 2018; Li et al., 2020; Wang et al., 2020). Monochamus saltuarius is univoltine in southern Europe (Hellrigl, 1970), while has a 2‐year life cycle in northern Asia (Cherepanov, 1990). Overwintering stage is the aged/mature larva, depending on voltinism, under the bark or in the wood. Adults emerge from 4 mm exit holes from April to June and can be found up end of August; their lifespan is about 2–3 months (Hellrigl, 1970; Cherepanov, 1990). Oviposition, female fecundity, feeding habits of adults on young shoots and larval development are very similar to other Monochamus species. No specific data are available on flight capability of M. saltuarius, but it could be similar to those of other Monochamus of similar size, as M. galloprovincialis and M. alternatus (EFSA, 2020b). Monochamus sutor is distributed throughout central and northern Eurasia from France, Great Britain and Norway to east Siberia, China, Sakhalin and Japan. The species is mainly associated with Picea abies in central Europe and Pinus sylvestris in northern parts of its European distribution range; trees with a stem diameter of 8–14 cm are preferred (Hellrigl, 1970). According to Cherepanov (1990) in north‐eastern Asia M. sutor is less common in pine stands, where it is replaced by M. galloprovincialis. Monochamus sutor is considered a potential vector of B. xylophilus (Evans et al., 1996); it is also known as a vector of the closely related species B. mucronatus and three other nematode species (CABI, online_g). Monochamus sutor has a univoltine life cycle in central Europe and a semi‐voltine life cycle in northern areas of its range. Depending on voltinism, M. sutor can hibernate as aged or fully mature larva, under the bark or in the wood. Adults emerge from 4–8 mm exit holes in June and July. Reproductive traits, feeding habits of adults, oviposition behaviour and larval development are similar to M. sartor/urussovi (Hellrigl, 1970; Cherepanov, 1990). According to Putz et al. (2016), Monochamus sutor shows a dispersal pattern similar to M. galloprovincialis; the mean distance per flight is 1,653 m and the maximum 5,556 m. |
| Symptoms | Main type of symptoms |
Susceptible pines infected by B. xylophilus can wilt and die very rapidly (30–40 days) in warmer regions, showing a not‐specific needle yellowing. In northern colder areas the infection may be slower, the discoloration of needles may appear gradually, and the death of pines may occur 1–2 years after infection (VKM, 2008; EFSA, 2019; CABI, online_a). Main symptoms of attack by Monochamus on pine shoots and twigs are the feeding scars and oviposition cavities bored by mandibles on thin bark, usually not easy to detect. Wilting or falling of shoots and needle falling may be occasionally observed as consequence of stronger feeding activity (maturation feeding by adults). Frass composed by wood shreds and larval excrements is expelled out of the galleries by larvae and can be frequently observed in bark crevices along the trunk and under the bark. Round exit holes from 4 to 12 mm diameter bored by emerging adults are easily detectable (Hellrigl, 1970; Cherepanov, 1990; CABI, online_e). |
| Presence of asymptomatic plants | The absence of symptoms usually occurs in early stage of infection of B. xylophilus (EPPO, online_l); when pines have been infected in autumn, symptoms can usually appear only in the following year (EFSA PLH Panel, 2019). Furthermore, pines infected by B. xylophilus may survive 1–2 years or even more without showing any external symptom. All asymptomatic plants should show oviposition scars or maturation feeding spots done by Monochamus spp., but these remain visible for short period and are generally difficult to see. | |
| Confusion with other pests |
Both needle discoloration and wilting are non‐specific symptoms of infection of B. xylophilus on pines, and not easily distinguishable from symptoms caused by other pests or diseases. For a reliable identification of B. xylophilus laboratory tests on symptomatic material are always needed (EFSA PLH Panel, 2019; EFSA, 2020a). Symptoms caused by feeding activity of Monochamus spp. adult beetles on pines (feeding scars on shoots/twigs, exit holes) are non‐specific, as they are common to other cerambycid species of similar size living on conifers. Moreover, Monochamus spp. are not always easily distinguishable from each other on the base of morphology, so that expert examination and/or molecular analysis may be needed to confirm the identification (EFSA, 2020b). |
|
| Host plant range |
Pinus species reported as host plants for B. xylophilus so far are Pinus armandii, P. ayacahuite, P. banksiana, P. brutia, P. bungeana, P. caribea, P. cembra, P. clausa, P. contorta, P. densiflora, P. echinata, P. elliottii, P. halepensis, P. hartwegii, P. jeffreyi, P. koraiensis, P. lambertiana, P. leiophylla, P. luchuensis, P. massoniana, P. monticola, P. mugo, P. nigra, P. oocarpa, P. palustris, P. parviflora, P. pinaster, P. pinea, P. ponderosa, P. pungens, P. radiata, P. resinosa, P. strobiformis, P. strobus, P. sylvestris, P. tabuliformis, P. taeda, P. thunbergii, P. virginiana, P. wallichiana, P. yunnanensis; other hosts of the nematode are: Abies amabilis, A. balsamea, A. firma, A. grandis, A. sachalinensis, Cedrus atlantica, C. deodara, Larix decidua, L. kaempferi, L. laricina, L. occidentalis, Picea abies, P. engelmannii, P. glauca, P. jezoensis, P. mariana, P. pungens, P. rubens, P. sitkensis, Pseudotsuga menziesii, Tsuga canadensis, Xanthocyparis nootkatensis (Koo et al., 2013; EFSA, 2019; CABI, online_d; EPPO, online_j). Monochamus species mentioned above are associated with pines and other conifers. The detailed information can be found in Hellrigl (1970); Cherepanov (1990); Yanovskii and Baranchikov (1999); EFSA PLH Panel (2018); CABI (online_b,c,e,f) and EPPO (online_m,n,u,v,w). |
|
| Reported evidence of impact | Bursaphelenchus xylophilus and its vectors (Monochamus spp. non‐European populations) are EU quarantine pests. | |
| Evidence that the commodity is a pathway | Plants for planting (bonsai included) are a possible pathway for B. xylophilus only when used for maturation feeding by Monochamus species vectoring the nematode; differently, large dying trees are required by these beetles to complete their life cycle. Plants for planting are not considered a pathway for non‐EU Monochamus species (EFSA PLH Panel, 2018). | |
| Surveillance information |
There is a specific surveillance protocol implemented for B. xylophilus and its vector M. alternatus in the nursery and its surrounding environment (radius of at least 2 km) (Dossier Section 4.0). Surveillance consists of inspections carried out at least six times/year (Dossier Section 4.0) by visual checking of symptoms on pine trees growing along a designated route (Dossier Section 4.0). Both green and woody material are sampled from symptomatic plants and submitted to laboratory tests (Dossier Section 4.0). In addition, random sampling inspections are planned on asymptomatic Pinus parviflora trees and other pines present in the area surrounding the nursery; samples are sent to the Zhejiang Academy of Science and Technology to check for the presence of pathogenic nematodes (Dossier Section 4.0). Asymptomatic plants are also randomly sampled within the nursery (Dossier Section 4.0). Monochamus alternatus is well‐known pest harmful to the Chinese forests and present also in Zhejiang. It is surveyed by a specific monitoring program based on light trapping, bait trapping and killing traps (without indication of the used lures) (Dossier Section 2.0). No pheromone traps are used. Monitoring is carried out by the Municipal Bureau of Forestry and Water Resources, which has stated the absence of the pest in the survey area of the nursery in recent years (Dossier Section 4.0). Monochamus saltuarius and M. sutor are both present in Zhejiang (Danilevsky, 2019; EPPO, online_s,t). Monochamus saltuarius is a vector of Bursaphelenchus xylophilus (Koo et al., 2013; EFSA PLH Panel, 2020; Li et al., 2020; Wang et al., 2020). Monochamus sutor is a potential vector of B. xylophilus (Evans et al., 1996). No specific surveillance is carried out for these pests. Monochamus urussovi and M. galloprovincialis are absent in Zhejiang (Danilevsky, 2019; CABI, online_c,e; EPPO, online_g,r); no specific surveillance for these pests is carried out. |
|
A.2.2. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Bursaphelenchus xylophilus and Monochamus group (M. alternatus, M. galloprovincialis, M. saltuarius, M. sutor and M. urussovi) between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
For the same period, there are 20 interceptions of Monochamus alternatus, five interceptions of M. galloprovincialis, six interceptions of M. sutor and 116 interceptions of Monochamus sp., all reported for wooden products (wood and bark, pallets, dunnage, etc.) (EUROPHYT/TRACES‐NT, online).
A.2.3. Evaluation of the implementation and relevance of specific measures in China
Commission Implementing Regulation (EU) 2019/2072 specifies in point 30 of Annex VII measures which are required for the import of the commodity from China.
The below overview provides special requirements for naturally or artificially dwarfed plants for planting other than seeds according to Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 including an assessment of whether or not the applicant country implements those measures with respect to Bursaphelenchus xylophilus and Monochamus group (M. alternatus, M. galloprovincialis, M. saltuarius, M. sutor and M. urussovi) identified in this Opinion. The Panel assumes that information on treatments required to be included in the phytosanitary certificate is provided by the applicant country according to the Article 71 of Regulation (EU) 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’.
| Special requirements as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 | Implementation of the special requirements in China according to information provided in the Dossier | Relevance of special requirements for the pest including uncertainties |
|---|---|---|
| ‘Official statement that: | – | – |
| a) the plants, including those collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime, | Yes | Yes |
| b) the plants in the nurseries referred to in point (a) of this entry: | – | – |
| i) at least during the period referred to in point (a) of this entry: | – | – |
| — were potted, in pots which are placed on shelves at least 50 cm above ground, |
Yes, partially. Pots are also reported to be kept on the ground. |
No |
| — have been subjected to appropriate treatments to ensure freedom from non‐European rusts, and the active ingredient, concentration and date of application of these treatments has been mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’. |
Yes. Treatments are appropriate. They are expected to reduce the likelihood of infection of the pathogens and the rate of colonisation of plant tissues, although it is uncertain if freedom from non‐European rusts could be reachable. Treatments used are listed in Table 6.
Uncertainties:
|
No |
| — have been officially inspected at least six times a year at appropriate intervals for the presence of Union quarantine pests of concern in accordance with Regulation (EU) No 2016/2031, and these inspections have also been carried out on plants in the immediate vicinity of the nurseries referred to in point (a) of this entry, at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus, | Yes |
Yes. It is known from the Dossier that asymptomatic plants are tested, however the number is not provided. |
| — have been found free, in these inspections, from the relevant Union quarantine pests of concern as specified in the previous indent, infested plants have been removed and the remaining plants, where appropriate, have been effectively treated and have been held for an appropriate period and inspected to ensure freedom from such pests, | Yes | Yes |
| — have been planted in either an unused artificial growing medium or in a natural growing medium, which has been treated by fumigation or by appropriate heat treatment and has been of any Union quarantine pests, | Yes | No |
| — have been kept under conditions which ensure that the growing medium has been maintained free from Union quarantine pests and within 2 weeks prior to dispatch, have been: | Yes | No |
| — shaken and washed with clean water to remove the original growing medium and kept bare rooted, or | – | – |
| — shaken and washed with clean water to remove the original growing medium and replanted in growing medium which meets the conditions laid down in (i) fifth indent, or | – | – |
| — subjected to appropriate treatments to ensure that the growing medium is free from Union quarantine pests, and the active ingredient, concentration and date of application of these treatments have been indicated on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031 under the rubric ‘Disinfestation and/or disinfection treatment’. | Yes | No |
| ii) were packed in closed containers which have been officially sealed and bear the registration number of the registered nursery, and this number has been indicated under the rubric ‘Additional declaration’ on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, enabling the consignments to be identified.’ | Yes | Yes |
A.2.4. Reference List
Akbulut S and Stamps W, 2012. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. Forest Pathology, 42, 89–99.
CABI (Centre for Agriculture and Bioscience International), online_a. Datasheet Bursaphelenchus xylophilus (pine wilt nematode). Available online: https://www.cabi.org/isc/datasheet/10448 [Accessed: 2 May 2021].
CABI (Centre for Agriculture and Bioscience International), online_b. Datasheet Monochamus alternatus (Japanese pine sawyer). Available online: https://www.cabi.org/isc/datasheet/34719 [Accessed: 2 May 2021].
CABI (Centre for Agriculture and Bioscience International), online_c. Datasheet Monochamus urussovi (white mottled sawyer). Available online: https://www.cabi.org/isc/datasheet/34737 [Accessed: 2 May 2021].
CABI (Centre for Agriculture and Bioscience International), online_d. Datasheet Bursaphelenchus xylophilus (pine wilt nematode). Available online: https://www.cabi.org/isc/datasheet/10448 [Accessed: 6 May 2021].
CABI (Centre for Agriculture and Bioscience International), online_e. Datasheet Monochamus galloprovincialis (pine sawyer). Available online: https://www.cabi.org/isc/datasheet/34722 [Accessed: 6 June 2021].
CABI (Centre for Agriculture and Bioscience International), online_f. Datasheet Monochamus saltuarius (Japanese pine sawyer). Available online: https://www.cabi.org/isc/datasheet/34733 [Accessed: 6 June 2021].
CABI (Centre for Agriculture and Bioscience International), online_g. Datasheet Monochamus sutor (small white‐marmorated longicorn). Available online: https://www.cabi.org/isc/datasheet/34735 [Accessed: 6 June 2021].
Cherepanov AJ, 1990. Cerambycidae of Northern Asia. Volume 3, Lamiinae, Part 1, EJ Brill, Leiden, New York, Kobenhavn, Koln. 395 pp.
Danilevsky ML, 2019. Catalogue of Palearctic Cerambycoidea. Updated 12.07.2019. 275 pp. Available online: https://www.zin.ru/animalia/coleoptera/rus/danlists.htm [Accessed 4 May 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online_a. UK Risk Register Details for Bursaphelenchus xylophilus. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=13891 [Accessed: 8 June 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online_b. UK Risk Register Details for Monochamus alternatus. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=9594 [Accessed: 8 June 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online_c. UK Risk Register Details for Monochamus galloprovincialis Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=16706&riskId=16706 [Accessed: 8 June 2021].
EFSA (European Food Safety Authority), Baker R, Gilioli G, Behring C, Candiani D, Gogin A, Kaluski T, Kinkar M, Mosbach‐Schulz O, Neri FM, Preti S, Rosace MC, Siligato R, Stancanelli G and Tramontini, 2019. Bursaphelenchus xylophilus ‐ Pest Report and Datasheet to support ranking of EU candidate priority pests [Data set]. Zenodo. https://doi.org/10.5281/zenodo.2788667
EFSA (European Food Safety Authority), Schenk M, Loomans A, den Nijs L, Hoppe B, Kinkar M and Vos S, 2020a. Pest survey card on Bursaphelenchus xylophilus. EFSA supporting publication 2020;EN‐1782, 32 pp. https://doi.org/10.2903/sp.efsa.2020.EN‐1782
EFSA (European Food Safety Authority), Schenk M, Loomans A, Kinkar M, Vos S, 2020b. Pest survey card on non‐European Monochamus spp. EFSA supporting publication 2020;EN‐1781, 24 pp. https://doi.org/10.2903/sp.efsa.2020.EN‐1781
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Fejer Justese n A, MacLeod A, Magnusson CS,Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A,Yuen J, Zappalà L, Grégoire J‐C, Kertész V and Milonas P, 2018. Scientific Opinion on the pest categorisation of non‐EU Monochamus spp. EFSA Journal 2018;16(11):5435, 35 pp. https://doi.org/10.2903/j.efsa.2018.5435
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Vettraino AM, Leuschner R, Mosbach‐Schulz O, Rosace MC and Potting R, 2019. Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. https://doi.org/10.2903/j.efsa.2019.5667
EPPO (European and Mediterranean Plant Protection Organization), 2009. Report of a Pest Risk Analysis for Bursaphelenchus xylophilus, 09/15449. 63 pp. Available online: https://pra.eppo.int/getfile/e575b4b0‐3f6c‐4195‐9245‐0b630d57dc58
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A2 List of pests recommended for regulation as quarantine pests, version 2019‐09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed: 2 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Bursaphelenchus xylophilus (BURSXY), Categorization. Available online: https://gd.eppo.int/taxon/BURSXY/categorization [Accessed: 2 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Monochamus alternatus (MONCAL), Categorization. Available online: https://gd.eppo.int/taxon/MONCAL/categorization [Accessed: 2 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Monochamus urussovi (MONCUR), Categorization. Available online: https://gd.eppo.int/taxon/MONCUR/categorization [Accessed: 2 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_e. Bursaphelenchus xylophilus (BURSXY), Distribution. Available online: https://gd.eppo.int/taxon/BURSXY/distribution [Accessed: 2 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_f. Monochamus alternatus (MONCAL), Distribution. Available online: https://gd.eppo.int/taxon/MONCAL/distribution [Accessed: 2 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_g. Monochamus urussovi (MONCUR), Distribution. Available online: https://gd.eppo.int/taxon/MONCUR/distribution [Accessed: 2 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_h. Situation of Bursaphelenchus xylophilus in Portugal. EPPO Reporting Service no. 04‐2011, Num. article:2011/070. Available online: https://gd.eppo.int/reporting/article‐186 [Accessed: 4 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_i. Isolated finding of Bursaphelenchus xylophilus in Castilla y Léon. EPPO Reporting Service no. 07‐2018, Num. article:2018/140. Available online: https://gd.eppo.int/reporting/article‐6334 [Accessed: 4 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_j. Bursaphelenchus xylophilus (BURSXY), Hosts. Available online: https://gd.eppo.int/taxon/BURSXY/hosts [Accessed: 6 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_k. Bursaphelenchus xylophilus (BURSXY), EPPO datasheet Available online: https://gd.eppo.int/taxon/BURSXY/datasheet [Accessed: 7 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_l. PM 9/1 (6) Bursaphelenchus xylophilus and its vectors: procedures for official control. Bulletin OEPP/EPPO Bulletin (2018), 48, 503–515. Available online: https://onlinelibrary.wiley.com/doi/10.1111/epp.12505 [Accessed: 9 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_m. Monochamus alternatus (MONCAL), Hosts. Available online: https://gd.eppo.int/taxon/MONCAL/hosts [Accessed: 4 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_n. Monochamus urussovi (MONCUR), Hosts. Available online: https://gd.eppo.int/taxon/MONCUR/hosts [Accessed: 4 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_o. Monochamus galloprovincialis (MONCGA), Categorization. Available online: https://gd.eppo.int/taxon/MONCGA/categorization [Accessed: 2 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_p. Monochamus saltuarius (MONCSL), Categorization. Available online: https://gd.eppo.int/taxon/MONCSL/categorization [Accessed: 2 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_q. Monochamus sutor (MONCSU), Categorization. Available online: https://gd.eppo.int/taxon/MONCSU/categorization [Accessed: 2 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_r. Monochamus galloprovincialis (MONCGA), Distribution. Available online: https://gd.eppo.int/taxon/MONCGA/distribution [Accessed: 2 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_s. Monochamus saltuarius (MONCSL), Distribution. Available online: https://gd.eppo.int/taxon/MONCSL/distribution [Accessed: 2 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_t. Monochamus sutor (MONCSU), Distribution. Available online: https://gd.eppo.int/taxon/MONCSU/distribution [Accessed: 2 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_u. Monochamus saltuarius (MONCSL), Hosts. Available online: https://gd.eppo.int/taxon/MONCSL/hosts [Accessed: 6 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_v Monochamus galloprovincialis (MONCGA), Hosts. Available online: https://gd.eppo.int/taxon/MONCGA/hosts [Accessed: 2 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_w. Monochamus sutor (MONCSU), Hosts. Available online: https://gd.eppo.int/taxon/MONCSU/hosts [Accessed: 2 June 2021].
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://ec.europa.eu/food/plants/plant‐health‐and‐biosecurity/European‐union‐notification‐system‐plant‐health‐interceptions‐en [Accessed: 17 June 2021].
Evans HF, McNamara DG, Braasch H, Chadoeuf J and Magnusson C, 1996. Pest Risk Analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. EPPO Bulletin, 26, 199–249.
Hellrigl KG, 1970. Die Bionomie der europaischen Monochamus‐Arten (Coleopt., Cerambycid.) und ihre Bedeutung fur die Forstund Holzwirtschaft. Redia, 52, 367–509.
Koo C‐D, Lee H‐Y, Han J‐H, Sung J‐H and Shin J‐H, 2013. Infection behavior and distribution of Bursaphelenchus xylophilus in Pinus densiflora trees, Forest Science and Technology, 9, 81–86. https://doi.org/10.1080/21580103.2013.801168
Putz J, Vorwagner EM and Hoch G, 2016. Flight performance of Monochamus sartor and Monochamus sutor, potential vectors of the pine wood neematode. Lesnicky Casopis – Forestry Journal, 62, 195–201. https://doi.org/10.1515/forj‐2016‐0024
State Forestry and Grassland Administration Government, online_a. Announcement of the State Forestry and Grassland Administration (No. 5 of 2021). Available online: https://www.forestry.gov.cn/main/3457/20210806/105838059235194.html [Accessed: 31 August 2021].
State Forestry and Grassland Administration Government, online_b. Announcement of the State Forestry and Grassland Administration (No. 14 of 2021). Available online: https://www.forestry.gov.cn/main/5461/20210812/102005939215795.html [Accessed: 31 August 2021].
Togashi K and Shigesada N, 2006. Spread of the pinewood nematode vectored by the Japanese sawyer: modeling and analytical approaches. Population Ecology, 48, 271–283.
Togashi K, Taga Y, Iguchi K and Aikawa T, 2008. Bursaphelenchus mucronatus (Nematoda: Aphelenchoididae) vectored by Monochamus urussovi (Coleoptera: Cerambycidae) in Hokkaido, Japan. Journal of Forest Research, 13, 127–131.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
VKM (Norwegian Scientific Committee for Food Safety), 2008. Pest risk assessment of the Pine Wood Nematode (PWN) Bursaphelenchus xylophilus in Norway ‐ Part 1. Opinion of the Panel on Plant Health of the Norwegian Committee of Food Safety. VKM, Oslo, Norway, 48 pp.
Yanovskii VM and Baranchikov YuN, 1999. On polyphagy of the fir sawyer beetle Monochamus urussovi (Colepotera, Cerambycidae). Zoologicheskii Zhurnal, 78(7), 889–890.
A.3. Ceroplastes rubens
A.3.1. Organism information
| Taxonomic information |
Current valid scientific name: Ceroplastes rubens Synonyms: Ceroplastes rubens minor Name used in the EU legislation: – Order: Hemiptera Family: Coccidae Common name: pink wax scale, red wax scale, ruby wax scale Name used in the Dossier: Ceroplastes rubens |
| Group | Insects |
| EPPO code | CERPRB |
| Regulated status |
The pest is neither regulated in the EU nor listed by EPPO. Ceroplastes rubens is a quarantine species in Mexico and Israel. It is reported on A1 list of Argentina, Brazil, Chile and Southern Africa. It is on A2 list of COSAVE (=Comite de Sanidad Vegetal del Cono Sur – Argentina, Brazil, Chile, Paraguay, Peru and Uruguay) (EPPO, online_a). |
| Pest status in China | Ceroplastes rubens is present in China in Anhui, Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hebei, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Quinghai, Shaanxi, Shanghai, Shanxim, Sichuan, Tibet, Xianggang (Hong Kong), Xizang, Yunnan and Zhejiang (Li et al., 1997; Dossier Section 4.0; CABI, online; EPPO, online_b; García Morales et al., online). |
| Pest status in the EU |
Ceroplastes rubens is absent in the EU (EPPO, online_b). However, it has been intercepted many times on plants to the EU. The scale was found in Hungary (greenhouse in Budapest Botanical Garden) on plants of Schefflera sp. in 2012 (Fetykó and Kozár, 2012; Kozár et al., 2013; CABI, online; García Morales et al. online) and in Germany (greenhouse in Brandenburg) (Schönfeld, 2015; CABI, online; García Morales et al., online). However, there is no information whether the scales were eradicated or acclimatised. |
| Host status on Pinus parviflora and P. thunbergii | Pinus parviflora and P. thunbergii are reported as hosts of C. rubens (García Morales et al., online). |
| PRA information |
Pest Risk Assessment currently available: – From the USA: Importation of Fresh Mango Fruit (Mangifera indica L.) from India into the Continental United States. A Qualitative, Pathway‐Initiated Pest Risk Assessment (USDA, 2006), – From the UK: Rapid Assessment of the need for a detailed Pest Risk Analysis for Ceroplastes rubens Maskell (Malumphy, 2011), – From New Zealand: Generic Pest Risk Assessment: armoured scale insects (Hemiptera: Coccoidea: Diaspididae) on the fresh produce pathway (Berry et al., 2014), – UK Risk Register Details for Ceroplastes rubens (DEFRA, online). |
| Other relevant information for the assessment | |
| Biology |
Ceroplastes rubens is a scale insect, native to Africa and widely distributed in the world – Africa, Asia, Caribbean islands, Europe, North America (Florida, Hawaii), South America (Columbia, Venezuela) and Oceania (Berry et al., 2014; EPPO, online_b; García Morales et al., online), particularly in tropical and subtropical areas. It is also extending into temperate areas (Malumphy, 2014). Adults and nymphs of C. rubens feed on leaves, twigs, stems (Malumphy, 2014) and very rarely on fruits (Berry et al., 2014). Like most Ceroplastes species, they prefer the upper side of leaves (Malumphy, 2014) and usually settle near to or on the leaf veins (Waterhouse and Sands, 2001). Young stages and adult females are covered by wax. Females develop through an egg, four nymphal instars and adult. Winged males are rare and have one additional instar compared to females (Malumphy, 2014). The species reproduces mainly parthenogenetically (Waterhouse and Sands, 2001; Berry et al., 2014). Females lay eggs under their bodies (Vithana et al., 2018), usually between 300 and 1187 eggs. The first instar is called crawler, which moves until it finds a suitable place on vegetation to settle in. Crawlers can be dispersed over longer distances by air currents or vector animals (Malumphy, 2014). Ceroplastes rubens has up to two generations annually (Camacho and Chong, 2015): usually one generation in Japan and China (Itioka and Inoue 1991; Xia et al., 2005) and two generations in Australia (Loch and Zalucki, 1997). In Shanghai it was reported that C. rubens overwinters as fertilised female (Xia et al., 2005). Slide mounted females are 1.2–1.5 mm wide and 1.8–2.5 mm long (Ben‐Dov et al., 2000). The scale is highly visible because it produces honeydew and females are covered by white, cream, pink, reddish or brownish thick wax, between 3.5 (Malumphy, 2014) and 5 mm long (Ben‐Dov et al., 2000). Eggs and nymphs are pink (Vithana et al., 2018). Ants are protecting scales from natural enemies in order to collect the honeydew and help the scales to aggregate (Itioka and Inoue, 1996). Possible pathways of entry for C. rubens are plants for planting, foliage and less likely fruits (Malumphy, 2011). |
| Symptoms | Main type of symptoms |
Main symptoms of infestation are the presence of the insect on the leaves, honeydew and sooty mould (Waterhouse and Sands, 2001; Prinsloo and Uys, 2015). Higher infestation can lead to yellowing of leaves, drop of leaves and fruits (Prinsloo and Uys, 2015). Ceroplastes rubens reduces photosynthesis and makes fruits unmarketable (Waterhouse and Sands, 2001). There is no information on the symptoms caused to Pinus plants. |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. | |
| Confusion with other pests | It can be confused with other Ceroplastes species of similar size. A morphological or molecular analysis is needed in order to distinguish them. | |
| Host plant range |
Ceroplastes rubens is highly polyphagous species of more than 80 families of both shrubs and trees. The main hosts are avocado (Persea americana), citrus (Citrus spp.), gardenia (Gardenia spp.), mango (Mangifera americana) and palms (Berry et al., 2014; García Morales et al., online). Conifer hosts are Agathis lanceolata, Cedrus deodara, Cephalotaxus, Nageia nagi, Pinus (P. caribaea, P. densiflora, P. elliottii, P. montezumae, P. parviflora, P. radiata, P. tabuliformis, P. taeda and P. thunbergii) and Podocarpus (García Morales et al., online). Broadleaves host plants are Acacia, Acer, Buxus, Chrysanthemum, Cycas, Cydonia, Euonymus, Euphorbia, Ficus, Hedera, Hibiscus, Ilex, Laurus, Ligustrum, Malus, Magnolia, Morus, Nerium, Olea, Prunus, Pyrus, Rhododendron, Rosa, Spiraea, Viburnum, Wisteria and many others (Berry et al., 2014; García Morales et al., online). DEFRA (online) reports C. rubens as very unlikely to be able to overwinter outdoors in the UK and therefore establishment will be restricted to protected ornamental plants. |
|
| Reported evidence of impact |
Ceroplastes rubens is a major pest of citrus in Australia, Hawaii, Korea, China and Japan (Malumphy, 2014) and of umbrella trees (Schefflera actinophylla) in Queensland of Australia (Loch and Zalucki, 1998). There is no evidence of impact on Pinus plants. |
|
| Evidence that the commodity is a pathway |
According to Malumphy (2011), C. rubens can travel with plants for planting. Ceroplastes rubens has been intercepted on bonsai plants of Ilex from China in 2018 (EUROPHYT, online) and on other tropical plants destined to the UK (Malumphy, 2010), the Netherlands (Jansen, 1995), Hungary (Fetykó and Kozár, 2012) and Germany (Schönfeld, 2015). |
|
| Surveillance information |
Ceroplastes rubens is recorded in Dossier Sections 4.0 and 5.0 as a pest occurring in Zhenjiang province where the nursery is located. No surveillance information for this pest is currently available from China. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. |
|
A.3.2. Possibility of pest presence in the nursery
A.3.2.1. Possibility of entry from the surrounding environment
Ceroplastes rubens is known to be present in several provinces of China. The nursery is located in Zhejiang province, where C. rubens is reported to be present (Dossier Section 4.0; García Morales et al., online). Ceroplastes rubens is polyphagous scale able to infest leaves, twigs, stems and fruits of plants. According to Dossier Sections 4.0 and 5.0, the nursery is surrounded by different plants, among which Acer buergerianum, Acer palmatum, Camellia japonica, Camellia sasanqua, Cedrus deodara, Chaenomeles, Cinnamomum, Citrus maxima, Citrus paradisi, Cycas revoluta, Diospyros kaki, Distylium racemosum, Eriobotrya japonica, Fatsia japonica, Hibiscus mutabilis, Ilex cornuta, Lagerstroemia indica, Laurus nobilis, Morus alba, Nandina domestica, Nerium oleander, Photinia glabra, Pinus elliottii, Pinus parviflora, Pinus thunbergia, Pittosporum tobira, Prunus domestica, Prunus mume, Punica granatum, Rhododendron indicum, Spiraea thunbergii are hosts of C. rubens (Berry et al., 2014; García Morales et al., online_a). Therefore, the Panel assumes that the pest can be present in the production area of bonsai plants destined for export to the EU.
The possibility of entry for C. rubens from surrounding environment to nursery is through crawler dispersal including air currents, human, ants and animal assisted spread. As stated in Dossier Section 4.0, the bonsai cultivation site is protected by a 40‐mesh insect‐proof net (0.4 mm). The body width of crawlers is not known although in other species of the same genus is around 0.4 mm (Tremblay, 1981). The Panel assumes that at least part of the crawlers can go through the net.
Uncertainties
-
–
There is no surveillance information on the presence or population pressure of C. rubens in the area where the nursery is located.
-
–
No information available on the distance of the nursery to sources of C. rubens in the surrounding environment.
-
–
The quantity of C. rubens crawlers that can go through the net, because precise information on their size is not available.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for C. rubens to enter the nursery. The pest can be present in the surrounding area because of the presence of suitable hosts and the transferring rate could be enhanced by movement by wind, animals, ants and humans.
A.3.2.2. Possibility of entry with new plants/seeds
All seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (P. thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triaimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
The growing media used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0). Possibility of entry with seeds or soil/growing media is not relevant for C. rubens.
Uncertainties
-
–
It is not clear if and how mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pest could enter the nursery with new plants/seeds or soil/growing media.
A.3.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of Pinus parviflora are taken (Dossier Section 4.0).
Ceroplastes rubens within the nursery can spread by hitchhiking on clothing of nursery staff, by ants, by wind and by scions from infested mother plants. In addition, at least part of the crawlers can go through the net.
Spread within the nursery through the movement of soil, water, equipment and tools is not relevant.
Uncertainties
-
–
There is no information on the presence or population pressure of the pest in the nursery.
-
–
The quantity of C. rubens crawlers that can go through the net, because precise information on their size is not available.
-
–
Whether the grafting time matches the crawler occurrence/presence.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pest within the nursery is possible due to the presence of suitable hosts (e.g. mother plants, commodity).
A.3.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Ceroplastes rubens between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.3.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on Ceroplastes rubens is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
Physical separation of the bonsai plants may have an effect on reducing risk of infestation with C. rubens, especially in the production base.
Uncertainties:
|
| 2 | Growing medium and its treatment | No | Not applicable. |
| 3 | Treatment of seeds | No | Not applicable. |
| 4 | Insecticide and acaricide treatments | Yes |
Spray of contact insecticides can only kill the crawlers that are present on the plants at the time of spraying. Once they are fixed and covered by wax they are not expected to be killed by the specified insecticides.
Uncertainties:
|
| 5 | Fungicide treatments | No | Not applicable. |
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | No | Not applicable. |
| 8 | Official inspections during production | Yes |
Ceroplastes rubens is generally visible.
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
Ceroplastes rubens is generally visible.
Uncertainties:
|
A.3.5. Overall likelihood of pest freedom for Ceroplastes rubens on grafted bonsai plants
A.3.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The population density around the nursery is low and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are effective. The detection before export is carefully done. Pinus is not a suitable host.
A.3.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
The population density around the nursery is high and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are only partially effective. The detection before export is not detailed enough to spot insects when they are present without obvious signs. Pinus is a suitable host.
A.3.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
Different combinations of population density around the nursery, effect of the net barrier and of the insecticide applications may result in an intermediate scenario as they are acting independently of one another on the presence of the insect in the commodity. Median is shift to the left side (lower infestation rate) because of the moderate susceptibility of Pinus and the relatively easy detection of scales.
A.3.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The signs of the insect occurrence (large sizes of females, honeydew) are generally detectable. We assume that a high infestation level is less likely to happen than having smaller number of infested plants where the insect density is low and difficult to detect.
A.3.5.5. Elicitation outcomes of the assessment of the pest freedom for Ceroplastes rubens on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.1) and pest freedom (Table A.2).
Table A.1.
Elicited and fitted values of the uncertainty distribution of pest infestation by Ceroplastes rubens per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 20 | 70 | 125 | 280 | 600 | ||||||||||
| EKE | 20.0 | 21.3 | 24.1 | 31.3 | 43.6 | 62.2 | 84.3 | 140 | 217 | 267 | 332 | 402 | 480 | 541 | 600 |
The EKE results are the BetaGeneral (0.73607,2.5254,19.5,740) distribution fitted with @Risk version 7.6.
Table A.2.
The uncertainty distribution of plants free of Ceroplastes rubens per 10,000 plants calculated by Table A.1
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,400 | 9,720 | 9,875 | 9,930 | 9,980 | ||||||||||
| EKE results | 9,400 | 9,459 | 9,520 | 9,598 | 9,668 | 9,733 | 9,783 | 9,860 | 9,916 | 9,938 | 9,956 | 9,969 | 9,976 | 9,979 | 9,980 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.2.
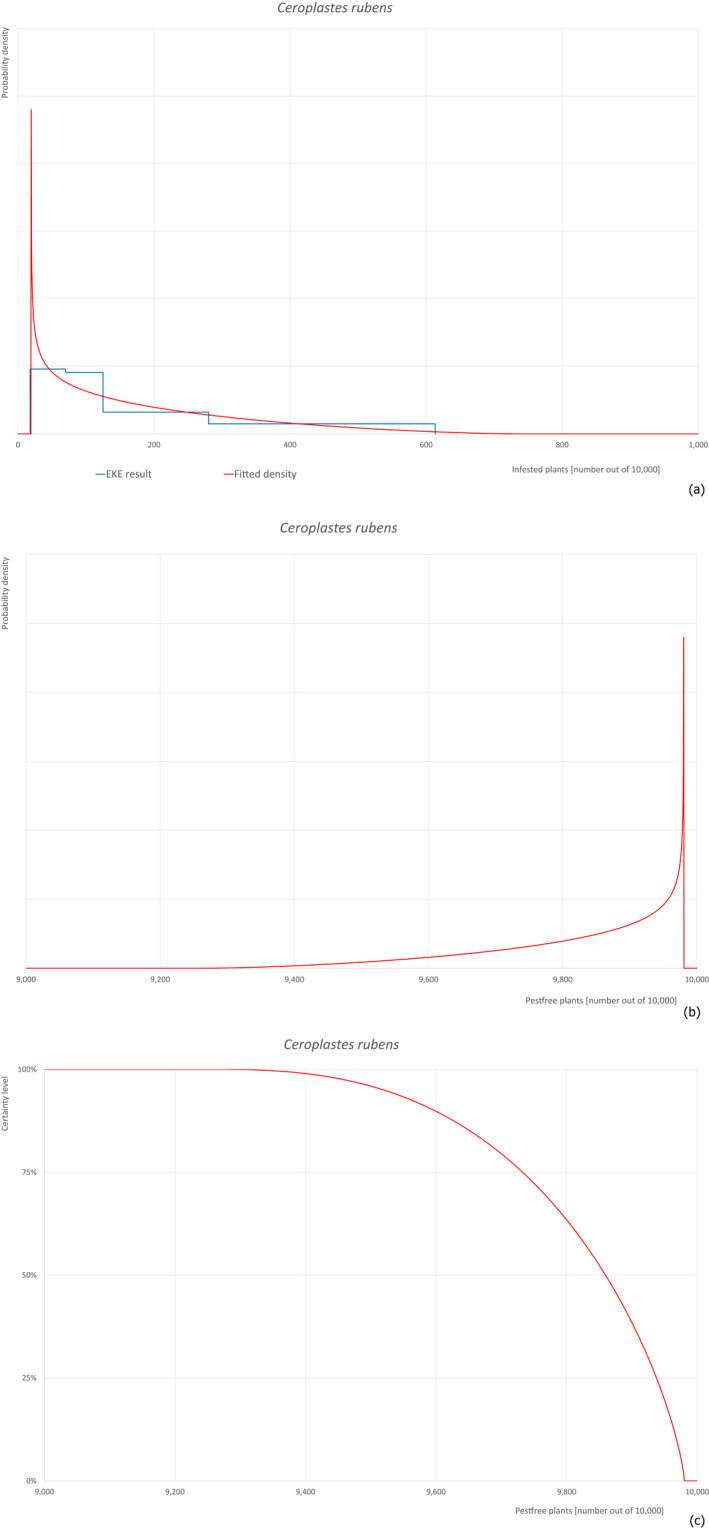
Figure A.1: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.3.6. Reference List
Ben‐Dov Y, Matile‐Ferrero D and Gafny R, 2000. Taxonomy of Ceroplastes rubens Maskell with description of a related new species (Hemiptera: Coccoidea: Coccidae) from Reunion, including DNA polymorphism analysis. Annales de la Société entomologique de France, 36, 423–433.
Berry JA, Bywater C, Siva A, Clark S, Crook K, McDonald C, Ormsby M, Richmond J, Dyck M, O’Neill B, Hill G and Zlotina M, 2014. Generic Pest Risk Assessment: Armoured scale insects (Hemiptera: Coccoidea: Diaspididae) on the fresh produce pathway. Ministry for Primary Industries. Wellington, New Zealand. 76 pp.
CABI (Centre for Agriculture and Bioscience International), online. Ceroplastes rubens (red wax scale). Available online: https://www.cabi.org/cpc/datasheet/12351 [Accessed: 13 May 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Ceroplastes rubens. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=8292 [Accessed: 8 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_a. Ceroplastes rubens (CERPRB), Categorization. Available online: https://gd.eppo.int/taxon/CERPRB/categorization [Accessed: 14 May 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Ceroplastes rubens (CERPRB), Distribution. Available online: https://gd.eppo.int/taxon/CERPRB/distribution [Accessed: 13 May 2021].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Fetykó K and Kozár F, 2012. Records of Ceroplastes Gray 1828 in Europe, with an identification key to species in the Palaearctic Region. Bulletin of Insectology, 65, 291–295.
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: A literature‐based model of scale insect biology and systematics, Ceroplastes rubens. Available online: http://scalenet.info/catalogue/Ceroplastes%20rubens/ [Accessed 13 May 2021].
Itioka T and Inoue T, 1991. Settling‐site selection and survival of two scale insects, Ceroplastes rubens and C. ceriferus, on citrus trees. Researches on Population Ecology, 33, 69–85. https://doi.org/10.1007/BF02514575
Itioka T and Inoue T, 1996. Density‐dependent ant attendance and its effects on the parasitism of a honeydew‐producing scale insect, Ceroplastes rubens. Oecologia, 106, 448–454. https://doi.org/10.1007/bf00329700
Jansen MGM, 1995. Scale insects (Homoptera: Coccinea) from import interceptions and greenhouses in the Netherlands. Israel Journal of Entomology, 29, 131–146.
Kozár F, Benedicty ZK, Fetykó K, Kiss B and Szita É, 2013. An annotated update of the scale insect checklist of Hungary (Hemiptera, Coccoidea). ZooKeys, 309, 49–66. https://doi.org/10.3897/zookeys.309.5318
Li L, Wang R and Waterhouse DF, 1997. The distribution and importance of arthropod pests and weeds of agriculture and forestry plantations in southern China. Australian Centre for International Agricultural Research (ACIAR). 201 pp.
Loch AD and Zalucki MP, 1998. Outbreaks of pink wax scale, Ceroplastes rubens Maskell (Hemiptera: Coccidae), on umbrella trees in south‐eastern Queensland: Patterns of parasitisation. Australian Journal of Entomology, 37, 328–334. https://doi.org/10.1111/j.1440‐6055.1998.tb01592.x
Malumphy C, 2010. The status of wax scales (Hemiptera: Coccidae: Ceroplastinae) in Britain. Entomologists Monthly Magazine, 146, 105–112.
Malumphy C, 2011. Rapid Assessment of the need for a detailed Pest Risk Analysis for Ceroplastes rubens Maskell. FERA (The Food and Environment Research Agency). 8 pp. Available online: https://www.fera.defra.gov.uk/plants/plantHealth/pestsDiseases/documents/ceroplastesRubens.pdf
Malumphy C, 2014. Pink wax scale, Ceroplastes rubens. FERA (The Food and Environment Research Agency). 6 pp. Available online: https://randd.defra.gov.uk/Document.aspx?Document=13218_08Appendix7CeroplastesrubensDatasheet.pdf
Prinsloo G and Uys V, 2015. Insects of Cultivated Plants and Natural Pastures in Southern Africa, first ed. Entomological Society of Southern Africa, Cape Town, Africa. 786 pp.
Schönfeld U, 2015. Coccoidea species in Brandenburg. Journal für Kulturpflanzen, 67, 337–341 (in German).
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
Tremblay E, 1981. Entomologia applicata. Second volume, First part. Liguori, Napoli. 310 pp (in Italian).
USDA (United States Department of Agriculture), 2006. Importation of Fresh Mango Fruit (Mangifera indica L.) from India into the Continental United States. A Qualitative, Pathway‐Initiated Pest Risk Assessment. United States Department of Agriculture, Animal and Plant Health Inspection Service, Plant Protection and Quarantine Center for Plant Health Science and Technology Plant Epidemiology and Risk Analysis Laboratory. 90 pp. Available online: https://www.cerambycoidea.com/titles/usda2006.pdf
Vithana KV, Sirisena UG and Hemachandra KS, 2018. Pink wax scale (Ceroplastes rubens) a growing threat to agriculture in Sri Lanka. Horticultural Crops Research and Development Institute, Gannoruwa, Sri Lanka. Tropical Agricultural Research, 30, 13–22. https://doi.org/10.4038/tar.v30i2.8305
Waterhouse DF and Sands DPA, 2001. Classical biological control of arthropods in Australia. ACIAR monograph No. 77. CSIRO Entomology and CSIRO Publishing: Canberra and Melbourne. 560 pp.
Xia C, Zhang W, Sun X and Li H, 2005. Observations on biological habits of Ceroplastes rubens Maskell in Shanghai. Journal of Shanghai Jiaotong University‐Agricultural Science, 23(4), 439–442.
A.4. Coleosporium group (C. asterum, C. eupatorii and C. phellodendri)
A.4.1. Organism information
| Taxonomic information |
1. Coleosporium asterum Current valid scientific name: Coleosporium asterum Synonyms: Coleosporium montanum, Coleosporium solidaginis, Peridermium montanum, Puccinia extensicola var. solidaginis, Stichopsora solidaginis, Uredo solidaginis Name used in the EU legislation: Coleosporium asterum (Dietel) Sydow & P. Sydow Order: Pucciniales Family: Coleosporiaceae Common name: rust of aster, needle cast of red pine, rust of Solidago Name used in the Dossier: Coleosporium asterum 2. Coleosporium eupatorii Current valid scientific name: Coleosporium eupatorii Synonyms: Coleosporium eupatorii Name used in the EU legislation: – Order: Pucciniales Family: Coleosporiaceae Common name: – Name used in the Dossier: Coleosporium eupatorii 3. Coleosporium phellodendri Current valid scientific name: Coleosporium phellodendri Synonyms: – Name used in the EU legislation: Coleosporium phellodendri Komarov Order: Pucciniales Family: Coleosporiaceae Common name: pine needle rust Name used in the Dossier: Coleosporium phellodendri |
| Group | Fungi |
| EPPO code |
COLSAS: Coleosporium asterum COLSEU: Coleosporium eupatorii COLSPH: Coleosporium phellodendri |
| Regulated status |
Coleosporium asterum is listed as harmful organism of concern according to EU Decision (2002/499/EC ‐ 2010/646/EU) about import of P. parviflora from Korea. The fungus is also included in Mexico’s quarantine list since 2018. Coleosporium eupatorii is not regulated in the EU. Coleosporium phellodendri is listed as harmful organism of concern according to EU Decision (Commission Implementing Regulation (EU) 2020/1217) about import of Pinus parviflora from Japan. Formerly EPPO Alert list (1999–2002) (EPPO, 1999). Coleosporium asterum and C. phellodendri are listed in the Commission Implementing Regulation (EU) 2020/1217 as pathogens of concern for P. thunbergii. |
| Pest status in China |
All three species are present in China (DEFRA, online_a,b,c; Farr and Rossmann, online). Coleosporium asterum is present in provinces of Anhui, Gansu, Guangdong, Guangxi, Hebei, Heilongjiang, Hubei, Jiangsu, Jiangxi, Jilin, Liaoning, Shaanxi, Shanxi, Shandong, Tibet, Yunnan (Dossier Section 2.0), Fujian (Zhuang, 1983) and Zhejiang (Dossier Sections 2.0 and 4.0; CABI, online). Coleosporium phellodendri is present in Fujian (Zhuang, 1983), Heilongjiang, Jilin, Liaoning (Dossier Section 2.0; EPPO, online), Shaanxi (Cao et al., 2000; Cao et al., 2017) and Sichuan (Zhang et al., 1997). Coleosporium eupatorii was recorded in the province of Anhui (Anhwei) in 1932 on Eupatorium lindleyanum (Cummins, 1956) and in Fujian on Ainsliaea (Zhuang, 1983). |
| Pest status in the EU |
All absent (DEFRA, online_a,b,c; Farr and Rossmann, online); old records of C. asterum for Europe were rectified (Voglmayr et al., 2020). Coleosporium solidaginis (synonymous of C. asterum) has been reported in the EU (Germany and Northern Spain) by Beenken et al. (2017). However, Beenken et al. (2017) consider C. solidaginis and C. asterum to be different species. |
| Host status of Pinus parviflora and P. thunbergii |
Only Pinus parviflora is considered, because Coleosporium species are only associated with pine needles and P. thunbergii is the rootstock of the commodity under consideration. Coleosporium asterum includes mainly 2‐ and 3‐needles pines as hosts, with the only exception of P. strobus (having 5 needles), Sansford (2015) excludes the 5‐needles P. parviflora from the host list of C. asterum. Nevertheless, P. parviflora belongs to the subsection Strobus (London), suggesting the possibility for P. parviflora to be a potential host of C. asterum. Pinus parviflora is reported as a host of C. asterum in Dossier Section 2.0. Coleosporium eupatorii is reported as pathogen of P. parviflora (Zinno and Endo, 1964; Hiratsuka et al., 1992; Farr and Rossman, online). Coleosporium phellodendri is reported as pathogen of P. parviflora in Dossier Section 2.0. |
| PRA information |
Pest Risk Assessment currently available:
|
| Other relevant information for the assessment | |
| Biology |
Coleosporium species are difficult to identify morphologically, and usually they are identified according to the taxonomic position of their telial hosts (Beenken et al., 2017). Coleosporium has five spore stages: pycnidial, aecial, uredinial, telial and basidial. The pycnidial and the aecial stages develop on the aecial host (pines), while the uredinial, telial and basidial stages develop on the telial hosts which are usually different for every Coleosporium species. Because the uredinial stage produces uredospores that infect other telial hosts, several Coleosporium species may survive and spread even without the aecial host’s presence (Beenken et al., 2017). In late summer, the basidiospores infect the current‐year needles of the host pines (aecial hosts); the pathogen overwinters in needles without producing symptoms until the early spring of the next year, when the pycnidial stage appears on the needle surface. Differently, C. phellodendri remains in the needles for about 18 months, with no external symptoms, a minimal development and an unclear infection potential during that period (Suzuki et al., 2018). In late spring the aecial stage appears on the needles and produces aeciospores that the wind will disperse on the telial hosts where the uredial stage will develop during summer. Uredospores produced by the uredial stage spread the pathogen through the wind on other telial hosts, with possibly multiple generations during summer. Each uredinial generation develops in about 16 days for C. asterum (Kim et al., 2017). At the end of summer, the telial stage appears on the telial hosts and produces the basidial stage. In late summer‐autumn the basidial stage will release the basidiospores returning on the pines and infecting the current‐year needles. There is no specific information about C. eupatorii biology. Dispersal factors include wind and insect vectors, such as Diptera of the genus Mycodiplosis (Henk et al., 2011), although many aspects of the insect lifecycle and their real role in spore dispersals are still uncertain. Wind and insect vectors are reported to allow a range of spore dispersal up to 800 metres (Sansford, 2015). Possible pathways of entry for C. asterum are plants for planting, leaves and cut flowers (Sansford, 2015). Coleosporium asterum was intercepted on cut flowers and foliage of Solidago sp. from Kenya to United Kingdom in 2016 (EUROPHYT, online). |
| Symptoms | Main type of symptoms |
Coleosporium usually has been described based on telia and uredia produced on telial hosts (Cummins, 1956). On Pinus, Coleosporium generally shows yellow‐brown droplets (pycnidial stage) or white and columnar blisters (aecial stage) on second‐year needles (3‐year needles for C. phellodendri), and these symptoms are easy to notice. On telial hosts (not Pinus), the main symptoms are yellow‐orange pustules occurring under the leaves, with possible yellow spotting occurring on the upper side of the leaves. |
| Presence of asymptomatic plants |
The pine needles infected by overwintering basidiospores are asymptomatic from late summer to early spring. Coleosporium phellodendri can remain asymptomatic for as long as 18 months (Suzuki et al., 2018). |
|
| Confusion with other pests |
Needle rust damages can be attributed to biotic factors (mites or scale insects) and abiotic factors (air pollution, drought, herbicide damages). Cankers and galls can be similar to ones caused by insects or other fungi. Spores examination easily identify the Coleosporium genus. Coleosporium species are difficult to determine morphologically, especially when occurring on the aecial host (Beenken et al., 2017). Moreover, the taxonomy of the Coleosporium genus is still under debate, and species identification often needs either molecular analysis or host analysis of the telial stage, which could be different among different Coleosporium species (Beenken et al., 2017). |
| Host plant range |
Aecial hosts for C. asterum are Pinus armandii, P. banksiana, P. contorta, P. densiflora, P. mugo, P. echinata, P. koraiensis, P. massoniana, P. nigra, P. palustris, P. ponderosa, P. radiata, P. resinosa, P. hartwegii, P. sylvestris, P. rigida, P. strobus, P. tabuliformis, P. kesiya var. langbianensis, P. taeda, P. taiwanensis and P. thunbergii (Farr and Rossman, online). Because aecial hosts of C. asterum include only 2‐ and 3‐needles pines, with the only exception of P. strobus (having 5 needles), Sansford (2015) excludes the 5‐needles P. parviflora from the host list of C. asterum. Nevertheless, P. parviflora belongs to the subsection Strobus (London), suggesting the possibility for P. parviflora to be a potential host of C. asterum. Telial hosts of C. asterum include more than 100 species from the family Compositae, mainly from the Aster, Kalimeris and Symphyotrichum genera. Coleosporium eupatorii hosts are less known, and a not comprehensive list of aecial hosts includes Pinus cembra, P. wallichiana, P. koraiensis, P. monticola, P. parviflora var. pentaphylla, P. peuce, P. strobus (Farr and Rossman, online), and other unspecified five‐needle pines (Saho, 1962). Coleosporium eupatorii telial hosts are plants from the genera Ainsliaea, Brickellia, Chromolaena, Eupatorium, Hebeclinium and Stevia (DEFRA, online_b). The aecial hosts of C. phellodendri are Pinus banksiana, P. cembra, P. contorta, P. densiflora, P. hartwegii, P. mugo, P. nigra, P. nigra subsp. laricio, P. nigra subsp. pallasiana, P. pallasiana, P. ponderosa, P. resinosa, P. sylvestris and P. tabuliformis. The telial hosts of C. phellodendri are Phellodendron amurense, P. chinense, P. chinense var. glabriusculum, P. lavallei, P. sachalinense (Farr and Rossman, online) and Zanthoxylum americanum (Back et al., 2012). |
| Reported evidence of impact |
Damages caused by C. asterum on host pines are mostly limited to small trees (less than 3 metres high) (Lowe, 1972), affecting needles and canopy. The pathogen needs few consecutive years to stunt the tree growth because, although the infected needles usually fall on the litter during the summer following the infection, they can often persist on the tree also for 3–5 years. The infection is lethal only on seedlings when combined with insect attacks (Lowe, 1972). Christmas and ornamental pines production can be damaged, as well as ornamental Compositae cultivations (Lowe, 1972). On unspecified five‐needle pine species attacked by C. eupatorii, a growth reduction of 30–40% is expected to occur (Saho, 1962). Coleosporium phellodendri seems to have the most damaging potential, because young pines, when heavily infected, can wither and die (Kishi, 1998). |
| Evidence that the commodity is a pathway |
The needles of the host pines can be infected both symptomatically and asymptomatically (asymptomatic during autumn and winter) (Suzuki et al., 2018). The commodity has needles at the time of export. Therefore, it is a pathway for the pathogen. According to Sanders (2015), plants for planting are pathway for the pathogen. |
| Surveillance information |
Coleosporium asterum is recorded in Dossier Section 5.0 as a pathogen occurring in the Zhenjiang province where the nursery is located. According to Dossier Section 4.0, C. phellodendri is pathogen occurring in China. A nursery survey is conducted by a combination of random samplings and targeted screening carried out for several pests, including C. asterum and C. phellodendri. The random sampling is performed in five points within the nursery. Symptomatic plant tissues are immediately sampled together with the root soil of the plants, and numbered, subject to DNA analysis and cultures. Sampling locations and date of sample collection are also recorded (Dossier Section 4.0). No information about surveillance of C. eupatorii is currently available. There is no information about survey carried out on telial hosts. There is no information on whether the pathogens have ever been found in the nursery or its surrounding environment. |
A.4.2. Possibility of pest presence in the nursery
A.4.2.1. Possibility of entry from the surrounding environment
These three Coleosporium species (C. asterum, C. eupatorii and C. phellodendri) are present in China and are known to infect Pinus parviflora and P. thunbergii. Coleosporium asterum is known to be present in the Zhenjiang province (Dossier Section 5.0). There is no specific survey measure set up against C. asterum or C. eupatorii. The screening protocol described in Annex EN could be able to detect the possible presence of all Coleosporium species eventually occurring on pines (even without pathogen identification at species level). Nonetheless, the great variability of the telial hosts, most of them are perennials, and their use as ornamental plants increase the potential fungal inoculum occurring in the surrounding environment and the risk of entry in the nursery. Finally, wind and insect vectors are reported to allow a range of spore dispersal up to 800 metres (Sansford, 2015).
According to Dossier Sections 4.0 and 5.0, the nursery is surrounded by different host plants, including Pinus densiflora, P. massoniana, P. parviflora and P. thunbergii. In addition, the following telial hosts of C. asterum and C. eupatorii are present within 1.5 km from the production site: Aster, Ainsliaea, Eupatorium and Kalimeris (Dossier Section 5.0).
Uncertainties
-
–
Level of susceptibility of P. parviflora to the pathogens.
-
–
Presence and/or abundance of pathogens in Zhejiang province and in the surrounding of the nursery.
-
–
Distance between the nursery and the sources of the pathogen in the surrounding environment.
-
–
The full host‐range (telial and aecial) of the pathogens.
-
–
The effective dispersal range of the pathogens.
-
–
The role of Mycodiplosis species as insect vectors in the spore dispersal.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for pathogens to enter the nursery. Pathogens could be present in the area on suitable host plants and could move into the nursery mainly by wind.
A.4.2.2. Possibility of entry with new plants/seeds
All pine seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (P. thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triaimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
The growing media used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0).
Possibility of entry with seeds or soil/growing media is not relevant for Coleosporium species.
Uncertainties
-
–
It is not clear if and how mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that pathogens could enter the nursery with new plants/seeds and soil/growing media.
A.4.2.3. Possibility of spread within the nursery
There are about 50 mother plants located in the exporting nursery, from which the scions of P. parviflora are taken (Dossier Section 4.0). The possibility of spread of the pathogen within the nursery depends on amount of spore inoculum produced by pine plants present in the nursery and by the presence of telial hosts within the nursery or in the surroundings.
Growing practices, media, tools and water are not relevant for the dispersal of these pathogens within the nursery.
Uncertainties
-
–
No information on the presence or population pressure of the pathogens in the nursery.
-
–
The presence and abundance of telial hosts in the nursery and in the surroundings.
-
–
Host suitability of Pinus parviflora to the three Coleosporium species.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of pathogens within the nursery is possible by natural dispersal of inoculum should telial hosts be present.
A.4.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii bonsai plants neither from China nor from other countries due to the presence of Coleosporium asterum, C. eupatorii and C. phellodendri between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.4.4. Evaluation of the risk mitigation options
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on Coleosporium group (C. asterum, C. eupatorii and C. phellodendri) is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
The net does not prevent the entry of airborne inoculum (basidiospores). However, it might reduce air currents thereby decreasing the amount of inoculum entering the production site. As it cannot be excluded that the pathogens could be dispersed by insect vectors, insect vectors, such as Diptera of the genus Mycodiplosis, the net is also expected to be effective against the entry of these vectors.
Uncertainties:
|
| 2 | Growing medium and its treatment | No | Not applicable. |
| 3 | Treatment of seeds | No | Not applicable. |
| 4 | Insecticide and acaricide treatments | Yes |
As it cannot be excluded that the pathogens could be dispersed by insect vectors, such as Diptera of the genus Mycodiplosis, insecticides could have some effects in reducing the insect vector populations.
Uncertainties:
|
| 5 | Fungicide treatments | Yes |
Fungicide treatments (most of active ingredients are systemic) are expected to reduce the likelihood of infection of the pathogens and the rate of colonisation of needles.
Uncertainties:
|
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | No | Not applicable. |
| 8 | Official inspections during production | Yes |
The official inspections are expected to have some effects in detecting the pathogens. However, asymptomatic periods of at least 6 months (18 months for C. phellodendri) are reported during which infection cannot be detected visually.
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
The official inspections are expected to have some effects in detecting the pathogen. However, asymptomatic periods of at least 6 months (18 months for C. phellodendri) are reported during which infection cannot be detected visually. The removal of 2 cm of surface growing medium 2 weeks before export and its replacement with new growing medium is expected to reduce pathogen inoculum because of the removal of fallen infected needles. However, the inoculum may persist on infected needles in the crown.
Uncertainties:
|
A.4.5. Overall likelihood of pest freedom for Coleosporium asterum on grafted bonsai plants
A.4.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected grafted bonsai plants
The scenario assumes a low inoculum pressure from outside the nursery because of the lack of telial hosts. The scenario also assumes that the pathogen is promptly detected because of signs of the fungus (vesicles on pine needles) either on needles which are still attached on the plants or dropped on the growing medium. Finally, the scenario assumes that the systemic fungicides kill the fungus inside needles and prevent new infections.
A.4.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected grafted bonsai plants
The scenario assumes a high inoculum pressure from outside the nursery because of the high prevalence of telial hosts. The scenario also assumes that the pathogen goes undetected because signs of the fungus (vesicles on pine needles) only develop in late winter and spring. Finally, the scenario assumes that the systemic fungicides do not kill the fungus inside needles and can delay the appearance of symptoms and signs of the disease, hence hampering its detection.
A.4.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected grafted bonsai plants (Median)
The median is closer to the lower values because there is little evidence that P. parviflora is an important host of the pathogen. In addition, the prevalence of telial host in the surrounding of the nursery is most likely low. Fungicides, some of which are systemic, are expected to reduce the infection.
A.4.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
There is a lack of quantitative information on the prevalence and abundance of the telial hosts in the surrounding of the nursery which results in high level of uncertainties for infection rates below the median. Signs of the pathogen are expected to be detectable during production which reduces uncertainties for rates above the median.
A.4.5.5. Elicitation outcomes of the assessment of the pest freedom for Coleosporium asterum on grafted bonsai plants
The following tables show the elicited and fitted values for pest infection (Table A.3) and pest freedom (Table A.4).
Table A.3.
Elicited and fitted values of the uncertainty distribution of pest infection by Coleosporium asterum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 20 | 75 | 130 | 300 | 500 | ||||||||||
| EKE | 20.1 | 21.0 | 23.3 | 30.0 | 42.7 | 63.1 | 88.1 | 152 | 236 | 286 | 344 | 400 | 450 | 480 | 502 |
The EKE results are the BetaGeneral (0.64175, 1.3189, 19.8, 525) distribution fitted with @Risk version 7.6.
Table A.4.
The uncertainty distribution of plants free of Coleosporium asterum per 10,000 plants calculated by Table A.3
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,500 | 9,700 | 9,870 | 9,925 | 9,980 | ||||||||||
| EKE results | 9,498 | 9,520 | 9,550 | 9,600 | 9,656 | 9,714 | 9,764 | 9,848 | 9,912 | 9,937 | 9,957 | 9,970 | 9,977 | 9,979 | 9,980 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.4.
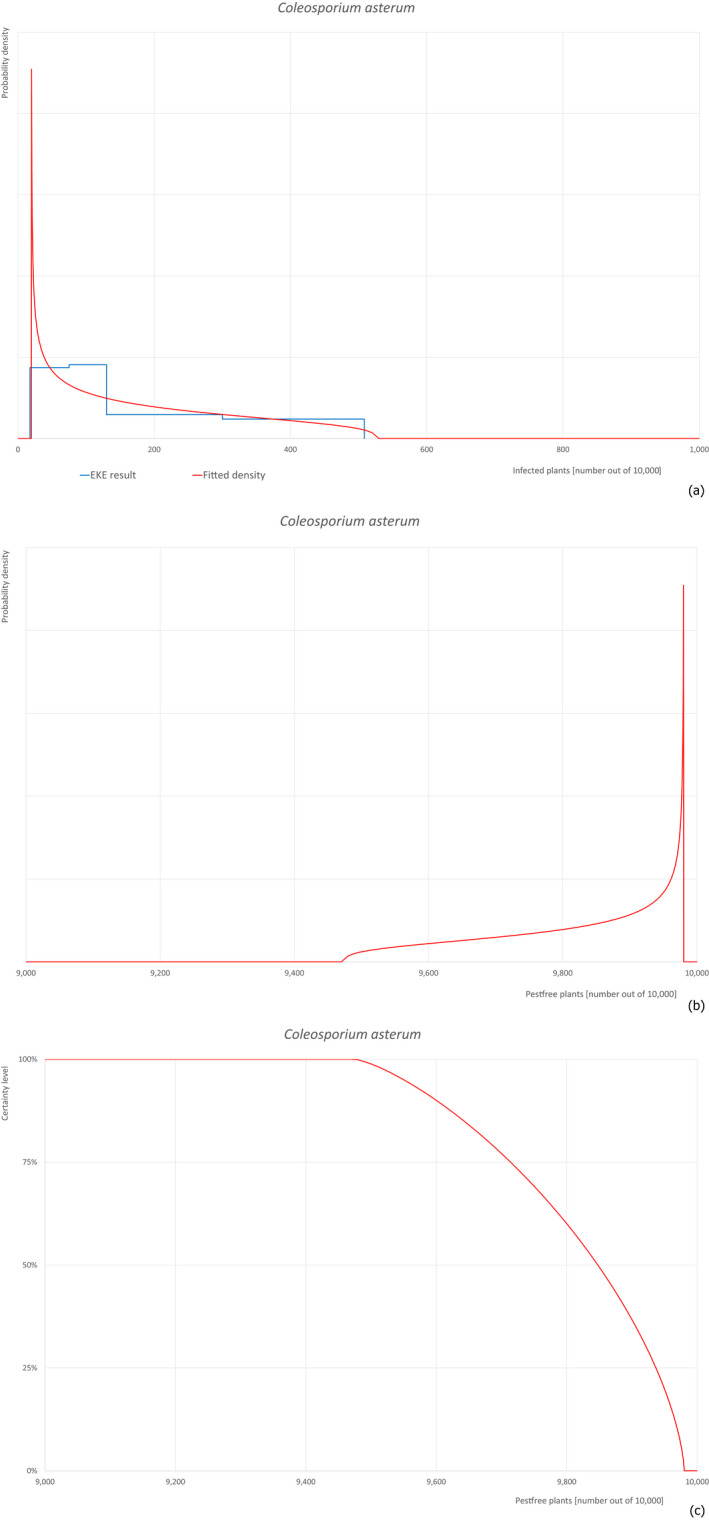
Figure A.2: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.4.6. Overall likelihood of pest freedom for Coleosporium eupatorii on grafted bonsai plants
A.4.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected grafted bonsai plants
The scenario assumes a very low inoculum pressure from outside the nursery because of the lack of telial hosts and as the presence of the pathogen has not been reported in the province. The scenario also assumes that the pathogen is promptly detected because of signs of the fungus (vesicles on pine needles) either on needles which are still attached on the plants or dropped on the growing medium. Finally, the scenario assumes that the systemic fungicides kill the fungus inside needles and prevent new infections.
A.4.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected grafted bonsai plants
The scenario assumes a moderate inoculum pressure from outside the nursery because of the presence of the pathogen in the province and on the prevalence of telial hosts in the surrounding of the nursery. The scenario also assumes that the pathogen goes undetected because signs of the fungus (vesicles on pine needles) only develops in late winter and spring. Finally, the scenario assumes that the systemic fungicides do not kill the fungus inside needles and can delay the appearance of symptoms and signs of the disease, hence hampering its detection.
A.4.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected grafted bonsai plants (Median)
Although P. parviflora is a confirmed host of C. eupatorii, the median is closer to the lower values because there is little evidence that the pathogen is present in the province and in the surrounding of the nursery. In addition, the prevalence of telial hosts in the surrounding of the nursery is most likely low. Fungicides, some of which are systemic, are expected to reduce the infection.
A.4.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
There is a lack of quantitative information on the prevalence and abundance of the pathogen and its telial hosts in the surrounding of the nursery which results in high level of uncertainties for infection rates below the median. Signs of the pathogen are expected to be detectable during production which reduces uncertainties for rates above the median.
A.4.6.5. Elicitation outcomes of the assessment of the pest freedom for Coleosporium eupatorii on grafted bonsai plants
The following tables show the elicited and fitted values for pest infection (Table A.5) and pest freedom (Table A.6).
Table A.5.
Elicited and fitted values of the uncertainty distribution of pest infection by Coleosporium eupatorii per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5 | 25 | 50 | 100 | 250 | ||||||||||
| EKE | 5.02 | 5.93 | 7.57 | 11.2 | 16.5 | 23.9 | 32.2 | 52.3 | 79.5 | 97.7 | 122 | 150 | 185 | 215 | 250 |
The EKE results are the BetaGeneral (0.9148, 5.1699, 4.5, 430) distribution fitted with @Risk version 7.6.
Table A.6.
The uncertainty distribution of plants free of Coleosporium eupatorii per 10,000 plants calculated by Table A.5
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,900 | 9,950 | 9,975 | 9,995 | ||||||||||
| EKE results | 9,750 | 9,785 | 9,815 | 9,850 | 9,878 | 9,902 | 9,921 | 9,948 | 9,968 | 9,976 | 9,983 | 9,989 | 9,992 | 9,994 | 9,995 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.6.
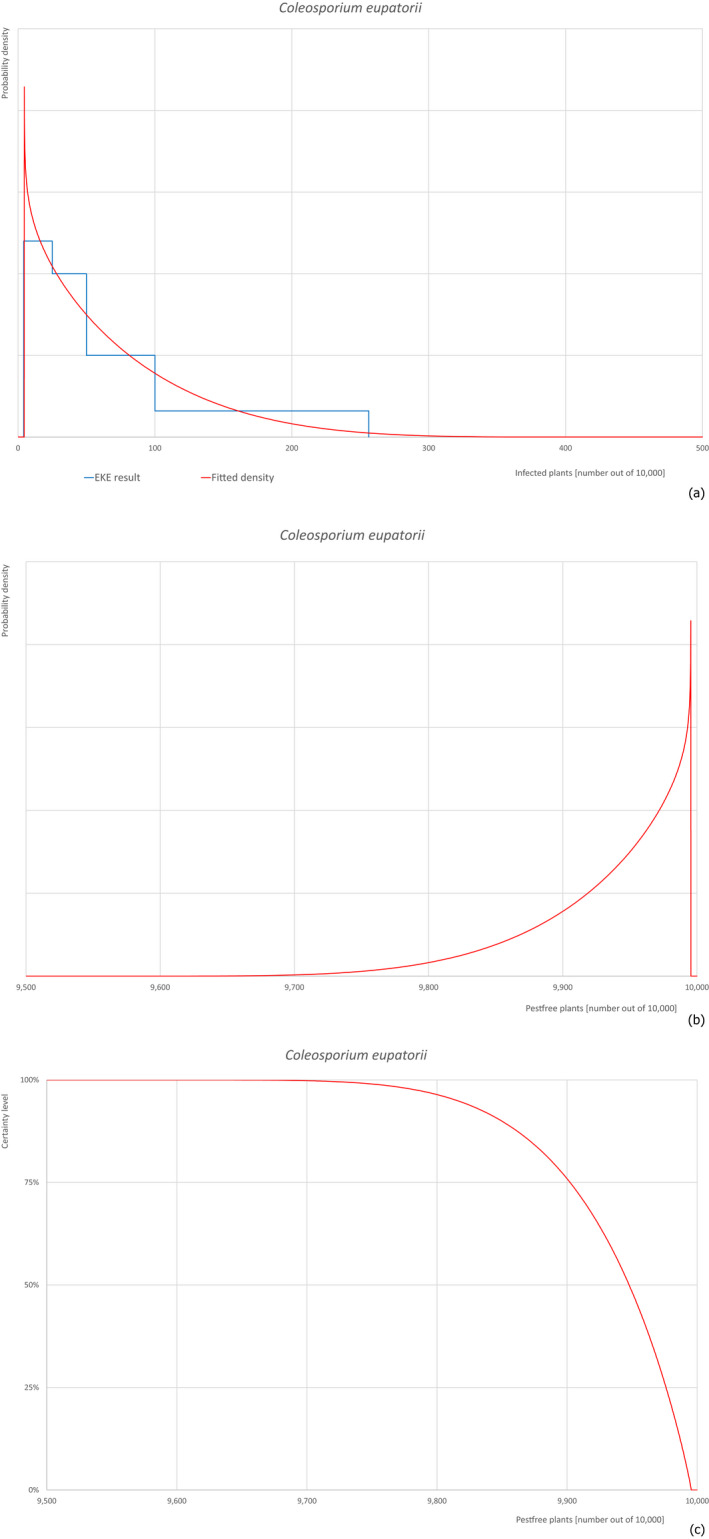
Figure A.3: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.4.7. Overall likelihood of pest freedom for Coleosporium phellodendri on grafted bonsai plants
A.4.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected grafted bonsai plants
The scenario assumes that the pathogen is not present in the province where the nursery is located and that the telial hosts are absent from the surrounding of the nursery.
A.4.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected grafted bonsai plants
The scenario assumes a low inoculum pressure from outside the nursery because of the undetected presence of the pathogen in the province and on the potential presence of telial hosts in the surrounding of the nursery. The scenario also assumes that the mitigation measures does drastically reduce the pathogen to very low levels.
A.4.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected grafted bonsai plants (Median)
The median is closer to the lower values because there is little evidence that the pathogen is present in the province and in the surrounding of the nursery. In addition, telial hosts are not reported to be present in the surrounding of the nursery. Fungicides, some of which are systemic, are expected to reduce the infection.
A.4.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
There is a lack of information on the presence of the pathogen and its telial hosts in the surrounding of the nursery which results in high level of uncertainties for infection rates below the median. Signs of the pathogen are expected to be detectable during production which reduces uncertainties for rates above the median.
A.4.7.5. Elicitation outcomes of the assessment of the pest freedom for Coleosporium phellodendri on grafted bonsai plants
The following tables show the elicited and fitted values for pest infection (Table A.7) and pest freedom (Table A.8).
Table A.7.
Elicited and fitted values of the uncertainty distribution of pest infection by Coleosporium phellodendri per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 2 | 5 | 15 | 50 | ||||||||||
| EKE | 0.009 | 0.038 | 0.117 | 0.363 | 0.847 | 1.67 | 2.74 | 5.80 | 10.6 | 14.1 | 19.1 | 25.4 | 33.4 | 41.0 | 50.0 |
The EKE results are the BetaGeneral (0.61663, 6.5786, 0, 115) distribution fitted with @Risk version 7.6.
Table A.8.
The uncertainty distribution of plants free of Coleosporium phellodendri per 10,000 plants calculated by Table A.7
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,950 | 9,985 | 9,995 | 9,998 | 10,000 | ||||||||||
| EKE results | 9,950 | 9,959 | 9,967 | 9,975 | 9,981 | 9,986 | 9,989 | 9,994 | 9,997 | 9,998 | 9,999.2 | 9,999.6 | 9,999.9 | 9,999.96 | 9,999.99 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.8.
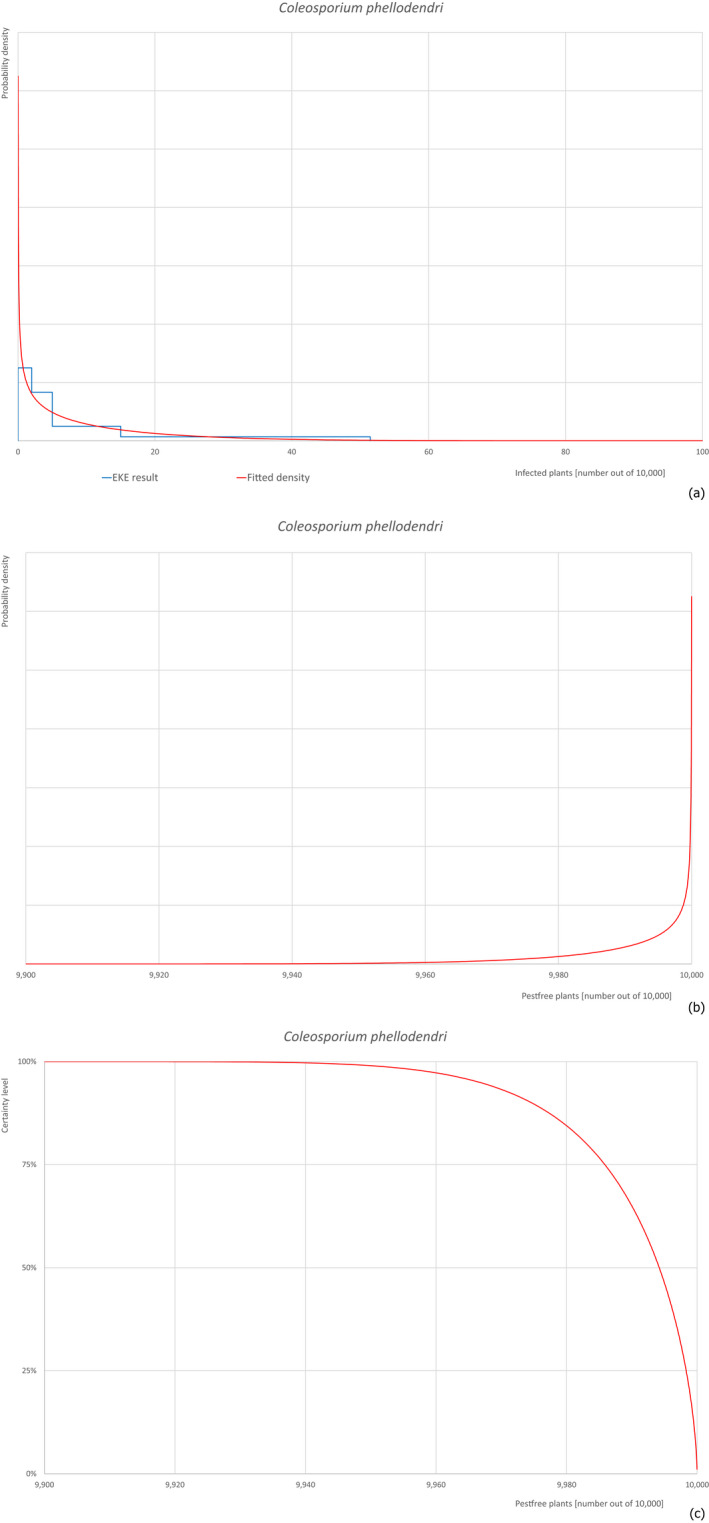
Figure A.4: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.4.8. Reference List
Back C, Nam G, Kyu Kyu Win N, Lee S, Kang I, Lee S, Jung H and Oga S, 2012. Characterization of Coleosporium phellodendri causing rust disease on Japanese prickly–ash tree. Journal of the Faculty of Agriculture, Kyushu University, 57, 379–382. https://doi.org/10.5109/25194
Beenken L, Lutz M and Scholler M, 2017. DNA barcoding and phylogenetic analyses of the genus Coleosporium (Pucciniales) reveal that the North American goldenrod rust C. solidaginis is a neomycete on introduced and native Solidago species in Europe. Mycological Progress, 16, 1073–1085. https://doi.org/10.1007/s11557‐017‐1357‐2
CABI (Centre for Agriculture and Bioscience International), online. Datasheet Coleosporium asterum (needle cast: red pine). Available online: https://www.cabi.org/cpc/datasheet/15502 [Accessed: 11 June 2021].
Cao Z‐m, Li Z‐Q and Zhuang J‐Y, 2000. Uredinales from the Qinling mountains. I. Mycosystema, 19, 13–23.
Cao J, Tian CM, Liang Y and You CJ, 2017. A new rust species of Diaphanopellis on Rhododendron oreodoxa from Southern China. Phytotaxa, 309, 55–65.
Cummins GB, 1956. Nomenclatural changes for some North American Uredinales. Mycologia, 48, 601–608. https://doi.org/10.1080/00275514.1956.12024571
DEFRA (Department for Environment, Food and Rural Affairs), online_a. UK Risk Register Details for Coleosporium asterum. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=12723 [Accessed: 11 June 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online_b. UK Risk Register Details for Coleosporium eupatorii. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=26762 [Accessed: 11 June 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online_c. UK Risk Register Details for Coleosporium phellodendri. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=240 [Accessed: 11 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), 1999. Addition to the EPPO Alert List: some Pinus pests and diseases from Far East Asia. EPPO Reporting Service no. 10 ‐ 1999 Num. article: 1999/163. Available online: https://gd.eppo.int/reporting/article‐3483
EPPO (European and Mediterranean Plant Protection Organization), online. Coleosporium phellodendri (COLSPH), Distribution. Available online: https://gd.eppo.int/taxon/COLSPH/distribution [Accessed: 11 June 2021].
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://ec.europa.eu/food/plants/plant‐health‐and‐biosecurity/European‐union‐notification‐system‐plant‐health‐interceptions‐en [Accessed: 17 June 2021].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars‐grin.gov/fungaldatabases [Accessed: 11 June 2021].
Henk DA, Farr DF and Aime MC, 2011. Mycodiplosis (Diptera) infestation of rust fungi is frequent, wide spread and possibly host specific. Fungal Ecology, 4, 284–289. https://doi.org/10.1016/j.funeco.2011.03.006
Hiratsuka N, Sato S, Katsuya K, Kakishima M, Hiratsuka Y, Kaneko S, Ono Y, Sato T, Harada Y, Hiratsuka T and Nakayama K, 1992. The rust flora of Japan. Tsukuba Shuppankai, Takezono, Ibaraki, 1205 pp.
Kim BS, Choi IY, Park MJ, Cho SE and Shin HD, 2017. First Report of Rust Caused by Coleosporium asterum on Aster spathulifolius in Korea. Plant Disease, 101, 1957–1957. https://doi.org/10.1094/PDIS‐12‐16‐1713‐PDN
Kishi K, 1998. Plant Diseases in Japan. Zenkoku Noson Kyoiku Kyokai, Tokyo, 1, 276 pp.
Lowe DP, 1972. Needle rust of lodgepole pine. Forest Insect and Disease Survey pest Leaflet no. 41. May 1972. Pacific Forest Research Centre, British Columbia. 7 pp.
Saho H, 1962. Preliminary list of pathogenic fungi of foreign forest trees planted in Tokyo University forest, Hokkaido, Japan. Plant Disease Reporter, 46, 34–35.
Sansford C, 2015. Pest Risk Analysis for Coleosporium asterum. Forestry Commission. Version 5 from 23 April 2015, 46 pp. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/downloadExternalPra.cfm?id=4047
Suzuki H, Hirose D and Yamaoka Y, 2018. Species composition and distribution of Coleosporium species on the needles of Pinus densiflora at a semi‐natural vegetation succession site in central Japan. Mycoscience, 59, 424–432. https://doi.org/10.1016/j.myc.2018.04.001
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
Voglmayr H, Krisai‐Greilhuber I and Kirisits T, 2020. First report of Coleosporium montanum on Symphyotrichum in Austria and Europe. New Disease Reports, 42, 24. https://doi.org/10.5197/j.2044‐0588.2020.042.024
Zhang N, Zhuang J‐Y and Wei S‐X, 1997. Fungal flora of the Daba Mountains: Uredinales. Mycotaxon, 61, 49–79.
Zhuang J‐Y, 1983. A provisional list of Uredinales of Fujian Province, China. Acta Mycologica Sinica, 2, 146–158.
Zinno Y and Endô A, 1964. Needle rust of Pinus pentaphylla MAYR caused by Coleosporium eupatorii ARTHUR. Journal of the Japanese Forestry Society, 46, 178–180.
A.5. Crisicoccus pini
A.5.1. Organism information
| Taxonomic information |
Current valid scientific name: Crisicoccus pini Synonyms: Dactylopius pini, Pseudococcus pini Name used in the EU legislation: – Order: Hemiptera Family: Pseudococcidae Common name: Japanese pine mealybug, Kuwana pine mealybug Name used in the Dossier: Crisicoccus pini |
| Group | Insects |
| EPPO code | DACLPI |
| Regulated status |
The pest is neither regulated in the EU, nor anywhere in the world. Crisicoccus pini is listed in the Commission Implementing Regulation (EU) 2020/1217 as a pest of concern for Pinus parviflora and P. thunbergii. Crisicoccus pini is included in the EPPO alert list (EPPO, online_a). |
| Pest status in China | In China, Crisicoccus pini is present in provinces of Hebei, Heilongjiang, Hubei, Hunan, Jilin, Jiangxi, Liaoning, Shandong, Taiwan, Xianggang (Hong Kong), Xizang (Tibet) and Zhejiang (Dossier Section 4.0; EPPO, online_b; García Morales et al., online). |
| Pest status in the EU | Crisicoccus pini is present in France (Côte d'Azur) and Italy (Cervia, Ravenna province) (Germain and Matile‐Ferrero, 2006; Boselli and Pellizzari, 2016; EPPO, online_b). |
| Host status on Pinus parviflora and P. thunbergii | Crisicoccus pini is reported as a pest of Pinus parviflora (Kuwana, 1902; EPPO, online_c; García Morales et al., online) and P. thunbergii (Ferris, 1919; EPPO, online_c; García Morales et al., online). |
| PRA information |
Available Pest Risk Assessment:
|
| Other relevant information for the assessment | |
| Biology |
Crisicoccus pini is a mealybug native to Japan, where it was first reported on Pinus pentaphylla (=P. parviflora) (Kuwana, 1902). From Japan it spread to China, South Korea, Taiwan and Russia (Boselli and Pellizzari, 2016). The pest was introduced to Italy, France, Monaco, California and District of Columbia (Germain and Matile‐Ferrero, 2006; Danzig and Gavrilov, 2010; Boselli and Pellizzari, 2016; EPPO, online_d). Females are reddish brown, covered by white cottony secretion, up to 4 mm long and 2 mm wide (Kuwana, 1902). Female body is up to 3.5 mm long (McKenzie, 1967; Danzig and Gavrilov‐Zimin, 2015; García Morales et al., online) and between 1.25 and 1.80 mm wide (McKenzie, 1967). Newly hatched larvae (crawlers) are oval, 0.35 mm long and half as wide as long (Kuwana, 1902). Eggs are oval and pink (EFSA PLH Panel, 2021, citing others). Crisicoccus pini can be found at the base of needles, between needles and in bark crevices (Chen et al., 2005), it feeds on developing pine needles (Boselli and Pellizzari, 2016). The optimum temperature for development of C. pini is 25°C and the development threshold temperature is about 13°C (Chen et al., 2006). The lifecycle consists of three stages – egg, nymph (three instars for females and four for males) and adult. Last two nymphal stages of male are called prepupa and pupa. After mating, females of C. pini lay around 50 eggs in waxy ovisac at the base of new shoots. Adult females are wingless. Males have wings (Chen et al., 2005), are weak flyers and short‐lived (EFSA PLH Panel, 2021, citing others). There is no information on development time of each life stage. In general, the eggs of mealybugs hatch in 4–10 days. Crawlers mature to adults in 3 or more weeks, depending on temperature. Females usually die right after laying eggs (Mani and Shivaraju, 2016). Crisicoccus pini overwinters as nymphs in bark cracks and crevices on branches or lower part of the trunk (EFSA PLH Panel, 2021, citing others). It is reported that in China C. pini has two generations annually, with two peak periods in May–June and in September–October (Chen et al., 2005). Possible pathways of entry for C. pini are plants for planting, bonsai plants and possibly cut branches, pinecones, bark, wood with bark and woodchip (Loyd, 2019). Possible pathways of entry for mealybugs in general are plant materials of any kind (hiding in a protected site – on the bark, roots, stems, leaves), human transportation, irrigation water, wind, animals and ants (Mani and Shivaraju, 2016). |
| Symptoms | Main type of symptoms | Main symptoms caused by C. pini are chlorosis, premature needle drop, drop of branches, needle size reduction, decline and mortality of trees, yellowing and partial necrosis of pine needles associated with white wax, honeydew and sooty mould (Chen et al., 2005; Boselli and Pellizzari, 2016; Boselli et al., 2018; Loyd, 2019). |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. | |
| Confusion with other pests | The pest can be confused with other Crisicoccus and Pseudococcus species, such as Pseudococcus citri and P. azalea (Ferris, 1919). A morphological (Danzig and Gavrilov, 2010; Son and Suh, 2017) or molecular analysis is needed in order to distinguish among them. | |
| Host plant range | Crisicoccus pini is a pest of conifers: Abies, Pinus (P. coulteri, P. densiflora, P. halepensis, P. koraiensis, P. massoniana, P. nigra, P. parviflora, P. pinaster, P. pinea, P. radiata, P. tabuliformis and P. thunbergii) (EPPO, online_c; García Morales et al., online), Keteleeria sp. and Larix sp. (Chen et al., 2005). | |
| Reported evidence of impact |
Crisicoccus pini is a major pest of Pinus densiflora and P. thunbergii in Chinese city Qingdao since 1998 (Chen et al., 2005). In California it is a pest of ornamental pines (Danzig and Gavrilov, 2010; Loyd, 2019; García Morales et al., online) and in Italy it caused death of Pinus pinaster and P. pinea (Boselli et al., 2018). |
|
| Evidence that the commodity is a pathway |
According to Loyd (2019), C. pini can travel with plants for planting including bonsai plants. The pest was found on Pinus pinaster in a Japanese garden in Monaco (Loyd, 2019; EPPO, online_d) and on dwarfed Pinus plants in California (McKenzie, 1967). Therefore, it is highly likely that the pathway of introduction in both cases was with plants for planting from Asia. |
|
| Surveillance information |
Crisicoccus pini is recorded in Dossier Sections 4.0 and 5.0 as a pest occurring in Zhenjiang province where the nursery is located. No surveillance information for this pest is currently available from China. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. |
|
A.5.2. Possibility of pest presence in the nursery
A.5.2.1. Possibility of entry from the surrounding environment
Crisicoccus pini is known to be present in several provinces of China. The nursery is located in Zhejiang province, where C. pini is reported to be present (Dossier Section 4.0; García Morales et al., online). According to Dossier Sections 4.0 and 5.0, the nursery is surrounded by different plants, among which Pinus massoniana, P. parviflora and P. thunbergii are hosts of C. pini.
The possibility of entry for C. pini from surrounding environment to nursery relies on the crawler dispersal capacity through air currents, human, animal and ant assisted spread (Mani and Shivaraju, 2016). As stated in Dossier Section 4.0, the bonsai cultivation site is protected by a 40‐mesh insect‐proof net (0.4 mm), which is wider than the body width of crawlers. Therefore, some of the crawlers can go through the net. There are potential sources of C. pini (pine trees) within the distance of 1.5 km.
Uncertainties
-
–
There is no surveillance information on the presence or population pressure of the pest in the area where the nursery is located.
-
–
The abundance of pine trees in the surrounding is unknown.
-
–
No information is available on the dispersal distance of the crawlers.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery. The pest can be present in the surrounding area because of suitable hosts and the transferring rate could be enhanced by animals, ants, humans and by wind.
A.5.2.2. Possibility of entry with new plants/seeds
All seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (P. thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triadimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
The growing media used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0). Possibility of entry with seeds or soil/growing media is not relevant for C. pini.
Uncertainties
-
–
It is not clear if and how new mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pest could enter the nursery with new plants/seeds or soil/growing media.
A.5.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of P. parviflora are taken (Dossier Section 4.0). Crawler can be taken inside the net protected area with scions from mother plants.
The pest within the nursery can spread by hitchhiking on clothing of nursery staff, by ants, by wind. In addition, the crawlers can go through the net.
Spread within the nursery through the movement of soil, water, equipment and tools is not relevant.
Uncertainties
-
–
There is no information on the presence or population pressure of the pest in the nursery.
-
–
Whether the grafting time matches the crawler occurrence/presence.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pest within the nursery is possible due to grafting operations; animals, ants and human assisted spread; by wind and due to the presence of suitable hosts (e.g. mother plants, commodity).
A.5.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Crisicoccus pini between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
In the EUROPHYT database there was one interception of Pseudococcidae not identified at species level on bonsai plants of unknown species from Japan in 2013 (EUROPHYT, online).
A.5.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on Crisicoccus pini is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
Physical separation of the bonsai plants may have an effect on reducing risk of infestation with C. pini, especially in the production base, because crawlers are smaller than the net mesh.
Uncertainties:
|
| 2 | Growing medium and its treatment | Yes |
The removal of the soil and treatment of Avermectin will eliminate resting stages associated with upper soils as with other mealybug species, although this is not verified for this mealybug.
Uncertainties:
|
| 3 | Treatment of seeds | No | Not applicable. |
| 4 | Insecticide and acaricide treatments | Yes |
Spray of contact insecticides can kill the mealybugs that are present on the plants at the time of spraying. Soaking treatment of Avermectin will eliminate resting stages associated with upper soils as with other mealybug species, although this is not verified for this mealybug.
Uncertainties:
|
| 5 | Fungicide treatments | No | Not applicable. |
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | No | Not applicable. |
| 8 | Official inspections during production | Yes |
Mealybugs are generally visible, although the resting stages in bark crevices can go undetected, especially if they are at the base of the tree. However, the presence of honeydew can alert inspectors for the presence of the pest. Uncertainties: – Non‐destructive samples can miss the insect, because they can be hidden at the base of the tree. – It is not clear how mealybugs can be detected at low density when they produce a small amount of honeydew. – There is no information about the prevalence of mealybug infested plants in the nursery and surroundings. |
| 9 | Official inspections and treatments before export | Yes |
Mealybugs are generally visible, although the resting stages in bark crevices can go undetected, especially if they are at the base of the tree. The change of the soil and treatment of Avermectin will eliminate resting stages associated with upper soils as with other mealybug species, although this is not verified for this mealybug.
Uncertainties:
|
A.5.5. Overall likelihood of pest freedom for Crisicoccus pini on grafted bonsai plants
A.5.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The population density around the nursery is low and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are highly effective. The detection before export is carefully done.
A.5.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
The population density around the nursery is high and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are only partially effective. The detection before export is not detailed enough to spot insects when they are present without obvious signs.
A.5.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
Different combinations of population density around the nursery, effect of the net barrier and of the insecticide applications may result in an intermediate scenario as they are acting independently from each other on the presence of the insect in the commodity.
A.5.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
As the signs of the insect occurrence (wax, honeydew) are generally detectable, the Panel assumes that a high infestation level is less likely to happen than having smaller number of infested plants where the insect density is low and difficult to detect.
A.5.5.5. Elicitation outcomes of the assessment of the pest freedom for Crisicoccus pini on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.9) and pest freedom (Table A.10).
Table A.9.
Elicited and fitted values of the uncertainty distribution of pest infestation by Crisicoccus pini per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 30 | 100 | 175 | 300 | 600 | ||||||||||
| EKE | 29.9 | 34.3 | 41.3 | 55.1 | 73.7 | 97.5 | 123 | 180 | 251 | 296 | 352 | 414 | 484 | 541 | 600 |
The EKE results are the BetaGeneral (1.0807, 3.4093, 26.7, 790) distribution fitted with @Risk version 7.6.
Table A.10.
The uncertainty distribution of plants free of Crisicoccus pini per 10,000 plants calculated by Table A.9
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,400 | 9,700 | 9,825 | 9,900 | 9,970 | ||||||||||
| EKE results | 9,400 | 9,459 | 9,516 | 9,586 | 9,648 | 9,704 | 9,749 | 9,820 | 9,877 | 9,903 | 9,926 | 9,945 | 9,959 | 9,966 | 9,970 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.10.
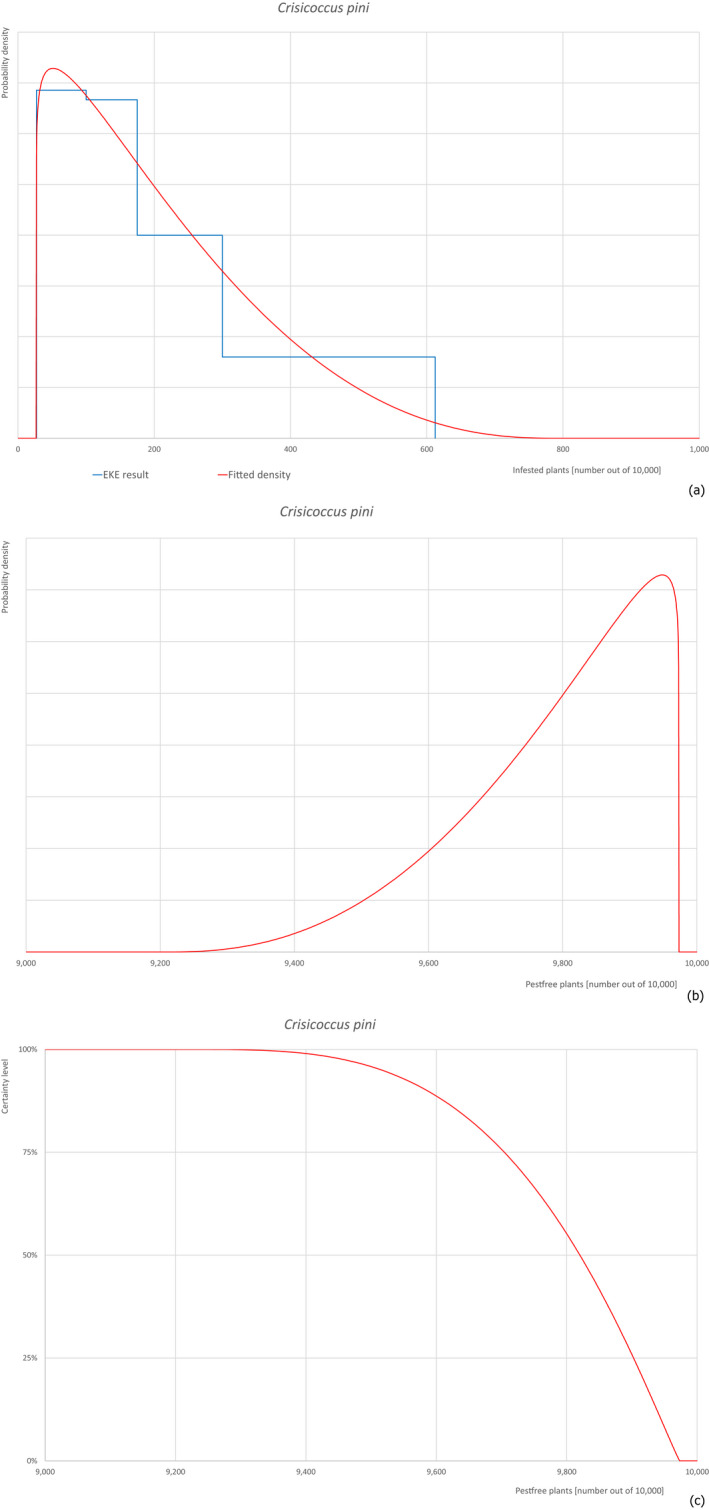
Figure A.5: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.5.6. Reference List
Boselli M and Pellizzari G, 2016. First record of the Kuwana pine mealybug Crisicoccus pini (Kuwana) in Italy: a new threat to Italian pine forests? Zootaxa, 4083, 293–296. https://doi.org/10.11646/zootaxa.4083.2.8
Boselli M, Vai N, Mirotti A, Mazzini F, Mazzoni F, Mosti M, Foschi S and Scapini C, 2018. Crisicoccus pini (Hemiptera, Pseudococcidae) in Emilia Romagna: delimitation of the infested area and control plan. Atti, Giornate Fitopatologiche, Chianciano Terme (SI), Italia, 6‐9 marzo 2018, Volume primo (2018), 265–272.
Chen SW, Chen R, Chen QD, He L and Liu ZX, 2005. Bionomics of Crisicoccus pini in Qingdao area. Forest Pest and Disease, 24, 8–11.
Chen S, Chen R, Yin T, Li B, Xu H and Zhang X, 2006. Influence of gradient constant temperatures on the experimental population of Crisicoccus pini. Zhongguo Senlin Bingchong, 25, 13–16.
Danzig EM and Gavrilov IA, 2010. Mealybugs of the genera Planococcus and Crisicoccus (Sternorrhyncha: Pseudococcidae) of Russia and adjacent countries. Zoosystematica Rossica, 19, 39–49. https://doi.org/10.31610/zsr/2010.19.1.39
Danzig EM and Gavrilov‐Zimin IA, 2015. Palaearctic mealybugs (Homoptera: Coccinea: Pseudococcidae), Part 2: Subfamily Pseudococcinae. Russian Academy of Sciences, Zoological Institute St. Petersburg. 619 pp.
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Crisicoccus pini. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=27689 [Accessed: 8 June 2021].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Vettraino AM, Leuschner R, Mosbach‐Schulz O, Rosace MC and Potting R, 2019. Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019, 17(5), 5667, 184 pp. https://doi.org/10.2903/j.efsa.2019.5667
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Di Serio F, Gonthier P, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke HH, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Grégoire J‐C, Malumphy C, Czwienczek E, Kertész V, Maiorano A and MacLeod A, 2021. Scientific Opinion on the pest categorisation of Crisicoccus pini. EFSA Journal 2021;19(11):6928, 20 pp. https://doi.org/10.2903/j.efsa.2021.6928
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO Alert List – Crisicoccus pini (Hemiptera: Coccidae). Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/alert_list_insects/crisicoccus_pini [Accessed: 13 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Crisicoccus pini (DACLPI), Distribution. Available online: https://gd.eppo.int/taxon/DACLPI/distribution [Accessed: 13 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Crisicoccus pini (DACLPI), Hosts. Available online: https://gd.eppo.int/taxon/DACLPI/hosts [Accessed: 14 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Crisicoccus pini (DACLPI), Distribution details in Monaco. Available online: https://gd.eppo.int/taxon/DACLPI/distribution/MC [Accessed: 31 May 2021].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Ferris GF, 1919. Observations on some mealy bugs. Journal of Economic Entomology, 12, 292–299.
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: A literature‐based model of scale insect biology and systematics, Crisicoccus pini. Available online: https://scalenet.info/catalogue/crisicoccus%20pini/ [Accessed 13 May 2021].
Germain JF and Matile‐Ferrero D, 2006. Comstockiella sabalis (Comstock), Crisicoccus pini (Kuwana) et Phenacoccus defectus Ferris, cochenilles nouvelles pour la France (Hem., Diaspididae et Pseudococcidae). Bulletin de la Société Entomologique de France, 111, 395–401.
Kuwana SI, 1902. Coccidae (scale insects) of Japan. Proceedings of the California Academy of Sciences, 3, 43–98. Available online: https://www.biodiversitylibrary.org/page/31547837#page/79/mode/1up
Loyd S, 2019. Rapid Pest Risk Analysis (PRA) for: Crisicoccus pini. DEFRA (Department for Environment Food and Rural Affairs). UK, York, pp. 33. Available online: https://planthealthportal.defra.gov.uk/assets/pras/Crisicoccus‐pini‐PRA.pdf
Mani M and Shivaraju C, 2016. Mealybugs and their management in agricultural and horticultural crops. Berlin, Germany, Springer. 655 pp.
McKenzie HL, 1967. Mealybugs of California with taxonomy, biology, and control of North American species (Homoptera: Coccoidea: Pseudococcidae). University of California Press, 526 pp.
Son AS and Suh SJ, 2017. Current status of Pseudococcidae (Hemiptera: Coccoidea) in South Korea. Insecta Mundi, 0581, 1–6.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
A.6. Cronartium group (C. coleosporioides, C. orientale and C. quercuum)
A.6.1. Organism information
| Taxonomic information |
1. Cronartium coleosporioides Current valid scientific name: Cronartium coleosporioides Synonyms: Uredo coleosporioides, Peridermium stalactiforme, Cronartium stalactiforme, Cronartium coleosporioides f. album Name used in the EU legislation: Cronartium spp. (non‐European) [1CRONG] Order: Pucciniales Family: Cronartiaceae Common name: pine cow wheat rust Name used in the Dossier: Cronartium coleosporioides; Cronartium spp. (non‐European) 2. Cronartium orientale Current valid scientific name: Cronartium orientale Synonyms: – Name used in the EU legislation: Cronartium spp. (non‐European) [1CRONG] Order: Pucciniales Family: Cronartiaceae Common name: – Name used in the Dossier: Cronartium spp. (non‐European) 3. Cronartium quercuum Current valid scientific name: Cronartium quercuum Synonyms: Cronartium asclepiadeum var. quercuum, Uredo quercus, Uromyces quercus, Puccinia quercus, Melampsora quercus, Dicaeoma quercus, Cronartium quercus, Peridermium cerebrum, Aecidium cerebrum, Cronartium cerebrum, Aecidium giganteum, Peridermium giganteum, Peridermium mexicanum, Peridermium fusiforme, Cronartium fusiforme Name used in the EU legislation: Cronartium spp. (non‐European) [1CRONG] Order: Pucciniales Family: Cronartiaceae Common name: eastern gall rust of pine Name used in the Dossier: Cronartium quercuum |
| Group | Fungi |
| EPPO code |
CRONCL: Cronartium coleosporioides CRONOR: Cronartium orientale CRONQU: Cronartium quercuum |
| Regulated status |
Cronartium coleosporioides, C. orientale and C. quercuum are listed in Annex II/A of Commission Implementing Regulation (EU) 2019/2072 as Cronartium spp. (non‐European) [1CRONG], currently not present in the EU territories. Cronartium orientale is listed in the Commission Implementing Regulation (EU) 2020/1217 as pests of concern for Pinus thunbergii. Cronartium quercuum is listed in the Commission Implementing Regulation (EU) 2020/1217 as a pest of concern for Pinus parviflora. Cronartium coleosporioides and C. quercuum are listed in the A1 EPPO list. |
| Pest status in China |
Even though Cronartium coleosporioides is not reported to be present in China according to EPPO (online_a) and GBIF (online), in the USDA Fungal Database there is one report of C. coleosporioides present in China on Pinus tabuliformis (Farr and Rossman, online). Cronartium orientale is present in China (Farr and Rossman, online). Cronartium quercuum is present and detected in Anhui, Gansu, Guangxi, Guizhou, Hubei, Heilongjiang, Henan, Jiangsu, Jiangxi, Sichuan, Shaanxi, Yunnan and Zhejiang provinces (Dossier Sections 2.0 and 4.0; EPPO, online_b). |
| Pest status in the EU | Cronartium coleosporioides, C. orientale and C. quercuum are absent in the EU (EPPO, online_a,b; Farr and Rossman, online; GBIF, online). |
| Host status on Pinus parviflora and P. thunbergii |
Pinus thunbergii is reported as a host of C. orientale and C. quercuum (Farr and Rossman, online). There is no information on whether C. orientale and C. quercuum can also attack P. parviflora. However, Pinus as a genus is reported as a host of both C. coleosporioides and C. quercuum (EPPO, online_c,d). |
| PRA information |
Pest Risk Assessment currently available:
|
| Other relevant information for the assessment | |
| Biology |
Cronartium coleosporioides is native to North America and it is present in the USA and Canada (EPPO, online_a), while C. quercuum is widely distributed on the American continent (the southernmost occurrence in Guyana) and in Eastern Asia (including the far East of Russia). Cronartium orientale is a species newly segregated in Japan from C. quercuum (Kaneko, 2000). As there is little information about C. orientale specific biology, in this opinion the lifecycle of this pathogen will be considered the same as C. quercuum. Cronartium spp. are heteroecious rusts that alternate their lifecycle between Pinus spp. (the aecial hosts) and a wide variety of telial hosts, both trees and grasses. Once the Pinus needles are infected, the pathogen takes one to several years to produce pycnia and aecia. Pycnospores are not infectious and serve as spermatia. Pycnia and aecia of C. coleosporioides appear in spring the same year, while aecia of C. quercuum appear the year after pycnia. Aecia then produce aeciospores, which are airborne and are able to travel long distances carried by wind. Once aeciospores reach a suitable telial host, infection occurs and uredinia will appear in about 2 weeks. Uredinia produce urediniospores which are airborne and able to re‐infect telial hosts during summer. In late summer and autumn (but earlier in C. quercuum which starts usually 2 weeks after the appearance of uredinia), telia are produced in which basidiospores are formed. Basidiospore can be carried by wind up to 1.5 km distance. Basidiospores will infect Pinus trees via first year needles. The mycelium will then develop into woody tissues, where it overwinters in bark or in the galls whose development is triggered by the presence of the pathogen (EPPO, 1997a,b). |
| Symptoms | Main type of symptoms |
Cronartium coleosporioides is part of the Blister Rust group, while C. quercuum (likely with the almost identical C. orientale) is included in the Gall Rust group (Wijesinghe et al., 2019). The main symptom of C. coleosporioides is dieback of branches. However, when the pathogen is present in association with the fungus Atropellis piniphila, bark necrosis and cankers may appear yellow/orange blisters form and develop vertically, upward and downward, along the stem from infected branches (EPPO, 1997a). It should be noted that A. piniphila is not reported to be present in China. Cronartium quercuum develops yellow/brown galls on stem or branches, until aecia develop on the gall in a cerebroid pattern (EPPO, 1997b). Infection of seedlings, results in severe stunting or rapid death. On telial hosts, Cronartium spp. produce yellow spots (uredinia) on the lower side of leaves. Once symptomatic, all these Cronartium species are easy to detect. |
| Presence of asymptomatic plants | Infected pines will be asymptomatic for one or more years. | |
| Confusion with other pests |
Symptoms are generic and can easily misidentified. The genus Cronartium can be identified by analysis of their spores. Cronartium coleosporioides can be distinguished from other Cronartium spp., including C. quercuum by sequence analysis of the ITS region (Vogler and Bruns, 1998; Wijesinghe et al., 2019). |
|
| Host plant range |
Aecial hosts for Cronartium coleosporioides are Pinus as a genus, Pinus attenuate, P. banksiana, P. contorta, P. coulteri, P. densiflora, P. echinata, P. halepensis, P. jeffreyi, P. leiophylla var. chihuahuana, P. mugo, P. nigra, P. ponderosa, P. radiata, P. sabiniana, P. sylvestris and P. tabuliformis. Telial hosts are Castilleja species, Cordylanthus sp., Lamourouxia dependens, L. rhinanthifolia, Melampyrum lineare, Orthocarpus luteus, Pedicularis bracteosa, P. groenlandica, Rhinanthus crista‐galli and R. borealis subsp. kyrollae (Farr and Rossman, online). Aecial hosts for Cronartium orientale are Pinus banksiana, P. densiflora, P. echinate, P. elliottii, P. kesiya, P. luchuensis, P. massoniana, P. mugo, P. nigra, P. nigra subsp. laricio, P. pinaster, P. ponderosa, P. sylvestris, P. sylvestris var. mongholica, P. tabuliformis, P. taiwanesis, P. thunbergii, P. virginiana and P. yunnanensis. Telial hosts are Castanea crenata, C. dentata, C. koreana, C. mollissima, Castanopsis cuspidata, C. sieboldin, Fagus crenata, Quercus acutissima, Q. aliena, Q. dentata, Q. fabrei, Q. glauca, Q. langbianensis, Q. microphylla, Q. mongolica, Q. mongolica subs. crispula, Q. myrsinifolia, Q. palustris, Q. petraea, Q. phellos, Q. rubra, Q. serrata, Q. spinosa, Q. suber, Q. variabilis, Q. wutaishanica, Q. x major and Q. x mccormickii (Farr and Rossman, online). Aecial host for Cronartium quercuum is Pinus as a genus, including P. parviflora and P. thunbergii. Telial hosts are Castanea mollissima, C. crenata, C. dentata, C. henryi, C. pumila, C. sativa, Castanopsis cuspidata, C. sieboldii, Fagus japonica, Notholithocarpus densiflorus, Rhus chinensis and a large number of Quercus species (Farr and Rossman, online). |
|
| Reported evidence of impact | Cronartium coleosporioides, C. orientale and C. quercuum are EU quarantine pests. | |
| Evidence that the commodity is a pathway |
Needles, branches and stems of Pinus spp. can be infected and carry overwintering mycelium (EPPO, 1997a), although infection could be asymptomatic. Cronartium sp. was intercepted in 2000 in the UK on Mahonia plants for planting coming from China (EUROPHYT, online). |
|
| Surveillance information |
Cronartium quercuum is recorded in Dossier Sections 2.0, 4.0 and 5.0 as a pathogen occurring in Zhejiang. The nursery and its immediate vicinity areas (at least 2 km) are inspected at least six times a year at appropriate intervals targeting pests of EU concern on their main and secondary hosts (Dossier Section 4.0). The survey shall be carried out at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus (Dossier Section 4.0). Inspection shall be conducted to examine the presence or absence of harmful organisms of EU concern (Dossier Section 4.0). |
|
A.6.2. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Cronartium coleosporioides, C. orientale and C. quercuum between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.6.3. Evaluation of the implementation and relevance of specific measures in China
Commission Implementing Regulation (EU) 2019/2072 specifies in point 30 of Annex VII measures which are required for the import of the commodity from China.
The below overview provides special requirements for naturally or artificially dwarfed plants for planting other than seeds according to Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 including an assessment of whether or not the applicant country implements those measures with respect to Cronartium group (C. coleosporioides, C. orientale and C. quercuum) identified in this Opinion. The Panel assumes that information on treatments required to be included in the phytosanitary certificate is provided by the applicant country according to the Article 71 of Regulation (EU) 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’.
| Special requirements as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 | Implementation of the special requirements in China according to information provided in the Dossier | Relevance of special requirements for the pest including uncertainties |
|---|---|---|
| ‘Official statement that: | – | – |
| a) the plants, including those collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime, | Yes | Yes |
| b) the plants in the nurseries referred to in point (a) of this entry: | – | – |
| i) at least during the period referred to in point (a) of this entry: | – | – |
| — were potted, in pots which are placed on shelves at least 50 cm above ground, |
Yes, partially. Pots are also reported to be kept on the ground. |
No |
| — have been subjected to appropriate treatments to ensure freedom from non‐European rusts, and the active ingredient, concentration and date of application of these treatments has been mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’. |
Yes. Treatments are appropriate. They are expected to reduce the likelihood of infection of the pathogens and the rate of colonisation of plant tissues, although it is uncertain if freedom from non‐European rusts could be reachable. Treatments used are listed in Table 6.
Uncertainties:
|
Yes |
| — have been officially inspected at least six times a year at appropriate intervals for the presence of Union quarantine pests of concern in accordance with Regulation (EU) No 2016/2031, and these inspections have also been carried out on plants in the immediate vicinity of the nurseries referred to in point (a) of this entry, at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus, | Yes |
Yes. The official inspections are expected to be effective in detecting the pathogens. However, the asymptomatic period can last from one to several years. During that period, infection cannot be detected visually.
Uncertainties:
|
| — have been found free, in these inspections, from the relevant Union quarantine pests of concern as specified in the previous indent, infested plants have been removed and the remaining plants, where appropriate, have been effectively treated and have been held for an appropriate period and inspected to ensure freedom from such pests, | Yes |
Yes The asymptomatic period can last several years.
Uncertainties:
|
| — have been planted in either an unused artificial growing medium or in a natural growing medium, which has been treated by fumigation or by appropriate heat treatment and has been of any Union quarantine pests, | Yes | No |
| — have been kept under conditions which ensure that the growing medium has been maintained free from Union quarantine pests and within 2 weeks prior to dispatch, have been: | Yes | No |
| — shaken and washed with clean water to remove the original growing medium and kept bare rooted, or | – | – |
| — shaken and washed with clean water to remove the original growing medium and replanted in growing medium which meets the conditions laid down in (i) fifth indent, or | – | – |
| — subjected to appropriate treatments to ensure that the growing medium is free from Union quarantine pests, and the active ingredient, concentration and date of application of these treatments have been indicated on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031 under the rubric ‘Disinfestation and/or disinfection treatment’. | Yes | No |
| ii) were packed in closed containers which have been officially sealed and bear the registration number of the registered nursery, and this number has been indicated under the rubric ‘Additional declaration’ on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, enabling the consignments to be identified.’ | Yes | Yes |
A.6.4. Reference List
DEFRA (Department for Environment, Food & Rural Affairs), online_a. UK Risk Register Details for Cronartium coleosporioides. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=11756 [Accessed: 11 June 2021].
DEFRA (Department for Environment, Food & Rural Affairs), online_b. UK Risk Register Details for Cronartium quercuum. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=12320 [Accessed: 11 June 2021].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Boberg J, Jeger M, Pautasso M and Dehnen‐Schmutz K, 2018. Scientific Opinion on the pest categorisation of Cronartium spp. (non‐EU). EFSA Journal 2018;16(12), 5511, 30 pp. https://doi.org/10.2903/j.efsa.2018.5511
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Vettraino AM, Leuschner R, Mosbach‐Schulz O, Rosace MC and Potting R, 2019. Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. https://doi.org/10.2903/j.efsa.2019.5667
EPPO (European and Mediterranean Plant Protection Organization), 1997a. Data sheets on quarantine pests: Cronartium coleosporioides. In: Smith IM, McNamara DG, Scott PR and Holderness M (eds.). Quarantine Pests for Europe, 2nd edition. CABI/EPPO, Wallingford, 5 pp.
EPPO (European and Mediterranean Plant Protection Organization), 1997b. Data sheets on quarantine pests: Cronartium quercuum. In: Smith IM, McNamara DG, Scott PR and Holderness M (eds.). Quarantine Pests for Europe, 2nd edition. CABI/EPPO, Wallingford, 5 pp.
EPPO (European and Mediterranean Plant Protection Organization), online_a. Cronartium coleosporioides (CRONCL), Distribution. Available online: https://gd.eppo.int/taxon/CRONCL/distribution [Accessed: 29 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Cronartium quercuum (CRONQU), Distribution. Available online: https://gd.eppo.int/taxon/CRONQU/distribution [Accessed: 29 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Pinus parviflora (PIUPF), Pests. Available online: https://gd.eppo.int/taxon/PIUPF/pests [Accessed: 29 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Pinus thunbergii (PIUTH), Pests. Available online: https://gd.eppo.int/taxon/PIUTH/pests [Accessed: 29 May 2021].
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://ec.europa.eu/food/plants/plant‐health‐and‐biosecurity/European‐union‐notification‐system‐plant‐health‐interceptions‐en [Accessed: 17 June 2021].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars‐grin.gov/fungaldatabases/ [Accessed: 29 May 2021].
GBIF (Global Biodiversity Information Facility), online. Cronartium orientale S. Kaneko. in GBIF Secretariat (2021). GBIF Backbone Taxonomy. Checklist dataset https://doi.org/10.15468/39omei. Available online: https://www.gbif.org/species/2517504 [Accessed: 29 May 2021].
Kaneko S, 2000. Cronartium orientale, sp. nov., segregation of the pine gall rust in eastern Asia from Cronartium quercuum. Mycoscience, 41, 115–122. https://doi.org/10.1007/bf02464319
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
Vogler DR and Bruns TD, 1998. Phylogenetic relationships among the pine stem rust fungi (Cronartium and Peridermium spp.). Mycologia, 90, 244–257. https://doi.org/10.1080/00275514.1998.12026904
Wijesinghe S, McKenzie E, Wanasinghe DN, Boonmee S and Jayawardena RS, 2019. The Genus Cronartium Revisited. Plant Pathology and Quarantine, 9, 219–238. https://doi.org/10.5943/ppq/9/1/20
A.7. Dendrolimus group (D. punctatus, D. sibiricus, D. spectabilis, D. superans and D. tabulaeformis)
A.7.1. Organism information
| Taxonomic information |
1. Dendrolimus punctatus Current valid scientific name: Dendrolimus punctatus Synonyms: – Name used in the EU legislation: – Order: Lepidoptera Family: Lasiocampidae Common name: Masson pine caterpillar Name used in the Dossier: Dendrolimus punctatus 2. Dendrolimus sibiricus Current valid scientific name: Dendrolimus sibiricus Synonyms: Dendrolimus laricis, Dendrolimus superans sibiricus Name used in the EU legislation: Dendrolimus sibiricus Chetverikov [DENDSI] Order: Lepidoptera Family: Lasiocampidae Common name: Siberian silk moth Name used in the Dossier: Dendrolimus sibiricus 3. Dendrolimus spectabilis Current valid scientific name: Dendrolimus spectabilis Synonyms: Odonestis spectabilis, Oeona segregatus Name used in the EU legislation: – Order: Lepidoptera Family: Lasiocampidae Common name: Japanese pine caterpillar Name used in the Dossier: Dendrolimus spectabilis 4. Dendrolimus superans Current valid scientific name: Dendrolimus superans Synonyms: Dendrolimus jezoensis, Dendrolimus superans albolineatus Name used in the EU legislation: – Order: Lepidoptera Family: Lasiocampidae Common name: Japanese hemlock caterpillar, Sakhalin silk moth Name used in the Dossier: Dendrolimus superans [also cited in the Dossier as Dendrolimus sibiricus, which however is not a synonym] 5. Dendrolimus tabulaeformis Current valid scientific name: Dendrolimus tabulaeformis Synonyms: – Name used in the EU legislation: – Order: Lepidoptera Family: Lasiocampidae Common name: Chinese pine caterpillar Name used in the Dossier: Dendrolimus tabulaeformis |
| Group | Insects |
| EPPO code |
DENDPU: Dendrolimus punctatus DENDSI: Dendrolimus sibiricus DENDSC: Dendrolimus spectabilis DENDSU: Dendrolimus superans DENDTA: Dendrolimus tabulaeformis |
| Regulated status |
Dendrolimus sibiricus is listed in Annex II/A of Regulation (EU) 2019/2072 as Dendrolimus sibiricus Chetverikov [DENDSI]. Dendrolimus sibiricus is also listed as a priority pest under Commission Delegated Regulation (EU) 2019/1702. Dendrolimus punctatus, D. spectabilis, D. superans and D. tabulaeformis are not regulated in the EU. Dendrolimus sibiricus, D. spectabilis and D. superans are listed in the Commission Implementing Regulation (EU) 2020/1217 as pests of concern for Pinus parviflora and P. thunbergii. EPPO categorisation: Dendrolimus sibiricus is included in A2 EPPO list. Dendrolimus sibiricus is also included in A1 list in Georgia, Turkey and Ukraine, in A2 list in Russia, Armenia, Belarus and Kyrgyzstan, and in both A1 and A2 list in Kazakhstan (EPPO, online_a). Dendrolimus spectabilis is currently listed in A1 list only for Chile (EPPO, online_b). It was in the EPPO Alert List from 1999 to 2004 (EPPO, 2004). Dendrolimus superans is included in A2 EPPO list, it is also included in A1 list for Georgia and Chile (EPPO, online_c). Dendrolimus punctatus and D. tabulaeformis are not listed by EPPO. |
| Pest status in China |
Dendrolimus punctatus, D. sibiricus, D. superans, D. spectabilis and D. tabulaeformis are present in China. Dendrolimus punctatus is present in Anhui, Beijing, Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hebei, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Shaanxi, Sichuan, Yunnan, Zhejiang, Hong Kong (Kononov et al., 2016; Dossier Section 4.0; CABI, online_a). Dendrolimus sibiricus and D. superans are present in China only in the northern provinces of Heilongjiang, Jilin, Liaoning and Neimenggu (Dai et al., 2012; Kononov et al., 2016; Dossier Section 2.0; CABI, online_b; EPPO, online_d). Dendrolimus spectabilis is present in Hebei, Heilongjiang, Jiangsu, Jilin, Liaoning and Shandong (Dai et al., 2012; Dossier Section 2.0; EPPO, online_e). Dendrolimus tabulaeformis is present in Beijing, Gansu, Guizhou, Hebei, Henan, Hubei, Jilin, Liaoning, Neimenggu, Shaanxi, Shandong, Shanxi and Sichuan (Dai et al., 2012; Kononov et al., 2016; Dossier Section 4.0; CABI, online_c). |
| Pest status in the EU | All the five considered species of Dendrolimus are absent in the EU (CABI, online_a,b,c; EPPO, online_d,e,f). |
| Host status on Pinus parviflora and Pinus thunbergii |
Pinus parviflora and P. thunbergii are both hosts for D. punctatus, D. sibiricus, D. spectabilis and D. superans (Dossier Section 2.0; CABI, online_d; EPPO, online_g,h,k). Pinus thunbergii is also host for D. tabulaeformis (CABI, online_e). |
| PRA information |
Pest Risk Assessment currently available:
|
| Other relevant information for the assessment | |
| Biology |
Dendrolimus spp. are lappet moths of medium–large size (40–100 mm wingspan) in the family Lasiocampidae; mature larvae (caterpillars) are 50–70 (110) mm long (CAPS, 2016a,b). No other information is available on body length of larvae at different instars. All species feed on conifers, of which they are among the most important defoliating pests in the world. Several species still have unclear or controversial taxonomic position (Dai et al., 2012). According to Kononov et al. (2016), six species of Dendrolimus widespread in Eurasia may be separated into two distinct clusters each including three taxa that show close phylogenetic relationships:
Dendrolimus sibiricus has a wide range distribution throughout northern Eurasia, from Russia to eastern Siberia and Sakhalin, northern China and Korea (Kononov et al., 2016; Jeong et al., 2018; VKM, 2018; EPPO, online_d). The distribution of the closely related species D. superans is instead limited to eastern Siberia and Sakhalin, Japan and northern China (Kononov et al., 2016; VKM, 2018). In the Dossier, D. superans is considered as synonym of D. sibiricus (Dossier Section 2.0). Dendrolimus punctatus has a large subtropical distribution from central‐eastern China to Taiwan, Vietnam and India (Manipur) (Li et al., 2019; CABI, online_a). Dendrolimus spectabilis is present in north‐eastern China, Korea and Japan (Kononov et al., 2016). Dendrolimus tabulaeformis is distributed in central and north‐eastern part of China, where is largely sympatric with both the two above mentioned similar species. Occasional hybridisations are reported among the three species (Dai et al., 2012); D. tabulaeformis is genetically not distinguishable from D. punctatus and could be considered as a subspecies (Kononov et al., 2016). General life history traits: Eurasian species of Dendrolimus have all similar biology and feeding habits, but the duration of the life cycle may be very variable depending on species, climatic zones, population density and larval diapause. Dendrolimus has 4 stages of development: egg, larva (6–10 larval instars), pupa and adult. Overwintering occurs in the ground at larval stage from 3rd to 5th instar (EPPO, 2005c; Choi et al., 2011; Luo et al., 2018; Shao et al., 2018). In spring, from March to June, the larvae emerge from the ground and climb the stems to feed on needles, causing intense defoliation especially in the last weeks of development. Pupation occurs in silky cocoons attached to needles or branches. Adults fly from March to October, depending on species and the number of generations per year; they are nocturnal and during the day remain on the stems (EFSA, 2020). Dendrolimus are multivoltine in warmer subtropical regions (up to 5 generations/year), univoltine in temperate regions and semivoltine in colder northern regions, where up to 4 years can be needed to complete the life cycle (EPPO, 2005c; Flø et al., 2020; CABI, online_a). Overlapping of generations is frequent and may be a contributing cause of outbreaks. Females lay 200–800 eggs (size of an egg is 1.9–2.2 mm) (EPPO, 2005c) on the needles and branches of the lower part of the crown. The young gregarious larvae hatch after 13–22 days and begin to feed on the needles, of which initially gnaw only the edges; larval development occurs rapidly in multivoltine populations; from 3rd to 4th instar onwards larvae feed individually and the last generation larvae descend to the ground to overwinter. In univoltine or semivoltine populations, the development of the larvae runs slower during summer, and overwintering usually occurs from 3rd to 5th instar. In the following spring, the emerged larvae resume feeding on foliage and complete their development, or eventually continue feeding activity during summer and overwinter for a second time. Under summer unfavourable conditions (hot temperatures, etc.), larvae can enter summer diapause and return to soil (Kirichenko et al., 2011). The adults of Dendrolimus have a short lifespan (7–18 days) but are generally able to cover tens of km in flight, so that they can easily spread naturally where suitable climate conditions and host plants are available (Wylie and Speight, 2012; Möykkynen and Pukkala, 2014; EFSA PLH Panel, 2018; EFSA, 2019; Flø et al., 2020). Furthermore, Dendrolimus can also passively spread in all stages of development by transport of wood products (as wood with bark, bark, fuel wood), living plants (plants for planting, Christmas trees, cut branches) and soil associated with plants (EPPO, 2005c; VKM, 2018; EFSA PLH Panel, 2019; Flø et al., 2020). Adults can also spread passively on long distances by transport vehicles (Dossier Section 2.0). Species specific life history traits: Dendrolimus sibiricus is considered ‘an integral part of taiga forest ecosystems’ (Sultson et al., 2021) feeding on all main Siberian coniferous species (Abies, Larix, Picea and Pinus) and severely damaging million hectares during cyclical outbreaks. The life cycle last 1–3 years, depending on climate conditions (EPPO, 2005c). However, extended life cycle up to 3–4 years is possible as consequence of extended larval life (Flø et al., 2020). Dendrolimus sibiricus is believed to be a strong flyer, but its flying capability has been recently revised by Flø et al. (2020) which judged not proven the natural spread rate of 50 km/year previously reported by EPPO (2005c) and by Möykkynen and Pukkala (2014). A lower spread rate of about 9.5 km/year (with a 95% uncertainty range of 1.2–33 km) is considered reliable by a recent EFSA assessment (EFSA, 2019). According to EFSA (2019), the larvae do not balloon. Dendrolimus superans is known as an important pest mainly for Larix spp. and Pinus spp. in north‐eastern China and Japan (Kamata, 2002; EPPO, 2005c; Fang et al., 2021). Its biological traits, including life cycle, are very similar to D. sibiricus (EPPO, 2005c; VKM, 2018). No specific information about the flight capability of D. superans is available, but it can be assumed that it is similar to that of D. sibiricus. Dendrolimus punctatus is the most important defoliating insect in Asian pine forests, infesting million hectares each year in China, Vietnam and Taiwan with outbreaks occurring every 3–5 years (Wylie and Speight, 2012; Fang et al., 2016). In most of its range, D. punctatus is mainly associated with previous year needles of Pinus massoniana as preferred food, ensuring fitness benefits in terms of larval development rapidity and survival, increased mating success of adults and female fecundity (Luo et al., 2018). The species has usually 2–4 generations/year and overwinters as 3rd/4th instar larvae. Mature larvae weave their cocoons at the tree top or the tip of branch (Luo et al., 2018). Dendrolimus punctatus is believed to be a sedentary species, unwilling to move on long distances when suitable food sources are available, and its migration ability is judged ‘weak’ by Du et al. (2020). However, the 1st and 2nd instar larvae can be easily dispersed by the wind on silk threads (ballooning), and adult moths are known to cover flight distances of 10–20 km, eventually even wind‐assisted (Wylie and Speight, 2012; Dossier Section 2.0). Dendrolimus spectabilis is mainly known as important defoliator of Pinus densiflora in Korea, P. thunbergii in Japan (Togashi and Takahashi, 1977; EPPO, 2004; Choi et al., 2011), and of both the mentioned conifers in China (Bao et al., 2019). For a long time known as univoltine species in Korea, D. spectabilis is recently turning to bivoltinism as consequence of both switching of main host tree (from Pinus thunbergii to P. rigida) and climate change (Choi et al., 2011). In Japan (Kyoto) an alternation of univoltine and bivoltine cycle was observed, so that adults can fly three times a year (Togashi and Takahashi, 1977). No specific information about the flight capability of D. spectabilis was found but can be assumed to be similar to D. punctatus. Dendrolimus tabulaeformis is considered a major pest for Pinus tabulaeformis in north China, significantly reducing the growth of monocultural pine plantations during frequent outbreaks (Shao et al., 2018). According to Kononov et al. (2016), this taxon should be considered as synonym of D. punctatus. The species has one generation/year and overwinters as larva 4th 5th instar; it is known as a chill tolerant species at the overwintering stage in northern China (Zeng et al., 2008; Shao et al., 2018). No detailed information about the flight capability of D. tabulaeformis was found in the literature but can be assumed that it is similar to that of D. punctatus. |
| Symptoms | Main type of symptoms |
Symptoms of Dendrolimus sp. easy to detect on the commodity are:
|
| Presence of asymptomatic plants | Infested plants are asymptomatic only when carrying eggs laid on needles and young larvae are newly hatched. | |
| Confusion with other pests | The identification of different species of Dendrolimus may be very uncertain due to the remarkable similarity among the species (especially between D. sibiricus and D. superans and within the cluster D. punctatus, D. spectabilis, D. tabulaeformis) and the possibility of hybridisation. For a reliable identification analysis by experts, eventually also with DNA barcoding support, is needed. |
| Host plant range |
The host plant range of D. sibiricus and D. superans includes: Abies (A. nephrolepis, A. sachalinensis, A. sibirica); Larix (L. cajanderi, L. gmelinii, L. kurilensis, L. sibirica); Picea (P. jezoensis, P. obovata); Pinus (P. bungeana, P. koraiensis, P. parviflora, P. pumila, P. sibirica P. sylvestris, P. thunbergii); Tsuga sp. and Pseudotsuga menziesii (CAPS, 2016a; EFSA, 2020; CABI, online_a; EPPO, online_g,h,i,j). Dendrolimus punctatus is known to feed only on Pinus spp. of which it is reported as a major pest of P. massoniana, and secondary pest of P. armandii, P. kesiya, P. luchiensis, P. merkusii, P. parviflora, P. taiwanensis, P. tabulaeformis, P. thunbergii (Wylie and Speight, 2012; CAPS, 2016b; Fang et al., 2016; Li et al., 2019) and other non‐Asian pine species as P. caribaea, P. elliottii, P. oocarpa and P. taeda (CAPS, 2016b). Dendrolimus spectabilis is a pest mainly for P. densiflora, P. thunbergii, P. tabulaeformis, P. bungeana, P. parviflora and P. rigida. It is also known to feed on Abies, Cedrus and Larix (EPPO, 2004; Choi et al., 2011; EPPO, online_g,h,k). Dendrolimus tabulaeformis is a pest for P. tabulaeformis, P. massoniana, P. armandii, P. bungeana, P. densiflora, P. sylvestris and P. thunbergii (Han et al., 2008; Shao et al., 2018; Bao et al., 2019; Wang et al., 2019; CABI, online_e). |
| Reported evidence of impact |
Dendrolimus sibiricus is EU quarantine pest. Dendrolimus spp. are very important defoliating insects in China, where more than 2 million ha of pine (Pinus spp.) plantations are annually damaged (Fang et al., 2016). Furthermore, toxic hairs of Dendrolimus spp. larvae and cocoons can cause irritation and arthritis to humans and livestock (Zhang et al., 2020). Dendrolimus superans is known as main pest in larch‐pine stands of Sakhalin, causing death of about 8,000 ha of forests in two distinct outbreaks in 1959–1964 and 1986–1989. Similar outbreaks also occurred in Japan, on Abies sachalinensis (Kamata, 2002; EPPO, 2005c). One million ha of Larix gmelinii/Betula platyphylla mixed forests have been severely damaged during an outbreak occurred in China (Heilongjiang) in 1989–1991 (Fang et al., 2021). Dendrolimus punctatus is the most destructive among all the pine caterpillar species in China, causing huge economic damage to the pine forests in southern China (> 1 million ha annually affected) during outbreaks lasting 3–5 years (Du et al., 2020; Dossier Section 4.0). It is also an important pest in Vietnam and Taiwan. Death of 25% of pine trees and 30% reduced growth in surviving trees have been reported after 100% defoliation (Wylie and Speight, 2012). Dendrolimus spectabilis caused in the past (1960–1970) severe defoliation damage on Pinus densiflora and P. thunbergii young plantations (< 20 years old) in Japan (Kamata, 2002) and in P. densiflora stands in Korea, but its importance in this country is progressively decreased and it is considered now only an occasional pest (Choi et al., 2011). Dendrolimus tabulaeformis is reported as the major pine pest in northern China (Han et al., 2008). Frequent outbreaks, often favoured by drought mainly in P. tabulaeformis monoculture plantations, cause serious growth reduction and important economic losses (Shao et al., 2018; Bao et al., 2019). |
| Evidence that the commodity is a pathway | Plants for planting, included Christmas trees and bonsais, are pathway for all Dendrolimus species. Bonsai plants in pots may carry eggs, larvae or cocoons of the pests, and overwintering larvae also might be present in the soil. |
| Surveillance information |
Dendrolimus punctatus is recorded in Dossier Sections 4.0 and 5.0 as a pest occurring in the Zhenjiang province where the nursery is located. And according to Dossier Section 4.0, D. sibiricus and D. spectabilis are present in China. No specific surveillance protocol for all the mentioned Dendrolimus species is described in the Dossier. However, D. sibiricus and D. spectabilis are listed as target pests (Dossier Section 4.0) for which monitoring activities (pheromone traps, light traps, etc.) are performed, together with inspections and samplings of insects on host plants in the survey area, which are carried out three times a year. |
A.7.2. Possibility of pest presence in the nursery
Information is provided only for Dendrolimus species (Dendrolimus punctatus, D. spectabilis, D. superans and D. tabulaeformis) evaluated using Expert Knowledge Elicitation.
A.7.2.1. Possibility of entry from the surrounding environment
Dendrolimus punctatus is the only species reported to be present in Zhejiang (Dossier Section 2.0; CABI, online_a). Dendrolimus spectabilis is found in the bordering province of Jiangsu (Dossier Section 2.0; EPPO, online_e). Dendrolimus superans is present in northern regions of China. Dendrolimus tabulaeformis is in the northeast. However, the Dossier states that none of the mentioned Dendrolimus species is present in the Hangzhou area.
According to Dossier Section 4.0, the following hosts for Dendrolimus spp. are present in parks and gardens of the Hangzhou area, where the nursery is located:
-
–
Cedrus deodara (D. spectabilis),
-
–
Pinus bungeana (D. superans, D. spectabilis, D. tabulaeformis),
-
–
Pinus densiflora (D. spectabilis, D. tabulaeformis),
-
–
Pinus elliottii (D. punctatus),
-
–
Pinus massoniana (D. punctatus, D. tabulaeformis),
-
–
Pinus parviflora (D. superans, D. punctatus, D. spectabilis),
-
–
Pinus thunbergii (D. superans, D. punctatus, D. spectabilis, D. tabulaeformis).
Adults of Dendrolimus punctatus (and D. tabulaeformis) are able to fly 10–20 km, and young larvae can be dispersed by the wind (Wylie and Speight, 2012; Dossier Section 2.0). All the Dendrolimus species can also move on long distance by passive transport of woody material, living plants and soil (EPPO, 2005c; VKM, 2018; EFSA PLH Panel, 2019; Flø et al., 2020; Dossier Section 2.0). The bonsai cultivation in the nursery is protected by a 40‐mesh insect‐proof net, only removed during winter (Dossier Section 4.0).
Uncertainties
-
–
There is no surveillance information on the presence or population pressure of the pests in the area where the nursery is located.
-
–
No information available on the distance of the nursery to sources of pests in the surrounding environment.
-
–
Pattern of the presence of D. punctatus in Zhejiang.
-
–
Number of generations of D. punctatus in Zhejiang and flight periods of moths.
-
–
Presence of other species of Dendrolimus in Zhejiang (it cannot be excluded, considering their spread capabilities both natural and human assisted).
-
–
Laying down and removal calendar of insect‐proof net on bonsai cultivation in relation to the flight periods of adults.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the Dendrolimus species to enter the nursery from the surrounding area. One of them (D. punctatus) is present in Zhejiang province, and the occurrence of the remaining four species cannot be excluded as consequence of both their high natural and human assisted spread capabilities and the presence of several suitable hosts in the area.
A.7.2.2. Possibility of entry with new plants/seeds
All seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (P. thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triaimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
Growing media used during bonsai production is coconut coir imported from abroad and not containing any soil (Dossier Section 5.0).
Possibility of entry with seeds or growing media is not relevant for Dendrolimus species.
Uncertainties
-
–
It is not clear if and how new mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers is not possible that the pests could enter the nursery with new plants/seeds or soil/growing media.
A.7.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of P. parviflora are taken (Dossier Section 4.0).
Dendrolimus species within the nursery can spread by scions from infested mother plants. Spread within the nursery through the movement of soil, water, equipment and tools is not relevant.
Uncertainties
-
–
There is no information on the presence or population pressure of the pests in the nursery.
-
–
There is no information about the possibility that small larvae are accidentally taken on clothes of nursery staff from mother plants when scions are collected to the bonsai cultivation area.
-
–
Host suitability of Pinus parviflora for some Dendrolimus species.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pests within the nursery is possible since the eggs or young larvae of Dendrolimus on scions can be taken to the bonsai cultivation area.
A.7.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there were two interceptions of Dendrolimus spectabilis on Pinus thunbergii bonsais from Japan occurred in Germany in 2018 (EUROPHYT/TRACES‐NT, online).
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii bonsai plants neither from China nor from other countries due to the presence of Dendrolimus punctatus, D. sibiricus, D. superans and D. tabulaeformis between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.7.4. Evaluation of the risk mitigation measures
According to Section 1.2, the Panel did not assess the effectiveness of measures for Dendrolimus sibiricus for which specific measures are specified in point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072. The assessment was restricted to whether or not the applicant country implements those measures (see Section 6 and A.7.9).
Therefore, effectiveness of risk mitigation measures was evaluated only for D. punctatus, D. spectabilis, D. superans and D. tabulaeformis.
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on D. punctatus, D. spectabilis, D. superans and D. tabulaeformis is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
Physical separation of the bonsai plants may have an effect on reducing risk of infestation with Dendrolimus species, especially in the production base.
Uncertainties:
|
| 2 | Growing medium and its treatment | No | Not applicable because the soaking is done when larvae are not present in the soil. |
| 3 | Treatment of seeds | No | Not applicable. |
| 4 | Insecticide and acaricide treatments | Yes |
Spray of contact and ingestion insecticides can be very effective in killing the larvae that are present on the plants.
Uncertainties:
|
| 5 | Fungicide treatments | No | Not applicable. |
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | No | Not applicable. |
| 8 | Official inspections during production | Yes |
The sampling and laboratory inspection of plant material may allow to identify plants infested by Dendrolimus species.
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
The sampling and laboratory inspection of plant material may allow to identify plants infested by Dendrolimus species. Export plants can be infested when they are moved from the bonsai production base to the storage and packaging place. The removal of the soil and soaking will kill the larvae hidden in the soil. Whereas some larvae can remain on the foliage.
Uncertainties:
|
A.7.5. Overall likelihood of pest freedom for Dendrolimus punctatus on grafted bonsai plants
A.7.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The pest is well‐known to the managers and is present in the nursery province, effective treatments are performed, net is preventing females for reaching the plants, inspection before export are effective, removal of the upper soil will eliminate the overwintering larvae.
A.7.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
Pest can reach very high density in the surroundings, because of outbreak dynamics, pressure can be very high. Mother plants can be fully exposed to females and their egg deposition. Infested scions can be introduced to the bonsai plantation under the net. Small larvae can be difficult to be reached by insecticides when they are hidden, especially in the resting stage.
A.7.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
Measures to contain the pest are generally effective and the pest is relatively easy to control.
A.7.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Uncertainty is high about the control methods performed because information provided in the Dossier is not complete. The behaviour of the larvae on the potted plants is unknown. Measures taken in the nature cannot be fully taken to the potted plants. Alternation of high and low density in the surrounding determines larger variation in the pest pressure.
A.7.5.5. Elicitation outcomes of the assessment of the pest freedom for Dendrolimus punctatus on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.11) and pest freedom (Table A.12).
Table A.11.
Elicited and fitted values of the uncertainty distribution of pest infestation by Dendrolimus punctatus per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5 | 25 | 40 | 100 | 200 | ||||||||||
| EKE | 5.00 | 5.41 | 6.35 | 8.87 | 13.3 | 20.0 | 28.1 | 48.7 | 76.6 | 94.6 | 117 | 141 | 165 | 184 | 200 |
The EKE results are BetaGeneral (0.70643, 2.0405, 4.85, 230) distribution fitted with @Risk version 7.6.
Table A.12.
The uncertainty distribution of plants free of Dendrolimus punctatus per 10,000 plants calculated by Table A.11
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,800 | 9,900 | 9,960 | 9,975 | 9,995 | ||||||||||
| EKE results | 9,800 | 9,816 | 9,835 | 9,859 | 9,883 | 9,905 | 9,923 | 9,951 | 9,972 | 9,980 | 9,987 | 9,991 | 9,994 | 9,995 | 9,995 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.12.
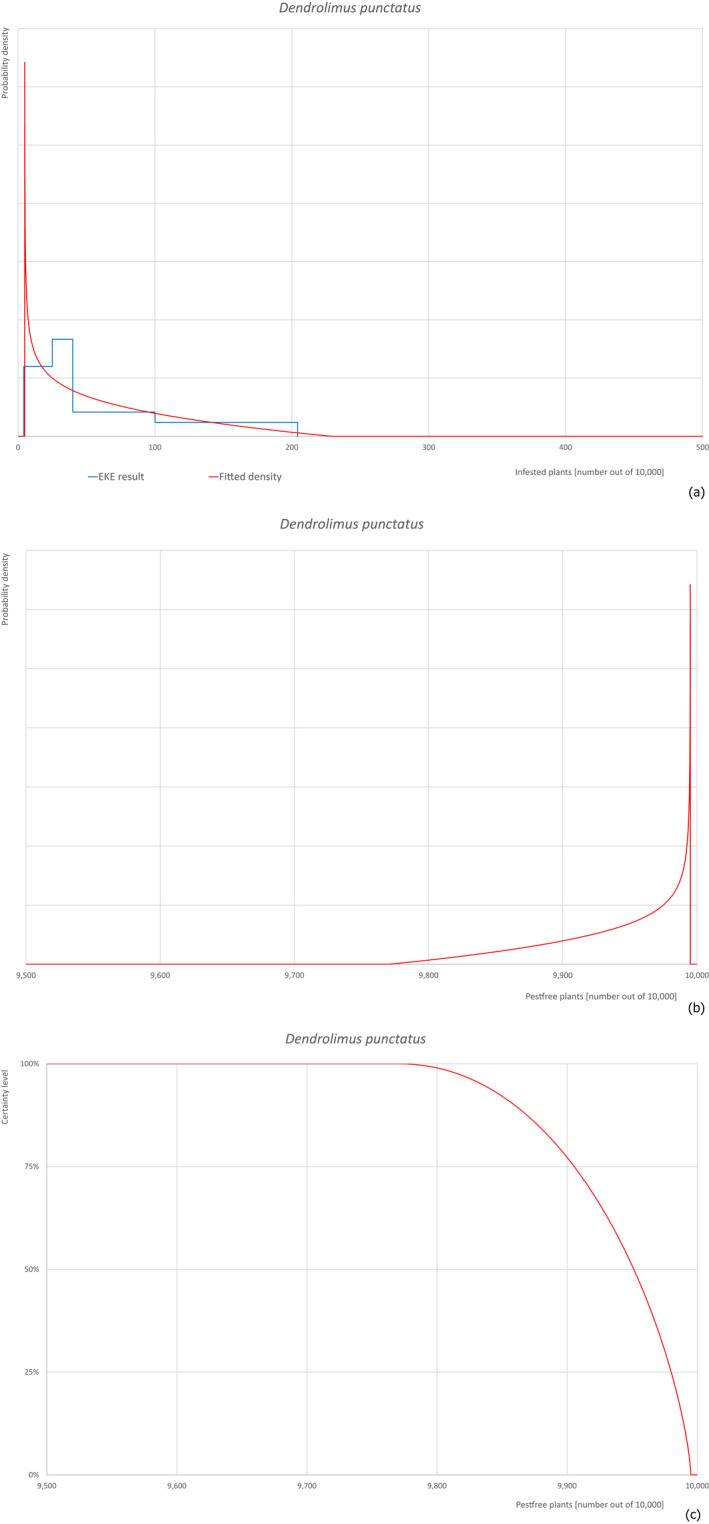
Figure A.6: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. =1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.7.6. Overall likelihood of pest freedom for Dendrolimus spectabilis on grafted bonsai plants
A.7.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
In comparison to D. punctatus, the other Dendrolimus species occur away from the nursery province and their arrival in the area is associated with accidental transportation. The species are not very well adapted to the local conditions.
A.7.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
In comparison to D. punctatus, the other Dendrolimus species occur away from the nursery province and their arrival in the area is associated with accidental transportation. The species can show good adaptation to the local conditions.
A.7.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
In comparison to D. punctatus, the other Dendrolimus species are much less likely to occur in the area of the nursery.
A.7.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
In comparison to D. punctatus, the other Dendrolimus species are not known in their capacity to exploit potted plants of P. parviflora.
A.7.6.5. Elicitation outcomes of the assessment of the pest freedom for Dendrolimus spectabilis on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.13) and pest freedom (Table A.14).
Table A.13.
Elicited and fitted values of the uncertainty distribution of pest infestation by Dendrolimus spectabilis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 2 | 4 | 10 | 20 | ||||||||||
| EKE | 0.017 | 0.061 | 0.162 | 0.434 | 0.906 | 1.63 | 2.50 | 4.69 | 7.63 | 9.50 | 11.8 | 14.2 | 16.6 | 18.4 | 19.9 |
The EKE results are the BetaGeneral (0.70703, 1.9011, 0, 22.5) distribution fitted with @Risk version 7.6.
Table A.14.
The uncertainty distribution of plants free of Dendrolimus spectabilis per 10,000 plants calculated by Table A.13
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,980 | 9,990 | 9,996 | 9,998 | 10,000 | ||||||||||
| EKE results | 9,980 | 9,982 | 9,983 | 9,986 | 9,988 | 9,991 | 9,992 | 9,995 | 9,998 | 9,998 | 9,999.1 | 9,999.6 | 9,999.8 | 9,999.9 | 10,000.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.14.
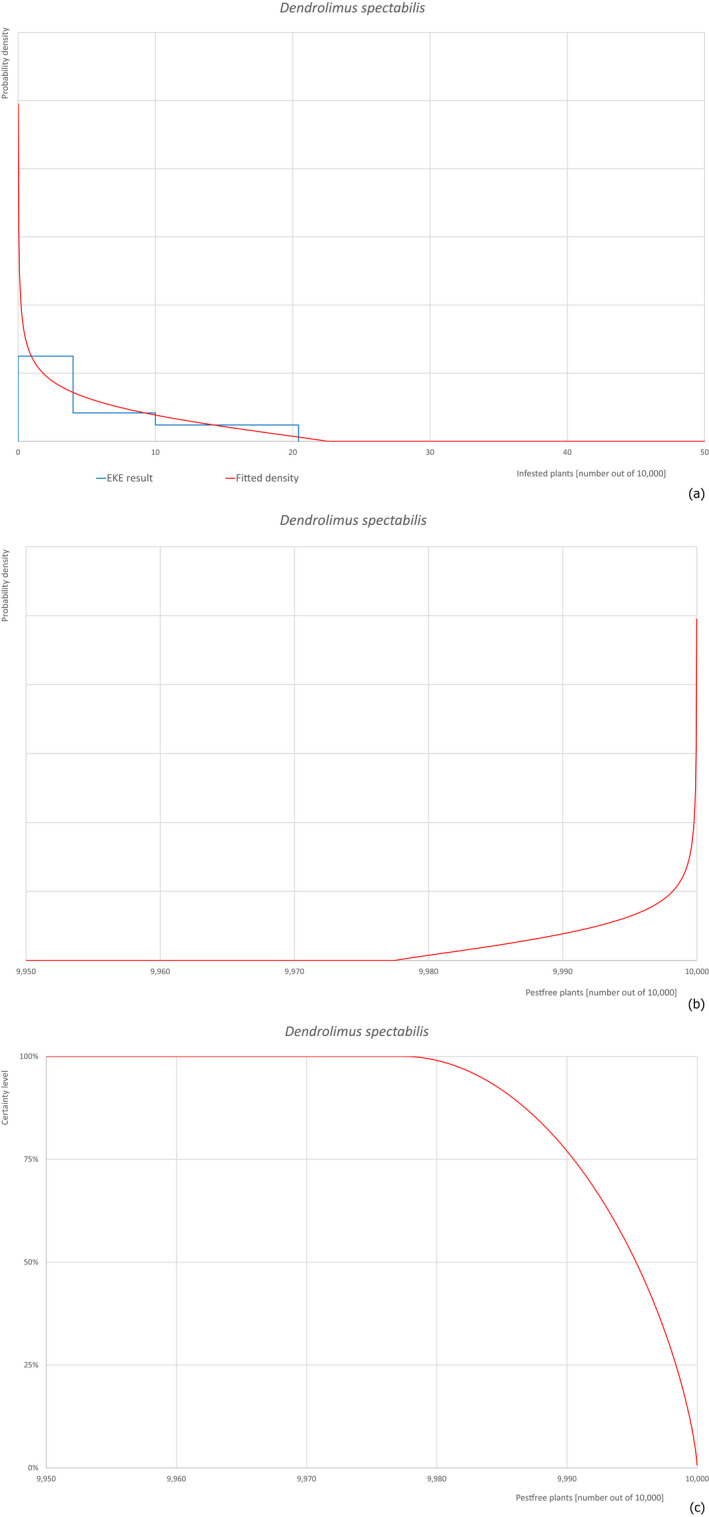
Figure A.7: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.7.7. Overall likelihood of pest freedom for Dendrolimus superans on grafted bonsai plants
Dendrolimus superans was evaluated in comparison to Dendrolimus punctatus as reference pest, as the pests share common characteristics, e.g. as the same genus. Dendrolimus spectabilis, D. superans and D. tabulaeformis are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overall likelihood of pest freedom can be found in Section A.7.6.
A.7.8. Overall likelihood of pest freedom for Dendrolimus tabulaeformis on grafted bonsai plants
Dendrolimus tabulaeformis was evaluated in comparison to Dendrolimus punctatus as reference pest, as the pests share common characteristics, e.g. as the same genus. Dendrolimus spectabilis, D. superans and D. tabulaeformis are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overall likelihood of pest freedom can be found in Section A.7.6.
A.7.9. Evaluation of the implementation and relevance of specific measures in China
Commission Implementing Regulation (EU) 2019/2072 specifies in point 30 of Annex VII measures which are required for the import of the commodity from China.
The below overview provides special requirements for naturally or artificially dwarfed plants for planting other than seeds according to Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 including an assessment of whether or not the applicant country implements those measures with respect to Dendrolimus sibiricus identified in this Opinion. The Panel assumes that information on treatments required to be included in the phytosanitary certificate is provided by the applicant country according to the Article 71 of Regulation (EU) 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’.
| Special requirements as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 | Implementation of the special requirements in China according to information provided in the Dossier | Relevance of special requirements for the pest |
|---|---|---|
| ‘Official statement that: | – | – |
| a) the plants, including those collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime, | Yes | Yes |
| b) the plants in the nurseries referred to in point (a) of this entry: | – | – |
| i) at least during the period referred to in point (a) of this entry: | – | – |
| — were potted, in pots which are placed on shelves at least 50 cm above ground, |
Yes, partially. Pots are also reported to be kept on the ground. |
Yes |
| — have been subjected to appropriate treatments to ensure freedom from non‐European rusts, and the active ingredient, concentration and date of application of these treatments has been mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’. |
Yes. Treatments are appropriate. They are expected to reduce the likelihood of infection of the pathogens and the rate of colonisation of plant tissues, although it is uncertain if freedom from non‐European rusts could be reachable. Treatments used are listed in Table 6.
Uncertainties:
|
No |
| — have been officially inspected at least six times a year at appropriate intervals for the presence of Union quarantine pests of concern in accordance with Regulation (EU) No 2016/2031, and these inspections have also been carried out on plants in the immediate vicinity of the nurseries referred to in point (a) of this entry, at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus, | Yes | Yes |
| — have been found free, in these inspections, from the relevant Union quarantine pests of concern as specified in the previous indent, infested plants have been removed and the remaining plants, where appropriate, have been effectively treated and have been held for an appropriate period and inspected to ensure freedom from such pests, | Yes | Yes |
| — have been planted in either an unused artificial growing medium or in a natural growing medium, which has been treated by fumigation or by appropriate heat treatment and has been of any Union quarantine pests, | Yes | Yes |
| — have been kept under conditions which ensure that the growing medium has been maintained free from Union quarantine pests and within 2 weeks prior to dispatch, have been: | Yes |
Yes. However, the timing of the treatment as provided in the Dossier, i.e. September, may not be appropriate. |
| — shaken and washed with clean water to remove the original growing medium and kept bare rooted, or | – | – |
| — shaken and washed with clean water to remove the original growing medium and replanted in growing medium which meets the conditions laid down in (i) fifth indent, or | – | – |
| — subjected to appropriate treatments to ensure that the growing medium is free from Union quarantine pests, and the active ingredient, concentration and date of application of these treatments have been indicated on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031 under the rubric ‘Disinfestation and/or disinfection treatment’. | Yes | Yes |
| ii) were packed in closed containers which have been officially sealed and bear the registration number of the registered nursery, and this number has been indicated under the rubric ‘Additional declaration’ on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, enabling the consignments to be identified.’ | Yes | Yes |
A.7.10. Reference List
Bao Y, Wang F, Tong S, Na L, Han A, Zhang J, Bao Y, Han Y and Zhang Q, 2019. Effect of Drought on Outbreaks of Major Forest Pests, Pine Caterpillars (Dendrolimus spp.), in Shandong Province, China. Forests, 10, 264, 1–16. https://doi.org/10.3390/f10030264
CABI (Centre for Agriculture and Bioscience International), online_a. Datasheet Dendrolimus punctatus (Masson pine caterpillar). Available online: https://www.cabi.org/isc/datasheet/18368 [Accessed: 12 June 2021].
CABI (Centre for Agriculture and Bioscience International), online_b. Datasheet Dendrolimus sibiricus (Siberian silk moth). Available online: https://www.cabi.org/isc/datasheet/18371 [Accessed: 12 June 2021].
CABI (Centre for Agriculture and Bioscience International), online_c. Datasheet Dendrolimus tabulaeformis (Chinese pine caterpillar). Available online: https://www.cabi.org/isc/datasheet/18373 [Accessed: 12 June 2021].
CABI (Centre for Agriculture and Bioscience International), online_d. Plantwise Knowledge bank. Species Page Masson pine caterpillar Dendrolimus punctatus. Available online: https://www.plantwise.org/knowledgebank/datasheet/18368 [Accessed: 12 June 2021].
CABI (Centre for Agriculture and Bioscience International), online_e. Plantwise knowledge bank. Species page Chinese pine caterpillar Dendrolimus tabulaeformis. Available online: https://www.plantwise.org/knowledgebank/datasheet/18373 [Accessed: 12 June 2021].
CAPS (Cooperative Agricultural Pest Survey) Purdue University, 2016a. CAPS pest tracker datasheet Dendrolimus sibiricus, 8 pp. Available online: https://pest.ceris.purdue.edu/ [Accessed: 12 June 2021].
CAPS (Cooperative Agricultural Pest Survey) Purdue University, 2016b. CAPS Pest Tracker Datasheet Dendrolimus punctatus, 8 pp. Available online: https://pest.ceris.purdue.edu/ [Accessed: 12 June 2021].
Choi WII, Park Y‐K, Park Y‐S, Ryoo NII and Lee H‐P, 2011. Changes in voltinism in a pine moth Dendrolimus spectabilis (Lepidoptera: Lasiocampidae) population: implications of climate change. Applied Entomology and Zoology, 46, 319–325. https://doi.org/10.1007/s13355‐011‐0046‐x
Dai Q‐Y, Gao Q, Wu C‐S, Chesters D, Zhu C‐D and Zhang A‐B, 2012. Phylogenetic reconstruction and DNA bar‐coding for closely related pine moth species (Dendrolimus) in China with multiple gene markers. PLoS ONE, 7, e32544. https://doi.org/10.1371/journal.pone.0032544
DEFRA (Department for Environment, Food and Rural Affairs), online_a. UK Risk Register Details for Dendrolimus sibiricus. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=20156 [Accessed: 14 June 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online_b. UK Risk Register Details for Dendrolimus spectabilis. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=3295 [Accessed: 14 June 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online_c. UK Risk Register Details for Dendrolimus superans. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=14807 [Accessed: 14 June 2021].
Du H, Liu M, Zhang S, Liu F, Zhang Z and Kong X, 2020. Lineage divergence of Dendrolimus punctatus in Southern China based on mitochondrial genome. Frontier in Genetics, 11, 65, 1–11. https://doi.org/10.3389/fgene.2020.00065
EFSA (European Food Safety Authority), Baker R, Gilioli G, Behring C, Gogin A, Kaluski T, Kinkar M, Mosbach‐Schulz O, Neri FM, Preti S, Rosace MC, Siligato R, Stancanelli G and Tramontini S, 2019. Dendrolimus sibiricus Pest Report and Datasheet to support ranking of EU candidate priority pests [Data set]. Zenodo. https://doi.org/10.5281/zenodo.2789555
EFSA (European Food Safety Authority), Wilstermann A, Schrader G, Kinkar M, Vos S, 2020. Pest survey card on Dendrolimus sibiricus. EFSA supporting publication 2020;EN‐1779, 23 pp. https://doi.org/10.2903/sp.efsa.2020.EN‐1779
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Kirichenko N, Kertész V and Grégoire J‐C, 2018. Scientific opinion on pest categorisation of Dendrolimus sibiricus. EFSA Journal 2018;16(6):5301, 29 pp. https://doi.org/10.2903/j.efsa.2018.5301
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Vettraino AM, Leuschner R, Mosbach‐Schulz O, Rosace MC and Potting R, 2019. Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. https://doi.org/10.2903/j.efsa.2019.5667
EPPO (European and Mediterranean Plant Protection Organization), 2000. Pest Risk Assessment Dendrolimus sibiricus 00/8481. 16 pp. Available online: https://gd.eppo.int/taxon/DENDSI/documents
EPPO (European and Mediterranean Plant Protection Organization), 2002. Report of a Pest Risk Assessment for Dendrolimus sibiricus 02/9138 3 pp. Available online: https://gd.eppo.int/taxon/DENDSI/documents
EPPO (European and Mediterranean Plant Protection Organization), 2004. Mini data sheet on Dendrolimus spectabilis. EPPO RS 99/163, 1 p.
EPPO (European and Mediterranean Plant Protection Organization), 2005a. Report of a Pest Risk Management: Dendrolimus superans 05/11595 4 pp. Available online: https://gd.eppo.int/taxon/DENDSU/documents
EPPO (European and Mediterranean Plant Protection Organization), 2005b. Report of a Pest Risk Assessment for Dendrolimus superans 05/11591 3 pp. Available online: https://gd.eppo.int/taxon/DENDSU/documents
EPPO (European and Mediterranean Plant Protection Organization), 2005c Data sheets on quarantine pests Dendrolimus sibiricus and Dendrolimus superans. EPPO Bulletin, 35, 390–395. Available online: https://gd.eppo.int/taxon/DENDSU/documents
EPPO (European and Mediterranean Plant Protection Organization), online_a. Dendrolimus sibiricus (DENDSI), Categorization. Available online: https://gd.eppo.int/taxon/DENDSI/categorization [Accessed: 12 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Dendrolimus spectabilis (DENDSC), Categorization. Available online: https://gd.eppo.int/taxon/DENDSC/categorization [Accessed: 12 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Dendrolimus superans (DENDSU), Categorization. Available online: https://gd.eppo.int/taxon/DENDSU/categorization [Accessed: 12 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Dendrolimus sibiricus (DENDSI), Distribution. Available online: https://gd.eppo.int/taxon/DENDSI/distribution [Accessed: 12 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_e. Dendrolimus spectabilis (DENDSC), Distribution. Available online: https://gd.eppo.int/taxon/DENDSC/distribution [Accessed: 12 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_f. Dendrolimus superans (DENDSU), Distribution. Available online: https://gd.eppo.int/taxon/DENDSU/distribution [Accessed: 12 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_g. Pinus parviflora (PIUPF), Pests. Available online: https://gd.eppo.int/taxon/PIUPF/pests [Accessed: 12 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_h. Pinus thunbergii (PIUTH), Pests. Available online: https://gd.eppo.int/taxon/PIUTH/pests [Accessed: 12 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_i. Dendrolimus sibiricus (DENDSI), Hosts. Available online: https://gd.eppo.int/taxon/DENDSI/hosts [Accessed: 14 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_j. Dendrolimus superans (DENDSU), Hosts. Available online: https://gd.eppo.int/taxon/DENDSU/hosts [Accessed: 14 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_k. Dendrolimus spectabilis (DENDSC), Hosts. Available online: https://gd.eppo.int/taxon/DENDSC/hosts [Accessed: 14 June 2021].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Fang X‐M, Christenson LM, Wang F‐C, Zemg J‐P and Chen F‐S, 2016. Pine caterpillar outbreak and stand density impacts on nitrogen and phosphorus dynamics and their stoichiometry in Masson pine (Pinus massoniana) plantations in subtropical China. Canadian Journal of Forest Research, 46, 601–609. https://doi.org/10.1139/cjfr‐2015‐0357
Fang L, Yu Y, Fang G, Zhang X, Yu Z, Zhang X, Crocker E and Yang J, 2021. Effects of meteorological factors on the defoliation dynamics of the larch caterpillar (Dendrolimus superans Butler) in the Great Xing’an boreal forests. Journal of Forest Research, 1–15. https://doi.org/10.1007/s11676‐020‐01277‐6
Flø D, Rafoss T, Wendell M and Sundheim L, 2020. The Siberian moth (Dendrolimus sibiricus), a pest risk assessment for Norway. Forest Ecosystems, 7, 1–11. https://doi.org/10.1186/s40663‐020‐00258‐9
Forestry Commission, 2016. Contingency plan for the Siberian Coniferous Silk Moth (Dendrolimus sibiricus), 26 pp. Available online: https://www.forestresearch.gov.uk/documents/7160/Contingency_Plan_Siberian_Silk_Moth_11_08_2016.pdf
Han R‐D, Gan YL, Kong X‐H and Ge F, 2008. Physiological and endocrine differences between diapausing and nondiapausing larvae of the pine caterpillar Dendrolimus tabulaeformis (Lepidoptera: Lasiocampidae). Zoological Studies, 47, 96–102.
Institut Ochrony Roslin, 2017. Podsumowanie Analizy Zagrożenia Agrofagiem (Ekspres PRA) dla Dendrolimus sibiricus. PRA ‐ Summary of the Express Pest Risk Analysis for Dendrolimus sibiricus (eppo.int). Available online: https://www.agrofagi.com.pl/plik,1305,dendrolimus‐sibiricus‐pdf.pdf
Jeong JS, Kim NJ, Kim S‐S, Choi S‐W and KIM I, 2018. DNA data and morphology suggest an occurrence of Dendrolimus sibiricus Tschetverikov, 1908 (Lepidoptera: Lasiocampidae) instead of D. superans Butler, 1877, in South Korea. Entomological Research, 48, 108–121.
Kamata N, 2002. Outbreaks of forest defoliating insects in Japan, 1950‐2000. Bulletin of Entomological Research, 92, 109–117. https://doi.org/10.1079/BER2002159
Kirichenko N, Flament J, Baranchikov Y and Grégoire JC, 2011. Larval performances and life cycle completion of the Siberian moth, Dendrolimus sibiricus (Lepidoptera: Lasiocampidae), on potential host plants in Europe: a laboratory study on potted trees. European Journal of Forest Research, 130, 1067–1074. https://doi.org/10.1007/s10342‐011‐0495‐3
Kononov A, Ustyantsev K, Wang B, Mastro VC, Fet V, Blinov A and Baranchikov Y, 2016. Genetic diversity among eight Dendrolimus species in Eurasia (Lepidoptera: Lasiocampidae) inferred from mitochondrial COI and COII, and nuclear ITS2 markers. BMC Genetics, 17(3), 157, 173–191. https://doi.org/10.1186/s12863‐016‐0463‐5
Li J, Jin Q, Zhu G‐P, Jiang C and Zhang A‐B, 2019. Phylogeography of Dendrolimus punctatus (Lepidoptera: Lasiocampidae): Population differentiation and last glacial maximum survival. Ecology and Evolution, 9, 7480–7496. https://doi.org/10.1002/ece3.5278
Luo D, Lai M, Xu C, Shi H and Liu X, 2018. Life history traits in a capital breeding pine caterpillar: effect of host species and needle age. BMC Ecology, 18, 1–8. https://doi.org/10.1186/s12898‐018‐0181‐0
Möykkynen T and Pukkala T, 2014. Modelling of the spread of a potential invasive pest, the Siberian moth (Dendrolimus sibiricus) in Europe. Forest ecosystems, 1, 10. https://doi.org/10.1186/s40663‐014‐0010‐7
Shao Y, Feng Y, Tian B, Wang T, He Y and Zong S, 2018. Cold hardiness of larvae of Dendrolimus tabulaeformis (Lepidoptera: Lasiocampidae) at different stages during the overwintering period. European Journal of Entomology, 115, 198–207. https://doi.org/10.14411/eje.2018.018
Sultson SM, Goroshko AA, Verkhovets SV, Mikhaylov PV, Ivanov VA, Demidko DA and Kulakov SS, 2021. Orographic factors as a predictor of the spread of the Siberian silk moth outbreak in the mountainous southern taiga forests of Siberia. Land, 10, 115, 1–16. https://doi.org/10.3390/land10020115
Togashi K and Takahashi F, 1977. Coadaptative preferential feeding of the pine moth, Dendrolimus spectabilis Butler (Lepidoptera, Lasiocampidae), on the old needles of Japanese black pine, Pinus thunbergii. The Entomological Society of Japan, Parl. Kontyu, 45, 399–414.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
USDA (United States Department of Agriculture), 1991. Pest Risk Assessment of the importation of larch from Siberia and the Soviet Far East. Miscellaneous Publication no. 1495, 263 pp. Available online: https://www.fs.usda.gov/treesearch/pubs/6632
VKM (Vitenskapskomiteen for mat og miljø), 2018. Pest risk assessment of Dendrolimus sibiricus and Dendrolimus superans. Opinion of the Panel on Plant Health of the Norwegian Scientific Committee for Food and Environment. VKM report 2018:08, Norwegian Scientific Committee for Food and Environment (VKM), Oslo, Norway, 69 pp.
Wang W, Gao C, Ren L and Luo Y, 2019. The Effect of Longwave Ultraviolet Light Radiation on Dendrolimus tabulaeformis Antioxidant and Detoxifying Enzymes. Insects, 11, 1–12. https://doi.org/10.3390/insects11010001
Wylie FR and Speight MR, 2012. Insect Pests in Tropical Forestry 2nd Edition. CABI Publishing Oxfordshire, UK, 409 pp.
Zeng J‐P, Feng GE, Su J‐W and Wang J, 2008. The effect of temperature on the diapause and cold hardiness of Dendrolimus tabulaeformis (Lepidoptera: Lasiocampidae). European Journal of Entomology, 105, 599–606.
Zhang S, Shen S, Peng J, Zhou X, Kong X, Ren P, Liu F, Han L, Zhan S, Huang Y, Zhang A and Zhang Z, 2020. Chromosome‐level genome assembly of an important pine defoliator, Dendrolimus punctatus (Lepidoptera; Lasiocampidae). Molecular Ecology Resources, 15 pp. https://doi.org/10.1111/1755‐0998.13169
A.8. Godronia zelleri
A.8.1. Organism information
| Taxonomic information |
Current valid scientific name: Godronia zelleri Synonyms: Atropellis pinicola Name used in the EU legislation: Atropellis spp. [1ATRPG] Order: Helotiales Family: Dermateaceae Common name: Twig blight of pine Name used in the Dossier: Atropellis pinicola |
| Group | Fungi |
| EPPO code | ATRPPC |
| Regulated status |
Godronia zelleri (A. pinicola) is listed in Annex II/A of Commission Implementing Regulation (EU) 2019/2072 as Atropellis spp. [1ATRPG]. Godronia zelleri is quarantine in Norway and Tunisia. It is on A1 list of EPPO (EPPO, online_a), Jordan, Kazakhstan and Russia (EPPO, online_b). |
| Pest status in China | Godronia zelleri has been reported to be present in China (Farr and Rossmann, online), but the only record is from Jilin Province in 1951 (Teng, 1996; Dossier Section 5.0). Besides this record, there are no other reports of G. zelleri in China (Dossier Section 5.0). The Panel cannot exclude that the pathogen is present in China. |
| Pest status in the EU | Godronia zelleri is absent in the EU (CABI, online; EPPO, online_c; Farr and Rossmann, online). |
| Host status of Pinus parviflora and P. thunbergii |
Pinus sp. is a host of G. zelleri (EPPO, online_d; Farr and Rossmann, online). There is no information on whether G. zelleri can also attack Pinus parviflora and P. thunbergii. However, in EPPO Godronia zelleri is on a list of pests for Pinus parviflora (EPPO, online_e) and P. thunbergii (EPPO, online_f). The Panel cannot exclude that P. parviflora and P. thunbergii can be hosts of G. zelleri. |
| PRA information |
Pest Risk Assessment currently available:
|
| Other relevant information for the assessment | |
| Biology |
Godronia zelleri is a fungus present in the US (mainly the western states) and Canada. It has been reported once in China by Teng (1996). The infection of the hosts starts with the ascospores. They are produced and dispersed in summer/early autumn mainly by wind. The role of rain in the dispersal of the ascospores is still unclear. When they reach a new host, under the right climatic conditions (between 4°C and 25°C, with relative humidity higher than 50%), ascospores germinate and mycelium penetrates the host via bark, possibly through microscopic cracks or leaf scars (Hopkins, 1963; EPPO, 1997; EFSA PLH Panel, 2014). Infection typically occurs in tissues between 5 and 30 years old (Hopkins, 1963). After the mycelium enters the sapwood and the xylem, an asymptomatic infection phase begins. It can last from 2 to 5 years on small and suppressed trees, up to 20 or more years on larger and vigorous ones, if the stem is infected (Hopkins, 1963; EPPO, 1997; EFSA PLH Panel, 2014). After the asymptomatic phase, a symptomatic phase begins which can be associated with production of inoculum after a long time. The inoculum increases on cankerous tissues of the bark as stromata harbouring fruiting bodies producing ascospores and conidia. The role of conidia for infection is still debated (EPPO, 1997; EFSA PLH Panel, 2014). The fungus will produce new inoculum every year until few years after the host’s death, or 1 year after cutting the tree and up to 3 or 4 years on logs left in the stands in the shade (Hopkins, 1969). |
| Symptoms | Main type of symptoms |
Symptoms include production of resin on the bark surface. The production of resin over the newly attacked tissue will continue each year in association with the colonisation by the pathogen, mainly along the longitudinal axis of the branch or stem (Hopkins and Callan, 1991; Callan, 1997). The bark over the infected area can crack (Hopkins and Callan, 1991; Callan, 1997). Needles may become chlorotic and small branches can die. The death of the entire tree is uncommon (EPPO, 1997; EFSA PLH Panel, 2014). |
| Presence of asymptomatic plants | After the mycelium enters the sapwood and the xylem, an asymptomatic infection phase begins. It can last from 2 to 5 years on small and suppressed trees, up to 20 or more years on larger and vigorous ones, if the stem is infected (Hopkins, 1963; EPPO, 1997; EFSA PLH Panel, 2014). | |
| Confusion with other pests |
Godronia zelleri can be confused with other fungal pathogens associated with bark tissues. For instance, Godronia/Atropellis can be confused with Cenangium spp. which are also reported on pines. Identification of Godronia spp. is done via chlorimetric response of the apothecia, and G. zelleri differs from the other species of Godronia/Atropellis by the shape, size and number of ascospores cells, and also by cultural characteristics. |
|
| Host plant range |
Hosts of G. zelleri are Pinus sp., P. albicaulis, P. contorta, P. contorta var. contorta, P. contorta var. latifolia, P. lambertiana, P. monticola, P. nigra, P. strobus and P. sylvestris (EPPO, online_d; Farr and Rossmann, online). In EPPO, G. zelleri is on a list of pests for P. parviflora (EPPO, online_e) and P. thunbergii (EPPO, online_f). |
|
| Reported evidence of impact | Damages caused by G. zelleri are known to be significant on Pinus contorta, where it can damage the wood value, otherwise are considered minor, rarely killing their host on other Pinus species (Baranyay et al., 1973). | |
| Evidence that the commodity is a pathway | Growing material and twigs are known to be pathway for G. zelleri (EPPO, 1997). | |
| Surveillance information |
According to Dossier Section 2.0, the inspection and quarantine organisation will conduct official inspection on the export registered plantation base no less than twice every year. The inspection focuses on: surrounding environment and conditions of the plantation base, cultivated varieties and their quantities, diseases and pests occurrence and prevention and control records, purchase and delivery logbook, production cancelling after verification, as well as other items that should be supervised. Supervision records are properly kept. The inspection and quarantine organisation are expected to conduct surveillance of pests of concern for the EU at least six times a year at reasonable time intervals. The infested plants are removed from the nursery. The nursery and its immediate vicinity areas (at least 2 km) are surveyed as follows: at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of 10% of the plants. According to Dossier Section 4.0, the pathogen is absent, not known to occur in China. |
|
A.8.2. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Atropellis pinicola (G. zelleri) between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.8.3. Evaluation of the implementation and relevance of specific measures in China
Commission Implementing Regulation (EU) 2019/2072 specifies in point 30 of Annex VII measures which are required for the import of the commodity from China.
The below overview provides special requirements for naturally or artificially dwarfed plants for planting other than seeds according to Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 including an assessment of whether or not the applicant country implements those measures with respect to Godronia zelleri (regulated as Atropellis spp.) identified in this Opinion. The Panel assumes that information on treatments required to be included in the phytosanitary certificate is provided by the applicant country according to the Article 71 of Regulation (EU) 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’.
| Special requirements as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 | Implementation of the special requirements in China according to information provided in the Dossier | Relevance of special requirements for the pest including uncertainties |
|---|---|---|
| ‘Official statement that: | – | – |
| a) the plants, including those collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime, | Yes | Yes |
| b) the plants in the nurseries referred to in point (a) of this entry: | – | – |
| i) at least during the period referred to in point (a) of this entry: | – | – |
| — were potted, in pots which are placed on shelves at least 50 cm above ground, |
Yes, partially. Pots are also reported to be kept on the ground. |
No |
| — have been subjected to appropriate treatments to ensure freedom from non‐European rusts, and the active ingredient, concentration and date of application of these treatments has been mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’. |
Yes. Treatments are appropriate. They are expected to reduce the likelihood of infection and the rate of colonisation of plant tissues by rust fungi, although it is uncertain if freedom from non‐European rusts could be reachable. Treatments used are listed in Table 6.
Uncertainties:
|
No. However, treatments could reduce the likelihood of infection by the pathogen. |
| — have been officially inspected at least six times a year at appropriate intervals for the presence of Union quarantine pests of concern in accordance with Regulation (EU) No 2016/2031, and these inspections have also been carried out on plants in the immediate vicinity of the nurseries referred to in point (a) of this entry, at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus, | Yes | Yes |
| — have been found free, in these inspections, from the relevant Union quarantine pests of concern as specified in the previous indent, infested plants have been removed and the remaining plants, where appropriate, have been effectively treated and have been held for an appropriate period and inspected to ensure freedom from such pests, | Yes | Yes |
| — have been planted in either an unused artificial growing medium or in a natural growing medium, which has been treated by fumigation or by appropriate heat treatment and has been free of any Union quarantine pests, | Yes | No |
| — have been kept under conditions which ensure that the growing medium has been maintained free from Union quarantine pests and within 2 weeks prior to dispatch, have been: | Yes | No |
| — shaken and washed with clean water to remove the original growing medium and kept bare rooted, or | ‐ | No |
| — shaken and washed with clean water to remove the original growing medium and replanted in growing medium which meets the conditions laid down in (i) fifth indent, or | ‐ | No |
| — subjected to appropriate treatments to ensure that the growing medium is free from Union quarantine pests, and the active ingredient, concentration and date of application of these treatments have been indicated on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031 under the rubric ‘Disinfestation and/or disinfection treatment’. | Yes | No |
| ii) were packed in closed containers which have been officially sealed and bear the registration number of the registered nursery, and this number has been indicated under the rubric ‘Additional declaration’ on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, enabling the consignments to be identified.’ | Yes | Yes |
A.8.4. Reference List
Baranyay JA, Szabo T and Hunt K, 1973. Effect of Atropellis canker on growth and utilization of lodgepole pine. Canadian Forestry Service, Pacific Forest Research Centre, Victoria, British Columbia, Information Report Bc‐X‐86, Department of the Environment. 24 pp.
CABI, online. Atropellis pinicola (twig blight of pine). Available online: https://www.cabi.org/isc/datasheet/7815 [Accessed: 5 August 2021].
Callan B, 1997. Atropellis cankers. In: Hansen EM and Lewis KJ (eds.). Compendium of conifer diseases. APS Press, St. Paul, MN, USA, 46–47.
EFSA PLH Panel, 2014. Scientific Opinion on the pest categorisation of Atropellis spp. EFSA Journal, 12, 3926, 33 pp. https://doi.org/10.2903/j.efsa.2014.3926
EFSA PLH Panel, Jeger M, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Grégoire J, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Boberg J, Porta Puglia A, Vettraino AM, Pautasso M and Rossi V, 2017. Pest risk assessment of Atropellis spp. for the EU territory. EFSA Journal 2017;15(7), 4877, 46 pp. https://doi.org/10.2903/j.efsa.2017.4877
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A1 List of pests recommended for regulation as quarantine pests, version 2020‐09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list [Accessed: 5 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Atropellis pinicola (ATRPPC), Categorization. Available online: https://gd.eppo.int/taxon/ATRPPC/categorization [Accessed: 31 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Atropellis pinicola (ATRPPC), Distribution. Available online: https://gd.eppo.int/taxon/ATRPPC/distribution [Accessed: 31 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Atropellis pinicola (ATRPPC), Host. Available online: https://gd.eppo.int/taxon/ATRPPC/hosts [Accessed: 31 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_e. Pinus parviflora (PIUPF), Pests. Available online: https://gd.eppo.int/taxon/PIUPF/pests [Accessed: 31 August 2021]
EPPO (European and Mediterranean Plant Protection Organization), online_f. Pinus thunbergii (PIUTH), Pests. Available online: https://gd.eppo.int/taxon/PIUTH/pests [Accessed: 31 August 2021].
EPPO (European and Mediterranean Plant Protection Organization), 1997. Data sheets on quarantine pests: Atropellis spp. In: Smith IM, McNamara DG, Scott PR and Holderness M (eds.). Quarantine Pests for Europe, 2nd edition. CABI/EPPO, Wallingford. 1425 pp.
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars‐grin.gov/fungaldatabases [Accessed: 5 August 2021].
Hopkins JC, 1963. Atropellis canker of lodgepole pine: etiology, symptoms, and canker development rates. Canadian Journal of Botany, 41, 1535–1545. https://doi.org/10.1139/b63‐135
Hopkins JC, 1969. Atropellis canker in Alberta: silvicultural control and its biological basis. Canadian Forestry Service, Department of Fisheries and Forestry, 14 pp.
Hopkins JC and Callan B, 1991. Atropellis Canker. Forestry Canada, Forest Insect and Disease Survey, Forest Pest Leaflet No 25, 4 pp.
Teng SC, 1996. Fungi of China. Mycotaxon, University of Michigan, USA, 586 pp.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
A.9. Grubs (Anomala testaceipes and Popillia quadriguttata)
A.9.1. Organism information
A.9.1.1 Anomala testaceipes
| Taxonomic information |
Current valid scientific name: Anomala testaceipes Synonyms: Mimela testaceipes, Rhombonyx testaceipes ussuriensis Name used in the EU legislation: – Order: Coleoptera Family: Scarabaeidae Common name: striated chafer, lineate chafer Name used in the Dossier: – |
|
| Group | Insects | |
| EPPO code | ANMLTE | |
| Regulated status | Anomala testaceipes is neither regulated in the world nor listed by EPPO. | |
| Pest status in China | Anomala testaceipes is present in China (Toepfer et al., 2014; CABI, online), in Liaoning province (Toepfer et al., 2014). | |
| Pest status in the EU | Anomala testaceipes is absent in the EU (Toepfer et al., 2014; CABI, online). | |
| Host status on Pinus parviflora and P. thunbergii |
Anomala testaceipes is pest of conifers, adults feed on pine needles (Furuno and Uenaka, 1976) and larvae on roots (Toepfer et al., 2014). Pinus thunbergii is reported as a host of A. testaceipes (EFSA PLH Panel, 2019). There is no information on whether A. testaceipes can also attack P. parviflora. |
|
| PRA information | No Pest Risk Assessment is currently available. | |
| Other relevant information for the assessment | ||
| Biology |
Anomala testaceipes is a grub, present in China, Japan (Hokkaido), South Korea (Toepfer et al., 2014; CABI, online), East Russia (Primorsky region and Kurile islands) and North Korea (Toepfer et al., 2014). Anomala testaceipes has four stages of development: egg, larva (there is no information on number of instars), pupa and adult. Larvae damage roots of host plants (including conifers) and their development requires between 1 and 3 years. Anomala testaceipes overwinters in the soil as larvae of different instars or as adults (Toepfer et al., 2014). The larvae overwinter 10–40 cm deep in soil and are quite sensitive to freezing, with the supercooling point at about –6.8°C (Hoshikawa et al., 1988). Adults are yellowish brown, 14.5–20.0 mm long. They emerge from June to September (Fuji Flavor CO., LTD., online). Adults can fly (Hisano, 2019), they feed on needles of conifers (Furuno and Uenaka, 1976) and are attracted by black‐light (Torikura, 1992). Females lay between 25 and 60 eggs. The flight activity was observed mainly at dusk in Japan (Torikura, 1991). There is no detailed information on the biology nor on the possible flight distance that A. testaceipes can cover. It can be assumed that possible pathways of entry for A. testaceipes are like the ones for Popillia japonica. According to EFSA PLH Panel (2018), the pathways are infested soil and growing media accompanying plants for planting (i.e. eggs, larvae and pupae); leaves and flowers on plants for planting, cut flowers and cut branches (i.e. adults) and hitch‐hiking adults, independent of host plants. |
|
| Symptoms | Main type of symptoms |
Main symptoms of adult feeding on conifers are cut down needles (Furuno and Uenaka, 1976). Larvae cause damage on roots of host plants (including conifers), which leads to yield losses and in worst cases death of plants, especially young plants, seedlings and nursery stock (Toepfer et al., 2014). |
| Presence of asymptomatic plants | Infested plants are asymptomatic only when carrying eggs laid in soil and when young larvae are newly hatched. | |
| Confusion with other pests | It can be confused with other Anomala species. A morphological or molecular analysis is needed in order to distinguish them. | |
| Host plant range |
Adults of A. testaceipes were reported to feed on needles of Chamaecyparis obtusa, Cryptomeria japonica, Larix leptolepis, Pinus banksiana, P. echinata, P. elliottii, P. muricata, P. pungens, P. rigida, P. taeda and P. virginiana (Furuno and Uenaka, 1976). Hosts of larvae are conifers, citrus, pasture and grassy crops (Toepfer et al., 2014). Pinus thunbergii is reported as a host of A. testaceipes (EFSA PLH Panel, 2019). |
|
| Reported evidence of impact | Larvae of A. testaceipes are reported to cause root damage on conifers, citrus, pasture and grassy crops (Toepfer et al., 2014). | |
| Evidence that the commodity is a pathway | Even though there are no reports of Anomala spp. being intercepted on plants for planting with soil, it can be assumed that the pathway with bonsai plants is possible, just like for other grubs. | |
| Surveillance information | No surveillance information for this pest is currently available from China. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. | |
A.9.1.2. Popillia quadriguttata
| Taxonomic information |
Current valid scientific name: Popillia quadriguttata Synonyms: Popillia uchidai According to Dunlap (2016), other synonyms are: Trichius biguttatus, Popillia bogdanowi, Popillia castanoptera, Popillia chinensis, Popillia dichroa, Popillia frivaldszkyi, Popillia purpurarescens, Popillia ruficollis, Popillia sordida, Popillia straminipennis, Popillia uchidai Name used in the EU legislation: – Order: Coleoptera Family: Scarabaeidae Common name: four‐spotted beetle Name used in the Dossier: Popillia quadriguttata [also cited in the Dossier as Anomala multistriata, which however is not a synonym but rather a species present in Japan (Yoshida and Umemura, 1973)] |
| Group | Insects |
| EPPO code | POPIQU |
| Regulated status | Popillia quadriguttata is neither regulated in the world nor listed by EPPO. |
| Pest status in China |
Popillia quadriguttata is present in north China. In China it was misidentified as P. japonica. Therefore, it can be assumed that the grub is present in provinces of Heilongjiang, Jilin and Xianggang as reported by EFSA PLH Panel (2018) and EPPO (online). Popillia quadriguttata is also present in Hebei and Liaoning provinces (Toepfer et al., 2014). According to Chen et al. (2014), this species is widely distributed throughout China, especially in Northeast where soybean production is located. |
| Pest status in the EU | Popillia quadriguttata is absent in the EU (Chen et al., 2014). |
| Host status on Pinus parviflora and P. thunbergii |
In Dossier Section 2.0, P. quadriguttata (cited as Anomala multistriata, which however is not a synonym, see above) is reported as a pest of Pinus parviflora. There is no information in the scientific literature on whether Popillia quadriguttata can attack P. parviflora, P. thunbergii or any other conifer. |
| PRA information | No Pest Risk Assessment is currently available. |
| Other relevant information for the assessment | |
| Biology |
Popillia quadriguttata is a grub, present in China, North and South Korea, Russia (Primorsky region, Sakhalin, South Chaba‐Rovskovo region) and Vietnam. For a long time, this species was misidentified as Popillia japonica in China and Korea (Chen et al., 2014; Toepfer et al., 2014). They are morphologically very similar but differ from each other in host range and life history (Lee et al., 2007). Popillia quadriguttata has four stages of development: egg, larva (there is no information on number of instars), pupa and adult. It requires 1 year for complete development (Toepfer et al., 2014). Adults are shining green with reddish sheen, 8.0–11.0 mm long (Dunlap, 2016). Adults occurred from late June to late July in Korea. Active adult flight was observed mainly during noon up to 2 h in the afternoon. In the night adults were not attracted to the light (Lee et al., 2002). Sex pheromones of P. japonica or floral kairomones are used for trapping of P. quadriguttata (Chen et al., 2014). The overwintering stage are larvae in the soil (Toepfer et al., 2014). It is above and below ground pest. The larvae feed on roots of apricot, chestnut, elm, maize, peanut, poplar, potato, soybean, wheat, occasionally on turf grass, meadows and legumes (Toepfer et al., 2014). There is no detailed information on the biology of P. quadriguttata nor on the possible flight distance that can cover. Even though P. quadriguttata was reported to have different life history compared to P. japonica (Lee et al., 2007) it can be assumed that it will be in some aspects similar. Popillia japonica completes its life cycle in 1 or 2 years, depending on the climate conditions. There are four stages of development: egg, larva (three instars), pupa and adult. Adults usually emerge between June and July. They are most active on sunny days and feed on foliage and fruits. Adults can fly and can cover between 3 and 24 km/year. Females lay between 40 and 60 eggs, 10 cm deep in the soil. Eggs hatch after about 2 weeks. First two larval instars are in the upper 7.5 cm soil. They feed on decaying matter and roots of plants, mainly grasses. Development of the first larval instar takes 2–3 weeks and the second larval instar 3–4 weeks. The third larval instar overwinters 10–20 cm deep in the soil. In spring the third larval instar goes up the soil and pupate. If the life cycle of P. japonica takes 2 years, the second larval instar overwinters during the first winter and the third larval instar during the second one (EFSA PLH Panel, 2018). It can be assumed that possible pathways of entry for P. quadriguttata are like the ones for P. japonica. According to EFSA PLH Panel (2018), the pathways are infested soil and growing media accompanying plants for planting i.e. eggs, larvae and pupae); leaves and flowers on plants for planting, cut flowers and cut branches (i.e. adults) and hitch‐hiking adults, independent of host plants. |
| Symptoms | Main type of symptoms |
Popillia quadriguttata was never reported on conifers. Therefore, there is no information on symptoms caused to Pinus species. On flowers, buds, immature leaves and shoots of soybeans feeding scars caused by adults can be observed. Larvae feed on soybean roots (Chen et al., 2014). Larvae cause damage on roots, which leads to yield losses and in worst cases death of plants, especially young plants, seedlings and nursery stock (Toepfer et al., 2014). |
| Presence of asymptomatic plants | Infested plants are asymptomatic only when carrying eggs laid in soil and when young larvae are newly hatched. | |
| Confusion with other pests | It can be confused with other Popillia species, such as P. japonica (Lee et al., 2014) and P. lewisi (Dunlap, 2016). A morphological or molecular analysis is needed in order to distinguish them. |
| Host plant range |
Popillia quadriguttata is a polyphagous pest. It has been reported to feed on approximately 20 families and 25 species (Chen et al., 2014). Family hosts of P. quadriguttata confirmed in golf courses are Asteraceae (Artemisia princeps var. orientalis, Rhapontica uniflora), Betulaceae (Corylus heterophylla var. thunbergii), Dioscoreaceae (Dioscorea septemloba), Euphorbiaceae (Acalypha australis), Fabaceae (Amorpha fruticosa, Glycine max, Lespedeza cyrtobotrya, Wistaria floribunda), Fagaceae (Quercus serrata), Gramineae (Chloris virgata, Zea mays), Lauraceae (Lindera erythrocarpa), Oleaceae (Ligustrum obtusifolium), Polygonaceae (Persicaria senticosa), Primulaceae (Lysimachia burystachys), Pteridaceae (Pteridium aquilinum), Rosaceae (Malus pumila var. dulcissima, Pyrus spp., Prunus sargentii, Rubus parvifolius), Rutaceae (Zanthoxylum spp.), Salicaceae (Salix koreansis), Sterculiaceae (Helicteres angustifolia), Ulmaceae (Ulmus pumila) and Solanaceae (Solanum lyratum) (Lee et al., 2002). In laboratory conditions P. quadriguttata was observed to feed on additional plant families of Aceraceae, Aquifoliaceae, Caprifoliaceae, Celastraceae, Cornaceae, Ebenaceae, Hamameliaceae, Laesalpinaceae, Magnoliaceae, Malvaceae, Mimosaceae, Moraceae, Platanaceae, Punicaceae, Rhamnaceae, Sabiaceae, Sapindaceae, Staphyleaceae, Styracaceae, Symplocaceae, Theaceae, Tiliaceae, Thymelaeaceae, Verbenaceae and Vitaceae (Lee et al., 2002). No conifers are reported in the host list of P. quadriguttata. However, the Dossier states that P. quadriguttata is a pest of P. parviflora. Therefore, the Panel cannot exclude that P. parviflora is a host. |
| Reported evidence of impact |
Popillia quadriguttata caused significant crop losses on turfgrass, flower crops, soybeans and tree fruits in China. It causes approximately 1% yield loss of soybeans per year (Chen et al., 2014). In South Korea it is a pest of golf courses and turfgrasses. Turf damage is mainly caused by birds and mammals, which are digging up the turf to find and eat grub larvae (Lee et al., 2007). |
| Evidence that the commodity is a pathway | Popillia sp. was intercepted in Germany with Cycas revoluta plants for planting already planted from Costa Rica in 1999 (EUROPHYT, online). Therefore, it can be assumed that plants with soil (i.e. bonsai) can be a pathway for the pest. |
| Surveillance information | No surveillance information for this pest is currently available from China. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. |
A.9.2. Possibility of pest presence in the nursery
A.9.2.1. Possibility of entry from the surrounding environment
Anomala testaceipes and Popillia quadriguttata are present in China. The nursery is located in Zhejiang province, where A. testaceipes is not reported to be present. However, it is present in Liaoning province (Toepfer et al., 2014). Popillia quadriguttata is reported to be widely distributed throughout China (Chen et al., 2014). The possibility of entry for both grubs to surrounding environment of nursery is through hitchhiking of adults and adult flight dispersal.
Anomala testaceipes is a pest of conifers, citrus, pasture and grassy crops (Toepfer et al., 2014). Popillia quadriguttata is polyphagous pest, but not reported to infest conifers (Lee et al., 2002; Chen et al., 2014). According to Dossier Sections 4.0 and 5.0, the nursery is surrounded by many different plants. From these plant species mentioned in the Dossier Citrus maxima, C. paradisi, Pinus elliottii and P. thunbergii are hosts of A. testaceipes. Hosts of P. quadricuttata present in the surrounding are Acer buergerianum, Albizia julibrissin, Camellia japonica, Cercis chinensis, Cinnamomum camphora, Diospyros kaki, Ligustrum obtusifolium, Osmanthus fragrans, Platanus orientalis, Prunus mume, P. persica, P. salicina, Punica granatum, Sophora japonica and Ulmus pumila. Moreover, there are lawns in the surrounding area of the nursery, which can be attacked by both grubs. Based on the presence of suitable hosts of the pests in the surrounding, the Panel assumes that the pests can be present in the production area of bonsai plants destined for export to the EU.
As stated in Dossier Section 4.0, the bonsai cultivation site is protected by a 40‐mesh insect‐proof net (0.4 mm), which the grub adults cannot pass unless there is opening in the net. Moreover, the adult grubs can feed on pine needles of mother trees and establish there.
Uncertainties
-
–
There is no surveillance information on the presence or population pressure of the pests in the area where the nursery is located.
-
–
No information available on the distance of the nursery to sources of pests.
-
–
Presence of A. testaceipes and P. quadriguttata in Zhejiang province is uncertain.
-
–
The host status of P. parviflora for A. testaceipes.
-
–
The host status of P. parviflora and P. thunbergii for P. quadriguttata.
-
–
No information available on the flight capacity of the pests.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pests to enter the nursery. The pests can be present in the surrounding area and the transferring rate could be enhanced by adult flight dispersal. Suitable hosts are present in the surrounding of the nursery.
A.9.2.2. Possibility of entry with new plants/seeds
All seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (P. thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triadimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
The growing media used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0). Anomala testaceipes and P. quadriguttata could potentially enter with infested soil/growing media. However, since the coconut coir is cleaned, it is not relevant.
Possibility of entry with seeds is not relevant for A. testaceipes and P. quadriguttata.
Uncertainties
-
–
It is not clear if and how new mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pests could enter the nursery with new plants/seeds or soil/growing media.
A.9.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of Pinus parviflora are taken (Dossier Section 4.0).
Anomala testaceipes and P. quadriguttata can spread within the nursery by adult fight. However, it cannot reach the bonsai plantation unless there is opening in the net. Moreover, the adult grubs can feed on pine needles of mother trees and establish there.
The nursery also grows ornamental plants including abelia, bamboo, camphor, loropetalum, photinia, pyracantha, wisteria and other plants (Dossier Section 4.0). Cinnamomum camphora is a host of P. quadricuttata. Therefore, other suitable hosts of some grubs are present in the nursery.
Spread within the nursery through water, equipment and tools is not relevant.
Uncertainties
-
–
There is no information on the presence or population pressure of the pests in the nursery.
-
–
The host status of Pinus parviflora for A. testaceipes.
-
–
The host status of Pinus parviflora and P. thunbergii for P. quadriguttata.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pest within the nursery is possible due to the presence of suitable hosts (i.e. mother plants and commodity).
A.9.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Anomala testaceipes and Popillia quadriguttata between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.9.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on grubs (Anomala testaceipes and Popillia quadriguttata) is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
Physical separation of the bonsai plants has effect on reducing risk of infestation with grubs, especially in the production base.
Uncertainties:
|
| 2 | Growing medium and its treatment | Yes |
Soaking is very effective in killing the larvae hidden in the soil.
Uncertainties:
|
| 3 | Treatment of seeds | No | Not applicable. |
| 4 | Insecticide and acaricide treatments | Yes |
Spray of contact and ingestion insecticides can be very effective in killing the adults that are present on the plants. Soaking is very effective in killing the larvae hidden in the soil.
Uncertainties:
|
| 5 | Fungicide treatments | No | Not applicable. |
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | No | Not applicable. |
| 8 | Official inspections during production | Yes |
The sampling and laboratory inspection of plant material may allow to identify infested plants by grubs.
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
The sampling and laboratory inspection of plant material may allow to identify infested plants by grubs. Export plants can be infested when they are moved from the bonsai production base to the storage and packaging place. The removal of the soil and soaking will kill the larvae hidden in the soil.
Uncertainties:
|
A.9.5. Overall likelihood of pest freedom for Anomala testaceipes on grafted bonsai plants
A.9.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The scenario assumes that the pest is absent in the surroundings of the nursery. The scenario also assumes that treatment consisting in soaking in Avermectin is fully effective against the pest.
A.9.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
The scenario assumes that the pest is present in the surroundings of the nursery and it could affect mother plants. The scenario also assumes that the net house is not completely sealed. Finally, the scenario assumes that the treatments consisting in soaking in Avermectin and in removing the upper part of the soil (2 cm) is not properly done, so that, some larvae could remain in the soil after treatments.
A.9.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
The median is closer to the lower values because high effectiveness is expected from soaking in Avermectin treatment and because it is not expected a high abundance of the pest in the surroundings of the nursery.
A.9.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The presence of the pest in the surroundings of the nursery is uncertain as pest is not reported to be present in the province. However, it can be present in the province and the surroundings since the hosts of the pest are present. Uncertainty is high about the control methods performed because information provided in the Dossier is not complete. Efficacy of the soaking is also uncertain, although a high efficacy is expected, what reduces uncertainties for rates above the median.
A.9.5.5. Elicitation outcomes of the assessment of the pest freedom for Anomala testaceipes on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.15) and pest freedom (Table A.16).
Table A.15.
Elicited and fitted values of the uncertainty distribution of pest infestation by Anomala testaceipes per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 2 | 5 | 15 | 50 | ||||||||||
| EKE | 0.009 | 0.038 | 0.117 | 0.363 | 0.847 | 1.67 | 2.74 | 5.80 | 10.6 | 14.1 | 19.1 | 25.4 | 33.4 | 41.0 | 50.0 |
The EKE results are the BetaGeneral (0.61663, 6.5786, 0, 115) distribution fitted with @Risk version 7.6.
Table A.16.
The uncertainty distribution of plants free of Anomala testaceipes per 10,000 plants calculated by Table A.15
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,950 | 9,985 | 9,995 | 9,998 | 10,000 | ||||||||||
| EKE results | 9,950 | 9,959 | 9,967 | 9,975 | 9,981 | 9,986 | 9,989 | 9,994 | 9,997 | 9,998 | 9,999.2 | 9,999.6 | 9,999.9 | 9,999.96 | 9,999.99 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.16.
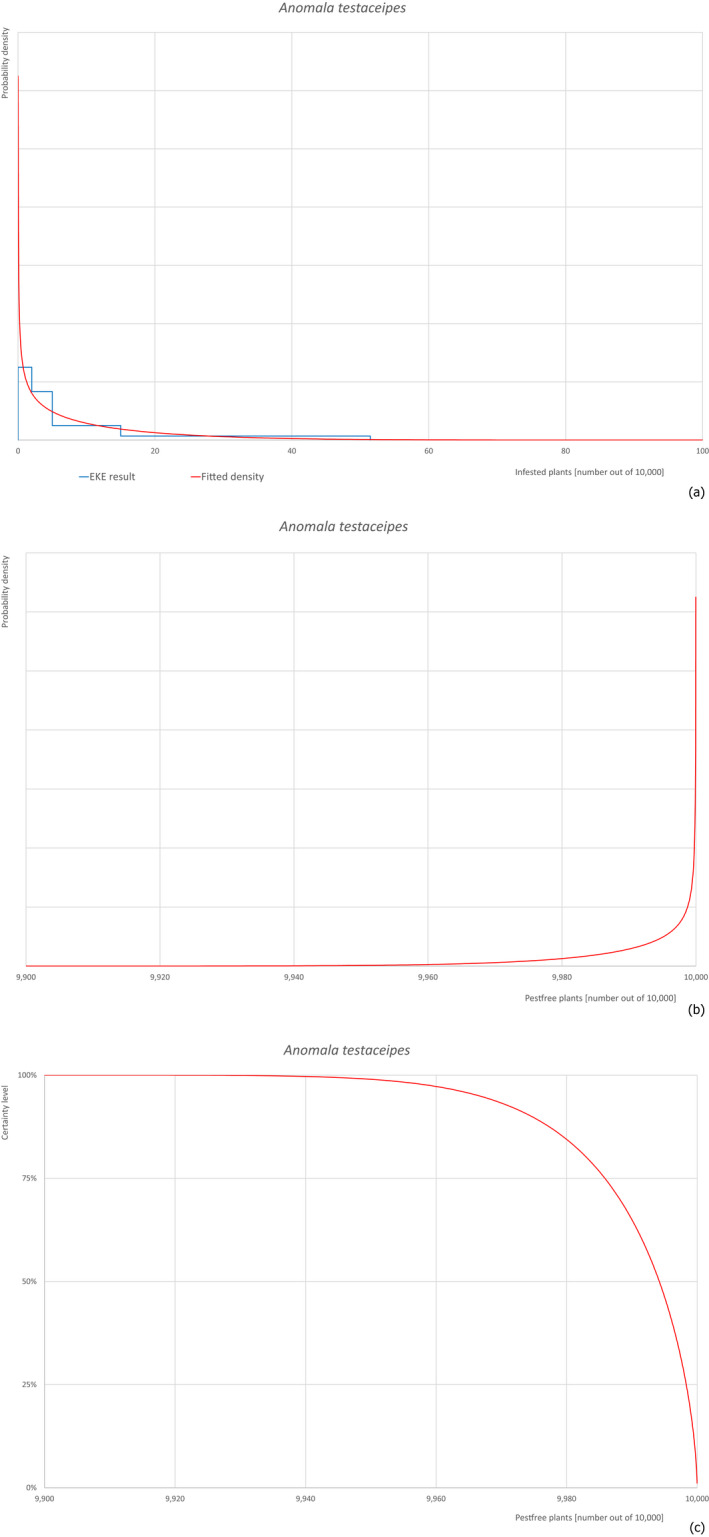
Figure A.13: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.9.6. Overall likelihood of pest freedom for Popillia quadriguttata on grafted bonsai plants
A.9.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The scenario assumes that the abundance of the pest is low in the surroundings of the nursery. The scenario also assumes that the commodity is not attractive for the pest. Finally, the scenario assumes that treatment consisting in soaking in Avermectin is fully effective against the pest.
A.9.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
The scenario assumes that, although Pinus plants are not attractive for the pest, it is a polyphagous pest and other hosts can be found inside the nursery and in the surroundings. The scenario also assumes that treatment consisting in soaking in Avermectin could be not fully effective.
A.9.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
The median is closer to the lower values because high effectiveness is expected from soaking in Avermectin treatment and because Pinus bonsais are not expected to be attractive to the pest.
A.9.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The attractiveness of Pinus bonsais to the pest is uncertain, although a poor attraction is expected. Uncertainty is high about the control methods performed because information provided in the Dossier is not complete. Efficacy of the soaking is also uncertain, although a high efficacy is expected, what reduces uncertainties for rates above the median.
A.9.6.5. Elicitation outcomes of the assessment of the pest freedom for Popillia quadriguttata on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.17) and pest freedom (Table A.18).
Table A.17.
Elicited and fitted values of the uncertainty distribution of pest infestation by Popillia quadriguttata per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 1 | 3 | 6 | 15 | ||||||||||
| EKE | 0.012 | 0.043 | 0.110 | 0.284 | 0.579 | 1.03 | 1.56 | 2.93 | 4.82 | 6.08 | 7.71 | 9.54 | 11.6 | 13.3 | 15.0 |
The EKE results are the BetaGeneral (0.73574, 2.8331, 0, 19.5) distribution fitted with @Risk version 7.6.
Table A.18.
The uncertainty distribution of plants free of Popillia quadriguttata per 10,000 plants calculated by Table A.17
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,985 | 9,994 | 9,997 | 9,999 | 10,000 | ||||||||||
| EKE results | 9,985 | 9,987 | 9,988 | 9,990 | 9,992 | 9,994 | 9,995 | 9,997 | 9,998 | 9,999 | 9,999.4 | 9,999.7 | 9,999.9 | 9,999.96 | 9,999.99 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.18.
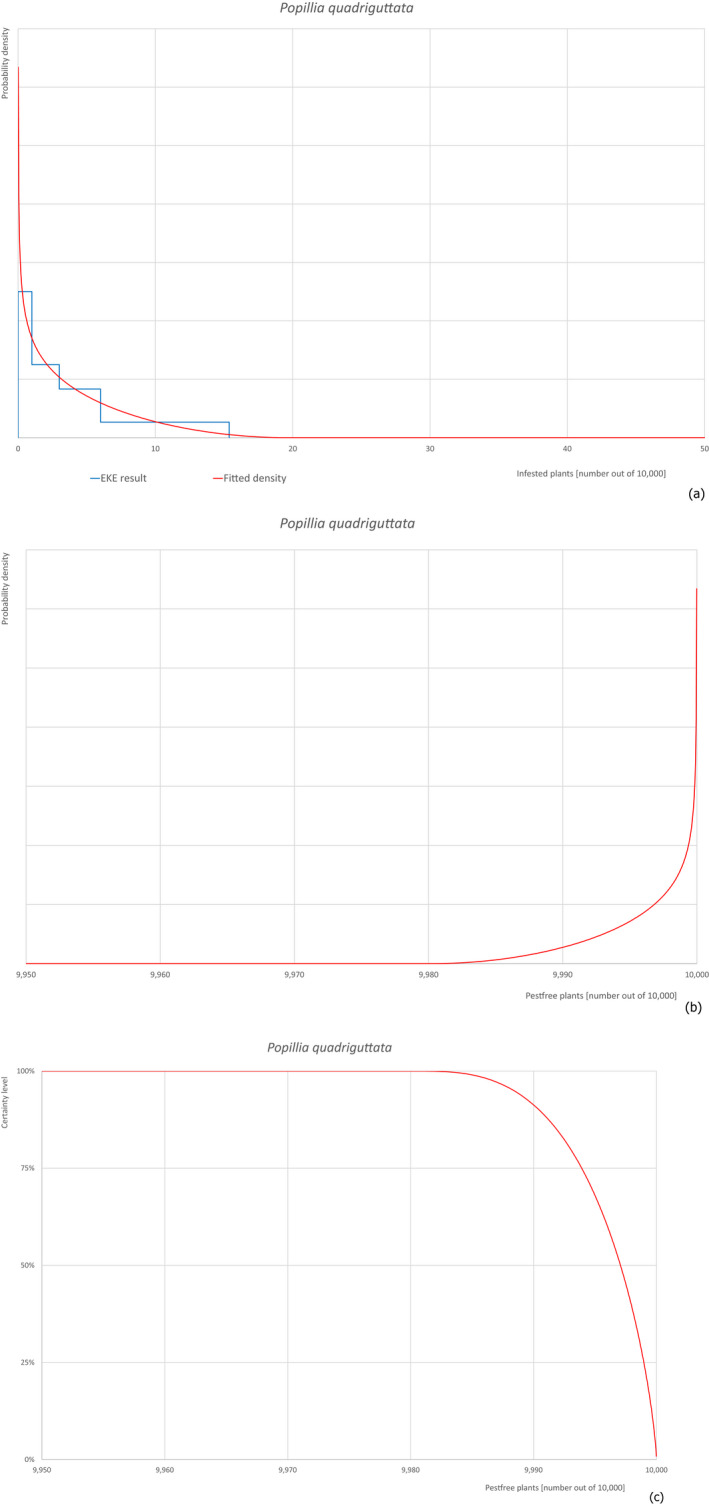
Figure A.14: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.9.7. Reference List
CABI (Centre for Agriculture and Bioscience International), online. Mimela testaceipes (chafer, striated). Available online: https://www.cabi.org/cpc/datasheet/5515 [Accessed: 24 June 2021].
Chen RZ, Klein MG, Li QY and Li Y, 2014. Mass trapping Popillia quadriguttata using Popillia japonica (Coleoptera: Scarabaeidae) pheromone and floral lures in Northeastern China. Environmental Entomology, 43, 774–781. https://doi.org/10.1603/en13319
Dunlap JB, 2016. Developing scarab beetle identification tools for Hawaii and the Pacific. Doctoral dissertation, Wichita State University. 310 pp.
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Czwienczek E and MacLeod A, 2018. Scientific Opinion on the pest categorisation of Popillia japonica. EFSA Journal 2018;16(11):5438, 30 pp. https://doi.org/10.2903/j.efsa.2018.5438
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Vettraino AM, Leuschner R, Mosbach‐Schulz O, Rosace MC and Potting R, 2019. Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. https://doi.org/10.2903/j.efsa.2019.5667
EPPO (European and Mediterranean Plant Protection Organization), online. Popillia japonica (POPIJA), Distribution. Available online: https://gd.eppo.int/taxon/POPIJA/distribution [Accessed: 25 June 2021].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Fuji Flavor CO., LTD., online. For agricultural insect pests (in Japan) New Windspack Material for monitoring scarab beetles ‐ Lineate cafer, Scientific name: Anomala testaceipes. Available online: https://www.fjf.co.jp/cn/ecomone/product/winspac.html [Accessed: 30 June 2021].
Furuno T and Uenaka K, 1976. Studies on the insect damage upon the pine‐species imported in Japan, 3: On the feeding of adult of striated chafer (Anomala testaceipes Motschulsky). Bulletin of the Kyoto University Forests. 14 pp.
Hisano M, 2019. Insectivory characteristics of the Japanese marten (Martes melampus): a qualitative review. Zoology and Ecology, 29, 71–77. https://doi.org/10.35513/21658005.2019.1.9
Hoshikawa K, Tsutsui H, Honma K and Sakagami SF, 1988. Cold resistance in four species of beetles overwintering in the soil, with notes on the overwintering strategies of some soil insects. Applied Entomology and Zoology, 23(3), 273–281. https://doi.org/10.1303/aez.23.273
Lee DW, Choo HY, Chung JM, Lee SM and Sagong Y‐B, 2002. Host plants of Popillia quadriguttata (Coleoptera: Scarabaeidae). Korean Journal of Applied Entomology, 41, 15–19.
Lee DW, Choo HY, Smitley DR, Lee SM, Shin HK, Kaya HK, Park CG and Park JK, 2007. Distribution and adult activity of Popillia quadriguttata (Coleoptera: Scarabaeidae) on golf courses in Korea. Journal of Economic Entomology, 100(1), 103–109. https://doi.org/10.1093/jee/100.1.103
Lee DW, Smitley DR, Lee SM, Kaya HK, Park CC and Choo HY, 2014. Seasonal phenology and diurnal activity of Promachus yesonicus (Diptera: Asilidae), a predator of scarabs, on Korean golf courses. Journal of Asia‐Pacific Entomology, 17, 169–174. https://doi.org/10.1016/j.aspen.2013.11.010
Toepfer S, Li H, Pak SG, Son KM, Ryang YS, Kang SI, Han R and Holmes K, 2014. Soil insect pests of cold temperate zones of East Asia, including DPR Korea: A review. Journal of Pest Science, 87, 567–595. https://doi.org/10.1007/s10340‐013‐0540‐8
Torikura H, 1991. On the flight of Mimela testaceipes between Sward and Forest, in relation to its internal conditions (Coleoptera, Scarabeidae). Japanese Journal of Entomology, 59, 199–211 (in Japanese).
Torikura H, 1992. What kind of chafers (Coleoptera, Scarabaeidae) are attracted to light? Annual Report of the Society of Plant Protection of North Japan (Japan), 43, 185–188.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
Yoshida M and Umemura T, 1973. Studies on May beetles injurious to the turfgrass. (1) On the kinds of species of the May beetles injurious to the turfgrass growing in seaside. Journal of Japanese Society of Turfgrass Science, 2, 19–25 (in Japanese). Available online: https://www.jstage.jst.go.jp/article/turfgrass1972/2/1/2_1_19/_pdf
A.10. Mycosphaerella gibsonii
A.10.1. Organism information
| Taxonomic information |
Current valid scientific name: Mycosphaerella gibsonii Synonyms: Cercoseptoria pini‐densiflorae (anamorph), Cercospora pini‐densiflorae (anamorph), Pseudocercospora pini‐densiflorae (teleomorph) Name used in the EU legislation: Pseudocercospora pini‐densiflorae (Hori & Nambu) Deighton [CERSPD] Order: Mycosphaerellales Family: Mycosphaerellaceae Common name: brown needle blight of pine, Cercospora blight of pine, needle blight of pine, brown needle disease, Cercospora needle blight Name used in the Dossier: Cercoseptoria pini‐densiflorae, Mycosphaerella gibsonii |
| Group | Fungi |
| EPPO code | CERSPD |
| Regulated status |
Mycosphaerella gibsonii is listed in Annex II/A of Commission Implementing Regulation (EU) 2019/2072 as Pseudocercospora pini‐densiflorae (Hori & Nambu) Deighton [CERSPD]. The pathogen is quarantine in Morocco, Norway and Tunisia. It is on A1 list of Argentina, Brazil, Chile, Georgia, Russia, Turkey, Ukraine and Uruguay (EPPO, online_a). It is included in EPPO A1 list (EPPO, online_b). |
| Pest status in China | In China, M. gibsonii is present in the provinces of Anhui, Fujian, Guangdong, Guangxi, Henan, Hunan, Jiangsu, Jiangxi and Hongkong (Dossier Section 4.0; EPPO, online_c). |
| Pest status in the EU | Mycosphaerella gibsonii is absent in the EU (EPPO, online_c; Farr and Rossman, online). |
| Host status on Pinus parviflora and P. thunbergii |
Reported hosts of M. gibsonii are Pinus parviflora (CABI, online; Farr and Rossman, online) and P. thunbergii (Quintero, 2015; CABI, online; EPPO, online_d; Farr and Rossman, online). However, only P. parviflora is considered because M. gibsonii is only associated with pine needles and P. thunbergii is the rootstock. |
| PRA information |
Pest Risk Assessment currently available:
|
| Other relevant information for the assessment | |
| Biology |
Mycosphaerella gibsonii is an ascomycete fungus considered to be indigenous to Central America (Evans, 1984) or to Himalayas (Ivory, 1994). It is also present in Africa, Eastern and South‐eastern Asia, South America and Papua New Guinea (CABI, online; EPPO, online_c). The disease was first observed in 1913 on seedlings of Pinus pinaster and P. thunbergii in Japan. In 1917 the anamorph state (Cercospora pini‐densiflorae/Cercoseptoria pini‐densiflorae) was described on Pinus densiflora in Japan (Itô, 1972). In 1984 the teleomorph M. gibsonii and spermogonia of Asteromella (spermatial anamorph) were found and named (Evans, 1984). According to Ivory (1994), there are three different ecotypes of M. gibsonii differing in the morphology of conidia: the first one from Asia, the second from Africa and Central America and the third from Philippines. In spring, conidia are produced from needles, which were infected the previous year. Conidia are spread by wind or water (rain or overhead irrigation). The distance that conidia can travel is unknown. However, their dispersal is usually more efficient in close proximity of nursery beds and less efficient between tree plantations (Ivory, 1987). Conidia require 2–3 days of moist humid conditions for dispersal and infection (Ivory, 1972; Sullivan, 2016). Conidia remain viable for approximately 1 month. They germinate on surfaces of needles within 24–40 h, with temperature between 10 and 35°C (Ivory, 1987) and penetrate through stomata. In stomatal cavities the fungus forms black stromata with conidiophores. Conidia are continuously produced during warm damp weather (Ivory and Wingfield, 1986). Symptoms usually appear in 5 weeks after the infection (Ivory, 1972), or on highly susceptible hosts within 2 weeks (Ivory, 1987). Mycosphaerella gibsonii in the form of Asteromella may also produce spermatia deemed to be important for fertilisation (EFSA PLH Panel, 2017). Ascomata producing sexual spores may also appear (Ivory, 1987), although the role of sexual spores in the development of epidemics is unknown (Diekmann et al., 2002). The fungus remains dormant during dry conditions (Gibson, 1979) and can survive for long periods in dry needles. It may overwinter as latent infection in green needles which may become symptomatic in the spring of the following year (Suto, 1982). According to Suto (1982) and Ivory (1987), M. gibsonii overwinters as mycelium or immature stromata in host needles. Possible pathways of entry for M. gibsonii are plants for planting, unclean seeds (with infected needles), cut branches, bark, leaves, stems and growing media (Quintero, 2015; EFSA PLH Panel, 2017). |
| Symptoms | Main type of symptoms |
Main symptoms of infection are lesions which appear as light yellow‐green spots or bands on needles. Later, the colour of infected tissues changes from yellow to brown then to grey‐brown. The fruiting bodies/stromata can be observed as lesions pustules on needles. The pathogen causes defoliation, stunt growth and mortality of nursery seedlings and young plants (Ivory and Wingfield, 1986; Sullivan, 2016). The most affected tissue is the foliage, especially in the lower crown. Dead needles usually remain on the tree for many months (Ivory and Wingfield, 1986). In 1‐ or 2‐year‐old plants, M. gibsonii infects mainly older foliage, causing lesions on needles (Sullivan, 2016) and later death (Itô, 1972). |
| Presence of asymptomatic plants |
The asymptomatic period can last from 2 to 5(6) weeks, depending on environmental conditions and susceptibility of the host to the pathogen (Ivory and Wingfield, 1986; Ivory, 1987; EPPO, 1997; Sullivan, 2016). However, if infections occur in autumn, the fungus may remain latent and plants will remain asymptomatic until the spring of the following year (Suto, 1982). |
|
| Confusion with other pests | The symptoms caused by M. gibsonii are similar to the ones caused by closely related pine pathogens (e.g. Lecanosticta acicola, Dothistroma septosporum, Dothistroma pini and Sphaeropsis sapinea) (Quintero, 2015; EFSA PLH Panel, 2017). The species can be identified and distinguished using morphological (Evans, 1984; EPPO, 2015) and molecular methods (Quaedvlieg et al., 2012; EPPO, 2015). |
| Host plant range |
Hosts of Mycosphaerella gibsonii are conifers: Abies procera, Larix kaempferi, Picea jezoensis, Pinus aristata, P. attenuata, P. canariensis, P. caribaea, P. contorta, P. densiflora, P. echinata, P. elliottii, P. flexilis, P. greggii, P. halepensis, P. jeffreyi, P. kesiya, P. lambertiana, P. luchuensis, P. massoniana, P. maximinoi, P. merkusii, P. morrisonicola, P. muricata, P. nigra, P. oocarpa, P. palustris, P. patula, P. pinaster, P. pinea, P. ponderosa, P. pseudostrobus, P. radiata, P. resinosa, P. roxburghii, P. strobus, P. sylvestris, P. taeda, P. taiwanensis, P. tecunumanii, P. thunbergii and P. wallichiana (Quintero, 2015). According to Farr and Rossman (online) and CABI (online), P. parviflora is a host of M. gibsonii. Other hosts listed by Farr and Rossman (online) and/or CABI (online) include Abies nobilis, Pinus armandii, P. ayacahuite, P. cembra, P. clausa, P. griffithii, P. hartwegii, P. kesiya var. langbianensis, P. mugo, P. rigida, P. tabulaeformis, P. tabuliformis and Tsuga canadensis. Abies sachalinensis, A. veitchii, Cedrus deodara, Picea glehnii and Pseudotsuga menziesii have been demonstrated as potential hosts through artificial inoculation (Quintero, 2015). According to Itô (1972), host susceptibility is as follows:
After Second World War, the disease caused severe loss of pine seedlings in nurseries of Japan (Itô, 1972). The damage is usually on 1‐ and 2‐year‐old seedlings of Pinus caribaea, P. densiflora, P. taeda and P. thunbergii. In epidemic conditions between 50% and 80% infected seedlings are killed by this disease. In young plantations, Pinus halepensis, P. pinaster and P. radiata are attacked (Itô, 1972). On older trees of Pinus canariensis, P. radiata and P. roxburghii, the fungus caused significant needle blight (Ivory, 1994). |
| Reported evidence of impact | Mycosphaerella gibsonii is EU quarantine pest. |
| Evidence that the commodity is a pathway | According to Quintero (2015) and EFSA PLH Panel (2017), plants for planting are the main pathway. The pathogen is associated, even asymptomatically, with pine needles, including needles of P. parviflora. Therefore, the commodity is a pathway. |
| Surveillance information |
Mycosphaerella gibsonii is recorded in Dossier Section 4.0 as a pathogen occurring in China. The nursery and its immediate vicinity area (at least 2 km) are inspected at least six times a year at appropriate intervals targeting pests of EU concern on their main and secondary hosts (Dossier Section 4.0). The survey shall be carried out at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus (Dossier Section 4.0). Inspection shall be conducted to examine the presence or absence of harmful organisms of EU concern (Dossier Section 4.0). |
A.10.2. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Mycosphaerella gibsonii between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.10.3. Evaluation of the implementation and relevance of specific measures in China
Commission Implementing Regulation (EU) 2019/2072 specifies in point 30 of Annex VII measures which are required for the import of the commodity from China.
The below overview provides special requirements for naturally or artificially dwarfed plants for planting other than seeds according to Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 including an assessment of whether or not the applicant country implements those measures with respect to Mycosphaerella gibsonii (regulated as Pseudocercospora pini‐densiflorae) identified in this Opinion. The Panel assumes that information on treatments required to be included in the phytosanitary certificate is provided by the applicant country according to the Article 71 of Regulation (EU) 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’.
| Special requirements as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 | Implementation of the special requirements in China according to information provided in the Dossier | Relevance of special requirements for the pest including uncertainties |
|---|---|---|
| ‘Official statement that: | – | – |
| a) the plants, including those collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime, | Yes | Yes |
| b) the plants in the nurseries referred to in point (a) of this entry: | – | – |
| i) at least during the period referred to in point (a) of this entry: | – | – |
| — were potted, in pots which are placed on shelves at least 50 cm above ground, |
Yes, partially. Pots are also reported to be kept on the ground. |
No |
| — have been subjected to appropriate treatments to ensure freedom from non‐European rusts, and the active ingredient, concentration and date of application of these treatments has been mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’. |
Yes. Treatments are appropriate. They are expected to reduce the likelihood of infection of the pathogens and the rate of colonisation of plant tissues, although it is uncertain if freedom from non‐European rusts could be reachable. Treatments used are listed in Table 6.
Uncertainties:
|
No. However, treatments could reduce the likelihood of infection by the pathogen. |
| — have been officially inspected at least six times a year at appropriate intervals for the presence of Union quarantine pests of concern in accordance with Regulation (EU) No 2016/2031, and these inspections have also been carried out on plants in the immediate vicinity of the nurseries referred to in point (a) of this entry, at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus, | Yes | Yes |
| — have been found free, in these inspections, from the relevant Union quarantine pests of concern as specified in the previous indent, infested plants have been removed and the remaining plants, where appropriate, have been effectively treated and have been held for an appropriate period and inspected to ensure freedom from such pests, | Yes | Yes |
| — have been planted in either an unused artificial growing medium or in a natural growing medium, which has been treated by fumigation or by appropriate heat treatment and has been of any Union quarantine pests, | Yes | No |
| — have been kept under conditions which ensure that the growing medium has been maintained free from Union quarantine pests and within 2 weeks prior to dispatch, have been: | Yes | No |
| — shaken and washed with clean water to remove the original growing medium and kept bare rooted, or | – | – |
| — shaken and washed with clean water to remove the original growing medium and replanted in growing medium which meets the conditions laid down in (i) fifth indent, or | – | – |
| — subjected to appropriate treatments to ensure that the growing medium is free from Union quarantine pests, and the active ingredient, concentration and date of application of these treatments have been indicated on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031 under the rubric ‘Disinfestation and/or disinfection treatment’. | Yes | No |
| ii) were packed in closed containers which have been officially sealed and bear the registration number of the registered nursery, and this number has been indicated under the rubric ‘Additional declaration’ on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, enabling the consignments to be identified.’ | Yes | Yes |
A.10.4. Reference List
CABI (Centre for Agriculture and Bioscience International), online. Mycosphaerella gibsonii (needle blight of pine). Available online: https://www.cabi.org/cpc/datasheet/12359 [Accessed: 7 June 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Pseudocercospora pini‐densiflorae. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=11729 [Accessed: 8 June 2021].
Diekmann M, Sutherland JR, Nowell DC, Morales FJ, Allard G, 2002. Pinus spp. International Plant Genetic Resources Institute (IPGRI), Rome, Italy. 90 pp. (FAO/IPGRI technical guidelines for the safe movement of germplasm no. 21).
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Grégoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Boberg J, Gonthier P and Pautasso M, 2017. Scientific opinion on the pest categorisation of Pseudocercospora pini‐densiflorae. EFSA Journal 2017;15(10):5029, 27 pp. https://doi.org/10.2903/j.efsa.2017.5029
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Vettraino AM, Leuschner R, Mosbach‐Schulz O, Rosace MC and Potting R, 2019. Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. https://doi.org/10.2903/j.efsa.2019.5667
EPPO (European and Mediterranean Plant Protection Organization), 1997. Data sheets on quarantine pests: Mycosphaerella gibsonii. In: Smith IM, McNamara DG, Scott PR and Holderness M (eds.). Quarantine Pests for Europe, 2nd Edition. CABI/EPPO, Wallingford, 1425 pp.
EPPO (European and Mediterranean Plant Protection Organization), 2015. PM 7/46 (3) Lecanosticta acicola (formerly Mycosphaerella dearnessii), Dothistroma septosporum (formerly Mycosphaerella pini) and Dothistroma pini. Bulletin OEPP/EPPO Bulletin, 45, 163–182. https://doi.org/10.1111/epp.12217
EPPO (European and Mediterranean Plant Protection Organization), online_a. Pseudocercospora pini‐densiflorae (CERSPD), Categorization. Available online: https://gd.eppo.int/taxon/CERSPD/categorization [Accessed: 7 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. EPPO A1 List of pests recommended for regulation as quarantine pests, version 2020‐09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list [Accessed: 7 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Pseudocercospora pini‐densiflorae (CERSPD), Distribution. Available online: https://gd.eppo.int/taxon/CERSPD/distribution [Accessed: 7 June 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Pseudocercospora pini‐densiflorae (CERSPD), Host plants. Available online: https://gd.eppo.int/taxon/CERSPD/hosts [Accessed: 7 June 2021].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Evans HC, 1984. The genus Mycosphaerella and its anamorphs Cercoseptoria, Dothistroma and Lecanosticta on pines. Mycological Papers, No. 153, Commonwealth Mycological Institute, Kew. 79 pp.
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars‐grin.gov/fungaldatabases/ [Accessed: 7 June 2021].
Gibson IAS, 1979. Diseases of forest trees widely planted as exotics in the tropics and southern hemisphere. Part II. The genus Pinus. Publ. Commonwealth Mycological Institute, Kew and Commonwealth Forestry Institute, Oxford. 135 pp.
Ivory MH, 1972. Pathological problems of fast‐growing exotic conifers in West Malaysia. Technical Report Number 6, Project FO:SFIMAL 12, UNDPIFAO, 299–308.
Ivory MH, 1987. Diseases and disorders of pines in the tropics: a field and laboratory manual. Overseas Research Publication No. 31, Overseas Development Administration, Oxford Forestry Institute, Oxford, UK. 92 pp.
Ivory MH, 1994. Records of foliage pathogens of Pinus species in tropical countries. Plant Pathology, 43, 511–518.
Ivory MJ and Wingfield MH, 1986. First report of Mycosphaerella gibsonii in South Africa. Phytophylactica, 18, 51–54.
Itô K, 1972. Cercospora needle blight of pines. Bulletin Government Forest Experiment Station, Meguro, 246, 21–33.
Quaedvlieg W, Groenewald JZ, de Jesús Yáñez‐Morales M and Crous PW, 2012. DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia: Molecular Phylogeny and Evolution of Fungi, 29, 101–115.
Quintero TG, 2015. New pest response guidelines. Pseudocercospora pini‐densiflorae (Hori & N. Nambu) Deighton. Brown Needle Fungus. USDA, Forest Service. 91 pp.
Sullivan M, 2016. CPHST Pest Datasheet for Pseudocercospora pini‐densiflorae. USDA‐APHISPPQ‐CPHST. Revised June 2015 by D. Z. Mackesy. 10 pp.
Suto Y, 1982. Fundamental studies on control of the needle blight in pines caused by Cercospora pini‐densijlorae Hori & Nambu. Bulletin Shimane Prefecture Forest Experiment Station, 32, 1–102.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
A.11. Pestalotiopsis group (P. disseminata and P. microspora)
A.11.1. Organism information
| Taxonomic information |
1. Pestalotiopsis disseminata Current valid scientific name: Pestalotiopsis disseminata Synonyms: Pestalotia disseminata Name used in the EU legislation: – Order: Amphisphaeriales (formely: Xylariales) Family: Amphisphaeriaceae Common name: leaf spot of eucalyptus Name used in the Dossier: – 2. Pestalotiopsis microspora Current valid scientific name: Pestalotiopsis microspora Synonyms: Pestalotia microspora Name used in the EU legislation: – Order: Amphisphaeriales (formely: Xylariales) Family: Amphisphaeriaceae Common name: – Name used in the Dossier: Pestalotiopsis microspore |
|
| Group | Fungi | |
| EPPO code |
PESTDI: Pestalotiopsis disseminata PESTDC: Pestalotiopsis microspora |
|
| Regulated status |
The pathogens are neither regulated in the EU nor listed by EPPO. Pestalotiopsis sp. is listed in the US regulated plant pest table (USDA APHIS, online). |
|
| Pest status in China |
Pestalotiopsis disseminata is present in China (Chen et al., 2013; Farr and Rossman, online). Pestalotiopsis microspora is present in China (Farr and Rossman, online; GBIF, online). According to Dossier Section 5.0, P. disseminata and P. microspora are present in Zhejiang province. |
|
| Pest status in the EU |
Pestalotiopsis disseminata has been reported in Portugal (Silva et al., 2020). Farr and Rossman (online) report one point‐data for P. microspora in Italy. |
|
| Host status on Pinus parviflora and P. thunbergii |
Pestalotiopsis disseminata is reported as associated with P. parviflora in China (Chen et al., 2013) and with P. pentaphylla (syn. P. parviflora var. pentaphylla) in Japan (Watanabe et al., 2010). Pestalotiopsis microspora is considered as a pathogen of Pinus parviflora in Dossier Sections 2.0 and 4.0. |
|
| PRA information | No pest risk assessment is currently available for P. disseminata and P. microspora. | |
| Other relevant information for the assessment | ||
| Biology |
Pestalotiopsis spp. are generally regarded as weak pathogens, entering the tissues through natural openings (stomata, lenticels, hydathodes) or wounds (Maharachchikumbura et al., 2011). Species of Pestalotiopsis have commonly been isolated as endophytes from asymptomatic plant tissues (Maharachchikumbura et al., 2011). This symptomless stage can last until the plant is stressed and symptomatic infection starts (Maharachchikumbura et al., 2011). However, the correlation between endophytic and pathogenic stages are still unclear for many Pestalotiopsis species. Once host tissue is infected, small dark pycnidia develop producing conidia. Pestalotiopsis disseminata is known to meet the optimal condition for sporulation at 25°C (±2) and 70% relative humidity (Das et al., 2010). The best environmental conditions for P. microspora are 16–20°C and 12 h of light per day, allowing reaching the sexual stage (identified as Pestalosphaeria hansenii) in 3–6 weeks and forming the perithecia that will produce the asci and ascospores (Metz et al., 2000). There is no information on overwintering stage of these pathogens nor on the possible distance that conidia and ascospores can travel. Pestalotiopsis spp. are easily dispersed by wind or water splash. Many insects (mainly Heteroptera and Lepidoptera) are known to spread these pathogens thereby aggravating their infections (Mitchell, 2004; Martínez and Plata‐Rueda, 2013). Pestalotiopsis spp. could also be spread by growing media, soil and contaminated tools (Ivanová, 2016). Some Pestalotiopsis species (i.e. P. sydowiana) have been isolated from soil and used growing media and pots (McQuilken and Hopkins, 2004). Based on Maharachchikumbura et al. (2011), the systematics of the genus Pestalotiopsis is not completely resolved, at least using ITS as a marker. Multiple genetic markers such as ITS, Beta‐Tubulin and Translation Elongation Factors are needed to clearly distinguish different species (Silva et al., 2020). |
|
| Symptoms | Main type of symptoms |
Main symptoms caused by Pestalotiopsis species on host plants are leaf spots, leaf blight, needle blight, severe chlorosis, shoot dieback, stem blight, tip blight, grey blight, scabby canker, canker lesions, fruit rot and post‐harvest diseases (Ivanová, 2016; Silva et al., 2020). On Pinus pinea, Silva et al. (2020) observed shoot blight, stem necrosis and wilted needles. On som (Persea bombycine), Pestalotiopsis spp. cause small oval lesions, irregularly scattered on leaves. As the disease progresses, the spots collapse and the entire leaf dries up (Das et al., 2010). Pestalotiopsis spp. causes leaf spots on palms (yellow, brown, black or grey with a black outline), petiole blight and bud rot. These pathogens attack all parts of the leaf (Elliott, 2018). |
| Presence of asymptomatic plants | Species of Pestalotiopsis have commonly been isolated as endophytes from asymptomatic plant tissues (Maharachchikumbura et al., 2011). This symptomless stage can last until the plant is stressed and symptomatic infection starts (Maharachchikumbura et al., 2011). | |
| Confusion with other pests |
Necrotic spots could be confused with those caused by other pathogens, but Pestalotiopsis spp. can be easily identified by examining conidia (Elliott, 2018). Based on Maharachchikumbura et al. (2011), the systematics of the genus Pestalotiopsis is not completely resolved, at least using ITS as a marker. Multiple genetic markers such as ITS, Beta‐Tubulin and Translation Elongation Factors are needed to clearly distinguish different species (Silva et al., 2020). |
|
| Host plant range |
The host range of Pestalotiopsis spp. is wide and several Pestalotiopsis species are associated with different hosts (Jeewon et al., 2004). Pestalotiopsis disseminata has been found on Eucalyptus diversicolor, E. globulus, E. lehmannii, E. alba, E. botryoides, E. citriodora, E. maidenii, E. robusta, E. saligna, Lagerstroemia indica, Leptospermum sp., Photinia serrulata, Podocarpus macrophylla, Psidium guajava, Rhizophora mangle, Sorghum vulgare, Terminalia arjuna, Acer laevigatum, Agropyron cristatum, Albizia odoratissima, Aleurites montana, Anacardium occidentale, Camellia sinensis, Cocos nucifera, Coffea arabica, Comocladia dentata, Dactylis glomerata, Desmodium floribundum, Desmodium ovalifolium, Elaeis guineensis, Eugenia sp., Euphorbia milii, Fragaria vesca, Helianthus annuus, Hemidesmus indicus, Hymenaea torreana, Ixora coccinea, Juniperus lucayana, Lagerstroemia sp., Litchi chinensis, Macadamia sp., Machilus bombycina, Malus pumila var. domestica, Manglietia fordiana, Musa, Oryza sativa, Persea americana, Persea bombycina, Pieris japonica, Pithecolobium bigeminum, Podocarpus imbricatus, P. macrophyllus, P. macrophyllus var. maki, Pyrus armeniaca, Rhizophora mucronata, Rhodomyrtus tomentosa, Saraca indica, Sideroxylon tomentosum, Stigmaphyllon sagraeanum, Strychnos sp., Syzygium cumini, Terminalia catappa, Terminalia ivorensis, Trachycarpus fortunei, Tripterygium wilfordii and Vicia faba (Farr and Rossman, online). The list of pines reported as hosts of Pestalotiopsis disseminata includes Pinus armandii, P. densiflora, P. radiata (Farr and Rossman, online), P. parviflora (Chen et al., 2013), P. pentaphylla (P. parviflora var. pentaphylla) (Watanabe et al., 2010; Farr and Rossman, online) and P. pinea (Silva et al., 2020). Pestalotiopsis microspora has been found on Abies beshanzuensis, Acer palmatum, Actinidia chinensis, Aegiceras corniculatum, Alnus rubra, Amomum tsao‐ko, Ananas comosus, Araucaria bidwillii, Archontophoenix alexandrae, Ardisia sp., Azadirachta indica, Biota orientalis, Bridelia monoica, Bridelia stipularis, Camellia sinensis, Campomanesia sp., Carya cathayensis, Carya illinoinensis, Carya pecan, Citrus limon, Copaifera sp., Corylus chinensis, Cunninghamia lanceolata, Cupressus funebris, Elaeis guineensis, Eriobotrya japonica, Hedera helix, Hedychium coronarium, Hymenaea sp., Hypericum androsaemum, Hypericum patulum, Juniperus bermudiana, Lagerstroemia speciosa, Lindera obtusiloba, Lithocarpus glaber, Machilus nanmu, Mahonia bealei, Mahonia confusa, Malus halliana, Myrica rubra, Nandina domestica, Oryza australiensis, Pandanus sp., Platanus orientalis, Podocarpus macrophyllus, Psidium guajava, Quercus acutissima, Quercus coccinea, Reineckea carnea, Sabina chinensis, Sorghum sp., Stanhopea bucephalus, Taxodium ascendens, Taxodium distinctum, Taxus cuspidata, Taxus wallichiana, Terminalia arjuna, Terminalia chebula, Torreya grandis, Torreya taxifolia, Vaccinium corymbosum (Farr and Rossman, online). Concerning pines, Pestalotiopsis microspora is reported as associated with Pinus radiata (Farr and Rossman, online) and as a pest of P. parviflora (Dossier Sections 2.0 and 4.0). |
|
| Reported evidence of impact | Pestalotiopsis disseminata and P. microspora (usually together with other Pestalotiopsis spp.) caused damages on leaves and fruit rot of guava (Psidium guajava) in Hawaii (Keith et al., 2006); grey blight disease on som (Persea bombycina) in India (Ray et al., 2016); postharvest rot in Chinese olive fruits (Canarium album) (Chen et al., 2018); leaf spot on oil palm (Elaeis guineensis) (Shen et al., 2014) and loquat root rot (Lu et al., 2016) in China; leaf blight on Japanese yew (Taxus cuspidata) in Korea (Jeon and Cheon, 2014); shoot blight and stem necrosis on Pinus pinea in Portugal (Silva et al., 2020). | |
| Evidence that the commodity is a pathway |
Needles and other plant tissues can be infected by the pathogens, even asymptomatically (Maharachchikumbura et al., 2011). Pestalotiopsis microspora was found during monitoring survey on needles of Pinus parviflora in the Zhenjiang province (Dossier Section 2.0). |
|
| Surveillance information |
Pestalotiopsis disseminata and P. microspora are recorded in Dossier Section 5.0 as pathogens occurring in Zhenjiang province where the nursery is located. No surveillance information for Pestalotiopsis spp is currently available from China. However, P. microspora was found on needles of Pinus parviflora in the Zhenjiang province during the monitoring survey in 2012 (Dossier Section 2.0). |
|
A.11.2. Possibility of pest presence in the nursery
A.11.2.1. Possibility of entry from the surrounding environment
Pestalotiopsis disseminata and P. microspora are present in China and they are both present in Zhenjiang (Dossier Section 5.0). During the survey in 2012 P. microspora was detected on Pinus parviflora at Liutong Gardening and Planting Base in Hangzhou and in the West Lake scenic spot (including Zhusu Garden, Hangzhou Flower Nursery, Botanical Garden and Viewing Fish at Flower Pond) (Dossier Section 2.0). As suitable hosts are present in the surroundings (see below), the pathogens could enter the nursery by dispersal of spores (conidia and ascospores) by wind, rain splash and insect vectors.
According to Dossier Sections 4.0 and 5.0, the following host species of P. disseminata are present in the surroundings: Camelia sinensis, Lagerstroemia indica, Musa basjoo, Photinia serrulata, Podocarpus imbricatus, P. macrophyllus and Trachycarpus fortunei.
The surroundings also harbour the following host species of P. microspora: Acer palmatum, Camelia sinensis, Eriobotrya japonica, Malus halliana, Nandina domestica, Platanus orientalis, Podocarpus macrophyllus, Quercus acutissima, Reineckea carnea, Sabina chinensis and Taxodium ascendens.
Some hosts of the pathogens, i.e. Lithocarpus glaber and Pinus spp., are present within 1.5 km from the production site (Dossier Section 5.0).
Uncertainties
-
–
The abundance of known hosts in the area surrounding the nursery.
-
–
The inoculum pressure of P. microspora in the surrounding of the nursery.
-
–
The presence and inoculum pressure of P. disseminata in the surrounding of the nursery.
-
–
The level of susceptibility of P. parviflora and P. thunbergii to P. disseminata and P. microspora.
-
–
The full host range of the pathogens.
-
–
The effective dispersal range of the pathogens.
-
–
Whether uncleaned contaminated tools coming from outside are used in the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogens to enter the nursery. Pathogens could be present in the area on suitable host plants and could move into the nursery mainly by wind, rain splash and insects.
A.11.2.2. Possibility of entry with new plants/seeds
All pine seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (Pinus thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triadimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
A new Pestalotiopsis species (i.e. P. pinicola sp. nov.) has been isolated as an endophyte from the endosperm of seeds of Pinus armandii (Tibpromma et al., 2019).
The growing medium used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0).
Uncertainties
-
‐
The capability of P. disseminata and P. microspora of infecting the endosperm of P. thunbergii seeds.
-
‐
The efficacy of seed treatments against Pestalotiopsis spp.
-
‐
It is not clear if and how mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible that pathogens could enter the nursery with seeds.
A.11.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of Pinus parviflora are taken (Dossier Section 4.0). The pathogens could be spread within the nursery by grafting asymptomatically infected scions.
The possibility of spread of the pathogen within the nursery depends on the number of spores produced by the pathogens on infected hosts in the nursery. Dispersal could occur by means of wind, rain splash and insect vectors.
The nursery also grows ornamental plants including abelia, bamboo, camphor, loropetalum, photinia, pyracantha, wisteria and other plants (Dossier Section 4.0). Photinia is a host of P. disseminata. Therefore, other suitable hosts of some Pestalotiopsis are present in the nursery.
Contaminated tools and soil/growing media, and possibly irrigation water are also relevant for the dispersal of these pathogens within the nursery.
Uncertainties
-
‐
Growing practices, media, tools and irrigation management in the nursery.
-
‐
The level of susceptibility of P. parviflora and P. thunbergii to P. disseminata and P. microspora.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of pathogens within the nursery is possible by anthropogenic, biotic (insect vectors) and abiotic (wind, rain) means.
A.11.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Pestalotiopsis disseminata and P. microspora between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.11.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on Pestalotiopsis group (P. disseminata and P. microspora) is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
The net does not prevent the entry of airborne inoculum. However, it might reduce air currents thereby decreasing the amount of inoculum entering the productions site. As it cannot be excluded that the pathogens, as reported for Pestalotiopsis spp., could be spread by insect vectors (mainly Heteroptera and Lepidoptera), the net is also expected to be effective against the entry of these vectors. Plants are cultivated separately in pots. Therefore, their density is expected to be lower than in other production systems, thereby reducing the spread of the pathogen by direct crown contacts and by creating conditions conducive to infections (high levels of relative humidity).
Uncertainties:
|
| 2 | Growing medium and its treatment | No | Not applicable. |
| 3 | Treatment of seeds | Yes |
Treatment of seeds with fungicides are expected to have some effects on the pathogens that may be present on the seed surface and the endosperm.
Uncertainties:
|
| 4 | Insecticide and acaricide treatments | Yes |
As it cannot be excluded that the pathogens, as reported for Pestalotiopsis spp., could be spread by insect vectors (mainly Heteroptera and Lepidoptera), insecticides could have some effects in reducing the insect vectors populations.
Uncertainties:
|
| 5 | Fungicide treatments | Yes |
Fungicide treatments (most of active ingredients are systemic) are expected to reduce the likelihood of infection of the pathogen and the rate of colonisation of plant tissues.
Uncertainties:
|
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | Yes | The removal of tea and potential other hosts is expected to reduce the inoculum pressure of the pathogens. |
| 8 | Official inspections during production | Yes |
The official inspections are expected to have some effects in detecting the pathogens. However, asymptomatic periods are commonly reported for Pestalotiopsis spp. During that period, infection cannot be detected visually.
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
The official inspections are expected to have some effects in detecting the pathogens. However, asymptomatic periods are commonly reported for Pestalotiopsis spp. During that period, infection cannot be detected visually. The removal of 2 cm of surface growing medium 2 weeks before export and its replacement with new growing medium is expected to reduce pathogen inoculum because of the removal of fallen infected needles. However, the inoculum may persist on infected needles in the crown and in other plant tissues.
Uncertainties:
|
A.11.5. Overall likelihood of pest freedom for Pestalotiopsis disseminata on grafted bonsai plants
A.11.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected grafted bonsai plants
The scenario assumes a low pressure of the pathogen in the environment and absence of infections in mother plants. Spread happens only via substrates contaminated with spores, e.g. needles, or dissemination of spores over short distances. Seeds are not a pathway for the entry of the pathogen in the nursery. Fungicides are effective in reducing infections and symptomatic plants are readily recognised during inspections.
A.11.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected grafted bonsai plants
The scenario assumes a high pressure of the pathogen in the environment and possible infections in mother plants. Spread happens via substrates contaminated with spores, e.g. needles, dissemination of spores and also via contaminated pruning tools. Seeds are a pathway for the entry of the pathogen in the nursery. Fungicides are not completely effective in reducing infections. Infected plants are not recognised during inspections because they are asymptomatic.
A.11.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected grafted bonsai plants (Median)
The median is closer to the lower values because fungicides treatments are applied frequently and are expected to be effective in reducing infections. Despite the pathogen has been reported in the province, the inoculum pressure in the environment is not expected to be extremely high. The potential range of dispersal of inoculum is expected to be moderate.
A.11.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
There is a lack of quantitative information on the presence and abundance of the pathogen and its hosts in the surrounding of the nursery which results in high level of uncertainties for infection rates below the median. The fungicides are expected to be rather effective in lowering the rates of infection which reduces uncertainties above the median.
A.11.5.5. Elicitation outcomes of the assessment of the pest freedom for Pestalotiopsis disseminata on grafted bonsai plants
The following tables show the elicited and fitted values for pest infection (Table A.19) and pest freedom (Table A.20).
Table A.19.
Elicited and fitted values of the uncertainty distribution of pest infection by Pestalotiopsis disseminata per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 20 | 110 | 200 | 350 | 600 | ||||||||||
| EKE | 19.9 | 25.0 | 33.5 | 50.7 | 74.4 | 105 | 137 | 209 | 293 | 343 | 402 | 462 | 522 | 564 | 601 |
The EKE results are the BetaGeneral (1.0098, 1.994, 16.5, 665) distribution fitted with @Risk version 7.6.
Table A.20.
The uncertainty distribution of plants free of Pestalotiopsis disseminata per 10,000 plants calculated by Table A.19
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,400 | 9,650 | 9,800 | 9,890 | 9,980 | ||||||||||
| EKE results | 9,399 | 9,436 | 9,478 | 9,538 | 9,598 | 9,657 | 9,707 | 9,791 | 9,863 | 9,895 | 9,926 | 9,949 | 9,967 | 9,975 | 9,980 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.20.
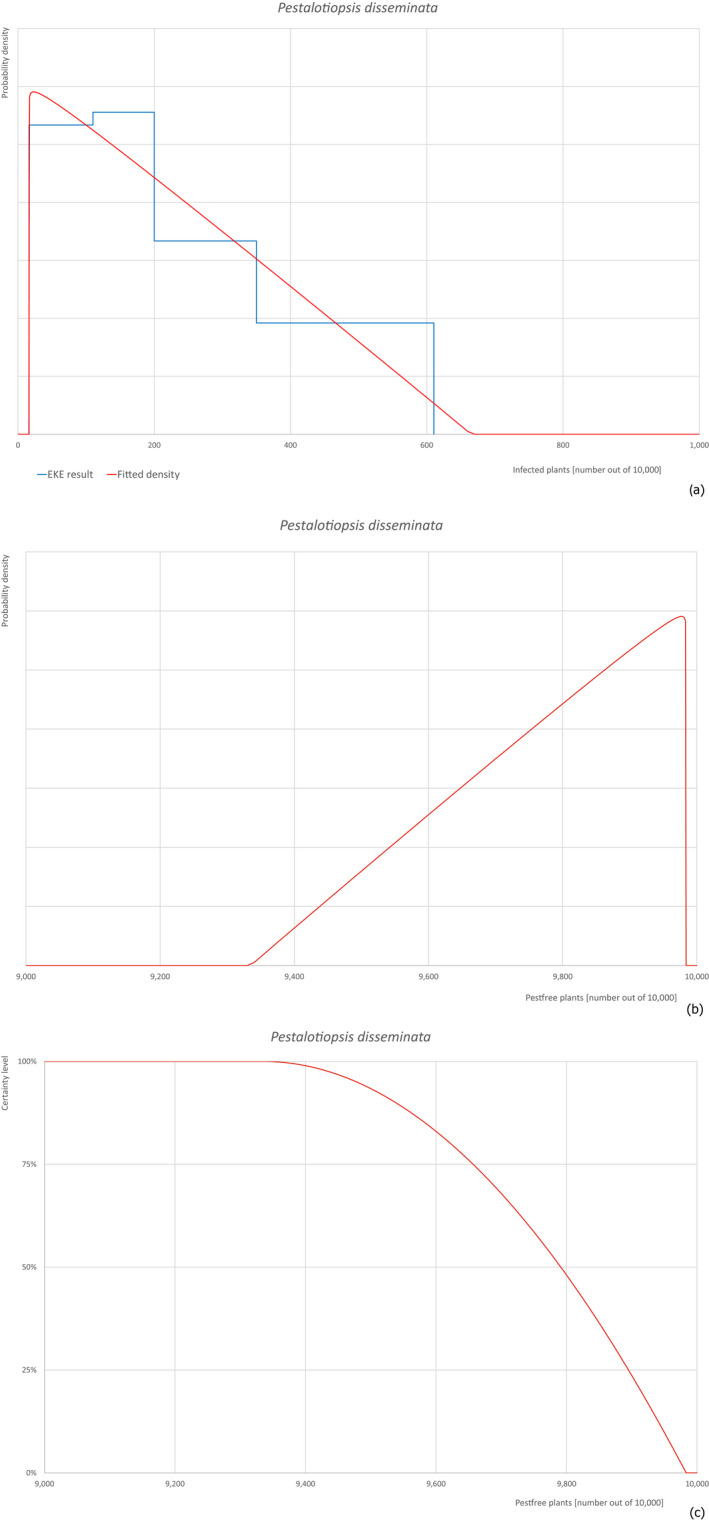
Figure A.10: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.11.6. Overall likelihood of pest freedom for Pestalotiopsis microspora on grafted bonsai plants
Pestalotiopsis disseminata and P. microspora are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overall likelihood of pest freedom can be found in Section A.11.5.
A.11.7. Reference List
Chen WJ, Gong Z, Wu Y, Zhong G, Wu X, Lin X and Zhu J, 2013. Investigation on the pests and pathogen identification in Pinus parviflora of Hangzhou in China. The Proceedings of Chinese Society of Plant Protection in 2013, Shandong, China, 327–330.
Chen T, Lu J, Kang B, Lin M, Ding L, Zhang L, Chen G, Chen S and Lin H, 2018. Antifungal activity and action mechanism of ginger oleoresin against Pestalotiopsis microspora isolated from Chinese olive fruits. Frontiers in Microbiology, 9, 2583. https://doi.org/10.3389/fmicb.2018.02583
Das R, Chutia M, Das K and Jha DK, 2010. Factors affecting sporulation of Pestalotiopsis disseminata causing grey blight disease of Persea bombycina Kost., the primary food plant of muga silkworm. Crop Protection, 29, 963–968. https://doi.org/10.1016/j.cropro.2010.05.012
Elliott ML, 2018. Pestalotiopsis (Pestalotia) diseases of palm. EDIS, Plant Pathology Department, UF/IFAS Extension, 2006, 1–3.
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars‐grin.gov/fungaldatabases/ [Accessed: 8 June 2021].
GBIF (Global Biodiversity Information Facility), online. Pestalotiopsis microspora (Speg.) GC Zhao & N. Li in GBIF Secretariat 2021. GBIF Backbone Taxonomy. Available online: https://www.gbif.org/species/2574966 [Accessed: 8 June 2021].
Ivanová H, 2016. Comparison of the fungi Pestalotiopsis funerea (Desm.) Steyaert and Truncatella hartigii (Tubeuf) Steyaert isolated from some species of the genus Pinus L. in morphological characteristics of conidia and appendages. Journal of Forest Science, 62, 279–284.
Jeewon R, Liew ECY and Hyde KD, 2004. Phylogenetic evaluation of species nomenclature of Pestalotiopsis in relation to host association. Fungal Diversity, 17, 39–55.
Jeon YH and Cheon W, 2014. First report of leaf blight of Japanese yew caused by Pestalotiopsis microspora in Korea. Plant Disease, 98, 691.
Keith LM, Velasquez ME and Zee FT, 2006. Identification and Characterization of Pestalotiopsis spp. Causing Scab Disease of Guava, Psidium guajava, in Hawaii. Plant Disease, 90, 16–23. https://doi.org/10.1094/PD‐90‐0016
Lu HJ, Wang CM, Zheng XL and Zhang YP, 2016. First report of loquat root rot disease caused by Pestalotiopsis microspora in China. Plant Disease, 100, 1008.
Maharachchikumbura SSN, Guo L‐D, Chukeatirote E, Bahkali AH and Hyde KD, 2011. Pestalotiopsis—morphology, phylogeny, biochemistry and diversity. Fungal Diversity, 50, 167–187. https://doi.org/10.1007/s13225‐011‐0125‐x
Martínez LC and Plata‐Rueda A, 2013. Lepidoptera vectors of Pestalotiopsis fungal disease: first record in oil palm plantations from Colombia. International Journal of Tropical Insect Science, 33, 239–246. https://doi.org/10.1017/s1742758413000283
McQuilken MP and Hopkins KE, 2004. Biology and integrated control of Pestalotiopsis on container‐grown ericaceous crops. Pest Management Science: formerly Pesticide Science, 60, 135–142.
Metz AM, Haddad A, Worapong J, Long DM, Ford EJ, Hess WM and Strobel GA, 2000. Induction of the sexual stage of Pestalotiopsis microspora, a taxol‐producing fungus. Microbiology, 146, 2079–2089. https://doi.org/10.1099/00221287‐146‐8‐2079
Mitchell PL, 2004. Heteroptera as vectors of plant pathogens. Neotropical Entomology, 33, 519–545. https://doi.org/10.1590/s1519‐566x2004000500001
Ray MK, Mishra PK and Baruah PK, 2016. Control of fungal pathogen Pestalotiopsis disseminata causing grey blight disease in Som (Persea bombycina Kost.): An In Vitro Study. International Journal of Pure and Applied Bioscience, 4, 180–185. https://doi.org/10.18782/2320‐7051.2412
Shen HF, Zhang JX, Lin BR, Pu XM, Zheng L, Qin XD, Li J and Xie CP, 2014. First report of Pestalotiopsis microspora causing leaf spot of oil palm (Elaeis guineensis) in China. Plant Disease, 98, 1429 pp. https://doi.org/10.1094/PDIS‐02‐14‐0163‐PDN
Silva AC, Diogo E, Henriques J, Ramos AP, Sandoval‐Denis M, Crous PW and Bragança H, 2020. Pestalotiopsis pini sp. nov., an emerging pathogen on stone pine (Pinus pinea L.). Forests, 11, 17 pp. https://doi.org/10.3390/f11080805
Tibpromma S, Mortimer PE, Karunarathna SC, Zhan F, Xu J, Promputtha I and Yan K, 2019. Morphology and multi‐gene phylogeny reveal Pestalotiopsis pinicola sp. nov. and a new host record of Cladosporium anthropophilum from edible pine (Pinus armandii) seeds in Yunnan province, China. Pathogens, 8, 285. https://doi.org/10.3390/pathogens8040285
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
USDA APHIS (Animal and Plant Health Inspection Service) Regulated Pest List, online. Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/import‐information/rppl/rppl‐table [Accessed: 8 June 2021].
Watanabe K, Motohashi K and Ono Y, 2010. Description of Pestalotiopsis pallidotheae: a new species from Japan. Mycoscience, 51, 182–188.
A.12. Pine bast scales (Matsucoccus massonianae and M. matsumurae)
A.12.1. Organism information
| Taxonomic information |
1. Matsucoccus massonianae Current valid scientific name: Matsucoccus massonianae Synonyms: – Name used in the EU legislation: – Order: Hemiptera Family: Matsucoccidae Common name: Massonian pine bast scale, Chinese pine bast scale Name used in the Dossier: Matsucoccus massonianae 2. Matsucoccus matsumurae Current valid scientific name: Matsucoccus matsumurae Synonyms: Matsucoccus resinosae, Matsucoccus thunbergianae, Xyllococcus matsumurae Name used in the EU legislation: – Order: Hemiptera Family: Matsucoccidae Common name: Japanese pine bast scale, red pine scale, pine bark scale Name used in the Dossier: Matsucoccus matsumurae The taxonomic status of M. massonianae is controversial. According to recent studies conducted by RAPD PCR, the populations of Matsucoccus of Zhejiang would all belong to the species M. matsumurae (Liu et al., 2014) so that M. massonianae would therefore be synonym of M. matsumurae (Ren et al., 2016). |
| Group | Insects |
| EPPO code |
MATSMS: Matsucoccus massonianae MATSRE: Matsucoccus matsumurae |
| Regulated status | Matsucoccus massonianae and M. matsumurae are not regulated in the EU. Neither species is listed by EPPO. While M. massonianae is not regulated anywhere in the world, M. matsumurae is a quarantine pest for China (Mudan et al., 2000; Zhang et al., 2007; Liu et al., 2014). |
| Pest status in China |
Matsucoccus massonianae and M. matsumurae are present in China. Matsucoccus massonianae is present only in the provinces of Anhui (Dossier Section 4.0) and Zhejiang (Dossier Sections 4.0 and 5.0; García Morales et al., online_a). Matsucoccus matsumurae is present in the provinces of Anhui, Beijing, Hebei, Jiangsu, Jilin, Liaoning, Shandong, Shanghai and Zhejiang (Foldi, 2004; Dossier Sections 4.0 and 5.0; García Morales et al., online_b). |
| Pest status in the EU |
Matsucoccus massonianae is absent in the EU (García Morales et al., online_a). Matsucoccus matsumurae is present in the EU in the Netherlands (Jansen, 1999), Sweden (García Morales et al., online_b), Portugal, Spain (Sierra, 1998; Soria et al., 1999) and Finland (Albrecht et al., 2015). Most of the records from Europe are doubtful and should be verified as they are not reported in García Morales et al. (online_b) with the only exception of Sweden. |
| Host status on Pinus parviflora and P. thunbergii |
There are no reports of Matsucoccus massonianae nor for M. matsumurae on Pinus parviflora. Pinus thunbergii is a host for both M. massonianae and M. matsumurae (Foldi, 2004; Dossier Section 4.0; García Morales et al., online_a,b). |
| PRA information | No Pest Risk Assessment nor Pest Categorisations for Matsucoccus massonianae and M. matsumurae are available. |
| Other relevant information for the assessment | |
| Biology |
Matsucoccus spp. are bast scales associated with Pinus spp. and they usually feed under the bark of branches, twigs and stems (only few species feed on needles). Matsucoccus scales are mainly distributed in the northern hemisphere in Eurasia and North America; only a few species have an oriental (China) and subtropical distribution (Central America) (Ben Dov, 2011). Some species can cause severe damage to pine trees by sucking sap, reducing growth and injecting toxic substances (Foldi, 2004). Matsucoccus massonianae is endemic to China, where it has restricted distribution in the eastern part of the country (Zhejiang and Anhui) (Dossier Section 4.0; García Morales et al., online_a). Matsucoccus matsumurae is probably native to Asia, where it is found in China, Korea and Japan, but its origin is not entirely clear (Anderson et al., 1976) as it is currently also found in North America (United States) and Europe (Jansen, 1999; Soria et al., 2000; Foldi, 2004; Albrecht et al., 2015; García Morales et al. online_b). Furthermore, the taxonomic status and the real distribution range of M. matsumurae are also still uncertain. A similar species, M. pini, widely distributed from North Africa (Morocco) to Russia, is believed to be synonym of M. matsumurae (Kosztarab and Kozár, 1998; Booth and Gullan, 2006), and according to Foldi (2004), these two taxa ‘may be a single species with a high capacity to exploit available hosts in variety of climatic areas, or a widespread complex of sibling species’. Matsucoccus species usually have 3 development stages in females: egg, nymph (3 instars) and adult and 4 development stages in males: egg, nymph (3 instars), pupa and adult (Foldi, 2004). Adult females of Matsucoccus massonianae and M. matsumurae are wingless, 2–4.6 mm long, 1.2–2.5 mm wide with a soft and wrinkled body and well‐developed legs; males are winged, midge‐like, 1.8–2 mm long, 0.4–0.8 mm wide. The 1st instar nymphs are 0.2–0.44 mm long and 0.15–0.20 mm wide (Bean and Godwin, 1971; Hu and Wang, 1976; Young et al., 1976). While the 1st instar nymph is mobile (crawlers) the intermediate stage nymphs are fixed and covered with white waxy filaments (cysts) (Anderson et al., 1976; Foldi, 2004). Matsucoccus are univoltine/bivoltine, rarely multivoltine (up to 5–6 generations per year) depending on species and climatic conditions. The overwintering stage is usually an aged sessile nymph (cyst) fixed within the bark crevices (Foldi, 2004) but can also be a 1st instar nymph in bivoltine species (Bean and Godwin, 1971; Anderson et al., 1976; Liu et al., 2014). In spring, after a last moult, mature nymphs turn to adult females, while adult males emerge after a prepupal and pupal stage. Females are mobile and crawl in search of suitable site for oviposition. Hundred eggs are laid in white waxy ovisacs and newly hatched 1st instar nymphs disperse on stems and branches in search of suitable sites for feeding on phloem and cambium under bark scales and in crevices. 2nd and 3rd instar nymphs are sessile and continue feeding during summer (Anderson et al., 1976; Foldi, 2004). High temperatures can cause summer diapause in some species. Matsucoccus scales can easily spread airborne to distances of about 500–1,600 m or more in mobile stages. Wind is considered the most important way of natural dispersal, but birds and mammals can also carry the pests. Fixed stages are frequently transported via wood with bark, or by plants for planting (Anderson et al., 1976; Stephens and Aylor, 1978). Matsucoccus massonianae is known in China as a pest of Pinus massoniana and to a less degree of P. thunbergii in Zhejiang. The scale is univoltine. Overwintering stage is 2nd instar nymph for females and pupa for males. As in Zhejiang there are favourable climatic conditions, the early appearance of adults (late January, with peak in the middle of February) is followed by dispersal of newly hatched nymphs from April to early May. Intermediate sessile stages causing damage can be found from April to March of the next year (Hu and Wang, 1976; Dossier Section 4.0). Matsucoccus matsumurae is a pest for several pine species in China, Korea and Japan (Foldi, 2004; Choi et al., 2019; García Morales et al., online_b) and also attack Pinus resinosa in the USA (Bean and Godwin, 1971; Anderson et al., 1976). Matsucoccus matsumurae is a bivoltine species either in North America or China (Anderson et al., 1976; Liu et al., 2014), whereas it is univoltine in South Korea (Choi et al., 2019). According to Liu et al. (2014) climatic conditions can cause delay in duration of development stages. In Zhejiang, the overwintering stage is the 1st instar nymph, which develops to the 2nd instar as early as the beginning of March, adults appear in late March–April and oviposition lasts until early May, starting the 1st generation (‘summer generation’ sensu Bean and Godwin (1971)). In autumn, the 2nd generation nymphs appear in Zhejiang in October, but start overwintering only in December, while in northern areas of China, where the life cycle is delayed for one and half month, overwintering starts as early as the end of October (Liu et al., 2014). |
| Symptoms | Main type of symptoms | The main symptoms of infestation by Matsucoccus massonianae and M. matsumurae on pine are needle discoloration and needle cast, progressing from pale green to yellow and red. White waxy masses covering the sessile scales can be visible in bark crevices by naked eye. |
| Presence of asymptomatic plants | Pine trees attacked by Matsucoccus massonianae and M. matsumurae can be asymptomatic in the early stage of infestation (April–June) before the beginning of feeding activity by fixed stages, or even all year around when infestation is very low. | |
| Confusion with other pests | The taxonomic status of Matsucoccus species, including M. massonianae and M. matsumurae, is still discussed and morphological or molecular analysis can be needed for reliable identification of the pests. | |
| Host plant range |
Matsucoccus massonianae is a pest of Pinus massoniana, P. thunbergii, Larix kaempferi and Pseudolarix amabilis (Dossier Section 4.0; García Morales et al., online_a; Ben‐Dov, 2011). Matsucoccus matsumurae is a pest of the following Pinus species: Pinus densiflora, P. massoniana, P. resinosa, P. tabulaeformis, P. thunbergii (García Morales et al., online_b), P. densiflora cv.umbraculifera, P. densiflora var. pendula, P. taiwanensis, P. massoniana × P. thunbergii, P. luchuensis (Xu et al., 2009), P. halepensis, P. mugo, P. nigra, P. pinaster, P. pinea, P. rigida, P. sylvestris (Foldi, 2004), P. banksiana, P. strobus, P. taeda (Kim and Ho, 1992), P. kesiya var. langbianensis (syn. P. insularis) (McClure, 1985) and P. koraiensis (Dossier Section 4.0). |
|
| Reported evidence of impact |
Matsucoccus massonianae has been reported as a pest of Pinus massoniana in Zhejiang (Yuwang Forest), causing however severe damage in 20 ha (Hu and Wang, 1976; Young et al., 1976). Matsucoccus matsumurae is known as a very important pest in pine stands in China and Korea, causing severe damage mainly to P. massoniana, P. tabulaeformis and P. thunbergii, on both natural and artificial forests (Foldi, 2004; Choi et al., 2019); over 70,000 km2/year have been damaged in China in recent years (Liu et al., 2014). Matsucoccus matsumurae is indeed of minor importance as a pest to Pinus densiflora and P. thunbergii in Japan, whereas it is reported as the most important pest in P. resinosa plantations in the USA (Bean and Godwin, 1971; Foldi, 2004). |
|
| Evidence that the commodity is a pathway | As M. massonianae and M. matsumurae live throughout the year under the bark of stems, branches and twigs of pines, bonsai plants are a possible pathway for both pests. The most likely life stage that can travel with the commodity is the 1st instar overwintering nymph of M. matsumurae, and all the development stages of M. massonianae. However, no interceptions of both pests on the commodity were reported so far. | |
| Surveillance information | Matsucoccus massonianae and M. matsumurae are recorded in Dossier Section 5.0 as pests occurring in Zhenjiang province where the nursery is located. The Dossier does not provide any information on surveillance for these pests. | |
A.12.2. Possibility of pest presence in the nursery
A.12.2.1. Possibility of entry from the surrounding environment
Matsucoccus massonianae and M. matsumurae are both present in Zhejiang province (Dossier Sections 4.0 and 5.0; García Morales et al., online_a,b) where the nursery is located. As stated in Dossier Section 4.0 in the area surrounding the nursery are present Pinus massoniana and P. thunbergii which are hosts for both pests; furthermore, Pseudolarix amabilis (host of M. massonianae) and Pinus densiflora (host of M. matsumurae) are also present. Based on the presence of suitable hosts of pests in the surrounding, the Panel assumes that pests can be present in the production area of bonsai plants destined for export to the EU.
The possibility that the pests could entry the nursery is mainly via air currents allowing the spread of mobile stages (adults and 1st instar nymphs) which may be easily transported to the crowns of mother plants; bird and human assisted spread of the same stages is also possible (Anderson et al., 1976; Stephens and Aylor, 1978).
As stated in Dossier Section 4.0, the bonsai cultivation site is protected by a 40‐mesh insect‐proof net (0.4 mm). According to Hu and Wang (1976) and Young et al. (1976), crawlers of Matsucoccus massonianae and M. matsumurae are on average 0.15–0.20 mm wide. Therefore, the crawlers are expected to go through the net.
Uncertainties
-
‐
The status of the pests in the surrounding area (no surveillance information).
-
‐
Laying down and removal calendar of insect‐proof net on bonsai cultivation in relation to the dispersal time of mobile stages.
-
‐
Use of insect‐proof net for protection of mother plants in the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pests to enter the nursery, because their absence in the surrounding area is not verified; host plants are present; pests have considerable capability of airborne dispersal and can be taken by birds and mammals and insect proof net is not enough.
A.12.2.2. Possibility of entry with new plants/seeds
All seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0). Seeds of black pine (P. thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triadimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
The growing media used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0).
Possibility of entry with seeds or soil/growing media is not relevant for M. massonianae and M. matsumurae.
Uncertainties
-
‐
It is not clear if and how new mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pests could enter the nursery with new plants/seeds or soil/growing media.
A.12.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of Pinus parviflora are taken (Dossier Section 4.0). The pest within the nursery can spread by scions taken from infested mother plants, by air currents, by crawling of mobile stages from plant to plant and eventually by passive transport on clothing of the staff.
Spread within the nursery through the movement of soil, water, equipment and tools is not relevant.
Uncertainties
-
‐
There is no information on the presence or population pressure of the pests in the nursery.
-
‐
The host status of P. parviflora for both scales.
-
‐
Whether the grafting time matches the crawler occurrence/presence.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pest within the nursery is possible due to the presence of suitable hosts and both active and passive spreading capability.
A.12.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Matsucoccus massonianae, M. matsumurae or Matsucoccus sp. between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.12.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on pine bast scales (Matsucoccus massonianae and M. matsumurae) is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
Physical separation of the bonsai plants may have limited effect on reducing risk of infestation with pine bast scales, especially in the production base, because the crawlers can go through the net.
Uncertainties:
|
| 2 | Growing medium and its treatment | No | Not applicable. |
| 3 | Treatment of seeds | No | Not applicable. |
| 4 | Insecticide and acaricide treatments | Yes |
Spray of contact insecticides can kill the scales that are mobile on the plants at the time of spraying.
Uncertainties:
|
| 5 | Fungicide treatments | No | Not applicable. |
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | No | Not applicable. |
| 8 | Official inspections during production | Yes |
Scales are only visible when they are mobile on the outer bark, although most of the life stages are hidden under the bark.
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
Scales are only visible when they are mobile on the outer bark, although most of the life stages are hidden under the bark.
Uncertainties:
|
A.12.5. Overall likelihood of pest freedom for Matsucoccus massonianae and M. matsumurae on grafted bonsai plants
A.12.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The scenario assumes the pest is present in the province, but its abundance is expected to be low in the surroundings of the nursery. The scenario also assumes that P. parviflora is not considered as a host. Finally, the scenario assumes that, although the protecting net is not fully effective in preventing the pest for reaching the plants, it reduces the flow of crawlers blowed by the wind, and that performed treatments can also reduce the population of the pest inside the nursery.
A.12.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
The scenario assumes that the pest can reach high density in the surroundings due to the presence of different hosts. The scenario also assumes that mother plants can be fully exposed to the pest and consequently crawlers can be taken inside the net protected area with infected scions from mother plants. Finally, the scenario assumes that different stages of the pest are hidden in crevices of the bark, and so they may be difficult to be reached by insecticides, and that these hidden stages will not be easy to detect in inspections.
A.12.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
The pest is present in province where the nursery is located. Crawlers can be easily spread by the wind, and its size is small enough to go through the protecting net, although some flow of insects may be reduced by the net. They also can be introduced by infected scions from mother plants. However, the median is closer to the lower values because some effectiveness is expected from insecticide treatments and from inspections, and because it is not expected a high abundance of the pest in the surroundings of the nursery.
A.12.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Uncertainty is high about the control methods performed because information provided in the Dossier is not complete. There is also a high uncertainty about the susceptibility of P. parviflora and about the pressure of the pest from around the nursery. Some stages of the pest are hidden in crevices and under the bark, so the effectiveness of insecticide treatments is also uncertain as pest may not be reached by the pesticide. And finally, effectiveness of inspections is uncertain due to the difficulties in detecting hidden stages of the pest.
A.12.5.5. Elicitation outcomes of the assessment of the pest freedom for Matsucoccus massonianae and M. matsumurae on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.21) and pest freedom (Table A.22).
Table A.21.
Elicited and fitted values of the uncertainty distribution of pest infestation by Matsucoccus massonianae and M. matsumurae per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 10 | 80 | 150 | 300 | 500 | ||||||||||
| EKE | 10.2 | 12.6 | 17.4 | 28.8 | 46.6 | 71.6 | 99.6 | 165 | 244 | 290 | 344 | 395 | 444 | 475 | 500 |
The EKE results are the BetaGeneral (0.81342, 1.5297, 9,530) distribution fitted with @Risk version 7.6.
Table A.22.
The uncertainty distribution of plants free of Matsucoccus massonianae and M. matsumurae per 10,000 plants calculated by Table A.21
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,500 | 9,700 | 9,850 | 9,920 | 9,990 | ||||||||||
| EKE results | 9,500 | 9,525 | 9,556 | 9,605 | 9,656 | 9,710 | 9,756 | 9,835 | 9,900 | 9,928 | 9,953 | 9,971 | 9,983 | 9,987 | 9,990 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.22.
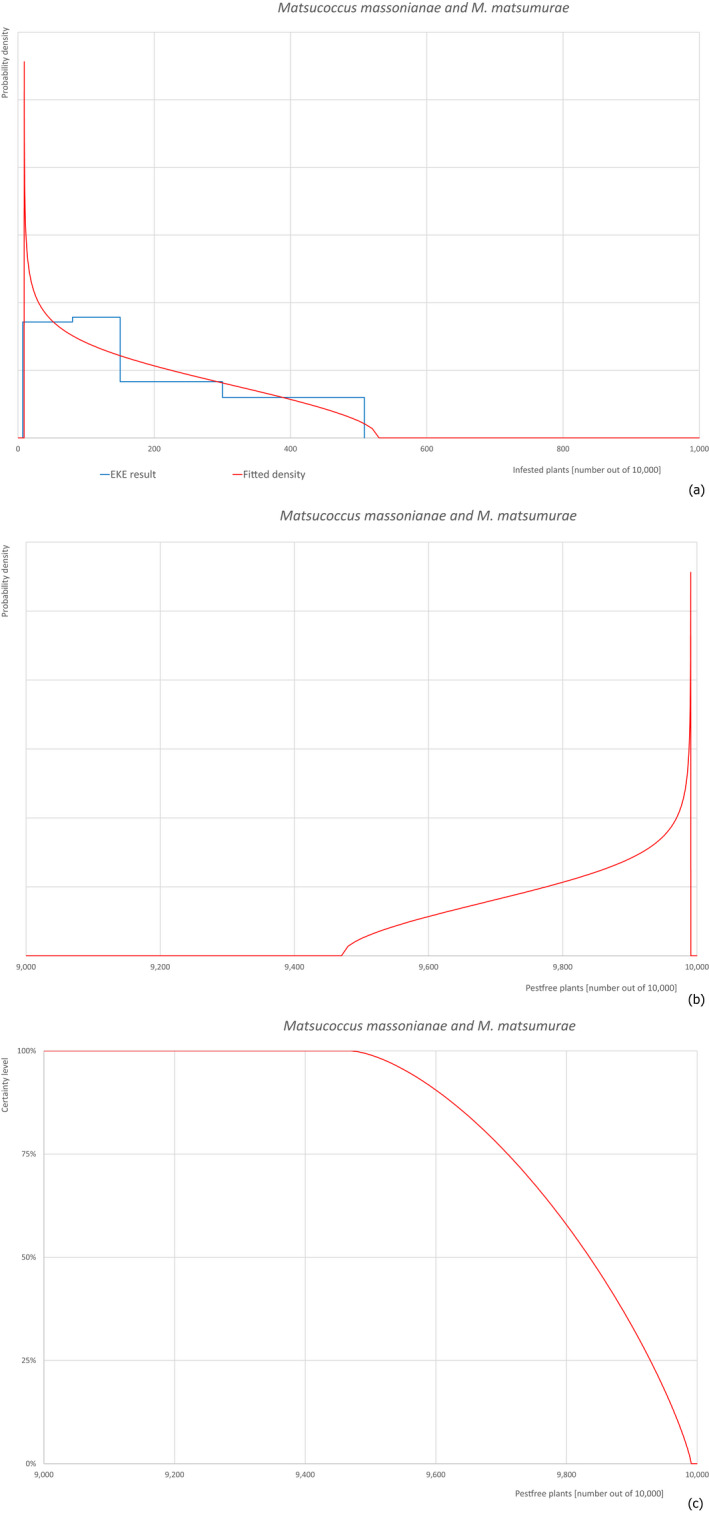
Figure A.11: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. =1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.12.6. Reference List
Albrecht A, Rinne V, Soderman G and Matila K, 2015. Check list of Finnish Hemiptera. Jalla, Bulletin of the Finnish Expert Group on Hemiptera, 1, 1–29.
Anderson JF, Ford RP, Kegg JD and Risley Jh, 1976. The Red Pine Scale in North America. A report to the 1975 Eastern Plant Board. The Connecticut Agricultural Experiment Station, New Haven, Bulletin, 765, 8.
Bean JL and Godwin AA, 1971. Red Pine Scale. USDA Forest Service, Forest Pest Leaflet, 10, 6.
Booth JM and Gullan PJ, 2006. Synonymy of three pestiferous Matsucoccus scale insects (Hemiptera: Coccoidea: Matsucoccidae) based on morphological and molecular evidence. Proceedings of the Entomological Society of Washington, 108, 749–760.
Choi J, Cha D, Kim D‐S and Lee S, 2019. Review of Japanese Pine Bast Scale, Matsucoccus matsumurae (Kuwana) (Coccomorpha: Matsucoccidae), Occurring on Japanese Black Pine (Pinus thunbergii Parl.) and Japanese Red Pine (P. densiflora Siebold & Zucc.) from Korea. Forests, 10, 639, 1–15. https://doi.org/10.3390/f10080639
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Foldi I, 2004. The Matsucoccidae in the Mediterranean basin with a world list of species (Hemiptera: Sternorrhyncha: Coccoidea), Annales de la Société Entomologique de France, 40, 145–168. https://doi.org/10.1080/00379271.2004.10697412
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_a. ScaleNet: A literature‐based model of scale insect biology and systematics, Matsucoccus massonianae. Available online: https://scalenet.info/catalogue/matsucoccus%20massonianae/ [Accessed 25 June 2021].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_b. ScaleNet: A literature‐based model of scale insect biology and systematics, Matsucoccus matsumurae. Available online: https://scalenet.info/catalogue/matsucoccus%20matsumurae/ [Accessed 25 June 2021].
Hu H and Wang L, 1976. Studies on the pine bast scale Matsucoccus massonianae Y. and H (I). Acta Entomologia Sinica, 19, 383–392.
Jansen MGM, 1999. An annotated list of the scale insects (Hemiptera: Coccoidea) of the Netherlands. Entomologica, 33, 197–206.
Kim KC and Oh KI, 1992. Bionomics, host range and analysis of damage aspects on the black pine bast scale, Matsucoccus thunbergianae (Homoptera: Coccoidea), in the coastal area of southwest Korea. Korean Journal of Applied Entomology, 31, 386–395.
Kosztarab M and Kozár F, 1988. Scale Insects of Central Europe. Akadémiai Kiadó, Budapest, 456 pp.
Liu W, Xie Y, Dong J, Yang Q, Xue J and Tian F, 2014. New Research on Matsucoccus matsumurae (Kuwana) (Hemiptera: Matsucoccidae) in China. Acta Zoologia Bulgarica, 6, 95–102.
McClure MS, 1985. Susceptibility of pure and hybrid stands of Pinus to attack by Matsucoccus matsumurae in Japan (Homoptera: Coccoidea: Margarodidae). Environmental Entomology, 14, 535–538.
Mudan Y, Jie S and Qingyu W, 1999. Quarantine Techniques for Matsucoccus matsumurae. Journal of Zhejiang Forestry Science, 19, 40–43.
Ren L, Li Z, Li Y and Guo Y, 2016. Revision of scientific names for the main insect species in the monograph ‘Forest Insects of China (2nd Edition, 1992)’. Scientia Silvae Sinicae, 52, 110–115. https://doi.org/10.11707/j.1001‐7488.20160413
Sierra JM, 1998. El control de plagas en las masas artificiales mediante la ordenacion de montes. Cuadernos de la Sociedad Espanola de Ciencias Forestales, 6, 67–74.
Soria S, Moreno M, Vinuela E and Del Estal P, 2000. Principales cochinillas en los pinos espanoles. Boletìn de sanidad vegetal. Plagas, 26, 335–348.
Stephens GR and Aylor DE, 1978. Aerial dispersal of red pine scale, Matsucoccus resinosae (Homoptera; Margarodidae). Environmental Entomology, 7, 556–563.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
Young BL, Hu JL and Ren ZY, 1976. Pine bast scales from China. Acta Entomologica Sinica, 19, 199–204.
Xu ZH, Wanna RC, Ren LJ, Yu HX, Huang J and Wang ZH, 2009. The role of parasitic wasps in biological control on exotic harmful scale insects. International Workshop on Biological Control of Invasive Species of Forests, 20–25 September 2007, Bejing, China, USDA Forest Service, 64–70.
Zhang S, Liu X, Wang M and Liu Q 2007. A survey method for monitoring epidemic situation of Matsucoccus matsumurae and quarantine measures. Jilin Forestry Science and Technology, 36, 39–44.
A.13. Pine needle scales (Fiorinia japonica, Hemiberlesia pitysophila, Lepidosaphes pineti, L. pini, L. piniphila and Parlatoria pinicola)
A.13.1. Organism information
A.13.1.1. Fiorinia japonica
| Taxonomic information |
Current valid scientific name: Fiorinia japonica Synonyms: Fiorinia fioriniae japonica, Fiorinia juniperi, Fiorinia iuniperi Name used in the EU legislation: – Order: Hemiptera Family: Diaspididae Common name: coniferous fiorinia scale, Japanese scale, juniper fiorinia scale Name used in the Dossier: Fiorinia japonica |
| Group | Insects |
| EPPO code | FIORJA |
| Regulated status |
The pest is neither regulated in the EU, nor listed by EPPO. In 2003, F. japonica was included in A1 list of Bahrain (EPPO, online_a) and is quarantine in Western Australia (Commonwealth of Australia, 2020). |
| Pest status in China | In China, F. japonica is present in provinces of Fujian, Guangdong, Hebei, Henan, Jiangsu, Jiangxi and Sichuan (Li et al., 1997; García Morales et al., online_a). |
| Pest status in the EU | The pest is present in France (Matile‐Ferrero, 1989; Foldi and Germain, 2018; García Morales et al., online_a). |
| Host status on Pinus parviflora and P. thunbergii | Pinus parviflora and P. thunbergii are reported as hosts of F. japonica (García Morales et al., online_a). |
| PRA information | No Pest Risk Assessment is currently available. |
| Other relevant information for the assessment | |
| Biology |
Fiorinia japonica is a scale insect native to Japan and in early 1900s it has been intercepted many times to the USA (Sasscer, 1912). It is also present in Australia, China, France, India, Mauritius, Philippines, South Korea, Sri Lanka and Taiwan (Matile‐Ferrero, 1989; Suh, 2012; García Morales et al., online_a). Females have brown to dark brown colour with white powdery cover. Males are white and smaller than females. The scale has three stages of development: egg, nymph (two instars) and adult. The pest can be mainly found on underside part of leaves (Sasscer, 1912). There is no information on the biology nor on the overwintering stage of the pest. |
| Symptoms | Main type of symptoms | The main symptoms of infestation are yellow stippling of leaves, defoliation of plants and reduction of plant growth (Arakelian, 2008). |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. | |
| Confusion with other pests | It can be confused with other Fiorinia species. It is very similar to Fiorinia fioriniae (Miller and Davidson, 2005). The first instar of F. japonica compared to the other Fiorinia species lacks dorsal thoracic ducts (Howell, 1977). A morphological (Howell, 1977) or molecular analysis is needed in order to distinguish them. |
| Host plant range |
Fiorinia japonica is mainly pest of conifers: Abies (A. alba, A. firma and A. veitchii), Cedrus atlantica, Cephalotaxus, Cephalotaxus harringtonii, Cupressus, Juniperus (J. bermudiana, J. chinensis and J. communis), Keteleeria davidiana, Picea, Picea pungens, Pinus (P. densiflora, P. koraiensis, P. parviflora and P. thunbergii), Sciadopitys, Sciadopitys verticillata, Taxus, Taxus mairei, Torreya, Torreya nucifera, Tsuga and Tsuga sieboldii (García Morales et al., online_a). It has been reported also on non‐coniferous plants such as Aucuba japonica, Camellia japonica, Dypsis lutescens, Eurya japonica, Ficus, Nageia nagi, Phoenix, Pittosporum tobira, Podocarpus, Podocarpus macrophyllus and Pyrus communis (García Morales et al., online_a). |
| Reported evidence of impact | Fiorinia japonica is a serious pest of pine trees in Beijing, China (Tang, 1984a), although in the USA it is reported as occasional pest (Miller and Davison, 1990). |
| Evidence that the commodity is a pathway | Fiorinia japonica was intercepted on dwarf pine from Japan in Pennsylvania in 1937 (USDA, 1939), therefore the commodity can be a pathway. |
| Surveillance information |
Fiorinia japonica is recorded in Dossier Section 4.0 as a pest occurring in China. No surveillance information for this pest is currently available from China. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. |
A.13.1.2. Hemiberlesia pitysophila
| Taxonomic information |
Current valid scientific name: Hemiberlesia pitysophila Synonyms: – Name used in the EU legislation: – Order: Hemiptera Family: Diaspididae Common name: pine needle hemiberlesian scale Name used in the Dossier: Hemiberlesia pitysophila |
| Group | Insects |
| EPPO code | HEBEPI |
| Regulated status |
The pest is neither regulated in the EU, nor listed by EPPO. In 1993 the pest was in the A1 list for China (EPPO, online_b) and it is quarantine in Australia (Commonwealth of Australia, 2020). |
| Pest status in China | In China, H. pitysophila is present in provinces of Fujian, Guangdong, Hainan, Hongkong, Liaoning, Macao/Macau (Aomen) and Shaanxi (Dossier Section 4.0; CABI, online; EPPO, online_c; Chen et al., 2004). |
| Pest status in the EU | The pest is absent in the EU (CABI, online; EPPO, online_c). |
| Host status on Pinus parviflora and P. thunbergii |
Pinus thunbergii is reported as host of H. pitysophila (Dossier Section 4.0; CABI, online). There is no information on whether H. pitysophila can also attack P. parviflora. |
| PRA information | No Pest Risk Assessment is currently available. |
| Other relevant information for the assessment | |
| Biology |
Hemiberlesia pitysophila is a scale insect native to Japan (Lv et al., 2011), it is also present in China, South Korea and Taiwan (García Morales et al., online_b). The scale has three stages of development: egg, nymph (2 instars for female and 4 for male) and adult. Eggs are yellowish and are laid under female’s body. Females lay between 35 and 137 eggs per generation. First nymph instar is called crawler, it is active for up to 76 h and moves on average 30–60 cm. The crawling distance is influenced by temperature, with an average distance of 145 cm for 2 h at 18.1°C and 236 cm for 2 h at 25.3°C. The last two male nymph instars are called prepupa and pupa. Adult female is yellowish with pear shaped‐like shield. Adult male is orange, has wings and flies up to 4–5 m. It lives from 10 to 55 h (Chen et al., 2004). Optimum temperature for the scale is between 18 and 23°C (FAO, 2007). The mortality of H. pitysophila is 100% when temperature is 43°C and higher or 1.5°C and lower. Rainfall when higher than 100 mm/month negatively influences development of H. pitysophila’s population (Tong et al., 1988). In China, the density of the population is the highest from April to July. There are between four (Chen et al., 2004) and five overlapping generations annually (Tong et al., 1988). There is no information on the overwintering stage of the pest. According to Chen et al. (2004) in Fuquing district during the month of December only females were present. In January and February, first and second instar nymphs appeared. Movement of infested plants and crawlers carried by wind (FAO, 2007), animals or people (CABI, online) are main possible ways of H. pitysophila dispersal. Possible pathways of entry with trade for H. pitysophila are plants for planting, seedlings, pine needles, cones, flowers, bark and woody material (branches and trunks) (FAO, 2007; CABI, online). |
| Symptoms | Main type of symptoms |
Main symptoms caused by H. pitysophila are yellowing of needles, premature needle drop, bending of branches, decline and mortality of trees (Lv et al., 2011, citing others; CABI, online). When trees are attacked by the pest, it can die in 3–5 years (Lv et al., 2011, citing others; CABI, online). |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. | |
| Confusion with other pests | It can be confused with other Hemiberlesia species. A morphological or molecular analysis is needed for a verified identification. | |
| Host plant range |
Hosts of H. pitysophila are conifers, such as Pinus (P. caribaea, P. elliottii, P. massoniana, P. taeda and P. thunbergii) (Dossier Section 4.0; CABI, online; García Morales et al., online_b). According to Dossier Section 4.0, the pest also attacks Picea glabra and Pinus (P. caribaea var. hondurensis, P. joocarpa, P. latteri, P. luchuensis, P. patula and P. serotina). |
|
| Reported evidence of impact | After H. pitysophila was introduced to China in 1980s, the pest caused serious damage and decline of Pinus massoniana in Guangdong province. It is considered as extremely harmful pest for pines in China (Feng et al., 2009; Lv et al., 2011, citing others). | |
| Evidence that the commodity is a pathway | According to FAO (2007), one of the main pathways of the scale are infested living plants. Therefore, the commodity (i.e. bonsai plants) can be a pathway for the scale. | |
| Surveillance information |
Hemiberlesia pitysophila is recorded in Dossier Section 4.0 as a pest occurring in China. No surveillance information for this pest is currently available from China. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. |
|
A.13.1.3. Lepidosaphes pineti, L. pini and L. piniphila
| Taxonomic information |
1. Lepidosaphes pineti Current valid scientific name: Lepidosaphes pineti Synonyms: Insulaspis pineti Name used in the EU legislation: – Order: Hemiptera Family: Diaspididae Common name: – Name used in the Dossier: Lepidosaphes pineti |
|
2. Lepidosaphes pini Current valid scientific name: Lepidosaphes pini Synonyms: Poliaspis pini, Chionaspis pini, Mytilococcus pinorum, Insulaspis pini Name used in the EU legislation: – Order: Hemiptera Family: Diaspididae Common name: Oriental pine scale, pine oystershell scale Name used in the Dossier: Lepidosaphes pini 3. Lepidosaphes piniphila Current valid scientific name: Lepidosaphes piniphila Synonyms: Lepidosaphes piniphilus, Parainsulaspis piniphila Name used in the EU legislation: – Order: Hemiptera Family: Diaspididae Common name: – Name used in the Dossier: Lepidosaphes piniphila |
|
| Group | Insects |
| EPPO code |
LEPSPT: Lepidosaphes pineti LEPSPN: Lepidosaphes pini LEPSPH: Lepidosaphes piniphila |
| Regulated status |
Lepidosaphes pineti and L. piniphila are not regulated anywhere in the world nor listed by EPPO. Lepidosaphes pini is not regulated in the EU nor listed by EPPO. It is quarantine in Australia (Australian Government Department of Agriculture, Water and the Environment, 2020). |
| Pest status in China |
Lepidosaphes pineti, L. pini and L. piniphila are present in China. Lepidosaphes pineti is present in Anhui, Beijing, Fujian, Guangdong, Hubei, Jiangsu, Shandong and Zhejiang (Jianying, 2002; Dossier Section 4.0; García Morales et al., online_c). Lepidosaphes pini is present in Anhui, Hebei, Jiangsu, Liaoning, Shandong and Shanghai (Dossier Section 4.0; García Morales et al., online_d). Lepidosaphes piniphila is present in Anhui, Guangdong, Hunan, Jiangsu and Jiangxi (Dossier Section 4.0; García Morales et al., online_e). |
| Pest status in the EU | Lepidosaphes pineti, L. pini and L. piniphila are absent in the EU (García Morales et al., online_c,d,e). |
| Host status on Pinus parviflora and P. thunbergii |
Pinus thunbergii is reported as a host of Lepidosaphes pineti (Jianying, 2002), L. pini (Takahashi, 1955; Takagi, 1970; Suh, 2020; García Morales et al., online_d) and L. piniphila (García Morales et al., online_e). Lepidosaphes pini was reported on P. parviflora var. pentaphylla (Malumphy et al., 2012). According to Dossier Section 4.0, L. pineti, L. pini and L. piniphila are reported on P. parviflora. |
| PRA information | No Pest Risk Assessment is currently available. |
| Other relevant information for the assessment | |
| Biology |
Lepidosaphes pineti, L. pini and L. piniphila are scale insects (García Morales et al., online_c,d,e). Lepidosaphes pineti is present only in China (García Morales et al., online_c), L. pini in Bonin Islands, China, Hawaiian Islands, Japan, South Korea, Taiwan and United States (García Morales et al., online_d) and L. piniphila in China, Japan and Malaysia (García Morales et al., online_e). Lepidosaphes pineti has three stages of development: egg, nymph (2 instars) and adult. Eggs are laid by overwintered females in mid‐April. Females lay between 10 and 36 eggs per generation below their shells. The eggs hatch in early May into 1st instar nymph (crawlers). Crawlers find suitable place to settle in on pine needles and secrete wax. The 2nd instar nymph starts to form shell. First adults emerge in mid‐June. In mid‐July, the second generation of juveniles hatches. Females are brown pear‐shaped, 2.0–2.4 mm long and 0.4 mm wide. Males have the same colour and shape as females, but they are smaller, 0.9 mm long and 0.24 mm wide. Males live for 1–2 days (Jianying, 2002). Lepidosaphes pineti overwinters as fertilised female or second larval instar. In China, there are one or two overlapping generations annually (Jianying, 2002). The life cycle of L. pini is poorly studied. Females are oyster‐shell shaped, brown with orange exuviae. Males are very similar to females, but smaller (García Morales et al., online_d). The size of the body is 2 mm long (Stimmel, 1994). Body of adult female, eggs and crawlers are white (Miller and Davidson, 2005). Females lay about 30 eggs per generation (Murakami, 1970). Lepidosaphes pini occurs on base of pine needles (Stimmel, 1994). It overwinters as fertilised female in Japan (Murakami, 1970) and New Jersey (Miller and Davidson, 2005) or possibly as eggs in Pennsylvania (Stimmel, 1994). There are one or two generations annually (Murakami, 1970; Stimmel, 1994). In New Jersey eggs are present in March and August, crawlers in June and September (Miller and Davidson, 2005). There is no information on the biology nor on the overwintering stage of L. piniphila. Females are narrow and brown. They are 1.7 mm long and 0.55 mm wide (Borchsenius, 1958). Possible pathways for Lepidosaphes species are plants for planting including bonsai plants and cut branches as reported for Lepidosaphes ussuriensis by EPPO Panel on Quarantine Pests for Forestry (2003). |
| Symptoms | Main type of symptoms |
According to Borchsenius (1958), Lepidosaphes species (including L. pineti and L. piniphila) cause early drop of needles. Main symptoms caused by L. pini are reduced vigour of pine needles, pallid and chlorotic spots near the base of needles. The scale does not produce honeydew (Stimmel, 1994). There is no information on symptoms caused by L. pineti and L. piniphila, but it can be assumed that they are similar to the ones described above. Symptoms caused by another species from North America (Lepidosaphes pallida) on conifers are chlorosis, branch dieback and death of host plants (Skvarla, 2020). |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. | |
| Confusion with other pests | Lepidosaphes pineti, L. pini and L. piniphila can be confused with other Lepidosaphes species. A morphological (Miller et al., 2006) or molecular analysis is needed in order to distinguish them. | |
| Host plant range |
Hosts of L. pineti are Pinus elliotti, P. taeda, P. thunbergii (Jianying, 2002) and P. massoniana (Jianying, 2002; García Morales et al., online_c). Hosts of L. pini are Cunninghamia lanceolata, Cycas revoluta, Abies, Pinus, P. densiflora, P. luchuensis, P. nigra, P. thunbergii, Podocarpus, P. macrophyllus, Taxus, Torreya (Murakami, 1970; Suh, 2020; García Morales et al., online_d), Pinus armandi (Miller and Davison, 1990) and P. parviflora var. pentaphylla (Malumphy et al., 2012). Hosts of L. piniphila are Pinus massoniana, P. thunbergii and Podocarpus (García Morales et al., online_e). |
|
| Reported evidence of impact |
Jianying (2002) reports L. pineti as a serious pest of pines in China. According to Borchsenius (1958), L. pineti and L. piniphila are considered serious pests of Pinus species in ornamental plantations of Beijing and other Chinese cities. Lepidosaphes pini is considered as an occasional pest (Miller and Davison, 1990), but along the coast of New Jersey it severely damaged P. thunbergii (Stimmel, 1994). |
|
| Evidence that the commodity is a pathway |
Lepidosaphes pini was intercepted in the UK on bonsai plants of P. parviflora var. pentaphylla from Japan in June 1986 (Malumphy et al., 2012). Adult females of L. pini were intercepted in Taiwan on P. thunbergii from Japan (Chen et al., 2014). |
|
| Surveillance information |
Lepidosaphes pineti is recorded in Dossier Sections 4.0 and 5.0 as a pest occurring in Zhejiang. According to Dossier Section 4.0, L. pini and L. piniphila are pests occurring in China. No surveillance information for this pest is currently available from China. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. |
|
A.13.1.4. Parlatoria pinicola
| Taxonomic information |
Current valid scientific name: Parlatoria pinicola Synonyms: – Name used in the EU legislation: – Order: Hemiptera Family: Diaspididae Common name: – Name used in the Dossier: Parlatoria pinicola |
|
| Group | Insects | |
| EPPO code | PARLPC | |
| Regulated status | Parlatoria pinicola is not regulated anywhere in the world nor listed by EPPO. | |
| Pest status in China | Parlatoria pinicola is present in China in Anhui (Dossier Section 4.0) and Zhejiang (García Morales et al., online_f). | |
| Pest status in the EU | Parlatoria pinicola is absent in the EU (García Morales et al., online_f). | |
| Host status on Pinus parviflora and P. thunbergii |
According to Dossier Section 4.0, Parlatoria pinicola is reported on Pinus parviflora. There is no information on whether Parlatoria pinicola can also attack Pinus thunbergii. |
|
| PRA information | No Pest Risk Assessment is currently available. | |
| Other relevant information for the assessment | ||
| Biology |
Parlatoria pinicola is a scale insect, native to China (García Morales et al., online_f). Females are dark yellow, oval, 1.70 mm long. Males are 1.0 mm long (Tang, 1984b; García Morales et al., online_f). There is no information on the biology nor on the overwintering stage of Parlatoria pinicola. Another species from the same genus, Parlatoria pittospori, which is from South hemisphere. It is polyphagous and infests also Pinus species. It has three stages of development: egg, nymph (there is no information on number of instars) and adult. All stages of the scale were found throughout the year in New Zealand. Females lay between 46 and 62 eggs per generation (Timlin, 1964). The dispersal of scales is caused by wind. The mobile crawlers are taken to other plants in the surroundings. Parlatoria pittospori was able to reach about 230 m (Timlin, 1964). |
|
| Symptoms | Main type of symptoms | There is no information on the main symptoms caused by Parlatoria pinicola. |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. | |
| Confusion with other pests | Parlatoria pinicola can be confused with other Parlatoria species. It is very similar to Parlatoria pini, which has been found on Pinus from India. A morphological (Tang, 1984b) or molecular analysis is needed in order to distinguish them. | |
| Host plant range |
The only reported host of Parlatoria pinicola is Pinus armandii (García Morales et al., online_f). According to Dossier Section 4.0, Parlatoria pinicola is reported on Pinus parviflora. |
|
| Reported evidence of impact | There is no information on the evidence of impact. However, Parlatoria pinicola is recorded in Dossier Section 4.0 as a pest having impact. | |
| Evidence that the commodity is a pathway | There is no information on the evidence that the commodity is a pathway. However, Parlatoria pinicola is recorded in Dossier Section 4.0 as a pest associated with the commodity. | |
| Surveillance information |
Parlatoria pinicola is recorded in Dossier Section 4.0 as a pest occurring in China. No surveillance information for this pest is currently available from China. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. |
|
A.13.2. Possibility of pest presence in the nursery
A.13.2.1. Possibility of entry from the surrounding environment
As long as many of pine needle scale species are common, very polyphagous and some of them invasive in China, high pressure is expected. The possibility of entry for pine needle scales from surrounding environment to nursery is through crawler dispersal including air currents, human and animal assisted spread. As stated in Dossier Section 4.0, the bonsai cultivation site is protected by a 40‐mesh insect‐proof net (0.4 mm), which is approximately the body width of crawlers. The Panel assumes that at least part of the crawlers can go through the net.
Pine needle scale insects are generally quite polyphagous, infesting different conifers. Based on the presence of suitable hosts of pests in the surrounding (Dossier Sections 4.0 and 5.0), the Panel assumes that pests can be present in the production area of bonsai plants destined for export to the EU.
Uncertainties
-
‐
There is no surveillance information on the presence or population pressure of scale insects in the area where the nursery is located.
-
‐
No information available on the distance of the nursery to sources of scale insects in the surrounding environment.
-
‐
There is no evidence that Fiorinia japonica and Hemiberlesia pitysophila are present in Zhejiang province. However, they are invasive species and it cannot be excluded that they will be present there in the future.
-
‐
The quantity of pine needle scale crawlers that can go through the net, because precise information on their size is not available.
-
‐
Host status of Pinus parviflora for Hemiberlesia pitysophila, Lepidosaphes pineti, L. piniphila and Parlatoria pinicola.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for pine needle scales to enter the nursery. The pests can be present in the surrounding area because of the presence of suitable hosts and the transferring rate could be enhanced by movement by wind, animals and humans.
A.13.2.2. Possibility of entry with new plants/seeds
All seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (Pinus thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triaimefon. Scions of Pinus parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
The growing media used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0). Possibility of entry with seeds or soil/growing media is not relevant for scale insects.
Uncertainties
-
‐
It is not clear if and how new mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pests could enter the nursery with new plants/seeds or soil/growing media.
A.13.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of Pinus parviflora are taken (Dossier Section 4.0).
The pine needle scales within the nursery can spread by hitchhiking on clothing of nursery staff, by wind and by scions from infested mother plants. In addition, at least part of the crawlers can go through the net.
Spread within the nursery through the movement of soil, water, equipment and tools is not relevant.
Uncertainties
-
–
There is no information on the presence or population pressure of the pests in the nursery.
-
–
The host status of Pinus parviflora for Hemiberlesia pitysophila, Lepidosaphes pineti, L. piniphila and Parlatoria pinicola.
-
–
Whether the grafting time matches the pine needle scales crawler occurrence/presence.
-
–
The quantity of pine needle scale crawlers that can go through the net, because precise information on their size is not available.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pests within the nursery is possible due to the presence of suitable hosts (e.g. mother plants, commodity).
A.13.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of pine needle scales between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.13.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on pine needle scales (Fiorinia japonica, Hemiberlesia pitysophila, Lepidosaphes pineti, L. pini, L. piniphila and Parlatoria pinicola) is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
Physical separation of the bonsai plants may have an effect on reducing risk of infestation with pine needle scales, especially in the production base.
Uncertainties:
|
| 2 | Growing medium and its treatment | No | Not applicable. |
| 3 | Treatment of seeds | No | Not applicable. |
| 4 | Insecticide and acaricide treatments | Yes |
Spray of contact insecticides can only kill the crawlers that are present on the plants at the time of spraying. Once they are fixed and covered by the scale they are not expected to be killed by the specified insecticides.
Uncertainties:
|
| 5 | Fungicide treatments | No | Not applicable. |
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | No | Not applicable. |
| 8 | Official inspections during production | Yes |
Pine needle scales are generally visible.
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
Pine needle scales are generally visible.
Uncertainties:
|
A.13.5. Overall likelihood of pest freedom for Fiorinia japonica on grafted bonsai plants
A.13.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The population density around the nursery is low and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are effective. The detection before export is carefully done.
A.13.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
The population density around the nursery is high and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are only partially effective. The detection before export is not detailed enough to spot insects when they are hidden between the needles.
A.13.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
Different combinations of population density around the nursery, effect of the net barrier and of the insecticide applications may result in an intermediate scenario as they are acting independently of one another on the presence of the insect in the commodity. Median is shift to the left side (lower infestation rate), because Lepidosaphes pineti is not known to be present in the area, the host status of P. parviflora is unclear and Fiorinia japonica has not yet been detected in Zhejiang.
A.13.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The signs of the insect occurrence (white wax covering the scales) are generally detectable. The Panel assumes that a high infestation level is less likely to happen than having smaller number of infested plants where the insect density is low and difficult to detect.
A.13.5.5. Elicitation outcomes of the assessment of the pest freedom for Fiorinia japonica on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.23) and pest freedom (Table A.24).
Table A.23.
Elicited and fitted values of the uncertainty distribution of pest infestation by Fiorinia japonica per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 10 | 55 | 100 | 200 | 500 | ||||||||||
| EKE | 10.0 | 12.5 | 16.5 | 24.7 | 36.2 | 51.3 | 67.9 | 107 | 159 | 193 | 240 | 294 | 362 | 423 | 495 |
The EKE results are the BetaGeneral (1.0347,6.9231,8.3,1000) distribution fitted with @Risk version 7.6.
Table A.24.
The uncertainty distribution of plants free of Fiorinia japonica per 10,000 plants calculated by Table A.23
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,500 | 9,800 | 9,900 | 9,945 | 9,990 | ||||||||||
| EKE results | 9,505 | 9,577 | 9,638 | 9,706 | 9,760 | 9,807 | 9,841 | 9,893 | 9,932 | 9,949 | 9,964 | 9,975 | 9,983 | 9,988 | 9,990 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.24.
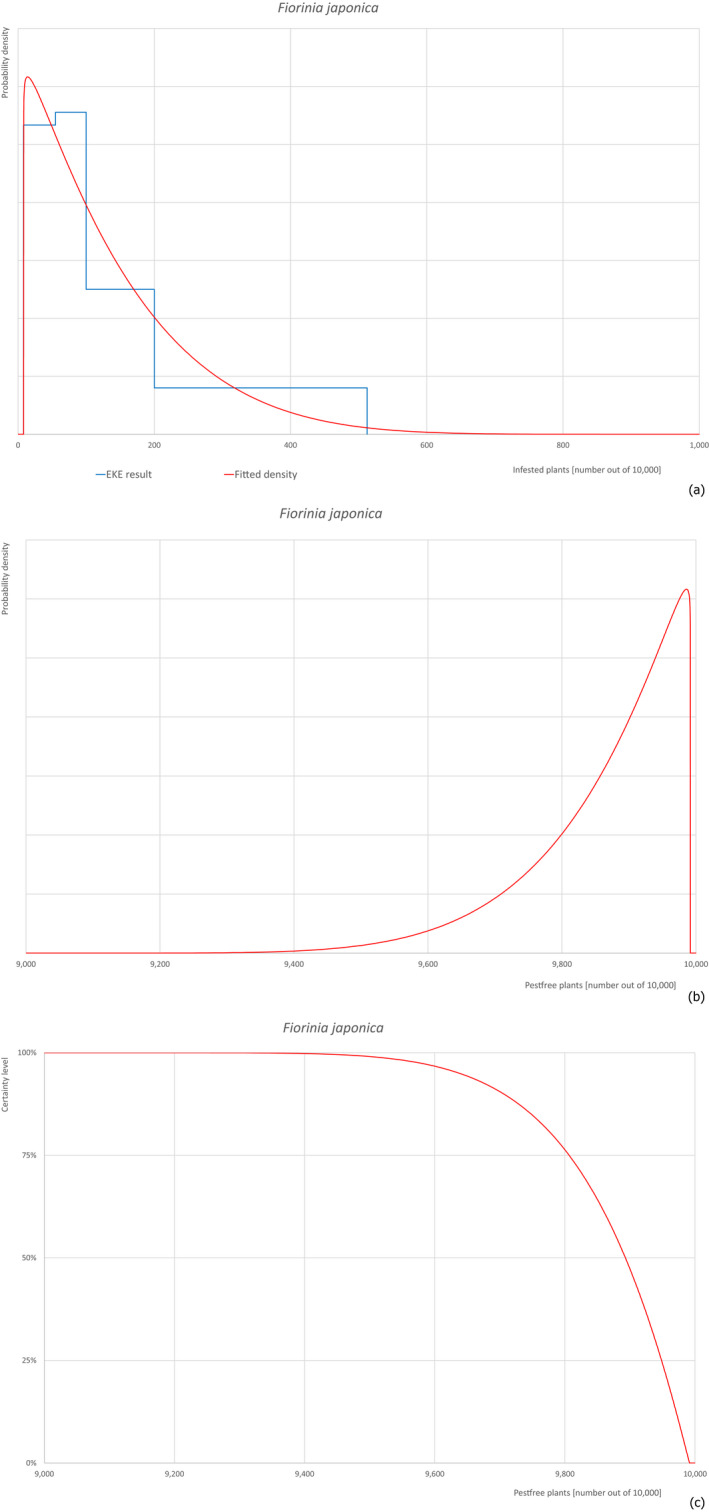
Figure A.12(a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. =1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.13.6. Overall likelihood of pest freedom for Hemiberlesia pitysophila on grafted bonsai plants
A.13.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The population density around the nursery is low and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are effective. The detection before export is carefully done.
A.13.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
The population density around the nursery is high and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are partly effective. The detection before export is not detailed enough to spot insects when they are hidden between the needles.
A.13.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
Different combinations of population density around the nursery, effect of the net barrier and of the insecticide applications may result in an intermediate scenario as they are acting independently of one another on the presence of the insect in the commodity. Median is shift to the left side (lower infestation rate), because the pest has not yet been detected in Zhejiang.
A.13.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The signs of the insect occurrence (white wax covering the scales) are generally detectable. The Panel assumes that a high infestation level is less likely to happen than having smaller number of infested plants where the insect density is low and difficult to detect.
A.13.6.5. Elicitation outcomes of the assessment of the pest freedom for Hemiberlesia pitysophila on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.25) and pest freedom (Table A.26).
Table A.25.
Elicited and fitted values of the uncertainty distribution of pest infestation by Hemiberlesia pitysophila per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5 | 35 | 65 | 120 | 300 | ||||||||||
| EKE | 5.01 | 7.18 | 10.4 | 16.4 | 24.1 | 33.8 | 44.0 | 67.3 | 97.6 | 118 | 144 | 176 | 216 | 253 | 298 |
The EKE results are the BetaGeneral (1.2287, 10.077, 3.1, 750) distribution fitted with @Risk version 7.6.
Table A.26.
The uncertainty distribution of plants free of Hemiberlesia pitysophila per 10,000 plants calculated by Table A.25
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,700 | 9,880 | 9,935 | 9,965 | 9,995 | ||||||||||
| EKE results | 9,702 | 9,747 | 9,784 | 9,824 | 9,856 | 9,882 | 9,902 | 9,933 | 9,956 | 9,966 | 9,976 | 9,984 | 9,990 | 9,993 | 9,995 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.26.
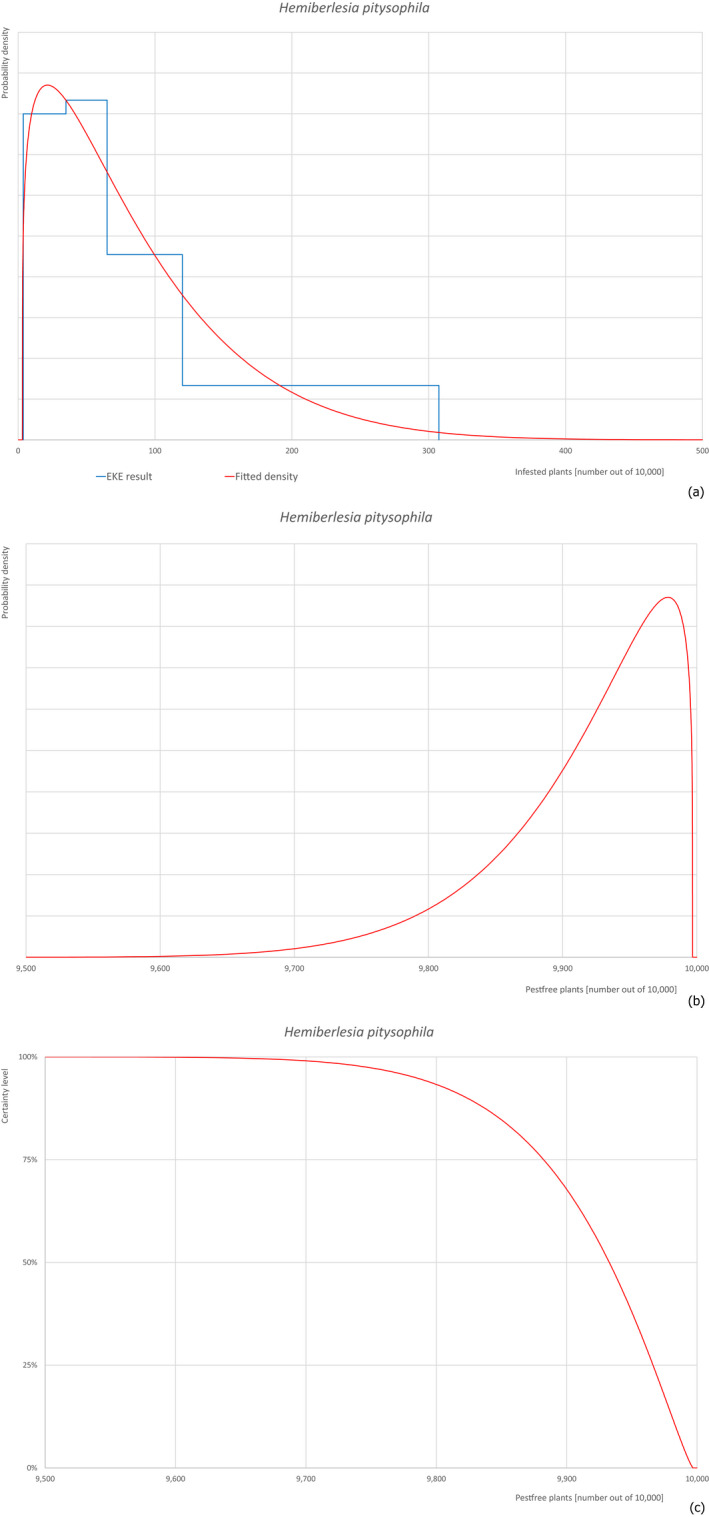
Figure A.13: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.13.7. Overall likelihood of pest freedom for grafted bonsai plants for Lepidosaphes pineti
Lepidosaphes pineti was evaluated in comparison to Ceroplastes rubens as reference pest, as the pests share common characteristics. Fiorinia japonica and Lepidosaphes pineti are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overall likelihood of pest freedom can be found in Section A.13.5
A.13.8. Overall likelihood of pest freedom for Lepidosaphes pini on grafted bonsai plants
A.13.8.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The population density around the nursery is low and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are effective. The detection before export is carefully done.
A.13.8.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
The population density around the nursery is high and the measures to prevent the colonisation of the bonsai plants and to suppress the insects eventually established are only partially effective. The detection before export is not detailed enough to spot insects when they are hidden between the needles.
A.13.8.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
Different combinations of population density around the nursery, effect of the net barrier and of the insecticide applications may result in an intermediate scenario as they are acting independently of one another on the presence of the insect in the commodity. Median is shift to the left side (lower infestation rate), because the presence of Lepidosaphes pini and L. piniphila is not reported in Zhejiang, and Parlatoria pinicola has unclear association with Pinus parviflora.
A.13.8.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The signs of the insect occurrence (white wax covering the scales) are generally detectable. The Panel assumes that a high infestation level is less likely to happen than having smaller number of infested plants where the insect density is low and difficult to detect.
A.13.8.5. Elicitation outcomes of the assessment of the pest freedom for Lepidosaphes pini on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.27) and pest freedom (Table A.28).
Table A.27.
Elicited and fitted values of the uncertainty distribution of pest infestation by Lepidosaphes pini per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5 | 40 | 80 | 200 | 500 | ||||||||||
| EKE | 4.99 | 5.83 | 7.73 | 12.7 | 21.3 | 34.4 | 50.3 | 91.4 | 150 | 190 | 243 | 304 | 376 | 437 | 502 |
The EKE results are the BetaGeneral (0.72706, 3.5262, 4.65, 730) distribution fitted with @Risk version 7.6.
Table A.28.
The uncertainty distribution of plants free of Lepidosaphes pini per 10,000 plants calculated by Table A.27
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,500 | 9,800 | 9,920 | 9,960 | 9,995 | ||||||||||
| EKE results | 9,498 | 9,563 | 9,624 | 9,696 | 9,757 | 9,810 | 9,850 | 9,909 | 9,950 | 9,966 | 9,979 | 9,987 | 9,992 | 9,994 | 9,995 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.28.
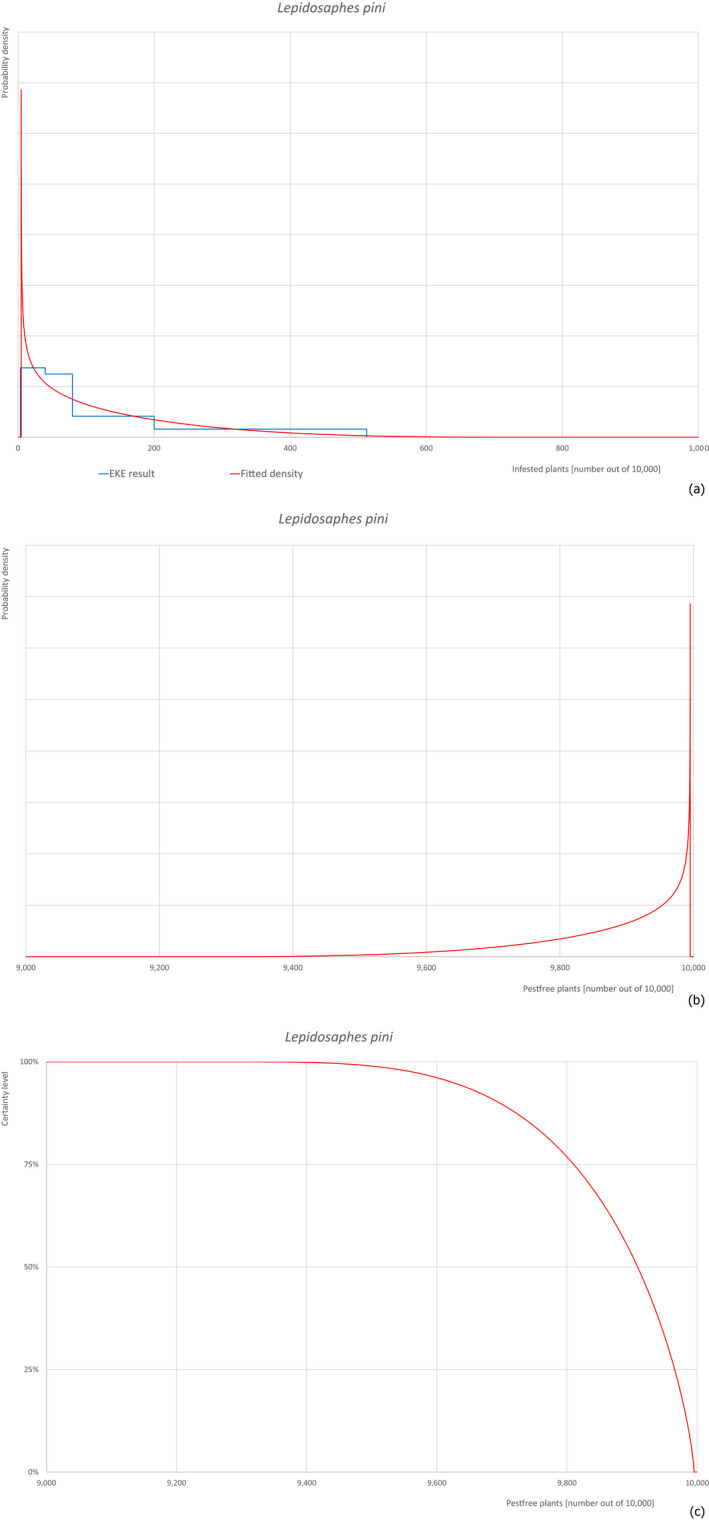
Figure A.14: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.13.9. Overall likelihood of pest freedom for Lepidosaphes piniphila on grafted bonsai plants
Lepidosaphes piniphila was evaluated in comparison to Lepidosaphes pineti as reference pest, as the pests share common characteristics. Lepidosaphes pini, Lepidosaphes piniphila and Parlatoria pinicola are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overall likelihood of pest freedom can be found in Section A.13.8.
A.13.10. Overall likelihood of pest freedom for Parlatoria pinicola on grafted bonsai plants
Parlatoria pinicola was evaluated in comparison to Lepidosaphes pineti as reference pest, as the pests share common characteristics. Lepidosaphes pini, Lepidosaphes piniphila and Parlatoria pinicola are evaluated in a combined assessment, as they have a similar risk of entry into the EU according to the evaluated evidence (see Section 2.3.4 for the methodology).
The overall likelihood of pest freedom can be found in Section A.13.8.
A.13.11. Reference List
Arakelian G, 2008. Coniferous Fiorinia Scale (Fiorinia japonica). County of Los Angeles Department of Agricultural Commissioner/Weights and Measure. 1 pp. Available online: https://www.researchgate.net/publication/270580138_Coniferous_Fiorinia_Scale_Fiorinia_japonica_Pest_bulletin_of_Los_Angeles_County_Department_of_Agricultural_Commissioner
Australian Government Department of Agriculture, Water and the Environment, 2020. Draft group pest risk analysis for scale insects on fresh fruit, vegetable, cut‐flower and foliage imports. CC BY 3.0. Canberra, Australia. 267 pp.
Borchsenius NS, 1958. Contribution to the coccid fauna of China. 3. Some new species of Lepidosaphini of coccid fauna of China (Homoptera, Coccoidea). Acta Entomologica Sinica, 8, 168–178 (in Chinese and Russian).
CABI (Centre for Agriculture and Bioscience International), online. Hemiberlesia pitysophila (pine needle hemiberlesian scale). Available online: https://www.cabi.org/cpc/datasheet/26650 [Accessed: 28 May 2021].
Camacho ER and Chong JH, 2015. General biology and current management approaches of soft scale pests (Hemiptera: Coccidae). Journal of integrated pest management, 6, 17, 22 pp. https://doi.org/10.1093/jipm/pmv016
Chen SL, Wu FH and Hou QW, 2004. A study on the biology of the Hemiberlesia pitysophila Takagi (Homoptera: Cccoidea) [J]. Journal of Fujian Forestry Science and Technology, 31, 1–5 (in Chinese).
Chen S, Wong J and Wu W, 2014. Armored scale insects (Hemiptera: Coccoidea: Diaspididae) intercepted from imported plant products to Taiwan. Journal of Taiwan Agricultural Research, 63, 129–142.
Commonwealth of Australia, 2020. Draft group pest risk analysis for soft and hard scale insects on fresh fruit, vegetable, cut‐flower and foliage imports. Australian Government Department of Agriculture, Water and the Environment, Canberra. 267 pp.
Danzig EM and Gavrilov IA, 2010. Mealybugs of the genera Planococcus and Crisicoccus (Sternorrhyncha: Pseudococcidae) of Russia and adjacent countries. Zoosystematica Rossica, 19, 39–49. https://doi.org/10.31610/zsr/2010.19.1.39
EPPO (European and Mediterranean Plant Protection Organization), online_a. Fiorinia japonica (FIORJA), Categorization. Available online: https://gd.eppo.int/taxon/FIORJA/categorization [Accessed: 14 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Hemiberlesia pitysophila (HEBEPI), Categorization. Available online: https://gd.eppo.int/taxon/HEBEPI/categorization [Accessed: 28 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Hemiberlesia pitysophila (HEBEPI), Distribution. Available online: https://gd.eppo.int/taxon/HEBEPI/distribution [Accessed: 28 May 2021].
EPPO Panel on Quarantine Pests for Forestry, 2003. Report of a Pest Risk Management: Lepidosaphes ussuriensis. 3 pp. Available online: https://gd.eppo.int/download/doc/1301_pra_prm_LEPSUS.pdf
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
FAO (Food and Agriculture Organization of the United Nations), 2007. Overview of forest pests – People’s Republic of China. Forest Resources Development Service, Forest Management Division, Forestry Department. Working Paper FBS/13E. FAO, Rome, Italy. 30 pp.
Feng Y, Liang J, Lu Q, and Zhang X, 2009. Potential suitability analysis of Hemiberlesia pitysophila Takagi in China. Forest Research, Beijing, 22, 563–567 (in Chinese).
Foldi I and Germain JF, 2018. Liste des Cochenilles de France (Hemiptera, Coccomorpha) [Checklist of the scale insects of France (Hemiptera, Coccomorpha)]. Bulletin de la Societe Entomologique de France, 123, 7–18. https://doi.org/10.32475/bsef_1973 (in French).
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_a. ScaleNet: A literature‐based model of scale insect biology and systematics, Fiorinia japonica. Available online: https://scalenet.info/catalogue/Fiorinia%20japonica/ [Accessed 14 May 2021].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_b. ScaleNet: A literature‐based model of scale insect biology and systematics, Hemiberlesia pitysophila. Available online: https://scalenet.info/catalogue/Hemiberlesia%20pitysophila/ [Accessed 28 May 2021].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_c. ScaleNet: A literature‐based model of scale insect biology and systematics, Lepidosaphes pineti. Available online: https://scalenet.info/catalogue/Lepidosaphes%20pineti/ [Accessed 14 June 2021].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_d. ScaleNet: A literature‐based model of scale insect biology and systematics, Lepidosaphes pini. Available online: https://scalenet.info/catalogue/Lepidosaphes%20pini/ [Accessed 14 June 2021].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_e. ScaleNet: A literature‐based model of scale insect biology and systematics, Lepidosaphes piniphila. Available online: https://scalenet.info/catalogue/Lepidosaphes%20piniphila/ [Accessed 14 June 2021].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_f. ScaleNet: A literature‐based model of scale insect biology and systematics, Parlatoria pinicola. Available online: https://scalenet.info/catalogue/Parlatoria%20pinicola/ [Accessed 14 June 2021].
Howell JO, 1977. Descriptions of the first instars of the North American species in the genus Fiorinia. Annals of the Entomological Society of America, 70, 829–836. https://doi.org/10.1093/aesa/70.6.829
Jianying S, 2002. Bionomics of Lepidosaphes pineti and its control. Forest Research, 15, 503–505 (in Chinese).
Li L, Wang R and Waterhouse DF, 1997. The distribution and importance of arthropod pests and weeds of agriculture and forestry plantations in southern China. Australian Centre for International Agricultural Research (ACIAR). 201 pp.
Lv C, Huang B, Qiao M, Wei J and Ding B, 2011. Entomopathogenic fungi on Hemiberlesia pitysophila. PLoS One, 6, 1–6. https://doi.org/10.1371/journal.pone.0023649
Malumphy C, Halstaed AJ and Salisbury A, 2012. First incursion of Chinese mussel scale Lepidosaphes chinensis (Hemiptera: Diaspididae) in Europe, with a review of Lepidosaphes species found in Britain. British Journal of Entomology and Natural History, 25, 65–74.
Matile‐Ferrero D, 1989. Sur Fiorinia japonica (Kuwana), cochenille nouvellement introduite en France, et description de sa larve mâle du deuxième stade [Hom. Coccoidea Diaspididae]. Bulletin de la Société Entomologique de France, 94, 205–211 (in French).
Miller DR and Davison JA, 1990. A list of armoured scale pests. In: Rosen D (eds.). Armoured scale insects. Amsterdam, Elsevier, 4B, 299–306.
Miller DR and Davidson JA, 2005. Armored Scale Insect Pests of Trees and Shrubs. Cornell Univ. Press Ithaca, NY, 206 pp.
Miller DR, Williams DJ and Davidson JA, 2006. Key to conifer‐infesting species of Lepidosaphes Shimer worldwide (Hemiptera: Coccoidea: Diaspididae), with descriptions of two new species and a redescription of L. pallidula (Williams). Zootaxa, 1362, 23–42. https://doi.org/10.11646/zootaxa.1362.1.2
Murakami Y, 1970. A review of biology and ecology of Diaspine scales in Japan (Homoptera: Coccoidea). Mushi, 43, 65–114.
Sasscer ER, 1912. The genus Fiorinia in the United States (No. 16). US Government Printing Office, Washington, US, 81–82.
Skvarla MJ, 2020. First Report of Scale Insects (Hemiptera: Diaspididae: Lepidosaphes pallida (Maskell) on Russian Arborvitae (Pinales: Cupressaceae: Microbiota Decussata Kom.). Proceedings of the Entomological Society of Washington, 122, 1035–1037.
Stimmel JF, 1994. Lepidosaphes pini, an armored scale on pines (Homoptera: Diaspididae). Regulatory Horticulture, Pennsylvania Department of Agriculture, 20, 19–20.
Suh SJ, 2012. Notes on pupillarial species of armored scale insects from Korea (Hemiptera: Diaspididae). Korean Journal of Applied Entomology, 51, 73–77. https://doi.org/10.5656/ksae.2012.01.1.081
Suh SJ, 2020. Host plant list of the scale insects (Hemiptera: Coccomorpha) in South Korea. Insecta Mundi, 0757, 1–26.
Takagi S, 1970. Diaspididae of Taiwan based on material collected in connection with the Japan‐US co‐operative science programme, 1965 (Homoptera: Coccoidea) Part 2. Insecta Matsumurana, 33, 1–142.
Takahashi R, 1955. Lepidosaphes of Japan (Diaspididae, Coccoidea, Homoptera). Bulletin of University of Osaka Perfecture, 5, 67–78.
Tang FT, 1984a. Observation on the scale insects injurious to forestry of North China. Shanxi Agricultural University Press Research Publication, 2, 122–133.
Tang FT, 1984b. The scale insects of horticulture and forests of China. Shanxi Agricultural University Press Research Publication, 2, 1–115.
Timlin JS, 1964. The biaology, bionomics, and control of Parlatoria pittospori Mask. (Hemiptera, Diaspididae): A pest on apples in New Zealand. New Zealand Journal of Agricultural Research, 7, 536–550.
Tong CJ, Tang ZY and Pan WY, 1988. Preliminary study on the fluctuation of natural populations of Hemiberlesia pitysophila. Forest Science and Technology, 2, 6–11 (in Chinese).
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
USDA (United States Department of Agriculture), 1939. List of intercepted plant pests, 1937 (List of pests recorded during the period July 1, 1936, to June 30, 1937, inclusive, as intercepted in, on, or with plants and plant products entering United States territory. US. 60 pp.
A.14. Pine weevils (Pissodes nitidus, P. punctatus, P. yunnanensis and Shirahoshizo patruelis)
A.14.1. Organism information
| Taxonomic information |
1. Pissodes nitidus Current valid scientific name: Pissodes nitidus Synonyms: – Name used in the EU legislation: Pissodes nitidus Roelfs [PISONI] Order: Coleoptera Family: Curculionidae Common name: yellow‐spotted pine weevil Name used in the Dossier: Pissodes nitidus 2. Pissodes punctatus Current valid scientific name: Pissodes punctatus Synonyms: – Name used in the EU legislation: Pissodes punctatus Langor & Zhang [PISOPU] Order: Coleoptera Family: Curculionidae Common name: Armand pine bark weevil Name used in the Dossier: Pissodes punctatus 3. Pissodes yunnanensis Current valid scientific name: Pissodes yunnanensis Synonyms: – Name used in the EU legislation: Pissodes yunnanensis Langor & Zhang [PISOYU] Order: Coleoptera Family: Curculionidae Common name: Yunnan pine weevil Name used in the Dossier: Pissodes yunnanensis 4. Shirahoshizo patruelis Current valid scientific name: Shirahoshizo flavonotatus Synonyms: Shirahoshizo patruelis, Cryptorhynchidius flavonotatus Name used in the EU legislation: – Order: Coleoptera Family: Curculionidae Common name: – Name used in the Dossier: Shirahoshizo patruelis |
| Group | Insects |
| EPPO code |
PISONI: Pissodes nitidus PISOPU: Pissodes punctatus PISOYU: Pissodes yunnanensis SHIRPA: Shirahoshizo patruelis |
| Regulated status |
Pissodes nitidus, P. punctatus and P. yunnanesis are listed in Annex II/A of Commission Implementing Regulation (EU) 2019/2072 as Pissodes nitidus Roelofs [PISONI], Pissodes punctatus Langor & Zhang [PISOPU], Pissodes yunnanensis Langor & Zhang [PISOYU]. Shirahoshizo patruelis is not regulated in the EU. For the export of Pinales living plants from third countries to the EU the places of production have to be free from nine Pissodes species, including Pissodes nitidus, P. punctatus and P. yunnanesis (Annex VII point 31, the Regulation 2019/2072). Pissodes nitidus is listed in the Commission Implementing Regulation (EU) 2020/1217 as a pest of concern for Pinus parviflora and P. thunbergii. None of the four weevil species is listed by EPPO or regulated anywhere in the world. |
| Pest status in China |
All three Pissodes species are present in China, with restricted distribution. Pissodes nitidus is present in Heilongjiang, Henan and Liaoning (Alonso‐Zarazaga et al., 2017; Dossier Section 2.0; EPPO, online_a), Hebei, Hubei, Jilin and Shaanxi (Alonso‐Zarazaga et al., 2017). Pissodes punctatus is present in Gansu, Guizhou, Sichuan and Yunnan (Dossier Section 2.0; EPPO, online_b). Pissodes yunnanensis is present in Guizhou, Sichuan and Yunnan (Dossier Section 2.0; EPPO, online_c). Shirahoshizo patruelis is present in China in Fujian, Guandong, Guizhou, Guangxi, Hubei, Hunan, Jiangsu, Jiangxi, Sichuan, Shaanxi, Shanghai, Yunnan, Zhejiang (Alonso‐Zarazaga et al., 2017; Dossier Section 2.0). |
| Pest status in the EU | All four pest species are absent in the EU (Alonso‐Zarazaga et al., 2017; EFSA, 2020). |
| Host status on Pinus parviflora and P. thunbergii |
Pissodes nitidus is known to be able to infest both Pinus parviflora and P. thunbergii (EFSA, 2020). Pinus parviflora and P. thunbergii are not included in the current lists of hosts of Pissodes punctatus and P. yunnanensis (Lei et al., 2003; Zhang et al., 2004; EFSA, 2020). According to Dossier Section 2.0, Pissodes nitidus, P. punctatus and P. yunnanensis are reported as pests of Pinus parviflora. However, Dossier Section 4.0 reports only P. nitidus as a pest of Pinus parviflora, as confirmed by scientific literature. The Panel is uncertain about the association of the two species Pissodes punctatus and P. yunnanensis with the commodity. The Panel decided to keep both species for the analyses. No information was found in literature on Pinus parviflora and P. thunbergii as hosts for Shirahoshizo patruelis. However, in Dossier Section 2.0, the weevil is reported as a pest for P. thunbergii (but not for P. parviflora). |
| PRA information |
Pest Risk Assessment currently available:
|
| Other relevant information for the assessment | |
| Biology |
Pissodes nitidus, P. punctatus and P. yunnanensis are weevil species native to Asia. Pissodes nitidus is present in China, Japan, Korea (EFSA, 2020) and it is also recorded from far east Russia (Lu et al., 2007). Pissodes punctatus and P. yunnanensis are both present only in China (EFSA, 2020; EPPO, online_b,c). Pissodes spp. live only on Pinaceae; depending on the species, they can develop in the stems or branches of dying trees or in terminal shoots of young healthy trees. The long‐lived adults (1–4 years) feed on the phloem of stems, branches and shoots. Larvae usually develop in the phloem and cambium, sometimes also in the pith of thinner shoots (Zhang et al., 2004). Mature larvae pupate inside typical pupal cells in the external sapwood, frequently strictly aggregated and covered with shredded wood fibres (chip cocoons). Pissodes spp. have four stages of development: egg, larva (4 instars), pupa and adult. They can be uni‐ or semivoltine (one generation over 2 years) depending on climatic conditions. In semivoltine species, a larval diapause and overlapping generations can be observed, with overwintering of both adults in the litter and larvae under the bark of the host plants. When developing on terminals, females lay eggs in cavities chewed with mandibles and covered with excrements and frass. Pissodes adults can move both crawling and flying. They are generally considered to be strong fliers at local scale capable of covering distances of at least 50–100 m per flight; passive spread of Pissodes weevils by human activity can occur in all stages of the life cycle, mainly with the transport of wood, wood with bark and living conifer plants (EFSA PLH Panel, 2019; EFSA, 2020). Pissodes nitidus has uni‐semivoltine life cycle depending on climate conditions (Jin, 1989; EFSA, 2020). Overwintering adults or immature adults emerge in spring and fly to top branches in order to feed on shoots and to mate. Females lay 1–2 eggs each time under the bark; 1‐year‐old shoots are preferred and about 30 eggs/year are laid. Larval development lasts about 65 days, from the end of May to early August. The threshold temperature for egg and larval development is 9.1°C (Jin, 1989), with the optimum at 25°C (Zhang et al., 2004). Pupal stage lasts from the end of June to August. Newly emerged adults can be found from July to September. Adults before overwintering are the most skilled in moving, as they can cover distance of 1,660 m (total of flying and crawling) (Jin, 1989). Pissodes punctatus is a mountain species, living on young pines located at elevations of 2,000–2,900 m; it is a univoltine species, with possible overlapping generations, so that both adults and larvae may overwinter (Langor et al., 1999). In spring and summer adults are primarily active during sunny days and prefer feeding on 1‐year‐old shoots (Chen et al., 2013). They live for 2 years (Langor et al., 1999) and are able to fly on average 50 m per flight (Chen et al., 2013). Larvae develop under the bark of the upper part of stems. Adult emergence and host colonisation last 6–9 months (Langor et al., 1999). According to Zhang et al. (2004), Pissodes yunnanensis is a univoltine species. Third‐instar larvae usually overwinter, and pupation occurs from March to May with adult emergence from April to July. Adults mate after a maturation feeding carried out on both new and 1‐year‐old shoots, where eggs are laid singly or in small groups of 2–3, for a total of 70–91 eggs per female. The pest prefers young pines 8–10 years old. Larvae develop in stems or branches from 0.5 to 4.8 cm diameter. Young larvae initially start to feed in the phloem (1st and 2nd instar) then enter the sapwood, but they can complete the development only in stems of 2.5 cm diameter or larger. In stems smaller than 1 cm, aged larvae are forced to move from sapwood into the pith. There are no specific data on the flight distance covered by P. yunnanensis, but it can be assumed that it is similar to that of P. punctatus, which shares some aspects of life cycle, distribution and host plant (Pinus yunnanensis). Pissodes nitidus, P. punctatus and P. yunnanensis are not known to be vectors of pathogenic fungi (Wondafresh, 2016). No specific information on the biology of Shirahoshizo patruelis was found. However, some biological traits on the species may be elicited from the studies carried out by Yoshikawa (1977, 1981, 1983, 1986) on three similar species (Shirahoshizo insidiosus, S. pini and S. rufescens) in Japan. Shirahoshizo is a genus of weevils belonging to the tribe Cryptorhynchini, including 18 species all distributed in Asia (China, Korea and Japan) (Alonso‐Zarazaga et al., 2017) many of which feed on conifers, mostly Pinus spp. Shirahoshizo spp. have feeding habits similar to that of Pissodes spp., developing under the bark of dying trees at larval stage; however, no information on the feeding habits of adults was found. Pissodes and Shirahoshizo frequently attack the same trees, but they have different preferences for bark thickness, as Shirahoshizo colonise the thickest bark (bark thickness in bait logs from 0.58 to 1.22 cm) preferably along the stem and near the ground, laying their eggs in crevices; for this reason they do not oviposit on the top where the bark is too thin (Yoshikawa, 1977, 1981, 1983). Furthermore, Shirahoshizo also need larger subcortical area than Pissodes for larval development and formation of pupal cells, which are dispersed and isolated (Yoshikawa, 1977). Shirahoshizo have 4 development stages: egg, larva (6 instars), pupa, adult, and are uni‐ or multivoltine (1–4 generations/year), as the females lay eggs from April to September (Yoshikawa, 1977, 1986; Dossier Section 4.0). No information on the overwintering stage was found. However, as Shirahoshizo begins to fly in April (Yoshikawa, 1977), it may be assumed that the pest overwinters at adult stage and/or as mature larva or pupa. Flight distance of Shirahoshizo adults has been estimated to be about 50 m (Yoshikawa, 1983). Adults are nocturnal. In Japan three flight peaks of Shirahoshizo adults were observed, in early spring (hibernate adults), after rainy season (hibernate larvae) and in early autumn (new adults) (Hagihara and Nakashima, 1970). |
| Symptoms | Main type of symptoms |
The most evident symptom of the advanced host colonisation by Pissodes species is yellowing and drying of terminal shoots as consequence of larval feeding in the phloem, cambium or pith; infested terminals can also be strongly deformed making symptoms even more evident. Circular exit holes of adults (3–5 mm diameter) may occur on infested shoots. Other symptoms present in the early phase of attack are feeding wounds and oviposition pits on both new and 1‐year‐old shoots caused by adults, often associated with the presence of resin droplets. Bark wounds produced by adult maturation feeding are small circular punctures 1–2.5 mm deep, usually located at the base of needles; the similar oviposition pits are less visible as they are protected from excrement and frass. These symptoms are generally not easy to detect and always require close examination. No information on the symptoms caused by Shirahoshizo patruelis was found. |
| Presence of asymptomatic plants | In the early phase of infestation, when adults, eggs and young larvae are present in the same host, the plants can be asymptomatic. This period may range between April and end June for Pissodes nitidus (Jin, 1989), between mid‐April and early July for P. yunnanesis (Zhang et al., 2004), while it is not defined for P. punctatus due to long emergence period of adults (Chen et al., 2013). | |
| Confusion with other pests |
Upon close examination, shoot wilting caused by Pissodes is characteristic and it cannot be confused with similar symptoms caused by other insects (e.g. bark beetles and shoot moths). Pissodes species adults are very similar and not easy to be identified. Taxonomic keys are useful for morphological identification but should be supported by genetic analysis and knowledge of insect ecology. Langor et al. (1999) provide a valid support for visual identification of Pissodes punctatus and P. yunnanensis. Belonging to a different tribe (Cryptorhynchini), Shirahoshizo patruelis is easily distinguishable from Pissodes at adult stage. However, as there are several species of Shirahoshizo feeding on pines, an examination by experts or the use of taxonomic keys is needed for a reliable identification. |
|
| Host plant range |
Pissodes nitidus is known to infest Pinus densiflora, P. koraiensis, P. parviflora, P. sylvestris var. mongolica, P. tabuliformis, P. thunbergii (Jin, 1989; EFSA, 2020) and P. parviflora (Dossier Section 2.0). Pissodes punctatus is recorded from Pinus armandii, P. yunnanensis (EFSA, 2020), P. massoniana, P. tabuliformis, Cedrus deodara, Pseudolarix amabilis (Lei et al., 2003) and P. parviflora (Dossier Section 2.0). Pinus yunnanensis is the only host known for Pissodes yunnanensis (Zhang et al., 2004; EFSA, 2020) and Pinus parviflora (Dossier Section 2.0). Shirahoshizo patruelis is recorded from Pinus densiflora, P. kesyia, P. massoniana (Yoshikawa, 1977; Duan et al., 2007; Huihua et al., 2013) and P. thunbergii (Dossier Section 2.0). |
|
| Reported evidence of impact |
Pissodes nitidus, P. punctatus and P. yunnanesis are EU quarantine pests. No evidence of impact was found for Shirahoshizo patruelis. However, S. patruelis is recorded in Dossier Section 4.0 as a pest having impact. |
|
| Evidence that the commodity is a pathway |
Since the only pathway for all Pissodes spp. infesting terminal shoots are living Pinaceae plants, bonsais are also a possible pathway, as confirmed by a recent interception of Pissodes sp. on Taxus cuspidata bonsai from Japan (EUROPHYT/TRACES‐NT, online). There is an uncertainty about the commodity being pathway for Shirahoshizo patruelis because of the thickness of the bark required for oviposition, although it cannot be excluded that bigger bonsai plants may have bark thick enough for oviposition. |
|
| Surveillance information |
Pissodes nitidus, P. nitidus, P. yunnanensis and Shirahoshizo patruelis are recorded in Dossier Section 4.0 as occurring in China. No specific surveillance protocol for the mentioned Pissodes species neither for Shirahoshizo patruelis is described in the Dossier. However, Pissodes are included in a list of target pests (Dossier Section 4.0) for which monitoring activities (sweeping) are performed, together with inspections and samplings to collect insects on host plants in the survey area, which are carried out three times a year. |
|
A.14.2. Possibility of pest presence in the nursery
Information is provided only for species (Shirahoshizo patruelis) evaluated using Expert Knowledge Elicitation.
A.14.2.1. Possibility of entry from the surrounding environment
Shirahoshizo patruelis is present in Zhejiang (Alonso‐Zarazaga et al., 2017), where the nursery is located. However, the Dossier states that S. patruelis is absent both in the Hangzhou area (Dossier Section 2.0) and in the Zhejiang province (Dossier Section 5.0).
According to Dossier Sections 4.0 and 5.0, the nursery is surrounded by different plants, among which Pinus massoniana, P. densiflora and P. thunbergii are also hosts for S. patruelis.
The possibility of entry for S. patruelis from surrounding environment to nursery relies on the flight capability, which for Shirahoshizo spp. is around 50 m (Yoshikhawa, 1983).
As stated in Dossier Section 4.0, the bonsai cultivation site is protected by a 40‐mesh insect‐proof net (0.4 mm). Adults of Shirahoshizo species have strong mandibles, capable of gnawing the wood and could be able to pierce the net.
At the date of export, the bonsai plants are 4–5 years old (Dossier Section 4.0), the height is between 30 and 50 cm, the stem diameter is 1.0–3.5 cm and the twig diameter is 0.5–3.0 cm (Dossier Section 5.0). The size of the plants is less suitable for Shirahoshizo, which requires thicker bark and larger subcortical space for both the larval development and the formation of pupal cells (Yoshikawa, 1977).
Uncertainties
-
–
Effectiveness of the protective nets against Shirahoshizo adults as beetles are potentially able to chew a hole in the plastic net.
-
–
No specific information on the biology and life cycle of S. patruelis is available.
-
–
No information about the pest status of S. patruelis in Zhejiang is available.
-
–
The survey by sweeping branches was done in 1 year (2012) and limited to few trees of P. parviflora.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the mentioned pests to enter the nursery from the surrounding area. Shirahoshizo patruelis is present in Zhejiang and its host plants are also present in the Hangzhou area.
A.14.2.2. Possibility of entry with new plants/seeds
All seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (P. thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triaimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
The growing media used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0). Possibility of entry with seeds or soil is not relevant for S. patruelis.
Uncertainties
-
‐
It is not clear if and how new mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers it is not possible that the weevil could enter the nursery with new plants/seeds and soil/growing media.
A.14.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of P. parviflora are taken (Dossier Section 4.0).
Shirahoshizo patruelis is not known to use P. parviflora as a host. However, P. thunbergii is reported as host for the pest in the Dossier. Shirahoshizo patruelis is able to fly for 50 m and can spread within the nursery.
Spread within the nursery through the movement of soil, water, equipment and tools is not relevant.
Uncertainties
-
‐
Host suitability of P. parviflora to S. patruelis.
-
‐
Effectiveness of the protective nets against Shirahoshizo adults as beetles are potentially able to chew a hole in the plastic net.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer within the nursery is possible as consequence of both the presence of host plants in the nursery and the spread capability of the pest.
A.14.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Pissodes spp. and Shirahoshizo patruelis between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
However, a recent interception of Pissodes sp. on Taxus cuspidata bonsais from Japan (EUROPHYT/Traces, online) confirms the possibility for Pissodes to infest also bonsais.
A.14.4. Evaluation of the risk mitigation measures
According to Section 1.2, the Panel did not assess the effectiveness of measures for Pissodes nitidus, P. punctatus and P. yunnanesis for which specific measures are specified in points 30 and 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 The assessment was restricted to whether or not the applicant country implements those measures (see Section 6 and A.14.6). Therefore, effectiveness of risk mitigation measures was evaluated only for Shirahoshizo patruelis.
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on Shirahoshizo patruelis is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
Physical separation of the bonsai plants may have effect on reducing risk of infestation with S. patruelis, especially in the production base.
Uncertainties:
|
| 2 | Growing medium and its treatment | No | Not applicable. |
| 3 | Treatment of seeds | No | Not applicable. |
| 4 | Insecticide and acaricide treatments | Yes |
Spray of contact insecticides can kill adult beetles that are present on the plants at the time of spraying.
Uncertainties:
|
| 5 | Fungicide treatments | No | Not applicable. |
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | No | Not applicable. |
| 8 | Official inspections during production | Yes |
The sampling and laboratory inspection of plant material may allow to identify infested plants by S. patruelis.
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
The sampling and laboratory inspection of plant material may allow to identify infested plants by S. patruelis.
Uncertainties:
|
A.14.5. Overall likelihood of pest freedom for Shirahoshizo patruelis on grafted bonsai plants
A.14.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The pest is present in the province where the nursery is located, effective treatments are performed, net is preventing adults from reaching the plants and inspections are effective.
A.14.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
There is high pest pressure from the surroundings. Mother plants can be attractive to adults and their egg deposition. The net is not completely sealed. Insecticide treatments are not completely effective. Bonsai bark is suitable for pest colonisation.
A.14.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
The median is shifted to the left (lower levels), because measures to contain the pest are generally effective.
A.14.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Uncertainty is high about the control methods performed because information provided in the Dossier is not complete. There is also a high uncertainty about the suitability of bonsai plants and about the pressure of the pest from around the nursery. Some stages of the pest are hidden inside the wood, so the effectiveness of insecticide treatments is also uncertain as pest may not be reached by the pesticide. High efficacy of the net is expected, which reduces uncertainties for rates above the median.
A.14.5.5. Elicitation outcomes of the assessment of the pest freedom for Shirahoshizo patruelis on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.29) and pest freedom (Table A.30).
Table A.29.
Elicited and fitted values of the uncertainty distribution of pest infestation by Shirahoshizo patruelis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2 | 9 | 17 | 40 | 80 | ||||||||||
| EKE | 2.00 | 2.14 | 2.48 | 3.43 | 5.12 | 7.79 | 11.0 | 19.4 | 30.8 | 38.1 | 47.2 | 56.8 | 66.6 | 73.7 | 80.0 |
The EKE results are the BetaGeneral (0.68003, 1.9159, 1.95, 90.5) distribution fitted with @Risk version 7.6.
Table A.30.
The uncertainty distribution of plants free of Shirahoshizo patruelis per 10,000 plants calculated by Table A.29
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,920 | 9,960 | 9,983 | 9,991 | 9,998 | ||||||||||
| EKE results | 9,920 | 9,926 | 9,933 | 9,943 | 9,953 | 9,962 | 9,969 | 9,981 | 9,989 | 9,992 | 9,995 | 9,996.6 | 9,997.5 | 9,997.9 | 9,998.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.30.
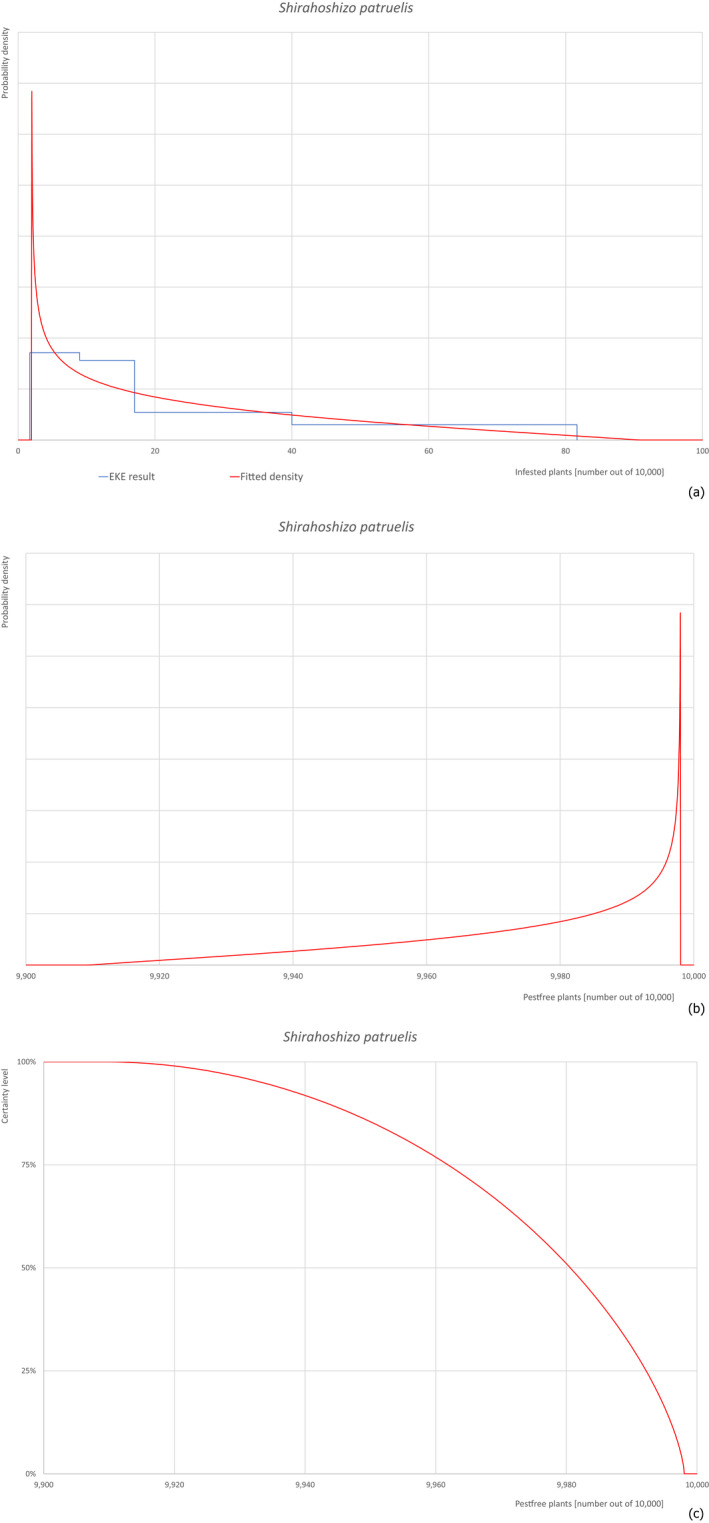
Figure A.15: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. =1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.14.6. Evaluation of the implementation and relevance of specific measures in China
Commission Implementing Regulation (EU) 2019/2072 specifies in point 30 of Annex VII measures which are required for the import of the commodity from China.
The below overview provides special requirements for naturally or artificially dwarfed plants for planting other than seeds according to Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 including an assessment of whether or not the applicant country implements those measures with respect to Pissodes nitidus, P. punctatus and P. yunnanesis identified in this Opinion. The Panel assumes that information on treatments required to be included in the phytosanitary certificate is provided by the applicant country according to the Article 71 of Regulation (EU) 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’.
| Special requirements as specified in Point 30 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 | Implementation of the special requirements in China according to information provided in the Dossier | Relevance of special requirements for the pest including uncertainties |
|---|---|---|
| ‘Official statement that: | – | – |
| a) the plants, including those collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime, | Yes | Yes |
| b) the plants in the nurseries referred to in point (a) of this entry: | – | – |
| i) at least during the period referred to in point (a) of this entry: | – | – |
| — were potted, in pots which are placed on shelves at least 50 cm above ground, |
Yes, partially. Pots are also reported to be kept on the ground. |
No |
| — have been subjected to appropriate treatments to ensure freedom from non‐European rusts, and the active ingredient, concentration and date of application of these treatments has been mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Disinfestation and/or disinfection treatment’. |
Yes. Treatments are appropriate. They are expected to reduce the likelihood of infection of the pathogens and the rate of colonisation of plant tissues, although it is uncertain if freedom from non‐European rusts could be reachable. Treatments used are listed in Table 6.
Uncertainties:
|
No |
| — have been officially inspected at least six times a year at appropriate intervals for the presence of Union quarantine pests of concern in accordance with Regulation (EU) No 2016/2031, and these inspections have also been carried out on plants in the immediate vicinity of the nurseries referred to in point (a) of this entry, at least by visual examination of each row in the field or nursery and by visual examination of all parts of the plant above the growing medium, using a random sample of at least 300 plants from a given genus where the number of plants of that genus is not more than 3,000 plants, or 10% of the plants if there are more than 3,000 plants from that genus, | Yes | Yes |
| — have been found free, in these inspections, from the relevant Union quarantine pests of concern as specified in the previous indent, infested plants have been removed and the remaining plants, where appropriate, have been effectively treated and have been held for an appropriate period and inspected to ensure freedom from such pests, | Yes | Yes |
| — have been planted in either an unused artificial growing medium or in a natural growing medium, which has been treated by fumigation or by appropriate heat treatment and has been of any Union quarantine pests, | Yes | No |
| — have been kept under conditions which ensure that the growing medium has been maintained free from Union quarantine pests and within 2 weeks prior to dispatch, have been: | Yes | No |
| — shaken and washed with clean water to remove the original growing medium and kept bare rooted, or | – | – |
| — shaken and washed with clean water to remove the original growing medium and replanted in growing medium which meets the conditions laid down in (i) fifth indent, or | – | – |
| — subjected to appropriate treatments to ensure that the growing medium is free from Union quarantine pests, and the active ingredient, concentration and date of application of these treatments have been indicated on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031 under the rubric ‘Disinfestation and/or disinfection treatment’. | Yes | No |
| ii) were packed in closed containers which have been officially sealed and bear the registration number of the registered nursery, and this number has been indicated under the rubric ‘Additional declaration’ on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, enabling the consignments to be identified.’ | Yes | Yes |
Point 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 specifies special requirements for Plants of Pinales, other than fruit and seeds as follows:
‘Official statement that the plants have been produced in a place of production free from Pissodes cibriani O'Brien, Pissodes fasciatus Leconte, Pissodes nemorensis Germar, Pissodes nitidus Roelofs, Pissodes punctatus Langor & Zhang, Pissodes strobi (Peck), Pissodes terminalis Hopping, Pissodes yunnanensis Langor & Zhang and Pissodes zitacuarense Sleeper.’
With respect to the point 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072 the Panel noted that from the above listed Pissodes species only Pissodes nemorensis, Pissodes nitidus, Pissodes punctatus, Pissodes strobi and Pissodes yunnanensis are associated with Pinus parviflora and/or Pinus thunbergii, and that Pissodes nemorensis and Pissodes strobi are not present in China (see Appendix E).
According to information in Dossier Section 5.0, Pissodes nitidus, Pissodes punctatus and Pissodes yunnanensis are absent in the production area and in the Zhenjiang province. The information sources for this statement are (i) China National pest quarantine information system (https://www.pestchina.com/SitePages/Home.aspx), (ii) Research institutions, universities, scientific societies, scientific and trade Journals and (iii) Crop Protection Compendium (https://cabi.org/cpc) (Dossier Section 5.0). In addition, in Dossier Section 4.0 Pissodes spp. (non‐European) are indicated as target pests for which a targeted survey was performed to demonstrate pest freedom of the area.
Based on the information provided by the NPPO of China, the Panel considers that China applies the relevant measures for Pissodes cibriani, Pissodes fasciatus, Pissodes nemorensis, Pissodes nitidus, Pissodes punctatus, Pissodes strobi, Pissodes terminalis, Pissodes yunnanensis and Pissodes zitacuarense as specified in Point 31 of Annex VII of Commission Implementing Regulation (EU) 2019/2072.
A.14.7. Reference List
Alonso‐Zarazaga MA, Barrios H, Borovec R, Bouchard P, Caldara R, Colonnelli E, Gültekin L, Hlaváč P, Korotyaev B, Lyal CHC, Machado A, Meregalli M, Pierotti H, Ren L, Sánchez‐Ruiz M, Sforzi A, Silfverberg H, Skuhrovec J, Trýzna M, Velázquez de Castro AJ and Yunakov NN, 2017. Cooperative catalogue of Palaearctic Coleoptera Curculionoidea. Monografías electrónicas Sociedad Entomológica Aragonesa, 8, 1–729.
Chen Y, Luo CW, Kuang RP, Li HW, Chen Z and Liu YJ, 2013. Phototactic behavior of the Armand pine bark weevil, Pissodes punctatus. Journal of Insect Science, 13, 10.
DEFRA (Department for Environment, Food & Rural Affairs), online_a. UK Risk Register Details for Pissodes nitidus. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=6123 [Accessed: 8 June 2021].
DEFRA (Department for Environment, Food and Rural Affairs), online_b. UK Risk Register Details for Pissodes punctatus. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=28969 [Accessed: 8 June 2021].
DEFRA (Department for Environment, Food & Rural Affairs), online_c. UK Risk Register Details for Pissodes yunnanensis. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=28968 [Accessed: 8 June 2021].
Duan Y, Xu Z and Zhang Q, 2007. Pest insects of Pinus kesiya var. langbianensis. Journal of Southwest Forestry College, 3, 52–57.
EFSA (European Food Safety Authority), 2020. Pest survey card on Pissodes cibriani, P. fasciatus, P. nemorensis, P. nitidus, P. punctatus, P. strobi, P. terminalis, P. yunnanensis and P. zitacuarense. EFSA supporting publication 2020;EN‐1910, 35 pp. https://doi.org/10.2903/sp.efsa.2020.EN‐1910
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Kenis M, Kertész V and Grégoire J‐C, 2018. Scientific Opinion on the pest categorisation of non‐EU Pissodes spp. EFSA Journal 2018;16(6):5300, 29 pp. https://doi.org/10.2903/j.efsa.2018.5300
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Vettraino AM, Leuschner R, Mosbach‐Schulz O, Rosace MC and Potting R, 2019. Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. https://doi.org/10.2903/j.efsa.2019.5667
EPPO (European and Mediterranean Plant Protection Organization), online_a. Pissodes nitidus (PISONI), Distribution. Available online: https://gd.eppo.int/taxon/PISONI/distribution [Accessed: 22 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Pissodes punctatus (PISOPU), Distribution. Available online: https://gd.eppo.int/taxon/PISOPU/distribution [Accessed: 22 May 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Pissodes yunnanensis (PISOYU), Distribution. Available online: https://gd.eppo.int/taxon/PISOYU/distribution [Accessed: 22 May 2021].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Hagihara Y and Nakashima Y, 1970. The general biology of the Pine bark beetle, Shirahoshizo spp. (Col. Curculionidae). 1, Flight pattern and host finding behavior. Bulletin of Fukuoka‐ken Forest Experiment Station, 21, 1–18 (in Japanese with English summary).
Huihua C, Jianwei Z and Zhihong X, 2013. Spatial pattern and its time series dynamics of Shirahoshizo patruelis adilts in forest. Journal of Northeast Forestry University, 41, 111–114 (in Chinese).
Jin L, 1989. Pissodes nitidus Roelofs, the yellow‐spotted pine weevil (Coleoptera: Curculionidae): a serious pest of Korean pine plantations in northeast China. In: Alfaro RI and Glover SG (eds.). Insects affecting reforestation: biology and damage. Forestry Canada, Pacific Forestry Centre, Victoria, British Columbia, 186–193.
Langor DW, Situ YX and Zhang R, 1999. Two new species of Pissodes (Coleoptera: Curculionidae) from China. The Canadian Entomologist, 131, 593–603.
Lei G L, Duan ZY, Feng ZW and Zheng Z, 2003. Pest risk analysis of Pissodes punctatus Langer Situ et Zhang. Journal of Northeast Forestry University, 31, 62–63 (in Chinese with English abstract).
Lu X, Zhang R, and Langor DW 2007. Two new species of Pissodes (Coleoptera: Curculionidae) from China, with notes on Palearctic species. Canadian Entomologist, 139, 179–188.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
Wondafrash M, 2016. Ecology and diversity of Pissodes (Coleoptera: Curculionidae) in South Africa. Chapter 1, PhD Thesis, University of Pretoria, South Africa, 51 pp.
Xu C, Zhang H and Zhang Z, 2004. Pest risk analysis of Pissodes yunnanensis. Forest Pest and Disease, 23, 30–32.
Yoshikawa K, 1977. Population study of pine bark weevils (Colepotera Curculionida) in bait logs. Applied Entomology and Zoology, 12, 9–17.
Yoshikawa K, 1981. Seasonal changes in number and distribution patterns of pine bark weevils (Shirahoshizo spp.) (Colepotera Curculionida) attracted to pine bait logs. Applied Entomology and Zoology, 16, 367–363.
Yoshikawa K, 1983. Habitat selection of pine bark weevils (Shirahoshizo spp.) (Colepotera Curculionida) attracted to pine bait logs. Applied Entomology and Zoology, 18, 149–160.
Yoshikawa K, 1986. A study of the subcortical insect community in pine trees I. Oviposition and emergence periods of each species. Applied Entomology and Zoology, 21, 258–268.
Zhang H, Ye H, Haack RA and Langor DW, 2004. Biology of Pissodes yunnanensis (Coleoptera: Curculionidae), a pest of Yunnan pine in southwestern China. The Canadian Entomologist, 136, 719–726.
A.15. Pyrrhoderma noxium
A.15.1. Organism information
| Taxonomic information |
Current valid scientific name: Pyrrhoderma noxium Synonyms: Fomes noxius, Phellinidium noxium, Phellinus noxius Name used in the EU legislation: – Order: Hymenochaetales Family: Hymenochaetaceae Common name: brown root rot Name used in the Dossier: – |
|
| Group | Fungi | |
| EPPO code | PHELNO | |
| Regulated status |
The pest is neither regulated in the EU nor listed by EPPO. Pyrrhoderma noxium is a New Zealand quarantine pest (MPI, online_a). |
|
| Pest status in China | Pyrrhoderma noxium is present in China with reports from Hainan island (Ann et al., 2002; Wang et al., 2016; Farr and Rossman, online), Hebei province (GBIF, online), Macao in Guangxi province (Wang et al., 2016) and Kinmen/Chinmen island (Watling et al., 2002). | |
| Pest status in the EU | Pyrrhoderma noxium is absent in the EU (Farr and Rossman, online; GBIF, online). | |
| Host status on Pinus parviflora and P. thunbergii |
Pinus thunbergii is reported as a host of Pyrrhoderma noxium (Farr and Rossman, online). There is no information on whether Pyrrhoderma noxium can also attack Pinus parviflora. However, the pathogen is known to be very polyphagous (Farr and Rossman, online). |
|
| PRA information |
Pest Risk Assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology |
Pyrrhoderma noxium is present in tropical areas of Southeast Asia, Africa, Central America and Oceania (CABI, online). The infection can follow two pathways: airborne basidiospores through wounds on trees or from tree to tree by mycelial growth through root contacts (Bolland, 1984). Basidiospores are produced in tropical regions during the rainy season. Basidiospores are dispersed by wind. While the majority of basidiospores travels only few metres from the fruiting bodies, their potential dispersal range, although uncertain, is likely to be of several kilometres (Chung et al., 2015). Infections by means of basidiospores are known to occur on stumps or wounded trees near the base (less than 2 metres from the ground) (Hsiao et al., 2019). In south‐eastern Queensland (Australia) (similar temperate climate as the one of the nursery), spores have been deemed of minor significance in the dissemination of P. noxium in hoop pine plantations (Bolland, 1984). In fact, after years of field observations, there was no evidence for spore infection of pruning or logging injuries to the stem (Bolland, 1984). On the other hand, population genetics analyses conducted in Taiwan revealed diverse MLGs, low geographical differentiation and lack of a clear pattern of isolation by distance thereby pointing to a potentially important role of basidiospore dispersal in the spread of P. noxium and colonisation of new habitats by the fungus (Chung et al., 2015). The most common way of infection is root contact in the soil with infected material, where P. noxium can remain active in fragments of contaminated roots up to 2 years, and inside the root system of dead trees up to 10 years (Chang, 1996), while bare soil from infected plants, although showing traces of P. noxium DNA, did not retain viable P. noxium (Wu et al., 2020). However, by performing experiments using soil artificially inoculated with basidiospores at 3.6 105 spores gr‐1 of dry soil, Chang (1996) reported that P. noxium basidiospores can survive up to 4.5 months in soils of varying moisture levels. Pyrrhoderma noxium colonises the root system growing towards the trunk, inside and outside the roots and it will girdle the stem, from there spreading to the healthy roots (Bolland, 1984). The mycelial growth on rubber trees has been estimated to be about 0.7 m/year (Nandris, 1987). The base of the stem of standing trees will be covered by brown mycelium where P. noxium will develop its basidiocarps. Fruiting bodies are bracket‐like when they develop on standing trees, while they are resupinate when they develop on the underside of fallen trees (Bolland, 1984). Pyrrhoderma noxium thrives in tropical climates and acidic conditions: the optimal growth temperature is 30°C, with growth range from 8 to 36°C (Ann et al., 2002), the soil pH can vary from 3.5 up to 7.5 (Ann et al., 1999). |
|
| Symptoms | Main type of symptoms |
The most evident symptom of P. noxium is brown encrustation of soil and mycelium occurring all around the infected roots that in time grows up all around the base of the trunk. Crown dieback, especially fast at the beginning of the growing season, is common (Bolland, 1984). |
| Presence of asymptomatic plants | The main symptoms are always present on the root system. Crown dieback and mycelium around the trunk represent a later stage of the infection. The mycelial growth on rubber trees has been estimated to be about 0.7 m/year (Nandris, 1987), therefore the asymptomatic period could depend on the plant size and the point of infection. | |
| Confusion with other pests | Aboveground symptoms can be misidentified as other rot fungi, but the soil encrusted mycelium is a typical symptom of Pyrrhoderma noxium. The only possible confusion is with Phellinus lamaensis. Phellinus lamaensis can be distinguished with a microscope due to the presence of hymenial setae (CABI, online). | |
| Host plant range |
The host plant range includes more than 250 species, from 174 genera, including the genus Pinus. Pinus thunbergii is reported as a host (Farr and Rossman, online). No information is available for Pinus parviflora as a potential host for Pyrrhoderma noxium. |
|
| Reported evidence of impact |
Pyrrhoderma noxium infection can have varying impacts due to the host variability. It is a well‐known pathogen of fruit orchards and forests (Brooks, 2002), causing mortality up to 50% in Araucaria cunninghamii plantations (Nandris, 1987) and threatening economically relevant crops like vineyards and pear plantations, with limited possibility of replanting due to the survival of the pathogen in small root fragments in the soil (MPI, online_b). Pyrrhoderma noxium has been reported as potentially invasive pathogen (Stewart et al., 2020). |
|
| Evidence that the commodity is a pathway | Although evidence for dwarfed plants to be a pathway is currently lacking, plants can be infected by means of airborne basidiospores through wounds. The commodity is grafted and pruned. Grafting and pruning wounds could offer entry points for the pathogen. | |
| Surveillance information | No surveillance information for this pathogen is currently available from China. There is no information on whether the pathogen has ever been found in the nurseries or their surrounding environment. | |
A.15.2. Possibility of pest presence in the nursery
A.15.2.1. Possibility of entry from the surrounding environment
Pyrrhoderma noxium is known to be present in China, with no reports from the province of Zhejiang where the nursery is located (Dossier Section 5.0). There is no surveillance information on the presence or population pressure of pathogen in the area where the nursery is located. The basidiospores are thought to have a dispersal range of several kilometres (Chung et al., 2015).
According to Dossier Section 4.0, the nursery is surrounded by different plant species. Among them Albizia julibrissim, Broussonetia papyrifera, Camellia japonica, C. sinensis, Celtis sinensis, Cinnamomun camphora, C. japonicum, Citrus maxima, C. paradisi, Eriobotrya japonica, Liquidambar formosana, Michelia figo, Nandina domestica, Nerium oleander, Osmanthus fragrans, Podocarpus macrophyllus, Prunus mume, P. persica and Salix babilonica are known to be hosts of Pyrrhoderma noxium. Based on the presence of suitable hosts, the Panel assumes that pathogen can be present in the production area of bonsai plants destined for export to the EU.
Uncertainties
-
–
The dispersal range of Pyrrhoderma noxium basidiospores.
-
–
The host status of Pinus parviflora.
-
–
The host range of Pyrrhoderma noxium.
-
–
Presence of Pyrrhoderma noxium in Zhejiang province.
-
–
No information available on the distance of the nursery to sources of pathogen in the surrounding environment.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nursery. The pathogen can be present in the surrounding area and the transferring rate of basidiospores could be enhanced by wind, while infection of plants could be triggered by wounds induced by grafting and pruning.
A.15.2.2. Possibility of entry with new plants/seeds
All seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (P. thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triadimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
The growing media used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0).
Possibility of entry with seeds or soil/growing media is not relevant for the pathogen.
Uncertainties
-
–
It is not clear if and how new mother plants are produced or introduced.
Taking into consideration the above evidence and uncertainties, the Panel considers it is not possible that the pathogen could enter the nursery with new plants/seeds or soil growing media.
A.15.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of Pinus parviflora are taken (Dossier Section 4.0).
The nursery also grows ornamental plants including abelia, bamboo, camphor, loropetalum, photinia, pyracantha, wisteria and other plants (Dossier Section 4.0). Cinnamomum camphora is a host to Pyrrhoderma noxium. Therefore, other suitable hosts of are present in the nursery.
Root contacts between the potted plants are not possible. Spread via basidiospores is possible if fruiting bodies are present in the nursery. The likelihood of development of fruiting bodies is expected to be higher on mother plants than on potted plants. Human mediated spread is not relevant for Pyrrhoderma noxium.
Uncertainties
-
–
If mother plants are injured at the base.
-
–
The host status of Pinus parviflora for Pyrrhoderma noxium.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pathogen within the nursery is possible, because fruiting bodies disseminating basidiospores may develop on mother plants.
A.15.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Pyrrhoderma noxium (Phellinus noxius) between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.15.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on Pyrrhoderma noxium is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
The net does not prevent the entry of airborne inoculum (i.e. basidiospores). However, it might reduce air currents thereby decreasing the amount of inoculum entering the productions site. Plants are cultivated separately in pots. Therefore, spread of the pathogen from plant to plant through root contacts is not possible.
Uncertainties:
|
| 2 | Growing medium and its treatment | Yes |
It cannot be excluded, as showed for other white rot fungi, that coconut coir could serve as a substrate for the establishment of the pathogen (Gupte et al., 2007) during production, although previous reports did not refer to establishment via basidiospores landing. Soaking with chemicals is not expected to be effective because chemicals are insecticides.
Uncertainties:
|
| 3 | Treatment of seeds | No | Not applicable. |
| 4 | Insecticide and acaricide treatments | No | Not applicable. |
| 5 | Fungicide treatments | Yes |
Although spraying may not be the best application method for such kind of pathogens, most fungicide treatments (most of active ingredients are systemic) may reduce the likelihood of infection of the pathogen and possibly the rate of colonisation of plant tissues. Fungicide treatments may also have some effects on inoculum present in coconut coir.
Uncertainties:
|
| 6 | Nematicide treatments | No | Not applicable. |
| 7 | Herbicide treatments and weed management | No | Not applicable. |
| 8 | Official inspections during production | Yes |
The official inspections are expected to have some effects. However, recently infected plants could be asymptomatic and fruiting bodies may not develop on small plants.
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
The official inspections before export are expected to have some effect. However, recently infected plants could be asymptomatic and fruiting bodies may not develop on small plants. The removal of 2 cm of surface growing medium could reduce the inoculum that might be present in coconut coir. Soaking with Avermectin will not be effective.
Uncertainties:
|
A.15.5. Overall likelihood of pest freedom for Pyrrhoderma noxium on grafted bonsai plants
A.15.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected grafted bonsai plants
The scenario assumes that the pathogen is not present in the province where the nursery is located being distributed exclusively in tropical regions. The scenario also assumes that has little chance to establish in the nursery in the nursery and in the surroundings because of the lack of suitable substrates.
A.15.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected grafted bonsai plants
The scenario assumes that the pathogen is present in the surrounding of the nursery because suitable hosts and substrates are present. The pathogen is also present in mother plants in the nursery, where it develops fruiting bodies. Infection of plants occurs by means of airborne basidiospores through wounds (pruning and grafting wounds). Fungicide treatments are not effective in reducing infection. At the time of export the commodity is asymptomatic hampering a prompt detection of the pathogen.
A.15.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected grafted bonsai plants (Median)
The median is closer to the lower values because the environment is not fully suitable for the pathogen. In addition, the likelihood of infection of small plants through wounds should not be high.
A.15.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
There is a lack of information whether the pathogen can find a suitable environment for establishment. In addition, there is lack of quantitative information on the presence of suitable hosts and substrates in the area where the nursery is located. This results in high level of uncertainty for infection rates below the median. The infection of small plants through wounds is deemed unlikely which reduces uncertainty above the median.
A.15.5.5. Elicitation outcomes of the assessment of the pest freedom for Pyrrhoderma noxium on grafted bonsai plants
The following tables show the elicited and fitted values for pest infection (Table A.31) and pest freedom (Table A.32).
Table A.31.
Elicited and fitted values of the uncertainty distribution of pest infection by Pyrrhoderma noxium per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 5 | 9 | 20 | 75 | ||||||||||
| EKE | 0.142 | 0.359 | 0.730 | 1.50 | 2.60 | 4.10 | 5.75 | 9.78 | 15.4 | 19.2 | 24.6 | 31.1 | 39.8 | 48.0 | 58.5 |
The EKE results are the BetaGeneral (0.99495, 17.241, 0, 250) distribution fitted with @Risk version 7.6.
Table A.32.
The uncertainty distribution of plants free of Pyrrhoderma noxium per 10,000 plants calculated by Table A.31
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,925 | 9,980 | 9,991 | 9,996 | 10,000 | ||||||||||
| EKE results | 9,942 | 9,952 | 9,960 | 9,969 | 9,975 | 9,981 | 9,985 | 9,990 | 9,994 | 9,996 | 9,997 | 9,998 | 9,999.3 | 9,999.6 | 9,999.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants, the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.32.
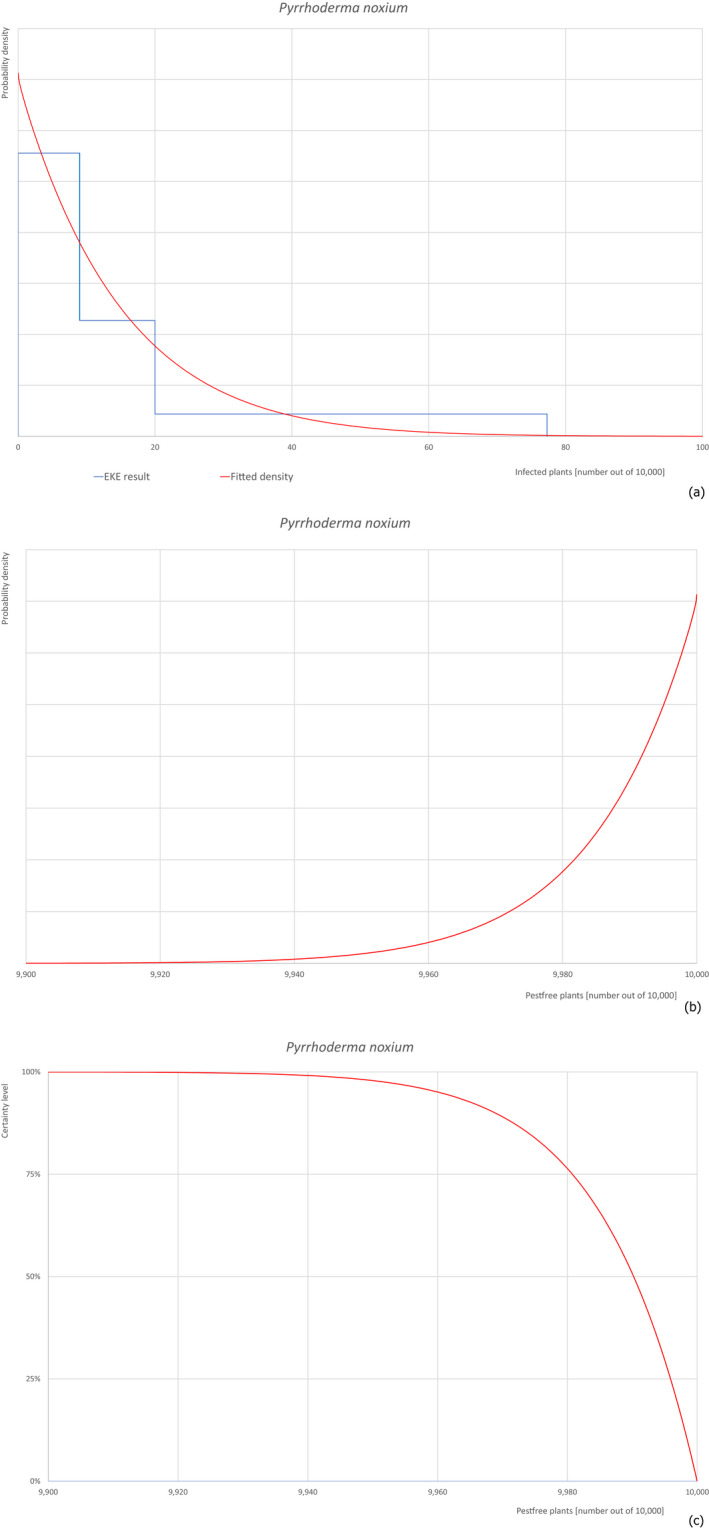
Figure A.16: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.15.6. Reference List
Ann P‐J, Lee H‐L and Huang T‐C, 1999. Brown Root Rot of 10 Species of Fruit Trees Caused by Phellinus noxius in Taiwan. Plant Disease, 83, 746–750. https://doi.org/10.1094/PDIS.1999.83.8.746
Ann P‐J, Chang T‐T and Ko W‐H, 2002. Phellinus noxius Brown Root Rot of Fruit and Ornamental Trees in Taiwan. Plant Disease, 86, 820–826. https://doi.org/10.1094/PDIS.2002.86.8.820
Bolland L, 1984. Phellinus noxius: cause of a significant root‐rot in Queensland hoop pine plantations, Australian Forestry, 47, 2–10, https://doi.org/10.1080/00049158.1984.10675972
Brooks FE, 2002. Brown Root Rot Disease in American Samoa’s Tropical Rain Forests. Pacific Science, 56, 377–387. https://doi.org/10.1353/psc.2002.0031
CABI (Centre for Agriculture and Bioscience International), online, Phellinus noxius (brown tea root disease) datasheet. Available online: https://www.cabi.org/cpc/datasheet/40154 [Accessed: 20 June 2021].
Chang T‐T, 1996. Survival of Phellinus noxius in soil and in the roots of dead host plants. Phytopathology, 86, 272–276. https://doi.org/10.1094/Phyto‐86‐272
Chung C‐L, Huang S‐Y, Huang Y‐C, Tzean S‐S, Ann P‐J, Tsai J‐N, Yang C‐C, Lee H‐H, Huang T‐W, Huang H‐Y, Chang T‐T, Lee H‐L and Liou R‐F, 2015. The genetic structure of Phellinus noxius and dissemination pattern of brown root rot disease in Taiwan. PLOS ONE, 10, 18. https://doi.org/10.1371/journal.pone.0139445
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars‐grin.gov/fungaldatabases/ [Accessed: 20 June 2021]
GBIF (Global Biodiversity Information Facility), online. Phellinus noxius (Corner) G. Cunn. in GBIF Secretariat (2021). GBIF Backbone Taxonomy. Available online: https://www.gbif.org/species/2520109 [Accessed: 20 June 2021].
Gupte A, Gupte S and Patel H, 2007. Ligninolytic enzyme production under solid‐state fermentation by white rot fungi. Journal of Scientific and Industrial Research, 66, 611–614.
Hsiao W‐W, Hung T‐H and Sun E‐J, 2019. Newly discovered basidiocarps of Phellinus noxius on 33 tree species with brown root rot disease in Taiwan and the basidiospore variations in growth rate. Taiwania, 64, 263–268. https://doi.org/10.6165/tai.2019.64.263
MPI (Ministry for Primary Industries), online_a, Ministry for Primary Industries standard 155.02.06. Available online: https://www.mpi.govt.nz/dmsdocument/1152/direct [Accessed: 20 June 2021].
MPI (Ministry for Primary Industries), online_b, Pest risk analysis: Phellinus noxius from all countries. Available online: https://www.mpi.govt.nz/dmsdocument/12681/direct [Accessed: 20 June 2021].
Nandris D, 1987. Root Rot Diseases of Rubber Trees. Plant Disease, 71, 298–306. https://doi.org/10.1094/PD‐71‐0298
Stewart JE, Kim MS, Ota Y, Sahashi N, Hanna JW, Akiba M, Ata JP, Atibalentja N, Brooks F, Chung CL, Dann EK, Mohd Farid A, Hattori T, Lee SS, Otto K, Pegg GS, Schlub RL, Shuey LS, Tang AMC, Tsai JN, Cannon PG and Klopfenstein NB, 2020. Phylogenetic and population genetic analyses reveal three distinct lineages of the invasive brown root‐rot pathogen, Phellinus noxius, and bioclimatic modeling predicts differences in associated climate niches. European Journal of Plant Pathology, 156, 751–766. https://doi.org/10.1007/s10658‐019‐01926‐5
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
Wang YF, Meng H, Gu VW and Gu JD, 2016. Molecular diagnosis of the brown root rot disease agent Phellinus noxius on trees and in soil by rDNA ITS analysis. Applied Environmental Biotechnology, 1, 81–91.
Watling R, Frankland JC, Ainsworth AM, Isaac S and Robinson CH, 2002. Tropical Mycology: Volume 1. Macromycetes. CABI Publishing, Wallingford, Oxon OX10 8DE, UK. 191 pp.
Wu Z‐C, Chang Y‐Y, Lai Q‐J, Lin H‐A, Tzean S‐S, Liou R‐F, Tsai IJ and Chung C‐L, 2020. Soil is not a reservoir for Phellinus noxius. Phytopathology, 110, 362–369. https://doi.org/10.1094/PHYTO‐08‐19‐0314‐R
A.16. Setoptus parviflorae
A.16.1. Organism information
| Taxonomic information |
Current valid scientific name: Setoptus parviflorae Synonyms: – Name used in the EU legislation: – Order: Acarida Family: Phytoptidae Common name: – Name used in the Dossier: – |
| Group | Mites |
| EPPO code | SETPPA |
| Regulated status | Setoptus parviflorae is not regulated anywhere in the world nor listed by EPPO. |
| Pest status in China |
The species has been described from a specimen found in the Nanjing Plant Garden, in the province of Jiangsu as vagrant on Pinus parviflora (Haiyuan, 1998). The author considers it close to S. strobacus. Another Setoptus species is present in China: S. koraiensis in Gansu, Henan, Jilin and Liaoning provinces (Song et al., 2008). |
| Pest status in the EU |
Setoptus parviflorae is absent in the EU. Setoptus multigranulatus and S. pini are from Europe (Chetverikov et al., 2019). Septopus strobacus was reported in Serbia (Petanović and Vidović, 2009). A new species, S. semiornatum, was described and reported in the UK and probably in Netherlands on Pinus parviflora bonsai plans introduced from Japan (Pye, 2011; NVWA, 2020). It is likely that S. parviflorae and S. semiornatum are the same species, but it requires taxonomic revision. |
| Host status on Pinus parviflorae and P. thunbergii |
Pinus parviflora is a host of Setoptus parviflorae (Haiyuan, 1998) and also S. semiornatum (Pye, 2011; NVWA, 2020). There is no information about the host status of P. thunbergii for this pest. Pinus thunbergii is a known host of Setoptus koraiensis (Song et al., 2008). |
| PRA information |
Pest Risk Assessment currently available that could be regarding also Setoptus parviflorae and S. semiornatum:
|
| Other relevant information for the assessment | |
| Biology |
Setoptus parviflorae is an eriophyoid mite, present in China (Haiyuan, 1998; NVWA, 2020). According to Chetverikov et al. (2019), there are currently sixteen species of Setoptus, which are known to inhabit Africa, Asia, Europe, North and South America. Eriophyoid mites are known to have variable life cycle. Usually passing through at least four development stages: egg, larva, nymph and adult, they also have a quiescent stage between larva and nymph (nymphocrysalis) and between nymph and adult (imagocrysalis). Eggs are laid among needles, difficult to see the first days as they soon change colour and subsequently hatch after few days. The shape of immature individuals is similar to the adult ones. Many immature forms have not been described, including those of the genus Setoptus. Adults are male and female, but for many species, males have never been observed (Setoptus allotype is present in Haiyuan (1998)). Setoptus are not known to show deuterogyny; therefore, males and females should be produced through the year, and their adult life, after shedding the imagocrysalis is short and focussed on reproduction. These species can remain active during winter, even in broadleaved trees, inside the bud scales. Adult life span and the duration of the reproductive period can be lengthened by suboptimal temperatures (Manson and Oldfield, 1996). On the other hand, Eriophyoid mites of the Phydoptidae family are known to have varying overwintering strategies, and Nalepella harlovi (close to the Setoptus genera) pest of Picea abies, overwinter in egg stage, showing the need of cold exposure to end the diapause, and hatching in 17 days at 10°C, and 1 week from 20 to 30°C (Manson and Oldfield, 1996). Two species were found to complete their lifecycle in 5 weeks in a 10°C environment, and 10 days in a 22°C one, and seems to be unaffected by photoperiod (Manson and Oldfield, 1996). Setoptus semiornatum was found on needles and needle sheaths (Pye, 2011). Main possible ways of eriophyoid mite dispersal are by wind, pollinators (phoresy), water (Navia et al., 2010) and pruning (NVWA, 2020). Possible pathways of entry for eriophyoid mites with trade are plants of any kind, propagation material, fresh fruits, cut flowers, buds and in some cases seeds (Navia et al., 2010). |
| Symptoms | Main type of symptoms | Main symptoms caused by Setoptus sp. are yellowing/chlorosis of needles and needle drop (Pye, 2011; NVWA, 2020). Traces of the mite bite can be observed with a magnifying glass. |
| Presence of asymptomatic plants | There is no information about the infestation level of asymptomatic plants. Considering that Setoptus sp. (later described as S. sermionatum) was imported on bonsai plants of Pinus parviflora from Japan to the UK (Pye, 2011). Seems plausible that low infestation levels can go undetected. | |
| Confusion with other pests |
Chlorosis and needle drop can be attributed to many biotic and abiotic factors, and while the presence of active mites is easy to detect, the taxonomic identification at species level is still debated (Chetverikov et al., 2019). It can be confused with other Setoptus species. It is very similar to S. strobacus (Haiyuan, 1998). A comprehensive morphological and molecular analysis is needed in order to clarify the taxonomy of the group (Chetverikov et al., 2019). |
|
| Host plant range |
Setoptus parviflorae has been described as a new species on Pinus parviflora (Haiyuan, 1998). Setoptus species are also linked to P. strobus, P. sylvestris, P. mugo, P. nigra (Ellis, online), P. thunbergii, P. koraiensis (Song et al., 2008), P. jefferi, P. muricata, P. ponderosa, P. torreyana (Keifer, 1952), Tsuga heterophylla (Chetverikov et al., 2019) and Tsuga chinensis (Huang and Boczek, 1996). |
|
| Reported evidence of impact | Setoptus semiornatum caused severe damage to 200 bonsai plants in a greenhouse in the UK (Pye, 2011). | |
| Evidence that the commodity is a pathway |
Setoptus sp. (later described as S. semiornatum) was imported on bonsai plants of Pinus parviflora from Japan to the UK (Pye, 2011). Therefore, it can be assumed that the commodity can be a pathway. Moreover, Setoptus spp. were detected on bonsai plants of Pinus parviflora in a greenhouse in the Netherlands (NVWA, 2020). |
|
| Surveillance information | No surveillance information for this pest is currently available from China. There is no information on whether the pest has ever been found in the nursery or its surrounding environment. | |
A.16.2. Possibility of pest presence in the nursery
A.16.2.1. Possibility of entry from the surrounding environment
Setoptus parviflorae is known to be present in provinces of China. The nursery is located in Zhejiang province, where S. parviflorae is not reported to be present. It was reported in Jiangsu (Haiyuan, 1998). According to Dossier Sections 4.0 and 5.0, the nursery is surrounded by different plants, from these plant species mentioned in the Dossier Pinus parviflora is a host of S. parviflorae. Other Pinus species, which can be potential hosts of this mite are also present, such as, P. bungeana, P. densiflora, P. elliottii, P. massoniana and P. thunbergii.
The possibility of entry for S. parviflorae from surrounding environment to nursery is through wind, pollinators and water. As stated in Dossier Section 4.0, the bonsai cultivation site is protected by a 40‐mesh insect‐proof net (0.4 mm), which the mite can easily get through, because of its small size.
Uncertainties
-
–
There is no surveillance information on the presence or population pressure of the pest in the area where the nursery is located.
-
–
No information available on the distance of the nursery to sources of pest in the surrounding environment.
-
–
No information is available on the dispersal distance of the mite.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery. The pest can be present in the surrounding area because of suitable hosts and the transferring rate could be enhanced by wind because mites can go through the net; pollinators, water and grafting operations.
A.16.2.2. Possibility of entry with new plants/seeds
All seedlings are cultivated and processed independently by export enterprises, and the cultivation site is located in the seedling cultivation area of export nursery (Dossier Section 4.0).
Seeds of black pine (P. thunbergii) are purchased from companies specialised in seed production and soaked with Potassium Permanganate and Triadimefon. Scions of P. parviflora are taken from mother plants located in the nursery. The same mother plants were used since 2006 (Dossier Section 4.0). In general mother plants have long life span and are rarely replaced.
The growing media used during production is coconut coir, which does not contain any soil. The coconut coir is imported from abroad and is cleaned thoroughly before using (Dossier Section 5.0).
Seeds are a possible pathway for some eriophyoid mites (Navia et al., 2010), therefore it cannot be excluded that the mite can enter the nursery with infested seeds.
Uncertainties
-
–
It is not clear if and how new mother plants are produced or introduced.
-
–
Whether seeds can be pathway for this mite.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery with seeds.
A.16.2.3. Possibility of spread within the nursery
There are around 50 mother plants located in the exporting nursery, from which the scions of P. parviflora are taken (Dossier Section 4.0).
The mite within the nursery can spread by hitchhiking on pollinators, by wind and by scions from infested mother plants. In addition, the mites can go through the net.
Spread within the nursery through the movement of soil, equipment and tools is not relevant.
Uncertainties
-
–
There is no information on the presence or population pressure of the pests in the nursery.
-
–
Whether the grafting time matches the mite occurrence/presence.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pest within the nursery is possible due to the presence of suitable hosts (e.g. mother plants, commodity).
A.16.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database, there are no records of notification of Pinus parviflora and P. thunbergii plants neither from China nor from other countries due to the presence of Setoptus parviflorae between the years 1995 and May 2021 (EUROPHYT/TRACES‐NT, online).
A.16.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in China are listed and an indication of their effectiveness on Setoptus parviflorae is provided. The description of the risk mitigation measures currently applied in China is provided in Table 15.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Separation and physical protection of the commodity during production and before export | Yes |
Physical separation of the bonsai plants may have limited effect on reducing risk of infestation with eriophyoid mite, especially in the production base, because the mites can go through the net.
Uncertainties:
|
| 2 | Growing medium and its treatment | No | Not applicable. |
| 3 | Treatment of seeds | No | Fungicides have no effect on the mite. |
| 4 | Insecticide and acaricide treatments | Yes |
Spray of contact acaricides can kill mites that are outside the needles at the time of spraying.
Uncertainties:
|
| 5 | Fungicide treatments | No | Fungicides have no effect on the mite. |
| 6 | Nematicide treatments | No | Nematicides have no effect on the mite. |
| 7 | Herbicide treatments and weed management | No | Herbicides have no effect on the mite. |
| 8 | Official inspections during production | Yes |
Mites are only visible under the strong magnifier (20x).
Uncertainties:
|
| 9 | Official inspections and treatments before export | Yes |
Mites are only visible under the strong magnifier (20x).
Uncertainties:
|
A.16.5. Overall likelihood of pest freedom for Setoptus parviflorae on grafted bonsai plants
A.16.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested grafted bonsai plants
The population density around the nursery is low and the measures to prevent the colonisation of the bonsai plants and to suppress the mites eventually established are effective.
A.16.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested grafted bonsai plants
The population density around the nursery is high and the measures to prevent the colonisation of the bonsai plants and to suppress the mites eventually established are only partially effective.
A.16.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested grafted bonsai plants (Median)
Different combinations of population density around the nursery and of the acaricide applications may result in an intermediate scenario as they are acting independently of one another on the presence of the mite in the commodity. Median is slightly shift to the left side (lower infestation rate) because the presence of the mite is not reported in Zhejiang.
A.16.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The signs of the mite occurrence (needle discoloration) are generally detectable, but very generic. The Panel assumes that a high infestation level is less likely to happen than having smaller number of infested plants where the mite density is low.
A.16.5.5. Elicitation outcomes of the assessment of the pest freedom for Setoptus parviflorae on grafted bonsai plants
The following tables show the elicited and fitted values for pest infestation (Table A.33) and pest freedom (Table A.34).
Table A.33.
Elicited and fitted values of the uncertainty distribution of pest infestation by Setoptus parviflorae per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 50 | 180 | 325 | 600 | 1,000 | ||||||||||
| EKE | 50.0 | 54.9 | 64.5 | 86.8 | 121 | 169 | 222 | 346 | 496 | 585 | 688 | 790 | 886 | 950 | 1,002 |
The EKE results are the BetaGeneral (0.83143, 1.616, 47.6, 1070) distribution fitted with @Risk version 7.6.
Table A.34.
The uncertainty distribution of plants free of Setoptus parviflorae per 10,000 plants calculated by Table A.33
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,000 | 9,400 | 9,675 | 9,820 | 9,950 | ||||||||||
| EKE results | 8,998 | 9,050 | 9,114 | 9,210 | 9,312 | 9,415 | 9,504 | 9,654 | 9,778 | 9,831 | 9,879 | 9,913 | 9,936 | 9,945 | 9,950 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.34.
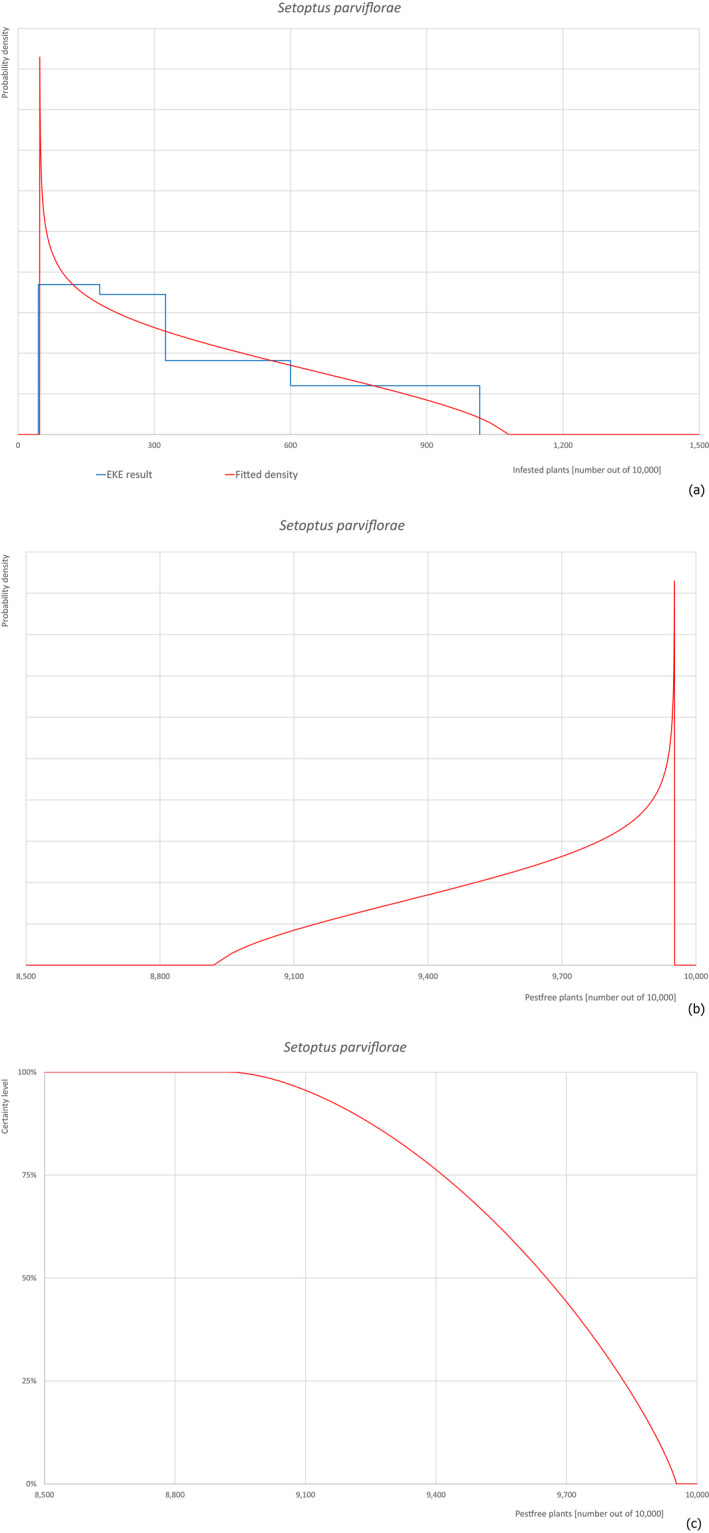
Figure A.17: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.16.6. Reference List
Chetverikov P, Desnitskaya EA, Efimov PG, Bolton S, Cvrkovic T, Petanovic R, Zukoff S, Klimov P, Amrine J and Klimov P, 2019. The description and molecular phylogenetic position of a new conifer‐associated mite, Setoptus tsugivagus n. sp. (Eriophyoidea, Phytoptidae, Nalepellinae). Systematic and Applied Acarology, 24, 683. https://doi.org/10.11158/saa.24.4.13
Ellis WN, online. Plant Parasites of Europe. Available online: https://bladmineerders.nl/parasites/animalia/arthropoda/acari/actinotrichida/prostigmata/eleutherengona/eriophyoidea/phytoptidae/nalepellinae/nalepellini/setoptus/ [Accessed: 2 July 2021].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 June 2021].
Haiyuan K, 1998. Four new eriophyid mites from forest plants in China (Acari: Eriophyoidea). Acta Entomologica Sinica, 41, 300–304.
Huang K‐W and Boczek J, 1996. Some eriophyoid mites on coniferous plants from high mountains in Taiwan (Acari: Eriophyoidea). Acarologia, 37, 217–227.
Keifer HH, 1952. The Eriophyid mites of California. Volume 2, NO. 1. Bulletin of the California Insect Survey, University of California Press, Berkeley and Los Angeles, California, 127 pp.
Manson DCM and Oldfield GN, 1996. Chapter 1.4 Biology and ecology 1.4.1 Life forms, deuterogyny, diapause and seasonal development. In: Eriophyoid mites: their biology, natural enemies and control. Lindquist EE, Bruin J and Sabelis MW (eds.). Elsevier, pp. 173–183.
Navia D, Ochoa R, Welbourn C and Ferragut F, 2010. Adventive eriophyoid mites: a global review of their impact, pathways, prevention and challenges. Experimental and applied Acarology, 51, 225–255. https://doi.org/10.1007/s10493‐009‐9327‐2
NVWA (Nederlandse Voedsel en Warenautoriteit), 2020. Quick scan answer for Setoptus sp. on Pinus parviflora. Available online: https://english.nvwa.nl/topics/pest‐risk‐analysis/documents/plant/plant‐health/pest‐risk‐analysis/documents/quick‐scan‐answer‐for‐setoptus‐sp.‐on‐pinus‐parviflora [Accessed: 2 July 2021].
Petanović R and Vidović B, 2009. New Acaricalus species (Acari: Eriophyoidea) from Turkey Oak, Quercus cerris L. (fagaceae) and the new records for the fauna of Serbia. Acta Entomologica Serbica, 14, 109–120.
Pye DRL, 2011. A new species of eriophyoid mite (Acari: Eriophyoidea: Phytoptidae) from Japan, causing damage to Pinus parviflora var. pentaphylla. International Journal of Acarology, 37, 122–130. https://doi.org/10.1080/01647954.2010.495352
Song Z‐W, Xue X‐F and Hong X‐Y, 2008. Eriophyoid mite fauna (Acari: Eriophyoidea) of Gansu Province, northwestern China with descriptions of twelve new species. Zootaxa, 1756, 1–48. https://doi.org/10.11646/zootaxa.1756.1.1
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
Appendix B – Web of Science All Databases Search Strings
In Table B.1, the search string for Pinus parviflora used in Web of Science is reported. Totally, 42 papers were retrieved. Titles and abstracts were screened, and 17 pests were added to the list of pests (see Appendix E).
Table B.1.
String for Pinus parviflora
| Web of Science All databases |
TOPIC: (“Pinus parviflora” OR “P. parviflora” OR “Japanese white pine”) AND TOPIC: (pathogen* OR pathogenic bacteria OR fung* OR oomycet* OR myce* OR bacteri* OR virus* OR viroid* OR insect$ OR mite$ OR phytoplasm* OR arthropod* OR nematod* OR disease$ OR infecti* OR damag* OR symptom* OR pest$ OR vector OR hostplant$ OR “host plant$” OR “host” OR “root lesion$” OR decline$ OR infestation$ OR damage$ OR symptom$ OR dieback* OR “die back*” OR “malaise” OR aphid$ OR curculio OR thrip$ OR cicad$ OR miner$ OR borer$ OR weevil$ OR “plant bug$” OR spittlebug$ OR moth$ OR mealybug$ OR cutworm$ OR pillbug$ OR “root feeder$” OR caterpillar$ OR “foliar feeder$” OR virosis OR viroses OR blight$ OR wilt$ OR wilted OR canker OR scab$ OR “rot” OR “rots” OR “rotten” OR “damping off” OR “damping‐off” OR blister$ OR “smut” OR “mould” OR “mold” OR “damping syndrome$” OR mildew OR scald$ OR “root knot” OR “root‐knot” OR rootknot OR cyst$ OR “dagger” OR “plant parasitic” OR “parasitic plant” OR “plant$parasitic” OR “root feeding” OR “root$feeding” OR “gall” OR “ambrosia beetle$” OR “gall$” OR “bark beetle$”) NOT TOPIC: (“winged seeds” OR metabolites OR *tannins OR climate OR “maple syrup” OR syrup OR mycorrhiz* OR “carbon loss” OR pollut* OR weather OR propert* OR probes OR spectr* OR antioxidant$ OR transformation OR RNA OR DNA OR “Secondary plant metabolite$” OR metabol* OR “Phenolic compounds” OR Quality OR Abiotic OR Storage OR Pollen* OR fertil* OR Mulching OR Nutrient* OR Pruning OR drought OR “human virus” OR “animal disease*” OR “plant extracts” OR immunological OR “purified fraction” OR “traditional medicine” OR medicine OR mammal* OR bird* OR “human disease*” OR biomarker$ OR “health education” OR bat$ OR “seedling$ survival” OR “anthropogenic disturbance” OR “cold resistance” OR “salt stress” OR salinity OR “aCER method” OR “adaptive cognitive emotion regulation” OR nitrogen OR hygien* OR “cognitive function$” OR fossil$ OR *toxicity OR Miocene OR postglacial OR “weed control” OR landscape) NOT TOPIC: (“Arceuthobium americanum” OR “Arceuthobium campylopodum” OR “Arceuthobium laricis” OR “Arceuthobium occidentale” OR “Arceuthobium pusillum” OR “Arceuthobium tsugense” OR “Arceuthobium vaginatum” OR “Atropellis pinicola” OR “Atropellis piniphila” OR “Bursaphelenchus mucronatus” OR “Bursaphelenchus xylophilus” OR “Chionaspis pinifoliae” OR “Choristoneura fumiferana” OR “Choristoneura lambertiana” OR “Coniferiporia weirii” OR “Crisicoccus pini” OR “Cronartium coleosporioides” OR “Cronartium comandrae” OR “Cronartium comptoniae” OR “Cronartium flaccidum” OR “Cronartium fusiforme” OR “Cronartium harknessii” OR “Cronartium himalayense” OR “Cronartium kamtschaticum” OR “Cronartium quercuum” OR “Dendroctonus adjunctus” OR “Dendroctonus brevicomis” OR “Dendroctonus frontalis” OR “Dendroctonus ponderosae” OR “Dendroctonus valens” OR “Dendrolimus sibiricus” OR “Dendrolimus spectabilis” OR “Dendrolimus superans” OR “Dothistroma septosporum” OR “Elasmopalpus lignosellus” OR “Epiphyas postvittana” OR “Gnathotrichus sulcatus” OR “Gremmeniella abietina” OR “Heterobasidion irregulare” OR “Hylurgus ligniperda” OR “Ips amitinus” OR “Ips calligraphus” OR “Ips confusus” OR “Ips duplicatus” OR “Ips grandicollis” OR “Ips hauseri” OR “Ips lecontei” OR “Ips pini” OR “Ips plastographus” OR “Ips subelongatus” OR “Lecanosticta acicola” OR “Leptoglossus occidentalis” OR “Malacosoma disstria” OR “Megaplatypus mutatus” OR “Melampsora medusae” OR “Melampsora medusae f. sp. deltoidis” OR “Monochamus alternatus” OR “Monochamus carolinensis” OR “Monochamus galloprovincialis” OR “Monochamus mutator” OR “Monochamus nitens” OR “Monochamus obtusus” OR “Monochamus saltuarius” OR “Monochamus scutellatus” OR “Monochamus sutor” OR “Monochamus titillator” OR “Monochamus urussovi” OR “Oemona hirta” OR “Ophiostoma wageneri” OR “Orgyia leucostigma” OR “Phytophthora cinnamomi” OR “Pissodes castaneus” OR “Pissodes nemorensis” OR “Pissodes strobi” OR “Platynota stultana” OR “Pseudocercospora pini‐densiflorae” OR “Rhyacionia buoliana” OR “Rotylenchus buxophilus” OR “Sirex ermak” OR “Sirex noctilio” OR “Tetropium gracilicorne” OR “Trichoferus campestris” OR “Tylenchorhynchus claytoni” OR “Xylosandrus compactus” OR “Xylosandrus germanus” OR “Cronartium ribicola” OR “Monochamus saltuarius” OR “Mycosphaerella gibsonii” OR “Dendrolimus punctatus” OR “Monochamus alternatus” OR “Pissodes strobi” OR “Cinara cembrae” OR “Cinara shinjii” OR “Cinara watanabei” OR “Eulachnus pumilae” OR “Pineus cembrae” OR “Pineus harakawai” OR “Pineus havrylenkoi” OR “Ceroplastes rubens” OR “Crisicoccus pini” OR “Fiorinia japonica” OR “Spulerina corticicola” OR “Conogethes punctiferalis” OR “Oligonychus ununguis” OR “Armillaria mellea” OR “Belonidium japonicum” OR “Ceratocystis piceae” OR “Cronartium ribicola” OR “Cucurbidothis pithyophila” OR “Cylindrocarpon destructans” OR “Cylindrocarpon sp.” OR “Cylindrocladium scoparium” OR “Fomes annosus” OR “Gloeophyllum sepiarium” OR “Helicobasidium mompa” OR “Hypoderma desmazieri” OR “Lophodermium pinastri” OR “Lophodermium pini‐excelsae” OR “Macrosporium sp.” OR “Ophiostoma clavatum” OR “Ophiostoma piceae” OR “Panus sp.” OR “Perenniporia subacida” OR “Pestalosphaeria gubae” OR “Pestalotiopsis foedans” OR “Pestalotiopsis lespedezae” OR “Pestalotiopsis sp.” OR “Phaeolus schweinitzii” OR “Phomopsis sp.” OR “Polyporus schweinitzii” OR “Pseudocercospora pini‐densiflorae” OR “Pythium sp.” OR “Racodium therryanum” OR “Rhizina undulata” OR “Rhizosphaera kalkhoffii” OR “Schizophyllum commune” OR “Scolecostigmina chibaensis” OR “Trichaptum fuscoviolaceum” OR “Waltonia pinicola”) |
In Table B.2, the search string for Pinus thunbergii used in Web of Science is reported. Totally, 216 papers were retrieved. Titles and abstracts were screened, and 36 pests were added to the list of pests (see Appendix E).
Table B.2.
String for Pinus thunbergii
| Web of Science All databases |
TOPIC: “Pinus thunbergii” OR “P. thunbergii” OR “Japanese black pine”) AND TOPIC: (pathogen* OR pathogenic bacteria OR fung* OR oomycet* OR myce* OR bacteri* OR virus* OR viroid* OR insect$ OR mite$ OR phytoplasm* OR arthropod* OR nematod* OR disease$ OR infecti* OR damag* OR symptom* OR pest$ OR vector OR hostplant$ OR “host plant$” OR “host” OR “root lesion$” OR decline$ OR infestation$ OR damage$ OR symptom$ OR dieback* OR “die back*” OR “malaise” OR aphid$ OR curculio OR thrip$ OR cicad$ OR miner$ OR borer$ OR weevil$ OR “plant bug$” OR spittlebug$ OR moth$ OR mealybug$ OR cutworm$ OR pillbug$ OR “root feeder$” OR caterpillar$ OR “foliar feeder$” OR virosis OR viroses OR blight$ OR wilt$ OR wilted OR canker OR scab$ OR “rot” OR “rots” OR “rotten” OR “damping off” OR “damping‐off” OR blister$ OR “smut” OR “mould” OR “mold” OR “damping syndrome$” OR mildew OR scald$ OR “root knot” OR “root‐knot” OR rootknot OR cyst$ OR “dagger” OR “plant parasitic” OR “parasitic plant” OR “plant$parasitic” OR “root feeding” OR “root$feeding” OR “gall” OR “ambrosia beetle$” OR “gall$” OR “bark beetle$”) NOT TOPIC: (“winged seeds” OR metabolites OR *tannins OR climate OR “maple syrup” OR syrup OR mycorrhiz* OR “carbon loss” OR pollut* OR weather OR propert* OR probes OR spectr* OR antioxidant$ OR transformation OR RNA OR DNA OR “Secondary plant metabolite$” OR metabol* OR “Phenolic compounds” OR Quality OR Abiotic OR Storage OR Pollen* OR fertil* OR Mulching OR Nutrient* OR Pruning OR drought OR “human virus” OR “animal disease*” OR “plant extracts” OR immunological OR “purified fraction” OR “traditional medicine” OR medicine OR mammal* OR bird* OR “human disease*” OR biomarker$ OR “health education” OR bat$ OR “seedling$ survival” OR “anthropogenic disturbance” OR “cold resistance” OR “salt stress” OR salinity OR “aCER method” OR “adaptive cognitive emotion regulation” OR nitrogen OR hygien* OR “cognitive function$” OR fossil$ OR *toxicity OR Miocene OR postglacial OR “weed control” OR landscape) NOT TOPIC: (“Acantholyda nipponica” OR “Alternaria alternata” OR “Amanitopsis vaginata” OR “Amphinema byssoides” OR “Amylostereum areolatum” OR “Anomala albopilosa” OR “Armillariella mellea” OR “Ascocalyx pinicola” OR “Aspidiotus cryptomeriae” OR “Basilepta pallidula” OR “Boletus granulatus” OR “Botryobasidium candicans var. latispora” OR “Botryobasidium microbotryosum” OR “Botryobasidium obtusisporum” OR “Botryobasidium subcoronatum” OR “Botryotinia fuckeliana” OR “Botrytis cinerea” OR “Bursaphelenchus doui” OR “Bursaphelenchus eproctatus” OR “Callidiellum rufipenne” OR “Calliteara argentata” OR “Calospora pini‐thunbergii” OR “Cecidomyia japonica” OR “Cenangium abietis” OR “Cenangium ferruginosum” OR “Cephalcia variegata” OR “Ceratocystis ips” OR “Ceratocystis minor” OR “Ceratosporium fuscescens” OR “Cercospora pini‐densiflorae” OR “Cinara etsuhoe” OR “Cinara formosana” OR “Cinara pinea” OR “Cinara pini” OR “Cinara pinidensiflora” OR “Cinara pinidensiflorae” OR “Cinara piniformosana” OR “Cinara sorini” OR “Cladosporium cladosporioides” OR “Climacodon pulcherrimus” OR “Coleosporium bletiae” OR “Coleosporium campanulae” OR “Coleosporium clematidis‐apiifoliae” OR “Coleosporium lycopi” OR “Coleosporium pedunculatum” OR “Coleosporium solidaginis” OR “Coleosporium tussilaginis” OR “Coleosporium xanthoxyli” OR “Coniochaeta malacotricha” OR “Conogethes pinicolalis” OR “Contarinia matsusintome” OR “Contarinia matusintome” OR “Cronartium orientale” OR “Cryphalus fulvus” OR “Cryphalus laricis” OR “Cryphodera brinkmani” OR “Cyclaneusma minus” OR “Dacrymyces capitatus” OR “Dacryobolus karstenii” OR “Dasyscyphus subtilissimus” OR “Dendroctonus terebrans” OR “Dendrosporium lobatum” OR “Desmazierella acicola” OR “Diaporthe conorum” OR “Diaspidiotus makii” OR “Dioryctria abietella” OR “Dioryctria sylvestrella” OR “Diprion nipponica” OR “Discosia pini” OR “Drosicha pinicola” OR “Epinotia pinivorana” OR “Essigella californica” OR “Eulachnus rileyi” OR “Eulachnus thunbergii” OR “Euwallacea interjectus” OR “Fomitopsis pinicola” OR “Fusarium avenaceum” OR “Fusarium circinatum” OR “Fusarium lateritium” OR “Fusarium moniliforme” OR “Fusarium oxysporum” OR “Fusarium oxysporum var. aurantiacum” OR “Fusarium solani f. sp. radicicola” OR “Fusoma parasiticum” OR “Ganoderma applanatum” OR “Ganoderma lucidum” OR “Ganoderma neojaponicum” OR “Gibberella baccata” OR “Gibberella circinata” OR “Glaucias subpunctatus” OR “Gloeophyllum subferrugineum” OR “Gyrothrix microsperma” OR “Heptophylla picea” OR “Heterobasidion insulare” OR “Humicola dimorphospora” OR “Hyloicus caligineus” OR “Hymenochaete yasudai” OR “Inonotus vallatus” OR “Ips acuminatus” OR “Jacksonomyces furfurellus” OR “Lentinus revelatus” OR “Lepidosaphes zhejiangensis” OR “Leptographium procerum” OR “Leptographium truncatum” OR “Leptosporomyces fuscostratus” OR “Leptostromella pini‐thunbergii” OR “Lloydella bicolor” OR “Lophodermium iwatense” OR “Macrophoma pinidensiflorae” OR “Macrophoma pini‐densiflorae” OR “Macrophomina phaseolina” OR “Matsucoccus resinosae” OR “Matsucoccus thunbergianae” OR “Mimela testaceipes” OR “Mortierella isabellina” OR “Mycosphaerella dearnessii” OR “Mycosphaerella pini” OR “Naemacyclus niveus” OR “Nanidorus minor” OR “Nectria haematococca” OR “Neodiprion sertifer” OR “Neolentinus suffrutescens” OR “Neottiosporella radicata” OR “Nesodiprion japonicus” OR “Nigrospora oryzae” OR “Nigrospora sphaerica” OR “Oligonychus clavatus” OR “Ophiostoma ips” OR “Ophiostoma minus” OR “Ophiostoma piliferum” OR “Ophiostoma pluriannulatum” OR “Oracella acuta” OR “Orthotomicus angulatus” OR “Orthotomicus tosaensis” OR “Parasympodiella longispora” OR “Pellicularia filamentosa” OR “Peridermium japonicum” OR “Peridermium pini‐thunbergii” OR “Peridermium praelongum” OR “Pestalotia sydowiana” OR “Pestalotiopsis glandicola” OR “Pestalotiopsis populi‐nigrae” OR “Petrova cristata” OR “Phellinus noxius” OR “Physarum lenticulare” OR “Phytophthora drechsleri” OR “Pineus laevis” OR “Pineus matsumurai” OR “Pineus orientalis” OR “Pineus sylvestris” OR “Pityophthorus jucundus” OR “Platyphytoptus thunbergii” OR “Polygraphus proximus” OR “Polyphylla albolineata” OR “Polyphylla laticollis” OR “Pseudococcus comstocki” OR “Pycnoporus coccineus” OR “Pyrenochaeta globosa” OR “Rhizoctonia solani” OR “Rhyacionia dativa” OR “Russula fragilis” OR “Russula livescens” OR “Scepticus griseus” OR “Scepticus insularis” OR “Schizolachnus orientalis” OR “Scleroderma geaster” OR “Seinura wuae” OR “Septoria pini‐thunbergii” OR “Septorioides pini‐thunbergii” OR “Sirococcus conigenus” OR “Sphaeropsis sapinea” OR “Spongipellis fissilis” OR “Stereum sanguinolentum” OR “Synanthedon sequoiae” OR “Thanatephorus cucumeris” OR “Thaumatographa eremnotorna” OR “Thelephora intybacea” OR “Thelephora terrestris” OR “Tomicus brevipilosus” OR “Trichaptum abietinum” OR “Trichodorus” OR “Trichodorus jeonjuensis” OR “Tricholoma ustale” OR “Tubulicrinis inornatus” OR “Tyromyces squalens” OR “Vesiculomyces citrinus” OR “Watabura nishiyae” OR “Wolfiporia cocos” OR “Xeris spectrum” OR “Xiphinema americanum sensu lato” OR “Xiphinema incognitum” OR “Aonidiella aurantti” OR “Aphrophora flavipes” OR “Arceuthobium americanum” OR “Arceuthobium campylopodum” OR “Arceuthobium laricis” OR “Arceuthobium occidentale” OR “Arceuthobium pusillum” OR “Arceuthobium vaginatum” OR “Armillaria mellea” OR “Arthrinium phaeospermum” OR “Atropellis pinicola” OR “Atropellis piniphila” OR “Bursaphelenchus mucronatus” OR “Bursaphelenchus xylophilus” OR “Ceratocystis piceae” OR “Cercoseptoria pini‐densiflorae” OR “Ceroplastes rubens” OR “Chionaspis pinifoliae” OR “Choristoneura fumiferana” OR “Choristoneura lambertiana” OR “Chrysomphalus dictyospermi” OR “Coleosporium asterum” OR “Colletotrichum gloeosporioides” OR “Coniferiporia weirii” OR “Crisicoccus pini” OR “Cronartium coleosporioides” OR “Cronartium comandrae” OR “Cronartium comptoniae” OR “Cronartium flaccidum” OR “Cronartium fusiforme” OR “Cronartium harknessii” OR “Cronartium himalayense” OR “Cronartium kamtschaticum” OR “Cronartium quercuum” OR “Cucurbidothis pithyophila” OR “Cylindrocarpon destructans” OR “Cylindrocladium scoparium” OR “Dendroctonus adjunctus” OR “Dendroctonus brevicomis” OR “Dendroctonus frontalis” OR “Dendroctonus micans” OR “Dendroctonus ponderosae” OR “Dendroctonus valens” OR “Dendrolimus punctatus” OR “Dendrolimus sibiricus” OR “Dendrolimus spectabilis” OR “Dendrolimus superans” OR “Dendrolimus tabulaeformis” OR “Dioryctria pryeri” OR “Diplodia pinea” OR “Dothistroma septosporum” OR “Elasmopalpus lignosellus” OR “Epiphyas postvittana” OR “Fiorinia fioriniae” OR “Fiorinia japonica” OR “Gloeophyllum sepiarium” OR “Gnathotrichus sulcatus” OR “Gravitarmata margarotana” OR “Gremmeniella abietina” OR “Helicobasidium mompa” OR “Hemiberlesia pitysophila” OR “Heterobasidion irregulare” OR “Hylurgus ligniperda” OR “Inonotus weirii” OR “Ips amitinus” OR “Ips calligraphus” OR “Ips confusus” OR “Ips duplicatus” OR “Ips grandicollis” OR “Ips hauseri” OR “Ips lecontei” OR “Ips pini” OR “Ips plastographus” OR “Ips sexdentatus” OR “Ips subelongatus” OR “Ips typographus” OR “Lecanosticta acicola” OR “Lepidosaphes pini” OR “Lepidosaphes piniphila” OR “Leptoglossus occidentalis” OR “Lophodermium conigenum” OR “Lophodermium kumaunicum” OR “Lophodermium pinastri” OR “Malacosoma disstria” OR “Matsucoccus massonianae” OR “Matsucoccus matsumurae” OR “Megaplatypus mutatus” OR “Melampsora medusae” OR “Melampsora medusae f. sp. deltoidis” OR “Monochamus alternatus” OR “Monochamus carolinensis” OR “Monochamus galloprovincialis” OR “Monochamus mutator” OR “Monochamus nitens” OR “Monochamus obtusus” OR “Monochamus saltuarius” OR “Monochamus scutellatus” OR “Monochamus sutor” OR “Monochamus titillator” OR “Monochamus urussovi” OR “Mycosphaerella gibsonii” OR “Oemona hirta” OR “Oligonychus ununguis” OR “Ophiostoma piceae” OR “Ophiostoma wageneri” OR “Orgyia leucostigma” OR “Paratrichodorus porosus” OR “Perenniporia subacida” OR “Pestalosphaeria gubae” OR “Pestalotia funerea” OR “Pestalotiopsis foedans” OR “Phaeolus schweinitzii” OR “Phytophthora cinnamomi” OR “Pineus cembrae” OR “Pineus harukawai” OR “Pineus pini” OR “Pissodes castaneus” OR “Pissodes nemorensis” OR “Pissodes nitidus” OR “Pissodes obscurus” OR “Pissodes strobi” OR “Platynota stultana” OR “Polyporus schweinitzii” OR “Pseudocercospora pini‐densiflorae” OR “Racodium therryanum” OR “Rhizina undulata” OR “Rhizosphaera kalkhoffii” OR “Rhyacionia buoliana” OR “Rhyacionia duplana” OR “Rotylenchus buxophilus” OR “Schizophyllum commune” OR “Scirrhia acicola” OR “Scirrhia pini” OR “Sirex ermak” OR “Sirex nitobei” OR “Sirex noctilio” OR “Tetropium gracilicorne” OR “Thecodiplosis japonensis” OR “Tomicus minor” OR “Tomicus piniperda” OR “Trichoferus campestris” OR “Tylenchorhynchus claytoni” OR “Urocerus japonicus” OR “Waltonia pinicola” OR “Xylosandrus compactus”) |
Appendix C – Plant taxa reported to be present within 15 km distance from the nursery
Table C.1: Plant taxa reported in Dossier Sections 4.0 and 5.0 to be present within 15 km distance from the nursery
| Number | Plant taxa | Number | Plant taxa |
|---|---|---|---|
| 1 | Abelia | 101 | Liriope cymbidiomorpha |
| 2 | Abelia biflora | 102 | Lithocarpus glaber |
| 3 | Acer buergerianum | 103 | Lonicera fragrantissima |
| 4 | Acer palmatum | 104 | Loropetalum |
| 5 | Acer palmatum cv. Dissectum | 105 | Loropetalum chinense |
| 6 | Acer palmatum ‘Atropurpureum’ | 106 | Loropetalum chinense var. rubrum |
| 7 | Acorus calamus | 107 | Lycium chinense |
| 8 | Acorus gramineus | 108 | Lythrum salicaria |
| 9 | Aesculus chinensis | 109 | Magnolia delavayi |
| 10 | Ainsliaea fragrans | 110 | Magnolia denudata |
| 11 | Ainsliaea macroclinidioides | 111 | Magnolia grandiflora |
| 12 | Albizia julibrissin | 112 | Magnolia soulangeana |
| 13 | Amygdalus persica | 113 | Mahonia fortunei |
| 14 | Aphananthe aspera | 114 | Malus ‘Flame’ |
| 15 | Arundo donax | 115 | Malus halliana |
| 16 | Aster ageratoides | 116 | Malus spectabilis |
| 17 | Aster sublatus | 117 | Melia azedarach |
| 18 | Aucuba japonica | 118 | Metasequoia glyptostroboides |
| 19 | Bambusa multiplex | 119 | Michelia chapensis |
| 20 | Bambusa multiplex cv. Alphonse‐Karr | 120 | Michelia figo |
| 21 | Berberis soulieana | 121 | Morus alba |
| 22 | Bombax malabaricum | 122 | Musa basjoo |
| 23 | Broussonetia papyrifera | 123 | Nandina domestica |
| 24 | Buxus megistophylla | 124 | Nelumbo nucifera |
| 25 | Buxus sinica | 125 | Nerium indicum |
| 26 | Camellia japonica | 126 | Nerium oleander |
| 27 | Camellia sasanqua | 127 | Nymphaea tetragona |
| 28 | Camellia sinensis | 128 | Oligostachyum lubricum |
| 29 | Camellia uraku | 129 | Orychophragmus violaceus |
| 30 | Camptotheca acuminata | 130 | Osmanthus fragrans |
| 31 | Canna indica | 131 | Othiopogon japanicus |
| 32 | Castanea mollissima | 132 | Paederia cavaleriei |
| 33 | Castanopsis carlesii | 133 | Paederia scandens |
| 34 | Castanopsis eyrei | 134 | Paulownia |
| 35 | Castanopsis sclerophylla | 135 | Paulownia fortunei |
| 36 | Cedrus deodara | 136 | Phoebe sheareri |
| 37 | Celtis sinensis | 137 | Phoenix canariensis |
| 38 | Cephalanthus tetrandrus | 138 | Photinia |
| 39 | Cerasus japonica | 139 | Photinia serrulata |
| 40 | Cerasus serrulata | 140 | Photinia x fraseri |
| 41 | Cerasus subhirtella | 141 | Phyllostachys bambusoides |
| 42 | Cercis chinensis | 142 | Phyllostachys heterocycla |
| 43 | Chaenomeles cathayensis | 143 | Phyllostachys nigra |
| 44 | Chaenomeles japonica | 144 | Phyllostachys praecox |
| 45 | Chaenomeles speciosa | 145 | Phyllostachys sulphurea |
| 46 | Chimonanthus praecox | 146 | Pinus bungeana |
| 47 | Chimonobambusa quadrangularis | 147 | Pinus densiflora |
| 48 | Cinnamomum bodinieri | 148 | Pinus elliottii |
| 49 | Cinnamomum camphora | 149 | Pinus massoniana |
| 50 | Cinnamomum japonicum | 150 | Pinus parviflora |
| 51 | Citrus maxima | 151 | Pinus thunbergii |
| 52 | Citrus paradisi | 152 | Pittosporum tobira |
| 53 | Cornus officinalis | 153 | Platanus orientalis |
| 54 | Cortaderia selloana | 154 | Podocarpus imbricatus |
| 55 | Cycas revoluta | 155 | Podocarpus macrophyllus |
| 56 | Cyclobalanopsis glauca | 156 | Podocarpus nagi |
| 57 | Dalbergia hupeana | 157 | Pontederia cordata |
| 58 | Diospyros kaki | 158 | Prunus |
| 59 | Diospyros oleifera | 159 | Prunus mume |
| 60 | Diospyros rhombifolia | 160 | Prunus persica 'Atropurpurea' |
| 61 | Distylium buxifolium | 161 | Prunus salicina |
| 62 | Distylium racemosum | 162 | Pseudolarix amabilis |
| 63 | Doellingeria scabra | 163 | Pterocarya stenoptera |
| 64 | Edgeworthia chrysantha | 164 | Punica granatum |
| 65 | Elaeagnus pungens | 165 | Pyracantha |
| 66 | Elaeocarpus decipiens | 166 | Pyracantha fortimeana ‘Harlequin’ |
| 67 | Erigeron annuus | 167 | Pyracantha fortuneana |
| 68 | Eriobotrya japonica | 168 | Quercus acutissima |
| 69 | Euonymus maackii | 169 | Quercus aliena |
| 70 | Eupatorium fortune | 170 | Reineckia carnea |
| 71 | Eupatorium japonicum | 171 | Rhododendron |
| 72 | Fatshedera lizei | 172 | Rhododendron pulchrum |
| 73 | Fatsia japonica | 173 | Rhododendron simsii |
| 74 | Firmiana platanifolia | 174 | Sabina chinensis |
| 75 | Forsythia viridissima | 175 | Sabina procumbens |
| 76 | Fortunella margarita | 176 | Salix babylonica |
| 77 | Ginkgo biloba | 177 | Salix magnifica |
| 78 | Hedera nepalensis | 178 | Salix matsudana |
| 79 | Heliciopsis terminalis | 179 | Sapindus mukorossi |
| 80 | Hemerocallis fulva | 180 | Sasa fortunei |
| 81 | Hibiscus mutabilis | 181 | Scirpus validus |
| 82 | Hypericum monogynum | 182 | Solidago canadensis |
| 83 | Ilex | 183 | Sophora japonica |
| 84 | Ilex corallina | 184 | Spiraea cantoniensis |
| 85 | Ilex cornuta | 185 | Spiraea salicifolia |
| 86 | Illicium verum | 186 | Spiraea thunbergii |
| 87 | Inula japonica | 187 | Taxodium ascendens |
| 88 | Iris tectorum | 188 | Taxus baccata |
| 89 | Jasminum mesnyi | 189 | Ternstroemia gymnanthera |
| 90 | Jasminum nudiflorum | 190 | Thalia dealbata |
| 91 | Juniperus procumbens | 191 | Trachycarpus fortunei |
| 92 | Kalimeris indica | 192 | Typha orientalis |
| 93 | Lagerstroemia | 193 | Ulmus pumila |
| 94 | Lagerstroemia indica | 194 | Viburnum macrocephalum |
| 95 | Laurus nobilis | 195 | Viburnum odoratissinum |
| 96 | Ligustrum lucidum | 196 | Vinca major |
| 97 | Ligustrum obtusifolium | 197 | Weigela florida |
| 98 | Liquidambar formosana | 198 | Wisteria |
| 99 | Liriodendron chinense | 199 | Wisteria sinensis |
| 100 | Liriope cymbidiomorpha | 200 | Ziziphus jujuba |
Appendix D – List of pests that can potentially cause an effect not further assessed
Table D.1: List of potential pests not further assessed
| Pest name | EPPO Code | Group | Pest present in China | Present in the EU | Pinus parviflora/Pinus thunbergii confirmed as a host (reference) | Pest can be associated with the commodity | Impact | Justification for inclusion in this list |
|---|---|---|---|---|---|---|---|---|
| Dioryctria pryeri | DIORPR | Insects | Yes | No | Pinus thunbergii (EFSA PLH Panel, 2019b; Dossier Section 4.0) | Yes | No data | There is no information about the impact. Uncertainty on the host status of P. parviflora. |
| Dioryctria rubella | DIORRU | Insects | Yes | No | Pinus thunbergii (Zhao et al., 2015; Dossier Section 4.0) | Yes | No data | There is no information about the impact. Uncertainty on the host status of P. parviflora. |
| Earliella scabrosa (synonym: Trametes sanguinea) | PYCPSA | Fungi | Yes | No | Pinus parviflora var. pentaphylla, Pinus thunbergii (Nagatomo, 1964) | Yes | No data | There is no information about the impact. |
| Glaucias subpunctatus | GLAUSU | Insects | Yes | No | Pinus thunbergii (EFSA PLH Panel, 2019b) | Yes | No data | There is no information on impact. |
| Hydnoporia yasudae (synonym: Hymenochaete yasudai) | HYDIYA | Fungi | Yes (one report) | No | Pinus thunbergii (Farr and Rossmann, online) | Yes | No data | There is no information about the impact. |
| Lophodermium kumaunicum | LOPHKU | Fungi | Yes | No | Pinus thunbergii (Dossier Section 4.0; Farr and Rossmann, online) | Yes | No data | There is no information about the impact. |
| Lophodermium pini‐excelsae | LOPHPX | Fungi | Yes | Restricted (Italy) | Pinus parviflora (Dossier Section 4.0; Farr and Rossmann, online) | Yes | No data | There is no information about the impact. |
| Poria cocos (synonym: Wolfiporia cocos) | WOLFCO | Fungi | Yes | No | Pinus thunbergii (Farr and Rossmann, online) | Yes | No data | There is no information about the impact. |
| Proiectus parviflis | POIEPA | Mites | Yes | No | Pinus parviflora (Zuo et al., 2017) | Yes | No data | There is no information about the impact. |
| Setoptus semiornatum | SETPSE | Mites | Uncertain | No | Pinus parviflora var. pentaphylla (Pye, 2011) | Yes | Yes | Damage and possibility of travelling with the commodity confirmed in the paper of Pye (2011). There is uncertainty about the presence in China (it is very likely that it is present since it is in Japan). |
Appendix E – Excel file with the pest list of Pinus parviflora and Pinus thunbergii
Appendix E can be found in the online version of this output (in the ‘Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2022.7077#support‐information‐section
Supporting information
Excel file with the pest list of Pinus parviflora and Pinus thunbergii
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F and Gonthier P, 2022. Scientific Opinion on the commodity risk assessment of bonsai plants from China consisting of Pinus parviflora grafted on Pinus thunbergii . EFSA Journal 2022;20(2):7077, 301 pp. 10.2903/j.efsa.2022.7077
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00277
Panel members: Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA Panel on Plant Health wishes to thank Světla Kozelská for the support during the whole process of the Scientific Opinion development.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 2: Provided by the Chinese National Plant Protection Organisation; Figure 3: © 2018 Google
Adopted: 16 December 2021
Note
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) No 228/2013, (EU) No 652/2014 and (EU) No 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC, OJ L 317/4, 23.11.2016.
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019, OJ L 319, 10.12.2019, p. 1–279.
EFSA Panel on Plant Health, 2019. Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. https://doi.org/10.2903/j.efsa.2019.5667
Commission Decision 2002/887/EC of 8 November 2002 authorising derogations from certain provisions of Council Directive 2000/29/EC in respect of naturally or artificially dwarfed plants of Chamaecyparis Spach, Juniperus L. and Pinus L., originating in Japan. OJ L 309, 12.11.2002, p. 8.
Commission Implementing Regulation (EU) 2020/1217 of 25 August 2020 on a derogation from Implementing Regulation (EU) 2019/2072 concerning the introduction into the Union of naturally or artificially dwarfed plants for planting of Chamaecyparis Spach, Juniperus L. and certain species of Pinus L., originating in Japan, and repealing Decision 2002/887/EC, OJ L 277/6, 26.8.2020.
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Protocol on Ireland/Northern Ireland in conjunction with Annex 2 to that Protocol, for the purposes of this Opinion, references to Member States include the United Kingdom in respect of Northern Ireland.
Commission Implementing Regulation (EU) (EU) 2018/2018 of 18 December 2018 laying down specific rules concerning the procedure to be followed in order to carry out the risk assessment of high risk plants, plant products and other objects within the meaning of Article 42(1) of Regulation (EU) 2016/2031 of the European Parliament and of the Council. OJ L 323, 19.12.2018, p. 7–9.
References
- CABI (Centre for Agriculture and Bioscience International) , online. CABI Crop Protection Compendium. Available online: https://www.cabi.org/cpc/ [Accessed: 23 July 2021].
- Chen S, Wong J and Wu W, 2014. Armored scale insects (Hemiptera: Coccoidea: Diaspididae) intercepted from imported plant products to Taiwan. Journal of Taiwan Agricultural Research, 63, 129–142. [Google Scholar]
- Choo HY, Harry KK and Shea P, 1989. Parasitylenchus orthotomici sp. n. (Tylenchida: Allantonematidae) from Orthotomicus angulatus (Coleoptera: Scolytidae), with note on Parasitism. Korean Journal of Applied Entomology, 28, 1–3. [Google Scholar]
- Devkota B and Schmidt GH, 1990. Larval development of Thaumetopoea pityocampa (Den. & Schiff.) (Lep., Thaumetopoeidae) from Greece as influenced by different host plants under laboratory conditions 1. Journal of Applied Entomology, 109, 321–330. [Google Scholar]
- DG SANTE (Directorate‐General for Health and Food Safety, European Commission) , 2016. Final report of an audit carried out in China from 01 December 2015 to 11 December 2015 in order to evaluate the system of official controls for the export of seeds and plants for planting to the European Union. DG(SANTE) 2015‐7645 – MR, 39 pp.
- EFSA (European Food Safety Authority) , 2020. Pest survey card on Pissodes cibriani, P. fasciatus, P. nemorensis, P. nitidus, P. punctatus, P. strobi, P. terminalis, P. yunnanensis and P. zitacuarense . EFSA Supporting Publication 2020;EN‐1910, 35 pp. 10.2903/sp.efsa.2020.EN-1910 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2019a. Guidance on commodity risk assessment for the evaluation of high risk plants dossiers. EFSA Journal 2019;17(4):5668, 20 pp. 10.2903/j.efsa.2019.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2019b. Commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. 10.2903/j.efsa.2019.5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018. Scientific Opinion on the principles and methods behind EFSA’s Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;6(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , 2014. Pest Risk Analysis for Oemona hirta (revised). EPPO, Paris. pp. 1–84. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , 2020. EPPO Technical Document No. 1081, EPPO Study on the risk of bark and ambrosia beetles associated with imported non‐coniferous wood, EPPO Paris. Available online: https://www.eppo.int/RESOURCES/eppo_publications [Accessed: 23 July 2021].
- EPPO (European and Mediterranean Plant Protection Organization) , online. EPPO Global Database. Available online: https://gd.eppo.int/ [Accessed: 23 July 2021].
- Esaki K, 1996. Emergence patterns and host wood diameter preference of seven cerambycid beetle species emerging from Pinus parviflora dead branches. Elytra, 24, 383–387. [Google Scholar]
- EUROPHYT (European Union Notification System for Plant Health Interceptions) , online. Available online: https://ec.europa.eu/food/plants/plant‐health‐and‐biosecurity/European‐union‐notification‐system‐plant‐health‐interceptions‐en [Accessed: 17 June 2021].
- FAO (Food and Agriculture Organization of the United Nations) , 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations) , 2017. ISPM (International standards for phytosanitary measures) No. 5. Glossary of phytosanitary terms. FAO, Rome. Available online: https://www.ippc.int/en/publications/622/
- FAO (Food and Agriculture Organization of the United Nations) , 2018. ISPM (International standards for phytosanitary measures) No. 15, Regulation of wood packaging material in international trade. FAO, Rome. Available online: https://www.fao.org/3/mb160e/mb160e.pdf
- FAO (Food and Agriculture Organization of the United Nations) , 2019. ISPM (International standards for phytosanitary measures) No. 36, Integrated measures for plants for planting. FAO, Rome. Available online: https://www.fao.org/3/k8114e/K8114E.pdf
- Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars‐grin.gov/fungaldatabases [Accessed: 23 July 2021].
- Fetykó K and Kozár F, 2012. Records of Ceroplastes Gray 1828 in Europe, with an identification key to species in the Palaearctic Region. Bulletin of Insectology, 65, 291–295. [Google Scholar]
- Gómez D, Martinez G and Beaver RA, 2012. First record of Cyrtogenius luteus (Blandford) (Coleoptera: Curculionidae: Scolytinae) in the Americas and its distribution in Uruguay. The Coleopterists Bulletin, 66, 362–364. [Google Scholar]
- Google Earth , online. Geographical coordinates of the nursery: 120°7' E 30°25' N, Imagery date: 4/18/18. Available online: https://earth.google.com/web/search/30.416870229580535,+120.116677426603/@30.41763162,120.11677027,3.7938463a,1497.57228051d,35y,0h,0t,0r/data=CmgaPhI4GU6tYwG4aj5AIatLfKR3B15AKiQzMC40MTY4NzAyMjk1ODA1MzUsIDEyMC4xMTY2Nzc0MjY2MDMYASABIiYKJAmu‐LD1AcdIQBGS2tPjhMJIQBnoLFDg_GgyQCEevfxPMVoyQA [Accessed: 5 August 2021].
- Hoebeke ER and Rabaglia RJ, 2008. Xyleborus seriatus Blandford (Coleoptera: Curculionidae: Scolytinae), an Asian ambrosia beetle new to North America. Proceedings of the Entomological Society of Washington, 110, 470–476. 10.4289/07-048.1 [DOI] [Google Scholar]
- Jansen MGM, 1995. Scale insects (Homoptera: Coccinea) from import interceptions and greenhouses in the Netherlands. Israel Journal of Entomology, 29, 131–146. [Google Scholar]
- Jin L, 1989. Pissodes nitidus Roelofs, the yellow‐spotted pine weevil (Coleoptera: Curculionidae): a serious pest of Korean pine plantations in northeast China. In: Alfaro RI and Glover SG (eds.). Insects Affecting Reforestation: Biology and Damage. Forestry Canada, Pacific Forestry Centre, Victoria, British Columbia. pp. 186–193. [Google Scholar]
- Koo C‐D, Lee H‐Y, Han J‐H, Sung J‐H and Shin J‐H, 2013. Infection behavior and distribution of Bursaphelenchus xylophilus in Pinus densiflora trees. Forest Science and Technology, 9, 81–86. 10.1080/21580103.2013.801168 [DOI] [Google Scholar]
- Kosztarab M and Kozár F, 1988. Scale Insects of Central Europe, Akadémiai Kiadó, Budapest, 456 pp. [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of Köppen‐Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Lei GL, Duan ZY, Feng ZW and Zheng Z, 2003. Pest risk analysis of Pissodes punctatus Langer Situ et Zhang. Journal of Northeast Forestry University, 31, 62–63 (in Chinese with English abstract). [Google Scholar]
- Malumphy C, 2010. The status of wax scales (Hemiptera: Coccidae: Ceroplastinae) in Britain. Entomologists Monthly Magazine, 146, 105–112. [Google Scholar]
- Malumphy C, Halstaed AJ and Salisbury A, 2012. First incursion of Chinese mussel scale Lepidosaphes chinensis (Hemiptera: Diaspididae) in Europe, with a review of Lepidosaphes species found in Britain. British Journal of Entomology and Natural History, 25, 65–74. [Google Scholar]
- Nagatomo I, 1964. On Trametes sanguinea (L. ex FR.) Lloyd causing the white‐rot of coniferous and broad‐leaved woods. Bulletin of the Kyoto Gakugei University Series B Mathematics and Natural Science, 25, 45–70 (in Japanese). [Google Scholar]
- NVWA (Nederlandse Voedsel en Warenautoriteit) , 2020. Quick scan answer for Setoptus sp. on Pinus parviflora . Available online: https://english.nvwa.nl/topics/pest‐risk‐analysis/documents/plant/plant‐health/pest‐risk‐analysis/documents/quick‐scan‐answer‐for‐setoptus‐sp.‐on‐pinus‐parviflora [Accessed: 2 July 2021].
- Pye DR, 2011. A new species of eriophyoid mite (Acari: Eriophyoidea: Phytoptidae) from Japan, causing damage to Pinus parviflora var. pentaphylla . International Journal of Acarology, 37, 122–130. [Google Scholar]
- Quintero TG, 2015. New pest response guidelines. Pseudocercospora pini‐densiflorae (Hori & N. Nambu) Deighton. Brown Needle Fungus. USDA, Forest Service. 91 pp.
- Schönfeld U, 2015. Coccoidea species in Brandenburg. Journal Für Kulturpflanzen, 67, 337–341 (in German). [Google Scholar]
- Stastny M, Battisti A, Petrucco‐Toffolo E, Schlyter F and Larsson S, 2006. Host‐plant use in the range expansion of the pine processionary moth. Thaumetopoea Pityocampa. Ecological Entomology, 31, 481–490. [Google Scholar]
- TRACES‐NT , online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 17 June 2021].
- USDA (United States Department of Agriculture) , 1939. List of intercepted plant pests, 1937 (List of pests recorded during the period July 1, 1936, to June 30, 1937, inclusive, as intercepted in, on, or with plants and plant products entering United States territory. US. 60 pp.
- USDA (United States Department of Agriculture) , 2016. Japanese Beetle Program Manual. 78 pp. Available online: https://www.aphis.usda.gov/import_export/plants/manuals/domestic/downloads/japanese_beetle.pdf [Accessed: 28 July 2021].
- Wang Z, Liu Y, Wang H, Meng X, Liu X, Decock C, Zhang X and Lu Q, 2020. Ophiostomatoid fungi associated with Ips subelongatus, including eight new species from northeastern China. IMA Fungus, 11, 1–29. 10.1186/s43008-019-0025-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SL and Bright DE, 1992. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: taxonomic index. Great Basin Naturalist Memoirs, 13, 1–1553. [Google Scholar]
- Xu YM and Zhao ZQ, 2019. Longidoridae and Trichodoridae (Nematoda: Dorylaimida and Triplonchida). Fauna of New Zealand, 79, 149. [Google Scholar]
- Zhang H, Ye H, Haack RA and Langor DW, 2004. Biology of Pissodes yunnanensis (Coleoptera: Curculionidae), a pest of Yunnan pine in southwestern China. The Canadian Entomologist, 136, 719–726. [Google Scholar]
- Zhao Z‐P, Ji B‐Z, Liu S‐W, Wang L‐P and Xu Z‐X, 2015. Selective preferences of Placusa pinearum to volatiles of pine shoot. Journal of Environmental Entomology, 37, 1118–1023. 10.3969/j.issn.1674-0858.2015.05.13 [DOI] [Google Scholar]
- Zuo Y, Wang Q and Xue X‐F, 2017. Three new eriophyoid mite species (Eriophyidae: Phyllocoptinae) from Nanling Mountain, China. International Journal of Acarology, 43, 22–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel file with the pest list of Pinus parviflora and Pinus thunbergii


